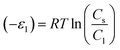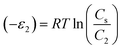Open Access Article
Open Access ArticleElimination of aspirin and paracetamol from aqueous media using Fe/N-CNT/β-cyclodextrin nanocomposite polymers: theoretical comparative survey via advanced physical models
Fatma Dhaouadi *a,
Fatma Aouainib,
Laila A. Al-Essac,
Noura Khemirid,
Alessandro Ertoe and
Abdelmottaleb Ben Laminea
*a,
Fatma Aouainib,
Laila A. Al-Essac,
Noura Khemirid,
Alessandro Ertoe and
Abdelmottaleb Ben Laminea
aLaboratory of Quantum and Statistical Physics, LR18ES18, Faculty of Sciences of Monastir, Monastir University, Monastir, Tunisia. E-mail: dhaouadifatma2018@gmail.com
bDepartment of Physics, College of Science, Princess Nourah Bint Abdulrahman University, P. O. Box 84428, Riyadh 11671, Saudi Arabia
cDepartment of Mathematical Sciences, College of Science, Princess Nourah Bint Abdulrahman University, P. O. Box 84428, Riyadh 11671, Saudi Arabia
dDepartment of Physics, College of Sciences at Yanbu, Taibah University, Yanbu, Medina 42353, Saudi Arabia
eDipartimento di Ingegneria Chimica, Dei Materiali Edella Produzione Industriale, Università Di Napoli Federico II, P.Le Tecchio 80, 80125 Napoli, Italy
First published on 17th May 2023
Abstract
The main purpose of this research is to theoretically investigate the adsorption of two pharmaceutical molecules, i.e. aspirin and paracetamol, using two composite adsorbents, i.e. N-CNT/β-CD and Fe/N-CNT/β-CD nanocomposite polymers. A multilayer model developed by statistical physics is implemented to explain the experimental adsorption isotherms at the molecular scale, so as to overpass some limitations of the classical adsorption models. The modelling results indicate that the adsorption of these molecules is almost accomplished by the formation of 3 to 5 adsorbate layers, depending on the operating temperature. A general survey of the number of adsorbate molecules captured by the adsorption site (npm) suggested that the adsorption process of pharmaceutical pollutants is multimolecular and that each adsorption site can capture several molecules simultaneously. Furthermore, the npm values demonstrated the presence of aggregation phenomena of aspirin and paracetamol molecules during adsorption. The evolution of the adsorbed quantity at saturation confirmed that the presence of Fe in the adsorbent enhanced the removal performance of the investigated pharmaceutical molecules. In addition, the adsorption of the pharmaceutical molecules aspirin and paracetamol on the N-CNT/β-CD and Fe/N-CNT/β-CD nanocomposite polymer surface involved weak physical type interactions, since the interaction energies do not overcome the threshold of 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 J mol−1.
000 J mol−1.
1. Introduction
Recently, research on water contamination by emerging pollutants such as pharmaceuticals has earned noteworthy consideration. These drugs are increasingly consumed due to the rising requests of human communities.1 Furthermore, in light of the wide availability of medical therapies and the large affordability of drugs, pharmaceutical products have experienced a rapid sales growth. This is also because most of them are over-the-counter drugs, hence useable without any medical prescription.2 Due to the large spread of use, the consequential excretion of pharmaceutical compounds, their corresponding feedback metabolites and conjugates in water effluents is simultaneously increasing. Therefore, several harmful effects, which are still not well comprehended, may influence human health and marine wildlife, because many of these pharmaceuticals are regarded as bioaccumulative and lingering in the environment.3Among the large variety of used drugs, non-steroidal anti-inflammatory pharmaceutical compounds are frequently identified in water resources matrices and the most diffused include aspirin and paracetamol.4 Consequently, their elimination from water matrixes is essential to maintain natural systems in healthy conditions. In general, wastewater matrixes present a broad variety of synthetic and poisonous organic and inorganic pollutants. Some of these molecules are inherently complex to eliminate due to their non-biodegradable structure; moreover, physical treatments are completely ineffective and civil wastewater plants have a limited use of specific chemical or advanced treatments.5 This means that pharmaceutical compounds or the derived metabolites are commonly not removed by typical wastewater plants, and the increasing finding of such substances in water bodies pushes to the individuation of new and effective technologies, able to reduce their concentration at acceptable levels. Hence, a wide number and type of treatment technologies have been tested to address the removal of such contaminants in wastewater, which can be easily and proficiently integrated in existing wastewater plants. Some common examples include precipitation, flotation, sedimentation, filtration, electrochemical and chemical oxidation, adsorption, ion exchange, catalysis and photocatalysis, and reverse osmosis, among many other possibilities.6,7 All the above-mentioned technologies have certain advantages and drawbacks. Nevertheless, adsorption has numerous advantages over the