DOI:
10.1039/D3RA03331G
(Paper)
RSC Adv., 2023,
13, 21991-22006
Thermal and bisphenol-A adsorption properties of a zinc ferrite/β-cyclodextrin polymer nanocomposite†
Received
19th May 2023
, Accepted 13th July 2023
First published on 20th July 2023
Abstract
The present study investigated the use of a nanocomposite, produced by reinforcing nanosize zinc ferrite (ZnFe2O4) in a porous β-CD based polymeric matrix (β-CD-E-T/ZnFe2O4), for the removal of Bisphenol A (BPA) from aqueous solutions via adsorption. The thermal stability of the β-CD-based polymer and β-CD-E-T/ZnFe2O4 nanocomposite were investigated using simultaneous thermal analysis at four heating rates. Non-isothermal isoconversion methods were employed to study the thermal degradation kinetics of the β-CD based polymer before and after ZnFe2O4 nano-filling. The results showed that ZnFe2O4 nano-reinforcement increased the activation energy barrier for the thermal degradation of the β-CD-based polymeric matrix. Adsorption experiments showed that the β-CD-E-T/ZnFe2O4 nanocomposite exhibited very high BPA adsorption within 5 minutes. Isotherm, kinetics, and thermodynamic investigations revealed that the adsorption of BPA was via multilayer adsorption on a heterogeneous β-CD-E-T/ZnFe2O4 surface. The thermodynamic studies indicated that BPA adsorption on β-CD-E-T/ZnFe2O4 was spontaneous and exothermic. Overall, the β-CD-E-T/ZnFe2O4 nanocomposite showed less thermal degradation and high efficiency for removing BPA from contaminated water, indicating its potential as a promising material for wastewater treatment applications.
1. Introduction
Bisphenol A (2,2-bis(4-hydroxyphenyl)propane; BPA) is a diphenylmethane derivative. BPA is known to exhibit toxic properties and can disrupt the normal function of endogenous hormones. Studies have shown that BPA exposure can lead to an increased risk of cancer and other adverse health effects.1–3 Polycarbonate plastic is commonly utilized in the packaging of food, beverages, medical equipment, dental supplies, and thermal paper. However, due to the susceptibility of plastic materials to release BPA under certain conditions such as high temperatures, and basic or acidic environments, these plastic products may contaminate food and beverages with BPA. A survey conducted by National Health and Nutrition Examination Survey (NHANES; United States; 2013–2014) revealed that BPA was detected in 95.7% of randomly selected urine samples. The median concentration of BPA in urine samples from adults and children in the United States was found to be 1.24 μg L−1 and 1.25 μg L−1, respectively. Notably, the concentration of BPA in these samples was significantly higher than its other derivatives, such as bisphenol F and bisphenol S.4 Analysis of samples collected by Fürhacker et al.5 from various wastewater sources, including metal/wood manufacturing, chemical industry, hospitals, paper production, cloth washing companies, household areas, and food industry, revealed that the concentration of BPA varied widely, ranging from 1 to 72 μg L−1. Notably, the highest concentration of BPA was found in samples collected from paper production sites. The harmful effects of BPA have prompted the development of efficient technologies for its removal from wastewater. Advanced oxidation, biodegradation, photocatalytic degradation, membrane filtration, and adsorption processes are among the methods that have been reported for the treatment of BPA. These techniques are often employed in combination to achieve more efficient removal of BPA. Further research is needed to identify the most effective and sustainable approach for the removal of BPA from different sources.6–12 Adsorption is a simple, cost-effective, and fast method for the removal of BPA from wastewater. The effectiveness of this process can be further improved by tailoring the functionality of the adsorbent material. Conventional adsorbent materials, such as activated carbons and clay, may have slower adsorption rates and limited tailoring properties, making it challenging to achieve optimal results. For instance, Martín-Lara et al.13 reported that it took activated carbon 2880 minutes to reach equilibrium during the removal of BPA. Another study demonstrated that activated carbons derived from biomass can exhibit a high adsorption capacity (>458 mg g−1) for both anionic and cationic adsorbates, as determined by Langmuir adsorption isotherm.14 Similarly, other studies investigating the use of activated carbon as a sorbent have also noted a lack of selectivity.15,16 Additionally, natural clays rich in smectite, when used as adsorbents, did not exhibit significant changes in selectivity between anionic and cationic adsorbates.17 Pleşa Chicinaş et al.18 utilized bentonite clay to remove cationic and anionic dyes, wherein the clay displayed a slightly higher adsorption capacity for the cationic dye compared to the anionic dye, albeit with a negligible difference (<15 mg g−1). Other potential adsorbent materials, such as metal–organic frameworks (MOFs), have also been explored.19–21 Although MOFs can demonstrate good adsorption performance, their stability is compromised under certain conditions, such as exposure to water or acidic/alkaline environments.22 Therefore, it is necessary to utilize adsorbent materials whose properties can be tailored according to the specific adsorbate being targeted for removal. By employing such tailored adsorbents, the efficacy of the adsorption process can be enhanced, leading to a more efficient BPA removal method.
Polysaccharides represent a substantial class of naturally occurring biological macromolecules, consisting of repeating units of monosaccharides, such as glucose and fructose. Due to their abundance, biodegradable nature, nontoxic properties, and cost-effectiveness, polysaccharides have gained significant attention for a broad range of applications. As a result, they have emerged as one of the most promising and versatile materials for various scientific and industrial applications.23 Starch is a widely available polysaccharide found naturally in sources such as potatoes, rice, wheat, sweet potatoes, and corn. One of the intriguing chemical compounds obtained from starch is cyclodextrins, which are oligosaccharides composed of 3 to 10 monosaccharides in their structure. Cyclodextrins and their derivatives are formed from glucopyranose units linked together through glycosidic bonds to form ring structures. These structures are further classified into α, β, and γ-cyclodextrins, consisting of 6, 8, and 10 glucopyranose units, respectively. β-Cyclodextrin (β-CD), composed of eight glucopyranose units, has a cone-shaped structure with an inner hydrophobic cavity (0.60–0.65 nm) and an outer hydrophilic portion, owing to the abundance of hydroxyl (–OH) groups.24 β-CD is a promising material for various scientific and industrial applications due to its unique shape and chemical properties. One of the distinctive properties of β-CD is its ability to host or trap various organic molecules within its cavity, β-CD, and its derivatives have been extensively investigated as potential drug delivery carriers, owing to their host–guest interaction properties. The host–guest interaction of β-CD with organic molecules makes it a promising material for wastewater treatment. However, the direct use of β-CD in wastewater treatment may not be efficient due to its partial solubility in water (18.6 mg mL−1) and the potential for creating secondary pollutants during its application.25 To address the issue, various β-CD based polymers and their nanocomposites are being extensively researched for the potential removal of BPA from the wastewater. Despite these efforts, effective removal of BPA remains a significant challenge, as β-CD based materials exhibit low adsorption capacity, slow adsorption rates, poor removal efficiency, and challenging regeneration processes.26–30 Previous studies have reported on the successful use of β-CD-based porous polymers, such as β-CD-epichlorohydrin (ECH; E)-tetrafluoroterephthalonitrile (TFTPN; T) and β-CD-4,4′-bis(chloromethyl) biphenyl, which contain aromatic moieties, for the rapid removal of BPA from aqueous solutions with high adsorption capacity.26,28 However, these polymers exhibited low equilibrium adsorption capacity, which limits their practical application. Thus, there is a need for further improvement in the design and synthesis of β-CD-based polymers to enhance their adsorption capacity and efficiency for the removal of BPA from wastewater.
The incorporation of nanosize (size < 100 nm) reinforcement materials is a common approach to enhance the adsorption capacity of polymers used in wastewater treatment.31–34 Among the widely used reinforcements for polymers: clays, metal oxides, and carbon-based nanomaterials have gained significant attention. Metal oxides such as zinc oxide (ZnO) and magnetite (Fe3O4) have been extensively studied for improving the adsorption performance of polymers. However, the selection of reinforcement materials for composite production should be based on their synergetic effect to improve the removal of BPA. For instance, previously reported composites, such as β-CD-capped graphene–magnetite nanocomposite and carboxymethyl-β-CD polymer/Fe3O4, exhibited a slow adsorption rate for BPA.27,29 Ferrites (MFe2O4; where M represents metal ions in a 2+ state) have been utilized in a variety of applications, including catalysis and semiconductor technology. Zinc ferrite (ZnFe2O4) has previously been employed to enhance the adsorption performance of polymeric films due to its high specific surface area and the synergistic effects between Fe and Zn.35 ZnFe2O4 can be synthesized using methods such as the co-precipitation, hydrothermal, sol–gel, and thermal decomposition to name a few. The hydrothermal method can provide better nanosized ZnFe2O4 particles with narrow particle size distributions. Therefore, the authors opted for the modified hydrothermal method to synthesize nanoscale ZnFe2O4.
The present study investigates the use of a nanocomposite, β-CD-E-T/ZnFe2O4 reported in our previous work36 for the removal of BPA from an aqueous solution. The thermal stability and degradation of the nanocomposite were examined at various heating rates (5, 10, 15, and 20 °C min−1) to evaluate the influence of ZnFe2O4 reinforcement. The adsorption of BPA onto β-CD-E-T/ZnFe2O4 was studied under different conditions, and the isotherm, kinetic, and thermodynamic properties of the adsorption process were investigated to determine its efficiency. Furthermore, the Fourier transform infrared spectroscopy (FTIR) spectra of the nanocomposite were obtained both before and after the adsorption process to evaluate potential interactions responsible for the adsorption of BPA. It should be noted that, to the best of the author's knowledge, the utilization of the β-CD-E-T/ZnFe2O4 nanocomposite for the removal of BPA has not been reported thus far.
2. Experimental
2.1. Synthesis of β-CD-E-T/ZnFe2O4 nanocomposite
All the chemicals used in this study were received from their respective suppliers (Table S1†) and used as received without additional modifications.
