Open Access Article
Open Access ArticleSpectral properties of B40 enhanced by small molecule adsorption†
Jia Wang a,
Yunkai Zhanga,
Meiqi Wanga,
Ming-Xing Songa,
Bo Wang*b and
Zhengkun Qin
a,
Yunkai Zhanga,
Meiqi Wanga,
Ming-Xing Songa,
Bo Wang*b and
Zhengkun Qin *a
*a
aCollege of Information Technology, Jilin Engineering Research Center of Optoelectronic Materials and Devices, Jilin Normal University, Siping, 136000, China. E-mail: qzkjlnu@163.com
bSchool of Science, Northeast Electric Power University, Jilin, 131200, China. E-mail: bowang@neepu.edu.cn
First published on 20th September 2023
Abstract
The luminescence characteristics of small molecule excited B40 have not been studied yet, and it may have a potential application value in quantum dot luminescence. Herein, the adsorption and fluorescence emission spectra of small molecules (pyridine, pyrazine and benzene) adsorbed on B40 are studied using first-principles. The results show that the absorption of pyridine and pyrazine on B40 can form stable chemisorption structures pyridine-B40 and pyrazine-B40, while benzene adsorption can form physisorption structure benzene-B40. Moreover, the adsorbed pyridine can enhance the intensity of emission spectra of B40. And the pyrazine adsorbed can obviously enhance the intensity of absorption and emission spectra of B40 and cause the spectra to redshift to the visible light range. And the adsorption of benzene has almost no enhancement effect on absorption and emission spectra of B40. In addition, the influence of different computational basis sets on spectra characteristics has also been discussed and the results show that the main peaks of absorption and emission spectra calculated by the diffuse function augmented basis sets are redshifted relatively. This finding provides a strategy for quantum dot luminescence and a theoretical reference for experimental research.
Introduction
All-boron fullerenes B40 were discovered by a combination of experiment and theory in 2014.1 Subsequent theoretical studies revealed that B40 is a superatom.2 And the chain-like assembly with B40 superatom has also been studied, which show that the assembly can decrease band gap and achieve transformation from insulator to a semiconductor.3 Moreover, due to the electron deficiency of boron fullerenes,4,5 adsorbed or doped additional atoms or small molecules on them may form more stable structures. The adsorption behaviors of B40 nanocage towards aniline is reported, and doping B40 with Mn and Fe atoms can be an efficient approach for the removal of aniline.6 And atom adsorbed or doped B40 can improve hydrogen storage capacity of B40.7–10 Therefore, adsorbed or doped atoms or small molecules on the surface of B40 fullerene may also enhance its other properties, such as fluorescence spectra.Previous studies have found that superatoms have unique spectral properties, such as C60,11 Na40,12 Al13−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 and Au20,14 etc. The spectra of B40 fullerene is also been investigated and distinguished the hollow cage structure from other quasi-planar structures.15 Moreover, the spectral properties of metalloborospherenes MB400/− (M = Cu, Ag, Au) are also studied, and the results suggest that doped metal atoms in borospherene B40 can change the spectral features since the extra metal atoms can modify the electronic structure of B40.16 Nonlinear optical (NLO) features of metals decorated B40 fullerene are studied and show remarkable electro-optical response.17,18 Can the small molecules with strong electronegativity, such as the benzene, enhance or change the spectral characteristics when they adsorbed on B40?
13 and Au20,14 etc. The spectra of B40 fullerene is also been investigated and distinguished the hollow cage structure from other quasi-planar structures.15 Moreover, the spectral properties of metalloborospherenes MB400/− (M = Cu, Ag, Au) are also studied, and the results suggest that doped metal atoms in borospherene B40 can change the spectral features since the extra metal atoms can modify the electronic structure of B40.16 Nonlinear optical (NLO) features of metals decorated B40 fullerene are studied and show remarkable electro-optical response.17,18 Can the small molecules with strong electronegativity, such as the benzene, enhance or change the spectral characteristics when they adsorbed on B40?
In this work, the adsorption of small molecules (pyridine, pyrazine and benzene) on all-boron fullerene B40 is investigated by density functional theory (DFT).19 The results show that the adsorbed small molecules can strengthen the absorption and emission spectra of B40. The absorption and emission spectra of pyrazine-B40 are in the visible light range. The purpose of this work is to gain the influence of small molecule on the spectra of B40, and how these effects could be used to design quantum dot luminescence.
