Open Access Article
Open Access ArticleRecent advances in the applications of graphene materials for the oil and gas industry
Yang Xuana,
Luo Zhaob,
Daqi Lia,
Shaocong Pangbc and
Yuxiu An *b
*b
aKey Laboratory of Shale Oil and Gas Enrichment Mechanism and Development, Sinopec Research Institute of Petroleum Engineering, Changping District, Beijing, 100101, China
bSchool of Engineering and Technology, China University of Geosciences (Beijing), Haidian District, Beijing, 100083, China. E-mail: anyx@cugb.edu.cn; 13522045597@163.com
cZhengzhou Institute, China University of Geosciences (Beijing), Ximei Building, High-tech Industrial Development Zone, Zhengzhou City, Henan Province 450001, China
First published on 1st August 2023
Abstract
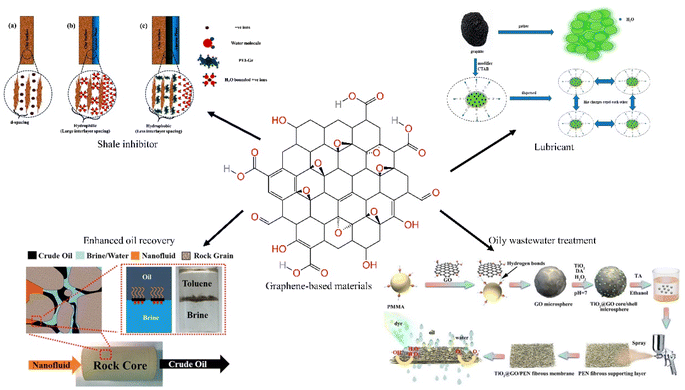
Graphene is a material formed with carbon atoms connected by sp2 hybridization. It is extremely strong and very ductile, and is superhydrophobic and superlipophilic. It has important application prospects in materials science, micro and nano processing, energy, aerospace and biomedicine. Graphene also has some applications in the petroleum industry. As nanoscale materials, graphene-based materials can plug nano-pores and prevent water intrusion into clay minerals during the drilling process, they are suitable for sliding between layers and can be used as lubricants due to the two-dimensional structure. The adsorption properties of graphene-based materials allow them to improve the treatment rate when treating oily wastewater. This paper compiles recent advances in the application of graphene and its derivatives in oilfield extraction, including improving drilling fluid performance, enhanced oil recovery and oily wastewater treatment. We compare the performance advantages of graphene-based materials over other additives, and summarize the mechanism of action of graphene-based materials. The shortcomings of current research are identified and future research and improvement directions are envisaged.
1. Introduction
Graphene is a material formed with carbon atoms connected by sp2 hybridization and has a single layer of two-dimensional honeycomb lattice structure. It is extremely strong and very ductile, and is superhydrophobic and superlipophilic. Graphene is more easily doped and chemically modified due to its longer edges compared to carbon nanotubes. And then it has important application prospects in materials science, micro and nano processing, energy, aerospace and biomedicine. Graphene oxide (GO) has increased dispersion in water due to the introduction of hydroxyl and carboxyl groups. The oxygen-containing functional groups located at the edges of GO sheets lead to hydrophilicity and make surface modification easier, leading to the creation of other graphene-based materials. Graphene and its derivatives are also used in the oil and gas industry due to their unique physical and chemical properties. The nanoscale size and flexible film structure of graphene-based materials allow them to plug nanopores and prevent water intrusion into clay minerals during the drilling process.1 Their two-dimensional structure, which slides easily between layers, allows they to be used as a drilling fluid lubricant.2 Their adsorption properties allow they to improve filtration performance in oily wastewater treatment.3 The amphiphilic nature of modified graphene materials allows them to modify wettability and reduce interfacial tension, which can be applied for enhanced oil recovery (EOR).4 In addition, the chemical and thermal stability of graphene-based materials allow them to perform well under extreme conditions, and they are reusable and environmentally friendly (Fig. 1).5In recent years, graphene and its derivatives have been used in the oil and gas industry due to their excellent physicochemical properties, and there are many reviews addressing related studies.6,7 However, some reviews only focus on one aspect of graphene-based materials in the oil recovery industry, focusing on the application of graphene nanocomposites in enhanced oil recovery (EOR),8 and some on the application in oil–water separation or in the removal of aromatic organic compounds from water.9–11 Some reviews do not include only graphene-based materials, but address the whole range of nanomaterials and are oriented only towards applications in drilling fluids, with a focus on functionalized nanomaterials,12 applications of materials whose feedstock is waste.13 As well as the influence of their performance factors.14 This paper compiles recent advances in the application of graphene and its derivatives in oilfield extraction, including improved drilling fluid performance, EOR, and wastewater treatment. This paper compares the performance advantages of graphene-based materials over other additives, summarizes the mechanism of action of graphene-based materials, identifies the shortcomings of current research, and envisages future research and improvement directions.
2. Applications in improving drilling fluid performance
Drilling fluid is usually a mixture of water, bentonite, and chemical treatment agent, usually called mud. In addition to the commonly used water-based drilling fluids (WBDF), there are also oil-based drilling fluids (OBDF) which use oil (crude oil or diesel oil) as the dispersing medium, and foam drilling fluids. Various treating agents are added to drilling fluids to provide the various properties required for the drilling process. Some common and important drilling fluid treating agents include shale inhibitor, fluid loss agent, viscosity reducer, adhesion enhancer and lubricant. In recent years, graphene and its derivatives have been gradually applied to improve drilling fluid performance due to their excellent physical and chemical properties.2.1 Shale inhibitor and shale plugging agent
Aftab et al. evaluated the performance of graphene nanosheets (GNP) in potassium chloride (KCl) mud systems to improve drilling fluid rheological properties and inhibit hydration of clay minerals.15 GNP showed superior inhibition in KCl mud systems compared to polymeric and the nanomaterials silicon dioxide (SiO2) and multi-walled carbon nanotubes (MWCNT). The change in shale volume in KCl mud systems containing GNP was reduced by at least 20% compared to the same concentration of SiO2 or MWCNT. Moreover, GNP also improved the rheological properties of KCl mud systems and reduced the filtration loss. Wang et al. investigated the improvement of GO in water-based drilling fluids to inhibit shale hydration.16 Compared with common shale inhibitors KCl and the nanomaterial SiO2, GO showed better performance in plugging nano- and micron-scale pores, preventing water intrusion into shale, inhibiting hydration swelling of clay minerals, and maintaining shale strength. GO sheets formed large, unbroken films to protect shale, and the soft GO sheets could be easily deformed to plug and fill different shapes of shale pores. Therefore, GO shows great potential to protect and stabilize shale in WBDF.Modification of GO to introduce more functional groups can add more chemical reaction sites. Rana et al. prepared several modified graphene as shale inhibitors, including polyethyleneimine (PEI) modified graphene (PEI-Gr), glucopyranose (Glu) modified graphene (Glu-Gr), and cetyltrimethylammonium (CTAB) modified graphene (CTAB-Gr).17–19 The inhibition properties of the modified graphene were evaluated by dispersion recovery tests and inhibition stability tests. The WBDF added the modified graphene exhibited better dispersion recovery and longer inhibition durability compared to the base WBDF and the KCl-added WBDF. The addition of modified graphene to the WBDF significantly improved the shale inhibition and enhanced the rheological properties. All three modified graphene still exhibited excellent inhibition at 65.5 °C. In addition to the physical plugging effect of graphene plugging the pores in the clay, the inhibitors also inhibited the hydration through the chemical interaction of the modified groups with the clay minerals. The hydroxyl groups of Glu bound to the surface of clay by hydrogen bonding. The positively charged amines contained in PEI helped PEI-Gr to interact with the hydroxyl groups on the clay surface. And the positively charged amines of the CTAB supported CTAB-Gr to bind the surface of clay through hydrogen bonding. The plugging, intercalation, and adsorption of modified graphene prevented water molecules from entering the clay nanopores, mitigating the swelling due to hydration (Fig. 2).
