Open Access Article
Open Access ArticleA novel n–p heterojunction Bi2S3/ZnCo2O4 photocatalyst for boosting visible-light-driven photocatalytic performance toward indigo carmine†
Nguyen Thi Mai Tho *a,
Nguyen Van Cuonga,
Viet Ha Luu Thi
*a,
Nguyen Van Cuonga,
Viet Ha Luu Thi a,
Nguyen Quoc Thang
a,
Nguyen Quoc Thang a and
Phuc Huu Dang*b
a and
Phuc Huu Dang*b
aFaculty of Chemical Engineering, Industrial University of Ho Chi Minh City, Ho Chi Minh, Vietnam. E-mail: nguyenthimaitho@iuh.edu.vn
bFaculty of Fundamental Science, Industrial University of Ho Chi Minh City, Ho Chi Minh, Vietnam. E-mail: danghuuphuc@iuh.edu.vn
First published on 31st May 2023
Abstract
An innovative p–n heterojunction Bi2S3/ZnCo2O4 composite was first fabricated via a two-step co-precipitation and hydrothermal method. By controlling the weight amount of Na2S and Bi(NO3)3 precursor, different heterogeneous xBi2S3/ZnCo2O4 were synthesized (x = 0, 2, 6, 12, and 20). The p–n heterojunction Bi2S3/ZnCo2O4 was characterized by structural, optical, and photochemical properties and the photocatalyst decoloration of indigo carmine. Mott–Schottky plots proved a heterojunction formed between n-Bi2S3 and p-ZnCo2O4. Furthermore, the investigation of the photocurrent response indicated that the Bi2S3/ZnCo2O4 composite displayed an enhanced response, which was respectively 4.6 and 7.3 times (4.76 μA cm−2) greater than that of the pure Bi2S3 (1.02 μA cm−2) and ZnCo2O4 (0.65 μA cm−2). Especially the optimized p–n Bi2S3/ZnCo2O4 heterojunction with 12 wt% Bi2S3 showed the highest photocatalyst efficacy of 92.1% at 40 mg L−1 solutions, a loading of 1.0 g L−1, and a pH of 6 within 90 min of visible light illumination. These studies prove that p–n Bi2S3/ZnCo2O4 heterojunction photocatalysts can greatly boost their photocatalytic performance because the inner electric field enhances the process of separating photogenerated electron–hole pairs. Furthermore, this composite catalyst showed good stability and recyclability for environmental remediation.
1. Introduction
Currently, environmental pollution in the world is at an alarming level, especially water pollution. Wastewater including pigments, reactive dyes, heavy metal ions, and organic substances is the main cause of pollution because it is difficult to decompose and highly resistant to light, heat, and oxidizing agents that can affect the health of humans and organisms.1,2 A typical indigo dye used in textile dyeing and other industries is indigo carmine (C16H8N2Na2O8S2). However, indigo carmine (IC) is a very dangerous agent that is categorized as being environmentally toxic; regular exposure to it can irritate the respiratory tract, the skin on the back of the eyes, and the cornea, in addition to causing acute toxicity. Therefore, the pollution control and treatment of IC dyes from textile dyeing wastewater are of great interest to many people.3–5 Over the past few years, advanced oxidation processes (AOPs) provide a possibility for completing the cleaning of wastewater polluted with recalcitrant organic chemicals.6 A number of these methodologies are frequently employed in Advanced Oxidation Processes (AOPs), including (i) Fenton oxidations, (ii) photocatalysts, (iii) plasma oxidation, and (iv) ozonation. Recently, metal oxide semiconductor photocatalysis has been widely explored. WO3, TiO2, ZnO, Fe2O3, ZrO2, CuO, and NiO are semiconductor photocatalysts.7 However, these metal oxide semiconductors only take advantage of the UV radiation spectrum (3–5% of the total solar spectrum) because of their wide bandgap.8 Moreover, hybrid metal chalcogenide compounds can have adequate redox potential while improving solar spectrum absorption.9–11 Among the hybrid metal chalcogenide compounds, n-type bismuth sulfide (Bi2S3), possesses unique properties such as wide light absorption, high dielectric properties, a narrow band gap (Eg) (approx. 1.3 eV), lamellar structure, and especially an adjustable band gap, so it is used in photocatalysis and photo electrochemistry (PEC).12–16 Unfortunately, the photogenerated electron–hole pair (eCB− − hVB+) recombines rapidly due to the narrow Eg of Bi2S3, resulting in low separation efficiency and challenging its reality-based applications.6 So, scientists look for solutions such as dopants, decoration with the plasmonic noble metal, novel (p–n/n–n) heterojunction formation, etc.17–21Recent research has focused on the transition metal p-type oxide ZnCo2O4 due to its narrow energy band, higher photoelectrochemical stabilization, higher electrical conductivity, and a larger amount of redox reaction sites than metal oxides (ZnO, Co3O4).22,23 It is noted that because of the specific energy band structure, the valence band (VB) is formed by the energy levels of O 2p, while the conduction band is formed by the energies of Co 3d. So, the electron inside the band gap can switch easily, thus increasing the photogenerated electron–hole lifetimes.24 Besides, ZnCo2O4 is a promising candidate for fabricating an advanced p–n heterojunction in conjunction with other photocatalysts.25 Some heterojunction structure was studied such as SnO2/ZnCo2O4,26 ZnCo2O4/Bi2O3,25 ZnO/ZnCo2O4,27 CaFe2O4/ZnCo2O4,28 BiVO4/ZnCo2O4 in the photodegradation of dye molecules from aqueous solution.
Based on the above discussion, p–n heterojunctions structure including an n-type narrow bandgap Bi2S3 was deposited on the surface of p-type ZnCo2O4, which is the solution to boost the photocatalyst efficacy of ZnCo2O4. The structure with 12 wt% Bi2S3 on ZnCo2O4 showed an improvement in visible light photocatalytic performance than that of pure Bi2S3 and ZnCo2O4. This is because ZnCo2O4 provides superior photoelectrochemical stability, whereas the low energy bandgap Bi2S3 boosts solar light absorption ability. The type-II heterojunction forms a built-in electric field between the interface of Bi2S3 and ZnCo2O4 semiconductors, which accelerates the separation of photocarriers and is good for applications related to energy harvesting. The process of degradation was thoroughly studied.
