Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
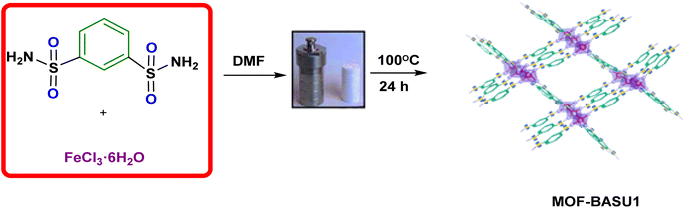
A sustainable protocol for selective alcohols oxidation using a novel iron-based metal organic framework (MOF-BASU1)†
Mahtab Yaghubzadeh,
Sedigheh Alavinia and
Ramin Ghorbani-Vaghei *
*
Department of Organic Chemistry, Faculty of Chemistry, Bu-Ali Sina University, Hamedan, 6517838683, Iran. E-mail: rgvaghei@yahoo.com; ghorbani@basu.ac.ir; Tel: +98-8138380647
First published on 17th August 2023
Abstract
The selective oxidation of active and inactive alcohol substrates is a highly versatile conversion that poses a challenge in controlling the functionality and adjustments on MOFs. On the other hand, it offers an attractive opportunity to expand their applications in designing the next generation of catalysts with improved performance. Herein, a novel iron-based MOF containing sulfonamide (MOF-BASU1) has been fabricated by the reaction of 1,3-benzene disulfonylchloride linker and FeCl3·6H2O. Based on the results, the active surface area of the synthesized MOF is large, which highlights its unique catalytic activity. Optimum conditions were reached after 0.5–2 h, with 15 mg loading of the synthesized MOF under optimal conditions. Furthermore, the turnover frequency was 18–77.6 h−1, which is comparable to values previously reported for this process. Overall, the high catalytic activity observed for MOF-BASU1 might be because of the obtained high surface area and the Lewis acidic Fe nodes. Furthermore, the MOF-BASU1 revealed a remarkable chemoselectivity for aldehydes in the presence of aliphatic alcohols. Overall, the high product yields, facile recovery of nanocatalysts, short reaction times, and broad substrate range make this process environmentally friendly, practical, and economically justified.
1. Introduction
Alcohol oxidation reactions play a vital role in industrial applications, the synthesis of synthetic intermediates, and natural products. In the pharmaceutical industry, the oxidation reactions of alcohols are used extensively.1 Therefore, the development of alcohol oxidations with an environmentally friendly and cost-effective strategy is one of the important and necessary concepts for the pharmaceutical and chemical industry. Alcohol oxidation is widely carried out traditionally with small organic-based reagents, such as Dess–Martin periodinane and Swern oxidation or metal-based systems, such as pyridinium chlorochromate, pyridinium dichromate, and ruthenium tetroxide.2–5 However, most of these have various limitations including moisture-sensitive, expensive, and not reusable. Therefore, new oxidation approaches are expected to be carried out selectively with green, cheap, and non-toxic oxidants, such as dioxygen or air in the presence of reusable catalysts.6Recently, various homogeneous catalysts have played an important role owing to their efficiency and all-purpose impact on the many catalytic reactions.7–9 However, despite the high efficiency of homogeneous catalysts, all these reactions suffer from poor catalyst recovery. The benefits of catalyst recovery include the economic and environmentally friendly concept of special transition metals; however, the key challenges are yet to be resolved.10–12 One of the solutions to overcome this limitation in oxidation reactions is the immobilization of homogeneous catalysts on supports that are not chemically active. In this regard, one of the most popular materials for a wide range of applications in oxidation reactions are metal–organic frameworks (MOFs) with tunable chemical and physical properties.13–19
Metal organic frameworks (MOFs) are a new class of organic–inorganic hybrids that, owing to their nature, have many applications in fields, such as gas storage,20 catalytic processes,21,22 encapsulate material, super capacitors, and the absorption of heavy metals.23 MOFs not only have a higher level of activation and stability than other classes of porous materials, but can also change the morphology and size of cavities uncomplicatedly, and this has become an advantage in terms of separation and greater selectivity in their applications.24–27
Designing and synthesizing new molecular scaffolds with unique structural and biological properties that increase their capability and selectivity is an interesting challenge. Nowadays, sulfonamides, with unique features, such as strong chemical/thermal stability, are considered as new ligands by the chemical community.28–30 The use of sulfonamide ligands in the synthesis of metal organic frameworks can create a revolution in the MOFs field and catalysis.
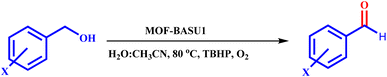
Regarding the points mentioned, this study presents the development and characterization of a new Fe-based MOF (MOF-BASU1) from the reaction of 1,3-benzenedisulfonamide (BDS) and FeCl3·6H2O (Scheme 1). The catalytic activity of the MOF-BASU1 was tested in aerobic alcohol oxidation without over oxidation. In the light of the results obtained, the high efficiency, high surface area, and easy recovery of the catalyst are strong points to justify its use. To the best of our knowledge, there are no reports on the use of a MOF-BASU1 catalyst in the synthesis of aldehyde/ketone compounds. The catalyst can usefully act as both acid and redox active sites platform. This study consistently has advantages, such as the availability of MOF, inexpensive catalyst, mild reaction conditions, reasonable yields, and simple experimental procedures. Such a potential catalytic utility of MOF-BASU1 make it quite attractive for sustainable industrial chemistry.
