Open Access Article
Open Access ArticleThe development of chiral metal–organic frameworks for enantioseparation of racemates†
Farzana Yasmeen ,
Uzma Yunus
,
Uzma Yunus *,
Moazzam H. Bhatti
*,
Moazzam H. Bhatti ,
Muhammad Sher and
Muhammad Nadeem
,
Muhammad Sher and
Muhammad Nadeem
Department of Chemistry, Allama Iqbal Open University, Islamabad, Pakistan. E-mail: uzma.yunus@aiou.edu.pk; uzma_yunus@yahoo.com; Tel: +9251-9057818 Tel: +9251-5975200
First published on 2nd June 2023
Abstract
MIL-101(Cr), an achiral metal–organic framework, made up of a terephthalic acid ligand and a metal chromium ion was selected as a template. Its structural features are unsaturated Lewis acid sites that can be easily activated and it has an extremely high specific surface area, big pore size, and good thermal/chemical/water stability. This achiral framework was modified to introduce chirality within the structure to develop chiral metal–organic frameworks (CMOFs). Here, natural chiral ligands, amino acids (L-proline, L-thioproline and L-tyrosine), were selected for post synthetic modification (PSM) of MIL-101(Cr). This is a very simple, clean and facile methodology with respect to the reactants and reaction conditions. CMOFs 1–3 abbreviated as MIL-101-L-proline (CMOF-1), MIL-101-L-thioproline (CMOF-2) and MIL-101-L-tyrosine (CMOF-3) were prepared by introducing L-proline, L-thioproline and L-tyrosine as chiral moieties within the framework of (Cr). These CMOFs were characterized by FTIR, PXRD, SEM, and thermo gravimetric analysis. Chirality within these CMOFs 1–3 was established by circular dichroism (CD) and polarimetric methods. These three CMOFs 1–3 showed enantioselectivity towards RS-ibuprofen, RS-mandelic acid and RS-1-phenylethanol to varying extents. Their enantioselectivity towards racemates was studied by chiral HPLC and polarimetry.
1. Introduction
The porous coordination networks known as metal–organic frameworks (MOFs) are created by combining metal ions and different organic linkers. These MOFs have been introduced as a large class of porous materials with extraordinary surface area, varied functionality, adaptable composition, and increased porosity. One of the profound features of MOFs is that their structure can be tuned according to applications.1–5 Due to these properties, MOFs have recently attracted significant attention in heterogeneous catalysis, optics, biomedical applications, gas storage and especially in gas chromatography (GC) and high-performance liquid chromatography (HPLC) for the enantioseparation of racemic compounds.6–16Knowledge of chirality is crucial in determining the origin of life since all chiral amino acids in enzymes are only found in their “L” form. In the field of pharmaceutical and agriculture, chiral enantiopure compounds are also important. Due to their potential use in catalysis and enantioselective separations, chiral and porous materials, like MOFs and chiral inorganic zeolites, are greatly coveted. Depending on their distinctive features, chiral metal–organic frameworks (CMOFs), in particular CMOFs with entangled systems, have demonstrated outstanding significance in recent years.17–20 More than 30 different types of CMOFs have been created and utilised to study enantioselective adsorption so far. CMOFs face additional difficulties because of their lower stability in severe and humid environments. These problems limit the practical use of CMOFs. So, there is still dire need to explore the synthesis and applications of CMOFs.21,22
To the best of our knowledge, direct synthesis and post-synthetic modification are the key areas of focus when designing CMOFs for enantiomers separation. In case of direct synthesis, enantiopure ligands are usually used such as chiral bridging ligand, (R)-6,6-dichloro-2,2-dihydroxy-1,1-binaphthyl-4,4-bipyridine, which possess the bipyridyl group as primary and orthogonal chiral 2,2-dihydroxy as secondary functionality in their structure. A chiral 3,3′,6,6′- or 4,4′,6,6′-tetra(benzoate) ligand prepared from 1,1′-binaphthyl-2,2′-phosphoric acid has been used to prepare a porous CMOF possessing chiral pockets just like enzymes.23,24 Many dipeptides and tripeptides also act as enantiopure ligands for the construction of CMOFs.25 Another example of synthesis of amino acid based CMOF is development of D-his-ZIF-8. In which D-histidine is added in the reaction mixture as an enantiopure ligand which is found to be responsible of chirality in the CMOF structure.26
CMOFs can also be obtained from spontaneous resolution as a result of entanglement of helical molecular chains developed from achiral components. These CMOFs usually possess helicity of chirality. One of the best examples of three dimensional CMOFs synthesis by the spontaneous resolution is synthesis of CMOFs from achiral ligand 5-(pyridine-3-yl)isophthalic acid with different metals. Axial chirality in these CMOFs is transmitted throughout the structure.27–29
But, direct synthesis often relies on the self-assembly of chiral building blocks but it is usually hampered by the difficult and expensive chiral precursor synthesis processes resulting unpredictable structure and performance of the CMOFs produced.
