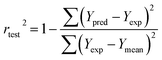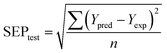Open Access Article
Open Access ArticleSynthesis of 3,4-dihydroisoquinolin-1(2H)-one derivatives and their antioomycete activity against the phytopathogen Pythium recalcitrans†
Delong Wang‡
 ,
Min Li‡,
Jing Li,
Yali Fang
,
Min Li‡,
Jing Li,
Yali Fang * and
Zhijia Zhang*
* and
Zhijia Zhang*
Department of Pharmaceutical Engineering, College of Plant Protection, Shanxi Key Laboratory of Integrated Pest Management in Agriculture, Shanxi Agricultural University, Taiyuan 030031, China. E-mail: fang_august@163.com; Zhang770504@126.com
First published on 3rd April 2023
Abstract
In an effort to exploit the bioactive natural scaffold 3,4-dihydroisoquinolin-1(2H)-one for plant disease management, 59 derivatives of this scaffold were synthesized using the Castagnoli–Cushman reaction. The results of bioassay indicated that their antioomycete activity against Pythium recalcitrans was superior to the antifungal activity against the other 6 phytopathogens. Compound I23 showed the highest in vitro potency against P. recalcitrans with an EC50 value of 14 μM, which was higher than that of the commercial hymexazol (37.7 μM). Moreover, I23 exhibited in vivo preventive efficacy of 75.4% at the dose of 2.0 mg/pot, which did not show significant differences compared with those of hymexazol treatments (63.9%). When the dose was 5.0 mg per pot, I23 achieved a preventive efficacy of 96.5%. The results of the physiological and biochemical analysis, the ultrastructural observation and lipidomics analysis suggested that the mode of action of I23 might be the disruption of the biological membrane systems of P. recalcitrans. In addition, the established CoMFA and CoMSIA models with reasonable statistics in the three-dimensional quantitative structure–activity relationship (3D-QSAR) study revealed the necessity of the C4-carboxyl group and other structural requirements for activity. Overall, the above results would help us to better understand the mode of action and the SAR of these derivatives, and provide crucial information for further design and development of more potent 3,4-dihydroisoquinolin-1(2H)-one derivatives as antioomycete agents against P. recalcitrans.
1. Introduction
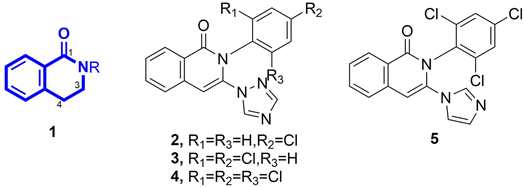
Natural products (NPs) have achieved a long tradition as a source of agrochemical active ingredients.1–3 However, NPs are often perceived as chemically complex molecules and synthetically intractable, have limited availability of resources, or possess undesired substructural elements that impede their suitability for direct application in agricultural settings.4 More often than not, a prominent exploitation strategy is the simplification of the NP structures and their molecular frameworks to provide a synthetically accessible bioactive scaffold, equivalent or mimic for semisynthetic optimization, ultimately leading to the establishment of the desired compounds for application in crop protection.4 Correspondingly, such an approach renders synthetic entities of NP mimics that account for 14% of the crop protection product market.1,4 Particularly, this exploitation strategy could be exemplified by the commercial fungicide florylpicoxamid5 and insecticide flupyradifurone6 that have been released on the market recently. Considering these aspects, NP-inspired synthetic scaffolds or mimics can afford feasible and innovative solutions to the aforementioned significant and enduring discovery challenges of crop protection agents.The fragment of 3,4-dihydroisoquinolin-1(2H)-one (1) (Fig. 1) is prevalently encountered in numerous NPs with various biological activities.7,8 Consequently, it has been utilized as a privileged scaffold in the discovery of synthetically accessible drug molecules, such as antitumor,9–13 antimicrobial,14–18 antiviral,19 and antifungal16 agents. Among the many synthetic approaches explored, the Castagnoli–Cushman reaction (CCR) between homophthalic anhydride and inimes is especially appealing because the reaction not only produces the desired products in high yields, but also offers a remarkably facile and often diastereoselective entry to derivatives of this scaffold that are 2,3-disubstituted and occur a carboxylic acid function at the C4 site.20,21 By simply drawing from pertinent pools of substrates, this robust, streamlined, straightforward, and flexible approach could enable independent change of substituents around the core scaffold in question, generating diverse chemical libraries for use in the screening of valuable molecules. However, as revealed by thorough literature retrieval, the derivatives of scaffold 1 regarding the bioactivity against phytopathogens have received far less attention. Nevertheless, despite the dearth of research, compounds 2–5 (Fig. 1), four isoquinolin-1(2H)-one derivatives sharing the same carbon skeleton with scaffold 1, showed moderate in vivo control efficacies against Blumeria graminis (DC.) Speer, Puccinia recondite, Botrytis cinerea, and Plasmopara viticola,22 indicating its potential as a starting point for the development of crop protection agents in the plant disease management.
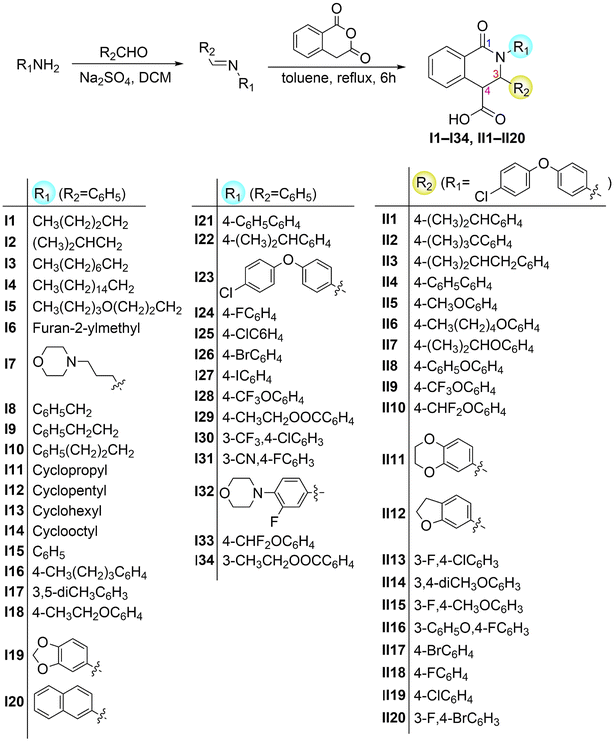
In the present study, we synthesized 59 derivatives of scaffold 1 by employing the CCR and esterification reaction (Scheme 1 and 2), and then we evaluated their antifungal or antioomycete activities. The obtained compounds exhibited high in vitro inhibition activity against the phytopathogen Pythium recalcitrans, and their antioomycete activity was optimized via sequential incorporation of various substituents to the N2, C3, and C4 sites. The three-dimensional quantitative structure–activity relationship (3D-QSAR) was further studied. Finally, the control efficacy of the most potent compound and its antioomycete effects on the physiological and morphological changes of P. recalcitrans were investigated as well.
2. Experimental
2.1 General remarks
The analytical grade reagents used were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. (China). Dichloromethane (DCM) and toluene, the two reaction solvents, were newly distilled in the reactions before drying under reflux over calcium hydride and sodium wires, respectively. The other starting materials, unless otherwise stated, were utilized without additional purification. On a Bruker AV-400 (or 500) instrument, 1H NMR and proton-decoupled 13C NMR spectra were recorded using CDCl3 or DMSO-d6 as the solvent. Chemical shifts (δ) are recorded in ppm, relative to the residual solvent peaks (CDCl3, δ 7.26 for 1H and δ 77.36 for 13C; DMSO-d6, δ 2.50 for 1H and δ 39.52 for 13C). The coupling constants (J) were shown in Hertz. Data of 1H NMR spectra are presented as follows: chemical shift (multiplicity, coupling constants, and the number of hydrogen). The types of peak splitting are as follows: s (singlet), d (doublet), t (triplet), q (quartet), m (multiplet), and bs (broadening singlet). High-resolution ESI mass experiments were operated on a Waters Xevo G2-XS instrument with a Quadrupole-Orbitrap mass analyzer. The X-ray diffraction dataset was collected on an XtaLAB Synergy-R single crystal X-ray diffractometer (Rigaku Corporation, Japan). Column chromatography was performed using silica gel (200–300 mesh) purchased from Qingdao Haiyang Chemical Co., China. Seven plant pathogens, Pythium recalcitrans, B. cinerea, Fusarium oxysporum f. sp. niveum, Trichothecium roseum, Colletotrichum gloeosporioides, Alternaria mali, and Rhizoctonia solani were used for the bioassay experiment. These isolates kept on sterile potato dextrose agar (PDA; aqueous extract of 200 g of peeled potato, 20 g of dextrose, and 20 g of agar in 1.0 L of distilled water sterilized in an autoclave at 121 °C for 15 min) plates at 4 °C are stored in Plant Pathology Laboratory, College of Plant Protection, Shanxi Agricultural University and are available from the authors upon request. Sterile potato dextrose broth (PDB; aqueous extract of 200 g of peeled potato and 20 g of dextrose in 1.0 L of distilled water sterilized in an autoclave at 121 °C for 15 min) was used for culture of P. recalcitrans mycelia. For the lipidomics experiment, mass spectrometry grade reagents methanol (CH3OH), acetonitrile (CH3CN), 2-propanol, and methyl-tert-butyl ether (MTBE) were purchased from Thermo Fisher and HPLC-grade formic acid and ammonium formate were purchased from Sigma.2.2 Synthesis of 3,4-dihydroisoquinolin-1(2H)-one derivatives
2-Butyl-1-oxo-3-phenyl-1,2,3,4-tetrahydroisoquinoline-4-carboxylic acid (I1): white powder, yield 508 mg (52.4%), mp 206–208 °C. 1H NMR (400 MHz, DMSO-d6) δH (ppm): 13.15 (s, 1H), 7.93–7.91 (m, 1H), 7.40–7.36 (m, 2H), 7.26–7.17 (m, 4H), 7.07–7.06 (m, 2H), 5.34 (s, 1H), 4.13 (s, 1H), 4.04–3.97 (m, 1H), 2.77–2.70 (m, 1H), 1.54–1.50 (m, 2H), 1.29–1.23 (m, 2H), 0.86 (t, J = 7.2 Hz, 3H). 13C NMR (100 MHz, DMSO-d6) δC (ppm): 172.6, 163.4, 140.0, 134.1, 132.1, 130.0, 129.6, 129.0, 128.2, 127.9, 127.2, 126.5, 61.1, 51.1, 46.0, 30.0, 20.1, 14.3. HRMS (ESI) m/z: [M + H]+, calcd for C20H22NO3: 324.1600, found: 324.1596.
2-Isobutyl-1-oxo-3-phenyl-1,2,3,4-tetrahydroisoquinoline-4-carboxylic acid (I2): white powder, yield 553 mg (57%), mp 176–178 °C. 1H NMR (400 MHz, DMSO-d6) δH (ppm): 13.18 (s, 1H), 7.98–7.96 (m, 1H), 7.41–7.38 (m, 2H), 7.27–7.18 (m, 4H), 7.08–7.06 (m, 2H), 5.36 (s, 1H), 4.18 (s, 1H), 3.96–3.91 (m, 1H), 2.49–2.45 (m, 1H), 2.06–1.99 (m, 1H), 0.89 (d, J = 6.4 Hz, 3H), 0.85 (d, J = 6.4 Hz, 3H). 13C NMR (100 MHz, DMSO-d6) δC (ppm): 172.3, 163.6, 139.4, 133.6, 131.7, 129.6, 129.1, 128.6, 127.8, 127.4, 126.9, 126.0, 61.1, 52.8, 50.7, 26.9, 20.3, 20.2. HRMS (ESI) m/z: [M + H]+, calcd for C20H22NO3: 324.1600, found: 324.1596.
2-Octyl-1-oxo-3-phenyl-1,2,3,4-tetrahydroisoquinoline-4-carboxylic acid (I3): white powder, yield 603 mg (53%), mp 212–214 °C. 1H NMR (400 MHz, DMSO-d6) δH (ppm): 13.04 (s, 1H), 7.92–7.91 (m, 1H), 7.41–7.36 (m, 2H), 7.25–7.16 (m, 4H), 7.07–7.05 (m, 2H), 5.33 (s, 1H), 4.11 (s, 1H), 3.99–3.93 (m, 1H), 2.77–2.72 (m, 1H), 1.54–1.52 (m, 2H), 1.22 (m, 10H), 0.85 (t, J = 5.2 Hz, 3H). 13C NMR (100 MHz, DMSO-d6) δC (ppm): 172.6, 163.4, 140.1, 134.1, 132.1, 130.0, 129.7, 129.0, 128.2, 127.9, 127.2, 126.5, 61.2, 51.2, 46.4, 31.7, 29.3, 29.1, 27.8, 26.9, 22.6, 14.4. HRMS (ESI) m/z: [M + H]+, calcd for C24H30NO3: 380.2226, found: 380.2224.
2-Hexadecyl-1-oxo-3-phenyl-1,2,3,4-tetrahydroisoquinoline-4-carboxylic acid (I4): white powder, yield 833 mg (56.5%), mp 224–226 °C. 1H NMR (400 MHz, DMSO-d6) δH (ppm): 13.02 (s, 1H), 8.03–8.02 (m, 1H), 7.54–7.48 (m, 2H), 7.44–7.41 (m, 1H), 7.21–7.18 (m, 3H), 7.01–6.99 (m, 2H), 5.09 (d, J = 4.8 Hz, 1H), 4.70 (d, J = 4.4 Hz, 1H), 3.84–3.78 (m, 1H), 2.86–2.81 (m, 1H), 1.53–1.52 (m, 1H), 1.44–1.43 (m, 1H), 1.20 (m, 26H), 0.84 (t, J = 5.2 Hz, 3H). 13C NMR (100 MHz, DMSO-d6) δC (ppm): 170.8, 163.1, 137.9, 133.9, 132.2, 129.6, 128.6, 128.5, 128.3, 128.2, 127.8, 127.7, 61.7, 48.7, 46.2, 31.8, 29.6, 29.5, 29.4, 29.2, 27.8, 26.9, 22.6, 14.4. HRMS (ESI) m/z: [M + H]+, calcd for C32H46NO3: 492.3478, found: 492.3484.
2-(3-Butoxypropyl)-1-oxo-3-phenyl-1,2,3,4-tetrahydroisoquinoline-4-carboxylic acid (I5): white powder, yield 789 mg (69%), mp 236–238 °C. 1H NMR (400 MHz, DMSO-d6) δH (ppm): 13.06 (s, 1H), 7.94–7.92 (m, 1H), 7.40–7.37 (m, 2H), 7.26–7.18 (m, 4H), 7.07–7.06 (m, 2H), 5.33 (s, 1H), 4.13 (s, 1H), 4.04–4.00 (m, 1H), 3.40–3.33 (m, 4H), 2.84–2.79 (m, 1H), 1.83–1.74 (m, 2H), 1.47–1.44 (m, 2H), 1.32–1.27 (m, 2H), 0.86 (t, J = 6.0 Hz, 3H). 13C NMR (100 MHz, DMSO-d6) δC (ppm): 172.1, 163.0, 139.5, 133.5, 131.7, 129.6, 129.1, 128.6, 127.8, 127.5, 126.8, 126.0, 69.7, 67.6, 60.9, 50.6, 43.6, 31.4, 27.9, 18.9, 13.8. HRMS (ESI) m/z: [M + H]+, calcd for C23H28NO4: 382.2018, found: 382.2022.
2-(Furan-2-ylmethyl)-1-oxo-3-phenyl-1,2,3,4-tetrahydroisoquinoline-4-carboxylic acid (I6): white powder, yield 781 mg (75%), mp 238–239 °C. 1H NMR (400 MHz, DMSO-d6) δH (ppm): 12.99 (s, 1H), 8.03–8.01 (m, 1H), 7.48 (s, 1H), 7.42–7.40 (m, 2H), 7.21–7.15 (m, 4H), 7.04–7.02 (m, 2H), 6.31 (s, 2H), 5.39 (s, 1H), 5.12 (d, J = 12.4 Hz, 1H), 4.23 (d, J = 12.4 Hz, 1H), 4.14 (s, 1H). 13C NMR (100 MHz, DMSO-d6) δC (ppm): 172.0, 163.0, 150.0, 142.6, 139.0, 133.7, 132.1, 129.6, 128.9, 128.5, 128.0, 127.4, 127.1, 125.9, 110.4, 108.9, 60.7, 50.8, 42.0. HRMS (ESI) m/z: [M + H]+, calcd for C21H18NO4: 348.1236, found: 348.1229.
2-(3-Morpholinopropyl)-1-oxo-3-phenyl-1,2,3,4-tetrahydroisoquinoline-4-carboxylic acid (I7): white powder, yield 869 mg (73.5%), mp 221–223 °C. 1H NMR (400 MHz, DMSO-d6) δH (ppm): 13.00 (s, 1H), 7.92–7.91 (m, 1H), 7.40–7.34 (m, 2H), 7.26–7.18 (m, 4H), 7.07–7.05 (m, 2H), 5.36 (s, 1H), 4.06 (s, 1H), 4.03–3.98 (m, 1H), 3.57 (t, J = 1.6 Hz, 4H), 2.81–2.76 (m, 1H), 2.40–2.35 (m, 6H), 1.80–1.71 (m, 2H). 13C NMR (100 MHz, DMSO-d6) δC (ppm): 172.3, 163.1, 139.8, 134.1, 131.6, 129.5, 129.1, 128.6, 127.6, 127.4, 126.7, 126.0, 65.9, 61.1, 55.2, 52.9, 51.1, 44.2, 24.1. HRMS (ESI) m/z: [M + H]+, calcd for C23H27N2O4: 395.1971, found: 395.1967.
