Open Access Article
Open Access ArticleUnveiling the effect of crystallinity and particle size of biogenic Ag/ZnO nanocomposites on the electrochemical sensing performance of carbaryl detection in agricultural products†
Nguyen Le Nhat Trang a,
Dao Thi Nguyet Nga
a,
Dao Thi Nguyet Nga a,
Lemma Teshome Tufae,
Van Tan Tranc,
Thuan-Tran Hungd,
Vu Ngoc Phanac,
Tuyet Nhung Pham
a,
Lemma Teshome Tufae,
Van Tan Tranc,
Thuan-Tran Hungd,
Vu Ngoc Phanac,
Tuyet Nhung Pham a,
Van-Tuan Hoang
a,
Van-Tuan Hoang *a and
Anh-Tuan Le
*a and
Anh-Tuan Le *ab
*ab
aPhenikaa University Nano Institute (PHENA), Phenikaa University, Hanoi 12116, Vietnam. E-mail: tuan.hoangvan@phenikaa-uni.edu.vn; tuan.leanh@phenikaa-uni.edu.vn
bFaculty of Materials Science and Engineering (MSE), Phenikaa University, Hanoi 12116, Vietnam
cFaculty of Biotechnology, Chemical and Environmental Engineering (BCEE), Phenikaa University, Hanoi 12116, Viet Nam
dCenter for Advanced Materials and Environmental Technology, National Center for Technological Progress, Hanoi 12116, Viet Nam
eDepartment of Chemical Engineering and Applied Chemistry, Chungnam National University, Daejeon, 34134, Republic of Korea
First published on 15th March 2023
Abstract
In this study, bio-Ag/ZnO NCs were synthesized via a microwave-assisted biogenic electrochemical method using mangosteen (Garcinia mangostana) peel extract as a biogenic reducing agent for the reduction of Zn2+ and Ag+ ions to form hybrid nanoparticles. The as-synthesized NC samples at three different microwave irradiation temperatures (Z70, Z80, Z90) exhibited a remarkable difference in size and crystallinity that directly impacted their electrocatalytic behaviors as well as electrochemical sensing performance. The obtained results indicate that the Z90 sample showed the highest electrochemical performance among the investigated samples, which is attributed to the improved particle size distribution and crystal microstructure that enhanced charge transfer and the electroactive surface area. Under the optimal conditions for carbaryl pesticide detection, the proposed nanosensor exhibited a high electrochemical sensitivity of up to 0.303 μA μM−1 cm−2 with a detection limit of LOD ∼0.27 μM for carbaryl pesticide detection in a linear range of 0.25–100 μM. Overall, the present work suggests that bio-Ag/ZnO NCs are a potential candidate for the development of a high-performance electrochemical-based non-enzymatic nanosensor with rapid monitoring, cost-effectiveness, and eco-friendly to detect carbaryl pesticide residues in agricultural products.
1. Introduction
Carbaryl (1-naphthyl methylcarbamate, C12H11NO2) is the main ingredient in many commercial pesticides that have been widely used in agriculture to increase crop yield and enhance quality. However, their excessive residue products in soil, fruits, vegetables, and water can cause many negative effects on human health and the environment. Especially, they inhibit the catalytic activity of acetylcholinesterase (AChE) in the central nervous system and cause serious effects on humans and animals.1,2 Because of the potential risks to people's health and animals, many countries have taken regulations on carbaryl limitation. For example, the carbaryl limit level in Europe is only in the concentration range from 0.01 mg kg−1 to 0.5 mg kg−1 for rice, fruits, and cereals. This allowable limit of Russia is from 0.02 mg kg−1 to 2.0 mg kg−1 in milk products, maize, and grains; and from 0.02 mg kg−1 to 25.0 mg kg−1 in olive oil.3 Hence, it is necessary to develop a sensitive and robust method to determine carbaryl levels in foods.Until now, many analytical methods have been developed for the detection of carbaryl in food, water, and drinks, including ultra-performance liquid chromatography with mass spectrometry (UPLC/MS),4 reaction-resonance scattering spectroscopy, gas chromatography-mass spectrometry (GC-MS),5 high-performance liquid chromatography (HPLC),6 colorimetry,7 fluorimetry,8 surface-enhanced Raman spectroscopy,9 and electrochemical methods.10 Among them, the electrochemical sensor has received significant attention for the detection of carbaryl, due to their low expense, easy use, fast response, and high sensitivity. In addition, electrochemical sensors are also convenient for on-site determination and point-of-care testing.11 For example, a novel electrochemical sensor based on graphene oxide-ionic liquid composite modified glassy carbon electrode (GCE) was used for the quick and ultra-sensitive detection of carbaryl with a detection limit (LOD) of 2 × 10−8 M.12 Glass carbon electrode (GCE) was modified by a new polyaniline/CoAl-layered double hydroxide composite (PANI/CoAl-LDH) with the carbaryl detection limit of 6.8 × 10−9 M.13 Reduced graphene oxide-wrapped silver nanoparticles (Ag@rGO) were utilized to modify the electrode surface with carbaryl being the detection target and the detection limit of 42 × 10−9 M.14 Electrocatalysts of Pd and ZnO nanoparticles (NPs) supported on a highly porous framework of activated carbons (APC) were synthesized for electrochemical detection of carbaryl with a detection limit of 10−8 M.15 Therefore, it is crucial to develop highly sensitive electrochemical sensors modified with eco-friendly materials to detect carbaryl.
Recently, the Ag/ZnO nanocomposites (Ag/ZnO NCs) were exposed as a candidate for the electrochemical nanosensor for organic agent trace detection.16–18 Particularly, ZnO nanoparticles (NPs) possess a range of desirable traits, including wide availability, non-toxicity, robust mechanical strength, high thermal stability, antifouling and antibacterial properties, high specific surface area, efficient catalytic performance, and strong adsorption capabilities.19,20 These properties make ZnO NPs an excellent choice for modifying working electrode surfaces in electrochemical sensors. However, ZnO NPs have poor charge transfer ability and rapid recombination of charge carriers, which are the main weak points of ZnO NPs for electrocatalysis applications.21 To address these challenges, researchers have employed several approaches, including doping, modification, and hybridization, etc. As an effective way, ZnO NPs were combined with noble metal NPs to form metal–metal oxide interfaces.22,23 The interesting studies of Ag/ZnO NCs have related to their morphology, size, component, and structure via some different synthesis methods, including bio-hydrothermal,15,24 sol–gel method,25 photo-chemical reduction,26 chem-hydrothermal method,27,28 and mechanochemical method.29 Almost them displayed the impressive enhancements in electrochemical sensors toward toxic organic compounds. Using the one-pot hydrothermal method, an electrochemical sensor based on Ag/ZnO NCs was developed onto the ITO electrode, with a low limit detection of phosphorothioate pesticides of 0.010 μM. In addition, the photocatalytic performance of Ag/ZnO NCs was demonstrated for the degradation of Phox, with higher performance than pure ZnO nanorods. Therefore, modifying electrodes with Ag/ZnO NCs shows promising potential for the electrochemical sensor and photocatalytic degradation of toxic pesticides in the food and environmental fields.30 N. Sebastian et al. have devoted to developing a sensitive electrochemical sensor based on silver-decorated zinc oxide (Ag/ZnO) material for the nanomolar-level detection of rhodamine B (RhB). The obtained result showed a low limit detection of 0.8 nM. Moreover, Ag/ZnO NCs was also found to be a remarkable photocatalyst for efficiently degrading RhB. The sensitive detection and degradation of dye are crucial to ensuring the safety of living being.31 A novel biosensor was also successfully developed to detect xanthine in both meat and fish samples corresponding to limit detection of 0.07 μM. The electrode was covered with nano Ag–ZnO, which played an important role in the performance of the biosensor by significantly increasing the porous structure of the conducting polymer (pyrrole) surface.32
In this work, for the first time, we reported a green approach for the synthesis of Ag/ZnO NCs via a combined microwave-assisted/biogenic electrochemical method. Biomolecules from mangosteen peel extract not only played the role of biogenic reducing agent for the reduction of Zn2+ and Ag+ ions to form ZnO and Ag nanoparticles but also acted as stabilizers. The present work has exposed eco-friendly, easy-to-scale-up, and cost-effective methods. More interestingly, by tuning particle size distribution and crystal microstructure of Ag/ZnO NCs, their systematic impacts on electrocatalytic activity, conductivity, and sensing performance in electrochemical sensors based on biogenic Ag/ZnO NCs were clarified. Finally, according to the proposed approach, a rapid electrochemical-based non-enzymatic nanoplatform was proposed for carbaryl detection in agricultural products.