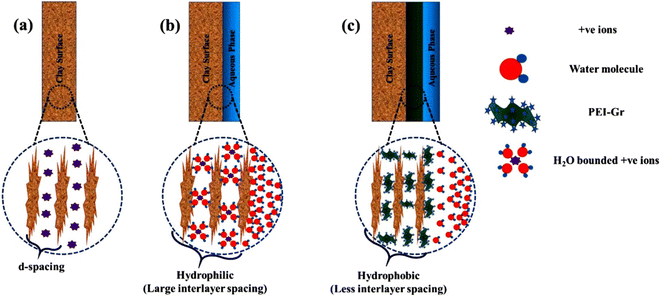 | ||
| Fig. 2 Mechanism of modified graphene to inhibit hydration of clay minerals.17 | ||
The performance of modified graphene in inhibiting hydration has been proven to be superior, and some scholars have investigated its inhibition effect at high temperatures. GO modified by 3-aminopropyltriethoxysilane exhibited good inhibition and can resist temperatures of up to 120 °C.20 Choline chloride-modified graphene (Ch-G) had better inhibition properties than conventional shale inhibitors and can resist temperatures up to 180 °C.21
Janus amphiphilic graphene oxide (JAGO) has been used as shale inhibitors due to the 2D nano-sheet amphiphilic structure. Lv et al. investigated JAGO modified by dodecylamine on one side of GO as a shale inhibitor.22 JAGO exhibited excellent inhibition, and greatly reduced filtration loss. Composite of polymers with GO has also been used as shale inhibitor. Zhao et al. synthesized hyperbranched polyethyleneimine/graphene composite (HPEI-G) as a shale inhibitor.1 As shown in Fig. 3, HPEI-G has a better effect of inhibiting hydration at lower concentrations. Compared with twice the concentration of KCl, HPEI-G reduced the swelling rate of bentonite by more than 10%. Moreover, HPEI-G still showed good inhibition at 150 °C. The inhibition mechanism of HPEI-G included chemical adsorption and physical blockage.
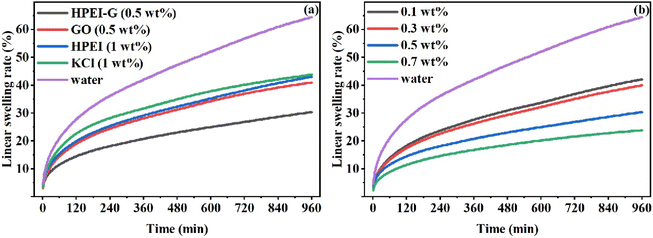 | ||
| Fig. 3 Linear swelling rate of bentonite at room temperature with (a) different inhibitors, and (b) different concentrations of HPEI-G.1 | ||
Graphene-based materials have also been used as shale plugging agents due to their excellent hydration inhibition and plugging properties. Yuxiu et al. developed ethylenediamine-modified graphene (EDA-G) as a shale plugging agent.23 Compared with inorganic nanomaterials and GO, EDA-G exhibited high performance in plugging nanopores and inhibiting hydration of clay minerals in shale formations. Under some specific conditions, the addition of EDA-G to the drilling fluid results in the lowest filtration. Zhang et al. prepared carboxylated GO (GO-COOH) using a modified method and evaluated its plugging properties.24 This GO-COOH maintained good dispersion properties after hydrothermal treatment at 180 °C. The pressure transfer time of 0.1 wt% GO-COOH was 12 times higher than that of 1 wt% silica, indicating that GO-COOH could be a new and efficient high-temperature resistant plugging agent for use in WBDF.
A variety of graphene-based materials have been developed as shale inhibitors or shale sealers. Inhibition of hydration by shale inhibitors and shale plugging agents containing graphene materials is shown in Table 1. The amphiphilic JAGO has the best inhibition effect, and the Janus particles can be studied in more depth in the future. Compared with conventional inhibitors and other nanomaterials, graphene-based materials exhibit superior inhibition and plugging properties and generally have good temperature resistance. GO inhibits hydration and prevents water intrusion by plugging nanopores. The inhibition mechanism of modified graphene generally includes the physical plugging effect of graphene sheets and the chemical interaction between grafting materials and clay minerals through hydrogen and ionic bonding adsorption. More research can be conducted on modified graphene in the future to make it inhibit well even under extreme conditions such as high temperature and high salt.
| Material | Author | Year | Ratioa/concentration (wt%) |
|---|---|---|---|
| a Ratio is the ratio of the linear swelling rate height in inhibitor solution to these in water. | |||
| EDA-G | Yuxiu et al. | 2016 | 0.39/0.2 |
| JAGO | Lv et al. | 2020 | 0.25/0.2 |
| PEI-Gr | Rana et al. | 2020 | 0.81/0.85 |
| Glu-Gr | Rana et al. | 2020 | 0.70/0.85 |
| CTAB-Gr | Rana et al. | 2020 | 0.66/0.85 |
| GO | Wang et al. | 2021 | 0.21/0.8 |
| Ch-G | Zhu et al. | 2022 | 0.37/4 |
| HPEI-G | Zhao et al. | 2022 | 0.47/0.5 |
2.2 Fluid loss agent
Many nanomaterials have been used in the development of fluid loss agents, and graphene-based materials were also being investigated in this area due to the special structure. Kosynkin et al. investigated the filtration loss reduction performance of powdered GO (PGO) and large flake GO (LFGO) in water-based drilling fluids, and methylated GO to improve its stability in brine environments.25 GO showed good filtration loss reduction even at carbon contents as low as 0.2 w/w%. The combination of LFGO and PGO can further improve the filtration loss reduction effect. Compared to clay-based fluid loss additives, GO solution exhibited better filtration performance and shear thinning, and was more resistant to high temperature.The composite use of graphene and other nanomaterials may significantly improve the filtration loss reduction. Aramendiz et al. evaluated the potential of using silica nanoparticles (SiO2-NPs) and GNPs to formulate nano water-based drilling fluids (NP-WBM).26 A total NP concentration of 0.75 wt% of NP-WBM produced the maximum filtrate volume reduction at ambient and high temperature/high pressure conditions and resulted in a 35.61% reduction in cutting erosion. NP-WBM provided an adequate plugging network between grain boundaries, resulting in the absence of microfractures and hence excellent filtration loss reduction performance. Ghayedi et al. investigated the effect of adding graphene oxide–zinc oxide (GO–ZnO) nanocomposite on the drilling fluid properties and H2S removal.27 GO–ZnO nanocomposite improved the drilling fluid performance by increasing the yield point, gel strength, and shear rate. It also decreased the filtration rate significantly. Besides, GO–ZnO nanocomposite can removed H2S from the drilling fluid in 15 min completely.
