Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Green solvents, materials, and lead-free semiconductors for sustainable fabrication of perovskite solar cells
Suresh K. Podapangi a,
Farshad Jafarzadeh
a,
Farshad Jafarzadeh a,
Sara Mattiello
a,
Sara Mattiello b,
Tulja Bhavani Korukondac,
Akash Singhd,
Luca Beverina
b,
Tulja Bhavani Korukondac,
Akash Singhd,
Luca Beverina b and
Thomas M. Brown
b and
Thomas M. Brown *a
*a
aCHOSE (Centre for Hybrid and Organic Solar Energy), Department of Electronic Engineering, University of Rome-Tor Vergata, via del Politecnico 1, 00133, Rome, Italy. E-mail: Thomas.brown@uniroma2.it
bDepartment of Materials Science, State University of Milano-Bicocca, Via Cozzi 55, I-20126 Milano, Italy
cDepartment of Centre for Energy Studies, Indian Institute of Technology Delhi, Hauz Khas, New Delhi-110016, India
dDepartment of Mechanical Engineering and Materials Science, Duke University, Durham, NC 27708, USA
First published on 15th June 2023
Abstract
Perovskite materials research has received unprecedented recognition due to its applications in photovoltaics, LEDs, and other large area low-cost electronics. The exceptional improvement in the photovoltaic conversion efficiency of Perovskite solar cells (PSCs) achieved over the last decade has prompted efforts to develop and optimize device fabrication technologies for the industrial and commercial space. However, unstable operation in outdoor environments and toxicity of the employed materials and solvents have hindered this proposition. While their optoelectronic properties are extensively studied, the environmental impacts of the materials and manufacturing methods require further attention. This review summarizes and discusses green and environment-friendly methods for fabricating PSCs, particularly non-toxic solvents, and lead-free alternatives. Greener solvent choices are surveyed for all the solar cell films, (i.e. electron and hole transport, semiconductor, and electrode layers) and their impact on thin film quality, morphology and device performance is explored. We also discuss lead content in perovskites, its environmental impact and sequestration routes, and progress in replacing lead with greener alternatives. This review provides an analysis of sustainable green routes in perovskite solar cell fabrication, discussing the impact of each layer in the device stack, via life cycle analysis.
1. Introduction
Metal Halide Perovskites (MHPs) have revolutionized the field of optoelectronics and garnered worldwide interest due to their outstanding semiconducting characteristics complemented by structure–property tunability and facile deposition techniques.1 Such attributes have made perovskite a ubiquitous material in the research domain with potential applications in fields of photovoltaics, light-emitting diodes, lasers, data storage and computing devices. PSCs have experienced an unparalleled swift rise in photovoltaic power conversion efficiency (PCE) from 3.8% to 25.7% in just over a decade2–4 surpassing other emerging photovoltaic technologies.4 High absorption coefficients over broad spectral ranges, defect tolerance to deep gap states, low exciton binding energy5 and excellent ambipolar charge transport6 are salient features of perovskites that make them suited for solar devices. In general, perovskites have structural framework of multiple dimensionality (3D, 2D, quasi-2D, 1D and 0D).7 However, the most studied systems are 3D and 2D with ABX3 and A′2BX4 compositions respectively where, A is a monovalent small cation, A′ is a bigger alkyl or aryl ammonium cation, B a divalent metal cation and X a halogen.8–10Although perovskite PV has great potential, research and development has major challenges to tackle before commercialization, mainly stability under operating conditions, and developing economic and scalable fabrication process that can be industrialized. In addition, it is necessary to make sure both the end product and the manufacturing process are environmentally friendly and with a low carbon footprint answering at the same time regulatory and environmental concerns. Nowadays, PSCs encounter two critical issues that are of the utmost importance. Primarily, the presence of lead in the photoactive semiconductor, and secondly the toxicity of solvents used in fabrication. Both need to be addressed. Attempts have been directed to replace the 3-D framework of lead with periodic table group elements 14 and 15, or transition metals.11,12 The incorporation of these alternative elements has come with a lowering in device stability and efficiency, as discussed in detail in Section 4. The toxicity of the solvents used in the processing of active and charge transport layers is the other and less frequently discussed concern. In large scale manufacturing, PSC possesses the advantage of deposition by solution processing. However, the majority of current methods use toxic solvents like N,N-dimethylformamide (DMF) and N-methylpyrrolidone (NMP) for perovskite deposition13 and chlorobenzene (CB), di-chlorobenzene (DCB), for hole transport materials such as spiro-OMeTAD and poly(triarylamine) (PTAA).14–16 To develop alternative green solvents three key factors to be considered are: (i) solute–solvent interactions i.e., solubility of the precursors, (ii) solution–substrate interactions i.e., wettability of the substrate and (iii) formation of high-quality conformal layers.17 Further, the deposition of individual layers in a complete device should not interfere with the quality of the already-deposited underlying layer for proper stacking of layers.
Recently, several articles have discussed recent advancements in the production of greener perovskite solar cells (PSCs).18–21 These advancements include the use and effects of solvents and antisolvents that can be classified as eco-friendly on perovskite synthesis, film quality and device performance, the potential environmental and health hazards from materials even for mass production, the sustainability and lead handling and recycling technologies. This review focuses on summarizing current progress in developing more environmentally-friendly perovskite solar cells from many angles. It not only covers recent advances in green fabrication, including the use of green solvents for the perovskite layer. It also provides a detailed description for each layer of the device including those for transport, and front, and back contacts including life cycle assessment considerations as well as lead free perovskite alternatives.
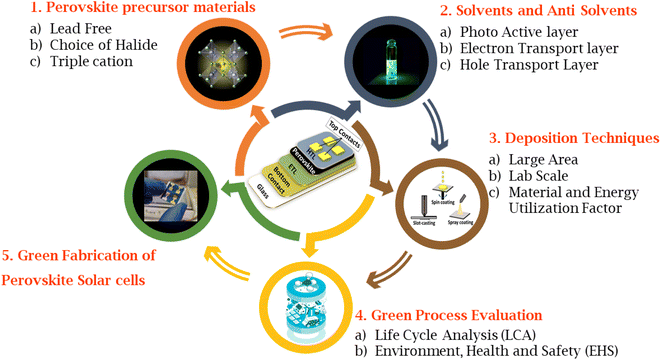
Fig. 1 summarizes different stages of green fabrication of PSCs system that include: (1) perovskite precursor materials, (2) solvents and anti-solvents, (3) deposition techniques, (4) green process evaluation, (5) complete green fabrication of PSCs. In this review, we focus on the environmentally friendly sustainable fabrication techniques and replacement of toxic elements of PSCs. Section 2 deals with the selection and description of green solvents. Sections 3 provides a comprehensive discussion of alternative solvents for replacing toxic solvents in the fabrication of PSCs. Section 4 supplies a focused literature survey on lead-free perovskites. We further explore alternative charge transport layers (Section 5), electrode materials (Section 6) and green solvents that can be used in their deposition process in dedicated subsections. Finally, an overview of life cycle analysis (LCA) of PSCs is discussed in Section 7 before arriving at the conclusions and outlook (section 8). This review aims to provide an overview of the materials and fabrication technologies which can be adapted to achieve fabrication of a greener perovskite solar cell, relevant to the current times when perovskite solar cell technology is evolving from lab to fab.
2. Categorization and key aspects in the quest for green solvent alternatives
2.1 General definitions and classification of green solvents according to Safety (S), Health (H), Environment (E)
Currently, many industries and research communities are addressing the effect of solvents on the health, safety, contamination, energy consumption, air quality and climate change.17 The European Chemical Agency, through REACH legislation (Registration, Evaluation, Authorization, and Restriction), targets chemical legislation to protect human health and the environment regulating substances of very high concern (SVHC) to progressively replace them with less toxic alternatives that are feasible, both technically and economically.22 Green solvents are environment-friendly solvents or bio solvents that make a product or process less taxing on the environment over its entire life cycle.23 General guidelines for the identification and selection of green solvents are based on the following factors: (i) risk factors which are analyzed using Environment, Health and Safety (EHS) methods and (ii) the energy required to produce and recover the solvent which is estimated using life cycle analysis (LCA) methods.24,25According to the twelve principles of green chemistry, when considering the sustainability of a process, a solvent should possess the following features to be considered green:24,26
• A solvent must have negligible or reduced toxicity to minimize risks when manipulated or accidently released in nature.
• A solvent must be inert in ambient conditions (to avoid reaction/decomposition risks) and non-flammable, making it easy to store and transport.
• Synthesis of solvents must have a high atom economy percentage (processes should be designed so that the maximum amount of raw materials ends up in the product and a minimum amount of waste is produced)24 and must follow an energy-saving process using substances obtained from renewable feedstocks.
• A solvent must be efficiently recyclable.
• It must be highly biodegradable (degradation through biological action)26 without producing toxic metabolites.
Furthermore, to be used in industries, a solvent should be available on a large scale, be affordable and meet the requirements for a specific application (e.g., vapour pressure, viscosity, polarity, solubilization, stabilization of the dissolved species, ability to favor a chemical process etc.). When all these requirements are taken into consideration, it becomes clear that no universal green solvent exists.26
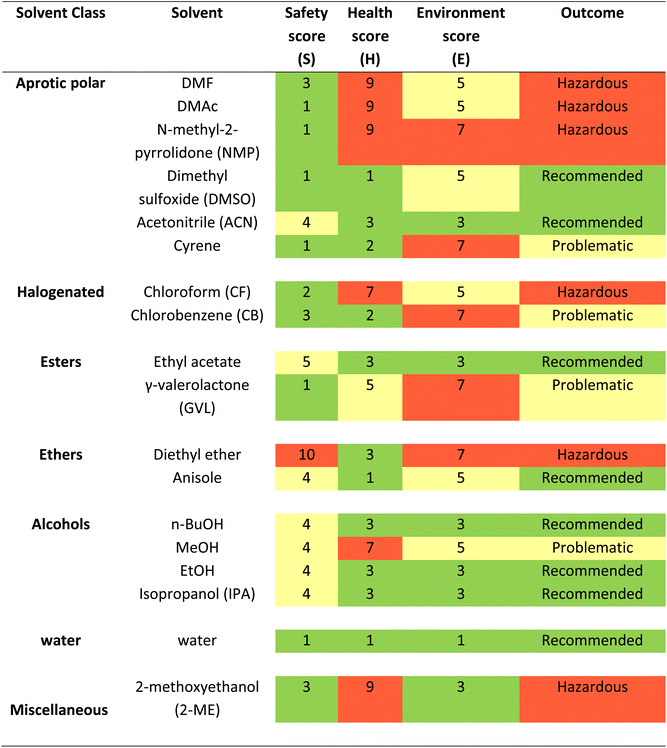
There have been various attempts to categorize solvents based on Safety (S), Health (H), Environment (E) scores which include Global Harmonized System (GHS) and European regulations. Several pharmaceutical companies, such as GlaxoSmithKline (GSK),27 Pfizer,28 Astra Zeneca29 and Sanofi,30 Charnwood Technical Consulting Ltd and the GCCE have ranked the sustainability of (organic) solvents and produced guides31 for selecting greener alternatives, in order to make evaluation and choice as simple as possible for the final user.32–35 Their efforts converged in 2016 with the publication of the CHEM21 (Chemical Manufacturing Methods for the 21st Century Pharmaceutical Industries) selection guide.36 In Table 1 and Fig. 2 we report list of solvents and their molecular structure respectively from the large CHEM21 database that are used in the fabrication of PSCs. The consensus is that simple alcohols, ketones, esters, and water should replace halogenated and polar aprotic solvents wherever possible. Conventional solvents produced from renewable sources, such as cellulose and starch, are also appropriate green candidates.23,35 This category comprises bio-based common solvents, such as bioethanol or bio-acetone, and recently assessed bio-renewable solvents, such as 2-methyltetrahydrofuran, γ-valerolactone (GVL) and dihydrolevoglucosenone (Cyrene).37–39
| a The final ranking is based on H, S, and E scores and other parameters like H phrases or occupational exposure limits, which are not listed here. |
|---|
 |
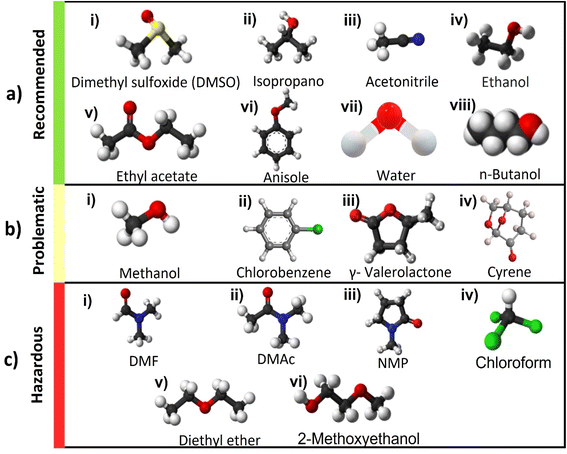 | ||
| Fig. 2 Chemical structure of various solvents used in the perovskite films classified based on outcome of Table 1 in the three categories recommended in green, problematic in yellow and hazardous in red33 (a) recommended solvents, (i) dimethyl sulfoxide (DMSO), (ii) IPA, (iii) acetonitrile (ACN), (iv) ethanol, (v) ethyl acetate, (vi) anisole, (vii) water, (viii) n-butanol, (b) problematic solvents, (i) methanol, (ii) CB, (iii) γ-valerolactone (GVL), (iv) Cyrene39, (c) hazardous solvents, (i) DMF, (ii) dimethylacetamide (DMAc), (iii) N-methyl-2-pyrrolidone (NMP), (iv) chloroform (CF), (v) diethyl ether (DE), (vi) 2-methoxyethanol (2-ME). | ||
Apart from the standard organic solvents, such as those reported in the Table 1, alternative classes of solvents called “neoteric” exist, which are considered green according to one or more favorable features including being non-volatile, and generally easily-recovered at the end of the process.35,40 Neoteric solvents comprise three classes of non-standard solvents: ionic liquids (ILs), deep eutectic mixtures, and supercritical fluids. Even if applications of such materials as a medium for the synthesis and processing of materials for energy have been documented,41 the very nature of such derivatives makes them highly impractical for the preparation of inks. ILs and deep eutectic solvents (as well as the closely associated polymeric solvents) are non-volatile.42–44 Supercritical fluids can only be used under relatively high-pressure conditions. The only exception regards the use of methylammonium carboxylates (such as formate, acetate, or propionate) as solvents for the preparation of the perovskite layer. In this case, however, these liquid salts do not only act as the solvent since the methylammonium cations are also incorporated in the final perovskite layer. Section 3.4 is specifically dedicated to this topic. It is however worth mentioning that ILs are gaining attention especially as additives in fabrication of PSCs, specifically to improve device stability.
2.2 Solubility considerations and solvent parameters useful for the selection of green solvents
For the preparation of perovskite precursor inks, as well as the transport layers, which need to dissolve in orthogonal solvents,45 the most critical parameter to bear in mind is the solubility in the solvent of choice. Many times, trial and error approaches are applied to find appropriate solvents (or solvent mixtures). The most common rule of thumb has always been “likes dissolves like”, meaning that solute and solvent should display similar polarity. Polarity scales exist based either on physical constants (such as the dielectric constant or the dipole moment), or on empirical constants (such as Hansen solubility parameters (HSP), the normalized polarity parameter ETN or other solvatochromic parameters); the latter are generally more predictive for solvency evaluation.46 Knowing and understanding solvent parameters related to polarity might be a very powerful approach to predict the capability of a liquid to actually behave as a good solvent for any specific solute. This might be particularly important in the case of green solvents, as new candidates for this role are studied and proposed with increasing frequency, to avoid trial and error evaluation campaigns.Among the many possible polarity rankings based on empirical constants, HSP have already been used to rationalize the solubility of both the materials used as Hole Transport Layer (HTL) and lead-containing precursors.17,47 Fig. 3a shows the determination of Hansen parameters δD, δH and δP (and the Hansen sphere) for three conjugated polymers (Alkoxy-PC8, Thiophenyl-PTEG and Alkoxy-PTEG) used as HTL in FTO/SnO2/perovskite/HTL/Au devices (the perovskite composition being Cs0.06FA0.78MA0.16Pb0.94I2.4Br0.48). Having such information allowed the authors to deposit their HTLs using nontoxic 2-methylanisole (2-MA) and 3-methylcyclohexanone (3-MC) instead of CB. While the application of HSP to predict the solubility of neutral organic molecules and polymers is generally appropriate, in the case of perovskite precursors they display two relevant limits: they do not account for ionic interactions and solvent complexation.48,49 Both phenomena occur during the nucleation of the MAPbX3/FAPbX3 phase and cannot be overlooked. Indeed, it is documented that the dissolution of Pb2+ ions is strongly influenced by the formation of a Lewis acid–base adduct with the solvent.50,51
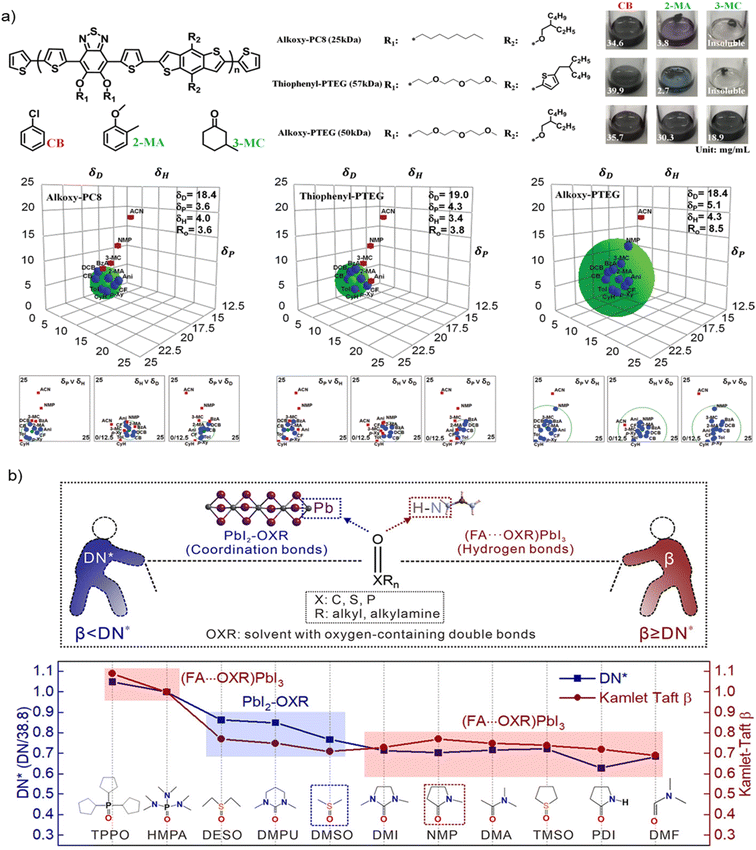 | ||
| Fig. 3 (a) Chemical structures of alkoxy-PC8, thiophenyl-PTEG, and alkoxy-PTEG; their solubility in CB, 2-methylanisole (2-MA) and 3-methylcyclohexanone (3-MC); and their HSP (and Hansen spheres), determined by dissolution in a set of different solvents, copyright 2020, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim63 (b) use of the normalized Gutmann's Donor Number (DN*) and Kamlet–Taft β parameter to rationalize the formation of PbI2–OXR or (FA⋯OXR)PbI3 structures in different solvents. When β < DN*, PbI2–OXR forms; when β ≥ DN*, (FA⋯OXR)PbI3 emerges from FAPbI3-based solutions, copyright 2022, American Chemical Society.58 | ||
Apart from the presence of a huge dipole moment (necessary to achieve the dissolution of MAX/FAX species), a critical feature that the solvent must possess is therefore the availability of a lone pair to be donated to the Pb2+ ion. In short, the most suitable solvent must be a good Lewis base. Lewis's basicity can be evaluated according to different models. Both the Gutmann's donor number DN and the Mayer bond order scales have been used in the context of hybrid perovskites, with some degree of predictability.52,53 Unfortunately, both scales have practical limitations. The DN scale is a measure of the enthalpy of formation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 SbCl5–base adduct in a dilute solution of an inert solvent (1,2-dichloroethane), therefore it is a solute scale and not a solvent scale. Moreover, it has been criticized for the choice of the reference Lewis acid and data reliability, reasons why other scales are now preferred, such as the Lewis affinity scale of BF3.54 Conversely, the Mayer bond order cannot be measured experimentally. Computational estimates are available, with limitations coming from a lack of experimental verification.
1 SbCl5–base adduct in a dilute solution of an inert solvent (1,2-dichloroethane), therefore it is a solute scale and not a solvent scale. Moreover, it has been criticized for the choice of the reference Lewis acid and data reliability, reasons why other scales are now preferred, such as the Lewis affinity scale of BF3.54 Conversely, the Mayer bond order cannot be measured experimentally. Computational estimates are available, with limitations coming from a lack of experimental verification.
The solvatochromic β,55,56 a well-established parameter introduced by Kamlet and Taft to describe linear solvation energy relationships, estimates the H-bond accepting capability of a solvent. This is the equivalent of measuring the capability of the same to act as a Lewis base. β is a known parameter for all industrial solvents and can be measured easily for new ones, including recently introduced green alternatives.57 So far, the possible predictiveness of the β scale for the solubility of PbX2 and MAX/FAX species has not been directly proven, but all the solvents already used to dissolve perovskite precursors are good Lewis bases, thus substantiating the choice of this scale as a suitable molecular descriptor. In this same direction, very recently Zheng et al. moved a big step forward in the rationalization of solvent effects on the perovskite formation from precursors solutions.58 They demonstrated that, for FAPbI3 perovskite, the intermediate structure formed in the chosen solvent (named OXR) depend on both the capability of the solvent to coordinate with PbI2 (measured by DN) and to assist the formation of hydrogen bonds in (FA⋯OXR)PbI3 structures (measured by β), as shown in Fig. 3b. The disclosed solvent chemistry behind the formation of perovskite intermediate structures allowed to understand the perovskite evolution from solution and finally to prepare defect-less films and highly stable final devices.
Table 2 lists the DN and β values for commonly used solvents used to solubilize lead halide precursors, alongside green alternatives: triethyl phosphate (TEP), N,N′-dimethyl propylene urea (1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone, DMPU), GVL, Cyrene and Polar Clean. TEP and DMPU are uncommon aprotic dipolar solvents of limited toxicity. GVL and Cyrene are classified as bio-renewable dipolar aprotic solvents,59,60 while 5-(dimethyl amino)-2-methyl-5-oxopentanoate (commercialized under the name of Rhodiasolv PolarClean)61 is a synthetic derivative obtained upcycling methylene glutarodinitrile (a by-product of nylon 66 manufacturing).62
| Solvent | Boiling point (°C) | DN (kcal mol−1) | β | OSHA hazards (as reported in safety data sheets) |
|---|---|---|---|---|
| a The asterisk * indicates that the value is unknown. | ||||
| DMF | 153 | 26.6 | 0.69 (ref. 56) | Flammable liquids, H226 |
| Acute toxicity, inhalation, H332 | ||||
| Acute toxicity, dermal, H312 | ||||
| Eye irritation, H319 reproductive toxicity, H360D | ||||
| DMAc | 165–166 | 27.8 | 0.76 (ref. 56) | Acute toxicity, inhalation, H332 |
| Acute toxicity, dermal, H312 | ||||
| Eye irritation, H319 | ||||
| Reproductive toxicity, H360D | ||||
| NMP | 202 | 27.3 | 0.77 (ref. 56) | Skin irritation, H315 |
| Eye irritation, H319 | ||||
| Reproductive toxicity, H360FD | ||||
| Specific target organ toxicity – single exposure, respiratory system, H335 | ||||
| DMSO | 189 | 29.8 (ref. 64) | 0.76 (ref. 56) | — |
| TEP | 215 | * | 0.77 (ref. 56) | Acute toxicity, oral, H302 |
| Eye irritation, H319 | ||||
| DMPU | 247 | 33.0 (ref. 58) | 0.90 (ref. 65) | Acute toxicity, oral, H302 |
| Serious eye damage, H318 | ||||
| Reproductive toxicity, H361f | ||||
| Rhodiasolv PolarClean | 281–282 | * | 0.62 (ref. 65) | Eye irritation, H319 |
| Cyrene | 227 | * | 0.61 (ref. 39) | Eye irritation, H319 |
| GVL | 207–208 | * | 0.70 (ref. 66) | — |
Understanding solvent–solute interactions and solvent chemistry makes possible to choose the appropriate solvent both to obtain solution of the starting materials and to find orthogonal solvents to avoid dissolution of the previously deposited layers. As such interactions are described by solvent parameters, knowing and understanding these parameters might be especially beneficial to the development of solution processed material systems and their devices. Moreover, it should be considered that the development of green solvents is a matter of intense research. The solvent parameters can therefore not only be useful to understand solvent–solute interactions and improve the quality of deposited films but might prove practical to predict the potential role of a newly developed solvents, thus facilitating the progresses in green development of PSCs.
3. Green solvents for the perovskite layer
Replacing toxic solvents with green solvents is one of the critical factors for commercialization of PSCs. This section discusses efforts towards greener approaches for depositing the main perovskite film. First, we briefly cover the standard solvents for lead halide perovskite precursors, i.e., DMSO and DMF, that enable the formation of high-quality films.For the preparation of perovskite precursors solutions, the proper choice of solvents becomes critical. The solvent in fact is not only a medium of the reaction, but it plays a fundamental role in the crystallization of the material, thus affecting its final morphology, defect densities, and performance when integrated in a device. So far, aprotic dipolar solvents have given the best results: DMF,67 NMP,68–70 GBL, DMSO,71,72 dimethylacetamide (DMAc), etc. They have long been popular choice due to their capability to dissolve the lead-containing precursor, relatively low volatility enabling morphological evolution on the substrate and miscibility with most other organic solvents (widening the choice for the anti-solvent).
