Open Access Article
Open Access ArticleApplication of functional metal anionic Lewis acid ionic liquids in the alkylation of chlorobenzene/SOCl2†
Jiru Guoa,
Yanxia Zhenga,
Yuchao Li *a,
Zijian Wena,
Xiuxu Shenb and
Yansong Zhao*c
*a,
Zijian Wena,
Xiuxu Shenb and
Yansong Zhao*c
aSchool of Chemistry and Chemical Engineering, Institute of Clean Chemical Technology, Shandong Collegial Engineering Research Center of Novel Rare Earth Catalysis Materials, Shandong University of Technology, Zibo 255049, P. R. China. E-mail: cyulee@126.com
bYantai Laiyang Central Hospital, No. 119 Changshan Road, Laiyang, Shandong 265200, China
cDepartment of Safety, Chemistry and Biomedical Laboratory Sciences, Faculty of Engineering and Science, Western Norway University of Applied Sciences (HVL), 5063 Bergen, Norway. E-mail: yansong.zhao@hvl.no
First published on 14th April 2023
Abstract
4,4′-Dichlorodiphenyl sulfoxide is the main raw material for the manufacture of polysulfone, polyether sulfone and other engineering plastics. It is also an intermediate for medicines, dyes and pesticides, which has been widely utilized in engineering plastics, fine chemicals and other fields. The alkylation of chlorobenzene with thionyl chloride can give 4,4′-dichlorodiphenyl sulfoxide as a product using Lewis acidic ionic liquids. In this work, metal-based methylimidazolium ionic liquids were synthesized, which were found to be efficient catalysts for alkylation reactions. The molar ratio of different metal chlorides to 1-butyl-3-methyl imidazole chloride and the influence of different metal chlorine additives on the catalytic Lewis acid center were investigated. The fissionable species of AlCl3 in ionic liquids will enhance the acidity of ionic liquids and, thus, promote the catalytic performance of ionic liquids. Under the optimized reaction conditions, the conversion rate of excess chlorobenzene was 45.3% and the selectivity of 4,4′-dichlorodiphenyl sulfoxide was 31.9%.
1 Introduction
4,4′-Dichlorodiphenyl sulfone is the main raw material for the manufacture of engineering plastics such as polysulfone and polyethersulfone.1–3 It is also an intermediate for medicines, dyes and pesticides, and it has a wide range of applications in fine chemicals and other fields.4–7 Polymer materials have the advantages of high specific strength, good toughness, and fatigue resistance, and most of them are inert and corrosion resistant. As an important raw material of engineering plastics, 4,4′-dichlorodiphenyl sulfone is crucial in the field of polymer materials.8,9At present, the main processes for the industrial production of 4,4′-dichlorodiphenyl sulfone are the chlorosulfonic acid process and sulfuric acid process. However, there are some problems such as environmental pollution and catalyst corrosion in the process of using sulfuric acid as a traditional catalyst.10,11 In the sulfoxide oxidation process, HCl as the main by-product is also one of the disadvantages of this process route over other production methods. The good catalytic effect of AlCl3 in the Friedel–Crafts alkylation process has gradually made it the main catalyst for the synthesis of 4,4′-dichlorodiphenyl sulfone. However, AlCl3 must be separated from the product by hydrolysis after the reaction, resulting in a large amount of acidic wastewater and causing serious environmental problems. Some solid acids such as composite oxide catalysts have also been recommended as alkylation catalysts.12,13 However, the main disadvantages of solid acid catalysts are the rapid inactivation due to coking and the limited accessibility of matrix-binding acid sites.14 The acidic sites of Brønsted acid and Lewis acid in the acidic ionic liquid are more conducive to inducing alkylation reactions. While it has better stability, it can also be recycled by membrane separation and other methods to reduce environmental pollution and improve economic benefits to a certain extent.15–17 Therefore, especially in the context of sustainable development, the IL has a good development prospect due to its catalytic property toward chlorobenzene/thionyl chloride alkylation.