Models and computational methods
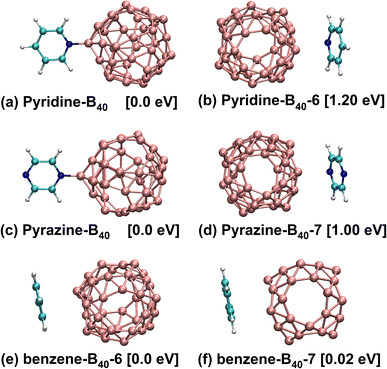
In this work, the model is built by adsorbing small molecules (pyridine, pyrazine and benzene) on the B40 cage. Due to B40 fullerene has two heptagons and four hexagons,1,2,20 the pyridine, pyrazine and benzene are adsorbed at hexagon, heptagon and B atom sites of B40 fullerene to form studied structures. After theoretical simulation and structural optimization, the six stable geometric structures are obtained, as presented in Fig. 1. Fig. 1a shows that the N atom of pyridine is bonded with the B atom of B40 (denoted as pyridine-B40), and Fig. 1b shows that pyridine is adsorbed at the hexagon of B40 (pyridine-B40-6), the former has the lowest energy. Fig. 1c and d show that the N atom of pyrazine is bonded with B atom of B40 and the pyrazine is adsorbed at heptagon of B40, denoted as pyrazine-B40 and pyrazine-B40-7, respectively, and the former have the lowest energy. It can be seen that the chemisorption structures are more stable than the physisorption structures. Besides, Fig. 1e and f show that the benzene are respectively adsorbed at hexagon and heptagon of B40 (denoted as benzene-B40-6 and benzene-B40-7), and they are physisorption structures. Compared with benzene-B40-7, the benzene-B40-6 has lower energy. In addition, we further verified it by adsorption energy decomposition, and the results show that the benzene-B40-6 is more stable than benzene-B40-7 structure. The detailed adsorption energy analysis is placed in the first part in the ESI.† The relative energies between each isomer and the lowest energy structure are listed in brackets. In the following, we mainly analysed the three structures with the lowest energy, that is, pyridine-B40, pyrazine-B40 and benzene-B40-6.The empirical dispersion-corrected density functional theory (DFT-D3)21 is used to fully optimize the geometric structures by hybrid functionals PBE0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22 with 6-31G* basis sets.23 All the optimized structures are confirmed to be local minima. The calculation details are placed in the second part in the ESI.† Simultaneously, based on the geometric structures, TD-DFT method19,24 is used to calculate the electronic transition, absorption and emission spectral properties. And we chose the range-separated hybrid functionals CAM-B3LYP25,26 to calculate the absorption and emission spectra with 6-31G*, 6-31+G* and 6-311+G* basis sets.27 For accurate calculation, we selected 30 and 10 electronic states in the adsorption and emission spectra for calculation. In addition, all the computations are carried out using the Gaussian16 software package.28
22 with 6-31G* basis sets.23 All the optimized structures are confirmed to be local minima. The calculation details are placed in the second part in the ESI.† Simultaneously, based on the geometric structures, TD-DFT method19,24 is used to calculate the electronic transition, absorption and emission spectral properties. And we chose the range-separated hybrid functionals CAM-B3LYP25,26 to calculate the absorption and emission spectra with 6-31G*, 6-31+G* and 6-311+G* basis sets.27 For accurate calculation, we selected 30 and 10 electronic states in the adsorption and emission spectra for calculation. In addition, all the computations are carried out using the Gaussian16 software package.28
