Open Access Article
Open Access ArticleWater soluble macrocyclic host for recognition of N-methylquinolinium salts in water†
Yuan-Hong Tian,
Han Qin,
Man-Hua Ding*,
Lin-Li Tang and
Fei Zeng *
*
Department of Biology and Chemistry, Hunan University of Science and Engineering, Yongzhou 425199, China. E-mail: zengfei@iccas.ac.cn; 42979930@qq.com
First published on 15th May 2023
Abstract
In this paper, we reported the synthesis of water soluble macrocyclic arenes 1 containing anionic carboxylate groups. It was found that host 1 could form a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex with N-methylquinolinium salts in water. Moreover, the complexation and decomplexation of the complexes between host and the guests could be achieved by changing the pH of the solution, and the process could also be observed by naked eye.
1 complex with N-methylquinolinium salts in water. Moreover, the complexation and decomplexation of the complexes between host and the guests could be achieved by changing the pH of the solution, and the process could also be observed by naked eye.
Macrocyclic arenes composed of hydroxyl-substituted aromatic rings bridged by methylene or methenyl groups have attracted considerable attention for their wide application in molecular recognition and assembly, biomedicine, materials science, and adsorption separation.1–9 Good examples are calixarenes,10–12 pillar[n]arenes,13–16 prism[5]arene,17 pagoda[n]arene,18,19 geminiarenes,20,21 tiara[5]arene,22,23 biphen[n]arenes,24–26 helic[6]arene27–29 and so on.30,31 Although a large number of macrocyclic arenes have been reported in the last decade. However, reports on the synthesis and applications of water soluble macrocyclic arenes are very limited.32–34 Water soluble macrocyclic arenes have the ability to recognize and assemble in water, which is of practical significance for understanding and mimicking biological processes.35–38 By introducing the water soluble groups into suitable positions on the macrocyclic arenes, water-soluble macrocyclics can be easily obtained. Water soluble carboxylatopillar[n]arenes39–42 and psulfonatocalix[n]arenes43–45 have gained maximum popularity for their easy preparation, π-electron rich hydrophobic cavity, ideal candidates for host–guest binding with cationic guests and application in fabricating responsive supramolecular systems and as nanovalves for trapping and releasing cargo molecules. Moreover, water-soluble carboxylatobiphen[n]arenes and cationic biphen[4,5]arenes46,47 were also been reported. Despite these seminal reports, exploring new water soluble macrocyclic arenes is still very urgent and of great value.
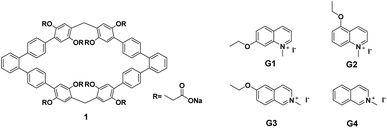
Recently, our group reported the easy synthesis of phenanthrene[2]arene and found that it has a larger cavity,5 which can encapsulate benzene molecules. Inspired by these results, we deduce that if the cavity can be further expanded and then water-soluble groups are introduced, water soluble hosts with the abilities to form different kinds of complexes with guests in water could be obtained. 1,1':2′,1′′-Terphenyl can be regarded as the product of broken triphenylene at position 12a,12b. Thus, using 1,1′:2′,1′′-terphenyl as the building block for the construction of macrocyclic arenes, the cavity should be larger than that of phenanthrene[2]arene. In addition, the introduction of anionic carboxylate groups into the synthesized macrocyclic arenes will certainly improve its water solubility. Herein, we report the synthesis of 1,1′:2′,1′′-terphenyl-based water soluble macrocyclic arenes 1 (Fig. 1). 1H NMR experiments demonstrated that host 1 could form 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes with N-methylquinolinium salts in water. Furthermore, the complexation and decomplexation of the complexes between host 1 and the guests could be chemically controlled by the addition of acid/base, and the process could also be observed by naked eye.
1 complexes with N-methylquinolinium salts in water. Furthermore, the complexation and decomplexation of the complexes between host 1 and the guests could be chemically controlled by the addition of acid/base, and the process could also be observed by naked eye.
