Open Access Article
Open Access ArticleIron/vanadium co-doped tungsten oxide nanostructures anchored on graphitic carbon nitride sheets (FeV-WO3@g-C3N4) as a cost-effective novel electrode material for advanced supercapacitor applications
Sajida Parveena,
Eric W. Cochran b,
Sonia Zulfiqarbc,
Mohammed A. Amind,
Muhammad Farooq Warsi
b,
Sonia Zulfiqarbc,
Mohammed A. Amind,
Muhammad Farooq Warsi *a and
Khadija Chaudhary*a
*a and
Khadija Chaudhary*a
aInstitute of Chemistry, Baghdad-ul-Jadeed Campus, The Islamia University of Bahawalpur, Bahawalpur 63100, Pakistan. E-mail: farooq.warsi@iub.edu.pk; khadija.chaudhary.kc@gmail.com
bDepartment of Chemical and Biological Engineering, Iowa State University, Sweeney Hall, 618 Bissell Road, Ames, Iowa 50011, USA
cDepartment of Chemistry, Faculty of Science, University of Ostrava, 30. Dubna 22, Ostrava 701 03, Czech Republic
dDepartment of Chemistry, College of Science, Taif University, PO Box 11099, Taif 21944, Saudi Arabia
First published on 6th September 2023
Abstract
In this work, we studied the effect of iron (Fe) and vanadium (V) co-doping (Fe/V), and graphitic carbon nitride (g-C3N4) on the performance of tungsten oxide (WO3) based electrodes for supercapacitor applications. The lone pair of electrons on nitrogen can improve the surface polarity of the g-C3N4 electrode material, which may results in multiple binding sites on the surface of electrode for interaction with electrolyte ions. As electrolyte ions interact with g-C3N4, they quickly become entangled with FeV-WO3 nanostructures, and the contact between the electrolyte and the working electrode is strengthened. Herein, FeV-WO3@g-C3N4 is fabricated by a wet chemical approach along with pure WO3 and FeV-WO3. All of the prepared samples i.e., WO3, FeV-WO3, and FeV-WO3@g-C3N4 were characterized by XRD, FTIR, EDS, FESEM, XPS, Raman, and BET techniques. Electrochemical performance is evaluated by cyclic voltammetry (CV), galvanic charge/discharge (GCD), and electrochemical impedance spectroscopy (EIS). It is concluded from electrochemical studies that FeV-WO3@g-C3N4 exhibits the highest electrochemical performance with specific capacitance of 1033.68 F g−1 at scan rate 5 mV s−1 in the potential window range from −0.8 to 0.25 V, that is greater than that for WO3 (422.76 F g−1) and FeV-WO3 (669.76 F g−1). FeV-WO3@g-C3N4 has the highest discharge time (867 s) that shows it has greater storage capacity, and its coulombic efficiency is 96.7%, which is greater than that for WO3 (80.1%) and FeV-WO3 (92.1%), respectively. Furthermore, excellent stability up to 2000 cycles is observed in FeV-WO3@g-C3N4. It is revealed from EIS measurements that equivalent series resistance and charge transfer values calculated for FeV-WO3@g-C3N4 are 1.82 Ω and 0.65 Ω, respectively.
1. Introduction
The indiscriminative use of non-renewable resources of energy such as coal, oil, and natural gas, etc. for the generation of power causes environmental issues like climate change and depletion of fossil fuels. Furthermore, the planet's population is expanding quickly, and also their social desire to speed up the energy-generation and storage processes even more.1,2 In view of this scenario, extensive work is being done on environment friendly and renewable technologies for energy storage to limit the use of traditional energy resources and reduce environmental pollution.3–5 Different energy storage technologies, such as lithium-ion batteries (LIBs), capacitors, supercapacitors, and other energy storage devices are commonly available to fulfill market requirements. LIBs are excellent electrochemical devices because of their large energy density,6 however, they have low energy output and short lifespan because of the thick and heavy electrodes, and poor electron transfer.7Supercapacitors as energy storage devices are classified into different categories, as electrical double layer capacitors (EDLCs), pseudo-capacitors, and hybrid capacitors. Pseudo-capacitors have higher capacitance values than EDLCs. Supercapacitors with efficient power density and higher sustainability are highly recommended for electric and hybrid vehicles as well as for electronics and backup energy systems.8–10 Although supercapacitors are ideal for purposes that need relatively efficient improvements of power, such as power supply in exigent circumstances and peak power output that assists batteries in electric and hybrid vehicles, the energy storage characteristics of supercapacitors still need improvement.11 Many of the transition metal oxides have been used as electrode material for supercapacitors, such as MoO3,12 ZnO,13 MnO2,14 NiO,15 Co3O4,16 V2O5,17 CuO,18 NiS,19 and CoS20 due to pseudo-capacitive behavior. These days, the majority of researchers in this field are working on improving the electrode durability and specific capacitance. The nature of the electrode material determines a supercapacitor's entire electrochemical behavior and the electrodes with organized morphology are capable of achieving high capacitance value.21–26
Supercapacitors based on WO3 have been thoroughly explored and frequently reported.27–29 WO3 demonstrates pseudo-capacitive behavior due to its various oxidation states, quick surface interactions, and favorable crystalline structure for the interactions with electrolyte ions. It has lately gained attention as a potential electrode material in the fabrication of pseudo-capacitors due to its outstanding capacitive performance and natural availability.30–33 Additionally, WO3 is known to be a potential contender for electro-catalytic performance,34 in gas sensors,35 electro-chromism,36 rechargeable LIBs,37 dye sensitized solar cells,38 and for energy storage. The prime choice for supercapacitors is that the active material should have high electrical conductance and be capable of providing excellent electrical performance. Hence, multiple attempts have been made to improve WO3 efficiency.
Several researchers have worked on different strategies to improve the properties of WO3. WO3 has been doped with various types of metals to enhance its efficiency as a photoactive material in photoelectrochemical water splitting mechanism.39–43 Further, the energy storage characteristics of WO3 can also be improved by doping with different transition metal elements. For instance, Gupta et al. used a hydrothermal technique to synthesize Pd@WO3 nanostructures with enhanced energy density for energy storage applications.44 Kumar et al. manufactured Ni@WO3 and NiWO4 nanostructures by a microwave method for supercapacitor applications.45 VWO3 electrodes have drawn more interest.46–49 In general, it has been demonstrated that increasing the energy and power density of W containing cathode layers through the doping of ecologically favorable and sustainable V elements is an effective strategy.49 Further, compositing WO3 with other metal oxides or carbon supports is another strategy to improve its performance. The selection of components for the manufacturing of composite depends on how efficiently they work together. In comparison to metal oxides, carbon materials usually have high conductivity, specific energy, and surface area. Therefore, active material can be deposited on highly conductive materials such as CNTs, graphene, and graphitic carbon nitride (g-C3N4) to increase the electrical conductivity of the bare samples. The nitrogen element of g-C3N4 renders it suitable for supercapacitor and battery uses.50 The lone pair of electrons on nitrogen improves the surface polarity of g-C3N4 based electrode material, resulting in multiple binding sites on the electrode surface, which in turn enhances the wettability and affinity of electrode material for electrolyte ions. In addition, enhancement in surface area after the addition of carbon-based g-C3N4 helps in better dispersion of active sites or improves the electroactive surface area for charge storage. Metal oxides with a pseudo-capacitive nature can be effectively integrated with g-C3N4 structure in order to enhance the electrochemical performance of the subsequent electrode material.50–54
Hence, in this work, we first time report the influence of Fe and V co-doping and g-C3N4 fabrication on the structural, functional, morphological, and electrochemical performance of the WO3 for supercapacitor study. WO3 and FeV-WO3 were synthesized by co-precipitation method and its composite with (g-C3N4) was successfully fabricated by ultra-sonication technique. The electrochemical efficiency of FeV-WO3@g-C3N4 was compared with pure WO3 and FeV-WO3, and prepared samples were analyzed by different characterization techniques i.e. XRD, FTIR, FESEM, EDS, XPS, Raman, and BET analysis. The electrochemical measurements were also carried out by CV, GCD, and EIS measurements.
2. Experimental part
2.1. Materials
Sodium tungstate (Na2WO4), ethylene glycol (C2H6O2), hydrochloric acid (HCl), ammonium metavanadate (NH4VO3), iron(III) nitrate (Fe(NO3)3), sodium sulfate (Na2SO4), sodium carboxy methyl cellulose (SCMC), and ethanol (C2H6O) were used. The chemicals were pure and used without further purification.2.2. Synthesis of FeV-WO3
Iron vanadium co-doped tungsten oxide (FeV-WO3) was prepared by adding 0.2 g of ammonium metavanadate (NH4VO3), 0.2 g of iron nitrate (Fe(NO3)3), and 2 g of sodium tungstate (Na2WO4) into the mixture of water and ethylene glycol (volumetric ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). Obtained solution was stirred at 75 °C for almost 10 min along with continuous addition of 10 mL conc. HCl until pH ∼ 2 was achieved. After that, reaction was allowed to age for 12 h. Precipitates were washed with distilled water and ethanol successively until it became neutral. Then, the brown green colored FeV-WO3 precipitates were dried in the electric oven at 85 °C. Next, dried precipitates were annealed at 250 °C for 3 h and ground well to fine powder. In addition, pure WO3 was also synthesized for comparison following the same methodology without adding NH4VO3 and Fe (NO3)3 during the first step.
2). Obtained solution was stirred at 75 °C for almost 10 min along with continuous addition of 10 mL conc. HCl until pH ∼ 2 was achieved. After that, reaction was allowed to age for 12 h. Precipitates were washed with distilled water and ethanol successively until it became neutral. Then, the brown green colored FeV-WO3 precipitates were dried in the electric oven at 85 °C. Next, dried precipitates were annealed at 250 °C for 3 h and ground well to fine powder. In addition, pure WO3 was also synthesized for comparison following the same methodology without adding NH4VO3 and Fe (NO3)3 during the first step.
