Open Access Article
Open Access ArticleMukaiyama aldol reaction: an effective asymmetric approach to access chiral natural products and their derivatives/analogues
Aqsa Mushtaq and
Ameer Fawad Zahoor *
*
Department of Chemistry, Government College University Faisalabad, 38000 Faisalabad, Pakistan. E-mail: fawad.zahoor@gcuf.edu.pk
First published on 8th November 2023
Abstract
The Mukaiyama aldol reaction is generally a Lewis-acid catalyzed cross-aldol reaction between an aldehyde or ketone and silyl enol ether. It was first described by Mukaiyama in 1973, almost 5 decades ago, to achieve the enantioselective synthesis of β-hydroxy carbonyl compounds in high percentage yields. Mukaiyama aldol adducts play a pivotal role in the synthesis of various naturally occurring and medicinally important organic compounds such as polyketides, alkaloids, macrolides, etc. This review highlights the significance of the Mukaiyama aldol reaction towards the asymmetric synthesis of a wide range of biologically active natural products reported recently (since 2020).
1. Introduction
The Mukaiyama aldol reaction was first reported by Mukaiyama in 1973, about 50 years ago.1 He carried out the aldol reaction of an aldehyde or ketone with trimethyl silyl enol ether by utilizing titanium chloride as a catalyst.2 The electrophilic nature of carbonyl compounds is enhanced using Lewis acid catalyst, thereby leading to a facile attack by the carbon nucleophile. Similarly, Mukaiyama and his coworker Ishida implemented the aldol reaction between silyl enol ether, which was obtained as a derivative of crotonaldehyde, and cinnamaldehyde dimethyl acetal. The aldol reaction, involving these substituents was named vinylogous Mukaiyama aldol reaction (VMAR).3–5 VMAR results in the synthesis of double bonds containing bulky structural skeletons, which can then be subjected to various reactions including organometallic reactions, dihydroxylation and epoxidation (Scheme 1).6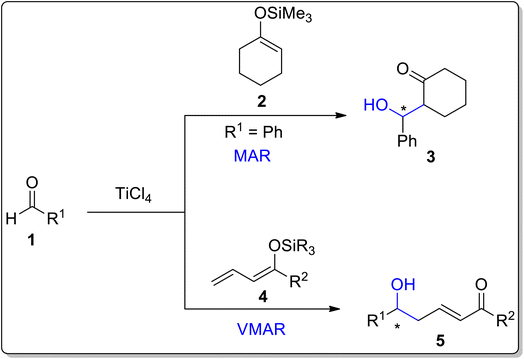 | ||
| Scheme 1 Titanium chloride catalyzed Mukaiyama and vinylogous Mukaiyama aldol reactions.6 | ||
The general mechanism of the Mukaiyama aldol reaction involves the attack of aldehyde oxygen to titanium followed by the release of a chloride ion. The chloride ion then attacks silylenol ether 6 to generate enolate and trimethylsilyl chloride. The enolate then attacks the electron-deficient carbonyl carbon followed by the addition of water to give the Mukaiyama aldol product 7 (Scheme 2).
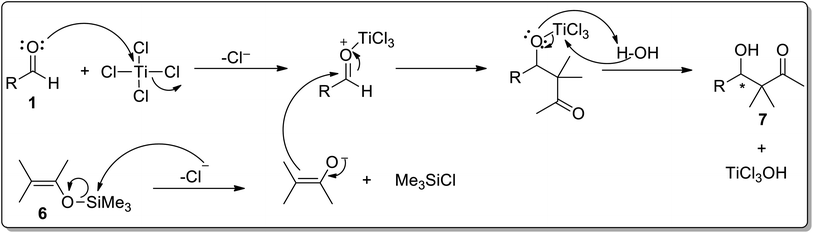 | ||
| Scheme 2 The general mechanism of the TiCl4 catalyzed Mukaiyama aldol reaction.1,7 | ||
Silicon7–9 and boron enolates10–12 were also employed by Mukaiyama and co-workers to execute the aldol reaction, which is highly stereoselective in nature. A strong base is employed to abstract a proton from the carbonyl compound to generate silicon enolate, which is then reacted with trialkylsilyl chloride.13 Then, this silicon enolate further undergoes reaction with a carbonyl compound by utilizing a Lewis acid as catalyst. In general, the Mukaiyama aldol adduct is formed by the involvement of an open transition state, however, boron enolates require the use of a weak base with boron Lewis acid and the reaction proceeds through a transition state that is cyclic in nature.14,15
Various other Lewis acids i.e., zinc chloride, iron chloride, tin chloride, boron trifluoride and aluminium trichloride have been utilized after the employment of titanium tetrachloride in Mukaiyama aldol reactions.16 Moreover, there have been further advancements in this reaction by the application of numerous other electrophiles such as acetals, thioacetals, ketimines, imines and ketones.17–20 Besides electrophiles, many other nucleophiles have now been employed in the Mukaiyama aldol reaction including allylsilanes, silicon dienolates and silyl cyanides.21,22
Moreover, a facile reaction between silicon enolates and acetals has been observed in trimethylsilyl trifluoromethanesulfonate, trityl perchlorate and trityl hexachloroantimonate mediated Mukaiyama aldol reactions.23 In addition, utilization of non-metallic Lewis acids i.e., triphenyl methyl salt has also been reported.24a Previously, almost all reported Mukaiyama aldol reactions were carried out in organic solvents. Despite water being an easily accessible and eco-friendly solvent, it was not considered for Mukaiyama aldol reactions due to the possibility of decomposition of silicon enolates. However, later studies revealed that lanthanide triflate catalyzed Mukaiyama aldol reactions were possible using water as solvent, resulting in enantioselective synthesis of aldol adducts in high yields.24b
β-Hydroxy carbonyl compounds with two asymmetric centers are produced as a result of the reaction between enolates and carbonyl compounds.25,26 C–C bonds are formed as a result of this reaction leading to the generation of complicated structures. Sakurai–Hosomo allylation27 and hetero-Diels–Alder reactions are examples of such C–C bond forming reactions. Moreover, chiral Lewis acid catalysts are used to carry out asymmetric transformations. Various natural products constitute β-hydroxy carbonyl groups in their structural framework. Specifically, naturally occurring macrolides and polyketides are known to be derived from β-hydroxy carbonyl compounds. The stereocontrol nature of the Mukaiyama aldol reaction with ambient reaction conditions paved the way towards the synthesis of various natural products.28–30 There have been several reports on the incorporation of MAR and VMAR in the synthesis of various natural products. Some of their structures are given in Fig. 1.31,32
 | ||
| Fig. 1 Structures of some naturally occurring compounds involving Mukaiyama aldol reactions in their total synthesis. | ||
The synthesis of natural products is a very appealing yet complicated process and there has been a great focus on the procurement of biologically important natural products via facile synthetic routes.33–36 The role of the Mukaiyama aldol reaction towards the total synthesis of organic compounds and natural products has been summarized by various research groups previously.37,38 The objective of our review is to provide the recent applications of Mukaiyama aldol reaction towards the synthesis of natural products/pharmaceutically important organic compounds, thereby covering the literature reported during the past three years (2020–2023).