Hence, due to the precise design of pore size/shape/surface, postsynthetic modifications (PSM) have recently been a substitute method to add chiral functions into MOFs.30–36 Various CMOFs are synthesized by PSM of achiral MOFs.37–43 Such as CMOF which is difficult to obtain by the simple solvothermal postsynthetic method, it is prepared alternatively by the introduction of two chiral saline moieties in UiO-68.44 Similarly, a very facile and economically favourable method is introduced for the Zr-based MOFs by the replacement of coordinated molecules with zirconium metal in MOF-808. Formate molecules in the MOF-808 are exchanged with the chiral linker such as L-histidine, L-glutamic acid and L-tartaric acid by simply soaking the MOF-808 in salt solutions of these amino acids.45 Other two important CMOFs, CMIL-1 and CMIL-2 are produced by the coordination of L-proline based ligands with metal centre using post synthetic strategy.46 But, still CMOFs development faces additional difficulties in tuning the structure and properties of CMOFs. Moreover, introduction of chirality in MOFs is also very challenging in this case. Therefore, to overcome these problems, we initially selected MIL-101 (MIL, Matérial Institut Lavoisier), as the parent achiral framework for the synthesis of CMOFs 1–3 following this postsynthetic strategy, because it has a thermally and chemically robust structure with large pores (2.6–2.8 nm). In MIL-101 structure, open metal coordination sites can be created to attach chiral organic ligands. The insoluble behaviour of MIL-101 in organic solvents and water make it a suitable candidate for solid stationary phase.47 In short, to find a simple and low-cost method for synthesizing CMOFs is challenging but also highly desired. Moreover, the right chiral additive to permit chiral transfer while maintaining the framework topology remains a difficult task.48 For this purpose, we chose natural amino acids such as L-proline, L-thioproline and L-tyrosine as chiral ligand due to easy accessibility, availability, stability, and their coordination ability with metal centre through strong intermolecular interactions.49 In addition, amino acids especially L-proline may also act as modulator in developing structure of CMOFs.50–57
Various CMOFs has been reported for enantioselective separation especially by using chromatographic techniques.58–63 Their porous functionalities and chirality have garnered a lot of interest in enantioseparation as chiral selectors because enantioseparation play a vital role in pharmacology, molecular biology, drug delivery.64–68 Therefore, to demonstrate a potential use of CMOFs in chiral separations, CMOFs are typically evaluated in enantioselective adsorption as chiral stationary phase. But, only a few numbers of CMOFs have been observed as chiral stationary phase in GC and LC for racemic substances. On the other hand, it is difficult to investigate further CMOFs as new stationary phase for enantioseparation because research on CMOFs employed as stationary phases using HPLC is still in its infancy.69–71
So, in our study, enantioselective adsorption capabilities of the three newly synthesized CMOFs 1–3 are tried to check for separation of different racemates. Three racemic compounds, RS-ibuprofen, RS-mandelic acid and RS-1-phenylethanol have been selected as sample. For this purpose, batch experiment method and simple glass column chromatography are used as enantioseparation technique as explained in experimental section in detail.72
2. Experimental
2.1 Materials and methods
L-Proline, L-thioproline, L-tyrosine, RS-ibuprofen, RS-mandelic acid, RS-1-phenylethanol, Cr(NO3)3·9H2O, benzene dicarboxylic acid (BDC), dimethylformamide (DMF) and all organic solvents were purchased from Sigma-Aldrich. All provided solvents such as n-hexane, 2-propanol (IPA) and trifluoroacetic acid (TFA) were of HPLC grade. All compounds and reagents were of laboratory grade and used without further purification. Powder-X-ray diffraction (PXRD) patterns were recorded using a PANalytical X'Pert pro diffractometer with a solid-state detector using a Cu Kα source (46 kV, 35 Am, 10 divergences, receiver of 0.3 mm, anti-scatter slits, and detector) and Fourier transform infrared spectroscopy (FTIR) in ATR mode over a range of 4000 to 450 cm−1 using Varian FTS-800 infrared spectrophotometer. Bruker 300 MHz spectrophotometer, CHNS analysis was done by Vario MICRO cube elemental analyzer. Thermo-gravimetric analyzer, Hi-Res TGA 2950 (flow rate = 0.1 L min−1) was used for TGA analysis. Other analysis such as ICP-MS was recorded using inductively coupled plasma (NexION 5000 ICP-MS) and circular dichroism studies were carried out with Jasco J-710 CD in the range of 300–220 nm, response time 0.25 sec, sensitivity 100 mdeg, resolution 1 nm, bandwidth 1.0 nm, accumulation 5 at a speed 500 nm min−1 in a cell length of standard 1 cm × 1 cm quartz cuvettes at 25 °C. Water was used as solvent for the preparation of samples for analysis. CMOFs were not completely dissolved in water, so very dilute water suspension were used for this purpose. The ADP400 polarimeter was used to find observed optical rotation of prepared known solutions. A sample glass tube of length 1.5 dm was used. The 3-flex surface area analyzer (Quantachrome Nova 2200e) was used to record N2 adsorption data.High performance liquid chromatography (HPLC) using Hitachi L-6200 and L-6000 pumps, a chiral column, CHIRALCEL® OD, 250 × 4.6 mm, 10 μm, a UV/vis detector model L-4200 Hitachi operating at (max) 268 nm, and an eluting mixture of n-hexane, 2-propanol and trifluoroacetic acid was used to acquire data. All measurements were done at room temperature (25 °C).
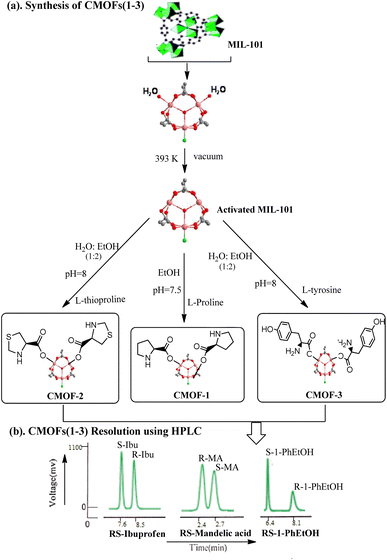
2.2 Synthesis of MIL-101 MOF
10 mmol each of Cr(NO3)3·9H2O and 1,4-benzenedicarboxylic acid (10 mmol) were dissolved in 50 mL of water by sonication (30 minutes) and then transferred to Teflon lined autoclave. The Teflon-lined steel autoclave was heated for 8 hours at 220 °C in a preheated oven. After the completion of reaction, a green-colour product was obtained which was separated by centrifugation. The product was washed three times with DMF (3 × 100 mL stirred for 20 minutes). After washing with DMF, it was stirred overnight at room temperature in chloroform and then separated by centrifugation. Finally, the green product was dried overnight at 80 °C in vacuum oven.The FT-IR was recorded in ATR mode, ν− (cm−1): 3407 (O–H stretching), 1619 (COO− stretching), 1398 (COO− bending), 1106 (C–O stretching), 744 (C–H bending). PXRD (2ϑ): 5.9, 9.11, and 16.6.
2.3 Synthesis of CMOFs 1–3 by post synthetic method (PSM)
For the synthesis of CMOFs (CMOF-1, CMOF-2 and CMOF-3), post synthetic modifications (PSM) of MIL-101 was done in two steps; in the first step the activation of (3.00 mmol) of MIL-101 was done by heating in a vacuum oven at 120 °C for 24 hours. The activated product was then cooled to room temperature and the vacuum was slowly filled with N2 gas. In second step three CMOFs 1–3 were developed from activated MIL-101. General procedure is reported here.7.0 mmol of ligand (L-proline/L-thioproline/L-tyrosine) was dissolved in ethanol and 3.00 mmol of activated MIL-101 was added in ligand solution. The reaction mixture was transferred in a screw capped glass vial and kept at 70 °C for three days. After the completion of reaction time, the product was recovered by centrifugation. The green product was washed three times with ethanol then with acetone and dried under vacuum at 80 °C for 8 hours before characterization as detail of synthesis is given in Scheme 1.
2.4 Enantioseparation by CMOFs 1–3 as solid stationary phase
Enantioseparation capabilities of synthesized CMOFs 1–3 as were examined by batch and simple glass column chromatographic methods. In these two techniques, CMOFs 1–3 used as solid stationary phase that selectively separate the enantiomers of three racemic compounds by the procedure given below.3. Results and discussion
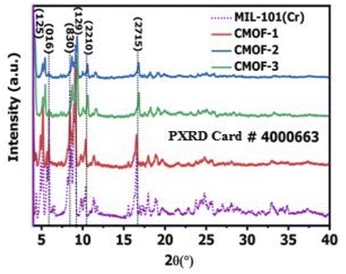
MIL-101 was selected as achiral template for the post synthetically modified MIL-101 chiral metal–organic frameworks (CMOFs 1–3). So, firstly, MIL-101 was synthesized by using method described in Experimental section.73 Various techniques were employed to confirm the structure of product e.g. PXRD, FTIR, 1HNMR and thermo gravimetric method. All the results completely matched the reported data of MIL-101 which confirmed the development of structure. The structural integrity of MIL-101 was confirmed by the position and intensities of PXRD diffractions peaks. The observed PXRD pattern was found consistent with the reported pattern of MIL-101.74 No additional signals were observed in the X-ray diffraction pattern as shown in Fig. 1.After the synthesis of MIL-101, open coordination sites were created by removing the water molecules attached to chromium metal in the architecture. These coordination sites were used to coordinate chiral ligands which induced chirality in the structure. The activation of MIL-101 by removing coordinated water molecules was also confirmed by FTIR analysis as given in ESI (Fig. S1†).