2. Experimental section
2.1. Chemicals and apparatus
Pure silver (Ag) bars were used as working electrodes in the electrochemical process, which were 10 and 15 cm high, 3 and 8 mm thick, respectively, and were purchased from a domestic jewellery company in Hanoi, Vietnam. Cabarryl (CBR, 98%), nitric acid (HNO3, 63%), iron(III) nitrate (Fe(NO3)3, 99%), copper(II) sulfate (CuSO4,99%), zinc nitrate (Zn(NO3)2, 99%), nickel(II) nitrate (Ni(NO3)2, 99%), potassium chloride (KCl, 99%), ascorbic acid (C6H8O6, 99%), urea (CH4N2O, 99%) amoxicillin (C16H19N3O5S, 99%), and D-glucose (C6H12O6, 99%) were purchased from Shanghai Chemical Reagent. Also, the double-distilled water used in the whole process was prepared using a Milli-Q® system (18.2 MΩ cm at 25 °C).The phosphate-buffered saline (PBS) buffer solution (0.1 M, pH 7.2) was prepared by using NaCl, KCl, Na2HPO4·12H2O, and KH2PO4 (>99%, Merck KGaA, Germany). The pH of PBS buffer was adjusted by using H3PO4(0.1 M) and NaOH (2 M). All chemicals were used as received without any further purification. Carbon screen-printed electrodes (SPEs-DS110) were provided from DS Dropsens, Spain.
2.2. Synthesis of bio-Ag/ZnO NCs and bio-Ag/ZnO NCs-modified SPE
20 mL of plant extract, namely mangosteen (Garcinia mangostana) peel was added to a 200 mL beaker and stirred for 15 min. Two zinc (Zn) rods were placed 7 cm apart and dipped about 10 cm deep into the beaker used as the cathode and anode. A 20 V DC voltage source was applied to the electrodes. The electrochemical synthetic process was carried out for 2 h at room temperature without using any chemical additives. Similarly, the electrolysis process of the bare Ag rods was carried out. However, here the voltage supplied was about 10 V for 30 min. Next, the homogenized solution was subjected using UWave-2000 multifunctional microwave aiming to heating for 15 min with 250 W at different temperature conditions of 70 °C, 80 °C, and 90 °C, which were denoted as Z70, Z80, and Z90, respectively.All carbon screen-printed electrodes (SPEs) modified with bio-Ag/ZnO NCs were prepared by a simple dropping method. Briefly, all SPEs were washed several times with DI and ethanol, then dried at room temperature. Next, a solution of biological (bio-Ag/ZnO NCs) with various volumes from 4 to 12 μL was dripped onto the surface of the SPE working electrode and dried naturally in the air. The modified electrode was used for subsequent electrochemical experiments.
2.3. Preparation of real samples
Real samples were prepared from cabbage samples, which were purchased from the local supermarket. 3 g of the homogenized cabbage samples was carefully mixed with 10 mL of phosphate buffer (0.1 M) using a vortex mixer. Then, the obtained solution was sonicated for 30 min. The resulted solution was centrifuged for 5 min at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm and filtered through Whatman filter paper to get clear liquid phase. Finally, the known different amounts (10, 25, and 50 μM) of CBR standard were added to the obtained liquid phase.
000 rpm and filtered through Whatman filter paper to get clear liquid phase. Finally, the known different amounts (10, 25, and 50 μM) of CBR standard were added to the obtained liquid phase.
2.4. Characterization techniques
The crystalline characterization and structural properties of bio-Ag/ZnO NCs were analyzed by a Bruker D5005 X-ray diffractometer (using Cu Kα radiation, λ = 0.154056 nm) under a voltage of 40 kV and a current of 30 mA. The absorption spectra of bio-Ag/ZnO NCs was measured by ultraviolet-visible (UV-vis) spectrophotometry (HP 8453 spectrophotometer). The morphology of bio-Ag/ZnO NCs was characterized by high-resolution transmission electron microscopy (HR-TEM, JEOL, JEM-3010, Tokyo, Japan). Particle size distribution was analyzed by dynamic light scattering (DLS) measurement (Horiba SZ-100). pH value was determined by an IC-PH60 pH tester kit. In addition, all electrochemical measurements were conducted on a three-electrode electrochemical cell (Palmsens4, PS Trace, PalmSens, The Netherlands) at room temperature.2.5. Electrochemical measurements
The electrochemical properties of the bare electrode (bare SPE) and bio-Ag/ZnO NCs-modified electrodes were investigated via cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) techniques. CV measurements were carried out in 0.1 M KCl containing 5 mM [Fe(CN)6]3−/4− as redox probes at a scan rate of 50 mV s−1 within the potential range from −0.3 V to 0.6 V. While, electrochemical impedance spectroscopy (EIS) was recorded in the frequency range of 0.01–50 kHz by applying an AC voltage of 10 mV amplitude. Electrolyte of 0.1 M phosphate buffer solution (PBS, pH 7.4) was prepared by dissolving 8 g NaCl, 0.2 g KCl, 1.44 g Na2HPO4, and 0.245 g KH2PO4 into 1000 mL DI water under the aid of magnetic stirring for 30 min at room temperature. The electrochemical behaviors of CBR on the modified electrodes were investigated using CV and differential pulse voltammetry (DPV) measurements in phosphate buffer (0.1 M PBS) containing different concentrations of CBR. The CV measurements were carried out in the potential range between 0 and 1.0 V, Tequilibrium = 120 s. The DPV measurements were completed as follows: scan rate of 6 mV s−1, Tequilibrium = 5 s, Epluse = 0.05 V, and Tpulse = 0.2 s in the potential range of 0.0 to 0.8 V.3. Results and discussion