2.3. Synthesis of graphitic carbon nitride (g-C3N4)
Appropriate amount of melamine was taken in a ceramic crucible and placed in the lab furnace for annealing at 500 °C for 2 h. The yellow-colored powder obtained was allowed to cool down and then ground in to fine powder. For exfoliation, as obtained powdered material was added in water and ultra-sonicated for 5 h and then dried in the electric oven. Hence, graphitic carbon nitride (g-C3N4) was obtained. It was used for the synthesis of the composite.2.4. Synthesis of FeV-WO3@g-C3N4
The composite of FeV-WO3 was prepared with g-C3N4 through ultra-sonication method. Briefly, FeV-WO3 and g-C3N4 were taken in 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio in 20 mL distilled water. After ultra-sonication of the solution for 2 h, the homogeneous suspension was dried in the electric oven at 60 °C. Finally, brown yellow colored powder of FeV-WO3@g-C3N4 was obtained.
2 ratio in 20 mL distilled water. After ultra-sonication of the solution for 2 h, the homogeneous suspension was dried in the electric oven at 60 °C. Finally, brown yellow colored powder of FeV-WO3@g-C3N4 was obtained.
2.5. Electrochemical tests
All the electrochemical measurements i.e., GCD, CV, and EIS were performed with the GAMRY interface/5000E consisting of three electrodes with 1 M Na2SO4 as an electrolyte. Platinum wire and Ag/AgCl were utilized as counter and reference electrodes. For working electrode, 0.005 g of the prepared samples were added into 3 μL of sodium carboxy methyl cellulose (SCMC) and then 1 mL of ethanol was added into it. Nickel foam was cut into a piece of 2 cm in length and 1 cm in width, washed with HCl, and dried in oven at 60 °C. The mixture for working electrode was loaded on Ni foam and dried at 60 °C. The whole synthesis process and fabrication of electrode are schematically shown in Fig. 1.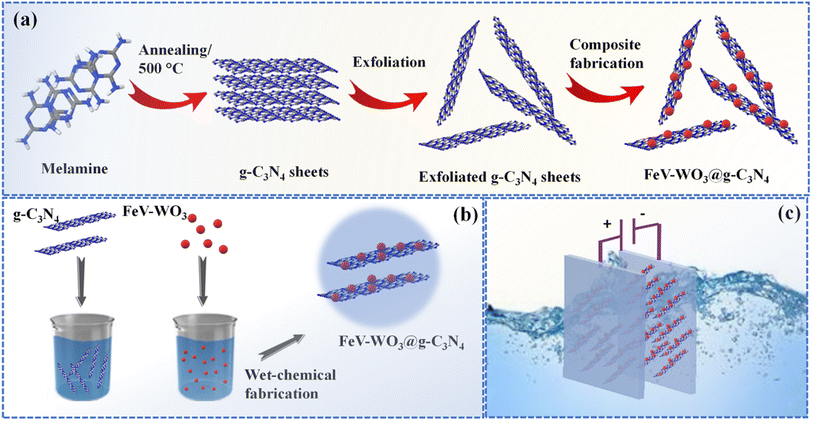 | ||
| Fig. 1 Schematic representation of (a and b) synthesis of FeV-WO3@g-C3N4 composite and (c) fabrication of electrode. | ||
3. Results and discussion
3.1. Phase analysis
The structural analysis of as fabricated samples i.e., WO3, FeV-WO3, and FeV-WO3@g-C3N4 was performed by X-ray diffraction technique, as shown in Fig. 2(a). XRD pattern of WO3 showed peaks at 2θ values of 23.13°, 24.05°, 28.91°, 33.83°, 42.02°, 45.41°, 47.37°, 49.45°, 50.76°, 55.03°, 60.05°, 61.25°, 63.10°, 69.77°, 71.51°, 74.113°, and 76.75°, which can be assigned to (101), (201), (111), (300), (211), (002), (220), (310), (112), (221), (400), (212), (401), (222), (312), (103), and (330) diffraction planes, respectively (JCPDS# 33-1387). It confirms the monoclinic crystal phase of WO3. High intensity of peaks showed the excellent crystalline nature of WO3.55 XRD pattern of FeV-WO3 is almost identical to the WO3, although there is slight peak shifting to higher 2θ values which may result from the contraction in lattice parameters after the doping of Fe and V ions in parent lattice of WO3 (Fig. 2(b)). In XRD pattern of FeV-WO3@g-C3N4, an additional peak appeared at 27.2° in correspondence to (002) plane which is the distinguishable peak of g-C3N4.56 It is noteworthy that the diffraction peaks of FeV-WO3@g-C3N4 shifted to a lower angle as compared to FeV-WO3, with increase in the breadth of peaks (indicated by peak corresponding to (300) plane in Fig. 2(b)). This peak shifting of (300) plane can be explained in terms of changes in d-spacing and other crystallite parameters as a result of inclusion of g-C3N4 sheets in FeV-WO3@g-C3N4 composite.57,58 Appearance of (002) plane and peak shifting confirms the successful assembly of FeV-WO3 with g-C3N4 sheets.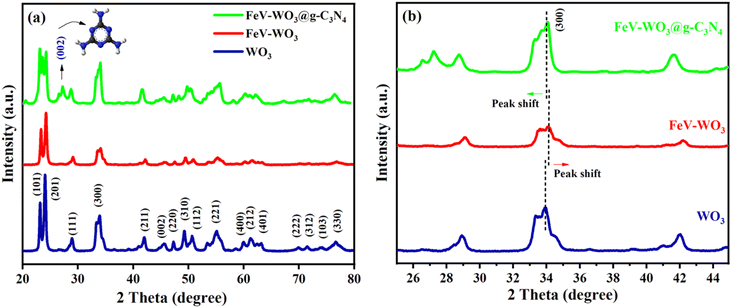 | ||
| Fig. 2 (a) XRD patterns of prepared WO3, FeV-WO3, and FeV-WO3@g-C3N4 composite, and (b) peak shift in XRD peaks of WO3, FeV-WO3, and FeV-WO3@g-C3N4 composite. | ||
3.2. Functional group analysis
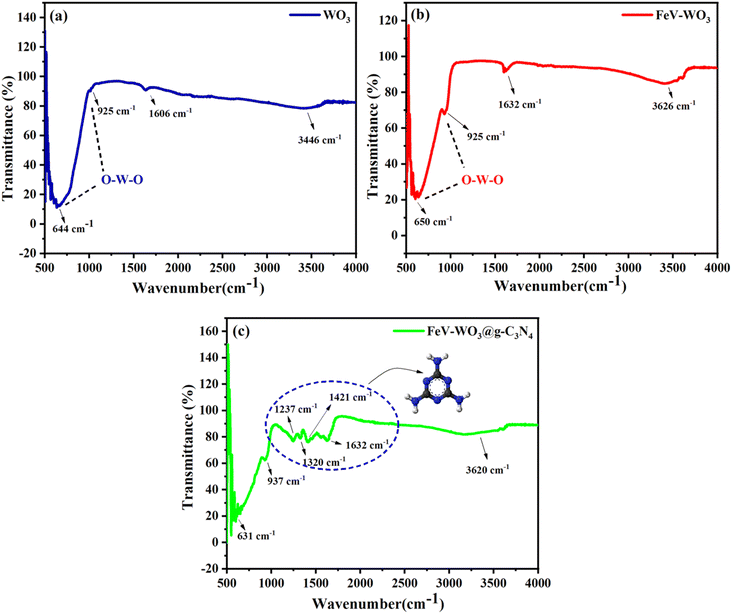
For the functional group analysis of all the prepared samples, FTIR technique was used. FTIR spectra of all the three prepared samples i.e., WO3, FeV-WO3, and FeV-WO3@g-C3N4 are exhibited in Fig. 3(a)–(c) in the wavenumber range of 500–4000 cm−1. FTIR spectrum of the WO3 show a broad band in the range of 500 cm−1 to 1000 cm−1. In Fig. 3(a), bands in the spectrum of WO3 at 644 cm−1 and 925 cm−1 clearly indicate the O–W–O vibrations and band that appeared at 3446 cm−1 is assigned to the stretching mode of the O–H group due to the adsorbed water.59,60 Similarly, in FTIR spectrum of FeV-WO3 two bands appeared at 650 cm−1 and 925 cm−1 show O–W–O vibrational modes (Fig. 3(b)).61 The band at 3626 cm−1 is due to the stretching vibration of O–H.62 In the FTIR spectrum of FeV-WO3@g-C3N4, various bands are shown in the region of 500–1632 cm−1 (Fig. 3(c)).63 Bands that appeared at 631 cm−1, and 937 cm−1 indicate the O–W–O vibrational modes. The bands at 1237 cm−1, 1320 cm−1, and 1421 cm−1 demonstrate different vibrational modes from g-C3N4 ring. The band that emerged at 3620 cm−1 is attributed to O–H stretching vibration.643.3. Morphological analysis
The apparent shape, particle size distribution, texture, and dimensional nature of the produced nanostructures and composite were examined by FESEM analysis. FESEM images of the prepared samples are shown in Fig. 4(a)–(f). Fig. 4(a) and (b) exhibits the FESEM micrographs of WO3 that shows asymmetrical 3D nanostructures with inhomogeneous particle sizes. The formation of asymmetric WO3 nanostructures in the acidic medium can be explained with the help of the following eqn (1) and (2):65| Na2WO4 + 2HCl → H2WO4 + 2NaCl | (1) |
| H2WO4 → WO3 + H2O | (2) |
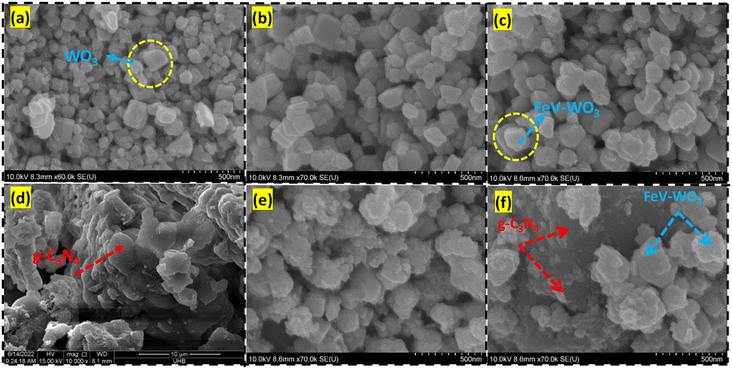 | ||
| Fig. 4 FESEM images of (a and b) WO3, (c) FeV-WO3, (d) g-C3N4, and (e and f) FeV-WO3@g-C3N4 composite. | ||
In the acidic medium, H+ and WO4−2 combine to form H2WO4. Nucleation and crystal growth largely depends on the reaction conditions i.e., pH, temperature, and reaction time.66,67 At low pH (high concentration of H+ ions) a large number of H2WO4 are formed. Supersaturation of H2WO4 is the driving force for nucleation.68 At supersaturation, when the decomposition temperature is attained, H2WO4 degrades to several small 0D WO3 nanoparticles. As the reaction time increases, these small nuclei in the absence of any surfactant aggregate together and grow into irregular shaped or asymmetric nanostructures.69 FESEM image of FeV-WO3 is represented in Fig. 4(c), which shows not much different morphology from WO3. FESEM image of g-C3N4 depicted in Fig. 4(d) clearly shows that g-C3N4 is comprised of sheet like structure with irregular surface that increases the surface area of the material. Fig. 4(e) and (f) for FeV-WO3@g-C3N4 shows that FeV-WO3 nanostructures were dispersed on the surface of g-C3N4 sheets. The particles of FeV-WO3 can easily attach to the surface of g-C3N4 due to the rough and uneven surface of g-C3N4. Such structures enhance the specific surface area that discloses more active sites and is more favorable for transfer of charges.70 It is revealed that the overall shape of the composite shows an assemblage of FeV-WO3 nanostructures with a loose stack of 2D sheets of g-C3N4.
