Open Access Article
Open Access ArticleA mechanistic study of gold nanoparticles catalysis of O2 reduction by ascorbate and hydroethidine, investigating reactive oxygen species reactivity†
Viacheslav Shcherbakov *,
Sergey A. Denisov
*,
Sergey A. Denisov and
Mehran Mostafavi
and
Mehran Mostafavi *
*
Institut de Chimie Physique (ICP), CNRS/Université Paris-Saclay, 91405 Orsay, France. E-mail: viacheslav.shcherbakov@universite-paris-saclay.fr; mehran.mostafavi@universite-paris-saclay.fr
First published on 15th March 2023
Abstract
In this work, the mechanism of dioxygen reduction catalysed by gold nanoparticles (AuNPs) by two electron donors was investigated, i.e., by sodium ascorbate and hydroethidine, focusing on potential ROS (reactive oxygen species) formation, such as O2˙− and H2O2. According to our results, when AuNPs catalyse the reduction of O2, ROS are formed only as intermediates on the surface of nanoparticles, and they are unavoidably reduced to water, catalysed by the AuNPs. Thus, the statement on ROS production in the presence of AuNPs often reported in the literature is excessive. The AuNPs can catalyze the oxidation of electron donors in the cell, e.g., antioxidants causing oxidative stress. Therefore we propose that when explaining damage in the living cells observed in the presence of AuNP, the catalysis of redox reactions by AuNPs must be considered.
Introduction
Reactive oxygen species (ROS) are essential actors in cell biochemistry.1 In biology, the reactivity of free radicals, including ROS, is a research domain under active investigation that has implications in, e.g., aging processes, stress-activated diseases, and radiotherapy.2–7Nanoparticles (NPs) are studied for different biomedical applications. One of the first examples was gold nanoparticles (AuNPs), which were considered an inert material and bio-compatible. However, it has been known for a long time that materials' properties at the nanoscale are different from the bulk ones. Therefore, many NPs, including AuNPs possess catalytic activity in different reactions.8 The idea that nanoparticles are not inert is gradually becoming more visible in biological research.
It is interesting to mention that the chemical activity of AuNPs is mainly raised in the context of ROS formation.9,10 The idea that nanoparticles cause ROS formation probably drifted from the radiosensitization research field (known as ROS overproduction),11,12 where nanoparticles are studied as radiosensitizing agents.9,10 Briefly, using the fluorescence probes for ROS detection, it was shown that in the presence of AuNPs under ionizing radiation, the quantity of formed fluorescent product was significantly increased, which was interpreted as ROS overproduction, taking into account the linear dependence of fluorescent product formation on ROS concentration. In our recent works, we showed that the increased formation of the fluorescent product could be explained not by additional ROS formation but by catalytic properties of AuNPs, which increase the yield of particular reaction products.13–15 Thus, not considering the catalytic properties of nanoparticles in reactions involving organic radicals led to an invalid interpretation of the experimental data. At the same time, it is often mentioned that in the absence of ionizing radiation NP could produce ROS via catalysis,9,10 e.g., catalytic reduction of dioxygen to superoxide radical or hydrogen peroxide. Due to the high reactivity of ROS, their detection in biological systems is not readily achievable. Therefore, in biological works, the statement about catalytic ROS production is used to explain the observed biological effects, e.g., oxidative stress.16
ROS formation and their reactivity in the presence of NPs are extensively discussed in the context of the enzymatic-like activity of nanomaterials. NPs, including gold, can catalyze reactions similar to natural enzymes.17–19 They exhibit oxidase-like activity, which includes reactions involving oxygen,20,21 hydrogen peroxide,22,23 and superoxide radicals.23 For example, ascorbate oxidation by dioxygen catalyzed by Au@Pt NPs was reported.24 Unlike, L-ascorbate oxidase, in the presence of the NPs, it is stated that oxygen is reduced to hydrogen peroxide. However, other works show that AuNPs catalyze H2O2 decomposition.22,23
The catalytic activity of NPs in biologically relevant reactions still does not receive proper attention, while it is crucial for further development of nanomedicine and understanding nanomaterials' toxicity. In our opinion, there is a demand for biologically orientated works in the chemistry of nanomaterials to provide a deeper and more convincing explanation of biological effects observed in the presence of nanomaterials. For example, it was shown that AuNPs affect living cells' antioxidant status by inhibiting enzyme activity, i.e., thioredoxin and glutathione reductases.25 That shows clearly that the chemical activity of AuNPs cannot be limited to ROS formation.