Graphene-based materials have also been studied in OBDF. Arain et al. and investigated the effect of GNP on OBDF properties at high temperature.28 The GNP improved the rheological and filtration properties of OBDF. At 120 °C, the filtration loss was reduced by 46% for 0.5 ppb concentration.
Currently only GO and GNP have been used as filtration loss reduction agents in WBDF and OBDF. As shown in Table 2, composites of GO with other nanomaterials have better filtration loss reduction performance, which may be attributed to the different microstructures of the materials. Graphene-based materials usually improved the rheological properties greatly and exert excellent filtration loss reduction at low concentrations. In addition, graphene-based materials had good temperature resistance due to their excellent thermal stability. Graphene-based materials can adsorb on the surface of clay layers due to their flake structure and prevent water intrusion into nanopores. The application of modified graphene materials as fluid loss agents can be investigated in the future.
| Material | Author | Year | API filtration loss (mL)/concentration |
|---|---|---|---|
| LFGO/PGO | Kosynkin et al. | 2012 | 10.8/2 g L−1 |
| SiO2-NP and GNP | Aramendiz et al. | 2019 | 7.4/0.75 wt% |
| GO–ZnO | Ghayedi et al. | 2020 | 5.7/0.3 w/w% |
| GNP on OBDF | Arain et al. | 2022 | 0.5/0.5 ppb |
2.3 Lubricant
Shear easily occurs between layers of graphene, since the bonding force between atoms in the same graphene layer is stronger than the bonding force between planes. The tangential friction is small if the graphene particles are attached on the interface, which ensures that the graphite provides a suitable lubricating effect. S. Q. Liu et al. investigated the effects of graphene and GO on the lubricating properties of drilling fluid.29 The addition of moderate amount of GO significantly improved the lubrication performance of drilling fluids, while graphene had limited effect on the lubrication performance of drilling fluids. In both indoor and field experiments, GO substantially reduced the coefficient of friction (CoF) and the wear volume of aluminum alloy disc. Zheng et al. investigated the improvement of graphene on the lubricating properties of three water-based drilling fluids: distilled water, artificial seawater (KCl brine), and KCl polymer WBDF.2 The presence of graphene resulted in significant improvements in the lubricity and wear resistance of different drilling fluids. 5.0 wt% of graphene reduced the CoF values of distilled water by 84%, of saturated KCl brine by 60%, and of KCl polymer WBDF by 33%. The effectiveness of graphene in improving lubricity and wear resistance continues to diminish as the graphene content of the KCl polymer WBDF increases. The higher improved lubricity of graphene in distilled water was attributed to the filling of the grinding trough with graphene flakes. In contrast, the presence of small particles in the wear track in the KCl brine and KCl polymer WBDF prevented this, resulting in a lower improvement in lubricity than in distilled water. Perumalsamy et al. investigated the improvement of graphene on the rheology and lubricity of water-based drilling fluids at different temperatures.30 Graphene still showed good improvement at 90 °C. Graphene nanoparticles do not participate in chemical bonding and reduce the CoF by rolling action on the surface and clay interface (Fig. 4).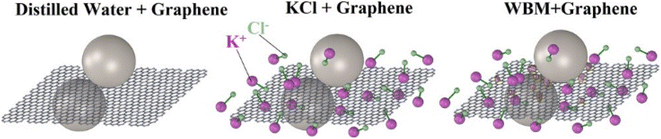 | ||
| Fig. 4 Lubrication mechanism of graphene in three water-based drilling muds.2 | ||
Modified graphene materials with higher chemical activity have also been used for lubricants. Kusrini et al. synthesized GO and phosphorylated GO (PGO) from graphite electrode waste or spent pot lining as additive in WBDF.31 The improvement of WBDF performances GO has been demonstrated. GO was phosphorylated to enhance its dispersion in water, but PGO as an additive showed a negative impact because the pH of the drilling fluid decreased significantly. In addition, in alkaline environments, GO may become negatively charged and repulsion with negatively charged bentonite particles may reduce plasticity. Wang et al. investigated the improvement of lubricity properties by highly dispersed graphene modified by cetyltrimethylammonium chloride (CTAC).32 The viscosity coefficient of the drilling fluid containing 0.05% modified graphene was reduced by 67% at 180 °C. The improved lubrication effect of this modified graphene compared to graphene stems from its high dispersion in water. The method of adsorption of CTAC on graphene is physical adsorption, which is highly affected by temperature, thus making it difficult to still have good lubrication performance at higher temperatures.
The lubrication effect of the complex of GO and deep eutectic solvent (GO/Gly-DES) in WBDF was investigated.33 These synthesized lubricants were all environmentally friendly. GO can exert a more obvious lubricating effect at much lower concentrations compared to the traditional commercial lubricant solid graphite (HY-202). And GO/Gly-DES can further increase the reduction rate of adhesion coefficient and friction coefficient.
Graphene-based materials can substantially improve the lubrication effect of WBDF. Graphene-based materials can easily generate shear at the sliding contact interface and thus have lubricating properties due to their two-dimensional structure. In the field of lubricants for drilling fluids, graphene and GO have been mainly studied, and less research has been done on modified graphene materials. Meanwhile, some of the modified graphene can lead to a decrease in pH of drilling fluids, and some of the modified graphene is strongly affected by temperature. As shown in Table 3, it is obvious that the lubricity of modified graphene is much better than other graphene materials. More research should be conducted in the future for modified graphene materials with temperature resistance and high dispersibility in water.
| Material | Author | Year | Reduction ratea (%)/concentration (wt%) |
|---|---|---|---|
| a The reduction rate is the reduction of coefficient of friction in the lubricant compared to it in the base mud. | |||
| GO | S. Q. Liu et al. | 2017 | 12.6/0.075 |
| GO | Zheng et al. | 2021 | 84/5 |
| GO/Gly-DES | Ma et al. | 2021 | 48.13/0.25 |
| GO modified by CTAC | Wang et al. | 2022 | 67/0.05 |
Compared to traditional additives and other nanomaterials, graphene-based materials greatly enhance performance. In addition to enhanced performance, graphene-based materials have the advantages of low dosage, environmental friendliness, and excellent performance under high temperature and high salt conditions. In addition, graphene-based materials played a complex role in drilling fluids, including inhibition of hydration, reduction of filtration loss, improvement of rheological properties, lubricity, and adsorption of harmful gases. Graphene-based materials improved performance in many aspects.