For the synthesis of β-CD-E-T polymer, the previously reported method was adopted with a few modifications.26 In our previous work,36 a detailed description of the synthesis procedure was provided. Briefly, 25 g of β-CD was dissolved in 37.5 mL of 6 N NaOH at 80 °C with vigorous stirring. Then, 3.75 g of TFTPN was added to the basic β-CD solution, and the resulting solution was stirred for 60 minutes before being irradiated with ultrasound in an ultrasound bath for 10 minutes. Afterward, the solution was stirred again, and 31.3 mL of ECH was slowly injected into the solution. Gelation was observed after 13 minutes of the complete addition of ECH. The obtained gel was washed with water several times to remove the soluble polymer fraction and then washed with acetone (Soxhlet) for 8 hours to remove impurities.
To synthesize ZnFe2O4, Zn(NO3)2·6H2O and Fe(NO3)3·9H2O were mixed in 100 mL of ethylene glycol in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 mole ratio. Later, sodium acetate (Fe3+
2 mole ratio. Later, sodium acetate (Fe3+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sodium acetate mole ratio = 1
sodium acetate mole ratio = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) was added. The mixture was vigorously stirred for 60 minutes and then subjected to ultrasound irradiation for 20 minutes. Next, 25 mL of ethylene diamine was added to the solution, and the resulting mixture was again irradiated with ultrasound for 20 minutes. After stirring the solution for 60 minutes, it was kept in an oven at 180 °C for 3 days until precipitations of ZnFe2O4 were obtained. The insoluble content was filtered and washed with water to remove any unreacted impurities and then dried in an oven at 60 °C. Finally, the material was calcined at 500 °C for 3 hours.
4) was added. The mixture was vigorously stirred for 60 minutes and then subjected to ultrasound irradiation for 20 minutes. Next, 25 mL of ethylene diamine was added to the solution, and the resulting mixture was again irradiated with ultrasound for 20 minutes. After stirring the solution for 60 minutes, it was kept in an oven at 180 °C for 3 days until precipitations of ZnFe2O4 were obtained. The insoluble content was filtered and washed with water to remove any unreacted impurities and then dried in an oven at 60 °C. Finally, the material was calcined at 500 °C for 3 hours.
To synthesize the β-CD-E-T/ZnFe2O4 nanocomposite, an appropriate amount of β-CD-E-T polymer (90% by mass) was dissolved in a mixture of tetrahydrofuran and water (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio). The solution was stirred at 60 °C until the β-CD-E-T was completely dissolved. Thereafter, ZnFe2O4 (10% by mass) was added to β-CD-E-T solution and subjected to ultrasound irradiation for 20 minutes, followed by stirring for 2 hours. Finally, the solvents were evaporated in an oven to obtain the β-CD-E-T/ZnFe2O4 nanocomposite.
1 ratio). The solution was stirred at 60 °C until the β-CD-E-T was completely dissolved. Thereafter, ZnFe2O4 (10% by mass) was added to β-CD-E-T solution and subjected to ultrasound irradiation for 20 minutes, followed by stirring for 2 hours. Finally, the solvents were evaporated in an oven to obtain the β-CD-E-T/ZnFe2O4 nanocomposite.
2.2. Characterization
FTIR spectroscopy was used to study structural changes in β-CD-E-T/ZnFe2O4 to confirm the formation of interaction between BPA and β-CD-E-T/ZnFe2O4. FTIR analysis was carried out between 400–4000 cm−1 wavenumber range (PerkinElmer, USA) using transmittance mode against KBr reference. For FTIR measurement, the samples were pre-heated in an oven to eliminate moisture. Specifically, 1 mg of the sample was thoroughly mixed with 190 mg of KBr using a mortar and pestle, resulting in a fine powder. Subsequently, the powder was compressed into pellets using a manual hydraulic press. Nova Nano FE-SEM 450 (FEI) was used to confirm the nanosize of ZnFe2O4 and reinforcement of ZnFe2O4 in the β-CD-E-T polymer matrix. Nova Touch LX2; Quantachrome Instruments was used to calculate the pore size, pore volume, and surface area of β-CD-E-T/ZnFe2O4. The sample was degassed at 150 °C for 5 hours before N2 physisorption analysis. To study the effect of ZnFe2O4 reinforcement on the thermal stability of β-CD-E-T polymer, the TG-DTG curves of the sample were obtained using 5000/2960 STA; TA instruments under 150 mL min−1 N2 gas flow between 30–600 °C in an alumina pan at 5, 10, 15, and 20 °C min−1 heating rates.
2.3. Adsorption and regeneration studies
The concentration of BPA before and after adsorption was monitored using SHIMADZU TCC-240 A UV-vis spectrophotometer taking water as a reference solvent. The calibration curve of BPA was generated by plotting the absorbance of BPA at λmax = 276 nm against concentration (3–30 mg L−1) (Fig. S1†). The before and after adsorption concentration of BPA was used to calculate the removal efficiency (R%; eqn (1)) and adsorption capacity (qe; eqn (2)) of β-CD-E-T/ZnFe2O4.| |
 | (1) |
| |
 | (2) |
where C0, Ce, V, and m represent initial BPA concentration (mg L−1), final BPA concentration (mg L−1), the volume of BPA solution (L), and mass of β-CD-E-T/ZnFe2O4 adsorbent (g), respectively.
Adsorption isotherms can provide information on the nature of the adsorption process (i.e., physical, or chemical adsorption), as well as the types of interactions that occur between the BPA and β-CD-E-T/ZnFe2O4 adsorbent. There are a total of four Langmuir linear equations given in the ESI File (eqn (S1)–(S4)).† Adsorption models like Freundlich (eqn (3)), Tempkin (eqn (4)), Langmuir (eqn (5)), and Dubinin–Radushkevich (eqn (6)) were tested to obtain isotherm parameters of adsorption of BPA on β-CD-E-T/ZnFe2O4. Moreover, the separation factor (RL), Polanyi potential (ε), and energy (E) of the adsorption were calculated using eqn (7), (8), and (9), respectively.
| |
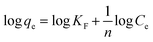 | (3) |
| |
 | (5) |
| |
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe = ln qe = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qm − δε2 qm − δε2
| (6) |
| |
 | (7) |
| |
 | (8) |
| |
 | (9) |
In this context, the symbols
R,
T,
qm,
KL,
KF,
n,
bT,
AT,
δ, and
Cs are employed to respectively denote the universal gas constant (8.314 J mol
−1 K
−1), the temperature during adsorption, Langmuir maximum adsorption capacity (mg g
−1), Langmuir constant, Freundlich constant, heterogeneity factor, Temkin constant, Temkin equilibrium binding constant (L g
−1), constant related to the energy of the adsorption (
eqn (9)), and saturation concentration of BPA. The value of
bT was calculated using the relation
B1 =
RT/
bT.
To describe the kinetics of BPA adsorption on β-CD-E-T/ZnFe2O4, we employed four different models: pseudo-second-order (eqn (10)), pseudo-first-order (eqn (11)), Elovich (eqn (12)), and intraparticle diffusion (eqn (13)). We then determined the best fitting model based on the experimental data to get information about the kinetics of the adsorption of BPA on β-CD-E-T/ZnFe2O4.
| |
 | (10) |
| |
 | (11) |
| |
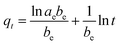 | (12) |
where
qt,
kp,
t,
k1,
k2, and
ae;
be represents adsorption capacity at ‘
t’ time (min), intraparticle diffusion constant, time (min), pseudo-first-order constant, pseudo second order constant, and Elovich constants, respectively. The width of the boundary layer and its impact on the rate of adsorption can be elucidated using the variable ‘
C’.
Moreover, the effect of temperature was used to investigate the nature of the adsorption of BPA on β-CD-E-T/ZnFe2O4. The slope and interception of the plot of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ke against 1000/T (K−1) were used to calculate thermodynamic parameters like enthalpy change (ΔH°) and entropy change (ΔS°) using eqn (14). The value of ΔH° and ΔS° was used to calculate free energy change (ΔG°) using eqn (15).
ke against 1000/T (K−1) were used to calculate thermodynamic parameters like enthalpy change (ΔH°) and entropy change (ΔS°) using eqn (14). The value of ΔH° and ΔS° was used to calculate free energy change (ΔG°) using eqn (15).
| |
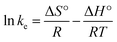 | (14) |
where
ke =
qe/
Ce.
To regenerate the adsorbent, the spent adsorbent, BPA-loaded β-CD-E-T/ZnFe2O4, was washed with acetone under continuous stirring and dried in an oven at 60 °C. The regenerated β-CD-E-T/ZnFe2O4 was reused for up to five cycles to evaluate the adsorption reusability of the nanocomposites.