2. Review of the literature
2.1. Synthesis of macrolide based natural products
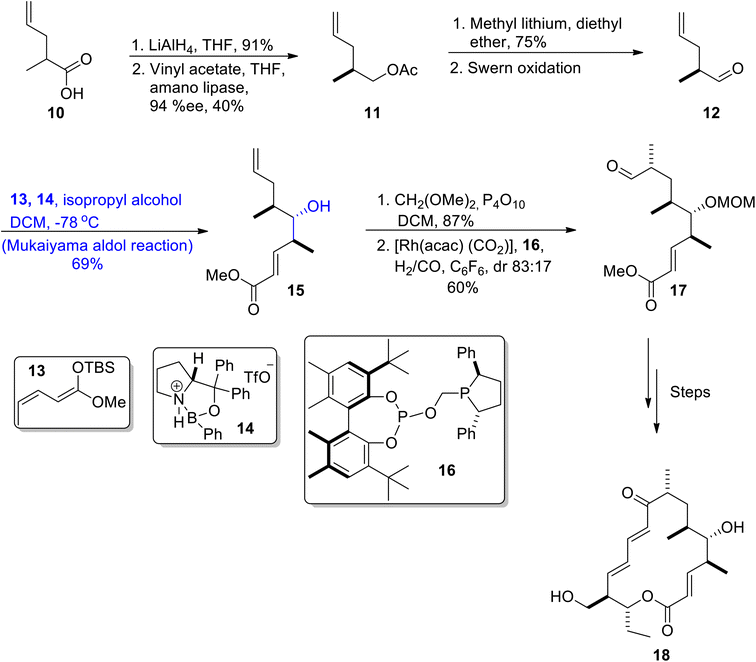
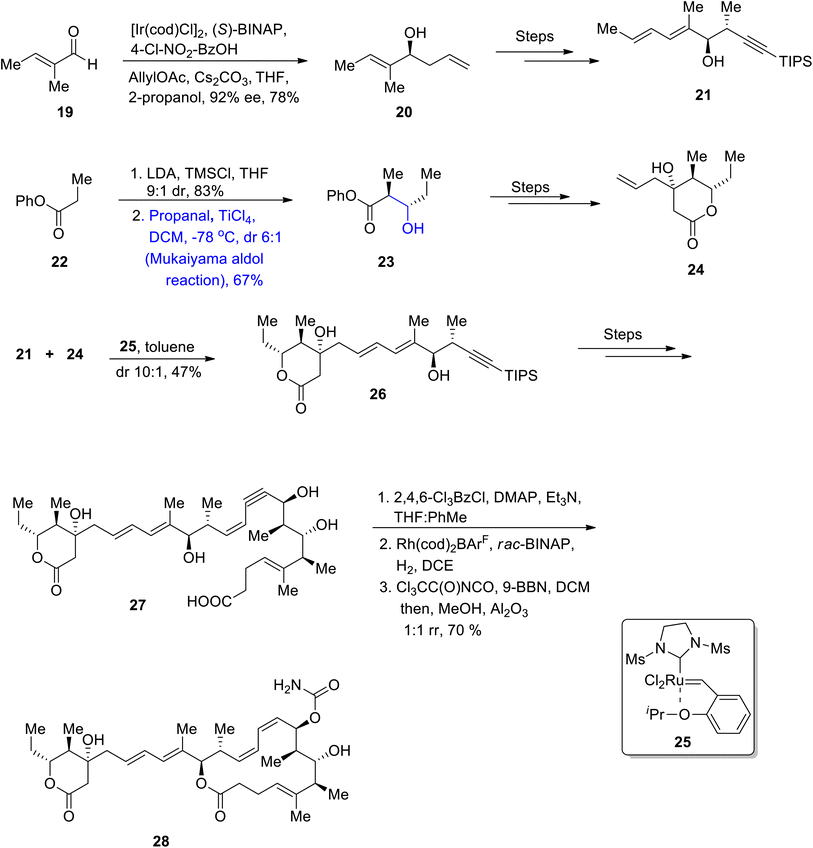
The antibacterial potential of Streptomyces species is well known, they are also source of various macrolides. Aldgamycin N has been isolated from Streptomyces strain HK-2006-1, and these macrolides have been found to be highly potent against numerous bacterial strains i.e., Staphylococcus aureus 209P.39,40 Aldgamycins are D-mycinose containing mycinolide macrolides having glycosylation at hydroxyl group on carbon 20.41,42 Herle et al.43 reported the total synthesis of mycinolide IV 18, which led to the synthetic pathway towards aldgamycin N. The first step of their synthetic route involves the reduction of compound 10 followed by its reaction with vinyl acetate and amino lipase in tetrahydrofuran, which resulted in compound 11 in 40% yield with 94% ee. Acetate 11 was then reacted with methyl lithium in the presence of diethyl ether followed by Swern oxidation to yield aldehyde 12. In the following step, compound 12 was subjected to the Mukaiyama aldol reaction by treatment with silyl enol ether 13 and compound 14 in isopropyl alcohol and DCM, which resulted in the synthesis of aldol adduct 15 in 69% yield. The compound 15 was further reacted with CH2(OMe)2, P4O10, dichloromethane followed by its reaction with [Rh(acac)(CO)2], and compound 16 to obtain aldehyde 17 in 60% yield. Aldehyde 17 was then reacted over a number of steps to synthesize target molecule 18 (Scheme 3).The Lithistid sponge of the genus Leiodermatium is the main source of anti-mitotic leiodermatolide A, which disrupts the arrangement of microtubules, thereby inhibiting uncontrolled cell division.44–46 This marine macrolide has found ample significance in medicinal fields; however, its usage is limited as it is not available in sufficient amounts. These factors have led to numerous efforts towards its total synthesis. In 2021, Siu et al.47 reported the efficient and facile synthesis of this structurally complex and medicinally important macrolide by utilizing catalytic enantioselective hydrogenative allylation, the Mukaiyama aldol reaction, crotylation and propargylation as key steps. For this purpose, they obtained homo allylic alcohol 20 in 78% yield with 92% enantiomeric excess as a result of the iridium catalyzed reaction of aldehyde 19 with 4-chloro-nitro benzyl alcohol using (S)-BINAP followed by allylation. Homoallylic alcohol 20 was further reacted via several steps to obtain compound 21. In the next step, ester 22 reacted with lithium diisopropylamide, TMSCl in the presence of THF followed by the titanium tetrachloride catalyzed Mukaiyama aldol reaction reacting with propanal in the presence of DCM, which resulted in the synthesis of adduct 23. Compound 23 was then transformed to compound 24 via several steps. Compounds 21 and 24 were then coupled in the presence of 25 and toluene to obtain dienyne 26 in 47% yield, which was then reacted via several steps to give conjugated enyne 27. Later, the conjugated enyne 27 was subjected to react over three distinct steps (including Yamaguchi lactonization) to accomplish the synthesis of leiodermatolide A 28 in 70% yield in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 racemic ratio (Scheme 4).
1 racemic ratio (Scheme 4).