As more efficient and environment-friendly catalysts, various ionic liquids (ILs) have been developed for their unique properties such as tunable Lewis and Brønsted acidity, negligible vapour pressure, good thermal stability and easy recycling to replace the traditional strong acid.18–23 Chloroaluminate ILs have been most frequently investigated to obtain excellent Lewis acidity and to catalyze alkylation reactions.24–26
Chauvin et al. first reported that isobutane/butene alkylation could be effectively catalyzed by the ionic liquid 1-butyl-3-methyl imidazolium chloride–aluminum chloride ([C4mim]Cl–AlCl3) and acidity, as well as catalytic activity, was strongly influenced by the molar fraction of AlCl3.27 Zhang et al. used triethylamine hydrochloride–aluminum chloride as a catalyst for the alkylation of isobutane/butene. Research results indicate that the active material in ionic liquids is a trace amount of AlCl3 or Al2Cl6.22 Wang et al. studied the effect of CuCl-modified composite metal-based ionic liquids on the catalytic activity of alkylation reactions on the basis of urea–AlCl3 ionic liquids, and observed the synergistic effect of Lewis acid and Brønsted acid sites on the alkylation reaction. Therefore, the synergistic effect of Lewis and Brønsted acid sites on product selectivity was verified. However, the catalytic synergistic effect of metal-based ionic liquids on the preparation of 4,4′-dichlorodiphenyl sulfoxide has hardly been investigated.14
In this work, imidazole/metal chloride-based ionic liquids were used in the alkylation of chlorobenzene/thionyl chloride. A series of metal-based ionic liquids were prepared based on metal chloride salts with different Lewis acids, and their catalytic performance in the alkylation of chlorobenzene/thionyl chloride was evaluated. The effects of metal chloride salts, imidazole/AlCl3 molar ratios, and metal salt additions on the catalytic performance, as well as the synergistic effect of Lewis and Brønsted acidity, were investigated.
2 Experimental
2.1 Materials
N-Methylimidazole, n-butane chloride, aluminum chloride, zinc chloride, ferric chloride, ferrous chloride, bismuth chloride copper, chlorobenzene and thionyl chloride were purchased from Aladdin Chemistry Co. Ltd. (Beijing, China). All the other chemical reagents were bought from commercial sources and of analytical reagent grade unless otherwise noted.2.2 Preparation of ionic liquids
ILs based on CuCl, FeCl3, ZnCl2 and FeCl2 were prepared following the same procedure. The molar ratio of metal chloride to [Bmim]Cl was 1.6.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MCly = 12
MCly = 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).2.3 Characterization
2.4 Chlorobenzene/thionyl chloride alkylation
A thermometer and an exhaust gas absorption device were installed in a flask. Chlorobenzene and ILs were added to the flask and heated by stirring. Thionyl chloride was gradually added, reacted at 65 °C for 2.5 h, cooled and then crystallized. The crystallization product was put into the flask, 10 mL acetic acid was added, and 1 g 35% hydrogen peroxide was added for oxidation within 0.5 h at 80 °C. The reaction was continued for 1 h, cooled to below 5 °C, filtered, washed and dried to obtain the crude product. Recrystallization with ethanol was used to purify the product.The alkylated products were analyzed by HPLC (LC2030C, Shim-pack VP-ODS 5 μm, 4.6 mm × 25 cm chromatography column, Shimadzu). Alkylated products were quantitatively analyzed by an area normalization method based on the peak area ratio. Chlorobenzene conversion was calculated as the percentage of chlorobenzene before and after the reaction. The selectivity of the product 4,4′-dichlorodiphenyl sulfoxide was calculated by the ratio of the actual yield to the theoretical yield.
3 Results and discussion
3.1 Characterizations of synthesized ILs
Metal chlorides such as zinc chloride have a certain degree of acidity themselves, and the presence of metal chlorides in ILs will influence the overall acidic properties of ILs.10 At the same time, the molar ratio of metal chloride to alkyl imidazole will change the existence of metal species in the ionic liquid.11Various metal-based ILs were obtained, respectively [Bmim]Cl–AlCl3, [Bmim]Cl–ZnCl2, [Bmim]Cl–FeCl3, [Bmim]Cl–FeCl2, [Bmim]Cl–CuCl.