2. Experimental section
2.1. Instrumentation
All the chemicals, reagents, and equipment used in this study are listed in Table 1.| Materials and equipment | Purity and brand |
|---|---|
| Iron(III) chloride hexahydrate (FeCl3·6H2O) | Sigma–Aldrich (≥98%) |
| Potassium carbonate (K2CO3) | Merck (98%) |
| N,N-Dimethylformamide (DMF) | Merck (99.8%) |
| n-Hexane | Sigma–Aldrich (95%) |
| Ethanol | Sigma–Aldrich (97%) |
| Acetonitrile | Merck (98%) |
| FT-IR analysis | Shimadzu IR-470 spectrometer |
| EDX analysis | Numerix DXP-X10P |
| SEM analysis | Sigma-Zeiss microscope |
| XRD analysis | JEOL JDX-8030 (30 kV, 20 mA) |
| NMR analysis | Varian Unity Inova 500 MHz |
| Ultrasound cleaning bath | KQ-250 DE (40 kHz, 250 W) |
| Melting point measurement | Electrothermal 9100, made in UK |
2.2. Synthesis of the MOF-BASU1 catalyst
MOF-BASU1 was produced by the solvothermal method. Briefly, 1 mmol of FeCl3·6H2O, 2 mmol of benzene disulfonamide (BDS), and 50 mL of DMF were poured into a beaker and dispersed in an ultrasonic bath for 10 min. Then, the mixture was stirred for 10 min at 100 °C. The resulting mixture was transferred to an autoclave and kept in an oven at 100 °C for 24 hours. Scheme 2 The obtained solution was centrifuged and the solid residues were washed several times with DMF and dried in an oven at 60 °C for 12 hours (Scheme 1).2.3. General method for the synthesis of benzaldehyde derivatives
Primary benzyl alcohol analogs (1 mmol), tertiary butylhydrogen peroxide (3 mmol), and MOF-BASU1 (15 mg) were stirred at 80 °C in H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3CN (2 mL, 1
CH3CN (2 mL, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, volume) for the suitable time, as illustrated in Table 3. After the reaction, the catalyst was filtered off. The carbonyl products and unreacted reactants in the reaction mixture were isolated using a facile extraction with water/ethyl acetate. The organic phase was then concentrated by full evaporation of the solvent. The residue was purified using column chromatography (n-hexane/EtOAc, 4
1, volume) for the suitable time, as illustrated in Table 3. After the reaction, the catalyst was filtered off. The carbonyl products and unreacted reactants in the reaction mixture were isolated using a facile extraction with water/ethyl acetate. The organic phase was then concentrated by full evaporation of the solvent. The residue was purified using column chromatography (n-hexane/EtOAc, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, volume) yielded the desired products.
1, volume) yielded the desired products.
3. Results and discussion
3.1. Catalyst characterization data analysis
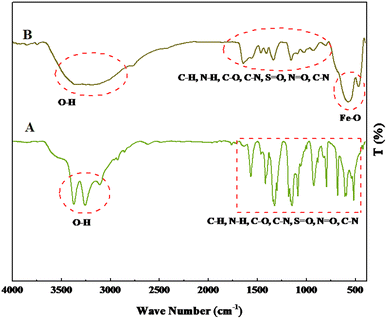
The FT-IR spectra of 1,3-benzenedisulfonamide and MOF-BASU1 catalyst are shown in Fig. 1. In 1,3-benzenedisulfonamide, the absorption bands at 1145 and 1332 cm−1 are attributed to S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching. The bands at 3344 cm−1 and 3248 cm−1 correspond to the NH2 stretching vibrations. After the synthesis of MOF-BASU1, a broad absorption band was observed at 3000–3500 cm−1 related to the interaction of amine groups and iron in the studied structure. Moreover, the absorption band at 526 cm−1 is associated with the Fe–O stretching in MOF-BASU1 (Fig. 1B).
O stretching. The bands at 3344 cm−1 and 3248 cm−1 correspond to the NH2 stretching vibrations. After the synthesis of MOF-BASU1, a broad absorption band was observed at 3000–3500 cm−1 related to the interaction of amine groups and iron in the studied structure. Moreover, the absorption band at 526 cm−1 is associated with the Fe–O stretching in MOF-BASU1 (Fig. 1B).
