Open Access Article
Open Access ArticleLewis acid catalyzed spiro annulation of (Z)-3-amino-acrylates with 2-amino arylbenzamides: one-pot synthesis of pyrrole–quinazoline hybrids†
Anil Kumar Soda*ab,
Krishna Prasad Chinthapallya,
Phani Krishna C. S.ab,
Sai Krishna Chilakaab and
Sridhar Madabhushi *ab
*ab
aFluoro-Agrochemicals Department, CSIR-Indian Institute of Chemical Technology, Hyderabad-500007, India. E-mail: sridharm@iict.res.in
bAcademy of Scientific and Innovative Research (AcSIR), Ghaziabad-201002, India
First published on 16th May 2023
Abstract
The one-pot domino reaction of ethyl (Z)-3-amino-3-phenylacrylates with 2-amino-N-alkyl/arylbenzamides under Lewis acid catalysis was described as an effective way to construct novel spiro [pyrrole-3,2′-quinazoline] carboxylate derivatives. By combining substituted alkyl/aryl amides with spiro annulated 1H-pyrrole-2,3-diones, this method provides a novel way for producing spiro pyrrole derivatives in good to excellent yields. The current procedure has a number of benefits, including quicker reaction times, a broad tolerance range for functional groups, and the ability to synthesize 2,3-dihydroquinazolin-4(1H)-ones that are of biological importance and take part in organic transformations. This is the first use of molecular hybridization involving linking with pyrrole derivatives and dihydroquinazolin-4(1H)-ones.
Introduction
Lewis acids are versatile catalysts in organic synthesis for carbon–carbon or carbon–nitrogen bond formation.1 Among them, bismuth(III) salts are efficient catalysts in various transformations;2 in particular, bismuth(III) triflate is the mainly proficient catalyst for diverse organic transformations.3 Predominantly, the annulation reactions of 2-amino N-arylbenzamides with alkynes,4 aldehydes,5 alcohols,6 ketones,7 imines8 or aryl halides9 also attracted more attention for C–N bond creation. Indeed, spiro annulation of ketones with 2-amino N-arylbenzamides in the presence of metal or non-metal catalysts has been playing a significant role in producing different cyclized products concerning two different pharmacophores. In particular, dihydroquinazolinones are more attractive scaffolds that have been established as key building blocks in diverse transformations. These occur in various natural products and in synthetic molecules that find applications in the pharmaceutical chemistry.10–17 Along with this, pyrrole is also one of the most significant heterocycles, for the reason that it exists in a large number of natural and synthetic compounds with significant pharmacological properties.18–22 Spirooxindole is a privileged scaffold commonly found in naturally occurring and synthetic biologically active compounds.23 Some of the important drugs with dihydroquinazolinone pharmacophore, pyrrole and spirooxindole dihydroquinazolin-4(1H)-one core structures are shown in Fig. 1.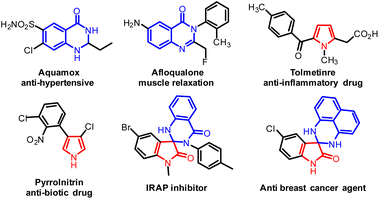 | ||
| Fig. 1 Some of the pharmacologically important dihydroquinazolinones, pyrroles and spirooxindole dihydroquinazolinones. | ||
In drug discovery, the molecular hybridization technique plays an important role in linking and expansion of different pharmacophores.24 Despite the expansion of the spiro annulated reactions with 2-amino-N-arylbenzamides were explored. In the literature studies, several methods are available for the linkage of isatins with 2-amino-N-phenylbenzamides to produce spirooxindole quinazolinone derivatives.