2. Experimental
2.1 Synthesis of ZnCo2O4
The ZnCo2O4 materials were fabricated by the coprecipitation method. A solution of 100 mL Zn(NO3)2 0.1 M and 100 mL Co(NO3)2 0.3 M with a Zn2+/Co2+ molar ratio of 1/3 was slowly added to 50 mL of 1 M NaOH with a rate of 10 mL min−1. The solution was unchanged at pH 10 using NaOH and was stirred. The after-precipitation solution was aged at 105 °C for 15 hours. The resulting powders were filtered, cleaned with de-ionized water numerous times, and heated at 100 °C for 10 hours. The black powder was thermally treated at 600 °C for 4 h to receive ZnCo2O4 (ZC).2.2 Synthesis of Bi2S3
The sample Bi2S3 was formed by slowly adding 10 mL Na2S dissolved in ethylene glycol (EG) to 50 mL EG containing Bi(NO3)3, with a molar ratio of Bi3+/S2− at 2/3. The Bi2S3 was collected, centrifuged, cleaned, and air-dried at 105 °C.2.3 Synthesis of Bi2S3/ZnCo2O4 heterostructures
First, the as-prepared 0.5 g ZnCo2O4 and Bi(NO3)3·5H2O which were diluted in a minimum amount of HNO3 (5%) before being dispersed, were inserted into 50 mL of EG. The solution of 50 mL Na2S with EG was added dropwise and slowly to the as-prepared solution. The suspension solution was stirred at 140 °C for 12 hours. Eventually, the Bi2S3/ZC samples were gathered following deionized water washing and 100 °C drying. By controlling the weight amounts of Na2S and Bi(NO3)3, different Bi2S3/ZnCo2O4 heterogeneous were obtained (x is the mass percentage of Bi2S3/ZnCo2O4). Four samples are labeled as 2.0Bi2S3/ZC; 6.0Bi2S3/ZC; 12.0Bi2S3/ZC and 20.0Bi2S3/ZC.The obtained Bi2S3, ZC, and x.0Bi2S3/ZC powder were identified by X-ray diffraction (XRD), Fourier-transform infrared spectroscopy (FT-IR) microscopy (TEM), scanning electron microscopy (SEM), and X-ray photoelectron spectroscopy (XPS); the UV-visible diffuse reflectance spectrum (DRS); the energy dispersive X-ray (EDX), high-resolution electron microscope image (HRTEM), BET data including N2 adsorption–desorption isotherms, inductively coupled plasma optical emission spectroscopy (ICP-OES), photocurrent response (j–t) and electrochemical impedance spectra (EIS). The ion Zn and Co leaching was detected by ICP-OES.
2.4 Evaluating photocatalytic performance
The catalytic activity of x.0Bi2S3/ZC powder was evaluated based on the decomposition of IC dye (λmax = 612 nm) using a 300 W halogen lamp (Osram, Germany). The catalytic reaction is carried out on a catalytic system consisting of two processes:Adsorption processes: The adsorption–desorption equilibrium of indigo carmine on the surface of x.0Bi2S3/ZC catalysts was in the dark for 1 hour.
Degradation processes: The light source is turned on to perform the photocatalytic reaction for 90 min. After every 15 minutes, 5 mL of suspension is placed in the centrifuge system to remove the catalyst. The solution was stirred using a magnetic stirrer and circulating water to keep the room temperature. The ultraviolet-visible spectrophotometer determines the concentration of the IC solution.
3. Results and discussion
3.1 Characterization
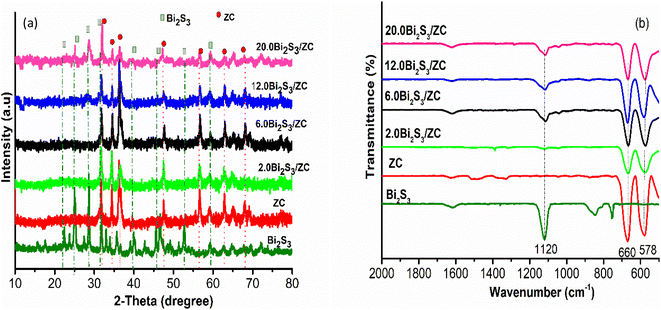
The structure of the prepared catalyst is studied based on XRD spectroscopy (Fig. 1a). The strong and sharp diffraction peaks of ZC at 31.85°; 36.17°; 47,38°, 56.76°, 62.77°, and 67.67° ascribed with (220); (311); (400); (422); (511) and (440) lattice planes of homogeneous cubic spinel phase ZnCo2O4 (JCPDS no. 23-1390), proving that the ZC was successfully synthesized by coprecipitation method. The diffraction peaks of pure Bi2S3 at 2θ values 22.46; 25.12; 28.35, 31.71; 39.8; 45.3; 46.5; 58.7 coincide with the orthorhombic Bi2S3 phase (JCPDS no. 17-0320).29 The XRD patterns of x.0Bi2S3/ZC heterostructures (x = 2, 6) are similar to those of ZC and do not appear to have diffraction peaks of pure Bi2S3. This can be caused by the low loading weight of Bi2S3 on ZC. However, x.0Bi2S3/ZC (x = 12, 20) heterostructures appear as main diffraction peaks of Bi2S3 the intensities of them increase according to the content percentage of Bi2S3 on heterostructures increasing. The above results suggest that the x.0Bi2S3/ZC heterostructures were successfully synthesized with good crystallinity and less impurity.The FTIR spectra of Bi2S3, ZC, and x.0Bi2S3/ZC heterostructure are presented in Fig. 1b. The Bi2S3 sample exhibited characteristic vibrational peaks at 1120 cm−1 relating to the stretching modes Bi–S groups.30 For x.0Bi2S3/ZC samples characteristic bands of the stretching modes Bi–S are also observed at 1120 cm−1 and this indicates the presence of Bi2S3 in the composites. This peak was observed with lower intensities in the composites which indicates the formation of x.0Bi2S3/ZC heterojunction nanocomposites. In addition, the characteristic peaks of the ZC and x.0Bi2S3/ZC (x = 2, 6, 12, 20) are observed in 660 cm−1 and the range of 578 cm−1 which are metal-oxy vibration such as the Co–O tensile and the Zn–O spinel.31
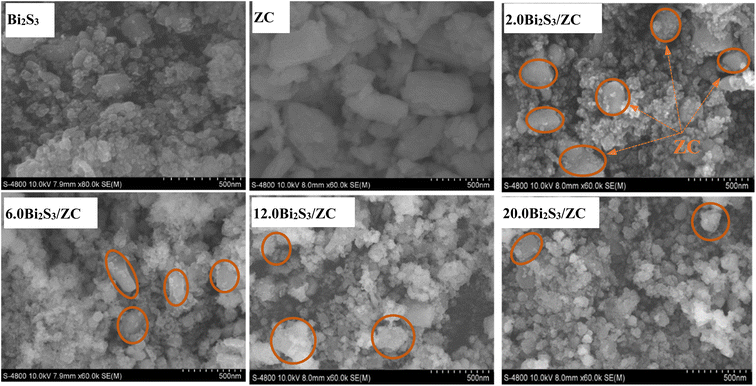
In Fig. 2a, the synthesized Bi2S3 was clearly shown to produce nanoparticles with a homogeneous array, and porous material. Bi2S3 nanoparticles aggregated loosely, and there were apparent pores, indicating that adding additional active sites might boost catalytic activity. Many ZC layers were stacked resulting in typical nanoplates with a homogeneous array with thin sheets. FESEM image of x.0Bi2S3/ZC shows that Bi2S3 are precipitated and wrapped on the surface of ZC. Nanoparticles of Bi2S3 were coated on a surface of ZC, forming a heterogeneous interface with intimate contact. The more Bi2S3 content, the better x.0Bi2S3/ZC nanosheets become rough and irregular gradually.