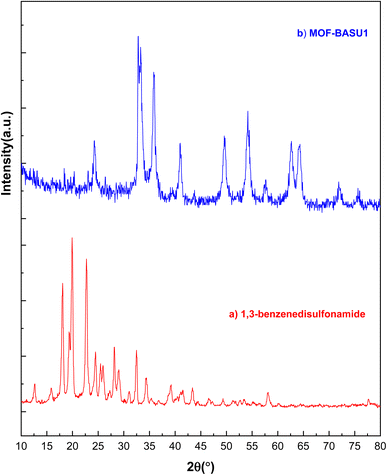
XRD analysis was used to check the crystal structures of 1,3-benzenedisulfonamide and MOF-BASU1 samples (Fig. 2). The XRD spectrum of 1,3-benzenedisulfonamide shows diffraction peaks at 2θ = 18°, 23°, 28°, 32°, and 40° (Fig. 2A). Also, the same peaks in the MOF-BASU1 sample were shifted compared to the pure 1,3-benzenedisulfonamide and appeared with less intensity (Fig. 2B). Moreover, the introduction of the iron into the MOF-BASU1 was well defined by the peaks at 52.46°, 58.40°, and 65.61° suggesting the clear growth in the crystal structure. The average distance and crystal size were then calculated by Scherrer and Bragg equations to determine 45 nm.
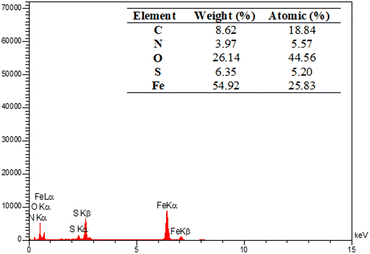
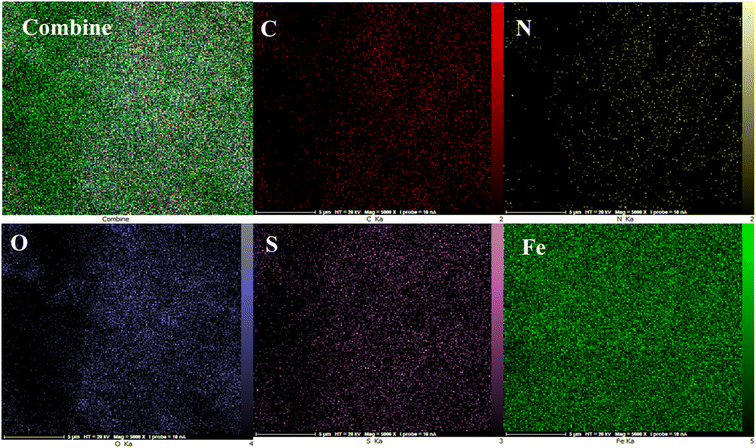
The energy-dispersive X-ray spectroscopy (EDX) elemental mapping of C, N, O, S, and Fe in MOF-BASU1 is depicted in Fig. 3. The analysis exhibited that all the required elements, including C (31.66%), O (22.10%), N (15.69%), S (8.55%), and Fe (3.46%) are present in the structure of MOF-BASU1. The elemental mapping studies of MOF-BASU1 show a uniform distribution of carbon, oxygen, iron, nitrogen, and sulfur components in the fabricated structure (Fig. 4).
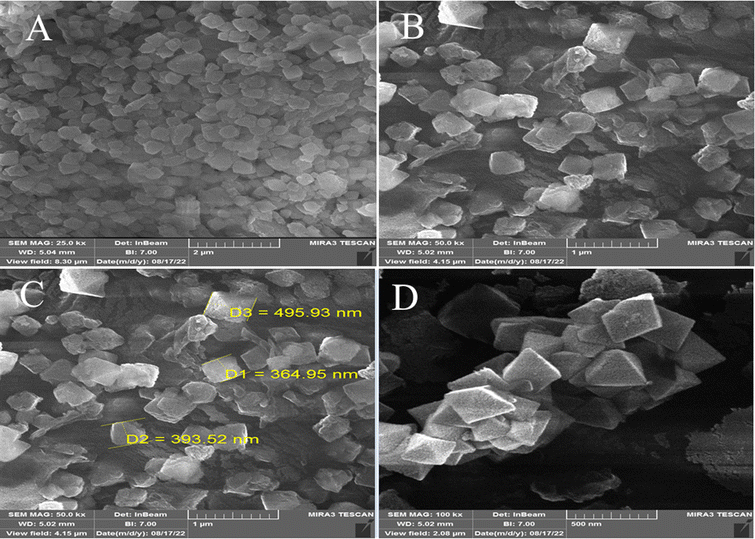
Fig. 5 shows the FE-SEM characterization of the size, shape, and morphology of the synthesized MOF-BASU1 nanocomposite. In the as-synthesized MOF-BASU1, we observed well distributed nearly-spherical uniform nanostructures (Fig. 5–D).
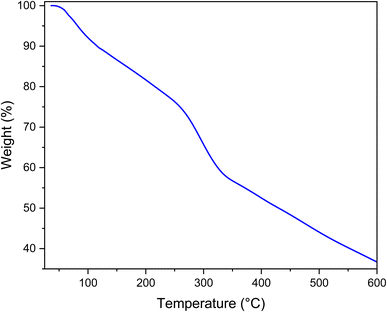
Fig. 6 shows the TGA carried out to study the stability of the prepared catalyst. The first step initiates from 25 to 200 °C. This step is attributed to solvent removal. According to Fig. 6, MOF-BASU1 decomposes at 309.14 °C, and generally loses 37.59% of its weight.