According to research by Hamill et al.53 the DN shows a better correlation with the precursor solute's capacity to dissolve. The lead halides can be efficiently dissolved by the solvents with a DN more than 18 kcal mol−1. This offers a standard by which to select appropriate solvents for making the precursor solution. Aprotic polar solvents can only successfully dissolve cations because they are unable to form hydrogen bonds73 The strength of the aprotic solvent's bonding to Pb2+ and the stability of the complex are both influenced by its polarity. This will significantly affect the quality and morphology of films. Despite their outstanding capacity to dissolve the perovskite precursors, all of them pose specific sustainability issues. DMF, NMP and DMAc are known reprotoxic substances (i.e., they damage human fertility) and are carcinogenic. Upon consumption, GBL is metabolized as γ-hydroxybutyric acid (GHB), a psychoactive drug: as a result, GBL has a limited legal status in many countries and strict regulations apply for its use.74 Furthermore, DMF, DMSO and GBL are considered hazardous solvents according to the 2012 OSHA Hazard Communication Standard (29 CFR 1910.1200).75 DMSO is not intrinsically toxic, but it does favour the permeation through the skin of all dissolved substances.76
3.1 Partial replacement of toxic solvent with non-toxic solvent
In 2016 Gardner et al. proposed a non-hazardous compound mixture based on HSP model. The HSP can be used to identify solvents that may be compatible with perovskite precursors (CH3NH3I, PbAc2, and PbCl2) while meeting non-hazard requirements.77 For cyclic carbonates, like GBL, greater solvent polarity enables higher salt miscibility, which can lead to denser film. Nevertheless, these solvents have high viscosity and boiling points, causing prolonged evaporation during the annealing process. By combining high boiling point cyclic carbonates with low flash point protic solvents like EtOH and PrOH, solubility can be improved, drying temperatures can be reduced. The work of Garder et al. showed that it is possible to design a safe and effective solvent system by using co-solvents engineering to overcome the drawbacks of individual solvents. This study examined the all the co-solvents systems with PbAc2/PbCl2/MAI precursors. Inks are considered soluble if their precursors dissolve at a concentration of at least 1 M, and insoluble if they precipitate at lower concentrations. They concluded that inks made with GBL/alcohol/acid are soluble when they contain at least 50 vol% GBL (Fig. 4a (ref. 78)) but show colloidal separation if the GBL content is below this threshold. The ink system with GBL, alcohol, and acid mixtures for perovskite inks are considerably less toxic than conventional alternatives. The best results, with a PCE of 15.1%, were obtained using a 60/20/20 vol% mixture of GBL, ethanol, and acetic acid.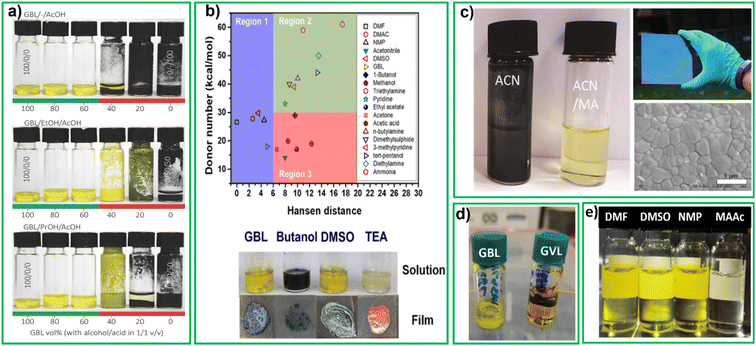 | ||
Fig. 4 (a) Photograph of inks based on decreasing vol% of γ-butyrolactone (GBL) with equal parts alcohol/acid,78 reproduced with permission. Copyright 2016, Wiley-VCH, (b) plot of DN versus Hansen distance of different solvents related to DMF, below pictures of the perovskite precursor solutions and films prepared using different solvents reproduced with the permission79, Copyright 2020, American Chemical Society (c) vials of perovskite precursors CH3NH3 I![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PbI2 (1 PbI2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.06 molar ratio) in neat ACN, and in the ACN/methylammonium (MA) gas mixture, perovskite coated film (above), SEM morphology of film (below), reproduced with the permission.81 copyright 2016, Royal Society of Chemistry, (d) GBL (left), γ-valerolactone (GVL) (right) precursor perovskite solutions, reproduced with the permission.92 Copyright 2021, Wiley online library, (e) perovskite solutions in DMF, DMSO, NMP and molten salt, methylammonium acetate (MAAc).118 1.06 molar ratio) in neat ACN, and in the ACN/methylammonium (MA) gas mixture, perovskite coated film (above), SEM morphology of film (below), reproduced with the permission.81 copyright 2016, Royal Society of Chemistry, (d) GBL (left), γ-valerolactone (GVL) (right) precursor perovskite solutions, reproduced with the permission.92 Copyright 2021, Wiley online library, (e) perovskite solutions in DMF, DMSO, NMP and molten salt, methylammonium acetate (MAAc).118 | ||
Huang et al. carried out a systematic study of HSP and DN of solvents used in cell fabrication.79 Based on solubility of perovskite precursors, solvents can be divided into the three regions shown in Fig. 4b. Solvents located in region 1 exhibit a low Hansen distance Ra (compared to DMF) and they can dissolve perovskite materials well, regardless of their DN values. With increasing Hansen distance, the effect of the DN values on the solubility of perovskite materials became obvious gradually. The solvents located in region 2 showed acceptable solubility for perovskite precursors with a DN value larger than 30. Finally, solvents in region 3 (with high Ra (>6) and low DN (<30)), showed poor solubility for perovskite precursors. The results indicate that CH3NH3I and PbI2 have good solubility in GBL, DMSO and TEA, as the solutes formed a clear solution in these solvents (see lower Fig. 4b). The high Ra (9.60) of 1-butanol indicates that it is a poor solvent for PbI2 resulting in a dark opaque solution. The authors determined that the solvent mixture that gave the highest efficiency was DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) GBL in 9
GBL in 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by volume. This mixture contains 90% of DMSO that is considered non-toxic according to the CHEM21 of 2016 solvent selection guide. Cells fabricated by blade coating in air with this mixture together with a green additive called polyethylene glycol (PEG) which passivated defects in perovskite film delivered a PCE of 17.0%.79
1 by volume. This mixture contains 90% of DMSO that is considered non-toxic according to the CHEM21 of 2016 solvent selection guide. Cells fabricated by blade coating in air with this mixture together with a green additive called polyethylene glycol (PEG) which passivated defects in perovskite film delivered a PCE of 17.0%.79
Replacement of solvents for perovskite precursor with, at least in part, non-toxic alternatives, leads to more difficulties in reaching high quality crystallization. Researchers have thus introduced gases, together with nontoxic solvents to improve film quality, even without further antisolvent treatment. For the first time Cui et al. reported methylamine induced defect-healing (MIDH) in CH3NH3PbI3 perovskite thin films. This process involves the ultrafast and reversible reaction of the films with CH3NH2 gas at room temperature, leading to the formation of defect-free films. This study provided a new approach in the development of hybrid perovskite films.80 Noel et al. study demonstrated the use of low boiling, low viscosity ACN/methylamine gas (MA) composite solvent system for dissolving CH3NH3PbI3. Upon spin-coating this precursor solution, the researchers obtained extremely smooth and pinhole-free perovskite films that crystallize at room temperature. The solution contained a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.06 ratio of MAI and PbI2 at a concentration of 0.5 M. After adding ACN, a black dispersion was observed (left vial, Fig. 4C). Subsequently, MA gas was bubbled through such dispersion until all the perovskite particles dissolved and formed a clear, light-yellow solution (right vial, Fig. 4C). The solution was spin coated onto a substrate resulting in uniform dense, smooth mirror-like film (top right, Fig. 4C). The SEM image (bottom right, Fig. 4C) shows individual domains that range from 500 nm to 700 nm in size. This study represented the first attempt to use a low-boiling point solvent for fabrication of large-area perovskite films with the one-step method.81 Other studies have shown enhanced carrier lifetime, crystallinity, and grain size through use of MA and ethylamine (EA) gas assisted recrystallization of methylammonium lead iodide films.16,82,83
1.06 ratio of MAI and PbI2 at a concentration of 0.5 M. After adding ACN, a black dispersion was observed (left vial, Fig. 4C). Subsequently, MA gas was bubbled through such dispersion until all the perovskite particles dissolved and formed a clear, light-yellow solution (right vial, Fig. 4C). The solution was spin coated onto a substrate resulting in uniform dense, smooth mirror-like film (top right, Fig. 4C). The SEM image (bottom right, Fig. 4C) shows individual domains that range from 500 nm to 700 nm in size. This study represented the first attempt to use a low-boiling point solvent for fabrication of large-area perovskite films with the one-step method.81 Other studies have shown enhanced carrier lifetime, crystallinity, and grain size through use of MA and ethylamine (EA) gas assisted recrystallization of methylammonium lead iodide films.16,82,83
Here we mentioned some more studies involved mixed non-volatile, coordinating solvents (NVCS) like DMF, DMSO and volatile, non-coordinating solvents (VNCS) like ACN, 2-ME, GBL etc. Normally coordinating solvents have strong bonding between perovskite precursor materials, particularly Pb2+. This strong bonding between the solvents and Pb2+ through the use of NVCS leads to the formation of a solid-state intermediate phase.84 Deng et al. studied the trade-off between rapid crystallization and large grain growth through solvent selection. Inks developed with combinations of VNCS (2-methoxy ethanol (2-ME) and ACN) and NVCS solvents like DMSO were used to fabricate modules via blade coating delivering a PCE of 16%.84 Further, Li et al. suggested that a 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 volume/volume composition of DMSO and ACN works best.85 Numata et al. fabricated formamidinium lead-bromide (FAPbBr3) perovskite absorber film made using a sequential deposition technique using a DMSO and tetramethylenesulfoxide (TMSO) mixed solvent, PSCs with an open-circuit voltage (VOC) of over 1.5 V were developed.91 Guerrero et al. optimized GBL and DMSO mixtures to enable blade-coating deposition.86 Galagan and co-workers demonstrated that a combination of DMSO, 2-methyl pyrazine and 1-pentanol was safer for industrial scale-up than devices showing comparable performances to ones processed in DMF.87 In 2020, Zhang et al. presented a double-cation perovskite solution that was deposited using pure dimethyl sulfoxide (DMSO) and fabricated 21.8% certified efficiency device.88 In 2022, X. Cao et al. introduced a green solvent called triethyl phosphate (TEP) to prepare perovskite solutions, and the non-toxic solvent dibutyl ether (DBE) as the anti-solvent and developed ((FAPbI3)1−x(MAPbBr3)x) based perovskite cells with a PCE of 20.1%.89
3 volume/volume composition of DMSO and ACN works best.85 Numata et al. fabricated formamidinium lead-bromide (FAPbBr3) perovskite absorber film made using a sequential deposition technique using a DMSO and tetramethylenesulfoxide (TMSO) mixed solvent, PSCs with an open-circuit voltage (VOC) of over 1.5 V were developed.91 Guerrero et al. optimized GBL and DMSO mixtures to enable blade-coating deposition.86 Galagan and co-workers demonstrated that a combination of DMSO, 2-methyl pyrazine and 1-pentanol was safer for industrial scale-up than devices showing comparable performances to ones processed in DMF.87 In 2020, Zhang et al. presented a double-cation perovskite solution that was deposited using pure dimethyl sulfoxide (DMSO) and fabricated 21.8% certified efficiency device.88 In 2022, X. Cao et al. introduced a green solvent called triethyl phosphate (TEP) to prepare perovskite solutions, and the non-toxic solvent dibutyl ether (DBE) as the anti-solvent and developed ((FAPbI3)1−x(MAPbBr3)x) based perovskite cells with a PCE of 20.1%.89
HSP and DN can help find suitable solvent or co-solvent systems as (partial) replacements for toxic solvents. By combining the right amount of high boiling point cyclic carbonates such as GBL with low flash point protic solvents like ethanol or propanol, the solubility is enhanced and the time for solvent evaporation can be reduced.17 Mixing low boiling point, low viscosity solvents with highly evaporative gases like MA gas or EA gas can lead to quick and room temperature crystallization of perovskite films. Alkylamines (EA or FA) can dissolve perovskite salts when in used in tandem with aprotic solvents like ACN.81 Using volatile solvents such as ethanol, tetrahydrofuran, or ACN often results in uncontrolled nucleation and formation, limiting the grain growth of perovskite. Therefore, a careful optimization of the solvent–antisolvent system is necessary for good perovskite film formation. We have discussed suitable anti solvent for high quality of perovskite film formation in Section 3.5. Mixture of non-volatile coordinating solvents (DMSO) and volatile non-coordinating solvents (ACN, GBL, 2-ME) helps rapid crystallization of perovskite films, which is useful for scaling up perovskite technology. Solvents systems like DMSO/ACN, DMSO/2-methyl pyrazine/1-pentanol and DMSO/TMSO and triethyl phosphate (TEP) are viewed as safer options compared to conventional solvents used for ink formulation. In 2020, Zhang et al. presented a double-cation perovskite solution that was deposited using pure dimethyl sulfoxide (DMSO) and fabricated a solar cell with a certified efficiency of 21.8%.88 But this study still used toxic antisolvents like CB or DEE which need to be replaced. Following Section 3.2 will describe ways in which research has processed perovskite films with only one solvent (and without antisolvent). Table 3 provides a concise overview of absorber materials, solvent choices, deposition techniques, device architectures, and key photovoltaic parameters for perovskite solar cells (PSCs) using environmentally friendly or low-hazard solvents in their fabrication.
| Absorber | Deposition method | Solvent | Device architecture | VOC [V] | JSC [mA cm−2] | FF [%] | PCE [%] | Ref. |
|---|---|---|---|---|---|---|---|---|
| Solvent mixtures | ||||||||
| MAPbICl2 | Spin-coating | GBL/ethanol/acetic acid (60/20/20 vol%) | Glass/ITO/TiO2/perovskite/spiro-OMeTAD/Au | 0.88 | 21.2 | 71 | 15.1 | 17 |
| MAPbI3 | Doctor blade coating | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (GBL 2 (GBL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO) DMSO) |
Glass/FTO/TiO2/perovskite/spiro-OMeTAD/Au | 1.01 | 20.6 | 75 | 15.6 | 86 |
| CH3NH3PbI3−xClx | Spin-coating | DMSO/2-MP/1-P | Glass/ITO/C–TiO2/perovskite/spiro-MeOTAD/Au | 1.04 | 22 | 72 | 16.5 | 87 |
| MAPbI3 | Spin-coating | ACN + MA gas | FTO/TiO2/C60/perovskite/spiro-OMeTAD/Au | 1.1 | 22.1 | 77 | 19 | 81 |
| MAPbI3 | Blade coating | ACN (60%, v/v)/2-ME (40%, v/v) | Glass/ITO/PTAA/perovskite/C60/BCP | 1.13 | 23 | 81.8 | 21.3 | 84 |
| CH3NH3PbI3−xClx | Spin-coating | DMSO + ACN (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
Glass/FTO/C–TiO2/m-TiO2/perovskite/spiro-MeOTAD/Au | 1.03 | 20.39 | 73 | 15.32 | 85 |
| MAPbI3 | Spin-coating | DMF + ACN | Glass/ITO/C–TiO2/perovskite/spiro-MeOTAD/Au | 1.11 | 22.49 | 75.09 | 18.8 | 90 |
| MAPbI3 | Blade coating | DMSO + GBL (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) 1 v/v) |
Glass/FTO/NiOx/perovskite/PCBM/C60/BCP/Ag | 1.09 | 21.29 | 73.49 | 17.02 | 79 |
| FAPbBr3 | Two step | DMSO + TMSO | FTO/TiO2/Li–m-TiO2/FAPbBr3/PMMA/spiro/Au | 1.53 | 6.96 | 74.0 | 7.88 | 91 |
| FA1−xMAxPbI3 | Spin-coating | DMSO | FTO/SnO2/m-TiO2/perovskite/spiro-OMeTAD/AU | 1.09 | 22.39 | 79.5 | 21.76 | 88 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| Single solvents | ||||||||
| (FAPbI3)1−x(MAPbBr3)x | Spin-coating | Triethyl phosphate (TEP) | FTO/SnO2/perovskite/spiro-OMeTAD/Au | 1.09 | 20.13 | 74.8 | 20.13 | 89 |
| MAPbI3 | Blade coating | GVL | Glass/FTO/C–TiO2/m-TiO2/perovskite/carbon | 0.89 | 23.42 | 62 | 12.91 | 92 |
| FA0.83Cs0.17Pb(I0.87Br0.13)3 | Blade coating | DMSO | Glass/ITO/PTAA/SiO2 NPs/perovskite/PCBM/BCP/Ag | 1.10 | 19.6 | 76.9 | 16.7 | 93 |
| CsPbIBr2 | Spin coating | DMSO | FTO/c-TiO2/perovskite/spiro-OMeTAD/Ag | 1.22 | 13.33 | 71.0 | 11.49 | 94 |
| FA0.85MA0.15PbI2.55Br0.45 | Blade coating | DMSO | FTO/C–TiO2/perovskite/PTAA/Ag | 1.10 | 23.20 | 78.58 | 20.05 | 95 |
| FA0.85MA0.15PbI2.55Br0.45 | Blade coating | DMSO | ITO/PTAA/perovskite/PCBM/BCP/In | 1.10 | 22.34 | 78.26 | 19.23 | 95 |
| FA0.75Cs0.25PbI2.7Br0.3 | Blade coating | DMSO | FTO/SnO2/perovskite/spiro/Au | 1.14 | 23.4 | 75.6 | 20.2 | 96 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| H2O as co-solvent | ||||||||
| MAPbI3 | Spin-coating/IPA bath | Pb(NO3)2·H2O & MAI·IPA | Glass/FTO/C–TiO2/m-TiO2/perovskite/spiro-MeOTAD/Au | 0.94 | 21.81 | 61 | 12.58 | 97 |
| MAPbI3 | Spin-coating/IPA bath | Pb(NO3)2·H2O & MAI·IPA | Glass/FTO/C–TiO2/m-TiO2/perovskite/spiro-MeOTAD/Au | 1.07 | 19.07 | 74 | 15.11 | 98 |
| MAPbI3 | Spin-coating/IPA bath | Pb(NO3)2·H2O & MAI·IPA | Glass/FTO/C–TiO2/m-TiO2/perovskite/spiro-MeOTAD/Au | 0.9 | 21.2 | 72 | 13.7 | 99 |
| Cs0.1FA0.9Pb(I0.83Br0.17)3 | Spin-coating/IPA bath | H2O & IPA | Glass/FTO/TiO2/perovskite/spiro-MeOTAD/Au | 0.96 | 18.8 | 66 | 11.7 | 100 |
| FAxMA1−xI0.9Br0.1 | Spin-coating/IPA bath | H2O &IPA | PEN/SnO2/TiO2/perovskite/spiro-MeOTAD/Au | 1.03 | 21.77 | 73.6 | 16.5 | 101 |
| MAPbI3 | Spin-coating/IPA bath | H2O & IPA | Glass/FTO/C–TiO2/m-TiO2/perovskite/spiro-MeOTAD/Au | 1.1 | 22.5 | 74 | 18.3 | 102 |
| FAxMA1−xI0.9Br0.1 | Chemical bath & spin coating | H2O & IPA | Glass/FTO/C–TiO2/m-TiO2/perovskite/spiro-MeOTAD/Au | 0.918 | 21.61 | 56.57 | 11.23 | 103 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| Ionic liquids (ILs) | ||||||||
| α-FAPbI3 | Spin coating | MAF | ITO/SnO2/perovskite/spiro-OMeTAD/MoO3/Au | 1.17 | 25.34 | 81.36 | 24.1 | 104 |
| (MA0.15FA0.85)Pb(I0.85Br0.15)3 | Spin coating | MAP/DMSO/ACN | Glass/FTO/C–TiO2/m-TiO2/perovskite/spiro-MeOTAD/Au | 1.07 | 23.08 | 62 | 15.46 | 105 |
| MAPbI3 | Spin coating | MAP | ITO/SnO2/perovskite/spiro-OMeTAD/MoO3/Ag | 1.12 | 23.39 | 79.52 | 20.56 | 106 |
| MAPbI3 | Spin coating | MAAc | ITO/SnO2/perovskite/spiro-OMeTAD/MoO3/Ag | 1.07 | 23.12 | 76.65 | 18.99 | 106 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| Anti-solvents | ||||||||
| MAPbI3 | Spin coating | Tetraethyl orthocarbonate (TEOC) | Glass/ITO/SnO2/perovskite/spiro-OMeTAD/PCBM/BCP/Ag | 1.06 | 21.9 | 78 | 18.15 | 107 |
| [CsPbI3]0.05[(FAPbI3)0.85(MAPbBr3)0.15]0.9 | Spin-coating | Anisole | Glass/FTO/TiO2/perovskite/spiro-OMeTAD/Ag | 1.15 | 21.98 | 78 | 19.76 | 108 |
| (FAPbI3)1−x(MAPbBr3)x | Spin coating | Anisole | PET/ITO/SnO2/perovskite/spiro-OMeTAD/Au | 1.11 | 22.48 | 68.49 | 17.09 | 109 |
| Cs0.05(MA0.17FA0.83)0.95Pb(I0.83Br0.17)3 | Spin-coating | Anisole | Glass/FTO/TiO2/perovskite/spiro-OMeTAD/Au | 1.14 | 23.5 | 76 | 20.14 | 110 |
| (FA0.85MA0.15)Pb(I0.85Br0.15)3 | Spin coating | Ethyl acetate | Glass/FTO/SnO2/perovskite/spiro-OMeTAD/Au | 1.22 | 23.13 | 73.7 | 20.77 | 111 |
| (FA0.85MA0.15)Pb(I0.85Br0.15)3 | Spin coating | Methyl benzoate (MB) | Glass/FTO/SnO2/perovskite/spiro-OMeTAD/Au | 1.21 | 24.46 | 74.95 | 22.37 | 111 |
| (FA0.85MA0.15)Pb(I0.85Br0.15)3 | Spin coating | Ethyl acetate | Glass/FTO/C–TiO2/m-TiO2/perovskite/spiro/Au | 1.12 | 22.89 | 75.6 | 19.43 | 112 |
3.2 Non-toxic solvents which can be used pure
Till now we have discussed co-solvents used to dissolve perovskite precursors the following paragraphs are going to describe various single solvents which can dissolve perovskite precursors by itself without any co-dependency. In the life cycle assessment study shown by Vidal et al.113 DMSO had the least human health and environmental impact compared to DMF, DMAC, NMP, DMEU, GBL, THF, and DMPU. DMSO is able to solubilize perovskite precursors due to its high DN (29.8 kcal mol−1). From CHEM21 Table 1 and Vidal LCA studies, DMSO can be considered a green solvent for perovskite device fabrication even though we noted above that it assists permeation through the skin of dissolved substances. Küffner et al.93 reported blade-coated devices in a single step process for inverted PSCs (p–i–n). The deposition process utilized only environmentally friendly green DMSO and carried out at low temperatures, which is making it a significant advancement towards commercialization of solution processed of PSCs. To produce the perovskite dry film from a wet film a state of supersaturation must be induced in the film. This is achieved through the rapid drying or quenching of the film. To do this there four widely used quenching methods are antisolvent, vacuum, heat, and gas-assisted quenching.114 Recently many of researchers reported highly efficient perovskite films by blade-coating followed by gas quenching.84,93,115 Jafarzadeh et al. used a two-step blade coating technique to deposit perovskite using DMSO as the solvent for the first step.116 They demonstrated that gas quenching in combination with additive engineering enables the deposition of perovskite on flexible substrates under ambient conditions without the usage of high deposition temperature. The all-blade coated flexible perovskite solar cells delivered 14% PCE.Xing et al. reported (FA)–cesium lead halide perovskite by heat-assisted blade coating with DMSO as a solvent. The use of DMSO has been shown to enhance the formation of α-phase crystals, resulting in improved crystallinity. Additionally, increasing the substrate temperature during the coating process leads to a more compact film, with a more pronounced preferred facet orientation and a desired phase transition.96 He et al. reported a new method in blade coating of perovskite films called meniscus-assisted solution printing (MASP) and showed dense perovskite films with large grains with pure DMSO as solvent. In this study they achieved a PCE of 19.23% in inverted planar and PCE of 20.05% in standard planar perovskite devices.95
In 2021, Worsley et al. introduced a new biodegradable green solvent, GVL, often used in foods and perfumes, which was as an alternative to GBL. PSCs fabricated with GVL delivered a PCE of 12.9% on a 1 cm2 active area (GBL and GVL perovskite solutions shown in Fig. 4d).92 According to CHEM21 solvent selection guide GVL is considered as problematic solvent. Many bio-based solvents are considered problematic because they have a high boiling point, making it challenging to separate the product and recycle the solvent. Some new solvents, such as γ-valerolactone, are only produced in limited quantities or only as intermediates, and have not yet been evaluated by REACH. As a result, these solvents are given a default score of at least 5 in health and environmental criteria.33 Recently, Kerkel et al. extensively studied HSP and COSMO-RS calculated on GVL. Toxicity tests on aquatic plants, bacteria, invertebrates, and a vertebrate cell line showed that GVL has low acute toxicity to aquatic organisms. Furthermore, GVL was found to be readily biodegradable, further reinforcing its potential as a environmentally friendly solvent.117 Some common perovskite solutions with DMF, DMSO, NMP and molten salt (MAAc) shown in Fig. 4e.118
3.3 Water (H2O) in manufacturing of PSCs
 | ||
| Fig. 5 SEM images of CH3NH3PbI3−xClx films based on (a) DMF, (b) DMF + 1% H2O, (c) DMF + 2% H2O, (d) DMF + 3% H2O, (e) DMF + 5% H2O, (f) DMF + 7% H2O, and (g) DMF + 10% H2O solvent.119 copyright 2015, Wiley online library (h) the photographs of PbI2/DMF solutions containing various amounts of H2O additive (the H2O content is volume ratio vs. DMF)67, copyright 2015, The Royal Society of Chemistry, (i) & (j) photograph of perovskite (CH3NH3PbI3−xClx) precursor solutions of fresh and overnight stored perovskite solutions with various H2O concentrations, reproduced and copyright 2018,120 published by Wiley-VCH. | ||
In 2015, Hsieh et al. introduced a low toxicity lead nitrate precursor [Pb (NO3)2], which can dissolve in water. By a two-step process for the deposition of the perovskite layer (shown in Fig. 6a) they achieved a PCE of 12.6%.97 The conversion mechanism of Pb (NO3)2 to MAPbI3 was considerably slower (700 s) than those with conventional precursors (20 s forPbI2 and MAI) needing an intermediate ion–exchange reaction.97
| [Pb (NO3)2] + 2CH3NH3I → PbI2 +2CH3NH3 (NO3) |
| PbI2 + CH3NH3I → CH3NH3PbI3 |
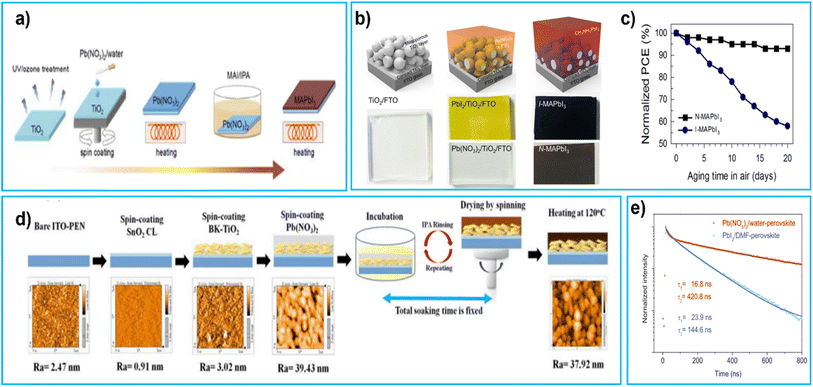 | ||
| Fig. 6 (a) Procedure for preparing MAPbI3 layer in the Pb(NO3)2/water system, reproduced with permission, Copyright 2015, The Royal Society of Chemistry,97 (b) schematic representation conversion of MAPbI3 formation from aqueous lead nitrate, structural schemes and optical views of mesoporous-TiO2, Pb (NO3)2 and PbI2, and MAPbI3 layers sequentially stacked on an FTO coated glass substrate, reproduced with permission, copyright 2017, American Chemical Society99 (c) normalized PCE values of the corresponding solar cells before and after storage in a dark chamber with 20% RH for various lengths of time, reproduced with permission, Copyright 2017, American Chemical Society99 (d) process flowchart of fabricating PEN-PSC in this study and the AFM topographic image of each step in the process, reproduced with permission, Copyright 2019, Elsevier101 (e) TRPL decay of the perovskite film fabricated using the Pb(NO3)2/water (red) and PbI2/DMF (blue) systems with biexponential decay fitting, reproduced with permission, Copyright 2020, Wiley.102 | ||
The slow incubation process led to a dissolution–recrystallisation effect (also known as Ostwald ripening)121 resulting in rough and non-uniform perovskite films98 which increases charge carrier recombination time. To solve this problem and shorten the synthesis time (500 s), in 2018 the same group introduced chloride ions in MAI/IPA solution. These chloride ions greatly improved efficiency to 15.1% as well as stability over 55 days.98 In 2017, V. Shinde et al. reported perovskite films formed by aqueous lead nitrate ([Pb (NO3)2]/H2O) and perovskite conversion schematic diagram shown in Fig. 6b. The structural layout as well as visual appearance of mp-TiO2, layers of Pb(NO3)2 and PbI2, and a stack of MAPbI3 layers on FTO shown in Fig. 6b. The PbI2 and Pb (NO3)2 layers, when coated on TiO2 surfaces through spin-coating, appear yellow and colourless respectively. The perovskite films are formed by combining different lead sources with MAI dissolved in IPA. The process of converting Pb(NO3)2 to N-MAPbI3 (with Pb(NO3)2)is slower than the typical direct method of converting PbI2 to I-MAPbI3 (with PbI2)because Pb(NO3)2 undergoes an intermediate ion–exchange reaction before becoming N-MAPbI3 and they observed that the conversion of Pb(NO3)2 to N-MAPbI3 takes 600 s (10 min) while PbI2-based I-MAPbI3 system takes only 60 s. N-MAPbI3 based devices maintained 95% of their initial PCE over 20 days.99 As shown in Fig. 6c solar cells before and after storage in a dark chamber with 20% RH for various lengths of time and solar cells operated under 1 Sun illumination. In 2017, K. Sveinbjörnsson et al. introduced mixed cation and halide Cs0.1FA0.9Pb(I0.83Br0.17)3 perovskites using aqueous CsNO3 and Pb(NO3)2 achieving a PCE of 13.0%.100 In 2019, P. Zhai et al. used a two-step process in which, after drying Pb(NO3)2, the film was immersed in IPA solutions containing either MAI or FAI/MAI/MABr (fabrication steps shown in Fig. 6d). With this method FAxMA1−xI0.9Br0.1 PSCs with PCE = 16.50% were obtained.101 In 2020, T. Hsieh et al. demonstrated Pb(NO3)2/water-based PSCs with a PCEs of 18.3% and a large VOC of 1.1 V, with longer lifetime compared to the cell fabricated with the PbI2/DMF precursor from the photoluminescence (PL) decay results showed that the Pb(NO3)2/water-based perovskite film had a fast component with a short lifetime, which represents the process of photogenerated carriers filling into subgap traps.102 It also had a slow component with a much longer lifetime, resulting from band-to-band recombination. The fast component corresponds to the first decay, while the slow component corresponds to the second decay as shown in Fig. 6e. In 2021, S. Gozalzadeh et al. used lead sulphide (PbS) produced by aqueous chemical bath deposition. Subsequently PbS films were chemically converted to PbI2 and finally transformed into mixed-cation mixed halide pinhole-free uniform perovskite films. The resulting cells delivered a PCE of 11.35%.103 Since toxic solvents have been completely substituted with eco-friendly water and IPA the results of these works are very encouraging. However, the devices processed with unconventional precursor like Pb(NO3)2 or PbS in water based solution are underperforming in comparison to conventionally processed devices. This could be possibly due to the formation of intermediate products which slows down the reaction and relative merits of the residual water during solvent evaporation and crystallization of the precursor into perovskite phase, leading to degradation of the perovskite itself.