Results and discussion
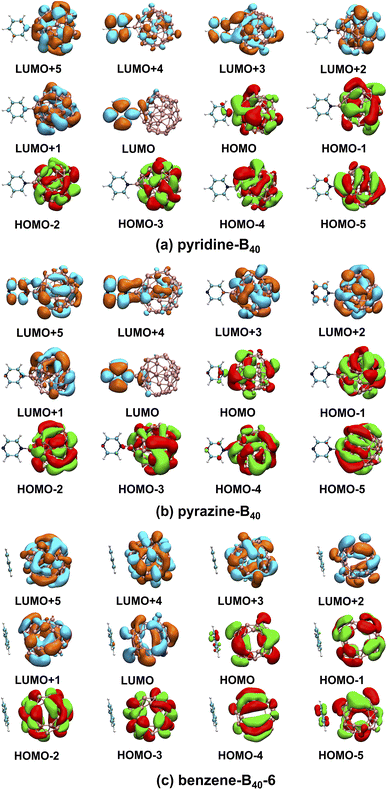
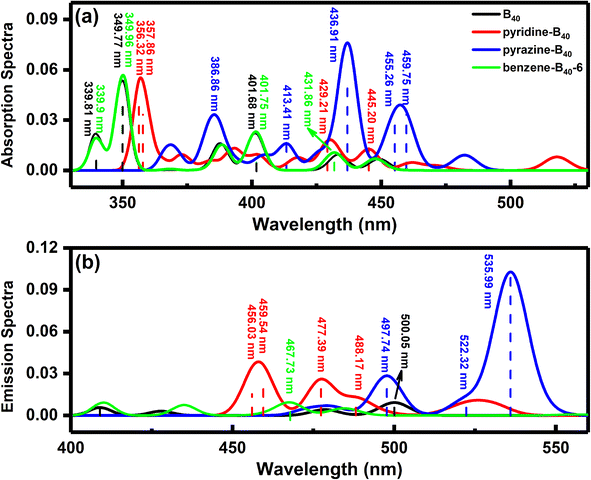
Our calculations show that the ground states of pyridine-, pyrazine-, and benzene-B40 are non-spin-polarized singlet, and the diagram of frontier molecular orbitals (MOs) is shown in Fig. 2. For pyridine-B40 structure (Fig. 2a), the lowest unoccupied molecular orbital plus 5 (LUMO+5), LUMO+2, LUMO+1, the highest occupied molecular orbital (HOMO), HOMO−1, HOMO−2, HOMO−3, HOMO−4 and HOMO−5 mainly occupies on B40, and the LUMO mainly occupy on pyridine. Moreover, the LUMO+4 and LUMO+3 are delocalization on B40 and pyridine. Similar to pyridine-B40, the LUMO+3, LUMO+2, LUMO+1, HOMO, HOMO−1, HOMO−2, HOMO−3, HOMO−4 and HOMO−5 of pyrazine-B40 mainly occupy on B40, the LUMO+4 and LUMO mainly occupy on pyridine (shown in Fig. 2b). And the LUMO+5 are delocalization on the whole structure. Different from pyridine-B40 and pyrazine-B40, the frontier MOs of benzene-B40-6 mainly occupy on B40 (as see Fig. 2c). Thus, the electrons in the pyridine-B40 and pyrazine-B40 structures transition from B40 to small molecules, while for the benzene-B40-6 structure, the electrons transition from B40 to B40.To analyze the spectral characteristics of small molecule excitation B40, the UV-vis absorption and emission spectra of the singlet excited states are shown in Fig. 3. The black, red, blue and green lines in the figure represent the absorption and emission spectra of the B40, pyridine-B40, pyrazine-B40, and benzene-B40-6 structures. Fig. 3a shows that the absorption spectrum of B40 is localized around 350 nm, in the ultraviolet (UV) light range. And small molecules adsorbed not only enhance the intensity of the adsorption spectrum of B40, but also redshift the spectrum from UV to visible light range. As see the red curve in the Fig. 3a, although the intensity of the absorption spectrum of pyridine-B40 is not stronger than that of B40 (black curve), the main absorption peak is relatively redshifted. Furthermore, the data of four typical absorption peaks for pyridine-B40 are listed in Table 1. The first strong absorption peak is localized near 356 nm and the lowest single excited transition S0 → S1 (S0 and S1 represent the ground state and first singlet excited state) mainly originates from the transition from the HOMO, HOMO−5 and HOMO−6 to the LUMO, LUMO+3 and LUMO+4, that is, the S0 → S1 originates from the transition from B40 to B40 and pyridine. The second absorption peak near 358 nm arises from the HOMO−5 to LUMO and LUMO+3 transitions, similar discussions way have also been reflected in other works.29 The other two typical absorption peaks are relatively weaker and localized near 429 nm and 445 nm, originating from the transition from HOMO, HOMO−1, HOMO−2, HOMO−3 and HOMO−4 to LUMO, LUMO+1, LUMO+2 and LUMO+3. Thus, the main adsorption peaks of pyridine-B40 originate from the transition from B40 to B40 and pyridine.