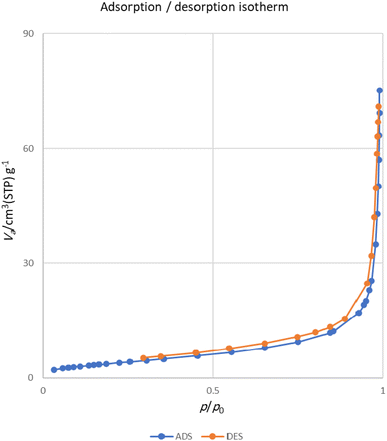
The important parameters associated with the porosity of MOF-BASU1 sample, including the surface area, average diameter of the pores, and total pore volume of the pores, are reported in Table 2. Fig. 7 indicates the N2 adsorption isotherms of the sample. Nitrogen adsorption and desorption curves of the sample show type I isotherm with H2 hysteresis curves, which confirms their microporous structure.
| Sample | Surface area (m2 g−1) | Mean pore diameter (nm) | Total pore volume (cm3 g−1) |
|---|---|---|---|
| MOF-BASU1 | 14.56 | 30.09 | 0.11 |
3.2. Optimization conditions
To optimize the reaction parameters for the best catalytic property, the oxidation reaction of p-chlorobenzyl alcohol was used as a model reaction, and several reaction conditions, such as the presence and absence of different catalysts, solvents, and catalyst concentrations, were investigated (Table 3). First, the experimental reaction was carried out without a catalyst for 12 hours under an acetonitrile:water solvent system (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, volume), and no product was generated at room temperature (entry 1). Next, the temperature of the reaction is a further area for optimization, and we increased the temperature from room temperature to reflux, but no reaction progressed (entry 2). The same reaction was subsequently tested with TBHP oxidant, in an attempt to improve the catalyst activity. No reaction was progressed at reflux condition for a prolonged period of time (entry 3). This proved that the catalyst was necessary for carrying out these reactions. The same reaction was then conducted using a variety of MOFs, including IRMOF-3, basolite (Fe), MIL (101)Fe, and UiO-66-NH2 in TBHP. However, even after a 6 hours reaction, these catalysts only produced modest yields (entries 4–7). Encouraged by the above results, and in an endeavor to enhance the efficiency of the pilot reaction, the reaction was repeated using MOF-BASU1. Remarkably, the reaction took 1 hour to reach a high product yield of 97% (entry 8). Next, the solvent plays a vital influence in chemical conversions in terms of reaction time and product yield. The impact of several solvents and solvent-free conditions on the pilot reaction was investigated (entries 9–14). The oxidation reaction did not proceed in toluene because of the low dispersion of the synthesized catalyst in toluene (entry 9). While utilizing different solvents, such ethanol, acetonitrile, and DMF, under identical reaction conditions led to higher yields. Surprisingly, the same reaction, when conducted in a mixture of H2O
1, volume), and no product was generated at room temperature (entry 1). Next, the temperature of the reaction is a further area for optimization, and we increased the temperature from room temperature to reflux, but no reaction progressed (entry 2). The same reaction was subsequently tested with TBHP oxidant, in an attempt to improve the catalyst activity. No reaction was progressed at reflux condition for a prolonged period of time (entry 3). This proved that the catalyst was necessary for carrying out these reactions. The same reaction was then conducted using a variety of MOFs, including IRMOF-3, basolite (Fe), MIL (101)Fe, and UiO-66-NH2 in TBHP. However, even after a 6 hours reaction, these catalysts only produced modest yields (entries 4–7). Encouraged by the above results, and in an endeavor to enhance the efficiency of the pilot reaction, the reaction was repeated using MOF-BASU1. Remarkably, the reaction took 1 hour to reach a high product yield of 97% (entry 8). Next, the solvent plays a vital influence in chemical conversions in terms of reaction time and product yield. The impact of several solvents and solvent-free conditions on the pilot reaction was investigated (entries 9–14). The oxidation reaction did not proceed in toluene because of the low dispersion of the synthesized catalyst in toluene (entry 9). While utilizing different solvents, such ethanol, acetonitrile, and DMF, under identical reaction conditions led to higher yields. Surprisingly, the same reaction, when conducted in a mixture of H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3CN (1
CH3CN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, volume), produced a notable result due to the catalyst surface's enhanced activity. The typical reaction was explored for the different catalytic loadings of MOF-BASU1 under moderate reaction conditions in the presence of H2O
1, volume), produced a notable result due to the catalyst surface's enhanced activity. The typical reaction was explored for the different catalytic loadings of MOF-BASU1 under moderate reaction conditions in the presence of H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3CN solvent (1
CH3CN solvent (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, volume) in order to improve reaction conditions for the influence of catalyst loading (entries 16–17). After investigating the reaction with 7 mg to 20 mg of catalyst, the product yields increased and reaction time decreased. Next, utilizing more than 15 mg of catalyst did not result in any appreciable improvements in yield or reaction time. Consequently, the ideal catalyst material, MOF-BASU1 (15 mg) (3 mg of iron from inductively coupled plasma (ICP) analysis. 0.075 mmol of Fe) was used to provide the desired result in 60 minutes reaction time. Finally, the same reaction was then conducted at 60 °C in H2O
1, volume) in order to improve reaction conditions for the influence of catalyst loading (entries 16–17). After investigating the reaction with 7 mg to 20 mg of catalyst, the product yields increased and reaction time decreased. Next, utilizing more than 15 mg of catalyst did not result in any appreciable improvements in yield or reaction time. Consequently, the ideal catalyst material, MOF-BASU1 (15 mg) (3 mg of iron from inductively coupled plasma (ICP) analysis. 0.075 mmol of Fe) was used to provide the desired result in 60 minutes reaction time. Finally, the same reaction was then conducted at 60 °C in H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3CN (1
CH3CN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, volume). However, even after a 6 hours reaction time, the catalyst only produced modest yields (entry 18). It is important to note that superior results were achieved using pure oxygen (entry 19) (10 hours, 80%). It should also be noted that using less than 3 equivalents does not provide significant improvements in the efficiency or reaction time (entry 20).
