Open Access Article
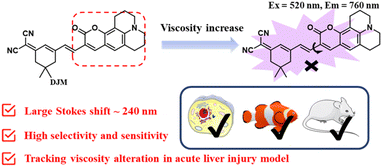
Open Access ArticleDevelopment of a novel near-infrared molecule rotator for early diagnosis and visualization of viscosity changes in acute liver injury models†
Manlin Fu ,
Fenglin He,
Zhelu Jiang,
Xue Chen,
Zhenda Xie* and
Jin-Feng Hu
,
Fenglin He,
Zhelu Jiang,
Xue Chen,
Zhenda Xie* and
Jin-Feng Hu *
*
Institute of Natural Medicine and Health Products, School of Pharmaceutical Sciences, Zhejiang Provincial Key Laboratory of Plant Evolutionary Ecology and Conservation, Taizhou University, Zhejiang 318000, China. E-mail: jfhu@tzc.edu.cn
First published on 4th September 2023
Abstract
Acute liver injury leading to acute liver failure can be a life-threatening condition. Therefore, timely and accurate early diagnosis of the onset of acute liver injury in vivo is critical. Viscosity is one of the key parameters that can accurately reflect the levels of relevant active analytes at the cellular level. Herein, a novel near-infrared molecule rotator, DJM, was designed and synthesized. This probe exhibited a highly sensitive (461-fold from PBS solution to 95% glycerol solution) and selective response to viscosity with a maximum emission wavelength of 760 nm and a Stokes shift of 240 nm. Furthermore, DJM has exhibited a remarkable capacity to discern viscosity changes induced by nystatin in viable cells with sensitivity and selectivity and further applied in the zebrafish and mouse model of acute liver injury. Additionally, DJM may potentially offer direction for the timely observation and visualization of viscosity in more relevant disease models in the future.
Acute liver injury has been identified as a potential trigger for various intracellular stress responses, including but not limited to metabolic stress, oxidative stress, and endoplasmic reticulum stress.1 Prolonged exposure to stress may elevate the probability of liver cell injury and death, thereby resulting in liver pathologies such as inflammation and fibrosis.2,3 Therefore, timely detection of acute liver injury is imperative for comprehensive prevention and treatment, ultimately mitigating the extent of liver damage. Notably, intracellular oxidative stress has been closely linked to acute liver injury. Hence, comprehending the interplay between oxidative stress and the onset and progression of acute liver injury holds significant value for clinical research and therapy.
Viscosity is a pivotal intracellular microenvironmental parameter that contributes significantly to the interaction of intracellular biomolecules, chemical signal transmission, and active metabolite diffusion.4,5 Deviations from normal viscosity values are strongly associated with numerous cellular malfunctions and the onset of pathologies such as Alzheimer's disease, hypertension, diabetes, and even cancer.6–8 Recently, it has been reported that viscosity can accurately reflect the level of oxidative stress in the body.9–11 Consequently, the development of a convenient tool for the precise monitoring of cellular viscosity fluctuations is imperative. In recent years, fluorescence imaging has gained significant attraction due to its real-time visualization, high sensitivity, and relative simplicity of operation.12,13 Therefore, fluorescence spectroscopy-based detection of intracellular viscosity could potentially emerge as a novel and effective method for the early diagnosis of acute liver injury.
To date, substantial progress has been made in imaging viscosity alterations in vivo.14–20 However, existing probes are not capable of accurately monitoring changes in viscosity within organisms due to their shorter wavelengths or lower sensitivity. In 2023, Li group reported a near-infrared fluorescence probe, designated as QX-V. The probe demonstrated a significant increase in fluorescence intensity, by approximately 42.8-fold, in response to changes in viscosity from 1.2 cP to 1023 cP.21 In 2022, Xu group reported a novel viscosity probe based on dicyanoisophorone, designated as NV1. The emission wavelength of probe NV1 is 590 nm.22 Henceforth, it is of great significance to fabricate fluorescent probes with superior sensitivity capabilities and near-infrared emission wavelengths, specifically tailored to selectively respond to changes in viscosity within various disease models.23–25
Herein, a highly-sensitive near-infrared probe (named DJM) was developed by modifying a coumarin derivative as a strong donor as well as the rotor, while dicyanoisophorone acted as an electron acceptor, building upon our prior research (Scheme 1).26 This probe possesses a remarkably larger Stokes shift (240 nm), and it displays more highly sensitive turn-on signal responses (461-fold at 760 nm) to viscosity. In addition, the probe DJM can be applied to detect viscosity in the presence of other interfering ions. Using DJM as a chemical tool, we successfully monitored the viscosity variation in Miha cells. Furthermore, the practicability of DJM has been further confirmed by the acute liver damage model of zebrafish and mouse liver tissues. We believe that DJM can provide a convenient and efficient method for more promising clinical applications.