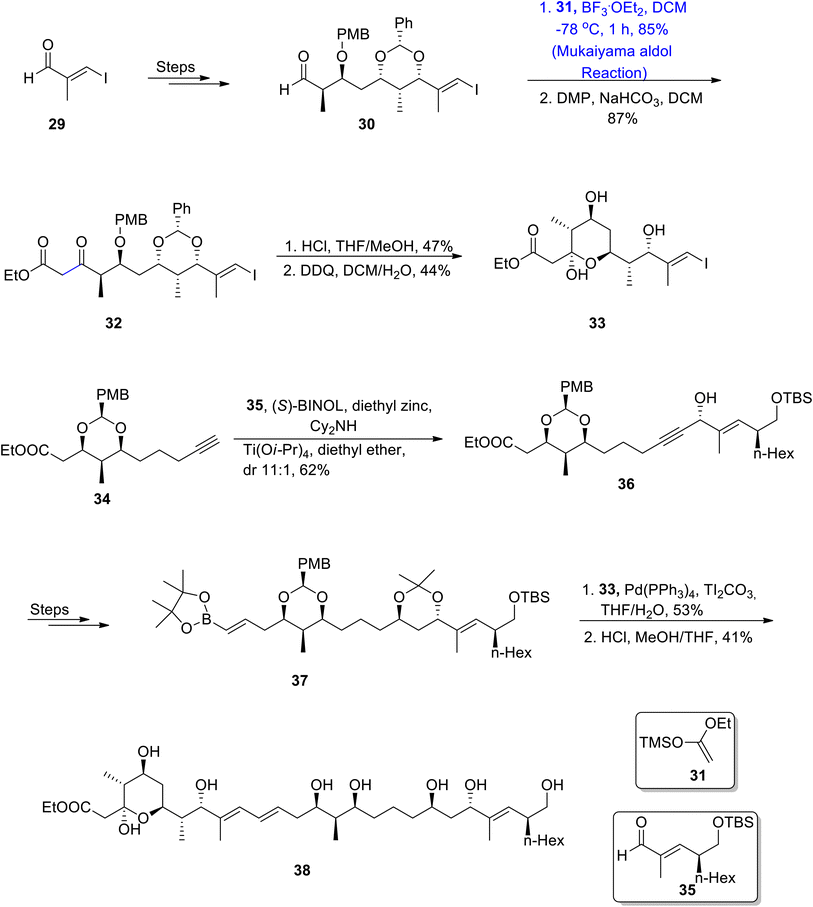
Stambomycins are naturally occurring and biologically active macrolides containing two chiral centers that are known to be obtained from Streptomyces ambofaciens.48 Confirmation of the structural framework and stereochemistry of natural products is one of the key factors in devising an efficient synthetic strategy for these molecules. Researchers have confirmed the planar structure of stambomycin D by employing NMR spectroscopy and various efforts have been carried out to get information about their stereochemistry via predictive sequence analysis.49,50 In order to ensure the correct stereochemistry of stambomycin D, Lim et al.51 in 2021 reported its total synthesis and then justified its structure through nuclear magnetic resonance spectroscopy. They employed the Mukaiyama aldol reaction in their synthetic approach towards the synthesis of C1–C27 fragment of stambomycin D. The synthesis was inaugurated with the procurement of compound 30 by reacting aldehyde 29 over a number of steps. Then, compound 30 was subjected to react with silyl enol ether 31 by employing the Mukaiyama aldol reaction, using boron trifluoride diethyl etherate catalyst in DCM, which gave the Mukaiyama aldol product in 85% yield. The next step involved the Dess–Martin periodinane mediated oxidation to obtain β-keto ester 32 in 87% yield. β-Keto ester 32 was reacted with HCl by using a suitable solvent ratio of methanol and THF, followed by deprotection of the alcoholic group to furnish C1–C11 fragment 33 in 44% yield. Furthermore, fragments C13–C22 34 and C23–C27 35 were prepared individually, which were coupled in the presence of (S)-BINOL, diethyl zinc and Cy2NH followed by the addition of Ti(Oi-Pr)4 and diethyl ether, which resulted in the synthesis of propargylic alcohol 36 in 62% yield. Propargylic alcohol 36 was later converted to vinylboronic ester 37. This synthesized ester 37 was then coupled with C1–C11 fragment 33 by using palladium tetrakistriphenyl phosphine and Tl2CO3, thereby leading to the synthesis of target molecule 38 in 41% yield by using the deprotection strategy (Scheme 5).
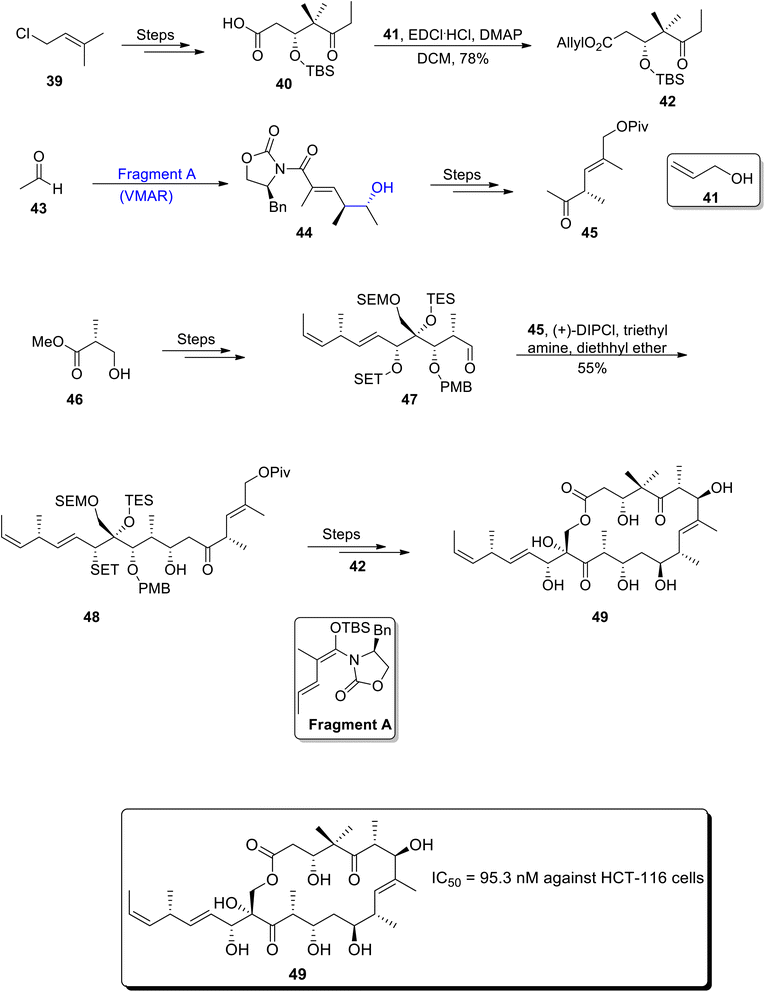
Tedanolide C is a naturally occurring macrolide extracted from sea sponges i.e. Ircinia sp. Excellent cytotoxic activity has been found to be demonstrated by tedanolide C against HCT-116 cell lines i.e., 95.3 nM.52 Moreover, it plays an important role in interrupting the biosynthesis of proteins.53 The synthesis of tedanolide C was considered to be a troublesome task due to the primary hydroxyl group containing few macrolactone moieties. Thus, Lucke et al.54 reported the total synthesis of desepoxy-tedanolide C by utilizing the Mukaiyama aldol reaction, Kiyooka aldol reaction and Julia–Kocienski olefination as key steps. The first step of the synthetic scheme involved the synthesis of carboxylic acid 40 which was further reacted with compound 41 in the presence of EDCl, dimethyl aminopyridine and dichloromethane to obtain allyl ester 42 in 78% yield. Fragment A was subjected to the Mukaiyama aldol reaction upon treatment with acetaldehyde 43, which resulted in the synthesis of aldol adduct 44. Compound 44 was later reacted over a number of steps to obtain fragment 45. In the second part of their synthetic scheme, ester 46 was reacted over number of steps, which gave aldehyde 47. Aldehyde 47 was further subjected to react with previously synthesized fragment 45 in the presence of DIPCl, triethyl amine and diethyl ether, which resulted in the synthesis of compound 48 in 55% yield. Compound 48 was then treated over a number of steps, thus leading to the synthesis of target molecule i.e., tedanolide C 49 (Scheme 6).