3.4. Elemental analysis
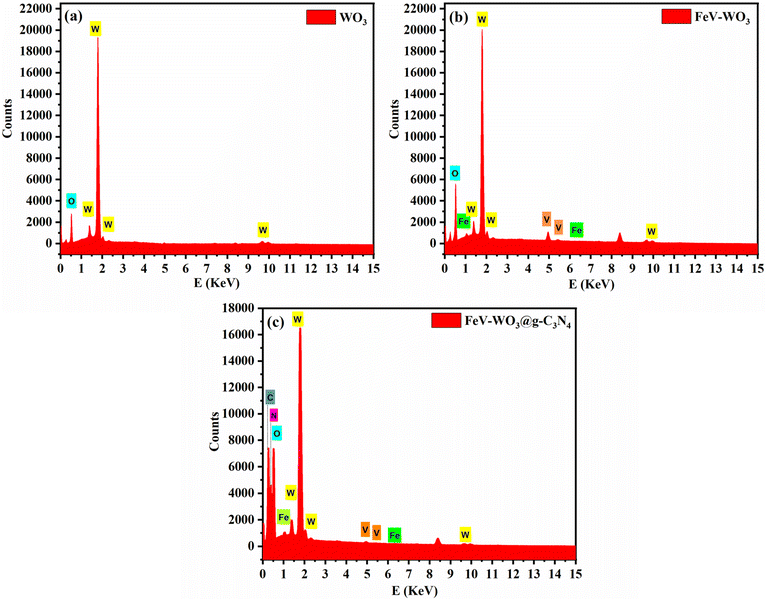
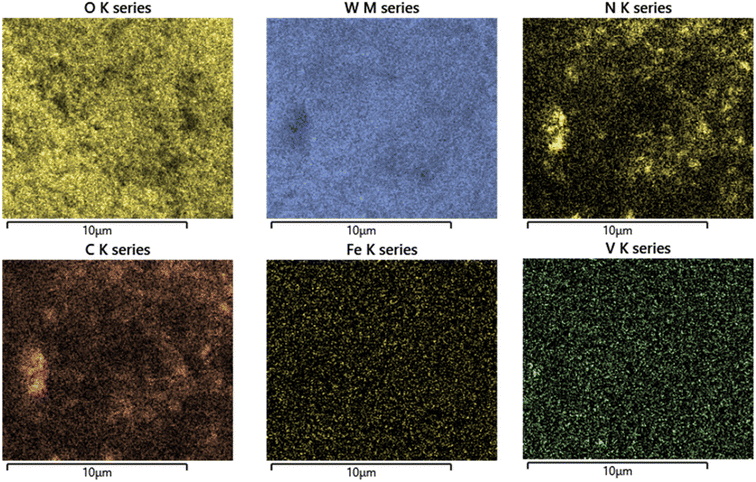
In Fig. 5(a)–(c) EDS spectra of WO3, FeV-WO3, and FeV-WO3@g-C3N4 are shown. EDS spectrum of WO3 consists of W (at E = 1.38 keV, 1.81 keV, and 9.6 keV) and O (0.53 keV) elements. After the doping of Fe and V ions, new peaks appeared at 0.79 keV and 6.39 keV for Fe, and 4.9 keV and 5.4 keV for V. Finally, for FeV-WO3@g-C3N4, C and N elements were observed at 0.23 keV and 0.38 keV. EDS mapping of FeV-WO3@g-C3N4 is shown in Fig. 6 which clearly represents the presence of all required elements i.e., O, W, N, C, Fe, and V.3.5. XPS analysis
XPS measurements were performed to further analyze the elemental composition of the prepared samples. XPS spectra of WO3, FeV-WO3, and FeV-WO3@g-C3N4 are depicted in Fig. 7(a)–(d). The overall survey spectrum of FeV-WO3@g-C3N4 in Fig. 7(a) confirms the presence of all elements i.e., W, O, Fe, V, C, and N. The primary doublet peaks of WO3 at binding energies of 35.7 and 37.8 eV are possessed by W 4f7/2 and W 4f5/2 core levels, respectively (Fig. 7(b)).71 These peaks represent that tungsten exhibits an oxidation state of W+6. The difference in binding energy between the W 4f7/2 and W 4f5/2 levels is around 2.1 eV, which demonstrates excellent purity of WO3. In XPS spectrum of FeV-WO3 the binding energy of FeV-WO3 nanoparticles was shifted slightly to lower energy side as compared to that of pure WO3, which is attributed to the strong interaction of Fe and V with WO3 (Fig. 7(c)). The XPS spectrum of FeV-WO3@g-C3N4 confirms the presence of g-C3N4 (Fig. 7(d)). The peaks that emerged at 284.9 eV and 288.9 eV are attributed to the C–C and N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds, respectively.72
N bonds, respectively.72
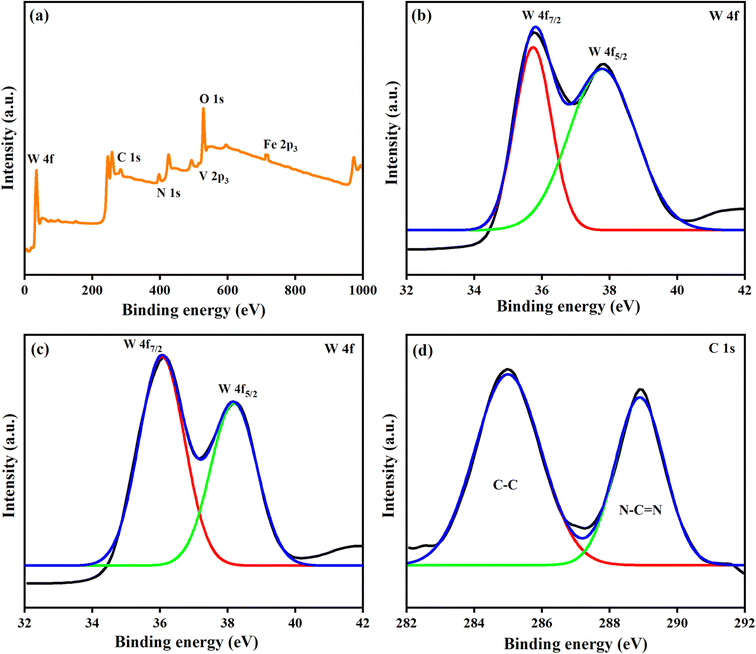 | ||
| Fig. 7 (a) XPS survey spectrum of FeV-WO3@g-C3N4. Deconvoluted high resolution XPS spectra of W 4f core levels of (b) WO3, (c) FeV-WO3, and C 1s core levels of (d) FeV-WO3@g-C3N4. | ||
3.6. Raman spectroscopy
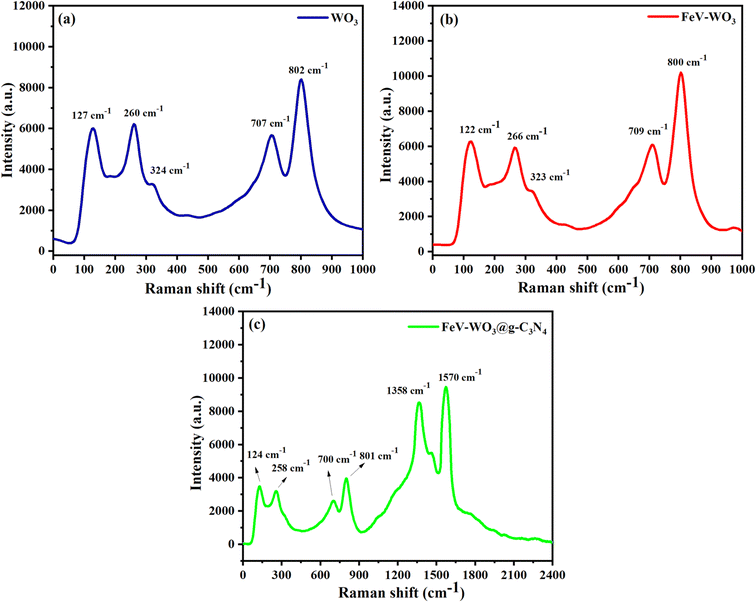
Raman spectroscopy was used to investigate the local symmetry of the prepared materials. Raman spectra of WO3, FeV-WO3, and FeV-WO3@g-C3N4 are exhibited in Fig. 8(a)–(c). In Fig. 8(a) peaks emerged at 127 cm−1, 260 cm−1, 324 cm−1, 707 cm−1, and 802 cm−1 in WO3 spectra confirmed that the synthesized material exhibits monoclinic phase.73 It is observed that peaks emerged in two pairs from 100–400 cm−1 and 600–900 cm−1 corresponding to O–W–O bending and stretching modes, respectively.74 The monoclinic phase WO3 exhibits a tilted and corner-shaped octahedral structure. The W atoms present on the off-center of the structure form 3 longer and 3 shorter bonds with the adjacent oxygen atom. This shows that the WO3 tungsten ion has a coordination number ‘6’ in the prepared sample.75 Raman spectra of FeV-WO3 showed similar pattern with almost same peak positions as exhibited by pure WO3 (Fig. 8(b)). However, after the addition of g-C3N4, two new peaks appeared in Fig. 8(c) at 1358 cm−1 and 1570 cm−1 corresponding to D and G bands of g-C3N4.76–783.7. BET analysis
The N2 adsorption/desorption isotherms with BJH pore size distribution plot are given in Fig. 9(a) and (b) for FeV-WO3@g-C3N4. N2 adsorption/desorption isotherms show typical type-IV behavior with a small H3 hysteresis loop in range of 0.8–1 (Fig. 9(a)).79 Presence of H3 hysteresis loop indicates the existence of mesopores in FeV-WO3@g-C3N4 which can also be verified from pore size distribution plot. Based on adsorption/desorption isotherms, the BET surface area for FeV-WO3@g-C3N4 was determined to be 40.2 m2 g−1. This significant specific surface area is certainly contributed by the g-C3N4 sheets.80 Pore size distribution plot in Fig. 9(b) shows that the mesopores are centralized at ∼3 nm and ∼35 nm. These mesopores in FeV-WO3@g-C3N4 develop ion diffusion channels and provide short ion diffusion paths with small resistance. The electrolyte ions can reach the interfacial sections of active electrode material through these mesopores which will lead to an enhancement of charge storage capacity of FeV-WO3@g-C3N4.81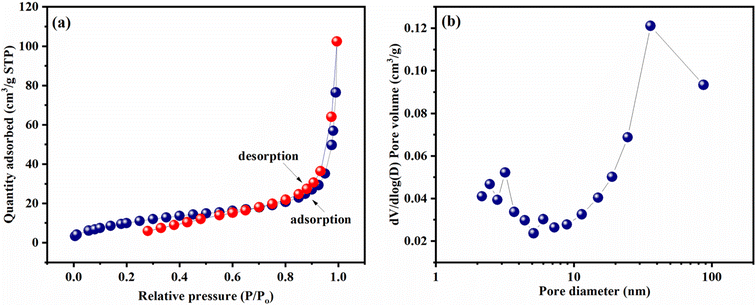 | ||
| Fig. 9 (a) N2 adsorption/desorption isotherms and (b) pore size distribution plot for FeV-WO3@g-C3N4 composite. | ||
4. Electrochemical analysis
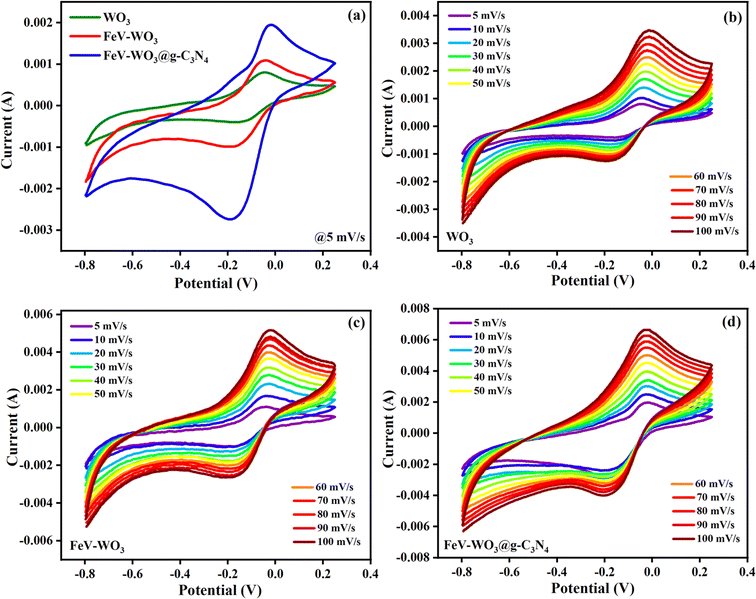
Electrochemical properties of all the prepared samples were tested by cyclic voltammetry (CV), galvanic charge–discharge (GCD), and electrochemical impedance spectroscopy (EIS) through a three electrode system in the electrolyte of 1 M Na2SO4. CV of WO3, FeV-WO3, and FeV-WO3@g-C3N4 electrodes was performed at room temperature (26 °C) at different scan rates from 5 mV s−1 to 100 mV s−1 in Fig. 10(a)–(d). The potential window range of the CV profile was from −0.8 to 0.25 V (vs. Ag/AgCl). Fig. 10(a) shows comparative CV profiles of all samples at 5 mV s−1. The CV curves exhibited duck shape with clear oxidation and reduction peaks which shows the pseudo-capacitive processes. During the charging process, Na+ ions from the electrolyte were successfully incorporated onto the surface of electrodes and detached during the discharging process. The whole response mechanism can be illustrated by the following reaction in eqn (3):| WO3 + xNa+ ↔ xNa+WO3 | (3) |