Experimental section
Materials and apparatus
The materials and instruments used in the work could be found in the ESI.†Synthesis of DJM
The synthetic route of probe DJM shows in Scheme S1,† the final steps are as follows: compound 1 and compound 2 were synthesized according to the previous methods.26,27 The detailed information of instruments used in this work were provided in ESI.†Compound 1 (22.4 mg, 1.2 mmol), compound 2 (26.9 mg, 1 mmol) and EtOH (5 mL) refluxed for 6 h at 80 °C in a 25 mL round bottomed flask with a few drops of piperidine. After cooling to room temperature, the solvent was removed under reduced pressure. Then, the crude product was purified by column chromatography using CH2Cl2/MeOH (v/v = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to obtain DJM, a purple solid (26.2 mg, yield 60%). 1H NMR (400 MHz, CDCl3): δ 7.69 (s, 1H), 7.16 (d, J = 6.8 Hz, 2H), 6.90 (s, 1H), 6.79 (s, 1H), 3.34 (q, J = 5.7 Hz, 4H), 2.89 (t, J = 6.3 Hz, 2H), 2.78 (t, J = 6.2 Hz, 2H), 2.56 (s, 2H), 2.46 (s, 2H), 1.98 (p, J = 6.1 Hz, 5H), 1.06 (s, 6H). 13C NMR (100 MHz, CDCl3): δ 169.24, 161.44, 154.80, 151.47, 147.21, 140.46, 132.13, 128.53, 125.94, 122.90, 119.38, 114.57, 113.11, 108.91, 106.22, 50.25, 49.84, 43.01, 39.09, 32.02, 28.02, 27.50, 21.26, 20.30, 20.19. LR-ESIMS: m/z 460.20 (C28H27N3O2, [M + Na]+).
1) to obtain DJM, a purple solid (26.2 mg, yield 60%). 1H NMR (400 MHz, CDCl3): δ 7.69 (s, 1H), 7.16 (d, J = 6.8 Hz, 2H), 6.90 (s, 1H), 6.79 (s, 1H), 3.34 (q, J = 5.7 Hz, 4H), 2.89 (t, J = 6.3 Hz, 2H), 2.78 (t, J = 6.2 Hz, 2H), 2.56 (s, 2H), 2.46 (s, 2H), 1.98 (p, J = 6.1 Hz, 5H), 1.06 (s, 6H). 13C NMR (100 MHz, CDCl3): δ 169.24, 161.44, 154.80, 151.47, 147.21, 140.46, 132.13, 128.53, 125.94, 122.90, 119.38, 114.57, 113.11, 108.91, 106.22, 50.25, 49.84, 43.01, 39.09, 32.02, 28.02, 27.50, 21.26, 20.30, 20.19. LR-ESIMS: m/z 460.20 (C28H27N3O2, [M + Na]+).
Monitoring viscosity in mouse model of acute liver injury
Prior to the start of the experiments, mice were acclimated for 7 days after arrival. All study groups contained n = 3 mice. After overnight fasting, the mice were intraperitoneally injected with APAP (300 mg kg−1, 200 μL) to establish an acute liver injury model. Mice in the control group were pretreated intraperitoneally with 200 μL PBS for 2 h. All animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of Taizhou University and approved by the Animal Ethics Committee of Taizhou University. During the experiments, all efforts were made to minimize animal suffering. After direct injection of the probe DJM (50 μM, 100 μL) into the livers of the acute liver injury model and control mice. The abdominal cavity was then opened and the liver was removed for fluorescence imaging.Results and discussion
Design strategy of the probe DJM
The viscosity-sensitive probe generally comprises a fluorophore and a rotor linked via a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond, based on the Twisted Intramolecular Charge Transfer (TICT) mechanism.28–30 In our previous work, we reported a NIR fluorescent probe DJH based on the D–π–A structure.26 However, the relatively short emission wavelength of DJH was inadequate for detecting living organisms. To overcome this limitation, we extended the conjugated system to red-shift the fluorescence wavelength. We utilized 9-formyl-8-julolidinol to synthesize a coumarin derivative (Scheme 1). The vinyl group, coupled to the isophorone derivatives via σ-bonds, served as a viscosity-sensitive group, enabling DJM to indicate viscosity changes. In low-viscosity environments, the unrestricted rotation of the rotor around the C–C bond led to the non-radiative quenching of the excited state. However, an increase in viscosity induced a constraint on the rotor's rotation, which triggered the activation of fluorescence.