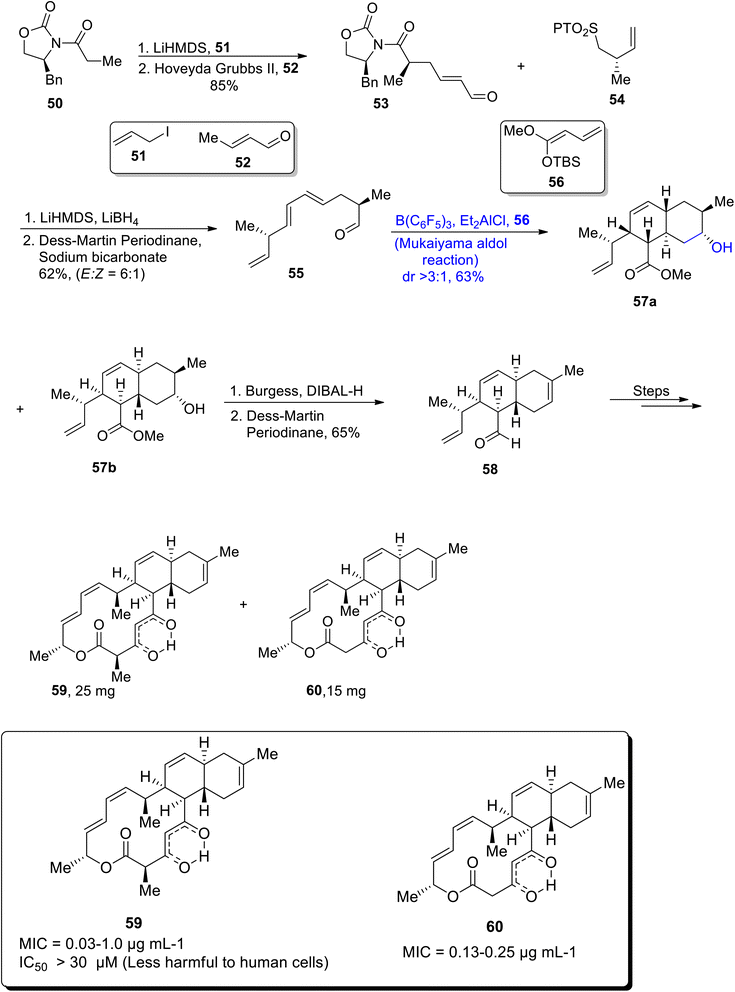
The ability of bacteria to develop resistance against numerous antibiotics has reached dangerous levels, which has led to emphasis on the synthesis and discovery of non-resistant antibiotics to treat bacterial diseases. Anthracimycin is a non-resistible, naturally occurring macrolide that is highly potent against various bacterial strains i.e. Mycobacterium tuberculosis, S. aureus and B. anthracis.55–57 They are not dangerous to normal human cells and perform their biological activities effectively. Taking into account the high demand of non-resistible antibiotics, Tian et al.58 in 2022 devised a concise and efficient synthetic strategy for the synthesis of pharmaceutically important macrolides i.e. anthracimycin and anthracimycin B in 25 mg and 14 mg, respectively. They carried out the Mukaiyama aldol reaction, intramolecular Diels–Alder reaction and olefin-cross metathesis as key steps in their synthetic approach. The synthesis began with the generation of aldehyde 53 in 85% yield from the Evan's symmetric alkylation of xazolidinone 50 followed by treatment with crotonaldehyde via olefin cross-metathesis. Sulfone 54 was then made to react with aldehyde 53 in the presence of lithium hexamethyldisilazide and lithium borohydride followed by Dess–martin periodinane oxidation to obtain diene 55 in 62% yield. Diene 55 further underwent triaryl borane catalyzed Mukaiyama aldol reaction with silyl ketene 56 in diethyl aluminium chloride which gave more than 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 diastereomeric ratio of trienes 57a and 57b in 63% yield. In the next step, reduction of ester with Burgess reagent in the presence of DIBAL-H took place, which gave aldehyde 58 in 65% yield on further treatment with Dess–Martin periodinane. This aldehyde 58 later reacted over three different steps to give anthracimycin 59 and anthracimycin B 60 in 25 mg and 14 mg, respectively (Scheme 7).
1 diastereomeric ratio of trienes 57a and 57b in 63% yield. In the next step, reduction of ester with Burgess reagent in the presence of DIBAL-H took place, which gave aldehyde 58 in 65% yield on further treatment with Dess–Martin periodinane. This aldehyde 58 later reacted over three different steps to give anthracimycin 59 and anthracimycin B 60 in 25 mg and 14 mg, respectively (Scheme 7).
