Open Access Article
Open Access ArticleComment on “Study of enantioselective metolachlor adsorption by activated carbons” by A. Gomis-Berenguer, I. Laidin, S. Rononcial and B. Cagnon, RSC Advances, 2020, 10, 40321
Diego D. Colasurdoa,
Javier G. Carrerasab and
Sergio L. Laurella*a
aCEDECOR (Centro de Estudio de Compuestos Orgánicos), Facultad de Ciencias Exactas, Universidad Nacional de La Plata (UNLP), Calle 115 y 47, (1900) La Plata, Argentina. E-mail: sllaurella@quimica.unlp.edu.ar
bCONICET (Consejo Nacional de Investigaciones Científicas y Técnicas), Argentina
First published on 31st March 2023
Abstract
A recent study on adsorption of metolachlor on activated carbons carried out by Gomis-Berenguer et al. has reported higher adsorption capacity for pure S-metolachlor compared with the adsorption capacity for the racemic mixture of this pesticide. The authors claim that the adsorption is enantioselective, with the activated carbon being more efficient for the adsorption of the S enantiomer compared with the R enantiomer. In this comment, we question this explanation (since the non-chiral surface of an activated carbon would never be selective towards one enantiomer instead of the other) and we offer some possible answers supported by theoretical calculations.
1. Comment
In this comment to Gomis-Berenguer et al.,1 we criticize some conclusions drawn from the experimental data regarding the claimed selectivity of the adsorbents (activated carbons) towards S-metolachlor. The authors studied the adsorption of the pesticide metolachlor on four different activated carbons under three different formulations: 100% S-metolachlor, 60% S-metolachlor (40% R-metolachlor) and racemic R/S-metolachlor. Adsorption kinetics and isotherms were evaluated in batch experiments. The authors found that in the case of two carbons (called L27 and AQ630), the adsorption capacity raises in the sequence racemic < 60%S < 100%S. This behavior is explained in terms of enantioselectivity: “the S metolachlor could adopt the adequate conformation to be grater retained in the mesoporous surface, compared with the R-metolachlor, favouring the dispersive interactions between the herbicide and the activated carbon surface and increasing the adsorption capacity”. This statement is wrong. The only thing proven by the experimental data is that the adsorption capacity obtained for the S enantiomer is greater than the adsorption capacity for the racemic, not for the R enantiomer. Activated carbon surfaces are non-chiral (at least to the best of our knowledge, since there are no reports of chiral carbon materials), and the question here is why a non-chiral surface would have greater affinity for one enantiomer over the other. The only way to verify the enantioselectivity of the surface towards S-metolachlor would be carrying out the adsorption experiments with 100% S- and 100% R-metolachlor.2. Possible explanations
Now supposing that the differences observed in adsorption capacity are not due to a difference in affinity of S vs. R enantiomers but to a difference between pure enantiomer vs. racemic, the question is why this difference exists. Several explanations are possible, and we will consider them one by one.2.1. Diastereomeric ratios
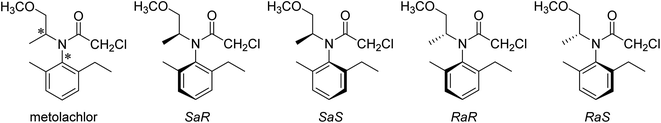
The first explanation requires a more detailed consideration on the structure of metolachlor. This molecule has two chiral elements: a stereocenter (that can be whether S or R) and a chiral axis (whose possible configuration will be called aS and aR). They are both marked with asterisks in Scheme 1, where the four possible stereoisomers are depicted.The four isomers are not interconvertible at room temperature in aqueous solution since the rotation energy barrier of the N-aryl axis is considerably high (near 37 kcal mol−1).2 Although the interconversion aS ↔ aR has only been observed in very specific solvent mixtures,3 in aqueous solution the four stereoisomers are stable and they have even been quantified by HPLC-MS.4 So, when we talk about S-metolachlor, we have to be aware that in fact we have a mixture of SaS and SaR diastereomers. Analogously, R-metolachlor is a mixture of RaR and RaS isomers.
The adsorption behaviors of SaS and RaR isomers are identical since they are enantiomers (and cannot have differences towards a non-chiral surface), and so will be the case of SaR and RaS for the same reason. Here we could find a possible explanation to an enantioselectivity if the diastereomers ratio SaS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SaR and RaR
SaR and RaR![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) RaS were different.