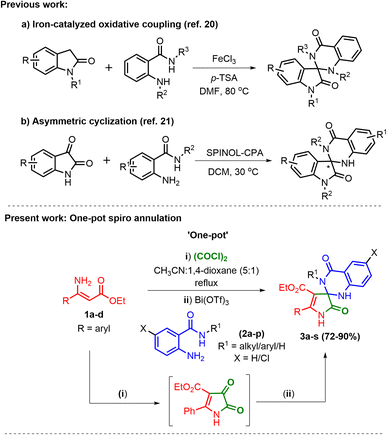
Iron-catalyzed oxidative coupling of indoline-2-ones with aminobenzamides under iron chloride catalysis;25 SPINOL-CPA catalyzed cyclization of 2-amino-N-substituted benzamides and isatins;26 asymmetric cyclization reactions between N-substituted anthranilamides and isatins;27 stannous chloride catalyzed reductive cyclization of 2-nitrobenzamides with isatins;28 alum catalyzed regioselective reaction of isatoic anhydride, isatins and aromatic/aliphatic amines;29 one pot synthesis of spirooxindole dihydroquinazolinone derivatives under nano cerium oxide catalysis.30 The synthesis of spirooxindole quinazoline derivatives uses all methods described in the literature; however, there is no literature on the synthesis of spiro[pyrrole-3,2′-quinazoline]carboxylates. Hybrids of quinazoline and pyrrole have not yet been reported.
This exacting reaction proceeds in two sequential steps; first, the cyclization of the (Z)-3-amino-3-phenylacrylates transpires to form 1H-pyrrole-2,3-diones,31 which are then converted to the desired products by spiro annulation with 2-amino-N-arylbenzamides (Scheme 1).
Moreover, one-pot synthesis reactions are helpful for avoiding several purification procedures at the same time and it can thus reduce chemical waste.32 The multicomponent reactions have attracted a high level of interest in the pharma industry due to their synthetic efficiency, high reactivity and atom economy.33
Herein, we have established the first Lewis acid catalyzed one-pot spiro annulation of in situ generated 1H-pyrrole-2,3-diones from ethyl (Z)-3-amino-3-phenylacrylates and 2-amino-N-alkyl/aryl benzamides for the synthesis of N-heterocyclic spiro [pyrrole-3,2′-quinazoline]carboxylates, as shown in Scheme 1. The prominent features of this work include multicomponent one-pot reactions, novel spiro annulation under Lewis acid catalysis, the construction of a C–N framework, and abundant substrate scope with amides.
To the best of our knowledge, there is no precedent in the literature for using Lewis acids as a catalyst and ethyl (Z)-3-amino-3-phenylacrylates for the preparation of novel spiro [pyrrole-3,2′-quinazoline]carboxylates.
Results and discussion
Chemistry
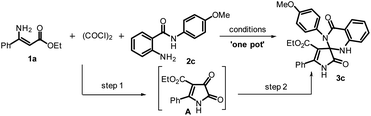
To begin the study for optimal conditions, ethyl (Z)-3-amino-3-phenylacrylate (1a), oxalyl chloride, and 2-amino-N-(4-methoxyphenyl) benzamides (2c) were taken as model substrates, and the results are mentioned in Table 1. In the beginning, 1a was reacted with oxalyl chloride in dichloromethane at reflux, and we observed the formation of the intermediate ethyl 4,5-dioxo-2-phenyl-4,5-dihydro-1H-pyrrole-3-carboxylate (A) in 68% yield (entry 1, Table 1). To get a better yield of A, we carried out the reaction in various solvents using acetonitrile in combination with a solvent such as 1,4-dioxane, toluene, tetrahydrofuran, 1,2-dichloro ethane, and diethyl ether in 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio (entries 2–6, Table 1).
1 ratio (entries 2–6, Table 1).