Further, it can be clearly observed that the composite FeV-WO3@g-C3N4 exhibited higher current and CV area as compared to WO3 and FeV-WO3 which is related to higher electrodeposition and charge storage capacity, indicating the advantages of g-C3N4 for enhanced electrochemical performance. The current of the CV curves increased by increasing the scan rate in all cases (Fig. 10(b)–(d)) which shows direct relation between scan rate and current. CV curves remained uniform along y-axis with the increase in scan rate, and it also shows the good reversibility of redox processes.82
Specific capacitance of all the prepared electrodes at different scan rates i.e., 5 mV s−1 to 100 mV s−1 was calculated by the following eqn (4):83,84
| CSP = ∫IdV/(2 × m × ΔV) | (4) |
In the above formula, IdV is the area of CV loop, m is the mass of material deposited on electrode, and ΔV is applied potential limits.
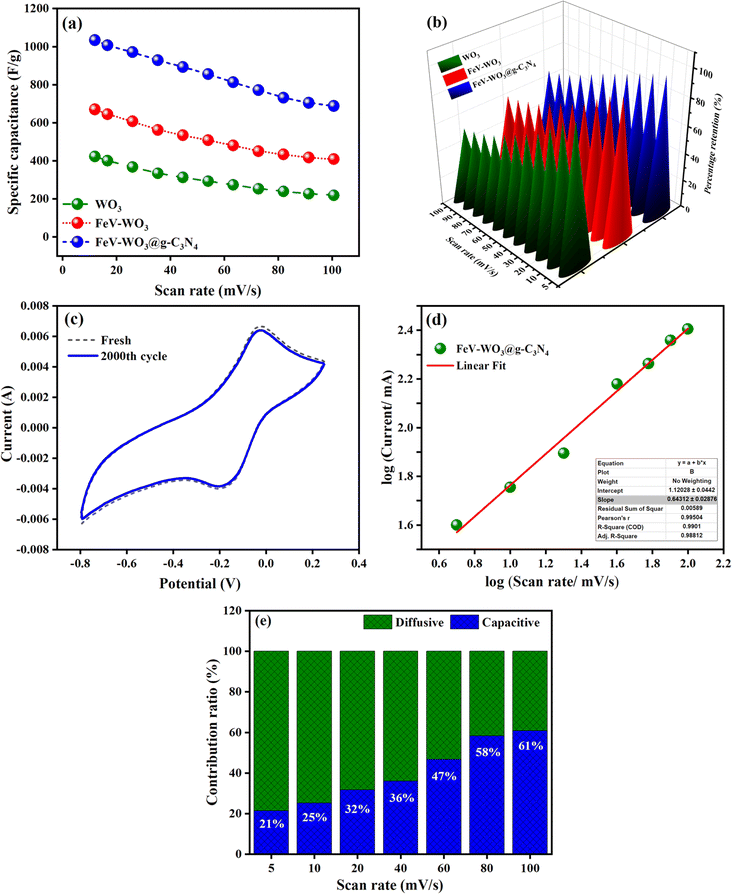
The specific capacitance values calculated for WO3, FeV-WO3, and FeV-WO3@g-C3N4 were found to be 422.76 F g−1, 669.76 F g−1, and 1033.68 F g−1, respectively. As expected, FeV-WO3@g-C3N4 has the highest specific capacitance value than WO3 and FeV-WO3 which is attributed to the increased interactions between electrolyte solution and active sites due to the presence of g-C3N4. The electrons interact with g-C3N4, they quickly become entangled with WO3 nanostructures, and the contact between the electrolyte and the working electrode is further strengthened by the structure of FeV-WO3@g-C3N4 due to their synergistic effects. The morphology of the g-C3N4 speeds up the charge transfer between FeV-WO3@g-C3N4 and current collector which reveals the efficient electrochemical performance of the FeV-WO3@g-C3N4. Fig. 11(a) shows change in specific capacitance of electrode with scan rate. Specific capacitance values at different scan rates for all samples are presented in Table 1. The specific capacitance of electrodes degraded with the scan rate that is because at high scan rate the charge storage process is mostly limited to the surface of electrode due to the limited time. Capacitance retention values at different scan rates for all samples are presented in Table 2. As found, FeV-WO3@g-C3N4 has highest capacitance retention value than WO3 and FeV-WO3 (Fig. 11(b)). The fundamental need of a supercapacitor for its real time implementation is the long-term cycle stability. In order to test the cyclic stability of the FeV-WO3@g-C3N4, CV measurement was repeated at constant scan rate of 100 mV s−1 in 1 M Na2SO4 electrolyte. The stability test of the electrode for 2000 cycles is shown in Fig. 11(c). It can be seen that our system can sustain approximately 2000 CV runs without any significant loss in the performance and distortion of CV shape. The percentage capacitance retention after 2000 cycles was determined to be 97.3%. A graph was plotted between log (scan rate) vs. log (current) for kinetic study of electrochemical measurements by Dunn's method (eqn (5) and (6)).85
| i = avb | (5) |
| Scan rate (mV s−1) | Specific capacitance (F g−1) | ||
|---|---|---|---|
| WO3 | FeV-WO3 | FeV-WO3@g-C3N4 | |
| 5 | 422.76 | 669.76 | 1033.68 |
| 10 | 400.38 | 645.06 | 1006.33 |
| 20 | 367.18 | 606.94 | 971.04 |
| 30 | 334.51 | 562.24 | 928.28 |
| 40 | 313.18 | 533.72 | 892.89 |
| 50 | 293.01 | 508.01 | 855.37 |
| 60 | 273.69 | 480.5 | 812.74 |
| 70 | 253.52 | 450.29 | 771.4 |
| 80 | 239.29 | 433.43 | 731.14 |
| 90 | 227.46 | 416.71 | 704.48 |
| 100 | 218.96 | 408.43 | 687.79 |
| Scan rate (mV s−1) | Specific capacitance retention (%) | ||
|---|---|---|---|
| WO3 | FeV-WO3 | FeV-WO3@g-C3N4 | |
| 5 | 100 | 100 | 100 |
| 10 | 94.7 | 96.31 | 97.35 |
| 20 | 86.85 | 90.62 | 93.94 |
| 30 | 79.12 | 83.94 | 89.8 |
| 40 | 74.08 | 79.68 | 86.38 |
| 50 | 69.31 | 75.85 | 82.75 |
| 60 | 64.73 | 71.74 | 78.62 |
| 70 | 59.97 | 67.23 | 74.62 |
| 80 | 56.6 | 64.71 | 70.73 |
| 90 | 53.8 | 62.21 | 68.15 |
| 100 | 51.79 | 60.98 | 66.53 |
Eqn (5) can be written as:
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) i = log i = log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a + b a + b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v v
| (6) |
By linear fitting, the slope value of anodic peak was found to be 0.643, represented in Fig. 11(d). This value of slope indicates that the dominant charge storage mechanism is diffusion controlled. Fig. 11(e) shows percentage contribution to charge storage at scan rates (5 to 100 mV s−1). The bar graph clearly indicates that at high scan rates diffusion controlled processes become limited and mostly the surface of electrode takes part in charge storage process, as discussed beforehand.