In addition, the HRTEM results also confirmed the formation of the 12.0Bi2S3/ZC heterojunction. The lattice fringes were approximately ∼0.310 nm which is ascribed to the (221) plane of orthorhombic Bi2S3,32,33 in which 0.239 nm belongs to the (311) plane of cubic spinel ZC.23,28 In addition, the TEM image also observed the appearance of an amorphous phase at the interface of the crystalline Bi2S3 and ZC phases. The association between the crystalline and amorphous phases helps speed up the charge migration due to the mobility of localization sites in the amorphous phase.34 The distribution of particles was around 20-60 nm, which was determined by the TEM image (Fig. S1†). Moreover, Fig. 3c and d displays that the EDX spectra of ZC consists of only three main elements Zn (19.04%), Co (23.01%), O (57.94%) while the 12.0Bi2S3/ZC heterojunction shows the presence of Zn (28.93%), Co (7.18%), O (57.59%), Bi (1.47%) and S (4.82%). This result proved the formation of the Bi2S3/ZC heterojunction. Furthermore, the Co/Zn atomic ratio in ZC and 12.0Bi2S3/ZC are about 2.17 and 2.3 by ICP-OES measurement, respectively (Table S1†).
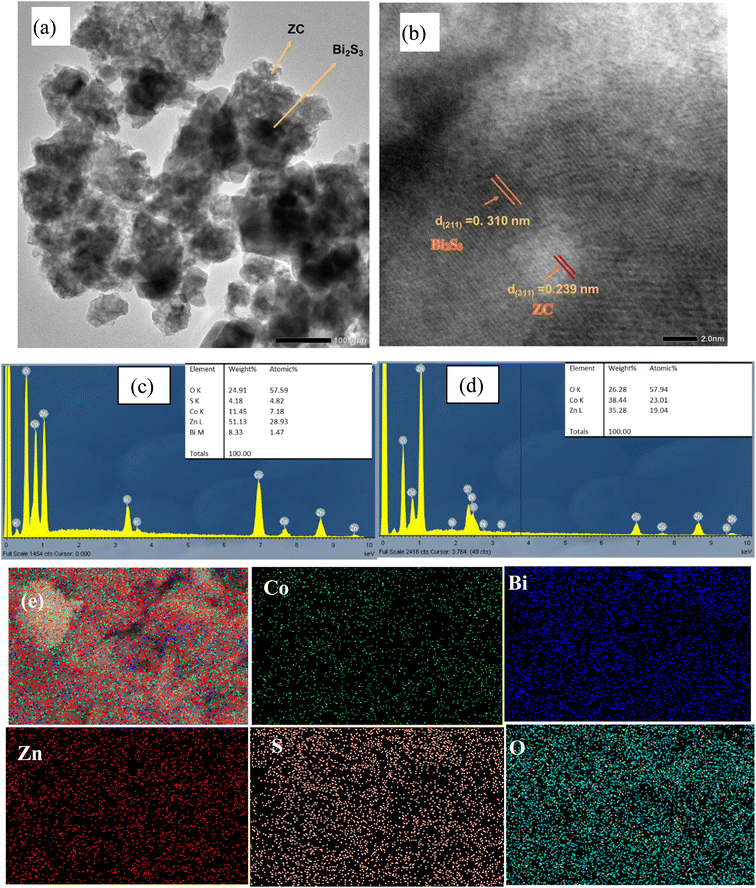 | ||
| Fig. 3 TEM (a) and HRTEM (b) diagram of 12.0Bi2S3/ZC samples; the EDX (c–e) of ZnCo2O4 and 12.0Bi2S3/ZC samples. | ||
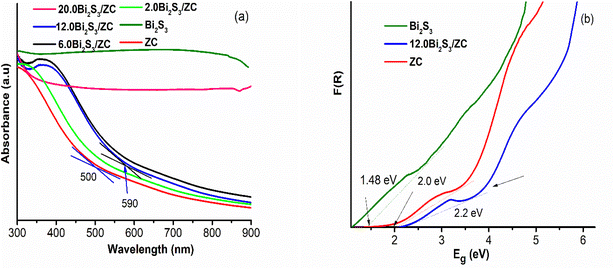
The optical absorption of Bi2S3, ZC, and x.0Bi2S3/ZC are shown in Fig. 4b. ZC shows good absorbance of light located at about 500 nm while pure Bi2S3 presents an optical absorption edge nearly in the visible light spectral region that extended even to the infrared region. For the x.0Bi2S3/ZC heterojunction, the absorption edges have changed compared to ZC, edges appeared at 590 nm and were blue-shifted in comparison with those of ZC. It has been discovered that the proportion of the amount of Bi2S3 causes a change in the absorption edge and absorbance density in the visible spectrum. However, the band gap of 20.0Bi2S3/ZC will be dominated by the narrower band gap of Bi2S3 because Bi2S3 covers the surface of ZC. This phenomenon was observed in the morphology image FESEM. Besides, Using the Kubelka–Munk function estimate the band gap energy of the powders. Results can be seen from Fig. 4b that the bandgap of ZC, Bi2S3, and 12.0Bi2S3/ZC are approximately 2.2 eV, 1.48 eV, and 2.0 eV, respectively. The development of Bi2S3 nanoparticles on the surface of ZC can interface in heterojunction which increases more photogenerated hole–electron pair, thereby enhancing the photocatalytic performance of 12.0Bi2S3/ZC.26,27
