 Open Access Article
Open Access ArticleAdvances in biomass derived low-cost carbon catalyst for biodiesel production: preparation methods, reaction conditions, and mechanisms
Gaurav Yadav,
Nidhi Yadav and
Md. Ahmaruzzaman *
*
Department of Chemistry, National Institute of Technology Silchar, 788010, Assam, India. E-mail: mda2002@gmail.com
First published on 3rd August 2023
Abstract
Biodiesel is a less hazardous, environmentally friendly biofuel that has been extensively investigated in modern years to ensure that we lessen our dependency on fossil fuels and mitigate climate change. While fossil fuel substitutes like biodiesel may help transition to a less polluted world, industrial-scale manufacturing still relies highly on chemical catalysis. However, heterogeneous solid catalysts result in less activity for biodiesel production due to their deactivation effects, porosity, surface area, material stability, and lower reactivity under moderate conditions. The “sulfonated carbons” are metal-free solid protonic acids distinguished by their distinctive carbon structure and Brønsted acidity (H0 = 8–11). Heterogeneous sulfonated catalysts derived from waste biomass were a significant focus of the most advanced biodiesel processing techniques for simple and low-cost manufacturing processes. This study discusses the advantages and disadvantages of various catalysts, biomass sources and properties, synthesis of catalysts, and factors influencing the insertion of active sulfonic sites on biomass surfaces. Additionally, transesterification and esterification reaction mechanisms and kinetics are discussed. At last, future directions are provided for young, dynamic researchers.
1. Introduction
According to contemporary energy legislation, climate change and energy efficiency must be tackled simultaneously through technical advancements. The consumption of renewable energy among the public and scientific community has piqued attention due to the faster depletion and consumption of fossil fuels and the high emissions of toxic gases. Due to their renewability and non-toxicity, the widely accepted techniques for alternative fuels that partly replace fossil fuels are bioethanol and biodiesel. According to ASTM D6751, biodiesel is defined as the fatty acid alkyl esters of animal fats and vegetable oils that are obtained from various feedstocks.1–3Biodiesel is a clean, renewable, non-toxic and biodegradable biofuel.4 A variety of feedstock based on agriculture, wood waste, energy crops, plant-oil based, and aquatic biomass are helpful to produce biodiesel. Numerous major countries have accessibility of prospective bio-resources as feedstock materials such as Calophyllum inophyllum (Malaysia and Australia), Pongamia pinnata (India, Bangladesh, and Australia), Sunflower (Argentina, Greece, Turkey, Italy, and Canada), Jatropha curcas (India, Thailand, Malaysia, Tanzania, Mali, Mozambique, Cuba, Peru, and Zimbabwe, Pakistan), rapeseed (Italy, Chile, Canada, Greece, and Turkey, China), oil seed palm (Thailand, Brazil, Iran, Peru, Malaysia, and Mexico), soyabean (Unites States, Italy, Argentina, and Canada), fish residues (Norway) and fish oil (Iran).5 These natural assets have ideal value for biofuels generation due to biodegradability, availability, and CO2 neutrality.6,7 Transesterification is the most often utilized process for producing biodiesel from vegetable oil.8,9 When an alcohol and triglycerides are reacted in absence or presence of catalyst resulted in formation of fatty acid methyl ester (FAME) and a byproduct glycerin. In comparison to other petro-diesel, biodiesel has high flashpoint and cetane number, and low cold flow properties and calorific values.10,11 Due to these properties, biodiesel is more secure for storage and transport. As energy source, biodiesel claimed to reduce 78% of CO2 emissions as petro-diesel.12 The biodiesel market in India reached 383.4 million US dollar in 2022 and expected to reach 643 million US dollar by 2028. Fig. 1 displays the data of biodiesel production (from 2011 to 2021) in India which is continuously growing.
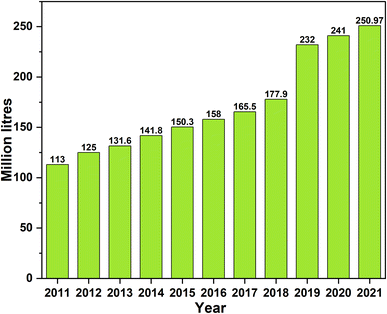 | ||
| Fig. 1 Biodiesel production in India over the years (data obtained from OECD https://stats.oecd.org/Index.aspx?QueryId=107145). | ||
Homogeneous catalysts are favorable catalysts for synthesis of biodiesel due to their simplicity and low energy utilization. However, homogeneous catalysts have several disadvantages like non-recoverable, non-biodegradable, corrosion of instruments and recyclability.13 These issues are easily resolved by heterogeneous catalyst. Heterogeneous catalysts are extensively investigated due to ease of separation, recyclability, non-corrosiveness of instruments and no soap formation.13,14 There are a variety of materials that may be used to make heterogeneous catalysts, such as biochar, biomass ash, waste shells, agriculture products and activated carbon.15 Researchers from all around the world have recently been interested in biomass-derived heterogeneous catalysts. Ecologically and economically, it makes sense to use a commercial waste product as a catalyst in the manufacturing of biodiesel.16 Consequently, the catalyst has to have a greater surface area in order to generate a solid acid catalyst of excellent quality (external catalytic sites, hydrophobicity, etc.) having a significant pore diameter.17 Table 1 discusses the various advantages and disadvantage of different catalyst for biodiesel production.
| Catalyst type | Example | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Homogeneous acidic and basic catalyst | H2SO4, HCl, H3PO4, HNO3, KOH, NaOH | High activity | Hard to separate from reaction mixture | 15 and 18 |
| Shorter reaction time | Soap formation | |||
| Lesser purification steps | Sensitive to free fatty acid (FFA) and H2O | |||
| Low cost | High temperature | |||
| High pressure | ||||
| Cannot utilized in animal fats and waste cooking oil | ||||
| Release in high quantity of wastewater | ||||
| Corrosiveness | ||||
| Heterogeneous catalyst | CaO, MgO, Hydrotalcite, ZrO2, TiO2, ion-exchange resin, mixed oxide | Non-corrosion | High methanol: oil ratio | 19 and 20 |
| Easy separation | Actives sites leaching | |||
| High reusability | High cost | |||
| Insensitive to FFA and H2O | Complex synthesis procedure | |||
| Diffusion limitations | ||||
| Low microporosity | ||||
| Low acidic or basic sites | ||||
| Enzyme | Lipase | Green approach | High cost | 21 and 22 |
| High specificity | Inhibition by glycerol and alcohol | |||
| High catalytic efficiency | Low reaction rate | |||
| High biodiesel purity | Enzyme denaturation | |||
| Fewer purification steps | ||||
| No soap formation |
Hara and his colleagues first developed the SO3H carbon sheets in 2004.23 The metal free sulfonated carbon catalyst is generally prepared using concentrated H2SO4 with the help of carbonized organic matter.24–26 In this review, we have added various strategies of developing low-cost catalyst for biodiesel production. Low-cost catalyst which is derived from biomass are well recognized for biodiesel formations. Although several review published on biodiesel production by utilization of carbon-based material.15,27–30 However, there is no recent review on biodiesel production including esterification and transesterification process from biomass by SO3H functionalized active site as per our knowledge. The authors try to summarizes the recent advancement in the biodiesel formation by esterification and transesterification reaction which include FFA and triglycerides. Along with this, biomass derived catalyst like sulfonated catalyst (acid catalyst) and magnetic catalyst are discussed. From this viewpoint, researchers are developed a novel and sustainable materials with the utilization of these waste and also, they have value addition for environment.
2. Biomass source and properties
Biomass is a readily available material that is found in nature abundantly through various agricultural activities. Recently, biomass has been considered a potential material for biodiesel production due to its low cost and easy availability. Biomass mainly contains carbon material that possesses lignin, hemicellulose, and cellulose. Due to these characteristics, researchers utilized biomass for high-value addition materials through various processes. Biomass char has a large pore size and uneven structure. Forestry wastes include bark, leaves, branches, and other wood fragments. In general, lignocellulosic biomass has 15–25% lignin, 25–40% hemicellulose, and 35–55% cellulose, with a minuscule quantity of protein, extractives, and ash. For the pyrolysis process, the content of water in the feedstock should be less than 10%. Agricultural leftovers such as rice husks can have an ash level of up to 20%, whereas most woody biomass, devoid of “dirt” (needles and leaves), has an ash concentration of less than 2%. In terms of elemental compositions, wood residues contain somewhat more hydrogen and carbon than agricultural leftovers.Pyrolysis, direct combustion, hydro gasification, gasification, anaerobic digestion, liquefaction, trans-esterification, esterification and alcoholic fermentation are the principal methods for getting energy from biomass. Relying on the bioresource and the type of energy required, each technique offers distinct benefits.31 Recently, there has been an upsurge in demand for farming residues as substitutes for woody cellulosic fibers because of growing awareness of environmental concerns, global deforestation about the low-cost residues as well as burning the residues, in comparison to plant fibers. Additionally, using agricultural residues can help nations with a dearth of forestry resources address their wood resource shortfall. Rice, wheat, maize stover, barley straw, coconut husk, sorghum stalks, sugarcane bagasse, banana, and pineapple leaves are the primary lignocellulosic agricultural leftovers. So, the demand for agricultural residue is continuously increasing instead of producing energy from woody biomass. Agro-industrial waste, pruned biomass, arable crop residues, and biomass derived from the farming of energy crops are all examples of solid agricultural feedstock. Table 2 provide the summary of various biomass resources obtained from agriculture, forest and other sources.