1, volume). However, even after a 6 hours reaction time, the catalyst only produced modest yields (entry 18). It is important to note that superior results were achieved using pure oxygen (entry 19) (10 hours, 80%). It should also be noted that using less than 3 equivalents does not provide significant improvements in the efficiency or reaction time (entry 20).
| Entry | Catalyst (mg) | Solvent | Temperature (°C) | Time (h) | TBHP (mmol) | Yieldb (%) |
|---|---|---|---|---|---|---|
| a Reaction condition: p-chlorobenzyl alcohol (1.0 mmol).b Isolated yields.c The reaction was investigated in the absence of O2. | ||||||
| 1 | — | H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3CN (1 CH3CN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
r.t. | 12 | — | N.R. |
| 2 | — | H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3CN (1 CH3CN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
80 | 12 | — | N.R. |
| 3 | — | H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3CN (1 CH3CN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
80 | 12 | 3 | Trace |
| 4 | IRMOF-3 (15) | H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3CN (1 CH3CN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
80 | 6 | 3 | 30 |
| 5 | UiO-66-NH2 (15) | H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3CN (1 CH3CN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
80 | 6 | 3 | 35 |
| 6 | Basolite (Fe) (15) | H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3CN (1 CH3CN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
80 | 6 | 3 | 55 |
| 7 | MIL (101)Fe (15) | H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3CN (1 CH3CN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
80 | 6 | 3 | 62 |
| 8 | MOF-BASU1 (15) | H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3CN (1 CH3CN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
80 | 1 | 3 | 98 |
| 9 | MOF-BASU1 (15) | Toluene | 80 | 1 | 3 | N.R. |
| 10 | MOF-BASU1 (15) | CH3CN | 80 | 1 | 3 | 80 |
| 11 | MOF-BASU1 (15) | H2O | 80 | 1 | 3 | 40 |
| 12 | MOF-BASU1 (15) | EtOH | 80 | 1 | 3 | 65 |
| 13 | MOF-BASU1 (15) | H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH (1 EtOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
80 | 1 | 3 | 75 |
| 14 | MOF-BASU1 (15) | Solvent-free | 80 | 1 | 3 | 52 |
| 15 | MOF-BASU1 (15) | DMF | 80 | 1 | 3 | 39 |
| 16 | MOF-BASU1 (7.5) | H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3CN (1 CH3CN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
80 | 1 | 3 | 82 |
| 17 | MOF-BASU1 (20) | H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3CN (1 CH3CN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
80 | 1 | 3 | 97 |
| 18 | MOF-BASU1 (15) | H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3CN (1 CH3CN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
60 | 1 | 3 | 79 |
| 19 | MOF-BASU1 (15)c | H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3CN (1 CH3CN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
80 | 10 | 3 | 80 |
| 20 | MOF-BASU1 (15) | H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3CN (1 CH3CN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
80 | 1 | 2 | 80 |
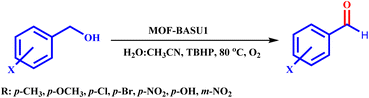
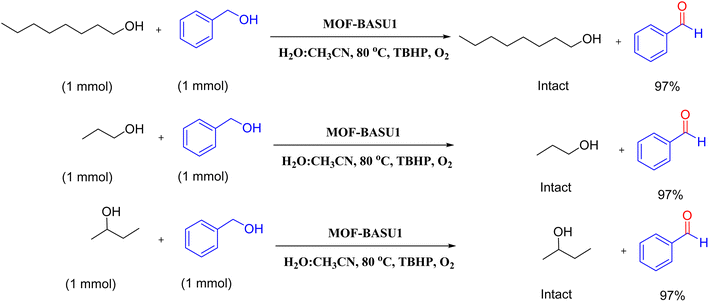
Optimized conditions in hand, the generality and limitations of the catalytic activity of MOF-BASU1 were confirmed by developing the oxidation of benzyl alcohol derivatives. The nanocatalyst carried out the reactions chemoselectively with high yields. A range of functional groups on the phenyl ring of the benzyl alcohol, such as bromo, chloro, nitro, hydroxyl, methyl, and methoxy, were compatible under this procedure, and the products were isolated in good to high yields. Furthermore, when the reaction was performed with substrates bearing meta-substituted aryl rings, the reaction occurred with high selectivity (Table 4). The chemoselectivity of benzyl alcohol was investigated in the presence of aliphatic alcohols (Scheme 3). The results strongly confirmed the chemoselectivity of the present methodology for aldehydes.