We have recently shown that in many works when gold nanoparticles are used for both therapeutic and diagnostic purposes, the chemical effect of the nanoparticles is not taken into account, and this effect can have a powerful impact on the interpretation of the results.14,15
In this work, we have chosen two molecules: sodium ascorbate and hydroethidine, the first one as a model of an antioxidant that can be present in the biological medium and the second one as a model of a fluorescent probe used in ROS detection experiments. Here we aim to provide the mechanism of catalytic oxidation in the presence of AuNPs for both chosen molecules investigating potential ROS formation.
Experimental
Gold nanoparticles synthesis and supernatant preparation
We used 20 nm AuNPs prepared by reduction of potassium gold(III) chloride (K[AuCl4], 98%, Sigma Aldrich) with sodium borohydride (NaBH4, 98%, Sigma Aldrich). Briefly, 3 ml of 100 mM K[AuCl4] solution was mixed with 87.7 ml of deionized water (Milli-Q, 18 MΩ cm), and 9.3 ml of 100 mM NaBH4 was quickly added to the solution while actively stirring with a magnetic stirrer for 15 min. The final concentration of gold atoms was 3 mM.AuNPs were characterized by several techniques: zeta potential of −20 mV was measured by Zetasizer Nano ZS, Malvern; UV-vis absorption spectra were measured on Hewlett Packard 8453 spectrophotometer (Fig. S1†); the microphotography of the nanoparticles were taken by transmission electron microscopy (STEM 1400, JEOL, Fig. S2†).
The supernatant of AuNPs used as reference was prepared by adding NaCl to provoke particles aggregation with further sedimentation by centrifugation. Centrifugation was done in 2 ml Eppendorf microtubes utilizing an Eppendorf MiniSpin centrifuge with F-45-12-11 rotor at a speed of 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 rpm for 30 min. Then, the transparent liquid above the solid precipitate was gently taken.
400 rpm for 30 min. Then, the transparent liquid above the solid precipitate was gently taken.
The quality of the nanoparticle's removal from the solution was checked spectroscopically. The pH of AuNPs' suspension and its supernatant was ca. 7.
Ascorbate oxidation
We studied the catalytic oxidation of sodium ascorbate by two oxidizers, oxygen, and hydrogen peroxide, in the presence of AuNPs. The concentration of sodium ascorbate (99%, Alfa Aesar) was determined by UV-vis absorption spectroscopy (Hewlett Packard 8453) using an extinction coefficient of 14.6 × 103 M−1 cm−1 at 265 nm measured in our laboratory. Studied solutions containing sodium ascorbate, oxygen or hydrogen peroxide, and AuNPs were placed into 1 mm quartz cuvette. The optical cuvette was covered by Parafilm M to prevent gas exchange. The kinetics of ascorbate oxidation were measured by following a decrease of its absorption band at 265 nm.We also measured ascorbate stability at 1 mM concentration in oxygen-saturated solutions in the absence of AuNPs.
For experiments with AuNPs, 3 ml of an aqueous solution containing 1.5 mM sodium ascorbate with 0.75 mM of H2O2 and 3 ml of AuNPs suspension with 3 mM of gold atoms were deoxygenated by bubbling with Ar gas for 15 min. Then, 260 μl of ascorbate solution and 130 μl of AuNPs suspension were mixed directly in a 1 mm quartz cuvette. Thus, the final solution contained 1 mM of sodium ascorbate, 0.5 mM of H2O2, and AuNPs with 1 mM of gold atoms under an argon atmosphere.