other processes mostly due to its effectiveness, cheapness, wide applicability, simplicity of deployment and exploitation, and flexibility, and for all these reasons it is one of the most widely employed processes.8 Furthermore, the performance of an adsorption process is strictly dependent on to the effectiveness of a certain adsorbent for the capture of a specific molecule. About this important point, various adsorbents have been investigated in order to remove different these emerging contaminants from wastewater, including activated carbons, raw agricultural solid wastes and wastes from forestry industries,9,10 while inorganic materials are generally ineffective. Due to the general low concentrations of pharmaceutical contaminants in wastewater, the adopted adsorbents must show a high adsorption capacity in the indicated conditions and must assure a high efficiency regardless the specific water composition. Activated carbons and carbonaceous adsorbents are usually few selective and the co-presence of other compounds can affect their performances. Moreover, the increase in adsorption capacity by means of tuned superficial functionalization can be a proficient mean factor in order to increase the cost-effectiveness of the treatment, because it allows to significantly reduce the extent of the intervention, mainly in terms of plant dimensions. As an example, cyclodextrins (CDs) are starch-based nanostructured materials that could potentially act as a good adsorbent for aspirin and paracetamol.11 Due to their solubility in water, CDs cannot be used directly for separation; they must be first made insoluble and supported on a porous material.12 In the literature, there are some attempts to use CDs for the removal of specific contaminants, such as heavy metals,13 endocrin disruptors,14 dyes,15 and some pharmaceutical compounds,4,16 all very recent. However, the properties of the produced adsorbent can significantly vary depending on the specific interactions between support and active phase, and the effects can be different depending on the probe molecule used for the adsorption tests. Hence, further studies are necessary, in order to assess an efficient adsorbent tuned for specific pharmaceutical compounds. In particular, the modelling of retrieved results and the deep understanding of the adsorption dynamics can provide invaluable information, which can be of great help in the design of real intervention based on adsorption devices.
In this work, CD polymers copolymerized with carbon nanotubes (CNTs), taken as porous support material, were considered as potential material for the synthesis of new adsorbents. The latter were doped with N nitrogen to obtain deficient and rough outer walls and to improve the covalent reaction with CD. Moreover, as iron (Fe) is known for its reducing properties and adsorption capacities,17,18 it was used as co-functionalizer for the doping of the CNT and another adsorbent was synthesized. Following these routes, such two new adsorbents (named N-CNT/β-CD and Fe/N-CNT/β-CD nanocomposite polymers) were individuated and their application to the removal of aspirin and paracetamol was considered.4 Subsequently, the research focused on the theoretical analysis of the adsorption mechanism of the two pharmaceutical molecules, i.e. aspirin and paracetamol, from aqueous solutions using the indicated adsorbents. To this aim, adsorption isotherms of both the compounds were retrieved on each of the synthesized adsorbents, at three different temperature levels. Three advanced analytical models developed by statistical physics, assuming the formation of one, two or more layers of pharmaceutical molecules on the adsorbent surface, were applied to the experimental adsorption isotherms data set, to infer important information about the adsorption mechanism.
2. Materials and methods
Adsorption isotherms of aspirin and paracetamol molecules on N-CNT/β-CD and Fe/N-CNT/β-CD nanopolymers were retrieved from the work of Mphahlele et al. 2015.4 The adsorbents tested in this work were obtained by microwave-assisted synthesis of nanocomposites.4 Additionally, batch equilibrium adsorption experiments were performed utilizing the bottle point test method. A mother solution of either aspirin or paracetamol aqueous solution (1000 mg L−1) was prepared and also diluted to the necessary initial concentrations. The aspirin and paracetamol adsorption capacity of the adsorbents was measured by contacting a constant mass (0.05 g) of adsorbent with a fixed volume (50 ml) of aspirin or paracetamol solution with initial concentrations varying from 10 to 100 mg L−1 in sealed plastic bottles. The bottles were stirred in an isothermal water bath agitator for 24 h to achieve equilibrium.43. Adsorption models
Adsorption isotherms were modeled in light of innovative models defined from the statistical physics; to this aim, different models assuming different hypotheses were set up and applied to the experimental data set.These models were defined and applied to explicate the adsorption mechanism at the microscopic level; subsequently, using the best-fitting model deriving from a fair comparison, further investigations were dedicated to analyze the steric and energetic parameters included in the model formulation.