| Entry | Reaction conditions for step 1 solvent (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Reaction conditions for step 2 Lewis acid (mol%) | % yielde | |
|---|---|---|---|---|
| A | 3c | |||
| a Reaction conditions: all the reactions were performed using 1 (1.0 mmol), oxalyl chloride (1.15 mmol), 2c (1.0 mmol) and Lewis acid (10 mol%) in 1.0 mL of the solvent at reflux for 2–4.b For entries 7–16, after confirming the formation of A by TLC, 10 mol% of Lewis acid and 2c (1.0 mmol) (step 2) were added and stirred for 2–4 h.c Bi(OTf)3 (5 mol%).d Bi(OTf)3 (20 mol%).e Isolated yields. | ||||
| 1 | DCM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,4-dioxane 1,4-dioxane |
— | 68 | — |
| 2 | Toluene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,4-dioxane 1,4-dioxane |
— | 75 | — |
| 3 | THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,4-dioxane 1,4-dioxane |
— | 78 | — |
| 4 | DCE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,4-dioxane 1,4-dioxane |
— | 81 | — |
| 5 | Ether![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,4-dioxane 1,4-dioxane |
— | 86 | — |
| 6 | CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,4-dioxane 1,4-dioxane |
— | 95 | — |
| 7b | CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,4-dioxane 1,4-dioxane |
Bi(OTf)3 (10) | Trace | 90 |
| 8 | CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,4-dioxane 1,4-dioxane |
In(OTf)3 (10) | Trace | 82 |
| 9 | CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,4-dioxane 1,4-dioxane |
Cu(OTf)2 (10) | 17 | 78 |
| 10 | CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,4-dioxane 1,4-dioxane |
ZnCl2 (10) | 20 | 75 |
| 11 | CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,4-dioxane 1,4-dioxane |
I2 (10) | 20 | 75 |
| 12 | CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,4-dioxane 1,4-dioxane |
Eu(OTf)2 (10) | 20 | 74 |
| 13 | CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,4-dioxane 1,4-dioxane |
InCl3 (10) | 25 | 70 |
| 14 | CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,4-dioxane 1,4-dioxane |
Zn(OTf)2 (10) | 27 | 68 |
| 15 | CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,4-dioxane 1,4-dioxane |
FeCl3 (10) | 27 | 65 |
| 16 | CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,4-dioxane 1,4-dioxane |
BF3·OEt2 (10) | 33 | 60 |
| 17c | CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,4-dioxane 1,4-dioxane |
Bi(OTf)3 (5) | 15 | 78 |
| 18d | CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,4-dioxane 1,4-dioxane |
Bi(OTf)3 (20) | Trace | 90 |
However, all these reactions provided the intermediate A exclusively, and the best yield was obtained in acetonitrile. To achieve the spiro[pyrrole-3,2′-quinazoline]-4-carboxylate 3c in one pot, we added 2-amino-N-arylbenzamide (2c) to the same reaction mixture, but no formation of 3c was observed. In view of this unsuccessful result in attaining 3c, the approach of the reaction was modified. Primarily, the formation of A under appropriate conditions (entry 6, Table 1) was recognized by thin-layer chromatography (TLC), and then, 10 mol% of Bi(OTf)3 was added along with 2c and stirred for 2 h. To our delight, the formation of 3c was observed in 90% yield (entry 7, Table 1). Next, we also studied the reaction with different catalysts in the optimized solvent medium, and the results are shown in Table 1. In a while, the replacement of Bi(OTf)3 with other Lewis acids in step 2 showed that indium triflate, copper triflate, zinc chloride, iodine, europium triflate, indium chloride, zinc triflate, ferric chloride, and BF3·OEt2 also gave the product 3c in yields of 82, 78, 75, 75, 74, 70, 68, 65 and 60%, respectively (entries 8–16, Table 1). In addition, we varied the loading of the catalyst Bi(OTf)3, and the reactions were conducted with 5 and 20 mol% of the catalyst, and a moderate yield of 3c was observed with a decrease in the catalyst loading, while no significant effect was observed on the product yield with an increasing amount of catalyst (entries 17–18, Table 1). From these observations, Bi(OTf)3 (10 mol%) is the best catalyst, and CH3CN-1,4-dioxane is the most suitable solvent medium for this transformation. Hence, the conditions used in entry 7 (Table 1) were found to be most favorable to exploring the preparation of differently substituted spiro[pyrrole-3,2′-quinazoline]-4-carboxylate from the reaction of (Z)-3-amino-acrylate with a 2-amino arylbenzamide.
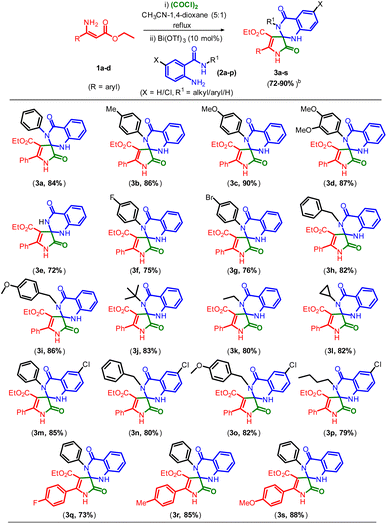
From the optimal reaction conditions (entry 7, Table 1), we next turned our interest to evaluate the overview of this conversion with various 2-amino-N-alkyl/arylbenzamides (2a–p) examined, and the results are shown in Table 2.
