Open Access Article
Open Access ArticleAcetamido-TEMPO mediated electrochemical oxidation of alcohols to aldehydes and ketones†
Chelsea M. Schroeder a,
Fabrizio Politano
a,
Fabrizio Politano ab,
Kristiane K. Ohlhorsta and
Nicholas E. Leadbeater
ab,
Kristiane K. Ohlhorsta and
Nicholas E. Leadbeater *a
*a
aDepartment of Chemistry, University of Connecticut, 55 North Eagleville Road, Storrs, Connecticut 06269, USA. E-mail: nicholas.leadbeater@uconn.edu
bInstituto de Investigaciones en Físico Química de Córdoba (INFIQC)-CONICET, Departamento de Química Orgánica, Facultad de Ciencias Químicas, Universidad Nacional de Córdoba, Ciudad Universitaria, X5000HUA Córdoba, Argentina
First published on 24th August 2023
Abstract
A protocol for the oxidation of alcohols to aldehydes and ketones employing an electrochemical aminoxyl-mediated reaction is presented. The approach employs a catalytic amount of the radical and the use of a base is not required. It is performed using readily available electrodes in a commercially available electrochemistry apparatus and does not require a reference electrode. The methodology is applicable to a range of structurally and electronically diverse substrates, including the oxidation of primary alcohols to aldehydes rather than the more commonly formed carboxylic acids.
Introduction
Using electricity as a tool for preparative organic chemistry has gained significant attention recently.1–4 This is because electrochemistry offers an attractive alternative to conventional approaches to performing known functional-group transformations as well as opening the door for exploring new chemical space.5,6 One area of particular interest is oxidation and reduction reactions where electrons can replace traditional oxidising or reducing agents that are often toxic, hazardous, or costly. As such, this makes electrochemistry attractive for both academic and industrial settings.7–10Most approaches to synthesis using electrochemistry have employed “homemade” equipment. This can result in issues with reproducibility due to variations in reactor dimensions, electrode sources, and current density. However, with the advent of commercially available, specifically designed equipment, more standardised methodologies can be developed.11,12
With an eye to oxidation reactions, aminoxyl compounds such as 2,2,6,6-tetramethylpiperidinyloxy (TEMPO), 4-acetamido-TEMPO (ACT, 1), and 9-azabicyclo[3.3.1]nonane N-oxyl (ABNO) have been explored as electrocatalysts.13–18 These bench-stable radical species are easy to use, recoverable, and non-toxic, making them attractive from a sustainability standpoint.19–21 Differing from direct electrolysis22 where the electron-transfer (ET) process occurs on the electrode surface, electrocatalytic reactions use indirect electrolysis.7 First, the electrocatalyst is activated by an ET on the electrode surface. It then diffuses into the solution to react with the substrate in a homogeneous manner. The electrocatalyst is then regenerated, and the cycle repeats. This process allows reactions to be performed at lower potentials than anodic oxidation which broadens functional-group compatibility.
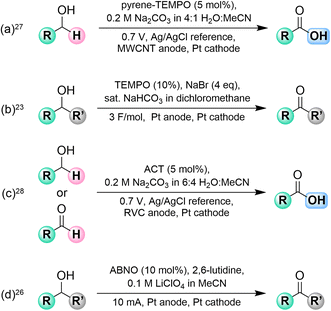
TEMPO, ACT, and ABNO have been employed by several groups as an electrocatalyst for the conversion of primary and secondary alcohols to aldehydes, ketones, or carboxylic acids (Fig. 1).23–27 The published approaches use an excess of base and often employ expensive electrode materials such as platinum,23–28 multi-walled carbon nanotubes (MWCNT),27 or reticulated vitreous carbon (RVC)28 to perform the transformations on what is often a narrow set of substrates, and expensive reference electrodes are sometimes required. A methodology for alcohol oxidation using an immobilized TEMPO catalyst has been developed (Fig. 1a)27 as well as one for Anelli and Pinnick oxidations in basic media with a reference electrode (Fig. 1c).28 Electrochemical oxidation of alcohols can also be achieved using TEMPO and a superstoichiometric quantity of sodium bromide (Fig. 1b)23 or, in the case of secondary alcohols, by using ABNO as the electrocatalyst (Fig. 1d).26
Compared to other aminoxyl radicals, 1 is less costly and has been proven to operate well, if not better, at a comparable overpotential.14 A case in point is the electrochemical oxidation of 5-hydroxymethylfurfural (HMF).29 1 also shows better electrocatalytic activity in the oxidation of alcohols compared with less hindered aminoxyls such as ABNO or 2-azaadamantane-N-oxyl (AZADO).30 Despite this, the use of 1 as an electrocatalyst for oxidation reactions has not been extensively explored. When it is employed, primary alcohols are converted to carboxylic acids rather than aldehydes.28