3. Applications in EOR
The oil field is generally extracted by first using the original energy of the formation, and after a certain stage, artificial water or gas injection is implemented to replenish the formation and drive the crude oil to the bottom of the well, which are primary and secondary oil recovery. After secondary oil recovery, part of the crude oil remains in the formation. Injecting substances not present in the formation to further enhance the recovery of crude oil is enhanced oil recovery, also known as EOR technology. Injection of hot steam, surfactant systems, polymer solutions, alkali solutions, concentrated sulfuric acid and carbon dioxide into the formation can significantly increase the recovery of crude oil. Nanofluids (NF) are liquids in which the nanoparticles are uniformly and steadily dispersed. Recently, the application of NF with thermal stability and high viscosity in EOR has been widely studied.Yusuff et al. investigated the effects of graphene concentrations (0.01, 0.05 & 0.1 wt%) on flowability of oil and its adsorption energy.34 Viscosity of the more viscous crude oil was reduced by 43–65%. The adsorption energy of graphene is moderately low and potentially viable for sandstone reservoir application. Pakharukov et al. found that GNP–oil interaction is affected by GNP concentration and temperature.35 At low concentrations of GNP (500 ppm), the dynamic viscosity of the base fluid decreased by 10–17%, and the greatest decrease in viscosity was observed at 50 °C. The viscosity of oil-based graphene NF (GNF) decreased with increasing nanoparticle concentration, while the viscosity of water-based GNF did not decrease with increasing nanoparticle concentration.
The hydrophobic properties of GO depend on the chain length of grafted alkylamines. Chen et al. investigated the effect of modified GO (M-GO) grafted with alkylamines of different chain lengths on EOR.36 Hexadecylamine and octadecylamine modified GO reduced the injection pressure and improved the aqueous phase permeability of low permeability reservoirs during water flooding.
The nanoparticle-stabilized emulsions remained stable under the harsh thermodynamic conditions of the reservoir. Rezaei Namin et al. functionalized nitrogen-doped graphene (NDG) with carboxyl and hydroxyl groups to enhance the structure at the molecular scale and obtained APH-NDG (acidified, functionalized, heated nitrogen-doped graphene).37 The APH-NDG in all aqueous phases leads to a decrease in contact angle, resulting in a more hydrophilic rock surface, which is shown in Fig. 5.
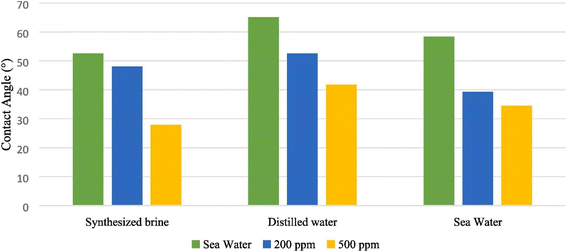 | ||
| Fig. 5 Contact angle of crude oil droplet onto carbonate rock in different aqueous phases with different NGD concentrations.37 | ||
Janus amphiphilic graphene-based materials exhibited excellent interfacial properties due to the amphiphilic characteristic, thus were selected for EOR. Luo et al. synthesized a nanofluid with graphene-based amphiphilic Janus nanosheets that was very effective at low concentrations, NF with 15.2% tertiary oil recovery at 0.01 wt% nanosheet loading.38 While nanofluids containing graphene-based amphiphilic Janus nanosheets can improve the secondary oil recovery rate by 7.5% at 0.005 wt% nanosheet loading, and their performance is also better than other homogeneous nanoparticles at higher concentrations.4 Regardless of the wettability of the solid surface, amphiphilic Janus nanosheets tend to move to the oil–water interface and reduce the interfacial tension (IFT). The climbing and interfacial films form under moderate and strong hydrodynamic conditions, respectively, and rapidly separate the oil and water phases. This results in a twofold increase in optimal performance under similar conditions compared to conventional nanofluid flooding methods. Nanofluids for secondary oil recovery can eliminate tertiary oil recovery to save significant water resources (Fig. 6).
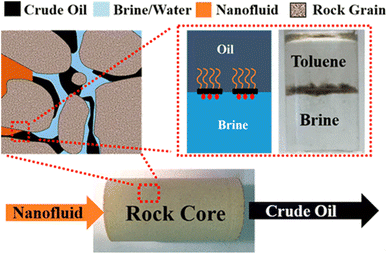 | ||
| Fig. 6 Mechanism of enhanced oil recovery by Janus amphoteric graphene material.4 | ||
Radnia et al. developed a novel NF containing an amphiphilic material (G-DS-Su) based on sulfonated graphene with a hydrophobic base and hydrophilic edges.39 The introduction of the sulfonate group resulted in excellent dispersion and stability under extreme conditions. The concentration of G-DS-Su at 0.5 and 2 mg mL−1 increased the oil recovery by 16% and 19%. G-DS-Su tended to migrate to the oil/water interface and form emulsions. The case of G-DS-Su decreased the IFT by about 11% but did not reach the ultra-low IFT. G-DS-Su changed the wettability of the sandstone surface from the oil-wet state to the intermediate state. The change in wettability may be due to the formation of wedge-shaped films in the oil/nanofluid/solid three-phase contact region and the adsorption of G-DS-Su onto the sandstone due to the interaction between the sand and the functional groups of G-DS-Su. Cao et al. prepared Janus sulfonated graphene oxide nanosheets (JSGO) by a simple method for large-scale production.40 JSGO can reduce the interfacial tension by 71% and has a strong wettability reversal ability, which can turn hydrophobic surfaces into fully hydrophilic ones.
Some scholars prepared amphiphilic GOs by introducing alkylamines with different chain lengths. Chen et al. introduced hexadecylamine on the GO surface to synthesize amphiphilic GOs (H-GO).41 The average droplet diameter of the Pickering emulsion formed by H-GO and oil was only 2.66 μm, and it was quite stable. In addition, the IFT between H-GO and oil is one order of magnitude lower than that between ordinary nanoparticles and oil. Due to these advantages of better wettability modification efficiency of H-GO and very high sweep and displacement efficiency for oil, a low concentration of H-GO (1 mg mL−1) can achieve 10.83% increment in ultra-low permeability cores. Jia et al. evaluated the interfacial properties of Janus graphene oxide (JGO) at liquid–liquid and liquid–solid interfaces using a series of dodecyl amine-modified Janus graphene oxide with different degrees of modification.42 Highly modified JGO can easily create three-dimensional structures to block the flow channels, while moderately modified JGO is more suitable for EOR applications with carefully controlled injection rates. Cao et al. prepared nanofluids containing a polyoxyethylene Janus graphene oxide based material (P-GO-O) with a hydrophilic surface of polyoxyethylene and a hydrophobic surface of octadecyl.43 P-GO-O nanofluid is able to reduce oil–water IFT to 12.2 mN m−1 and has excellent wettability reversal ability. The oil recovery rate of P-GO-O nanofluid (17.2%) is higher than other NFs due to its good stability and wettability reversal ability. In addition, P-GO-O nanofluid exhibited excellent resistance to high temperature and high salinity.