Pleasingly, a wide range of 2-amino-N-alkyl/arylbenzamides are well companionable with the present transformation to provide the corresponding spiro [pyrrole-3,2′-quinazoline]carboxylates in good yields. 2-Amino-N-arylbenzamides having electron donating groups 2b to 2d were well reacted with 1a and oxalyl chloride to give the products methyl (–CH3, 3b), methoxy (–OCH3, 3c) and di-methoxy (3,4-OCH3, 3d) in 86, 90, and 87% yields, respectively. Interestingly, having free amide functionality compound 2e underwent reaction smoothly to provide the corresponding product 3e in a 72% yield. 2-Amino-N-arylbenzamides 2f and 2g which bear halogens were also found to be equally consenting in reaction with 1a and oxalyl chloride under similar reaction conditions to give the products fluoro (–F, 3f) and bromo (–Br, 3g) in 75 and 76% yields, respectively. In addition, 2-amino-N-alkylbenzamides benzyl (2h), 4-OCH3 benzyl (2i), tert-butyl (2j), ethyl (2k) and cyclopropyl (2l) were also well tolerated to react with 1a and oxalyl chloride under optimal conditions to give the corresponding products 3h, 3i, 3j, 3k and 3l in 82, 86, 83, 80, and 82% yields, respectively. Further, other 5-Cl substituted 2-amino-N-aryl/alkyl benzamides such as phenyl (2m), benzyl (2n), 4-OCH3 benzyl (2o) and n-butyl (2p) were found to react with 1a and oxalyl chloride to give the desired products 3m, 3n, 3o and 3p in 85, 80, 82 and 79% yields, respectively. To further expand the substrate scope, the reactivity of diversely substituted phenyl (4-F, 1b), (4-CH3, 1c) and (4-OCH3, 1d) was well tolerated to react with oxalyl chloride under optimized conditions and followed by a reaction with 2a to obtain the corresponding products 3q, 3r and 3s in 73, 85, and 88% yields, respectively.
To analyze the expediency of this method, a gram-scale reaction of compound 1a with 2p was carried out under standard reaction conditions (Scheme 2a). To our delight, the formation of 3p was observed in 76% yield, and moreover, it was further utilized for the synthetic transformations, as shown in Scheme 2b. Palladium-catalyzed C–C coupling reaction of compound 3p with phenylboronic acid 4 in toluene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (1
water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the solvent medium delivered the ethyl 3′-butyl-2,4′-dioxo-5,6′-diphenyl-1,2,3′,4′-tetrahydro-1′H-spiro[pyrrole-3,2′-quinazoline]-4-carboxylate 5 in 82% yield.34 Next, we turned our attention towards the generality of this transformation with o-phenylenediamine 6 in the presence of standard reaction conditions. Delightfully, the reaction progressed well and gave the ethyl 2-phenyl-1H-pyrrolo[2,3-b]quinoxaline-3-carboxylate 7 in an 88% yield (Scheme 2c).
1) as the solvent medium delivered the ethyl 3′-butyl-2,4′-dioxo-5,6′-diphenyl-1,2,3′,4′-tetrahydro-1′H-spiro[pyrrole-3,2′-quinazoline]-4-carboxylate 5 in 82% yield.34 Next, we turned our attention towards the generality of this transformation with o-phenylenediamine 6 in the presence of standard reaction conditions. Delightfully, the reaction progressed well and gave the ethyl 2-phenyl-1H-pyrrolo[2,3-b]quinoxaline-3-carboxylate 7 in an 88% yield (Scheme 2c).
