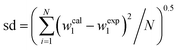Open Access Article
Open Access ArticleExtraction of some essential amino acids using aqueous two-phase systems made by sugar-based deep eutectic solvents
İnci SÖĞÜTLÜa,
Shakir Mahmood Saeedb,
Mohaned Adilc,
Anupam Yadav d,
Evan Abdulkareem Mahmood*e and
Mohamed J. Saadh
d,
Evan Abdulkareem Mahmood*e and
Mohamed J. Saadh fg
fg
aRepublic of Turkey Ministry of Agriculture and Forestry, Turkey
bDepartment of Pharmacy, Al-Noor University College, Nineveh, Iraq
cPharmacy College, Al-Farahidi University, Iraq
dDepartment of CEA, GLA University, Mathura-281406, India
eMedical Laboratory Sciences Department, College of Health Sciences, University of Human Development, Sulaymaniyah, Iraq. E-mail: shakir.mahmood@alnoor.edu.iq; evan.mahmood@uhd.edu.iq
fFaculty of Pharmacy, Middle East University, Amman, 11831, Jordan
gApplied Science Research Center, Applied Science Private University, Amman, Jordan
First published on 29th June 2023
Abstract
Aqueous two-phase systems (ATPSs) have long been recognized as versatile and efficient tools for the extraction of biomolecules, including amino acids. Recent advancements in the field have introduced a novel approach by utilizing deep eutectic solvents (DES) to form ATPs. This study aimed to determine the phase diagrams for an ATPS made of polyethylene glycol dimethyl ether 250 and two types of NADESs, namely choline chloride as a hydrogen bond acceptor (HBA), and either sucrose or fructose as a hydrogen bond donor (HBD) with a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. The measured tie-line results revealed that the hydrogen bonds of NADES may not be entirely disrupted in aqueous solutions, and thus, these ATPSs act as ternary-like systems. Additionally, the binodal data were fitted using two semi-empirical equations, namely Merchuk and Zafarani-Moattar et al. equations. Furthermore, the ATPSs mentioned above were applied to extract three amino acids, namely L-arginine, L-phenylalanine, and L-tyrosine, and demonstrated good extraction levels. Finally, the Diamond–Hsu equation and its modified version were utilized to correlate the experimental partition coefficients of the amino acids. These advancements pave the way for the development of improved extraction methodologies and the exploration of new applications in the field of biotechnology, pharmaceuticals, and beyond.
2. The measured tie-line results revealed that the hydrogen bonds of NADES may not be entirely disrupted in aqueous solutions, and thus, these ATPSs act as ternary-like systems. Additionally, the binodal data were fitted using two semi-empirical equations, namely Merchuk and Zafarani-Moattar et al. equations. Furthermore, the ATPSs mentioned above were applied to extract three amino acids, namely L-arginine, L-phenylalanine, and L-tyrosine, and demonstrated good extraction levels. Finally, the Diamond–Hsu equation and its modified version were utilized to correlate the experimental partition coefficients of the amino acids. These advancements pave the way for the development of improved extraction methodologies and the exploration of new applications in the field of biotechnology, pharmaceuticals, and beyond.
1. Introduction
With the advent of green chemistry, many investigators have been paying considerable attention to the discovery and application of green solvents.1 Aqueous two-phase systems (ATPSs) are environmentally friendly alternatives for traditional organic water solvent extraction systems. An ATPS is mostly composed of water and non-volatile components. These systems have been used for many years in biotechnological applications such as the separation of amino acids, enzymes, proteins, etc.2–4Recently, ATPSs based on deep eutectic solvents (DESs)5–8 have been created to extraction of biomolecules. This innovation brings forth several distinctive features that enhance the partitioning process of amino acids. First, DES is composed of a hydrogen bond donor and acceptor, which facilitates the formation of ATPS with water. This unique property allows for the creation of DES-based ATPS with tailored physicochemical properties, such as tunable polarity and viscosity, offering a wide range of design options for specific amino acid partitioning. Furthermore, DES-based ATPS exhibit excellent biocompatibility, making them highly suitable for biomolecule separation applications. The inherent low toxicity and biodegradability of DES ensure minimal interference with the integrity and functionality of the partitioned amino acids. Moreover, DES possesses a wide liquid range, enabling their application over a broad temperature range, expanding the operational flexibility of the partitioning process. Overall, the integration of deep eutectic solvents into aqueous two-phase systems for amino acid partitioning introduces a novel and promising approach that combines the unique properties of DES with the well-established advantages of ATPS, offering a platform for efficient and environmentally friendly separation and purification of amino acids.9 However, ATPs made by natural deep eutectic solvents (NADES) have some advantages like low toxicity, and more stability in comparison of ATPs based on DESs. Natural deep eutectic solvents are bio-based deep eutectic solvents made up of two or more compounds derived from plants, such as organic acids, sugars, alcohols, amines, and amino acids.5,10–12 Xu and co-workers6,7,13,14 were used DESs for first time as a phase forming component of ATPS. Investigation on these kinds of ATPSs15 provided convincing evidence about impairing interactions of the HBA and HBD in presence of water and that DES complexes can be entirely broken at high water concentrations. In other words, in DES-based aqueous two-phase systems, the HBA and HBD molar ratio is destroyed in the coexisting phases, demonstrating that the HBA and HBD act as phase forming ATPS components separately and ATPS is a quaternary mixture.15 In this line of research, Farias et al.8 have made a study about the