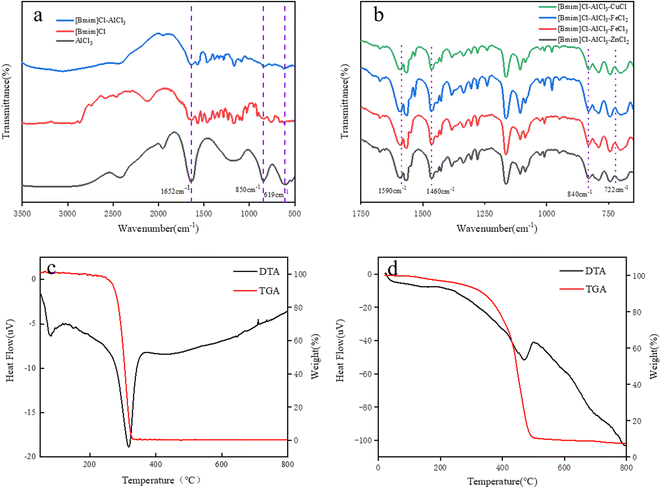
The FT-IR spectra of pure AlCl3, [Bmim][Cl] and [Bmim][Cl]–AlCl3 are shown in Fig. 1a. It can be seen from that the peaks at 1650 and 1560 cm−1 may be ascribed to the C–O and C–N stretching vibrations of the imidazole ring, respectively, while the peak at 1460 cm−1 is assigned to the C–H bending vibration of the imidazole ring.28 In addition, it was found that the peaks at 1652 cm−1, 850 cm−1 and 619 cm−1 shown in Fig. 1a belong to the infrared stretching vibration peaks of pure AlCl3. Successful synthesis of [Bmim][Cl]–AlCl3 can be confirmed by FT-IR analysis results. Similarly, in Fig. 1b, in addition to the stretching vibration peak with the imidazole ring, it was found that the infrared peak at 722 cm−1 may be attributed to FeCl3 and other metal salts. Therefore, successful synthesis of [Bmim][Cl]–AlCl3–MCly can be confirmed by FT-IR analysis results.
The thermal stability of ILs was studied by TGA. The TGA curve of ILs is shown in Fig. 1c and d. The IL samples showed mass loss in the temperature range of 20–190 °C, which was due to the desorption of water. It can be seen from Fig. 1c that [Bmim][Cl] begins to decompose at 190 °C and becomes completely decomposed at 330 °C. As shown in Fig. 1d, the initial decomposition temperature of ILs increased from 190 to 200 °C after the addition of AlCl3. Mass loss in the range of 200–470 °C was due to the decomposition of alkyl chains and imidazole rings, and the mass loss in the range of 470–490 °C was due to the sublimation of partial AlCl3.29,30
3.2 Effects of different metal chlorides
The synthesized metal-based ionic liquid catalyst was evaluated, and its catalytic effect on the alkylation reaction of chlorobenzene/thionyl chloride was tested, as shown in Fig. 2a. The alkylation of chlorobenzene catalyzed by metal-based ionic liquids has a certain effect. However, 4,4′-dichlorodiphenyl sulfoxide was not observed in the catalytic products of [Bmim]Cl–FeCl3, [Bmim]Cl–FeCl2, and [Bmim]Cl–CuCl. Ionic liquids based on AlCl3 and ZnCl2 exhibited good catalysis and excellent selectivity for the alkylation reaction of chlorobenzene/SOCl2. This may be due to the acidity of the anionic types of ZnCl2 and AlCl3 in the ionic liquids in the acidic range suitable for initiating the chlorobenzene/SOCl2 alkylation reaction.14,31 Blank controls were also performed using FeCl3, FeCl2 and CuCl as catalysts for the chlorobenzene/SOCl2 alkylation reaction, and the experimental results are shown in Table S3.† The results showed that FeCl3, FeCl2 and CuCl did not exhibit good catalytic performance in the chlorobenzene/SOCl2 alkylation reaction.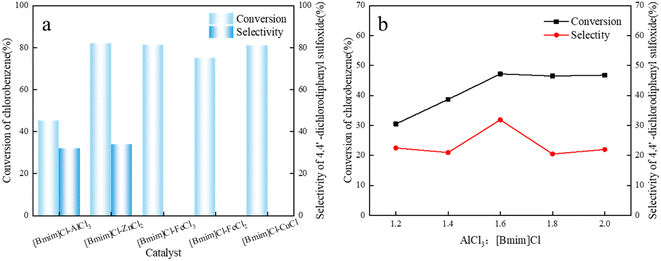 | ||
| Fig. 2 (a) Effect of different metal bases on the conversion and selectivity of chlorobenzene. (b) Effect of catalysts with different AlCl3 contents on conversion and selectivity. | ||
Since [Bmim]Cl–AlCl3 has better catalytic performance, lower cost and lower humidity sensitivity, [Bmim]Cl–AlCl3 was selected for further research.