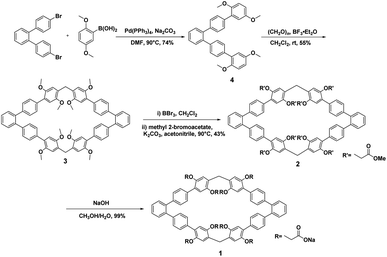
The synthetic route for host 1 was outlined in Scheme 1 and contains four steps. Firstly, compound 4 was obtained by the Suzuki coupling reaction of the commercially available 4,4′′-dibromo-1,1′:2′,1′′-terphenyl with 2,5-dimethoxy phenyl borate in the presence of Pd(PPh3)4 as the catalyst in 74% yield. Then, macrocyclic arenes 3 was synthesized in moderate yield by treatment of 4 with paraformaldehyde and boron trifluoride diethyl etherate in dichloromethane at room temperature. per-Hydroxylated macrocyclic arenes can be quantitatively obtained by the cleavage of the methyl groups in macrocyclic arenes 3 through the reaction with excess BBr3 in CH2Cl2. 2 was prepared in 43% yield by nucleophilic substitution reaction of per-hydroxylated macrocyclic arenes and methyl bromoacetate in the presence of excess of K2CO3. Finally, water soluble host 1 was obtained nearly quantitatively by hydrolysis of 2 in the presence of NaOH in CH3OH/H2O. The structure of host 1 was first confirmed by 1H NMR, 13C NMR as well as HRMS spectra.
We tried to obtain the single crystals of host 1 that suitable for X-ray analysis, but failed. Luckily, single crystals of 3 that suitable for X-ray analysis was obtained by slow evaporation of the solution in CHCl3, providing unambiguous evidence for the construction of new 1,1′:2′,1′′-terphenyl-based macrocyclic arenes. As shown in Fig. 2, the formed macrocyclic arenes 3 has a large cavity with the size of 7.630 Å × 7.249 Å. The distance between the two dimethoxybenzene units that attached to the 1,1′:2′,1′′-terphenyl is measured to be 10.532 Å. Moreover, from the c-axis direction of 3 packing structure, a channel-like architecture is formed. Since macrocyclic arenes 3 has larger cavity, therefore, macrocyclic arenes 1 should also have a large cavity that can be used to complex guest molecules.
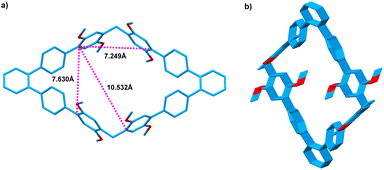 | ||
| Fig. 2 Crystal structures of 3 (a) cavity size; (b) packing of 3 view along the c axis; Solvents and hydrogen atoms not involved in the noncovalent interactions are omitted for clarity. | ||
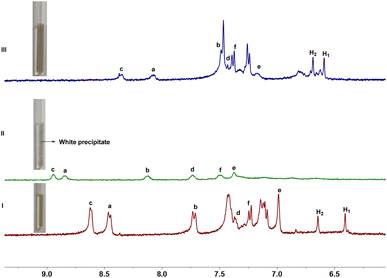
Consequently, the complex ability of host 1 toward N-methylquinolinium salt G1 were first investigated by 1H NMR experiments. As shown in Fig. 3, when 1 and G1 were mixed at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio in D2O, a new set of proton signals that different from 1 and G1 were observed on 1H NMR spectrum, indicating that a new complex 1@G1 was formed. The protons H1 and H2 corresponding to 1 shift upfield by 0.05 and 0.16 ppm respectively, which could be attributed to the strong shielding effect of G1. Meanwhile, the a–f proton signals corresponding to G1 also showed a significantly upfield shift, which might be due to the strong shielding effect of the aromatic rings in host 1. These results indicating that a new complex 1@G1 could be formed. In addition, the spectrum of complex 1@G1 showed only one set of resonances, which indicated that the complexation and decomplexation between host 1 and G1 were a fast exchange on the NMR time scale at room temperature. Similar to the G1, G2–G4 can also form 1
1 molar ratio in D2O, a new set of proton signals that different from 1 and G1 were observed on 1H NMR spectrum, indicating that a new complex 1@G1 was formed. The protons H1 and H2 corresponding to 1 shift upfield by 0.05 and 0.16 ppm respectively, which could be attributed to the strong shielding effect of G1. Meanwhile, the a–f proton signals corresponding to G1 also showed a significantly upfield shift, which might be due to the strong shielding effect of the aromatic rings in host 1. These results indicating that a new complex 1@G1 could be formed. In addition, the spectrum of complex 1@G1 showed only one set of resonances, which indicated that the complexation and decomplexation between host 1 and G1 were a fast exchange on the NMR time scale at room temperature. Similar to the G1, G2–G4 can also form 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex with host 1 in the similar complexation mode to that of complex 1@G1. To further get insights into the complexation process between 1 and G1, 1H NMR spectroscopic titrations experiments were then carried out. By monitoring the change of proton H1 corresponding to 1 upon the addition of G1, a 1
1 complex with host 1 in the similar complexation mode to that of complex 1@G1. To further get insights into the complexation process between 1 and G1, 1H NMR spectroscopic titrations experiments were then carried out. By monitoring the change of proton H1 corresponding to 1 upon the addition of G1, a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex between 1 and G1 was formed by the mole ratio plot. The binding constant Ka of the complex 1@G1 was determined to be 375.5 ± 23.1 M−1 using the BindFit software. The binding constant Ka of the complex 1@G2, 1@G3 and 1@G4 were calculated to be 421.7 ± 19.5 M−1, 385.4 ± 28.4 M−1 and 193.5 ± 19.7 M−1, respectively.