The generalized expression of these models is provided by this equation:19
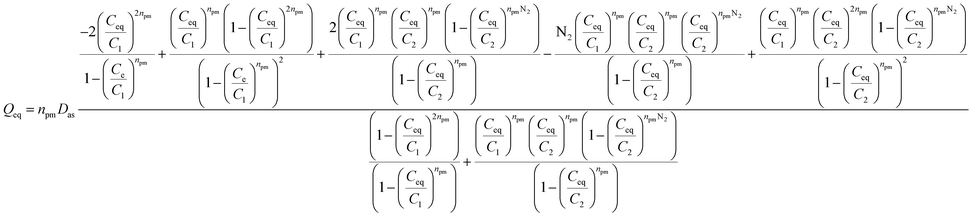 | (1) |
• If NTL = 1, a single layer of molecules on the surfaces of N-CNT/β-CD and Fe/N-CNT/β-CD nanocomposite polymers is formed, and each adsorption site can accept several pharmaceutical molecules simultaneously. Therefore, the previous equation becomes as (monolayer model, MM):20,21
 | (2) |
• If NTL = 2, two layers of pharmaceutical molecules are formed on the adsorbent surfaces, by two different adsorption energies. The first energy (−ε1) is associated to the interactions between the first layer of adsorbates and the adsorption sites and the second energy (−ε2) characterizes the interactions between the two layers of adsorbate molecules. The model can be referred to as double layer model with two types of adsorption sites (DLMTTAS), and the corresponding equation specifies as follows:7
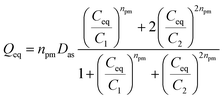 | (3) |
• If NTL = 1 + N2 > 2, in this case a multilayer model with saturation (MMS), which requires the formation of a finished number of layers of molecules on the adsorption sites, is hypothesized. A first layer is described by an energy (−ε1) and the other N2 layers are characterized by a second energy (−ε2). This is the case of generalized expression (1).
4. Results and discussion
4.1. Adsorption isotherms
The equilibrium adsorption isotherms retrieved at different temperatures (298, 308, and 318 K) for the adsorption of both aspirin and paracetamol using either N-CNT/β-CD or Fe/N-CNT/β-CD nanocomposite polymers, are displayed in Fig. 1.4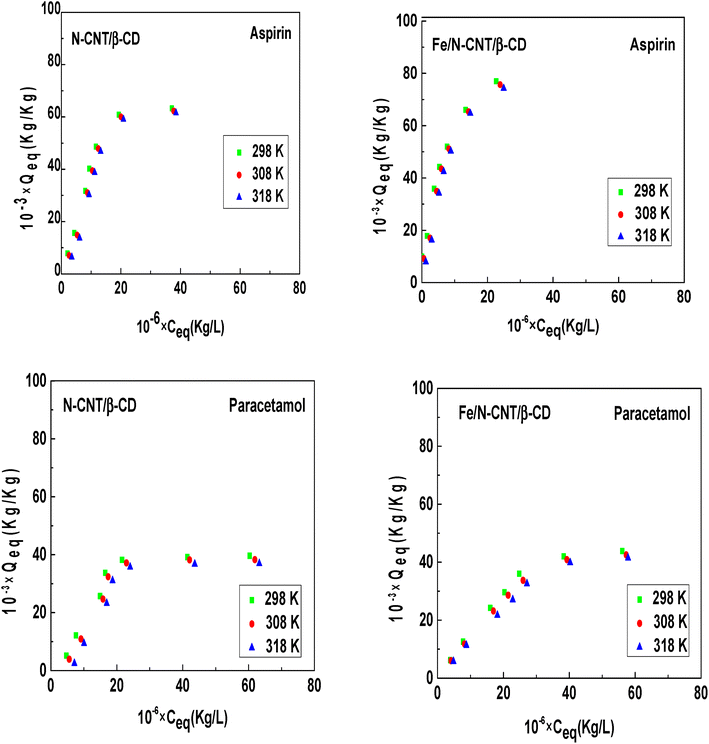 | ||
| Fig. 1 Equilibrium curves for aspirin and paracetamol adsorption on N-CNT/β-CD and Fe/N-CNT/β-CD nanocomposite polymers at three temperatures (298, 308, and 318 K).4 | ||
The experimental adsorption isotherms of the pharmaceutical molecules aspirin and paracetamol can be categorized as L-type curves in which a steeper initial part at low equilibrium concentrations can be recognized, testifying a strong affinity between the adsorbents and the tested pharmaceutical molecules. However, at higher equilibrium concentrations, in all the experimental systems, the adsorbed quantities tend towards a saturation plateau. This result may be the result of a complete adsorption sites occupation and the formation of one or more layers of molecules on the surface of N-CNT/β-CD and Fe/N-CNT/β-CD nanocomposite polymers. Concerning the impact of temperature, it is possible observing that the adsorption capacity of aspirin and paracetamol slightly decreased with the rise of temperature, for both the tested adsorbents N-CNT/β-CD and Fe/N-CNT/β-CD nanocomposite polymers. Finally, a fair comparison between the two synthesized adsorbents indicates that for aspirin adsorption no significant differences in the adsorption capacities can be retrieved, while for paracetamol it seems that Fe/N-CNT/β-CD nanocomposite shows slightly better performances.