| Structures | States | E/λ | f | Main configuration (transitions) | Assignment |
|---|---|---|---|---|---|
| B40 | S28 | 3.65/339.81 | 0.0163 | HOMO−7 → LUMO+1 (34%) | B40 → B40 |
| HOMO−7 → LUMO+2 (48%) | |||||
| S26 | 3.54/349.77 | 0.0795 | HOMO−6 → LUMO+2 (44%) | B40 → B40 | |
| HOMO−5 → LUMO+1 (44%) | |||||
| S10 | 3.09/401.68 | 0.0194 | HOMO−3 → LUMO+2 (21%) | B40 → B40 | |
| HOMO−2 → LUMO+1 (21%) | |||||
| HOMO → LUMO+3 (59%) | |||||
| S4 | 2.86/433.54 | 0.0121 | HOMO−1 → LUMO (66%) | B40 → B40 | |
| Pyridine-B40 | S30 | 3.48/356.32 | 0.0485 | HOMO−6 → LUMO+3 (25%) | B40 → B40 and pyridine |
| HOMO−5 → LUMO (23%) | |||||
| HOMO → LUMO+4 (29%) | |||||
| S29 | 3.46/357.86 | 0.0253 | HOMO−5 → LUMO (21%) | B40 → B40 and pyridine | |
| HOMO−5 → LUMO+3 (22%) | |||||
| S9 | 2.89/429.21 | 0.0153 | HOMO−4 → LUMO (27%) | B40 → B40 and pyridine | |
| HOMO−4 → LUMO+1 (31%) | |||||
| HOMO−3 → LUMO+2 (22%) | |||||
| HOMO → LUMO + 2 (26%) | |||||
| HOMO → LUMO + 3 (24%) | |||||
| S5 | 2.78/445.20 | 0.0159 | HOMO−2 → LUMO+1 (21%) | B40 → B40 and pyridine | |
| HOMO−1 → LUMO (21%) | |||||
| HOMO−1 → LUMO+1 (32%) | |||||
| HOMO → LUMO+2 (38%) | |||||
| Pyrazine-B40 | S12 | 3.00/413.41 | 0.0203 | HOMO−1 → LUMO+3 (53%) | B40 → B40 |
| S9 | 2.84/436.91 | 0.0957 | HOMO−4 → LUMO (27%) | B40 → pyrazine | |
| HOMO−3 → LUMO (46%) | |||||
| HOMO−1 → LUMO (22%) | |||||
| S6 | 2.72/455.26 | 0.0232 | HOMO−5 → LUMO (27%) | B40 → pyrazine | |
| HOMO−2 → LUMO (42%) | |||||
| S5 | 2.70/459.75 | 0.0298 | HOMO−2 → LUMO (22%) | B40 → B40 and pyrazine | |
| HOMO → LUMO+2 (55%) | |||||
| Benzene-B40-6 | S29 | 3.65/339.9 | 0.0133 | HOMO−7 → LUMO+1 (51%) | B40 → B40 |
| HOMO−7 → LUMO+2 (27%) | |||||
| S26 | 3.54/349.96 | 0.0834 | HOMO−6 → LUMO+1 (43%) | B40 → B40 | |
| HOMO−5 → LUMO+2 (46%) | |||||
| S10 | 3.09/401.75 | 0.0199 | HOMO → LUMO+3 (56%) | B40 → B40 | |
| S4 | 2.87/431.86 | 0.0135 | HOMO−1 → LUMO (66%) | B40 → B40 |
Further, the UV-vis absorption spectrum of S0 → S1 for pyrazine-B40 is shown in the blue curve in Fig. 3a, and the data of four typical absorption peaks are also listed in Table 1. The first strong absorption peak near 437 nm originates from the HOMO−1, HOMO−3 and HOMO−4 to LUMO transition. The other three typical absorption peaks are weaker, and localized around 413 nm, 455 nm and 460 nm, respectively. They are arising from the HOMO−5, HOMO−2, HOMO−1 and HOMO to LUMO, LUMO+2, and LUMO+3 transitions. The results indicate that the main adsorption peaks of pyrazine-B40 originate from the B40 to pyrazine transition. Compared with the absorption spectra of B40 and pyridine-B40 structures, the intensity of the absorption spectrum of pyrazine-B40 is distinctly enhanced, and the absorption peak wavelength is redshifted to the visible light range. The redshift is caused by the main adsorption peak transition from HOMO−5 to LUMO+6. Compared to the B40 (transition from HOMO−7 to LUMO+3), the transition MOs of pyrazine-B40 transition to higher MO energy levels.