2-Benzyl-1-oxo-3-phenyl-1,2,3,4-tetrahydroisoquinoline-4-carboxylic acid (I8): white powder, yield 772 mg (72%), mp 171–173 °C. 1H NMR (400 MHz, DMSO-d6) δH (ppm): 13.03 (s, 1H), 8.07–8.06 (m, 1H), 7.44–7.42 (m, 2H), 7.30–7.19 (m, 9H), 7.08–7.07 (m, 2H), 5.35 (s, 1H), 5.32 (d, J = 12.0 Hz, 1H), 4.14 (s, 1H), 3.91 (d, J = 12.0 Hz, 1H). 13C NMR (100 MHz, DMSO-d6) δC (ppm): 172.1, 163.5, 139.2, 137.2, 133.6, 132.0, 129.6, 129.0, 128.7, 128.2, 128.0, 128.0, 127.6, 127.1, 127.0, 126.1, 61.3, 50.9, 49.4. HRMS (ESI) m/z: [M + H]+, calcd for C23H20NO3: 358.1443, found: 358.1433.
1-Oxo-2-phenethyl-3-phenyl-1,2,3,4-tetrahydroisoquinoline-4-carboxylic acid (I9): white powder, yield 855 mg (76.8%), mp 168–170 °C. 1H NMR (400 MHz, DMSO-d6) δH (ppm): 13.07 (s, 1H), 7.96–7.94 (m, 1H), 7.43–7.38 (m, 2H), 7.29–7.18 (m, 9H), 7.12–7.10 (m, 2H), 5.54 (s, 1H), 4.19 (s, 1H), 4.18–4.12 (m, 1H), 3.05–2.92 (m, 2H), 2.80–2.74 (m, 1H). 13C NMR (100 MHz, DMSO-d6) δC (ppm): 172.1, 162.8, 139.5, 138.9, 133.7, 131.8, 129.6, 129.1, 128.6, 128.4, 128.2, 127.8, 127.4, 126.8, 126.2, 126.1, 60.8, 50.6, 48.0, 33.6. HRMS (ESI) m/z: [M + H]+, calcd for C24H22NO3: 372.1600, found: 372.1589.
1-Oxo-3-phenyl-2-(3-phenylpropyl)-1,2,3,4-tetrahydroisoquinoline-4-carboxylic acid (I10): white powder, yield 811 mg (70.2%), mp 164–166 °C. 1H NMR (400 MHz, DMSO-d6) δH (ppm): 13.09 (s, 1H), 7.97–7.95 (m, 1H), 7.42–7.37 (m, 2H), 7.28–7.17 (m, 9H), 7.09–7.07 (m, 2H), 5.39 (s, 1H), 4.16 (s, 1H), 4.12–4.06 (m, 1H), 2.83–2.78 (m, 1H), 2.61–2.56 (m, 2H), 1.88 (t, J = 6.4 Hz, 2H). 13C NMR (100 MHz, DMSO-d6) δC (ppm): 172.2, 163.0, 141.9, 139.5, 133.5, 131.8, 129.6, 129.1, 128.6, 128.3, 127.8, 127.5, 126.8, 126.1, 125.7, 60.6, 50.6, 45.7, 32.7, 29.5. HRMS (ESI) m/z: [M + H]+, calcd for C25H24NO3: 386.1756, found: 386.1754.
2-Cyclopropyl-1-oxo-3-phenyl-1,2,3,4-tetrahydroisoquinoline-4-carboxylic acid (I11): white powder, yield 733 mg (79.5%), mp 155–157 °C. 1H NMR (400 MHz, DMSO-d6) δH (ppm): 13.01 (s, 1H), 7.94–7.92 (m, 1H), 7.40–7.36 (m, 2H), 7.27–7.21 (m, 3H), 7.19–7.17 (m, 1H), 7.12–7.11 (m, 2H), 5.30 (s, 1H), 4.17 (s, 1H), 2.80–2.77 (m, 1H), 0.92–0.90 (m, 1H), 0.77–0.74 (m, 1H), 0.67–0.56 (m, 2H). 13C NMR (100 MHz, DMSO-d6) δC (ppm): 172.0, 164.6, 139.8, 133.5, 131.9, 129.7, 129.1, 128.6, 127.7, 127.3, 126.8, 125.8, 61.6, 50.7, 29.7, 8.2, 5.3. HRMS (ESI) m/z: [M + H]+, calcd for C19H18NO3: 308.1287, found: 308.1283.
2-Cyclopentyl-1-oxo-3-phenyl-1,2,3,4-tetrahydroisoquinoline-4-carboxylic acid (I12): white powder, yield 784 mg (78%), mp 172–175 °C. 1H NMR (400 MHz, DMSO-d6) δH (ppm): 13.04 (s, 1H), 7.93–7.91 (m, 1H), 7.37–7.35 (m, 2H), 7.22–7.19 (m, 2H), 7.15–7.14 (m, 2H), 7.09–7.08 (m, 2H), 5.32 (s, 1H), 4.90–4.83 (m, 1H), 4.10 (s, 1H), 1.78–1.74 (m, 2H), 1.66–1.63 (m, 1H), 1.57–1.50 (m, 2H), 1.45–1.37 (m, 2H), 1.27–1.18 (m, 1H). 13C NMR (100 MHz, DMSO-d6) δC (ppm): 171.9, 163.1, 141.0, 133.1, 131.5, 130.1, 129.2, 128.4, 127.7, 127.1, 126.8, 125.9, 57.1, 55.2, 51.3, 28.8, 28.0, 23.3, 22.7. HRMS (ESI) m/z: [M + H]+, calcd for C21H22NO3: 336.1600, found: 336.1591.
2-Cyclohexyl-1-oxo-3-phenyl-1,2,3,4-tetrahydroisoquinoline-4-carboxylic acid (I13): white powder, yield 852 mg (81.3%), mp 188–190 °C. 1H NMR (400 MHz, DMSO-d6) δH (ppm): 13.03 (s, 1H), 7.95–7.93 (m, 1H), 7.37–7.36 (m, 2H), 7.20–7.07 (m, 6H), 5.40 (s, 1H), 4.49–4.46 (m, 1H), 4.03 (s, 1H), 1.76–1.74 (m, 1H), 1.66–1.52 (m, 4H), 1.34–1.31 (m, 2H), 1.21–1.16 (m, 1H), 1.08–0.99 (m, 2H). 13C NMR (100 MHz, DMSO-d6) δC (ppm): 171.9, 162.5, 141.3, 133.0, 131.5, 130.3, 129.2, 128.2, 127.8, 127.1, 126.9, 126.1, 56.8, 53.2, 51.7, 30.1, 29.9, 25.5, 25.4, 24.9. HRMS (ESI) m/z: [M + H]+, calcd for C22H24NO3: 350.1756, found: 350.1750.
2-Cyclooctyl-1-oxo-3-phenyl-1,2,3,4-tetrahydroisoquinoline-4-carboxylic acid (I14): white powder, yield 877 mg (77.5%), mp 185–188 °C. 1H NMR (400 MHz, DMSO-d6) δH (ppm): 13.08 (s, 1H), 7.96–7.94 (m, 1H), 7.38–7.35 (m, 2H), 7.20–7.07 (m, 6H), 5.35 (s, 1H), 3.99 (s, 1H), 1.97–1.93 (m, 1H), 1.71–1.44 (m, 14H). 13C NMR (100 MHz, DMSO-d6) δC (ppm): 172.2, 162.2, 141.3, 133.0, 131.4, 130.5, 129.1, 128.3, 127.8, 127.2, 126.9, 126.2, 58.6, 55.1, 51.8, 31.3, 30.3, 26.1, 26.0, 25.5, 24.8, 24.2. HRMS (ESI) m/z: [M + H]+, calcd for C24H28NO3: 378.2069, found: 378.2067.
1-Oxo-2,3-diphenyl-1,2,3,4-tetrahydroisoquinoline-4-carboxylic acid (I15): white powder, yield 690 mg (67%), mp 202–203 °C. 1H NMR (400 MHz, DMSO-d6) δH (ppm): 13.03 (s, 1H), 8.09–8.07 (m, 1H), 7.62–7.57 (m, 2H), 7.52–7.48 (m, 1H), 7.33–7.29 (m, 2H), 7.20–7.16 (m, 6H), 7.05–7.03 (m, 2H), 5.50 (d, J = 5.6 Hz, 1H), 4.96 (d, J = 5.6 Hz, 1H). 13C NMR (100 MHz, DMSO-d6) δC (ppm): 170.5, 162.9, 141.7, 137.3, 134.3, 132.3, 129.2, 128.6, 128.2, 128.0, 127.9, 127.8, 127.6, 127.4, 126.6, 64.4, 49.0. HRMS (ESI) m/z: [M + H]+, calcd for C22H18NO3: 344.1287, found: 344.1277.
2-(4-Butylphenyl)-1-oxo-3-phenyl-1,2,3,4-tetrahydroisoquinoline-4-carboxylic acid (I16): white powder, yield 650 mg (64.3%), mp 186–188 °C. 1H NMR (400 MHz, DMSO-d6) δH (ppm): 13.03 (s, 1H), 8.10–8.08 (m, 1H), 7.62–7.53 (m, 2H), 7.48–7.45 (m, 1H), 7.15–7.04 (m, 9H), 5.46 (d, J = 4.0 Hz, 1H), 4.97 (d, J = 4.0 Hz, 1H), 2.50 (t, J = 6.0 Hz, 2H), 1.51–1.48 (m, 2H), 1.28–1.24 (m, 2H), 0.86 (t, J = 5.6 Hz, 3H). 13C NMR (100 MHz, DMSO-d6) δC (ppm): 170.4, 162.9, 140.7, 139.4, 137.3, 134.1, 132.2, 129.3, 128.4, 128.1, 128.0, 127.8, 127.8, 127.6, 127.1, 64.6, 49.0, 34.4, 33.0, 21.8, 13.8. HRMS (ESI) m/z: [M + H]+, calcd for C26H26NO3: 400.1913, found: 400.1903.
2-(3,5-Dimethylphenyl)-1-oxo-3-phenyl-1,2,3,4-tetrahydroisoquinoline-4-carboxylic acid (I17): white powder, yield 702 mg (63%), mp 189–191 °C. 1H NMR (400 MHz, DMSO-d6) δH (ppm): 13.09 (s, 1H), 8.12–8.11 (m, 1H), 7.66–7.47 (m, 3H), 7.18–7.17 (m, 3H), 7.06–6.85 (m, 5H), 5.47 (d, J = 4.8 Hz, 1H), 5.02 (d, J = 4.8 Hz, 1H), 2.18 (s, 6H). 13C NMR (100 MHz, DMSO-d6) δC (ppm): 170.5, 162.8, 141.8, 137.8, 137.3, 134.1, 132.3, 129.4, 128.3, 128.2, 128.1, 127.9, 127.9, 127.8, 127.6, 125.1, 64.7, 48.9, 20.8. HRMS (ESI) m/z: [M + H]+, calcd for C24H22NO3: 372.1600, found: 372.1589.
2-(4-Ethoxyphenyl)-1-oxo-3-phenyl-1,2,3,4-tetrahydroisoquinoline-4-carboxylic acid (I18): white powder, yield 872 mg (75.1%), mp 207–209 °C. 1H NMR (400 MHz, DMSO-d6) δH (ppm): 13.04 (s, 1H), 8.09–8.07 (m, 1H), 7.62–7.55 (m, 2H), 7.50–7.47 (m, 1H), 7.18–7.16 (m, 3H), 7.09–7.03 (m, 2H), 7.03–7.01 (m, 2H), 6.85–6.82 (m, 2H), 5.42 (d, J = 5.6 Hz, 1H), 4.97 (d, J = 5.6 Hz, 1H), 3.96 (q, J = 6.8 Hz, 2H), 1.29 (t, J = 6.8 Hz, 3H). 13C NMR (100 MHz, DMSO-d6) δC (ppm): 170.9, 163.4, 157.4, 137.8, 134.8, 134.7, 132.7, 129.7, 129.0, 128.6, 128.4, 128.4, 128.3, 128.2, 128.0, 114.7, 65.2, 63.6, 49.4, 15.1. HRMS (ESI) m/z: [M + H]+, calcd for C24H22NO4: 388.1549, found: 372.1549.
2-(Benzo[d][1,3]dioxol-5-yl)-1-oxo-3-phenyl-1,2,3,4-tetrahydroisoquinoline-4-carboxylic acid (I19): white powder, yield 779 mg (67.1%), mp 217–219 °C. 1H NMR (400 MHz, DMSO-d6) δH (ppm): 13.03 (s, 1H), 8.09–8.07 (m, 1H), 7.61–7.56 (m, 2H), 7.50–7.47 (m, 1H), 7.19–7.18 (m, 3H), 7.05–7.04 (m, 2H), 6.84–6.78 (m, 2H), 6.63–6.61 (m, 1H), 6.00 (s, 1H), 5.98 (s, 1H), 5.42 (d, J = 4.8 Hz, 1H), 4.95 (d, J = 4.8 Hz, 1H). 13C NMR (100 MHz, DMSO-d6) δC (ppm): 170.5, 163.1, 147.1, 145.8, 137.2, 135.6, 134.3, 132.3, 129.2, 128.2, 128.1, 128.0, 127.8, 127.6, 120.8, 118.1, 108.9, 107.8, 101.5, 64.8, 49.0. HRMS (ESI) m/z: [M + H]+, calcd for C23H18NO5: 388.1185, found: 388.1195.
2-(Naphthalen-2-yl)-1-oxo-3-phenyl-1,2,3,4-tetrahydroisoquinoline-4-carboxylic acid (I20): white powder, yield 866 mg (73.4%), mp 195–198 °C. 1H NMR (400 MHz, DMSO-d6) δH (ppm): 13.08 (s, 1H), 8.15–8.13 (m, 1H), 7.87–7.79 (m, 4H), 7.66–7.60 (m, 3H), 7.54–7.51 (m, 1H), 7.49–7.47 (m, 2H), 7.39–7.37 (m, 1H), 7.15–7.11 (m, 4H), 5.70 (d, J = 4.8 Hz, 1H), 5.01 (d, J = 4.8 Hz, 1H). 13C NMR (100 MHz, DMSO-d6) δC (ppm): 170.6, 163.2, 139.3, 137.2, 134.4, 132.9, 132.4, 131.4, 129.2, 128.2, 128.0, 128.0, 127.9, 127.9, 127.7, 127.7, 127.5, 126.3, 126.2, 125.5, 64.5, 49.2. HRMS (ESI) m/z: [M + H]+, calcd for C26H20NO3: 394.1443, found: 394.1444.
2-([1,1′-Biphenyl]-4-yl)-1-oxo-3-phenyl-1,2,3,4-tetrahydroisoquinoline-4-carboxylic acid (I21): white powder, yield 864 mg (68.7%), mp 191–193 °C. 1H NMR (400 MHz, DMSO-d6) δH (ppm): 13.06 (s, 1H), 8.16–8.14 (m, 1H), 7.66–7.58 (m, 7H), 7.51–7.50 (m, 1H), 7.45–7.42 (m, 2H), 7.36–7.32 (m, 3H), 7.20–7.19 (m, 2H), 7.12–7.11 (m, 2H), 5.59 (d, J = 4.4 Hz, 1H), 5.03 (d, J = 4.4 Hz, 1H). 13C NMR (100 MHz, DMSO-d6) δC (ppm): 170.5, 163.0, 141.1, 139.4, 138.3, 137.3, 134.2, 132.4, 129.2, 129.0, 128.3, 128.1, 127.9, 127.8, 127.7, 127.6, 126.9, 126.7, 64.4, 49.1. HRMS (ESI) m/z: [M + H]+, calcd for C28H22NO3: 420.1600, found: 420.1602.
2-(4-Isopropylphenyl)-1-oxo-3-phenyl-1,2,3,4-tetrahydroisoquinoline-4-carboxylic acid (I22): white powder, yield 811 mg (70.2%), mp 214–216 °C. 1H NMR (400 MHz, DMSO-d6) δH (ppm): 13.18 (s, 1H), 8.02–8.0 (m, 1H), 7.48–7.42 (m, 3H), 7.27–7.22 (m, 8H), 7.18–7.17 (m, 1H), 5.69 (s, 1H), 4.25 (s, 1H), 2.89–2.84 (m, 1H), 1.18 (d, J = 5.2 Hz, 6H). 13C NMR (100 MHz, DMSO-d6) δC (ppm): 172.1, 162.7, 146.7, 140.0, 139.3, 133.7, 132.3, 129.7, 129.2, 128.6, 128.0, 127.4, 127.3, 126.6, 126.2, 64.3, 51.1, 33.0, 23.8, 23.8. HRMS (ESI) m/z: [M + H]+, calcd for C25H24NO3: 386.1756, found: 386.1754.