| Category | Example | Characteristics | References |
|---|---|---|---|
| Agricultural activities | Rice husk, rice straw, wheat straw, corn stover, begasse | Low cost | 32, and 33 |
| Easy availability | |||
| High photosynthesis ability | |||
| High cellulose content | |||
| Low harvest time | |||
| Less nitrogen and sulfur than coal | |||
| Forest | Wood | Higher carbon content | 34 |
| Fewer ash contents | |||
| High cellulose content | |||
| A high density of biomass | |||
| Less nitrogen and sulfur than coal | |||
| Perennial grass | Indian grass, sugarcane bagasse, reed canary | Improve soil quality | 35 |
| Carbon sequestering | |||
| Easy and fast growth | |||
| Low fertilizers dependance | |||
| Decrease dependency on forest biomass | |||
| Aquatic plant | Brown algae, green algae | Bioremediation capability | 35 |
| Low nutrition requirement | |||
| Short life cycle | |||
| High biomass potential yield |
3. Catalyst synthesis by biomass
The sulfonated catalysts are fabricated using various techniques by utilization of biomass. The biomass contains a large amount of oxygen, carbon, and less amount of calcium, potassium, magnesium, and sodium.36 Generally, sulfonated carbon catalysts can be fabricated by two processes known as hydrothermal carbonization (HTC) and pyrolysis, followed by sulfonation.Araujo et al.37 created the catalyst through the utilization of acai seed. The powder of acai seed was heated at 600 °C in a tubular furnace under nitrogen for 2 h. About 35 weight percent of carbon was produced when acai stone debris was carbonized. The obtained carbonaceous material was sulfonated with H2SO4 and stirred for effective mixing. After that, the solution was washed with distilled water several times until no sulfate was detected by the BaCl2 solution. In another similar type of study, the corn straw was heated at various temperatures, followed by sulfonation.38 A 100 mL flask was filled with 10 mL of fuming sulfuric acid and heated in an oil bath at 80 °C for 240 min. The mixture was cooled and washed to remove impurities using a vacuum pump. Finally, the resulting material was dried at 80 °C for 4 h in a vacuum before using an esterification reaction. Microalgal biomass was also utilized for the esterification reaction. First, the microalgal biomass was de-oiled by lipid extraction via the Soxhlet extraction process.39 The biomass was de-oiled and then dried in an oven to remove all traces of solvents before the carbon catalyst was prepared. Synthesis of the de-oiled microalgal biomass (DMB) catalyst required two steps: first, carbonization of DMB, and then, sulfonation of the resulting biochar using sulfuric acid. In another work, a solid acid catalyst was produced by sulfonating three different carbonaceous materials (algal biomass, moringa, and rice husk).40 Previously to sulfonation, the material was ground into powder form. 20 mL of 2 M H2SO4 was added to a 100 mL Teflon autoclave and heated in a microwave at 200 °C for 30 min while 5 g of sample were stirred at a slow pace. The microwave temperature ramped for 5 min at 200 °C, held for 30 min at 200 °C, and then decreased to 40 °C. The sample was then dried overnight at 60 °C in the oven before being hand-milled into a fine powder. The fine powder was then mixed in the microwave reactor with 20 mL of 20% SO3 fuming H2SO4. For 30 min, sulfonation was done in a microwave. The material was dried at 150 °C for 12 hours after being cooled to 40 °C.
HTC's approach of creating carbonaceous materials from naturally occurring biomass combines the use of renewable resources with attractive porosity and stability. Gaurav and Ahmaruzzaman successfully synthesized a sulfonated carbon material via the facile hydrothermal carbonization (HTC) method in a single step at a low temperature.14 The as-synthesized material is fabricated using waste citrus limetta peel. The successful insertion of active sulfonic sites was observed using FTIR spectroscopy. EDS analysis also supports the data of FTIR. In another similar type of study, areca nut husk was used as a carbon precursor for the fabrication of sulfonated carbon catalyst by the HTC method.10 The author used two temperatures, 80 °C and 100 °C. The crushed areca nut husk was prepared using a batch experiment in the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 and 1
10 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20. The author observed that increasing the temperature results in the breakage of the carbon structure. Carbonizing biomass in supercritical ethanol and then sulfonating the resulting alcohothermal chars has resulted in the first-ever synthesis of a novel solid acid with enhanced stability. Alcohothermal chars were more stable after being sulfonated because oxygenated functional groups and sulfonic groups were simpler to load on them than on hydrothermal or pyrolytic chars.41
20. The author observed that increasing the temperature results in the breakage of the carbon structure. Carbonizing biomass in supercritical ethanol and then sulfonating the resulting alcohothermal chars has resulted in the first-ever synthesis of a novel solid acid with enhanced stability. Alcohothermal chars were more stable after being sulfonated because oxygenated functional groups and sulfonic groups were simpler to load on them than on hydrothermal or pyrolytic chars.41
4. Factors influencing the SO3H acid density
4.1. Effect of temperature
Temperature plays a significant impact on the insertion of SO3H actives sites on the biomass surface. High temperature causes biomass dehydration and bond breaking of numerous carbon skeletons, which results in the formation of amorphous structures includes polycyclic aromatic skeleton.42 Gaurav et al. noticed that the high temperature of the areca nut husk causes the breakage of cellulose, hemicellulose, and lignin part of biomass which forms polycyclic aromatic hydrocarbon.10 As further the temperature rises, these groups disintegrate, resulting in a decrease in functionalization efficiency. The anchoring of the SO3H functional group may be in jeopardy due to the rise in carbonization temperature.43 However, the degree of functionalization increases with decreasing carbonization temperature. This means that materials functionalized at lower temperatures will likely have a larger density of acid sites. The carbon structure was expected to enhance reactant diffusion, allowing for more excellent anchoring of acid groups and the generation of active SO3H sites. Biomass components were decomposed at higher temperatures which do not form an appropriate skeleton for insertion of SO3H actives site. For example, Joyleene et al. fabricated woody biomass at a temperature of 450, 675 and 875 °C. The biomass pyrolyzed at 450 °C (2.6 mmol g−1) has the highest total acid density followed by 675 °C (1.2 mmol g−1) and 875 °C (0.43 mmol g−1).444.2. Effect of sulfonation
The insertion of the SO3H active site is done with the help of various sulfonating agents like conc. sulfuric acid, fuming sulfuric acid, aminobenzene sulfonic acid, chlorosulfonic acid, p-toluene sulfonic acid, hydroxyethyl sulfonic acid, sulfosalicylic acid, and other.45 Among all these acids, concentrated sulfuric acid is used for insertion of SO3H acidic sites due to low cost.As aforementioned, temperature significantly affects the stability and activity of sulfonic acid. Limited sulfonation leads to the formation of unstable and readily decomposed material. As the temperatures increase, an undesired multi-sulfonated group may reduce catalytic activity. At low carbonization temperatures, biomass structure does not deteriorate. Consequently, surface groups are produced that can more effectively react with sulfuric acid, promoting increasing inclusion of sulfonic groups on the carbon catalysts surface. The reduction in sulfonation surface areas was caused by carbon network breakdown and high-temperature acid group dehydration. The adverse impact on the S content of the catalyst may be ascribed to the low thermal robustness of SO3H groups.46 Sulfonic groups were inserted via various pathways, i.e., functionalization method and chemical modification. Depending upon these techniques and carbon precursors, the sulfonic density ranges from 0.05 to 7.3 mmol g−1.23,45 At the initial point, the sulfonic acid insertion in the biomass surface increase by increasing the wt% of sulfuric acid. The higher rate of carbonization-sulfonation reaction as the sulfuric acid wt% increases could explain the increase in insertion of sulfonic active sites. After reaching an optimum value, there was no effect on the reaction rate, but at high sulfuric acid wt%, the rate of carbonization-sulfonation decreases resulting in decrease in the S-content.10
4.3. Effect of time
Time also plays an essential role in the insertion of sulfonic acid active sites. In comparison to the wt% of sulfuric acid reaction temperature, reaction time has a significant influence on carbonization-sulfonation. The increase in the reaction time has a positive effect on the catalyst procedure, leading to a rise in its S-content, resulting in an increase in total acid density until the optimum time is reached. However, more increase in reaction time does not effect on s-content;46 hence the acid density will be the same. Lancer and Kucera stated that sulfonation-desulfonation is a reversible reaction.47 Prolonged time at high temperatures leads to a decrease in total acid density. As a result, the substantial decrease in strong acid density seen throughout the protracted sulfonation process can always be accounted by the desulfonation process. In another study, the increase in the time from 1 h to 4 h resulted in increase the acid density to 4.2 mmol g−1, but in the same reaction condition, the decrease in the acid density has noticed in 8 h (2.9 mmol g−1).48 This change in the acid density beyond the optimum value is due to subsequent decrease and functionalization phase of the pyrolyzed carbon material.5. Process of biodiesel formation
5.1. Esterification reactions
The high cost of producing biodiesel is directly related to the current high price of oil. Waste cooking oil for transesterification processes is common but not used because of the oil's high impurity levels, particularly its high FFA content. The esterification of an acid catalyst would be preferable.Memon et al. evaluated the potential of Mangifera indica peels for biodiesel production by varying the amount of concentrated sulfuric acid at various time intervals.49 The maximum catalyst yield for biodiesel production was obtained when six hours of time was taken to sulfonate the Mangifera indica peel due to highly acidic groups like –OH, –COOH, and –SO3H. The as-synthesized catalyst has a wide surface area (42.13 m2 g−1), high pore volume, and porosity. The reusability study of the catalyst was observed up to 4 cycles showing the excellent stability of the catalyst. In the esterification process between methanol and oleic acid (OA), researchers used an acid catalyst derived from Sargassum horneri carbon.50 The lower amount of lignin and a more significant amount of total cellulose (hemicellulose and cellulose) in Sargassum horneri relative to terrestrial plants indicate modest pyrolysis conditions. Due to this, the carbonization synthesis temperature was varied from 250 to 550 °C. The Sargassum horneri gives maximum biodiesel at 300 °C having conversion of 96.4%. Due to the high carbon concentration in its elemental composition, it is well suited for producing carbon-based materials. Catalytic efficiency in the esterification process required for biodiesel manufacture may be increased by the availability of sulfonic acid groups and carbonization for forming amorphous carbon layers. After the four-cycle, the recovered catalyst was sulfonated again, which gave the maximum yield of 95.4%. MnFe2O4 and CoFe2O4 ferrite nanoparticles loaded with sulfonated lignin (SL) were used for the esterification of OA.51 SL was deliberately produced by reacting sugarcane bagasse lignin with acetyl sulfate (SO3H agent). In order to create the magnetic nanoparticles, two distinct solids were created using differing reaction proportions. In the esterification processes, MnFe2O4-SL and CoFe2O4-SL displayed the highest conversion of OA into FAME, at around 80%.