Hydrogen peroxide stability in the presence of AuNPs
The stability of hydrogen peroxide in the presence of AuNPs was measured by titanium(IV) oxysulfate solution (1.9–2.1% TiOSO4, Sigma-Aldrich) titration method. Briefly, 0.5 ml of 20 mM H2O2 was added to 5 ml of water or supernatant or AuNPs suspension with a gold atomic concentration of 3 mM. Then, the solution's volume was adjusted to 10 ml. 1.6 ml of each solution was taken every 10 min for 1 hour and mixed with 80 μl of 10 wt% NaCl solution to cause AuNPs aggregation. Then, samples were centrifuged to precipitate AuNPs. Then, 1.5 ml of the samples were mixed with 1.5 ml of 1.9–2.1 wt% TiOSO4 solution and UV-vis absorption spectra were measured in 1 cm quartz optical cuvette. H2O2 concentrations were calculated using an extinction coefficient of 689 M−1 cm−1 at 407 nm of the yellow-colored complex of pertitanic acid (H2TiO4).27Dihydroethidium oxidation by oxygen
Hydroethidine (HE, ≤95%, Sigma-Aldrich) was dissolved in DMSO to get 1 mM solution. The exact concentration was determined by absorption measurement at 345 nm using extinction coefficient of 9750 M−1 cm−1. Prior the measurement HE solution in DMSO was diluted 10 times by deionized water.For the experiment on HE oxidation by oxygen in the presence of AuNPs, 1.5 ml of 110 μM HE solution was mixed with 1.5 ml of AuNPs suspension ([Au] = 300 μM) directly in 1 cm quartz cuvette. Then, the evolution of absorption spectra was recorded.
For the experiment on the reduction of the oxidized product obtained in the previous experiment, AuNPs were removed from the solution by centrifugation. Then, 10 μl of 30 mM NaBH4 solution was added to reduce the oxidized product. Then, the evolution of absorption spectra was recorded.
Results
Ascorbate oxidation by oxygen
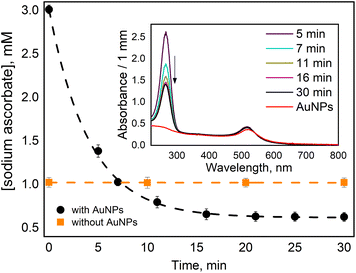
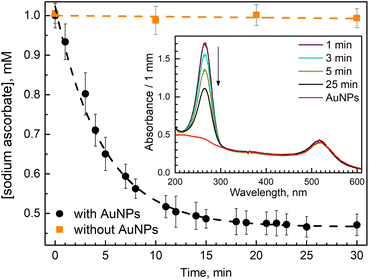
In our work, we measured ascorbate (H2A) oxidation to dihydro ascorbate (DHA) by following the disappearance of its absorption band at 265 nm (Fig. 1 insert). When ascorbate was added to AuNPs suspension, it caused a slight blue shift and a little increase in absorption of AuNPs plasmon band (Fig. 1 insert). A similar observation was reported in work utilizing surface plasmon spectroscopy of a single gold nanocrystals.28 It has been suggested that the nanoparticle oxidizes the ascorbate ions, becoming negatively charged, which causes a blue shift in surface plasmon absorption. Then, when dioxygen molecules reach the AuNP surface, they uptake electrons, reducing the overall negative charge of AuNP, which causes a red shift of the plasmon band. However, we did not observe ascorbate oxidation by AuNPs in deoxygenated solution. Thus, we think that the reason for the observed blue shift is that ascorbate molecules work as surfactants interacting with AuNPs' surface, causing a change in nanoparticles absorption. For example, it was shown that the formation of Cl− complexes with AuNPs affects the position of the surface plasmon resonance peak.29We observed that the absorption band of ascorbate (H2A) at 265 nm decreased within tens of minutes only when the solution contained both AuNPs and O2 (Fig. 1 insert). The experiment was designed in such a way as to observe the termination of the reaction when the oxidant (oxygen) is depleted.