3.1 Characterization of synthesized CMOFs 1–3
Chiral ligands were incorporated in activated MIL-101 and three naturally occurring amino acids were used as chiral ligands (L-proline, L-thioproline, and L-tyrosine) for the synthesis of post synthetically modified MIL-101 chiral metal–organic frameworks as carboxylic group present in their structures possess maximum probability to coordinate with the active chromium metal node. After the development of three CMOFs 1–3 by post synthetic modification as given in Section 2.3, all three CMOFs 1–3 were characterized by PXRD, FTIR, BET, CD, SEM, TGA and polarimetric methods.In PXRD analysis, same pattern for newly synthesized chiral metal–organic frameworks, CMOF-1, CMOF-2 and CMOF-3 with some decreased intensities were observed as that of MIL-101. It showed that basic architecture of MIL-101 remained intact after the coordination with chiral ligands. Moreover, appearance of high intensity peaks below 10, 2ϑ-value as observed at 5.4, 5.9, 8.6, 9.3, 10.5 and 16.7 for CMOF-1, at 5.4, 5.9, 8.6, 9.3, 10.5 and 16.7 for CMOF-2.
And for CMOF-3 at 5.4, 5.9, 8.5, 9.4 positions also indicated development of the architecture of three CMOFs 1–3 as shown in Fig. 1.
These 2ϑ values confirmed that the structures of these CMOFs are highly porous in nature due to presence of empty spaces within the architecture of CMOFs 1–3.
The phase purity and crystallinity of structures is also determined which showed that decrease in crystallinity occurred by involvement of chiral ligands. The different value of decrease in crystallinity also indicated the incorporation of new chiral ligand within the architecture of MIL-101 as given in Table 1. It is also observed that average crystallite size decreases as larger chiral moiety was introduced within the structure as calculated in CMOF-1, CMOF-2 and CMOF-3. In case of CMOF-3, crystallite size becomes smaller as compared to CMOF-1 and CMOF-2 due to large size of L-tyrosine as compared to L-proline and L-thioproline ligands.
| Compounds | 2θ (°) | FWHM (rad) | Interplanar spacing (nm) | Crystallite size (nm) | Average crystallite size (nm) | Area of crystalline peaks (nm2) | Area of all peaks (nm2) | Crystallinity (%) |
|---|---|---|---|---|---|---|---|---|
| MIL-101 | 5.1 | 0.001 | 1.734 | 95.4 | 33.5 | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 508 508 |
41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 301 301 |
61.8 |
| 5.7 | 0.007 | 1.549 | 19.5 | |||||
| 5.8 | 0.015 | 1.520 | 9.1 | |||||
| 5.9 | 0.008 | 1.499 | 18.1 | |||||
| 9.0 | 0.009 | 0.977 | 15.0 | |||||
| 9.1 | 0.004 | 0.975 | 31.7 | |||||
| 16.5 | 0.004 | 0.536 | 36.5 | |||||
| 18.1 | 0.003 | 0.489 | 42.9 | |||||
| CMOF-1 | 3.5 | 0.001 | 2.503 | 95.416 | 24.1 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 906 906 |
28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 509 509 |
45.3 |
| 5.2 | 0.007 | 1.712 | 19.460 | |||||
| 5.5 | 0.015 | 1.598 | 9.134 | |||||
| 8.5 | 0.004 | 1.042 | 31.676 | |||||
| 9.1 | 0.004 | 0.968 | 36.768 | |||||
| 9.2 | 0.003 | 0.960 | 43.312 | |||||
| 16.5 | 0.076 | 0.537 | 1.797 | |||||
| 18.0 | 0.094 | 0.494 | 1.460 | |||||
| 18.9 | 0.111 | 0.469 | 1.229 | |||||
| 19.7 | 0.129 | 0.451 | 1.061 | |||||
| CMOF-2 | 6.4 | 0.056 | 3.677 | 2.157 | 12.3 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 895 895 |
56.2 |
| 9.3 | 0.081 | 0.272 | 29.130 | |||||
| 12.2 | 0.106 | 5.077 | 1.556 | |||||
| 12.3 | 0.107 | 4.804 | 1.644 | |||||
| 16.7 | 0.146 | 0.243 | 32.326 | |||||
| 16.7 | 0.146 | 576.870 | 0.014 | |||||
| 19.2 | 0.167 | 0.269 | 29.136 | |||||
| 31.1 | 0.271 | 3.208 | 2.386 | |||||
| 6.4 | 0.056 | 3.677 | 2.157 | |||||
| CMOF-3 | 5.3 | 0.046 | 0.631 | 12.568 | 8.9 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 492 492 |
21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 137 137 |
59.1 |
| 8.7 | 0.076 | 0.242 | 32.755 | |||||
| 9.2 | 0.081 | 0.368 | 21.501 | |||||
| 9.2 | 0.081 | 10.824 | 0.732 | |||||
| 9.2 | 0.081 | 10.879 | 0.728 | |||||
| 9.2 | 0.081 | 15.887 | 0.498 | |||||
| 19.8 | 0.172 | 8.171 | 0.958 | |||||
| 20.8 | 0.181 | 5.813 | 1.344 |
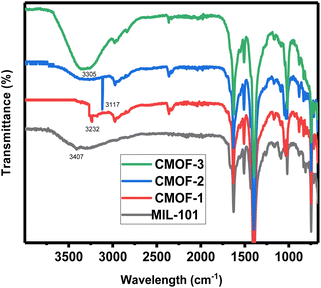
FTIR analysis was done for characterization of coordination complex of chiral ligands with the activated sites (metal node) of MIL-101 after removal of water molecules. In FTIR spectra, peaks relevant to chiral organic ligands were observed in CMOF-1, CMOF-2 and CMOF-3. For CMOF-1, –NH absorption peaks appeared at 3232 cm−1; 3117 for CMOF-2 at; or CMOF-2 but in case of CMOF-2 this peak is somewhat sharp as compared to CMOF-1. In FTIR spectrum of CMOF-3, N–H peak is not more visible due to presence of –OH group of L-tyrosine. In CMOF-3, broader peak was observed as compared to CMOF-1 and CMOF-2 because in CMOF-3, free hydroxyl of L-tyrosine is present. Moreover, shifting of peaks from 1627 to 1619 cm−1 (COO− asymmetric stretching vibrations) and 1398 to 1391 cm−1 (COO− bending vibrations) also observed in comparison with the spectrum of achiral MIL-101 and CMOFs 1–3 as mentioned in Fig. 2.