Waste Citrus limetta peels (SCLPB) were used for the production of biodiesel from oleic acid. The EDS and FTIR data show the insertion of sulfonic actives site, which were helpful for the reaction process. SCLPB catalyst's porous nature was beneficial for the conversion of oleic acid into methyl ester. The self-oxidation of oleic acid at higher temperature result in the formation of multiple esters, as shown by the GC-MS data.14 Due to the catalyst's high surface area (32.210 m2 g−1), OA diffusion occurs on the catalyst's surface, enhancing catalytic activity. Mesoporous materials, which enable the reactants to diffuse into the pores, may facilitate faster reaction rates. In another similar study, areca nut husk was synthesized using the HTC method. Due to the active sulfonic group, high S-content (3.12%) and high acid density (1.88 mmol g−1) of the fabricated catalyst were noticed. Acid-base back titration was used to calculate the acid density of the fabricated catalyst. The as-synthesized catalyst was reusable for up to 4 catalytic cycles. The insertion of sulfonic actives sites into the catalyst surface shows a nucleophilic substitution reaction. The formation of covalent bonds between the sulfonic group (SO3H) and the catalyst surface increases the acid density. The conversion of OA into methyl oleate was fast-forwarded by weak (–OH, –COOH) as well as strong acidic groups (SO3H), thus increasing the reaction process.10
Tang compared the catalytic efficiency of biomass waste generated (corncob biomass, empty fruit bunch, and papaya seed) carbon-based acid catalysts used in the esterification reaction of methanol and palm fatty acid distillate (PFAD) to assess the feasibility in the manufacturing of biodiesel.52 An effective sulfonation of the catalyst support was achieved by the arylation of 4-benzene diazonium sulfonate. The synthesized carbon catalyst had a high porosity and a surface area that varied from 639.68 to 972.66 m2 g−1. At optimum conditions, corncob shows the highest FFA conversion of 93.49% and FAME production of 72.09%. The FAME production was found to be reasonably high after just two reaction cycles when using a sulfonated AC catalyst produced from corncob waste. It was determined that the esterification process was irreversible pseudo-homogeneous. In a similar study, biomass obtained from marine algae, moringa oleifera seed, and rice husk (RH) surfaces were incorporated with the SO3H group and assessed their activity for the esterification process. It was noticed from the ion exchange titration method that the rice husk was bearing the most active sulfonic group having a value of 4.24 mmol g−1.40 Algal biomass surface contains lower sites for binding the SO3H group; as a consequence, the acid site density found was 0.27 mmol g−1. The result shows that high SO3H site insertion was due to greater surface area and high porous carbon structure. The surface of SO3H-RH has a more significant potential to connect SO3H groups and create active sites for FFA to biodiesel conversion. EDS analysis also confirms the presence of sulfur along with carbon and oxygen, which actively participate in the reaction. Monk fruit seeds (Siraitia grosvenorii) were also used to develop sulfonated carbon catalysts for biodiesel synthesis.53 The efficiency of the fabricated catalyst was assessed using palmitic acid. The catalyst shows 98.5% efficiency under various conditions (4 wt%, 120 °C, 6 h). Phenylation with trimethoxy phenyl silane and sulfonation with chlorosulfonic acid were the two methods used for surface functionalization. Phenyl groups added by trimethoxy phenyl silane phenylation would intercalate on the monk fruit seed pore and surface. The SO3H groups produced by the chlorosulfonic acid would then be replaced with the phenyl rings during the sulfonation process, creating strong covalent bonds. Table 3 showing the summary of various catalyst for production of biodiesel from esterification process.
| Biomass source | Acid density (mmol g−1) | Optimization parameters | Yield (%) | Kinetics | References |
|---|---|---|---|---|---|
| Mangifera indica peels | 3.46 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (OA 10 (OA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3OH), 5 wt%, 65 °C, 4 h CH3OH), 5 wt%, 65 °C, 4 h |
98.6 | 1st | 49 |
| Sargassum horneri | 1.40 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 (OA 15 (OA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3OH), 10 wt%, 70 °C, 5 h CH3OH), 10 wt%, 70 °C, 5 h |
96.4 | — | 50 |
| Sugarcane bagasse | 1.8 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (OA 10 (OA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3OH), 100 °C, 6 h CH3OH), 100 °C, 6 h |
79.50, 78.5 | — | 51 |
| Citrus limetta | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (OA 20 (OA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3OH), 5 wt%, 70 °C, 3 h CH3OH), 5 wt%, 70 °C, 3 h |
96 | — | 14 |
| Areca nut husk | 1.88 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 (OA 25 (OA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3OH), 9 wt%, 80 °C, 1 h CH3OH), 9 wt%, 80 °C, 1 h |
96.4 | 1st | 10 |
| Corncob | 2.07 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 (OA 30 (OA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3OH), 5 wt%, 100 °C, 4 h CH3OH), 5 wt%, 100 °C, 4 h |
72.09 | 1st | 52 |
| Rice husk | 4.24 | — | 99.9 | — | 40 |
| Siraitia grosvenorii | 7.61 | −4 wt%, 120 °C, 6 h | 98.5 | — | 53 |
| Orange peel | 0.93 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 2.15 wt%, 3 h 10, 2.15 wt%, 3 h |
92.8 | — | 54 |
| Corncob | 2.05 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12, 10 wt%, 65 °C, 4 h 12, 10 wt%, 65 °C, 4 h |
98.5 | — | 55 |
| Cacao shell | 4.56 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, 5 wt%, 45 °C, 4 h 7, 5 wt%, 45 °C, 4 h |
93 | — | 56 |
| Acai seed | 0.74 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12, 5 wt%, 100 °C, 1 h 12, 5 wt%, 100 °C, 1 h |
88 | — | 57 |
| Palm oil empty fruit bunch | 1.96 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 (OA 50 (OA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CH3COOCH3), 10 wt%, 100 °C, 8 h CH3COOCH3), 10 wt%, 100 °C, 8 h |
50.5 | — | 58 |
| Acai stone | 1.97 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12, 5 wt%, 100 °C, 1 h 12, 5 wt%, 100 °C, 1 h |
93 | — | 37 |
| Sargassum horneri | 1.62 | —, 2 h | 96.6 | — | 59 |
| Potato peel | 1.6 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12, 5 wt%, 80 °C, 2.5 h 12, 5 wt%, 80 °C, 2.5 h |
97.2 | 1st | 60 |
| Rice husk | 2.26 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24, 8 wt%, 80 °C, 1 h 24, 8 wt%, 80 °C, 1 h |
99.6 | 1st | 32 |
| Walnut shell | 2.06 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16, 9 wt%, 85 °C, 1 h 16, 9 wt%, 85 °C, 1 h |
99.01 | 1st | 4 |
| Corn straw | 2.64 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, 7 wt%, 60 °C, 4 h 7, 7 wt%, 60 °C, 4 h |
98 | — | 38 |
| Soldier fly larvae shells | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, 10 wt%, 65 °C, 6 h 8, 10 wt%, 65 °C, 6 h |
93.2 | — | 61 |
| Ginger straw | 1.05 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, 7 wt%, 64 °C, 3.5 h 9, 7 wt%, 64 °C, 3.5 h |
93.2 | — | 62 |
| Murumuru kernel shell | 4.2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 5 wt%, 90 °C, 4 h 10, 5 wt%, 90 °C, 4 h |
97.2 | — | 48 |
(1) Generation of carbocation and protonation of oleic acid carbonyl oxygen.
(2) Formation of unstable intermediate due to nucleophilic attack by hydroxyl group at positive carbon and deprotonation from unstable intermediate.
(3) Formation of water and methyl oleate.
Since methanol is a weak alkali and SO3H is a strong acidic group, the esterification reaction does not begin until the second step in this well-established esterification process. As a result, protonating the methanol molecule is challenging due to the very acidic nature of SO3H. In contrast, the oxygen in methanol molecules receives a small amount of “negative charge” through the addition of weak acid groups like –COOH, the deprotonated version of which may establish a hydrogen bond with the –OH group in the methanol molecule {Fig. 2}. Because of this “negative charge,” the nucleophilic nature of the methanol is enhanced, which speeds up the esterification process and improves the conversion.