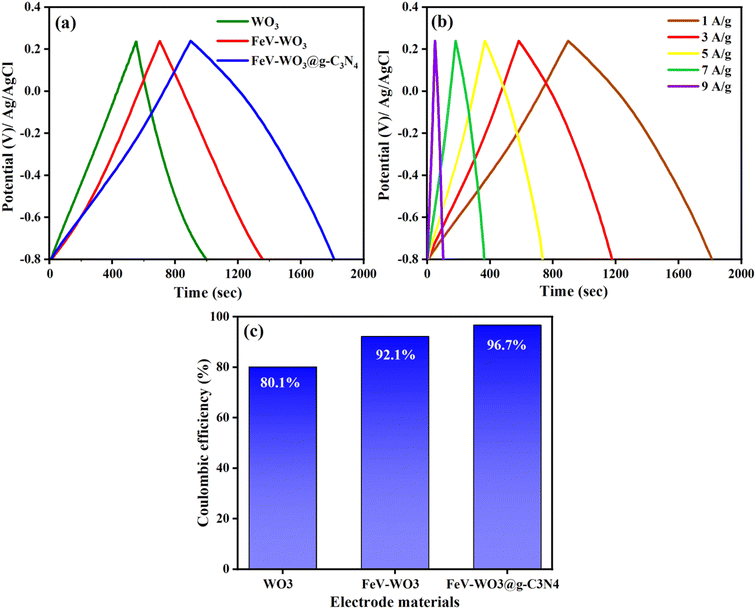
The GCD plots of the prepared electrodes WO3, FeV-WO3, and FeV-WO3@g-C3N4 at current density of 1 A g−1 and potential range of −0.8 to 0.25 V are represented in Fig. 12(a). GCD plots integrate to form quasi-triangular shape that represents electrochemical redox reactions at the electrode–electrolyte interface. Discharge time of the prepared electrodes WO3, FeV-WO3, and FeV-WO3@g-C3N4 was 440 s, 649 s, and 867 s, respectively. FeV-WO3@g-C3N4 exhibits a much longer discharge time than WO3 and FeV-WO3 indicating that it has greater charge storage capacity. The GCD plots of FeV-WO3@g-C3N4 at different current densities i.e., 1 A g−1, 3 A g−1, 5 A g−1, 7 A g−1, and 9 A g−1 are represented in Fig. 12(b). GCD plots demonstrate the good symmetry of the curves with no distortion in the shape of curves at high current densities. The coulombic efficiency for all electrodes was calculated by the following eqn (7):
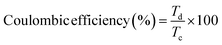 | (7) |
The calculated coulombic efficiency of WO3, FeV-WO3, and FeV-WO3@g-C3N4 at 1 A g−1 was 80.1%, 92.1%, and 96.7%, respectively, as shown in Fig. 12(c). FeV-WO3@g-C3N4 exhibits higher value of coulombic efficiency than WO3 and FeV-WO3 because of the presence of g-C3N4. Generally, higher value of internal resistance disturbs the coulombic efficiency value of energy storing material by consumption of a small amount of energy during the discharge time, that's why the energy released is always less than the amount of energy stored. The greater discharge current is related to the greater redox reactions that also tend to increase the internal resistance. The best coulombic efficiency value for composite illustrates uniformed charge and discharge current values and low internal resistance.3 Further, a comparison of different performance parameters (specific capacitance, cyclic stability, and coulombic efficiency) for FeV-WO3@g-C3N4 with already reported similar electrode materials in Table 3 shows comparable or even greater electrochemical response of FeV-WO3@g-C3N4.
| Electrode | Specific capacitance (F g−1) | Cyclic stability (%) | Coulombic efficiency (%) | Ref. |
|---|---|---|---|---|
| h-WO3 nanoflakes | 588 | 95.5%@5000 cycles | 90.7% | 86 |
| WO3 nanofibers | 436 | 98%@5000 cycles | 98% | 87 |
| Nb doped WO3//MnO2 | 126 | 90%@2000 cycles | 96% | 88 |
| WO3/graphene | 761 | 99.2%@5000 cycles | 98.2% | 89 |
| WO3/carbon fiber | 385 | 88%@1000 cycles | — | 90 |
| RGO–CNT-WO3 | 691.38 | 89.09%@5000 cycles | 98.4% | 91 |
| FeV-WO3@g-C3N4 | 1033.68 | 97.3%@2000 cycles | 96.7% | Current work |
EIS study is performed for the analysis of ion transfer and electrical conductivity of the prepared electrodes i.e., WO3, FeV-WO3, and FeV-WO3@g-C3N4. EIS measurements of the WO3, FeV-WO3, and FeV-WO3@g-C3N4 in 1 M Na2SO4 were performed in the frequency range of 0.1 Hz–100 kHz, and corresponding Nyquist plots are presented in Fig. 13(a)–(c). Nyquist plot has two separate zones, the high frequency portion usually represented by an arc, and the low frequency zone by a sloped line.92 The real impedance axis in Nyquist provides internal resistance of active material and the capacitance behavior of the electrode can be easily analyzed by the imaginary component of the Nyquist plot. The internal resistance or equivalent series resistance (ESR) is represented by Nyquist's intersect with the real part of Nyquist plot. ESR values of WO3, FeV-WO3, and FeV-WO3@g-C3N4 were 2.22 Ω, 1.92 Ω, and 1.82 Ω, respectively. FeV-WO3@g-C3N4 has low value of ESR which shows it contains low internal resistance and efficient electrical conductance. The charge-transfer resistance (RCT) at electrode–electrolyte interface can be determined by the diameter of semicircle at high frequency region. In case of distorted semicircle, RCT value can be determined by the extrapolation of arc on real axis. RCT values for WO3, FeV-WO3, and FeV-WO3@g-C3N4 were 1.53 Ω, 1.28 Ω, and 0.65 Ω, respectively. FeV-WO3@g-C3N4 has a smaller value of charge transfer resistance than WO3 and FeV-WO3. The line at 45 °C parallel to the imaginary part at a higher frequency zone defines the diffusion or Warburg resistance (Rw) that is primarily related to the diffusive resistance of the electrolyte ions in electrode. Electrodes showing a vertical line towards the low frequency zone of the Nyquist plot illustrate the ideal behavior of the electrode for supercapacitors.93 Equivalent circuit fitted to EIS data of prepared electrodes is shown in Fig. 13(d).