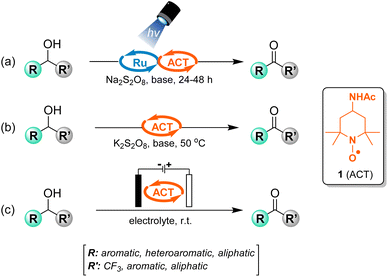
Our group has significant experience using 1 in a variety of oxidation and oxidative functionalisation reactions, such as the transformation of alcohols and aldehydes to esters,31 amides,32,33 and nitriles.34,35 Focusing on the oxidation of alcohols to aldehydes or ketones, we have used 1 in conjunction with visible-light photocatalysis (Fig. 2a).36 Employing a dual catalytic system of Ru(bpy)3(PF6)2 as a photocatalyst, 1 as the primary oxidant, and sodium persulfate as a terminal oxidant, the oxidation of a wide range of alcohols, including trifluoromethyl alcohols, is possible. More recently, we have developed an allied methodology employing only potassium persulfate and a catalytic amount of 1 under mild conditions (Fig. 2b).37 However, the reactions still require super-stoichiometric quantities of a terminal oxidant.
Spurred on by the possibility of substituting chemical oxidants with electrons, we envisioned an electrocatalytic approach for the oxidation of alcohols to aldehydes and ketones using 1 (Fig. 2c). We report the results from this study here. Highlights and novel aspects of our approach are that:
• the transformation is base-free;
• inexpensive electrode materials and a commercially-available electrochemistry unit are used, thereby ensuring reproducibility, and offering greater utility to the synthetic chemist;
• a simple two-electrode undivided cell is employed; there is no need for a reference electrode;
• the substrate scope of the methodology is diverse, including sensitive propargylic and α-trifluoromethyl alcohols;
• the aldehyde product is generated, in the case of primary alcohols, without overoxidation to the corresponding carboxylic acid;
• product isolation is simple and does not require column chromatography.
Results and discussion
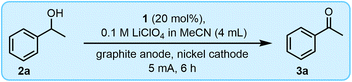
As a starting point for developing reaction conditions for the electrochemical oxidation of alcohols to aldehydes and ketones using 1, we decided to focus attention on the conversion of 1-phenylethanol (2a) to acetophenone (3a). When performing the reaction using 20 mol% of 1, lithium perchlorate in acetonitrile (0.1 M, 4 mL) as the electrolyte and solvent, and a graphite anode and nickel cathode, we obtained a 54% conversion of 2a to 3a after 6 h at a constant current of 5 mA (Table 1, entry 1). The addition of base to the reaction mixture had no observable effect on product conversion, thereby showing that it is not a required component (entries 2 and 3). This is noteworthy given that previous electrocatalytic approaches to the oxidation of alcohols using either TEMPO or 1 have involved the use of a base in excess.23–25| Entry | Change from conditions above | 3a (%) |
|---|---|---|
| a Reactions performed on the 1 mmol scale unless stated otherwise.b Product conversion determined by 1H-NMR spectroscopy.c Significant electrode fouling observed. | ||
| 1 | None | 54% |
| 2 | Addition of 5 eq. pyridine | 55% |
| 3 | Addition of 5 eq. 2,6-lutidine | 53% |
| 4 | 30 mol% 1 | 55% |
| 5 | 20 mol% TEMPO in place of 1 | 52% |
| 6 | No 1 added | 25% |
| 7 | 10 mA | —c |
| 8 | No current | 0% |
| 9 | 0.1 M [TBA][PF6] in place of LiClO4 | 20% |
| 10 | 0.1 M [TBA][BF4] in place of LiClO4 | 13% |
| 11 | 0.1 M [TBA][ClO4] in place of LiClO4 | 21% |
| 12 | 0.1 M NaClO4 in place of LiClO4 | 42% |
| 13 | 0.5 mmol 2a and reaction time of 3 h | 43% |
| 14 | 0.5 mmol 2a and reaction time of 6 h | 98% |
| 15 | 2 mmol 2a and reaction time of 24 h | 99% |
| 16 | 0.5 mmol 2a, 3h, Pt anode, Ni cathode | 46% |
| 17 | 0.5 mmol 2a, 3h, glassy carbon anode, Ni cathode | 41% |
| 18 | 0.5 mmol 2a, 3h, graphite anode, Pt cathode | 35% |
Increasing the quantity of 1 from 20 mol% to 30 mol% did not improve the outcome of the oxidation reaction, and replacing 1 with TEMPO resulted in a lower product conversion, proving that the former is a better catalyst (entries 4 and 5). This is consistent with the knowledge that 1 is a more active electrocatalyst than TEMPO and other less sterically hindered aminoxyls like and ABNO or AZADO.30 In the absence of 1, only a small amount of 3a is observed, this occurring as a result of direct oxidation of the substrate, 2a, on the surface of the anode (entry 6). In addition, the absence of 1 significantly increases resistance and hence the requisite voltage required to maintain the reaction at constant current. This indicates that 1 is essential for performing the reaction under mild conditions. Cyclic voltammetry also supports the assertion that 1 acts as mediator, it being oxidised at lower potential than the substrate and the process being chemically reversible (See ESI†).