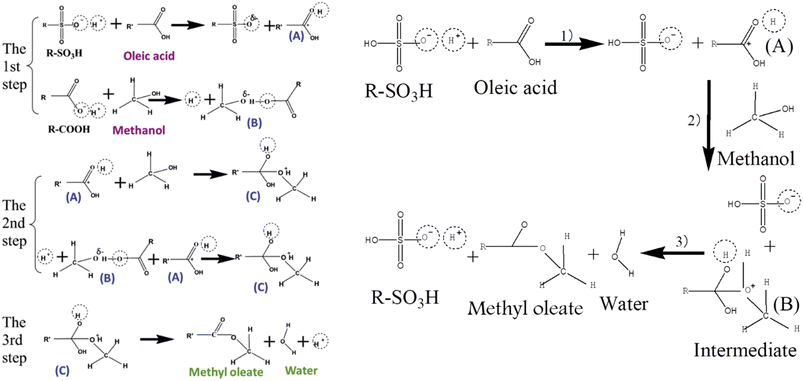 | ||
| Fig. 2 Reaction mechanism of esterification process by SO3H functionalized carbon material.63 | ||
Generally, the esterification reaction follows pseudo-1st order kinetics due to excess of methanol.4,10,66–68 Following factors should be considered for kinetics of esterification reaction-
1. The esterification process is the only responsible in biodiesel production.
2. The non-catalytic process is not important.
3. The reversible process was little affected by the addition of more methanol to the reaction mixture.
However, some researcher used the pseudo 2nd order kinetics69,70 and pseudo homogeneous third order kinetics.65,71
5.2. Transesterifications reactions
Due to high oil prices, the transesterification process is still used for biodiesel production. The esterification process for biodiesel production requires high methanol and free fatty acid molar ratio. The biodiesel production process from waste cooking oil is gaining attention due to its value addition. Waste cooking oil contains high-free fatty acids.32 An acid catalyst might be used to transform the feedstock's high FFA content into biodiesel.Leesing et al. used an integrated process for biodiesel formation by yeast-based catalyst and catalyst derived from durian peel (DP).72 Catalyst derived from durian peel shows enhanced activity on insertion of sulfonic sites, and FAME production efficiency reaches 81.57%. Before sulfonation, the acid density of the catalyst was just 0.01 mmol g−1, whereas, after sulfonation, the acid density reached 1.22 mmol g−1 showing the incorporation of active sulfonic sites on the durian peel surface. The greater heat capacity of FAME generated led to an estimate of energy production between 32.44 - 34.90 MJ kg−1. From 1000 kilograms of waste DP, the suggested bio-refinery idea produced 16.0 kg of yeast lipid, 101.2 kg of yeast biomass, 717 kg of DP-SO3H catalyst, 70.4 kg of yeast wastes, and 79.7–82.6 kg FAME. In another study, microalgal biomass was de-oiled and then sulfonated to produce biodiesel. The catalyst was characterized by various techniques to check the morphology, incorporation of sulfonic sites, and surface area. The as-synthesized catalyst shows high efficiency using waste cooking oil (WCO) (96.25%) and microalgal oil (AO) (94.23%). The catalyst reusability study shows that the catalyst can be used for up to four cycles (≥90%) with a little loss in activity.39 Orange peel is a primarily generated waste material obtained from food industries. Carbonization followed by sulfonation treatment was used to make the solid acid carbon catalyst from waste orange peel—the SO3H group attached to the orange peel with a covalent bond. SEM images show that sulfonation has altered the surface of the orange peel with some cracks, partial disintegration, and partial oxidation. The fabricated catalyst has a large surface area of 44 m2 g−1 and an acid density of 1.57 mmol g−1. Catalyst efficiency is demonstrated using the transesterification of maize acid oil, yielding cost-effective biodiesel. Maize acid oil is a low-cost feedstock with an acid value of 127.21 mg KOH/g. Under optimal circumstances, Box–Behnken design achieves 91.68% biodiesel conversion.73 In another study, corncob as a carbon precursor was used for transesterification reaction from soyabean oil. The optimal reaction conditions consisted of a microwave power range of 0–600 W, an oil-to-alcohol molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, a catalyst-to-oil mass ratio of 20%, and a reaction period of 20 min. The catalyst effectively accelerated the transesterification process in as little as 20 min, resulting in an 88.7% yield of pure biodiesel. The catalyst was utilized five times in succession without losing its catalytic activity.74
6, a catalyst-to-oil mass ratio of 20%, and a reaction period of 20 min. The catalyst effectively accelerated the transesterification process in as little as 20 min, resulting in an 88.7% yield of pure biodiesel. The catalyst was utilized five times in succession without losing its catalytic activity.74
Bastos et al.75 used the murumuru kernel shell (an agricultural waste) to develop an acid catalyst in biodiesel production from jupati oil. Based on a 23-central composite design, the response-surface approach was applied to determine the optimal reaction conditions. The as-fabricated catalyst was characterized by various techniques such as SEM-EDS, FTIR, TGA, XRD, and total acid density. Methanol/oil molar ratio, catalyst concentration, and temperature were optimized in jupati biodiesel synthesis, yielding optimum reaction conditions of 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 6, and 135 °C, respectively. The FAME production under these optimization conditions reaches 91.8%. The reusability studies of fabricated catalysts have shown less activity due to chlorophyll presence. Purified jupati oil also helps in the high catalytic activity, whereas unpurified vegetable oil results in catalytic loss of up to 60%76—the presence of pheophytin resulting in a decrease in activity. Strong adsorption of oil contaminants on sulfonated catalyst renders the active acid sites inactive after one cycle.77 The problem was fixed by utilizing an adsorbent made from the murumuru nuts shell, which successfully removed 92.5% of the chlorophyll, left 0.9 ppm pheophytin level. As a result, the author proposes using the acid solid extracted from the murumuru kernel as a bifunctional catalyst for both biodiesel synthesis and chlorophyll eradication from vegetable oils. The synthesized catalyst shows excellent catalytic activity in four cycles in jupati oil. There was only a little decline in catalytic activity over time due to the leaching of acid sites.
1, 6, and 135 °C, respectively. The FAME production under these optimization conditions reaches 91.8%. The reusability studies of fabricated catalysts have shown less activity due to chlorophyll presence. Purified jupati oil also helps in the high catalytic activity, whereas unpurified vegetable oil results in catalytic loss of up to 60%76—the presence of pheophytin resulting in a decrease in activity. Strong adsorption of oil contaminants on sulfonated catalyst renders the active acid sites inactive after one cycle.77 The problem was fixed by utilizing an adsorbent made from the murumuru nuts shell, which successfully removed 92.5% of the chlorophyll, left 0.9 ppm pheophytin level. As a result, the author proposes using the acid solid extracted from the murumuru kernel as a bifunctional catalyst for both biodiesel synthesis and chlorophyll eradication from vegetable oils. The synthesized catalyst shows excellent catalytic activity in four cycles in jupati oil. There was only a little decline in catalytic activity over time due to the leaching of acid sites.
During transesterification reaction, microalgal oil was purified with the help of various treatment method which results in high cost and energy loss. Lipid-rich wet algae obtained from Nannochloropsis salina can be converted into biodiesel without losing nutrient algae nutrient content by in situ transesterification process under microwave-assisted supercritical ethanol optimization conditions.78 The lipids in wet algae may be extracted and trans-esterified into biodiesel in a single step using this method. In order to assess the oxidative and thermal stability of wet algae-derived ethyl esters, a thermogravimetric analysis was carried out in both oxygen and nitrogen atmospheres. The catalyst-free and green solvent in situ transesterification process shows promise as a practical method for producing algae biodiesel. In another study, microalgal oil was used untreated to produce biodiesel. First, rice husk was sulfonated with the help of microwave acid treatment. The sulfonated rice husk was employed in situ transesterifications of Parachlorella kessleri (microalgal) biomass.79 After optimizing the catalyst dosage, it was determined that a reaction duration of 30 min at room temperature (25 °C) yields the maximum FAME production with 30 mg catalyst. The author found that the surface area of rice husk fluorocarbon was high than sulfonated rice husk. However, the efficiency of the biodiesel production of sulfonated rice husk was better than rice husk fluorocarbon, which means that the surface area is not enough for the transesterification reaction. Due to the large area of rice husk fluorocarbon, actives sites become inactive due to the adsorption of triglyceride on its surface. These results demonstrated that the cell wall of the microalgae was ruptured by the sulfonated rice husk solid acid catalyst and that the triglyceride contents were released. These results demonstrated that the cell wall of the microalgae was ruptured by the sulfonated rice husk catalyst and that the triglyceride contents were released.
Quah et al.80 fabricated the magnetic biochar from waste palm kernel shell (PMB) followed by sulfonation to generate active sulfonic sites—the optimization study was done with the help of response surface methodology. The biodiesel yield reaches up to 90.2% under optimum conditions. The incorporation of an iron layer, which may significantly enhance the catalyst separation performance in the biodiesel manufacturing process, allows PMB-SO3H to be developed as a potential heterogeneous catalyst, which has significant practical implications. In another study, woody biomass was pyrolyzed at 450, 675, and 875 °C and sulfonated at 150 °C using fuming H2SO4.81 Canola oil was used to assess the transesterification process of the synthesized catalyst. The author observed that the efficiency of biodiesel formation was dependent on total acid density and surface area, suggesting the highest efficiency of biodiesel was obtained at 675 and 875 °C. The biochar's carbon sheets become more reoriented toward a more graphite-like structure at higher carbonization temperatures, resulting in a decrease in the overall acid density despite an increase in surface area. Due to increased stiffness, sulfonic acid groups were more challenging to incorporate into activated carbon sheets at 875 °C. Carbonization at high temperatures helps form linked rigid carbon frameworks; may address this lack of activity by tuning the carbonization temperature to promote the creation of polycyclic aromatic carbons, which can then be sulfonated.82 Table 4 demonstrate the various biomass derived catalyst for biodiesel formation.