3.1. Characterization of bio-Ag/ZnO NCs
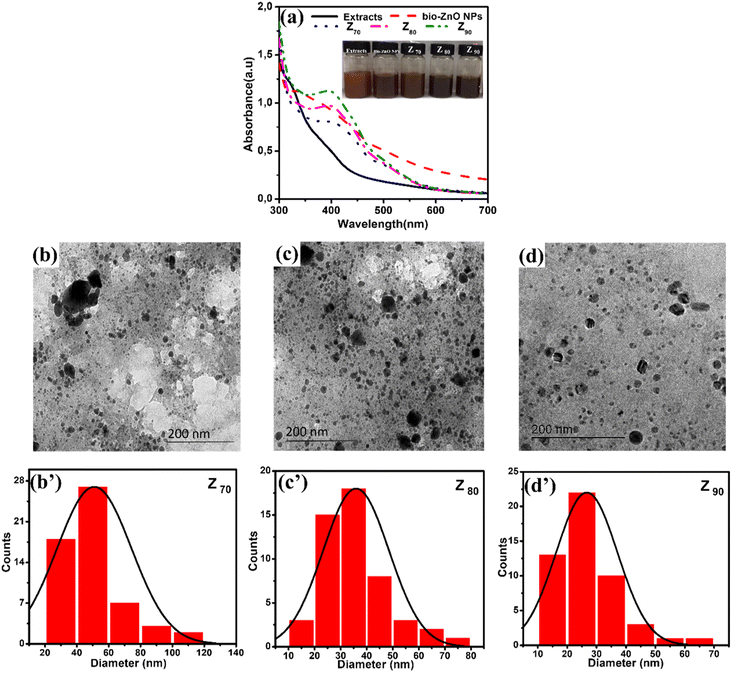
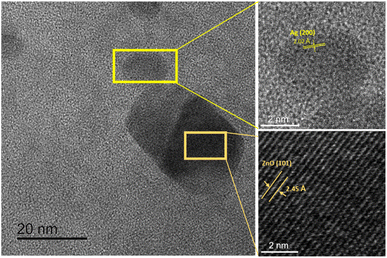
Bio-Ag/ZnO NCs were synthesized by a combined microwave-assisted/biogenic electrochemical method using mangosteen peel extracts. UV-vis spectroscopy and TEM were used to confirm the characteristic absorption peak and the morphology of bio-Ag/ZnO NCs (see Fig. 1). Fig. 1a shows the UV-vis spectra of the as-synthesized samples and the color of their solutions. After the electrochemical process, the color of the extracted mixture turned yellow-brown due to the formation of bio-Ag/ZnO NCs under microwave radiation. The UV-vis spectra of ZnO nanoparticles (NPs) showed a characteristic absorption peak centered at 334 nm, which is the characteristic peak for hexagonal wurtzite ZnO.33 The formation of Ag NPs was confirmed by the characteristic surface plasmon resonance absorption band at 400–420 nm in the UV-vis region. As the increase in microwave temperature, the absorption peak intensity increased clearly meanwhile the absorption peak wavelength remained unchanged.The morphology of bio-Ag/ZnO NCs could be viewed in Fig. 1b–d. It can be observed that NPs with different sizes were separately dispersed on the phytochemical matrix in the as-synthesized solution. The uniform near-spherical nanoparticle (average size of ∼20 nm) could correspond to Ag NPs. The others (average size of ∼50 nm) could correspond to bio-Ag/ZnO NCs due to Ag NPs decorated on ZnO NPs. The Z90 nanoparticles had small size, relatively uniform dispersion. However, Z70 and Z80 nanoparticles had large size, uneven distribution, and showed the phenomenon of agglomeration. The results of Fig. 1b’–d’ show that the nanoparticle size distribution of the samples Z70, Z80, Z90 the range of 20 to 80 nm, 20 to 50 nm and 10 to 40 nm, respectively. The particle size distribution of these composite samples is further confirmed through the results of DLS spectroscopy (Fig. S1†). For more convincing evidence, the result of the HRTEM image analysis was shown in Fig. 2. Two different contrasting bands can be found within the boundary of bio-Ag/ZnO NCs. The 2.45 Å inter-plane distance corresponds to the (101) plane of ZnO, while the 2.02 Å inter-plane distance was assigned to the (200) lattice plane of Ag. The observed result confirmed that bio-Ag/ZnO NCs have formed in the as-synthesized sample.
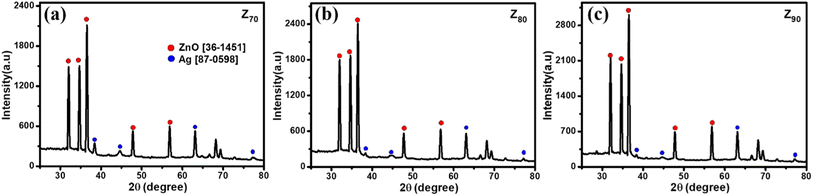
Fig. 3 shows the crystallographic microstructure and particle size distribution of bio-Ag/ZnO NCs. As shown in Fig. 3, all three bio-Ag/ZnO NCs samples exhibited the appearance of characteristic peaks at 31.7°, 34.4°, 36.2°, 47.5°, and 56.6° that fix well with (100), (002), (101), (102), and (110) planes, respectively, of hexagonal wurtzite structure of ZnO (JCPDS standard card no. 36-1451). Furthermore, the other diffraction peaks were also observed at around 37.2°, 40.5°, 54.8°, and 76.1°, revealing the presence of Ag, according to JCPDS (87-0598) within the bio-Ag/ZnO NCs crystalline lattice structure. The average crystal size of ZnO NPs was calculated by the Debye–Scherrer equation, corresponding to 44 nm, 32 nm, and 23 nm for Z70, Z80, and Z90, respectively. For Ag NPs, the average crystal size of sample Z70 was estimated to be 25 nm, the values of Z80 and Z90 samples were 21 nm and 15 nm, respectively. The temperature favored the crystallization of bio-Ag/ZnO NCs nanocrystals. Obviously, increasing microwave temperature decreased the particle size. Smaller particles produced at higher temperatures could be attributed to the higher supersaturation ratio of metal atoms in the solution at faster temperature ramp rates.34 Herein, the significant effect of the synthesis temperature on the size distribution was considered due to the impact of temperature on the motivation of ions within the solution. Indeed, at higher temperatures, the ions tend to move faster within the solution, leading to a low ability for collision and contact among ions. Thus, it reduces and inhibits the crystallization ability as well as the formation of particle size.35,36 Clearly, Z90 showed more unique features in not only size and crystallinity. Thus, Z90 was expected as a key candidate possessing a high potential for electrocatalytic activity and electron conductivity in the sensing electrode. Crystal structure and particle size distribution were the factors that were reported a decisive influence on their electrocatalytic activity, conductivity, and electrochemical sensing performance.37–39 This relationship was further studied and discussed in more detail in the following sections.