3.3 Effect of the AlCl3/[Bmim]Cl molar ratio
The anion species and acidity of [Bmim]Cl–AlCl3-based ionic liquids are directly related to the ratio of the AlCl3/[Bmim]Cl substrate, which is crucial for the catalytic progress of chlorobenzene alkylation. The effect of the AlCl3/[Bmim]Cl molar ratio on the acidity and alkylation properties of alkylimidazole–AlCl3 was investigated.Alkylimidazole–xAlCl3-based ILs with different AlCl3 concentrations were used to catalyze the alkylation of chlorobenzene with thionyl chloride, conversion of chlorobenzene as the main component in the alkylation product and 4,4′-dichlorobenzene conversion. The selectivity of chlorodiphenyl sulfoxide is shown in Fig. 2b.
When the molar ratio of AlCl3/[Bmim]Cl was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2, the conversion rate of chlorobenzene was 30.5%, and ILs were generally considered to be neutral at this molar ratio. The conversion of chlorobenzene increased significantly with the increase in AlCl3/[Bmim]Cl molar ratio. When the molar ratio of AlCl3/[Bmim]Cl was changed from 1.2
1.2, the conversion rate of chlorobenzene was 30.5%, and ILs were generally considered to be neutral at this molar ratio. The conversion of chlorobenzene increased significantly with the increase in AlCl3/[Bmim]Cl molar ratio. When the molar ratio of AlCl3/[Bmim]Cl was changed from 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 2
1 to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the conversion rate of chlorobenzene fluctuated between 30.5% and 46.8%. As the molar ratio of AlCl3/[Bmim]Cl increases, the selectivity of product 4,4′-dichlorodiphenyl sulfoxide also changes. For alkylimidazole–xAlCl3 ILs, changes in AlCl3 molar fractions can easily disrupt the equilibrium between molecular Al and ionic Al species.31 As the mole fraction of AlCl3 increases, the degree of asymmetric cleavage of AlCl3 increases, and molecular species are converted into ionic species. Meanwhile, with the increase in AlCl3 molar fraction, acidity of ILs also increases.14 When molar fraction of AlCl3 increased from 1.2 to 1.6, the selectivity of the target product 4,4′-dichlorodiphenyl sulfoxide increased. When the molar ratio of AlCl3 increased further, the selectivity of the target product 4,4′-dichlorodiphenyl sulfoxide decreased slightly. This result indicates that ILs with a certain acidity range can significantly catalyze the isobutane alkylation reaction. Lower or higher molar ratios of AlCl3/[Bmim]Cl can lead to side reactions. Regarding the conversion of chlorobenzene and the selectivity of the target product 4,4′-dichlorodiphenyl sulfoxide, the rate of AlCl3 and [Bmim]Cl was 1.6, which was optimal.
1, the conversion rate of chlorobenzene fluctuated between 30.5% and 46.8%. As the molar ratio of AlCl3/[Bmim]Cl increases, the selectivity of product 4,4′-dichlorodiphenyl sulfoxide also changes. For alkylimidazole–xAlCl3 ILs, changes in AlCl3 molar fractions can easily disrupt the equilibrium between molecular Al and ionic Al species.31 As the mole fraction of AlCl3 increases, the degree of asymmetric cleavage of AlCl3 increases, and molecular species are converted into ionic species. Meanwhile, with the increase in AlCl3 molar fraction, acidity of ILs also increases.14 When molar fraction of AlCl3 increased from 1.2 to 1.6, the selectivity of the target product 4,4′-dichlorodiphenyl sulfoxide increased. When the molar ratio of AlCl3 increased further, the selectivity of the target product 4,4′-dichlorodiphenyl sulfoxide decreased slightly. This result indicates that ILs with a certain acidity range can significantly catalyze the isobutane alkylation reaction. Lower or higher molar ratios of AlCl3/[Bmim]Cl can lead to side reactions. Regarding the conversion of chlorobenzene and the selectivity of the target product 4,4′-dichlorodiphenyl sulfoxide, the rate of AlCl3 and [Bmim]Cl was 1.6, which was optimal.