3. Results and discussions
3.1. Structural and surface characteristics
FTIR spectrum of β-CD-E-T and β-CD-E-T/ZnFe2O4 is given in Fig. 1. In the FTIR spectrum, a broad peak between 3000–3700 cm−1 was observed because of the large number of the hydroxyl group present in the β-CD moiety giving rise to O–H stretching vibrations. The peak between 3000–2800 cm−1 was observed because of C–H stretching vibrations. The peak at ∼2381–2291 cm−1 confirmed the presence of the nitrile group in the TFTPN moiety, thereby indicating the presence of the TFTPN fragment in the synthesized β-CD-E-T/ZnFe2O4 nanocomposite. The characteristic band at 1700–1550 cm−1 corresponds to carbohydrates (from β-CD) present in the β-CD-E-T polymer matrix. O–H and C–H bending vibrations, C–O, C–O–C, and aromatic C–C stretching vibrations give rise to peaks between 1530–1230 cm−1. Additionally, peaks corresponding to C–O–C at 1087 cm−1 and C–O/C–C stretching vibrations at 1041 cm−1 were observed in the FTIR spectrum of β-CD-E-T/ZnFe2O4. Peaks below 1000 cm−1 were complex and could not be accurately assigned, but were thought to arise from various bending vibrations in the glucopyranose moiety (from β-CD) and ZnFe2O4.36,37
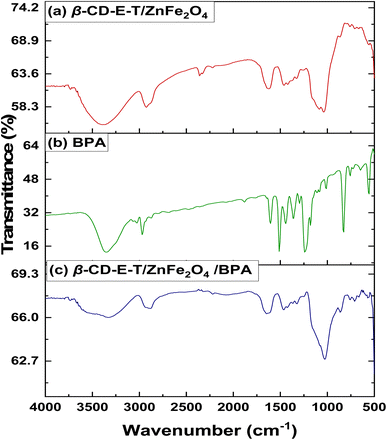 |
| | Fig. 1 FTIR spectrum of (a) β-CD-E-T/ZnFe2O4, (b) BPA, and (c) β-CD-E-T/ZnFe2O4 after BPA adsorption. | |
To investigate potential interactions between β-CD-E-T/ZnFe2O4 and BPA, the FTIR spectra of β-CD-E-T/ZnFe2O4 and β-CD-E-T/ZnFe2O4 loaded with BPA (Fig. 1b) were analyzed. The FTIR spectrum of BPA exhibited bands that were consistent with prior findings in the literature.38 There were no major changes in the FTIR spectrum of β-CD-E-T/ZnFe2O4 before and after BPA adsorption. Most peaks in β-CD-E-T/ZnFe2O4 maintained the same position and width before and after BPA adsorption. However, the –O–H stretching vibration band between 3700–3000 cm−1 in β-CD-E-T/ZnFe2O4 was broadened after BPA adsorption. A similar peak broadening for the –OH group (3700–3000 cm−1) was observed in BPA-adsorbed covalent organic frameworks.19 According to previous publications, –OH was crucial in the formation of hydrogen bonds between adsorbent and BPA, which promoted the adsorption of organic pollutants.19,39,40 This suggested that –OH probably had a significant impact on the adsorption procedure. Therefore, hydrogen bonding may have played a significant role in the adsorption of BPA onto β-CD-E-T/ZnFe2O4. The peak corresponding to nitrile stretching vibration (∼2381–2291 cm−1) was eliminated in BPA-loaded β-CD-E-T/ZnFe2O4, indicating a strong interaction between BPA and β-CD-E-T/ZnFe2O4 nanocomposite. Moreover, BPA is a diphenylmethane derivative with a π–electron cloud. This allows the π–π interactions between BPA and TFTPN moiety from β-CD-E-T/ZnFe2O4 nanocomposite. Additionally, the hydroxyl groups in BPA make the electron-rich and polar areas more prominent, resulting in enhanced π–π interaction between BPA and β-CD-E-T/ZnFe2O4.40 The observed π–π interaction could have been responsible for the reduction in the peak within the range of ∼2381–2291 cm−1. Additionally, the peaks at 1087 and 1041 cm−1 in β-CD-E-T/ZnFe2O4 shifted to lower wavenumber values (i.e., 1080 and 1034 cm−1) suggesting possible interactions between BPA and glucopyranose fragment (C–O–C/C–O) of β-CD. However, the hydrophobic/host guest interactions between β-CD from β-CD-E-T/ZnFe2O4 and BPA is also possible.41,42
β-CD-E-T polymer (Fig. 2a) exhibited an irregular morphology which could be attributed to different arrangements of TFTPN, ECH, and β-CD during the polymerization. The polymer appeared as a micron-sized material. The SEM image of β-CD-E-T polymer showed both low and high sphericity angular grain shapes. On the other hand, ZnFe2O4 (Fig. 2b) was observed to form aggregates of particles, giving the appearance of a micron-sized particle. Although the SEM image of ZnFe2O4 revealed that it was agglomerated, it is worth noting that nano-sized particles tend to agglomerate due to surface attractive interactions. The presence of smaller spherical particles within the aggregate indicated that the size of ZnFe2O4 particles was ∼30–50 nm. Bright, nano-sized spherical spots were observed on micron-sized granules (Fig. 2c; 200 μm scale), suggesting successful reinforcement of nano-sized ZnFe2O4 in the β-CD-E-T polymer matrix. The enlarged image confirmed the reinforcement of ZnFe2O4 in the polymer matrix.
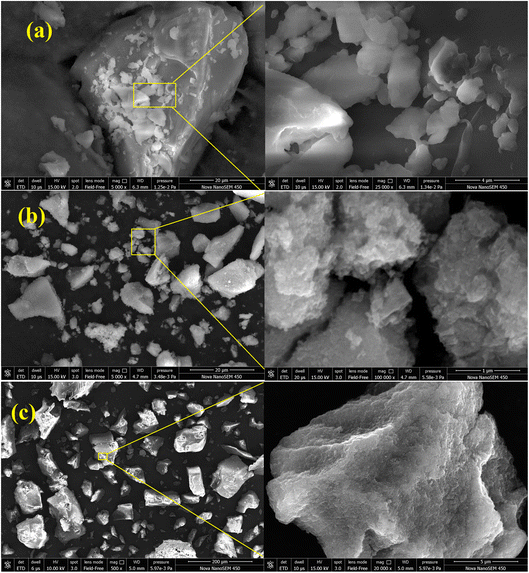 |
| | Fig. 2 SEM images of (a) β-CD-E-T, (b) ZnFe2O4, and (c) β-CD-E-T/ZnFe2O4. | |
From BET and Barrett–Joyner–Halenda (BJH) analysis the surface area, pore volume, and pore size of β-CD-E-T/ZnFe2O4 were found to be 6.97 m2 g−1, 0.0164 cm3 g−1, and 1.70 nm, respectively.36 The BJH pore size distribution of β-CD-E-T/ZnFe2O4 is given in Fig. S2.† Depending on pore size, the material can be classified as (i) microporous (pore size < 2 nm), (ii) mesoporous (2 nm < pore size < 50 nm), and (iii) macroporous (pore size > 50 nm).43 However, from BJH pore size distribution, it was found that both pore size <2 nm and pore size between 2–14 nm were present in the β-CD-E-T/ZnFe2O4 nanocomposite. Despite the presence of pores, β-CD-E-T/ZnFe2O4 has a low specific surface area than other porous materials. For example, Li et al.,44 reported that β-CD-Decafluorobiphenyl based polymer show micropores between 1.1–1.7 nm with a high specific surface area of 261.1 m2 g−1. However, the specific surface area of β-CD-E-T/ZnFe2O4 was higher than that reported for β-CD polymer containing ECH and TFTPN (0.31 m2 g−1).26 The wide number of pores and moderate surface area can allow a better interaction between BPA and β-CD-E-T/ZnFe2O4, leading to significant adsorption.
3.2. Thermal studies
The thermal stability of β-CD-E-T and β-CD-E-T/ZnFe2O4 was studied at four heating rates and the corresponding TG-DTG curves are provided in Fig. 3(a–d). Two decomposition steps were observed in the TG curve of β-CD-E-T and β-CD-E-T/ZnFe2O4. The TG curve of both β-CD-E-T (as shown in Fig. 3a) and β-CD-E-T/ZnFe2O4 (as illustrated in Fig. 3c) indicated a substantial mass loss occurring below 230 °C. This mass loss can be attributed to the loss of moisture and volatile content from the surfaces of β-CD-E-T and β-CD-E-T/ZnFe2O4. The first decomposition curve was a common occurrence in the TG curve of β-CD.45,46 The second step, which exhibited a high mass loss, was identified as the main decomposition step and was attributed to the decomposition of glucopyranose units present in β-CD. The second decomposition step for both β-CD-E-T and β-CD-E-T/ZnFe2O4 occurred between 200–400 °C, followed by a slow, gradual mass loss up to 600 °C. The first decomposition of β-CD-E-T occurred between 30–220 °C, resulting in a mass loss of 15%. The primary decomposition of β-CD-E-T took place between 221–370 °C, exhibiting a mass loss of 55%. Subsequently, an additional mass loss of 12% was observed up to a temperature of 600 °C. Upon subjecting β-CD-E-T to a higher heating rate, both the TG and DTG curves (as depicted in Fig. 3a and b) shifted towards higher temperatures. The mass loss observed for the first and second decomposition step of β-CD-E-T at a heating rate of 10 °C min−1 was 10% and 50%, respectively. At a heating rate of 15 °C min−1, mass losses of 6% and 55% were observed for the first and second decomposition steps, respectively. Similarly, at a heating rate of 20 °C min−1, mass losses of 7% and 55% were observed for the first and second decomposition steps.
 |
| | Fig. 3 (a) TG curve of β-CD-E-T, (b) DTG curve of β-CD-E-T, (c) TG curve of β-CD-E-T/ZnFe2O4, and (d) DTG curve of β-CD-E-T/ZnFe2O4. | |
The thermal decomposition of pristine β-CD-E-T and β-CD-E-T/ZnFe2O4 was compared, and it was observed that the latter occurred at a slightly lower temperature. As previously discussed, the decomposition pattern of β-CD-E-T/ZnFe2O4 was like that of β-CD-E-T, as ZnFe2O4 is a stable compound and does not undergo additional decomposition. The mass loss in the second decomposition step of β-CD-E-T/ZnFe2O4 was found to be 7–9% less than that of β-CD-E-T polymer at heating rates of 10, 15, and 20 °C min−1, which agreed with the ZnFe2O4 reinforcement content. However, a slightly higher mass loss was observed at a heating rate of 5 °C min−1. The peak temperature of the derivative thermogravimetric (DTG) curves of β-CD-E-T/ZnFe2O4 (Fig. 3d) was found to shift to a lower temperature than β-CD-E-T by 5, 5, 6, and 7 K at heating rates of 5, 10, 15, and 20 °C min−1, respectively. This observation indicates that the addition of nano ZnFe2O4 destabilized the β-CD-E-T matrix. Table 1 provides the peak decomposition temperature of the DTG curves of β-CD-E-T and β-CD-E-T/ZnFe2O4 at the four heating rates. It was observed that β-CD-E-T/ZnFe2O4 nanocomposite decomposed at a lower temperature than β-CD-E-T at all heating rates, suggesting that the reinforcement of ZnFe2O4 in β-CD-E-T resulted in matrix destabilization.