| Entry | Substrate | Product | Time (h) | Yieldb (%) | TOFc (h−1) | TONd | Melting point–boiling point |
|---|---|---|---|---|---|---|---|
| a Reaction condition: benzyl alcohol derivatives (1.0 mmol), TBHP (3 mmol).b Isolated yields.c TOF (turnover frequency) = TON per time (h).d TON = yield (%)/catalyst (mol%). | |||||||
| 1 | 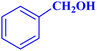 |
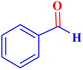 |
0.5 | 97 | 77.6 | 38.8 | 172 |
| 2 | 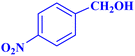 |
 |
1.5 | 97 | 25.86 | 38.8 | 103–104 |
| 3 |  |
 |
1.5 | 98 | 26.13 | 39.2 | 58–60 |
| 4 |  |
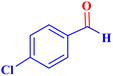 |
1 | 98 | 39.2 | 39.2 | 48–50 |
| 5 | 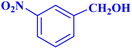 |
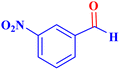 |
2 | 90 | 18 | 36 | 59 |
| 6 | 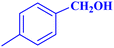 |
 |
0.5 | 85 | 68 | 34 | 200 |
| 7 |  |
 |
1 | 96 | 38.4 | 38.4 | 244–246 |
| 8 |  |
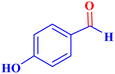 |
1.5 | 90 | 24 | 36 | 113–115 |
| 9 | 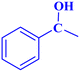 |
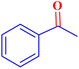 |
1 | 88 | 35.2 | 35.2 | 200 |
| 10 | 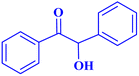 |
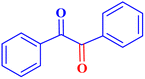 |
1 | 96 | 38.4 | 38.4 | 90–92 |
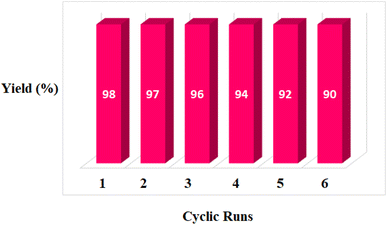
In addition to catalyst activity, its reusability is a significant point in practical application. To investigate its recovery performance, MOF-BASU1 was examined by successive cycles of catalytic oxidation reactions. In a general experiment, the catalyst was isolated from an as-completed reaction solution and used directly for the next reaction cycle. Results of these studies exhibited that the catalyst could successfully be recycled and reused for 6 sequential runs without diminution in catalytic activity (98, 97, 96, 94, 92, and 90).
According to a literature survey, the catalyst sites are the weak Brønsted acid sites, which may be attributed to uncoordinated sulfonamide groups, the redox-active,31 and Lewis acid iron sites.32 So, this catalyst can act usefully both as acid and redox active sites platform (Fig. 8).
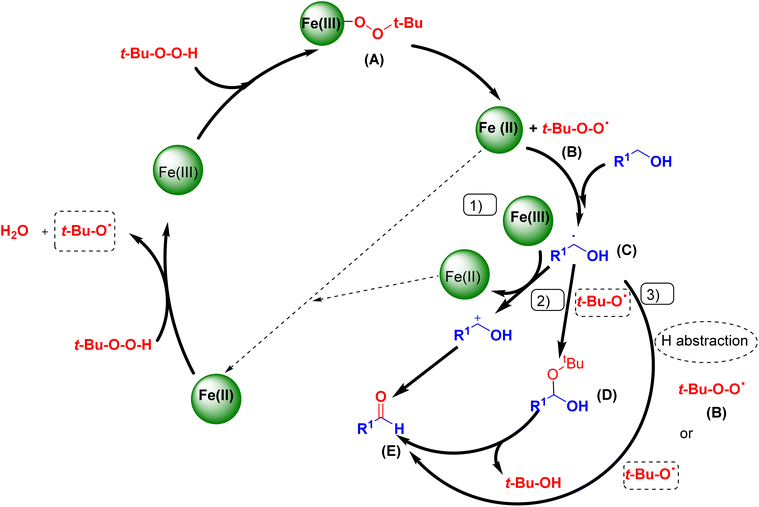
Based on the literature survey and the above argument, we introduce a mechanism for the oxidation process as shown in Scheme 4. At first, the coordination of t-BuOOH to the Fe(III) sites affords t-Bu–OO–Fe(III) species (A) which subsequently produces the active species (t-Bu–O–O˙(II)). Then, the abstraction of a hydrogen-atom from the benzyl alcohol gives a radical intermediate (C). As the proposed mechanism shows, the three pathways: (1), (2), and (3) give the aldehyde product (E). The pathway (1) passes through the carbocation formation with the concomitant reduction of Fe(III) to Fe(II). The pathway (2) proceeds via a gem-diol-like structure formation and dehydration. The third pathway (3) involves a hydrogen-atom abstraction from (C) by the t-BuOO˙ and t-BuO˙ radicals.33 Moreover, H2 can be produced from methanol reforming in four different systems: MD, POM, SRM, and OSRM. Methoxy, formaldehyde, dioxymethylene, formate, and methyl formate are the commonly reported surface species detected on Cu-based catalysts.34
The advantages of the nanocomposites produced are summarized in Table 5 by comparing the results of the present study with the different catalytic systems used to produce aldehyde/ketone derivatives. According to these data, the catalytic system proposed herein, is more efficient in terms of reaction yield and time compared to other systems for this reaction. High yields and easy work-up are some advantages of this procedure (entry 7).