ability of natural deep eutectic solvents to create ternary-like aqueous biphasic systems. Their ATPS was consisted of PPG400 and the NADES (choline chloride as HBA and glucose or urea as HBD). This investigation indicated that a combination of factors, including the nature of the ATPS components and the hydrophobicity/hydrophilicity of the HBD, enables the formation of systems in which the HBA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HBD stoichiometry used in creation of NADES is retained in the co-existing phases, thus these ATPSs behave as ternary-like systems.9 Similar studies on phase behavior and stability of natural deep eutectic solvents in aqueous solutions have been carried out.16–18 Also, to investigation of DESs applications for extraction of macromolecules and amino acids the DES-based ATPSs have been applied for partitioning of proteins,6,7,13,14 dyes,8 phenolic compounds, amino acids and alkaloids.19–22
HBD stoichiometry used in creation of NADES is retained in the co-existing phases, thus these ATPSs behave as ternary-like systems.9 Similar studies on phase behavior and stability of natural deep eutectic solvents in aqueous solutions have been carried out.16–18 Also, to investigation of DESs applications for extraction of macromolecules and amino acids the DES-based ATPSs have been applied for partitioning of proteins,6,7,13,14 dyes,8 phenolic compounds, amino acids and alkaloids.19–22
In this work, aqueous two-phase systems composed of polyethylene glycol dimethyl ether with a molar mass of the 250 g mol−1 (PEGDME250), and a natural deep eutectic solvent (ChCl:sucrose or ChCl:fructose with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio), were studied at 298.15 K and under pressure of 85 kPa. For these aqueous two-phase systems, the binodal and tie-line values were determined. Merchuk23 and Zafarani-Moattar et al.24 equations were used to fit the binodal data. Tie-lines for { PEGDME250 + ChCl:sucrose + water} and { PEGDME250 + ChCl:fructose + water} ATPSs were also determined to see whether stoichiometry of initial components of NADES is maintained in the top and bottom phases or not, and from these data it can be considered that studied ATPSs act as ternary or quaternary systems.
2 molar ratio), were studied at 298.15 K and under pressure of 85 kPa. For these aqueous two-phase systems, the binodal and tie-line values were determined. Merchuk23 and Zafarani-Moattar et al.24 equations were used to fit the binodal data. Tie-lines for { PEGDME250 + ChCl:sucrose + water} and { PEGDME250 + ChCl:fructose + water} ATPSs were also determined to see whether stoichiometry of initial components of NADES is maintained in the top and bottom phases or not, and from these data it can be considered that studied ATPSs act as ternary or quaternary systems.
Also, the application of these aqueous two-phase systems was studied for partitioning of some amino acids namely L-arginine, L-phenylalanine, and L-tyrosine. The partitioning coefficients, K, and the corresponding extraction efficiency, EE%, at each tie-line were calculated to the investigation of effect of amino acid nature and the kind of HBD in structure of NADES on the separation of mentioned amino acids. Finally, the Diamond–Hsu25 equation and its modified form were used to fit the partitioning coefficient values.
2. Chemicals and methods
2.1. Chemicals
The choline chloride (>99.0% w/w) was provided from Daejung. The sucrose (>99.5% w/w), fructose (>99.5% w/w), PEGDME with a molar mass of 250 g mol−1 (>99.0% w/w), L-arginine (>99.0% w/w), L-phenylalanine (>99.0% w/w), and L-tyrosine (>99.0% w/w) were purchased from Merck. All of the materials were utilized without additional purification and also the solutions were prepared with double distilled deionized water. To determine the water content of compounds, the Karl-Fischer technique was utilized.2.2. Preparation of NADES
In this study, two kinds of NADES (ChCl:sucrose and ChCl:fructose) with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio were prepared. According the procedure described previously,26,27 for preparing NADES, first choline chloride as HBA and sucrose or fructose as HBD were mixed in a 50 mL round-bottom flask. After that, the flask was immersed in a tiny paraffin oil bath heated by a hot plate stirrer. Flask contents were stirred for 2 h at 353.15 K until a colorless, homogeneous liquid formed. A thermometer (0.01 K) was used to continually check the temperature of the NADES. After processing, all samples were stored in well-sealed vials in a moisture-controlled environment.
2 molar ratio were prepared. According the procedure described previously,26,27 for preparing NADES, first choline chloride as HBA and sucrose or fructose as HBD were mixed in a 50 mL round-bottom flask. After that, the flask was immersed in a tiny paraffin oil bath heated by a hot plate stirrer. Flask contents were stirred for 2 h at 353.15 K until a colorless, homogeneous liquid formed. A thermometer (0.01 K) was used to continually check the temperature of the NADES. After processing, all samples were stored in well-sealed vials in a moisture-controlled environment.
The temperature of the NADES was continuously monitored with a thermometer (±0.01 K). The samples were provided in a moisture controlled environment and then after preparation they kept in well-sealed vials. The Karl-Fischer analysis indicated the mass fraction water content of the prepared NADES to be 0.0008.
2.3. Methods
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) were used. Throughout the experiment, the solution was constantly stirred. During the preparation of the solutions water contents of chemicals, which were measured by Karl Fischer coulometer (Metrohm 751 GPD) were taken into account. An analytical balance (Shimadzu, 321-34553, Shimadzu Co., Japan) with a precision of ±1 × 10−7 kg was used to determine the mass fraction of the components. The maximum uncertainty in determining the mass fraction of polymer and NADES was found to be 0.002.