3.4 Influence of reaction conditions
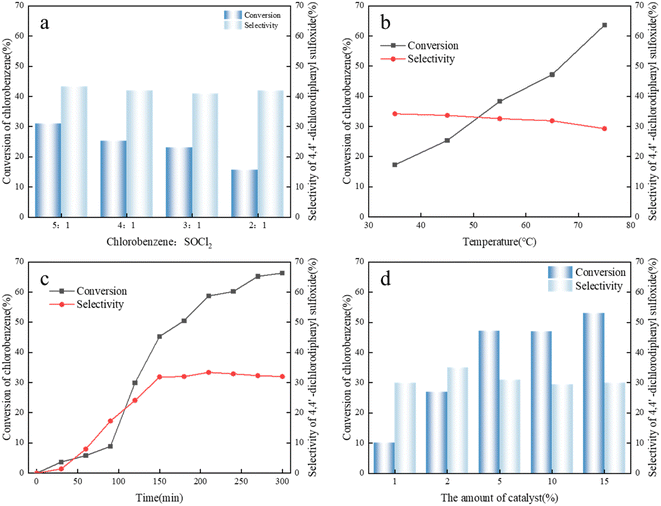
Strong liquid acid-catalyzed alkylation is a typical fast exothermic reaction process that occurs at the acid catalyst–hydrocarbon interface. Using alkylimidazole–AlCl3 as a catalyst, the effects of reaction temperature, stirring rate, raw material molar ratio and other parameters on the diffusion of hydrocarbons in catalysts, conversion of butene, and product selectivity were investigated. Another variable that directly influences 4,4′-dichlorodiphenyl sulfoxide product selectivity, namely, reaction time, was also optimized.The limited solubility of chlorobenzene and SOCl2 in the putty liquid and the instability of SOCl2 will result in a reduction in the quality of the reactants. Therefore, excessive chlorobenzene is used to dilute SOCl2 to avoid the decomposition of SOCl2. The effect of chlorobenzene/SOCl2 feed molar ratios on the reaction system is shown in Fig. 3a. With the increase in molar ratio of chlorobenzene/thionyl chloride, the conversion of chlorobenzene increased to a certain extent, and the selectivity of the product 4,4′-dichlorodiphenyl sulfoxide also increased. This indicated that increasing the molar ratio of chlorobenzene/thionyl chloride is beneficial to the formation of 4,4′-dichlorodiphenyl sulfoxide and inhibited the occurrence of side reactions to a certain extent.
The effect of reaction temperature on chlorobenzene/thionyl chloride alkylation was investigated. As shown in Fig. 3b, the influence of temperature on chlorobenzene/SOCl2 alkylation was studied by changing the temperature from 35 °C to 75 °C. It was found that with the increase in reaction temperature from 35 °C to 75 °C, the conversion of thionyl chloride increased and the selectivity of 4,4′-dichlorodiphenyl sulfoxide gradually decreased. The alkylation reaction of chlorobenzene and thionyl chloride is an exothermic reaction, and a higher temperature is not conducive to the progress of the forward reaction. At 35 °C, the conversion rate of chlorobenzene is 17.3%, but the selectivity is 34.2%. With the increase in temperature, the product selectivity basically stays the same, and the conversion of chlorobenzene did not reach the maximum value. The reason for this phenomenon may be that mass transfer and reaction rate are low when the temperature is low, and the conversion rate increases with the increase in temperature. The conversion rate reaches a maximum value at 75 °C, where the selectivity is 63.6%. With the further increase in temperature, the selectivity continued to decrease, indicating that high temperatures would exacerbate the occurrence of side reactions, resulting in a decrease in the selectivity of 4,4′-dichlorodiphenyl sulfoxide.
The alkylation reaction of chlorobenzene and thionyl chloride is not a fast process, and the reaction rate can be controlled by mass transfer. With the prolonged reaction time, the occurrence of side reactions may be promoted. The effect of reaction time on the butylimidazole–AlCl3-catalyzed chlorobenzene/thionyl chloride alkylation reaction was investigated, as shown in Fig. 3c. When the reaction time was 2.5 h, the conversion rate of chlorobenzene was 45.3%, and the reaction was incomplete. When the reaction time was 3.5 h, the conversion rate of thionyl chloride increased to 66.3%, but the selectivity of 4,4′-dichlorodiphenyl sulfone did not improve much from about 32%. The reason is that with the prolongation of reaction time, other by-products of chlorobenzene are formed, or alkylation product 4,4′-dichlorodiphenyl sulfoxide participates in the reaction again. Therefore, the optimal reaction time is 2.5 h.
Chlorobenzene and thionyl chloride have limited solubility in AlCl3-based ILs. Therefore, the alkylation reaction occurs at the liquid–liquid interface (hydrocarbons are in liquid state under reaction pressure), and a larger interfacial area is conducive to the alkane free radical reaction. It can be seen from the experimental results that the conversion of chlorobenzene increases with the increase in the amount of chloroaluminate, but the selectivity of 4,4′-dichlorodiphenyl sulfoxide remains basically unchanged. The reason is that excessive use of chlorobenzene makes the surface of ionic liquid flooded with chlorobenzene, and cannot make chlorobenzene and thionyl chloride enter the surface of the ionic liquid at the same time, which eventually leads to the selection of 4,4′-dichlorodiphenyl sulfoxide. Selectivity did not increase significantly. The effect of the amount of catalyst dosage on the conversion and selectivity is shown in Fig. 3d.