Table 1 The peak decomposition temperature of β-CD-E-T and β-CD-E-T/ZnFe2O4 at 5, 10, 15, and 20 °C min−1 heating rates
| Sample |
DTG peak decomposition temperature (K) at four heating rates |
| 5 K min−1 |
10 K min−1 |
15 K min−1 |
20 K min−1 |
| β-CD-E-T |
606 |
617 |
623 |
629 |
| β-CD-E-T/ZnFe2O4 |
601 |
612 |
617 |
622 |
However, there are non-isothermal isoconversion methods are available to confirm the thermal degradation of β-CD-E-T and β-CD-E-T/ZnFe2O4 from the activation energy of the decomposition process. To study the thermal degradation, the activation energy of both β-CD-E-T and β-CD-E-T/ZnFe2O4 nanocomposite was calculated using two non-isothermal isoconversion methods namely Flynn–Wall–Ozawa (FWO; eqn (16)) and Kissinger–Akahira–Sunose (KAS; eqn (17)) and two iterative methods (it-FWO; eqn (18) and it-KAS; eqn (19)). The DTG curve of β-CD-E-T at 5 °C min−1 heating rate was deconvoluted before the calculations.
| |
 | (16) |
| |
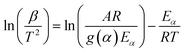 | (17) |
| |
 | (18) |
| |
 | (19) |
where
α,
β,
A,
Eα, and
g(
α) represent the extent of conversion, heating rate (°C min
−1), pre-exponential factor, activation energy at a given extent of conversion (kJ mol
−1), and mathematical model, respectively. The value of
α,
H(x), and
h(x) was calculated using
eqn (20),
(21), and
(22), respectively. The extent of conversion at different heating rates is reported in Fig. S3.
†| |
 | (20) |
| |
 | (21) |
| |
 | (22) |
where,
m0,
mt, and
m∞ represents initial mass, mass at a given time/temperature, and mass at end of the decomposition step, respectively. The value of previously calculated activation energy (using the KAS/FWO method) was used to determine the value of
x (
x =
Eα/
RT) and iteration was used until the change in the
Eα was minimal (Δ
Eα < 0.001 kJ mol
−1). The value of
Eα was calculated from the slope of the plot of ln
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β
β, ln
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β
β/
T2, ln
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β
β/
H(x), and ln
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β
β/
h(x)T2 against 1000/
T. All fitted data were calculated with a resolution of
α = 0.025 across the range of
α = 0.1–0.9. The plots of KAS and FWO for β-CD-E-T polymer and β-CD-E-T/ZnFe
2O
4 nanocomposite are depicted in Fig. S4.
†
The variation in the activation energy of the decomposition of β-CD-E-T and β-CD-E-T/ZnFe2O4 with the extent of conversion along with standard error is given in Fig. 4. Although the thermal decomposition temperature of β-CD-E-T/ZnFe2O4 was lower than that of β-CD-E-T polymer, the activation energy of β-CD-E-T/ZnFe2O4 was found to be higher than that of β-CD-E-T polymer at all extents of conversions between α = 0.1–0.9. Regardless of the isoconversion method used, the change in activation energy was the same, ascending with reaction progress. This suggests that a single mechanism exists for the thermal decomposition of both pristine β-CD-E-T and β-CD-E-T/ZnFe2O4 nanocomposite. The average activation energy of β-CD-E-T and its composite with ZnFe2O4 was calculated using four methods, and the results are presented in Fig. S5.† The average Eα for β-CD-E-T using FWO, KAS, it-FWO, and it-KAS was found to be 180 ± 9, 179 ± 9, 171 ± 9, and 179 ± 9 kJ mol−1, respectively. The incorporation of nanosize ZnFe2O4 into the β-CD-E-T polymer matrix resulted in increased activation energy. The average Eα for β-CD-E-T using FWO, KAS, it-FWO, and it-KAS was found to be 218 ± 14, 219 ± 15, 210 ± 14, and 219 ± 15 kJ mol−1, respectively. The value of Eα suggested that the thermal degradation of β-CD-E-T/ZnFe2O4 nanocomposite was harder compared to β-CD-E-T polymer, as it possessed a higher activation energy barrier than β-CD-E-T polymer. The results of the thermo-kinetic investigations suggest that β-CD-E-T/ZnFe2O4 was less prone to thermal degradation than β-CD-E-T polymer. ZnFe2O4 may have significantly interacted with the functional groups of β-CD-E-T polymer, leading to the formation of a new β-CD-E-T/ZnFe2O4 phase with delayed thermal degradation. ZnFe2O4 may have decreased the mobility of the β-CD-E-T polymer chains, physically interacted with the surface of β-CD-E-T, and generated a highly stable zone that aided in decreasing the activation energy of thermal decomposition.47
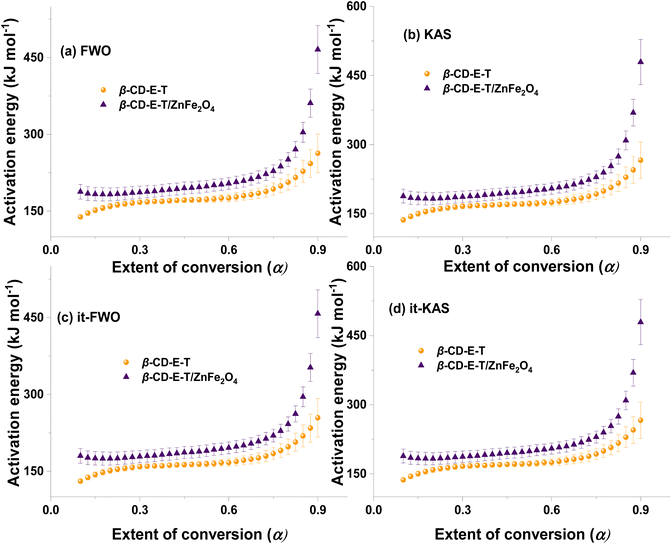 |
| | Fig. 4 Variation in the activation energy of the thermal decomposition of β-CD-E-T and β-CD-E-T/ZnFe2O4 calculated using (a) FWO, (b) KAS, (c) it-FWO, and (d) it-KAS with the extent of conversion. | |
3.3. Effect of different parameters
The pH level plays a crucial role in determining the adsorption of contaminants onto the adsorbent surface. Variations in pH have a direct impact on the surface charge of the adsorbent, which in turn influences the ionic attraction and repulsion forces between the adsorbent and adsorbate, thereby significantly contributing to the adsorption process. To investigate the impact of pH on the adsorption process, several parameters including adsorbent dosage, contact time, the volume of BPA, temperature, and concentration were kept constant, while only the pH levels were varied (pH values of 3, 4, 6, 8, and 10 were used).
The investigation of various pH levels (as shown in Fig. 5a) revealed that the β-CD-E-T/ZnFe2O4 nanocomposite displayed a negative surface charge across all conditions (pH > 2.44; negative charge).36 It is worth noting that the pKa range of BPA was estimated to be between 9.6 and 10.2.48,49 When the pH value exceeded the pKa of BPA, the hydroxyl groups of BPA underwent deprotonation, resulting in a predominantly negative charge. Conversely, at pH levels below the pKa of BPA, the compound did not exhibit any charge, either positive or negative, and remained neutral. Effect of pH (BPA solution volume = 20 mL; T = 28 °C; β-CD-E-T/ZnFe2O4 dose = 10 mg; BPA solution concentration = 32 mg L−1; t = 30 min) on the removal of BPA from the aqueous solution is depicted in Fig. 5a. Initially, there were no significant changes in the adsorption behavior of BPA onto the β-CD-E-T/ZnFe2O4 nanocomposite observed up to a pH of 8, except for a 7% drop in removal percentage observed at pH 4. The adsorption of BPA at pH values of 3, 6, and 8 was approximately 87%, corresponding to a qe value of around 56 mg g−1. However, a substantial decrease in BPA adsorption was observed at pH 10 compared to pH values between 3 and 8. Specifically, the β-CD-E-T/ZnFe2O4 nanocomposite exhibited only ∼55% BPA adsorption (qe of ∼35 mg g−1) at pH 10. The observed decrease in the removal percentage of BPA at pH 10 can be plausibly explained by the negatively charged deprotonated BPA species. Due to the repulsive forces between the negative surface of β-CD-E-T/ZnFe2O4 and the negative charge of BPA, it becomes more difficult for the deprotonated BPA molecules to approach and interact with the adsorbent, leading to a reduced removal efficiency.26,39,40,50,51 This repulsive force is absent between the neutral BPA molecules and β-CD-E-T/ZnFe2O4 adsorbent at pH levels below 10. Since the maximum adsorption of BPA occurred when it was not charged, it is reasonable to infer that the interaction between BPA and β-CD-E-T/ZnFe2O4 was not primarily driven by ionic attraction.52
 |
| | Fig. 5 Effect of (a) pH, (b) ionic salt concentration, (c) contact time, and (d) temperature on the adsorption of BPA by β-CD-E-T/ZnFe2O4 from aqueous solution. | |
Moreover, the effect of ionic salt on the removal of BPA was tested (Fig. 5b). The removal percentage of BPA was studied in the presence of 0.1, 0.2, 0.3, and 0.4 mol L−1 sodium chloride (NaCl) solution and the rest of the parameters were unchanged during the measurement (BPA solution volume = 10 mL; T = 28 °C; β-CD-E-T/ZnFe2O4 dose = 5 mg; BPA solution concentration = 32 mg L−1; t = 30 min). The adsorption of BPA onto β-CD-E-T/ZnFe2O4 was found to be higher in the absence of any ionic salt i.e., NaCl, which was attributed to the strong interaction between the β-CD-E-T/ZnFe2O4 surface and BPA due to availability of more adsorption sites. However, as the ionic concentration of NaCl was increased, the adsorption of BPA decreased. This can be plausibly explained by the fact that as the concentration of ionic salt increases, Na1+, and Cl1− ions can occupy the active adsorption sites and pores on β-CD-E-T/ZnFe2O4 nanocomposite, resulting in fewer active sites available for the adsorption of BPA, and hence, a decreased adsorption percentage of BPA was observed. Nevertheless, it is noteworthy that the decrease in BPA removal was relatively minor, with the removal percentage dropping from 93% (59.6 mg g−1) in the absence of NaCl to approximately 82% (52.2 mg g−1) in the presence of 0.4 mol L−1 NaCl. These results indicate that BPA can still be effectively removed even in the presence of Na1+ and Cl1− ions, which are often present in wastewater. Furthermore, the outcomes of the ionic salt effect experiments suggest that the absorption of BPA by β-CD-E-T/ZnFe2O4 was not driven by electrostatic attraction, which is consistent with the findings obtained from the pH experiments. Had the electrostatic interaction between β-CD-E-T/ZnFe2O4 and BPA been substantial, a marked reduction in the adsorption capacity would have been observed. For example, in our prior research,36 we observed a substantial decline in the removal of malachite green dye as the sodium chloride concentration increased, which was attributed to the electrostatic interaction between the adsorbent and malachite green. No such decline was observed in the case of BPA adsorption onto β-CD-E-T/ZnFe2O4. Hence, electrostatic interaction between BPA and β-CD-E-T/ZnFe2O4 nanocomposite was insignificant.