Despite the promising results of water-based perovskite solar cells (PSCs) with a reported highest efficiency of 18.3%, reproducibility remains a major challenge in the field. Trace amounts of nitrate and residual water can lead to an increased number of defects in films. The conversion process for creating a complete perovskite film also requires a longer time frame and multiple rigorous IPA rinsing steps.
However, the use of water as a solvent in PSCs offers numerous benefits in the field of solar energy. This relatively simple method of fabrication would have several advantages compared to traditional methods, a water-based solvent that is more environmentally friendly than other solvents, as well as cost-effective.
3.4 Ionic liquids as solvents
ILs have been researched as a potential solvent for perovskite solar cells. ILs are liquid salts that are composed of positively and negatively charged ions. ILs refer to salts that exist in a liquid state and possess a low melting point, typically less than 100 °C.122 They are often used as solvents in PSCs due to their interesting chemical and physical properties, such as high conductivity, thermal stability, low vapour pressure, adjustable acidity, and high solubilizing power compared to traditional solvents.123–126 In addition, the role of IL in homogenous nucleation is significant as it modifies surface defects, adjusts energy levels, and influences the kinetics of crystal growth and charge transportation of the emerging PSCs fields.127MAF is an effective solvent for dissolving PbI2 and MAI due to its ionic nature and strong solvation interactions. The COO– groups in MAF interact strongly with Pb2+ ions, and CH3NH3+ and I− ions, making MAF more effective in dissolving PbI2 and MAI than other common organic solvents like DMF and DMSO.128 In 2015, Moore et al. reported, the first use of ILs as precursor solvents, i.e. methylamine formate (MAF) to deposit highly crystalline and uniform MAPbI3 films129 ILs like MAF are non-volatile because they have very low vapor pressures, meaning they do not easily evaporate at ambient temperatures. This is due to the fact that they are composed of ions rather than molecules, and the forces holding the ions together are much stronger than the forces holding molecules together in a traditional liquid. To remove MAF residue from the perovskite films they have treated perovskite films with butanol resulting highly crystallized MAPbI3 without any residual precursors. Cho et al. reported the thermal gradient-assisted directional crystallization process is a technique used for perovskite films.128 In this process, a solution of MAI and PbI2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio) in MAF (25 wt%) is prepared and then spread on a substrate using blade-coating method. The liquid film is then exposed to a thermal gradient, causing the perovskite crystals to form in a specific direction. This process improves the uniformity and quality of the perovskite film.128
1 molar ratio) in MAF (25 wt%) is prepared and then spread on a substrate using blade-coating method. The liquid film is then exposed to a thermal gradient, causing the perovskite crystals to form in a specific direction. This process improves the uniformity and quality of the perovskite film.128
Protic Ionic Liquids (PILs) are a class of ILs that contain protons (H+) as part of their cation, which can participate in hydrogen bonding and other types of interactions.130,131 PILs possess lower thermal stability and chemical stability as compared to other classes of ILs. They also exhibit non-negligible vapor pressure, which can be considered an advantage when it comes to solution processing of hybrid perovskites.132 This is because the removal of residual solvents is a critical criteria for achieving high material purity in perovskite solar cells. The vapor pressure of PILs allows for easy removal of the solvent by simple evaporation, which reduces the risk of contamination and impurities in the perovskite film.105 Additionally, PILs can also be chosen based on their solubility and ability to dissolve the precursors of perovskite, which can help to improve the quality and uniformity of the perovskite film. It's worth mentioning that the choice of IL and its properties depend on the specific application and the perovskite material used, and it is important to carefully consider the trade-offs between the properties of the IL and the requirements of the application. PILs have a high viscosity that can make it difficult to create uniform thin films using spin-coating at ambient temperatures. One way to overcome this challenge is to blend the PIL with a co-solvent, such as water, ethanol, isopropanol, or ACN. These co-solvents can help to lower the viscosity of the ink, making it easier to process into a uniform thin film.
S. Öz et al.105 prepared perovskite solutions using Protic ILs (PILs) which contains methylammonium cation and different anions like MAF, methylammonium acetate (MAAc) and methylammonium propionate (MAP). PILs have high viscosities (136 mPa s for MAAc and 94.2 mPa s for MAP) because of this film formation was initially poor. S. Öz et al. subsequently applied co-solvent engineering with IPA, ethanol, water, and ACN (as shown in Fig. 7), and reaching a PCE of 15% with (MA0.15FA0.85)Pb(I0.85Br0.15)3 as active layer, PIL/ACN as solvent system.105 Hoang and colleagues demonstrated a benign method to synthesize green emissive MAPbBr3 by using an environmentally friendly solvent based on ionic liquids. They conducted a study on a range of methylammonium carboxylate protic ionic liquids, which differed in their alkyl chain lengths. The liquids explored included methylammonium formate (MAF), methylammonium propionate (MAP), and methylammonium butyrate (MAB). Perovskite NCs with controlled sizes and shapes (nanocubes, nanorods) and subsequently high photoluminescence were obtained by controlling the alkyl chain length of the carboxylate group and reaction time.133 L. Gu et al. fabricated PSCs from perovskite ink formulations MAF, MAAc, MAP and methylammonium isobutyrate (MAIB) ILs. They studied that molecular structure of MAP led to the formation of strong Lewis adducts, providing sufficient time for large crystal formation (SEM images shown in Fig. 8a–d) resulting in solar cells with a PCE of 20.56%, also retained 88% of initial efficiency for up to 1000 hours of storage in N2 atmosphere.106 Hui et al. fabricated a stable α-FAPbI3 based PSCs using an IL, MAF. Lead iodide reacted with the MAF solvent forming N–H⋯I hydrogen bonds, which lowered the formation energy between Formamidinium iodide (FAI) and the PbI2 leading to a rapid transformation to the stable α-FAPbI3 black-phase. PbI2-MAF and PbI2-DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO solutions and interactions in the solutions are shown in Fig. 8e. In this study, device with PCE of 24.1% in ambient air, with 93% of the initial efficiency maintained up to 500 hours as shown in Fig. 8f and g were fabricated.104
DMSO solutions and interactions in the solutions are shown in Fig. 8e. In this study, device with PCE of 24.1% in ambient air, with 93% of the initial efficiency maintained up to 500 hours as shown in Fig. 8f and g were fabricated.104
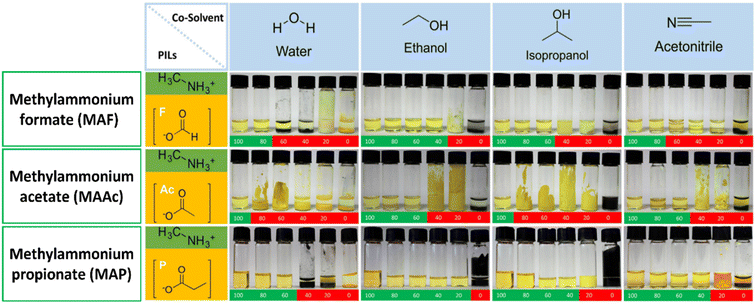 | ||
| Fig. 7 Photographs of binary perovskite ink-systems (protic ILs/co-solvent) with increasing co-solvent volume. (Top-row, methylamine formate (MAF), middle-row, methylammonium acetate (MAAc), bottom-row, methylammonium propionate (MAP).) Green indicates: soluble, red indicates: separation or formation of solids105, reproduced, copyright 2018 published by Elsevier Ltd. | ||
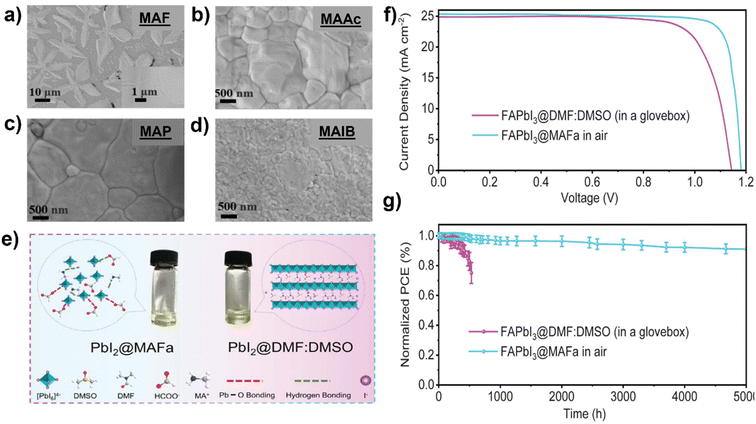 | ||
Fig. 8 SEM images of the perovskite films fabricated by (a) MAF, (b) MAAc, (c) MAP, and (d) MAIB solvents,106 reproduced and copyright 2022, American Chemical Society, (e) images of PbI2-MAF and PbI2-DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO solutions and schematic diagram of interactions in the solutions104 reproduced and copyright 2021, Science. (f) J–V curves of champion device based on FAPbI3@MAF and FAPbI3@DMF DMSO solutions and schematic diagram of interactions in the solutions104 reproduced and copyright 2021, Science. (f) J–V curves of champion device based on FAPbI3@MAF and FAPbI3@DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO films, (g) stability of unencapsulated devices stored in an N2-filled glove box in the dark,104 reproduced and copyright 2021, Science. DMSO films, (g) stability of unencapsulated devices stored in an N2-filled glove box in the dark,104 reproduced and copyright 2021, Science. | ||
Chatterjee et al. investigated the use of ionic liquids (ILs) for synthesizing lead halide perovskite nanocrystals (NCs). They found that carboxylic acid chain length affects the emissive properties of the NCs. The researchers also discovered that lauric acid–based ILs can improve the moisture and environmental stability of the NCs. Furthermore, ILs have the potential to enhance the efficiency, stability, and synthetic toxicity of perovskite NCs.134 Chatterjee et al. utilized menthol-based deep eutectic solvents (DESs) to produce cesium lead halide (CsPbX3) nanocrystals (NCs) and nanoplates (NPLs) that exhibit high photoluminescence quantum yield (PLQY). These DESs possess environmentally-friendly, non-toxic, and low volatility properties, making them advantageous for use as a solvent media for the synthesis of perovskite NCs. The DESs also offer exceptional solubility for lead halide salts in the presence of oleylamine (OAm), which is critical in creating highly luminescent CsPbX3 NCs and 2D Ruddlesen-Popper (RP) NPLs.135 Zhang et al. used utilized natural deep eutectic solvents (NADESs) as both solvents and surface ligands for the green synthesis of cesium lead bromide perovskite nanocrystals (CsPbBr3). The –COOH and –OH groups in the NADESs can poly-chelate with Pb2+ and provide a strong interaction with CsPbBr3 NCs, making them suitable as both solvents and surface ligands for the NCs. The use of NADESs resulted in highly stable, bright-luminous NCs with a high photoluminescence quantum yield (PLQY) of approximately 96.8%.136
ILs are considered promising alternative green solvents to traditional polar aprotic solvents to produce efficient and stable PSCs. Additionally, perovskite films prepared by ILs do not require antisolvent treatments and films deposited in ambient environments are more durable, which would simplify the manufacturing of PSCs in an industrial environment. They form strong interactions with the perovskite materials, which helps to improve their stability and efficiency. ILs can provide a better environment for the perovskite materials to form more efficient films with improved performance, making them ideal solvents for this type of solar cell.
3.5 Solvent free deposition techniques
Mechanochemistry and melt processing are emerging solid-state synthetic techniques that show great promise in the synthesis of perovskite materials. These methods offer several advantages over traditional solution-based methods, including higher reaction rates, better control over the crystal structure, reduced environmental impact, and the ability to obtain high purity materials without the need for solvents. Overall, the potential of mechanochemistry and melt processing in perovskite synthesis is tremendous and these techniques have the ability to drive new advances in materials science and technology, paving the way for the development of innovative and sustainable technologies.
3.6 Green antisolvents for perovskite layer engineering
Especially with the spin coating procedure, the deposition of the precursor ink is often followed by application of an anti-solvent over the film. This antisolvent aids in crystallization of the layer to yield superior perovskite film, that is conducive to achieve high efficiencies in solar cells.107,109–112,148,149 Antisolvent use has also been recently reported for some scalable or large area deposition techniques.150,151 Antisolvent treatment brings out smooth, large grain and low defect thin films of high quality. As the name suggests, the antisolvent must be a non-solvent for the perovskite and there are many solvents for this purpose. Commonly used antisolvents, like diethyl ether, chlorobenzene (CB), chloroform and toluene, have low polarity and lower boiling points than that of the solvent used to dissolve precursors.60 The most widely used antisolvents like halogen-based chemicals (for example, CB) are hazardous to the environment as they pollute water bodies and deplete the ozone layer. Environment-friendly alcohols like ethanol and IPA have been tested as antisolvents, but the high polarity imparted by the –OH group suppresses the growth of larger grains and defect-free perovskite films.152 As various solvents of low polarity exist, such toxic aromatics and chlorinated species are easily replaceable with less hazardous ones. Esters or ethers might represent good candidates and considering the efficiencies and CHEM21 solvent selection guide33 (Table 1), the main green solvents are ethyl acetate, methyl benzoate (MB) and anisole as well as tetraethyl orthocarbonate (TEOC).Zhao et al. conducted a systematic study on the effect of volume of anisole and dripping time for formation of uniform perovskite layer over large areas.108 Yavari et al. proved the efficacy of anisole as anti-solvent by fabricating devices with PCEs of over 20%.110 While efficiencies greater than 20% were obtained for cells fabricated on glass/ITO substrates using anisole as antisolvent, 17% efficiency was achieved for flexible cells.109
Recently Bu group rationalized scattered literature results and reported a general approach to achieve high efficiency PSCs independently on the polarity of the antisolvent. Their study demonstrates how properly exploit mutual solvent-antisolvent miscibility, and the solubility of perovskite precursors in the antisolvent, to obtain high quality films of material. Polarity of the anti-solvent should be high enough to miscible with perovskite precursor solvent and sufficiently low to dissolve precursors with an optimum in the 2 to 4.5D range.112 For example, ethyl acetate has a polarity of 4.4 and a boiling point of 77 °C, which is good enough to remove the precursor solvent, providing uniform pinhole-free film (Fig. 9a) and 19.4% efficient solar cells.112 M. Wang et al. tested TEOC as a non-toxic anti-solvent, as along with the PCBM dissolved in an anisole, fabricating p–i–n PSCs with a PCE of 18.15%.107 As shown in Fig. 9b, the perovskite film is denser and with larger grains when applying antisolvent engineering with TEOS rather than CB reducing the density of electron trap states improving PV performance.107 Anisole, a frequent additive in the food and cosmetic industry, has also proven to be an effective anti-solvent.32,108 Tan et al. studied the process of creating a two-layer structured perovskite film. They began by using an antisolvent i.e., ethyl acetate, on top of the first perovskite film (MAPbI3) to promote fast nucleation. Next, a solution of methylammonium chloride (MACI) dissolved in IPA was added to influence crystal growth, resulting in the formation of a bottom layer of MAPbI3 and an upper layer of mixed MAPbI3/MAPbIxCl3−x. The upper layer exhibited a higher degree of crystallinity and fewer trap states due to the higher LUMO level of MAPbIxCl3−x in comparison to MAPbI3. This allowed for more efficient charge transfer without significantly impacting the overall absorption of the perovskite.148 Y. Yun et al. used a digestive-ripening agent used in the food industry called MB, to help the rapid crystallization of perovskite film and prevent the loss of organic components during the thermal-annealing step (schematic diagram shown in the Fig. 9c) to fabricate PSCs with a PCE of 22.37% and >1300 h stability.111 L. Xu conducted a cumulative study, analyzing the effect of CB, toluene and ethyl acetate on bromide-based perovskites and LEDs. The study demonstrated that ethyl acetate produced films with higher carriers decay time and lower defect concentrations.153 Ethyl acetate is also suitable as antisolvent for relatively stable cesium-based perovskites.154
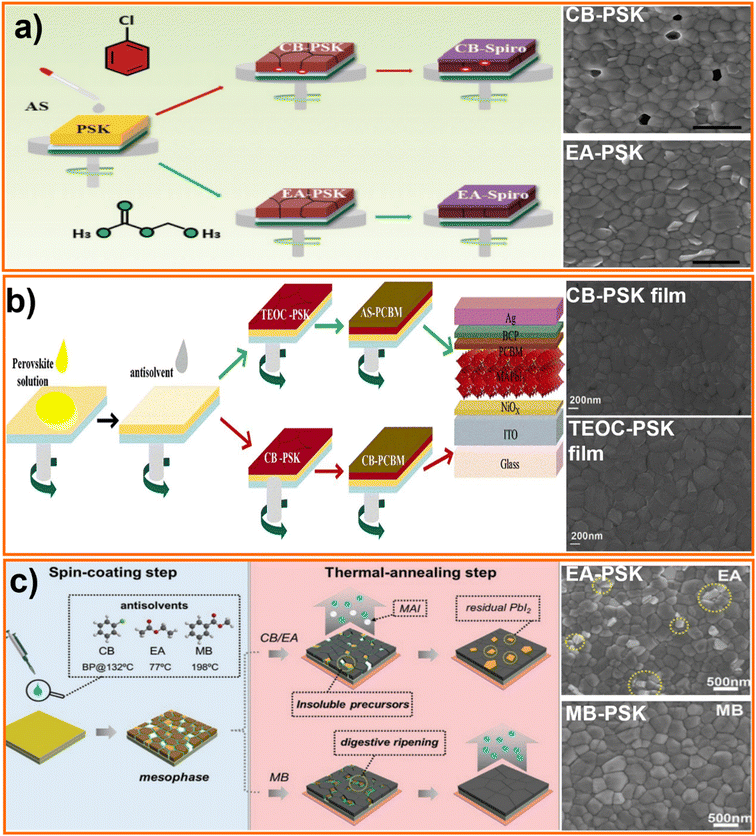 | ||
| Fig. 9 Schematic processing scheme for perovskite films treated by different antisolvents, (a) PSCs fabrication process. (a) Schematic processing scheme for perovskite films, surface SEM images of Chloro Benzene (CB) and Ethyl Acetate (EA) solvents processed perovskite films. Top view SEM images of the (a) CB-perovskite and (b) EA-perovskite films, and the scale bar is 1 μm,112 reproduced and copyright 2017, WILEY-VCH. (b) Tetraethyl orthocarbonate (TEOC)/anisole (AS) and chloro benzene (CB)/CB solvent systems, surface SEM images of the CB-PSK film (above) and TEOC-PSK film (below),107 reproduced and copyright 2020 Elsevier, (c) schematic processing scheme for perovskite films treated by different antisolvents (CB, EA, and MB). Top-view SEM images of Ethyl Acetate (EA)-PSK, and Methyl Benzoate (MB)-PSK films after annealing step111, reproduced and copyright 2020 WILEY-VCH. | ||
Thus, to choose a greener antisolvent, the first and foremost property to be considered is polarity as described above. Apart from the green anti-solvents mentioned above, various alcohols, including n-butyl alcohol,155 sec-pentyl alcohol,156 ethanol157 and IPA150,157 have been used. However, their performance remains lower compared to cells treated with anisole, ethyl acetate and methyl benzoate and further research needs to be carried out for alternatives. Also, most of the antisolvents explored are studied on conventional single-cation perovskite (MAPbX3 or FAPbX3 or CsPbX3), and there is a need to identify and greener antisolvent system for triple cation perovskite, which is capable of producing highly efficient devices. In conclusion, several green alternatives to the common halogenated and aromatic antisolvents have been reported. Among all anti solvents ethyl acetate, anisole, methyl benzoate (MB) and tetraethyl orthocarbonate (TEOC) are proven to be promising candidates.
4. Tackling the content of lead in the active layer
The toxicity of lead and its compounds together with the vulnerability of perovskites to humidity, heat, and light raises concerns over the commercialization of PSCs. It has been suggested that these issues can be resolved by careful device encapsulation. However, accidental damage to encapsulated solar cells is at times inevitable. Environmental fate modelling has revealed that in the case of water exposure, lead compounds can end up in water systems resulting in lead-related toxicity.158 Furthermore, according to Li et al., perovskite contaminated soil proved to be more hazardous than other types of lead sources and it can be a threat to the food chain.159 The inorganic part of perovskites facilitated the absorption of lead by plants and Pb in the perovskite composition was ten times more likely to be absorbed by mint plants via contaminated soil. Thus, the toxicity of lead and its compounds and vulnerability of perovskites to humidity, heat, and light raises concerns over the commercialization of PSCs. Research for tackling this issue has been directed in three main directions.Firstly, understand the true environmental and safety issues of lead, the content of which is extremely small per square meter, compared to the benefits of large-scale production of low-cost solar power, and compared to alternative power generation. No technology comes without risks. Thus, benefits and risks must be well laid out and investigated.
Secondly, devise ways to limit the escape of lead from the solar cell during its operational lifetime (especially in the event of damage) and at its end of life (e.g., via special encapsulation/sequestration strategies).
Thirdly, design and practically fabricate solar cells with perovskite derived semiconductors that replace Pb with less problematic elements. The investigation of different lead-free perovskites to discover stable and non-toxic alternatives to lead perovskites has become a popular topic recently. The ideal lead-free perovskite suitable for commercialization must have: (i) optical and electronic properties comparable with lead-based perovskites, (ii) stability when exposed to air, moisture, light, and heat, (iii) low toxicity, and (iv) environmentally friendly fabrication routes utilizing non-toxic solvents. The emphasis of this section is to review lead sequestration techniques and then summarize lead-free PCSs in terms of efficiency, stability, and toxicity with an overview of environmentally friendly fabrication techniques of these PSCs.
4.1 Lead sequestration techniques
The issue of lead leaching into the environment has raised concern in the scientific community and search for alternative non-toxic divalent metal cations has begun. However, the actively researched alternative, tin (Sn) shows limited degree of success due to its ambivalency and propensity towards oxidative degradation.160,161 Recently, such an issue has been given an out-of-box approach wherein lead can continue to be used without sacrificing photovoltaic efficiency. This unique confluence has been brought by using lead-absorbing chelating agents like transparent P,P′-di(2-ethylhexyl) methane di phosphonic acid (DMDP) and N,N,N′,N′-ethylene diamine tetrakis (methylene phosphonic acid), or EDTMP films.162 Such films on either side of a perovskite photovoltaic cell can offer on-device sequestration of more than 96 per cent of lead leakage caused by severe device damage (Fig. 10) due to inadvertent mechanical breakage or under harsh or acidic rainy conditions. Such techniques can offer solutions to lead leaking even at higher temperatures and heavy rain. After this first report, another group demonstrated the use of a porous metal–organic framework (MOF) polymer composite where the MOF scaffold contains a metal binding agent that stops the lead leaching.163 Contaminated water derived from damaged PSCs with such layer was brought below the drinkable standards set by the Environmental Protection Agency (EPA). Recently, the lead sequestration is heavily researched with multiple groups working towards turning this into a viable technology to tackle the challenge of lead leaching from PSCs using bioinspired hydroxyapatite/TiO2 nanoparticle (NPs) blend,164 thiol-functionalized NPs,165 and polyoxometalates-metal–organic frameworks host–guest nanostructured dopants.166 Fig. 10a shows the schematic of an encapsulated perovskite solar cell in which the lead absorbing material, (3-mercaptopropyl)trimethoxysilane (MPTMS)-capped nanospheres (MPTMS-ns), is incorporated into the silicone-based adhesive.165 This low-cost thiol-functionalized delivered 90% of Pb sequestration efficiency (Fig. 10b).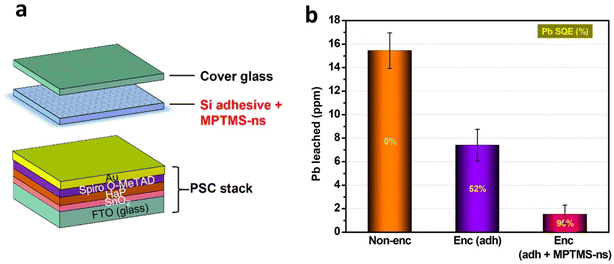 | ||
| Fig. 10 (a) Schematic of perovskite solar cell encapsulated using Si adhesive combined with lead MPTMS-ns. (b) Average Pb sequestration efficiency of non-encapsulated, encapsulated, and encapsulated + MNTMS-ns devices.165 | ||
4.2 Lead-free perovskites
There are a growing number of materials that have been investigated as a substitution for lead, including but not limited to group 14 elements Sn and Ge, group 15 elements Bi and Sb, and some transition metals. Depending on the ionic radius and oxidation state, perovskites form in 3D AB2+X3, 2D A2B2+X4, and 2D A3B23+X9 phases. It is also possible to use two ion-splitting (e.g., a B3+ and a B+) or ordered vacancy (e.g., a B4+ and a vacancy) approaches to form double perovskites. In addition, some chalcogenide perovskite and perovskite-like structures are also explored as potential replacements of lead halide perovskites.152,167 Fig. 11a shows the classification of lead-free perovskites used in solar cells and Fig. 11b. The comparison of power conversion efficiency (PCE) record of lead-based and lead-free PSCs. Perovskite PV has been shown to have remarkable efficiency indoors reaching and surpassing 30% PCEs.168–172 Lead free alternatives,173 have started to be developed for indoor light harvesting.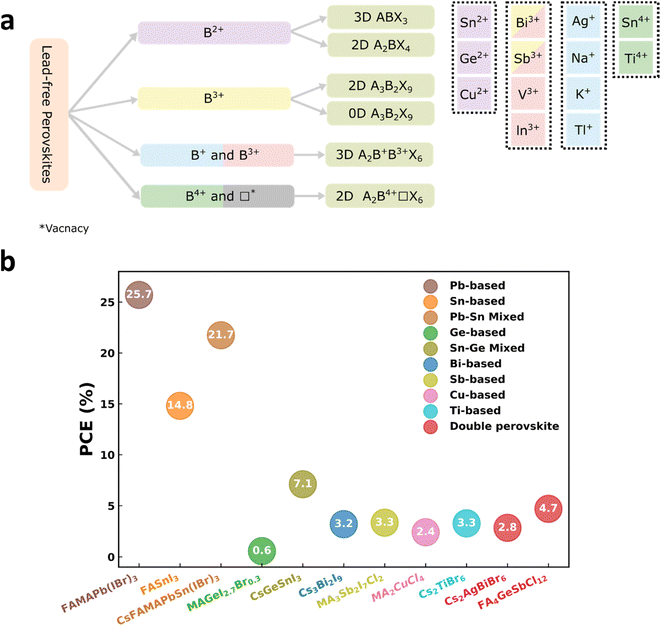 | ||
| Fig. 11 (a) Types of lead-free perovskites. (b) Efficiency record of lead-based and lead-free PSCs.4,12,174–181 | ||
One of the main drawbacks of Sn-based PSCs is their stability as Sn2+ tends to oxidize to Sn4+ easily in the presence of oxygen or the widespread used DMSO solvent.185–187 As shown in the Fig. 12a, the color of SnI2 and FASnI3 in DMSO changes after heating at 100 °C. In addition, even the commercial batches of 99.999% SnI2 have found to contain considerable amounts of Sn4+.188 The Sn4+, which is present in the perovskite layers, acts as a p-type dopant and adversely affects efficiency by decreasing lifetime of photocarriers in the perovskite layer.189,190 Various approaches have been adopted to enhance stability. One is to add reducing agents such as Sn powder, SnX2 (X= F, Cl, Br, I, SCN), hydrazine compounds, and gallic acid to prevent Sn oxidation.191 However, the poor conductivity of most of these additives reduce the charge transfer in the fabricated devices. Another strategy to enhance stability is to replace the “A” cation with larger cations, to form 2D perovskites since, in 2D perovskites, organic spacing layers protect the perovskite from moisture. 2D-quasi-2D-3D Sn perovskites demonstrate superior stability by sacrificing efficiency, reaching the PCE of 9.4%.192 It appears that this low-dimensional perovskite compromises voltage in favour of stability as devices have the VOC of 0.6 V compared to 0.86 V for CsSnI3-based cells, and other photovoltaic characteristics remain almost the same. As the stability is due to the tendency of Sn2+ to oxidize to Sn4+, one potential solution is to use Sn4+-based perovskites as a light absorber. Cs2SnI6, a 2D Sn(IV)-based double perovskite is very stable and retained 95% of its efficiency after 45 days of exposure to air, yet its efficiency is limited to 2.1%.193 Hence, the stability issue remains unanswered to date as these perovskites are more unstable than Pb-based ones, and it is difficult to enhance their stability without sacrificing the device's efficiency.