2) were used. Throughout the experiment, the solution was constantly stirred. During the preparation of the solutions water contents of chemicals, which were measured by Karl Fischer coulometer (Metrohm 751 GPD) were taken into account. An analytical balance (Shimadzu, 321-34553, Shimadzu Co., Japan) with a precision of ±1 × 10−7 kg was used to determine the mass fraction of the components. The maximum uncertainty in determining the mass fraction of polymer and NADES was found to be 0.002.Five different solutions of each ATPS consist of {PEGDME250 + NADES (ChCl:sucrose or ChCl:fructose with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio) + water} were prepared to determination of tie-lines. A mixture of NADES, PEGDME250 and water for each tie-line was prepared gravimetrically within ±10−7 kg and at the biphasic region. The prepared solutions to reach equilibrium for 30 min were vigorously stirred, then centrifuged and placed in water bath with temperature of 298.15 K. The compositions of coexisting phases were analytically determined. The phenol–sulfuric acid method31,32 was used to quantify the sucrose and fructose compositions. For this purpose, first in a test tube a 2 mL aliquot of a carbohydrate solution was mixed with 1 mL of 5% aqueous solution of phenol. Then, 5 mL of concentrated sulfuric acid was added rapidly to the mixture; the test tubes allowed standing for 10 min, and they were vortexed for 30 seconds before being placed in a room temperature water bath for 20 minutes for color development. Finally, a spectrophotometer (Model: SPECORD 40-Series Analytik Jena Germany) was used to record light absorption at 490 nm. For preparation of reference solutions the same method as explained above was used, except that the double distilled deionized water was used instead of the 2 mL aliquot of carbohydrate.
2 molar ratio) + water} were prepared to determination of tie-lines. A mixture of NADES, PEGDME250 and water for each tie-line was prepared gravimetrically within ±10−7 kg and at the biphasic region. The prepared solutions to reach equilibrium for 30 min were vigorously stirred, then centrifuged and placed in water bath with temperature of 298.15 K. The compositions of coexisting phases were analytically determined. The phenol–sulfuric acid method31,32 was used to quantify the sucrose and fructose compositions. For this purpose, first in a test tube a 2 mL aliquot of a carbohydrate solution was mixed with 1 mL of 5% aqueous solution of phenol. Then, 5 mL of concentrated sulfuric acid was added rapidly to the mixture; the test tubes allowed standing for 10 min, and they were vortexed for 30 seconds before being placed in a room temperature water bath for 20 minutes for color development. Finally, a spectrophotometer (Model: SPECORD 40-Series Analytik Jena Germany) was used to record light absorption at 490 nm. For preparation of reference solutions the same method as explained above was used, except that the double distilled deionized water was used instead of the 2 mL aliquot of carbohydrate.
Mohr method,33 known as argentometric method, is one of the significant methods for determination of chloride in water. Choline chloride is a quaternary ammonium salt with choline cation and chloride anion. This method determines the chloride ion concentration of a solution by titration with silver nitrate using potassium chromate as indicator.
There are some common chemical indicators that are utilized with argentometric titrations: (I) the chromate ion, CrO2−4 (the Mohr method); (II) adsorption indicators such as fluorescein (the Fajans method); (III) the ferric ion, Fe3+ (the Volhard method).34,35
Mohr indicator reaction is based on the following reactions:
| Cl− + AgNO3 → AgCl + NO−3 |
| 2Ag+ + CrO2−4 → Ag2CrO4 |
The concentration of titrant rises sharply near the equivalence point, and the solubility of Ag2CrO4 is exceeded. The appearance of red precipitate marks the endpoint.
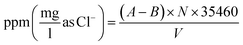 | (1) |
To determination of the polymer concentration in both phases the refractive index method was used which proposed for first time by Cheluget et al.36 In our work a refractometer (ATAGO DR-A1, Japan) with a precision of 0.0001 was applied to measure the prepared solutions. The refractive index uncertainty was estimated to be 0.0002.
According to the refractive index method,36 there is a relation between the refractive index of solution, nD, and the corresponding components mass fractions in dilute aqueous solutions of each phase of an ATPS. The relation between nD and mass fractions of polymer, wp, choline chloride, wc and sucrose, wsu, takes the following form:16
| nD = n0+apwp + acwc + asuwsu | (2) |
| Material | Constant | Value | C Range (w/w) | aR2 |
|---|---|---|---|---|
| a Where, R2, represented respective correlation coefficient value of the linear calibration plot of the refractive index against mass fraction of choline chloride, polymer and sugars at the mass fraction range (C range) of each material. | ||||
| ChCl | ac | 0.1452 | 0 to 0.08 | 0.9987 |
| PEGDME250 | ap | 0.1323 | 0 to 0.10 | 0.9994 |
| Sucrose | asu | 0.1486 | 0 to 1.80 | 0.9968 |
| Fructose | asu | 0.1492 | 0 to 1.70 | 0.9908 |
The partitioning coefficient, K, and extraction efficiency, EE%, were calculated respectively by eqn. (3) and (4) as below:
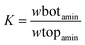 | (3) |
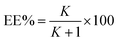 | (4) |
3. Results and discussion
3.1. Phase diagrams
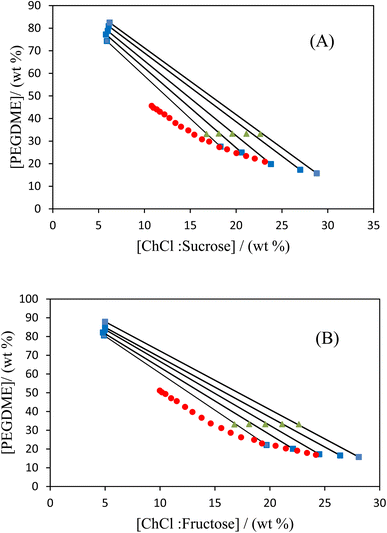
For each studied ATPS made by PEGDME250, NADES (ChCl:sucrose and ChCl:fructose (with molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)), and water phase diagrams at 298.15 K are plotted in Fig. 1, and the experimental mass fractions are also presented in Table 2.