Remaining focused on the electrochemical component of the reaction, we increased the current from 5 mA to 10 mA. This resulted in a concomitant increase in product conversion (entry 7), but was at the cost of substantial electrode fouling and so did not prove advantageous overall. When no current was applied, the reaction did not occur, thereby proving that the process is electrochemically mediated (entry 8). Next, we explored the effect of the electrolyte on the reaction by screening different ion pairs systematically. Three different tetrabutylammonium (TBA) salts were used to probe the effect of the anion (entries 9–11). The use of the perchlorate salt (entry 11) resulted in better performance than the hexafluorophosphate and tetrafluoroborate salts (entries 9 and 10). This could be related to the hydrogen-bond acceptor capacity of perchlorate,38 suggesting that this anion is non-innocent and assist the oxidation process itself. Turning to the cation, using sodium or TBA perchlorate instead of the lithium congener reduced the effectiveness of the reaction (entries 11 and 12). Since it showed the best performance in the oxidation of 2a, and because of the ease of separation from 3a at the end of the reaction, lithium perchlorate remained the electrolyte of choice.
We wanted to probe the effect of substrate concentration on the effectiveness of the oxidation reaction. When reduced from 0.250 M to 0.125 M, a 43% conversion to 3a was obtained after 3 h, and the reaction was essentially complete after 6 h (entries 13 and 14). When raised to 0.500 M, the reaction took 24 h to reach full conversion, indicating that the reaction time required is proportional to the quantity of starting material used.
Platinum, glassy carbon, and graphite are common electrodes used in preparative organic electrochemistry, so we screened various combinations of these (entries 16–18). In each case, product conversions were comparable. As a result, we opted to remain with our initial selection of a graphite anode and a nickel cathode because of their low cost and easy accessibility. Thus, our optimised conditions were: alcohol substrate (2 mmol), ACT (20 mol%), lithium perchlorate in acetonitrile (0.1 M, 4 mL), a graphite anode and a nickel cathode, constant current (5 mA), for 24–48 h.