Relying on these insights, Mphahlele et al. 2015 (ref. 4) applied two standard models, Langmuir and Freundlich, to explain the experimental adsorption data of aspirin and paracetamol on N-CNT/β-CD and Fe/N-CNT/β-CD nanocomposite polymers adsorbents. These classical models represent a typical first approach to the modelling analysis but they can lead to erroneous conclusions because of the straightforwardness of their basic underlying hypotheses. Thus, to surmount these limitations, three advanced models developed in statistical physics, which suppose the formation of one, two or more layers of the studied pharmaceutical molecules on the surface of the adsorbents, were fitted to the experimental data set, as reported in the following section.
4.2. Modelling analysis
The physical adsorption models previously described were utilized to fit all the experimental isotherms of aspirin and paracetamol, in order to predict the probable number of molecule layers formed on the surface of the N-CNT/β-CD and Fe/N-CNT/β-CD nanocomposite polymers during adsorption of the studied compounds. Moreover, a subsequent analysis of model parameters was dedicated to the determination of the number of molecules captured per adsorption site and its density, the adsorption capacities at saturation and the corresponding interaction energies. A multivariate nonlinear regression was carried out for the fitting of the experimental data by the Levenberg–Marquardt algorithm and the identification of the optimal model was based on the coefficient of determination R2. The modeling results demonstrate that the MMS model, which supposes the formation of several layers of pharmaceutical molecules on the surface of the tested adsorbents, showed the highest R2 values which ranged between 0.997 and 0.999 for N-CNT/β-CD and Fe/N-CNT/β-CD nanocomposites polymers respectively. In addition, the behavior of their physicochemical parameters versus temperature permitted to gain important insight into the adsorption mechanism of the tested pharmaceutical molecules. The adsorption isotherm curves of the pharmaceutical molecules fitted by the adapted model (MMS) are represented Fig. 2; the corresponding fitting coefficients and the adjustable parameters are listed in Table 1.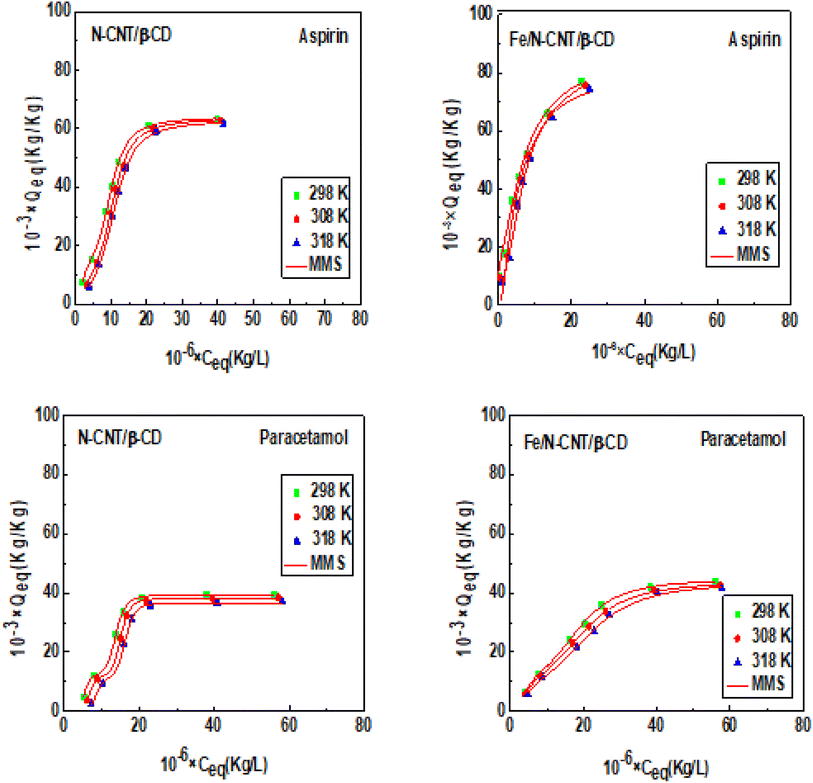 | ||
| Fig. 2 MMS Model adsorption data of aspirin and paracetamol on N-CNT/-CD and Fe/N-CNT/-CD nanocomposite polymers surface at different temperatures. | ||
| T (K) | R2 | npm | 10−3 × Das (kg kg−1) | N2 | 10−6 × C1 (kg L−1) | 10−6 × C2 (kg L−1) | 10−3 × Qs (kg kg−1) |