2)), and water phase diagrams at 298.15 K are plotted in Fig. 1, and the experimental mass fractions are also presented in Table 2.
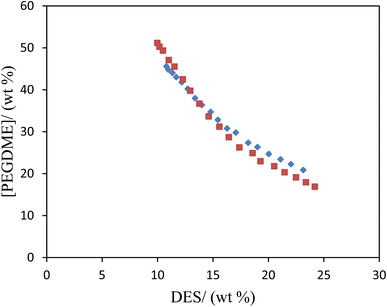 | ||
Fig. 1 Experimental binodal data of ATPSs composed of mixtures of ChlCl:sucrose and ChCl:fructose at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio, PEGDME250, and water at 298.15 K: ChlCl:sucrose (♦); ChCl:fructose (■). 2 molar ratio, PEGDME250, and water at 298.15 K: ChlCl:sucrose (♦); ChCl:fructose (■). | ||
| wNADES | wp | wNADES | wp |
|---|---|---|---|
ChCl:sucrose 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
ChCl:fructose 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
||
| a The standard uncertainties σ for temperature, pressure, and mass fraction are: σ (T) = 0.05 K; σ (p) = 0.5 kPa; and σ (wi) = 0.005, respectively. | |||
| 10.79 | 45.59 | 9.98 | 51.16 |
| 10.97 | 44.80 | 10.17 | 50.27 |
| 11.34 | 44.03 | 10.49 | 49.35 |
| 11.68 | 43.03 | 11.01 | 47.09 |
| 12.18 | 41.81 | 11.52 | 45.53 |
| 12.72 | 40.22 | 12.28 | 42.49 |
| 13.38 | 38.00 | 12.95 | 39.76 |
| 13.99 | 36.39 | 13.78 | 36.69 |
| 14.78 | 34.73 | 14.61 | 33.64 |
| 15.44 | 32.83 | 15.58 | 31.18 |
| 16.27 | 30.80 | 16.43 | 28.68 |
| 17.06 | 29.80 | 17.38 | 26.25 |
| 18.17 | 27.35 | 18.57 | 24.9 |
| 19.01 | 26.35 | 19.28 | 22.97 |
| 20.03 | 24.70 | 20.52 | 21.77 |
| 21.09 | 23.41 | 21.45 | 20.32 |
| 22.03 | 22.26 | 22.49 | 19.12 |
| 23.14 | 20.87 | 23.38 | 17.94 |
| 24.19 | 16.9 | ||
The binodal data in Table 2 were fitted by Merchuk,23 eqn (5), and Zafarani-Moattar et al.,24 eqn (6) using a nonlinear least-square regression method.
wp = a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(bw0.5NADES − cw3NADES) exp(bw0.5NADES − cw3NADES)
| (5) |
wp = α + β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln(wNADES) + γwNADES ln(wNADES) + γwNADES
| (6) |
| Zafarani-Moattar et al. (eqn (6)) | ||||
|---|---|---|---|---|
| α | β | γ | 100.sd | |
| ChCl:sucrose | −0.7178 | −0.4824 | 0.9492 | 0.24 |
| ChCl:fructose | −1.3033 | −0.7065 | 1.9455 | 0.45 |
3.2. Analysis of tie-lines
The tie-lines together with binodal curves are presented in Fig. 2 for ATPSs composed of NADES (ChCl:sucrose and ChCl:fructose) with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio, PEGDME250, and water. For mentioned systems five tie-lines and their corresponding length (TLL) were characterized. The related data are listed in Table 4. It can be seen from this table, the top phase is always rich in PEGDME250, and this behavior happens for both of studied ATPS and different tie-line length. The majority of bottom phase is composed of higher concentration of ChCl and sucrose or fructose than polymer and water. Therefore, bottom phase denoted as ChCl-rich phase.
2 molar ratio, PEGDME250, and water. For mentioned systems five tie-lines and their corresponding length (TLL) were characterized. The related data are listed in Table 4. It can be seen from this table, the top phase is always rich in PEGDME250, and this behavior happens for both of studied ATPS and different tie-line length. The majority of bottom phase is composed of higher concentration of ChCl and sucrose or fructose than polymer and water. Therefore, bottom phase denoted as ChCl-rich phase.