With optimised conditions in hand, we screened a range of alcohol substrates to evaluate the scope of our methodology (Fig. 3). Different 1-phenylethanol derivatives, with substituents in ortho-, meta- and para-positions were successfully oxidised to the corresponding ketones in good to excellent yields (3a–3j). A representative propargylic benzylic alcohol was effectively oxidised in good yield (3k). This is noteworthy because it represents a class of sensitive substrates that typically decompose or generate off-target products when oxidised using conventional approaches.39
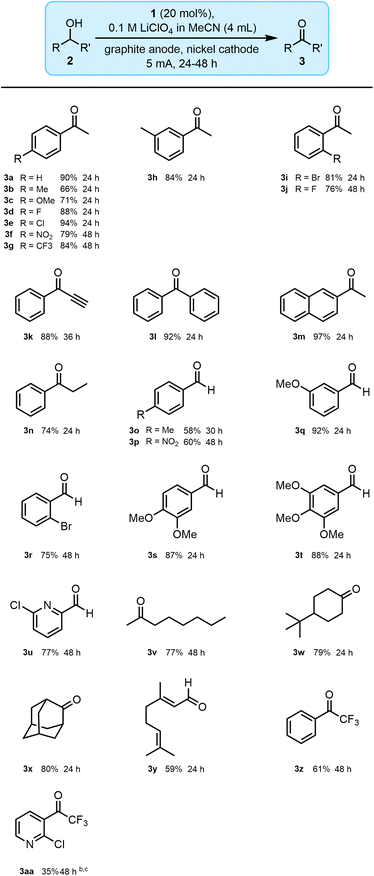 | ||
| Fig. 3 Substrate scope for the oxidation of alcohols.a Performed on the 2 mmol scale; isolated yield after purification.b Performed on the 1 mmol scale.c Product conversion. | ||
Biphenyl, naphthyl, and phenylpropyl alcohols could also be oxidised successfully to the ketones (3l–3n). Benzylic and heteroaromatic primary alcohols were oxidised to the corresponding aldehydes and not further transformed to carboxylic acids (3o–3u). Aliphatic alcohols were also amenable to the procedure (3v–3x), as was geraniol (3y). Two representative trifluoromethyl ketones (TFMK) were also prepared with conversions near 90% (3z and 3aa). However, due to their low polarity, separating the desired TFMK product from the remaining α-trifluoromethyl alcohol was not trivial and came at a cost to the isolated yield obtained. As is generally the case when using oxoammonium cations as oxidants, electron-deficient substrates require longer reaction times than their electron-rich congeners, as do stating materials bearing substituents in ortho-positions.21 Due to its extreme electron-withdrawing nature of the substrate, 3aa was synthesised on a smaller, 1 mmol scale to avoid a significantly extended reaction time. Overall, 27 representative alcohols were screened, with isolated yields of 58–97% being obtained.
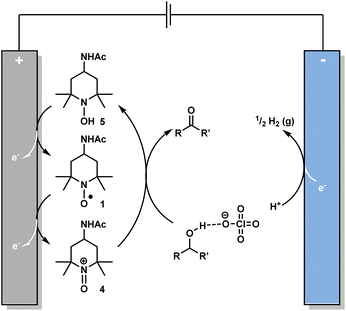
A proposed mechanism for the oxidation process is shown in Fig. 4. The first step is the electrochemical generation of oxoammonium cation 4 from 1. This takes place at the anode through a single electron transfer (SET) process. Facilitated by the perchlorate anion from the electrolyte, 4 then oxidises the alcohol substrate to form the carbonyl product. The hydroxylamine species, 5, generated from this reaction is converted back into 1 at the anode by another SET process, thereby completing the catalytic cycle. At the cathode, the proton released from the alcohol oxidation step is reduced to H2 (hydrogen evolution reaction).18,26–28,40 This is supported by the observation of bubbles formed on the cathode surface over the course of the reaction.
Conclusion
We have developed a methodology for the nitroxide-mediated electrochemical oxidation of alcohols to aldehydes and ketones. The reaction is performed in the absence of base, this representing an advance in terms of ease of use compared to similar procedures. Our approach also allows for the selective oxidation of primary alcohols to aldehydes rather than the corresponding carboxylic acids that are traditionally formed when using 1 as an electrocatalyst. The methodology uses inexpensive, widely available electrode materials, a simple electrolyte, and a small quantity of solvent. It is performed using a commercially available electrochemistry unit, making it reliable and reproducible. The approach proves effective for the oxidation of a range of aromatic, heteroaromatic, and aliphatic alcohols and can be applied to sensitive propargylic alcohols and to α-trifluoromethyl alcohols, which are classes of compounds that are notoriously hard to oxidise.Experimental
General considerations