Moreover, the UV-vis absorption spectrum of S0 → S1 for benzene-B40-6 is shown in the green curve in Fig. 3a, and the data of four typical absorption peaks are also listed in Table 1. The first strong absorption peak is localized near 349.96 nm and originates from the HOMO−6 and HOMO−5 to LUMO+2 and LUMO+1 transition. The second strong absorption peak is localized near 401.75 nm and originates from the HOMO to LUMO+3 transitions. The other two typical absorption peaks are weaker, and locate around 339.90 nm and 431.86 nm, respectively. They originate from the HOMO−7 and HOMO−1 to LUMO, LUMO+1 and LUMO+2 transitions. The results show that the adsorption peaks of benzene-B40-6 mainly transition from B40 to B40. Compared with the absorption spectra of pyridine-B40 and pyrazine-B40 structures, the intensity of the absorption spectrum of benzene-B40-6 is weaker.
From the absorption spectra, it can be seen that pyridine adsorption can slightly redshift the main absorption peaks. The adsorbed pyrazine not only enhances the absorption spectrum of B40, but also causes the absorption peaks of B40 to redshift. However, the benzene adsorption has almost no effect on the absorption spectrum of B40. This is because pyridine-B40 and pyrazine-B40 are chemisorption structures, the N atoms of pyridine and pyrazine are bonded to the B atoms of B40. Further, the analysis of electron density difference indicates that there is electron accumulation at the bonding region and small molecules (pyridine and pyrazine), while there is electron dissipation on the B40 that is close to the small molecules. However, the benzene-B40-6 is physisorption structure, there is both electron accumulation and electron dissipation between benzene and B40. The detailed diagram of electron density difference is shown in Fig. S1 in the ESI.† In other words, electrons are transferred from B40 to small molecules for the pyridine-B40 and pyrazine-B40 structures, while for the benzene-B40-6, there is no electron transfer between B40 and benzene. This is consistent with the results of frontier MOs analysis.
To obtain the fluorescence emission properties of small molecule adsorbed on B40, the single excited state of B40, pyridine-B40, pyrazine-B40, and benzene-B40-6 are also studied. The fluorescence emission spectra curves fitted by Gaussian function are shown in Fig. 3b. The lowest energy fluorescence emission wavelength of B40 is around 500 nm (black curve), and the intensity of emission spectra of B40 is weaker. For the pyridine-B40 structure, the intensity of its emission spectrum is stronger than that of B40. There are four typical emission spectra peaks of pyridine-B40 around 456.03 nm, 459.54 nm, 477.39 nm and 488.17 nm, respectively, as see the red curve and dash lines in Fig. 3b. And the fluorescence emission peaks of pyridine-B40 arises from the LUMO, LUMO+1, LUMO+2 and LUMO+3 to HOMO, HOMO−1, HOMO−2, HOMO−3 and HOMO−5 transition, which originates from the pyridine and B40 to B40 transition. The fluorescence emission wavelength and corresponding transition properties are listed in Table 2.