2.2. Synthesis of polyketide-based natural products
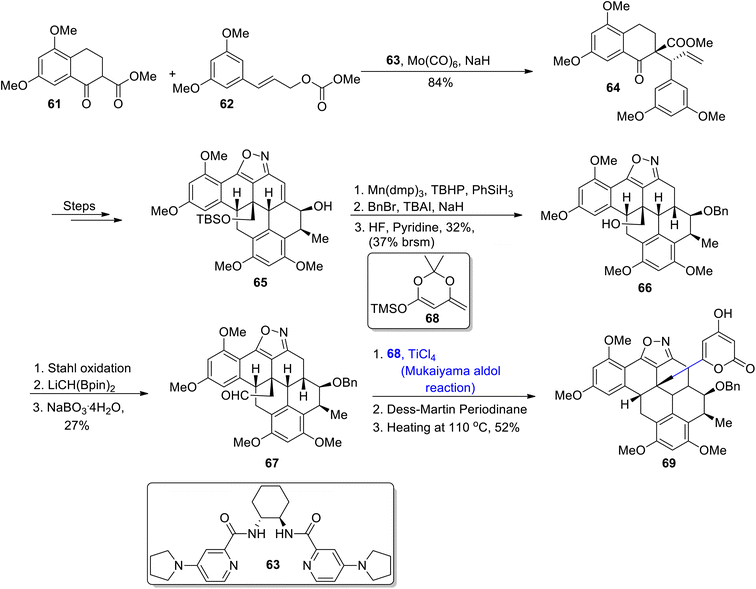
A-74528 is a naturally occurring polyketide, that has been isolated from Streptomyces strains. They are highly remarkable in medicinal chemistry as they restrain the release of 2′-phosphodiesterase, hence exhibiting high potency against viral and cancerous cells. Their structure comprises six fused rings along with a pyrone side chain and six asymmetric centers.59 Despite their significant biological activity, there have been minor contributions towards their total synthesis. Maeir et al.,60 in 2022 attempted to report the total synthesis of A-74528 by employing the Mukaiyama aldol reaction, 1,3-dipolar cycloaddition, spiro epoxide annulation and hydroarylation as major steps. In the first step, tetralone 61 was treated with carbonate 62 by employing a bis-pyridine ligand to obtain ketoester 64 in 84% yield. Later, compound 65 was obtained in 52% yield by reacting keto ester 64 over a number of different steps. Compound 65 was treated with Shenvi's HAT hydrogenation, followed by treatment with benzyl bromide in tetrabutyl aluminium iodide and sodium hydride. Further treatment with HF in the presence of pyridine gave neopentylic alcohol 66 in 32% yield with 37% brsm. The synthesized alcohol 66 was further subjected to Stahl oxidation, then subsequent treatment with LiCH(BPin)2 and NaBO3·4H2O resulted in the synthesis of aldehyde 67 in 27% yield. The synthesized aldehyde 67 was subjected to titanium chloride catalyzed Mukaiyama aldol reaction with silyl enol ether 68. Thus, the carbon skeleton of A-74528 69 was obtained in 52% yield on subsequent oxidation with Dess–Martin periodinane (Scheme 8).It has been discovered that many coloured substances isolated from coloured aphids are highly biologically active and are found to be highly potent against a number of bacterial diseases. They are also found to inhibit the proliferation of cancerous cells.61 In a similar way, Inai et al., isolated pharmacologically important organic compounds from Cryptomyzus species, as a result four different polyketides i.e., caprolactones A1, A2, B1, and B2 were obtained. These polyketides are α,β-unsaturated compounds that have been derived from δ-lactones.62 These are found to be effective against various diseases as they inhibit the human immunodeficiency virus activity and interrupt the uncontrolled cell division. Inai et al.,63 reacted compound 70 through a number of steps to obtain aldehyde 71, which was further subjected to react with TMS-substituted moiety 72 via a boron trifluoride diethyl etherate catalyzed Mukiyama aldol reaction to give 73a, 73b and 73c in 59%, 52% and 40% yields, respectively. Each of them was reacted with a Grubbs second generation catalyst in DCM to synthesize a mixture of 74a and 75a (caprolactones A1 and A2), 74b and 75b (caprolactones B1 and B2) and 74c and 75c in 79%, 69% and 60% yields, respectively (Scheme 9).
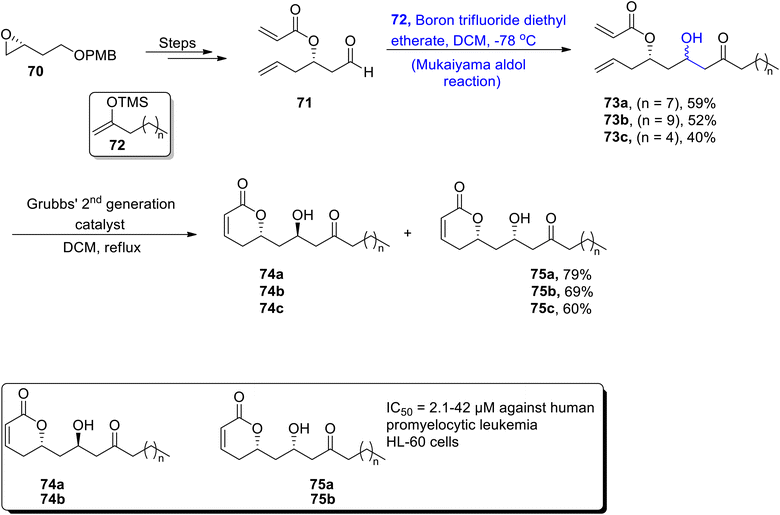 | ||
| Scheme 9 Synthesis of caprolactones A1 and A2, caprolactones 74a and 75a, B1 and B2, 74b and 75b and their derivatives 74c and 75c. | ||
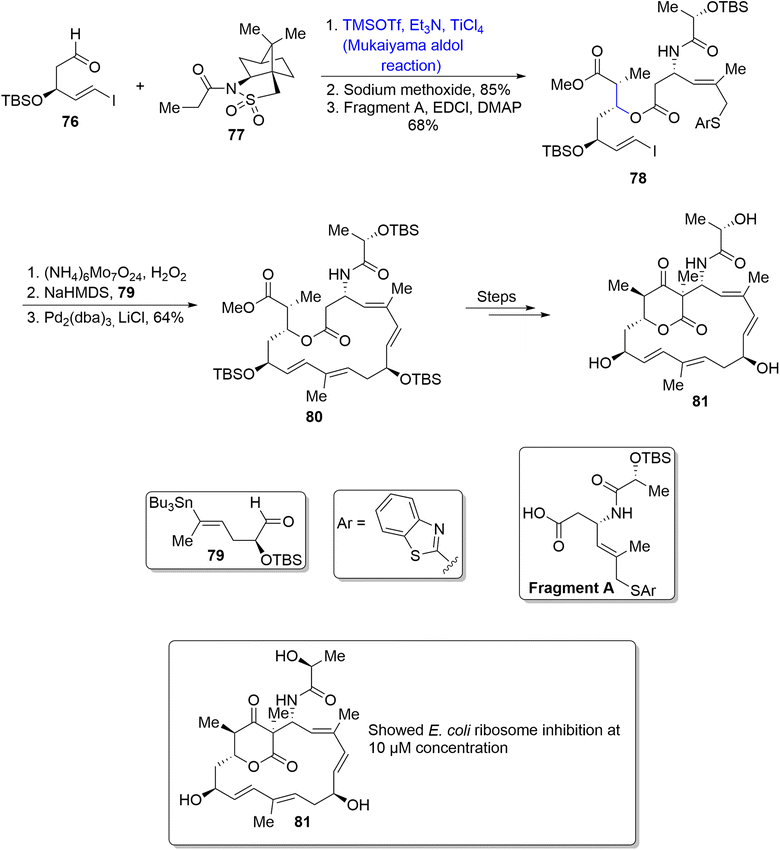
Soil bacteria i.e. Streptomyces rochei are a source of numerous naturally occurring compounds including lankacidins. Lankacidins are pharmacologically important polyketides which are found to be highly potent against bacterial diseases. Their structural formulae contain huge 17-membered cyclic rings.64,65 Cai et al.66 in 2020 reported the synthesis of naturally occurring members of lankacidin family i.e., iso- and seco-lankacidin and evaluated their efficacy against bacterial strains. Iso-lankacidin was synthesized by involving the Mukaiyama aldol rection as one of the main steps in their synthetic scheme. The synthesis began with treatment of l-camphorsultam derivative 76 with aldehyde 77 via titanium chloride catalyzed Mukaiyama aldol reaction (dr = more than 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Furthermore, reaction of the Mukaiyama aldol product with sodium methoxide followed by esterification with EDCl in the presence of DMAP resulted in the synthesis of compound 78 in 68% yield. Compound 78 was further subjected to three different steps; oxidation, Julia–Kocienski olefination and Stille coupling to obtain the macrocyclic ring 80 in 64% yield. Compound 80 was then reacted again over three steps to synthesize iso-lankacidin 81 (Scheme 10).