|---|---|---|---|---|---|---|---|
| Aspirin on N-CNT/β-CD | |||||||
| 298 | 0.997 | 1.78 | 7.21 | 4.04 | 2.48 | 10.47 | 64.68 |
| 308 | 0.999 | 2.48 | 5.56 | 3.53 | 4.07 | 11.46 | 62.46 |
| 318 | 0.997 | 2.85 | 4.80 | 3.47 | 4.90 | 12.42 | 61.14 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| Aspirin on Fe/N-CNT/β-CD | |||||||
| 298 | 0.999 | 2.51 | 6.15 | 3.13 | 2.14 | 10.12 | 63.75 |
| 308 | 0.998 | 2.16 | 5.93 | 3.96 | 3.42 | 11.04 | 63.53 |
| 318 | 0.999 | 2.03 | 6.13 | 4.06 | 4.76 | 11.77 | 62.96 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| Paracetamol on N-CNT/β-CD | |||||||
| 298 | 0.999 | 7.69 | 1.61 | 2.15 | 2.15 | 13.74 | 38.99 |
| 308 | 0.999 | 8.96 | 1.30 | 2.24 | 6.74 | 15.03 | 37.73 |
| 318 | 0.998 | 9.03 | 1.23 | 2.28 | 8.45 | 16.23 | 34.43 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| Paracetamol on Fe/N-CNT/β-CD | |||||||
| 298 | 0.997 | 2.44 | 5.62 | 2.27 | 4.33 | 19.46 | 44.84 |
| 308 | 0.998 | 2.21 | 5.83 | 2.42 | 4.52 | 21.01 | 44.06 |
| 318 | 0.998 | 2.12 | 5.93 | 2.51 | 5.32 | 23.13 | 44.12 |
4.3. Analysis of pharmaceutical molecules adsorption mechanism via MMS
A dedicated analysis of the physicochemical model parameters deriving by the experimental data fitting by MMS model at different temperature was carried out to reach a clearer comprehension of the adsorption mechanism of aspirin and paracetamol on the investigated adsorbents, at the microscopic scale.Computations with MMS revealed that NTL values ranged between 3.15 and 5.06 for the two pharmaceutical molecules. It can be deduced that the adsorption of these molecules was almost accomplished by the formation of 3 to 5 adsorbate layers, according to the operating temperature. Furthermore, it was feasible to identify the relative proportions of adsorbed molecules forming either three, four or five layers on the surface of the adsorbents. As an example, a value of NTL = 4.53 for aspirin adsorption onto N-CNT/β-CD at 308 K can be interpreted as an average contribution of molecules binding in four layers and molecules binding in five layers, according to this simple algorithm: k × 4 + (1 − k) × 5, where k is the percentage of molecules that formed four layers on the N-CNT/β-CD surface and (1 − k) is the percentage of adsorbed molecules corresponding to five layers, respectively.19 Under these assumptions, in the previous example, 47% of the aspirin molecules formed four layers and the remaining 53% formed five layers on the N-CNT/β-CD surface.