| Structures | States | E/λ | f | Main configuration (transitions) | Assignment |
|---|---|---|---|---|---|
| B40 | S1 | 2.48/500.05 | 0.0108 | LUMO → HOMO−1 (69%) | B40 → B40 |
| Pyridine-B40 | S10 | 2.72/456.03 | 0.0227 | LUMO → HOMO−5 (38%) | Pyridine → B40; B40 and pyridine → B40 |
| LUMO+1 → HOMO−3 (32%) | |||||
| LUMO+1 → HOMO−1 (25%) | |||||
| S9 | 2.70/459.54 | 0.0281 | LUMO → HOMO−5 (27%) | Pyridine → B40; B40 and pyridine → B40 | |
| LUMO+1 → HOMO−2 (24%) | |||||
| LUMO+1 → HOMO−1 (41%) | |||||
| S8 | 2.60/477.39 | 0.0302 | LUMO → HOMO−5 (23%) | Pyridine → B40; B40 and pyridine → B40 | |
| LUMO → HOMO−3 (39%) | |||||
| LUMO → HOMO−2 (33%) | |||||
| LUMO+3 → HOMO (33%) | |||||
| S7 | 2.54/488.17 | 0.0122 | LUMO+2 → HOMO (57%) | B40 → B40; B40 and pyridine → B40 | |
| LUMO+3 → HOMO (22%) | |||||
| Pyrazine-B40 | S7 | 2.49/497.74 | 0.0332 | LUMO+5 → HOMO (65%) | B40 and pyrazine → B40 |
| S6 | 2.37/522.32 | 0.0131 | LUMO → HOMO−2 (43%) | Pyrazine → B40 | |
| LUMO → HOMO−1 (51%) | |||||
| S4 | 2.31/535.99 | 0.1132 | LUMO → HOMO−5 (29%) | Pyrazine → B40 | |
| LUMO → HOMO−2 (43%) | |||||
| LUMO → HOMO−1 (42%) | |||||
| Benzene-B40-6 | S4 | 2.65/467.73 | 0.0108 | LUMO → HOMO−1 (67%) | B40 → B40 |
Furthermore, the lowest energy fluorescence emission of pyrazine-B40 (blue curve in Fig. 3b) is significantly stronger than that of B40 and the data of three typical emission peaks of pyrazine-B40 is listed in Table 2. The first strong emission peak is near 536 nm and originates from LUMO to HOMO−1, HOMO−2 and HOMO−5 transition. The other two typical emission spectra peaks are near 497.74 nm and 522.32 nm, and they originate from the LUMO and LUMO+5 to HOMO, HOMO−1 and HOMO−2 transition. So the main emission peaks of pyrazine-B40 originate from pyrazine to B40 transition. For benzene-B40-6 structure, the fluorescence emission is near 467.73 nm (green curve) and originates from the LUMO to HOMO−1 transition. And its fluorescence emission spectrum intensity is similar to that of B40, but weaker than that of pyridine-B40 and pyrazine-B40. Thus, the absorption of pyridine and pyrazine can enhance the emission spectrum of B40, and they all in the visible light range. The fluorescence emission peaks of pyridine-B40 and pyrazine-B40 originate from small molecules to B40 transition, while the fluorescence emission peak of benzene-B40-6 originates from B40 to B40 transition.
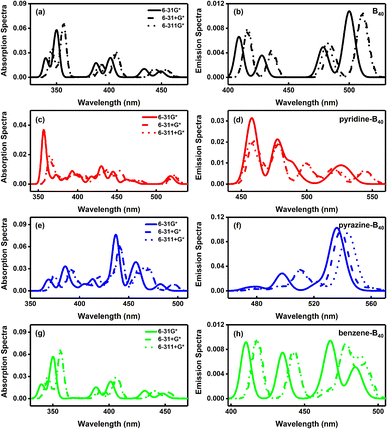
Finally, we discussed the effect of the basis sets on the absorption and emission spectra. The absorption and emission spectra of B40, pyridine-B40, pyrazine-B40, benzene-B40-6 calculated by CAM-B3LYP/6-31G*, CAM-B3LYP/6-31+G* and CAM-B3LYP/6-311+G* are shown in Fig. 4. The results show that the intensity of absorption and emission spectra of B40 calculated by 6-31+G* and 6-311+G* is stronger than that calculated by 6-31G*, and the main peaks of absorption and emission spectra calculated using 6-31+G* and 6-311+G* exhibit redshift relatively, as seen in Fig. 4a and b. And the absorption and emission spectra of B40 calculated by 6-31+G* and 6-311+G* are almost identical. For pyridine-B40, the intensity of absorption and emission spectra calculated by 6-31+G* and 6-311+G* is weaker than that calculated by 6-31G*, and the absorption peaks are also relative redshift (Fig. 4c and d). The absorption and emission spectra of pyrazine-B40 calculated by 6-31G* are stronger than that calculated by 6-31+G* and 6-311+G* basis sets. And the main peaks of absorption and emission spectra calculated using 6-31+G* and 6-311+G* are redshift relatively, as shown in Fig. 4e and f. For benzene-B40-6, the absorption and emission spectra calculated by 6-31+G* and 6-311+G* are stronger than that calculated by 6-31G*, and the main peaks occur to redshift (as seen in Fig. 4g and h). Thus, for different structures, the basis sets have different influence on the calculation of absorption and emission. But the main peaks of absorption and emission spectra calculated by the diffuse function augmented basis sets almost all undergo redshift. For the same structure, the influence of 6-31+G* and 6-311+G* basis sets on the calculation of absorption and emission spectra is almost same.