3.2. Electrochemical characteristics of the modified electrodes
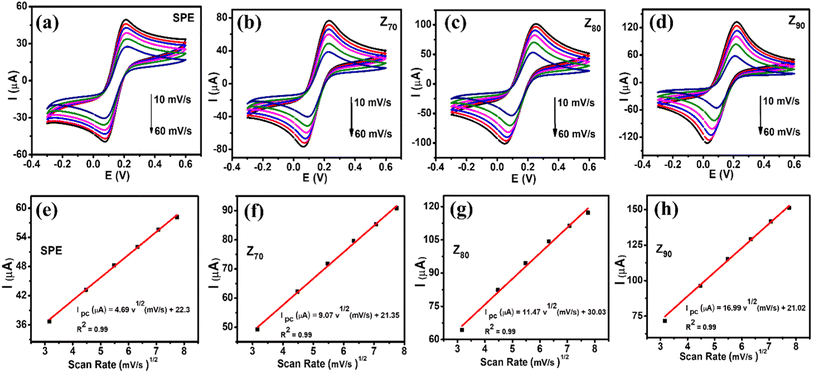
The electrochemical properties of the modified electrodes were investigated by CV and EIS measurements using a standard redox probe, [Fe(CN)6]3−/4−, aiming to explore the role of the crystal structure of bio-Ag/ZnO NCs on CBR electrochemical sensing performance. Fig. 4a shows the CV curves of bare SPE and modified electrodes (Z70/SPE, Z80/SPE, and Z90/SPE) in 0.1 M KCl containing 5 mM [Fe(CN)6]3−/4− at a scan rate of 60 mV s−1. The obtained CV curves exhibited a pair of oxidations–reduction peaks, corresponding to the electron transfer of Fe2+ reversibly into Fe3+. Notably, all the modified electrodes showed a remarkable enhancement in current response, compared to bare SPE. Indeed, the reduction peak currents were about 58.16 μA, 90.15 μA, 117.24 μA, and 151.2 μA for bare SPE, Z70/SPE, Z80/SPE, and Z90/SPE, respectively. Modified electrodes clearly exhibited higher electrochemical properties than the bare electrode. Among them, Z90/SPE had the highest reduction/oxidation current response, suggesting that crystal structure and particle size distribution of bio-Ag/ZnO NCs have a strong influence on the resistivity of the modified electrodes. Accordingly, the obtained results reveal that Z90/SPE promises a better electrochemical sensing performance than others.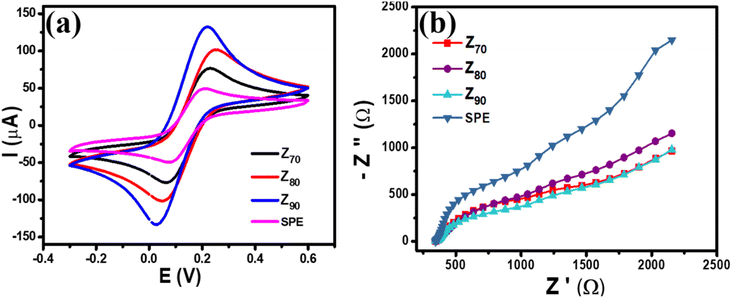 | ||
| Fig. 4 (a) CV profiles of various modified electrodes at scan rate 60 mV s−1 in 0.1 M KCl containing 5 mM [Fe(CN)6]3−/4− (b) EIS profiles of various modified electrodes. | ||
To investigate the charge transfer capacity of the variable electrodes, electrochemical impedance spectroscopy (EIS) measurements were performed. Nyquist plots of Z70/SPE, Z80/SPE, and Z90/SPE in 0.1 M KCl containing 5 mM [Fe(CN)6]3−/4− in the frequency range 50 kHz–0.01 Hz with amplitude 10 mV of a.c. voltage have been shown in Fig. 4b. For all electrodes, for the EIS measurements, the Rct values of SPE, Z70/SPE, Z80/SPE, and Z90/SPE were calculated of (731 ± 1.0) Ω, (620 ± 0.71) Ω, (473 ± 0.81) Ω, and (347 ± 0.9) Ω, respectively. Obviously, there is a notable decrease in Rct in modified electrodes with the smallest Rct of Z90/SPE. The decrease in Rct of smaller samples could be attributed to both higher volume fractions of smaller NPs packed inside the film and higher crystallinity which facilitated the charge transfer.
Fig. 5a–d shows CV curves recorded at bare SPE and modified electrodes in 0.1 M KCl containing 5 mM [Fe(CN)6]3−/4− with various scan rates from 10 to 60 mV s−1. It can be seen that the observed peak current increased with an increase in scan rate from 10 to 60 mV s−1. Their corresponding linear plots of current response (I, which is the average of three consecutive measurements I1, I2, I3 of the amperage value: I = (I1 + I2 + I3)/3) against the square root of scan rate were presented in Fig. 5e–h, suggesting the redox reaction onto all the electrode surfaces was a diffusion-controlled process.37,40 To further explain the enhancement of the current intensity, the electroactive surface area (EASA) value, which reflects the total electrochemically active area, was calculated based on the Randles–Sevick equation:
| Ip = 2.69 × 105An3/2D1/2Cν 1/2 | (1) |
3.3. Electrochemical sensing performance of the modified electrodes
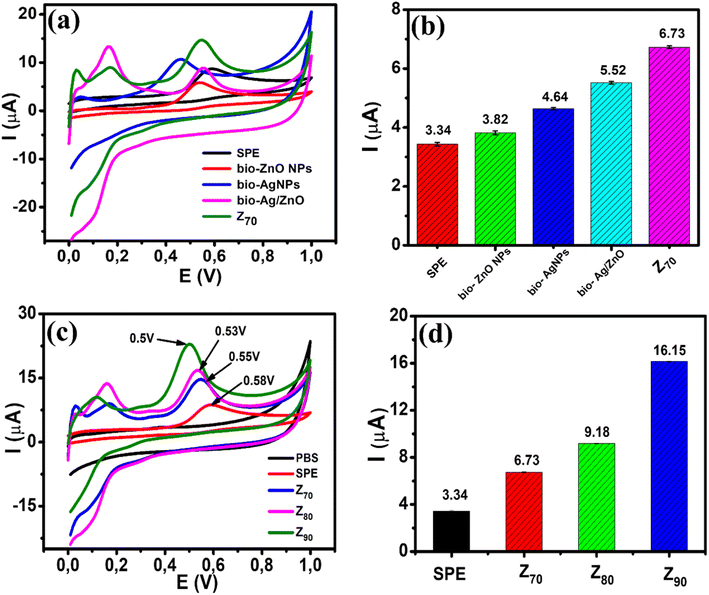
The electrochemical behavior of CBR at bio-ZnO NPs, bio-AgNPs, bio-Ag/ZnO, and Z70-modified electrodes in PBS buffer solution (pH 3) was considered by typical CV at a scan rate of 50 mV s−1 (Fig. 6a). It can be seen that the strongly increased oxidation peak occurs at 0.58 V for the SPE, 0.55 V for the three electrodes modified with bio-ZnO NPs, bio-Ag/ZnO, and Z70, particularly the lowest potential at 0.46 V for the electrode modified with bio-AgNPs. This peak could be assigned to the carbaryl oxidation which is consistent with some previous reports regarding the oxidation peak of CBR.12,41 Fig. 6b shows a bar plot of the irreversible oxidation peak current response for CBR for all modified electrodes. Indeed, the oxidation peak current response of CBR at bio-ZnO NPs (3.82 μA), bio-AgNPs (4.64 μA), bio-Ag/ZnO (5.52 μA), and Z70 (6.73 μA) was 1.1-fold, 1.4-fold, 1.7-fold and 2.0-fold higher as compared with bare SPE (3.34 μA), respectively. Among them, Z70 offered the highest anodic peak current compared with other modified electrodes. It is clear that the Z70 sample with microwave assistance enhanced significantly the amperage signal compared with the rest of the samples. Therefore, the Z70 sample was selected to conduct further experiments.To evaluate the electrochemical activity of different electrodes, the CV response of SPE and SPE modified with Z70/SPE, Z80/SPE, and Z90/SPE were measured in PBS buffer solution (pH 3.0) at a scan rate of 50 mV s−1. Fig. 6c shows the CV curves of all four electrodes in 0.1 M PBS (pH 3) containing 100 μM CBR. It can be seen that a sharp increase in oxidation peak occurred at 0.58 V, 0.55 V, 0.53 V, and 0.5 V for SPE, Z70/SPE, Z80/SPE, and Z90/SPE, respectively. For modified electrodes, the sharp oxidation peaks were recorded as the oxidation process of CBR at the potential. It is found that the oxidation peak potential on Z90 shifted to a more positive direction, compared to Z70 and Z80, consuming less energy required for the electrochemical oxidation of CBR. The difference in oxidation peak potential may be due to the change in crystallinity. Fig. 6d shows a bar chart diagram of irreversible oxidation peak current responses towards CBR for all three modified electrodes. Compared to the bare SPE, all the modified SPEs showed an increase in the oxidation peak current, indicating that the bio-nanocomposites significantly improved the electrochemical activity for CBR oxidation. Among them, Z90 offered the highest anodic peak current of about 16.15 μA, which is ∼1.8 times higher than Z80 (9.18 μA), 2.4 times higher than Z70 (6.73 μA), and 4.8 times higher than bare electrode (3.34 μA). It is clear that the size and crystallinity of Ag/ZnO NCs were important factors, affecting the electrochemical sensing performance for detecting CBR.