In the oxygen-saturated solution, 2.4 ± 0.1 mM of H2A was consumed (Fig. 1), considering oxygen concentration of 1.22 mM, ascorbate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2 ratio is 2
O2 ratio is 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. However, if we consider oxygen reduction only to hydrogen peroxide (reaction (1)) as it was proposed elsewhere,24 then the ascorbate
1. However, if we consider oxygen reduction only to hydrogen peroxide (reaction (1)) as it was proposed elsewhere,24 then the ascorbate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2 ratio should be 1
O2 ratio should be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which does not satisfy our experimental results; meaning that hydrogen peroxide is further reduced to water (reaction (2)), giving an overall reaction (3).
1, which does not satisfy our experimental results; meaning that hydrogen peroxide is further reduced to water (reaction (2)), giving an overall reaction (3).
 | (R. 1) |
 | (R. 2) |
 | (R. 3) |
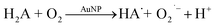
It was confirmed by ESR measurements at an ambient temperature that H2A oxidation by oxygen catalysed by Pt@Au nanorods occurs through ascorbate radical formation (HA˙).24 The same was observed for palladium concave nanocrystals.30 Therefore, we can consider that reaction (1) includes two following steps:
 | (R. 1.1) |
 | (R. 1.2) |
Reaction (1.1), where ascorbate and superoxide radicals are formed, is catalysed by AuNPs because, in their absence, this reaction is very slow. Reaction (1.2) is not necessarily catalyzed by AuNPs since O2˙− is able to oxidize ascorbate radical. An important point is that reaction (1.1) is a limiting step. Therefore, the steady-state concentration of O2˙− is low, and it is localized at NPs' surface.
Reaction of superoxide radical with ascorbate radical is not specific and occurs by superoxide radical attachment to the ascorbate radical followed by cyclization and hydrolysis yielding DHA and H2O2.31 Thus, simply by measuring the disappearance of ascorbate, we cannot understand whether the reaction between superoxide and the ascorbate radical occurs directly or whether it is catalyzed by nanoparticles. Moreover, it was reported that AuNPs possess superoxide dismutase-like activity.23 Therefore, we can propose the disproportionation of two superoxide radicals into dioxygen and hydrogen peroxide catalyzed by gold (reaction (1.3)) as an alternative reaction mechanism.
 | (R. 1.3) |
Both proposed mechanisms, superoxide radical reduction (reaction (1.2)) and its disproportionation (reaction (1.3)), satisfy experimental observation that one O2 oxidizes two H2A molecules in the presence of AuNPs. To examine the reactivity of O2˙− in the presence of AuNPs, we used another molecule probe, which is discussed in the next section.
Superoxide radical fate in the presence of AuNPs
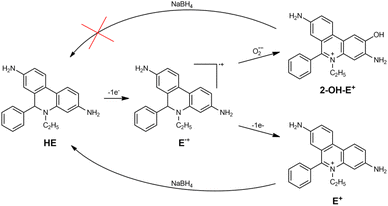
Superoxide radical formation via catalysis has a great interest for the biomedical application of NPs. The O2˙− formation in the presence of different NPs, including gold was even proposed as a chemical mechanism of AuNPs radiosensitizing effect.9,10We used hydroethidine (HE) to study possible superoxide formation during the catalytic reduction of oxygen. HE is a fluorescent dye widely used in biological studies.32 It has two oxidation products, ethidium (E+) and 2-hydroxyethidium cation (2-OH-E+) (Scheme 1). 2-OH-E+ is considered a specific product of HE˙+ radical oxidation by O2˙−.
However, many studies showed that since fluorescent spectra of both oxidation products are similar, HE should not be considered a specific fluorescent probe for intercellular O2˙− detection.33 Therefore, in our work, instead of fluorescence measurements, we used only absorption spectra which enabled us to differentiate between the two compounds. The experiments were based on the fact that E+ can be reduced to HE, but 2-OH-E+ cannot (Scheme 1).
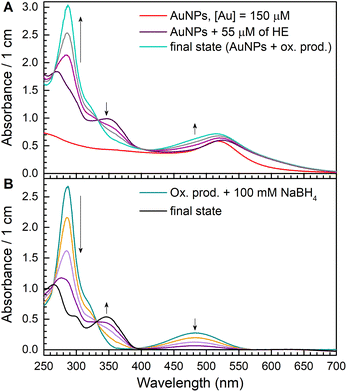
We observed a fast (minutes) transformation of the absorption spectrum of the sample containing HE and AuNPs (Fig. 2A). An increase at 290 nm and a decrease at 350 nm bear witness to HE oxidation. Similar to ascorbate experiments, HE oxidation does not occur in the absence of AuNPs or oxygen. This experiment was performed in an air atmosphere. Thus, the oxygen concentration in the solution is ca. 0.26 mM,26 which is greater than the HE concentration allowing its total oxidation.