| Source | Acid density | Optimization parameters | Efficiency (%) | References |
|---|---|---|---|---|
| Durian peel | 1.22 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 9 wt%, 65 °C, 10 min 10, 9 wt%, 65 °C, 10 min |
81.57 | 72 |
| Micro-algal biomass | 3.25 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11, 3 wt%, 60 °C, 6 h (WCO)/1 11, 3 wt%, 60 °C, 6 h (WCO)/1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11, 4 wt%, 70 °C, 8 h (AO) 11, 4 wt%, 70 °C, 8 h (AO) |
96.25, 94.23 | 39 |
| Orange peel | 1.57 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15, 5 wt%, 65 °C, 4.5 h 15, 5 wt%, 65 °C, 4.5 h |
91.68 | 73 |
| Corncob | 0.89 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, 75 °C, 0.4 h 6, 75 °C, 0.4 h |
88.7 | 74 |
| Murumuru shell | 4.19 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, 6 wt%, 135 °C, 4 h 30, 6 wt%, 135 °C, 4 h |
91.8 | 75 |
| Rice husk | 4.25 | —, 30 mg, 25 °C, 0.5 h | 100 | 79 |
| Palm kernel shell | 1.92 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13, 3.66 wt%, 65 °C, 1.7 h 13, 3.66 wt%, 65 °C, 1.7 h |
90.2 | 80 |
| Woody biomass | 1.2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15, 65 °C, 5 wt%, 3 h 15, 65 °C, 5 wt%, 3 h |
44.2 | 81 |
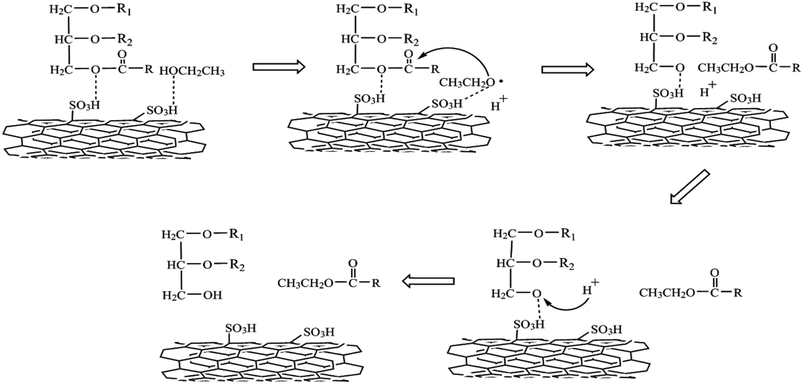 | ||
| Fig. 3 Probable reaction pathways for transesterification reaction by SO3H active sites.83 | ||
1. Triglyceride adsorbs on the catalyst surface, and SO3H provides the sites through interaction betwixt ester linkage and acid sites/protons.
2. Further, two electrons from oxygen atoms in C–O–C are thought to have been donated to SO3H groups. The oxygen atom attack most likely weakens the C–O–C bond, forming the carbonyl carbon OR1.
3. The adsorption of ethanol or alcohol follows a similar pattern. Sulfate ions increase ethanol's nucleophilicity to give protons (H+). The attack on C–O–C by sulfate ions reduces the binding strength of the carbonyl carbon –OR. The carbonyl carbon is subsequently attacked by nucleophilic ethanol, yielding CH3CH2COOR. Finally, the ethanol protons (H+) replace SO3H in R–OSO3H to generate glycerin, diglycerides, or monoglycerides.
Pseudo-homogeneous first-order84 and second-order reaction models85 for acidic catalyzed reactions are often used in heterogeneous catalyzed processes. Moreover, the Langmuir–Hinshelwood86 and Eley–Rideal model,65 both of which investigated the limiting factor in the chemical reaction process, have been published in the literature. The main difference between the Eley–Rideal model and the Langmuir–Hinshelwood model was that the Langmuir–Hinshelwood model assumes that two reactants (alcohol and triglyceride) were simultaneously adsorbed on the catalyst surface for reaction, while the Eley–Rideal model assumes that only one reactant (alcohol and triglyceride) was adsorbed on surface of catalyst for reaction.
6. Discussion and future perspectives
Biomass (a carbon material) is the most studied comprehensive material due to its low cost and easy availability. Among the family of solid protonic acids, the SO3H-functionalized carbon compounds, also known as “sulfonated carbons,” are a recent addition due to their high Brønsted acidity and low manufacturing cost.24 These acidic carbons with SO3H groups are typically generated by sulfonating amorphous carbon sources. SO3H functionalized materials are used as a substitute of liquid sulfuric acid due to their high mechanical, thermal, and chemical stability, as well as their surface and pore structure.25,87 Due to these properties, SO3H catalysts are used in multiple applications, including esterification, transesterification, electrocatalysis, water treatment, CO2 reduction, and energy storage.45 A wide range of carbonaceous material is obtained depending on the carbon precursor, having varying textural features and functionalized SO3H group. Due in part to dwindling resources of novel and rare earth metals typically utilized as catalysts in industrial processes, the application of heterogeneous carbon catalysts in electrochemical and organic transformation processes has significantly advanced over the last decade. The sulfonated carbons with high specific surface areas are potential catalysts for the transformation of large hydrophobic and hydrophilic compounds since they are water-tolerant and compatible with hydrothermal reaction conditions.Fig. 4(a) shows the effect of temperature and time on the total acid density caused due to OH, COOH, and SO3H. The total acid density is directly linked to biodiesel production. High total acid density results in an increase in the biodiesel production yield. Among the reaction methods, microwave and ultrasonic methods cause a high biodiesel yield in less time {Fig. 4(b)}. There are numerous approaches for the functionalization of carbonaceous material for SO3H grafting. Sulfonation coupled with carbonization (HTC) is an effective and straightforward approach for the fabrication of SO3H functionalized carbon catalyst, which possesses high SO3H acid density. Despite the high SO3H acid density, these carbonaceous materials possess high oxygen functional sites, less porosity, and low thermal stability. Consequently, the carbonaceous materials fabricated using the HTC method cannot be used at high temperatures and hydrophobic mediums. Additionally, these carbons exhibit low levels of polycondensation and a soft (less rigid) structure. Moreover, HTC methods cause serious environmental issues due to the utilization of significant amount of H2SO4, which results in the emission of hazardous acid gases like SO2 and SO3 and the production of neutralizing waste generated during washing and carbonizing.
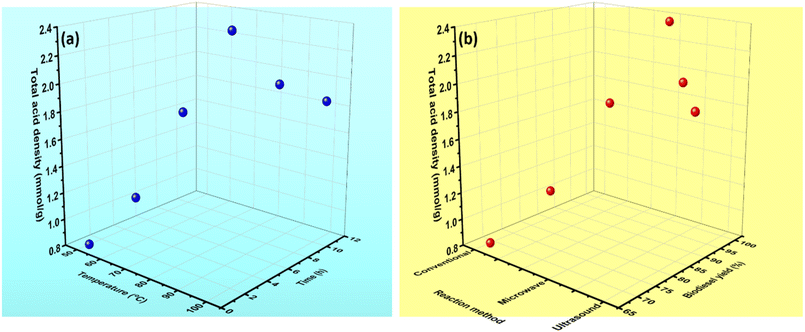 | ||
| Fig. 4 (a) Effect of temperature and time on total acid density, and (b) effect of total acid density on biodiesel production. | ||
In another method, biomass is pyrolyzed in the absence of oxygen (in the presence of N2 or Ar) to form biochar (carbonaceous material).88 The carbonization temperature has a direct influence on the surface of biochar. The catalyst synthesized at low carbonization temperature (400–600 °C) has a significant impact on the structure. Several acids, like H3PO4, HCl, and ZnCl2, improve the dehydration process, which results in an increase in the surface area and pore diameter of the synthesized carbonaceous material. A high temperature (greater than 600 °C) results in the breakage of carbonaceous structure. Then the synthesized biochar was functionalized with various activating agents such as sulfuric acid, toluene sulfonic acid, chlorosulfonic acid, fuming SO3, etc. The biochar has aromatic carbon structure in which the aromatic hydrogens are substituted by the SO3H groups during the sulfonation process. The incorporation of the SO3H active site on the biochar surface results in a slight decrease in the surface area of the carbonaceous material. The simultaneous carbonization and sulfonation process preserve the meso/nanostructure of the carbonaceous material, which bonded covalently with SO3H sites. The insertion of SO3H sites depends on a number of factors, including sulfonation time, temperature, amount, and type of sulfonating agent {Fig. 4(a)}. After grafting, a number of SO3H functionalized materials are obtained, which differs in terms of SO3H density, textural properties, and surface chemistry ((hydrophilicity/hydrophobicity). The catalyst activity directly depends on the total acid density, SO3H acid density, surface area, pore size, and the number of functional groups. However, the reusability study shows that the sulfonated carbon catalysts show effective reusability up to 4 to 5 cycles. The loss of total acid density due to SO3H site leaching is the reason for the loss of activity. On the other hand, the catalysts often experience activity loss due to reversible deactivation by ion exchange and pore blockage brought on by impurity adsorption. Biochar, hydrochar, and partly carbonized materials, all of which exhibit a modest polycondensation degree after being functionalized by sulfonic acid treatment, are known to undergo fast irreversible deactivation under high-temperature, high-pressure conditions. The carbonaceous material is deactivated in the alcohol medium due to the creation of sulfonate ester under high pressure.
The exact mechanism of deactivation of SO3H functionalized carbon catalyst is not clear till now. Additional experiment investigations like NMR, mass, and FTIR spectroscopic methods and molecular modelling may give insights details of deactivation, activation, and stability of actives sites under various conditions. The large-scale production of biodiesel from the SO3H functionalized carbon catalyst requires reaction kinetics by mathematical modeling. Density functional theory and molecular modeling elucidate the adsorption–desorption of products, reactants, and impurities (H2O and humins) on the catalyst surface. The selectivity, activity, activation, and deactivation of sulfonated carbon catalysts can be better understood by these modeling methods. Further research in flow fixed-bed reactors, in particular, should be conducted to assess catalyst durability beneath commercially acceptable conditions. More study should be carried out to assess the stability of SO3H active sites in the presence of oxidizing and reducing environment as the carbonaceous material has shown promising for bifunctional metal fabrication by selective deposition on oxygenate surface.