HBA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HBD molar ratio 1 HBD molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Overall composition/wt% | Polymer-rich phase composition/wt% | ChCl-rich phase composition/wt% | TLL | |||||
|---|---|---|---|---|---|---|---|---|---|
[HBA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HBD] HBD] |
[PEGDME] | [HBA] | [HBD] | [PEGDME] | [HBA] | [HBD] | [PEGDME] | ||
| a The standard uncertainty of mass percent for each component is 0.8. | |||||||||
| ChCl:sucrose | 16.77 | 33.11 | 1.09 | 4.8 | 74.29 | 3.59 | 14.71 | 27.56 | 47.84 |
| ChCl:fructose | 1.51 | 3.4 | 80.6 | 5.61 | 14.11 | 22.16 | 60.55 | ||
| ChCl:sucrose | 18.11 | 33.3 | 1.02 | 4.85 | 77.18 | 4.43 | 16.2 | 24.95 | 53.57 |
| ChCl:fructose | 1.47 | 3.39 | 82.18 | 7.34 | 14.92 | 20.15 | 63.36 | ||
| ChCl:sucrose | 19.59 | 33.25 | 1.12 | 4.89 | 78.75 | 5.03 | 18.81 | 19.83 | 60.66 |
| ChCl:fructose | 1.52 | 3.39 | 83.75 | 7.93 | 16.62 | 17.23 | 68.12 | ||
| ChCl:sucrose | 21.12 | 33.24 | 1.12 | 5.03 | 80.89 | 5.33 | 21.65 | 17.29 | 65.89 |
| ChCl:fructose | 1.52 | 3.5 | 84.89 | 9.15 | 17.31 | 16.59 | 70.07 | ||
| ChCl:sucrose | 22.64 | 33.19 | 1.09 | 5.12 | 82.49 | 6.14 | 22.69 | 15.78 | 69.21 |
| ChCl:fructose | 1.48 | 3.58 | 87.89 | 9.58 | 18.69 | 15.75 | 74.14 | ||
Fig. 2 shows that, for the studied ATPSs, the ChCl-rich phase is more affected than the polymer-rich phase by variation of the TLL; so that by increasing the TLL the amount of water in bottom phase decreases remarkably leading to increase of the ChCl:sucrose or ChCl:fructose concentrations. But, the polymer-rich phase composition is only slightly changed by variations of the TLL or overall compositions. To investigate the final stoichiometry of HBA: HBD in the coexisting phases, the molar ratios for the NADESs (ChCl:sucrose and ChCl:fructose) were computed in each phases, and the results are listed in Table 5. This table enables us to compare the final HBA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HBD molar ratios in both phases with those selected initially as 1
HBD molar ratios in both phases with those selected initially as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2; the fairly good agreement between initial and calculated final molar ratios indicates that the initial molar ratios are approximately retained and both studied ATPSs may be acted as a ternary-like system. In fact, this behavior can happen when the HBA and HBD both show extremely hydrophilic properties and poor solubility in the polymer-rich phase.8
2; the fairly good agreement between initial and calculated final molar ratios indicates that the initial molar ratios are approximately retained and both studied ATPSs may be acted as a ternary-like system. In fact, this behavior can happen when the HBA and HBD both show extremely hydrophilic properties and poor solubility in the polymer-rich phase.8
HBA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HBD molar ratio 1 HBD molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Overall composition/wt% | HBA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HBD (mol HBD (mol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mol) mol) |
TLL | ||
|---|---|---|---|---|---|
[HBA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HBD] HBD] |
[PEGDME] | Polymer-rich phase | ChCl-rich phase | ||
| ChCl:sucrose | 16.77 | 33.11 | 0.562 | 0.602 | 47.84 |
| ChCl:fructose | 0.569 | 0.512 | 60.55 | ||
| ChCl:sucrose | 18.11 | 33.3 | 0.511 | 0.666 | 53.57 |
| ChCl:fructose | 0.531 | 0.632 | 63.36 | ||
| ChCl:sucrose | 19.59 | 33.25 | 0.550 | 0.652 | 60.66 |
| ChCl:fructose | 0.569 | 0.614 | 68.12 | ||
| ChCl:sucrose | 21.12 | 33.24 | 0.539 | 0.599 | 65.89 |
| ChCl:fructose | 0.553 | 0.679 | 70.07 | ||
| ChCl:sucrose | 22.64 | 33.19 | 0.529 | 0.659 | 69.21 |
| ChCl:fructose | 0.538 | 0.656 | 74.14 | ||
Also, the data reported in Table 4 indicate that in polymer-rich phase (in both studied ATPSs) there are only very small amounts of NADES components. This behavior can be related to sucrose, fructose, and choline chloride high hydrophilic properties (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow values are −3.70,-1.55, and −5.16, respectively);38 so that these components have a low solubility in the hydrophobic polymer-rich phase and therefore prefer to stay in the bottom phase.8
Kow values are −3.70,-1.55, and −5.16, respectively);38 so that these components have a low solubility in the hydrophobic polymer-rich phase and therefore prefer to stay in the bottom phase.8
3.3. The performance of NADES-PEGDME ATPSs in the partition behavior of amino acids
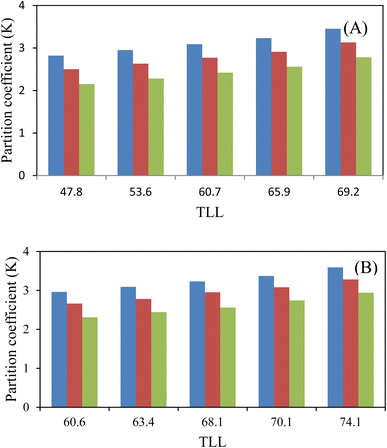
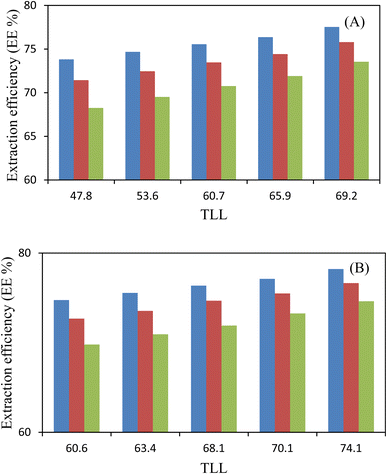
The experimental partition coefficients, K, and the percentage extraction efficiencies, EE%, of investigated amino acids (L-arginine, L-tyrosine, and L-phenylalanine) are presented in.Table 6 and their variation with TLL can be seen in Fig. 3 and 4, respectively. For both the studied ATPSs the observed trend is as follows: K (L-arginine) > K (L-tyrosine) > K (L-phenylalanine). It is observed from Table 6 that amino acids effectively partitioned to the ChCl-rich phase (more hydrophilic phase).