The broadening of the plasmon band of AuNPs around 500–600 nm is due to the aggregation of the particles in the presence of HE. This process was instantaneous and did not affect HE oxidation.
After the absorption spectrum stopped changing, AuNPs were removed from the solution by centrifugation. Then, we added 100 μM of NaBH4 to the solution obtained after the particles removal and recorded the evolution of the absorption spectrum (Fig. 4B). We observed that the absorption maximum at 345 nm appeared back, and final absorption spectrum is similar to HE. Using ε345nm = 9750 M−1 cm−1, we calculated HE concentration as 55 μM, which equals to the initial HE concentration. Thus, we can conclude that the main product of HE oxidation by oxygen in the presence of AuNPs is E+, and 2-OH-E+ is not formed.
These experimental results mean that potentially formed O2˙− radicals do not react directly with the molecule or its radical. AuNPs catalyse reactions involving superoxide radicals. They either catalyse O2˙− reduction (like reaction (1.2)) or disproportionation (reaction (1.3)). We tend to think that nanoparticles work like an electron relay transferring an electron from an electron donor to electron acceptors. In this case, all transient species are formed and further reduced or oxidized depending on their RedOX potential on the nanoparticle's surface, being unable to interact with any other molecules. AuNPs could be considered as a microelectrode with adjustable RedOx potential depending on the potentials of reacting RedOx couples. This idea was proposed to explain corrosion and discussed elsewhere.34
Hydrogen peroxide reactivity in the presence of AuNPs
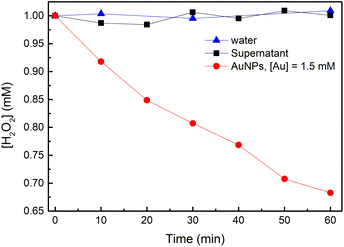
To study H2O2 reactivity in the presence of AuNPs, first, we studied its stability by TiOSO4 titration (Fig. 3 and S3†).Partial H2O2 decomposition was observed only in the presence of AuNPs within 60 min. Thus, we can conclude that AuNPs catalyse the disproportionation of H2O2 into dioxygen and water (reaction (4)).
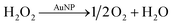 | (R. 4) |
However, this reaction is slow to explain ascorbate oxidation by dioxygen, which is achieved within 10 min in our conditions (Fig. 1). Only 30% of H2O2 was decomposed in 1 hour, which means that reaction (2) does not include H2O2 disproportionation. Similar conclusion was made in work on glucose oxidation catalysed by AuNPs.22
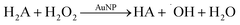
It seems that AuNPs catalyse oxidation of electron donors like ascorbate by H2O2. To show that, we performed an experiment for ascorbate oxidation by H2O2 in deoxygenated solution in the presence of AuNPs (Fig. 4). Similar to ascorbate oxidation by oxygen, the experiment was design to have ascorbate in excess.
0.5 mM of H2O2 oxidized equal amount (0.53 ± 0.03 mM) of ascorbate in the presence of AuNPs. Thus, the ratio of H2O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O2 is 1
O2 is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (reaction (5)).
1 (reaction (5)).
 | (R. 5) |
 | (R. 5.1) |
 | (R. 5.2) |
Taking these results into account, we cannot consider H2O2 as a stable product of oxygen reduction in the presence of AuNPs.