NMR spectra (1H-, 13C-, and 19F-) were performed at 300 K using either a Brüker Avance Ultra Shield 300 MHz, or Brüker DRX-400 400 MHz spectrometer. 1H-NMR spectra were referenced to residual CHCl3 (7.26 ppm) in CDCl3. 13C-NMR spectra were referenced to CDCl3 (77.16 ppm). 19F-NMR spectra were referenced to hexafluorobenzene (−161.64 ppm).41 Reactions were monitored by 1H- or 19F-NMR, and/or by TLC on silica gel plates (60 Å porosity, 250 μm thickness). TLC plates were visualised using UV light. Electrochemistry reactions were performed using an IKA ElectraSyn 2.0 single vial holder and carousel units.Chemicals
Deuterated chloroform (CDCl3) was purchased from Cambridge Isotope Laboratories. Hexafluorobenzene was purchased from Oakwood Chemicals. 4-Acetamido-TEMPO (ACT, 1) was prepared and recrystallised using a previously reported protocol.42 All the alcohols used were purchased from Oakwood Chemicals, Sigma-Aldrich, or Alfa Aesar.General procedure for the preparation of ketones and aldehydes
Without precautions to exclude air or moisture; alcohol (2 mmol, 1 eq.), 1 (0.4 mmol, 0.2 eq.), lithium perchlorate in acetonitrile (4 mL, 0.1 M), and a stir bar were placed in an ElectraSyn 2.0 vial (5 mL capacity). The ElectraSyn vial cap was equipped with a graphite anode (working electrode) and a nickel cathode (counter electrode) and then secured onto the vial. The reaction mixture was stirred at 500 rpm. A constant current of 5 mA was passed through the reaction mixture until the oxidation was deemed complete, as monitored by 1H-NMR spectroscopy (24–48 h). On completion, the product mixture was filtered through a pad of silica. The silica was washed with diethyl ether to elute the product and then the organic solvent removed from the filtrate under vacuum to afford the pure product.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The University of Connecticut Office of Undergraduate Research and the Department of Chemistry Charles Waring Fund are acknowledged. We thank Dr Vitaliy Gorbatyuk for NMR spectroscopy equipment support.Notes and references
- H. Kolbe, Ann. Chem. Pharm., 1849, 69, 257–294 CrossRef.
- M. M. Baizer, Tetrahedron Lett., 1963, 973–977 CrossRef CAS.
- M. M. Baizer, in Electrochemistry, Past and Present, American Chemical Society, 1989, vol. 390, pp. 172–175 Search PubMed.
- J. Yoshida, K. Kataoka, R. Horcajada and A. Nagaki, Chem. Rev., 2008, 108, 2265–2299 CrossRef CAS PubMed.
- T. Gieshoff, S. R. Waldvogel, M. Zirbes, E. Rodrigo, A. Wiebe and S. Möhle, Angew. Chem., Int. Ed., 2018, 57, 5594–5619 CrossRef PubMed.
- M. Yan, Y. Kawamata and P. S. Baran, Chem. Rev., 2017, 117, 13230–13319 CrossRef CAS PubMed.
- R. Francke and R. D. Little, Chem. Soc. Rev., 2014, 43, 2492–2521 RSC.
- B. A. Frontana-Uribe, R. D. Little, J. G. Ibanez, A. Palma and R. Vasquez-Medrano, Green Chem., 2010, 12, 2099–2119 RSC.
- D. S. P. Cardoso, B. Šljukić, D. M. F. Santos and C. A. C. Sequeira, Org. Process Res. Dev., 2017, 21, 1213–1226 CrossRef CAS.
- E. J. Horn, B. R. Rosen and P. S. Baran, ACS Cent. Sci., 2016, 2, 302–308 CrossRef CAS PubMed.
- M. Yan, Y. Kawamata and P. S. Baran, Angew. Chem., Int. Ed., 2018, 57, 4149–4155 CrossRef CAS PubMed.
- S. B. Beil, D. Pollok and S. R. Waldvogel, Angew. Chem., Int. Ed., 2021, 60, 14750–14759 CrossRef CAS PubMed.
- J. E. Nutting, M. Rafiee and S. S. Stahl, Chem. Rev., 2018, 118, 4834–4885 CrossRef CAS PubMed.
- F. Wang and S. S. Stahl, Acc. Chem. Res., 2020, 53, 561–574 CrossRef CAS PubMed.
- J. B. Gerken, Y. Q. Pang, M. B. Lauber and S. S. Stahl, J. Org. Chem., 2018, 83, 7323–7330 CrossRef CAS PubMed.
- R. Ciriminna, M. Ghahremani, B. Karimi and M. Pagliaro, ChemistryOpen, 2017, 6, 5–10 CrossRef CAS PubMed.
- H. Hou, X. Ma, Y. Ye, M. Wu, S. Shi, W. Zheng, M. Lin, W. Sun and F. Ke, RSC Adv., 2022, 12, 5483–5488 RSC.
- B. Karimi, M. Ghahremani, R. Ciriminna and M. Pagliaro, Org. Process Res. Dev., 2018, 22, 1298–1305 CrossRef CAS.