1 complex between 1 and G1 was formed by the mole ratio plot. The binding constant Ka of the complex 1@G1 was determined to be 375.5 ± 23.1 M−1 using the BindFit software. The binding constant Ka of the complex 1@G2, 1@G3 and 1@G4 were calculated to be 421.7 ± 19.5 M−1, 385.4 ± 28.4 M−1 and 193.5 ± 19.7 M−1, respectively.
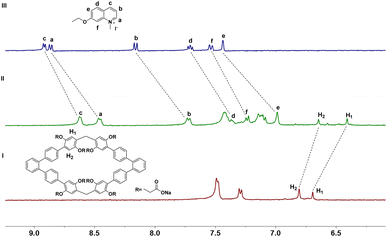 | ||
| Fig. 3 Partial 1H NMR spectra (400 MHz, D2O, 298 K) of (I) free 1, (II) 1 and 1.0 equiv. of G1, and (III) free G1. [1]0 = 1.0 mM. | ||
Since the conversion of anionic carboxylate groups and neutral carboxylic groups can be controlled by the addition of acid and base. Therefore, we wonder whether the complexation and decomplexation of complex 1@G1 can be regulated by acid and base. In order to verify our hypothesis, 1H NMR experiments were carried out. After the addition of aqueous DCl to the solution of complex 1@G1, the solution changed from neutral to acidic, the protons of H1 and H2 corresponding to 1 disappeared, while the a-f proton signals corresponding to G1 shifted downfield to their original values, which suggested the decomplexation of complex 1@G1. Moreover, white precipitates were observed, indicating the formation of water-insoluble protonated host 1. When aqueous NaOD was added into the above mixture, the solution changed from acidic to neutral, the signals of protons a-f in G1 shifted upfield to their complexed values, while the signal of protons H1 and H2 in host 1 appeared again and the white precipitates disappeared, which indicated the reformation of complex 1@G1. After the addition of NaOD, the chemical shift of host and guest protons could not be completely restored, which may due to the formation of NaCl in solution. Since the anion in the guest molecule is iodine, after adding DCl and NaOD, there are iodine and chlorine anions in the solution, resulting in changes in the chemical shift of the formed complex. The appearance and disappearance of the precipitates might be mainly due to the protonation and deprotonation of carboxylate groups on host 1. Addition of aqueous DCl resulted in the precipitation of water-insoluble protonated host 1, and led to the decomplexation of complex 1@G1. However, upon the addition of aqueous NaOD, the carboxylic acid groups of host 1 were converted to carboxylate groups, and complex 1@G1 was reformed. Thus, the complexation and decomplexation of complex 1@G1 could be reversibly controlled by acid/based and observed by naked eye (Fig. 4). Similar to the case of complex 1@G1, the complexation and decomplexation process of complexes 1@G2, 1@G3 and 1@G4 can also be reversibly controlled by acid/based and observed by naked eye.
Conclusions
In summary, we have successfully synthesized 1,1′:2′,1′′-terphenyl-based water soluble macrocyclic arenes 1 that containing of anionic carboxylate groups, and studied its binding ability toward N-methylquinolinium salts in water. It was found that host 1 could form 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex with N-methylquinolinium salts in water. Since the conversion of anionic carboxylate groups and neutral carboxylic groups can be controlled by the addition of acid and base. Thus, the complexation and decomplexation of formed complexes can be achieved by the addition of acid and base and the process can be observed by naked eye. The high affinity ability in water and controllable host–guest system of this new water-soluble host could be utilized to fabricate functional supramolecular assemblies, which are ongoing in our laboratory.