Discussion
All reactions discussed in previous sections are generalized in Scheme 2 (I). It includes complete step-by-step reduction of dioxygen to water and ROS such as O2−˙, H2O2, and ˙OH that are formed as intermediates. In a living cell, the ROS cause non-specific damage (i.e., oxidation), defend the organism against pathogens and are involved in the signaling pathways, where they specifically react with bio-molecules.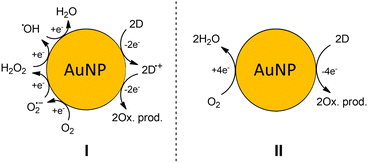 | ||
| Scheme 2 The generalized mechanism of oxygen reduction on the surface of AuNP. H+ is omitted for simplicity. (I) – step-by-step reduction of dioxygen (II) – overall reaction of dioxygen reduction. | ||
The lifetime of ROS in the cellular environment is very short (e.g., for ˙OH radical, it does not exceed a few nanoseconds). Therefore, one should consider the stationary concentration of ROS, which is dependent on the rate of its production and elimination. When the stationary concentration of ROS becomes higher, this is called “oxidative stress”. In other words, oxidative stress is an imbalance of ROS, antioxidants, and enzymatic systems responsible for ROS elimination. Thus if the concentration of antioxidants drops, oxidative stress is achieved.
Therefore, in our experiments with AuNPs we observe the catalysis of oxidation reactions by oxygen. We want to stress that ROS reactivity was not observed, even though they are formed as intermediates. It was clearly shown in the experiment on HE oxidation. Therefore, the mechanism could be simplified to 4 electron dioxygen reduction (Scheme 2 (II)). Now, if we translate our observation to the biological systems, we can state if one observes more oxidative damage in the presence of AuNP it does not necessarily mean ROS stationary concentration increases. The AuNP can catalyze the oxidation of electron donors, e.g., antioxidants causing oxidative stress and detectable damage in the cell.
Despite the fact that ROS are formed during 4 electrons step-by-step reduction of dioxygen, these reactions occur locally on the surface of AuNP, and thus the release of ROS from the surface is unlikely to occur.
Conclusions
In this work, we studied the mechanism of dioxygen reduction by two electron donors: sodium ascorbate and hydroethidine catalysed by AuNPs, focusing on potential ROS formation such as O2˙− and H2O2. According to our results, when AuNPs catalyse the reduction of O2, ROS are formed only as intermediates on the surface of NPs, and they are unavoidably reduced on-site to water catalysed by AuNPs. Thus, the statement on ROS production in the presence of AuNPs often reported in the literature is inaccurate. When AuNPs are absent, ROS can be generated and diffuse in the solution; in the presence of AuNPs, the catalytic effect of AuNPs causes the transformation of ROS into water. We also discussed the importance of catalytic activity of AuNPs in biological systems. Briefly, the main effect of AuNPs is catalysis of RedOx reactions, which includes O2 reduction, leading to the oxidation of electrons donors such as antioxidants. That eventually leads to oxidative stress. The RedOx reactions in biological systems are complex and not only include oxygen and H2O2 as oxidants. Therefore, we suppose that biological observations of damage in the cells in the presence of AuNP should consider catalytic properties of AuNP in redox reactions.Author contributions
The contributions from V. Shcherbakov, according to ‘CRediT – Contributor Roles Taxonomy’, include conceptualization, data curation, formal analysis, investigation, methodology, validation, visualization, writing – original draft, writing – review & editing. The contributions from S. A. Denisov include conceptualization, formal analysis, validation, project administration, supervision, visualization, writing – original draft, writing – review & editing. The contributions from M. Mostafavi include conceptualization, formal analysis, validation, resources, project administration, supervision, visualization, writing – original draft, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Notes and references
- B. Halliwell, Reactive oxygen species in living systems: Source, biochemistry, and role in human disease, Am. J. Med., 1991, 91, S14–S22 CrossRef PubMed.
- B. Perillo, M. Di Donato, A. Pezone, E. Di Zazzo, P. Giovannelli, G. Galasso, G. Castoria and A. Migliaccio, ROS in cancer therapy: the bright side of the moon, Exp. Mol. Med., 2020, 52, 192–203 CrossRef CAS PubMed.
- U. S. Srinivas, B. W. Q. Tan, B. A. Vellayappan and A. D. Jeyasekharan, ROS and the DNA damage response in cancer, Redox Biol., 2019, 25, 101084 CrossRef CAS PubMed.