For further confirmation of ligands involvement within the CMOFs 1–3, 1HNMR technique was employed by dissociating the CMOFs 1–3 in KOH solution. In NMR analysis, peaks at 3.59 (1H), 2.78 (2H), 1.65 (2H), 1.62 (2H) ppm appeared for CMOF-1, at 3.59 (1H), 2.78 (2H), 1.65 (2H) for CMOF-2 and at 7.6 (2H), 4.7 (1H), 3.1 (2H) in case of CMOF-3 as given in ESI (Fig. S2–S4†).
As architectures of CMOFs 1–3 were dissociated, some fragments of chiral ligands indicated by 1HNMR spectra, but no prominent coupling was observed in 1HNMR analysis.
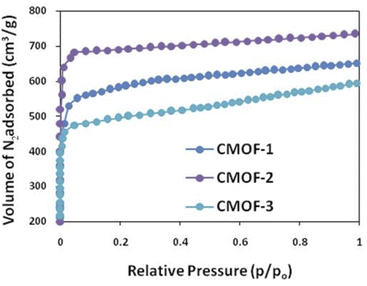
MIL-101 was selected due to pore size 2.81 nm width and after the incorporation of L-amino acids as ligands, decrease in pore size was observed. CMOF-1 has pore size of 2.21 nm with surface area 832 m2 g−1, CMOF-2 has pore size of 2.16 nm with surface area of 811 m2 g−1 and CMOF-3 has a pore size of 2.18 nm and surface area of 1056 m2 g−1. All other parameters of BET analysis are given in detail in Table 2.
| Adsorbent | BET (m2 g−1) | Pore volume | Pore size (nm) |
|---|---|---|---|
| CMOF-1 | Single point surface area at P/Po = 0.200279717 = 813 m2 g−1 | Single point adsorption total pore volume of pores less than 78.2840 nm diameter at P/Po = 0.974635832 = 0.89 cm3 g−1 | Adsorption average pore width (4 V/A by BET) = 2.21 |
| BET surface area = 832 m2 g−1 | BJH adsorption cumulative volume of pores between 1.7000 nm and 300.0000 nm diameter = 0.8714 cm3 g−1 | BJH adsorption average pore diameter (4 V/A) = 2.60 nm | |
| Langmuir surface area = 967 m2 g−1 | BJH desorption cumulative volume of pores between 1.7000 nm and 300.0000 nm diameter = 0.88 cm3 g−1 | BJH desorption average pore diameter (4 V/A) = 2.01 nm | |
| CMOF-2 | Single point surface area at P/Po = 0.200279717 = 815 m2 g−1 | Single point adsorption total pore volume of pores less than 78.2840 nm diameter at P/Po = 0.974635832 = 0.80 cm3 g−1 | Adsorption average pore width (4 V/A by BET) = 2.16 nm |
| BET surface area = 811 m2 g−1 | BJH adsorption cumulative volume of pores between 1.7000 nm and 300.0000 nm diameter = 0.821 cm3 g−1 | BJH adsorption average pore diameter (4 V/A) = 2.19 nm | |
| Langmuir surface area = 887 m2 g−1 | BJH desorption cumulative volume of pores between 1.7000 nm and 300.0000 nm diameter = 0.814 cm3 g−1 | BJH desorption average pore diameter (4 V/A) = 2.1 nm | |
| CMOF-3 | Single point surface area at P/Po = 0.200279717 = 957 m2 g−1 | Single point adsorption total pore volume of pores less than 78.2840 nm diameter at P/Po = 0.974635832 = 0.79 cm3 g−1 | Adsorption average pore width (4 V/A by BET) = 2.18 nm |
| BET surface area = 1056 m2 g−1 | BJH adsorption cumulative volume of pores between 1.7000 nm and 300.0000 nm diameter = 0.72 cm3 g−1 | BJH adsorption average pore diameter (4 V/A) = 2.31 nm | |
| Langmuir surface area = 1067 m2 g−1 | BJH desorption cumulative volume of pores between 1.7000 nm and 300.0000 nm diameter = 0.792 cm3 g−1 | BJH desorption average pore diameter (4 V/A) = 2.30 nm |
The BET analysis of these three CMOFs 1–3 showed that these are mesoporous in nature and have the capability for adsorption. It has been observed that CMOF-3 possess greater adsorption ability of N2 as compared to CMOF-1 and CMOF-2 as shown in Fig. 3. CMOF-1 and CMOF-2 have comparable adsorption due to minor size difference of incorporated amino acids in these CMOFs.
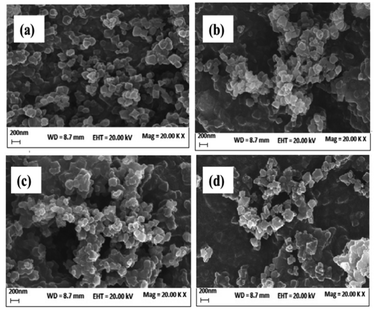
The scanning electron microscopy (SEM) showed non uniformity in MIL-101 and CMOFs 1–3 due to some coagulation of particles as shown in Fig. 4. Moreover, particle size calculation by using SEM image showed that particles exist in the range of 134 nm to 236 nm for CMOF-1, in the range of 62 nm to 255 nm for CMOF-2 and in the range of 97 nm to 255 nm for CMOF-3.
The chiral nature of CMOFs 1–3 was confirmed by polarimetric method and circular dichroism (CD). The specific optical rotation calculated for CMOF-1 and CMOF-2 were −38° and −40° respectively but for CMOF-3 was −60°.
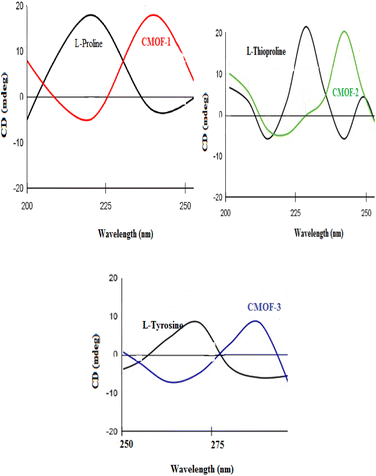
For further confirmation, CD spectrum was recorded as pure L-proline showed positive cotton effect at 222 nm in water solution but a suspension of CMOF-1 in water shifted the value from 222 nm to 241 nm as shown in Fig. 5(a) which confirmed the coordination of L-proline ligand with chromium centre and retained the chirality, showing positive cotton effect.