HBA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HD molar ratio 1 HD molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Overall composition/wt% | K | EE% | |
|---|---|---|---|---|
[HBA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HBD] HBD] |
[PEGDME] | |||
| a The standard uncertainties σ for partitioning coefficient, temperature and pressure are: σ (K) = 0.15, σ (T) = 0.05 K and σ (p) = 0.5 kPa respectively.b The standard uncertainty σ for partitioning coefficient is: σ (K) = 0.1. | ||||
| L-Arginine | ||||
| ChCl:sucrose | 16.77 | 33.11 | 2.82 | 73.82 |
| 18.11 | 33.30 | 2.95 | 74.68 | |
| 19.59 | 33.25 | 3.09 | 75.55 | |
| 21.12 | 33.24 | 3.23 | 76.36 | |
| 22.64 | 33.19 | 3.45 | 77.53 | |
| ChCl:fructose | 16.77 | 33.11 | 2.96 | 74.75 |
| 18.11 | 33.30 | 3.09 | 75.55 | |
| 19.59 | 33.25 | 3.23 | 76.36 | |
| 21.12 | 33.24 | 3.37 | 77.12 | |
| 22.64 | 33.19 | 3.59 | 78.21 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| L-Tyrosine | ||||
| ChCl:sucrose | 16.77 | 33.11 | 2.50 | 71.43 |
| 18.11 | 33.30 | 2.63 | 72.45 | |
| 19.59 | 33.25 | 2.77 | 73.47 | |
| 21.12 | 33.24 | 2.91 | 74.42 | |
| 22.64 | 33.19 | 3.13 | 75.79 | |
| ChCl:fructose | 16.77 | 33.11 | 2.66 | 72.68 |
| 18.11 | 33.30 | 2.78 | 73.54 | |
| 19.59 | 33.25 | 2.95 | 74.68 | |
| 21.12 | 33.24 | 3.08 | 75.49 | |
| 22.64 | 33.19 | 3.28 | 76.64 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| L-Phenylalanine | ||||
| ChCl:sucrose | 16.77 | 33.11 | 2.15 | 68.25 |
| 18.11 | 33.30 | 2.28 | 69.51 | |
| 19.59 | 33.25 | 2.42 | 70.76 | |
| 21.12 | 33.24 | 2.56 | 71.91 | |
| 22.64 | 33.19 | 2.78 | 73.54 | |
| ChCl:fructose | 16.77 | 33.11 | 2.31 | 69.79 |
| 18.11 | 33.30 | 2.44 | 70.93 | |
| 19.59 | 33.25 | 2.56 | 71.91 | |
| 21.12 | 33.24 | 2.74 | 73.26 | |
| 22.64 | 33.19 | 2.94 | 74.62 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio of HBA
2 molar ratio of HBA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HBD indicate that, the K values for system containing (NADES (ChCl:fructose) + PEGDME250 + water) are slightly higher than the K values for system containing (NADES (ChCl:sucrose) + PEGDME250 + water) and these values are very close to each other, this behavior maybe is because of the biphasic region of studied systems. Also, the studied amino acids in both mentioned ATPSs transferred preferentially to the bottom ChCl-rich phase.
HBD indicate that, the K values for system containing (NADES (ChCl:fructose) + PEGDME250 + water) are slightly higher than the K values for system containing (NADES (ChCl:sucrose) + PEGDME250 + water) and these values are very close to each other, this behavior maybe is because of the biphasic region of studied systems. Also, the studied amino acids in both mentioned ATPSs transferred preferentially to the bottom ChCl-rich phase.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow); so that lower log
Kow); so that lower log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow values show that the solute have more hydrophilicity and higher tendency to water molecules.28 The log
Kow values show that the solute have more hydrophilicity and higher tendency to water molecules.28 The log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow values of amino acids are −4.20, −1.49 and −1.18 (ref. 39) for L-arginine, L-tyrosine, and L-phenylalanine, respectively. Therefore the amino acid extraction efficiency is in the order: L-arginine > L-tyrosine > L-phenylalanine. This study and previous one39 indicate that the hydrophobicity of amino acids can be an important criteria to predict of solute partitioning between two-phases of an ATPS.
Kow values of amino acids are −4.20, −1.49 and −1.18 (ref. 39) for L-arginine, L-tyrosine, and L-phenylalanine, respectively. Therefore the amino acid extraction efficiency is in the order: L-arginine > L-tyrosine > L-phenylalanine. This study and previous one39 indicate that the hydrophobicity of amino acids can be an important criteria to predict of solute partitioning between two-phases of an ATPS.
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K = AΔw(PEGDME250) + BΔw(PEGDME250)2 K = AΔw(PEGDME250) + BΔw(PEGDME250)2
| (7) |
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K = A1 + B1Δw(PEGDME250) + C1Δw(PEGDME250)2 K = A1 + B1Δw(PEGDME250) + C1Δw(PEGDME250)2
| (8) |
For each amino acid the results of fitting experimental partition coefficients values to eqn (7) and (8) together with the corresponding standard deviations, sd are reported in Table 7. According to the sd values in Table 7, we conclude that correlation with all of the eqn (7) and (8) is satisfactory; and excellent performance is obtained with the eqn (8).