1 complex with N-methylquinolinium salts in water. Since the conversion of anionic carboxylate groups and neutral carboxylic groups can be controlled by the addition of acid and base. Thus, the complexation and decomplexation of formed complexes can be achieved by the addition of acid and base and the process can be observed by naked eye. The high affinity ability in water and controllable host–guest system of this new water-soluble host could be utilized to fabricate functional supramolecular assemblies, which are ongoing in our laboratory.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for the financial support from the National Natural Science Foundation of China (No. 21602055 and 51772091); Natural Science Foundation of Hunan Province (No. 2017JJ3094). Research Project on Teaching Reform of Ordinary Colleges and Universities in Hunan Province: [2018] (No. 436: 670).Notes and references
- C. F. Chen and Y. Han, Acc. Chem. Res., 2018, 51, 2093–2106 CrossRef CAS PubMed.
- T. Schrader and A. D. Hamilton, Functional Synthetic Receptors, Wiley-VCH, Weinheim, Germany, 2005 Search PubMed.
- F. Zeng, L. Cheng, G.-C. Ou, L.-L. Tang and M.-H. Ding, J. Org. Chem., 2022, 87, 3863–3867 CrossRef CAS PubMed.
- M.-H. Ding, J. Liao, L.-L. Tang, G.-C. Ou and F. Zeng, Chin. Chem. Lett., 2021, 32, 1665–1668 CrossRef CAS.
- F. Zeng, L. Cheng, W.-J. Zhang, L.-L. Tang and X.-F. Wang, Org. Chem. Front., 2022, 9, 3307–3311 RSC.
- F. Zeng, X.-S. Xiao, S.-F. Gong, L. Yuan and L.-L. Tang, Org. Chem. Front., 2022, 9, 4829–4833 RSC.
- Q. Shi, X. Wang, B. Liu, P. Qiao, J. Li and L. Wang, Chem. Commun., 2021, 57, 12379–12405 RSC.
- H. Yao, S.-Y. Li, H. Zhang, X.-Y. Pang, J.-L. Lu, C. Chen, W. Jiang, L.-P. Yang and L.-L. Wang, Chem. Commun., 2023, 59, 5411–5414 RSC.
- F. Zeng, L.-L. Tang, H. Yu, F.-P. Xu and L. Wang, Chin. Chem. Lett., 2023 DOI:10.1016/j.cclet.2023.108304.
- P. Neri, J. L. Sessler and M.-X. Wang, Calixarenes and Beyond, Springer, Cham, Switzerland, 2016 Search PubMed.
- W. Sliwa and C. Kozlowski, Calixarenes and Resorcinarenes: Synthesis, Properties and Applications, Wiley-VCH, Weinheim, Germany, 2009 Search PubMed.
- Y. Ding, J. Jiao, B. Sun, Z. Yang, L. Chen and L. Wang, Chin. Chem. Lett., 2021, 32, 3539–3543 CrossRef CAS.
- T. Ogoshi, S. Kanai, S. Fujinami, T.-A. Yamagishi and Y. Nakamoto, J. Am. Chem. Soc., 2008, 130, 5022–5023 CrossRef CAS PubMed.
- T. Ogoshi, Pillararenes, Royal Society of Chemistry, Cambridge, U.K., 2016 Search PubMed.
- T. Ogoshi, T.-A. Yamagishi and Y. Nakamoto, Chem. Rev., 2016, 116, 7937–8002 CrossRef CAS PubMed.
- M. Xue, Y. Yang, X. Chi, Z. Zhang and F. Huang, Acc. Chem. Res., 2012, 45, 1294–1308 CrossRef CAS PubMed.
- P. D. Sala, R. D. Regno, C. Talotta, A. Capobianco, N. Hickey, S. Geremia, M. De Rosa, A. Spinella, A. Soriente, P. Neri and C. Gaeta, J. Am. Chem. Soc., 2020, 142, 1752–1756 CrossRef PubMed.
- X.-N. Han, Y. Han and C.-F. Chen, J. Am. Chem. Soc., 2020, 142, 8262–8269 CrossRef CAS PubMed.
- X.-N. Han, Q.-S. Zong, Y. Han and C.-F. Chen, CCS Chem., 2021, 3, 738–750 Search PubMed.
- J. R. Wu and Y. W. Yang, J. Am. Chem. Soc., 2019, 141, 12280–12287 CrossRef CAS PubMed.
- J. R. Wu, G. Wu and Y. W. Yang, Acc. Chem. Res., 2022, 55, 3191–3204 CrossRef CAS PubMed.