RaS were different.
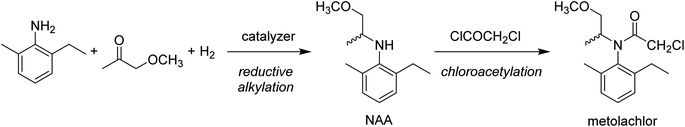
In order to consider the possibility of such a difference, we have to take account of the synthesis of metolachlor. In the last step shown in Scheme 2, a chiral N-alkyl aniline (NAA) is acetylated by chloroacetyl chloride in toluene.
Some industries produce metolachlor from racemic NAA, and they therefore commercialize the product as racemic metolachlor. Some others use an enantioselective process to obtain S-NAA and they so produce S-metolachlor.5
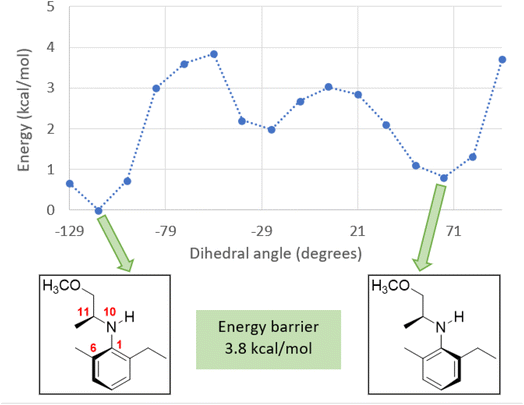
We have carried out an energy scan for the rotation of the N-aryl axis in S-NAA, finding two conformers of minimum energy. The theoretical calculations were carried out using the Gaussian 03 package6 at the DFT level with the hybrid B3LYP exchange and correlation functional and the 6-31G(d) basis set. The energy scan was performed around the dihedral angle C6–C1–N10–C11 (the atom numbering is shown in Fig. 1), taking 24 steps of 15° each and considering the effect of solvent (toluene) by means of the self-consistent reaction field (SCRF)-polarizable continuum model (PCM) version of the polarization continuum model.7
When acetylated, one conformer leads to SaS-metolachlor, and the other produces the SaR isomer. The energy barrier between the two most stable conformers of NAA is 3.8 kcal mol−1 in toluene (Fig. 1), suggesting a fast rotation around the N-aryl axis in NAA and a fast equilibrium between the two conformers. This fact leads to the conclusion that S-NAA and R-NAA exist in two mirrored conformational equilibria around the N-aryl axis, which would produce metolachlor in identical proportions SaS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SaR and RaR
SaR and RaR![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) RaS.
RaS.
In fact, these two proportions (SaS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SaR and RaR
SaR and RaR![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) RaS) have been observed and also calculated to be identical and close to 69
RaS) have been observed and also calculated to be identical and close to 69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31.3
31.3
In conclusion: if the proportions SaS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SaR and RaR
SaR and RaR![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) RaS are equal, then the ratio of diastereomers (SaS + RaR)
RaS are equal, then the ratio of diastereomers (SaS + RaR)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (SaR + RaS) is constant and we cannot explain the difference in adsorption claiming that R and S-metolachlor diastereomers are adsorbed in a different way.
(SaR + RaS) is constant and we cannot explain the difference in adsorption claiming that R and S-metolachlor diastereomers are adsorbed in a different way.
2.2. Racemic effects
Therefore, it is clear that the difference in adsorption capacity between S-metolachlor and racemic metolachlor is not due to enantioselectivity but to some effect between the stereoisomers present in the racemic mixture. This S–R effect could exist in solution, on the surface, or in both sites.An enantiomer interaction in solution could be conceivable, and we have considered possible configurations (by means of theoretical calculations) in order to find some strong interaction between R and S metolachlor, even including water molecules (as we have done in similar systems8), but we could not find any specific low-energy array that justify such a hypothesis.
On the other hand, theoretical calculations on the adsorption of metolachlor (and other pesticides) on activated carbons9 show that R- and S-metolachlor have a preferred orientation towards the surface when being adsorbed. Fig. 2 shows a top and side view for adsorbed SaS and RaR isomers (the other two are quite similar and do not affect the analysis). It is clear that, when seen from above, the two enantiomers give mirror images that are not superimposable.