HBA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HBD molar ratio 1 HBD molar ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Diamond–Hsu (eqn (7)) | ||
|---|---|---|---|
| A | 104 × B | sd | |
| L-Arginine | |||
| ChCl:sucrose | 0.0303 | −1.8232 | 0.58 |
| ChCl:fructose | 0.0220 | −0.6173 | 0.42 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| L-Tyrosine | |||
| ChCl:sucrose | 0.0253 | −1.2838 | 0.50 |
| ChCl:fructose | 0.0176 | −0.1635 | 0.10 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| L-Phenylalanine | |||
| ChCl:sucrose | 0.0188 | −0.5853 | 0.71 |
| ChCl:fructose | 0.0114 | −0.4697 | 0.68 |
| Modified Diamond–Hsu (eqn (8)) | ||||
|---|---|---|---|---|
| A1 | B1 | 104 × C1 | sd | |
| L-Arginine | ||||
| ChCl:sucrose | 1.4621 | −0.0219 | 2.7535 | 0.09 |
| ChCl:fructose | 1.9717 | −0.0387 | 4.0318 | 0.05 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| L-Tyrosine | ||||
| ChCl:sucrose | 1.3694 | −0.0236 | 3.0029 | 0.10 |
| ChCl:fructose | 1.4306 | −0.0265 | 3.2097 | 0.04 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| L-Phenylalanine | ||||
| ChCl:sucrose | 1.2501 | −0.0258 | 3.3279 | 0.31 |
| ChCl:fructose | 1.9534 | −0.0487 | 5.0755 | 0.52 |
4. Conclusion
This study focused on the applications and composition of aqueous two-phase systems (ATPS) utilizing natural eutectic solvents as environmentally friendly alternatives. Specifically, phase diagrams were constructed for two ATPSs consisting of polyethylene glycol dimethyl ether (with a molar mass of 250 g mol−1) and two types of natural deep eutectic solvents (NADESs). The NADESs were composed of choline chloride as the hydrogen bond acceptor (HBA), and either sucrose or fructose as the hydrogen bond donor (HBD), in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio. The phase diagrams were studied at a temperature of 298.15 K and atmospheric pressure (approximately 85 kPa). Thorough analysis of the tie-lines revealed that the initial stoichiometry of HBA
2 molar ratio. The phase diagrams were studied at a temperature of 298.15 K and atmospheric pressure (approximately 85 kPa). Thorough analysis of the tie-lines revealed that the initial stoichiometry of HBA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HBD in each NADES was approximately maintained in the coexisting phases. Consequently, the ATPSs exhibited properties resembling a ternary system, indicating that the hydrogen bonding interactions between the components of the NADESs were largely preserved within the ATPSs. Furthermore, the performance of these ATPSs in partitioning certain amino acids, namely L-arginine, L-tyrosine, and L-phenylalanine, was investigated. The experimental partition coefficients (K) and percentage extraction efficiencies (EE%) for the amino acids exhibited the following trend: L-arginine > L-tyrosine > L-phenylalanine. These results indicate that the chemical structures and polarities of the amino acids influence the observed variations in K and EE% values, with amino acids showing a preference for extraction into the ChCl-rich bottom phase.
HBD in each NADES was approximately maintained in the coexisting phases. Consequently, the ATPSs exhibited properties resembling a ternary system, indicating that the hydrogen bonding interactions between the components of the NADESs were largely preserved within the ATPSs. Furthermore, the performance of these ATPSs in partitioning certain amino acids, namely L-arginine, L-tyrosine, and L-phenylalanine, was investigated. The experimental partition coefficients (K) and percentage extraction efficiencies (EE%) for the amino acids exhibited the following trend: L-arginine > L-tyrosine > L-phenylalanine. These results indicate that the chemical structures and polarities of the amino acids influence the observed variations in K and EE% values, with amino acids showing a preference for extraction into the ChCl-rich bottom phase.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.Conflicts of interest
The authors declare that they have no conflict of interest.References
- R. t. Zhao, D. Pei, P. l. Yu, J. t. Wei, N. l. Wang, D. L. Di and Y. w. Liu, J. Sep. Sci., 2020, 43, 348–359 CrossRef CAS PubMed.
- P.-Å. Albertsson, Nature, 1958, 182, 709–711 CrossRef CAS PubMed.
- C.-Y. Yeh and J. C.-W. Lan, J. Taiwan Inst. Chem. Eng., 2014, 45, 1119–1125 CrossRef CAS.
- M. C. Hespanhol, B. M. Fontoura, J. C. Quintao and L. H. da Silva, J. Taiwan Inst. Chem. Eng., 2020, 115, 218–222 CrossRef CAS.
- Y. Song, R. P. Chandra, X. Zhang and J. N. Saddler, Carbohydr. Polym., 2020, 250, 116956 CrossRef CAS PubMed.
- K. Xu, Y. Wang, Y. Huang, N. Li and Q. Wen, Anal. Chim. Acta, 2015, 864, 9–20 CrossRef CAS PubMed.
- Q. Zeng, Y. Wang, Y. Huang, X. Ding, J. Chen and K. Xu, Analyst, 2014, 139, 2565–2573 RSC.
- F. O. Farias, H. Passos, A. l. S. Lima, M. R. Mafra and J. o. A. Coutinho, ACS Sustainable Chem. Eng., 2017, 5, 9402–9411 CrossRef CAS.