2-(4-(4-Chlorophenoxy)phenyl)-1-oxo-3-phenyl-1,2,3,4-tetrahydroisoquinoline-4-carboxylic acid (I23): white powder, yield 1.17 g (83%), mp 236–238 °C. 1H NMR (400 MHz, DMSO-d6) δH (ppm): 13.01 (s, 1H), 8.09–8.07 (m, 1H), 7.61–7.58 (m, 3H), 7.51–7.48 (m, 1H), 7.43–7.41 (m, 2H), 7.21–7.19 (m, 4H), 7.06–7.05 (m, 2H), 6.99–6.95 (m, 4H), 5.51 (d, J = 4.8 Hz, 1H), 4.93 (d, J = 4.8 Hz, 1H). 13C NMR (100 MHz, DMSO-d6) δC (ppm): 170.5, 163.0, 155.5, 154.4, 137.3, 137.2, 134.3, 132.3, 129.9, 129.1, 128.2, 128.0, 128.0, 127.8, 127.8, 127.6, 127.3, 120.3, 118.7, 64.4, 49.0. HRMS (ESI) m/z: [M + H]+, calcd for C28H21ClNO4: 470.1159, found: 470.1147.
2-(4-Fluorophenyl)-1-oxo-3-phenyl-1,2,3,4-tetrahydroisoquinoline-4-carboxylic acid (I24): white powder, yield 821 mg (75.8%), mp 193–195 °C. 1H NMR (400 MHz, DMSO-d6) δH (ppm): 12.97 (s, 1H), 8.10–8.09 (m, 1H), 7.62–7.57 (m, 2H), 7.52–7.49 (m, 1H), 7.24–7.21 (m, 2H), 7.19–7.18 (m, 3H), 7.15–7.11 (m, 2H), 7.07–7.05 (m, 2H), 5.51 (d, J = 4.8 Hz, 1H), 4.93 (d, J = 4.8 Hz, 1H). 13C NMR (100 MHz, DMSO-d6) δC (ppm): 170.4, 163.1, 160.2 (d, 1JC–F = 245.5 Hz), 137.8 (d, 4JC–F = 2.5 Hz), 137.1, 134.4, 132.3, 129.5 (d, 3JC–F = 8.8 Hz), 129.0, 128.1, 128.0, 128.0, 127.8, 127.7, 127.6, 115.3 (d, 2JC–F = 23.0 Hz), 64.4, 49.1. HRMS (ESI) m/z: [M + H]+, calcd for C22H17FNO3: 362.1192, found: 362.1189.
2-(4-Chlorophenyl)-1-oxo-3-phenyl-1,2,3,4-tetrahydroisoquinoline-4-carboxylic acid (I25): white powder, yield 752 mg (66.4%), mp 200–201 °C. 1H NMR (400 MHz, DMSO-d6) δH (ppm): 13.03 (s, 1H), 8.08–8.06 (m, 1H), 7.60–7.59 (m, 2H), 7.52–7.48 (m, 2H), 7.38–7.35 (m, 2H), 7.23–7.18 (m, 4H), 7.06–7.04 (m, 2H), 5.54 (d, J = 5.6 Hz, 1H), 4.88 (d, J = 5.6 Hz, 1H). 13C NMR (100 MHz, DMSO-d6) δC (ppm): 170.5, 163.1, 140.4, 137.1, 134.5, 132.5, 130.8, 129.3, 128.9, 128.6, 128.2, 128.1, 128.0, 127.9, 127.8, 127.7, 64.1, 49.1. HRMS (ESI) m/z: [M + H]+ calcd for C22H17ClNO3: 378.0897, found: 378.0888.
2-(4-Bromophenyl)-1-oxo-3-phenyl-1,2,3,4-tetrahydroisoquinoline-4-carboxylic acid (I26): white powder, yield 920 mg (72.7%), mp 211–213 °C. 1H NMR (400 MHz, DMSO-d6) δH (ppm): 12.99 (s, 1H), 8.09–8.08 (m, 1H), 7.60–7.49 (m, 4H), 7.19–7.15 (m, 6H), 7.07–7.05 (m, 2H), 5.55 (d, J = 4.8 Hz, 1H), 4.89 (d, J = 4.8 Hz, 1H). 13C NMR (100 MHz, DMSO-d6) δC (ppm): 170.4, 163.0, 140.8, 137.0, 134.5, 132.5, 131.5, 129.6, 128.9, 128.0, 127.9, 127.9, 127.7, 127.7, 119.2, 64.0, 49.1. HRMS (ESI) m/z: [M + H]+, calcd for C22H17BrNO3: 422.0392, found: 422.0387.
2-(4-Iodophenyl)-1-oxo-3-phenyl-1,2,3,4-tetrahydroisoquinoline-4-carboxylic acid (I27): white powder, yield 1.13 g (80.3%), mp 208–210 °C. 1H NMR (400 MHz, DMSO-d6) δH (ppm): 12.99 (s, 1H), 8.08–8.07 (m, 1H), 7.66–7.59 (m, 4H), 7.51–7.48 (m, 1H), 7.18–7.17 (m, 3H), 7.05–7.01 (m, 4H), 5.53 (d, J = 4.4 Hz, 1H), 4.89 (d, J = 4.4 Hz, 1H). 13C NMR (100 MHz, DMSO-d6) δC (ppm): 170.9, 163.4, 141.8, 137.9, 137.5, 134.9, 133.0, 130.3, 129.4, 128.7, 128.6, 128.4, 128.3, 128.2, 125.8, 92.6, 64.5, 49.6. HRMS (ESI) m/z: [M + H]+, calcd for C22H17INO3: 470.0253, found: 470.0246.
1-Oxo-3-phenyl-2-(4-(trifluoromethoxy)phenyl)-1,2,3,4-tetrahydroisoquinoline-4-carboxylic acid (I28): white powder, yield 956 mg (74.6%), mp 211–213 °C. 1H NMR (400 MHz, DMSO-d6) δH (ppm): 13.03 (s, 1H), 8.10 (m, 1H), 7.61–7.60 (m, 2H), 7.52–7.49 (m, 1H), 7.35–7.30 (m, 4H), 7.19–7.18 (m, 3H), 7.09–7.07 (m, 2H), 5.58 (d, J = 4.4 Hz, 1H), 4.93 (d, J = 4.4 Hz, 1H). 13C NMR (100 MHz, DMSO-d6) δC (ppm): 170.5, 163.1, 146.3, 140.6, 137.0, 134.5, 132.5, 129.3, 128.9, 128.2, 128.1, 128.0, 127.9, 127.8, 127.7, 121.1 (q, 1JC–F = 256.2 Hz), 64.2, 49.2. HRMS (ESI) m/z: [M + H]+, calcd for C23H17F3NO4: 428.1110, found: 428.1100.
2-(4-(Ethoxycarbonyl)phenyl)-1-oxo-3-phenyl-1,2,3,4-tetrahydroisoquinoline-4-carboxylic acid (I29): white powder, yield 869 mg (69.8%), mp 187–189 °C. 1H NMR (400 MHz, DMSO-d6) δH (ppm): 13.03 (s, 1H), 8.11–8.10 (m, 1H), 7.89–7.87 (m, 2H), 7.61–7.60 (m, 2H), 7.53–7.50 (m, 1H), 7.39–7.37 (m, 2H), 7.18–7.17 (m, 3H), 7.10–7.08 (m, 2H), 5.67 (d, J = 4.4 Hz, 1H), 4.87 (d, J = 4.4 Hz, 1H), 4.28 (q, J = 5.6 Hz, 2H), 1.28 (t, J = 5.6 Hz, 3H). 13C NMR (100 MHz, DMSO-d6) δC (ppm): 170.5, 165.2, 163.1, 145.7, 137.0, 134.5, 132.6, 129.4, 128.9, 128.2, 128.0, 128.0, 127.9, 127.8, 127.7, 127.5, 127.4, 63.8, 60.7, 49.3, 14.1. HRMS (ESI) m/z: [M + H]+, calcd for C25H22NO5: 416.1498, found: 416.1496.
2-(4-Chloro-3-(trifluoromethyl)phenyl)-1-oxo-3-phenyl-1,2,3,4-tetrahydroisoqui-noline-4-carboxylic acid (I30): white powder, yield 1.01 g (76.1%), mp 221–223 °C. 1H NMR (400 MHz, DMSO-d6) δH (ppm): 13.01 (s, 1H), 8.10–8.08 (m, 1H), 7.74–7.73 (m, 1H), 7.65–7.58 (m, 3H), 7.53–7.50 (m, 1H), 7.46–7.44 (m, 1H), 7.21–7.19 (m, 3H), 7.11–7.09 (m, 2H), 5.69 (d, J = 4.4 Hz, 1H), 4.86 (d, J = 4.4 Hz, 1H). 13C NMR (100 MHz, DMSO-d6) δC (ppm): 170.6, 163.4, 140.6, 136.8, 134.8, 133.1, 132.8, 131.8, 128.6, 128.1, 128.0, 127.9, 127.8, 127.0, 126.6 (q, 2JC–F = 31.4 Hz), 122.5 (q, 1JC–F = 273.2 Hz), 63.7, 49.2. HRMS (ESI) m/z: [M + H]+, calcd for C23H16ClF3NO3: 446.0771, found: 446.0760.
2-(3-Cyano-4-fluorophenyl)-1-oxo-3-phenyl-1,2,3,4-tetrahydroisoquinoline-4-carboxylic acid (I31): white powder, yield 759 mg (65.5%), mp 230–233 °C. 1H NMR (400 MHz, DMSO-d6) δH (ppm): 13.03 (s, 1H), 8.10–8.09 (m, 1H), 7.87–7.85 (m, 1H), 7.62–7.56 (m, 3H), 7.53–7.50 (m, 1H), 7.46–7.42 (m, 1H), 7.21–7.19 (m, 3H), 7.12–7.10 (m, 2H), 5.67 (d, J = 4.4 Hz, 1H), 4.84 (d, J = 4.4 Hz, 1H). 13C NMR (100 MHz, DMSO-d6) δC (ppm): 170.6, 163.4, 160.4 (d, 1JC–F = 255.2 Hz), 138.2 (d, 4JC–F = 3.1 Hz), 136.6, 135.7 (d, 3JC–F = 9.0 Hz), 134.8, 132.8, 128.5, 128.3, 128.2, 128.1, 127.9, 127.8, 116.7 (d, 2JC–F = 20.5 Hz), 113.5, 100.2 (d, 3JC–F = 16.3 Hz), 63.8, 49.3. HRMS (ESI) m/z: [M + H]+, calcd for C23H16FN2O3: 387.1145, found: 387.1134.
2-(3-Fluoro-4-morpholinophenyl)-1-oxo-3-phenyl-1,2,3,4-tetrahydroisoquino-line-4-carboxylic acid (I32): white powder, yield 843 mg (63%), mp 239–241 °C. 1H NMR (400 MHz, DMSO-d6) δH (ppm): 13.05 (s, 1H), 8.11–8.10 (m, 1H), 7.63–7.56 (m, 2H), 7.50–7.47 (m, 1H), 7.20–7.18 (m, 3H), 7.10–7.06 (m, 3H), 6.94–6.92 (m, 2H), 5.50 (d, J = 4.4 Hz, 1H), 4.97 (d, J = 4.4 Hz, 1H), 3.71 (t, J = 3.6 Hz, 4H), 2.96 (t, J = 3.6 Hz, 4H). 13C NMR (100 MHz, DMSO-d6) δC (ppm): 170.4, 163.0, 154.0 (d, 1JC–F = 244.7 Hz), 138.2 (d, 3JC–F = 8.3 Hz), 137.2, 135.8 (d, 3JC–F = 9.7 Hz), 134.2, 132.4, 129.1, 128.2, 128.1, 127.9, 127.6, 123.6 (d, 4JC–F = 2.7 Hz), 118.4 (d, 4JC–F = 3.7 Hz), 115.5 (d, 2JC–F = 22.2 Hz), 66.2, 64.4, 50.4, 49.0. HRMS (ESI) m/z: [M + H]+, calcd for C26H24FN2O4: 447.1720, found: 447.1711.
2-(4-(Difluoromethoxy)phenyl)-1-oxo-3-phenyl-1,2,3,4-tetrahydroisoquinoline-4-carboxylic acid (I33): white powder, yield 859 mg (70%), mp 205–208 °C. 1H NMR (400 MHz, DMSO-d6) δH (ppm): 13.01 (s, 1H), 8.10–8.09 (m, 1H), 7.62–7.59 (m, 2H), 7.52–7.49 (m, 1H), 7.27–7.25 (m, 5H), 7.21–7.06 (m, 5H), 5.52 (d, J = 4.4 Hz, 1H), 4.95 (d, J = 4.4 Hz, 1H). 13C NMR (100 MHz, DMSO-d6) δC (ppm): 170.5, 163.1, 149.1, 138.5, 137.1, 134.4, 132.4, 129.1, 129.0, 128.2, 128.1, 127.9, 127.9, 127.8, 127.7, 118.8, 116.3 (t, 1JC–F = 257.2 Hz), 64.4, 49.0. HRMS (ESI) m/z: [M + H]+, calcd for C23H18F2NO4: 410.1204, found: 410.1192.
2-(3-(Ethoxycarbonyl)phenyl)-1-oxo-3-phenyl-1,2,3,4-tetrahydroisoquinoline-4-carboxylic acid (I34): white powder, yield 908 mg (72.9%), mp 196–198 °C. 1H NMR (400 MHz, DMSO-d6) δH (ppm): 13.03 (s, 1H), 8.11–8.09 (m, 1H), 7.84–7.77 (m, 2H), 7.61–7.60 (m, 2H), 7.53–7.50 (m, 1H), 7.44–7.43 (m, 2H), 7.19–7.18 (m, 3H), 7.08–7.06 (m, 2H), 5.60 (d, J = 4.4 Hz, 1H), 4.96 (d, J = 4.4 Hz, 1H), 4.28 (q, J = 5.6 Hz, 2H), 1.28 (t, J = 5.6 Hz, 3H). 13C NMR (100 MHz, DMSO-d6) δC (ppm): 170.5, 165.2, 163.1, 137.1, 134.5, 132.5, 132.1, 130.3, 129.0, 128.9, 128.3, 128.2, 128.1, 128.0, 127.9, 127.8, 127.7, 127.2, 64.1, 60.9, 49.1, 14.1. HRMS (ESI) m/z: [M + H]+, calcd for C25H22NO5: 416.1498, found: 416.1496.
2-(4-(4-Chlorophenoxy)phenyl)-3-(4-isopropylphenyl)-1-oxo-1,2,3,4-tetrahydro-isoquinoline-4-carboxylic acid (II1): white powder, yield 1.15 g (75.3%), mp 225–227 °C. 1H NMR (400 MHz, DMSO-d6) δH (ppm): 13.00 (s, 1H), 8.09–8.08 (m, 1H), 7.64–7.62 (m, 1H), 7.59–7.47 (m, 2H), 7.42–7.21 (m, 4H), 7.06–7.05 (m, 2H), 7.00–6.96 (m, 6H), 5.46 (d, J = 4.4 Hz, 1H), 4.96 (d, J = 4.4 Hz, 1H), 2.80–2.75 (m, 1H), 1.11 (d, J = 5.2 Hz, 6H). 13C NMR (100 MHz, DMSO-d6) δC (ppm): 170.4, 162.9, 155.5, 154.4, 147.9, 137.4, 134.4, 134.3, 132.3, 129.9, 129.2, 129.0, 127.8, 127.5, 127.3, 126.1, 120.2, 118.8, 64.3, 48.9, 32.8, 23.6. HRMS (ESI) m/z: [M + H]+, calcd for C31H27ClNO4: 512.1629, found: 512.1626.
3-(4-(tert-Butyl)phenyl)-2-(4-(4-chlorophenoxy)phenyl)-1-oxo-1,2,3,4-tetrahydroisoquinoline-4-carboxylic acid (II2): white powder, yield 1.14 g (72.7%), mp. 219–221 °C 1H NMR (400 MHz, DMSO-d6) δH (ppm): 13.04 (s, 1H), 8.11–8.09 (m, 1H), 7.65–7.64 (m, 1H), 7.59–7.56 (m, 1H), 7.50–7.47 (m, 1H), 7.41–7.39 (m, 2H), 7.24–7.19 (m, 4H), 6.99–6.95 (m, 6H), 5.47 (d, J = 4.4 Hz, 1H), 4.98 (d, J = 4.4 Hz, 1H), 1.18 (s, 9H). 13C NMR (100 MHz, DMSO-d6) δC (ppm): 170.4, 162.9, 155.5, 154.4, 150.3, 137.5, 134.3, 134.1, 132.3, 129.9, 129.2, 128.9, 127.9, 127.6, 127.5, 127.4, 125.0, 120.3, 118.7, 118.0, 64.3, 48.8, 34.2, 31.0, 31.0. HRMS (ESI) m/z: [M + H]+, calcd for C32H29ClNO4: 526.1785, found: 526.1786.