The effect of β-CD-E-T/ZnFe2O4 dose and BPA dose was investigated, and the results are shown in Fig. S6 (ESI File).† The β-CD-E-T/ZnFe2O4 dose was varied between 5 and 100 mg while keeping other parameters constant (BPA solution volume = 20 mL; T = 28 °C; BPA solution concentration = 32 mg L−1; t = 30 min; pH = 6; β-CD-E-T/ZnFe2O4 dose = varied). Similarly, to study the effect of BPA dose on the adsorption of BPA on β-CD-E-T/ZnFe2O4, the BPA dose was varied between 0.2 and 1.6 mg while keeping other parameters constant (T = 28 °C; t = 30 min; pH = 6; β-CD-E-T/ZnFe2O4 dose = 5 mg; BPA dose = varied). At low β-CD-E-T/ZnFe2O4 doses (i.e., 5 mg and 10 mg), nanocomposite exhibited significant adsorption for BPA (>85%). However, thereafter, the removal % of BPA drops significantly with increasing β-CD-E-T/ZnFe2O4 dose. This could be attributed to the fact that at low β-CD-E-T/ZnFe2O4 doses, the adsorbent particles are not agglomerated, resulting in more interaction between the active surface of β-CD-E-T/ZnFe2O4 and a larger number of pores being available for adsorption of BPA. However, at higher β-CD-E-T/ZnFe2O4 doses, a greater number of moles of β-CD-E-T/ZnFe2O4 are present in the same volume of BPA solution, which can lead to increased collision/interaction between β-CD-E-T/ZnFe2O4 adsorbent–adsorbent molecules. This may result in the formation of agglomerates of β-CD-E-T/ZnFe2O4, leading to a decrease in available surface area and pores for BPA-β-CD-E-T/ZnFe2O4 interaction in the solution, thereby decreasing the overall BPA removal percentage at higher β-CD-E-T/ZnFe2O4 loadings. Therefore, since the BPA removal efficiency was almost the same for both 5 mg and 10 mg β-CD-E-T/ZnFe2O4 loading, the lower loading of 5 mg β-CD-E-T/ZnFe2O4 was found to be the most appropriate dose for β-CD-E-T/ZnFe2O4.
The R% of BPA was decreased by increasing the BPA dose in the solution from 0.2 to 1.2 mg (Fig. S6b†). However, the adsorption capacity of β-CD-E-T/ZnFe2O4 was continuously increased between 0.2 and 1.2 mg BPA dose. The adsorption capacity of β-CD-E-T/ZnFe2O4 for BPA varied from 27.8 mg g−1 at 0.2 mg BPA dose to 204.6 mg g−1 at 1.2 BPA dose. After 1.2 mg BPA content, a decline in the adsorption capacity was observed. The removal % of BPA remained >85% up to 0.64 mg BPA dose. The decrease in the adsorption capacity observed at high BPA concentrations was attributed to the saturation of active adsorption sites available on β-CD-E-T/ZnFe2O4 for BPA adsorption.
The effect of contact time on the R% of BPA is depicted in Fig. 5c. The effect of contact time was investigated under defined parameters (BPA solution volume = 20 mL; T = 28 °C; BPA solution concentration = 32 mg L−1; β-CD-E-T/ZnFe2O4 dose = 5 mg; t = 0–180 minutes; pH = 6). Very rapid adsorption of BPA onto the β-CD-E-T/ZnFe2O4 nanocomposite surface was observed, with over 85% BPA adsorbed within 5 minutes. This can be attributed to the higher availability of BPA molecules, resulting in more collisions between the adsorbent–adsorbate and adsorbate–adsorbate molecules. At pH = 6, the BPA molecules could easily approach the surface of the β-CD-E-T/ZnFe2O4 nanocomposite because of their neutral charge and get adsorbed, leading to an increase in BPA concentration on the nanocomposite's surface and a decrease in freely movable BPA molecules in the aqueous solution. The adsorbed BPA molecules occupy active sites on the β-CD-E-T/ZnFe2O4's surface, reducing the availability of active sites for approaching BPA molecules in the solution. As a result, BPA adsorption on β-CD-E-T/ZnFe2O4 slowed down significantly after 5 minutes, with only a 4% increase in removal observed between 5 and 30 minutes. Equilibrium was established thereafter, and no significant variation in BPA removal percentage was observed up to 180 minutes. Consequently, a contact time of 30 minutes was selected for the subsequent experimental measurements. The contact time data was then fitted using four kinetic models to obtain a more comprehensive understanding of the adsorption kinetics of BPA onto the β-CD-E-T/ZnFe2O4 nanocomposite.
To calculate the thermodynamic parameters concerning the adsorption process, the effect of temperature on the adsorption of BPA on β-CD-E-T/ZnFe2O4 adsorbent was investigated under standard conditions (BPA solution volume = 20 mL; T = varied; BPA solution concentration = 32 mg L−1; β-CD-E-T/ZnFe2O4 dose = 5 mg; time = 30 minutes; pH = 6). Fig. 5d illustrates the changes in the BPA R% at three different temperatures. It was observed that the BPA R% decreased with an increase in temperature, indicating an exothermic nature of the adsorption process. The decline in the BPA removal percentage with increasing temperature was attributed to a greater number of BPA molecules being desorbed from the β-CD-E-T/ZnFe2O4's surface than those being adsorbed onto it.53 A decrease in BPA removal efficiency with rising temperatures was noted in prior investigations examining the efficacy of covalent organic frameworks and mesoporous-activated carbon for BPA removal.19,53 This behavior is typically observed in physical adsorption processes, where the desorption rate increases with temperature. It can be inferred that the adsorption of BPA onto β-CD-E-T/ZnFe2O4 nanocomposite was primarily governed by physical interactions. The thermodynamic parameters like ΔS°, ΔH°, and ΔG° were calculated by fitting experimental data to get more insight into the adsorption nature.
3.4. Kinetics, isotherm, and thermodynamic studies
The kinetics of the adsorption process was used to define the rate of BPA adsorption by β-CD-E-T/ZnFe2O4 adsorbent, and various kinetic models were employed to analyze experimental data to explain the adsorption process. A total of four kinetic models were used to get insight into the kinetic process of adsorption. The kinetic plots are given in Fig. 6(a–d) and the corresponding results are reported in Table 2. Among the four kinetic models, the pseudo-second-order kinetic model was the best-suited (correlation coefficient R2 = 0.999) model to describe the kinetics of the uptake of BPA by β-CD-E-T/ZnFe2O4. A very good equilibrium adsorption capacity of 121 ± 1 mg g−1 was obtained using a pseudo-second-order fit, which was closer to the experimental value (122 ± 1 mg g−1). The correlation coefficient for BPA adsorption by β-CD-E-T/ZnFe2O4 was poor for the intraparticle diffusion model and Elovich model suggesting that the intraparticle diffusion was not rate-determining the adsorption process. The high value of k2 suggested a very fast adsorption of BPA onto β-CD-E-T/ZnFe2O4.
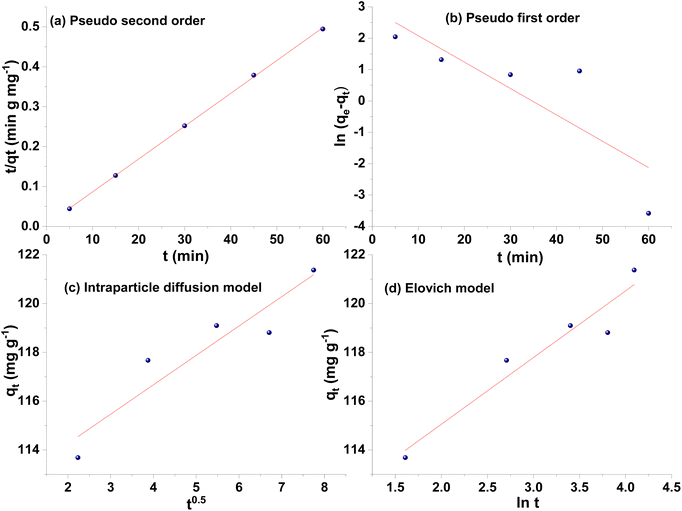 |
| | Fig. 6 Adsorption kinetics plots of BPA adsorption onto β-CD-E-T/ZnFe2O4: (a) pseudo-second-order, (b) pseudo-first-order, (c) intraparticle diffusion, and (d) Elovich plot. | |