The elements and chemical states in photocatalyst heterojunction were studied by X-ray photoelectron spectroscopy (XPS). As depicted in Fig. 5a Zn, Co, O, and S elements of both ZnCo2O4 and x.0Bi2S3/ZC were detected from characteristic peaks of O 1s; Co 2p and Zn 2p; x.0Bi2S3/ZC samples also have characteristic peaks of S 2p and Bi 4f.22 The high-resolution spectrum for the S 2p and Bi 4f region shows (Fig. 5b) the number of deconvoluted peaks at 158.16 and 163.71 eV ascribed to Bi 4f7/2 and Bi 4f5/2 orbital, meanwhile, the peak at 161.22 eV and 162.64 eV can be ascribed to S 2p3/2 and S 2p1/2.32,35 The first group at 779.37; 794.47 eV and the second group at 780.61; 795.88 eV binding energy (Fig. 5c) which can be ascribed to Co 2p in ZC indicating that the valence of Co3+ and Co2+ of ZC spinel, these peaks move to the direction of large binding energy with 780.53; 796.55 eV and 783.12; 802.14 eV in 12.0Bi2S3/ZC, respectively.36 Moreover, a multivalent in ZC was observed by the two weaks satellite peaks, as mentioned in the literature. Fig. 5d shows the peaks of ZC are Zn 2p3/2 (1021.38 eV) and Zn 2p1/2 (1044.45 eV), respectively. Moreover, the existence of Zn2+ in the ZC structure was identified by the distance between two peaks Zn2p3/2 and Zn2p5/2 (23.07 eV). Besides, the shift of Zn2p orbitals peaks to the higher binding energies for the 12.0Bi2S3/ZC heterojunction, compared to ZC. The deconvolution O 1 s peak (Fig. 5e) using the Gaussian-based included 530.48 and 532.65 components, which were attributed to metal–oxygen bonds (O2−), the surface hydroxyl groups, and the O–H species absorbed water on the surface of ZC.37 The positive shift of binding energy of O 1s for 12.0Bi2S3/ZC heterojunction compared to ZC indicates a change of the electron density, establishing an effective charge transfer between ZC and Bi2S3. This could be explained due to the interaction effect of the strong chemical bond between ZC and Bi2S3 changing the outer electron cloud densities of Co.22,25,36 Finally, from the XPS results, we can conclude the 12.0Bi2S3/ZC photocatalyst contains the elements Bi, S, O, Co, and Zn, indicating that Bi2S3 was synthesized successfully and loaded on the surface of spinel phase ZnCo2O4 nanoparticles.
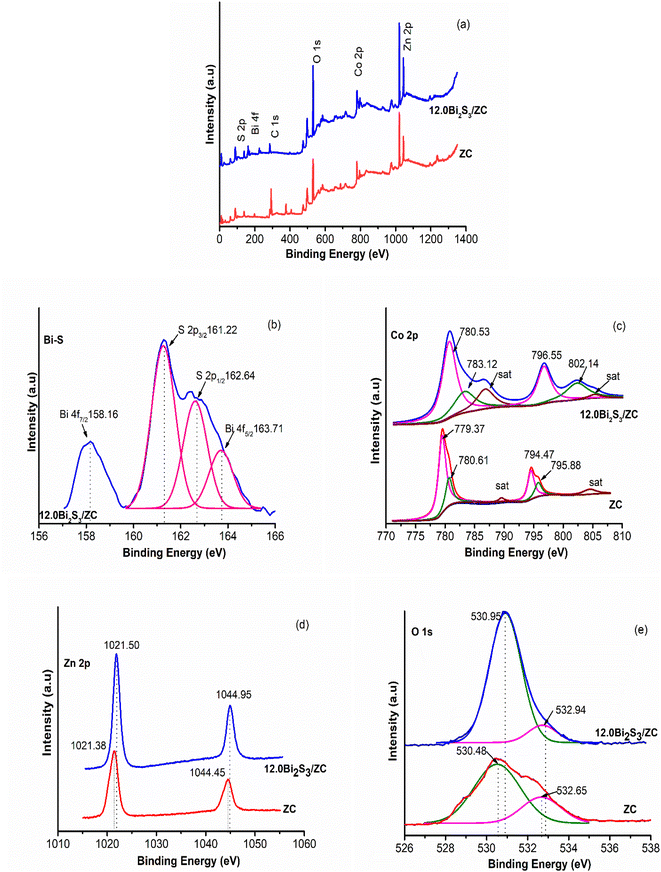 | ||
| Fig. 5 XPS spectra (a) survey; (b) Bi–S 2p; (c) Co 2p; (d) Zn 2p; (e) O 1s of pristine ZnCo2O4 and 12.0Bi2S3/ZC samples. | ||
Fig. 6 shows the result of the N2 adsorption/desorption isotherm and pore-size distribution of ZC and 12.0Bi2S3/ZC that all samples exhibited a type IV isotherm, and the hysteresis loops were the type of H3. The specific surface area, pore volume, and average pore size of the ZC and 12.0Bi2S3/ZC were shown in Table 1. The Specific surface area calculated from the Brunauer–Emmett–Teller (BET) isotherms is 15.49 m2 g−1 and 21.33 m2 g−1 for ZC and 12.0Bi2S/ZC, respectively. The increased surface area of 12.0Bi2S/ZC could be attributed to the loading of pores Bi2S3 nanoparticles. The pore size distribution of ZC is approximately 13.07 nm, while that of 12.0Bi2S/ZC is 15.76 nm. The significant specific surface area and pore constructed help increase active sites, enhance organic dye adsorption, and effectively transfer charge carriers, thus enhancing the photocatalytic activity of 12.0Bi2S3/ZC.