Graphene quantum dots (GQDs) are graphene structures with dimensions below a few nanometers, usually obtained by restricting graphene in all three dimensions. Shayan Nasr et al. investigated the EOR performance of NF with synthetic nitrogen-doped GQDs (N-GQDs) with different GQDs sizes and concentrations.44 NF containing N-GQDs with the lowest average GQDs size and concentration of 0.5 mg mL−1 had a greater effect on the altered wettability of carbonate rock. AfzaliTabar et al. proposed a simple method of N-GQDs/MoS2 quantum dots (MQDs) nanomixes for the preparation of different ratios of nanoemulsions.5 GQDs/MQDs at 10% in cationic surfactants and 50% in anionic surfactants have good stability with fine emulsified droplets. GQDs/MQDs can change the wettability of reservoir rocks to water wetting and still have 22% oil recovery efficiency at very low concentrations.
Nanocomposites containing graphene-based materials have more reactive groups than pure graphene-based materials. Nguyen et al. synthesized highly heat and salt resistant nanocomposite GO–P(AM-NVP) by conjugating a copolymer of acrylamide (AM) and N-vinylpyrrolidone (NVP) (P(AM-NVP)) on GO sheets.45 The temperature and salt resistance of GO–P(AM-NVP) was higher than that of P(AM-NVP), and the structure was more stable due to the addition of graphene. Hamdi et al. prepared functionalized GNPs by two different methods of mixing and grafting with natural gum arabic.46 Polymer grafted GNPs (PG-GNPs) showed higher resistance to high temperature and salt compared to polymer dispersed GNPs (PD-GNPs) at low concentrations. The overall results of PG-GNPs showed better performance due to their higher stability compared to PD-GNPs. Yuh Tiong et al. investigated the application of amphiphilic graphene oxide-based Janus nanosheets (AMGO) with cellulose nanocrystals (CNC) and composition of a novel fused nanofluid (FN) for EOR.47 FN shows better dispersion compared to CNC, probably due to the action of hydrogen bonding between CNC and AMGO. FN is more able to modify the wettability of the rock. FN obtained higher additional oil recovery compared to AMGO.
The addition of nanomaterials to polymer solutions can improve their oil repellent ability as well as high temperature and salt resistance. B. D. Nguyen et al. improved the viscosity stability of dilute polymer/seawater solutions under reservoir conditions by adding graphene-oxide particles (GOs).48 300 ppm GOs improved the viscosity of 1700 ppm acrylamide-based polymers in seawater solutions stability from 92 °C to 135 °C. The oxygen-containing functional groups on the surface of GOs combined with hydrophilic polymer chains resulted in good dispersion of GOs in polymeric liquids.
Aliabadian et al. studied the effect of modified GOs with OH groups mainly on the basal plane (S-GO) and modified GOs with OH groups mainly on the edges (E-GO) on the oil recovery efficiency of partially hydrolyzed polyacrylamide (HPAM) solutions improvement.49 S-GO has better dispersion in HPAM solutions. The oil recovery rate in HPAM solution containing 0.2 wt% of S-GO was improved by 7.8% compared to HPAM solution. de Vasconcelos et al. investigated the improvement of oil recovery efficiency in HPAM solution by GO and aminated graphene oxide (GOA-EDA).50 The NF containing GOA-EDA initially promoted a smaller increase in viscosity than with the GO-containing NF. However, after 90 aging days, the NF containing GOA-EDA showed higher viscosity increase. The aminated graphene oxide (DDGO) modified by ethylenediamine improved HPAM solution in EOR.51 The introduction of DDGO significantly improved the resistance of HPAM solution to high temperature and salt resistance. The enhanced oil recovery of the HPAM/DDGO system was improved by 13.8% compared to the HPAM solution. The group also investigated the improvement of acrylamide-based copolymers (PM) in EOR by GO and hydroxyl-functionalized GO (OGO) modified by diethanolamine.52 The introduction of OGO significantly improved the high temperature and salt resistance of PM solutions due to the introduction of more hydroxyl groups, which provided higher dispersion and stability and enhanced interaction with the polymer. The enhanced oil recovery of the PM/OGO-2 system was improved by 7.1% compared to the PM solution.
NFs based on graphene-based materials are able to modify the wettability and significantly reduce the IFT, thus providing excellent performance in terms of enhanced oil recovery. EOR of nanofluids containing graphene materials is shown in Table 4, and higher EOR of amphiphilic Janus particles at low concentrations can be observed. Janus particles are widely investigated for EOR applications due to their amphiphilic nature. Janus particles tend to move to the oil–water interface thus modifying the wettability and are partially resistant to high temperature and salt due to their chemical and thermal stability. Nanocomposites containing graphene have better resistance to high temperatures and high salinity. The addition of graphene materials to polymer solutions enhances their oil repellency as well as high temperature and salt resistance. As shown in Table 5, the addition of graphene materials to polymer solutions has a high effect on improving EOR, and future research in this area is promising. Hydroxyl groups on the surface of GO and modified graphene materials are bridged to the polymer through hydrogen bonding to improve the network structure and obtain higher dispersion and stability. Janus amphiphilic graphene-based materials and graphene-containing nanocomposites can be investigated more thoroughly in the future to obtain oil repellents with better performance, high temperature and salt resistance.
| Material | Author | Year | Enhanced oil recovery (%)/concentration |
|---|---|---|---|
| Graphene-based Janus nanosheets | Luo et al. | 2016 | 15.2/0.01 wt% |
| G-DS-Su | Radnia et al. | 2018 | 19/2 mg mL−1 |
| GO with hexadecylamine | Chen et al. | 2018 | 10.83/1 mg mL−1 |
| Nitrogen-doped GQDs/MoS2 quantum dots | Afzali Tabar et al. | 2020 | 22/10 wt% |
| Nitrogen-doped GQDs | Shayan Nasr et al. | 2021 | 18/0.5 mg mL−1 |
| Polymer grafted GNPs | Hamdi et al. | 2022 | 15/0.05 mg mL−1 |
| Polyoxyethylated GO | Cao et al. | 2022 | 17.2/100 mg L−1 |
| Octadecylaminated GO | Cao et al. | 2022 | 6.7/100 mg L−1 |
| Janus sulfonated GO | Cao et al. | 2022 | 18.4/100 mg L−1 |
| Janus GO | Jia et al. | 2022 | 5.38/50 mg L−1 |
| Material | Author | Year | Enhanced oil recovery (%) | Increasea (%)/concentration |
|---|---|---|---|---|
| a Increase is the difference in the enhanced oil recovery of the polymer solutions with or without graphene-based material. | ||||
| S-GO in HPAM | Aliabadian et al. | 2020 | 36.13 | 7.8/0.2 wt% |
| AMGO with CNC | Yuh Tiong et al. | 2022 | 22.96 | 16.04/50 ppm |
| HPAM/DDGO | Cao et al. | 2022 | 21.5 | 13.8/44 mg mL−1 |
| PM/OGO-2 | Cao et al. | 2022 | 20.9 | 7.1/50 mg mL−1 |
4. Applications in oily wastewater treatment
For every 1 t of crude oil produced, about 2–3 m3 of water is injected, which is separated from the crude oil when it is extracted to the surface and becomes the oily wastewater. Re-injecting oily wastewater into the ground for recycling is the best way to avoid wasting water and polluting the environment. Before reinjection, it must be treated to eliminate impurities to meet the requirements of water quality for injection.Hydrophobic polymeric membranes such as polybenzimidazole (PBI), polydopamine (PDA), polyetherimide (PEI), polyether sulfone (PES), polyvinylidene fluoride (PVDF) and polyacrylonitrile (PAN) have been widely studied for the treatment of oily wastewater. However, the hydrophobicity of the membranes leads to fouling and hinders the penetration of impurities and oils. The introduction of GO and modified graphene with hydrophilic properties can improve the membrane performance and enhance filtration performance and reusability.