Table 2 Kinetics parameters of adsorption of BPA onto β-CD-E-T/ZnFe2O4 nanocomposite
| Kinetic model |
Parameter |
BPA |
| Pseudo-first order |
k1 (min−1) |
0.194 ± 0.073 |
| qe,cal (mg g−1) |
18.5 ± 12.8 |
| R2 |
0.701 |
| Pseudo-second order |
qe,exp |
122 ± 1 |
| k2 (g mg−1 min−1) |
0.0157 ± 0.0062 |
| qe,cal (mg g−1) |
121 ± 1 |
| R2 |
0.999 |
| Intraparticle diffusion |
kp (mg g−1 min−0.5) |
1.20 ± 0.26 |
| C (mg g−1) |
112 ± 1 |
| R2 |
0.881 |
| Elovich |
a (mg g−1 min−1) |
24.7 ± 4.3 |
| b (g mg−1) |
(7.07 ± 2.9) × 1017 |
| R2 |
0.926 |
The knowledge of adsorption isotherm is critical for planning and optimizing BPA adsorption on β-CD-E-T/ZnFe2O4 nanocomposite, as well as understanding the underlying adsorption mechanism (i.e., multilayer, monolayer, chemosorption, physisorption, favourability of the BPA adsorption).54 Adsorption isotherm models were used among the four isotherm models, all models were showing good linear fit (Fig. 7a–d; R2 = 0.967–0.994). Hence, the adsorption of BPA could have been due to many possible mechanisms. A high Langmuir maximum adsorption capacity of 270 ± 36 mg g−1 (Table 3) was obtained for the removal of BPA using β-CD-E-T/ZnFe2O4 nanocomposite. The Freundlich isotherm proved to be the most suitable model (R2 = 0.994) for describing the adsorption process, indicating that the adsorption of BPA onto the heterogeneous β-CD-E-T/ZnFe2O4 surface occurred via multilayer adsorption. The high value of Freundlich constant n (1.63 ± 0.07) suggested the adsorption process was highly favorable (n > 1).29 The obtained factor n value provided further support for the high Langmuir adsorption capacity observed. Additionally, the calculated RL value of 0.155 (0 < RL < 1), indicated favorable adsorption of BPA onto the β-CD-E-T/ZnFe2O4 surface. Based on the results obtained from the Dubinin–Radushkevich model, the calculated adsorption energy value of 5.87 ± 0.19 kJ mol−1 was found to be lower than the threshold of 8 kJ mol−1, suggesting that the adsorption of BPA onto the β-CD-E-T/ZnFe2O4 nanocomposite was primarily a physical process.54
 |
| | Fig. 7 Adsorption isotherms plots of BPA adsorption onto β-CD-E-T/ZnFe2O4: (a) Langmuir, (b) Freundlich, (c) Temkin, and (d) Dubinin–Radushkevich plot. | |
Table 3 Isotherms parameters of the adsorption of BPA onto β-CD-E-T/ZnFe2O4 nanocomposite
| Model |
Parameter |
BPA |
| Langmuir |
qm,exp (mg g−1) |
205 ± 2 |
| qm (mg g−1) |
270 ± 36 |
| KL (L mg−1) |
0.171 ± 0.012 |
| R2 |
0.984 |
| Freundlich |
KF (mg g−1 (L mg−1)1/n) |
44.1 ± 2.0 |
| 1/n |
0.613 ± 0.028 |
| R2 |
0.994 |
| Temkin |
AT (L g−1) |
1.24 ± 0.20 |
| bT (kJ mol−1) |
35.4 ± 3.4 |
| R2 |
0.967 |
| Dubinin–Radushkevich |
qm (mol g−1) |
315 ± 23 |
| δ (mol2 J2) |
(1.45 ± 0.10) × 10−8 |
| E (kJ mol−1) |
5.87 ± 0.19 |
| R2 |
0.987 |
The analysis of the temperature effects revealed that the adsorption of BPA onto the β-CD-E-T/ZnFe2O4 adsorbent was characterized by an exothermic nature. To confirm this ΔS° and ΔH° were calculated from the interception and slope of the plot given in Fig. S7† (eqn (12)), respectively. From the calculation of the thermodynamic parameters, it was found that the value of ΔH° and ΔS° for the adsorption of BPA onto the β-CD-E-T/ZnFe2O4 was −77.1 ± 16.0 kJ mol−1 and −0.213 ± 0.051 kJ mol−1 K−1, respectively. 0 > ΔH° (negative) confirms the exothermic nature of the adsorption process. The negative value of ΔS° (0 > ΔS°) indicated a reduction in randomness or an increase in the orderliness of the system after BPA adsorption onto the β-CD-E-T/ZnFe2O4 adsorbent. The calculated values of ΔG°, ranging from −11.1 to −8.36 kJ mol−1 with an average of −9.78 kJ mol−1, were also found to be negative, indicating that the adsorption of BPA onto the β-CD-E-T/ZnFe2O4 surface occurred spontaneously without the requirement of additional energy. The high negative value ΔG° at the low temperature suggested that the adsorption of BPA was more spontaneous at low temperatures.29 Fu et al. has reported that adsorption of BPA on imine-based covalent organic frameworks was an exothermic process with ΔH° = −37.1 kJ mol−1, ΔS° = −0.109 kJ mol−1 K−1, and ΔG° = −4.24 to −1.95 kJ mol−1.19 The more negative value of ΔG° implied more favorable adsorption of BPA onto β-CD-E-T/ZnFe2O4 than imine based covalent organic frameworks, which was confirmed by the higher adsorption of BPA onto β-CD-E-T/ZnFe2O4.
3.5. Plausible adsorption mechanism
Based on the results obtained from FTIR, isotherm, kinetics, and thermodynamics analyses, it was evident that the high adsorption capacity of β-CD-E-T/ZnFe2O4 for BPA was primarily attributed to physical adsorption. Moreover, the adsorption of BPA by β-CD-E-T/ZnFe2O4 was found to be unaffected by pH levels ranging from 3–8 and the presence of ionic salts, indicating that ionic interactions between the β-CD-E-T/ZnFe2O4 adsorbent and BPA adsorbate were not the main driving force responsible for the adsorption. Instead, the adsorption of BPA onto β-CD-E-T/ZnFe2O4 was primarily driven by hydrogen bonding, π–π interaction, and host–guest/hydrophobic interactions, as suggested by the FTIR studies (Scheme 1).19,27,29 Furthermore, it is possible that the cross-linking material caused the formation of secondary cavities within the polymer network, which facilitated the sequestration of BPA molecules within these cavities. In addition, the moderate specific surface area of β-CD-E-T/ZnFe2O4 provided a greater number of available adsorption sites for the binding of BPA.27
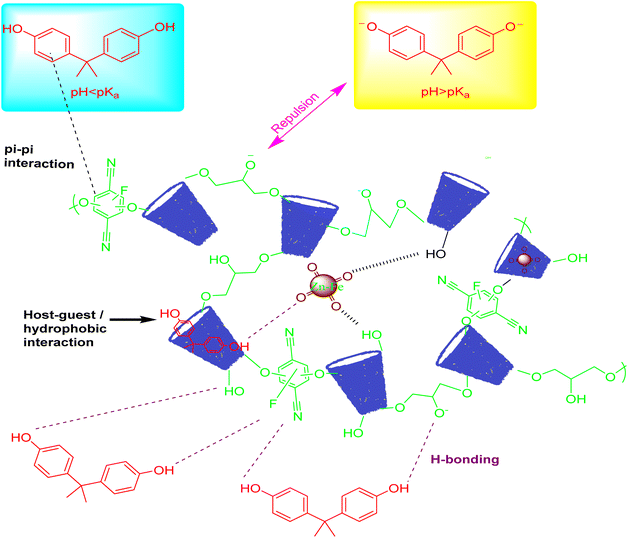 |
| | Scheme 1 Proposed interactions responsible for the adsorption of BPA onto β-CD-E-T/ZnFe2O4. | |
3.6. Regeneration performance of β-CD-E-T/ZnFe2O4 nanocomposite
The β-CD-E-T/ZnFe2O4 nanocomposite was subjected to five regeneration–reuse cycles to evaluate its stability and reusability. As shown in Fig. 8, a significant decrease in the adsorption capacity of β-CD-E-T/ZnFe2O4 for BPA was observed after each regeneration–reuse cycle. The adsorption capacity decreased by 25.6%, 49.1%, and 57.0% after the first, third, and fifth cycles, respectively, with a final adsorption capacity of only 85.6 ± 3.3 mg g−1. This reduction in adsorption capacity may be attributed to the stronger binding between BPA and β-CD-E-T/ZnFe2O4 adsorbent. The strong interaction between the adsorbent and adsorbate resulted in reduced desorption of BPA molecules, leading to a high decrement in adsorption capacity with subsequent regeneration/reuse cycles. Despite this decrease, the adsorption capacity of β-CD-E-T/ZnFe2O4 after the fifth cycle (qe > 85 mg g−1) was still higher than that reported for other β-CD-containing adsorbents, such as carboxymethyl-β-CD/Fe3O4 composite, β-CD capped graphene-magnetite nanocomposite, and polyethylenimine–polyethylene glycol-β-CD polymer.27,29,30 These findings indicate that the β-CD-E-T/ZnFe2O4 nanocomposite could be reused to a certain extent.