In CD spectrum of CMOF-2, positive cotton effect at 230 nm was observed for L-thioproline in water solution and at 245 nm for CMOF-2. The comparison of both cures showed that shifting of ligand peak to high wavelength value from 230 nm to 245 nm with same positive cotton effect in case of CMOF-2 which confirmed the incorporation of ligand within the structure in Fig. 5(b).
In CD spectrum of CMOF-3, positive cotton effect at 271 nm was observed for L-tyrosine ligand in water solution and at 287 nm for CMOF-3.
The comparison of both curves showed that shifting of ligand peak to high wavelength value from 271 nm to 287 nm with same positive cotton effect in case of CMOF-3 which confirmed presence of chirality due to attachment of chiral ligand within the structure as indicated in Fig. 5(c).
3.2 Determination of CMOFs 1–3 purity and stability
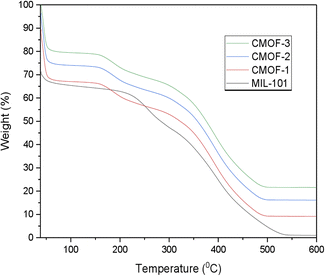
After the complete characterization of CMOFs, their purity and stability was also examined because purity and stability of architecture is highly concerned while performing enantioseparation. The stability of CMOFs 1–3 was determined by thermal gravimetric measurements. The results of TGA also justify the purity of MIL-101 and synthesized CMOFs 1–3. TGA analysis showed firstly ethanol molecules removal at 82 °C and then trapped water molecules 12% removal occurred at 243 °C. The removal of water molecules at this high temperature of 243 °C indicated that these water molecules have very strong coordination with the metal centre. The further weight loss of chiral ligands and benzene dicarboxylic acid ligand was observed in the temperature range of 378 °C to 484 °C.At the end, 24% of Cr2O3 remained as residue at 600 °C in case of CMOFs 1–3 as given Fig. 6. The FTIR spectra also clarify the purity of CMOFs 1–3 because no extra peaks appeared in FTIR spectra of CMOFs 1–3.
3.3 Enantioseparations studies by synthesized CMOFs 1–3
Resolution of racemates by using chiral stationary phases is considered one of the best strategies to get enantiopure compounds. For this purpose, porous CMOFs having maximum stability in humid environment are very fascinating material as chiral stationary phase. The enantioselective properties of synthesized CMOFs 1–3 as chiral stationary phase were examined by using two methods, batch experiment method and simple glass chromatography. Racemate such as RS-ibuprofen (IBU), RS-mandelic acid (MA) and RS-1-phenylethanol (PhEtOH) were selected to find the enantioselectivities of synthesized CMOFs 1–3 depending on easy availability, high thermal stability, possible accessible molecular size, or suitable minimum kinetic diameter (MKD) and the polar nature of these compounds due to presence of polar functional groups. The main reason of selection of these polar groups containing racemate is their maximum interactions possibility with chiral stationary phase as CMOFs 1–3 are also polar in nature.Batch experiment was performed to calculate enantioselectivities of CMOFs 1–3 as given in Scheme 2. Second method of simple glass column chromatography was also employed to check the enantioselective nature of CMOFs 1–3 however; best results were obtained by batch experiment.
It was observed that best resolution was shown by RS-1-phenylethanol as compared to RS-ibuprofen and RS-mandelic acid in case of all three synthesized CMOFs 1–3. To evaluate the enantioselectivities of CMOFs 1–3 for RS-1-phenylethanol, firstly HPLC analysis for racemic solution was done. The enantiomeric excess (%ee) value was calculated for racemate and for CMOFs 1–3 one by one using chiral HPLC and compared their values as shown in ESI (Fig. S5–S7†).
To explain the mechanism of chiral separation by these CMOFs 1–3 used as chiral stationary phase employed both in batch experiment and glass chromatography, nature of chiral points within the pockets of CMOFs stationary phases was studied. The FTIR spectra of these CMOFs clearly showed that amino group in the structure is available for interactions with the incoming chiral moieties as given in Fig. 2. As a result of these interactions, diastereomeric relationship may develop. The HPLC results as given in ESI† proved that these interactions of chiral points present within the CMOFs stationary phases with chiral groups exist in racemates are very weak which develop temporarily then detach resulting elution of one enantiomer than the other one.
In case of enantioseparation of 1-phenylethanol, the enhanced enantioselective ability for CMOFs 1–3 may be attributed to the small volume of RS-1-phenylethanol (125.45 cubic angstrom) and optimum orientation of hydroxyl group of 1-phenylethanol with the functional groups present in the structure of CMOFs 1–3.75–79 CMOF-1 and CMOF-2 resolved the enantiomers of 1-phenylethanol with 19% ee value.
Whereas CMOF-3 showed greater enantioselectivities for this racemic compound with 46% enantiomeric excess as shown in HPLC spectrum which may be attributed to maximum interactions with free hydroxyl group available in CMOF-3 however its exact mechanism is still under research. Dalgliesh's three-point interaction theory may also explain this enantioselective recognition as this theory states that one enantiomer of a chiral compound should interact with at least three points around the chiral centre of the adsorbent via hydrogen bonding, dipole–dipole interactions, or other intermolecular forces, resulting in a transient diastereomeric interaction that leads to chiral recognition.80 As these interactions, most probably three-point hydrogen bonding and other intermolecular interactions strongly occurs in case of 1-phenylethanol and L-tyrosine ligand causing high enantioselectivity as compared to other two CMOFs 1–2 as given in Fig. 7. This theory also supports the retention of chirality while performing the enantioseparation by CMOFs 1–3.
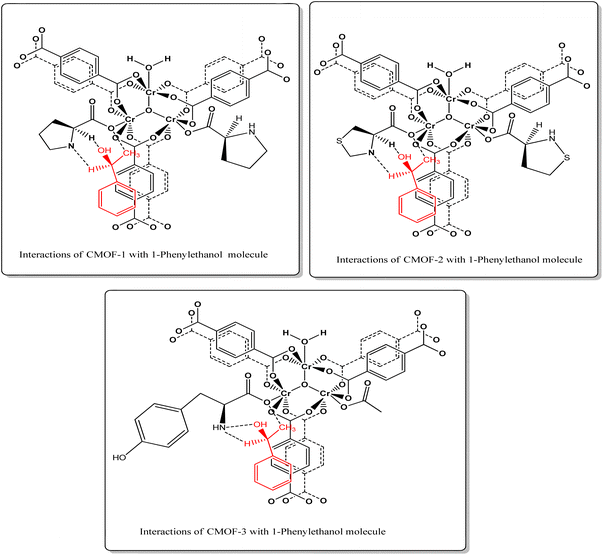 | ||
| Fig. 7 Three-points electrostatic interactions illustrated by the dotted lines between the chiral centres of CMOFs 1–3 and 1-phenylethanol. | ||
CMOFs 1–3 also showed enantioselective adsorption for other two racemate such as RS-ibuprofen and RS-mandelic acid but showed best resolution for RS-ibuprofen as given in ESI.†
CMOF-1 and CMOF-2 resolved enantiomers of RS-ibuprofen with 18% enantiomeric excess, however CMOF-3 showed 15% enantiomeric excess. As ibuprofen possess 7.4 Å minimum kinetic diameter (MKD) as calculated by Gaussian software which is accessible molecular size for these porous CMOFs 1–3.