 | ||
| Fig. 12 (a) Vials containing A: FAI in DMSO; B: SnI2 in DMSO; C: FASnI3 in DMF; D: FASnI3 in DMSO with 10 mol% SnF2; E: CsSnI3 in DMSO before and after heating at 100 °C for 30 min.185 (b) The 16 non-sulfoxide solvents examined for Sn-based perovskites from 6 different classes.190 (c) The Start and the end of drop casting FASnI3 solution in mentioned solvents.190 | ||
However, when it comes to environmental impacts, the oxidation of tin to Sn4+ can be an advantage since when it reacts with water and oxygen, it results in the production of the inert, non-toxic SnO2,194 according to the following equations:
| 8SnI2 + 4H2O + O2 → 2SnI4 + 6Sn(OH)I + 2HI |
| SnI4 + 2H2O → SnO2 + 4HI |
Tin and its compounds are generally less toxic than lead compounds and are believed to be non-carcinogenic. The permissible workday exposure limit of tin is 2.0 mg in 1 m3 of air, 40 times higher than that of lead,195 and the impacts of tin in human health is insignificant compared to lead.196 One factor in measuring the toxicity is bioavailability, meaning to what extent living organisms absorb a material. Li and colleagues proved that mint plant tends to absorb a significantly lower amount of Sn from perovskite contaminated soil.159 In addition, Tin halides can potentially be more harmful to living organisms than lead halides with the same concentration in the aqueous environment.197 Nevertheless, the rapid oxidation of Sn2+ to Sn4+ leads to less water solubility and could reduce the bioavailability of tin. Overall, in the case of accidental leachate, tin is less likely to enter the food chain than lead.159
Life cycle assessment (LCA) is one of the best methodologies to evaluate the environmental impacts of solar cells from very first production stages to the end of their life. We found two studies on LCA of tin-based PSCs, with both considering a lower PCE for Sn-based solar cells compared to their current record. Zhang et al. assumed that MASnI3−xBrx and MAPbI3 PSCs had PCEs of 5.73% and 20% respectively, while the Krebs group assumed 15.4% efficient CH3NH3PbI3−xClx and 6.4% efficient MASnI3 solar cells.196,198 They concluded that when fabricating PSCs with 1 cm2 active area, tin-based PSCs have a lower environmental impact, energy consumption, and material consumption than lead-based ones. Considering power output, however, the environmental impacts of these PSCs change. Zhang and co-workers estimated that generating 1 kW h of electricity, tin-based PSCs emit 2.5 times higher greenhouse gasses than lead-based ones: due to their lower efficiency a larger active area is needed for tin perovskites to generate 1 kW h energy. Serrano-Lujan and colleagues concluded that to have a similar environmental footprint in large-scale production, tin-based PSCs must have at least 12% efficiency.196 In light of the fact that they considered a PCE of 15.4% for lead-based PSCs and the current PCE record is 25.7%, we surmise that unless tin-based PSCs surpass 20% efficiencies at the laboratory scale, they cannot be considered a strong competitor to lead-based ones. Nevertheless, it is possible to achieve promising efficiencies with Sn–Pb alloys, which are often called lead-less perovskites. For instance, Kapil and co-workers demonstrated that 21.74% efficiency is viable with tin-lead PSCs,181 which contain 50% less lead than conventional PSCs.
Few studies have focused on the fabrication of tin-based perovskites with non-hazardous or less hazardous solvents; typically, tin-perovskites are being deposited using DMSO or DMSO/DMF mixture. Unlike lead halides, tin halides are partially soluble in alcohol. Taking this as an advantage, Greul et al. fabricated lead-free perovskite solar cells based on MASnI3 in methanol. Methanol dissolves low concentrations of MASnI3 (0.1 M); still, they managed to triple the concentration by using 1,4-dioxane as a co-solvent and obtained 1.05% PCE.13 Although dioxane is classified as a hazardous solvent, the presence of 50% methanol is a positive step toward less-hazardous solvents in Sn-based perovskites. Another study used formic acid as a co-solvent with DMSO to fabricate FASnI3 solar cells with 10.37% efficiency.199 Formic acid is a less hazardous solvent compared to DMF and NMP, but cannot be considered as a green solvent.200 They suggested that acidic conditions might enhance the stability of Sn2+, as encapsulated devices maintained 95% of their efficiency after 200 hours of light soaking. Sn halides are soluble in acidic aqueous solutions. Abate group did a systematic solvent investigation by studying the solubility, thermal stability, and perovskite formability of FAI and SnO2 precursors in different solvents and found 12 non-sulfoxide solvents in which formation of FASnI3 occurs with no oxidation.190 As shown in Fig. 12b, they identified 16 non-sulfoxide solvents for FASnI3, by estimating solubility in 80 solvents based on HSP, dielectric constant, and calculation of the binding energy of the solvents to tin ion by density functional theory. Fig. 12c shows the drop casted films based on these 16 solvents before and after annealing comparing them with DMSO-based ink. The formation of perovskite was not observed in two of solvents and the perovskite was not stable in other two solvents after 3 hours when exposed at 100 °C. Finally, a mixture of Diethylformamide (DEF) and DMPU was selected as an optimal solvent to deposit large-grain perovskite films and a PCE of 6.2% obtained. We believe further research should also focus on the environmental impact of selected solvents for tin-based perovskites.
Regarding environmental impacts and toxicity, germanium is generally less harmful and less toxic than lead and its compounds. Germanium compounds are not carcinogenic, and some of its organic compounds are used for cancer treatment,202 but its inorganic compounds found to be damaging to the liver and lethal in some cases.203 However, germanium is phenomenally expensive as its price is 800 times of that of lead.204 This presents difficulty in producing low-cost Ge-based PSCs. It is possible to synthesize CsSnGeI3 by mixing CsI, SnI2, and GeI2 powders and maintaining in vacuum for 72 h at 450 °C.175 Solid-state reactions that eliminate solvents can be considered as an environmental-friendly fabrication route for Ge-based perovskites. It should be noted that although this method is solvent-free, the energy required can be higher than that of solution-processed synthesis methods due to prolonged exposure to high temperatures. In addition, for the fabrication of the solar cells, the obtained CsSnGeI3 was deposited with the energy-demanding thermal evaporation technique (Fig. 13a).
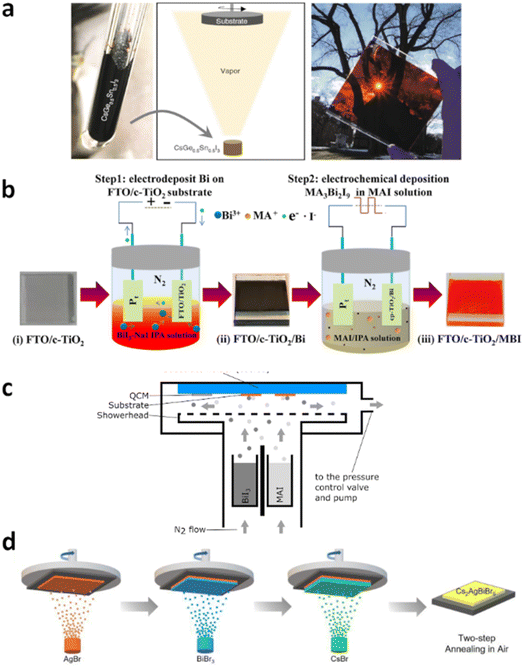 | ||
| Fig. 13 (a) Mixed Sn–Ge perovskite synthesized with melt crystallization method and deposited with thermal evaporation.175 (b) Schematic of two-stage electrodeposition of MBI films in isopropyl alcohol.210 (c) Sequential vapour deposition of Cs2AgBiBr6 double perovskites.218 (d) Schematic of chemical vapour deposition of Bi-based perovskites under N2 atmosphere.212 | ||
The primary solvents used in solution-processed Bi-based perovskites are DMF and DMSO, yet several studies focused on the fabrication of these perovskites with less hazardous solvents. One of the most recommended non-toxic solvents is ethanol, but BiI3 is not soluble enough in ethanol to deposit films with several hundred nanometers thicknesses that are required for solar cell absorbers.207 Li et al. managed to successfully dissolve BiI3 and MAI in ethanol with the addition of methylamine to the solution, and fabricate MBI-based PSCs.208 The single-step fabricated MBI thin-films were dense with equiaxed grains, far preferable to porous and rough films obtained by DMF solution with the same concentration. The green solvent-based MBI PSC outperformed DMF-based solar cells, delivering a PCE of 0.053% while devices with the same architecture using DMF as solvent delivered a PCE of 0.022%. These solar cells maintained their performance after 5 days of exposure to 50% humidity. Methyl-acetate (MeOAc), is another non-toxic solvent used to produce MBI thin-films, which is ranked in between recommended and problematic solvent systems.200 Bi-based perovskites fabricated with MeOAc demonstrated a PCE of 1.62%, the highest record among non-toxic solvent-based bismuth PSCs, and also no evidence degradation after being soaked under one sun illumination over 30 days.209 Korukonda et al. demonstrated the fabrication of pinhole free bismuth perovskite layers. Employing a greener solvent system which consists of acetone and DMF and deposition carrying out through electric-field assisted spray technique reveals the potential application of the process for eco-friendly and commercialization of Bi-based PSCs.335 A novel two-stage approach for deposition of MBI-films by electrodeposition in IPA bath was introduced by Want et al.210 This method did not require annealing, and after the deposition of Bi on the working electrode, the perovskite was formed in a methylammonium iodide IPA solution electrodeposition bath. Fig. 13b illustrates this two-step deposition method. Electrodeposited MBI-based solar cells have managed to reach 0.042% PCE.
Solvent-free synthesis of bismuth perovskites is also viable. El Ajjouri et al. demonstrated the solid-state synthesis of fifteen different A3Bi2I9 compounds with K+, Rb+, Cs+, MA and FA as “A” cation, and I−, Cl−, and Br− as “X” anion by dry ball-milling of AX and BiX3 salts under nitrogen atmosphere.211 This approach opens opportunities for the green synthesis of bismuth-based perovskites by the elimination of solvents and the annealing process. However, this solid-state synthesis has not been adopted for the fabrication Bi-based of devices yet. Another solvent-free fabrication route which is widely used in semiconductor industries is chemical vapor deposition (CVD). For the first time, Sanders and colleagues fabricated MBI layers with the CVD method under an N2 atmosphere and total gas pressure of 1 kPa (see Fig. 13c).212 This method is solvent-free, requires a low vacuum, and is large-scale compatible. However, solar cells fabricated delivered only 0.016% PCE, indicating that much more optimization is required.
MA3Sb2I9 (MASI) crystallizes in a 0D structure consisting of (Sb2I9)−3 octahedral, which are surrounded by three MA+ cations. Having an optical bandgap of 2.14 eV, the first attempts to fabricate PSCs based on this material resulted in 0.49% efficiency.214 Various strategies have been developed to synthesize 2D-structured Sb-based perovskites, including composition engineering and altering precursors. For instance, by adding chlorine, MASI transforms into its 2D phase. Recent work led by Yang et al. showed that uniform, pinhole-free, and low-defect MA3Sb2I7Cl2 thin-films could be obtained by incorporating bis(trifluoro methane) sulfonimide lithium (LiTFSI) additive in DMF solution of MASI perovskite.179 This approach led to PSCs with 3.34% PCE, the JSC almost doubled, and devices-maintained stability after remaining 1400 hours in ambient conditions.
Just recently, the MASI synthesis form antimony(III) acetate (Sb(OAc)3) precursor in different solvent systems, namely DMSO, DMF, tetrahydrothiophene-1-oxide (THTO), ethanol, and methanol has been investigated.215 It was shown that the 2D MASI forms in alcohol solvents, but the use of other solvents resulted in the formation of the 0D MASI phase. The 2D MASI has better stability than 0D phase. It was also revealed that methanol is a better solvent for this purpose as the solubility of Sb (OAc)3 is 0.25 mmol ml−1 in methanol, which is double the number of that of ethanol. Consequently, solar cells fabricated by methanol as a solvent for the perovskite layer perform better, achieving 0.54% efficiency. In another attempt, Zou et al. discovered a new group of Sb-based perovskites with (NH4)+ as A+ cation that are soluble in ethanol.216 The optical bandgap of (NH4)3Sb2I9−xBrx was tuned from 2.27 eV to 2.78 eV by varying the halide in the composition. Devices based on (NH4)3Sb2I9 delivered 0.051% efficiency, with 1.03 V open-circuit voltage. Even though the use of ethanol seems promising, the authors employed hazardous CF as anti-solvent, which raises a question about the environmental impact of this method. In addition, these devices stopped working after two days of exposure to ambient air with 50% humidity.
Although there are a limited number of works on Cu-based PSCs, most of them use non-toxic solvents for synthesis and deposition of these perovskites. Cui and colleagues reported the first Cu-based perovskites used in solar cells with the chemical formula of (p-F–C6H5C2H4–NH3)2–CuBr4 and (CH3(CH2)3NH3)2–CuBr4 which have the bandgaps of 1.74 eV and 1.76 eV respectively and achieved 0.51% and 0.63% PCEs.217 These perovskites were synthesized in the aqueous HBr (40 wt%) and were dissolved in the non-toxic ethanol solvent for film deposition. Another Cu-based perovskite (C6H5CH2NH3)2CuBr4, synthesized, and deposited with the same method, has a bandgap of 1.81 eV, and the fabricated cells reached 0.2% PCE.11 This copper-based perovskite material showed high stability exposed to humidity, heat, and UV-light, the stability of devices is not reported though. Later, Elseman et al. demonstrated that it is possible to synthesize Cu-based perovskites by grinding precursor powders for 40 minutes.12 Although the perovskite powder was obtained via solvent-free synthesis, it was dissolved in DMF to be deposited via spin coating, which gave a PCE of 2.41%.
4.3 Double perovskites
An additional method to synthesize perovskites is replacing 2+ cations with 1+ and 3+ cations or a 4+ cation with a vacancy (□) to form A2B′B′′X6 or A2B□X6 structure. As a result, the overall charge balance is similar to that of ABX3 perovskites. Examples of common ions used in double perovskites are Ag+, Na+, K+, Tl+, Sb3+, Bi3+, In3+, V3+, Ti4+, and Sn4+. These perovskites have been synthesized with both organic and inorganic A+ cations; the former results in the 3D structure while the latter often yields a 2D perovskite structure. Due to the wide range of materials available to produce these perovskites, the bandgap can be tuned over a wide range. Until now, the majority of discovered double perovskites had high bandgaps, and the highest PCE achieved is 4.7% by the 2D FA4GeSbCl12 perovskite.178 However, there are a growing number of studies in the area of double perovskite materials, and their full potential is yet to be discovered. Double perovskite powders are often synthesized with the hydrothermal method, in the acidic aqueous environments as the precursors are soluble in these solutions, but the perovskites are partially soluble in these solvent systems. For instance, only 0.05 mol of Cs2AgBiBr6 dissolved in 1 L HBr, while its solubility in DMSO was 0.6 mol L−1.219 Although there are multitudes of reports in the synthesis of double perovskites with the hydrothermal method, very few works to the best of our knowledge fabricated thin-films with non-hazardous or less hazardous solvents. However, there are reports of solid-state, solvent-free double perovskite film deposition by evaporation methods (Fig. 14d). Despite being solvent-free, evaporation techniques could be more energy-intensive than solution-processed methods as highlighted in Section 7. This section covers the current state of double perovskites in photovoltaic applications with the focus on their environmental impacts.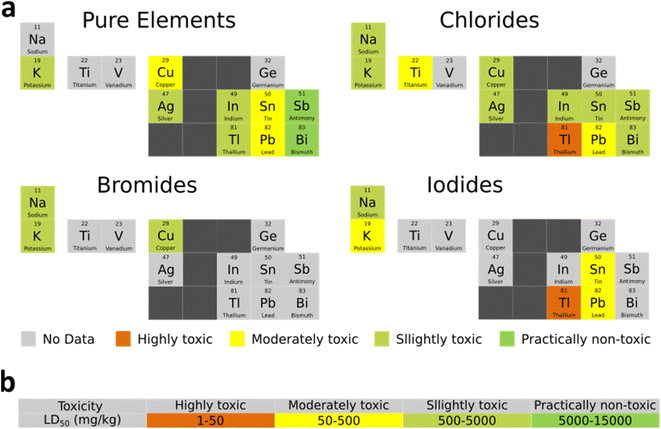 | ||
| Fig. 14 (a) The toxicity of lead and its potential replacements in perovskites and double perovskites, their chlorides, bromides, and iodides. The estimation is based on the reported LD50 amounts for rats in mg kg−1 from safety data sheets provided by Fisher Scientific. (b) The toxicity rating system of elements and compounds are based-on LD50 values.226 | ||
4.4 Lead-free perovskites; greener or not?
Table 4 and 5 summarize solvents used, deposition method, device architecture, and photovoltaic parameters of the reported lead-free PSCs in which the absorber is deposited by greener fabrication routes. This section discusses the question of the superiority or inferiority of lead-free perovskites. To address lead-free perovskites as a greener alternative to lead-based ones, several factors must be considered such as toxicity, abundance, ease of supply, cost, stability, and performance. For instance, Grancini group elaborated on lead zirconate titanate (PZT) and CdTe case studies in their perspective article on the lead-free perovskites.225 The most used piezoelectric material in the world, PZT, has 60% lead(II) oxide in its compound and remained in the market so far because rival materials either lack performance, stability or competitiveness in terms of price. Similarly, CdTe solar cells are exempt from Restriction of Hazardous Substances Directive (RoHS) directives limit on the amount of Cd in consumer products. However, they could not outcompete silicon photovoltaics due to small supply of Te which restricted the annual production and consequently the market share of CdTe solar cells.225 One of the factors with which one can compare toxicity of elements and compounds is the oral mean lethal dose (LD50). Fig. 15 illustrates the toxicity of elements and halides which are used in perovskites solar cells based on the reported LD50. It should be noted that there are not sufficient data for measuring the toxicity of most bromide salts, and further measures to create a comprehensive toxicity database have to be taken. Based on current data, Bi and Sb could be the most non-toxic alternatives to Pb in perovskite materials. However, less toxicity alone does not make a perovskite material superior to lead-based ones. Due to its abundant supply, low price, and global production, Pb has a competitive advantage over Sb and Bi when it comes to production. Table 6 compares Pb and other candidates in terms of abundance, cost, global production, global warming potential (GWP), and whether they are listed as critical raw materials. Based on this information, tin and copper are the only viable options with relatively low GWP, low price, and large supply.| Absorber | Solvent | Deposition method | Device architecture | VOC [V] | JSC [mA cm−2] | FF [%] | PCE [%] | Ref. |
|---|---|---|---|---|---|---|---|---|
| FASnI3 | Formic acid/DMSO (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
Spin-coating | PEDOT:PSS/perovskite/C60/BCP/Ag | 0.59 | 20.6 | 71 | 8.58 | 199 |
| FASnI3 | Formic acid/DMSO (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Spin-coating | PEDOT:PSS/perovskite/C60/BCP/Ag | 0.63 | 22.3 | 74 | 10.37 | 199 |
| FASnI3 | Formic acid | Spin-coating | PEDOT:PSS/perovskite/C60/BCP/Ag | 0.61 | 20.3 | 73 | 9.01 | 199 |
| FASnI3 | DEF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMPU DMPU |
Spin-coating | PEDOT:PSS/perovskite/C60/BCP/Ag | 0.53 | 21.9 | 53 | 6.2 | 190 |
| MASnI3 | Methanol/1,4-dioxane (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : 1) : 1) |
Spin-coating | c-TiO2/m-TiO2/perovskite/spiro/Au | 0.18 | 15.44 | 38 | 1.05 | 13 |
| MA3Bi2I9 | Ethanol | Spin-coating | c-TiO2/m-TiO2/perovskite/spiro/Au | 0.84 | 0.17 | 35 | 0.053 | 208 |
| MA3Bi2I9 | IPA | Electrodeposition | c-TiO2/perovskite/spiro/Au | 0.55 | 0.17 | 44 | 0.042 | 224 |
| MA3Bi2I9 | Methyl-acetate | Spin-coating | c-TiO2/m-TiO2/perovskite/P3HT/carbon | 0.87 | 2.70 | 69 | 1.62 | 209 |
| (NH4)3Sb2I9 | Ethanol | Spin-coating | PEDOT:PSS/perovskite/PC61BM/Al | 1.03 | 1.15 | 43 | 0.51 | 216 |
| MA3Sb2I9 | Methanol | Spin-coating | c-TiO2/m-TiO2/perovskite/spiro/Au | 0.46 | 2.21 | 52 | 0.54 | 215 |
| (p-F–C6H5C2H4–NH3)2–CuBr4 | Ethanol | Spin-coating | c-TiO2/perovskite/spiro/Ag | 0.87 | 1.46 | 40 | 0.51 | 217 |
| (CH3(CH2)3NH3)2–CuBr4 | Ethanol | Spin-coating | c-TiO2/perovskite/spiro/Ag | 0.88 | 1.18 | 40 | 0.63 | 217 |
| (C6H5CH2NH3)2CuBr4 | Ethanol | Spin-coating | c-TiO2/m-TiO2/perovskite/P3HT/Au | 0.68 | 0.73 | 41 | 0.2 | 11 |
| Absorber | Synthesis method | Deposition method | Device architecture | VOC [V] | JSC [mA cm−2] | FF [%] | PCE [%] | Ref. |
|---|---|---|---|---|---|---|---|---|
| CsSnGeI3 | Melt crystallization | Thermal evaporation | PCBM/perovskite/spiro/Au | 0.63 | 18.61 | 61 | 7.11 | 175 |
| MA3Bi2I9 | Chemical vapor deposition | c-TiO2/m-TiO2/perovskite/spiro/Au | 0.39 | 0.13 | 39 | 0.02 | 212 | |
| MA2CuCl4 | Grinding milling | Spin coating in DMF | c-TiO2/m-TiO2/perovskite/spiro/Au | 0.56 | 8.12 | 52 | 2.41 | 12 |
| Cs2AgBiBr6 | Sequential vapor deposition | c-TiO2/perovskite/P3HT/Au | 1.12 | 1.79 | 68 | 1.37 | 218 | |
| Cs2TiBr6 | Sequential vapor deposition | C60/TiO2/perovskite/P3HT/Au | 1.02 | 5.69 | 56 | 3.28 | 176 | |
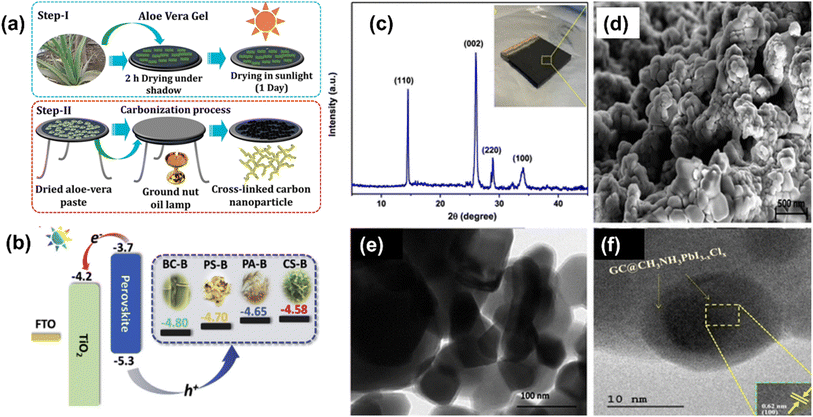 | ||
| Fig. 15 (a) Schematic representation of the preparation of aloe-vera processed cross-linked carbon nanoparticles using an ancient Indian method.315 (b) Energy level diagram of PSC devices based on different bio-carbon electrodes.314 (c)–(f) Structural and morphological properties of the bio-inspired naturally extracted graphitic carbon from an invasive plant species of Eichhornia crassipes. (c) XRD pattern (inset shows the digital photograph), (d) field emission microscopic SEM image, (e) high-resolution microscopic TEM image, and (f) magnified HRTEM image with lattice fringes of the deposited GC@CH3NH3PbI3−xClx thin film.317 | ||
| Element | Abundance in Earth's crust (ppm) | Average price in 2016–2020 (USD per kg) | Global production in 2017 (tonnes) | GWP (kg CO2-eq. per kg) | Critical raw material for the EU in 2020 |
|---|---|---|---|---|---|
| Pb | 14 | 2.25 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 059 059![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 133 133 |
1.3 | No |
| Sn | 2.3 | 19.23 | 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 947 947 |
17.1 | No |
| Ge | 1.5 | 1189.60 | 98 | 170 | Yes |
| Sb | 0.2 | 8.42 | 130![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 751 751 |
12.9 | Yes |
| Bi | 0.0085 | 8.81 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 521 521 |
58.9 | Yes |
| Ti | 5650 | 8.31 | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 027 027![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 450 450 |
8.1 | Yes |
| Cu | 60 | 6.03 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 939 825 939 825 |
2.8 | No |
| Ag | 0.075 | 560.64 | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 469 469 |
196 | No |
| In | 0.25 | 374.6 | 790 | 102 | Yes |
| Tl | 0.85 | 7733.33 | N/A | 376 | No |
Lead-free perovskites are not likely to enter the market with their current PCE records. At the moment, the major competitor to the Pb-based perovskites, tin, achieved just a little more than half of that of Pb-based solar cells, and has problematic stability issues. Considering this, the most viable alternative is mixed Pb–Sn PSCs which can achieve more than 21% efficiency while having half amount of Pb.181 In the short term, the deployment of lead-less PSCs with durable and rugged encapsulation, on-device sequestration and legitimate recycling policy could be a satisfactory solution towards decreasing the environmental impacts of Pb-based solar cells. On the contrary, to completely eliminate these environmental problems, lead-free perovskites made of non-toxic elements with high stability and efficiency should be developed in the long run.