C bond, based on the Twisted Intramolecular Charge Transfer (TICT) mechanism.28–30 In our previous work, we reported a NIR fluorescent probe DJH based on the D–π–A structure.26 However, the relatively short emission wavelength of DJH was inadequate for detecting living organisms. To overcome this limitation, we extended the conjugated system to red-shift the fluorescence wavelength. We utilized 9-formyl-8-julolidinol to synthesize a coumarin derivative (Scheme 1). The vinyl group, coupled to the isophorone derivatives via σ-bonds, served as a viscosity-sensitive group, enabling DJM to indicate viscosity changes. In low-viscosity environments, the unrestricted rotation of the rotor around the C–C bond led to the non-radiative quenching of the excited state. However, an increase in viscosity induced a constraint on the rotor's rotation, which triggered the activation of fluorescence.
The response of DJM to viscosity
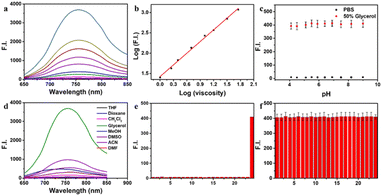
As mentioned above, the probe DJM has the properties of a molecular rotor as a viscosity-sensitive group. Thus, we studied the spectral properties of DJM in water–glycerol mixtures. The absorption maxima of DJM (λabs) is about 520 nm and increases gradually with the addition of glycerol (Fig. S1†). When excited at 520 nm, a fluorescence emission peak (λem) near 760 nm was observed, which was red-shifted compared to our previous work (675 nm), as depicted in Scheme 1. This could be the reason for the enlarged conjugated structure of the chromophore system favoring electron delocalization. It is worth mentioning that DJM shows a remarkable viscosity-dependent effect from water (∼0.893 cP) to glycerol (∼945 cP). The fluorescence intensity at 760 nm is significantly increased by a factor of ∼461-fold (when excited at 520 nm). By plotting the log(I760 nm) and log(viscosity) across the range of 1–458 cP (glycerol content 0–80%), as shown in Fig. 1a and b, good linearity can be observed (R2 = 0.9965). Regarding the fluorescence quantum yield, it was enhanced dramatically by about 386 folds in glycerol (Φ = 0.197) compared to that in water (Φ < 0.001), using Rhodamine B as a reference. This characteristic makes the probe DJM highly applicable due to its low background fluorescence. Based on the aforementioned data, DJM exhibits a favorable response to changes in viscosity.Evaluation of the selectivity of DJM towards viscosity
To confirm the specificity of DJM as a viscosity detector, we evaluated its response to changes in pH. We added the probe to PBS buffer with varying pH values (resulting in a final pH range of 4.2 to 10.8). Then fluorescence intensity at an emission wavelength of 670 nm was measured (Ex = 520 nm), as shown in Fig. 1c. The results demonstrate that the fluorescence intensity remained consistent across the entire pH range, indicating that DJM is highly resistant to changes in pH and exhibits good photostability.Evaluating the selectivity of fluorescent probes is crucial to assess their performance, ensuring specificity and avoiding interference from complex cellular components. To this end, we investigated the effect of solvent polarity on the probe DJM. We examined the probe's response to various polarities of solvents, including THF, dioxane, DCM, glycerol, MeOH, DMSO, DMF, and ACN. As shown in Fig. 1d, the probe exhibited a much stronger fluorescence intensity in 95% glycerol at 760 nm than in solvents with a small polarity, suggesting that solvent polarity has minimal effect on the fluorescence properties of the probe DJM. Furthermore, we measured the fluorescence emission intensity of the probe DJM in the presence of high concentrations (100 μM) of interfering ions, disruptors, including biological RSS (Cys, Hcy, GSH, SO32−), reactive oxygen species (HOCl, H2O2), and various types of anions. As demonstrated in Fig. 1e, the fluorescence intensity of the probe DJM in PBS solution in the presence of a high concentration of interfering ions remained almost unchanged compared to that of the pure probe. Remarkably, even in the PBS/glycerol (v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v = 1
v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture, the probe DJM maintained excellent stability towards these ions (Fig. 1f), suggesting that the probe DJM can be used to monitor viscosity changes in complex cell environments and has broader applications.