Regarding the temperature effect, the total number of layers of aspirin molecules formed on the surface of N-CNT/β-CD decreases by increasing the temperature, indicating that a classical effect of thermal agitation on this parameter was detected. However, temperature has a positive effect on this number for the other cases studied (slight for paracetamol). For the adsorption of aspirin on Fe-CNT/β-CD, the increase in adsorbed layers could be ascribed to different (stronger) interactions on the adsorbent surface, which propagates in the subsequent layers and make possible the establishment of a higher number of layers.
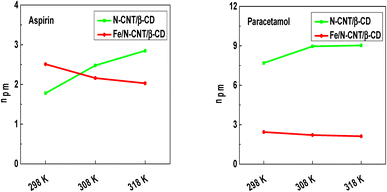 | ||
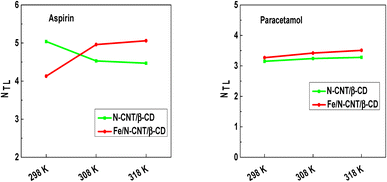
| Fig. 4 Evolution of the number of aspirin and paracetamol molecules captured by adsorption site versus temperature. | ||
A general overview allows noting that all npm values are exceeding unity, which suggests that the adsorption of pharmaceutical molecules is multimolecular and each adsorption site can capture simultaneously several molecules.22 In addition, the npm values demonstrate the presence of aggregation phenomena of aspirin and paracetamol molecules upon adsorption. Concerning the effect of temperature, modelling data suggests that it has a favorable influence on the number of aspirin and paracetamol molecules captured by the N-CNT/β-CD nanocomposite polymer adsorbent sites, which varies from 1.78 to 2.85 and from 7.69 to 9.03, respectively forming dimer, trimer, tetramer, pentamer, and hexamer etc. In fact, the increment of the number of molecules captured by each adsorption site according to the temperature indicates that the exothermal behavior (i.e. the reduction of adsorption capacity with temperature) is counter balanced by molecular aggregation.
On the contrary, for the Fe/N-CNT/β-CD nanocomposite polymer adsorbent, the number of aspirin and paracetamol molecules captured by adsorption site decreases with the temperature, passing from 2.51 to 2.03 and from 2.44 to 2.12, respectively. These obtained findings confirmed that thermal agitation caused the breakdown of the bonds between the molecules in the solution.
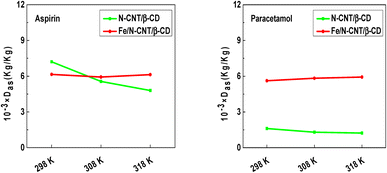 | ||
| Fig. 5 Impact of tested adsorption temperature on the density of adsorption site of N-CNT/β-CD and Fe/N-CNT/β-CD nanocomposite polymers adsorbents at saturation. | ||
From Fig. 5, it can be observed that the trend of the density of adsorption sites as a function of temperature is the inverse of that estimated for the number of molecules captured by adsorption site. For aspirin, adsorption density is similar for both the adsorbents, while for paracetamol the value retrieved for N-CNT/β-CD is by far higher. Together with data about molecular aggregation, this result testifies that the two adsorbents are characterized by different active sites, both in number and adsorption properties. However, for both the adsorbents, the slight dependence on adsorption temperature, characterized by a slight decrease of the adsorption site density, is perfectly in line with the slight exothermic behavior depicted in Fig. 1. In fact, as temperature rises, the decrease in adsorption capacity depends on the reduction of the number of adsorption sites, in some cases counterbalanced by the aggregation process of molecules on the adsorbent surface.
To qualify and compare the performances of the two tested adsorbents N-CNT/β-CD and Fe/N-CNT/β-CD nanocomposite polymers, the adsorption capacity at saturation was determined, based on this relationship:
| Qs = NTL × npm × Das | (4) |
The evolution of the adsorbed quantities at saturation associated with the two adsorbents N-CNT/β-CD and Fe/N-CNT/β-CD nanocomposite polymers as a function of temperature is provided in Fig. 6.