Alammar et al. developed nanocomposite membranes composed of PBI, GO, and reduced GO (RGO) with or without PDA coating.3 The introduction of GO down to 0.5–1.5 wt% in the polymer matrix improves the performance of the membrane in several ways. Compared to pristine PBI membranes, the nanocomposite membranes showed increased permeability and oil removal efficiency, good long-term performance in oil-in-water emulsion separation as well as antifouling and antibacterial properties, ability to de-oil high salinity emulsions, and good reusability. Abdullahi et al. synthesized hydrophobic alkylamines using GO and three different alkylamines (hexylamine, dodecylamine, and hexadecylamine) functionalized GO (Cn-FGO) for the treatment of oily wastewater.53 The hexadecylamine-functionalized GO (C16-FGO) has good hydrophobicity and it showed ≈100% separation of oil from water. In addition, C16-FGO has good oil adsorption and reusability. H. Liang et al. prepared new CMCD@GO/PES hybrid matrix membranes (CMCDGOM) using graphene oxide nanosheets (CMCD@GO) modified by chemically bonded β-cyclodextrin.54 The modified CMCDGOM showed larger and continuous pore size and lower surface roughness compared to the original GO-loaded membrane (GOM). The modified CMCDGOM showed superior filtration, fouling resistance and reusability. G. N. et al. modified ultrafiltration PEI polymer membranes by adding GO and Pluronic F-127 (PF127).55
Mehranbod et al. performed surface modification of highly hydrophobic PVDF membrane by coating it with chitosan–graphene oxide nanostructures.56 The oil separation efficiency of the modified membrane was up to 99%. In addition, it could separate oil/water mixtures in a broad pH range with a high flux recovery ratio. The hybrid matrix membranes had increased porosity with higher improved permeability and oil treatment rates, superior fouling resistance and reusability. Ashraf et al. improved hydrophilicity and porosity by adding GO to PES nanofiltration membranes.57 The GO-containing PES nanofiltration membranes exhibited higher permeability and fouling resistance, and greatly improved rejection of chloride salts and oil. Chen et al. prepared a bifunctional electrospun polymer fibrous composite membrane (FCM) by simple spraying of the hierarchical 3D TiO2@crumpled GO core/shell sphere.58 The membrane had high separation efficiency (99.69%) and superior separation flux. In addition, it showed efficient photocatalytic degradation ability of soluble dyes (Fig. 7).
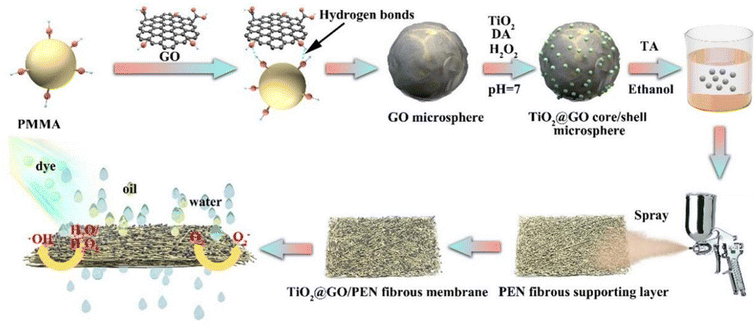 | ||
| Fig. 7 Schematic diagram of preparation process of TiO2@GO/PEN fibrous composite membrane.58 | ||
In addition to treating wastewater by filtering to form membranes, graphene-based materials can also be used as adsorbents to treat wastewater. Alghunaimi et al. synthesized a new hydrophobic material (POG) based on 9-octadecenoic acid grafted graphene (OG) for oil/water separation.59 POG has good oil removal and reusability. The higher the OG, the oil absorption efficiency and adsorption rate of the material increases with the increase of OG content. He et al. prepared a nano-adsorbent (PEG-g-EDTA/Fe3O4/GO) by grafting magnetic GO modified with ethylenediaminetetraacetic acid with polyethylene glycol for the removal of Ca2+ and Cr6+ from Jidong oilfield wastewater.60 Kudaibergenova et al. obtained RGO by reducing GO with hydrazine hydrate, and elaborated a new superhydrophobic magnetic sponge named PU/MgFe2O4/RGO/PDMS with magnetic MgFe2O4 nanoparticles, polyurethane (PU) sponge and polydimethylsiloxane (PDMS).61 The addition of RGO improved the absorption of oil and various organic solvents, while reducing the absorption of water. This sorbent had excellent mechanical/superhydrophobic properties, and it was easily separated from the water because of the magnet. Its separation efficiency was up to 99.7%, and it can be reused for more than 20 cycles.
Graphene foams have also been applied for oil–water separation. Liu et al. loaded gold nanoparticles onto superhydrophobic and superoleophilic graphene foams by ion sputtering.62 The prepared graphene foam has excellent oil–water separation ability and recoverability due to the rough structure of gold nanoparticles in it, which is a promising treatment agent for oil–water separation. Xu et al. prepared magnetic graphene oxide composite as emulsifier, with beetle-like structures that endowed anomalous wettability.63 It showed excellent separation properties for oil-in-water emulsion. In addition, Fe3O4 and C-SR enhanced solar-assisted recyclability of GO.
The main application of graphene-based materials in oily wastewater treatment is the preparation of filter membranes. The introduction of graphene-based materials with hydrophilic properties can improve the performance of membranes. On the one hand, graphene-based materials enhance the filtration effect due to their adsorption properties. On the other hand, graphene-based materials improve the stability of filter membranes and enhance reusability. Graphene-based membranes can achieve oily separation efficiency of 99% and above, so future research could focus on improving the separation flux. In addition, graphene-based materials can be used as adsorbent and graphene foam to treat oily wastewater. Compared with other water treatment agents, graphene-based materials can significantly reduce the oil and scaling ions in oily wastewater. Further research on filter membranes containing graphene-based materials can be conducted in the future to improve the removal of oil and scaling ions, fouling resistance and reusability.
5. Discussion
There have been many studies on the application of graphene and its derivatives in the oil and gas industry. Compared with conventional materials, graphene-based materials have superior performance and environmental performance due to their unique physical and chemical properties. However, at the same time, the existing studies still have some shortcomings and limitations.Graphene-based materials applied to improve the performance of drilling fluids generally play a complex role including improving the rheological properties and reducing the filtration loss of drilling fluids, inhibiting the hydration swelling of clay minerals, and increasing the lubricity. One of the main research objects is modified graphene materials, which not only have excellent improved properties, but also partially have high temperature resistance. However, some of the modified graphene materials are tedious and expensive to prepare, and some of the modified graphene are greatly affected by temperature, which is not ideal as additives for drilling fluids. In the future, we can research on the modified graphene with simple preparation methods to obtain drilling fluid additives with better performance and resistance to temperature and salt. In addition, composites of graphene materials with other nanomaterials as fluid loss agent have some research prospects due to the excellent performance.