1). Furthermore, reaction of the Mukaiyama aldol product with sodium methoxide followed by esterification with EDCl in the presence of DMAP resulted in the synthesis of compound 78 in 68% yield. Compound 78 was further subjected to three different steps; oxidation, Julia–Kocienski olefination and Stille coupling to obtain the macrocyclic ring 80 in 64% yield. Compound 80 was then reacted again over three steps to synthesize iso-lankacidin 81 (Scheme 10).
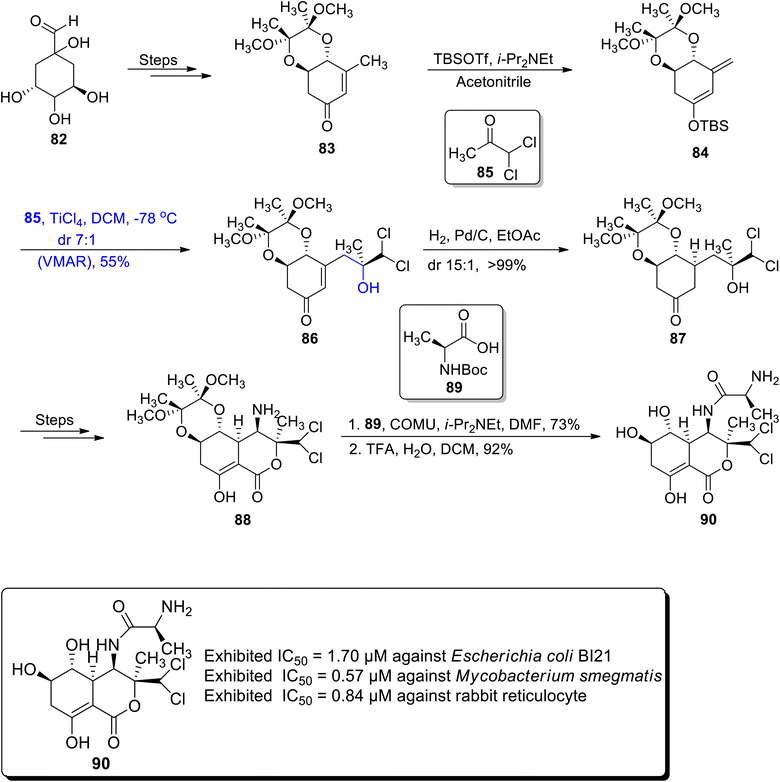
The naturally occurring polyketide i.e., bactobolin A is extracted from Pseudomonas sp. It is employed as a secondary metabolite as it exhibits a wide range of biological activities against both types of bacterial and tumour cell lines. Their structural skeleton comprises a lactone moiety along with five chiral centers.67,68 Considering the wide ranging significance of bactobolin A, Vojackova et al.69 in 2020 reported their total synthesis by employing the Mukaiyama aldol reaction, rhodium catalyzed amination and alkoxy carbonylation as major steps. The first step of their efficient synthetic strategy involved the preparation of enone 83 by the reaction of quinic acid 82 over a number of steps. In the next step, this enone 83 was made to react with TBSOTf and i-Pr2NEt by using acetonitrile as a solvent to obtain TMS-silyl enol ether 84. Silyl enol ether 84 was further subjected to react with compound 85 via a titanium chloride catalyzed vinylogous Mukaiyama aldol reaction (VMAR) by using dichloromethane as a solvent to obtain Mukaiyama aldol product 86 in 55% yield with a separable diastereoselectivity ratio of 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Later, aldol adduct 86 was hydrogenated in the presence of palladium to obtain ketone 87 in >99% yield. The ketone based compound 87 was further reacted over several steps to furnish amine moiety 88. Amine 88 was then coupled with acid 89 followed by deprotection of protecting groups in acidic conditions, which led to the synthesis of bactobolin A 90 in 92% yield (Scheme 11).
1. Later, aldol adduct 86 was hydrogenated in the presence of palladium to obtain ketone 87 in >99% yield. The ketone based compound 87 was further reacted over several steps to furnish amine moiety 88. Amine 88 was then coupled with acid 89 followed by deprotection of protecting groups in acidic conditions, which led to the synthesis of bactobolin A 90 in 92% yield (Scheme 11).
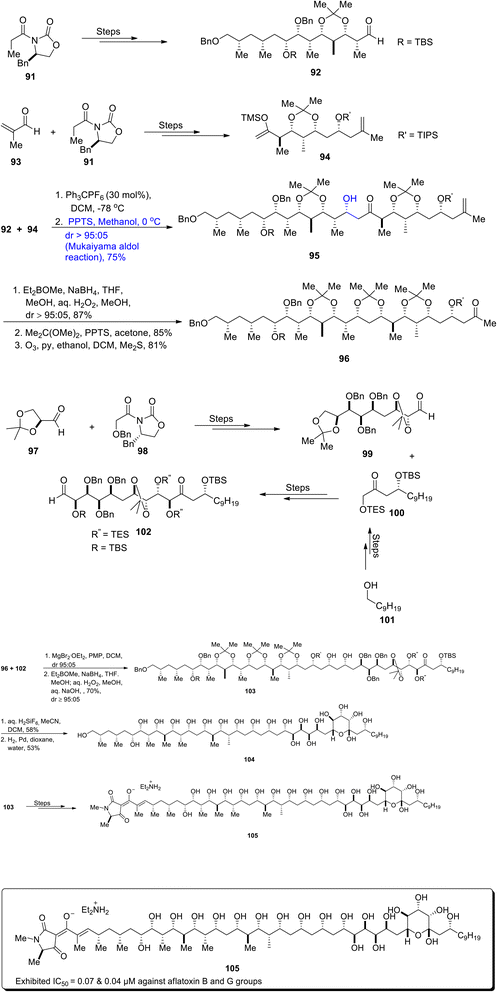
Aflatoxins are extremely detrimental as they contaminate food which results in the proliferation of cancer cells in animals and humans.70 In order to prevent the toxic effects of aflatoxins, there is a dire need for aflatoxin growth inhibitors. Aflastatin A, which was obtained from Streptomyces sp. MRI142 in 1996, was found to interrupt the growth of aflatoxins.71,72 Aflastatin A is a naturally occurring and structurally complex polyketide, which contains 29 optically active carbons along with numerous hydroxyl groups. Its structure also contains a derivative of tetramic acid on its lengthy chains. There have been numerous reports on the total synthesis of aflastatin A to date. Recently, Evans et al.73 reported the asymmetric and efficient approach for the total synthesis of aflastatin A in 0.69% overall yield by employing various aldol reactions including the Mukaiyama aldol reaction. The C3–C15 aldehyde 92 was synthesized in 94% yield by treating oxazolidinone 91 over numerous steps. Similarly, oxazolidinone 91 was made to react with aldehyde 93 through different reactions to synthesize C16–C26 enolsilane 94. Aldehyde 92 and enolsilane 94 were then made to react via a trityl-catalyzed Mukaiyama aldol reaction in the presence of Ph3CPF6 and DCM, followed by reaction with PPTS and methanol to obtain Mukaiyama aldol product 95 in 75% yield in a diastereoselectivity ratio of more than 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 05. It was inferred that the trityl-catalyzed Mukaiyama aldol reaction is highly efficient as compared to the BF3·OEt2 mediated Mukaiyama aldol reaction in this case, as the latter resulted in aldol adduct 95 with a lower diastereoselectivity ratio (dr = 57
05. It was inferred that the trityl-catalyzed Mukaiyama aldol reaction is highly efficient as compared to the BF3·OEt2 mediated Mukaiyama aldol reaction in this case, as the latter resulted in aldol adduct 95 with a lower diastereoselectivity ratio (dr = 57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 43). Compound 95 was further subjected to Prasad reduction, followed by preparation of acetonide and finally leading to the synthesis of ketone 96 via ozonolysis in 81% yield. In order to synthesize C27–C35 aldehyde 99, aldehyde 97 and oxazolidinone 98 were treated over a number of steps. Aldehyde 99 was then made to react with ketone 100 (which was synthesized by using 1-decanol 101 as the starting reagent) to furnish C27–C48 aldehyde fragment 102 in 80% yield over several steps. C27–C48 aldehyde 102 and C3–C26 ketone 96 were subsequently coupled in the presence of magnesium bromide diethyl etherate, PMP and dichloromethane followed by Prasad's reduction to furnish diol 103 in 70% yield with a diastereoselectivity ratio of more than 95
43). Compound 95 was further subjected to Prasad reduction, followed by preparation of acetonide and finally leading to the synthesis of ketone 96 via ozonolysis in 81% yield. In order to synthesize C27–C35 aldehyde 99, aldehyde 97 and oxazolidinone 98 were treated over a number of steps. Aldehyde 99 was then made to react with ketone 100 (which was synthesized by using 1-decanol 101 as the starting reagent) to furnish C27–C48 aldehyde fragment 102 in 80% yield over several steps. C27–C48 aldehyde 102 and C3–C26 ketone 96 were subsequently coupled in the presence of magnesium bromide diethyl etherate, PMP and dichloromethane followed by Prasad's reduction to furnish diol 103 in 70% yield with a diastereoselectivity ratio of more than 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 05. In the next step, diol 103 was reacted with aq. H2SiF6 and acetonitrile in DCM, followed by reaction with a hydrogen molecule in the presence of palladium to afford naturally occurring C3–C48 fragment 104 of aflastatin A in 53% yield. Furthermore, diol 103 was made to react under a number of steps to synthesize diethylamine salt of aflastain A 105 (Scheme 12).