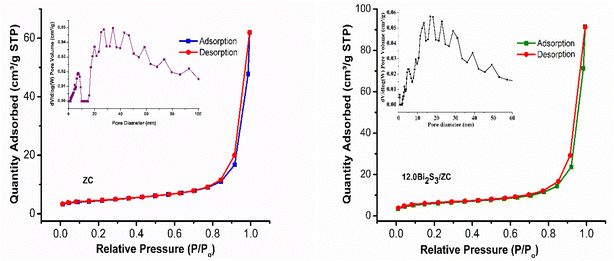 | ||
| Fig. 6 Nitrogen adsorption–desorption isotherm and Barrett–Joyner–Halenda (BJH) pore-size distribution (a) ZC and 12.0Bi2S3/ZC. | ||
| Sample | SBET (m2 g−1) | Pore volume (cm3 g−1) | Average pore size (nm) |
|---|---|---|---|
| ZC | 15.49 | 0.03566 | 13.07 |
| 12.0Bi2S3/ZC | 21.33 | 0.04342 | 15.76 |
3.2 Photoelectrochemical performance
The photoelectrochemical operation of the x.0Bi2S3/ZC in 0.5 M Na2SO4 solution was investigated to determine the separation and charge transfer efficiency. The photoelectrochemical efficiency of Bi2S3, ZC, and 12.0Bi2S3/ZC is shown in Fig. 7. Transient photocurrent response was tested in all samples (On and off interval time being 10 s). According to the transient photoelectric response (I–t curve) in Fig. 6a, the 10%-Bi2S3/ZC composite has a greater photocurrent intensity (4.76 μA cm−2) than the pure Bi2S3 (1.02 μA cm−2) and ZC (0.65 μA cm−2). It is generally agreed that the separation of photogenerated charges is more effective, and its photocatalytic performance is the greater intensity of photocurrent.35,38,39 Moreover, when the lamp is switched on, the photocurrent of 12.0Bi2S3/ZC continually rises, showing that the photogenerated electron–hole pairs separate continuously.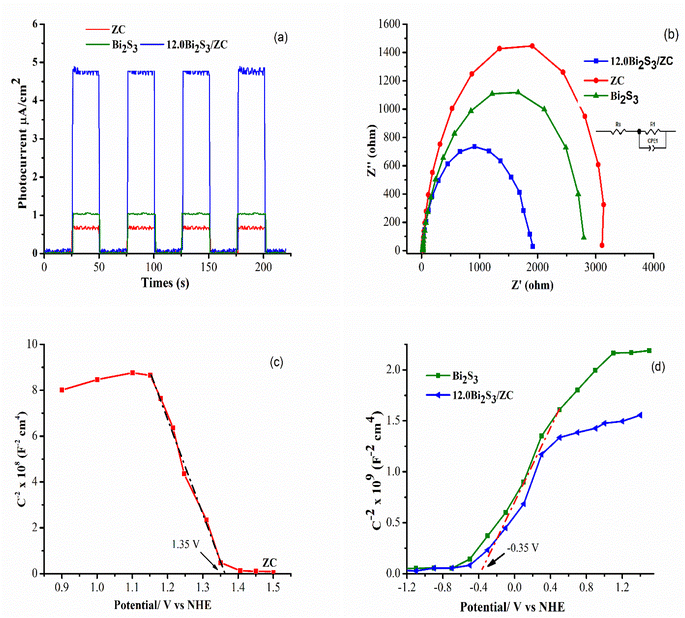 | ||
| Fig. 7 (a) Transient photocurrent response; (b) EIS curves, (c and d) Mott–Schottky plot of ZC, 12.0Bi2S3, and 12.0Bi2S3/ZC. | ||
Electrochemical impedance spectroscopy (EIS) experiments correlate the charge transport resistance of a catalyst with the Nyquist plot, where a significant shift in the arc radius of the Nyquist plot indicates effective charge transport in the preparation of Bi2S3 – ZC. EIS of synthesized catalysts is displayed in Fig. 7b, and the equivalent circuit is shown in the upper right corner of Fig. 7b. Pure Bi2S3, ZC, and 12.0Bi2S3/ZC samples all have Rs values of 2627, 3012, and 1794 Ω, whereas the corresponding Rct values are 43.34, 53.84, and 42.04 Ω. There is less resistance to charge transfer and more efficient carrier transfer when the semicircle's radius is smaller.40,41 It can be observed from the specific resistance values that the 12.0Bi2S3/ZC has the lowest resistance value, is more photo-catalytically active, and outperforms pure Bi2S3 and ZnCo2O4 in terms of surface charge transfer abilities. The EIS finding agreed with those from the UV-DRS characterization research and photocatalytic measurements.