2-(4-(4-Chlorophenoxy)phenyl)-3-(4-isobutylphenyl)-1-oxo-1,2,3,4-tetrahydroisoquinoline-4-carboxylic acid (II3): white powder, yield 1.09 g (69.1%), mp 234–236 °C. 1H NMR (400 MHz, DMSO-d6) δH (ppm): 12.99 (s, 1H), 8.10–8.09 (m, 1H), 7.62–7.56 (m, 2H), 7.50–7.47 (m, 1H), 7.41–7.19 (m, 4H), 6.98–6.93 (m, 8H), 5.48 (d, J = 4.0 Hz, 1H), 4.90 (d, J = 4.0 Hz, 1H), 2.33 (d, J = 5.6 Hz, 2H), 1.76–1.70 (m, 1H), 0.77 (d, J = 5.2 Hz, 6H). 13C NMR (100 MHz, DMSO-d6) δC (ppm): 170.5, 163.0, 155.5, 154.4, 140.9, 137.4, 134.5, 134.5, 132.3, 129.9, 129.1, 128.7, 127.8, 127.8, 127.6, 127.3, 120.2, 118.7, 64.3, 49.1, 44.1, 29.4, 22.1, 22.0. HRMS (ESI) m/z: [M + H]+, calcd for C32H29ClNO4: 526.1785, found: 526.1796.
3-([1,1′-Biphenyl]-4-yl)-2-(4-(4-chlorophenoxy)phenyl)-1-oxo-1,2,3,4-tetrahydroisoquinoline-4-carboxylic acid (II4): white powder, yield 1.15 g (70.7%), mp 241–243 °C. 1H NMR (400 MHz, DMSO-d6) δH (ppm): 13.07 (s, 1H), 8.12–8.10 (m, 1H), 7.64–7.59 (m, 4H), 7.52–7.50 (m, 2H), 7.43–7.33 (m, 4H), 7.26–6.96 (m, 10H), 5.57 (d, J = 4.0 Hz, 1H), 4.98 (d, J = 4.0 Hz, 1H). 13C NMR (100 MHz, DMSO-d6) δC (ppm): 170.5, 163.0, 155.5, 154.4, 139.5, 139.1, 137.4, 136.4, 134.4, 132.4, 129.9, 129.1, 128.9, 128.6, 127.9, 127.8, 127.7, 127.6, 127.3, 126.9, 126.5, 126.3, 120.2, 118.8, 64.2, 49.0. HRMS (ESI) m/z: [M + H]+ calcd for C34H25ClNO4: 546.1472, found: 546.1467.
2-(4-(4-Chlorophenoxy)phenyl)-3-(4-methoxyphenyl)-1-oxo-1,2,3,4-tetrahydroisoquinoline-4-carboxylic acid (II5): white powder, yield 1.25 g (83.7%), mp 228–230 °C. 1H NMR (400 MHz, DMSO-d6) δH (ppm): 12.93 (s, 1H), 8.09–8.07 (m, 1H), 7.63–7.57 (m, 2H), 7.50–7.48 (m, 1H), 7.43–7.19 (m, 4H), 7.00–6.73 (m, 8H), 5.44 (d, J = 4.4 Hz, 1H), 4.90 (d, J = 4.4 Hz, 1H), 3.66 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δC (ppm): 170.5, 162.9, 158.8, 155.5, 154.4, 137.4, 134.4, 132.3, 129.9, 129.1, 129.0, 127.8, 127.8, 127.6, 127.3, 120.3, 118.8, 113.5, 64.0, 54.9, 49.1. HRMS (ESI) m/z: [M + H]+, calcd for C29H23ClNO5: 500.1265, found: 500.1265.
2-(4-(4-Chlorophenoxy)phenyl)-1-oxo-3-(4-(pentyloxy)phenyl)-1,2,3,4-tetrahydroisoquinoline-4-carboxylic acid (II6): white powder, yield 1.31 g (79%), mp 244–246 °C. 1H NMR (400 MHz, DMSO-d6) δH (ppm): 12.97 (s, 1H), 8.08–8.07 (m, 1H), 7.63–7.56 (m, 2H), 7.50–7.47 (m, 1H), 7.42–7.19 (m, 4H), 6.99–6.71 (m, 8H), 5.44 (d, J = 4.4 Hz, 1H), 4.88 (d, J = 4.4 Hz, 1H), 3.84 (t, J = 5.2 Hz, 2H), 1.67–1.62 (m, 2H), 1.36–1.28 (m, 4H), 0.86 (t, J = 5.6 Hz, 3H). 13C NMR (100 MHz, DMSO-d6) δC (ppm): 170.5, 162.9, 158.3, 155.6, 154.3, 137.4, 134.4, 132.2, 129.9, 129.8, 129.1, 128.8, 127.8, 127.8, 127.5, 127.2, 118.8, 113.9, 67.2, 63.9, 49.1, 28.3, 27.7, 21.8, 13.8. HRMS (ESI) m/z: [M + H]+, calcd for C33H31ClNO5: 556.1891, found: 556.1890.
2-(4-(4-Chlorophenoxy)phenyl)-3-(4-isopropoxyphenyl)-1-oxo-1,2,3,4-tetrahydroisoquinoline-4-carboxylic acid (II7): white powder, yield 1.14 g (72.4%), mp 238–240 °C. 1H NMR (400 MHz, DMSO-d6) δH (ppm): 12.93 (s, 1H), 8.08–8.06 (m, 1H), 7.62–7.57 (m, 2H), 7.50–7.40 (m, 3H), 7.20–6.69 (m, 10H), 5.42 (d, J = 4.4 Hz, 1H), 4.87 (d, J = 4.4 Hz, 1H), 4.51–4.47 (m, 1H), 1.18 (d, J = 4.8 Hz, 6H). 13C NMR (100 MHz, DMSO-d6) δC (ppm): 170.5, 162.9, 157.1, 155.6, 154.3, 137.4, 134.5, 132.2, 129.8, 129.2, 129.1, 128.7, 127.8, 127.6, 127.3, 120.2, 118.8, 114.9, 69.0, 64.0, 49.1, 21.8, 21.7. HRMS (ESI) m/z: [M + H]+, calcd for C31H27ClNO5, 528.1578, found: 528.1577.
2-(4-(4-Chlorophenoxy)phenyl)-1-oxo-3-(4-phenoxyphenyl)-1,2,3,4-tetrahydroisoquinoline-4-carboxylic acid (II8): white powder, yield 1.29 g (76.7%), mp 246–248 °C. 1H NMR (400 MHz, DMSO-d6) δH (ppm): 13.03 (s, 1H), 8.10–8.08 (m, 1H), 7.63–7.59 (m, 2H), 7.51–7.49 (m, 1H), 7.42–7.10 (m, 6H), 7.07–6.97 (m, 7H), 6.91–6.80 (m, 4H), 5.52 (d, J = 4.4 Hz, 1H), 4.93 (d, J = 4.4 Hz, 1H). 13C NMR (100 MHz, DMSO-d6) δC (ppm): 170.6, 163.0, 156.3, 156.2, 155.5, 154.5, 137.3, 134.4, 132.4, 132.1, 130.0, 129.9, 129.7, 129.3, 129.1, 127.9, 127.7, 127.4, 123.7, 120.3, 118.7, 118.0, 63.9, 49.1. HRMS (ESI) m/z: [M + H]+, calcd for C34H25ClNO5: 562.1421, found: 562.1416.
2-(4-(4-Chlorophenoxy)phenyl)-1-oxo-3-(4-(trifluoromethoxy)phenyl)-1,2,3,4-tetrahydroisoquinoline-4-carboxylic acid (II9): white powder, yield 1.31 g (79.4%), mp 249–251 °C. 1H NMR (400 MHz, DMSO-d6) δH (ppm): 12.99 (s, 1H), 8.10–8.08 (m, 1H), 7.62–7.59 (m, 2H), 7.42–7.39 (m, 3H), 7.22–7.19 (m, 6H), 6.99–6.96 (m, 4H), 5.61 (d, J = 4.4 Hz, 1H), 4.93 (d, J = 4.4 Hz, 1H). 13C NMR (100 MHz, DMSO-d6) δC (ppm): 170.5, 163.0, 155.5, 154.5, 137.0, 136.6, 134.2, 132.5, 130.0, 129.9, 129.3, 128.9, 128.3, 128.3, 128.0, 127.8, 127.4, 121.0, 120.5, 120.3, 118.8, 63.5, 49.0. HRMS (ESI) m/z: [M + H]+, calcd for C29H20ClF3NO5: 554.0982, found: 554.0982.
2-(4-(4-Chlorophenoxy)phenyl)-3-(4-(difluoromethoxy)phenyl)-1-oxo-1,2,3,4-tetrahydroisoquinoline-4-carboxylic acid (II10): white powder, yield 1.19 g (74.2%), mp 248–250 °C. 1H NMR (400 MHz, DMSO-d6) δH (ppm): 13.04 (s, 1H), 8.09–8.08 (m, 1H), 7.60–7.50 (m, 3H), 7.43–7.41 (m, 2H), 7.23–7.09 (m, 2H), 7.01–6.97 (m, 9H), 5.54 (d, J = 4.4 Hz, 1H), 4.93 (d, J = 4.4 Hz, 1H). 13C NMR (100 MHz, DMSO-d6) δC (ppm): 170.5, 163.0, 155.5, 154.5, 150.5, 137.2, 134.2, 133.9, 132.4, 129.9, 129.7, 129.2, 129.0, 127.9, 127.8, 127.7, 127.4, 120.3, 118.8, 117.1 (t, 1JC–F = 260.1 Hz), 63.7, 49.0. HRMS (ESI) m/z: [M + H]+, calcd for C29H21ClF2NO5: 536.1076, found: 536.1077.
2-(4-(4-Chlorophenoxy)phenyl)-3-(2,3-dihydrobenzo[b][1,4]dioxin-6-yl)-1-oxo-1,2,3,4-tetrahydroisoquinoline-4-carboxylic acid (II11): white powder, yield 1.11 g (69.9%), mp 251–253C. 1H NMR (400 MHz, DMSO-d6) δH (ppm): 13.03 (s, 1H), 8.06–8.04 (m, 1H), 7.64–7.62 (m, 1H), 7.60–7.57 (m, 1H), 7.50–7.43 (m, 3H), 7.21–7.20 (m, 2H), 7.02–6.99 (m, 4H), 6.67–6.48 (m, 3H), 5.36 (d, J = 3.6 Hz, 1H), 4.91 (d, J = 3.6 Hz, 1H), 4.14 (s, 2H), 4.13 (s, 2H). 13C NMR (100 MHz, DMSO-d6) δC (ppm): 170.5, 162.9, 155.6, 154.4, 143.1, 142.7, 137.4, 134.4, 132.3, 130.1, 129.9, 129.1, 129.0, 127.9, 127.8, 127.6, 127.3, 120.9, 120.3, 118.8, 116.7, 116.5, 63.9, 63.9, 48.9. HRMS (ESI) m/z: [M + H]+, calcd for C30H23ClNO6: 528.1214, found: 528.1211.
2-(4-(4-Chlorophenoxy)phenyl)-3-(2,3-dihydrobenzofuran-5-yl)-1-oxo-1,2,3,4-tetrahydroisoquinoline-4-carboxylic acid (II12): white powder, yield 1.05 g (68.3%), mp 252–254 °C. 1H NMR (400 MHz, DMSO-d6) δH (ppm): 13.04 (s, 1H), 8.01–8.00 (m, 1H), 7.49–7.46 (m, 1H), 7.44–7.35 (m, 2H), 7.32–7.04 (m, 3H), 7.07–7.03 (m, 6H), 6.89–6.59 (m, 2H), 5.60 (s, 1H), 4.42 (t, J = 7.2 Hz, 2H), 4.18 (s, 1H), 3.04 (t, J = 7.2 Hz, 2H). 13C NMR (100 MHz, DMSO-d6) δC (ppm): 172.2, 162.6, 159.2, 155.5, 154.4, 138.0, 133.9, 132.4, 130.9, 129.9, 129.8, 129.1, 128.2, 128.0, 127.8, 127.4, 127.4, 125.8, 123.0, 120.4, 118.9, 108.7, 71.0, 64.1, 51.4, 28.9. HRMS (ESI) m/z: [M + H]+, calcd for C30H23ClNO5: 512.1265, found: 512.1273.
3-(4-Chloro-3-fluorophenyl)-2-(4-(4-chlorophenoxy)phenyl)-1-oxo-1,2,3,4-tetrahydroisoquinoline-4-carboxylic acid (II13): white powder, yield 1.19 g (76%), mp 243–245 °C. 1H NMR (400 MHz, DMSO-d6) δH (ppm): 12.97 (s, 1H), 8.10–8.08 (m, 1H), 7.60–7.51 (m, 3H), 7.45–7.39 (m, 4H), 7.25–7.24 (m, 2H), 7.07–6.97 (m, 5H), 5.63 (d, J = 4.4 Hz, 1H), 4.92 (d, J = 4.4 Hz, 1H). 13C NMR (100 MHz, DMSO-d6) δC (ppm): 170.5, 163.0, 156.6 (d, 1JC–F = 247.0 Hz), 155.5, 154.6, 139.1 (d, 3JC–F = 6.0 Hz), 136.9, 134.1, 132.6, 130.5, 130.0, 129.9, 129.3, 128.9, 128.3, 128.0, 128.0, 127.7, 127.4, 125.4 (d, 4JC–F = 3.0 Hz), 120.5, 120.3, 119.1 (d, 3JC–F = 17.3 Hz), 118.9, 118.8, 116.6 (d, 2JC–F = 21.6 Hz), 63.1, 49.1. HRMS (ESI) m/z: [M + H]+, calcd for C28H19Cl2FNO4: 522.0675, found: 522.0676.
2-(4-(4-Chlorophenoxy)phenyl)-3-(3,4-dimethoxyphenyl)-1-oxo-1,2,3,4-tetrahydroisoquinoline-4-carboxylic acid (II14): white powder, yield 1.25 g (78.6%), mp 238–240 °C. 1H NMR (400 MHz, DMSO-d6) δH (ppm): 12.98 (s, 1H), 8.08–8.07 (m, 1H), 7.64–7.48 (m, 5H), 7.42–7.40 (m, 2H), 7.24–6.96 (m, 4H), 6.76–6.60 (m, 3H), 5.47 (d, J = 4.0 Hz, 1H), 4.84 (d, J = 4.0 Hz, 1H), 3.66 (s, 3H), 3.51 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δC (ppm): 170.7, 163.1, 155.7, 154.3, 148.3, 147.9, 137.4, 134.6, 132.3, 129.9, 129.3, 127.8, 127.6, 127.3, 120.4, 120.1, 118.8, 111.6, 111.1, 64.0, 55.3, 55.0, 49.4. HRMS (ESI) m/z: [M + H]+, calcd for C30H25ClNO6: 530.1370, found: 530.1379.
2-(4-(4-Chlorophenoxy)phenyl)-3-(3-fluoro-4-methoxyphenyl)-1-oxo-1,2,3,4-tetrahydroisoquinoline-4-carboxylic acid (II15): white powder, yield 1.14 g (73.3%), mp 241–244 °C. 1H NMR (400 MHz, DMSO-d6) δH (ppm): 13.02 (s, 1H), 8.08–8.07 (m, 1H), 7.59–7.41 (m, 5H), 7.22–6.81 (m, 9H), 5.49 (d, J = 3.6 Hz, 1H), 4.88 (d, J = 3.6 Hz, 1H), 3.74 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δC (ppm): 170.6, 163.0, 155.5, 154.5, 150.6 (d, 1JC–F = 243.9 Hz), 146.7 (d, 3JC–F = 10.5 Hz), 137.1, 134.3, 132.5, 129.9, 129.2, 129.0, 127.9, 127.8 (d, 4JC–F = 2.7 Hz), 127.4, 124.4 (d, 4JC–F = 2.6 Hz), 120.3, 118.2, 115.5 (d, 2JC–F = 19.1 Hz), 113.3, 63.3, 55.8, 49.1. HRMS (ESI) m/z: [M + H]+, calcd for C29H22ClFNO5: 518.1171, found: 518.1174.
2-(4-(4-Chlorophenoxy)phenyl)-3-(4-fluoro-3-phenoxyphenyl)-1-oxo-1,2,3,4-tetrahydroisoquinoline-4-carboxylic acid (II16): white powder, yield 1.29 g (74.1%), mp 253–255 °C. 1H NMR (400 MHz, DMSO-d6) δH (ppm): 13.07 (s, 1H), 8.00–7.99 (m, 1H), 7.60–7.58 (m, 2H), 7.48–7.41 (m, 3H), 7.32–7.20 (m, 5H), 7.18–6.97 (m, 6H), 6.87–6.72 (m, 3H), 5.60 (d, J = 4.4 Hz, 1H), 4.84 (d, J = 4.4 Hz, 1H). 13C NMR (100 MHz, DMSO-d6) δC (ppm): 170.6, 163.0, 156.4, 155.5, 154.5, 154.0, 152.0, 142.1, 142.0, 136.9, 134.8, 134.8, 134.4, 132.5, 130.0, 129.9, 129.4, 128.9, 127.9, 127.8, 127.6, 125.3, 125.2, 123.3, 121.9, 120.3, 118.7, 117.0, 116.9, 116.5, 63.2, 49.2. HRMS (ESI) m/z: [M + H]+, calcd for C34H24ClFNO5: 580.1327, found: 580.1322.
3-(4-Bromophenyl)-2-(4-(4-chlorophenoxy)phenyl)-1-oxo-1,2,3,4-tetrahydroisoquinoline-4-carboxylic acid (II17): white powder, yield 1.29 g (78.4%), mp 249–251 °C. 1H NMR (400 MHz, DMSO-d6) δH (ppm): 13.03 (s, 1H), 8.09–8.07 (m, 1H), 7.59–7.58 (m, 2H), 7.43–7.39 (m, 5H), 7.22–7.21 (m, 2H), 7.02–6.96 (m, 6H), 5.54 (d, J = 4.4 Hz, 1H), 4.92 (d, J = 4.4 Hz, 1H). 13C NMR (100 MHz, DMSO-d6) δC (ppm): 170.5, 163.0, 155.5, 154.5, 137.1, 136.7, 134.2, 132.5, 131.1, 130.2, 129.9, 129.9, 129.2, 128.9, 128.6, 128.2, 127.9, 127.8, 127.4, 121.3, 120.4, 120.3, 118.8, 63.7, 49.0. HRMS (ESI) m/z: [M + H]+, calcd for C28H20BrClNO4: 548.0264, found: 548.0254.