5. Charge transport layers
5.1 Solvents for the deposition of the electron transport layer (ETL)
The electron transport layer (ETL) is the least concerning material in the preparation of PSCs. For n–i–p device architectures, inorganic ETL deposition is well established. Indeed, in the most common embodiment of the n–i–p architecture, the perovskite layer is stacked on top of semiconducting oxides like TiO2, SnO2 or ZnO.231 Often ETLs come as a combination of a compact layer and scaffolding layer (typically mesoporous TiO2). Compact layers can be prepared via sol–gel methods starting from the appropriate organometallic precursor. Sol–gel chemistry works best when performed on mixtures of water and lower alcohols, including ethanol, IPA, and butanol. Compact layers now are of the deposited in nanocrystalline form directly, at low temperatures, compatible with flexible substrates, using water and alcohol mixtures which tend to outperform equivalent compact layers deposited from liquid precursors.232 Studies suggest ZnO233 or SnO2 (ref. 234 and 235) may have a less energetical impact on fabrication considering the fact that conventional TiO2 needs high-temperature processing. Despite consuming a large amount of energy, TiO2 based ETLs with ethanol as solvent are widely used due to their higher stabilities and good performance. Low-temperature-processed TiO2, such as the anatase processed at temperature as low as 150 °C, is an attractive option to lower embodied energies.236 Low temperature processing offers the benefit of fabrication of flexible devices and scaling up the technology.60 Light induced sintering of TiO2 layers is yet another energy efficient method that can be employed for plastic perovskite devices. Di Giacomo et al. sintered the mesoporous TiO2 layer first via UV irradiation which has been shown to get rid of solvents/binders as well as induce some sintering between nanoparticles.237 Different sintering procedures require different energies. A study on sintering of nanocrystalline TiO2 showed that the embodied energies (EE) for the processes were very high for the laboratory hotplate (37 kW h m−2), about half for conventional oven and belt furnace (16–18 kW h m−2) and down to (15 kW h m−2) for laser irradiation given the wall plug efficiency of the laser system was 3.5%. Obviously employing thinner substrates for conventional procedures would bring the energies down whereas designing more efficient laser systems could bring down the EE even further.238In general, most oxide ETL deposition methods can be considered green. Studies revealed that ZnO and SnO2 processing is energy efficient and greener compared to that of TiO2 (ref. 239–241). In an LCA conducted over a module of 70 cm2 area, the energy consumption for manufacturing a TiO2-based module was twice compared to that of a ZnO based module as a result of the low-T processing of the latter (usually less than 200 °C).239 Similar conclusions can be determined when using SnO2 as ETL which can be processed at T < 150 °C. Najafi et al. reported a simple synthesis procedure of ZnO and its deposition using green solvents essentially alcohols and aqueous solutions. Interestingly, the HTL was NiOx which is also green processed.240 In fact, the latter can be used in developing solar modules on PET plastic substrates.242,243 In another study by Gong and colleagues, two different perovskite modules with TiO2 and ZnO as ETLs were compared.239 Because they had different front and back contacts it was impossible to directly compare two ETLs however, the ZnO modules showed less energy payback time than TiO2 based modules. Sarialtin et al. performed LCA on mesoporous TiO2 and planar SnO2-based PSCs with HTM-free architecture and carbon electrodes.241 They concluded that using SnO2 as ETL, reduces the environmental impact to up to 20% compared to TiO2 devices (taking up 23% of the total energy required for the fabrication).
PCBM and modified PCBM are potential candidates among organic ETLs, but these fullerene-based ETLs are mostly soluble in toxic solvents like toluene and CB.248,249 M. Wang et al. showed that the greener alternative, anisole can be used to dissolve and deposit PCBM leading to devices with a PCE of 18.15%, equivalent to that with CB.107 Perylene diimides (PDI) based non-fullerene ETLs are another class of ETLs that are soluble in simple alcohols. Meng and co-workers studied the green solvent 2,2,2-trifluoroethanol to dissolve perylene diimides derivative(ETL).247 Table 7 presents a summary of the photovoltaic characteristics of PSCs with different ETLs casts from a variety of solvents. SnO2 is currently considered a better option for low-temperature processing as it is also suitable for flexible devices and water as the solvent makes the fabrication greener.232
| ETL | Solvent | Device architecture | VOC [V] | JSC [mA cm−2] | FF [%] | PCE [%] | Ref. |
|---|---|---|---|---|---|---|---|
| SnO2 | H2O | ITO/SnO2/PVSK/PEAI/spiro-OMeTAD/Au | 1.16 | 24.90 | 81.40 | 23.56 | 157 |
| SnO2 | Ethanol | ITO/SnO2/PVSK/spiro-OMeTAD/Au | 1.11 | 23.27 | 67.0 | 17.21 | 244 |
| SnO2 | H2O | ITO/SnO2/PVSK/spiro-OMeTAD/Au | 1.12 | 23.86 | 80.60 | 21.64 | 245 |
| SnO2 | H2O | FTO/SnO2/PVSK/spiro-OMeTAD/Au | 1.18 | 25.74 | 83.2 | 25.5 | 245 |
| SnO2 | IPA | FTO/SnO2/PVSK/spiro-OMeTAD/Au | 1.14 | 21.72 | 76.0 | 19.21 | 246 |
| TiO2 | Ethanol, methoxy ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) terpineol = 3.5 terpineol = 3.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w 1 w/w |
FTO/C–TiO2/m-TiO2/PVSK/spiro-OMeTAD/Au | 1.14 | 23.7 | 78.0 | 21.6 | 245 |
| TiO2 | Ethanol, methoxy ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) terpineol = 3.5 terpineol = 3.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w 1 w/w |
FTO/C–TiO2/m-TiO2/PVSK/spiro-OMeTAD/Au | 1.14 | 23.30 | 79.6 | 22.7 | 245 |
| PCBM | Anisole | Glass/ITO/SnO2/perovskite/spiro-OMeTAD/PCBM/BCP/Ag | 1.06 | 21.9 | 78 | 18.15 | 107 |
| ZnO | 1-Butanol | Glass/ITO/NiOx/perovskite/PCBM/ZnO/Al | 1.00 | 20.80 | 84 | 17.3 | 240 |
| ZnO | 1-Butanol | PEN/ITO/NiOx/perovskite/PCBM/ZnO/Al | 1.02 | 20.6 | 73.0 | 15.3 | 240 |
| PDIN | 2,2,2-Trifluoroethanol | Glass/TIO2/PTAA/perovskite/PDIN/Ag | 1.03 | 20.34 | 73.31 | 15.28 | 247 |
| PDIN | 2,2,2-Trifluoroethanol | Glass/TIO2/PTAA/perovskite/PDIN/Ag | 1.09 | 20.75 | 75.09 | 17.00 | 247 |
5.2 Solvents for the deposition of the hole transport layer
Solvent requirements for the deposition of the hole transport layer (HTL) are quite different from those necessary for the deposition of the active material. In the conventional n–i–p device architecture, the HTL is deposited directly on the active layer. Therefore, the ideal solvent should dissolve the hole transport material (HTM) without affecting the underlying perovskite layer.As HTMs are generally neutral organic molecules/polymers, HSP can be used for solubility predictions. This approach has already been applied to fullerene derivatives and organic polymers for organic solar cells,238,250–252 although with variable results.251,253 Instead, determining HSP binary via a solvent gradient method has proven to be a powerful tool for investigating solvent systems for OSC processing.236,253 Examples of application of this method to HTMs, including spiro-OMeTAD, are reported in the literature.63,236,254–256 The existence of software specific for HSPs calculation41 might make identifying solvents and solvent mixtures suitable for HTMs deposition faster, including those that are green, by expanding the solvent database.
The main factors for selecting a HTL for PSCs are: (1) an appropriate energy level to block the electrons and promote hole transport, (2) large hole mobility, even in the absence of additives/dopants (3) high solubility in non-harmful solvents.257
Spiro-OMeTAD has historically been the most popular HTL used in conventional n–i–p architectures delivering efficient devices, with PCEs of up to 25.5%.258 Spiro-OMeTAD and similar related molecules are typically cast from chlorinated and highly aromatic solvents such as CB and DCB or in toluene which are toxic. Nevertheless, aromatic solvents with a moderately green profile exist. Among bio-renewable aromatic solvents displaying low toxicity, p-xylene and anisole have been successfully employed for PSC fabrication. Isabelli et al. in particular used the former for HTL processing, producing devices having PCE of ∼14%, essentially matching the values obtained from analogous devices processed with CB.255 Jiang et al. demonstrated that THF can be used instead of CB to obtain efficient devices (PCE of 17%),259 thus other greener ethers (such as 2-MeTHF, or 2-MA) can be studied for the deposition of spiro-OMeTAD HTL. In 2022, L. Beverina group synthesized spiro-OMeTAD by green processing route, the performance of the new spiro showed equal performance as commercial spiro-OMeTAD. It significantly reduced the overall E-factor a green chemistry metric measuring the synthesis's waste/purified product ratio, from 5299 to 555, eliminating chlorinated solvents and hazardous chemicals in the synthesis process.260
LCA studies on HTMs are limited to considering spiro-OMeTAD and CuSCN for n–i–p structures, and PEDOT:PSS for p–i–n cells. Maranghi et al. reported six different LCA studies with the cradle-to-gate approach concluding the environmental impacts of different device architectures and deposition processes.261 They realized that the environment impact of the CuSCN is smaller than that of spiro-OMeTAD. The main contributor to the high environmental impacts of spiro-OMeTAD is the energy consumption during the deposition. Furthermore, the tedious synthesis procedure followed by rigorous purification makes it expensive. Also, spiro-OMeTAD is unstable when exposed to the ambient atmosphere. This has triggered the need for identifying substitutes for spiro-OMeTAD. There are a growing number of HTMs used in PSCs, as can be seen in the following sections. The use of non-hazardous solvents for organic HTMs deposited over the perovskite layer is rarely reported. The challenging task for this is to identify solvents with which the underlying perovskite film remains undisturbed if not even aiding its crystallization. At the moment, significant variations in batch-to-batch production and lower number of purification steps required as compared to polymers promote the use of small molecules as HTMs.262 Nevertheless, more LCA studies are required to compare the environmental impact of different HTMs.
2-MA, which is used as food additives, is an excellent solvent for modified polymers with benzo[1,2-b:4,5:b′]dithiophene (BDT) backbone. Lee et al. synthesized asymmetric benzothiadiazole (BT) and BDT based polymer (asy-PBTBDT) which is highly soluble in 2-MA and displayed similar performances to conventional spiro-OMeTAD for a meso-porous n–i–p PSC.257 Also asy-PBTBDT with higher molecular weight (MW) exhibited better solubility and superior properties than lower MW asy-PBTBDT in 2-MA solvent.276 BDT based polymers with lead capturing ability due to presence of alkoxy-tetraethylene glycol chains (also improving their solubility in the green solvents 2-MA and 3-methylcyclohexanone (3-MC)) triggered development of lead leakage from perovskite lattice green fabrication systems.277 Thiophene based P3HT is extensively used in organic photovoltaics, owing to its ease of processability and tunable optical properties via varying side chains and or molecular weight.278,279 Recently, Jeong et al. identified gallium acetylacetonate-doped P3HT as HTL to improve the stability of the device (which remained stable for 2000 hours at RH of 85%). Although the efficiencies are reported to be 22.9% and 24.6% for undoped and doped P3HT HTL based devices, use of CB as solvent for P3HT is concerning and has to be addressed.280 Table 8 provides a concise overview of HTMs, solvent choices, device architectures, and key photovoltaic parameters for perovskite solar cells (PSCs).
| HTL | Solvent | Device architecture | VOC [V] | JSC [mA cm−2] | FF [%] | PCE [%] | Ref. |
|---|---|---|---|---|---|---|---|
| TPE-NMe | CB | FTO/TiO2/CH3NH3PbI3−xClx/HTM/Ag | 0.87 | 21.69 | 73 | 13.78 | 268 |
| TPE-4DPA | CB | FTO/TiO2/CH3NH3PbI3−xClx/HTM/Ag | 1.02 | 19.63 | 0.65 | 13.04 | 268 |
| Dopant-free spiro | CB | ITO/C60/MAPbI3−xClx/dopant-free spiro/MoO3/Ag | 0.99 | 20.87 | 73.83 | 15.27 | 266 |
| Dopant-free spiro | THF | ITO/C60/MAPbI3−xClx/dopant-free spiro/MoO3/Ag | 1.02 | 21.29 | 77.78 | 16.94 | 266 |
| Po-TPE-4DPA | CB | FTO/TiO2/CH3NH3PbI3−xClx/HTM/Ag | 1.02 | 19.23 | 41 | 8.08 | 267 |
| Pm-TPE-4DPA | CB | FTO/TiO2/CH3NH3PbI3−xClx/HTM/Ag | 1.07 | 20.04 | 72 | 15.44 | 267 |
| Pp-TPE-4DPA | CB | FTO/TiO2/CH3NH3PbI3−xClx/HTM/Ag | 1.03 | 19.63 | 65 | 13.04 | 267 |
| CJ-01 | CB | FTO/TiO2/perovskite/HTM/Au | 1.11 | 22.32 | 74.7 | 18.56 | 269 |
| CJ-02 | CB | FTO/TiO2/perovskite/HTM/Au | 1.06 | 18.5 | 63.5 | 12.5 | 269 |
| Spiro | CB | FTO/TiO2/perovskite/HTM/Au | 1.08 | 22.72 | 76.0 | 18.69 | 269 |
| Di-TPA | Toluene | FTO/TiO2/MAPbI3/HTM/Au | 1.03 | 20.5 | 73.3 | 15.5 | 281 |
| Tri-TPA | Toluene | FTO/TiO2/MAPbI3/HTM/Au | 1.03 | 21.4 | 74.4 | 16.4 | 281 |
| Tetra-TPA | Toluene | FTO/TiO2/MAPbI3/HTM/Au | 1.05 | 22.0 | 78.0 | 18.0 | 281 |
| CuGaO2 | Iso-proponal | FTO/c-TiO2/perovskite/CuGaO2 | 1.11 | 21.66 | 77 | 18.51 | 282 |
| Spiro | CB | FTO/c-TiO2/perovskite/spiro | 1.08 | 21.45 | 74 | 17.14 | 282 |
| DPIE | — | FTO/bl-TiO2/mp-TiO2/CH3NH3PbI3/HTL/Au | 0.85 | 15.8 | 58 | 7.75 | 283 |
| Dopant free spiro-MeOTAD | — | FTO/bl-TiO2/mp-TiO2/CH3NH3PbI3/HTL/Au | 0.86 | 14.9 | 61 | 7.87 | 283 |
| DPIO | — | FTO/bl-TiO2/mp-TiO2/CH3NH3PbI3/HTL/Au | 0.96 | 15.08 | 70 | 10.14 | 283 |
| asy-PBTBDT | 2-MA | FTO/TiO2/m-TiO2/perovskite/HTM/Au | 1.14 | 22.4 | 73.2 | 18.2 | 276 |
| asy-PBTBDT | 2-MA | ITO/PEDOT:PSS/perovskite/asy-PBTBDT/Au | 1.11 | 22.4 | 73.2 | 18.3 | 257 |
| Spiro-OMeTAD | CB | ITO/PEDOT:PSS/perovskite/spiro/Au | 1.11 | 22.1 | 74.9 | 18.5 | 257 |
| Alkoxy-PTEG | 2-MA | FTO/SnO2/perovskite/alkoxy-PTEG/Au | 1.14 | 23.2 | 79.8 | 21.2 | 277 |
| Alkoxy-PTEG | 3-MC | FTO/SnO2/perovskite/spiro-OMeTAD/Au | 1.13 | 23.3 | 75.7 | 19.9 | 277 |
| Spiro-OMeTAD | CB | FTO/SnO2/perovskite/spiro-OMeTAD/Au | 1.13 | 23.3 | 78.3 | 20.6 | 277 |
| CuSCN | Propyl-sulfide | FTO/TiO2/CH3NH3PbI3/CuSCN/Au | 1.02 | 19.2 | 58 | 11.4 | 284 |
| CuSCN | Propyl-sulfide | FTO/bl-TiO2/m-TiO2/CH3NH3PbI3/CuSCN/Au | 0.92 | 18.7 | 56 | 9.79 | 285 |
| CuSCN | Propylsulfide + IPA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) + MAI (10 mg ml−1) 2) + MAI (10 mg ml−1) |
FTO/bl-TiO2/m-TiO2/CH3NH3PbI3/CuSCN/Au | 0.92 | 19.4 | 56 | 10.07 | 285 |
| CuI | ACN | FTO/TiO2/CH3NH3PbI3/CuI/Au | 0.73 | 32.72 | 31 | 7.4 | 286 |
| Cu2O | Evaporation | FTO/TiO2/CH3NH3PbI3−xClx/Cu2O/Au | 15.8 | 0.96 | 59 | 8.93 | 286 |
| Spiro-OMeTAD | CB | FTO/TiO2/CH3NH3PbI3−xClx/spiro-OMeTAD/Au | 17.0 | 0.99 | 68 | 11.5 | 286 |
| PEDOT:PS | CB | FTO/PEDOT:PS/CH3NH3PbI3/PCBM/BCP/Ag | 0.91 | 20.0 | 81.0 | 14.8 | 287 |
| Spiro-OMeTAD | THF | Glass/ITO/C60/MAPbI3−xClx/spiro-OMeTAD/MoO3/Ag | 1.023 | 21.29 | 77.78 | 16.94 | 266 |
| NiOx | H2O | Glass/ITO/NiOx/perovskite/PCBM/ZnO/Al | 1.00 | 20.80 | 84 | 17.3 | 240 |
| NiOx | H2O | PEN/ITO/NiOx/perovskite/PCBM/ZnO/Al | 1.02 | 20.6 | 73.0 | 15.3 | 240 |
| NiOx | IPA | ITO/NiOx/DEA/CH3NH3PbI3/C60(CH2)(Ind)/PN4N/Ag | 1.13 | 20.4 | 80.0 | 18.1 | 288 |
| NiOx | — | Glass/ITO/NiOx@NaCl/perovskite/PCBM/ZrAcac | 1.14 | 22.83 | 79.6 | 20.71 | 288 |
| NiOx | — | Glass/ITO/NiOx@KCl/perovskite/PCBM/ZrAcac | 1.15 | 22.89 | 79.5 | 20.96 | 288 |
| NiOx | EtOH | ITO/NiOx/MSs/perovskite/PC61BM/BCP/Ag | 1.12 | 22.34 | 80.8 | 20.34 | 289 |
| PTAA | Toluene | Glass/FTO/C–TiO2/m-TiO2/PVSK/PTAA/Au | 0.91 | 19.30 | 70.20 | 12.3 | 290 |
| PTAA | Toluene | Glass/FTO/C–TiO2/m-TiO2/PVSK/PTAA/Au | 0.99 | 16.5 | 72.7 | 12.0 | 291 |
| PTAA | Toluene | Glass/FTO/C–TiO2/m-TiO2/PVSK/PTAA/Au | 1.06 | 24.7 | 77.50 | 20.1 | 292 |
Copper oxides are yet another p-type semiconductor, mostly used in p–i–n PSCs with organic ETLs. A report suggested solvent free sputtering for deposition of high quality Cu2O as HTM. The device delivered an efficiency of 9%.293 When it comes to solution processing, aqueous solutions of nitrate/iodine/copper are coated followed by diluted NaOH treatment and methanol wash.287,294 Sun et al. achieved a PCE of 17.1% in PSCs by optimizing the spin-coating deposition of CuOx film using 1,2-dichlorobenzene as the solvent, suggesting potential for further improvement.295
Nickel oxide is a versatile inorganic HTM for PSCs owing to its flexibility in being deposited with either chemical or physical methods. Similarly, to CuOx, NiOx is obtained by reacting nitrates, acetates, or halide salts of nickel with NaOH or certain amines in polar solvents like water or ethanol. Once the process yields NiOx nanoparticles, the particles are dispersed in suitable green solvents, usually water of ethanol and are either spin-coated or printed. Otherwise, electro-deposition, RF sputtering, e-beam evaporator, pulsed laser deposition (PLD) are alternative advanced deposition techniques for NiOx that have been reported. In p–i–n solar cells, with NiOx as HTL, efficiencies of over 20% have been reached.289,292,296 Xie et al. spray coated a nickel precursor in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ACN and ethanol, to manufacture a FTO/NiOx/FAPbI3/PCBM/TiOx/Ag device with a PCE of 20%.297 Chang et al. fabricated a PSC with a low-cost interface layer material (18–22 USD per g), MS-OC in NiOx based HTL gave a promising efficiency of 20.34%.289
1 ACN and ethanol, to manufacture a FTO/NiOx/FAPbI3/PCBM/TiOx/Ag device with a PCE of 20%.297 Chang et al. fabricated a PSC with a low-cost interface layer material (18–22 USD per g), MS-OC in NiOx based HTL gave a promising efficiency of 20.34%.289
In summary, although dopant-free organic HTMs have attracted more attention in recent years, researchers have rarely reported processing these materials in green solvents. As an alternative, inorganic HTMs offer advantages of choice of processing, deposition, and solvents. NiOx based inorganic HTL is a good choice among all other HTLs regarding its stability and green processing.
6. Electrode materials
Selection criteria for top electrodes in perovskite solar cells generally focus on electrical conductivity and stability.298 For industrialization purposes cost and sustainability become also fundamental.6.1 Top metallic electrodes
Au can still be considered as the metal of choice for PSCs. Considering the environmental impact of acquiring, processing and discarding (if not recovered) the metal, makes the use of Au a concern.299 In fact, a LCA study carried out by Gong and co-workers revealed that gold electrodes contribute to more than half of both prime energy consumption and carbon footprint of raw material production of perovskite solar modules.239 The extraction and purification process of gold requires a great deal of energy and hence is expensive. Moreover, the process involves toxic chemicals such as mercury and cyanide.300 It has been shown that Au can be replaced by other metals such as Ag or Al, which would reduce energy consumption related to the material but are far from ideal choice especially because they are known to diffuse through the transport layers and interact with the perovskite semiconductor and lead to its degradation. In p–i–n, people often use bathocuproine (BCP) to avoid metals diffusion and also BCP cast from the green solvents.301 Introduction of MoOx/Al as back contact has resulted in around a 9% reduction in energy demand as well as global warming potential when compared to silver.302 Alternative choices for metallic top electrodes such as Cu, Ni, W, and Mo, have been explored by Wang et al.298 These materials were deposited by sputter coating and the energy requirement for these materials is less in comparison with metals discussed earlier. There have been notable attempts to identify a stable top electrode material that can be processed at lower energy.Carbon black, graphite/amorphous carbon, graphene, and carbon nanotubes are the most frequently used carbon materials in carbon-PSCs, though their preparation processes can be complex and expensive. As a result, bio-based carbon derived from various types of biomass waste has emerged as a promising carbon catalyst due to its abundance and ease of accessibility. In 2014 the porous carbon with a high surface area, which is prepared by pyrolysis and chemical activation of rice husk, is being examined as a counter electrode in dye-sensitized solar cells by Wang et al. A dye-sensitized solar cells (DSSCs) with this porous carbon counter electrode exhibited a conversion efficiency of 6.32%, which is similar to that of a cell with a Pt electrode (6.69%).312 These encouraging results with rice huck porous carbon opened a new door to apply a potential electrode for DSSCs. Later in 2017 Yu et al. created bio-based porous carbon from quince leaves as a catalyst for DSSC fabrication and achieved a high PCE of 5.52%.313
The first report on the use of bio-based carbon as electrodes in PSCs (perovskite solar cells) was published by Mali et al. in 2018.315 They introduced an aloe-vera processed carbon electrode made from naturally extracted cross-linked carbon nanoparticles, traditionally used in ancient Indian process, for mesoscopic perovskite solar cells. In the experiment as shown the Fig. 15a, the aloe vera gel was first extracted from the leaves and dried in sunlight for one day (Step I). Next, the dried gel was heated overnight using an oil lamp (Step II), which converted it into a black powder. The black powder was then scraped from a SS (stainless steel) substrate and ground using a ball mill. The fine activated carbon (AV-C) powder was washed with 2 M HCl and annealed in an argon atmosphere at 1000 °C. The carbon paste was coated by mixing the powder with chlorobenzene solvent (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 wt%/vol%) using screen-printing technique. Resulting more than 1000 hours stable PSC with PCE of 12.58%. In 2020 Gao et al. prepared bio-carbons using four different types of biomasses bamboo chopsticks (BC-B), peanut shell bio-carbon (PS-B), corn stalk (CS-B), and phragmites australis (PA-B) bio-carbons have been selected.314 It has been observed that the open-circuit voltage (Voc) loss in the J–V curve was primarily determined by the energy level alignment between PVK and bio-carbon CEs, as shown in Fig. 15b as a result Voc of the devices decreased with the energy level mismatch. The PSCs based on BC-B CEs had the optimal energy band and highest Voc when compared to the others. Which resulting ultra-low cost shown in Table 9 bio-carbon materials are significantly cheaper than other commercially available carbon materials and cost compared to other carbon-based materials as they are three orders of magnitude cheaper than the least expensive carbon material. Which resulting ultra-low cost BC-B CEs with 2000 hours stable PCE of 12.82%. In 2022 Xie et al. reported, a N, O co-doped porous composite carbon electrode was prepared using a spraying method. The electrode, named N-KSDC, was made from KOH-activated soybean dregs, conductive carbon black, and polymethylmethacrylate.316 This method was employed to enhance the interface qualities between the perovskite and carbon and improve the efficiency and stability of C–PSCs. The best power conversion efficiencies of N-KSDC-based C–PSCs were 13.45% and 11.08% for active areas of 0.08 cm2 and 1 cm2, respectively.
2 wt%/vol%) using screen-printing technique. Resulting more than 1000 hours stable PSC with PCE of 12.58%. In 2020 Gao et al. prepared bio-carbons using four different types of biomasses bamboo chopsticks (BC-B), peanut shell bio-carbon (PS-B), corn stalk (CS-B), and phragmites australis (PA-B) bio-carbons have been selected.314 It has been observed that the open-circuit voltage (Voc) loss in the J–V curve was primarily determined by the energy level alignment between PVK and bio-carbon CEs, as shown in Fig. 15b as a result Voc of the devices decreased with the energy level mismatch. The PSCs based on BC-B CEs had the optimal energy band and highest Voc when compared to the others. Which resulting ultra-low cost shown in Table 9 bio-carbon materials are significantly cheaper than other commercially available carbon materials and cost compared to other carbon-based materials as they are three orders of magnitude cheaper than the least expensive carbon material. Which resulting ultra-low cost BC-B CEs with 2000 hours stable PCE of 12.82%. In 2022 Xie et al. reported, a N, O co-doped porous composite carbon electrode was prepared using a spraying method. The electrode, named N-KSDC, was made from KOH-activated soybean dregs, conductive carbon black, and polymethylmethacrylate.316 This method was employed to enhance the interface qualities between the perovskite and carbon and improve the efficiency and stability of C–PSCs. The best power conversion efficiencies of N-KSDC-based C–PSCs were 13.45% and 11.08% for active areas of 0.08 cm2 and 1 cm2, respectively.
| Materials | Supplier | Price ($ per kg) |
|---|---|---|
| a The corresponding calculation of bio-carbon cost was shown in ref. 314.b Carbon paste from DYCOTEC added to original table. | ||
| Graphene | Sigma-Aldrich | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 271 271![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 660.01 660.01 |
| Carbon nanotubes | XFNano | 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 030.38 030.38 |
| Fullerene-C60 | Alfa Aesar | 421![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 204.57 204.57 |
| Fullerene-C70 | Alfa Aesar | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 929 929![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 026.52 026.52 |
| Carbon paste | DYCOTEC | 399.10b |
| Carbon black | Alfa Aesar | 227.15 |
| Graphite powder | Sigma-Aldrich | 78.16 |
| Bio-carbon | Waste recycling | 0.49a |
Pitchaiya et al. used graphitic carbon extracted from a plant species called Eichhornia crassipes, which served as both hole transporter and top electrode for the device.317 Properties of graphitic carbon extracted from the invasive plant species Eichhornia crassipes shown Fig. 15c that the XRD analysis of the graphitic carbon-encapsulated perovskite thin film (GC@CH3NH3PbI3−xClx) revealed the presence of tetragonal perovskite structure with peaks at 2θ = 13.97°, 28.32°, 31.78°, and 40.39°, and hexagonal graphitic carbon at 2θ = 26.12°, seen in the apical sites of the perovskite structure. Fig. 15d The FESEM image in Fig. 1b reveals a coagulated island-like structure, which is likely the result of strong coordination between Pb2+ ions and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group found in porous graphitic carbon. This observation supports the formation mechanism of the GC@CH3NH3PbI3−xClx structure that is encapsulated. Fig. 15e and f high-resolution TEM images reveal perovskite crystals in a darker region, which are trapped inside capsules of graphitic carbon (appearing in lighter contrast). Resulting devices shown 20% PCE under 200 lux of light and device also had a maximum PCE of 6.32% under 1 Sun illumination.