3.5 Stability and recyclability
Consumption of Lewis acid may lead to the inactivation of ILs, which is a challenge for ILS-catalyzed alkylation.32 The optimum reaction conditions of [Bmim]Cl–1.6AlCl3 (T = 65 °C, chlorobenzene/thionyl chloride = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
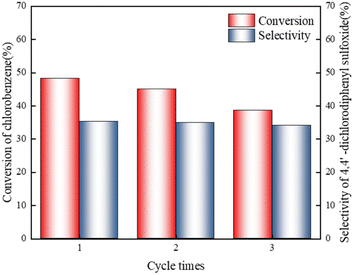
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, t = 150 min). Feedstock was pre-dried to prevent accumulation of water in the catalyst system and to avoid the loss of active species. At the end of reaction, the catalyst was separated from the reaction system, and HPLC analysis was performed. Catalyst was put back into the reactor for the next experiment. The results in Fig. 4 show that under given conditions, [Bmim]Cl–1.6AlCl3 can be reused three times, and the conversion rate of chlorobenzene and selectivity of 4,4′-dichlorodiphenyl sulfoxide do not decrease significantly.
1, t = 150 min). Feedstock was pre-dried to prevent accumulation of water in the catalyst system and to avoid the loss of active species. At the end of reaction, the catalyst was separated from the reaction system, and HPLC analysis was performed. Catalyst was put back into the reactor for the next experiment. The results in Fig. 4 show that under given conditions, [Bmim]Cl–1.6AlCl3 can be reused three times, and the conversion rate of chlorobenzene and selectivity of 4,4′-dichlorodiphenyl sulfoxide do not decrease significantly.
3.6 Alkylation reactions using composite metal-based ILs
Based on the reaction results, the alkylation reaction path (as show in Scheme 1) of chlorobenzene with thionyl chloride in the presence of Lewis acidic ionic liquids was proposed. The effect of Lewis acidity on the alkylation reaction catalyzed by ionic liquids was studied. When chlorobenzene is adsorbed by ionic liquids, sulfoxide chloride will also be complexed with the acidic site of an ionic liquid anion. After the anions of the ionic liquid snatch Cl atoms, the resulting S+ acts as a nucleophilic reagent to attack chlorobenzene and undergo nucleophilic substitution. Then, Cl from SOCl2 combines with H from chlorobenzene to form the HCl dissociation reaction. When HCl is shed, ILs will participate in the next round of alkylation reactions. HCl produced at the same time can restore the acidity of ionic liquids to a certain extent and improve the recyclability of ionic liquids. The specific reaction principle needs further study. | ||
| Scheme 1 Probable mechanism of the alkylation process catalyzed by metal-based Lewis acidic ionic liquids. | ||
3.7 Effect of composite metal-based ionic liquids
It has been reported that catalysts used in alkane/butene alkylation reactions, namely, ionic liquids with metal chlorides added to [Et3N]HCl–xAlCl3, can significantly improve the selectivity of target products.21 In this work, FeCl3, ZnCl2, FeCl2 and CuCl were used as metal chloride additives to investigate the catalytic activity of [Bmim]Cl–1.6AlCl3. FeCl3, ZnCl2, FeCl2 or CuCl were added to [Bmim]Cl–1.6AlCl3 (AlCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MCly = 12
MCly = 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The catalytic effect is shown in Table 1.
1). The catalytic effect is shown in Table 1.
| Catalyst | Conversion/% | Selectivity/% |
|---|---|---|
| [Bmim]Cl–AlCl3–FeCl3 | 54.1 | 48.6 |
| [Bmim]Cl–AlCl3–ZnCl2 | 42.3 | 42.5 |
| [Bmim]Cl–AlCl3–FeCl2 | 14.8 | 9.0 |
| [Bmim]Cl–AlCl3–CuCl | 100 | 4.2 |
The results indicated that [Bmim]Cl–1.6AlCl3–FeCl3 had the best catalytic effect, the conversion rate of chlorobenzene increased from 45.3% to 54.1%, and the selectivity of 4,4′-dichlorobenzene sulfone increased from 31.9% to 48.6%. Similarly, the catalytic effect of [Bmim]Cl–1.6AlCl3–ZnCl2 on the alkylation of chlorobenzene/SOCl2 was also improved. Compared with the previous two, [Bmim]Cl–1.6AlCl3–FeCl2 and [Bmim]Cl–1.6AlCl3–CuCl showed no good catalytic activity and selectivity for chlorobenzene/SOCl2 alkylation.