2-(4-(4-Chlorophenoxy)phenyl)-3-(4-fluorophenyl)-1-oxo-1,2,3,4-tetrahydroisoquinoline-4-carboxylic acid (II18): white powder, yield 1.20 g (82.1%), mp 246–248 °C. 1H NMR (400 MHz, DMSO-d6) δH (ppm): 13.02 (s, 1H), 8.09–8.08 (m, 1H), 7.60–7.59 (m, 2H), 7.52–7.49 (m, 1H), 7.43–7.41 (m, 2H), 7.21–6.96 (m, 10H), 5.55 (d, J = 4.4 Hz, 1H), 4.91 (d, J = 4.4 Hz, 1H). 13C NMR (100 MHz, DMSO-d6) δC (ppm): 170.5, 163.0, 162.6, 160.7, 155.5, 154.5, 137.1, 134.3, 133.5, 132.4, 130.1, 130.0, 129.9, 129.3, 129.0, 127.9, 127.8, 127.7, 127.4, 120.3, 118.8, 115.1, 114.9, 63.6, 49.1. HRMS (ESI) m/z: [M + H]+, calcd for C28H20ClFNO4: 488.1065, found: 488.1063.
2-(4-(4-Chlorophenoxy)phenyl)-3-(4-chlorophenyl)-1-oxo-1,2,3,4-tetrahydroisoquinoline-4-carboxylic acid (II19): white powder, yield 1.22 g (80.9%), mp 248–250 °C. 1H NMR (400 MHz, DMSO-d6) δH (ppm): 13.09 (s, 1H), 8.10–8.08 (m, 1H), 7.58–7.40 (m, 5H), 7.27–7.21 (s, 4H), 7.09–6.96 (m, 6H), 5.56 (d, J = 4.4 Hz, 1H), 4.93 (d, J = 4.4 Hz, 1H). 13C NMR (100 MHz, DMSO-d6) δC (ppm): 170.5, 163.0, 155.5, 154.5, 137.1, 136.3, 134.2, 132.7, 132.5, 129.9, 129.9, 129.2, 129.0, 128.6, 128.2, 128.2, 127.9, 127.8, 127.5, 127.4, 120.3, 118.8, 63.6, 49.0. HRMS (ESI) m/z: [M + H]+, calcd for C28H20Cl2NO4: 504.0769, found: 504.0770.
3-(4-Bromo-3-fluorophenyl)-2-(4-(4-chlorophenoxy)phenyl)-1-oxo-1,2,3,4-tetrahydroisoquinoline-4-carboxylic acid (II20): white powder, yield 1.32 g (77.6%), mp 253–255 °C. 1H NMR (400 MHz, DMSO-d6) δH (ppm): 13.10 (s, 1H), 8.09–8.07 (m, 1H), 7.61–7.51 (m, 4H), 7.42–7.24 (m, 3H), 7.23–7.05 (m, 2H), 7.00–6.87 (m, 5H), 5.61 (d, J = 4.4 Hz, 1H), 4.91 (d, J = 4.4 Hz, 1H). 13C NMR (100 MHz, DMSO-d6) δC (ppm): 170.5, 163.0, 158.6, 156.7, 155.5, 154.6, 136.9, 134.2, 133.3, 132.6, 130.0, 129.9, 129.3, 128.9, 128.9, 128.3, 128.2, 128.0, 128.0, 127.7, 127.4, 125.8, 116.6, 116.4, 107.7, 107.5, 63.1, 49.1. HRMS (ESI) m/z: [M + H]+, calcd for C28H19BrClFNO4: 566.0170, found: 566.0167.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
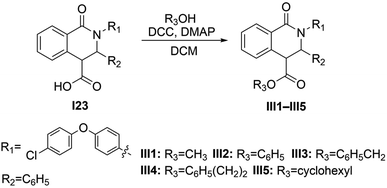
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), to yield ester derivatives III1–III5.
1), to yield ester derivatives III1–III5.Methyl 2-(4-(4-chlorophenoxy)phenyl)-1-oxo-3-phenyl-1,2,3,4-tetrahydroisoquinoline-4-carboxylate (III1): white powder, yield 406 mg (83.9%), mp 146–148 °C. 1H NMR (400 MHz, CDCl3) δH (ppm): 8.25–8.22 (m, 1H), 7.46–7.44 (m, 2H), 7.31–7.27 (m, 3H), 7.26–7.18 (m, 5H), 7.16–7.14 (m, 2H), 6.95–6.93 (m, 4H), 5.61 (s, 1H), 4.02 (d, J = 0.8 Hz, 1H), 3.74 (s, 3H). 13C NMR (100 MHz, CDCl3) δC (ppm): 171.5, 163.8, 155.9, 155.9, 132.8, 132.5, 130.1, 129.8, 129.7, 129.2, 129.0, 128.8, 128.8, 128.6, 128.4, 126.7, 120.7, 119.4, 65.3, 53.3, 52.0. HRMS (ESI) m/z: [M + H]+, calcd for C29H23ClNO4: 484.1316, found: 484.1303.
Phenyl 2-(4-(4-chlorophenoxy)phenyl)-1-oxo-3-phenyl-1,2,3,4-tetrahydroisoquinoline-4-carboxylate (III2): white powder, yield 465 mg (85.2%), mp 167–169 °C. 1H NMR (400 MHz, CDCl3) δH (ppm): 8.30–8.28 (m, 1H), 7.51–7.49 (m, 2H), 7.36–7.31 (m, 5H), 7.27–7.21 (m, 8H), 7.00–6.98 (m, 2H), 6.94–6.91 (m, 4H), 5.69 (s, 1H), 4.28 (d, J = 0.8 Hz, 1H). 13C NMR (100 MHz, CDCl3) δC (ppm): 169.4, 163.4, 155.7, 155.6, 150.5, 138.8, 137.7, 132.7, 131.8, 129.8, 129.6, 129.5, 129.0, 128.9, 128.7, 128.5, 128.4, 128.2, 126.5, 126.4, 121.2, 120.3, 119.1, 64.9, 51.8. HRMS (ESI) m/z: [M + H]+, calcd for C34H25ClNO4: 546.1472, found: 546.1472.
Benzyl 2-(4-(4-chlorophenoxy)phenyl)-1-oxo-3-phenyl-1,2,3,4-tetrahydroisoquinoline-4-carboxylate (III3): white powder, yield 457 mg (81.6%), mp 172–174 °C. 1H NMR (400 MHz, CDCl3) δH (ppm): 8.26–8.24 (m, 1H), 7.45–7.43 (m, 2H), 7.31–7.30 (m, 3H), 7.27–7.25 (m, 2H), 7.22–7.19 (m, 6H), 7.16–7.14 (m, 4H), 6.93–6.92 (m, 2H), 6.87–6.85 (m, 2H), 5.58 (s, 1H), 5.22 (d, J = 8.4 Hz, 1H), 5.16 (d, J = 8.4 Hz, 1H), 4.05 (d, J = 0.8 Hz, 1H). 13C NMR (100 MHz, CDCl3) δC (ppm): 170.8, 163.8, 155.9, 155.8, 139.4, 138.0, 135.5, 132.8, 132.5, 130.1, 129.8, 129.8, 129.2, 129.0, 129.0, 128.8, 128.8, 128.80, 128.5, 128.5, 128.4, 126.7, 120.6, 119.4, 67.9, 65.3, 52.2. HRMS (ESI) m/z: [M + H]+, calcd for C35H27ClNO4: 560.1629, found: 560.1628.
Phenethyl 2-(4-(4-chlorophenoxy)phenyl)-1-oxo-3-phenyl-1,2,3,4-tetrahydroisoquinoline-4-carboxylate (III4): light yellow oily liquid, yield 478 mg (83.3%). 1H NMR (400 MHz, CDCl3) δH (ppm): 8.24–8.23 (m, 1H), 7.42–7.40 (m, 2H), 7.27–7.22 (m, 4H), 7.22–7.18 (m, 6H), 7.13–7.10 (m, 3H), 7.02–7.01 (m, 2H), 6.94–6.91 (m, 4H), 5.56 (s, 1H), 4.38–4.33 (m, 2H), 3.96 (d, J = 0.8 Hz, 1H), 2.86 (t, J = 5.2 Hz, 2H). 13C NMR (100 MHz, CDCl3) δC (ppm): 170.8, 163.8, 155.9, 155.8, 139.4, 138.1, 137.7, 132.8, 132.5, 130.1, 129.9, 129.7, 129.1, 128.9, 128.9, 128.8, 128.8, 128.7, 128.5, 128.3, 127.0, 126.7, 120.6, 119.4, 66.7, 65.2, 52.1, 35.2. HRMS (ESI) m/z: [M + H]+, calcd for C36H29ClNO4: 574.1785, found: 574.1784.
Cyclohexyl 2-(4-(4-chlorophenoxy)phenyl)-1-oxo-3-phenyl-1,2,3,4-tetrahydroisoquinoline-4-carboxylate (III5): white powder, yield 456 mg (82.7%), mp 158–160 °C. 1H NMR (400 MHz, CDCl3) δH (ppm): 8.24–8.23 (m, 1H), 7.45–7.43 (m, 2H), 7.32–7.30 (m, 2H), 7.27–7.26 (m, 2H), 7.23–7.16 (m, 6H), 6.94–6.92 (m, 4H), 5.61 (s, 1H), 4.86–4.84 (m, 1H), 3.99 (d, J = 0.8 Hz, 1H), 1.77–1.76 (m, 1H), 1.66–1.65 (m, 1H), 1.55–1.45 (m, 1H), 1.44–1.41 (m, 3H), 1.36–1.24 (m, 4H). 13C NMR (100 MHz, CDCl3) δC (ppm): 170.4, 163.8, 156.0, 155.7, 133.0, 132.6, 130.0, 129.7, 129.6, 129.1, 128.8, 128.7, 128.7, 128.5, 128.3, 126.8, 120.6, 119.4, 74.4, 65.3, 52.5, 31.6, 31.4, 25.5, 23.5, 23.4. HRMS (ESI) m/z: [M + H]+ calcd for C34H31ClNO4: 552.1942, found: 552.1939.
2.3 Evaluation of in vitro antifungal and antioomycete activities of compounds I1–I34, II1–II20, and III1–III5
The in vitro antifungal and antioomycete activities of compounds I1–I34, II1–II20, and III1–III5 were determined using the mycelial growth method. The stock solutions of the synthesized compounds (200 mg) were prepared by dissolving them in 800 μL of dimethyl sulfoxide (DMSO) containing 5% N,N-dimethyl-1-dodecylamine N-oxide (OA-12), respectively. The prepared sterile PDA medium was melted by heating to 50 °C and 20 μL of the stock solution was added to the 50 mL of melted PDA medium and they were well-mixed to give the concentration of 100 μg mL−1. The above PDA medium was immediately poured into three sterilized Petri dishes (90 mm diameter). After the medium was solidified, a mycelial disc (5 mm diameter) of the fungal or oomycete isolate listed above was inoculated in the center of each Petri plate. The control group was used by the same treatment with DMSO containing 5% OA-12 without tested compounds. All the plates were incubated at 25 ± 2 °C in darkness for certain days listed in Table 1. Each treatment was replicated three times. The experiment was replicated three times for 7 microorganisms. The mycelial growth diameter (D) was the mean length (mm) of two orthogonal diameter axes passing through the center of the disc. The inhibition rate (I) was calculated by the formula: I (%) = (DC − DT)/DC × 100, where DC and DT are the mycelial growth diameters of the control and treatment groups, respectively. The result was presented as the mean value of three independent replicates ± standard error of the mean (SEM).| Compd. | Inhibition rate (%) | ||||||
|---|---|---|---|---|---|---|---|
| Bo. (6 d) | Fu. (6 d) | Co. (7 d) | Tr. (7 d) | Al. (7 d) | Rh. (3 d) | Py. (2 d) | |
| a Bo.: Botrytis cinerea; Fu.: Fusarium oxysporum f. sp. niveum; Co.: Colletotrichum gloeosporioides; Tr.: Trichothecium roseum; Al.: Alternaria mali; Rh.: Rhizoctonia solani; Py.: Pythium recalcitrans. The number of days in each bracket is the culture duration of the strains on PDA plates before measuring the diameters of mycelial colonies. The data in the table are expressed as the mean ± standard error of mean (SEM) (n = 3). | |||||||
| I1 | 71.9 ± 2.2 | 86.7 ± 2.1 | 86.3 ± 1.0 | 28.6 ± 1.3 | 69.2 ± 1.6 | 96.5 ± 3.5 | 99.2 ± 0.4 |
| I2 | 69.6 ± 0.9 | 70.3 ± 1.4 | 82.3 ± 0.7 | 27.8 ± 1.5 | 66.0 ± 0.9 | 81.2 ± 1.6 | 98.4 ± 0.3 |
| I3 | 70.2 ± 1.0 | 81.5 ± 0.3 | 88.3 ± 0.5 | 39.6 ± 3.3 | 73.0 ± 1.0 | 88.4 ± 0.7 | 99.7 ± 0.3 |
| I4 | 65.3 ± 0.6 | 55.1 ± 0.7 | 76.1 ± 1.1 | 33.3 ± 3.6 | 55.3 ± 1.8 | 55.8 ± 2.1 | 97.2 ± 0.1 |
| I5 | 72.2 ± 1.2 | 82.6 ± 1.2 | 78.3 ± 0.8 | 44.9 ± 1.3 | 71.3 ± 0.5 | 87.9 ± 2.1 | 98.9 ± 0.2 |
| I6 | 72.6 ± 0.7 | 73.9 ± 2.4 | 80.4 ± 0.5 | 39.6 ± 0.7 | 64.0 ± 3.9 | 89.8 ± 3.5 | 98.4 ± 0.1 |
| I7 | 68.6 ± 1.7 | 67.5 ± 1.4 | 79.1 ± 0.4 | 37.6 ± 0.8 | 61.7 ± 1.0 | 93.3 ± 3.4 | 96.7 ± 0.5 |
| I8 | 69.6 ± 1.4 | 64.7 ± 0.4 | 85.7 ± 0.1 | 44.4 ± 1.2 | 71.0 ± 1.2 | 80.3 ± 2.7 | 98.3 ± 0.4 |
| I9 | 72.6 ± 1.3 | 53.5 ± 2.9 | 86.5 ± 0.4 | 41.9 ± 0.5 | 73.0 ± 1.3 | 71.5 ± 1.1 | 98.2 ± 0.2 |
| I10 | 73.4 ± 1.5 | 80.6 ± 1.0 | 86.8 ± 0.3 | 36.3 ± 1.6 | 74.5 ± 0.8 | 79.8 ± 1.2 | 99.1 ± 0.3 |
| I11 | 74.5 ± 0.7 | 72.5 ± 0.7 | 78.8 ± 0.5 | 38.3 ± 1.3 | 65.7 ± 0.3 | 66.9 ± 1.6 | 94.7 ± 0.7 |
| I12 | 71.6 ± 2.4 | 74.4 ± 1.0 | 83.8 ± 0.7 | 39.1 ± 3.0 | 64.9 ± 0.8 | 81.4 ± 1.7 | 96.7 ± 0.2 |
| I13 | 65.3 ± 1.2 | 67.0 ± 0.5 | 84.3 ± 0.4 | 35.6 ± 1.0 | 65.4 ± 1.5 | 79.3 ± 0.3 | 97.2 ± 0.1 |
| I14 | 73.5 ± 0.9 | 73.1 ± 0.9 | 90.2 ± 0.3 | 41.4 ± 2.3 | 71.8 ± 0.8 | 82.8 ± 1.2 | 97.3 ± 0.1 |
| I15 | 68.9 ± 2.0 | 82.3 ± 1.2 | 87.5 ± 0.5 | 44.0 ± 0.4 | 68.4 ± 1.2 | 82.2 ± 0.8 | 98.9 ± 0.3 |
| I16 | 76.2 ± 0.6 | 86.8 ± 1.3 | 92.5 ± 0.1 | 45.9 ± 1.2 | 75.0 ± 0.8 | 81.7 ± 1.5 | 100.0 |
| I17 | 74.5 ± 1.7 | 85.5 ± 0.7 | 90.3 ± 0.4 | 44.4 ± 0.8 | 75.6 ± 3.1 | 78.7 ± 1.0 | 99.6 ± 0.1 |
| I18 | 80.8 ± 0.9 | 84.8 ± 1.4 | 89.7 ± 0.5 | 49.1 ± 1.8 | 70.7 ± 0.6 | 90.6 ± 0.7 | 99.6 ± 0.1 |
| I19 | 79.2 ± 2.1 | 90.8 ± 1.0 | 92.9 ± 0.4 | 50.1 ± 0.5 | 74.2 ± 0.3 | 93.8 ± 1.2 | 100.0 |
| I20 | 82.1 ± 0.6 | 87.6 ± 0.5 | 93.3 ± 0.8 | 43.0 ± 1.2 | 78.5 ± 1.6 | 73.1 ± 1.0 | 100.0 |
| I21 | 74.0 ± 0.4 | 86.3 ± 0.3 | 90.3 ± 0.1 | 39.3 ± 2.8 | 80.5 ± 1.5 | 85.7 ± 2.8 | 100.0 |
| I22 | 74.5 ± 0.9 | 78.3 ± 0.3 | 90.3 ± 0.4 | 36.5 ± 1.9 | 71.0 ± 1.8 | 100.0 | 100.0 |
| I23 | 76.4 ± 3.1 | 87.2 ± 0.4 | 88.5 ± 0.3 | 46.9 ± 1.0 | 81.4 ± 0.8 | 100.0 | 100.0 |
| I24 | 56.4 ± 1.5 | 37.9 ± 2.6 | 68.1 ± 1.1 | 42.3 ± 1.8 | 66.3 ± 1.3 | 93.7 ± 10.1 | 100.0 |
| I25 | 63.6 ± 2.9 | 42.7 ± 1.0 | 73.1 ± 1.5 | 45.9 ± 3.7 | 69.5 ± 1.0 | 77.2 ± 7.8 | 100.0 |
| I26 | 59.7 ± 1.2 | 39.4 ± 0.4 | 74.6 ± 1.5 | 42.3 ± 2.1 | 72.4 ± 0.3 | 78.6 ± 10.0 | 100.0 |
| I27 | 67.6 ± 0.3 | 39.3 ± 1.7 | 73.6 ± 2.2 | 50.0 ± 7.6 | 74.7 ± 1.3 | 68.2 ± 4.7 | 100.0 |
| I28 | 61.6 ± 0.3 | 42.3 ± 2.2 | 65.6 ± 1.6 | 49.5 ± 2.2 | 71.8 ± 2.8 | 76.2 ± 0.6 | 100.0 |
| I29 | 55.0 ± 1.2 | 41.8 ± 0.6 | 63.4 ± 0.8 | 46.4 ± 5.8 | 63.7 ± 2.8 | 62.5 ± 3.9 | 97.0 ± 0.4 |
| I30 | 65.9 ± 1.8 | 51.4 ± 0.7 | 77.1 ± 1.0 | 57.1 ± 6.3 | 76.2 ± 1.1 | 69.4 ± 2.8 | 100.0 |
| I31 | 60.2 ± 1.6 | 41.4 ± 2.1 | 64.6 ± 0.3 | 52.2 ± 6.3 | 70.7 ± 1.1 | 83.2 ± 10.1 | 98.8 ± 0.7 |
| I32 | 57.7 ± 1.4 | 42.7 ± 0.7 | 64.1 ± 0.1 | 53.3 ± 4.0 | 66.6 ± 0.6 | 67.7 ± 0.6 | 100.0 |
| I33 | 63.6 ± 1.6 | 41.4 ± 1.3 | 68.2 ± 0.2 | 41.6 ± 0.5 | 67.5 ± 1.1 | 94.5 ± 1.4 | 98.3 ± 0.3 |
| I34 | 60.6 ± 1.2 | 40.4 ± 0.5 | 68.9 ± 1.6 | 47.9 ± 1.8 | 64.0 ± 1.8 | 85.7 ± 5.8 | 95.9 ± 0.1 |
To obtain the toxicity of the compounds, 2 mg, 5 mg, 10 mg, 20 mg, 40 mg, 100 mg and 200 mg of each test compound were dissolved in 800 μL of DMSO containing 5% OA-12, respectively. The following mix of stock solution (20 μL) with PDA medium (50 mL) rendered the concentrations of 1 μg mL−1, 2 μg mL−1, 5 μg mL−1, 10 μg mL−1, 20 μg mL−1, 50 μg mL−1 and 100 μg mL−1. The following operations were performed by following the same procedures described above. The inhibition rate of each concentration was calculated. The concentration was log-transformed value (y) and the inhibition rate was transformed into probit value (x). A linear regression equation was obtained by fitting y against x using Microsoft Excel 2013 and the median effective concentration value (EC50) was calculated from the obtained equation. The experiment was replicated three times and the result was presented as the mean value of three independent replicates ± SD.