O group found in porous graphitic carbon. This observation supports the formation mechanism of the GC@CH3NH3PbI3−xClx structure that is encapsulated. Fig. 15e and f high-resolution TEM images reveal perovskite crystals in a darker region, which are trapped inside capsules of graphitic carbon (appearing in lighter contrast). Resulting devices shown 20% PCE under 200 lux of light and device also had a maximum PCE of 6.32% under 1 Sun illumination.
In conclusion, various bio-based carbon materials have been successfully prepared from biomass waste such as aloe-vera peel, fallen quince leaves, rice husk, bamboo chopsticks, peanut shell bio-carbon, corn stalk, and phragmites australis, Eichhornia crassipes, and soybean dregs. These bio-based carbon materials have been effectively utilized as superior ultra-low cost counter electrode catalysts for DSSCs and PSCs. The stability of bio-carbon based perovskite solar cells is an active area of research. However, initial studies have shown promising results in terms of stability, with some bio-carbon based perovskite solar cells showing good stability under various environmental conditions such as high humidity and heat. However, long-term stability and durability of these cells still need to be studied more extensively. Additionally, the stability of bio-carbon based perovskite solar cells also depend on the method of preparation, properties of bio-carbon materials, and device architecture. Therefore, more research is needed to fully understand the stability of bio-carbon based perovskite solar cells and to improve their performance and durability. The PCE of bio-carbon based perovskite solar cells is an active area of research. Bio-carbon based perovskite solar cells have shown promising results in terms of PCE. Above studies have reported PCE reach nearly 13%, however, it's worth to mention that the PCE of bio-carbon based perovskite solar cells can vary depending on the method of preparation, properties of bio-carbon materials, and device architecture. Additionally, it's important to note that the PCE is not the only metric to evaluate the performance of a solar cell, other parameters such as stability, durability, cost, and scalability also play an important role. Therefore, more research is needed to improve the PCE, and overall performance of bio-carbon based perovskite solar cells.
7. General directions from life cycle assessment of PSCs
Life cycle assessment (LCA) is a standard procedure to estimate and assess the environmental impacts of manufactured products, processes, or services throughout their lifetime.45 We have included LCA information for the different layers used in perovskite cell fabrication. Here we touch open broader aspects. In the assessments of devices like solar cells, environmental impacts can be evaluated from the first stage of raw material extraction and processing (cradle) to the manufacturing stage, consumer usage, and finally, the disposal or recycling stage (grave). The environmental footprints are estimated by considering different factors such as greenhouse gas emissions, consumed energy, used materials, waste, human toxicity, environmental toxicity, etc. Functional units such as kW h of generated electricity or constant module area are used to provide consistent comparisons of solar modules. Several studies performed LCA on PSCs and modules.158,196,239,302,324–330 These studies can be helpful in the comparison of different perovskite device architectures as well as perovskite modules with other photovoltaic technologies to point researchers toward more environmentally friendly solutions.Leccisi et al. compared the electricity consumption of perovskite solar cells with FTO/SnO2/MAPbI3/CuSCN/MoOx/Al with three different fabrication methods of gravure printing (GP), spray coating (SC), and vapor deposition (VD) (Fig. 16a–c). They studied all the layers including transparent conductive layer (TCL), ETL, perovskite absorber layer, HTL, and back contact layer (BCL). A comparison between these methods revealed that roll-to-roll (R2R) printing with GP method has the lowest environmental impact.302 In the spray-coated PSC the deposition of the front and back electrode, assumed to be deposited via vacuum-based techniques, contribute about 88% to the total required energy. R2R fabrication, which assumes that also the electrodes are deposited via solution processing, is expected to lower the energy consumption of manufacturing substantially from 21 kW h m−2 to 7.5 kW h m−2. The latter is around 22% of that of vapor deposited devices (33.8 kW h m−2) according to the same study. It is apparent that vapor deposition increases energy consumption. The difference is much greater compared to studies by Serrano-Lujan et al.,196 Espinosa et al.,329 and Celik et al.324 for which the estimated lowering in direct electricity inputs (kW h m−2) for thermally evaporated cell manufacturing to solution processing were around 26%, 26%, 8% respectively.331 Such a notable dissimilarity needs further detailed investigation. Use of flexible substrate also reduces the total mass of devices up to 97%.319 Finally, sometimes overlooked in studies, due to stability issues, PSCs require encapsulants which can be glass for rigid modules to more complex multilayer barriers for flexible cells.332 Encapsulation can contribute between 37% and 61% of the total energy footprint302 which is a significant number. Of course, the more stable the intrinsic material of the solar cells, then less sophisticated solutions for encapsulation required, especially for flexible devices.332
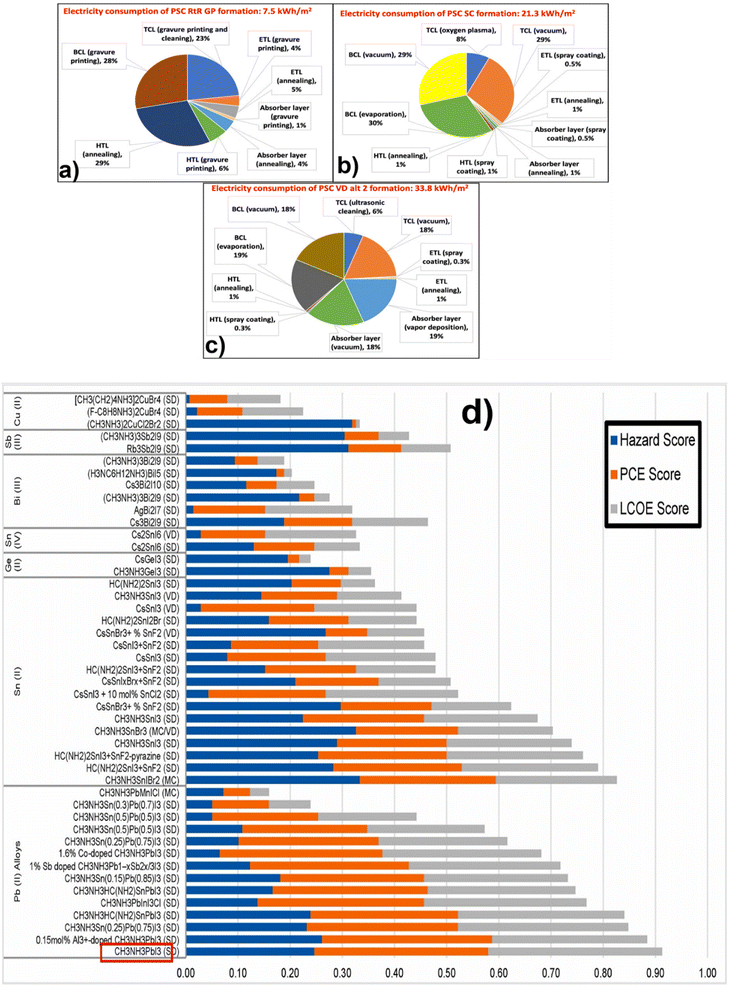 | ||
| Fig. 16 (a–c) Electricity consumption breakdown for the fabrication of a typical perovskite solar cell (b) with roll-to-roll fabrication method, (c) with sheet-to-sheet solution-processed method, (d) with vapor deposition method. Reproduced with permission from ref. 302. (d) Alternative perovskite composition ranking. The closer a perovskite's summed score is to 1, the better its aggregated performance.333 SD = Solution deposition, MC = Mechanochemical deposition, VD = Vapor deposition, RtR = Roll-to-roll, GP = Gravure printing, SC = Spray Coating, TCL = Transparent conductive layer, BCL = Back contact layer, ETL = Electron transport layer, HTL = Hole transport layer. | ||
Alberola-Borra et al. investigated HTM-free PSCs with screen printed mesoporous TiO2/ZrO2 ETL and carbon electrode to reach a conclusion about pre-industrial PSCs.336 They measured different categories, namely abiotic depletion (ADP), abiotic depletion-fossil fuels (ADPF), GWP, ozone layer depletion (ODP), photochemical oxidation (POP), acidification (AP), cumulative energy demand (CED), human toxicity-cancer effects (HTC), human toxicity-non cancer effects (HTNC), and fresh water ecotoxicity (FET). According to their research, in all categories except for POP the perovskite layer has a significant percentage of impact together with other layers. The preparation and annealing of the perovskite layer was responsible for the high impact of this layer rather than its Pb content. Thus, the optimization of the deposition of the perovskite film could be an effective route for reducing environmental impact of PSCs.
Using the ‘USEtox’ method, in 2021 Vidal et al. assessed human health and environmental impacts of 8 solvents used in the deposition of the PSCs.113 According to their estimations, to produce 1 GW power from PSCs with 15% of efficiency, 3500 litres of solvents are required. They concluded that use of DMSO has lowest human health toxicity and environmental impact expressed in DALYs per kg of a substance emitted for the scenario of emission to urban air among DMF, DMAc, NMP, THF, DMEU, GBL, and DMPU solvents.
To compare PVMs to other PV technologies, a practical approach is to estimate the energy payback time (EPBT), which is the time that it takes solar modules to generate enough energy to surpass the energy required to produce and install them. Solution-processing and low-temperature fabrication lead to lower EPBT of perovskite solar modules than other PV technologies.239,241,302,324 The estimated EPBT of PVMs varies from study to study depending on factors such as efficiency and lifespan. One study revealed EPBT of 0.3–0.4 years for single-junction perovskite with 19.3% PCE and all-perovskite tandem solar cells with a PCE of 28%.302 This EPBT is about 0.9 years for sc-Si PV with 20% PCE and 0.7 years for 2-terminal Si-perovskite tandems with 28% PCE. Taking into account cradle to gate LCA studies (from cradle to exiting the factory gate), PSCs can be considered greener than silicon PVs.329 However, when cradle to grave studies are taken into account, because of the shorter lifetime of PVMs (1–5 years), their environmental impacts are estimated to be higher than current commercial PV technologies that last more than 20 years.239,241,324 Vidal et al. collected the global warming potential (GWP) and cumulative energy demand (CED) resulting from different LCA studies of single-junction and tandem PSCs.334 GWP represents the equivalent mass of CO2 emission in kg unit, and CED is the sum of direct and indirect energy usage during the life cycle of products. For 1 kW power production, the mean values of GWP and CED were 2000 kg CO2 eq and 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 MJ for single-junction PSCs whereas for tandem were 2942 kg CO2, 13
000 MJ for single-junction PSCs whereas for tandem were 2942 kg CO2, 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 744 MJ.334 These mean values are lower than those of other PV technologies, but the range of this data is broad due to the lack of real manufacturing data for the perovskite technology. Llanos et al.333 and the U.S. National Research Council (NRC) studied 45 perovskite materials and their fabrication processes according to human and ecological hazards, efficiency, and cost analysis to find safer materials and solvents. As shown in Fig. 16d, perovskite materials, including lead-free alternatives, and their deposition technique were ranked 0 to 1.333 The scores involve hazards, power conversion efficiency (PCE), and the Levelized cost of electricity (LCOE). If the score is close to 1, it represents the better choice of materials and deposition method. This study concluded that the mechanochemical deposited lead-free CH3NH3SnIBr2 would be the most suitable perovskite material considering the combination of the above factors. Additionally, it states that among all lead-based perovskite materials, CH3NH3PbI3 (SD) is the better option. This suggests that these specific materials have been found to have the highest performance in terms of hazard, PCE, and LCOE among their respective groups.
744 MJ.334 These mean values are lower than those of other PV technologies, but the range of this data is broad due to the lack of real manufacturing data for the perovskite technology. Llanos et al.333 and the U.S. National Research Council (NRC) studied 45 perovskite materials and their fabrication processes according to human and ecological hazards, efficiency, and cost analysis to find safer materials and solvents. As shown in Fig. 16d, perovskite materials, including lead-free alternatives, and their deposition technique were ranked 0 to 1.333 The scores involve hazards, power conversion efficiency (PCE), and the Levelized cost of electricity (LCOE). If the score is close to 1, it represents the better choice of materials and deposition method. This study concluded that the mechanochemical deposited lead-free CH3NH3SnIBr2 would be the most suitable perovskite material considering the combination of the above factors. Additionally, it states that among all lead-based perovskite materials, CH3NH3PbI3 (SD) is the better option. This suggests that these specific materials have been found to have the highest performance in terms of hazard, PCE, and LCOE among their respective groups.
To conclude, LCA studies demonstrate that apart from material selection, the key to reducing the environmental footprint of PVMs is not only increasing the PCE but also simplifying the fabrication process and obtaining long-term stability.
8. Conclusion and outlook
In this review, we started by discussing solvents that can be considered “green”. Selection guides such as GSK and CHEM21 helped categorize solvents. Together with Safety (S), Health (H), Environment (E) scores, it is clear that the worst solvents for the perovskite layer are DMF, DMAc, NMP whereas DMSO and ACN fare better, with IPA and water the greenest alternatives already used for some precursors (Pb (NO3)2). For antisolvents, ethyl acetate and anisole are green solvents that have been used to engineer high quality polycrystalline films with cell efficiencies surpassing the 20% efficiency threshold. LCA studies using the ‘USEtox’ method concluded that the use of DMSO has the less human health toxicity and environmental impact. Further discussed is a new set of solvents called neoteric solvents such as ionic liquids (ILs), methylammonium carboxylates (such as formate, acetate, or propionate) in the preparation of the perovskite layer. In this case, liquid salts don't act as solvents as the methylammonium cations are also incorporated in the final perovskite. ILs are considered promising alternative green solvents to traditional polar aprotic solvents to produce efficient and stable PSCs (e.g. cells with FAPbI3 combined with methylammonium formate have reached PCEs of 24.1%). Additionally, perovskite films prepared by ILs don't need antisolvent treatments, and films deposited in ambient environments are more durable making the manufacturing of PSCs simpler in the industries.Solvents are important aspects, but also the perovskite composition must be considered for environmental and safety concerns, trying to either deal with the Pb content, e.g., by lead sequestration/encapsulation strategies, or by synthesizing lead-free perovskites. Bi and Sb are less-toxic alternatives to Pb. However, less toxicity alone does not make a perovskite material superior to lead-based ones. Due to its abundant supply, low price, and global production, Pb has a competitive advantage over Sb and Bi when it comes to production. The efforts to fabricate lead-free perovskites solar cells have proven that no best alternative material can replace the lead completely at the moment. In terms of abundance, cost, global production, global warming potential, and efficiency, Pb is currently the right choice of material. The international community here must continue to work on the containment and replacement of Pb as well as liaise with regulatory bodies to understand how/if Pb-containing perovskite solar panels are able to be deployed for future commercialization.
Among the electron transport layer materials SnO2 with a record efficiency of 25.7% is currently considered the best option for low-temperature processing, for compatibility with flexible substrates and for being cast from water-based inks. The choice for hole transport material is less straightforward since most of them are organic with concerns over their stability. Currently, research is going on testing different interface layers with HTLs for stabilization. Inorganic NiOx with efficiencies of over 20% have also been proven to be a good HTL. Easy processing Inorganic HTL NiOx in p–i–n structures may be a better choice when compared to spiro and PTAA in n–i–p structures. Also, NiOx is cast from green solvents like water, IPA, and ethanol.
Currently, many research groups use expensive Au, Ag, and Al top electrodes. Gold electrodes can contribute to more than 50% of prime energy consumption and carbon footprint in the fabrication of perovskite solar modules. Metallic top electrodes such as Cu, Ni, W, and Mo, have been explored, but are still sputtered or evaporated as well as less stable than Au. Carbon-based electrodes are one of the best electrode materials considering both stability and environmental concerns. Solvents and binders used for processing carbon materials like-ethanol, ethyl acetate, terpineol, cellulose, organic acetates, and IPA are non-toxic. Carbon is deposited by solution-processed methods such as screen printing and spray coating, which reduces the embedded energy of perovskite solar modules. For instance, deposition of carbon front contacts by spray coating leads to 35% less process energy consumption than evaporated MoOx/Al electrode. Further research into this field should close the efficiency gap that still exists compared to devices with Au electrodes. Recent developments in bio-carbons produced from biomass waste such as aloe-vera peel, fallen quince leaves, rice husk, bamboo chopsticks, peanut shell bio-carbon, corn stalk, and phragmites australis, Eichhornia crassipes, and soybean dregs further reduces the cost of carbon electrodes.
According to the life cycle assessment of a typical glass/FTO/TiO2/spiro-OMeTAD/Au perovskite solar cell architecture, FTO preparation accounts for 28% of device fabrication energy and 44% of embedded material energy. The presence of tin in ITO and FTO presents toxicity and supply concerns. Using aluminum-doped zinc oxide (AZO) as front contact is a prospective replacement with less environmental impact. Glass accounts for around 98% of the total mass of PSCs because of its relatively higher thickness than other layers. To reduce the embedded energy of the substrate, alternatives such as flexible PET, PEN, paper, and cellulose-based substrates can be considered. Plastic based substrates have surpassed the 21% efficiency threshold with exceptional power-to-weight ratios. However flexible technology can only be considered for products which do not require long lifetimes, until the performance and cost of encapsulation and intrinsic materials enable long term use. More research should be funneled for this aim.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support from the Italian Ministry of University and Research (MIUR) through the PRIN2017 BOOSTER (project no. 2017YXX8AZ) grant is gratefully acknowledged.References
- D. B. Mitzi, Prog. Inorg. Chem., 1999, 1–121 CAS
.
- A. Kojima, K. Teshima, Y. Shirai and T. Miyasaka, J. Am. Chem. Soc., 2009, 131, 6050–6051 CrossRef CAS PubMed
.
- N. J. Jeon, H. Na, E. H. Jung, T.-Y. Yang, Y. G. Lee, G. Kim, H.-W. Shin, S. Il Seok, J. Lee and J. Seo, Nat. Energy, 2018, 3, 682–689 CrossRef CAS
.
- M. A. Green, E. D. Dunlop, J. Hohl-Ebinger, M. Yoshita, N. Kopidakis, K. Bothe, D. Hinken, M. Rauer and X. Hao, Prog. Photovoltaics, 2022, 30, 687–701 Search PubMed
.
- G. W. Kim and A. Petrozza, Adv. Energy Mater., 2020, 10, 2001959 CrossRef CAS
.
- G. Giorgi and K. Yamashita, J. Mater. Chem. A, 2015, 3, 8981–8991 RSC
.
- B. Saparov and D. B. Mitzi, Chem. Rev., 2016, 116, 4558–4596 CrossRef CAS PubMed
.
- J. S. Manser, J. A. Christians and P. V Kamat, Chem. Rev., 2016, 116, 12956–13008 CrossRef CAS PubMed
.
- C. C. Stoumpos and M. G. Kanatzidis, Adv. Mater., 2016, 28, 5778–5793 CrossRef CAS PubMed
.
- A. Singh, M. K. Jana and D. B. Mitzi, Adv. Mater., 2021, 33, 2005868 CrossRef CAS PubMed
.
- X. Li, B. Li, J. Chang, B. Ding, S. Zheng, Y. Wu, J. Yang, G. Yang, X. Zhong and J. Wang, ACS Appl. Energy Mater., 2018, 1, 2709–2716 CrossRef CAS
.
- A. M. Elseman, A. E. Shalan, S. Sajid, M. M. Rashad, A. M. Hassan and M. Li, ACS Appl. Mater. Interfaces, 2018, 10, 11699–11707 CrossRef CAS PubMed
.
- E. Greul, P. Docampo and T. Bein, Z. Anorg. Allg. Chem., 2017, 643, 1704–1711 CrossRef CAS
.
- M. Petrović, T. Maksudov, A. Panagiotopoulos, E. Serpetzoglou, I. Konidakis, M. M. Stylianakis, E. Stratakis and E. Kymakis, Nanoscale Adv., 2019, 1, 3107–3118 RSC
.
- S. Wang, A. Cabreros, Y. Yang, A. S. Hall, S. Valenzuela, Y. Luo, J. P. Correa-Baena, M. cheol Kim, Ø. Fjeldberg, D. P. Fenning and Y. S. Meng, Cell Rep. Phys. Sci., 2020, 1, 100103 CrossRef
.
- A. Singh, A. S. Chouhan and S. Avasthi, Mater. Res. Express, 2019, 6, 085519 CrossRef CAS
.
- K. L. Gardner, J. G. Tait, T. Merckx, W. Qiu, U. W. Paetzold, L. Kootstra, M. Jaysankar, R. Gehlhaar, D. Cheyns, P. Heremans, J. K. P. L. Gardner, J. G. Tait, T. Merckx, W. Qiu, U. W. Paetzold, L. Kootstra, M. Jaysankar, R. Gehlhaar, D. Cheyns, P. Heremans, J. Poortmans and K. L. Gardner, Adv. Energy Mater., 2016, 6, 1–8 Search PubMed
.
- M. Zhang, D. Xin, X. Zheng, Q. Chen and W. H. Zhang, ACS Sustainable Chem. Eng., 2020, 8, 13126–13138 CrossRef CAS
.
- G. Ding, Y. Zheng, X. Xiao, H. Cheng, G. Zhang, Y. Shi and Y. Shao, J. Mater. Chem. A, 2022, 10, 8159–8171 RSC
.
- M. T. Hoang, F. Ünlü, W. Martens, J. Bell, S. Mathur and H. Wang, Green Chem., 2021, 23, 5302–5336 RSC
.
- H.-S. Kim, Y.-J. An, J. I. Kwak, H. J. Kim, H. S. Jung and N.-G. Park, ACS Energy Lett., 2022, 7, 1154–1177 CrossRef CAS
.
- European Chemicals Agency (ECHA), https://echa.europa.eu/, accessed November 16, 2020.
- M. Abraham, Encyclopedia of Sustainable Technologies, Elsevier, 1st edn, 2017 Search PubMed
.
- F. G. Calvo-Flores, M. J. Monteagudo-Arrebola, J. A. Dobado and J. Isac-García, Top. Curr. Chem., 2018, 376, 18 CrossRef PubMed
.
- C. Capello, U. Fischer and K. Hungerbühler, Green Chem., 2007, 9, 927–934 RSC
.
- H. C. Erythropel, J. B. Zimmerman, T. M. de Winter, L. Petitjean, F. Melnikov, C. H. Lam, A. W. Lounsbury, K. E. Mellor, N. Z. Janković, Q. Tu, L. N. Pincus, M. M. Falinski, W. Shi, P. Coish, D. L. Plata and P. T. Anastas, Green Chem., 2018, 20, 1929–1961 RSC
.
- https://www.gsk.com/en-gb/, accessed August 21, 202.
- https://www.pfizer.com, accessed August 21, 2022.
- https://www.astrazeneca.com/, accessed August 21, 2022.
- https://www.sanofi.com/, accessed August 21, 2022.
- D. Prat, J. Hayler and A. Wells, Green Chem., 2014, 16, 4546–4551 RSC
.
- S. Li, H. Zhang, W. Zhao, L. Ye, H. Yao, B. Yang, S. Zhang and J. Hou, Adv. Energy Mater., 2016, 6, 1501991 CrossRef
.
- D. Prat, A. Wells, J. Hayler, H. Sneddon, C. R. McElroy, S. Abou-Shehada and P. J. Dunn, Green Chem., 2016, 18, 288–296 RSC
.
- Dihydrolevoglucosenone, American Chemical Society, https://www.acs.org/content/acs/en/molecule-of-the-week/archive/d/dihydrolevoglucosenone.html, accessed May 3, 2022 Search PubMed.
- P. Pollet, E. A. Davey, E. E. Ureña-Benavides, C. A. Eckert and C. L. Liotta, Green Chem., 2014, 16, 1034–1055 RSC
.
- http://chem21.eu/, accessed July 26, 2022.
- V. Pace, P. Hoyos, L. Castoldi, P. Domínguez de María and A. R. Alcántara, ChemSusChem, 2012, 5, 1369–1379 CrossRef CAS PubMed
.
- D. M. Alonso, S. G. Wettstein and J. A. Dumesic, Green Chem., 2013, 15, 584 RSC
.
- J. Sherwood, M. De bruyn, A. Constantinou, L. Moity, C. R. McElroy, T. J. Farmer, T. Duncan, W. Raverty, A. J. Hunt and J. H. Clark, Chem. Commun., 2014, 50, 9650–9652 RSC
.
- C. J. Clarke, W.-C. Tu, O. Levers, A. Bröhl and J. P. Hallett, Chem. Rev., 2018, 118, 747–800 CrossRef CAS PubMed
.
- HSPiP | Hansen Solubility Parameters, https://www.hansen-solubility.com/HSPiP/, accessed November 2, 2021 Search PubMed.
- D. J. Heldebrant, H. N. Witt, S. M. Walsh, T. Ellis, J. Rauscher and P. G. Jessop, Green Chem., 2006, 8, 807–815 RSC
.
- Y. Dai, J. van Spronsen, G.-J. Witkamp, R. Verpoorte and Y. H. Choi, Anal. Chim. Acta, 2013, 766, 61–68 CrossRef CAS PubMed
.
- R. Sheldon, Chem. Commun., 2001, 2399–2407 RSC
.
- C. Zhang, B. Wang, W. Zheng, S. Huang, L. Kong, Z. Li, G. He and L. Li, Nano Energy, 2018, 51, 358–365 CrossRef CAS
.
- C. Reichardt, Chem. Rev., 1994, 94, 2319–2358 CrossRef CAS
.
- A. Babaei, L. Albero-Blanquer, A. M. Igual-Muñoz, D. Pérez-Del-Rey, M. Sessolo, H. J. Bolink and R. Tadmouri, Polyhedron, 2018, 147, 9–14 CrossRef CAS
.
- S. Venkatram, C. Kim, A. Chandrasekaran and R. Ramprasad, J. Chem. Inf. Model., 2019, 59(10), 4188–4194 CrossRef CAS PubMed
.
- M. J. Louwerse, A. Maldonado, S. Rousseau, C. Moreau-Masselon, B. Roux and G. Rothenberg, ChemPhysChem, 2017, 18, 2999–3006 CrossRef CAS PubMed
.
- J.-W. Lee, H.-S. Kim and N.-G. Park, Acc. Chem. Res., 2016, 49, 311–319 CrossRef CAS PubMed
.
- N. Ahn, D.-Y. Son, I.-H. Jang, S. M. Kang, M. Choi and N.-G. Park, J. Am. Chem. Soc., 2015, 137, 8696–8699 CrossRef CAS PubMed
.
- J. Stevenson, B. Sorenson, V. H. Subramaniam, J. Raiford, P. P. Khlyabich, Y.-L. Loo and P. Clancy, Chem. Mater., 2016, 29, 2435–2444 CrossRef
.
- J. C. Hamill, J. Schwartz and Y.-L. Loo, ACS Energy Lett., 2017, 3, 92–97 CrossRef
.
- C. Laurence, J. Graton and J.-F. Gal, J. Chem. Educ., 2011, 88, 1651–1657 CrossRef CAS
.
- M. J. Kamlet, J. L. M. Abboud, M. H. Abraham and R. W. Taft, J. Org. Chem., 1983, 48, 2877–2887 CrossRef CAS
.
- Y. Marcus, Chem. Soc. Rev., 1993, 22, 409 RSC
.
- P. G. Jessop, D. A. Jessop, D. Fu and L. Phan, Green Chem., 2012, 14, 1245 RSC
.
- X. Huang, G. Deng, S. Zhan, F. Cao, F. Cheng, J. Yin, J. Li, B. Wu and N. Zheng, ACS Cent. Sci., 2022, 8, 1008–1016 CrossRef CAS PubMed
.
- E. Ismalaj, G. Strappaveccia, E. Ballerini, F. Elisei, O. Piermatti, D. Gelman and L. Vaccaro, ACS Sustainable Chem. Eng., 2014, 2, 2461–2464 CrossRef CAS
.
- A. J. Doolin, R. G. Charles, C. S. P. De Castro, R. G. Rodriguez, E. V. Péan, R. Patidar, T. Dunlop, C. Charbonneau, T. Watson and M. L. Davies, Green Chem., 2021, 23, 2471–2486 RSC
.
- L. Cseri and G. Szekely, Green Chem., 2019, 21, 4178–4188 RSC
.
- F. Ferlin, L. Luciani, O. Viteritti, F. Brunori, O. Piermatti, S. Santoro and L. Vaccaro, Front. Chem., 2019, 659 CrossRef PubMed
.