3.4. The effect of scan rate and pH
The influence of experimental parameters, including pH value, scanning speed, accumulation time, and electrode coating volume on the sensing performance were investigated and optimized. To understand the mechanism of electron transfer reaction occurring onto the modified electrode surface, the effect of scanning speed and pH value on the oxidation potential as well as the current response of CBR were investigated by CV measurements. Based on relationship between sweep rate and peak amperage or voltage response, kinetic studies calculated from CV measurements were performed. Fig. S2† illustrates the effect of different sweep rates (10–60 mV s−1) on the electrochemical behaviors of CBR in 0.1 M PBS (pH 3) containing 100 μM CBR for modified electrodes. Apparently, the increases in both anode and cathode peak currents were observed along with the increase in the scan rate for the investigated electrodes. Calibration graphs between CBR irreversible peak oxidation current and sweep rate were described with linear regression equations:| Z70: Ip (μA) = 0.14v (mV s−1) + 0.137; R2 = 0.99 |
| Z80: Ip (μA) = 0.18v (mV s−1) + 0.194; R2 = 0.99 |
| Z90: Ip (μA) = 0.29v (mV s−1) + 2.06; R2 = 0.99 |
Accordingly, CBR oxidation was a typical surface adsorption-controlled process rather than a diffusion process. More interestingly, the adsorption capacity of CBR on the electrode surface of Z90/SPE was also determined through the equation:42,43
| Ip = n2F2AνΓ/4RT | (2) |
Z70: Epa = 0.029![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ν (V s−1) + 0.634 (R2 = 0.99) ν (V s−1) + 0.634 (R2 = 0.99) |
Z80: Epa = 0.031![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ν (V s−1) + 0.627 (R2 = 0.99) ν (V s−1) + 0.627 (R2 = 0.99) |
Z90: Epa = 0.033![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ν (V s−1) + 0.608 (R2 = 0.99) ν (V s−1) + 0.608 (R2 = 0.99) |
Based on the Laviron equation:44,45
| Epa = E0 + ((RT/(1 − α)nF) × ln((1 − α)nF)/RTket) + (RT/(1 − α)nF)ln(ν) | (3) |
According to that, the slope of a straight–line plot yielded by E = f(ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ν) equaled to RT/((1 − α)nF), where R is the gas constant (R = 8.314 J mol−1 K−1), T = 300 K, a is the electron transfer coefficient, F is the Faraday constant (F = 96
ν) equaled to RT/((1 − α)nF), where R is the gas constant (R = 8.314 J mol−1 K−1), T = 300 K, a is the electron transfer coefficient, F is the Faraday constant (F = 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 485.33 C mol−1), n is the number of electrons transferred, and ket is the electron transfer rate constant. From the determined slope values, the number of transfer electrons (n) in the electron transfer reaction could be determined to be 1 for CBR, corresponding to the charge transfer coefficient (α) found of 0.1, 0.16, and 0.21 for Z70/SPE, Z80/SPE, and Z90/SPE, respectively. More importantly, the electron transfer rate constant (ket) was calculated approximately 1.56 s−1 for Z90, 0.74 s−1 for Z80, and 0.34 s−1 for Z70. As we know, the electron transfer number in the redox reaction directly affects the current intensity and they are usually proportional to each other.42 Thus, Z90 exhibited the largest electron transfer rate constant (ket), demonstrating high electron conduction and faster response than the other electrodes.
485.33 C mol−1), n is the number of electrons transferred, and ket is the electron transfer rate constant. From the determined slope values, the number of transfer electrons (n) in the electron transfer reaction could be determined to be 1 for CBR, corresponding to the charge transfer coefficient (α) found of 0.1, 0.16, and 0.21 for Z70/SPE, Z80/SPE, and Z90/SPE, respectively. More importantly, the electron transfer rate constant (ket) was calculated approximately 1.56 s−1 for Z90, 0.74 s−1 for Z80, and 0.34 s−1 for Z70. As we know, the electron transfer number in the redox reaction directly affects the current intensity and they are usually proportional to each other.42 Thus, Z90 exhibited the largest electron transfer rate constant (ket), demonstrating high electron conduction and faster response than the other electrodes.
The impact of pH on the amperage and oxidative peak potential of CBR for bio-Ag/ZnO NCs was investigated by the DPV technique. The DPV curve of Z90 for 100 μM CBR in 0.1 M PBS with different pHs between pH 2 and pH 7 was shown in Fig. S3.† Obviously, the peak current response of CBR increased gradually as the pH varies from 2 to 3. However, beyond a pH value of 3, a further increase in pH led to a decrease in the peak current response. Obviously, at a pH value of 3, the CBR oxidation reaction reached a maximum. The obtained results could be explained by the fact that at low pH, the naphthol molecules can be protonated. This caused the diminution of peak current and affected the oxidation process. These results are similar to some previous reports.11 Therefore, pH 3 was chosen for CBR detection, which is consistent with some previous publications.46
Furthermore, with increasing solution pH, the peak potential turned negative, which infers that the oxidation of CBR involves protons. According to the regression equation of E = −0.0393 pH + 0.547 (R2 = 0.99) in Fig. S2b,† the slope value of −0.0393 V pH−1 was the same as the theoretical value of Nernst −(m*0.59)/n (V pH−1), where m and n represent the number of protons and electrons transferred. As a result, one proton and electron were involved in the electrochemical oxidation that occurred at the electrode surface. The Z90-modified SPE electrode was investigated from 30 to 150 s to determine the optimal accumulation time. The results are shown in Fig. S4.† The peak amperage (Ip) obviously increased with increasing accumulation time and peaked at 120 s. Therefore, the accumulation time was optimized to be 120 s for the subsequent experiments.
To optimize the Z90 coating volume on SPE, the electrochemical response of Z90/SPE with different dropping volumes from 4 to 12 μL was investigated by DPV technique towards 100 μM CBR detecting in 0.1 M PBS at a high sweep rate 60 mV s−1 (Fig. S5†). The oxidation peak current increased significantly with increasing coating volume from 4 to 8 μL. After that, the peak current gradually decreased as the adjusted coating volume increased. It is possible that when the increased volume caused the formation of thick layers of material on the electrode surface, this not only hindered the electron transfer within the electrode and the access of the electrolyte to the electrode surface but also led to easy peeling, breaking the working electrode during operation. Therefore, the optimal value of Z90 volume was suggested to be 8 μL for subsequent electrochemical measurements.