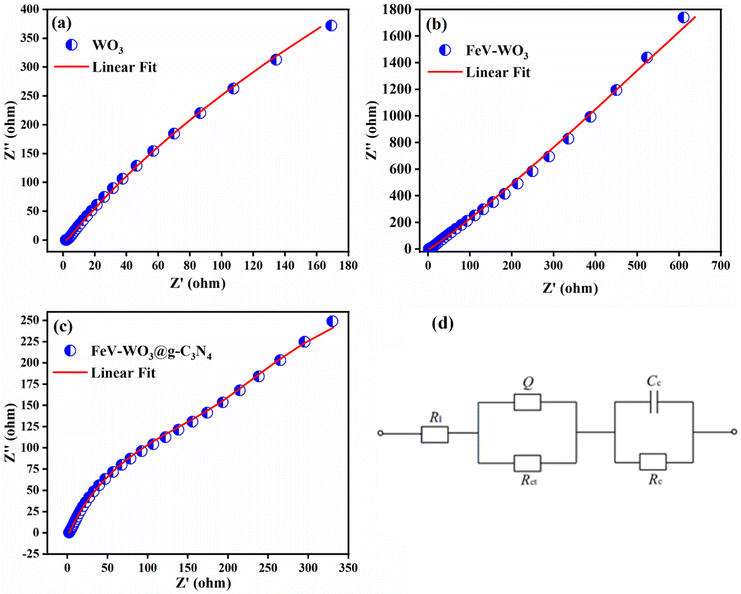 | ||
| Fig. 13 Nyquist plot of (a) WO3, (b) FeV-WO3, (c) FeV-WO3@g-C3N4, and (d) equivalent circuit diagram. | ||
5. Conclusions
In short, the conductive material FeV-WO3@g-C3N4 was prepared by wet chemical approach for supercapacitor study. When electrochemical studies were performed, prepared electrodes WO3, FeV-WO3, and FeV-WO3@g-C3N4 revealed specific capacitance of 422.76 F g−1, 669.76 F g−1, and 1033.68 F g−1 at scan rate 5 mV s−1, respectively in 1 M Na2SO4 electrolyte solution. FeV-WO3@g-C3N4 exhibited a higher value of specific capacitance and also maintained its shape without any significant change in current even after 2000 cycles which confirms that it is the potential material for supercapacitor application. GCD study showed composite exhibited greater discharge time than other electrodes of about 867 s which reveals that it has greater charge storage ability. It is concluded from EIS measurements that FeV-WO3@g-C3N4 has less electrochemical impedance than WO3 and FeV-WO3. The results demonstrated that FeV-WO3@g-C3N4 has the best structural, morphological, and componential properties for optimal efficiency. Therefore, the remarkable electrochemical behavior of FeV-WO3@g-C3N4 suggests that it can be employed as an effective supercapacitor electrode.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The researchers would like to acknowledge Deanship of Scientific Research, Taif University for funding this work. Authors are thankful to the Islamia University of Bahawalpur and HEC-Islamabad (Pakistan). Prof. Dr Sonia Zulfiqar is highly thankful for the support provided by the Statutory City of Ostrava, Czechia through Research Grant “Global Experts”. Prof. Cochran and Zulfiqar are grateful for FESEM imaging, EDS mapping services, and XRD provided by Drs Warren Straszheim and Carolina Selvati of the Materials Analysis and Research Laboratory of the Iowa State University Office of Biotechnology.References
- N. Shaheen, M. Aadil, S. Zulfiqar, H. Sabeeh, P. O. Agboola, M. F. Warsi, M. F. Aly Aboud and I. Shakir, Fabrication of different conductive matrix supported binary metal oxides for supercapacitors applications, Ceram. Int., 2021, 47, 5273–5285 CrossRef CAS.
- S. Munir, A. Rasheed, S. Zulfiqar, M. Aadil, P. O. Agboola, I. Shakir and M. F. Warsi, Synthesis, characterization and photocatalytic parameters investigation of a new CuFe2O4/Bi2O3 nanocomposite, Ceram. Int., 2020, 46, 29182–29190 CrossRef CAS.
- K. Chaudhary, S. Zulfiqar, H. H. Somaily, M. Aadil, M. F. Warsi and M. Shahid, Rationally designed multifunctional Ti3C2 MXene@Graphene composite aerogel integrated with bimetallic selenides for enhanced supercapacitor performance and overall water splitting, Electrochim. Acta, 2022, 431, 141103 CrossRef CAS.
- L. Dai, D. W. Chang, J. B. Baek and W. Lu, Carbon nanomaterials for advanced energy conversion and storage, Small, 2012, 8, 1130–1166 CrossRef CAS PubMed.
- L. Qie, W. Chen, H. Xu, X. Xiong, Y. Jiang, F. Zou, X. Hu, Y. Xin, Z. Zhang and Y. Huang, Synthesis of functionalized 3D hierarchical porous carbon for high-performance supercapacitors, Energy Environ. Sci., 2013, 6, 2497–2504 RSC.
- B. Ahmed, M. Shahid, D. H. Nagaraju, D. H. Anjum, M. N. Hedhili and H. N. Alshareef, Surface Passivation of MoO3 Nanorods by Atomic Layer Deposition toward High Rate Durable Li Ion Battery Anodes, ACS Appl. Mater. Interfaces, 2015, 7, 13154–13163 CrossRef CAS PubMed.
- T. Vignal, P. Banet, M. Pinault, R. Lafourcade, J. Descarpentries, L. Darchy, H. Hauf, C. Reynaud, M. Mayne-L’Hermite and P.-H. Aubert, Electropolymerized poly(3-methylthiophene) onto high density vertically aligned carbon nanotubes directly grown on aluminum substrate: application to electrochemical capacitors, Electrochim. Acta, 2020, 350, 136377 CrossRef CAS.
- D.-W. Kim, K.-Y. Rhee and S.-J. Park, Synthesis of activated carbon nanotube/copper oxide composites and their electrochemical performance, J. Alloys Compd., 2012, 530, 6–10 CrossRef CAS.
- A. Rudge, I. Raistrick, S. Gottesfeld and J. P. Ferraris, A study of the electrochemical properties of conducting polymers for application in electrochemical capacitors, Electrochim. Acta, 1994, 39, 273–287 CrossRef CAS.
- K. S. Ryu, K. M. Kim, N.-G. Park, Y. J. Park and S. H. Chang, Symmetric redox supercapacitor with conducting polyaniline electrodes, J. Power Sources, 2002, 103, 305–309 CrossRef CAS.
- H. Wang, H. S. Casalongue, Y. Liang and H. Dai, Ni(OH)2 Nanoplates Grown on Graphene as Advanced Electrochemical Pseudocapacitor Materials, J. Am. Chem. Soc., 2010, 132, 7472–7477 CrossRef CAS PubMed.
- J. Noh, C.-M. Yoon, Y. K. Kim and J. Jang, High performance asymmetric supercapacitor twisted from carbon fiber/MnO2 and carbon fiber/MoO3, Carbon, 2017, 116, 470–478 CrossRef CAS.
- Y. Zhang, H. Li, L. Pan, T. Lu and Z. Sun, Capacitive behavior of graphene–ZnO composite film for supercapacitors, J. Electroanal. Chem., 2009, 634, 68–71 CrossRef CAS.
- S.-I. Kim, J.-S. Lee, H.-J. Ahn, H.-K. Song and J.-H. Jang, Facile route to an efficient NiO supercapacitor with a three-dimensional nanonetwork morphology, ACS Appl. Mater. Interfaces, 2013, 5, 1596–1603 CrossRef CAS PubMed.
- S. I. Kim, J. S. Lee, H. J. Ahn, H. K. Song and J. H. Jang, Facile route to an efficient NiO supercapacitor with a three-dimensional nanonetwork morphology, ACS Appl. Mater. Interfaces, 2013, 5, 1596–1603 CrossRef CAS PubMed.
- S. K. Meher and G. R. Rao, Ultralayered Co3O4 for high-performance supercapacitor applications, J. Phys. Chem. C, 2011, 115, 15646–15654 CrossRef CAS.
- M. You, W. Zhang, X. Yan, H. Jiang, J. Miao, Y. Li, W. Zhou, Y. Zhu and X. Cheng, V2O5 nanosheets assembled on 3D carbon fiber felt as a free-standing electrode for flexible asymmetric supercapacitor with remarkable energy density, Ceram. Int., 2021, 47, 3337–3345 CrossRef CAS.
- S. K. Shinde, S. M. Mohite, A. A. Kadam, H. M. Yadav, G. S. Ghodake, K. Y. Rajpure, D. S. Lee and D. Y. Kim, Effect of deposition parameters on spray pyrolysis synthesized CuO nanoparticle thin films for higher supercapacitor performance, J. Electroanal. Chem., 2019, 850, 113433 CrossRef CAS.
- D. Wu, X. Xie, Y. Ma, J. Zhang, C. Hou, X. Sun, X. Yang, Y. Zhang, H. Kimura and W. Du, Morphology controlled hierarchical NiS/carbon hexahedrons derived from nitrilotriacetic acid-assembly strategy for high-performance hybrid supercapacitors, Chem. Eng. J., 2022, 433, 133673 CrossRef CAS.
- J. Li, D. Chen and Q. Wu, Facile synthesis of CoS porous nanoflake for high performance supercapacitor electrode materials, J. Energy Storage, 2019, 23, 511–514 CrossRef.
- X. Lu, D. Zheng, T. Zhai, Z. Liu, Y. Huang, S. Xie and Y. Tong, Facile synthesis of large-area manganese oxide nanorod arrays as a high-performance electrochemical supercapacitor, Energy Environ. Sci., 2011, 4, 2915–2921 RSC.
- X.-H. Xia, J.-P. Tu, Y.-J. Mai, X.-L. Wang, C.-D. Gu and X.-B. Zhao, Self-supported hydrothermal synthesized hollow Co3O4 nanowire arrays with high supercapacitor capacitance, J. Mater. Chem., 2011, 21, 9319–9325 RSC.
- D. Feng, Y. Lv, Z. Wu, Y. Dou, L. Han, Z. Sun, Y. Xia, G. Zheng and D. Zhao, Free-Standing Mesoporous Carbon Thin Films with Highly Ordered Pore Architectures for Nanodevices, J. Am. Chem. Soc., 2011, 133, 15148–15156 CrossRef CAS PubMed.
- H. Jiang, L. Yang, C. Li, C. Yan, P. S. Lee and J. Ma, High–rate electrochemical capacitors from highly graphitic carbon–tipped manganese oxide/mesoporous carbon/manganese oxide hybrid nanowires, Energy Environ. Sci., 2011, 4, 1813–1819 RSC.
- S. Guo, S. Dong and E. Wang, Constructing carbon-nanotube/metal hybrid nanostructures using homogeneous TiO2 as a spacer, Small, 2008, 4, 1133–1138 CrossRef CAS PubMed.
- H. Jiang, T. Zhao, J. Ma, C. Yan and C. Li, Ultrafine manganese dioxide nanowire network for high-performance supercapacitors, Chem. Commun., 2011, 47, 1264–1266 RSC.
- X. Lu, T. Zhai, X. Zhang, Y. Shen, L. Yuan, B. Hu, L. Gong, J. Chen, Y. Gao and J. Zhou, WO3−x@ Au@ MnO2 core–shell nanowires on carbon fabric for high-performance flexible supercapacitors, Adv. Mater., 2012, 24, 938–944 CrossRef CAS PubMed.
- J. H. Lee, K.-W. Kim, J. K. Kim and U. Jeong, DC Voltage Modulation for Integrated Self-Charging Power Systems of Triboelectric Nanogenerators and Ion Gel/WO3 Supercapacitors, ACS Appl. Electron. Mater., 2020, 2, 2550–2557 CrossRef CAS.