- J. Lee, G.-W. W. Kim, M. Kim, S. A. Park and T. Park, Adv. Energy Mater., 2020, 10, 1902662 CrossRef CAS
.
- V. Gutmann, Electrochim. Acta, 1976, 21, 661–670 CrossRef CAS
.
- A. Mouret, L. Leclercq, A. Mühlbauer and V. Nardello-Rataj, Green Chem., 2013, 16, 269–278 RSC
.
- S. Aparicio and R. Alcalde, Phys. Chem. Chem. Phys., 2009, 11, 6455–6467 RSC
.
- C.-G. Wu, C.-H. Chiang, Z.-L. Tseng, Md. K. Nazeeruddin, A. Hagfeldt and M. Grätzel, Energy Environ. Sci., 2015, 8, 2725–2733 RSC
.
- Y. Zhou, M. Yang, W. Wu, A. L. Vasiliev, K. Zhu and N. P. Padture, J. Mater. Chem. A, 2015, 3, 8178–8184 RSC
.
- W. Nie, H. Tsai, R. Asadpour, J.-C. Blancon, A. J. Neukirch, G. Gupta, J. J. Crochet, M. Chhowalla, S. Tretiak, M. A. Alam, H.-L. Wang and A. D. Mohite, Science, 2015, 347, 522–525 CrossRef CAS PubMed
.
- L. A. Malley, G. L. Kennedy, G. S. Elliott, T. W. Slone, W. Mellert, K. Deckardt, K. Kuttler, B. Hildebrand, M. I. Banton, R. J. Parod and J. C. Griffiths, Drug Chem. Toxicol., 2001, 24, 315–338 CrossRef CAS PubMed
.
- J. Galvao, B. Davis, M. Tilley, E. Normando, M. R. Duchen and M. F. Cordeiro, FASEB J., 2013, 28, 1317–1330 CrossRef PubMed
.
- J. H. Noh and S. I. Seok, MRS Bull., 2015, 40, 648–653 CrossRef CAS
.
- B. J. Kim, D. H. Kim, S. L. Kwon, S. Y. Park, Z. Li, K. Zhu and H. S. Jung, Nat. Commun., 2016, 7, 11735 CrossRef CAS PubMed
.
- F. Busardo and A. Jones, Curr. Neuropharmacol., 2015, 13, 47–70 CrossRef CAS PubMed
.
- Occupational Safety and Health Administration, https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.1200, accessed January 8, 2022.
- S. K. Chandrasekaran, P. S. Campbell and A. S. Michaels, AIChE J., 1977, 23, 810–816 CrossRef CAS
.
- C. M. Hansen, Hansen Solubility Parameters: A User's Handbook, CRC Press, 2nd edn, 2007 Search PubMed
.
- K. L. Gardner, J. G. Tait, T. Merckx, W. Qiu, U. W. Paetzold, L. Kootstra, M. Jaysankar, R. Gehlhaar, D. Cheyns, P. Heremans, J. K. P. L. Gardner, J. G. Tait, T. Merckx, W. Qiu, U. W. Paetzold, L. Kootstra, M. Jaysankar, R. Gehlhaar, D. Cheyns, P. Heremans, J. Poortmans and K. L. Gardner, Adv. Energy Mater., 2016, 6, 1–8 Search PubMed
.
- S.-H. Huang, K.-Y. Tian, H.-C. Huang, C.-F. Li, W.-C. Chu, K.-M. Lee, Y.-C. Huang and W.-F. Su, ACS Appl. Mater. Interfaces, 2020, 12, 26041–26049 CrossRef CAS PubMed
.
- Z. Zhou, Z. Wang, Y. Zhou, S. Pang, D. Wang, H. Xu, Z. Liu, N. P. Padture and G. Cui, Angew. Chem., Int. Ed., 2015, 54, 9705–9709 CrossRef CAS PubMed
.
- N. K. Noel, S. N. Habisreutinger, B. Wenger, M. T. Klug, M. T. Hörantner, M. B. Johnston, R. J. Nicholas, D. T. Moore and H. J. Snaith, Energy Environ. Sci., 2017, 10, 145–152 RSC
.
- H. Fan, F. Li, P. Wang, Z. Gu, J.-H. Huang, K.-J. Jiang, B. Guan, L.-M. Yang, X. Zhou and Y. Song, Nat. Commun., 2020, 11, 5402 CrossRef CAS PubMed
.
- A. Singh, S. Kumar Podapangi and S. Avasthi, Integr. Ferroelectr., 2018, 194, 107–114 CrossRef CAS
.
- Y. Deng, C. H. Van Brackle, X. Dai, J. Zhao, B. Chen and J. Huang, Sci. Adv., 2019, 5, eaax7537 CrossRef CAS PubMed
.
- R. Li, H. Zhang, M. Zhang and M. Guo, Appl. Surf. Sci., 2018, 458, 172–182 CrossRef CAS
.
- Z. Bi, X. Rodríguez-Martínez, C. Aranda, E. Pascual-San-José, A. R. Goñi, M. Campoy-Quiles, X. Xu and A. Guerrero, J. Mater. Chem. A, 2018, 6, 19085–19093 RSC
.
- J. Wang, F. Di Giacomo, J. Brüls, H. Gorter, I. Katsouras, P. Groen, R. A. J. Janssen, R. Andriessen and Y. Galagan, Sol. RRL, 2017, 1, 1700091 CrossRef
.
- Y. Zhang, M. Chen, Y. Zhou, W. Li, Y. Lee, H. Kanda, X. Gao, R. Hu, K. G. Brooks, R. Zia, S. Kinge, N. P. Padture and M. K. Nazeeruddin, Adv. Energy Mater., 2020, 10, 2001300 CrossRef CAS
.
- X. Cao, L. Hao, Z. Liu, G. Su, X. He, Q. Zeng and J. Wei, Chem. Eng. J., 2022, 437, 135458 CrossRef CAS
.
- L. Li, Y. Chen, Z. Liu, Q. Chen, X. Wang and H. Zhou, Adv. Mater., 2016, 28, 9862–9868 CrossRef CAS PubMed
.
- Y. Numata, N. Shibayama and T. Miyasaka, J. Mater. Chem. A, 2022, 10, 672–681 RSC
.
- C. Worsley, D. Raptis, S. Meroni, A. Doolin, R. Garcia-Rodriguez, M. Davies and T. Watson, Energy Technol., 2021, 9, 2100312 CrossRef CAS
.
- J. Küffner, J. Hanisch, T. Wahl, J. Zillner, E. Ahlswede and M. Powalla, ACS Appl. Energy Mater., 2021, 4, 11700–11710 CrossRef
.
- H. Wang, J. Sun, Y. Gu, C. Xu, Y. Lu, J. Hu, T. Chen, C. Zhu and P. Luo, Sol. Energy Mater. Sol. Cells, 2022, 238, 111640 CrossRef CAS
.
- M. He, B. Li, X. Cui, B. Jiang, Y. He, Y. Chen, D. O'Neil, P. Szymanski, M. A. EI-Sayed, J. Huang and Z. Lin, Nat. Commun., 2017, 8, 16045 CrossRef CAS PubMed
.
- Y. Zheng, X. Xu, S. Liu, G. Xu, Z. Bi, Y. Zhu, K. Wang, S. Liu, A. Guerrero and G. Xing, Sol. RRL, 2022, 2200737 CrossRef CAS
.
- T.-Y. Hsieh, T.-C. Wei, K.-L. Wu, M. Ikegami and T. Miyasaka, Chem. Commun., 2015, 51, 13294–13297 RSC
.
- T.-Y. Hsieh, T.-S. Su, M. Ikegami, T.-C. Wei and T. Miyasaka, Mater. Today Energy, 2019, 14, 100125 CrossRef
.
- D. V Shinde, L. Pyeon, M. Pei, G.-W. Kim, H. Yang and T. Park, ACS Appl. Mater. Interfaces, 2017, 9, 14023–14030 CrossRef PubMed
.
- K. Sveinbjörnsson, N. K. Kyi Thein, Z. Saki, S. Svanström, W. Yang, U. B. Cappel, H. Rensmo, G. Boschloo, K. Aitola and E. M. J. Johansson, Sustainable Energy Fuels, 2018, 2, 606–615 RSC
.
- P. Zhai, T.-S. Su, T.-Y. Hsieh, W.-Y. Wang, L. Ren, J. Guo and T.-C. Wei, Nano Energy, 2019, 65, 104036 CrossRef CAS
.
- T.-Y. Hsieh, M. Pylnev, E. Palomares and T.-C. Wei, Adv. Funct. Mater., 2020, 30, 1909644 CrossRef CAS
.
- S. Gozalzadeh, F. Nasirpouri and S. Il Seok, Sci. Rep., 2021, 11, 18561 CrossRef CAS PubMed
.
- H. Wei, C. Lingfeng, L. Hui, X. Fei, W. Qi, S. Zhenhuang, N. Tingting, T. Lei, D. Bin, L. Deli, W. Yue, D. He, Z. Shouwei, L. Bixin, S. Wei, R. Xueqin, L. Ping, Z. Hui, W. Zhongbin, R. Chenxin, S. Lin, X. Guichuan, G. Xingyu, Z. Jing, X. Yingdong, C. Yonghua and H. Wei, Science, 2021, 371, 1359–1364 CrossRef PubMed
.
- S. Öz, J. Burschka, E. Jung, R. Bhattacharjee, T. Fischer, A. Mettenbörger, H. Wang and S. Mathur, Nano Energy, 2018, 51, 632–638 CrossRef
.
- L. Gu, C. Ran, L. Chao, Y. Bao, W. Hui, Y. Wang, Y. Chen, X. Gao and L. Song, ACS Appl. Mater. Interfaces, 2022, 14(20), 22870–22878 CrossRef CAS PubMed
.
- M. Wang, Q. Fu, L. Yan, J. Huang, Q. Ma, M. Humayun, W. Pi, X. Chen, Z. Zheng and W. Luo, Chem. Eng. J., 2020, 387, 123966 CrossRef CAS
.
- P. Zhao, B. J. Kim, X. Ren, D. G. Lee, G. J. Bang, J. B. Jeon, W. Bin Kim and H. S. Jung, Adv. Mater., 2018, 30, 1802763 CrossRef PubMed
.
- D. Xin, Z. Wang, M. Zhang, X. Zheng, Y. Qin, J. Zhu and W.-H. Zhang, ACS Sustainable Chem. Eng., 2019, 7, 4343–4350 CrossRef CAS
.
- M. Yavari, M. Mazloum-Ardakani, S. Gholipour, M. M. Tavakoli, S.-H. Turren-Cruz, N. Taghavinia, M. Grätzel, A. Hagfeldt and M. Saliba, Adv. Energy Mater., 2018, 8, 1800177 CrossRef
.
- Y. Yun, F. Wang, H. Huang, Y. Fang, S. Liu, W. Huang, Z. Cheng, Y. Liu, Y. Cao, M. Gao, L. Zhu, L. Wang, T. Qin and W. Huang, Adv. Mater., 2020, 32, 1907123 CrossRef CAS PubMed
.
- T. Bu, L. Wu, X. Liu, X. Yang, P. Zhou, X. Yu, T. Qin, J. Shi, S. Wang, S. Li, Z. Ku, Y. Peng, F. Huang, Q. Meng, Y.-B. B. Cheng and J. Zhong, Adv. Energy Mater., 2017, 7, 1–10 Search PubMed
.
- R. Vidal, J.-A. Alberola-Borràs, S. N. Habisreutinger, J.-L. Gimeno-Molina, D. T. Moore, T. H. Schloemer, I. Mora-Seró, J. J. Berry and J. M. Luther, Nat. Sustainability, 2021, 4, 277–285 CrossRef
.
- J. Küffner, T. Wahl, M. Schultes, J. Hanisch, J. Zillner, E. Ahlswede and M. Powalla, ACS Appl. Mater. Interfaces, 2020, 12, 52678–52690 CrossRef PubMed
.
- J. Zhong, W. Wu, L. Ding and D. Kuang, Energy Environ. Mater., 2021, 4, 277–283 CrossRef CAS
.
- F. Jafarzadeh, L. A. Castriotta, F. De Rossi, J. Ali, F. Di Giacomo, A. Di Carlo, F. Matteocci and F. Brunetti, Sustainable Energy Fuels, 2023, 7, 2219–2228 RSC
.
- F. Kerkel, M. Markiewicz, S. Stolte, E. Müller and W. Kunz, Green Chem., 2021, 23, 2962–2976 RSC
.
- L. Chao, Y. Xia, B. Li, G. Xing, Y. Chen and W. Huang, Chem, 2019, 5, 995–1006 CAS
.
- X. Gong, M. Li, X.-B. Shi, H. Ma, Z.-K. Wang and L.-S. Liao, Adv. Funct. Mater., 2015, 25, 6671–6678 CrossRef CAS
.
- D. Liu, C. J. Traverse, P. Chen, M. Elinski, C. Yang, L. Wang, M. Young and R. R. Lunt, Adv. Sci., 2017, 5, 1700484 CrossRef PubMed
.
- P. W. Voorhees, J. Stat. Phys., 1985, 38, 231–252 CrossRef
.
- J. S. Wilkes, P. Wasserscheid and T. Welton, Ionic Liquids in Synthesis, Wiley Online Books, 2007, pp. 1–6 Search PubMed
.
- M. Kunze, M. Montanino, G. B. Appetecchi, S. Jeong, M. Schönhoff, M. Winter and S. Passerini, J. Phys. Chem. A, 2010, 114, 1776–1782 CrossRef CAS PubMed
.
- J. Reiter, S. Jeremias, E. Paillard, M. Winter and S. Passerini, Phys. Chem. Chem. Phys., 2013, 15, 2565–2571 RSC
.
- X. Duan, J. Ma, J. Lian and W. Zheng, CrystEngComm, 2014, 16, 2550–2559 RSC
.
- I. Katsounaros, S. Cherevko, A. R. Zeradjanin and K. J. J. Mayrhofer, Angew. Chem., Int. Ed., 2014, 53, 102–121 CrossRef CAS PubMed
.
- X. Deng, L. Xie, S. Wang, C. Li, A. Wang, Y. Yuan, Z. Cao, T. Li, L. Ding and F. Hao, Chem. Eng. J., 2020, 398, 125594 CrossRef CAS
.
- N. Cho, F. Li, B. Turedi, L. Sinatra, S. P. Sarmah, M. R. Parida, M. I. Saidaminov, B. Murali, V. M. Burlakov, A. Goriely, O. F. Mohammed, T. Wu and O. M. Bakr, Nat. Commun., 2016, 7, 13407 CrossRef CAS PubMed
.
- D. T. Moore, K. W. Tan, H. Sai, K. P. Barteau, U. Wiesner and L. A. Estroff, Chem. Mater., 2015, 27, 3197–3199 CrossRef CAS
.
- T. L. Greaves, D. F. Kennedy, S. T. Mudie and C. J. Drummond, J. Phys. Chem. B, 2010, 114, 10022–10031 CrossRef CAS PubMed
.
- T. L. Greaves and C. J. Drummond, Chem. Rev., 2008, 108, 206–237 CrossRef CAS PubMed
.
- B.-E. Cohen and L. Etgar, Front. Optoelectron., 2016, 9, 44–52 CrossRef
.
- M. T. Hoang, N. D. Pham, Y. Yang, V. T. Tiong, C. Zhang, K. Gui, H. Chen, J. Chang, J. Wang, D. Golberg, J. Bell and H. Wang, Green Chem., 2020, 22, 3433–3440 RSC
.
- S. Chatterjee, T. Khan, A. Sen, N. Das and P. Sen, Mater. Adv., 2022, 3, 7360–7369 RSC
.
- S. Chatterjee, A. Sen and P. Sen, Mater. Chem. Front., 2023, 7, 753–764 RSC
.
- H. Lu, X. Tan, G. Huang, S. Wu, Y. Zhou, J. Zhang, Q. Zheng, T. Chen, F. Li, Z. Cai, J. Zeng and M. Zhang, Nanoscale, 2022, 14, 17222–17229 RSC
.
- T. Friščić, C. Mottillo and H. M. Titi, Angew. Chem., Int. Ed., 2020, 59, 1018–1029 CrossRef PubMed
.
- D. Tan and F. García, Chem. Soc. Rev., 2019, 48, 2274–2292 RSC
.
- S. L. James, C. J. Adams, C. Bolm, D. Braga, P. Collier, T. Friščić, F. Grepioni, K. D. M. Harris, G. Hyett, W. Jones, A. Krebs, J. Mack, L. Maini, A. G. Orpen, I. P. Parkin, W. C. Shearouse, J. W. Steed and D. C. Waddell, Chem. Soc. Rev., 2012, 41, 413–447 RSC
.
- D. Prochowicz, M. Saski, P. Yadav, M. Grätzel and J. Lewiński, Acc. Chem. Res., 2019, 52, 3233–3243 CrossRef CAS PubMed
.
- F. Gomollón-Bel, Chem. Int., 2019, 41, 12–17 Search PubMed
.
- D. Prochowicz, M. Franckevičius, A. M. Cieślak, S. M. Zakeeruddin, M. Grätzel and J. Lewiński, J. Mater. Chem. A, 2015, 3, 20772–20777 RSC
.
- P. Ferdowsi, E. Ochoa-Martinez, U. Steiner and M. Saliba, Chem. Mater., 2021, 33, 3971–3979 CrossRef CAS
.
- N. Rodkey, S. Kaal, P. Sebastia-Luna, Y. A. Birkhölzer, M. Ledinsky, F. Palazon, H. J. Bolink and M. Morales-Masis, Chem. Mater., 2021, 33, 7417–7422 CrossRef CAS PubMed
.
- T. Li, W. A. Dunlap-Shohl, Q. Han and D. B. Mitzi, Chem. Mater., 2017, 29, 6200–6204 CrossRef CAS
.
- T. Li, W. A. Dunlap-Shohl, E. W. Reinheimer, P. Le Magueres and D. B. Mitzi, Chem. Sci., 2019, 10, 1168–1175 RSC
.
- D. B. Mitzi, D. R. Medeiros and P. W. DeHaven, Chem. Mater., 2002, 14, 2839–2841 CrossRef CAS
.
- W.-Y. Tan, P.-P. Cheng, Y.-W. Zhang, J.-M. Liang, X. Chen, Y. Liu and Y. Min, J. Mater. Chem. C, 2019, 7, 6004–6011 RSC
.
- M. Zhang, Z. Wang, B. Zhou, X. Jia, Q. Ma, N. Yuan, X. Zheng, J. Ding and W. Zhang, Sol. RRL, 2018, 2, 1700213 CrossRef
.
- L. Vesce, M. Stefanelli, J. P. Herterich, L. A. Castriotta, M. Kohlstädt, U. Würfel and A. Di Carlo, Sol. RRL, 2021, 5, 2100073 CrossRef CAS
.
- N. Zarabinia, G. Lucarelli, R. Rasuli, F. De Rossi, B. Taheri, H. Javanbakht, F. Brunetti and T. M. Brown, iScience, 2022, 25, 103712 CrossRef CAS PubMed
.
- D. S. Ahmed and M. K. A. A. Mohammed, Sol. Energy, 2020, 207, 1240–1246 CrossRef CAS
.
- L. Xu, S. Che, J. Huang, D. Xie, Y. Yao, P. Wang, P. Lin, H. Piao, H. Hu, C. Cui, F. Wu, D. Yang and X. Yu, Appl. Phys. Lett., 2019, 115, 33101 CrossRef
.
- C. Dong, X. Han, Y. Zhao, J. Li, L. Chang and W. Zhao, Sol. RRL, 2018, 2, 1800139 CrossRef
.
- S. Tian, J. Li, S. Li, T. Bu, Y. Mo, S. Wang, W. Li and F. Huang, Sol. Energy, 2019, 183, 386–391 CrossRef CAS
.
- S. Li, F. Zhang, Q. Sun, Z. Li, Y. Cui, T. Ji, W. Qin, F. Zhu and Y. Hao, Org. Electron., 2018, 57, 133–139 CrossRef CAS
.
- A. D. Taylor, Q. Sun, K. P. Goetz, Q. An, T. Schramm, Y. Hofstetter, M. Litterst, F. Paulus and Y. Vaynzof, Nat. Commun., 2021, 12, 1878 CrossRef CAS PubMed
.
- P. Billen, E. Leccisi, S. Dastidar, S. Li, L. Lobaton, S. Spatari, A. T. Fafarman, V. M. Fthenakis and J. B. Baxter, Energy, 2019, 166, 1089–1096 CrossRef CAS
.
- J. Li, H.-L. L. Cao, W.-B. Bin Jiao, Q. Wang, M. Wei, I. Cantone, J. Lü and A. Abate, Nat. Commun., 2020, 11, 310 CrossRef CAS PubMed
.
- A. Singh and S. Avasthi, in 2018 IEEE 7th World Conference on Photovoltaic Energy Conversion, WCPEC 2018 – A Joint Conference of 45th IEEE PVSC, 28th PVSEC and 34th EU PVSEC, Institute of Electrical and Electronics Engineers Inc., 2018, pp. 518–521 Search PubMed
.
- W. A. Dunlap-Shohl, E. T. Barraza, A. Barrette, K. Gundogdu, A. D. Stiff-Roberts and D. B. Mitzi, ACS Energy Lett., 2018, 3, 270–275 CrossRef CAS
.
- X. Li, F. Zhang, H. He, J. J. Berry, K. Zhu and T. Xu, Nature, 2020, 578, 555–558 CrossRef CAS PubMed
.
- A. J. Huckaba, D. T. Sun, A. A. Sutanto, M. Mensi, Y. Zhang, W. L. Queen and M. K. Nazeeruddin, Energy Technol., 2020, 8, 2000239 CrossRef CAS
.
- M. Z. Mokhtar, J. He, M. Li, Q. Chen, J. C. R. Ke, D. J. Lewis, A. G. Thomas, B. F. Spencer, S. A. Haque and B. R. Saunders, Chem. Commun., 2021, 57, 994–997 RSC
.
- R. D. Mendez L., B. N. Breen and D. Cahen, ACS Appl. Mater. Interfaces, 2022, 14, 29766–29772 CrossRef CAS PubMed
.
- Y. Dong, J. Zhang, Y. Yang, J. Wang, B. Hu, W. Wang, W. Cao, S. Gai, D. Xia, K. Lin and R. Fan, Nano Energy, 2022, 97, 107184 CrossRef CAS
.
- R. Nie, R. R. Sumukam, S. H. Reddy, M. Banavoth and S. Il Seok, Energy Environ. Sci., 2020, 13, 2363–2385 RSC
.
- V. Pecunia, L. G. Occhipinti and R. L. Z. Hoye, Adv. Energy Mater., 2021, 11, 2100698 CrossRef CAS
.
- S. Castro-hermosa, M. Top, J. Fahlteich, T. M. Brown, S. Castro-hermosa, G. Lucarelli, M. Top, M. Fahland and J. Fahlteich, Cell Rep. Phys. Sci., 2020, 100045 CrossRef
.
- J. Dagar, S. Castro-Hermosa, G. Lucarelli, F. Cacialli and T. M. Brown, Nano Energy, 2018, 49, 290–299 CrossRef CAS
.
- C.-Y. Chen, J.-H. Chang, K.-M. Chiang, H.-L. Lin, S.-Y. Hsiao and H.-W. Lin, Adv. Funct. Mater., 2015, 25, 7064–7070 CrossRef CAS
.
- F. Di Giacomo, V. Zardetto, G. Lucarelli, L. Cinà, A. Di Carlo, M. Creatore and T. M. Brown, Nano Energy, 2016, 30, 460–469 CrossRef CAS
.
- V. Pecunia, L. G. Occhipinti, A. Chakraborty, Y. Pan and Y. Peng, APL Mater., 2020, 8, 100901 CrossRef CAS
.
- I. Kopacic, B. Friesenbichler, S. F. Hoefler, B. Kunert, H. Plank, T. Rath and G. Trimmel, ACS Appl. Energy Mater., 2018, 1, 343–347 CrossRef CAS
.
- M. Chen, M.-G. Ju, H. F. Garces, A. D. Carl, L. K. Ono, Z. Hawash, Y. Zhang, T. Shen, Y. Qi, R. L. Grimm, D. Pacifici, X. C. Zeng, Y. Zhou and N. P. Padture, Nat. Commun., 2019, 10, 16 CrossRef CAS PubMed
.
- M. Chen, M. G. Ju, A. D. Carl, Y. Zong, R. L. Grimm, J. Gu, X. C. Zeng, Y. Zhou and N. P. Padture, Joule, 2018, 2, 558–570 CrossRef CAS
.
- X. Yang, Y. Chen, P. Liu, H. Xiang, W. Wang, R. Ran, W. Zhou and Z. Shao, Adv. Funct. Mater., 2020, 30, 2001557 CrossRef CAS
.
- W. B. Dai, S. Xu, J. Zhou, J. Hu, K. Huang and M. Xu, Sol. Energy Mater. Sol. Cells, 2019, 192, 140–146 CrossRef CAS
.
- Y. Yang, C. Liu, M. Cai, Y. Liao, Y. Ding, S. Ma, X. Liu, M. Guli, S. Dai and M. K. Nazeeruddin, ACS Appl. Mater. Interfaces, 2020, 12, 17062–17069 CrossRef CAS PubMed
.
- B. Yu, Z. Chen, Y. Zhu, Y. Wang, B. Han, G. Chen, X. Zhang, Z. Du and Z. He, Adv. Mater., 2021, 33, 2102055 CrossRef CAS PubMed
.
- G. Kapil, T. Bessho, T. Maekawa, A. K. Baranwal, Y. Zhang, M. A. Kamarudin, D. Hirotani, Q. Shen, H. Segawa and S. Hayase, Adv. Energy Mater., 2021, 11, 2101069 CrossRef CAS
.
- H. Fu, Sol. Energy Mater. Sol. Cells, 2019, 193, 107–132 CrossRef CAS
.
- H. yu Zhang, R. Li, W. wu Liu, M. Zhang and M. Guo, Int. J. Miner. Metall. Mater., 2019, 26, 387–403 CrossRef
.
- L. M. Herz, ACS Energy Lett., 2017, 2, 1539–1548 CrossRef CAS
.
- J. Pascual, G. Nasti, M. H. Aldamasy, J. A. Smith, M. Flatken, N. Phung, D. Di Girolamo, S.-H. Turren-Cruz, M. Li, A. Dallmann, R. Avolio and A. Abate, Mater. Adv., 2020, 1, 1066–1070 RSC
.
- M. I. Saidaminov, I. Spanopoulos, J. Abed, W. Ke, J. Wicks, M. G. Kanatzidis and E. H. Sargent, ACS Energy Lett., 2020, 1153–1155 CrossRef CAS
.
- J. Pascual, G. Nasti, M. H. Aldamasy, J. A. Smith, M. Flatken, N. Phung, D. Di Girolamo, S.-H. Turren-Cruz, M. Li, A. Dallmann, R. Avolio and A. Abate, Mater. Adv., 2020, 1, 1066–1070 RSC
.
- R. Lin, K. Xiao, Z. Qin, Q. Han, C. Zhang, M. Wei, M. I. Saidaminov, Y. Gao, J. Xu, M. Xiao, A. Li, J. Zhu, E. H. Sargent and H. Tan, Nat. Energy, 2019, 4, 864–873 CrossRef CAS
.
- Y. Takahashi, R. Obara, Z. Z. Lin, Y. Takahashi, T. Naito, T. Inabe, S. Ishibashi and K. Terakura, Dalton Trans., 2011, 40, 5563–5568 RSC
.
- D. Di Girolamo, J. Pascual, M. H. Aldamasy, Z. Iqbal, G. Li, E. Radicchi, M. Li, S.-H. Turren-Cruz, G. Nasti, A. Dallmann, F. De Angelis and A. Abate, ACS Energy Lett., 2021, 959–968 CrossRef CAS
.
- J. Cao and F. Yan, Energy Environ. Sci., 2021, 14, 1286–1325 RSC
.
- F. Wang, X. Jiang, H. Chen, Y. Shang, H. Liu, J. Wei, W. Zhou, H. He, W. Liu and Z. Ning, Joule, 2018, 2, 2732–2743 CrossRef CAS
.