| Entry | Reaction condition | T (°C) | Time (h) | Yield (%) | Ref. |
|---|---|---|---|---|---|
| 1 | CuPc nanoparticle 5 mol%, n-Bu4NHSO5 (0.7 g), H2O | 85 | 168 | 79 | 35 |
| 2 | Fe(II) phthalocyanines/TBHP (500/1) | 70 | 3 | 94 | 36 |
| 3 | Al-MCM-41(10)-CuPc (0.055 g) | 100 | 4 | 47.5 | 37 |
| 4 | CoPc@cell (0.05 g), KOH, o-xylene, O2 | r.t. | 8.5 | 83 | 38 |
| 5 | MIL-101-NH2/CoTSPc (0.42 mol%), p-xylene, KOH, air (300 mL min−1) | 100 | 8 | 95 | 39 |
| 6 | TACoPc/Si–Cl (20 mg), H2O2, CH3CN, visible light | r.t. | 6 | 60 | 40 |
| 7 | MOF-BASU1 (20 mg), TBHP (3 mmol), CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O H2O |
80 | 1 | 98 | This work |
4. Conclusion
In this study, a novel iron-based MOF containing a sulfonamide (MOF-BASU1) was developed and synthesized for the oxidation of benzyl alcohol derivatives. Furthermore, the MOF-BASU1 nanocomposite was characterized as a multifunctional MOF with robustness and stability under reaction conditions. Hence, it can be reused several times without losing its catalytic activity and structural integrity. Other advantages of the proposed mesoporous catalyst include high efficiency, easy separation of the catalyst from the mixture, and clean reaction profile. This research depicts a bright future in the use of porous MOFs and their functionalized analogs as multifunctional catalysts.Author contributions
Mahtab Yaghubzadeh: doing laboratory work and preparing data. Sedigheh Alavinia: involved in conceptualization, methodology, resources, writing—original draft, reviewing and editing, formal analysis. Ramin Ghorbani-Vaghei: took part in conceptualization, investigation, supervision, writing—review and editing.Conflicts of interest
There are no conflicts to declare.References
- A. Dhakshinamoorthy, A. M. Asiri and H. Garcia, Chem. Commun., 2017, 53, 10851–10869 RSC.
- G. Tojo and M. Fernández, Selective Oxidations of Secondary Alcohols in Presence of Primary Alcohols, in Oxidation of Alcohols to Aldehydes and Ketones: A Guide to Current Common Practice, Springer, 2006, pp. 339–349 Search PubMed.
- S. Ataie, M. Lohoar, L. P. Mangin and R. T. Baker, Chem. Commun., 2023, 59, 4044–4046 RSC.
- M. Wang, G. Liang, Y. Wang, T. Fan, B. Yuan, M. Liu, Y. Yin and L. Li, Eurasian J. Chem., 2021, 27, 9674–9685 CAS.
- L. Li, R. Matsuda, I. Tanaka, H. Sato, P. Kanoo, H. J. Jeon, M. L. Foo, A. Wakamiya, Y. Murata and S. Kitagawa, J. Am. Chem. Soc., 2014, 136, 7543–7546 CrossRef CAS PubMed.
- H. Keypour, J. Kouhdareh, S. Alavinia, R. Karimi-Nami and İ. Karakaya, ACS Omega, 2023, 24, 22138–22149 CrossRef PubMed.
- M. Fereydooni, S. Alavinia and R. Ghorbani-Vaghei, New J. Chem., 2023, 47, 8410–8425 RSC.
- H. Keypour, J. Kouhdareh, R. Karimi-Nami, S. Alavinia, I. Karakaya, S. Babaei and A. Maryamabadi, New J. Chem., 2023, 47, 6730–6738 RSC.
- S. Alavinia, R. Ghorbani-Vaghei, R. Ghiai and A. Gharehkhani, RSC Adv., 2023, 13, 10667–10680A RSC.
- D. J. Constable, et al., Green Chem., 2007, 9, 411–420 RSC.
- R. A. Sheldon and J. M. Woodley, Chem. Rev., 2018, 118, 801–838 CrossRef CAS PubMed.
- R. A. Sheldon, Chem. Soc. Rev., 2012, 41, 1437–1451 RSC.
- M. B. Chambers, X. Wang, L. Ellezam, O. Ersen, M. Fontecave, C. Sanchez, L. Rozes and C. Mellot-Draznieks, J. Am. Chem. Soc., 2017, 139, 8222–8228 CrossRef CAS PubMed.