3.5. Effect of crystallinity and size distribution of bio-Ag/ZnO NCs on electrochemical sensing performance
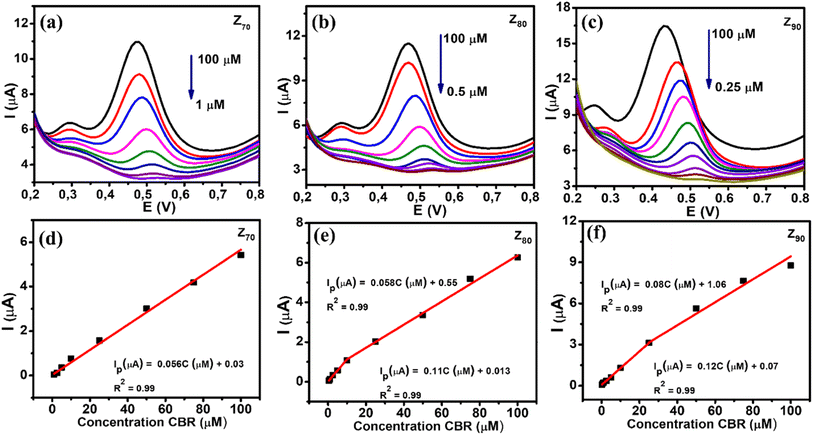
The DPV technique was used to evaluate the CBR sensing performance of various biological AgNPs-based electrochemical sensors. Fig. 7 shows the DPV curves of CBR with different concentrations on electrodes modified with Z70/SPE (a), Z80/SPE (b), and Z90/SPE (c) in 0.1 M PBS (pH 3), suboptimal conditions, and calibration graphs of peak amperage, corresponding to different concentrations of CBR. It is clear that the amperage increased as the CBR concentration increased for all samples. Calibration plots were constructed for each modified electrode with a correlation coefficient R2 greater than 0.99, as shown in Fig. 7d–f. The regression equation was found to be Ip (μA) = 0.056C (μM) + 0.03 (R2 = 0.99) for Z70/SPE in the CBR concentration range of 1–100 μM. For sample Z80: Ip (μA) = 0.058C (μM) + 0.05 (R2 = 0.99) in CBR concentration range from 10–100 μM and Ip (μA) = 0.11C (μM) + 0.013 (R2 = 0.99) in the shorter CBR concentration range of 0.5–10 μM. And Ip (μA) = 0.08C (μM) + 0.106 (R2 = 0.99) in the CBR concentration range of 25–100 μM and Ip (μA) = 0.12C (μM) + 0.07 (R2 = 0.99) in the CBR concentration range from 0.25–25 μM for Z90/SPE. Furthermore, it was observed that the oxidation potential slightly shifted to a more negative direction with increasing CBR concentration, which was attributed to proton involvement and the change in pH value around the electrode surface.Compared to the other samples, the Z90 exhibited the highest electrochemical sensitivity up to 0.303 μA μM−1 cm−2 with a CBR lowest detection limit of about 0.27 μM over a wider linear range (0.25–100 μM). The electrochemical sensitivity of Z80 was calculated as 0.27 μA μM−1 cm−2 with a detection limit of about 0.34 μM with a linear range of 0.5–100 μM. While sample Z70 the lowest electrochemical sensitivity of about 0.21 μA μM−1 cm−2 with detection limit of 0.64 μM, linear range 1–100 μM. In summary, Z90 achieved a higher sensitivity with lower LOD over a wider linear range for CBR detection than Z70 and Z80.
The difference in size, crystallinity, and distribution are important parameters that decisively influence the conductivity, electrocatalytic activity, and adsorption efficiency of the transformed electrodes change. The order of grain size is Z90 < Z80 < Z70, respectively, and crystallinity increases with increasing microwave temperature. Besides, the EASA value and Rct value were recorded in the order Z70 < Z80 < Z90 and Z90 < Z80 < Z70. The higher the temperature, the smaller the particle size, and the greater the crystallinity. In general, the smaller particle size is the origin of the larger electroactive surface area and is vital for enhancing electrochemical performance. There have been a number of publications indicating that the electrocatalytic properties increase with decreasing size and crystallinity of nanoparticles.37,47,48 Such potential characteristics have contributed to the enhancement of EASA value, electronic conductivity, and electrochemical response for CBR electrochemical detection. These results emphasize the synergistic effects of size, crystallinity, and distribution on electrochemical sensing performance.
The oxidation of CBR in the presence of Ag/ZnO NCs was proposed by two processes as follows:11,49,50
(1) In alkaline media with adding of NaOH, CBR molecules were hydrolyzed to 1-naphthol.
(2) Then, the [OH−] functional group within 1-naphthol was oxidized with removing of 1 electron and 1H+, corresponding to the appearance of a well-defined oxidation peak at 0.5 V.
The electrochemical mechanism of CBR was displayed as Fig. 8.
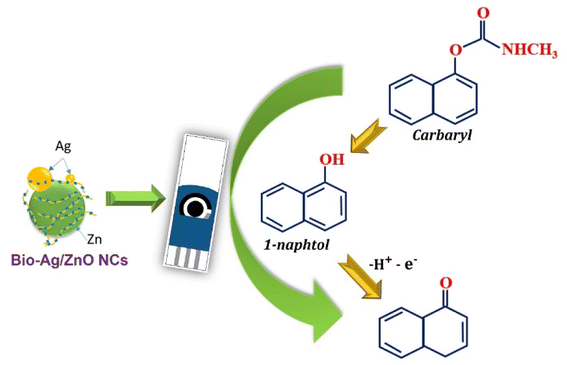 | ||
| Fig. 8 Schematic illustration of the enhanced mechanism of electrochemical sensing performance of CBR using SPE electrode modified. | ||
3.6. Repeatability, stability, reproducibility, and selectivity studies of the electrochemical sensing system
Under the optimal conditions, the repeatability of the modified electrodes was evaluated by recording the current response in the presence of 100 μM CBR for 10 consecutive times on the same electrode (Fig. S6†). The obtained results demonstrate that this sensor has good repeatability with relatively low standard deviation (RSD) values of 1.14%, 1.03%, and 1.02% for Z70/SPE, Z80/SPE, and Z90/SPE, respectively. The selectivity of this modified electrode was analyzed by the DPV technique in the presence of several different interfering compounds and ions. The test results were shown in Fig. S7.† The obtained results showed the presence of 200 μM of some ions (Ni2+, Fe2+, Cu2+, NO3−, and SO42−) and 200 μM of compounds such as D-glucose, ascorbic acid, urea, thiram, amoxicillin, chloramphenicol, and furazolidone did not interfere in the determination of CBR. To evaluate the practical applicability of an electrochemical sensor based on bio-nanocomposites in a real sample, cabbage samples with adding CBR were used. Different concentrations of CBR of 10 μM, 25 μM, and 50 μM were spiked into cabbage samples and then analyzed by the DPV technique. The final concentration of CBR was calculated according to the regression equation of the calibration curves, as summarized in Table 1. The mean recoveries for Z70 were found to range from 91% to 97% with deviations. The relative standard (RSD) was within 1.2–2.3% (n = 3). Z80/SPE, and Z90/SPE show average recovery between 93% and 98% and RSD around 2.2%. The calculation results also demonstrate that the modification with this composite nanomaterial can be used effectively to control and monitor CBR in cabbage samples.| Electrode | Amount added (μM) | Amount found (μM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| Z70/SPE | 10 | 48.46 | 96.93 | 2.27 |
| 25 | 22.61 | 90.45 | 1.22 | |
| 50 | 9.31 | 93.10 | 1.69 | |
| Z80/SPE | 10 | 46.72 | 93.45 | 1.41 |
| 25 | 24.75 | 98.99 | 1.58 | |
| 50 | 9.64 | 96.39 | 2.15 | |
| Z90/SPE | 10 | 49.16 | 98.33 | 1.35 |
| 25 | 23.99 | 95.98 | 0.93 | |
| 50 | 9.33 | 93.33 | 2.22 |
4. Conclusions
In summary, bio-Ag/ZnO NCs were successfully synthesized using a combined microwave-assisted/biogenic electrochemical method with mangosteen (Garcinia mangostana) peel extract. The obtained results show that microwave temperature impacted the characterization of as-synthesized samples. The relationship between tuning particle size distribution and crystal structure of bio-Ag/ZnO NCs on the electrochemical response of nanosensors was considered. It found that Z90/SPE exhibited the best electrochemical performance among the investigated modified electrodes. This provides an insight that enhanced electrochemical activity can be achieved by controlling the crystallinity as well as the particle size distribution of their nanomaterial-assembled layer on the electrode. For carbaryl detection, the Z90/SPE exhibited the highest sensing performance, good reproducibility, and reliability with electrochemical sensitivity up to 0.303 μA μM−1 cm−2 with a detection limit of ∼0.27 μM in wider linear range (0.25–100 μM). The proposed electrochemical-based non-enzymatic sensor can reliably detect carbaryl in cabbage samples with an average recovery of 98%. The present study revealed that bio-Ag/ZnO NCs offered great promise for designing a high-performance electrochemical-based non-enzymatic nanosensor.Author contributions
N. L. N. Trang: conceptualization, formal analysis, writing-original draft; D. T. N. Nga: formal analysis, writing-original draft; T. H. Thuan: methodology, formal analysis; L. T. Tufa: methodology, formal analysis; T. V. Tan: methodology, validation formal analysis, writing-review & editing; V. N. Phan: methodology, formal analysis; P. T. Nhung: investigation, formal analysis, writing-review & editing; H. V. Tuan: funding acquisition, formal analysis, writing-review & editing; L. A. Tuan: conceptualization, methodology, project administration, writing-review & editing.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This research was supported by Phenikaa University under grant number 2-04.2021.03. The authors would like to acknowledge the support for Raman, Electrochemical & UV-vis measurements from NEB Lab (Phenikaa University) and Zeta potential, DLS measurements from DKSH Lab at the Phenikaa University.References
- R. Krieger and W. Krieger, Index, Academic Press, San Diego, 2nd edn, 2001, pp. 1897–1908, DOI:10.1016/B978-012426260-7/50092-6
.