- P. Davalli, T. Mitic, A. Caporali, A. Lauriola and D. D'Arca, ROS, Cell Senescence, and Novel Molecular Mechanisms in Aging and Age-Related Diseases, Oxid. Med. Cell. Longevity, 2016, 2016, e3565127 Search PubMed.
- G.-Y. Liou and P. Storz, Reactive oxygen species in cancer, Free Radical Res., 2010, 44, 479–496 CrossRef CAS PubMed.
- H. Kawagishi and T. Finkel, Unraveling the truth about antioxidants: ROS and disease: finding the right balance, Nat. Med., 2014, 20, 711–713 CrossRef CAS PubMed.
- M. Dumont and M. F. Beal, Neuroprotective strategies involving ROS in Alzheimer disease, Free Radicals Biol. Med., 2011, 51, 1014–1026 CrossRef CAS PubMed.
- C. D. Pina, E. Falletta and M. Rossi, Update on selective oxidation using gold, Chem. Soc. Rev., 2012, 41, 350–369 RSC.
- S. Her, D. A. Jaffray and C. Allen, Gold nanoparticles for applications in cancer radiotherapy: Mechanisms and recent advancements, Adv. Drug Delivery Rev., 2017, 109, 84–101 CrossRef CAS PubMed.
- D. Howard, S. Sebastian, Q. V.-C. Le, B. Thierry and I. Kempson, Chemical Mechanisms of Nanoparticle Radiosensitization and Radioprotection: A Review of Structure-Function Relationships Influencing Reactive Oxygen Species, IJMS, 2020, 21, 579 CrossRef CAS PubMed.
- C. Sicard-Roselli, E. Brun, M. Gilles, G. Baldacchino, C. Kelsey, H. McQuaid, C. Polin, N. Wardlow and F. Currell, A New Mechanism for Hydroxyl Radical Production in Irradiated Nanoparticle Solutions, Small, 2014, 10, 3338–3346 CrossRef CAS PubMed.
- M. Gilles, E. Brun and C. Sicard-Roselli, Quantification of hydroxyl radicals and solvated electrons produced by irradiated gold nanoparticles suggests a crucial role of interfacial water, J. Colloid Interface Sci., 2018, 525, 31–38 CrossRef CAS PubMed.
- V. Shcherbakov, S. A. Denisov and M. Mostafavi, On the Primary Water Radicals' Production in the Presence of Gold Nanoparticles: Electron Pulse Radiolysis Study, Nanomaterials, 2020, 10, 2478 CrossRef CAS PubMed.
- V. Shcherbakov, S. A. Denisov and M. Mostafavi, Selective Oxidation of Transient Organic Radicals in the Presence of Gold Nanoparticles, Nanomaterials, 2021, 11, 727 CrossRef CAS.
- V. Shcherbakov, S. A. Denisov and M. Mostafavi, The mechanism of organic radical oxidation catalysed by gold nanoparticles, Phys. Chem. Chem. Phys., 2021, 23, 26494–26500 RSC.
- Y. Pan, A. Leifert, D. Ruau, S. Neuss, J. Bornemann, G. Schmid, W. Brandau, U. Simon and W. Jahnen-Dechent, Gold Nanoparticles of Diameter 1.4 nm Trigger Necrosis by Oxidative Stress and Mitochondrial Damage, Small, 2009, 5, 2067–2076 CrossRef CAS PubMed.
- Y. Chong, Q. Liu and C. Ge, Advances in oxidase-mimicking nanozymes: Classification, activity regulation and biomedical applications, Nano Today, 2021, 37, 101076 CrossRef CAS.
- H. Wei and E. Wang, Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes, Chem. Soc. Rev., 2013, 42, 6060 RSC.
- J. Wu, X. Wang, Q. Wang, Z. Lou, S. Li, Y. Zhu, L. Qin and H. Wei, Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes (II), Chem. Soc. Rev., 2019, 48, 1004–1076 RSC.
- M. Comotti, C. Della Pina, R. Matarrese and M. Rossi, The Catalytic Activity of “Naked” Gold Particles, Angew. Chem., Int. Ed., 2004, 43, 5812–5815 CrossRef CAS PubMed.