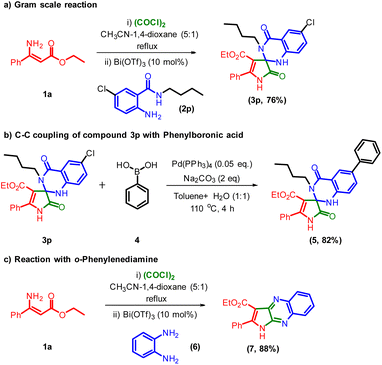 | ||
| Scheme 2 (a) Gram scale synthesis of 3p; (b) synthetic application of compound 3p; (c) reaction of compound 3a with o-phenylenediamine. | ||
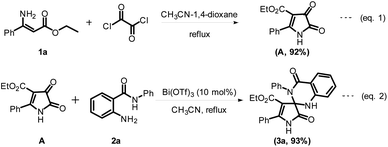
The obtained results from control experiments are shown in Scheme 3. In this observation, when the reaction was carried out with CH3CN-1,4-dioxane as a solvent medium, we got intermediate A from the reaction of ethyl (Z)-3-amino-3-phenylacrylate 1a and oxalyl chloride (Scheme 3, eqn (1)). Then intermediate A was transformed into ethyl 2,4′-dioxo-3′,5-diphenyl-1,2,3′,4′-tetrahydro-1′H-spiro[pyrrole-3,2′-quinazoline]-4-carboxylate 3a when it reacted with 2-amino N-phenylbenzamide 2a under Lewis acid catalysis (Scheme 3, eqn (2)). The noteworthy observation in this study is that the compound 1a transformed into 3a when 1a reacted with oxalyl chloride and then into 2a under Lewis acid catalysis.
On the basis of the results illustrated above and previous experiments, we suggest a feasible mechanism for the spiro annulation of ethyl (Z)-3-amino-3-phenylacrylate 1a and 2-amino N-phenylbenzamide 2a, as shown in Scheme 4. Initially, the intermediate compound A is formed by the cyclization of 1a and oxalyl chloride in the presence of the solvent medium. Then, the compound 3a was generated from the compound A by spiro annulation with 2a in the presence of Lewis acid catalysis. This route for the formation of 3a from compound A probably involves some transient stages.
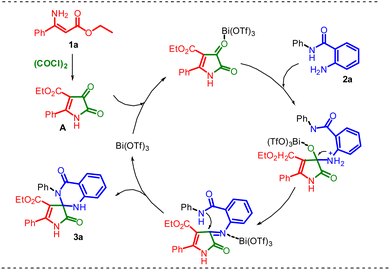 | ||
| Scheme 4 Plausible mechanism for the formation of ethyl 2,4′-dioxo-3′,5-diphenyl-1,2,3′,4′-tetrahydro-1′H-spiro[pyrrole-3,2′-quinazoline]-4-carboxylate 3a. | ||
Conclusions
In conclusion, we have demonstrated a highly efficient Lewis acid promoted spiro annulation for the one-pot synthesis of spiro[pyrrole-3,2′-quinazoline]-4-carboxylate derivatives using ethyl-(Z)-3-amino-3-phenylacrylate with oxalyl chloride and 2-amino-N-alkyl/arylbenzamides. This work established the first application of hybridization of pyrroles with dihydroquinazolinones by spiro annulation. The short reaction times and uncomplicated reaction conditions make this one pot method particularly attractive for the efficient preparation of biologically and medicinally interesting spiro[pyrrole-3,2′-quinazoline]-4-carboxylate molecules.Conflicts of interest
The authors declare that they have no conflict of interest.Acknowledgements
Authors A. K. S. is thankful to UGC, S. K. C. is thankful to CSIR, for financial support in the form of research fellowship. I am thankful to DKIM Division (IICT Communication No. IICT/Pubs./2022/367) for the support.Notes and references
- (a) H. Huang, T. Zhang and J. Sun, Angew. Chem., Int. Ed., 2021, 60, 2668 CrossRef CAS PubMed; (b) S. Yamazaki, T. Naito, M. Niina and K. Kakiuchi, J. Org. Chem., 2017, 82, 6748 CrossRef CAS PubMed; (c) A. Wang, M. Lu, X. Xie and Y. Liu, Org. Lett., 2022, 24, 2944 CrossRef CAS PubMed; (d) A. Borah, A. Sharma, H. Hazarika, K. Sharma and P. Gogoi, J. Org. Chem., 2017, 82, 8309 CrossRef CAS PubMed; (e) R. K. Chellu, S. Kurva, A. K. Soda, S. K. Chilaka, J. B. Nanubolu and S. Madabhushi, Asian J. Org. Chem., 2021, 10, 1432 CrossRef CAS.