Glycerol formation during the transesterification process resulted in an increase in the cost of biodiesel synthesis. A simple approach is considered to remove glycerol from the reaction mixture. Nearly 10% of glycerol was produced from transesterification reactions. Glycerol can be valorized by numerous methods, such as acetalization, dehydration (acrolein), esterification (acetin formation), etherification, and transesterification (glycerol carbonate), which are used further for fuel additives. The heterogeneous acid catalysts are useful for the glycerol valorization and reduce the expense of biodiesel production.
Although biomass is a cheap carbon precursor for the development of biodiesel. However, highly refined vegetable oil resulted in a high biodiesel cost, which retard the growth of biodiesel synthesis. The waste cooking oil should be beneficiary, but the high free fatty acid content should not be neglected. So, it is better to use waste cooking oil after pre-treatment. FFA such as oleic acid and others are also good choices for biodiesel production.
Nanoparticle incorporation into sulfonated carbon catalyst significantly enhance the activity of the catalyst thus improving the biodiesel production. In addition, the use of nano-catalysts eliminates the need for cleansing, thereby reducing the expense of biodiesel production.
7. Conclusion
In this review, the authors discuss the utilization of various biomass-derived heterogeneous catalysts for biodiesel production. The economically viable biodiesel manufacturing needs moderate reaction conditions and an acceptable molar alcohol ratio. The SO3H functionalized carbon catalysts are a novel type of solid acid catalysts for biodiesel generation with a unique carbon structure, chemical inertness, Brønsted acidity, structural diversity, surface hydrophobicity, and high thermal and mechanical durability. The catalytic activity of the SO3H functionalized carbon material depends on the temperature, sulfonating agent, and time. The sulfonation process parameters determine the acid density and biodiesel production. The higher the total acid density of the catalyst, the higher the biodiesel production. The heterogeneous catalyst derived from biomass shows excellent reusability for up to four or five cycles. In addition, the mechanism and kinetics of biodiesel production were investigated throughout this study. In addition to being a cheap, readily manufactured, stable, and effective biodiesel production catalyst, the catalyst also benefits from being synthesized from biomass, making it a more environmentally friendly option.Conflicts of interest
There are no conflicts to declare.References
- H. M. Mahmudul, F. Y. Hagos, R. Mamat, A. A. Adam, W. F. W. Ishak and R. Alenezi, Production, characterization and performance of biodiesel as an alternative fuel in diesel engines – A review, Renewable Sustainable Energy Rev., 2017, 72, 497–509, DOI:10.1016/J.RSER.2017.01.001.
- E. K. Sitepu, K. Heimann, C. L. Raston and W. Zhang, Critical evaluation of process parameters for direct biodiesel production from diverse feedstock, Renewable Sustainable Energy Rev., 2020, 123, 109762, DOI:10.1016/J.RSER.2020.109762.
- S. B. Živković, M. V. Veljković, I. B. Banković-Ilić, I. M. Krstić, S. S. Konstantinović, S. B. Ilić, J. M. Avramović, O. S. Stamenković and V. B. Veljković, Technological, technical, economic, environmental, social, human health risk, toxicological and policy considerations of biodiesel production and use, Renewable Sustainable Energy Rev., 2017, 79, 222–247, DOI:10.1016/J.RSER.2017.05.048.
- N. Yadav, G. Yadav and M. Ahmaruzzaman, Microwave-assisted biodiesel production using –SO3H functionalized heterogeneous catalyst derived from a lignin-rich biomass, Sci. Rep., 2023, 13, 1–17, DOI:10.1038/s41598-023-36380-1.
- A. Alagumalai, O. Mahian, F. Hollmann and W. Zhang, Environmentally benign solid catalysts for sustainable biodiesel production: A critical review, Sci. Total Environ., 2021, 768, 144856, DOI:10.1016/J.SCITOTENV.2020.144856.
- M. Ahmad, R. Farhana, A. A. A. Raman and S. K. Bhargava, Synthesis and activity evaluation of heterometallic nano oxides integrated ZSM-5 catalysts for palm oil cracking to produce biogasoline, Energy Convers. Manage., 2016, 119, 352–360, DOI:10.1016/J.ENCONMAN.2016.04.069.
- F. M. Wako, A. S. Reshad, M. S. Bhalerao and V. V. Goud, Catalytic cracking of waste cooking oil for biofuel production using zirconium oxide catalyst, Ind. Crops Prod., 2018, 118, 282–289, DOI:10.1016/J.INDCROP.2018.03.057.
- M. M. K. Bhuiya, M. G. Rasul, M. M. K. Khan, N. Ashwath and A. K. Azad, Prospects of 2nd generation biodiesel as a sustainable fuel—Part: 1 selection of feedstocks, oil extraction techniques and conversion technologies, Renewable Sustainable Energy Rev., 2016, 55, 1109–1128, DOI:10.1016/J.RSER.2015.04.163.
- S. Basumatary, B. Nath and P. Kalita, Application of agro-waste derived materials as heterogeneous base catalysts for biodiesel synthesis, J. Renewable Sustainable Energy, 2018, 10, 043105, DOI:10.1063/1.5043328.
- G. Yadav, N. Yadav and M. Ahmaruzzaman, Microwave-assisted synthesis of biodiesel by a green carbon-based heterogeneous catalyst derived from areca nut husk by one-pot hydrothermal carbonization, Sci. Rep., 2022, 12, 1–14, DOI:10.1038/s41598-022-25877-w.
- N. I. Mohammed, N. A. Kabbashi, M. Z. Alam and M. E. Mirghani, Jatropha curcas oil characterization and its significance for feedstock selection in biodiesel production, catalyst, 2014, 6, 7, DOI:10.7763/IPCBEE.
- J. Van Gerpen, Biodiesel processing and production, Fuel Process. Technol., 2005, 86, 1097–1107, DOI:10.1016/J.FUPROC.2004.11.005.
- N. Yadav, G. Yadav and M. Ahmaruzzaman, Biomass-derived sulfonated polycyclic aromatic carbon catalysts for biodiesel production by esterification reaction, Biofuels, Bioprod. Biorefin., 2023 DOI:10.1002/BBB.2486.
- G. Yadav and M. Ahmaruzzaman, Citrus limetta Peel-Derived Catalyst for Sustainable Production of Biodiesel, ACS Omega, 2022, 7, 28534–28544, DOI:10.1021/ACSOMEGA.2C03314.
- S. H. Y. S. Abdullah, N. H. M. Hanapi, A. Azid, R. Umar, H. Juahir, H. Khatoon and A. Endut, A review of biomass-derived heterogeneous catalyst for a sustainable biodiesel production, Renewable Sustainable Energy Rev., 2017, 70, 1040–1051, DOI:10.1016/J.RSER.2016.12.008.
- F. Cheng and X. Li, Preparation and Application of Biochar-Based Catalysts for Biofuel Production, Catal, 2018, 8, 346, DOI:10.3390/CATAL8090346.
- A. MacArio and G. Giordano, Catalytic conversion of renewable sources for biodiesel production: A comparison between biocatalysts and inorganic catalysts, Catal. Lett., 2013, 143, 159–168, DOI:10.1007/S10562-012-0949-3.
- D. Y. C. Leung, X. Wu and M. K. H. Leung, A review on biodiesel production using catalyzed transesterification, Appl. Energy, 2010, 87, 1083–1095, DOI:10.1016/J.APENERGY.2009.10.006.
- Z. Helwani, M. R. Othman, N. Aziz, J. Kim and W. J. N. Fernando, Solid heterogeneous catalysts for transesterification of triglycerides with methanol: A review, Appl. Catal., A, 2009, 363, 1–10, DOI:10.1016/J.APCATA.2009.05.021.
- M. Tariq, S. Ali and N. Khalid, Activity of homogeneous and heterogeneous catalysts, spectroscopic and chromatographic characterization of biodiesel: A review, Renewable Sustainable Energy Rev., 2012, 16, 6303–6316, DOI:10.1016/J.RSER.2012.07.005.
- J. M. Marchetti, V. U. Miguel and A. F. Errazu, Possible methods for biodiesel production, Renewable Sustainable Energy Rev., 2007, 11, 1300–1311, DOI:10.1016/J.RSER.2005.08.006.
- A. Guldhe, B. Singh, T. Mutanda, K. Permaul and F. Bux, Advances in synthesis of biodiesel via enzyme catalysis: Novel and sustainable approaches, Renewable Sustainable Energy Rev., 2015, 41, 1447–1464, DOI:10.1016/J.RSER.2014.09.035.
- M. Hara, T. Yoshida, A. Takagaki, T. Takata, J. N. Kondo, S. Hayashi and K. Domen, A carbon material as a strong protonic acid, Angew. Chem., Int. Ed., 2004, 43, 2955–2958, DOI:10.1002/ANIE.200453947.
- M. Hara, Biomass conversion by a solid acid catalyst, Energy Environ. Sci., 2010, 3, 601–607, 10.1039/B922917E.
- K. Nakajima and M. Hara, Amorphous carbon with SO3H groups as a solid brønsted acid catalyst, ACS Catal., 2012, 2, 1296–1304, DOI:10.1021/CS300103K.
- M. Toda, A. Takagaki, M. Okamura, J. N. Kondo, S. Hayashi, K. Domen and M. Hara, Biodiesel made with sugar catalyst, Nat, 2005, 438, 178, DOI:10.1038/438178a.
- Z. E. Tang, S. Lim, Y. L. Pang, H. C. Ong and K. T. Lee, Synthesis of biomass as heterogeneous catalyst for application in biodiesel production: State of the art and fundamental review, Renewable Sustainable Energy Rev., 2018, 92, 235–253, DOI:10.1016/J.RSER.2018.04.056.