- I. B. Krylov, A. S. Budnikov, E. R. Lopat'eva, O. O. Segida, S. A. Paveliev and A. O. Terent'ev, J. Phys.: Conf. Ser., 2021, 1942, 012007 CrossRef CAS.
- H. A. Beejapur, Q. Zhang, K. Hu, L. Zhu, J. Wang and Z. Ye, ACS Catal., 2019, 9, 2777–2830 CrossRef CAS.
- J. M. Bobbitt, C. Brückner and N. Merbouh, in Organic Reactions, John Wiley & Sons, Ltd, 2010, pp. 103–424 Search PubMed.
- D. Wang, P. Wang, S. Wang, Y. H. Chen, H. Zhang and A. Lei, Nat. Commun., 2019, 10, 1–8 CrossRef PubMed.
- Y. Demizu, H. Shiigi, T. Oda, Y. Matsumura and O. Onomura, Tetrahedron Lett., 2008, 49, 48–52 CrossRef CAS.
- M. Okimoto, T. Yoshida, M. Hoshi, T. Chiba and K. Maeo, Synth. Commun., 2011, 41, 3134–3139 CrossRef CAS.
- M. F. Semmelhack, C. S. Chou and D. A. Cortes, J. Am. Chem. Soc., 1983, 105, 4492–4494 CrossRef CAS.
- P. Niu, X. Liu, Z. Shen and M. Li, Molecules, 2019, 24, 100 CrossRef PubMed.
- A. Das and S. S. Stahl, Angew. Chem., Int. Ed., 2017, 56, 8892–8897 CrossRef CAS PubMed.
- M. Rafiee, Z. M. Konz, M. D. Graaf, H. F. Koolman and S. S. Stahl, ACS Catal., 2018, 8, 6738–6744 CrossRef CAS.
- A. C. Cardiel, B. J. Taitt and K.-S. Choi, ACS Sustain. Chem. Eng., 2019, 7, 11138–11149 CrossRef CAS.
- M. Rafiee, K. C. Miles and S. S. Stahl, J. Am. Chem. Soc., 2015, 137, 14751–14757 CrossRef CAS PubMed.
- A. León Sandoval, F. Politano, M. L. Witko and N. E. Leadbeater, Org. Biomol. Chem., 2021, 19, 2986–2990 RSC.
- J. Nandi, M. Z. Vaughan, A. L. Sandoval, J. M. Paolillo and N. E. Leadbeater, J. Org. Chem., 2020, 85, 9219–9229 CrossRef CAS PubMed.
- F. Politano, A. León Sandoval, M. L. Witko, K. E. Doherty, C. M. Schroeder and N. E. Leadbeater, Eur. J. Org Chem., 2022, 2022, e202101239 CrossRef CAS.
- J. Nandi and N. E. Leadbeater, Org. Biomol. Chem., 2019, 17, 9182–9186 RSC.
- A. León Sandoval, F. Politano, M. L. Witko and N. E. Leadbeater, Org. Biomol. Chem., 2022, 20, 667–671 RSC.
- V. A. Pistritto, J. M. Paolillo, K. A. Bisset and N. E. Leadbeater, Org. Biomol. Chem., 2018, 16, 4715–4719 RSC.
- F. Politano, W. P. Brydon and N. E. Leadbeater, Synthesis, 2023, 55, 1517–1524 CrossRef CAS.
- S. J. Pike, J. J. Hutchinson and C. A. Hunter, J. Am. Chem. Soc., 2017, 139, 6700–6706 CrossRef CAS PubMed.
- C. E. Hatch, M. I. Martin, P. H. Gilmartin, L. Xiong, D. J. Beam, G. P. A. Yap, M. J. Von Bargen, J. Rosenthal and W. J. Chain, Org. Lett., 2022, 24, 1423–1428 CrossRef CAS PubMed.
- A. Badalyan and S. Stahl, Nature, 2016, 535, 406–410 CrossRef CAS PubMed.
- C. P. Rosenau, B. J. Jelier, A. D. Gossert and A. Togni, Angew. Chem., Int. Ed., 2018, 57, 9528–9533 CrossRef CAS PubMed.
- M. A. Mercadante, C. B. Kelly, J. M. Bobbitt, L. J. Tilley and N. E. Leadbeater, Nat. Protoc., 2013, 8, 666–676 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra04608g |
| This journal is © The Royal Society of Chemistry 2023 |