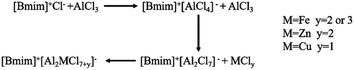
In the metal-based ionic liquid [Bmim]Cl–1.6AlCl3, the addition of FeCl3 and ZnCl2 significantly improved the conversion of chlorobenzene and the selectivity of 4,4′-dichlorodiphenyl sulfone. However, the catalytic effects of FeCl2 and CuCl on ionic liquids had little change. The difference in the catalytic activity of different metal matrix ionic liquids was explained by the difference in the overall Lewis acid strength of the complex metal matrix ionic liquids resulting from the binding reaction of different MCly and AlCl3. As shown in Scheme 2, with the increase of AlCl3 content in ionic liquids, the anion form changes from [AlCl4]− to [Al2Cl7]−. The introduction of bimetal leads to a greater change in anion morphology. For example, the introduction of FeCl3 creates a new anion [Al2FeCl10]−, which has a more dispersed charge density and is more stable and acidic than the [Bmim]Cl–AlCl3 catalyst.25 The Lambert–Beer law was used to calculate the acid strength of composite ILs. The results indicated that the acidity of the composite ionic liquid modified by FeCl3 was the strongest, and the intensity order is as follows: FeCl3 > ZnCl2 > FeCl2 > CuCl. In the presence of metal chlorides, the Lewis acidity of ILs becomes stronger and the catalytic activity increases due to electronic effects.33 At the same time, the introduction of bimetals improves the acidity of Brønsted acids in ILs.32 The synergistic effect of Brønsted and Lewis acid sites is the key to improve the catalytic performance of low eutectic ionic liquids. The enhancement of Brønsted and Lewis acids provides more active acid sites for alkylation, thus improving the catalytic activity and selectivity of ILs.14
4 Conclusions
The alkylation of chlorobenzene/sulfoxide chloride was carried out with a Lewis acidic butyl imidazole–AlCl3 metal ionic liquid as a catalyst. The presence of AlCl3 makes the ionic liquid have the adjustable Lewis acidity and ability to catalyze the alkylation of chlorobenzene/sulfoxide chloride. This may be because AlCl3 forms a new acidic species in the ionic liquid, which makes the ionic liquid have better catalytic performance for chlorobenzene/sulfoxide chloride alkylation. A possible reaction mechanism was also proposed. The final conversion rate of excess chlorobenzene was 45.3%, and the selectivity of 4,4′-dichlorodiphenyl sulfoxide was 31.9%.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Natural Science Foundation of China (21908132) and the Zibo City Integration Development Project (2021). We would also thank the Research Institute of the Yellow River Delta Development Project (2020) and the Innovation Research Fund Project of Zhaoyuan Industrial Technology Research Institute (2021).Notes and references
- H. Münstedt and H. Zeiner, Kunststoffe, 1989, 79, 993–996 Search PubMed.
- A. Lücke, Kunststoffe, 1990, 80, 1154–1158 Search PubMed.
- C. Chiriac and J. Stille, Macromolecules, 1977, 10, 712–713 CrossRef CAS.
- W. Xu, S. Yang, P. Bhadury, J. He, M. He, L. Gao, D. Hu and B. Song, Pestic. Biochem. Physiol., 2011, 101, 6–15 CrossRef CAS.
- F. Wang, M. Hickner, Q. Ji, W. Harrison, J. Mecham, T. A. Zawodzinski and J. E. McGrath, Macromol. Symp., 2001, 175, 387–396 CrossRef CAS.
- B. R. Einsla, Y. T. Hong, Y. S. Kim, F. Wang, N. Gunduz and J. E. McGrath, J. Polym. Sci., Part A: Polym. Chem., 2004, 42, 862–874 CrossRef CAS.
- S. C. Sutradhar, F. Ahmed, T. Ryu, S. Yoon, S. Lee, M. M. Rahman, J. Kim, Y. Lee, W. Kim and Y. Jin, Int. J. Hydrogen Energy, 2019, 44, 11321–11331 CrossRef.