Most of the graphene-based materials used in EOR not only have excellent oil recovery efficiency, but also have high temperature and salt resistance. One of the main research targets is Janus graphene materials with amphiphilic properties and the improvement of graphene-based materials in terms of enhanced oil recovery from polymer solutions, which have excellent results for improving EOR. However, the evaluation of graphene-based materials in EOR is still mostly under laboratory simulations and few field tests are available. In the future, while developing graphene-based materials with higher oil recovery, field tests can be added to evaluate their practicality.
Graphene-based materials applied to oily wastewater treatment are mainly used in filter membranes. Compared with ordinary hydrophobic membranes, filter membranes containing graphene materials have higher permeability, better fouling resistance and repeatability, and some of them can be applied to high salt conditions. Graphene-based membranes can achieve oily separation efficiency of 99% and above, so future research could focus on improving the separation flux. More graphene-based materials for oil and impurity treatment in high-salt conditions can be investigated in the future. In addition, research on the application of graphene materials outside of filter membranes needs to be increased.
6. Conclusions
This paper compiles recent advances in the application of graphene and its derivatives in oilfield extraction, including improving drilling fluid performance, EOR, and oily wastewater treatment. We compare the performance advantages of graphene-based materials over other additives and summarizes the mechanism of action of graphene-based materials. These graphene-based materials exhibited high performance compared to conventional materials, but they still exist some problems. Firstly, the synthesis of graphene-based materials is complicated, which limited the wide application. How to prepare these materials with simple method is the way to broaden their application. Secondly, the cost of these materials is high. The cost reduction is the key issue. Thirdly, application in extreme situations such as high temperature and high salt and field experiments is still lacking. It needs to be evaluated in depth. Finally, the application areas need to be extended in oil and gas industry.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We would like to thanks to the financial support of the Basic Research Program on Deep Petroleum Resource Accumulation and Key Engineering Technologies (U19B6003), the National Natural Science Foundation of China (52274011) and Science and Technology Innovation Special Project of Xiong'an New Area (2022XAGG0500) for this work.References
- L. Zhao, H. Zhu, G. Tian and Y. An, RSC Adv., 2023, 13, 2611–2619 RSC.
- Y. Zheng, A. Asif, A. Amiri and A. A. Polycarpou, ACS Appl. Nano Mater., 2021, 4, 1243–1251 CrossRef CAS.
- A. Alammar, S. H. Park, C. J. Williams, B. Derby and G. Szekely, J. Membr. Sci., 2020, 603, 118007 CrossRef CAS.
- D. Luo, F. Wang, J. Zhu, L. Tang, Z. Zhu, J. Bao, R. C. Willson, Z. Yang and Z. Ren, Ind. Eng. Chem. Res., 2017, 56, 11125–11132 CrossRef CAS.
- M. AfzaliTabar, A. Rashidi, M. Alaei, H. Koolivand, S. Pourhashem and S. Askari, J. Mol. Liq., 2020, 307, 112984 CrossRef CAS.
- L. Fu, K. Liao, B. Tang, L. Jiang and W. Huang, Nanomaterials, 2020, 10, 1013 CrossRef CAS PubMed.
- N. Neuberger, H. Adidharma and M. Fan, J. Pet. Sci. Eng., 2018, 167, 152–159 CrossRef CAS.
- S. Sikiru, A. Rostami, H. Soleimani, N. Yahya, Y. Afeez, O. Aliu, J. Y. Yusuf and T. L. Oladosu, J. Mol. Liq., 2020, 321, 114519 CrossRef.
- X. Luo, Z. He, H. Gong and L. He, Chem. Eng. Process., 2022, 170, 108678 CrossRef CAS.
- M. M. Paixão, M. T. Gomes Vianna and M. Marques, Mater. Res. Express, 2018, 5, 012002 CrossRef.
- S. Elhenawy, M. Khraisheh, F. Almomani, M. K. Hassan, M. A. Al-Ghouti and R. Selvaraj, Nanomaterials, 2018, 12, 87 CrossRef.
- T. A. Saleh and M. A. Ibrahim, Energy Rep., 2019, 5, 1293–1304 CrossRef.
- R. Ikram, B. Mohamed Jan, A. Sidek and G. Kenanakis, Materials, 2021, 14, 1944–1996 CrossRef PubMed.
- C. Zamora-Ledezma, C. Narváez-Muñoz, V. H. Guerrero, E. Medina and L. Meseguer-Olmo, ACS Omega, 2022, 7, 20457–20476 CrossRef CAS PubMed.
- A. Aftab, A. R. Ismail and Z. H. Ibupoto, Egypt. J. Pet., 2017, 26, 291–299 CrossRef.
- K. Wang, G. Jiang, X. Li and P. F. Luckham, Colloids Surf., A, 2020, 606, 125457 CrossRef CAS.
- T. A. Saleh, A. Rana and M. K. Arfaj, Environ. Nanotechnol., Monit. Manage., 2020, 14, 100348 Search PubMed.
- A. Rana, M. K. Arfaj, A. S. Yami and T. A. Saleh, J. Environ. Chem. Eng., 2020, 8, 103802 CrossRef CAS.
- A. Rana, M. K. Arfaj and T. A. Saleh, Appl. Clay Sci., 2020, 199, 105806 CrossRef CAS.
- X. Wang, W. Du, W. Hu, J. Zhang and G. Chen, Inorg. Nano-Met. Chem., 2021, 1–7 Search PubMed.
- H. Zhu, D. Li, X. Zhao, S. Pang and Y. An, RSC Adv., 2022, 12, 30328–30334 RSC.
- K. Lv, P. Huang, Z. Zhou, X. Wei, Q. Luo, Z. Huang, H. Yan and H. Jia, Front. Chem., 2021, 8, 201 CrossRef.
- A. Yuxiu, J. Guancheng, Q. Yourong, H. Xianbin and S. He, J. Nat. Gas Sci. Eng., 2016, 32, 347–355 CrossRef.
- H. Zhang, J. Yao, S. Zhang and H. Chen, Appl. Surf. Sci., 2021, 558, 149901 CrossRef CAS.
- D. V. Kosynkin, G. Ceriotti, K. C. Wilson, J. R. Lomeda, J. T. Scorsone, A. D. Patel, J. E. Friedheim and J. M. Tour, ACS Appl. Mater. Interfaces, 2012, 4, 222–227 CrossRef CAS PubMed.
- J. Aramendiz and A. Imqam, J. Pet. Sci. Eng., 2019, 179, 742–749 CrossRef CAS.
- A. Ghayedi and A. Khosravi, J. Pet. Sci. Eng., 2020, 184, 106684 CrossRef CAS.
- A. H. Arain, S. Ridha, S. U. Ilyas, M. E. Mohyaldinn and R. R. Suppiah, J. Pet. Explor. Prod. Technol., 2022, 12, 2467–2491 CrossRef CAS.