1) mixture, the probe DJM maintained excellent stability towards these ions (Fig. 1f), suggesting that the probe DJM can be used to monitor viscosity changes in complex cell environments and has broader applications.
Intracellular fluorescence imaging
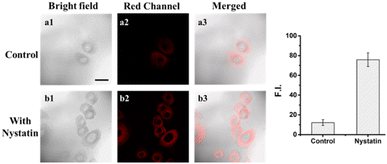
First, to assess the biological toxicity of DJM, we performed a 2,5-diphenyl-2H-tetrazolium bromide (MTT) assay to establish the cytotoxicity test in Miha cells. As shown in Fig. S2,† Miha cells still maintain high viability after incubation with different concentrations of DJM (0, 1, 2, 5, 10, 20 μM) for 24 h, indicating that the probe DJM has good biocompatibility advantages and is suitable for living cell imaging.In order to investigate the potential of the probe DJM to monitor changes in intracellular viscosity, the ionophore nystatin was used to induce mitochondrial dysfunction and increase intracellular viscosity.31 Miha cells were divided into two groups: a control group and a nystatin-induced group. As shown in Fig. 2, the control group treated with only the probe DJM exhibited weak background fluorescence in the red channel (700–800 nm). In contrast, the fluorescence intensity of the red channel was significantly stronger when cells were cultured with nystatin for 30 minutes and then incubated with the probe DJM for another 30 minutes. Furthermore, the mean fluorescence intensity of cell regions (Fig. 2, red channel) was calculated by ROI measurement. The fluorescence intensity in the nystatin-treated cells was 5.4 times higher than that in the non-treated cells. These results clearly indicate that the probe DJM is capable of responding to changes in viscosity induced by nystatin in living cells.
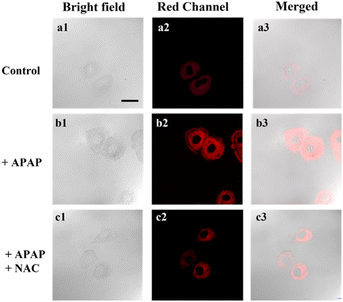
Acetaminophen (APAP) is a classical non-steroidal anti-inflammatory drug (NSAID), which is one of the most commonly used antipyretic drugs. At therapeutic doses, APAP is safe and effective, but its excessive use may cause liver damage and result in acute liver failure (ALF). Building on the excellent optical properties of DJM, we employed it to detect changes in viscosity in a model of acute liver injury based on Miha cells (Fig. 3). The addition of APAP significantly increased the fluorescence intensity of the cells by approximately three-fold compared to the control group (as per Fig. S3†), indicating an elevation in viscosity when normal liver cells were damaged. We subsequently introduced N-acetylcysteine (NAC), a liver-protecting agent that counteracts liver damage caused by APAP. Notably, the fluorescence intensity was significantly reduced to only 1.8-fold that of the control group (Fig. 3c). Collectively, our imaging experiments demonstrate that DJM can accurately track viscosity changes in living cells.
Fluorescence imaging in zebrafish model of acute liver injury
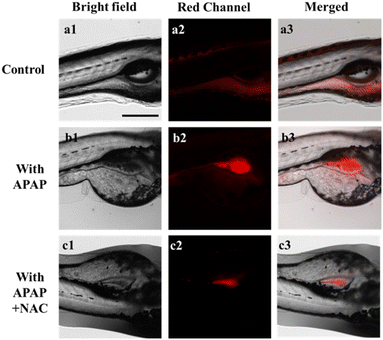
In the following study, the application of the DJM in vivo was further investigated. Zebrafish has been widely used as a biological model due to its strong optical transparency, fast growth, and high genetic similarity to humans.32,33 To test the ability of DJM to detect viscosity changes during liver injury, 3 day-old zebrafish were treated with probe DJM (10 μM) for 15 min. In the control group, weak red fluorescence was observed. However, in zebrafish treated with APAP (lipopolysaccharide, an inflammatory stimulator, 200 μM), the fluorescence intensity was significantly higher, indicating an increase in viscosity due to liver injury caused by APAP (Fig. 4b). The fluorescence quantification diagram (Fig. S4†) further revealed that the fluorescence intensity was approximately 4 times higher in the presence of APAP. Conversely, treatment with NAC significantly reduced the fluorescence intensity, with only a 1.6-fold increase compared to the control group (Fig. S4†). These results corroborate those obtained from living cells and provide evidence that DJM can be employed to image viscosity changes in zebrafish.Intracellular fluorescence imaging in mouse model of acute liver injury