- (a) T. Ollevier and T. M. Mwene-Mbeja, Tetrahedron Lett., 2006, 47, 4051 CrossRef CAS; (b) R. F. Lambert, R. J. Hinkle, S. E. Ammann, Y. Lian, J. Liu, S. E. Lewis and R. D. Pike, J. Org. Chem., 2011, 76, 9269 CrossRef CAS; (c) T. Ollevier, J.-E. Bouchard and V. Desyroy, J. Org. Chem., 2008, 73, 331 CrossRef CAS PubMed; (d) T. Ollevier and E. Nadeau, J. Org. Chem., 2004, 69, 9292 CrossRef CAS PubMed.
- (a) A. K. Soda, I. R. Bontha, S. K. Chilaka, R. K. Chellu and S. Madabhushi, Asian J. Org. Chem., 2022, 11 DOI:10.1002/ajoc.202200193; (b) J. Jaratjaroonphong, S. Tuengpanya and S. Ruengsangtongkul, J. Org. Chem., 2015, 80, 559 CrossRef CAS; (c) A. E. Schneider and G. Manolikakes, J. Org. Chem., 2015, 80, 6193 CrossRef CAS PubMed; (d) A. Kamal, S. K. Ahmed, M. Sandbhor, M. N. A. Khan and M. Arifuddin, Chem. Lett., 2005, 34, 1142 CrossRef CAS; (e) A. E. Schneider, T. Beisel, A. Shemet and G. Manolikakes, Org. Biomol. Chem., 2014, 12, 2356 RSC.
- (a) M. Abdullaha, S. Mohammed, M. Ali, A. Kumar, R. A. Vishwakarma and S. B. Bharate, J. Org. Chem., 2019, 84, 5129 CrossRef CAS PubMed; (b) E. Feng, Y. Zhou, D. Zhang, L. Zhang, H. Sun, H. Jiang and H. Liu, J. Org. Chem., 2010, 75, 3274 CrossRef CAS PubMed.
- (a) M. Prakash and V. Kesavan, Org. Lett., 2012, 14, 1896 CrossRef CAS PubMed; (b) P. Singh, N. Kaur and P. Banerjee, J. Org. Chem., 2020, 85, 3393 CrossRef CAS PubMed.
- H. Hikawa, Y. Ino, H. Suzuki and Y. Yokoyama, J. Org. Chem., 2012, 77, 7046 CrossRef CAS PubMed.
- Y.-p. Zhu, Z. Fei, M.-c. Liu, F.-c. Jia and A.-x. Wu, Org. Lett., 2013, 15, 378 CrossRef CAS PubMed.
- Q. Li, Y. Huang, T. Chen, Y. Zhou, Q. Xu, S.-F. Yin and L.-B. Han, Org. Lett., 2014, 16, 3672 CrossRef CAS PubMed.
- A. K. Soda, V. Sriramoju, R. K. Chellu, S. K. Chilaka, S. Kurva, S. Bansod and S. Madabhushi, ChemistrySelect, 2021, 6, 896 CrossRef CAS.
- (a) M.-J. Hour, L.-J. Huang, S.-C. Kuo, Y. Xia, K. Bastow, Y. Nakanishi, E. Hamel and K.-H. Lee, J. Med. Chem., 2000, 43, 4479 CrossRef CAS PubMed; (b) G. M. Chinigo, M. Paige, S. Grindrod, E. Hamel, S. Dakshanamurthy, M. Chruszcz, W. Minor and M. L. Brown, J. Med. Chem., 2008, 51, 4620 CrossRef CAS PubMed.
- Z. Xu, Y. Zhang, H. Fu, H. Zhong, K. Hong and W. Zhu, Bioorg. Med. Chem. Lett., 2011, 21, 4005 CrossRef CAS PubMed.
- A. A. Mohammadi, R. Ahdenov and A. A. Sooki, Heterocycl. Commun., 2017, 23, 105 CrossRef CAS.
- E. Manivannan and S. C. Chaturvedi, Bioorg. Med. Chem., 2012, 20, 7119 CrossRef CAS PubMed.
- C. Mustazza, A. Borioni, I. Sestili, M. Sbraccia and A. Rodomonte, Chem. Pharm. Bull., 2006, 54, 611 CrossRef CAS PubMed.