Conclusions
This work, the adsorption and fluorescence emission spectra of pyridine-B40, pyrazine-B40 and benzene-B40 are investigated by first principles. The results show that the adsorption of pyridine enhances the emission spectra of B40. And the pyrazine adsorbed on B40 not only enhance the adsorption and emission spectra of B40, but also redshift the spectra to the visible light range. Moreover, the adsorbed benzene has almost no effect on the absorption and emission spectra of B40. And the main adsorption peaks originate from the B40 to small molecules and B40 transition, and the main fluorescence emission peaks arise from small molecules to B40 transition. However, benzene-B40-6 is physisorption structure, its main adsorption and emission peaks originate from B40 to B40 transition. Finally, the influence on the calculation of 6-31G*, 6-31+G* and 6-311+G* basis sets on absorption and emission spectra are also studied, and the results indicate that the main peaks of absorption and emission spectra calculated by 6-31+G* and 6-311+G* basis sets occur to redshift.It is well known that the emission spectra of quantum dots can cover the entire visible light region by changing the size and chemical composition of quantum dots.30–33 The emission spectra of pyridine-B40, pyrazine-B40 and benzene-B40-6 are in the visible range, so we believed that this work has potential applications in quantum dot luminescence, especially in the pyrazine-B40 structure. To find inorganic and organic optical materials with good luminescent properties in the visible light range, many researchers focus on the electron absorption and emission of transition metal complexes.34–36 While we studied luminescent materials from the perspective of the unique spectrum of superatoms. We hope this work provides a new perspective for luminescent materials.
Author contributions
Jia Wang calculated and analyzed the results. All authors contributed to the general discussion.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the Natural Science Foundation Project of Jilin Province (grant number YDZJ202101ZYTS075), the National Natural Science Foundation of China (grant number 11947039), the Education Department of Jilin Province (grant number JJKH20230510KJ).Notes and references
- H.-J. Zhai, Y.-F. Zhao, W.-L. Li, Q. Chen, H. Bai, H.-S. Hu, Z. A. Piazza, W.-J. Tian, H.-G. Lu, Y.-B. Wu, Y.-W. Mu, G.-F. Wei, Z.-P. Liu, J. Li, S.-D. Li and L.-S. Wang, Nat. Chem., 2014, 6, 727–731 CrossRef CAS PubMed.
- J. Wang, T. Yu, Y. Gao and Z. Wang, Sci. China Mater., 2017, 60, 1264–1268 CrossRef CAS.
- J. Wang, W. Jiang, W. Xie, J. Wang and Z. Wang, Sci. China Mater., 2019, 62, 416–422 CrossRef CAS.
- N. Gonzalez Szwacki, A. Sadrzadeh and B. I. Yakobson, Phys. Rev. Lett., 2007, 98, 166804 CrossRef PubMed.
- N. Gonzalez Szwacki, Nanoscale Res. Lett., 2007, 3, 49 CrossRef.
- M. Keyhanian and D. Farmanzadeh, J. Mol. Liq., 2019, 294, 111638 CrossRef CAS.
- C. Tang and X. Zhang, Int. J. Hydrogen Energy, 2016, 41, 16992–16999 CrossRef CAS.
- H. Dong, T. Hou, S. T. Lee and Y. Li, Sci. Rep., 2015, 5, 9952 CrossRef CAS PubMed.
- Y. Zhang, X. Han and X. Cheng, Chem. Phys. Lett., 2020, 739, 136961 CrossRef CAS.
- J. Mao, P. Guo, T. Zhang, S. Zhang and C. Liu, Comput. Theor. Chem., 2020, 1181, 112823 CrossRef CAS.
- M. Feng, J. Zhao and H. Petek, Science, 2008, 320, 359–362 CrossRef CAS PubMed.
- W. D. Knight, K. Clemenger, W. A. De Heer, W. A. Saunders, M. Y. Chou and M. L. Cohen, Phys. Rev. Lett., 1984, 53, 510 CrossRef.