05. In the next step, diol 103 was reacted with aq. H2SiF6 and acetonitrile in DCM, followed by reaction with a hydrogen molecule in the presence of palladium to afford naturally occurring C3–C48 fragment 104 of aflastatin A in 53% yield. Furthermore, diol 103 was made to react under a number of steps to synthesize diethylamine salt of aflastain A 105 (Scheme 12).
2.3. Synthesis of terpenoids based natural products
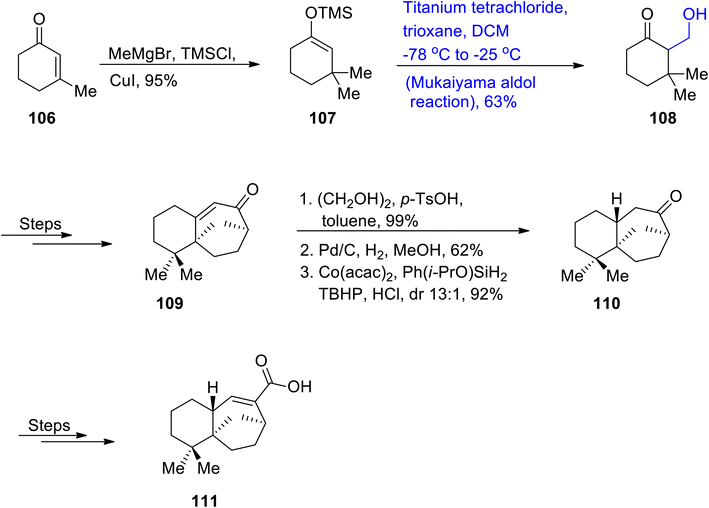
Omphaline sesquiterpenoids are generally obtained from land and sea organisms, and their structure is composed of two consistent quaternary carbon cores. Omphalic acid was the former omphalene sesquiterpenoid, which was extracted from Omphalanthus filiformis, that is a Colombian liverwort.74 Omphalic acid and its corresponding sesquiterpenoids hold prime importance in the natural product family, but owing to their complex carbon framework, it was considered a highly arduous task to successfully carry out their total synthesis. In this regard, Chen et al.75 in 2021 attempted to report the total synthesis of omphalic acid for the first time. For this purpose, they reacted cyclic ketone 106 with methyl magnesium bromide in the presence of trimethylsilyl chloride and cuprous iodide via conjugate addition to obtain compound 107 in 95% yield. Conjugate addition product 107 was further subjected to the Mukaiyama aldol reaction by reacting with titanium tetrachloride and trioxane in dichloromethane to obtain compound 108 in 63% yield. Mukaiyama aldol product 108 was then transformed to intermediate 109 by undergoing several steps. Intermediate 109 was later subjected to ketone synthesis and isomerization steps followed by palladium catalyzed hydrogenation and HAT hydrogenation to obtain compound 110 in 92% yield with a 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 diastereoselectivity ratio. In the next step, cyclic ketone 110 was made to react under various conditions, finally leading to the synthesis of target molecule i.e. (±)-omphalic acid 111 (Scheme 13).
1 diastereoselectivity ratio. In the next step, cyclic ketone 110 was made to react under various conditions, finally leading to the synthesis of target molecule i.e. (±)-omphalic acid 111 (Scheme 13).
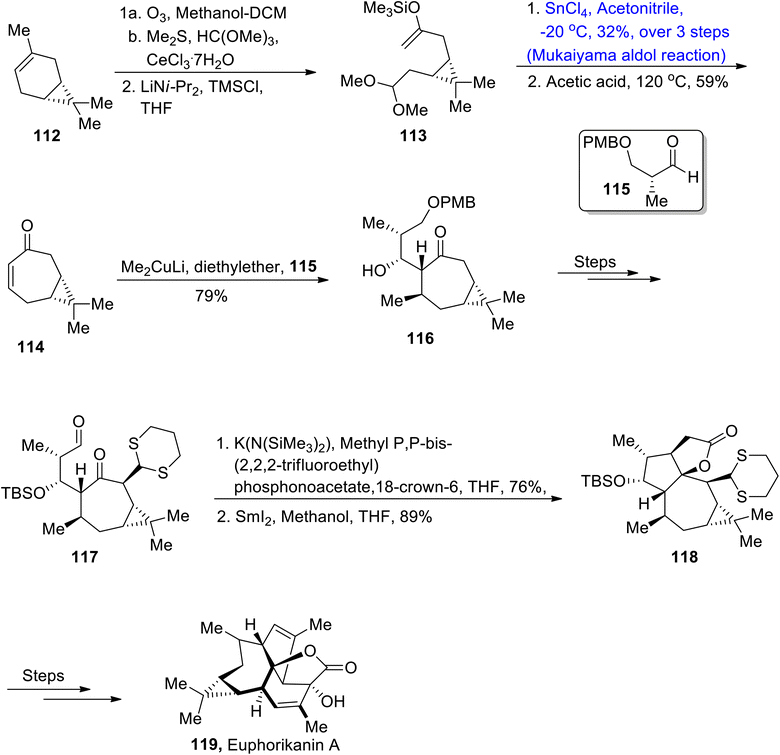
Most of the natural products are found to be isolated from Euphorbiaceae.76,77 Among them, euphorikanin A is a naturally occurring diterpenoid, which is composed of four fused cyclic rings with 8 chiral centers, obtained from Euphorbia kansui. These are found to be highly active against cancerous cell lines i.e., NCI-446 and HeLa. Classen et al.78 attempted to describe the total synthesis of euphorikanin A by utilizing Mukaiyama aldol reaction in their synthetic strategy. In this regard, they treated (+)-3-carene 112 with ozone, followed by its reaction with dimethyl sulfide and HC(OMe)3 and CeCl3·7H2O. In the next step, enol ether 113 was obtained as a result of reaction with LiNiPr2 and TMSCl in tetrahydrofuran. Compound 113 was further made to react with SnCl4 in acetonitrile at −20 °C via the Mukaiyama aldol reaction followed by reaction with acetic acid, which gave compound 114 in 59% yield. Aldol adduct 114 was then converted to alcohol containing compound 116 in 79% yield as a result of conjugate addition with aldehyde 115 in the presence of diethyl ether. Alcohol 116 was further treated through several steps to obtain compound 117. Aldehyde 117 then underwent Still-Gennari olefination followed by reaction with SmI2 using methanol/THF, which resulted in the synthesis of compound 118 in 89% yield. Compound 118 was then treated over a number of steps, thus leading to the synthesis of euphorikanin A 119 (Scheme 14).