- B. Lee, A. Krenselewski, S. Il Baik, D. N. Seidman and R. P. H. Chang, Sustainable Energy Fuels, 2017, 1, 710–724 RSC
.
- W. Ke and M. G. Kanatzidis, Nat. Commun., 2019, 10, 965 CrossRef PubMed
.
- Pocket Guide to Chemical Hazards, NIOSH | CDC, https://www.cdc.gov/niosh/npg/pgintrod.html, accessed April 11, 2020 Search PubMed.
- L. Serrano-Lujan, N. Espinosa, T. T. Larsen-Olsen, J. Abad, A. Urbina and F. C. Krebs, Adv. Energy Mater., 2015, 5, 1501119 CrossRef
.
- A. Babayigit, D. Duy Thanh, A. Ethirajan, J. Manca, M. Muller, H. G. Boyen and B. Conings, Sci. Rep., 2016, 6, 1–11 CrossRef PubMed
.
- J. Zhang, X. Gao, Y. Deng, Y. Zha and C. Yuan, Sol. Energy Mater. Sol. Cells, 2017, 166, 9–17 CrossRef CAS
.
- X. Meng, T. Wu, X. Liu, X. He, T. Noda, Y. Wang, H. Segawa and L. Han, J. Phys. Chem. Lett., 2020, 2965–2971 CrossRef CAS PubMed
.
- F. P. Byrne, S. Jin, G. Paggiola, T. H. M. Petchey, J. H. Clark, T. J. Farmer, A. J. Hunt, C. Robert McElroy and J. Sherwood, Sustainable Chem. Processes, 2016, 4, 7 CrossRef
.
- T. Krishnamoorthy, H. Ding, C. Yan, W. L. Leong, T. Baikie, Z. Zhang, M. Sherburne, S. Li, M. Asta, N. Mathews and S. G. Mhaisalkar, J. Mater. Chem. A, 2015, 3, 23829–23832 RSC
.
- G. B. Gerber and A. Léonard, Mutat. Res., Rev. Mutat. Res., 1997, 387, 141–146 CrossRef CAS PubMed
.
- E. Rosenberg, Rev. Environ. Sci. Bio/Technol., 2008, 8, 29 CrossRef
.
- Chemical elements by market price, http://www.leonland.de/elements_by_price/en/list, accessed April 18, 2020 Search PubMed.
- R. Mohan, Nat. Chem., 2010, 2, 336 CrossRef CAS PubMed
.
- S. M. Jain, D. Phuyal, M. L. Davies, M. Li, B. Philippe, C. De Castro, Z. Qiu, J. Kim, T. Watson, W. C. Tsoi, O. Karis, H. Rensmo, G. Boschloo, T. Edvinsson and J. R. Durrant, Nano Energy, 2018, 49, 614–624 CrossRef CAS
.
- U. H. Hamdeh, R. D. Nelson, B. J. Ryan, U. Bhattacharjee, J. W. Petrich and M. G. Panthani, Chem. Mater., 2016, 28, 6567–6574 CrossRef CAS
.
- H. Li, C. Wu, Y. Yan, B. Chi, J. Pu, J. Li and S. Priya, ChemSusChem, 2017, 10, 3994–3998 CrossRef CAS PubMed
.
- S. M. Jain, T. Edvinsson and J. R. Durrant, Commun. Chem., 2019, 2, 1–7 CrossRef CAS
.
- Y. Wang, Y. Liu, Y. Xu, C. Zhang, H. Bao, J. Wang, Z. Guo, L. Wan, D. Eder and S. Wang, Electrochim. Acta, 2020, 329, 135173 CrossRef CAS
.
- Y. El Ajjouri, V. S. Chirvony, N. Vassilyeva, M. Sessolo, F. Palazon and H. J. Bolink, J. Mater. Chem. C, 2019, 7, 6236–6240 RSC
.
- S. Sanders, D. Stümmler, P. Pfeiffer, N. Ackermann, G. Simkus, M. Heuken, P. K. Baumann, A. Vescan and H. Kalisch, Sci. Rep., 2019, 9, 9774 CrossRef CAS PubMed
.
- S. Sundar and J. Chakravarty, Int. J. Environ. Res. Public Health, 2010, 7, 4267–4277 CrossRef CAS PubMed
.
- J.-C. Hebig, I. Kühn, J. Flohre and T. Kirchartz, ACS Energy Lett., 2016, 1, 309–314 CrossRef CAS
.
- N. Giesbrecht, A. Weis and T. Bein, J. Phys.: Energy, 2020, 2, 024007 CAS
.
- C. Zuo and L. Ding, Angew. Chem., Int. Ed., 2017, 56, 6528–6532 CrossRef CAS PubMed
.
- X.-P. Cui, K.-J. Jiang, J.-H. Huang, Q.-Q. Zhang, M.-J. Su, L.-M. Yang, Y.-L. Song and X.-Q. Zhou, Synth. Met., 2015, 209, 247–250 CrossRef CAS
.
- M. Wang, P. Zeng, S. Bai, J. Gu, F. Li, Z. Yang and M. Liu, Sol. RRL, 2018, 2, 1800217 CrossRef
.
- E. Greul, M. L. Petrus, A. Binek, P. Docampo and T. Bein, J. Mater. Chem. A, 2017, 5, 19972–19981 RSC
.
- CRC Handbook of Chemistry and Physics, ed. W. M. Haynes, CRC Press, 97th edn, 2016, DOI:10.1201/9781315380476
.
- C. Zhang, L. Gao, S. Teo, Z. Guo, Z. Xu, S. Zhao and T. Ma, Sustainable Energy Fuels, 2018, 2, 2419–2428 RSC
.
- J. Luo, S. Li, H. Wu, Y. Zhou, Y. Li, J. Liu, J. Li, K. Li, F. Yi, G. Niu and J. Tang, ACS Photonics, 2018, 5, 398–405 CrossRef CAS
.
- J. Euvrard, X. Wang, T. Li, Y. Yan and D. B. Mitzi, J. Mater. Chem. A, 2020, 8, 4049–4054 RSC
.
- Y. Wang, Y. Liu, Y. Xu, C. Zhang, H. Bao, J. Wang, Z. Guo, L. Wan, D. Eder and S. Wang, Electrochim. Acta, 2020, 329, 135173 CrossRef CAS
.
- G. Schileo and G. Grancini, J. Mater. Chem. C, 2021, 9, 67–76 RSC
.
- K. S. Egorova and V. P. Ananikov, Organometallics, 2017, 36, 4071–4090 CrossRef CAS
.
- D. M. Flanagan, I. Ore, I. O. Pigments, P. Rock, Q. Crystal, R. Earths and S. Ash, Mineral Commodity Summaries 2021, 2021 Search PubMed
.
- C. Reichl and M. Schatz, International Organizing Committee for the World Mining Congresses, 2019, vol. 34, p. 262 Search PubMed
.
- P. Nuss and M. J. Eckelman, PLoS One, 2014, 9, 1–12 Search PubMed
.
- V. V. Popov, M. L. Grilli, A. Koptyug, L. Jaworska, A. Katz-Demyanetz, D. Klobčar, S. Balos, B. O. Postolnyi and S. Goel, Materials, 2021, 14, 909 CrossRef CAS PubMed
.
- K. Mahmood, S. Sarwar and M. T. Mehran, RSC Adv., 2017, 7, 17044–17062 RSC
.
- M. Dkhili, G. Lucarelli, F. De Rossi, B. Taheri, K. Hammedi, H. Ezzaouia, F. Brunetti and T. M. Brown, ACS Appl. Energy Mater., 2022, 5, 4096–4107 CrossRef CAS PubMed
.
- L.-C. Chen and Z.-L. Tseng, Nanostructured Solar Cells, 2017 Search PubMed
.
- Y. Yang, J. Wu, P. Guo, X. Liu, Q. Guo, Q. Liu and H. Luo, J. Mater. Sci.: Mater. Electron., 2018, 29, 13138–13147 CrossRef CAS
.
- J. Song, E. Zheng, X.-F. Wang, W. Tian and T. Miyasaka, Sol. Energy Mater. Sol. Cells, 2016, 144, 623–630 CrossRef CAS
.
- J. X. Song, X. X. Yin, Z. F. Li and Y. W. Li, Rare Met., 2021, 40, 2730–2746 CrossRef CAS
.
- F. Di Giacomo, V. Zardetto, A. D'Epifanio, S. Pescetelli, F. Matteocci, S. Razza, A. Di Carlo, S. Licoccia, W. M. M. Kessels, M. Creatore and T. M. Brown, Adv. Energy Mater., 2015, 5, 1401808 CrossRef
.
- G. Mincuzzi, M. Schulz-Ruhtenberg, L. Vesce, A. Reale, A. Di Carlo, A. Gillner and T. M. Brown, Prog. Photovoltaics, 2014, 22, 308–317 CAS
.
- J. Gong, S. B. Darling and F. You, Energy Environ. Sci., 2015, 8, 1953–1968 RSC
.
- M. Najafi, F. Di Giacomo, D. Zhang, S. Shanmugam, A. Senes, W. Verhees, A. Hadipour, Y. Galagan, T. Aernouts, S. Veenstra and R. Andriessen, Small, 2018, 14, 1702775 CrossRef PubMed
.
- H. Sarialtin, R. Geyer and C. Zafer, J. Renewable Sustainable Energy, 2020, 12, 023502 CrossRef CAS
.
- C. Liu, L. Zhang, X. Zhou, J. Gao, W. Chen, X. Wang and B. Xu, Adv. Funct. Mater., 2019, 29, 1807604 CrossRef CAS
.
- F. Bouhjar, L. Derbali and B. Marí, Nano Res., 2020, 13(9), 2546–2555 CrossRef
.
- W. Ke, G. Fang, Q. Liu, L. Xiong, P. Qin, H. Tao, J. Wang, H. Lei, B. Li, J. Wan, G. Yang and Y. Yan, J. Am. Chem. Soc., 2015, 137, 6730–6733 CrossRef CAS PubMed
.
- Q. Jiang, Y. Zhao, X. Zhang, X. Yang, Y. Chen, Z. Chu, Q. Ye, X. Li, Z. Yin and J. You, Nat. Photonics, 2019, 13, 460–466 CrossRef CAS
.
- E. H. Anaraki, A. Kermanpur, L. Steier, K. Domanski, T. Matsui, W. Tress, M. Saliba, A. Abate, M. Grätzel, A. Hagfeldt and J.-P. Correa-Baena, Energy Environ. Sci., 2016, 9, 3128–3134 RSC
.
- J. Miao, Z. Hu, M. Liu, M. Umair Ali, O. Goto, W. Lu, T. Yang, Y. Liang and H. Meng, Org. Electron., 2018, 52, 200–205 CrossRef CAS
.
- O. Fernandez-Delgado, P. S. Chandrasekhar, N. Cano-Sampaio, Z. C. Simon, A. R. Puente-Santiago, F. Liu, E. Castro and L. Echegoyen, J. Mater. Chem. C, 2021, 9, 10759–10767 RSC
.
- T. Zheng, H. Zhou, B. Fan, Y. Zhao, B. Jin, L. Fan and R. Peng, Chem. Eng. J., 2021, 415, 128816 CrossRef CAS
.
- B. Walker, A. Tamayo, D. T. Duong, X.-D. Dang, C. Kim, J. Granstrom and T.-Q. Nguyen, Adv. Energy Mater., 2011, 1, 221–229 CrossRef CAS
.
- F. MacHui, S. Abbott, D. Waller, M. Koppe and C. J. Brabec, Macromol. Chem. Phys., 2011, 212, 2159–2165 CrossRef CAS
.
- C. Do Park, T. A. Fleetham, J. Li and B. D. Vogt, Org. Electron., 2011, 12, 1465–1470 CrossRef
.
- D. Sharp, S. Taylor, M. Andrews and D. Boucher, Macromol. Chem. Phys., 2019, 220, 1800406 CrossRef
.
- F. Isabelli, F. Di Giacomo, H. Gorter, F. Brunetti, P. Groen, R. Andriessen and Y. Galagan, ACS Appl. Energy Mater., 2018, 1, 6056–6063 CrossRef CAS
.
- F. Isabelli, F. Di Giacomo, H. Gorter, F. Brunetti, P. Groen, R. Andriessen, Y. Galagan, F. Di Giacomo, H. Gorter, F. Brunetti, P. Groen, R. Andriessen and Y. Galagan, ACS Appl. Energy Mater., 2018, 1, 6056–6063 CrossRef CAS
.
- Y. Takebayashi, N. Morii, K. Sue, T. Furuya, S. Yoda, D. Ikemizu and H. Taka, Ind. Eng. Chem. Res., 2015, 54, 8801–8808 CrossRef CAS
.
- J. Lee, M. Malekshahi Byranvand, G. Kang, S. Y. Son, S. Song, G. W. Kim and T. Park, J. Am. Chem. Soc., 2017, 139, 12175–12181 CrossRef CAS PubMed
.
- H. Min, D. Y. Lee, J. Kim, G. Kim, K. S. Lee, J. Kim, M. J. Paik, Y. K. Kim, K. S. Kim, M. G. Kim, T. J. Shin and S. Il Seok, Nature, 2021, 598, 444–450 CrossRef CAS PubMed
.
- K. Jiang, F. Wu, G. Zhang, L. Zhu and H. Yan, Sol. RRL, 2019, 3, 1900061 CrossRef
.
- S. Mattiello, G. Lucarelli, A. Calascibetta, L. Polastri, E. Ghiglietti, S. K. Podapangi, T. M. Brown, M. Sassi and L. Beverina, ACS Sustainable Chem. Eng., 2022, 10, 4750–4757 CrossRef CAS
.
- S. Maranghi, M. L. Parisi, R. Basosi and A. Sinicropi, Energies, 2019, 12, 3746 CrossRef CAS
.
- P. Vivo, J. K. Salunke and A. Priimagi, Materials, 2017, 10, 1–45 CrossRef PubMed
.
- H. Lu, B. He, Y. Ji, Y. Shan, C. Zhong, J. Xu, J. LiuYang, F. Wu and L. Zhu, Chem. Eng. J., 2020, 385, 123976 CrossRef
.
- A. Pellis, F. P. Byrne, J. Sherwood, M. Vastano, J. W. Comerford and T. J. Farmer, Green Chem., 2019, 21, 1686–1694 RSC
.
- B. Chaudhary, A. Kulkarni, A. K. Jena, M. Ikegami and T. Miyasaka, Energy Technol., 2019, 8, 1900990 CrossRef
.
- K. Jiang, F. Wu, G. Zhang, L. Zhu and H. Yan, Sol. RRL, 2019, 3, 1900061 CrossRef
.
- F. Wu, J. Liu, G. Wang, Q. Song and L. Zhu, Chem. –Eur. J., 2016, 22, 16636–16641 CrossRef CAS PubMed
.
- F. Wu, Y. Shan, X. Li, Q. Song and L. Zhu, Org. Electron., 2016, 39, 323–327 CrossRef CAS
.
- J. Chen, J. Xia, H.-J. Yu, J.-X. Zhong, X.-K. Wu, Y.-S. Qin, C. Jia, Z. She, D.-B. Kuang and G. Shao, Chem. Mater., 2019, 31, 5431–5441 CrossRef CAS
.
- S. A. Carter, M. Angelopoulos, S. Karg, P. J. Brock and J. C. Scott, Appl. Phys. Lett., 1997, 70, 2067–2069 CrossRef CAS
.
- T. M. Brown, J. S. Kim, R. H. Friend, F. Cacialli, R. Daik and W. J. Feast, Appl. Phys. Lett., 1999, 75, 1679–1681 CrossRef CAS
.
- M. Helgesen, R. Søndergaard and F. C. Krebs, J. Mater. Chem., 2010, 20, 36–60 RSC
.
- Y. Xia and S. Dai, J. Mater. Sci.: Mater. Electron., 2021, 32, 12746–12757 CrossRef CAS
.
- W. Yu, K. Wang, B. Guo, X. Qiu, Y. Hao, J. Chang and Y. Li, J. Power Sources, 2017, 358, 29–38 CrossRef CAS
.
- S. H. Reddy, F. Di Giacomo and A. Di Carlo, Adv. Energy Mater., 2022, 12, 2103534 CrossRef CAS
.
- J. Lee, T. H. Lee, M. M. Byranvand, K. Choi, H. Il Kim, S. A. Park, J. Y. Kim and T. Park, J. Mater. Chem. A, 2018, 6, 5538–5543 RSC
.
- J. Lee, G. Kim, M. Kim, S. A. Park and T. Park, Adv. Energy Mater., 2020, 10, 1902662 CrossRef CAS
.
- A. Gadisa, W. D. Oosterbaan, K. Vandewal, J.-C. Bolsée, S. Bertho, J. D'Haen, L. Lutsen, D. Vanderzande and J. V Manca, Adv. Funct. Mater., 2009, 19, 3300–3306 CrossRef CAS
.
- R. Colle, G. Grosso, A. Ronzani and C. M. Zicovich-Wilson, Phys. Status Solidi B, 2011, 248, 1360–1368 CrossRef CAS
.
- M. J. Jeong, K. M. Yeom, S. J. Kim, E. H. Jung and J. H. Noh, Energy Environ. Sci., 2021, 14, 2419–2428 RSC
.
- S. Park, J. H. Heo, J. H. Yun, T. S. Jung, K. Kwak, M. J. Ko, C. H. Cheon, J. Y. Kim, S. H. Im and H. J. Son, Chem. Sci., 2016, 7, 5517–5522 RSC
.
- H. Zhang, H. Wang, W. Chen and A. K. Y. Jen, Adv. Mater., 2017, 29, 1604984 CrossRef PubMed
.
- S. Jeon, U. K. Thakur, D. Lee, Y. Wenping, D. Kim, S. Lee, T. K. Ahn, H. J. Park and B.-G. Kim, Org. Electron., 2016, 37, 134–140 CrossRef CAS
.
- P. Qin, S. Tanaka, S. Ito, N. Tetreault, K. Manabe, H. Nishino, M. K. Nazeeruddin and M. Grätzel, Nat. Commun., 2014, 5, 1–6 Search PubMed
.
- G. Murugadoss, R. Thangamuthu and S. M. Senthil Kumar, Sol. Energy Mater. Sol. Cells, 2017, 164, 56–62 CrossRef CAS
.
- S. Gharibzadeh, B. A. Nejand, A. Moshaii, N. Mohammadian, A. H. Alizadeh, R. Mohammadpour, V. Ahmadi and A. Alizadeh, ChemSusChem, 2016, 9, 1929–1937 CrossRef CAS PubMed
.
- S. Jeong, S. Seo and H. Shin, RSC Adv., 2018, 8, 27956–27962 RSC
.
- Q. Xue, Y. Bai, M. Liu, R. Xia, Z. Hu, Z. Chen, X.-F. Jiang, F. Huang, S. Yang, Y. Matsuo, H.-L. Yip and Y. Cao, Adv. Energy Mater., 2017, 7, 1602333 CrossRef
.
- Y.-M. Chang, C.-W. Li, Y.-L. Lu, M.-S. Wu, H. Li, Y.-S. Lin, C.-W. Lu, C.-P. Chen and Y. J. Chang, ACS Appl. Mater. Interfaces, 2021, 13, 6450–6460 CrossRef CAS PubMed
.
- J. H. Noh, S. H. Im, J. H. Heo, T. N. Mandal and S. Il Seok, Nano Lett., 2013, 13, 1764–1769 CrossRef CAS PubMed
.
- J. H. Heo, S. H. Im, J. H. Noh, T. N. Mandal, C.-S. Lim, J. A. Chang, Y. H. Lee, H. Kim, A. Sarkar, Md. K. Nazeeruddin, M. Grätzel and S. Il Seok, Nat. Photonics, 2013, 7, 486–491 CrossRef CAS
.
- W. S. Yang, J. H. Noh, N. J. Jeon, Y. C. Kim, S. Ryu, J. Seo and S. Il Seok, Science, 2015, 348, 1234–1237 CrossRef CAS PubMed
.
- B. A. Nejand, V. Ahmadi, S. Gharibzadeh and H. R. Shahverdi, ChemSusChem, 2016, 9, 302–313 CrossRef CAS PubMed
.
- S. Chatterjee and A. J. Pal, J. Phys. Chem. C, 2016, 120, 1428–1437 CrossRef CAS
.
- W. Sun, Y. Li, S. Ye, H. Rao, W. Yan, H. Peng, Y. Li, Z. Liu, S. Wang, Z. Chen, L. Xiao, Z. Bian and C. Huang, Nanoscale, 2016, 8, 10806–10813 RSC
.
- W. Chen, Y. Zhou, G. Chen, Y. Wu, B. Tu, F.-Z. Liu, L. Huang, A. M. C. Ng, A. B. Djurišić and Z. He, Adv. Energy Mater., 2019, 9, 1803872 CrossRef
.
- F. Xie, C.-C. Chen, Y. Wu, X. Li, M. Cai, X. Liu, X. Yang and L. Han, Energy Environ. Sci., 2017, 10, 1942–1949 RSC
.
- L. Wang, G.-R. Li, Q. Zhao and X.-P. Gao, Energy Stor. Mater., 2017, 7, 40–47 Search PubMed
.
- R. L. Moss, E. Tzimas, H. Kara, P. Willis and J. Kooroshy, Critical Metals in Strategic Energy Technologies Assessing Rare Metals as Supply-Chain Bottlenecks in Low-Carbon Energy Technologies, Netherlands, 2011 Search PubMed
.
- T. Norgate and N. Haque, J. Cleaner Prod., 2012, 29(30), 53–63 CrossRef
.
- S. Castro-Hermosa, L. Wouk, I. S. Bicalho, L. de Queiroz Corrêa, B. de Jong, L. Cinà, T. M. Brown and D. Bagnis, Nano Res., 2020, 14, 1034–1042 CrossRef
.
- E. Leccisi and V. Fthenakis, Prog. Photovoltaics, 2021, 29, 1078–1092 CAS
.
- E. Kasparavicius, A. Magomedov, T. Malinauskas and V. Getautis, Chem. –Eur. J., 2018, 24, 9910–9918 CrossRef CAS PubMed
.
- B. Rivkin, P. Fassl, Q. Sun, A. D. Taylor, Z. Chen and Y. Vaynzof, ACS Omega, 2018, 3, 10042–10047 CrossRef CAS PubMed
.
- F. Zhang, X. Yang, M. Cheng, J. Li, W. Wang, H. Wang and L. Sun, J. Mater. Chem. A, 2015, 3, 24272–24280 RSC
.
- L. Fagiolari and F. Bella, Energy Environ. Sci., 2019, 12, 3437–3472 RSC
.
- I. Jeon, S. Seo, Y. Sato, C. Delacou, A. Anisimov, K. Suenaga, E. I. Kauppinen, S. Maruyama and Y. Matsuo, J. Phys. Chem. C, 2017, 121, 25743–25749 CrossRef CAS
.
- H. Tao, Y. Li, C. Zhang, K. Wang, J. Wang, B. Tan, L. Han and J. Tao, Solid State Commun., 2018, 271, 71–75 CrossRef CAS
.
- Z. Liu, T. Shi, Z. Tang, B. Sun and G. Liao, Nanoscale, 2016, 8, 7017–7023 RSC
.
- L. Zhang, T. Liu, L. Liu, M. Hu, Y. Yang, A. Mei and H. Han, J. Mater. Chem. A, 2015, 3, 9165–9170 RSC
.
- Z. Wei, H. Chen, K. Yan and S. Yang, Angew. Chem., Int. Ed., 2014, 53, 13239–13243 CrossRef CAS PubMed
.
- G. Wang, D. Wang, S. Kuang, W. Xing and S. Zhuo, Renewable Energy, 2014, 63, 708–714 CrossRef CAS
.
- S. M. Cha, G. Nagaraju, S. C. Sekhar, L. K. Bharat and J. S. Yu, J. Colloid Interface Sci., 2018, 513, 843–851 CrossRef CAS PubMed
.
- L. Gao, Y. Zhou, F. Meng, Y. Li, A. Liu, Y. Li, C. Zhang, M. Fan, G. Wei and T. Ma, Carbon, 2020, 162, 267–272 CrossRef CAS
.
- S. S. Mali, H. Kim, J. V. Patil and C. K. Hong, ACS Appl. Mater. Interfaces, 2018, 10, 31280–31290 CrossRef CAS PubMed
.
- H. Liu, C. Geng, P. Wei, H. Chen, S. Zheng, H. Wang and Y. Xie, J. Alloys Compd., 2022, 912, 165123 CrossRef CAS
.
- S. Pitchaiya, N. Eswaramoorthy, V. Madurai Ramakrishnan, M. Natarajan and D. Velauthapillai, ACS Appl. Mater. Interfaces, 2022, 14, 43050–43066 CrossRef CAS PubMed
.
- T. Ibn-Mohammed, S. C. L. Koh, I. M. Reaney, A. Acquaye, G. Schileo, K. B. Mustapha and R. Greenough, Renewable Sustainable Energy Rev., 2017, 80, 1321–1344 CrossRef CAS
.
- J. M. Kadro and A. Hagfeldt, Joule, 2017, 1, 29–46 CrossRef
.
- K. Kawajiri, K. Tahara and S. Uemiya, Resour. Environ. Sustainability, 2022, 7, 100047 CrossRef
.
- F. Brunetti, A. Operamolla, S. Castro-Hermosa, G. Lucarelli, V. Manca, G. M. Farinola and T. M. Brown, Adv. Funct. Mater., 2019, 29, 1806798 CrossRef
.
- S. Castro-Hermosa, J. Dagar, A. Marsella and T. M. Brown, IEEE Electron Device Lett., 2017, 38, 1278–1281 CAS
.
- S. Castro-Hermosa, G. Lucarelli, M. Top, M. Fahland, J. Fahlteich and T. M. Brown, Cell Rep. Phys. Sci., 2020, 1, 100045 CrossRef
.
- I. Celik, Z. Song, A. J. Cimaroli, Y. Yan, M. J. Heben and D. Apul, Sol. Energy Mater. Sol. Cells, 2016, 156, 157–169 CrossRef CAS
.
- R. Itten and M. Stucki, Energies, 2017, 10, 841 CrossRef
.
- J.-A. A. Alberola-Borràs, R. Vidal, E. J. Juárez-Pérez, E. Mas-Marzá, A. Guerrero and I. Mora-Seró, Sol. Energy Mater. Sol. Cells, 2018, 179, 169–177 CrossRef
.
- J.-A. A. Alberola-Borràs, R. Vidal and I. Mora-Seró, Sustainable Energy Fuels, 2018, 2, 1600–1609 RSC
.
- S. Sánchez, M. Vallés-Pelarda, J. A. Alberola-Borràs, R. Vidal, J. J. Jerónimo-Rendón, M. Saliba, P. P. Boix and I. Mora-Seró, Mater. Today, 2019, 31, 39–46 CrossRef
.
- N. Espinosa, L. Serrano-Luján, A. Urbina and F. C. Krebs, Sol. Energy Mater. Sol. Cells, 2015, 137, 303–310 CrossRef CAS
.
- J. Zhang, X. Gao, Y. Deng, B. Li and C. Yuan, ChemSusChem, 2015, 8, 3882–3891 CrossRef CAS PubMed
.
- E. Leccisi and V. Fthenakis, Prog. Energy, 2020, 2, 032002 CrossRef
.
- S. Castro-Hermosa, M. Top, J. Dagar, J. Fahlteich and T. M. Brown, Adv. Electron. Mater., 2019, 5, 1800978 CrossRef CAS
.
- M. Llanos, R. Yekani, G. P. Demopoulos and N. Basu, Renewable Sustainable Energy Rev., 2020, 133, 110207 CrossRef CAS
.
- R. Vidal, J.-A. Alberola-Borràs, N. Sánchez-Pantoja and I. Mora-Seró, Adv. Energy Sustainability Res., 2021, 2, 2000088 CrossRef CAS
.
- T. B. Korukonda, D. N. Joshi and V. Dutta, Mater. Lett., 2021, 289, 129343 CrossRef CAS
.
- J.-A. Alberola-Borràs, J. A. Baker, F. D. Rossi, R. Vidal, D. Beynon, K. E. A. Hooper and T. M. Watson, Mora-Seró, iScience, 2018, 9, 542–551 CrossRef PubMed
.
| This journal is © The Royal Society of Chemistry 2023 |