- S. Daliran, A. Santiago-Portillo, S. Navalón, A. R. Oveisi, M. Álvaro, R. Ghorbani-Vaghei, D. Azarifar and H. García, J. Colloid Interface Sci., 2018, 532, 700–710 CrossRef CAS PubMed.
- D. Azarifar, R. Ghorbani-Vaghei, S. Daliran and A. R. Oveisi, ChemCatChem, 2017, 9, 1992–2000 CrossRef CAS.
- C.-W. Ding, W. Luo, J.-Y. Zhou, X.-J. Ma, G.-H. Chen, X.-P. Zhou and D. Li, ACS Appl. Mater. Interfaces, 2019, 11, 45621–45628 CrossRef CAS PubMed.
- H. Alamgholiloo, S. Rostamnia, K. Zhang, T.-H. Lee, Y.-S. Lee, R. S. Varma, H. W. Jang and M. Shokouhimehr, ACS Omega, 2020, 5(10), 5182 CrossRef CAS PubMed.
- H. Ghasempour, F. ZareKarizi, A. Morsali and X.-W. Yan, Appl. Mater. Today, 2021, 24, 101157 CrossRef.
- L. Zhang, J. Qiu, G. Xia, D. Dai, X. Zhong and J. Yao, J. Mater. Sci. Technol., 2023, 138, 214 CrossRef.
- M. Duan, L. Jiang, G. Zeng, D. Wang, W. Tang, J. Liang, H. Wang, D. He, Z. Liu and L. Tang, Appl. Mater. Today, 2020, 19, 100564 CrossRef.
- D. N. Dybtsev and K. P. Bryliakov, Coord. Chem. Rev., 2021, 437, 213845 CrossRef CAS.
- H. Keypour, J. Kouhdareh, S. Alavinia, K. Rabiei, M. Mohammadi, A. Maryamabadi and S. Babaei, J. Organomet. Chem., 2023, 989, 122646 CrossRef CAS.
- P. Rocío-Bautista, I. Taima-Mancera, J. Pasán and V. Pino, Separations, 2019, 6, 33 CrossRef.
- A. Nikseresht, R. Bagherinia, M. Mohammadi and R. Mehravar, RSC Adv., 2023, 13, 674–687 RSC.
- A. Ghorbani-Choghamarani, Z. Taherinia and M. Mohammadi, Environ. Technol. Innov., 2021, 24, 102050 CrossRef CAS.
- S. M. Ramish, A. Ghorbani-Choghamarani and M. Mohammadi, Sci. Rep., 2022, 12, 1479 CrossRef CAS PubMed.
- J. Babamoradi, S. Alavinia, R. Ghorbani-Vaghei and R. Azadbakht, New J. Chem., 2022, 46, 23394–23403 RSC.
- R. Ghiai, S. Alavinia, R. Ghorbani-Vaghei and A. Gharakhani, RSC Adv., 2022, 12, 34425–34437 RSC.
- S. Alavinia and R. Ghorbani-Vaghei, J. Phys. Chem. Solids, 2020, 146, 109573 CrossRef CAS.
- S. Alavinia, R. Ghorbani-Vaghei, J. Rakhtshah, J. Yousefi Seyf and I. Ali Arabian, Appl. Organomet. Chem., 2020, 34, e5449 CrossRef CAS.
- A. Dhakshinamoorthy, M. Alvaro and H. Garcia, J. Catal., 2012, 289, 259–265 CrossRef CAS.
- A. Dhakshinamoorthy, M. Alvaro and H. Garcia, Chem.–Eur. J., 2010, 16, 8530–8536 CrossRef CAS PubMed.
- A. R. Oveisi, A. Khorramabadi-zad and S. Daliran, RSC Adv., 2016, 6, 1136–1142 RSC.
- S. T. Yong, C. W. Ooi, S. P. Chai and X. S. Wu, Int. J. Hydrogen Energy, 2013, 38, 9541–9552 CrossRef CAS.
- S. Kheirjou, R. Kheirjou, A. H. Rezayan, M. Shakourian-Fard and M. Mahmoudi Hashemi, C. R. Chim., 2016, 19, 314–319 CrossRef.
- V. Çakır, E. T. Saka, Z. Bıyıklıoğlu and H. Kantekin, Synth. Met., 2014, 197, 233–239 CrossRef.
- A. Hamza and D. Srinivas, Catal. Lett., 2009, 128, 434–442 CrossRef CAS.
- A. Shaabani, S. Keshipour, M. Hamidzad and S. Shaabani, J. Mol. Catal. A: Chem., 2014, 395, 494–499 CrossRef CAS.
- A. Shaabani, M. M. Amini, M. Shadi, F. Bahri, M. K. Alavijeh and H. Farhid, J. Taiwan Inst. Chem. Eng., 2021, 126, 211–222 CrossRef CAS.
- S. Farahmand, R. Ayazi-Nasrabadi and M. A. Zolfigol, Tetrahedron Lett., 2023, 118, 154403 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra03058j |
| This journal is © The Royal Society of Chemistry 2023 |