 |
| | Fig. 8 Adsorption capacity of β-CD-E-T/ZnFe2O4 nanocomposite adsorbent for BPA after number of regeneration reuse cycles. | |
3.7. Comparative adsorption performance
The adsorption performance of β-CD-E-T/ZnFe2O4 nanocomposite was compared with previously reported adsorbents for the removal of BPA (Table 4).13,19,26–30,39,40,50–53,55–58 As reported in Table 4, β-CD based adsorbents like carboxymethyl-β-CD polymer/Fe3O4 composite, β-CD/graphene-magnetite nanocomposite, and polyethylenimine-polyethylene glycol-β-CD polymer were not very effective for the BPA elimination from the aqueous solution.27,29,30 The β-CD based polymer and composites were having a maximum uptake of 65–139 mg BPA per ‘g’ of adsorbent used. Compared to that the β-CD-E-T/ZnFe2O4 nanocomposite synthesized in the present work exhibited a very good BPA adsorption (195–205 mg g−1 more adsorption than reported β-CD based adsorbents in Table 4).27,29,30 The reported β-CD based adsorbents exhibited very slow adsorption compared to β-CD-E-T/ZnFe2O4 nanocomposite, except β-CD-4,4′-bis(chloromethyl) biphenyl and TFTPN-ECH-β-CD polymer.28 N/S doped magnetic carbon aerogel, iron oxide/graphene aerogel, partially reduced GO, L-glutamic acid modified GO and porous aromatic framework (PAF) were reported to show a very good adsorption efficiency for BPA.40,55–58 However, very slow adsorption was observed for partially reduced GO, iron oxide/graphene aerogel and N/S doped magnetic carbon aerogel. PAF-82 exhibited a faster and high good adsorption capacity for BPA removal. PAF-82 reported by Mo et al.56 exhibited a very high adsorption performance due to its high specific surface area (1073 mg g−1) and higher porous structure than β-CD-E-T/ZnFe2O4 nanocomposite. It is important to emphasize that a high specific surface area alone does not necessarily translate into greater adsorption efficiency. Effective adsorption relies on the presence of proper interactions between the adsorbate (BPA) and the adsorbent. For instance, despite having a significantly higher specific surface area (1200 m2 g−1; ∼172 times), the activated carbon reported by Martín-Lara et al.13 exhibited lower BPA adsorption compared to the β-CD-E-T/ZnFe2O4 nanocomposite described in this study. Furthermore, Supong et al.59 also reported low adsorption capacity (qm = 15.7 mg g−1) for the activated carbon, despite its surface area being about ∼123 times greater than that of the β-CD-E-T/ZnFe2O4 adsorbent. This discrepancy suggests that specific interactions between BPA and activated carbon may be lacking, unlike those observed between BPA and the β-CD-E-T/ZnFe2O4 nanocomposite. However, it is worth noting that within adsorbent materials possessing a similar composition, a high specific surface area can indeed contribute to enhanced BPA adsorption.56,60
Table 4 Comparison of adsorption performance of various adsorbents towards BPA removal
| Adsorbent |
qm (mg g−1) |
qe (mg g−1) |
Equilibrium time (min) |
Ref. |
| NA = not available. |
| β-CD-E-T/ZnFe2O4 nanocomposite |
270 ± 36 |
122 ± 1 |
30 |
Present work |
| Zeolite-imidazolate-framework-8@mesoporous silica (SBA-15) |
135 |
123 |
2 |
52 |
| Activated carbon from hydro-char |
21.3 |
14.2 |
240 |
50 |
| Partially reduced GO |
346 |
aNA |
1080 |
55 |
| Mesoporous silica nanoparticles functionalized with hexadecyltrimethylammonium |
128 |
80.5 ± 3.7 |
60 |
51 |
| Imine-based covalent organic framework (COF-2) |
149 |
116 |
60 |
19 |
| PAF-82 |
689 |
aNA |
15 |
56 |
| Polyethylenimine-polyethylene glycol-β-CD polymer |
65.3 |
60.1 |
1140 |
30 |
| Sludge-based mesoporous activated carbon (SS-HP) |
153 |
88.8 |
60 |
53 |
| Carboxymethyl-β-CD polymer/Fe3O4 |
74.6 |
60.8 |
250 |
27 |
| L-Glutamic acid modified GO (GO-Glu) |
237 ± 40 |
aNA |
<60 |
57 |
| GO/cross-linked chitosan/magnetite (GO–CSm) |
86.2 |
81.3 |
60 |
39 |
| N/S doped magnetic carbon aerogel |
200 |
197 |
120 |
58 |
| β-CD capped graphene-magnetite nanocomposite |
66.0 |
NA |
240 |
29 |
| Iron oxide/graphene aerogel |
254 |
216 |
480 |
40 |
| β-CD-4,4′-bis(chloromethyl) biphenyl polymer |
139 |
38.0 |
10 |
28 |
| TFTPN-ECH-β-CD polymer |
118 |
21.9 |
10 |
26 |
| Activated carbon |
91.9 |
43.1 |
2880 |
13 |
| Magnetic reduced GO (MRGO-1) |
93.0 |
60.9 |
240 |
60 |
In conclusion, despite the moderate surface area and decrease in adsorption performance observed after the fifth cycle, the β-CD-E-T/ZnFe2O4 nanocomposite demonstrated a higher maximum adsorption capacity than many pristine materials reported in Table 4. This makes β-CD-E-T/ZnFe2O4 nanocomposite a better adsorbent for the faster removal of BPA from an aqueous solution.
4. Conclusions
The thermo-kinetic investigations suggested that the β-CD-E-T/ZnFe2O4 nanocomposite was less prone to thermal degradation than the β-CD-E-T polymeric matrix due to the high activation energy barrier required for its thermal degradation. The adsorption studies confirmed that β-CD-E-T/ZnFe2O4 exhibited a high affinity towards BPA (qm = 270 ± 36 mg g−1) and achieved a high equilibrium adsorption capacity within a short time of 30 minutes. However, the removal of BPA decreased under highly alkaline conditions and at higher temperatures. The thermodynamic and isotherm investigations suggested that BPA adsorption onto the heterogeneous β-CD-E-T/ZnFe2O4 surface was a spontaneous, exothermic, multilayer physical adsorption process. Furthermore, β-CD-E-T/ZnFe2O4 was effective in removing BPA from aqueous solutions, even in the presence of ionic salt like sodium chloride. The ionic effect and pH studies indicated that ionic interaction was not the main interaction between β-CD-E-T/ZnFe2O4 and BPA responsible for the adsorption. The FTIR studies suggested that the adsorption of BPA onto β-CD-E-T/ZnFe2O4 was accompanied by strong hydrogen bonding, π–π interaction, and hydrophobic/host–guest interaction. One disadvantage of using the β-CD-E-T/ZnFe2O4 adsorbent for BPA removal was a drastic decrease in the adsorption efficiency of recycled β-CD-E-T/ZnFe2O4. However, despite this decrease, β-CD-E-T/ZnFe2O4 still exhibited a higher adsorption capacity than some of the as-synthesized adsorbent materials, making it a cost-effective adsorbent material.
Data availability
The data supporting the study's conclusions are included in the publication and its ESI.†
Author contributions
RS contributed to the conceptualization, methodology, data collection, data curation, and writing the original draft. PND supervised the research, edited and reviewed the original draft, and finalized the manuscript.
Conflicts of interest
The authors declare no known conflict of interest.
Acknowledgements
RS is grateful to NMDFC and UGC for UGC-MANF Junior Research Fellowship (no. F. 82-27/2019 (SA-III) dated July 31, 2020). The authors are thankful to the Department of Chemistry, Sardar Patel University for the research facilities. The authors are grateful to the MRC, MNIT, and Jaipur for SEM, BET, and XRD facilities. We thank UGC, New Delhi for the central thermal measurement facility sponsored under UGC-CAS (Phase-II) program grant vide sanction letter no. F. 540/5/CAS-II/2018 (SAP-I) dated 25th July 2018.
References
- A. Shafei, M. Matbouly, E. Mostafa, S. Al Sannat, M. Abdelrahman, B. Lewis, B. Muhammad, S. Mohamed and R. M. Mostafa, Environ. Sci. Pollut. Res., 2018, 25, 23624–23630 CrossRef CAS.
- J. Moreman, O. Lee, M. Trznadel, A. David, T. Kudoh and C. R. Tyler, Environ. Sci. Technol., 2017, 51, 12796–12805 CrossRef CAS PubMed.
- S. A. Krieg, L. K. Shahine and R. B. Lathi, Fertil. Steril., 2016, 106, 941–947 CrossRef CAS PubMed.
- H.-J. Lehmler, B. Liu, M. Gadogbe and W. Bao, ACS Omega, 2018, 3, 6523–6532 CrossRef CAS PubMed.
- M. Fürhacker, S. Scharf and H. Weber, Chemosphere, 2000, 41, 751–756 CrossRef.
- Y. Xu, Y. Wu, S. Hyun Hur, S. Ho Hong, W.-S. Choe and I.-K. Yoo, ChemistrySelect, 2022, 7, e202204102 CAS.
- C. Brahmi, M. Benltifa, M. Ghali, F. Dumur, C. Simonnet-Jégat, V. Monnier, F. Morlet-Savary, L. Bousselmi and J. Lalevée, J. Appl. Polym. Sci., 2021, 138, 50864 CrossRef CAS.
- Z. Pan, F. Yu, L. Li, C. Song, J. Yang, C. Wang, Y. Pan and T. Wang, Sep. Purif. Technol., 2019, 227, 115695 CrossRef CAS.
- Y. Jia, A. Eltoukhy, J. Wang, X. Li, T. S. Hlaing, M. M. Aung, M. T. Nwe, I. Lamraoui and Y. Yan, Int. J. Mol. Sci., 2020, 21, 3588 CrossRef CAS PubMed.
- R. Frankowski, A. Zgoła-Grześkowiak, W. Smułek and T. Grześkowiak, Appl. Biochem. Biotechnol., 2020, 191, 1100–1110 CrossRef CAS PubMed.
- Y. Huang, Q. Li, Z. Guan, D. Xia and Z. Wu, J. Water Process Eng., 2023, 52, 103564 CrossRef.
- S. Wang, Q. Wu, B. Yan, Y. Guo, W. Xia, J. Li, F. Cui and J. Tian, Sep. Purif. Technol., 2022, 291, 120874 CrossRef CAS.
- M. A. Martín-Lara, M. Calero, A. Ronda, I. Iáñez-Rodríguez and C. Escudero, Water, 2020, 12, 2150 CrossRef.
- N. U. M. Nizam, M. M. Hanafiah, E. Mahmoudi, A. A. Halim and A. W. Mohammad, Sci. Rep., 2021, 11, 8623 CrossRef CAS PubMed.
- H. Sayğılı, F. Güzel and Y. Önal, J. Cleaner Prod., 2015, 93, 84–93 CrossRef.
- C. Djilani, R. Zaghdoudi, F. Djazi, B. Bouchekima, A. Lallam, A. Modarressi and M. Rogalski, J. Taiwan Inst. Chem. Eng., 2015, 53, 112–121 CrossRef CAS.
- I. Chaari, E. Fakhfakh, M. Medhioub and F. Jamoussi, J. Mol. Struct., 2019, 1179, 672–677 CrossRef CAS.
- R. Pleşa Chicinaş, H. Bedelean, R. Stefan and A. Măicăneanu, J. Mol. Struct., 2018, 1154, 187–195 CrossRef.
- D. Fu, Q. Zhang, P. Chen, X. Zheng, J. Hao, P. Mo, H. Liu, G. Liu and W. Lv, RSC Adv., 2021, 11, 18308–18320 RSC.
- A. Elaouni, M. E. Ouardi, M. Zbair, A. BaQais, M. Saadi and H. A. Ahsaine, RSC Adv., 2022, 12, 31801–31817 RSC.
- J. Li, X. Wang, G. Zhao, C. Chen, Z. Chai, A. Alsaedi, T. Hayat and X. Wang, Chem. Soc. Rev., 2018, 47, 2322–2356 RSC.
- L. Jiao, J. Y. R. Seow, W. S. Skinner, Z. U. Wang and H.-L. Jiang, Mater. Today, 2019, 27, 43–68 CrossRef CAS.
- P. N. Dave and A. Gor, in Handbook of Nanomaterials for Industrial Applications, ed. C. Mustansar Hussain, Elsevier, 2018, pp. 36–66 Search PubMed.
- M. E. Davis and M. E. Brewster, Nat. Rev. Drug Discovery, 2004, 3, 1023–1035 CrossRef CAS PubMed.
- T. Loftsson and H. Friðriksdóttir, Int. J. Pharm., 1998, 163, 115–121 CrossRef CAS.
- G. Xu, X. Xie, L. Qin, X. Hu, D. Zhang, J. Xu, D. Li, X. Ji, Y. Huang, Y. Tu, L. Jiang and D. Wei, Green Chem., 2019, 21, 6062–6072 RSC.