- J. C. Bergmann, D. D. Tupinambá, O. Y. A. Costa, J. R. M. Almeida, C. C. Barreto and B. F. Quirino, Biodiesel production in Brazil and alternative biomass feedstocks, Renewable Sustainable Energy Rev., 2013, 21, 411–420, DOI:10.1016/J.RSER.2012.12.058.
- J. M. Fonseca, L. Spessato, A. L. Cazetta, C. da Silva and V. de C. Almeida, Sulfonated carbon: synthesis, properties and production of biodiesel, Chem. Eng. Process., 2022, 170, 108668, DOI:10.1016/J.CEP.2021.108668.
- Z. Zailan, M. Tahir, M. Jusoh and Z. Y. Zakaria, A review of sulfonic group bearing porous carbon catalyst for biodiesel production, Renewable Energy, 2021, 175, 430–452, DOI:10.1016/J.RENENE.2021.05.030.
- A. V. Bridgwater, A. J. Toft and J. G. Brammer, A techno-economic comparison of power production by biomass fast pyrolysis with gasification and combustion, Renewable Sustainable Energy Rev., 2002, 6, 181–246, DOI:10.1016/S1364-0321(01)00010-7.
- G. Yadav, N. Yadav and M. Ahmaruzzaman, Microwave-assisted sustainable synthesis of biodiesel on Oryza sativa catalyst derived from agricultural waste by esterification reaction, Chem. Eng. Process., 2023, 187, 109327, DOI:10.1016/J.CEP.2023.109327.
- M. Ahmaruzzaman, Proximate analyses and predicting HHV of chars obtained from cocracking of petroleum vacuum residue with coal, plastics and biomass, Bioresour. Technol., 2008, 99, 5043–5050, DOI:10.1016/J.BIORTECH.2007.09.021.
- S. Adhikari, H. Nam and J. P. Chakraborty, Conversion of Solid Wastes to Fuels and Chemicals Through Pyrolysis, Waste Biorefinery Potential Perspect, 2018, pp. 239–263, DOI:10.1016/B978-0-444-63992-9.00008-2.
- M. Antar, D. Lyu, M. Nazari, A. Shah, X. Zhou and D. L. Smith, Biomass for a sustainable bioeconomy: An overview of world biomass production and utilization, Renewable Sustainable Energy Rev., 2021, 139, 110691, DOI:10.1016/J.RSER.2020.110691.
- R. Luque, A. Pineda, J. C. Colmenares, J. M. Campelo, A. A. Romero, J. C. Serrano-Riz, L. F. Cabeza and J. Cot-Gores, Carbonaceous residues from biomass gasification as catalysts for biodiesel production, J. Nat. Gas Chem., 2012, 21, 246–250, DOI:10.1016/S1003-9953(11)60360-5.
- R. O. Araujo, J. da S. Chaar, L. S. Queiroz, G. N. da Rocha Filho, C. E. F. da Costa, G. C. T. da Silva, R. Landers, M. J. F. Costa, A. A. S. Gonçalves and L. K. C. de Souza, Low temperature sulfonation of acai stone biomass derived carbons as acid catalysts for esterification reactions, Energy Convers. Manage., 2019, 196, 821–830, DOI:10.1016/J.ENCONMAN.2019.06.059.
- T. Liu, Z. Li, W. Li, C. Shi and Y. Wang, Preparation and characterization of biomass carbon-based solid acid catalyst for the esterification of oleic acid with methanol, Bioresour. Technol., 2013, 133, 618–621, DOI:10.1016/J.BIORTECH.2013.01.163.
- M. Roy and K. Mohanty, Valorization of de-oiled microalgal biomass as a carbon-based heterogeneous catalyst for a sustainable biodiesel production, Bioresour. Technol., 2021, 337, 125424, DOI:10.1016/J.BIORTECH.2021.125424.
- A. Rana, M. S. M. Alghazal, M. M. Alsaeedi, R. S. Bakdash, C. Basheer and A. A. Al-Saadi, Preparation and Characterization of Biomass Carbon–Based Solid Acid Catalysts for the Esterification of Marine Algae for Biodiesel Production, BioEnergy Res., 2019, 12, 433–442, DOI:10.1007/S12155-019-9965-0.
- Y. T. Yang, X. X. Yang, Y. T. Wang, J. Luo, F. Zhang, W. J. Yang and J. H. Chen, Alcohothermal carbonization of biomass to prepare novel solid catalysts for oleic acid esterification, Fuel, 2018, 219, 166–175, DOI:10.1016/J.FUEL.2018.01.072.
- Z. Gao, S. Tang, X. Cui, S. Tian and M. Zhang, Efficient mesoporous carbon-based solid catalyst for the esterification of oleic acid, Fuel, 2015, 140, 669–676, DOI:10.1016/J.FUEL.2014.10.012.
- W. W. Mar and E. Somsook, Sulfonic-Functionalized Carbon Catalyst for Esterification of High Free Fatty Acid, Procedia Eng., 2012, 32, 212–218, DOI:10.1016/J.PROENG.2012.01.1259.
- J. T. Yu, A. M. Dehkhoda and N. Ellis, Development of Biochar-based Catalyst for Transesterification of Canola Oil, Energy Fuels, 2010, 25, 337–344, DOI:10.1021/EF100977D.
- L. Konwar, P. Mäki-Arvela and J.-P. Mikkola, SO3H-Containing Functional Carbon Materials: Synthesis, Structure, and Acid Catalysis, Chem. Rev., 2019, 119, 11576–11630, DOI:10.1021/acs.chemrev.9b00199.
- K. Malins, J. Brinks, V. Kampars and I. Malina, Esterification of rapeseed oil fatty acids using a carbon-based heterogeneous acid catalyst derived from cellulose, Appl. Catal., A, 2016, 519, 99–106, DOI:10.1016/J.APCATA.2016.03.020.
- F. Kučera and J. Jančář, Homogeneous and heterogeneous sulfonation of polymers: A review, Polym. Eng. Sci., 1998, 38, 783–792, DOI:10.1002/PEN.10244.
- A. P. da Luz Corrêa, R. R. C. Bastos, G. N. da Rocha Filho, J. R. Zamian and L. R. V. da Conceição, Preparation of sulfonated carbon-based catalysts from murumuru kernel shell and their performance in the esterification reaction, RSC Adv., 2020, 10, 20245–20256, 10.1039/D0RA03217D.
- S. S. Memon, N. Memon, S. Memon and A. Lachgar, An excellent sulfonated hydrothermal carbon catalyst from Mangifera indica L. (mango peels) for biodiesel production: preparation, characterization, optimization, and kinetic study, Biomass Convers. Biorefin., 2022, 12, 141–151, DOI:10.1007/S13399-021-01535-5/FIGURES/6.
- M. Cao, L. Peng, Q. Xie, K. Xing, M. Lu and J. Ji, Sulfonated Sargassum horneri carbon as solid acid catalyst to produce biodiesel via esterification, Bioresour. Technol., 2021, 324, 124614, DOI:10.1016/J.BIORTECH.2020.124614.
- L. H. R. Varão, T. A. L. Silva, H. D. Z. Zamora, L. C. de Morais and D. Pasquini, Synthesis of methyl biodiesel by esterification using magnetic nanoparticles coated with sulfonated lignin, Biomass Convers. Biorefin., 2022, 1, 1–14, DOI:10.1007/S13399-021-02214-1/TABLES/7.
- Z. E. Tang, S. Lim, Y. L. Pang, S. H. Shuit and H. C. Ong, Utilisation of biomass wastes based activated carbon supported heterogeneous acid catalyst for biodiesel production, Renewable Energy, 2020, 158, 91–102, DOI:10.1016/J.RENENE.2020.05.119.
- S. Lim, Y. L. Pang, S. H. Shuit, K. H. Wong and C. K. Leong, Synthesis and characterization of monk fruit seed (Siraitia grosvenorii)-based heterogeneous acid catalyst for biodiesel production through esterification process, Int. J. Energy Res., 2020, 44, 9454–9465, DOI:10.1002/ER.5007.
- H. Pan, J. Sun, J. Liu, Y. Zhang and S. Zhou, Preparation of sulfonated carbon derived from orange peel and its application in esterification, Chem. Phys. Lett., 2021, 770, 138395, DOI:10.1016/J.CPLETT.2021.138395.
- M. Cao, M. Lu, H. Yin, Q. Zhu, K. Xing and J. Ji, Effect of hemicellulose extraction pretreatment on sulfonated corncob biochar for catalytic biodiesel production, J. Environ. Chem. Eng., 2023, 11, 109058, DOI:10.1016/J.JECE.2022.109058.
- G. M. A. Bureros, A. A. Tanjay, D. E. S. Cuizon, A. W. Go, L. K. Cabatingan, R. C. Agapay and Y. H. Ju, Cacao shell-derived solid acid catalyst for esterification of oleic acid with methanol, Renewable Energy, 2019, 138, 489–501, DOI:10.1016/J.RENENE.2019.01.082.
- R. O. Araujo, V. O. Santos, F. C. P. Ribeiro, J. da S. Chaar, A. M. Pereira, N. P. S. Falcão and L. K. C. de Souza, Magnetic acid catalyst produced from acai seeds and red mud for biofuel production, Energy Convers. Manage., 2021, 228, 113636, DOI:10.1016/J.ENCONMAN.2020.113636.
- W. Y. Wong, S. Lim, Y. L. Pang, S. H. Shuit, W. H. Chen and K. T. Lee, Synthesis of renewable heterogeneous acid catalyst from oil palm empty fruit bunch for glycerol-free biodiesel production, Sci. Total Environ., 2020, 727, 138534, DOI:10.1016/J.SCITOTENV.2020.138534.