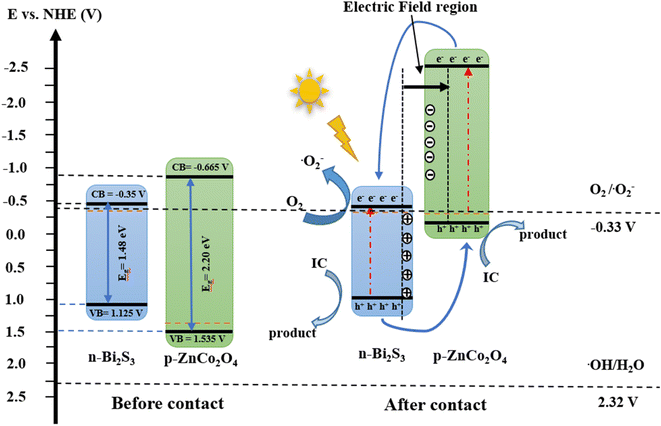
The semiconductor and energy-gap structure of the photocatalyst was analyzed using the Mott–Schottky (M–S) measurement at 1000 Hz in 0.5 M Na2SO4 solution at pH 7. The acquired curve for ZnCo2O4 has a negative slope, suggesting that it is an n-type semiconductor, whereas the obtained curve for Bi2S3 has a positive slope, showing that it is a p-type semiconductor (see Fig. 7c). This study reveals that the CB potential of Bi2S3 and the VB potential of ZnCo2O4 are identified at −0.355 V vs. NHE and 1.535 V vs. NHE (ENHE = EAg/AgCl-3.5M + 0.24), respectively. With the aforementioned results, it has been determined that the VB of Bi2S3 and CB of ZnCo2O4 are approximately 1.125 V and −0.665 V, respectively, as calculated by the formula ECB = EVB − Eg. To understand the mechanism of photocatalysis in the Bi2S3/ZnCo2O4 heterojunction, an M–S plot has been measured. The results indicate a flat band potential shift positive for the Bi2S3/ZnCo2O4 heterostructure photocatalyst, suggesting the formation of a p-ZC/n-Bi2S3 heterojunction. This finding is supported by previous research.1,42
3.3 Photocatalytic activity
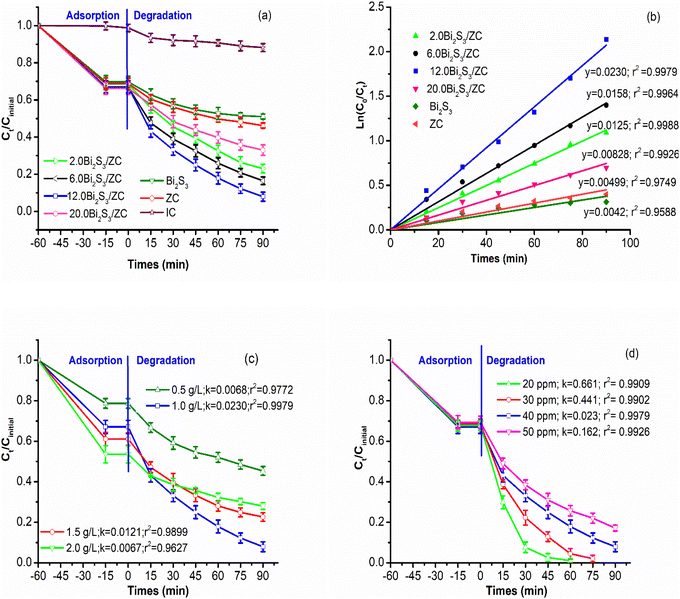
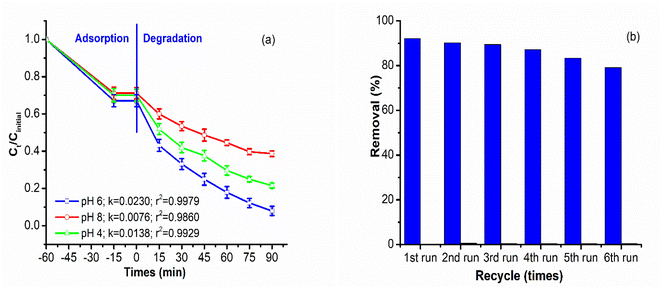
After a 60 minutes absorption process, the quantity of IC dye adsorbed from 29–33% for the adsorption equilibrium while after 90 minutes under the degradation process, the amount of IC degraded from 67% to 92.1% by x.0Bi2S3/ZC. The x.0Bi2S3/ZC heterostructures exhibit significantly increased photocatalytic performance as compared with ZC. The photodegradation efficiency of IC reached 92.1% for 12.0Bi2S3/ZC and only 53.8% for ZC. The photocatalytic experimental results of x.0Bi2S3/ZC obey the pseudo-first-order kinetic as indicated by the good values of the correlation coefficient (R2 > 0.9588). The photocatalyst performance was determined by the values of k, which were subsequently arranged in descending order of 12.0Bi2S3/ZC (0.0230 min−1) > 6.0Bi2S3/ZC (0.0158 min−1) > 2.0Bi2S3/ZC (0.0126 min−1) > 20.0Bi2S3/ZC (0.00828 min−1) > ZC (0.00499 min−1) > Bi2S3 (0.0042 min−1).
The photocatalytic activity of x.0Bi2S3/ZC heterostructures was affected by Bi2S3 content. The photocatalytic activity of x.0Bi2S3/ZC heterostructures first increased and then decreased with increasing Bi2S3 content. The interaction between Bi2S3 and ZC boosts that improve the efficiency of charge separation, because of the contribution of the internal electric field between the junction surface between Bi2S3 and ZC. However, for the 20.0Bi2S3/ZC sample, the catalytic efficiency decreased markedly because the amount of Bi2S3 completely covered the ZC surface, leading to the loss of the number of light photons in the visible light region. This agrees with the UV-Vis DRS spectra and FESEM image.26
The amount of 0.5–2.0 g L−1 12.0Bi2S3/ZC influence on photocatalytic degradation of IC was studied with unchanged the concentration of IC at 40 mg L−1 at pH 6.0 (Fig. 8c). When the loading of 12.0Bi2S3/ZC was changed from 0.5 to 1.0 g L−1, the rate constant k of IC degradation increased significantly from 0.0068 to 0.0230 min−1. However, the degradation efficiency decreases from 77.4% (k = 0.0121 min−1) to 72% (k = 0.0067 min−1) with catalyst loading of 1.5–2.0 g L−1. The above results can be explained as follows: the overall amount of photon absorption and activity centers on the surface of the catalyst increases with increasing the catalyst loading. However, the catalyst loading considerably increases, and the increased turbidity prevented light transmission into the solution, thereby decreasing the activity of the catalyst.43,44
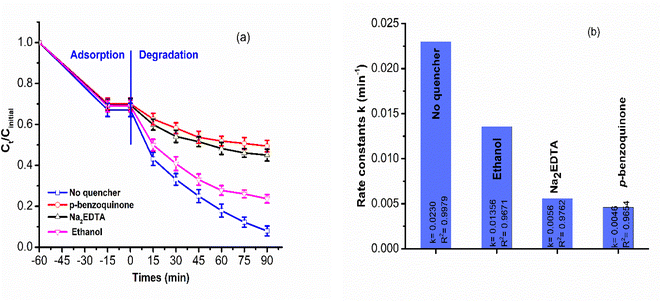
n-Bi2S3 nanoparticles warped on the ZC nanosheet formed a significant amount of interfacial sites, allowing for easy migration of the photoinduced charge carriers through the junction from one side to another. After contact, the Fermi level between p-ZC and n-Bi2S3 was equal, 12.0Bi2S3/ZC heterojunction generated electrons and holes pair at both CB and VB of ZC and Bi2S3 under light illumination. The movement of photogenerated electrons from the CB of ZnCo2O4 to the CB of Bi2S3 is facilitated. This result agrees with XPS, Co2p peak of 12.0Bi2S3/ZC shift to higher binding compared to ZC due to electron cloud density in ZC decrease. In particular, the internal electric field region is formed at the interface of the junction, which is accelerated and separated whole photoexcited electron–hole charge pairs, and that restricted the possibility of a photogenerated charge carrier recombining. This transfer leads to an increase in richer electrons in the CB of Bi2S3. The CB electrons of Bi2S3 are capable of reacting with O2 absorbed on the surface, resulting in the production of the one-electron reduction of dioxygen O2 such as superoxide radical anion (˙O2−). This is because CB Bi2S3 has a greater negative potential than O2/˙O2− (−0.33 V vs. NHE),46,47 as follows;