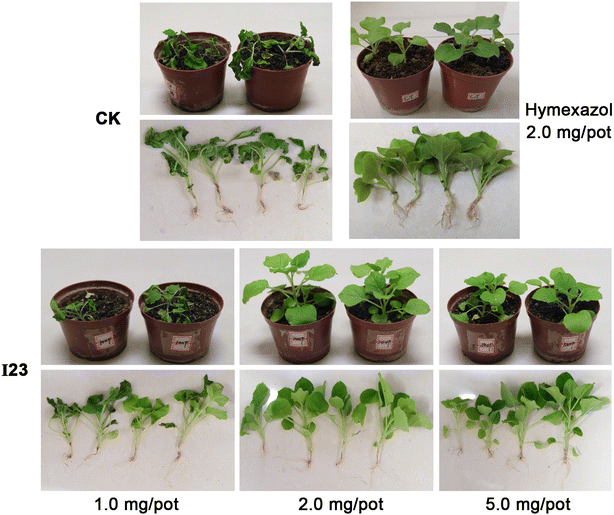
2.4 Evaluating in vivo control efficacy of compound I23 against P. recalcitrans by the pot experiment
Nicotiana benthamiana was used to evaluate in vivo control efficacy of compound I23 against P. recalcitrans. The PINDSTRUP substrate (Pindstrup, Denmark) and vermiculite in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio were mixed thoroughly and autoclaved at 120 °C for 4 h to give a sterile mixture. The N. benthamiana seeds were planted using the above mixed soil in plastic pots (top diameter × height × bottom diameter, 10 cm × 7 cm × 7 cm). All of the pots were placed in a greenhouse at 25 °C under ambient light. After 3 weeks of planting, 50 mL of the I23 solutions containing active ingredients (a.i) of 1.0 mg, 2.0 mg and 5.0 mg were applied into shallow circular trenches around the base of the N. benthamiana seedlings, respectively. In the preventive assay, after 1 day of treatment, 50 mL of a mycelial solution of P. recalcitrans (three 5 mm pellets inoculated in PDB at 25 °C in darkness for 2 d with a shaking speed of 120 rpm) was inoculated into the same shallow circular trench. In the curative assay, the mycelial solution was inoculated 2 d before the treatment of I23 solution. Two N. benthamiana plants were in each pot and each treatment contained 10 pots. After 1 week, the diseased plants were counted for each treatment. The incidence of disease (ID%) of each treatment was calculated by the following formula: (the number of diseased plants)/20 × 100. The experiment was repeated three times and the results were expressed as the mean ± SEM of three independent replicates.
1 ratio were mixed thoroughly and autoclaved at 120 °C for 4 h to give a sterile mixture. The N. benthamiana seeds were planted using the above mixed soil in plastic pots (top diameter × height × bottom diameter, 10 cm × 7 cm × 7 cm). All of the pots were placed in a greenhouse at 25 °C under ambient light. After 3 weeks of planting, 50 mL of the I23 solutions containing active ingredients (a.i) of 1.0 mg, 2.0 mg and 5.0 mg were applied into shallow circular trenches around the base of the N. benthamiana seedlings, respectively. In the preventive assay, after 1 day of treatment, 50 mL of a mycelial solution of P. recalcitrans (three 5 mm pellets inoculated in PDB at 25 °C in darkness for 2 d with a shaking speed of 120 rpm) was inoculated into the same shallow circular trench. In the curative assay, the mycelial solution was inoculated 2 d before the treatment of I23 solution. Two N. benthamiana plants were in each pot and each treatment contained 10 pots. After 1 week, the diseased plants were counted for each treatment. The incidence of disease (ID%) of each treatment was calculated by the following formula: (the number of diseased plants)/20 × 100. The experiment was repeated three times and the results were expressed as the mean ± SEM of three independent replicates.
2.5 Three-dimensional quantitative structure–activity relationship (3D-QSAR) analysis
The biological activities (EC50 (mM)) in Table 2 were transformed into pEC50 (−log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EC50). 3D structures of all the compounds were constructed using Sybyl X-2.1 software and they were energy minimized using Tripos force field with Powell conjugate gradient descent method (max iterations of 1000 and a convergence criterion of 0.05 kcal mol−1 Å−1). Meanwhile, Gasteiger–Huckel charges were added to the molecules. Compounds were ranked in descending order by their pEC50 values and the fourth, eighth, twelfth, …, were picked out as the test set (compounds I1, I3, I8, I14, I15, I17, I30, II1, II5, II12, II15, II16, II19 and III4). The remaining compounds were used as the training set. The molecular alignment was performed using the common backbone of the most potent compound I23 and the result was shown in Fig. S1.†
EC50). 3D structures of all the compounds were constructed using Sybyl X-2.1 software and they were energy minimized using Tripos force field with Powell conjugate gradient descent method (max iterations of 1000 and a convergence criterion of 0.05 kcal mol−1 Å−1). Meanwhile, Gasteiger–Huckel charges were added to the molecules. Compounds were ranked in descending order by their pEC50 values and the fourth, eighth, twelfth, …, were picked out as the test set (compounds I1, I3, I8, I14, I15, I17, I30, II1, II5, II12, II15, II16, II19 and III4). The remaining compounds were used as the training set. The molecular alignment was performed using the common backbone of the most potent compound I23 and the result was shown in Fig. S1.†
| Compd. | EC50 (μM) | Compd. | EC50 (μM) |
|---|---|---|---|
| a The data in the table are presented as the mean ± standard derivation (SD) of three repeated experiments. | |||
| I1 | 46.6 ± 2.9 | I32 | 26.9 ± 0.3 |
| I2 | 45.8 ± 2.3 | I33 | 43.1 ± 0.8 |
| I3 | 29.5 ± 2.0 | I34 | 40.9 ± 2.3 |
| I4 | 45.4 ± 2.8 | II1 | 18.2 ± 0.2 |
| I5 | 53.7 ± 1.0 | II2 | 27.7 ± 0.1 |
| I6 | 46.8 ± 2.6 | II3 | 32.0 ± 0.5 |
| I7 | 43.6 ± 0.5 | II4 | 38.5 ± 1.8 |
| I8 | 59.8 ± 0.6 | II5 | 45.3 ± 2.6 |
| I9 | 57.4 ± 0.5 | II6 | 43.0 ± 2.0 |
| I10 | 54.0 ± 0.7 | II7 | 31.5 ± 5.4 |
| I11 | 46.5 ± 2.1 | II8 | 47.1 ± 1.5 |
| I12 | 67.8 ± 0.1 | II9 | 31.3 ± 2.3 |
| I13 | 65.1 ± 0.1 | II10 | 41.3 ± 0.2 |
| I14 | 40.6 ± 1.5 | II11 | 16.9 ± 0.4 |
| I15 | 42.8 ± 0.7 | II12 | 35.8 ± 3.2 |
| I16 | 20.9 ± 0.2 | II13 | 29.6 ± 0.4 |
| I17 | 23.2 ± 1.1 | II14 | 47.8 ± 1.1 |
| I18 | 25.1 ± 1.7 | II15 | 31.3 ± 0.1 |
| I19 | 21.5 ± 0.5 | II16 | 48.7 ± 0.9 |
| I20 | 23.2 ± 1.0 | II17 | 30.3 ± 0.3 |
| I21 | 14.7 ± 0.3 | II18 | 33.1 ± 0.1 |
| I22 | 20.8 ± 0.1 | II19 | 32.2 ± 0.1 |
| I23 | 14.0 ± 0.3 | II20 | 34.0 ± 2.8 |
| I24 | 38.9 ± 4.0 | III1 | 951.4 ± 41.4 |
| I25 | 33.2 ± 1.1 | III2 | 1258.1 ± 76.3 |
| I26 | 29.6 ± 1.9 | III3 | 874.3 ± 65.2 |
| I27 | 26.0 ± 0.1 | III4 | 895.7 ± 58.7 |
| I28 | 29.4 ± 1.2 | III5 | 1055.2 ± 80.1 |
| I29 | 41.7 ± 3.5 | Hymexazol | 37.7 ± 3.8 |
| I30 | 26.0 ± 0.6 | Dimethomorph | >258.4 |
| I31 | 36.3 ± 2.7 | ||
For CoMFA studies, a cubic lattice with a grid spacing of 4.0 Å was generated to calculate steric and electrostatic fields using the sp3 hybridized carbon as the probe atom with a +1.0 charge. Cut-off values for both fields were set to 30.0 kcal mol−1. For CoMSIA studies, the same lattice with the parameters (probe atom with a +1.0 charge and attenuation factor of 0.3) was used to calculate steric and electrostatic, hydrophobic, and hydrogen bond donor and acceptor fields.
For the partial least squares (PLS) analysis, the leave-one-out (LOO) method was used to carry out a cross-validation analysis, giving the square of the cross-validation coefficient (qcv2) and the optimum number of components (Nopt). Using Nopt, a final model was generated with the non-cross-validated correlation coefficient (rncv2), standard error of estimation (SEEncv), and F-test value (F) using Sybyl software.
For the test set, the predictive correlation coefficient (rtest2) was calculated using the following formula:
where n is the number of samples in the test set.
2.6 Effects of I23 on cell membrane permeability of P. recalcitrans
Ten mycelial plugs taken from the edge of a 2-day-old colony of P. recalcitrans on PDA plates were transferred to 150 mL of PDB in a 250 mL flask. After incubation on a shaker (120 rpm in darkness at 25 °C) for 2 d, compound I23 was added to obtain the concentrations of 8.7 μM (EC30), 14.0 μM (EC50) and 20.0 μM (EC70). After shaking for a further 12 h, the mycelia were collected by two layers of filter paper and washed with distilled water three times. Afterward, 0.2 g mycelia of P. recalcitrans were suspended in 20 mL distilled water in a 20 mL centrifuge tube and the electrical conductivity of the suspensions was measured after 0, 0.5, 1, 2, 4, and 6 h using a laboratory conductivity meter digital conductivity meter (DDS-318, Shanghai Leici Xinjing Instrument Co., Ltd., China). At last, the mycelia were boiled for 5 min and the final electrical conductivity (σf) was recorded. The relative conductivity at different times was calculated by σt/σf × 100, where σt is the electrical conductivity at different times. The experiment was repeated three times.2.7 Fluorescence microscopy observation
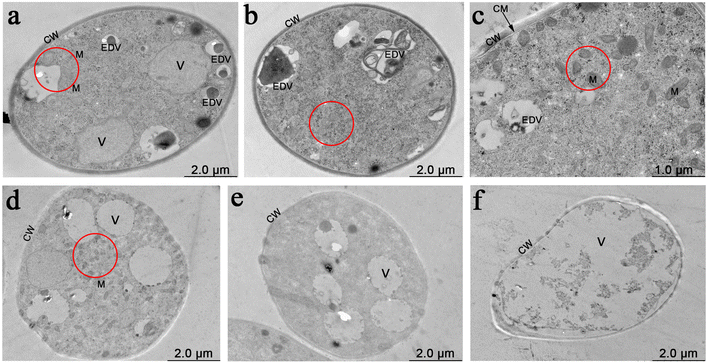
The vital dye FM4-64 (Catalog number: T13320, Invitrogen™, Thermo Fisher Scientific Inc.) was used to stain membrane systems of mycelia of P. recalcitrans. The mycelia of P. recalcitrans were collected from the above PDB after shaking for a further 12 h. Then, the mycelia were placed on a slide and a drop of FM4-64 (Catalog number: T13320, Invitrogen™, Thermo Fisher Scientific Inc.) working solution (10 μM) or JC-1 (Catalog number: C2005, Beyotime Biotechnology, China) working solution (10 μM) was added. The slice was then covered with a coverslip, and the samples were kept at room temperature in the dark for 10 min and analyzed using an Olympus IX71 inverted fluorescent microscope. Images were obtained using DP2-BSW software.2.8 Transmission electron microscopy (TEM) observation
The apical portions of the mycelial pellets from the above PDB after shaking for a further 12 h were cut and fixed in 2.5% glutaraldehyde at 4 °C overnight. Then samples were washed twice with 0.1 M PBS (pH 7.0), were post-fixed in 1% osmium tetroxide for 2 h, and washed twice with 0.1 M PBS (pH 7.0) again. After that, samples were dehydrated in an ascending ethanol series (30, 50, 70, 80, 90 and 100%, v/v) for 15 min each concentration. Subsequently, samples were embedded in Spurr resin and polymerized at 70 °C overnight. Blocks were sectioned (80 nm) using a LEICA EM UC7 ultratome (Leica UC7) and the sections were mounted on copper grids. Staining was performed with uranyl acetate and alkaline lead citrate for 10 min, respectively. Finally, micrographs were obtained using a transmission electron microscope (Hitachi Model H-7650, Tokyo, Japan) at an accelerating voltage of 80 kV.2.9 Lipidomics analysis
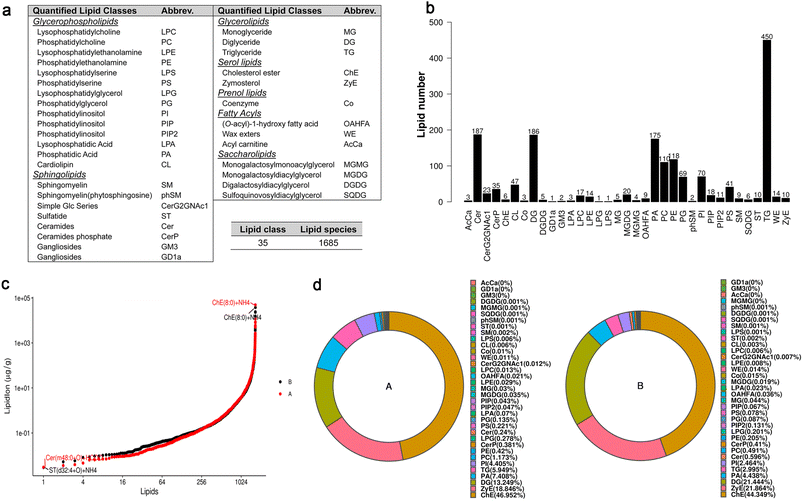
The mycelia of P. recalcitrans were collected from the above PDB after shaking for a further 6 h. The control and the treated groups contained each four independent replicates. Lipid extraction was followed the reported MTBE method.25 Briefly, appropriate amount of internal lipid standards were added to the samples and then they were homogenized with 200 μL H2O and 240 μL CH3OH. After that, 800 μL of MTBE was added and the mixture was ultrasound for 20 min at 4 °C followed by continually sitting for 30 min at room temperature. The obtained solution was subjected to centrifugation separation (14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g for 15 min at 10 °C) to give the upper organic solvent layer, which was dried under nitrogen atmosphere.