- Y. S. Sadanandam, K. R. M. Reddy and A. B. Rao, Eur. J. Med. Chem., 1987, 22, 169 CrossRef CAS.
- S. R. Steinmuller and J. B. Puschett, Kidney Int., 1972, 1, 169 CrossRef CAS PubMed.
- V. Alagarsamy, V. R. Solomon and M. Murugan, Bioorg. Med. Chem., 2007, 15, 4009 CrossRef CAS PubMed.
- V. Estevez, M. Villacampa and J. C. Menendez, Chem. Soc. Rev., 2014, 43, 4633 RSC.
- A. A. Moroz, V. E. Zhulanov, M. V. Dmitriev and A. N. Maslivets, Tetrahedron, 2019, 76, 130880 CrossRef.
- O. Bakhanovich, V. Khutorianskyi, V. Motornov and P. Beier, Beilstein J. Org. Chem., 2021, 17, 504 CrossRef CAS.
- S. S. Fatahala, S. Hasabelnaby, A. Goudah, G. I. Mahmoud and R. H. A.-E. Hameed, Molecules, 2017, 22, 461 CrossRef PubMed.
- S.-G. Zhang, C.-G. Liang, Y.-Q. Sun, P. Teng, J.-Q. Wang and W.-H. Zhang, Mol. Diversity, 2019, 23, 915 CrossRef CAS.
- R. Sridhar, B. Srinivas, B. Madhav, V. P. Reddy, Y. V. D. Nageswar and K. R. Rao, Can. J. Chem., 2009, 87, 1704 CrossRef CAS.
- (a) A. K. Soda, C. S. P. Krishna, S. K. Chilaka, E. V. Krishna, S. Misra and S. Madabhushi, RSC Adv., 2022, 12, 16589 RSC; (b) P. S. Singu, U. Chilakamarthi, N. S. Mahadik, B. Keerti, N. Valipenta, S. N. Mokale, N. Nagesh and R. M. Kumbhare, RSC Med. Chem., 2021, 12, 416 RSC.
- Y.-H. Lai, R.-S. Wu, J. Huang, J.-Y. Huang and D.-Z. Xu, Org. Lett., 2020, 22, 3825 CrossRef CAS PubMed.
- Y. Hu, M.-M. Wang, H. Chen and D.-Q. Shi, Tetrahedron, 2011, 67, 9342 CrossRef CAS.
- L.-L. Wang, T. Jiang, P.-H. Li, R.-J. Sun and Z. Zuo, Adv. Synth. Catal., 2018, 360, 4832–4836 CrossRef CAS.
- M. Abdi, S. Rostamizadeh and N. Zekri, Polycyclic Aromat. Compd., 2017, 39, 413 CrossRef.
- A. A. Mohammadi, M. Dabiri and H. Qaraat, Tetrahedron, 2009, 65, 3804 CrossRef CAS.
- J. Zhang, J. Zhao, L. Wang, J. Liu, D. Ren and Y. Ma, Tetrahedron, 2015, 72, 936 CrossRef.
- (a) M. Y. Belikov, A. G. Milovidova and M. Y. Ievlev, New J. Chem., 2022, 46, 11030 RSC; (b) T. Sano, Y. Horiguchi, J. Toda, K. Imafuku and Y. Tsuda, Chem. Pharm. Bull., 1984, 32, 47 Search PubMed.
- (a) A. V. Chate, P. P. Rudrawar, G. M. Bondle and J. N. Sangeshetti, Synth. Commun., 2020, 50, 226 CrossRef CAS; (b) S. K. Chilaka, R. K. Chellu, A. K. Soda, S. Kurva, J. B. Nanubolu and S. Madabhushi, Adv. Synth. Catal., 2022, 364, 2944–2950 CrossRef CAS.
- S. Singh, M. Saquib, S. B. Singh, M. Singha and J. Singh, RSC Adv., 2015, 5, 45152–45157 RSC.
- I. Hiroki, S. Ryuichi, M. Noritake, S. Yuki, K. Osamu, T. Tomoko, S. Shingo and O. Daichi, Photoreactive polymer, method for manufacturing of photoreactive polymer, optical film, liquid crystal alignment film and resin composition thereof, JP2022142780, 2022.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra02639f |
| This journal is © The Royal Society of Chemistry 2023 |