- S. Q. Liu, Z. R. Chen, Q. N. Meng, H. L. Zhou, C. Li and B. C. Liu, Nanosci. Nanotechnol. Lett., 2017, 9, 446–452 CrossRef.
- J. Perumalsamy, P. Gupta and J. S. Sangwai, J. Pet. Sci. Eng., 2021, 204, 108680 CrossRef CAS.
- E. Kusrini, F. Oktavianto, A. Usman, D. P. Mawarni and M. I. Alhamid, Appl. Surf. Sci., 2020, 506, 145005 CrossRef CAS.
- Q. Wang, M. Slaný, X. Gu, Z. Miao, W. Du, J. Zhang and C. Gang, Materials, 2022, 15, 1083 CrossRef CAS PubMed.
- J. Ma, J. Xu, S. Pang, W. Zhou, B. Xia and Y. An, Energy Fuels, 2021, 35, 8153–8162 CrossRef CAS.
- A. O. Yusuff, N. Yahya, M. A. Zakariya and S. Sikiru, J. Pet. Sci. Eng., 2021, 198, 108250 CrossRef CAS.
- Y. Pakharukov, F. Shabiev, R. Safargaliev, V. Mavrinskii, S. Vasiljev, B. Ezdin, B. Grigoriev and R. Salihov, J. Mol. Liq., 2022, 361, 119551 CrossRef CAS.
- H. Chen, L. Xiao, Y. Xu, X. Zeng and Z. Bin Ye, J. Nanomater., 2016, 2016, 8716257 Search PubMed.
- A. Rezaei Namin, M. Rajabi-Kochi, A. Rashidi, E. Yazdi, M. Montazeri and A. Asghar Gharesheikhlou, Fuel, 2023, 335, 127033 CrossRef CAS.
- D. Luo, F. Wang, J. Zhu, F. Cao, Y. Liu, X. Li, R. C. Willson, Z. Yang, C. W. Chu and Z. Ren, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 7711–7716 CrossRef CAS PubMed.
- H. Radnia, A. Rashidi, A. R. Solaimany Nazar, M. M. Eskandari and M. Jalilian, J. Mol. Liq., 2018, 271, 795–806 CrossRef CAS.
- J. Cao, Y. Chen, X. Wang, J. Zhang, Y. Li, S. Wang, X. Wang and C. Liu, Chem. Eng. J., 2022, 443, 136391 CrossRef CAS.
- L. Chen, X. Zhu, L. Wang, H. Yang, D. Wang and M. Fu, Energy Fuels, 2018, 32, 11269–11278 CrossRef CAS.
- H. Jia, X. Wei, Q. X. Wang, Y. B. Wang, S. J. Wen, F. N. Fan, Q. Wang, Z. Wang, D. X. Liu and P. Huang, Pet. Sci., 2022, 20, 1217–1224 CrossRef.
- J. Cao, Y. Chen, J. Zhang, X. Wang, J. Wang, C. Shi, Y. Ning and X. Wang, Chem. Eng. Sci., 2022, 247, 117023 CrossRef CAS.
- M. Shayan Nasr, E. Esmaeilnezhad, A. Allahbakhsh and H. J. Choi, J. Mol. Liq., 2021, 330, 115715 CrossRef CAS.
- T. L. Nguyen, A. Q. Hoang, P. T. Nguyen, A. T. Luu, D. K. Pham, V. P. Dinh, Q. H. Nguyen, V. T. Le, H. N. Tran and T. B. Luong, Colloids Surf., A, 2021, 628, 127343 CrossRef CAS.
- S. S. Hamdi, H. H. Al-Kayiem, M. S. Alsabah and A. S. Muhsan, J. Pet. Sci. Eng., 2022, 210, 110004 CrossRef CAS.
- A. C. Yuh Tiong, I. S. Tan, H. C. Yew Foo, M. K. Lam, H. Ben Mahmud, K. T. Lee and P. L. Show, J. Pet. Sci. Eng., 2022, 221, 111242 Search PubMed.
- B. D. Nguyen, T. K. Ngo, T. H. Bui, D. K. Pham, X. L. Dinh and P. T. Nguyen, Adv. Nat. Sci.: Nanosci. Nanotechnol., 2015, 6, 015012 CAS.
- E. Aliabadian, S. Sadeghi, A. Rezvani Moghaddam, B. Maini, Z. Chen and U. Sundararaj, Fuel, 2020, 265, 116918 CrossRef CAS.
- C. K. B. de Vasconcelos, F. S. Medeiros, B. R. S. Diniz, M. M. Viana, V. Caliman and G. G. Silva, Fuel, 2022, 310, 122299 CrossRef CAS.
- J. Cao, G. Xu, X. Wang, H. Wang, J. Zhang and C. Liu, Colloids Surf., A, 2022, 653, 129909 CrossRef CAS.
- J. Cao, G. Xu, X. Wang, K. Liu, J. Zhang, H. Wang, X. Wang and Z. Wu, J. Mol. Liq., 2022, 360, 119501 CrossRef CAS.
- B. O. Abdullahi, E. Ahmed, H. Al Abdulgader, F. Alghunaimi and T. A. Saleh, J. Mol. Liq., 2021, 325, 119501 CrossRef.
- H. Liang, C. Zou and W. Tang, J. Taiwan Inst. Chem. Eng., 2021, 118, 215–222 CrossRef CAS.
- G. N. Manikandan and M. Helen Kalavathy, Chem. Eng. Res. Des., 2021, 168, 214–226 CrossRef CAS.
- N. Mehranbod, M. Khorram, S. Azizi and N. Khakinezhad, J. Environ. Chem. Eng., 2021, 9, 106245 CrossRef CAS.
- T. Ashraf, N. Alfryyan, M. Nasr, S. Ahmed and M. Shaban, Polymers, 2022, 14, 2572 CrossRef CAS PubMed.
- X. Chen, Y. Zhan, A. Sun, Q. Feng, W. Yang, H. Dong, Y. Chen and Y. Zhang, Sep. Purif. Technol., 2022, 298, 121605 CrossRef CAS.
- F. I. Alghunaimi, D. J. Alsaeed, A. M. Harith and T. A. Saleh, J. Cleaner Prod., 2019, 233, 946–953 CrossRef CAS.
- L. He, Y. Dai, Z. Wang, L. Yang, L. Zhang, P. Hu, Y. Tian, H. Mo, H. Zhu and J. Zhang, Korean J. Chem. Eng., 2022, 39, 2229–2238 CrossRef CAS.
- R. Kudaibergenova, O. Ualibek, E. Sugurbekov, G. Demeuova, C. Frochot, S. Acherar and G. Sugurbekova, Int. J. Environ. Sci. Technol., 2022, 19, 8491–8506 CrossRef CAS.
- S. Liu, S. Wang, H. Wang, C. Lv, Y. Miao, L. Chen and S. Yang, Sci. Total Environ., 2021, 758, 143660 CrossRef CAS PubMed.
- Y. Xu, G. Wang, L. Zhu, W. Deng, C. Wang, T. Ren, B. Zhu and Z. Zeng, Chem. Eng. J., 2021, 427, 130904 CrossRef.
| This journal is © The Royal Society of Chemistry 2023 |