- P. Beltrame, M. Comotti, C. D. Pina and M. Rossi, Aerobic oxidation of glucose I. Enzymatic catalysis, J. Catal., 2004, 228, 282–287 CrossRef CAS.
- M. Comotti, C. Della Pina, E. Falletta and M. Rossi, Aerobic Oxidation of Glucose with Gold Catalyst: Hydrogen Peroxide as Intermediate and Reagent, Adv. Synth. Catal., 2006, 348, 313–316 CrossRef CAS.
- W. He, Y.-T. Zhou, W. G. Wamer, X. Hu, X. Wu, Z. Zheng, M. D. Boudreau and J.-J. Yin, Intrinsic catalytic activity of Au nanoparticles with respect to hydrogen peroxide decomposition and superoxide scavenging, Biomaterials, 2013, 34, 765–773 CrossRef CAS PubMed.
- Y.-T. Zhou, W. He, W. G. Wamer, X. Hu, X. Wu, Y. M. Lo and J.-J. Yin, Enzyme-mimetic effects of gold@platinum nanorods on the antioxidant activity of ascorbic acid, Nanoscale, 2013, 5, 1583 RSC.
- N. Daems, S. Penninckx, I. Nelissen, K. Van Hoecke, T. Cardinaels, S. Baatout, C. Michiels, S. Lucas and A. Aerts, Gold nanoparticles affect the antioxidant status in selected normal human cells, IJN, 2019, 14, 4991–5015 CrossRef CAS PubMed.
- W. Xing, M. Yin, Q. Lv, Y. Hu, C. Liu and J. Zhang, in Rotating Electrode Methods and Oxygen Reduction Electrocatalysts, ed. W. Xing, G. Yin and J. Zhang, Elsevier, Amsterdam, 2014, pp. 1–31 Search PubMed.
- Z. Machala, B. Tarabova, K. Hensel, E. Spetlikova, L. Sikurova and P. Lukes, Formation of ROS and RNS in Water Electro-Sprayed through Transient Spark Discharge in Air and their Bactericidal Effects, Plasma Processes Polym., 2013, 10, 649–659 CrossRef CAS.
- C. Novo, A. M. Funston and P. Mulvaney, Direct observation of chemical reactions on single gold nanocrystals using surface plasmon spectroscopy, Nat. Nanotechnol., 2008, 3, 598–602 CrossRef CAS PubMed.
- A. B. Dahlin, R. Zahn and J. Vörös, Nanoplasmonic sensing of metal–halide complex formation and the electric double layer capacitor, Nanoscale, 2012, 4, 2339 RSC.
- Y. Chong, X. Dai, G. Fang, R. Wu, L. Zhao, X. Ma, X. Tian, S. Lee, C. Zhang, C. Chen, Z. Chai, C. Ge and R. Zhou, Palladium concave nanocrystals with high-index facets accelerate ascorbate oxidation in cancer treatment, Nat. Commun., 2018, 9, 4861 CrossRef PubMed.
- Y.-J. Tu, D. Njus and H. B. Schlegel, A theoretical study of ascorbic acid oxidation and HOO˙/O2˙− radical scavenging, Org. Biomol. Chem., 2017, 15, 4417–4431 RSC.
- A. Gomes, E. Fernandes and J. L. F. C. Lima, Fluorescence probes used for detection of reactive oxygen species, J. Biochem. Biophys. Methods, 2005, 65, 45–80 CrossRef CAS PubMed.
- J. Zielonka and B. Kalyanaraman, Hydroethidine- and MitoSOX-derived red fluorescence is not a reliable indicator of intracellular superoxide formation: Another inconvenient truth, Free Radicals Biol. Med., 2010, 48, 983–1001 CrossRef CAS PubMed.
- C. Wagner and W. Traud, “On the Interpretation of Corrosion Processes through the Superposition of Electrochemical Partial Processes and on the Potential of Mixed Electrodes,” with a Perspective by F. Mansfeld, Corrosion, 2006, 62, 843–855 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra00443k |
| This journal is © The Royal Society of Chemistry 2023 |