- A. P. Abbott, G. Capper, D. L. Davies, R. K. Rasheed and V. Tambyrajah, Chem. Commun., 2003, 70–71 RSC.
- Y. H. Choi, J. van Spronsen, Y. Dai, M. Verberne, F. Hollmann, I. W. Arends, G.-J. Witkamp and R. Verpoorte, Plant Physiol., 2011, 156, 1701–1705 CrossRef CAS PubMed.
- M. Marchel, J. Niewisiewicz, A. S. Coroadinha and I. M. Marrucho, Sep. Purif. Technol., 2020, 252, 117480 CrossRef CAS.
- V. Selvanathan, A. D. Azzahari, A. A. A. Halim and R. Yahya, Carbohydr. Polym., 2017, 167, 210–218 CrossRef CAS PubMed.
- N. Li, Y. Wang, K. Xu, Y. Huang, Q. Wen and X. Ding, Talanta, 2016, 152, 23–32 CrossRef CAS PubMed.
- H. Zhang, Y. Wang, K. Xu, N. Li, Q. Wen, Q. Yang and Y. Zhou, Anal. Methods, 2016, 8, 8196–8207 RSC.
- Y. Dai, G.-J. Witkamp, R. Verpoorte and Y. H. Choi, Food Chem., 2015, 187, 14–19 CrossRef CAS PubMed.
- F. Ghaffari, M. T. Zafarani-Moattar and H. Shekaari, Fluid Phase Equilib., 2021, 113348 Search PubMed.
- M. T. Zafarani-Moattar, H. Shekaari and F. Ghaffari, J. Chem. Eng. Data, 2019, 64, 4754–4762 CrossRef CAS.
- M. T. Zafarani-Moattar, H. Shekaari and F. Ghaffari, J. Mol. Liq., 2020, 311, 113347 CrossRef CAS.
- Y. Dai, G.-J. Witkamp, R. Verpoorte and Y. H. Choi, Anal. Chem., 2013, 85, 6272–6278 CrossRef CAS PubMed.
- F. O. Farias, F. H. B. Sosa, L. Igarashi-Mafra, J. A. P. Coutinho and M. R. Mafra, Fluid Phase Equilib., 2017, 448, 143–151 CrossRef CAS.
- F. O. Farias, H. Passos, M. G. Sanglard, L. Igarashi-Mafra, J. A. Coutinho and M. R. Mafra, Sep. Purif. Technol., 2018, 200, 84–93 CrossRef CAS.
- F. O. Farias, H. Passos, J. o. A. Coutinho and M. R. Mafra, Ind. Eng. Chem. Res., 2018, 57, 16917–16924 CrossRef CAS.
- J. C. Merchuk, B. A. Andrews and J. A. Asenjo, J. Chromatogr. B: Biomed. Sci. Appl., 1998, 711, 285–293 CrossRef CAS PubMed.
- M. T. Zafarani-Moattar and E. Nemati-Kande, Calphad, 2010, 34, 478–486 CrossRef CAS.
- A. D. Diamond and J. T. Hsu, Biotechnol. Bioeng., 1989, 34, 1000–1014 CrossRef CAS PubMed.
- Y. Dai, J. van Spronsen, G.-J. Witkamp, R. Verpoorte and Y. H. Choi, Anal. Chim. Acta, 2013, 766, 61–68 CrossRef CAS PubMed.
- A. Hayyan, F. S. Mjalli, I. M. AlNashef, T. Al-Wahaibi, Y. M. Al-Wahaibi and M. A. Hashim, Thermochim. Acta, 2012, 541, 70–75 CrossRef CAS.
- H. Passos, D. J. Tavares, A. M. Ferreira, M. G. Freire and J. o. A. Coutinho, ACS Sustainable Chem. Eng., 2016, 4, 2881–2886 CrossRef CAS.
- M. T. Zafarani-Moattar, H. Shekaari and P. Jafari, J. Mol. Liq., 2021, 323, 115072 CrossRef CAS.
- M. T. Zafarani-Moattar, H. Shekaari and E. Pourbagherian, Fluid Phase Equilib., 2020, 514, 112536 CrossRef CAS.
- A. A. Albalasmeh, A. A. Berhe and T. A. Ghezzehei, Carbohydr. Polym., 2013, 97, 253–261 CrossRef CAS PubMed.
- M. Dubois, K. A. Gilles, J. K. Hamilton, P. t. Rebers and F. Smith, Anal. Chem., 1956, 28, 350–356 CrossRef CAS.
- Y. Román-Leshkov and J. A. Dumesic, Top. Catal., 2009, 52, 297–303 CrossRef.
- B. An, W. Zhang, J. Han, Y. Wang and L. Ni, J. Solution Chem., 2016, 45, 1811–1825 CrossRef CAS.
- P.-A. Albertsson, Methods Biochem. Anal., 1962, 10, 229–262 CAS.
- E. Cheluget, S. Marx, M. Weber and J. Vera, J. Solution Chem., 1994, 23, 275–305 CrossRef CAS.
- G. M. Ferreira, G. M. Ferreira, A. O. Maldaner, L. H. M. da Silva and M. C. Hespanhol, Fluid Phase Equilib., 2020, 506, 112367 CrossRef CAS.
- S. Pradhan, P. Kumar and I. Mehrotra, J. Environ. Eng., 2014, 140, 06013001 CrossRef.
- S.-W. Nam, D.-J. Choi, S.-K. Kim, N. Her and K.-D. Zoh, J. Hazard. Mater., 2014, 270, 144–152 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2023 |