- S. Wang, H. Xu, T. Hao, P. Wang, X. Zhang, H. Zhang, J. Xue, J. Zhao and Y. Li, In situ XRD and operando spectra-electrochemical investigation of tetragonal WO3−x nanowire networks for electrochromic supercapacitors, NPG Asia Mater., 2021, 13, 51 CrossRef CAS.
- J. Xu, T. Ding, J. Wang, J. Zhang, S. Wang, C. Chen, Y. Fang, Z. Wu, K. Huo and J. Dai, Tungsten oxide nanofibers self-assembled mesoscopic microspheres as high-performance electrodes for supercapacitor, Electrochim. Acta, 2015, 174, 728–734 CrossRef CAS.
- P. Yang, P. Sun, L. Du, Z. Liang, W. Xie, X. Cai, L. Huang, S. Tan and W. Mai, Quantitative Analysis of Charge Storage Process of Tungsten Oxide that Combines Pseudocapacitive and Electrochromic Properties, J. Phys. Chem. C, 2015, 119, 16483–16489 CrossRef CAS.
- M. Qiu, P. Sun, L. Shen, K. Wang, S. Song, X. Yu, S. Tan, C. Zhao and W. Mai, WO3 nanoflowers with excellent pseudo-capacitive performance and the capacitance contribution analysis, J. Mater. Chem. A, 2016, 4, 7266–7273 RSC.
- J. Chu, D. Lu, X. Wang, X. Wang and S. Xiong, WO3 nanoflower coated with graphene nanosheet: synergetic energy storage composite electrode for supercapacitor application, J. Alloys Compd., 2017, 702, 568–572 CrossRef CAS.
- D. J. Ham, A. Phuruangrat, S. Thongtem and J. S. Lee, Hydrothermal synthesis of monoclinic WO3 nanoplates and nanorods used as an electrocatalyst for hydrogen evolution reactions from water, Chem. Eng. J., 2010, 165, 365–369 CrossRef CAS.
- J. Li, X. Liu, J. Cui and J. Sun, Hydrothermal synthesis of self-assembled hierarchical tungsten oxides hollow spheres and their gas sensing properties, ACS Appl. Mater. Interfaces, 2015, 7, 10108–10114 CrossRef CAS PubMed.
- H. Zhang, J. Yang, D. Li, W. Guo, Q. Qin, L. Zhu and W. Zheng, Template-free facile preparation of monoclinic WO3 nanoplates and their high photocatalytic activities, Appl. Surf. Sci., 2014, 305, 274–280 CrossRef CAS.
- W.-J. Li and Z.-W. Fu, Nanostructured WO3 thin film as a new anode material for lithium-ion batteries, Appl. Surf. Sci., 2010, 256, 2447–2452 CrossRef CAS.
- N. Prabhu, S. Agilan, N. Muthukumarasamy and T. S. Senthil, Enhanced photovoltaic performance of WO3 nanoparticles added dye sensitized solar cells, J. Mater. Sci.: Mater. Electron., 2014, 25, 5288–5295 CrossRef CAS.
- P. M. Rao, I. S. Cho and X. Zheng, Flame synthesis of WO3 nanotubes and nanowires for efficient photoelectrochemical water-splitting, Proc. Combust. Inst., 2013, 34, 2187–2195 CrossRef CAS.
- Y. Li, X. Zhai, Y. Liu, H. Wei, J. Ma, M. Chen, X. Liu, W. Zhang, G. Wang and F. Ren, WO3-based materials as electrocatalysts for hydrogen evolution reaction, Front. Mater., 2020, 7, 105 CrossRef.
- A. K. Nayak, E. Enhtuwshin, S. J. Kim and H. Han, Facile Synthesis of N-Doped WS2 Nanosheets as an Efficient and Stable Electrocatalyst for Hydrogen Evolution Reaction in Acidic Media, Catalysts, 2020, 10, 12–38 CrossRef.
- W. Liu, P. Geng, S. Li, W. Liu, D. Fan, H. Lu, Z. Lu and Y. Liu, Tuning electronic configuration of WP2 nanosheet arrays via nickel doping for high-efficiency hydrogen evolution reaction, J. Energy Chem., 2021, 55, 17–24 CrossRef CAS.
- J. Zhang, H. Ma and L. Zhifeng, Highly efficient photocatalyst based on all oxides WO3/Cu2O heterojunction for photoelectrochemical water splitting, Appl. Catal., B, 2017, 201, 84–91 CrossRef CAS.
- S. P. Gupta, V. B. Patil, N. L. Tarwal, S. D. Bhame, S. W. Gosavi, I. S. Mulla, D. J. Late, S. S. Suryavanshi and P. S. Walke, Enhanced energy density and stability of self-assembled cauliflower of Pd doped monoclinic WO3 nanostructure supercapacitor, Mater. Chem. Phys., 2019, 225, 192–199 CrossRef CAS.
- R. D. Kumar, Y. Andou and S. Karuppuchamy, Synthesis and characterization of nanostructured Ni-WO3 and NiWO4 for supercapacitor applications, J. Alloys Compd., 2016, 654, 349–356 CrossRef CAS.
- A. Martin and V. N. Kalevaru, Heterogeneously catalyzed ammoxidation: a valuable tool for one-step synthesis of nitriles, ChemCatChem, 2010, 2, 1504–1522 CrossRef CAS.
- Y.-Y. Yeh, W.-H. Chiang and W.-R. Liu, Synthesis of few-layer WS2 by jet cavitation as anode material for lithium ion batteries, J. Alloys Compd., 2019, 775, 1251–1258 CrossRef CAS.
- K. Peng, H. Wang, X. Li, J. Wang, Z. Cai, L. Su and X. Fan, Emerging WS(2)/montmorillonite composite nanosheets as an efficient hydrophilic photocatalyst for aqueous phase reactions, Sci. Rep., 2019, 9, 16325 CrossRef PubMed.
- S. Karthikeyan, M. Selvapandiyan, P. Sasikumar, M. Parthibavaraman, S. Nithiyanantham and V. T. Srisuvetha, Investigation on the properties of vanadium doping WO3 nanostructures by hydrothermal method, Mater. Sci. Energy Technol., 2022, 5, 411–415 CAS.
- M. Devi, S. Yesmin, B. Das, S. S. Dhar and R. Dasgupta, Efficient Oxygen Doping of Graphitic Carbon Nitride by Green Microwave Irradiation for High-Performance Supercapacitor Electrode Material, Energy Fuels, 2023, 37, 3247–3259 CrossRef CAS.
- B. S. Reghunath, S. Rajasekaran, S. D. KR, B. Saravanakumar, J. J. William, D. Pinheiro, D. Govindarajan and S. Kheawhom, Fabrication of bismuth ferrite/graphitic carbon nitride/N-doped graphene quantum dots composite for high performance supercapacitors, J. Phys. Chem. Solids, 2022, 171, 110985 CrossRef.
- B. Rani, A. K. Nayak and N. K. Sahu, Electrochemical supercapacitor application of CoFe2O4 nanoparticles decorated over graphitic carbon nitride, Diamond Relat. Mater., 2021, 120, 108671 CrossRef CAS.
- C. T. Altaf, T. O. Colak, F. Lufrano, G. S. Unal, N. D. Sankir and M. Sankir, Graphitic carbon nitride/zinc oxide composite electrodes for all-solid-state photo-supercapacitor with ion exchange membrane separator, J. Energy Storage, 2022, 55, 105784 CrossRef.
- R. Santos, R. S. Babu, M. Devendiran, D. Haddad and A. De Barros, Facile synthesis of transition metal (M = Cu, Co) oxide grafted graphitic carbon nitride nanosheets for high performance asymmetric supercapacitors, Mater. Lett., 2022, 308, 131156 CrossRef CAS.
- S. M. El-Bahy, J. Arshad, S. Munir, K. Chaudhary, D. Alhashmialameer, D. R. Eddy, M. F. Warsi and M. Shahid, Improved photocatalytic performance of a new silver doped BiSbO4 photocatalyst, Ceram. Int., 2022, 48, 23914–23920 CrossRef CAS.
- A. Irshad, H. H. Somaily, S. Zulfiqar, M. F. Warsi, M. I. Din, K. Chaudhary and M. Shahid, Silver doped NiAl2O4 nanoplates anchored onto the 2D graphitic carbon nitride sheets for high-performance supercapacitor applications, J. Alloys Compd., 2023, 934, 167705 CrossRef CAS.
- I. Mustafa, B. Basha, S. Zulfiqar, A. Tahir, F. Hanif, M. Al-Buriahi, M. Akhtar and K. Chaudhary, L-Cysteine functionalized coral-like Ag doped MnO2 nanostructures for the real-time electrochemical detection of lead and cadmium ions, Mater. Chem. Phys., 2023, 305, 127991 CrossRef CAS.
- K. Chaudhary, M. Aadil, S. Zulfiqar, S. Ullah, S. Haider, P. O. Agboola, M. F. Warsi and I. Shakir, Graphene oxide and reduced graphene oxide supported ZnO nanochips for removal of basic dyes from the industrial effluents, Fullerenes, Nanotubes Carbon Nanostruct., 2021, 29, 915–928 CrossRef CAS.
- T. Tahir, K. Chaudhary, M. F. Warsi, M. S. Saif, I. A. Alsafari, I. Shakir, P. O. Agboola, S. Haider and S. Zulfiqar, Synthesis of sponge like Gd3+ doped vanadium oxide/2D MXene composites for improved degradation of industrial effluents and pathogens, Ceram. Int., 2022, 48, 1969–1980 CrossRef CAS.
- H. Chaudhary, K. Chaudhary, S. Zulfiqar, M. S. Saif, I. A. Alsafari, I. Shakir, P. O. Agboola, M. Safdar and M. F. Warsi, Fabrication of reduced Graphene Oxide supported Gd3+ doped V2O5 nanorod arrays for superior photocatalytic and antibacterial activities, Ceram. Int., 2021, 47, 32521–32533 CrossRef CAS.