000 g for 15 min at 10 °C) to give the upper organic solvent layer, which was dried under nitrogen atmosphere.
The lipid absolute quantification analysis was performed using liquid chromatography with tandem mass spectrometry (LC-MS-MS) method. Reverse phase chromatography was used for LC separation (charged surface hybrid C18 column: pore size 130 Å, particle size 1.7 μm, 2.1 mm × 100 mm, Waters). The eluting solution A was comprised of CH3CN/H2O (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, v/v) with 0.1% formic acid and 0.1 Mm ammonium formate and B was comprised of CH3CN/isopropanol (1
4, v/v) with 0.1% formic acid and 0.1 Mm ammonium formate and B was comprised of CH3CN/isopropanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, v/v) with 0.1% formic acid and 0.1 Mm ammonium formate. In brief, the lipid extract solutions (in 200 μL 90% isopropanol/CH3CN) were centrifuged at 14
9, v/v) with 0.1% formic acid and 0.1 Mm ammonium formate. In brief, the lipid extract solutions (in 200 μL 90% isopropanol/CH3CN) were centrifuged at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g for 15 min at 10 °C. An aliquot of 3.0 μL of sample was injected into the chromatographic instrument. The initial eluting solvent system was 30% B in A at a flow rate of 300 μL min−1. It was kept for 2 min, and then linearly increased to 100% solvent B in 23 min, followed by equilibrating at 5% solvent B for 10 min. MS was carried out by Q-Exactive Plus in positive and negative mode, respectively. ESI parameters were optimized and preset for all measurements as follows: source temperature, 300 °C; capillary temperature, 350 °C; the ion spray voltage, 3000 V; S-Lens RF level, 50%; and the scan range, m/z 200–1800.
000 g for 15 min at 10 °C. An aliquot of 3.0 μL of sample was injected into the chromatographic instrument. The initial eluting solvent system was 30% B in A at a flow rate of 300 μL min−1. It was kept for 2 min, and then linearly increased to 100% solvent B in 23 min, followed by equilibrating at 5% solvent B for 10 min. MS was carried out by Q-Exactive Plus in positive and negative mode, respectively. ESI parameters were optimized and preset for all measurements as follows: source temperature, 300 °C; capillary temperature, 350 °C; the ion spray voltage, 3000 V; S-Lens RF level, 50%; and the scan range, m/z 200–1800.
Lipid Search, a search engine, was used for the identification of lipid species based on MS/MS math. Lipid Search contains more than 30 lipid classes and more than 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500
500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 fragment ions in the database. Both mass tolerance for precursor and fragment were set to 5 ppm.
000 fragment ions in the database. Both mass tolerance for precursor and fragment were set to 5 ppm.
2.10 Statistical analysis
A one-way analysis of variance (ANOVA) was used for the statistical analysis using the software of SPSS 16.0 software package. Data were expressed as mean standard error of the mean (SEM) and compared using a Fisher's least-significant difference test at the 95% confidence interval.3. Results & discussion
3.1 Synthesis of 3,4-dihydroisoquinolin-1(2H)-one derivatives
The reaction of imines with homophthalic anhydride in refluxing dry toluene is outlined in general terms in Scheme 1. The imines were prepared in situ by condensation of the aromatic aldehydes with aliphatic and aromatic amines in anhydrous DCM using anhydrous Na2SO4 as desiccant agent. The imine intermediates are unstable and were therefore not isolated but used immediately in the subsequent CCR to afford the desired isoquinolinic acids I1–I34 and II1–II20. The imines employed in the present study generally resulted in good to excellent isolated product yields. Noticeably, in our unsuccessful cases, when the ortho-substituted aromatic amines were o-toluidine, 2-ethylaniline, 2-phenoxyaniline, 2-benzylaniline, 2-fluoroaniline, 2,3-dimethylaniline, 2,6-dimethylaniline, the corresponding products could not be obtained from the reaction system, suggesting that the CCR has a limited tolerance for the ortho-substituted aromatic amines. Remarkably, there is scarce literature that employs the substrates of ortho-substituted aromatic amines in CCR. Esters III1–III5 were readily synthesized by reaction of compound I23 with the corresponding hydroxyl substrates using DCC as a coupling reagent with yields higher than 80% (Scheme 2).Generally, the CCR generates racemic products. The relative configuration assignment was initially performed on the basis of the chemical shifts of H-4 and the coupling constants between H-3 and H-4. The high field signals (<4.30 ppm) were found for the H-4 signals of 3-phenyl trans-diastereomers, while the relative low field signals (>4.50 ppm) occurred for the cis-isomers.24,26–29 According to the reported molecular models,24,26–28 this might be ascribed to the shielded effects of the aromatic cloud on H-4 of trans-isomers. Meanwhile, a relative larger H-3/H-4 coupling constant of cis-diastereomers usually occurred higher than 4.0 Hz (Fig. 2). Conversely, trans-configured products generally show H-3/H-4 coupling constant of less than 2.5 Hz. The relative configuration analysis of trans-I6 was exemplified via its single-crystal X-ray results (Fig. 2 and Table S1†). Thereby, compounds I4, I5–I21, I23, I24, I26–I34, II1–II3, II5, II7, II8, II11, II14–II16, and II18 were obtained predominantly as a single and racemic cis-isomer. However, the only trans-isomer was formed for compounds I1–I3, I5–I8, I10–I14, I22, II12, and III1–III5, since their H-3/H-4 signals appeared singlet (or unresolved doublets). Collectively, the relative configuration ratios of the other compounds were shown in Table S2.† Particularly notable is the configuration inversion of the trans-esters III1–III5 from cis-I23. It has been demonstrated that the base could promote totally isomerization of cis- to trans-configuration when cis-products of CCR were exposed to aqueous NaOH solution30 or the organic base N,N′-carbonyldiimidazole (CDI) was used in the trans-ester synthesis from the cis-precursor.21 The cis-products of CCR are conceived as the kinetic products and they were removed from the reaction system by precipitation at room temperature and the isomerization to more stable trans-configured counterpart might occur under some conditions, such as the existence of bases. In the present study, we found the general coupling reagent DCC could lead to the configuration inversion as well.
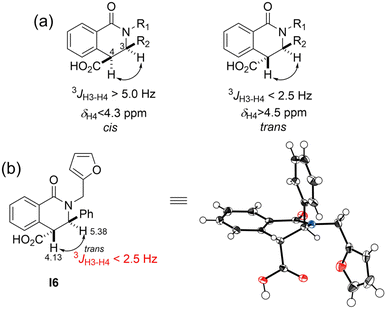 | ||
| Fig. 2 The reported δH4 values and H-3/H-4 coupling constants of cis- and trans-diastereomers of 3-phenyl tetrahydroisoquinolone-4-carboxylic acid compounds (a); and the δH4 value and H-3/H-4 coupling constant of cis-I6 and its single-crystal structure (CCDC Deposition No. 2180985†) (b). | ||
3.2 In vitro antifungal and antioomycete activities of 3,4-dihydroisoquinolin-1(2H)-one derivatives
Firstly, 7 phytopathogens were used to determine the in vitro antifungal and antioomycete activities of compounds I1–I34 against mycelial growth at the concentration of 100 μg mL−1. As shown in Table 1, in spite of their broad spectrum of bioactivities, they varied in activity against different pathogens. Generally, I1–I34 showed much better activity against oomycete P. recalcitrans than other 6 fungi, with all the inhibition rates higher than 95%. Meanwhile, the inhibition rates of the title compounds against B. cinerea, F. oxysporum f. sp. niveum, C. gloeosporioides, T. roseum, A. mali and R. solani were within the range of 55%–82.1%, 37.9%–90.8%, 63.4%–93.3%, 27.8%–57.1%, 55.3%–81.4%, and 55.8%–100%, respectively. Considering the distinguished differences between oomycetes and fungi and the conspicuous activity of I1–I34 against P. recalcitrans, we chose P. recalcitrans as the target strain for the following toxicity assay and SAR analysis.It is noteworthy that the oomycete P. recalcitrans is a soil-borne phytopathogen with a wide host-range, such as tobacco,31 soybean,32 corn,33 alfalfa,34 grape, beet35 and carrot,36 causing seed decay, seedling collapse and root rot, cavity spot, and significantly reducing crop yield. Due to its easy recovery profile from flooding soil37 and insensitivity to many commercial fungicides,33,36 there is a need for exploring effective antioomycete agents to control this pathogen. The toxicity of compounds I1–I34,II1–II20 and III1–III5 against mycelial growth of P. recalcitrans was further determined and the results were shown in Table 2. I23 stood out as the most potent activity with the EC50 value of 14.0 μM (Fig. S2†), which was superior over hymexazol (EC50 = 37.7 μM) and dimethomorph (EC50 > 258.4 μM). Generally, the EC50 values of 4-carboxyl derivatives I1–I34 and II1–II20 ranged from 14.0 μM to 67.8 μM. Nevertheless, loss of a free carboxyl group (III1–III5) triggered a significant decrease in toxicity, indicating the necessity of unsubstituted carboxyl group for the activity.
3.3 3D-QSAR analysis
| Parameters | CoMFA | CoMSIA |
|---|---|---|
| a “—” indicates that the data has not been determined. | ||
| qcv2 | 0.609 | 0.711 |
| Nopt | 4 | 5 |
| rncv2 | 0.940 | 0.945 |
| SEEncv | 0.111 | 0.107 |
| F-value | 171.652 | 147.469 |
| rtest2 | 0.989 | 0.989 |
| SEPtest | 0.130 | 0.131 |
| Steric | 0.581 | 0.110 |
| Electrostatic | 0.419 | 0.187 |
| Hydrophobic | — | 0.178 |
| H-bond donor | — | 0.135 |
| H-bond acceptor | — | 0.391 |
The internal validation parameters revealed that these two models were statistically significant of predicating the activity. As shown in Fig. S3,† the actual and predicated pEC50 values of the training and test set molecules (Table S3†) were strongly correlated in a linear fashion. Meanwhile, the external validation parameters of the test set reflect the good predicative capacity of the models. The rtest2 (and SEPtest) of the CoMFA and CoMSIA models were 0.989 (0.130) and 0.989 (0.131), respectively.
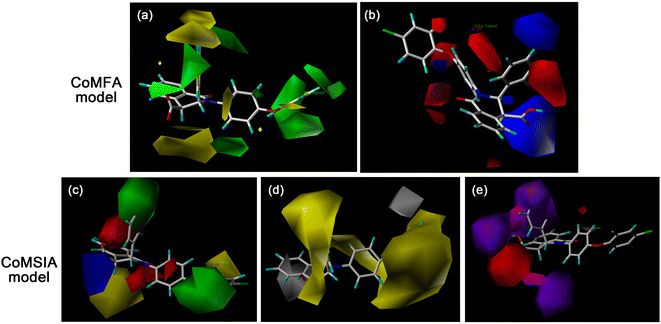
In the steric contour maps of CoMFA model (Fig. 3a), it can be observed that a big green contour was near the N2 site, indicating that bulky groups at that position might be more favorable for the activity. This was identical with the following experimental results: I21 (R1 = 4-C6H5C6H4) > I16 (4-CH3(CH2)3C6H4) > I15 (C6H5) > I1 ((CH2)3CH3) ≈ I11 (cyclopropyl), I28 (4-CF3OC6H4) > I33 (4-OCHF2C6H4), II13 (3-F,4-ClC6H3) > II19 (4-ClC6H4). The other two big yellow contour regions around the phenyl group at C3 site and the carboxyl group at C4 site suggest that bulky groups at those sites furnish negative effects to the bioactivity. This could be exemplified by the comparison of compounds I23 and II1–II20. Meanwhile, the above analysis have confirmed that the substitution of carboxyl group at C4 site significantly decreased the activity. It is found that a blue contour map overlaid on the para-position of the C-3 phenyl group in the electrostatic contour maps (Fig. 3b), signifying that the superior substituents at that site should cause fairly lesser electron density. The situation can be proved by the activity order: II1 (R2 = 4(CH3)2CHC6H4) > II2 (4-(CH3)3CC6H4) > II17 (4-BrC6H4). The presence of a red block near the meta-position of the C-3 phenyl group denotes that the electron-rich substituents attached there are harmful to activity, for instances, II15 (R2 = 3-F,4-CH3OC6H3) > II14 (3,4-diCH3OC6H3) and II13 (3-F,4-ClC6H3) > II19 (4-ClC6H4).
As shown in Fig. 3c, the steric and electrostatic contour maps of the CoMSIA model are similar to those of the CoMFA model, showing the similar trends of the influences of these two fields on activity in the CoMSIA model. For the hydrophobic field (Fig. 3d), most of the yellow contour is covered around R1 and R2 groups, revealing that the general hydrophobic substituents are advantageous. Besides, it is well known that the hydrophobicity would enhance with the increase of the alkane chain length, which is consistent with the sequences of activity: I3 (R1 = (CH2)7CH3) > I1 ((CH2)3CH3) and I10 ((CH2)3C6H5) > I9 ((CH2)2C6H5) > I8 (CH2C6H5). This may also explain that compounds with hydrophobic substituent such as –X (I24–I27, II13, and II17–II20), alkyl (I16, I17, I21, I22, and II1–II3) and ether group (I18 and II7) at these positions possess the desirable activity.
The hydrogen bond donor contour map (Fig. 3e) identifies a purple contour at the N2 position, implying that the R2 groups with H-bond donor are not welcome. Simultaneously, a magenta region near the same sites in hydrogen bond acceptor contour map (Fig. 3e) suggests that compounds with H-bond acceptor can ameliorate the activity, which is basically in line with the circumstance reflected in the H-bond donor field. The potency of compound II15 (R2 = 3-F,4-CH3OC6H3) with an H-bond acceptor at this site was raised compared with compound II5 (4-CH3OC6H4). On the contrary, the red polyhedron is nearest to the C4 position, suggesting that compounds I and II series with H-bond donor (R3 = H) have fantastic activity than esterified compounds.
3.4 Control efficacy of compound I23 in pot experiment
The control efficacy of compound I23 against P. recalcitrans on tobacco seedlings was determined by the pot experiment. As shown in Fig. 4, P. recalcitrans caused the obvious damping-off and plant shriveling of infected tobacco seedlings in the control group and I23-treated group (1.0 mg per pot), while the undiseased seedlings were healthy and vigorous. As can be seen from Table S4,† the preventive efficacy was superior over the curative efficacy, indicating that taking preventive measures to reduce is more effective to control the disease caused by P. recalcitrans before the onset of the disease outbreak. In practice, just like the common saying ‘an ounce of prevention is worth a pound of cure’, the preventive means, such as fungicide seed treatment, is a common and effective practice to control soilborne diseases worldwide in agriculture.38 Meanwhile, both of these control efficacies were improved with the applied dose of I23 being increased. Particularly, at the dose of 2.0 mg per pot, the preventive and curative efficacies of I23 were 75.4% and 33.3%, respectively, which did not show significant differences with those of hymexazol treatments. When the dose was 5.0 mg per pot, I23 achieved an obvious increase in the preventive efficacy (96.5%). These results demonstrated that compound I23 is a promising antioomycete agent to control the disease caused by P. recalcitrans.3.5 Effects of compound I23 on P. recalcitrans
As aforementioned by the 3D-QSAR analysis, the C4 carboxyl group is of great importance to the scaffold 1 and apparently the functional carboxylic acids represents their intrinsic profile of organic acid. Given the well-established fact that acid stress could result in the damaged lipidic cytoplasm membrane and decreased membrane fluidity,39 the impact of compound I23 on the cell membrane permeability of P. recalcitrans was evaluated. As can be seen from Fig. S4,† I23 caused the increase of the relative electrical conductivity at its EC30 (8.7 μM), EC50 (14.0 μM) and EC70 (20.0 μM), which was concentration-dependent. These results revealed that I23 impaired cell membrane integrity of P. recalcitrans, leading to the easy leakage of intracellular electrolytes and thus enhancing the relative electrical conductivity. Interestingly, it has also been found that cinnamic acid, a known small antifungal molecular organic acid, could significantly increase cell membrane permeability of Sclerotinia sclerotiorum (Lib.) de Bary.40Notely, when P. recalcitrans was exposed to the EC50 and EC70, their relative electrical conductivities were significantly greater than those of the EC30 and the control. Therefore, the EC50 was chosen to detect the effect of I23 on the ultrastructural changes of P. recalcitrans by TEM. The untreated control remained intactness of cellular ultrastructures, including dense and even cell walls, clear and complete plasma membranes, mitochondria with visible cristae, distinct electron-dense vesicles (EDVs) and organelles (Fig. 5a–c). After treatment with I23 at the EC50, moderate to extreme ultrastructural alternations occurred in the cells of P. recalcitrans. The density of the cell walls decreased and the membranous organelles became disorganized, with abnormal and indiscernable morphologies. Conspicuously, mitochondrial size was reduced and the incidence of empty vacuoles increased compared with those of cells from the untreated control (Fig. 5d and e). Even worse, the organelles degraded severely, the cell walls were not intact, and the inner wall layer of the original hyphae was retracted into cell lumen (Fig. 5f). Considering the ultrastructural changes of the cellular membranous systems, it is supposed that I23 had profound impacts on the biological membrane.