Moreover, the possible interactions of RS-ibuprofen with three synthesized CMOFs 1–3 according to three-point theory are illustrated in Fig. 8.
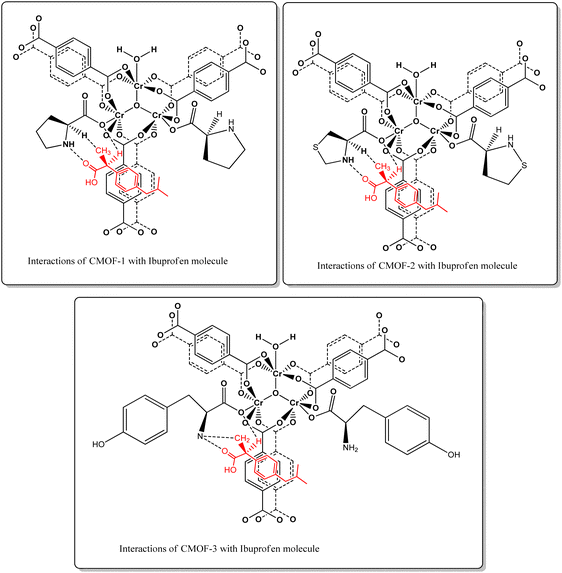 | ||
| Fig. 8 Three-points electrostatic interactions illustrated by the dotted lines between the chiral centres of CMOFs 1–3 and ibuprofen molecule. | ||
CMOFs 1–3 enantioselectivity for RS-mandelic acid was very lethargic with 5–11% enantiomeric excess.
It means that presence of number of functional groups and size of racemic compounds affects the enantioselectivities of CMOFs 1–3. The presence of two hydroxyl groups in the mandelic acid may cause steric hindrance resulting less enantioselectivity.
It is also observed that various properties of ligands incorporated in CMOFs 1–3 affected the enantioselectivities of CMOFs 1–3 as given in Table 4. The polar surface area of ligand and BET surface area of resultant CMOF greatly has determined the enantioselectivities of CMOFs 1–3.
| Enantiomers | CMOF-1 | CMOF-2 | CMOF-3 |
|---|---|---|---|
| a The %ee value was calculated by the formula, %ee = [(R − S)/(R + S) × 100]. | |||
 |
18% | 18% | 15% |
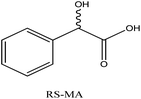 |
6% | 5% | 11% |
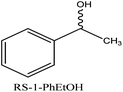 |
19% | 19% | 46% |
| Molar volume of ligand (cm3) | Polar surface area of ligand (A2) | BET surface area of CMOF (m2 g−1) | % ee for racemates | |||
|---|---|---|---|---|---|---|
| IBU | MA | 1-PhEtOH | ||||
| CMOF-1 | 97.0 ± 3.0 | 49 | 832 | 18 | 6 | 19 |
| CMOF-2 | 96.5 ± 3.0 | 75 | 811 | 18 | 5 | 19 |
| CMOF-3 | 135.9 ± 3.0 | 84 | 1056 | 15 | 11 | 46 |
The polar groups present in ligands interact with the functional group of racemic molecules and promote enantioseparation. It also showed by the results that increasing BET surface area of CMOFs 1–3 also increased enantioselectivity for selected racemic molecules.
As polar surface area of racemic ibuprofen and racemic 1-PhEtOH found in the range of 20–37 A2, so CMOF-1 showed comparable %ee value (18–19%) but racemic mandelic acid possess 58 A2 polar surface area and resulted poor enantioseparation (6%).
In case of CMOF-2, molar volume is approximately similar but polar surface area increased due to presence of sulphur group. Sulphur didn't interact efficiently with functional groups of racemate, that's why CMOF-2 also has comparably similar enantioseparation values as given in Table 3.
CMOF-3 has maximum polar surface area, so showed best resolution for all three racemic molecules especially for 1-PhEtOH (%ee, 46%). The %ee value also showed that.
Orientation of functional groups also enhanced the enantioselectivity as shown in Fig. 7. In case of racemic ibuprofen and racemic mandelic acid, carboxyl group may create hindrance leading less enantioseparation as compared to 1-PhEtOH.
In all these CMOFs 1–3, CMOF-3 showed best resolution for 1-phenylethanol with 46% enantiomeric excess. Moreover, enantioselectivity of three synthesized CMOFs 1–3 also compared with other physical properties especially BET surface area of CMOFs 1–3.
The retention of chirality in CMOFs 1–3 also observed by CD spectra, as no change in sign of cotton effect appeared as given in Fig. 5.
Moreover, these CMOFs are prepared in clean environment and showed maximum stability in humid conditions.81 These CMOFs also showed improved results for 1-phenylethanol as previously synthesized Zr based CMOFs using same enantioseparation strategy. The previously reported Zr based CMOFs also derived from naturally occurring amino acids and MOF-808, however their enantioselective capability towards these racemates was not attractive as shown by the present work. So, these CMOFs 1–3 works as good stationary material as compared to previously reported following the same enantioseparation methodology.
4. Conclusions
In conclusion, we have synthesized new chiral materials by the post synthetic modification of MIL-101(Cr), which can be employed for the enantioseparation of racemic compounds. All these three CMOFs 1–3 were prepared by the post synthetic strategy, which is very easy, clean, and economically favourable method because naturally occurring amino acids have used as ligands under mild conditions. The enantioselective properties of these CMOFs were evaluated by selecting RS-ibuprofen, RS-mandelic acid and RS-1-phenylethanol racemate. For this purpose, batch experiment method and simple glass column chromatography was used, and results were further confirmed by using polarimetric and HPLC techniques. In all these CMOFs 1–3, CMOF-3 showed best resolution for 1-phenylethanol with 46% enantiomeric excess. Moreover, enantioselectivity of three synthesized CMOFs 1–3 also compared with other physical properties especially BET surface area of CMOFs 1–3.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The Authors would like to thank Allama Iqbal Open University, Islamabad, Pakistan for providing research facilities.Notes and references
- K. K. Gangu, S. Maddila, S. B. Mukkamala and S. B. Jonnalagadda, Inorg. Chim. Acta, 2016, 446, 61–74 CrossRef CAS.
- K. K. Gangu and S. B. Jonnalagadda, Front. Sci. Ser., 2021, 9, 747615 CAS.
- A. Rubab, A. Altaf, S. Ishtiaq and M. Sohail, Nano Sci, 2022, 106–132 Search PubMed.
- D. Zhao, W. Zhang, Z. Wu and H. Xu, Front. Chem., 2021, 9, 834171 CrossRef CAS PubMed.
- A. Karmakar, A. V. Desai and S. K. Ghosh, Coord. Chem. Rev., 2016, 307, 313–341 CrossRef CAS.