- W. Yang, K. Samanta, X. Wan, T. U. Thikekar, Y. Chao, S. Li, K. Du, J. Xu, Y. Gao, H. Zuilhof and A. C.-H. Sue, Angew. Chem., Int. Ed., 2020, 59, 3994–3999 CrossRef CAS PubMed.
- M. Guo, X. Wang, C. Zhan, P. D. Drouhard, W. Li, K. Du, M. A. Olson, H. Zuilhof and A. C.-H. Sue, J. Am. Chem. Soc., 2018, 140, 74–77 CrossRef CAS PubMed.
- H. Chen, J. Fan, X. Hu, J. Ma, S. Wang, J. Li, Y. Yu, X. Jia and C. Li, Chem. Sci., 2015, 6, 197–202 RSC.
- Z.-Y. Zhang and C. Li, Acc. Chem. Res., 2022, 55, 916–929 CrossRef CAS.
- K. Xu, Z. Y. Zhang, Z. Zhou and C. Li, Chin. Chem. Lett., 2022, 33, 2451–2454 CrossRef CAS.
- G.-W. Zhang, P.-F. Li, Z. Meng, H.-X. Wang, Y. Han and C.-F. Chen, Angew. Chem., Int. Ed., 2016, 55, 5304–5308 CrossRef CAS PubMed.
- Q. Shi and C.-F. Chen, Org. Lett., 2017, 19, 3175–3178 CrossRef CAS PubMed.
- Q. Shi, Y. Han and C.-F. Chen, Chem.–Asian J., 2017, 12, 2576–2582 CrossRef CAS PubMed.
- J. Q. Wang, Y. Han and C.-F. Chen, Chem. Commun., 2021, 57, 3987–3990 RSC.
- J. Li, H.-Y. Zhou, Y. Han and C.-F. Chen, Angew. Chem., Int. Ed., 2021, 60, 21927–21933 CrossRef CAS PubMed.
- L. Escobar and P. Ballester, Chem. Rev., 2021, 121, 2445–2514 CrossRef CAS PubMed.
- F. Zeng and C.-F. Chen, Org. Biomol. Chem., 2015, 13, 1988–1991 RSC.
- S. Kubik, Chem. Soc. Rev., 2010, 39, 3648–3663 RSC.
- J. Murray, K. Kim, T. Ogoshi, W. Yao and B. C. Gibb, Chem. Soc. Rev., 2017, 46, 2479–2496 RSC.
- W. Wang, Z. Li, C. Song, J. Yang and Y. W. Yang, Molecules, 2022, 23, 8554 CrossRef PubMed.
- J. Mendoza, L. Cruz, V. Freitas, F. Pina and N. Basilio, Molecules, 2021, 26, 5389 CrossRef CAS.
- J.-R. Wu, G. Wu, Z. Cai, D. Li, M. H. Li, Y. Wang and Y.-W. Yang, Molecules, 2022, 27, 6259 CrossRef CAS PubMed.
- S. Dasgupta and P. S. Mukherjee, Org. Biomol. Chem., 2017, 15, 762–772 RSC.
- T. Ogoshi, M. Hashizume, T. Yamagishi and Y. Nakamoto, Chem. Commun., 2010, 46, 3708–3710 RSC.
- Z. Li, J. Yang, G. Yu, J. He, Z. Abliz and F. Huang, Chem. Commun., 2014, 50, 2841–2843 RSC.
- J. Yang, X. Chi, Z. Li, G. Yu, J. He, Z. Abliz, N. Li and F. Huang, Org. Chem. Front., 2014, 1, 630–633 RSC.
- D. S. Guo and Y. Liu, Acc. Chem. Soc., 2014, 47, 1925–1934 CrossRef CAS.
- Z. Liu, X. Sun, X. Dai, J. Li, P. Li and Y. Liu, J. Mater. Chem. C, 2021, 9, 1958–1965 RSC.
- L. Memmi, A. Lazar, A. Brioude, V. Ball and A. W. Coleman, Chem. Commun., 2001, 2474–2475 RSC.
- X. Huang, X. Zhang, T. Qian, J. Ma, L. Cui and C. Li, Beilstein J. Org. Chem., 2018, 14, 2236–2241 CrossRef CAS PubMed.
- J. Ma, Q. Meng, X. Hu, B. Li, S. Ma, B. Hu, J. Li, X. Jia and C. Li, Org. Lett., 2016, 18, 5740–5743 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis of 1–4; the 1H and 13C NMR spectra of 1–4; CCDC: 2255801 for 3. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3ra02447d |
| This journal is © The Royal Society of Chemistry 2023 |