- T. Gong, Y. Zhou, L. Sun, W. Liang, J. Yang, S. Shuang and C. Dong, RSC Adv., 2016, 6, 80955–80963 RSC.
- C. Yang, H. Huang, T. Ji, K. Zhang, L. Yuan, C. Zhou, K. Tang, J. Yi and X. Chen, Polym. Int., 2019, 68, 805–811 CrossRef CAS.
- K. V. Ragavan and N. K. Rastogi, Carbohydr. Polym., 2017, 168, 129–137 CrossRef CAS PubMed.
- J. H. Lee and S.-Y. Kwak, J. Appl. Polym. Sci., 2020, 137, 48475 CrossRef CAS.
- S. R. Patel and M. P. Patel, Polym. Bull., 2022, 79, 11079–11101 CrossRef CAS.
- S. R. Patel, R. H. Patel and M. P. Patel, J. Macromol. Sci., Part A, 2021, 58, 97–110 CrossRef CAS.
- P. N. Dave, B. Kamaliya, P. M. Macwan and J. H. Trivedi, Curr. Res. Green Sustainable Chem., 2023, 6, 100349 CrossRef CAS.
- P. N. Dave, L. V. Chopda and B. P. Kamaliya, Chem. Eng. Technol., 2023, 46, 997–1004 CrossRef CAS.
- R. Ramadan and A. M. Ismail, J. Inorg. Organomet. Polym. Mater., 2022, 32, 984–998 CrossRef CAS.
- R. Sirach and P. N. Dave, J. Hazard. Mater. Adv., 2023, 10, 100300 CrossRef CAS.
- M. A. Medeleanu, D. I. Hădărugă, C. V. Muntean, G. Popescu, M. Rada, A. Hegheş, S. E. Zippenfening, C. A. Lucan (Banciu), A. B. Velciov, G. N. Bandur, N. G. Hădărugă and M. Riviş, Carbohydr. Polym., 2021, 265, 118079 CrossRef CAS PubMed.
- G. G. Hacıosmanoğlu, T. Doğruel, S. Genç, E. T. Oner and Z. S. Can, J. Hazard. Mater., 2019, 374, 43–49 CrossRef PubMed.
- K. Rekos, Z.-C. Kampouraki, C. Sarafidis, V. Samanidou and E. Deliyanni, Materials, 2019, 12, 1987 CrossRef CAS PubMed.
- L. D. Quan, N. H. Dang, T. H. Tu, V. N. Phuong Linh, L. T. Mong Thy, H. M. Nam, M. T. Phong and N. H. Hieu, Synth. Met., 2019, 255, 116106 CrossRef CAS.
- M. Mondal, S. Basak, S. Ali, D. Roy, M. S. Haydar, K. Sarkar, N. N. Ghosh, K. Roy and M. N. Roy, Environ. Sci. Pollut. Res., 2023, 30, 43300–43319 CrossRef CAS PubMed.
- A. Z. M. Badruddoza, A. S. H. Tay, P. Y. Tan, K. Hidajat and M. S. Uddin, J. Hazard. Mater., 2011, 185, 1177–1186 CrossRef CAS PubMed.
- G. Leofanti, M. Padovan, G. Tozzola and B. Venturelli, Catal. Today, 1998, 41, 207–219 CrossRef CAS.
- Y. Li, P. Lu, J. Cheng, X. Zhu, W. Guo, L. Liu, Q. Wang, C. He and S. Liu, Talanta, 2018, 187, 207–215 CrossRef CAS PubMed.
- L. Ai, J. Hu, X. Ji and H. Zhao, RSC Adv., 2019, 9, 26224–26229 RSC.
- M. Kotronia, E. Kavetsou, S. Loupassaki, S. Kikionis, S. Vouyiouka and A. Detsi, Bioengineering, 2017, 4, 74 CrossRef PubMed.
- S. M. R. Paran, H. Vahabi, M. Jouyandeh, F. Ducos, K. Formela and M. R. Saeb, J. Appl. Polym. Sci., 2019, 136, 47483 CrossRef.
- L. Joseph, Q. Zaib, I. A. Khan, N. D. Berge, Y.-G. Park, N. B. Saleh and Y. Yoon, Water Res., 2011, 45, 4056–4068 CrossRef CAS.
- M. H. Dehghani, A. H. Mahvi, N. Rastkari, R. Saeedi, S. Nazmara and E. Iravani, Desalin. Water Treat., 2015, 54, 84–92 CrossRef CAS.
- R. V. P. Antero, A. C. F. Alves, P. de T. Ferreira Sales, S. B. de Oliveira, S. A. Ojala and S. S. Brum, Chem. Eng. Commun., 2019, 206, 1498–1514 CrossRef.
- S. Rovani, J. J. Santos, S. N. Guilhen, P. Corio and D. A. Fungaro, RSC Adv., 2020, 10, 27706–27712 RSC.
- J. Peng, Y. Li, X. Sun, C. Huang, J. Jin, J. Wang and J. Chen, ACS Appl. Mater. Interfaces, 2019, 11, 4328–4337 CrossRef CAS PubMed.
- W. Xin, X. Li and Y. Song, J. Chem. Technol. Biotechnol., 2020, 95, 1666–1674 CrossRef CAS.
- B. Mahanty, S. K. Behera and N. K. Sahoo, Sep. Sci. Technol., 2023, 58, 1275–1282 CrossRef CAS.
- C. V. Floare-Avram, O. Marincas, I. Feher, F.-D. Covaciu, C. G. Floare, M. D. Lazar and D. A. Magdas, Anal. Lett., 2023, 56, 272–285 CrossRef CAS.
- C. Mo, M. Faheem, S. Aziz, S. Jian, W. Xue, T. Yuyang, D. Shuang and Z. Guangshan, RSC Adv., 2020, 10, 26335–26341 RSC.
- S. Mantovani, T. Dorina Marforio, S. Khaliha, A. Pintus, A. Kovtun, F. Tunioli, L. Favaretto, A. Bianchi, M. Luisa Navacchia, V. Palermo, M. Calvaresi and M. Melucci, Environ. Sci.: Water Res. Technol., 2023, 9, 1030–1040 RSC.
- T. Ahamad, M. Naushad, A. N. Alhabarah and S. M. Alshehri, Int. J. Biol. Macromol., 2019, 132, 1031–1038 CrossRef CAS.
- A. Supong, P. C. Bhomick, M. Baruah, C. Pongener, U. B. Sinha and D. Sinha, Sustainable Chem. Pharm., 2019, 13, 100159 CrossRef.
- P. Wang, X. Zhou, Y. Zhang, L. Wang, K. Zhi and Y. Jiang, RSC Adv., 2016, 6, 102348–102358 RSC.
|
| This journal is © The Royal Society of Chemistry 2023 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article *
*
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 mole ratio. Later, sodium acetate (Fe3+
2 mole ratio. Later, sodium acetate (Fe3+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sodium acetate mole ratio = 1
sodium acetate mole ratio = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) was added. The mixture was vigorously stirred for 60 minutes and then subjected to ultrasound irradiation for 20 minutes. Next, 25 mL of ethylene diamine was added to the solution, and the resulting mixture was again irradiated with ultrasound for 20 minutes. After stirring the solution for 60 minutes, it was kept in an oven at 180 °C for 3 days until precipitations of ZnFe2O4 were obtained. The insoluble content was filtered and washed with water to remove any unreacted impurities and then dried in an oven at 60 °C. Finally, the material was calcined at 500 °C for 3 hours.
4) was added. The mixture was vigorously stirred for 60 minutes and then subjected to ultrasound irradiation for 20 minutes. Next, 25 mL of ethylene diamine was added to the solution, and the resulting mixture was again irradiated with ultrasound for 20 minutes. After stirring the solution for 60 minutes, it was kept in an oven at 180 °C for 3 days until precipitations of ZnFe2O4 were obtained. The insoluble content was filtered and washed with water to remove any unreacted impurities and then dried in an oven at 60 °C. Finally, the material was calcined at 500 °C for 3 hours.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio). The solution was stirred at 60 °C until the β-CD-E-T was completely dissolved. Thereafter, ZnFe2O4 (10% by mass) was added to β-CD-E-T solution and subjected to ultrasound irradiation for 20 minutes, followed by stirring for 2 hours. Finally, the solvents were evaporated in an oven to obtain the β-CD-E-T/ZnFe2O4 nanocomposite.
1 ratio). The solution was stirred at 60 °C until the β-CD-E-T was completely dissolved. Thereafter, ZnFe2O4 (10% by mass) was added to β-CD-E-T solution and subjected to ultrasound irradiation for 20 minutes, followed by stirring for 2 hours. Finally, the solvents were evaporated in an oven to obtain the β-CD-E-T/ZnFe2O4 nanocomposite.

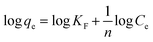
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AT + B1
AT + B1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce
Ce

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe = ln
qe = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qm − δε2
qm − δε2





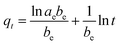
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ke against 1000/T (K−1) were used to calculate thermodynamic parameters like enthalpy change (ΔH°) and entropy change (ΔS°) using eqn (14). The value of ΔH° and ΔS° was used to calculate free energy change (ΔG°) using eqn (15).
ke against 1000/T (K−1) were used to calculate thermodynamic parameters like enthalpy change (ΔH°) and entropy change (ΔS°) using eqn (14). The value of ΔH° and ΔS° was used to calculate free energy change (ΔG°) using eqn (15).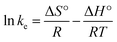



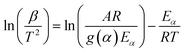





![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β, ln
β, ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β/T2, ln
β/T2, ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β/H(x), and ln
β/H(x), and ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β/h(x)T2 against 1000/T. All fitted data were calculated with a resolution of α = 0.025 across the range of α = 0.1–0.9. The plots of KAS and FWO for β-CD-E-T polymer and β-CD-E-T/ZnFe2O4 nanocomposite are depicted in Fig. S4.†
β/h(x)T2 against 1000/T. All fitted data were calculated with a resolution of α = 0.025 across the range of α = 0.1–0.9. The plots of KAS and FWO for β-CD-E-T polymer and β-CD-E-T/ZnFe2O4 nanocomposite are depicted in Fig. S4.†