- X. J. Hu, Z. X. Li, H. Xue, X. Huang, R. Cao and T. F. Liu, CCS Chem., 2020, 2(1), 616–622 CrossRef CAS.
- Y. Keum, S. Park, Y. P. Chen and J. Park, Angew. Chem., Int. Ed., 2018, 130(45), 15068–15072 CrossRef.
- F. Rouhani, F. Rafizadeh-Masuleh and A. Morsali, J. Am. Chem. Soc., 2019, 141(28), 11173–11182 CrossRef CAS PubMed.
- Y. Jiao, J. Yin, H. He, X. Peng, Q. GAO and C. Duan, J. Am. Chem. Soc., 2018, 140(18), 5882–5885 CrossRef CAS PubMed.
- R. B. Lin, S. Xiang, H. Xing, W. Zhou and B. Chen, Coord. Chem. Rev., 2019, 378, 87–103 CrossRef CAS.
- S. Yuan, L. Feng, K. Wang, J. Pang, M. Bosch, C. Lollar and H. C. Zhou, Adv. Mater., 2018, 30(37), 1704303 CrossRef PubMed.
- S. M. Xie, M. Zhang, Z. X. Fei and L. M. Yuan, J. Chromatogr. A, 2014, 1363, 137–143 CrossRef CAS PubMed.
- L. Li, J. D. Yi, Z. B. Fang, X. S. Wang, N. Liu, Y. N. Chen and R. Cao, Chem. Mater., 2019, 31(18), 7584–7589 CrossRef CAS.
- G. Huang, L. Yang, Q. Yin, Z. B. Fang, X. J. Hu, A. A. Zhang and R. Cao, Angew. Chem., Int. Ed., 2020, 59(11), 4385–4390 CrossRef CAS PubMed.
- C. Zhuo, Y. Wen, S. Hu, T. Sheng, R. Fu, Z. Xue and X. Wu, Inorg. Chem., 2017, 56(11), 6275–6280 CrossRef CAS PubMed.
- Z. G. Gu, C. Zhan, J. Zhang and X. Bu, Chem. Soc. Rev., 2016, 45(11), 3122–3144 RSC.
- S. Tanase-Grecea, E. Sharon, W. Sander and D. David, Chem.–A Eur. J., 2020, 26 Search PubMed.
- F. Wang, H.-R. Fu and J. Zhang, Cryst. Growth Des., 2015, 15(4), 1568–1571 CrossRef CAS.
- G.-L. Wen, G. P. Yang, P. Liu, B. Liu and Y. Y. Wang, Inorg. Chem. Commun., 2018, 94, 104–107 CrossRef CAS.
- M. Zhou, D. Yan, Y. Dong, X. He and Y. Xu, Inorg. Chem., 2017, 56(15), 9036–9043 CrossRef CAS PubMed.
- Z.-G. Gu, S. Grosjean, S. Brase, C. Woll and L. Heinke, Chem. Commun., 2015, 51(43), 8998–9001 RSC.
- X. Chen, H. Jiang, B. Hou, W. Gong, Y. Liu and Y. Cui, J. Am. Chem. Soc., 2017, 139(38), 13476–13482 CrossRef CAS PubMed.
- C.-D. Wu, A. Hu, L. Zhang and W. Lin, J. Am. Chem. Soc., 2005, 127(25), 8940–8941 CrossRef CAS PubMed.
- M. Zheng, Y. Liu, C. Wang, S. Liu and W. Lin, Chem. Sci., 2012, 3(8), 2623–2627 RSC.
- J. Bonnefoy, A. Legrand, E. A. Quadrelli, J. Canivet and D. Farrusseng, J. Am. Chem. Soc., 2015, 137(29), 9409–9416 CrossRef CAS PubMed.
- J. Zhao, H. Li, Y. Han, R. Li, X. Ding, X. Feng and B. Wang, J. Mat. Chem. A, 2015, 3(23), 12145–12148 RSC.
- T. Ezuhara, K. Endo and Y. Aoyama, J. Am. Chem. Soc., 1999, 121(14), 3279–3283 CrossRef CAS.
- X. Tan, J. Zhan, J. Zhang, L. Jiang, M. Pan and C.-Y. Su, CrystEngComm, 2012, 14(1), 63–66 RSC.
- B. Gil-Hernández, H. A. Höppe, J. K. Vieth, J. Sanchiz and C. Janiak, Chem. Comm., 2010, 46(43), 8270–8272 RSC.
- Y. Liu, W. Xuan and Y. Cui, Adv. Mater., 2010, 22(37), 4112–4135 CrossRef CAS PubMed.
- Y. Lu, H. Zhang, J. Y. Chan, R. Ou, H. Zhu, M. Forsyth and H. Wang, Angew. Chem., Int. Ed., 2019, 131(47), 17084–17091 CrossRef.
- W. T. Kou, C. X. Yang and X. P. Yan, J. Mat. Chem. A, 2018, 6(37), 17861–17866 RSC.
- M. Xue, B. Li, S. Qiu and B. Chen, Mat. Today, 2016, 19(9), 503–515 CrossRef CAS.
- X. Feng, H. S. Jena, K. Leus, G. Wang, J. Ouwehand and P. Van Der Voort, J. Catal., 2018, 365, 36–42 CrossRef CAS.
- W.-T. Kou, C.-X. Yang and X.-P. Yan, J. Mater. Chem. A, 2018, 6(37), 17861–17866 RSC.
- Z. Hu, Y. Peng, Y. Gao, Y. Qian, S. Ying and D. Yuan, Chem. Mater., 2016, 28(8), 2659–2667 CrossRef CAS.
- X. Ma, Y. Guo, L. Zhang, K. Wang, A. Yu, S. Zhang and G. Ouyang, Talanta, 2022, 239, 123143 CrossRef CAS PubMed.
- S. Li, Y. Zhou and B. Yan, Inorg. Chem., 2022, 61(25), 9615–9622 CrossRef CAS PubMed.
- M. Ma, C. Jiahuan, H. Liu, Z. Huang, H. Fuhong, L. Quanliang and X. Yuan, Nanoscale, 2022, 14, 13405–13427 RSC.
- A. Gheorghe, B. Strudwick, D. M. Dawson, S. E. Ashbrook, S. Woutersen, D. Dubbelda and S. Tanase, Chem.–A Eur. J., 2020, 26(61), 13957–13965 CrossRef CAS PubMed.
- K. Berijani and A. Morsali, Inorg. Chem., 2020, 60(1), 206–218 CrossRef PubMed.
- W. T. Kou, C. X. Yang and X. P. Yan, J. Mater. Chem. A, 2018, 6(37), 17861–17866 RSC.
- C. Tan, X. Han, Z. Li, Y. Liu and Y. Cui, J. Am. Chem. Soc., 2018, 140(47), 16229–16236 CrossRef CAS PubMed.