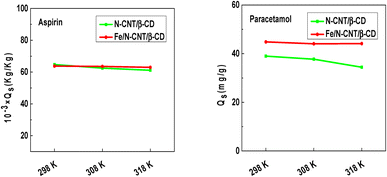 | ||
| Fig. 6 Adsorption capacities at saturation of aspirin and paracetamol molecules on the N-CNT/β-CD and Fe/N-CNT/β-CD nanocomposite polymers surface as a function of temperature. | ||
The adsorption capacities at saturation of the tested pharmaceutical molecules slightly decrease as a function of the temperature reaching 0.061 and 0.062 kg kg−1 for aspirin, and 0.034 and 0.044 kg kg−1 for paracetamol at the highest tested temperature, on N-CNT/β-CD and Fe/N-CNT/β-CD nanocomposite polymers surface, respectively. The results allow concluding that both the investigated adsorbents have a higher affinity towards aspirin, also confirming that, in the case of paracetamol, the inserting of Fe in the adsorbent formulation has a higher beneficial effects.
where Cs is the solubility of the pharmaceutical molecules in water and R is the ideal gas constant. Table 2 recaps the calculated adsorption energies (−ε1) and (−ε2) of the tested molecules.
| T (K) | (−ε1) (J mol−1) | (−ε2) (J mol−1) |
|---|---|---|
a It is evident that the adsorption of the pharmaceutical molecules aspirin and paracetamol on the N-CNT/β-CD and Fe/N-CNT/β-CD nanocomposite polymers surface involved weak physical type interactions, since the interaction energies do not overcome the threshold of 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 J mol−1. Accordingly, electrostatic forces, hydrogen bonding and van der Waals forces may be responsible for the adsorption of these molecules. It is remarkable that the interaction energies between adsorbent surface and adsorbate molecules for both N-CNT/β-CD and Fe/N-CNT/β-CD were significantly higher than those corresponding to the molecule–molecule interactions of the subsequent layers. In addition, the estimated interaction energies for the paracetamol molecules were stronger than those of aspirin, suggesting more effective adsorption forces. 000 J mol−1. Accordingly, electrostatic forces, hydrogen bonding and van der Waals forces may be responsible for the adsorption of these molecules. It is remarkable that the interaction energies between adsorbent surface and adsorbate molecules for both N-CNT/β-CD and Fe/N-CNT/β-CD were significantly higher than those corresponding to the molecule–molecule interactions of the subsequent layers. In addition, the estimated interaction energies for the paracetamol molecules were stronger than those of aspirin, suggesting more effective adsorption forces. |
||
| Aspirin on N-CNT/β-CD | ||
| 298 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 580 580 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 030 030 |
| 308 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 920 920 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 280 280 |
| 318 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 060 060 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 610 610 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Aspirin on Fe/N-CNT/β-CD | ||
| 298 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 950 950 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 110 110 |
| 308 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 370 370 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 380 380 |
| 318 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 140 140 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 750 750 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Paracetamol on N-CNT/β-CD | ||
| 298 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 680 680 |
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
| 308 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 470 470 |
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 430 430 |
| 318 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 560 560 |
17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 840 840 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Paracetamol on Fe/N-CNT/β-CD | ||
| 298 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 960 960 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 240 240 |
| 308 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 490 490 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 570 570 |
| 318 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 790 790 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 910 910 |
5. Conclusions
A theoretical assessment of the adsorption mechanism of aspirin and paracetamol molecules using N-CNT/β-CD and Fe/N-CNT/β-CD nanocomposite polymers adsorbents was performed using statistical physics theory. The calculations showed that the adsorption of these pharmaceutical molecules involved the formation of a high number of layers on the adsorbent surface (3 to 5 layers). The presence of molecular aggregates upon adsorption for these pharmaceuticals was also possible. The adsorption of these pharmaceuticals was associated to physical interaction forces. The findings also revealed that the adsorption capacities of aspirin and paracetamol on the Fe/N-CNT/β-CD adsorbent were higher than those of N-CNT/β-CD due to the presence of Fe in the nanocomposites, which allow establishing specific interactions with the investigated adsorbates. This study provides new insights into the adsorption mechanism of pharmaceutical molecules, which are important and priority pollutants in water treatment.Conflicts of interest
There are no conflicts of interest.Acknowledgements
The authors express their gratitude to Princess Nourah bint Abdulrahman University Researchers Supporting Project (Grant No. PNURSP2023R46), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.References
- A. C. Fröhlich, G. S. dos Reis, F. A. Pavan, É. C. Lima, E. L. Foletto and G. L. Dotto, Environ. Sci. Pollut. Res., 2018, 25, 24713–24725 CrossRef PubMed.