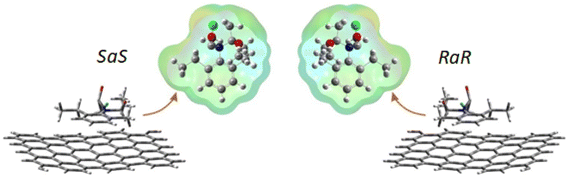 | ||
| Fig. 2 Minimum energy adsorption orientations for metolachlor.7 | ||
The adsorption of pure S-metolachlor (where all molecules are orientated in the same way) could have a more efficient packing, thus resulting in higher adsorption capacity. The adsorption of an R/S mixture results in a less efficient array (Fig. 3 gives a schematic explanation about this point).
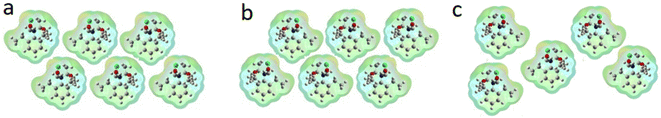 | ||
| Fig. 3 Schematic comparison of packing efficiency of S-metolachlor (a), R-metolachlor (b) and racemic metolachlor (c). Racemic molecules adsorb in a less packing-efficient array. | ||
But it is clear that adsorption of R-metolachlor molecules should give (exactly) the same adsorption capacity than S-metolachlor, and then there would not be enantioselectivity between S- and R-isomers of this pesticide in the adsorption on activated carbon.
3. Conclusions
Based on the discussions, we suggest that the differences observed between the (higher) adsorption capacity of S-metolachlor and the (lowest) adsorption capacity of racemic metolachlor is not due to enantioselectivity but to a less efficient packing of adsorbed molecules in the racemic mixture.Conflicts of interest
There are no conflicts to declare.References
- A. Gomis-Berenguer, I. Laidin, S. Renoncial and B. Cagnon, RSC Adv., 2020, 10, 40321–40328 RSC.
- H. Moser, G. Rihs and H. Sauter, Z. Naturforsch., 1982, 87, 451–462 CrossRef.
- S. Jayasundera, W. Schmidt, C. Hapeman and A. Torrens, J. Agric. Food Chem., 1999, 47, 4435–4447 CrossRef CAS PubMed.
- T. Poiger, M. Müller and H. Buser, Chimia, 2002, 56, 300–303 CrossRef CAS.
- H. Blaser, Adv. Synth. Catal., 2002, 344, 17–31 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. B. Scuseria, M. A. Robb, J. R. Cheeseman, J. A. Montgomery Jr., T. Vreven, K. Kudin, J. Burant, J. Millam, S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G. Petersson, H. Nakatsuji, M. Hada, M. Ehara, R. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J. Knox, H. Hratchian, J. Cross, C. Adamo, J. Jaramillo, R. Gomperts, R. Stratmann, A. Yazyev, R. Austin, C. Cammi, J. W. Pomelli, P. Y. Ochterski, K. Ayala, G. A. Morokuma, P. Voth, J. J. Salvador, V. G. Dannenberg, S. Zakrzewski, A. D. Dapprich, M. C. Daniels, O. Strain, D. K. Farkas, A. D. Malick, K. Rabuck, J. B. Raghavachari, J. V. Foresman, Q. Ortiz, A. G. Cui, S. Baboul, J. Clifford, B. B. Cioslowski, G. Stefanov, A. Liu, P. Liashenko, I. Piskorz, R. L. Komaromi, D. J. Martin, T. Fox, M. A. Keith, C. Y. Al-Laham, A. Peng, M. Nanayakkara, P. M. W. Challacombe, B. Gill, W. Johnson, M. W. Chen, C. Wong, R. Gonzalez and J. A. Pople, Gaussian 03, Revision B.03, Gaussian, Inc., Pittsburgh PA, 2003 Search PubMed.
- M. T. Cancs, B. Mennucci and J. Tomasi, J. Chem. Phys., 1997, 107, 3032–3041 CrossRef.
- M. Caputo, D. Colasurdo, P. Allegretti and S. Laurella, J. Phys. Org. Chem., 2020, 33, e4108 CrossRef CAS.
- M. Pila, D. Colasurdo, S. Simonetti, G. Dodero, P. Allegretti, D. Ruiz and S. Laurella, Korean Chem. Eng. Res., 2023, 61, 97–108 CAS.
| This journal is © The Royal Society of Chemistry 2023 |