| CB of Bi2S3 (e−) + O2 → ˙O2− |
| ˙O2− + IC → degradation products |
In addition, the holes of Bi2S3 migrate towards the VB of ZnCo2O4 with the helpful support of a built-in electric field for can directly decompose IC.25,43 The decoloration of IC can also be contributed by the remaining holes (h+) at the VB of Bi2S3.30,33
The reasons why Bi2S3/ZC heterostructure shows excellent photocatalyst performance are as follows: (i) the Bi2S composited with ZnCo2O4 improved photon absorption; (ii) an n–p heterojunction constructs inner built-in potential at the interface between Bi2S3 and ZC. Consequently, an increased quantity of free charges accumulates at the junction, as proven by the M-S tests; (iii) the HRTEM image demonstrates that the Bi2S3 amorphous layer, which is deposited onto the ZnCo2O4 structure, functions as a catalyst that enables charge injection, thus generating more photogenerated pathways. This is a critical factor in enhancing the activity of the photocatalyst. Therefore, the heterostructure formed between Bi2S3 and ZC exhibits potential as a photocatalyst for the decomposition of diverse water pollutants in the presence of natural sunlight.
4. Conclusion
In summary, p–n heterojunction x.0Bi2S3/ZC (x = 1, 2, 6, 12, 20) photocatalysts were successful. The chemical structure, morphology, optical, and photoelectrical properties of x.0Bi2S3/ZC powder were analyzed. By controlling the molar amounts of Na2S and BiNO3, we have found that the Bi2S3 modification can tremendously boost the photocatalytic performance of ZC. When compared to pure ZC, the self-biased photocurrent density of 12.0Bi2S3/ZC is 7.3 times higher. The EIS results offer enhanced comprehension of the function participated by Bi2S3 in the efficient separation and transfer of photogenerated charge carriers due to the contribution of an internal electric field. The results find that the 12.0Bi2S3/ZC showed a higher efficiency of photodegradation than that of ZC and Bi2S3 for the degradation of IC and the degradation rate is 5 times higher than that of ZC. A pseudo-first-order kinetics equation is perfectly suitable for the IC decomposition of an x.0Bi2S3/ZC catalyst. Additionally, the results of different scavengers indicated that the important active species for IC decomposition were O2˙− and h+.Author contributions
Nguyen Thi Mai Tho: methodology, writing – original draft, validation, writing – review & editing, project administration. Nguyen Van Cuong: investigation, resources. Luu Thi Viet Ha: data curation, software. Nguyen Quoc Thang: investigation, resources. Dang Huu Phuc: writing – review & editing.Conflicts of interest
The authors declare that they have no known competing interests that could have appeared to influence the work reported in this paper.Acknowledgements
This work is supported by Industrial University of Ho Chi Minh City (IUH), Ho Chi Minh, Vietnam under grant number 22/1HH01.References
- S. Wang, T. He, J. Yun, Y. Hu, M. Xiao, A. Du and L. Wang, Adv. Funct. Mater., 2018, 28, 1802685 CrossRef.
- S. Lodha, A. Jain and P. B. Punjabi, Arabian J. Chem., 2011, 4, 383–387 CrossRef CAS.
- M. N. Chong, B. Jin, C. W. K. Chow and C. Saint, Water Res., 2010, 44, 2997–3027 CrossRef CAS PubMed.
- S. Ammar, R. Abdelhedi, C. Flox, C. Arias and E. Brillas, Environ. Chem. Lett., 2006, 4, 229–233 CrossRef CAS.
- A. Hernández-Gordillo, V. Rodríguez-González, S. Oros-Ruiz and R. Gómez, Catal. Today, 2016, 266, 27–35 CrossRef.
- M. Cheng, G. Zeng, D. Huang, C. Lai, P. Xu, C. Zhang and Y. Liu, Chem. Eng. J., 2016, 284, 582–598 CrossRef CAS.
- Md. T. Uddin, M. Z. Bin Mukhlish and Md. R. H. Patwary, Desalin. Water Treat., 2021, 212, 311–322 CrossRef CAS.
- K. Maeda, K. Ishimaki, Y. Tokunaga, D. Lu and M. Eguchi, Angew. Chem., Int. Ed., 2016, 55, 8309–8313 CrossRef CAS PubMed.
- M. Shao, Y. Shao, S. Ding, R. Tong, X. Zhong, L. Yao, W. F. Ip, B. Xu, X.-Q. Shi, Y.-Y. Sun, X. Wang and H. Pan, ACS Sustainable Chem. Eng., 2019, 7, 4220–4229 CrossRef CAS.
- S. S. Wong, M. J. Hülsey, H. An and N. Yan, Catal. Sci. Technol., 2022, 12, 5217–5228 RSC.
- S. Song, J. Qu, P. Han, M. J. Hülsey, G. Zhang, Y. Wang, S. Wang, D. Chen, J. Lu and N. Yan, Nat. Commun., 2020, 11, 4899 CrossRef CAS.
- D. R. Kumar, S. Kesavan, M. L. Baynosa, V. Q. Nguyen and J.-J. Shim, J. Colloid Interface Sci., 2018, 530, 361–371 CrossRef CAS PubMed.
- W. Dai, J. Yu, S. Luo, X. Hu, L. Yang, S. Zhang, B. Li, X. Luo and J. Zou, Chem. Eng. J., 2020, 389, 123430 CrossRef CAS.
- K. Ai, Y. Liu, J. Liu, Q. Yuan, Y. He and L. Lu, Adv. Mater., 2011, 23, 4886–4891 CrossRef CAS PubMed.