- H. Zeng, M. Gao, T. Shen and F. Ding, J. Taiwan Inst. Chem. Eng., 2018, 93, 329–335 CrossRef CAS.
- A. F. M. Streit, G. C. Collazzo, S. P. Druzian, R. S. Verdi, E. L. Foletto, L. F. S. Oliveira and G. L. Dotto, Chemosphere, 2021, 262, 128322 CrossRef CAS PubMed.
- K. Mphahlele, M. S. Onyango and S. D. Mhlanga, J. Environ. Chem. Eng., 2015, 3, 2619–2630 CrossRef CAS.
- A. C. Fröhlich, E. L. Foletto and G. L. Dotto, J. Cleaner Prod., 2019, 229, 828–837 CrossRef.
- S. Sharma and A. Bhattacharya, Appl. Water Sci., 2017, 7, 1043–1067 CrossRef CAS.
- C. Yanan, J. Ali, L. Sellaoui, F. Dhaouadi, M. Naeem, D. S. P. Franco, J. Georgin, A. Erto and M. Badawi, Chemosphere, 2023, 313, 137355 CrossRef PubMed.
- G. L. Dotto and G. McKay, J. Environ. Chem. Eng., 2020, 8, 103988 CrossRef CAS.
- N. Yeddou and A. Bensmaili, Desalination, 2005, 185, 499–508 CrossRef CAS.
- I. Ali, M. Asim and T. A. Khan, J. Environ. Manage., 2012, 113, 170–183 CrossRef CAS PubMed.
- M. Kitaoka and K. Hayashi, J. Incl. Phenom., 2002, 44, 429–431 CrossRef CAS.
- E. M. M. Del Valle, Process Biochem., 2004, 39, 1033–1046 CrossRef CAS.
- W. Xu, X. Liu and K. Tang, Carbohydr. Polym., 2022, 294, 119806 CrossRef CAS PubMed.
- T. Wei, H. Wu and Z. Li, J. Hazard. Mater., 2023, 441, 129969 CrossRef CAS.
- J. Yan, K. Li, J. Yan, Y. Fang and B. Liu, Appl. Surf. Sci., 2022, 602, 154338 CrossRef CAS.
- A. Skwierawska, D. Nowacka and K. Kozłowska-Tylingo, Water Resour. Ind., 2022, 28, 100186 CrossRef CAS.
- Y. Wang, R. Cheng, Z. Wen and L. Zhao, J. Chem. Eng., 2012, 203, 277–284 CrossRef CAS.
- V. Nahuel Montesinos, N. Quici, E. Beatriz Halac, A. G. Leyva, G. Custo, S. Bengio, G. Zampieri and M. I. Litter, J. Chem. Eng., 2014, 244, 569–575 CrossRef CAS.
- F. Dhaouadi, L. Sellaoui, T. Sonia, F. Louis, A. El Bakali, M. Badawi, J. Georgin, D. Franco, L. Silva, A. Bonilla-Petriciolet and S. Rtimi, J. Chem. Eng., 2022, 445, 136773 CrossRef CAS.
- F. Dhaouadi, L. Sellaoui, B. Chavez-Gonzalez, H. E. Reynel-Ávila, L. L. Diaz-Muñoz, D. I. Mendoza-Castillo, A. Bonilla-Petriciolet, E. C. Lima, J. C. Tapia-Picazo and A. B. Lamine, J. Chem. Eng., 2021, 417, 127975 CrossRef CAS.
- F. Dhaouadi, L. Sellaoui, H. E. Reynel-Ávila, V. Landín-Sandoval, D. I. Mendoza-Castillo, J. E. Jaime-Leal, E. C. Lima, A. Bonilla-Petriciolet and A. B. Lamine, Environ. Sci. Pollut. Res., 2021, 28, 30943–30954 CrossRef CAS PubMed.
- L. Sellaoui, D. I. Mendoza-Castillo, H. E. Reynel-Ávila, A. Bonilla-Petriciolet, A. Ben Lamine and A. Erto, J. Chem. Eng., 2018, 343, 544–553 CrossRef CAS.
- L. Sellaoui, F. Dhaouadi, H. E. Reynel-Avila, D. I. Mendoza-Castillo, A. Bonilla-Petriciolet, R. Trejo-Valencia, S. Taamalli, F. Louis, A. El Bakali and Z. Chen, Environ. Sci. Pollut. Res., 2021, 28, 67248–67255 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2023 |