These impacts could be exemplified by the FM4-64 staining and mitochondrial membrane potential vanishing by JC-1 staining as well. The lipophilic vital styryl dye FM4-64 primarily stains the plasma membrane, endosomes and vesicles in the untreated fine cells of P. recalcitrans (Fig. 6Aa and b). In contrast, after P. recalcitrans was treated with I23 at the EC50 for 12 h, no definitive staining pattern was observed either at the plasma membrane or in the cytosol, up to 60 min after exposure to the dye (Fig. 6Ac and d). Further JC-1 staining showed that the decreasing and vanishing status of mitochondrial membrane potential occurred as early as 6 h post-treatment (Fig. 6B), indicating the mitochondria damaged or dead states. As a matter of fact, it has been found that organic acids could serve as uncouplers that generally dissipate pH and electrical gradients across biological membranes.41 Taken together, it has been speculated that I23 might exert the antioomycete activity against P. recalcitrans by interfering with cytoplasmic membrane structure and membrane proteins or membrane uncoupling capabilities.
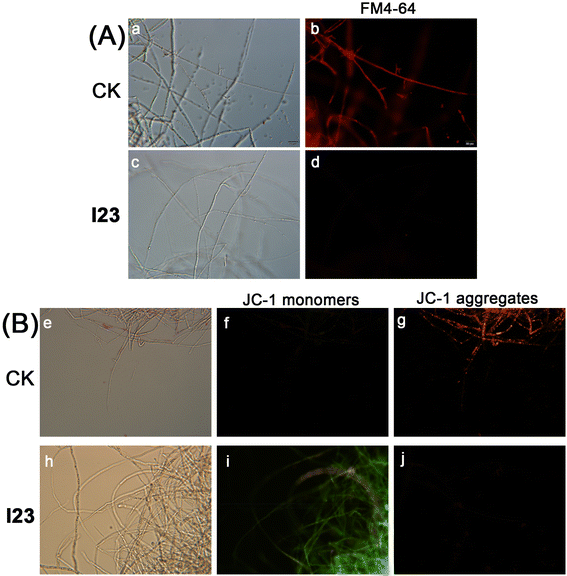 | ||
| Fig. 6 The fluorescence microscopy photos of S. sclerotiorum mycelia stained by FM4-64 (A) and JC-1 dyes (B). | ||
3.6 Lipidomics analysis
As is known, a lipid bilayer is the foundational part of all cellular membranes. In order to gain an insight into the effects of I23 on the biological membranes of P. recalcitrans, lipidomics strategy was carried out to identify and quantify the lipid constituents of the control and the I23-treated groups, respectively. A total of 1685 individual lipid species were quantified; these include different glycerophospholipid, sphingolipid, sterol lipid, prenol lipid, fatty acyl, and saccharolipid classes (Fig. 7a and b). Generally, the basic lipid bilayer is composed of three main types of lipids—phospholipids, sphingolipids, and cholesterol, in which glycerophospholipid serves as a basically structural component of cell membranes.42,43 The results of the lipid composition analysis indicated that I23 did not alter the composition of the membraneous lipids but caused their proportional changes. The dynamic range analysis results (Fig. 7c) of the detected lipid species demonstrated that both the control and the treated groups spanned a similar dynamic range, reflecting the less impacts on the lipid composition. Nevertheless, after treatment with I23, there were increases in the total proportions of sterol lipids (ChE and ZyE) and sphingolipids (Fig. 7d). Significantly, sterol lipids are thought to play a role in ensuring the stability of the lipid bilayer and resisting the permeabilization under osmotic stress.44,45 Simultaneously, it is perceived that sphingolipids help stabilize the membrane bilayer.46,47 Therefore, the increases might be the salvage response to the negative effects of I23 on the cell membrane integrity and function of P. recalcitrans. Notably, glycerolipids are a large group of storage biological molecules necessary for membrane formation, metabolic energy, and fat acids in most living organisms.48,49 In the present study, I23 increased the proportion of glycerolipids, which might be also associated with different degrees of defects in membrane integrity and function.The results of the lipid absolute quantification analysis (Fig. S5†) showed that I23 could lead to the increase of the total lipid content but this difference did not reach significance. Notwithstanding, among the cell membrane components with the contents higher than 1%, Cer class, and PI and PC classes showed significant increase and decreases, respectively. To have a specific overview of how changes in the three lipid classes, the carbon chain length and the degree of unsaturation of the corresponding lipid species were further analyzed. As depicted in Fig. S6,† the Cer content enhanced at the different chain length and the degree of unsaturation levels, while PI and PC decreased. The alternation of Cer, PI and PC confirmed the instability and the integrity changes of the cell membrane induced by I23, which might be responsible for the malfunction and the increased permeability of cellular membrane P. recalcitrans. Besides, the 3D-QSAR analysis indicated that the hydrophobic R1 and R2 groups are favorable, suggesting the necessity of the hydrophobicity of the potent compounds. It has been demonstrated that the small hydrophobic molecules can easily enter into the cellular membranes, which might induce detrimental actions of I23 on cellular membranes.
4. Conclusions
In conclusion, CCR was employed to synthesize a series of 3,4-dihydroisoquinolin-1(2H)-one derivatives as new antioomycete agents against P. recalcitrans. Compound I23 showed the highest in vitro potency with an EC50 value of 14 μM, which was higher than the commercial hymexazol (37.7 μM). Moreover, I23 exhibited the in vivo preventive efficacy of 75.4% at the dose of 2.0 mg per pot, which did not show significant differences with those of hymexazol treatments. When the dose was 5.0 mg per pot, I23 achieved a preventive efficacy of 96.5%. The toxicity study indicated that the I23 might exert the antioomycete activity via disruption of the biological membrane systems. In addition, the established CoMFA and CoMSIA models with reasonable statistics revealed the necessity of C4-carboxyl group and other structural requirements for activity. Therefore, our developed models can help to understand the SAR, and thus aid in the design and development of more potent 3,4-dihydroisoquinolin-1(2H)-one derivatives as antioomycete agents against P. recalcitrans. Design and synthesis of more new compounds are currently in progress.Author contributions
D. Wang, Y. Fang, and Z. Zhang conceived the idea and designed the research. D. Wang, M. Li, and J. Li performed the research. M. Li, J. Li, Y. Fang, and Z. Zhang analyzed the data. D. Wang and M. Li wrote the original manuscript. Y. Fang, and Z. Zhang reviewed the manuscript. D. Wang was responsible for the funding acquisition.Conflicts of interest
The authors declare that there are no conflicts of interest.Acknowledgements
The study was supported by the National Natural Science Foundation of China (No. 31901909), and the Doctoral Research Startup Fund of Shanxi Agricultural University. The authors expressed gratitude to Professor Yan He, Northwest A&F University, for his molecular simulation in this work, and Professor Wang Tuhong, Institute of Bast Fiber crops, Chinese Academy of Agricultural Science, for identifying and providing the phytopathogen Pythium recalcitrans.References
- B. C. Gerwick and T. C. Sparks, Pest Manage. Sci., 2014, 70, 1169–1185 CrossRef CAS PubMed.
- C. L. Cantrell, F. E. Dayan and S. O. Duke, J. Nat. Prod., 2012, 75, 1231–1242 CrossRef CAS PubMed.
- F. E. Dayan, C. L. Cantrell and S. O. Duke, Bioorg. Med. Chem., 2009, 17, 4022–4034 CrossRef CAS PubMed.
- T. C. Sparks and S. O. Duke, J. Agric. Food Chem., 2021, 69, 8324–8346 CrossRef CAS PubMed.
- K. G. Meyer, K. Bravo-Altamirano, J. Herrick, B. A. Loy, C. Yao, B. Nugent, Z. Buchan, J. F. Daeuble, R. Heemstra, D. M. Jones, J. Wilmot, Y. Lu, K. DeKorver, J. DeLorbe and J. Rigoli, Bioorg. Med. Chem., 2021, 50, 116455 CrossRef CAS PubMed.
- R. Nauen, P. Jeschke, R. Velten, M. E. Beck, U. Ebbinghaus-Kintscher, W. Thielert, K. Wölfel, M. Haas, K. Kunz and G. Raupach, Pest Manage. Sci., 2015, 71, 850–862 CrossRef CAS PubMed.
- D. K. Yadav, N. Singh, K. Dev, R. Sharma, M. Sahai, G. Palit and R. Maurya, Fitoterapia, 2011, 82, 666–675 CrossRef CAS PubMed.
- Y. Wang, D. Wang, J. Zhang, D. Liu, Z. Wang and D. Meng, Phytochemistry, 2018, 155, 93–99 CrossRef CAS PubMed.
- A. Chiarugi, E. Meli, M. Calvani, R. Picca, R. Baronti, E. Camaioni, G. Costantino, M. Marinozzi, D. E. Pellegrini-Giampietro, R. Pellicciari and F. Moroni, J. Pharmacol. Exp. Ther., 2003, 305, 943 CrossRef CAS PubMed.
- R. K. Morgan, I. Carter-O’Connell and M. S. Cohen, Bioorg. Med. Chem. Lett., 2015, 25, 4770–4773 CrossRef CAS PubMed.
- R. Pellicciari, E. Camaioni, G. Costantino, L. Formentini, P. Sabbatini, F. Venturoni, G. Eren, D. Bellocchi, A. Chiarugi and F. Moroni, ChemMedChem, 2008, 3, 914–923 CrossRef CAS PubMed.
- M. T. M. Nemr and A. M. AboulMagd, Bioorg. Chem., 2020, 103, 104134 CrossRef CAS PubMed.
- M. T. M. Nemr, M. Teleb, A. M. AboulMagd, M. E. El-Naggar, N. Gouda, A. A. Abdel-Ghany and Y. A. M. M. Elshaier, J. Mol. Struct., 2023, 1272, 134216 CrossRef CAS.
- S. Armstrong, J.-H. Li, J. Zhang and A. Rod Merrill, J. Enzyme Inhib. Med. Chem., 2002, 17, 235–246 CrossRef CAS PubMed.
- N. Jegham, M. B. Braiek, Y. Kacem and B. B. Hassine, J. Chem. Res., 2016, 40, 687–690 CrossRef CAS.
- A. R. Saundane, V. A. Verma and K. Vijaykumar, Med. Chem. Res., 2013, 22, 3787–3793 CrossRef CAS.
- V. V. Dabholkar and D. R. Tripathi, J. Heterocycl. Chem., 2011, 48, 529–532 CrossRef CAS.
- M. T. M. Nemr, A. M. AboulMagd, H. M. Hassan, A. A. Hamed, M. I. A. Hamed and M. T. Elsaadi, RSC Adv., 2021, 11, 26241–26257 RSC.
- B. Dorothee, B. Emilie, C. Patrick, K. Mohamed, L. Pieter, N. Johan, M. Arnaud and V. Inge, US2011224208A1, 2011.
- N. Guranova, D. Dar’in and M. Krasavin, Synthesis, 2018, 50, 2001–2008 CrossRef CAS.
- A. Mikheyev, G. Kantin and M. Krasavin, Synthesis, 2018, 50, 2076–2086 CrossRef CAS.
- P. J. Henry, H. R. George, L. S. Elizabeth, P. Albert, B. A. David, G. D. Eric and M. Michael, EP0247760A2, 1987.
- S. W. Laws, S. Y. Howard, R. Mato, S. Meng, J. C. Fettinger and J. T. Shaw, Org. Lett., 2019, 21, 5073–5077 CrossRef CAS PubMed.
- E. Chupakhin, D. Dar' and M. Krasavin, Tetrahedron Lett., 2018, 59, 2595–2599 CrossRef CAS.
- V. Matyash, G. Liebisch, T. V. Kurzchalia, A. Shevchenko and D. Schwudke, J. Lipid Res., 2008, 49, 1137–1146 CrossRef CAS PubMed.
- M. A. Haimova, N. M. Mollov, S. C. Ivanova, A. I. Dimitrova and V. I. Ognyanov, Tetrahedron, 1977, 33, 331–336 CrossRef CAS.
- N. Yu, L. Bourel, B. Deprez and J.-C. Gesquiere, Tetrahedron Lett., 1998, 39, 829–832 CrossRef CAS.
- S. Y. Howard, M. J. Di Maso, K. Shimabukuro, N. P. Burlow, D. Q. Tan, J. C. Fettinger, T. C. Malig, J. E. Hein and J. T. Shaw, J. Org. Chem., 2021, 86, 11599–11607 CrossRef CAS PubMed.
- Y. A. A. M. Elshaier, M. T. M. Nemr, M. S. Refaey, W. A. A. Fadaly and A. Barakat, New J. Chem., 2022, 46, 13383–13400 RSC.
- A. Safrygin, O. Bakulina, D. Dar’in and M. Krasavin, Synthesis, 2020, 52, 2190–2195 CrossRef CAS.
- C. Bian, S. Zhao, K. Jang and Y. Kang, Australas. Plant Dis. Notes, 2016, 11, 7 CrossRef.
- N. Li, Q. Zhou, K.-F. Chang, H. Yu, S.-F. Hwang, R. L. Conner, S. E. Strelkov, D. L. McLaren and G. D. Turnbull, Crop Protect., 2019, 118, 36–43 CrossRef.
- L. Radmer, G. Anderson, D. M. Malvick, J. E. Kurle, A. Rendahl and A. Mallik, Plant Dis., 2016, 101, 62–72 CrossRef PubMed.
- L. E. Berg, S. S. Miller, M. R. Dornbusch and D. A. Samac, Plant Dis., 2017, 101, 1860–1867 CrossRef CAS PubMed.
- E. Moralejo, A. Clemente, E. Descals, L. Belbahri, G. Calmin, F. Lefort, C. F. J. Spies and A. McLeod, Mycologia, 2008, 100, 310–319 CrossRef CAS PubMed.
- X. H. Lu, R. Michael Davis, S. Livingston, J. Nunez and J. J. Hao, Plant Dis., 2011, 96, 384–388 CrossRef PubMed.
- M. T. Kirkpatrick, J. C. Rupe and C. S. Rothrock, Plant Dis., 2006, 90, 592–596 CrossRef CAS PubMed.
- J. R. Lamichhane, M. P. You, V. Laudinot, M. J. Barbetti and J.-N. Aubertot, Plant Dis., 2019, 104, 610–623 CrossRef PubMed.
- N. Guan and L. Liu, Appl. Microbiol. Biotechnol., 2020, 104, 51–65 CrossRef CAS PubMed.
- Y. Wang, Y. Sun, J. Wang, M. Zhou, M. Wang and J. Feng, Plant Dis., 2019, 103, 944–950 CrossRef CAS PubMed.
- S. C. Ricke, Poult. Sci., 2003, 82, 632–639 CrossRef CAS PubMed.
- P. J. Quinn, Prog. Lipid Res., 2010, 49, 390–406 CrossRef CAS PubMed.
- G. van Meer, D. R. Voelker and G. W. Feigenson, Nat. Rev. Mol. Cell Biol., 2008, 9, 112–124 CrossRef CAS PubMed.
- A. M. Farnoud, A. M. Toledo, J. B. Konopka, M. Del Poeta and E. London, in Current Topics in Membranes, ed. A. K. Kenworthy, Academic Press, 2015, vol. 75, pp. 233–268 Search PubMed.
- S. Dupont, L. Beney, T. Ferreira and P. Gervais, Biochim. Biophys. Acta Biomembr., 2011, 1808, 1520–1528 CrossRef CAS PubMed.
- L. V. Michaelson, J. A. Napier, D. Molino and J.-D. Faure, Biochim. Biophys. Acta Mol. Cell Biol. Lipids, 2016, 1861, 1329–1335 CrossRef CAS PubMed.
- F. M. Goñi and A. Alonso, Biochim. Biophys. Acta Biomembr., 2006, 1758, 1902–1921 CrossRef PubMed.
- D. R. Voelker, in Encyclopedia of Biological Chemistry, ed. W. J. Lennarz and M. D. Lane, Academic Press, Waltham, 2nd edn., 2013, pp. 412–418 Search PubMed.
- C.-L. E. Yen, S. J. Stone, S. Koliwad, C. Harris and R. V. Farese Jr, J. Lipid Res., 2008, 49, 2283–2301 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2180985. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3ra00855j |
| ‡ These authors contributed equally to this work and share the first authorship. |
| This journal is © The Royal Society of Chemistry 2023 |