- K. Chaudhary, N. Shaheen, S. Zulfiqar, M. I. Sarwar, M. Suleman, P. O. Agboola, I. Shakir and M. F. Warsi, Binary WO3–ZnO nanostructures supported rGO ternary nanocomposite for visible light driven photocatalytic degradation of methylene blue, Synth. Met., 2020, 269, 116526 CrossRef CAS.
- M. F. Warsi, K. Chaudhary, S. Zulfiqar, A. Rahman, I. A. Al Safari, H. M. Zeeshan, P. O. Agboola, M. Shahid and M. Suleman, Copper and silver substituted MnO2 nanostructures with superior photocatalytic and antimicrobial activity, Ceram. Int., 2022, 48, 4930–4939 CrossRef CAS.
- S. Sunasee, K. H. Leong, K. T. Wong, G. Lee, S. Pichiah, I. Nah, B.-H. Jeon, Y. Yoon and M. Jang, Sonophotocatalytic degradation of bisphenol A and its intermediates with graphitic carbon nitride, Environ. Sci. Pollut. Res., 2019, 26, 1082–1093 CrossRef CAS PubMed.
- I. A. Alsafari, K. Chaudhary, M. F. Warsi, A.-Z. Warsi, M. Waqas, M. Hasan, A. Jamil and M. Shahid, A facile strategy to fabricate ternary WO3/CuO/rGO nano-composite for the enhanced photocatalytic degradation of multiple organic pollutants and antimicrobial activity, J. Alloys Compd., 2023, 938, 168537 CrossRef CAS.
- F. Mehmood, J. Iqbal, T. Jan, W. Ahmed, W. Ahmed, A. Arshad, Q. Mansoor, S. Z. Ilyas, M. Ismail and I. Ahmad, Effect of Sn doping on the structural, optical, electrical and anticancer properties of WO3 nanoplates, Ceram. Int., 2016, 42, 14334–14341 CrossRef CAS.
- C. Pang, J. Luo, Z. Guo, M. Guo and T. Hou, Inhibition of tungsten particle growth during reduction of V-doped WO3 nanoparticles prepared by co-precipitation method, Int. J. Refract. Met. Hard Mater., 2010, 28, 343–348 CrossRef CAS.
- C.-S. Wu, Hydrothermal fabrication of WO3 hierarchical architectures: structure, growth and response, Nanomaterials, 2015, 5, 1250–1255 CrossRef CAS PubMed.
- G. Cao, Nanostructures & Nanomaterials: Synthesis, Properties & Applications, Imperial College Press, 2004 Search PubMed.
- Y. Yu, W. Zeng, M. Xu and X. Peng, Hydrothermal synthesis of WO3·H2O with different nanostructures from 0D to 3D and their gas sensing properties, Phys. E, 2016, 79, 127–132 CrossRef CAS.
- J. Yu, S. Xiong, B. Wang, R. Wang, B. He, J. Jin, H. Wang and Y. Gong, Constructing boron-doped graphitic carbon nitride with 2D/1D porous hierarchical architecture and efficient N2 photofixation, Colloids Surf., A, 2023, 656, 130481 CrossRef CAS.
- Y. Huang, Y. Li, G. Zhang, W. Liu, D. Li, R. Chen, F. Zheng and H. Ni, Simple synthesis of 1D, 2D and 3D WO3 nanostructures on stainless steel substrate for high-performance supercapacitors, J. Alloys Compd., 2019, 778, 603611 CrossRef.
- S. Gong, Z. Jiang, S. Zhu, J. Fan, Q. Xu and Q. Min, The synthesis of graphene-TiO2/g-C3N4 super-thin heterojunctions with enhanced visible-light photocatalytic activities, J. Nanopart. Res., 2018, 20, 1–13 CrossRef CAS.
- S. V. Mohite, V. V. Ganbavle and K. Y. Rajpure, Solar photoelectrocatalytic activities of rhodamine-B using sprayed WO3 photoelectrode, J. Alloys Compd., 2016, 655, 106–113 CrossRef CAS.
- L. Mohan, A. V. Avani, P. Kathirvel, R. Marnadu, R. Packiaraj, J. R. Joshua, N. Nallamuthu, M. Shkir and S. Saravanakumar, Investigation on structural, morphological and electrochemical properties of Mn doped WO3 nanoparticles synthesized by co-precipitation method for supercapacitor applications, J. Alloys Compd., 2021, 882, 160670 CrossRef CAS.
- H. Habazaki, Y. Hayashi and H. Konno, Characterization of electrodeposited WO3 films and its application to electrochemical wastewater treatment, Electrochim. Acta, 2002, 47, 4181–4188 CrossRef CAS.
- M. Sumathi, A. Prakasam and P. Anbarasan, Fabrication of hexagonal disc shaped nanoparticles g-C3N4/NiO heterostructured nanocomposites for efficient visible light photocatalytic performance, J. Cluster Sci., 2019, 30, 757–766 CrossRef CAS.
- G. Wu, Q. Liu, J. Wang, Z. Cai, H. Li, T. Zhang, R. Lu, P. Li, J. Han and W. Xing, Synthesis of silver-based composite photocatalysis material and its visible-light-driven photocatalytic degradation of dye pollutants, Fresenius Environ. Bull., 2021, 30, 9696–9706 CAS.
- M. Sumathi, A. Prakasam and P. Anbarasan, High capable visible light driven photocatalytic activity of WO3/g-C3N4 hetrostructure catalysts synthesized by a novel one step microwave irradiation route, J. Mater. Sci.: Mater. Electron., 2019, 30, 3294–3304 CrossRef CAS.
- K. Chaudhary, B. Basha, S. Zulfiqar, S. Yousaf, E. W. Cochran, M. Al-Buriahi, M. F. Warsi and M. Shahid, 3D cellular lattice like-Ti3C2 MXene based aerogels embedded with metal selenides particles for energy storage and water splitting applications, Fuel, 2023, 351, 128856 CrossRef CAS.
- Z. Zhao, H. Ma, M. Feng, Z. Li, D. Cao and Z. Guo, In situ preparation of WO3/g-C3N4 composite and its enhanced photocatalytic ability, a comparative study on the preparation methods, Eng. Sci., 2019, 7, 52–58 Search PubMed.
- A. M. Patil, X. An, S. Li, X. Yue, X. Du, A. Yoshida, X. Hao, A. Abudula and G. Guan, Fabrication of three-dimensionally heterostructured rGO/WO3·0.5 H2O@ Cu2S electrodes for high-energy solid-state pouch-type asymmetric supercapacitor, Chem. Eng. J., 2021, 403, 126411 CrossRef CAS.
- T. Tahir, D. Alhashmialameer, S. Zulfiqar, A. M. Atia, M. F. Warsi, K. Chaudhary and H. M. El Refay, Wet chemical synthesis of Gd3+ doped vanadium Oxide/MXene based mesoporous hierarchical architectures as advanced supercapacitor material, Ceram. Int., 2022, 48, 24840–24849 CrossRef CAS.
- M. Mahmood, K. Chaudhary, M. Shahid, I. Shakir, P. O. Agboola and M. Aadil, Fabrication of MoO3 Nanowires/MXene@ CC hybrid as highly conductive and flexible electrode for next-generation supercapacitors applications, Ceram. Int., 2022, 48, 19314–19323 CrossRef CAS.
- S. Rafiq, A. K. Alanazi, S. Bashir, A. Y. Elnaggar, G. A. Mersal, M. M. Ibrahim, S. Yousaf and K. Chaudhary, Optimization studies for nickel oxide/tin oxide (NiO/Xg SnO2, X: 0.5, 1) based heterostructured composites to design high-performance supercapacitor electrode, Phys. B, 2022, 638, 413931 CrossRef CAS.
- J. Gao, Y. Zhou, Z. Liu, H. Wang and Y. He, NiCo-Se nanoparticles encapsulated N-doped CNTs derived from prussian blue analogues for high performance supercapacitors, Electrochim. Acta, 2022, 411, 140064 CrossRef CAS.
- F. Zheng, J. Wang, W. Liu, J. Zhou, H. Li, Y. Yu, P. Hu, W. Yan, Y. Liu and R. Li, Novel diverse-structured h-WO3 nanoflake arrays as electrode materials for high performance supercapacitors, Electrochim. Acta, 2020, 334, 135641 CrossRef CAS.
- F. Zheng, C. Xi, J. Xu, Y. Yu, W. Yang, P. Hu, Y. Li, Q. Zhen, S. Bashir and J. L. Liu, Facile preparation of WO3 nano-fibers with super large aspect ratio for high performance supercapacitor, J. Alloys Compd., 2019, 772, 933–942 CrossRef CAS.
- V. Lokhande, T. Hussain, A. Shelke, A. Lokhande and T. Ji, Substitutional doping of WO3 for Ca-ion based supercapacitor, Chem. Eng. J., 2021, 424, 130557 CrossRef CAS.
- P. Nagaraju, A. Alsalme, A. M. Alkathiri and R. Jayavel, Rapid synthesis of WO3/graphene nanocomposite via in-situ microwave method with improved electrochemical properties, J. Phys. Chem. Solids, 2018, 120, 250–260 CrossRef CAS.
- H. Peng, G. Ma, K. Sun, J. Mu, M. Luo and Z. Lei, High-performance aqueous asymmetric supercapacitor based on carbon nanofibers network and tungsten trioxide nanorod bundles electrodes, Electrochim. Acta, 2014, 147, 54–61 CrossRef CAS.
- F. Nasreen, A. W. Anwar, A. Majeed, M. A. Ahmad, U. Ilyas and F. Ahmad, High performance and remarkable cyclic stability of a nanostructured RGO–CNT-WO3 supercapacitor electrode, RSC Adv., 2022, 12, 11293–11302 RSC.
- İ. İnce and S. Ersoy, Phase transformation of α-MnO2 to β-MnO2 induced by Cu doping: improved electrochemical performance for next generation supercapacitor, Mater. Sci. Eng. B, 2023, 295, 116580 CrossRef.
- N. M. Shinde, A. D. Jagadale, V. S. Kumbhar, T. R. Rana, J. Kim and C. D. Lokhande, Wet chemical synthesis of WO3 thin films for supercapacitor application, Korean J. Chem. Eng., 2015, 32, 974–979 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2023 |