- R. Gupta, Index, Academic Press, Burlington, 2006, pp. 735–763. DOI:10.1016/B978-012088523-7/50051-X
.
- The WHO Recommended Classification of Pesticides by Hazard, 2019 Search PubMed
.
- N. Abdullah, J. Zaidan, Z. A. Alothman, S. M. Wabaidur, A. A. Ghafar and T. Saleh, Journal of King Saud University – Science Development of a sensitive liquid-liquid extraction and ultra-performance liquid chromatography-tandem mass spectrometry method for the analysis of carbaryl residues in fresh vegetables sold in Riyadh, J. King Saud Univ., Sci., 2020, 4, DOI:10.1016/j.jksus.2020.03.030
.
- K. Elmamoun, A. Ibrahim and D. Şolpan, Removal of carbaryl pesticide in aqueous solution by UV and UV/hydrogen peroxide processes, Int. J. Environ. Anal. Chem., 2020, 1–15, DOI:10.1080/03067319.2020.1767091
.
- X. Song, Y. Shi and J. Chen, Carbon nanotubes-reinforced hollow fibre solid-phase microextraction coupled with high performance liquid chromatography for the determination of carbamate pesticides in apples, Food Chem., 2013, 139, 246–252, DOI:10.1016/j.foodchem.2013.01.112
.
- X. Jing, H. Wang, X. Huang, Z. Chen, J. Zhu and X. Wang, Digital image colorimetry detection of carbaryl in food samples based on liquid phase microextraction coupled with a microfluidic thread-based analytical device, Food Chem., 2021, 337(2020), 127971, DOI:10.1016/j.foodchem.2020.127971
.
- A. Ratiometric, A Ratiometric Fluorescence Nanoprobe Using CdTe QDs for Fast Detection of Carbaryl Insecticide in Apple, Talanta, 2020, p. 121467, DOI:10.1016/j.talanta.2020.121467
.
- Y. Fan, K. Lai, B. A. Rasco and Y. Huang, LWT – Food Science and Technology Determination of carbaryl pesticide in Fuji apples using surface-enhanced Raman spectroscopy coupled with multivariate analysis, LWT–Food Sci. Technol., 2015, 60(1), 352–357, DOI:10.1016/j.lwt.2014.08.011
.
- T. Rahmani and H. Bagheri, Modified 3D Graphene-Au as a Novel Sensing Layer for Direct and Sensitive Electrochemical Determination of Carbaryl Pesticide in Fruit, Vegetable and Water Samples, Food Anal. Methods, 2018, 3005–3014 CrossRef
.
- F. E. Salih, et al., Electrochemical Sensor Based On Low Silica X Zeolite Modified Carbon Paste For Carbaryl Determination, J. Adv. Res., 2017, 8, 669–676, DOI:10.1016/j.jare.2017.08.002
.
- B. Liu, B. Xiao and L. Cui, Journal of Food Composition and Analysis Electrochemical analysis of carbaryl in fruit samples on graphene oxide-ionic liquid composite modified electrode, J. Food Compos. Anal., 2015, 40, 14–18, DOI:10.1016/j.jfca.2014.12.010
.
- W. Jiao, G. Ding, L. Wang, Y. Liu and T. Zhan, Polyaniline functionalized CoAl-layered double hydroxide nanosheets as a platform for the electrochemical detection of carbaryl and isoprocarb, Microchim. Acta, 2022, 189(2), 78, DOI:10.1007/s00604-022-05183-y
.
- P. N. Minh, H. V. Tuan, N. X. Dinh, O. V. Hoang, N. V. Cuong, D. T. B. Hop, T. Q. Tuan, N. T. Khi, T. Q. Huy and L. A. Tuan, Reduced graphene oxide-wrapped silver nanoparticles for applications in ultrasensitive colorimetric detection of Cr(VI) ions and the carbaryl pesticide, New J. Chem., 2020, 44, 7611–7620, 10.1039/D0NJ00947D
.
- R. Jemai, et al., Activated porous carbon supported Pd and ZnO nanocatalysts for trace sensing of carbaryl pesticide in water and food products, New J. Chem., 2022, 46(29), 13880–13895 RSC
.
- T. H. V. Kumar, S. K. Raman Pillai, M. B. Chan-Park and A. K. Sundramoorthy, Highly selective detection of an organophosphorus pesticide, methyl parathion, using Ag–ZnO–SWCNT based field-effect transistors, J. Mater. Chem. C, 2020, 8(26), 8864–8875, 10.1039/C9TC07043E
.
- J. Yoon, D. Lee, E. Lee, Y. S. Yoon and D.-J. Kim, Ag/ZnO Catalysts with Different ZnO Nanostructures for Non-enzymatic Detection of Urea, Electroanalysis, Jan. 2019, 31(1), 17–21, DOI:10.1002/elan.201800595
.
- A. Zno, M. M. Rahman, H. B. Balkhoyor and A. M. Asiri, Removal of a melamine contaminant with Ag-doped ZnO nanocomposite
materials, New J. Chem., 2019, 18848–18859, 10.1039/c9nj04638k
.
- M. T. Noman, N. Amor and M. Petru, Synthesis and applications of ZnO nanostructures (ZONSs): a review, Crit. Rev. Solid State Mater. Sci., 2021, 1–43, DOI:10.1080/10408436.2021.1886041
.
- K. M. Lee, C. W. Lai, K. S. Ngai and J. C. Juan, Recent developments of zinc oxide based photocatalyst in water treatment technology: a review, Water Res., 2015, 88, 428–448, DOI:10.1016/j.watres.2015.09.045
.
- M. Nie, et al., Study on electrocatalytic property of ZnO and Ag/ZnO, Mater. Lett., 2020, 271, 1277851–1277854, DOI:10.1016/j.matlet.2020.127785
.
- G. R. S. Andrade, et al., ZnO/Au nanocatalysts for enhanced decolorization of an azo dye under solar, UV-A and dark conditions, J. Alloys Compd., 2017, 710, 557–566, DOI:10.1016/j.jallcom.2017.03.295
.
- W. He, Unraveling the Enhanced Photocatalytic Activity and Phototoxicity of ZnO/Metal Hybrid Nanostructures from Generation of Reactive Oxygen Species and Charge Carriers, ACS Appl. Mater. Interfaces, 2014, 6(17), 15527–15535 CrossRef CAS PubMed
.
- M. Zare, K. Namratha, S. Ilyas, A. Hezam, S. Mathur and K. Byrappa, Smart Fortified PHBV-CS Biopolymer with ZnO–Ag Nanocomposites for Enhanced Shelf Life of Food Packaging, ACS Appl. Mater. Interfaces, 2019, 11(51), 48309–48320, DOI:10.1021/acsami.9b15724
.
- L. Sun, R. Shao, L. Tang and Z. Chen, Synthesis of Ag/ZnO Nanocomposite with Excellent Photocatalytic Performance Via a Facile Sol–Gel Method, Adv. Mater. Res., 2014, 877, 251–256, DOI:10.4028/www.scientific.net/AMR.875-877.251
.