- K. Gotham and S. Turner, Polymer, 1974, 15, 665–670 CrossRef CAS.
- A. H. Wagner, J. S. Yu and D. M. Kalyon, Polym. Eng. Sci., 1989, 29, 1298–1307 CrossRef CAS.
- W. Zheng, H. Wang, W. Xie, L. Zhao and W. Sun, AIChE J., 2018, 64, 950–960 CrossRef CAS.
- H. Zhang, R. Liu, Z. Yang, F. Huo, R. Zhang, Z. Li, S. Zhang and Y. Wang, Fuel, 2018, 211, 233–240 CrossRef CAS.
- S. O. Omarov, E. Vlasov, D. Sladkovskiy, K. Semikin, A. Matveyeva, S. Fedorov, G. Oganesyan and D. Y. Murzin, Appl. Catal., B, 2018, 230, 246–259 CrossRef CAS.
- E. Vlasov, S. Myakin, M. Sychov, A. Aho, A. Y. Postnov, N. Mal'Tseva, A. Dolgashev, S. O. Omarov and D. Y. Murzin, Catal. Lett., 2015, 145, 1651–1659 CrossRef CAS.
- H. Wang, S. Ma, Z. Zhou, M. Li and H. Wang, Fuel, 2020, 269, 117419 CrossRef CAS.
- L. Zhang, T.-H. Tsui, J. Fu, Y. Dai and Y. W. Tong, Carbon Neutrality, 2022, 1, 8 CrossRef.
- N. Yan, Science, 2022, 378, 132–133 CrossRef CAS PubMed.
- G. k. Gözaydın, Q. Sun, M. Oh, S. Lee, M. Choi, Y. Liu and N. Yan, ACS Sustainable Chem. Eng., 2023, 11(6), 2511–2519 CrossRef.
- Y. Liu, R. Hu, C. Xu and H. Su, Appl. Catal., A, 2008, 346, 189–193 CrossRef CAS.
- P. Kumar, W. Vermeiren, J.-P. Dath and W. F. Hoelderich, Appl. Catal., A, 2006, 304, 131–141 CrossRef CAS.
- X. Sheng, H. Gao, Y. Zhou, B. Wang and X. Sha, Appl. Organomet. Chem., 2019, 33, e4979 CrossRef.
- Y. Liu, R. Li, H. Sun and R. Hu, J. Mol. Catal. A: Chem., 2015, 398, 133–139 CrossRef CAS.
- J. Zhang, C. Huang, B. Chen, P. Ren and M. Pu, J. Catal., 2007, 249, 261–268 CrossRef CAS.
- X. Xing, G. Zhao and J. Cui, Sci. China Chem., 2012, 55, 1542–1547 CrossRef CAS.
- Z. Song, X. Li, H. Chao, F. Mo, T. Zhou, H. Cheng, L. Chen and Z. Qi, Green Energy Environ., 2019, 4, 154–165 CrossRef.
- X. Sheng, B. Wang, C. Mao, X. Sha and Y. Zhou, Appl. Organomet. Chem., 2021, 35, e6055 CrossRef CAS.
- S. Liu, S. Tan, B. Bian, H. Yu, Q. Wu, Z. Liu, F. Yu, L. Li, S. Yu and X. Song, RSC Adv., 2018, 8, 19551–19559 RSC.
- Y. Chauvin, A. Hirschauer and H. Olivier, J. Mol. Catal., 1994, 92, 155–165 CrossRef CAS.
- Z. Liu, X. Meng, R. Zhang, C. Xu, H. Dong and Y. Hu, AIChE J., 2014, 60, 2244–2253 CrossRef CAS.
- Y. Cao and T. Mu, Ind. Eng. Chem. Res., 2014, 53, 8651–8664 CrossRef CAS.
- Y. Hao, J. Peng, S. Hu, J. Li and M. Zhai, Thermochim. Acta, 2010, 501, 78–83 CrossRef CAS.
- G. Wu, Y. Liu, G. Liu, R. Hu and G. Gao, J. Catal., 2021, 396, 54–64 CrossRef CAS.
- S. Liu, C. Chen, F. Yu, L. Li, Z. Liu, S. Yu, C. Xie and F. Liu, Fuel, 2015, 159, 803–809 CrossRef CAS.
- T. L. T. Bui, W. Korth, S. Aschauer and A. Jess, Green Chem., 2009, 11, 1961–1967 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra00935a |
| This journal is © The Royal Society of Chemistry 2023 |