- D. E. Bergeron, A. W. Castleman, T. Morisato and S. N. Khanna, Science, 2004, 304, 84–87 CrossRef CAS PubMed.
- J. Li, X. Li, H. J. Zhai and L. S. Wang, Science, 2003, 299, 864–867 CrossRef CAS PubMed.
- R. He and X. C. Zeng, Chem. Commun., 2015, 51, 3185–3188 RSC.
- S.-X. Li, Z.-P. Zhang, Z.-W. Long and S.-J. Qin, RSC Adv., 2017, 7, 38526–38537 RSC.
- E. Shakerzadeh, M. Yousefizadeh and M. Bamdad, Inorg. Chem. Commun., 2020, 112, 107692 CrossRef CAS.
- E. Shakerzadeh, Z. Biglari and E. Tahmasebi, Chem. Phys. Lett., 2016, 654, 76–80 CrossRef CAS.
- R. G. Parr, Annu. Rev. Phys. Chem., 1983, 34, 631–656 CrossRef CAS.
- Y. Yang, Z. Zhang, E. S. Penev and B. I. Yakobson, Nanoscale, 2017, 9, 1805–1810 RSC.
- S. Grimme, J. Antony, S. Ehrlich and H. Krieg, J. Chem. Phys., 2010, 132, 154104 CrossRef PubMed.
- C. Adamo and V. Barone, J. Chem. Phys., 1999, 110, 6158–6170 CrossRef CAS.
- W. J. Hehre, R. Ditchfield and J. A. Pople, J. Chem. Phys., 1972, 56, 2257–2261 CrossRef CAS.
- M. A. Marques and E. K. Gross, Annu. Rev. Phys. Chem., 2004, 55, 427–455 CrossRef CAS PubMed.
- A. D. Laurent and D. Jacquemin, Int. J. Quantum Chem., 2013, 113, 2019–2039 CrossRef CAS.
- T. Yanai, D. P. Tew and N. C. Handy, Chem. Phys. Lett., 2004, 393, 51–57 CrossRef CAS.
- D. Kannar, A. Tajti and P. G. Szalay, J. Chem. Theory Comput., 2017, 13, 202–209 CrossRef CAS PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. V. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, F. D. Williams, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, T. A. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, J. B. Foresman, O. Farkas and D. J. Fox, Gaussian 16, Revision C.01, Gaussian, Inc., Wallingford, CT, 2016 Search PubMed.
- Y. Gao, B. Wang, Y. Y. Lei, B. K. Teo and Z. G. Wang, Nano Res., 2016, 9, 622–632 CrossRef CAS.
- H. V. Han, H. Y. Lin, C. C. Lin, W. C. Chong, J. R. Li, K. J. Chen, P. Yu, T. M. Chen, H. M. Chen, K. M. Lau and H. C. Kuo, Opt. Express, 2015, 23, 32504–32515 CrossRef CAS PubMed.
- J. Owen and L. Brus, J. Am. Chem. Soc., 2017, 139, 10939–10943 CrossRef CAS PubMed.
- L. Qian, Y. Zheng, J. Xue and P. H. Holloway, Nat. Photonics, 2011, 5, 543–548 CrossRef CAS.
- Y. X. Yang, Y. Zheng, W. R. Cao, A. Titov, J. Hyvonen, J. R. Manders, J. G. Xue, P. H. Holloway and L. Qian, Nat. Photonics, 2015, 9, 259–266 CrossRef CAS.
- T. Liu, B.-H. Xia, X. Zhou, Q.-C. Zheng, Q.-J. Pan and H.-X. Zhang, Theor. Chem. Acc., 2008, 121, 155–164 Search PubMed.
- K. J. Chen, H. V. Han, H. C. Chen, C. C. Lin, S. H. Chien, C. C. Huang, T. M. Chen, M. H. Shih and H. C. Kuo, Nanoscale, 2014, 6, 5378–5383 RSC.
- J. Shinar and R. Shinar, J. Phys. D: Appl. Phys., 2008, 41, 133001 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: The adsorption energy analysis, the calculation details about structural optimization and the analysis of electronic density difference. See DOI: https://doi.org/10.1039/d3ra04631a |
| This journal is © The Royal Society of Chemistry 2023 |