- M. Y. Malca, H. Bao, T. Bastaille, N. K. Saadé, J. M. Kinsella, T. Friščić and A. Moores, Chem. Mater., 2017, 29, 7766–7773 CrossRef CAS.
- B. Shao, X. Liu, Z. Liu, G. Zeng, Q. Liang, C. Liang, Y. Cheng, W. Zhang, Y. Liu and S. Gong, Chem. Eng. J., 2019, 368, 730–745 CrossRef CAS.
- A. Pandikumar, K. Jothivenkatachalam and S. Moscow, Heterojunction Photocatalytic Materials, Jenny Stanford Publishing, New York, 2022 Search PubMed.
- A. Galán-González, A. K. Sivan, J. Hernández-Ferrer, L. Bowen, L. Di Mario, F. Martelli, A. M. Benito, W. K. Maser, M. U. Chaudhry, A. Gallant, D. A. Zeze and D. Atkinson, ACS Appl. Nano Mater., 2020, 3, 7781–7788 CrossRef PubMed.
- Y. Liu, X. Yan, Z. Kang, Y. Li, Y. Shen, Y. Sun, L. Wang and Y. Zhang, Sci. Rep., 2016, 6, 29907 CrossRef CAS PubMed.
- J. Jian, R. Kumar and J. Sun, ACS Appl. Energy Mater., 2020, 3, 10408–10414 CrossRef CAS.
- K. Kim and J. H. Moon, ACS Appl. Mater. Interfaces, 2018, 10, 34238–34244 CrossRef CAS PubMed.
- W. Zhang, C. Xu, E. Liu, J. Fan and X. Hu, Appl. Surf. Sci., 2020, 515, 146039 CrossRef CAS.
- X. Wang, P. Wu, Z. Zhao, L. Sun, Q. Deng, Z. Yin and X. Chen, J. Mater. Sci.: Mater. Electron., 2020, 31, 4895–4904 CrossRef CAS.
- J. Chen, J. Zhan, E. Lu, Y. Wan, Z. Jin and H. Qi, Mater. Lett., 2018, 220, 66–69 CrossRef CAS.
- J. Chen, J. Zhan, Y. Zhang and Y. Tang, Chin. Chem. Lett., 2019, 30, 735–738 CrossRef CAS.
- H. Benhebal, C. Wolfs, S. Kadi, R. G. Tilkin, B. Allouche, R. Belabid, V. Collard, A. Felten, P. Louette, S. D. Lambert and J. G. Mahy, Inorganics, 2019, 7, 77 CrossRef CAS.
- L. Liu, G. Zhao, C. Li, S. Zhou, Y. Wang and F. Jiao, Desalin. Water Treat., 2021, 217, 411–421 CrossRef CAS.
- B. Tan, Y. Fang, Q. Chen, X. Ao and Y. Cao, Opt. Mater., 2020, 109, 110470 CrossRef CAS.
- S. Bera, S. Ghosh and R. N. Basu, New J. Chem., 2018, 42, 541–554 RSC.
- S. Jiang, K. Zhou, Y. Shi, S. Lo, H. Xu, Y. Hu and Z. Gui, Appl. Surf. Sci., 2014, 290, 313–319 CrossRef CAS.
- I. Ahmad, M. S. Akhtar, E. Ahmed and M. Ahmad, Sep. Purif. Technol., 2020, 245, 116892 CrossRef CAS.
- X. Gao, G. Huang, H. Gao, C. Pan, H. Wang, J. Yan, Y. Liu, H. Qiu, N. Ma and J. Gao, J. Alloys Compd., 2016, 674, 98–108 CrossRef CAS.
- S. Sharma and N. Khare, Colloid Polym. Sci., 2018, 296, 1479–1489 CrossRef CAS.
- H. Han, H. Choi, S. Mhin, Y.-R. Hong, K. M. Kim, J. Kwon, G. Ali, K. Y. Chung, M. Je, H. N. Umh, D.-H. Lim, K. Davey, S.-Z. Qiao, U. Paik and T. Song, Energy Environ. Sci., 2019, 12, 2443–2454 RSC.
- Y. Liu, Y. Zhang and L. Shi, Colloids Surf., A, 2022, 641, 128577 CrossRef CAS.
- X. Li, L. Youji, X. Guo and Z. Jin, Front. Chem. Sci. Eng., 2023, 17, 606–616 CrossRef CAS.
- T. V. M. Sreekanth, R. Ramaraghavulu, S. V. Prabhakar Vattikuti, J. Shim and K. Yoo, Mater. Lett., 2019, 253, 450–453 CrossRef CAS.
- H. Song, J. Sun, T. Shen, L. Deng and X. Wang, Catalysts, 2021, 11, 489 CrossRef CAS.
- Q.-Y. Tang, X.-L. Luo, S.-Y. Yang and Y.-H. Xu, Sep. Purif. Technol., 2020, 248, 117039 CrossRef CAS.
- X. Zhang, L. Shi and Y. Zhang, J. Taiwan Inst. Chem. Eng., 2022, 132, 104111 CrossRef CAS.
- T. Liu, L. Shi, Z. Wang and D. Liu, Colloids Surf., A, 2022, 632, 127811 CrossRef CAS.
- X. Chang, T. Wang, P. Zhang, J. Zhang, A. Li and J. Gong, J. Am. Chem. Soc., 2015, 137, 8356–8359 CrossRef CAS.
- M. I. A. Abdel Maksoud, G. S. El-Sayyad, N. Mamdouh and W. M. A. El Rouby, J. Inorg. Organomet. Polym. Mater., 2022, 32, 3621–3639 CrossRef CAS.
- D. Zhang, S. Lv and Z. Luo, RSC Adv., 2020, 10, 1275–1280 RSC.
- R. Abdel-Aziz, M. A. Ahmed and M. F. Abdel-Messih, J. Photochem. Photobiol., A, 2020, 389, 112245 CrossRef CAS.
- F. Xu, Y. Yuan, H. Han, D. Wu, Z. Gao and K. Jiang, CrystEngComm, 2012, 14, 3615 RSC.
- V. S. Kirankumar and S. Sumathi, Mater. Res. Bull., 2017, 93, 74–82 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra02803h |
| This journal is © The Royal Society of Chemistry 2023 |