- C. Tan, X. Han, Z. Li, Y. Liu and Y. Cui, J. Am. Chem. Soc., 2018, 140(47), 16229–16236 CrossRef CAS PubMed.
- X. J. Hu, G. Huang, S. Zhang, Z. B. Fang, T. F. Liu and R. Cao, Chem. Comm., 2020, 56(54), 7459–7462 RSC.
- M. Banerjee, S. Das, M. Yoon, H. J. Choi, M. H. Hyun, S. M. Park and K. Kim, J. Am. Chem. Soc., 2009, 131(22), 7524–7525 CrossRef CAS PubMed.
- G. Férey, C. Mellot-Draznieks, C. Serre, F. Millange, J. Dutour, S. Surblé and I. Margiolaki, Science, 2005, 309(5743), 2040–2042 CrossRef PubMed.
- R. A. Sheldon, Chirotechnology: Industrial Syn. of Optically Active Compounds, CRC press, 1993 Search PubMed.
- R. E. Morris and X. Bu, Nat. Chem., 2010, 2(5), 353–361 CrossRef CAS PubMed.
- P. Deria, J. E. Mondloch, E. Tylianakis, P. Ghosh, W. Bury, R. Q. Snurr and O. K. Farha, J. Am. Chem. Soc., 2013, 135(45), 16801–16804 CrossRef CAS PubMed.
- G. Lidi, H. Xingfang, Q. Shili, C. Hongtao, Z. Xuan and W. Bingbing, RSC Adv., 2022, 12(10), 6063–6075 RSC.
- B. Ding, M. B. Solomon, C. F. Leong and D. M. D'Alessandro, Coord. Chem. Rev., 2021, 439, 213891 CrossRef CAS.
- R. K. Gupta, M. Riaz, M. Ashfaq, Z. Y. Gao, R. S. Varma, D. C. Li and D. Sun, Coord. Chem. Rev., 2022, 464, 214558 CrossRef CAS.
- Q. Shili, S. Yangyang, H. Xudong, C. Hongtao, G. Lidi and H. Zhongyu, RSC Adv., 2021, 11(59), 37584–37594 RSC.
- C. E. Bien, Z. Cai and C. R. Wade, Inorg. Chem., 2021, 60(16), 11784–11794 CrossRef CAS PubMed.
- M. Banerjee, S. Das, M. Yoon, H. J. Choi, M. H. Hyun and K. Park, J. Am. Chem. Soc., 2009, 131(22), 7524–7525 CrossRef CAS PubMed.
- S. M. Xie, Z. J. Zhang, Z. Y. Wang and L. M. Yuan, J. Am. Chem. Soc., 2011, 133(31), 11892–11895 CrossRef CAS PubMed.
- A. L. Nuzhdin, D. N. Dybtsev, K. P. Bryliakov, E. P. Talsi and V. P. Fedin, J. Am. Chem. Soc., 2007, 129(43), 12958–12959 CrossRef CAS PubMed.
- M. Padmanaban, P. Müller, C. Lieder, K. Gedrich, R. Grünker, V. Bon and S. Kaskel, Chem. Commun., 2011, 47(44), 12089–12091 RSC.
- K. Tanaka, T. Muraoka, D. Hirayama and A. Ohnish, Chem. Commun., 2012, 48(68), 8577–8579 RSC.
- J. K. Chen, N. Y. Xu, P. Guo, B. J. Wang, J. H. Zhang, S. M. Xie and L. M. Yuan, J. Sep. Sci., 2021, 44(21), 3976–3985 CrossRef CAS PubMed.
- Y. Zhang, X. Jin, X. Ma and Y. Wang, Anal. Methods, 2021, 13(1), 8–33 RSC.
- Enantiomer separation: fundamentals and practical methods, ed. F. Toda, Springer Science and Business Media, 2007 Search PubMed.
- T. A. Goetjen, J. Liu, Y. Wu, J. Sui, X. Zhang and J. T. Hupp, Chem. Commun., 2020, 56(72), 10409–10418 RSC.
- J. Li, Y. Ren, C. Qi and H. Jiang, Dalton Trans., 2017, 46(24), 7821–7832 RSC.
- B. Shen, Y. Kim and M. Lee, Adv. Mater., 2020, 32(41), 1905669 CrossRef CAS PubMed.
- C.-X. Yang and X.-P. Yan, Anal. Chem., 2011, 83(18), 7144–7150 CrossRef CAS PubMed.
- Z.-L. Fang, S. R. Zheng, J. B. Tan, S. L. Cai, J. Fan and X. Yan, J. Chromatogr. A, 2013, 1285, 132–138 CrossRef CAS PubMed.
- D. Bradshaw, J. B. Claridge, E. J. Cussen, T. J. Prior and M. J. Rosseinsky, Acc. Chem. Res., 2005, 38(4), 273–282 CrossRef CAS PubMed.
- Y. Liu, W. Xuan and Y. Cui, Adv. Mater., 2010, 22(37), 4112–4135 CrossRef CAS PubMed.
- X. J. Hu, G. Huang, S. Zhang, Z. B. Fang, T. F. Liu and R. Cao, Chem. Commun., 2020, 56(54), 7459–7462 RSC.
- P. B. Rallapalli, M. C. Raj, S. Senthilkumar, R. S. Somani and H. C. Bajaj, Environ. Prog. Sustainable Energy, 2016, 35(2), 461–468 CrossRef CAS.
- D. Jiang, L. L. Keenan, A. D. Burrows and K. J. Edler, Chem. Commun., 2012, 48(99), 12053–12055 RSC.
- J. Bonnefoy, J. Canivet, D. Farrusseng, and Q. Alessandra, in Inter. Symp. Nonporous Materials 7 (Nano7), 2014 Search PubMed.
- S. Yuan, W. Lu, Y. P. Chen, Q. Zhang, T. F. Liu, D. Feng and H. C. Zhou, J. Am. Chem. Soc., 2015, 137(9), 3177–3180 CrossRef CAS PubMed.
- X. Hou, T. Xu, Y. Wang, S. Liu, J. Tong and B. Liu, ACS Appl. Mater. Interfaces, 2017, 9(37), 32264–32269 CrossRef CAS PubMed.
- L. Zhang and J. Jiang, J. Membr. Sci., 2011, 367(1–2), 63–70 CrossRef CAS.
- S. C. Xiang, Z. Zhang, C. G. Zhao, K. Hong, X. Zhao, D. R. Ding and B. Chen, Nat. Commun., 2011, 2(1), 1–7 Search PubMed.
- C. E. Dalgliesh, J. Chem. Soc., 1952, 3940–3942 RSC.
- V. A. Davankov, Chirality, 1997, 9(2), 99–102 CrossRef CAS.
- M. Banerjee, S. Das, M. Yoon, H. J. Choi, M. H. Hyun and S. M. Park, J. Am. Chem. Soc., 2009, 131(22), 7524–7525 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra02489j |
| This journal is © The Royal Society of Chemistry 2023 |