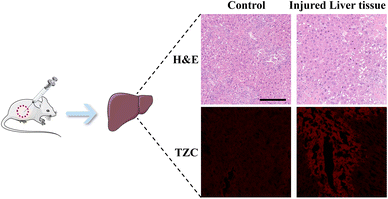
Inspired by the promising results obtained in previous experiments, we further assessed the capability of DJM to detect viscosity changes in mice. To this end, an acute liver injury model was induced in mice through intraperitoneal injection of APAP, while PBS was used as the control group.28,29 H&E staining showed significant hepatocyte swelling in the APAP-treated mice, whereas the liver tissues of PBS-treated control mice appeared aligned (Fig. 5a). Fig. 5b demonstrates that the fluorescence intensity of the APAP group was lower than that of the PBS-treated mice (control group). However, the fluorescence intensity of the APAP-treated group was markedly increased upon adding the DJM probe, indicating a higher liver viscosity and more severe liver damage in the APAP model group. The experimental results demonstrate that DJM can effectively detect viscosity in the imaging of the mouse liver injury model. Furthermore, the high sensitivity of DJM enables the acquisition of clear and accurate fluorescence images, which has significant implications for early diagnosis.Conclusion
In this paper, a novel NIR viscosity fluorescent probe DJM was successfully designed and synthesized. The probe displayed strong fluorescence enhancement at 760 nm with a large Stokes shift (240 nm). Notably, DJM exhibited high sensitivity with a 461-fold increment and fine specificity for viscosity in the presence of other analytes. Utilizing DJM, we have demonstrated, for the first time, enhanced viscosity levels in acute liver injury models of live cells, zebrafish, and mice tissues. These findings highlight the effectiveness of constructing a NIR viscosity-sensitive rotor and offer a promising direction for developing new diagnostic methods for viscosity-related diseases.Author contributions
Manlin Fu: design and synthesis of the probe; Fenglin He: writing – original draft; Zhelu Jiang: data curation; Xue Chen: cell imaging assay; Zhenda Xie: synthesis; Jin-Feng Hu: supervision.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was financially supported by the Natural Science Foundation of Zhejiang Province (LY23H300001) and the National Natural Science Foundation of China (No. 21937002).References
- A. Ramachandran and H. Jaeschke, Curr. Opin. Toxicol., 2018, 7, 17–21 CrossRef PubMed.
- R. Jalan, P. Gines, J. C. Olson, R. P. Mookerjee, R. Moreau, G. Garcia-Tsao, V. Arroyo and P. S. Kamath, J. Hepatol., 2012, 57, 1336–1348 CrossRef PubMed.
- P. Angeli, P. Gines, F. Wong, M. Bernardi, T. D. Boyer, A. Gerbes, R. Moreau, R. Jalan, S. K. Sarin, S. Piano, K. Moore, S. S. Lee, F. Durand, F. Salerno, P. Caraceni, W. R. Kim, V. Arroyo, G. Garcia-Tsao and A. International Club of, Gut, 2015, 64, 531–537 CrossRef CAS PubMed.
- G. C. Berry, J. Rheol., 1996, 40, 1129–1154 CrossRef CAS.
- Y. He, B. X. Wang, L. K. Wen, F. Z. Wang, H. S. Yu, D. X. Chen, X. Su and C. Zhang, Food Sci. Hum. Wellness, 2022, 11, 1–10 CrossRef.
- Z. Wen, J. Xie, Z. Guan, D. Sun, W. Yao, K. Chen, Z. Y. Yan and Q. Mu, Clin. Hemorheol. Microcirc., 2000, 22, 261–266 CAS.
- M. R. Fedde and R. F. Wideman Jr, Poult. Sci., 1996, 75, 1261–1267 CrossRef CAS PubMed.
- G. Sloop, R. E. Holsworth Jr, J. J. Weidman and J. A. St Cyr, Ther. Adv. Cardiovasc. Dis., 2015, 9, 19–25 CrossRef PubMed.
- A. Kasperczyk, L. Słowińska-Łożyńska, M. Dobrakowski, J. Zalejska-Fiolka and S. Kasperczyk, Clin. Hemorheol. Microcirc., 2014, 56, 187–195 CAS.
- P. Gyawali, R. S. Richards, E. U. Nwose and P. T. Bwititi, Clin. Lipidol., 2017, 7, 709–719 CrossRef.