- M. J. Jiménez Toro, X. Dou, I. Ajewole, J. Wang, K. Chong, N. Ai, G. Zeng and T. Chen, Preparation and Optimization of Macroalgae-Derived Solid Acid Catalysts, Waste Biomass Valorization, 2019, 10, 805–816, DOI:10.1007/S12649-017-0101-0/FIGURES/6.
- M. F. Hussein, A. O. Abo El Naga, M. El Saied, M. M. AbuBaker, S. A. Shaban and F. Y. El Kady, Potato peel waste-derived carbon-based solid acid for the esterification of oleic acid to biodiesel, Environ. Technol. Innovation, 2021, 21, 101355, DOI:10.1016/J.ETI.2021.101355.
- S. Tang, X. Duan, Q. Zhang, C. Wang, W. Wang, W. Feng and T. Wang, Biodiesel production using a novel surface functionalized biomass residue solid green catalyst, Biomass Convers. Biorefin., 2022, 1, 1–10, DOI:10.1007/S13399-022-02576-0/FIGURES/12.
- H. Yu, Y. Cao, H. Li, G. Zhao, X. Zhang, S. Cheng and W. Wei, An efficient heterogeneous acid catalyst derived from waste ginger straw for biodiesel production, Renewable Energy, 2021, 176, 533–542, DOI:10.1016/J.RENENE.2021.05.098.
- H. Zhang, X. Luo, K. Shi, T. Wu, F. He, H. Yang, S. Zhang and C. Peng, Nanocarbon-based catalysts for esterification: Effect of carbon dimensionality and synergistic effect of the surface functional groups, Carbon, 2019, 147, 134–145, DOI:10.1016/J.CARBON.2019.02.079.
- J. Li, Y. J. Fu, X. J. Qu, W. Wang, M. Luo, C. J. Zhao and Y. G. Zu, Biodiesel production from yellow horn (Xanthoceras sorbifolia Bunge.) seed oil using ion exchange resin as heterogeneous catalyst, Bioresour. Technol., 2012, 108, 112–118, DOI:10.1016/J.BIORTECH.2011.12.129.
- R. Tesser, L. Casale, D. Verde, M. Di Serio and E. Santacesaria, Kinetics and modeling of fatty acids esterification on acid exchange resins, Chem. Eng. J., 2010, 157, 539–550, DOI:10.1016/J.CEJ.2009.12.050.
- A. B. Fadhil, A. M. Aziz and M. H. Al-Tamer, Biodiesel production from Silybum marianum L. seed oil with high FFA content using sulfonated carbon catalyst for esterification and base catalyst for transesterification, Energy Convers. Manage., 2016, 108, 255–265, DOI:10.1016/J.ENCONMAN.2015.11.013.
- Z. T. Alismaeel, A. S. Abbas, T. M. Albayati and A. M. Doyle, Biodiesel from batch and continuous oleic acid esterification using zeolite catalysts, Fuel, 2018, 234, 170–176, DOI:10.1016/J.FUEL.2018.07.025.
- H. R. Mahmoud, S. A. El-Molla and M. M. Ibrahim, Biodiesel production via stearic acid esterification over mesoporous ZrO2/SiO2 catalysts synthesized by surfactant-assisted sol-gel auto-combustion route, Renewable Energy, 2020, 160, 42–51, DOI:10.1016/J.RENENE.2020.06.005.
- Q. Shu, W. Zou, J. He, H. Lesmana, C. Zhang, L. Zou and Y. Wang, Preparation of the F−-SO4 2-/MWCNTs catalyst and kinetic studies of the biodiesel production via esterification reaction of oleic acid and methanol, Renewable Energy, 2019, 135, 836–845, DOI:10.1016/J.RENENE.2018.12.067.
- R. Tesser, M. Di Serio, M. Guida, M. Nastasi and E. Santacesaria, Kinetics of Oleic Acid Esterification with Methanol in the Presence of Triglycerides, Ind. Eng. Chem. Res., 2005, 44, 7978–7982, DOI:10.1021/IE050588O.
- M. Dokić, Ž. Kesić, J. Krstić, D. Jovanović and D. Skala, Decrease of free fatty acid content in vegetable oil using silica supported ferric sulfate catalyst, Fuel, 2012, 97, 595–602, DOI:10.1016/J.FUEL.2012.03.039.
- R. Leesing, S. Siwina and K. Fiala, Yeast-based biodiesel production using sulfonated carbon-based solid acid catalyst by an integrated biorefinery of durian peel waste, Renewable Energy, 2021, 171, 647–657, DOI:10.1016/J.RENENE.2021.02.146.
- D. R. Lathiya, D. V. Bhatt and K. C. Maheria, Synthesis of sulfonated carbon catalyst from waste orange peel for cost effective biodiesel production, Bioresour. Technol. Rep., 2018, 2, 69–76, DOI:10.1016/J.BITEB.2018.04.007.
- P. D. Rocha, L. S. Oliveira and A. S. Franca, Sulfonated activated carbon from corn cobs as heterogeneous catalysts for biodiesel production using microwave-assisted transesterification, Renewable Energy, 2019, 143, 1710–1716, DOI:10.1016/J.RENENE.2019.05.070.
- R. R. C. Bastos, A. P. da Luz Corrêa, P. T. S. da Luz, G. N. da Rocha Filho, J. R. Zamian and L. R. V. da Conceição, Optimization of biodiesel production using sulfonated carbon-based catalyst from an amazon agro-industrial waste, Energy Convers. Manage., 2020, 205, 112457, DOI:10.1016/J.ENCONMAN.2019.112457.
- P. Rechnia-Gorący, A. Malaika and M. Kozłowski, Acidic activated carbons as catalysts of biodiesel formation, Diamond Relat. Mater., 2018, 87, 124–133, DOI:10.1016/J.DIAMOND.2018.05.015.
- C. Baroi and A. K. Dalai, Simultaneous esterification, transesterification and chlorophyll removal from green seed canola oil using solid acid catalysts, Catal. Today, 2013, 207, 74–85, DOI:10.1016/J.CATTOD.2012.07.003.
- P. D. Patil, H. Reddy, T. Muppaneni, T. Schaub, F. O. Holguin, P. Cooke, P. Lammers, N. Nirmalakhandan, Y. Li, X. Lu and S. Deng, In situ ethyl ester production from wet algal biomass under microwave-mediated supercritical ethanol conditions, Bioresour. Technol., 2013, 139, 308–315, DOI:10.1016/J.BIORTECH.2013.04.045.
- A. Wadood, A. Rana, C. Basheer, S. A. Razzaq and W. Farooq, In situ Transesterification of Microalgae Parachlorella kessleri Biomass Using Sulfonated Rice Husk Solid Catalyst at Room Temperature, BioEnergy Res., 2020, 13, 530–541, DOI:10.1007/S12155-019-10060-3/TABLES/3.
- R. V. Quah, Y. H. Tan, N. M. Mubarak, J. Kansedo, M. Khalid, E. C. Abdullah and M. O. Abdullah, Magnetic biochar derived from waste palm kernel shell for biodiesel production via sulfonation, Waste Manage., 2020, 118, 626–636, DOI:10.1016/J.WASMAN.2020.09.016.
- J. T. Yu, A. M. Dehkhoda and N. Ellis, Development of biochar-based catalyst for transesterification of canola oil, Energy Fuels, 2011, 25, 337–344, DOI:10.1021/EF100977D/ASSET/IMAGES/LARGE/EF-2010-00977D_0005.JPEG.
- R. Xing, Y. Liu, Y. Wang, L. Chen, H. Wu, Y. Jiang, M. He and P. Wu, Active solid acid catalysts prepared by sulfonation of carbonization-controlled mesoporous carbon materials, Microporous Mesoporous Mater., 2007, 105, 41–48, DOI:10.1016/J.MICROMESO.2007.06.043.
- Q. Guan, Y. Li, Y. Chen, Y. Shi, J. Gu, B. Li, R. Miao, Q. Chen and P. Ning, Sulfonated multi-walled carbon nanotubes for biodiesel production through triglycerides transesterification, RSC Adv., 2017, 7, 7250–7258, 10.1039/C6RA28067F.
- P. Andreo-Martínez, N. García-Martínez, M. del M. Durán-del-Amor and J. Quesada-Medina, Advances on kinetics and thermodynamics of non-catalytic supercritical methanol transesterification of some vegetable oils to biodiesel, Energy Convers. Manage., 2018, 173, 187–196, DOI:10.1016/J.ENCONMAN.2018.07.069.
- L. J. Konwar, J. Wärnå, P. Mäki-Arvela, N. Kumar and J. P. Mikkola, Reaction kinetics with catalyst deactivation in simultaneous esterification and transesterification of acid oils to biodiesel (FAME) over a mesoporous sulphonated carbon catalyst, Fuel, 2016, 166, 1–11, DOI:10.1016/J.FUEL.2015.10.102.
- E. G. Al-Sakkari, S. T. El-Sheltawy, N. K. Attia and S. R. Mostafa, Kinetic study of soybean oil methanolysis using cement kiln dust as a heterogeneous catalyst for biodiesel production, Appl. Catal., B, 2017, 206, 146–157, DOI:10.1016/J.APCATB.2017.01.008.
- M. Okamura, A. Takagaki, M. Toda, J. N. Kondo, K. Domen, T. Tatsumi, M. Hara and S. Hayashi, Acid-catalyzed reactions on flexible polycyclic aromatic carbon in amorphous carbon, Chem. Mater., 2006, 18, 3039–3045, DOI:10.1021/CM0605623.
- G. Yadav, N. Yadav, M. Sultana and M. Ahmaruzzaman, A comprehensive review on low-cost waste-derived catalysts for environmental remediation, Mater. Res. Bull., 2023, 164, 112261, DOI:10.1016/J.MATERRESBULL.2023.112261.
| This journal is © The Royal Society of Chemistry 2023 |