- B. Divband, M. Khatamian, G. R. K. Eslamian and M. Darbandi, Applied Surface Science Synthesis of Ag/ZnO nanostructures by different methods and investigation of their photocatalytic efficiency for 4-nitrophenol degradation, Appl. Surf. Sci., 2013, 284, 80–86, DOI:10.1016/j.apsusc.2013.07.015
.
- D. Y. Nadargi, M. S. Tamboli, S. S. Patil, I. S. Mulla and S. S. Suryavanshi, Development of Ag/ZnO nanorods and nanoplates at low hydrothermal temperature and time for acetone sensing application : an insight into spillover mechanism, SN Appl. Sci., 2019, 1, 1564, DOI:10.1007/s42452-019-1573-2
.
- Y. Wei, et al., Materials Science in Semiconductor Processing Hydrothermal synthesis of Ag modi fi ed ZnO nanorods and their enhanced ethanol-sensing properties, Mater. Sci. Semicond. Process., 2017, 1–7, DOI:10.1016/j.mssp.2017.11.007
.
- E. Mendoza-Mendoza, et al., One-step synthesis of ZnO and Ag/ZnO heterostructures and their photocatalytic activity, Ceram. Int., 2018, 44, 6176–6180, DOI:10.1016/j.ceramint.2018.01.001
.
- X. Zhai, et al., Analytica Chimica Acta A highly selective and recyclable sensor for the electroanalysis of phosphothioate pesticides using silver-doped ZnO nanorods arrays, Anal. Chim. Acta, 2021, 1152, 338285, DOI:10.1016/j.aca.2021.338285
.
- N. Sebastian, et al., Ultrasensitive Electrochemical Detection and Plasmon-Enhanced Photocatalytic Degradation of Rhodamine B Based on Dual-Functional, 3D, Hierarchical Ag/ZnO Nanoflowers, Sensors, 2022, 22(13), 5049, DOI:10.3390/s22135049
.
- B. Y. Sahyar, M. Kaplan, M. Ozsoz, E. Celik and S. Otles, Bioelectrochemistry, 2019, 107327, DOI:10.1016/j.bioelechem.2019.107327
.
- H. D. Kiriarachchi, K. M. Abouzeid, L. Bo and M. S. El-Shall, Growth Mechanism of Sea Urchin ZnO Nanostructures in Aqueous Solutions and Their Photocatalytic Activity for the Degradation of Organic Dyes, ACS Omega, 2019, 4(9), 14013–14020, DOI:10.1021/acsomega.9b01772
.
- S. Kwon, D. Kim, S. Jung and Y. Hwu, Microwave synthesis of gold nanoparticles : Effect of applied microwave power and solution pH, Mater. Chem. Phys., 2011, 131(1–2), 331–335, DOI:10.1016/j.matchemphys.2011.09.050
.
- Z. Zou, et al., Opposite Particle Size Effect on Amorphous Calcium Carbonate Crystallization in Water and during Heating in Air, Chem. Mater., 2015, 27(12), 4237–4246, DOI:10.1021/acs.chemmater.5b00145
.
- G. P. Barreto, G. Morales and M. L. L. Quintanilla, Microwave Assisted Synthesis of ZnO Nanoparticles: Effect of Precursor Reagents, Temperature, Irradiation Time, and Additives on Nano-ZnO Morphology Development, J. Mater., 2013, 2013(1), 1–11, DOI:10.1155/2013/478681
.
- N. T. Anh, et al., PAPER electrochemical nanosensors for the detection of furazolidone in pork and shrimp samples : exploring the role of crystallinity , phase ratio , and, New J. Chem., 2022, 46, 7090–7102, 10.1039/D1NJ05837A
.
- G. Kesavan, M. Pichumani and S. Chen, Influence of Crystalline, Structural, and Electrochemical Properties of Iron Vanadate Nanostructures on Flutamide Detection, ACS Appl. Nano Mater., 2021, 4(6), 5883–5894, DOI:10.1021/acsanm.1c00802
.
- A. de la Escosura-Muñiz, C. Parolo, F. Maran and A. Mekoçi, Size-dependent direct electrochemical detection of gold nanoparticles: application in magnetoimmunoassays, Nanoscale, 2011, 3(8), 3350, 10.1039/c1nr10377f
.
- A. Sangili, V. Vinothkumar, S. Chen, P. Veerakumar and K. Lin, Gold Nanoparticle Embedded on a Reduced Graphene Oxide/polypyrrole Nanocomposite: Voltammetric Sensing of Furazolidone and Flutamide, Langmuir, 2020, 36(46), 13949–13962, DOI:10.1021/acs.langmuir.0c02448
.
- M. Saqib, E. V. Dorozhko, J. Barek, V. Vyskocil, E. I. Korotkova and A. V. Shabalina, A Laser Reduced Graphene Oxide Grid Electrode for the Voltammetric Determination of Carbaryl, Molecules, 2021, 26(16), 5050, DOI:10.3390/molecules26165050
.
- T. N. Pham, et al., Insight into the Influence of Analyte Molecular Structure Targeted on MoS2-GO-Coated Electrochemical Nanosensors, Langmuir, 2021, 37(41), 12059–12070, DOI:10.1021/acs.langmuir.1c01853
.
- H. Zhai, Z. Liu, Z. Chen, Z. Liang, Z. Su and S. Wang, A sensitive electrochemical sensor with sulfonated graphene sheets/oxygen-functionalized multi-walled carbon nanotubes modified electrode for the detection of clenbuterol, Sens. Actuators, B, 2015, 210, 483–490, DOI:10.1016/j.snb.2014.12.121
.
- E. Laviron, General expression of the linear potential sweep voltammogram in the case of diffusion less electrochemical systems, J. Electroanal. Chem. Interfacial Electrochem., 1979, 101, 19–28 CrossRef CAS
.
- T. Yin, et al., The catalytic effect of TiO2 nanosheets on extracellular electron transfer of Shewanella loihica PV-4, Phys. Chem. Chem. Phys., 2016, 18, 29871–29878, 10.1039/C6CP04509J
.
- P. Hashemi, N. Karimian, H. Khoshsafar, F. Arduini and M. Mesri, Materials Science & Engineering C Reduced graphene oxide decorated on Cu/CuO-Ag nanocomposite as a high- performance material for the construction of a non-enzymatic sensor : Application to the determination of carbaryl and fenamiphos pesticides, Mater. Sci. Eng., C, 2019, 102, 764–772, DOI:10.1016/j.msec.2019.05.010
.
- N. Le et al., Bio-AgNPs-based electrochemical nanosensors for the sensitive determination of 4-nitrophenol in in physicochemical parameters and sensing,” pp. 6007–6017, 2022, 10.1039/d1ra09202b.
- D. B. Priya and I. V. Asharani, Size Dependent Catalytic Activity of Actinodaphne madraspatana Bedd Leaves Mediated Silver Redox potential sil, J. Cluster Sci., 2017, 28, 1837–1856, DOI:10.1007/s10876-017-1185-1
.
- M. Zhang, Z. Zhang, Y. Yang, Y. Zhang, Y. Wang and X. Chen, Ratiometric Strategy for Electrochemical Sensing of Carbaryl Residue in Water and Vegetable Samples, Sensors, 2020, 20(5), 1524, DOI:10.3390/s20051524
.
- F. C. Moraes, L. H. Mascaro, S. A. S. Machado and C. M. A. Brett, Direct electrochemical determination of carbaryl using a multi-walled carbon nanotube/cobalt phthalocyanine modified electrode, Talanta, 2009, 79, 1406–1411, DOI:10.1016/j.talanta.2009.06.013
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra00399j |
| This journal is © The Royal Society of Chemistry 2023 |