- D. Konukoglu, M. Ercan and H. Hatemi, Clin. Hemorheol. Microcirc., 2002, 27, 107–113 CAS.
- M. Fu, Y. Sun, Kenry, M. Zhang, H. Zhou, W. Shen, Y. Hu and Q. Zhu, Chem. Commun., 2021, 57, 3508–3511 RSC.
- W. Shen, P. Wang, Z. Xie, H. Zhou, Y. Hu, M. Fu and Q. Zhu, Talanta, 2021, 234, 122621 CrossRef CAS PubMed.
- S. Wang, W. X. Ren, J. T. Hou, M. Won, J. An, X. Chen, J. Shu and J. S. Kim, Chem. Soc. Rev., 2021, 50, 8887–8902 RSC.
- Z. Yang, J. Cao, Y. He, J. H. Yang, T. Kim, X. Peng and J. S. Kim, Chem. Soc. Rev., 2014, 43, 4563–4601 RSC.
- Y. Q. Lai, Z. D. Xie, M. L. Fu, X. Chen, Q. Zhou and J. F. Hu, Prog. Chem., 2022, 34, 2024–2034 CAS.
- M. Fu, K. Wang, Q. Ma, J. Zhu, M. Bian and Q. Zhu, Org. Biomol. Chem., 2022, 20, 672–677 RSC.
- M. Fu, K. Wang, J. Xue, Y. Li, M. Bian and Q. Zhu, Org. Biomol. Chem., 2022, 20, 3359–3364 RSC.
- G. Q. Fu, Q. T. Liao, Z. Q. Wang, Z. K. Tan, G. J. Mao, B. Yang and C. Y. Li, Anal. Chim. Acta, 2022, 1226, 340192 CrossRef CAS PubMed.
- Y. Wang, S. Li, X. Zhu, X. Shi, X. Liu and H. Zhang, Anal. Chim. Acta, 2022, 1202, 339670 CrossRef CAS PubMed.
- J. J. Chao, H. Zhang, Z. Q. Wang, Q. R. Liu, G. J. Mao, D. H. Chen and C. Y. Li, Anal. Chim. Acta, 2023, 1242, 340813 CrossRef CAS PubMed.
- X. Pan, C. Wang, C. Zhao, T. Cheng, A. Zheng, Y. Cao and K. Xu, Chem. Commun., 2022, 58, 4663–4666 RSC.
- C. Chen, R. Tian, Y. Zeng, C. Chu and G. Liu, Bioconjugate Chem., 2020, 31, 276–292 CrossRef CAS PubMed.
- Z. Guo, S. Park, J. Yoon and I. Shin, Chem. Soc. Rev., 2014, 43, 16–29 RSC.
- C. X. Yan, Y. T. Zhang and Z. Q. Guo, Coord. Chem. Rev., 2021, 427, 213556 CrossRef CAS.
- M. Fu, W. Shen, Y. Chen, W. Yi, C. Cai, L. Zhu and Q. Zhu, J. Mater. Chem. B, 2020, 8, 1310–1315 RSC.
- J. Li, L. Tu, Q. Ouyang, S. G. Yang, X. Liu, Q. Zhai, Y. Sun, J. Yoon and H. Teng, J. Mater. Chem. B, 2022, 10, 1427–1433 RSC.
- C. Ma, W. Sun, L. Xu, Y. Qian, J. Dai, G. Zhong, Y. Hou, J. Liu and B. Shen, J. Mater. Chem. B, 2020, 8, 9642–9651 RSC.
- W. Miao, C. Yu, E. Hao and L. Jiao, Front. Chem., 2019, 7, 825 CrossRef CAS PubMed.
- H. Xiao, P. Li and B. Tang, Chemistry, 2021, 27, 6880–6898 CrossRef CAS PubMed.
- Z. Xie, Y. Zhou, M. Fu, L. Ni, Y. Tong, Y. Yu, N. Li, Z. Yang, Q. Zhu and J. Wang, Talanta, 2021, 231, 122365 CrossRef CAS PubMed.
- A. V. Kalueff, A. M. Stewart and R. Gerlai, Trends Pharmacol. Sci., 2014, 35, 63–75 CrossRef CAS PubMed.
- Y. J. Dai, Y. F. Jia, N. Chen, W. P. Bian, Q. K. Li, Y. B. Ma, Y. L. Chen and D. S. Pei, Environ. Toxicol. Chem., 2014, 33, 11–17 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra04391f |
| This journal is © The Royal Society of Chemistry 2023 |