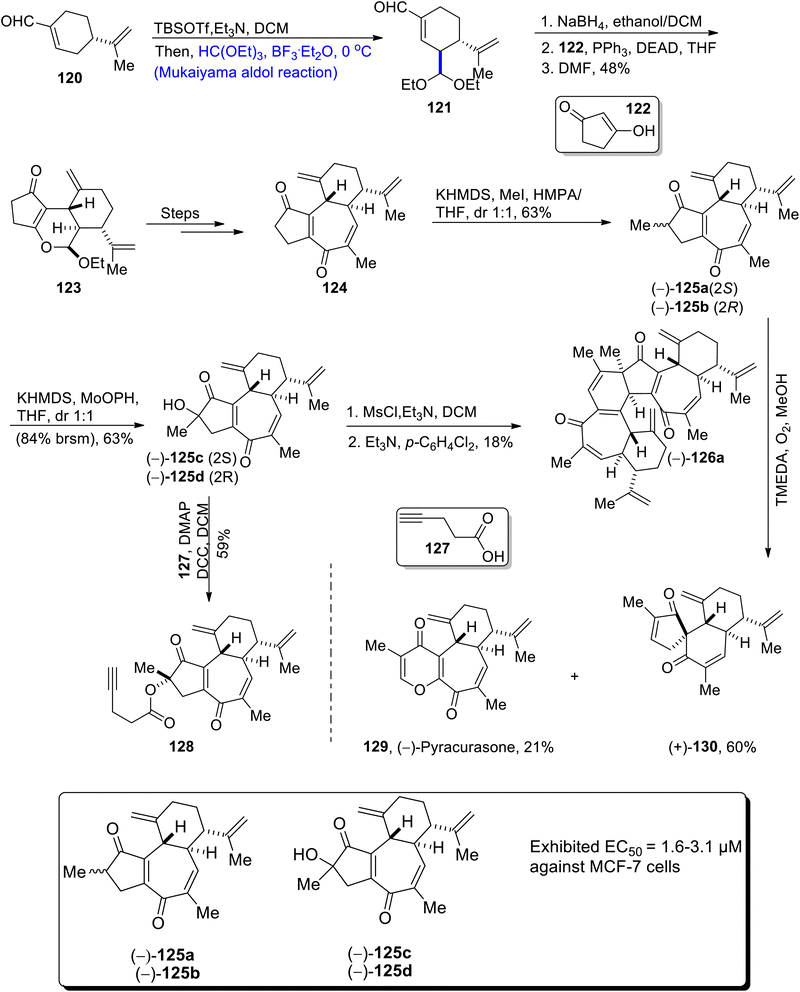
Jatropha curcas is the source of curcusone diterpenes. These diterpenes are used to cure a number of diseases. Their structure is composed of three carbon containing cyclic rings.79 Owing to the biological significance of curcusone diterpenes, Cui et al.80 in 2021, for the first time reported their total synthesis. The first step of total synthesis involved the treatment of perillaldehyde 120 with TBSOTf, triethyl amine and dichloromethane via the Mukaiyama aldol reaction, which further involved the Lewis acid catalyzed reaction with HC(OET)3. Mukaiyama aldol product 121 was subjected to reduction followed by treatment with compound 122 in the presence of triphenyl phosphine, DEAD and tetrahydrofuran, which resulted in the synthesis of compound 123 in 48% yield. Compound 123 was then treated via a number of steps to obtain enone 124. Compound 124 was further treated with KHMDS, methyl iodide in the presence of tetrahydrofuran to obtain curcusone A 125a and curcusone B 125b in 63% yield. In the next step, curcusone C 125c and curcusone D 125d were synthesized from 125a and 125b, as a result of the reaction with KHMDS and MoOPh in tetrahydrofuran. 125c and 125d were then subjected to react through two different routes to obtain dimericursone (−)-126a and curcusone analog 128 in 18% and 59% yields respectively. Furthermore, curcusone A and B 125a and 125b were made to react with TMEDA in the presence of oxygen and methanol, which resulted in 21% yield of pyracurcasone 129 and 60% yield of compound 130 (Scheme 15).
An efficient methodology for treating cyclic dienol silanes with chiral aldehydes has been put forward by using Lewis acid i.e., Yb(OTf)3 or Zn(OTf)2 as catalysts for known Mukaiyama aldol reactions. This efficient methodology was then employed to synthesize various naturally occurring terpenoids. These naturally occurring terpenoids have been found to be active against various inflammatory and cancerous diseases.81,82 These are also used as effective pain killers. In this regard, Adamkiewicz et al.83 reacted sclareolide 131 with CH3NHOCH3 in the presence of HCl, AlMe3 and dichloromethane, followed by its reaction with thionyl chloride and then subsequent reduction resulted in the synthesis of compound 132 in 90% yield. Compound 132 was further reacted with silyl enol ether 133 via the Yb(OTf)3 catalyzed Mukaiyama aldol reaction, which resulted in the mixture of curcucomosin 134 and vitexolide 135 in 70% yield (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). This was then reacted with aluminium oxide and pyridine to obtain villosin 136 in 84% yield. Villosin 136 was then subjected to reduction in THF and DCM one by one, thus leading to the synthesis of coronarin E 137 and chinensine C 138 in 80% and 76% yield, respectively. Similarly, sclareolide 131 was transformed into mixture of aldehydes 139 and 140. Aldehyde 139 was subjected to Mukaiyama aldol reaction to obtain compound 141, which was further reacted with aluminium oxide and pyridine to synthesize (E)-labda-7,11,13-trien-16,15-olide 142 in 78% yield (Scheme 16).
1). This was then reacted with aluminium oxide and pyridine to obtain villosin 136 in 84% yield. Villosin 136 was then subjected to reduction in THF and DCM one by one, thus leading to the synthesis of coronarin E 137 and chinensine C 138 in 80% and 76% yield, respectively. Similarly, sclareolide 131 was transformed into mixture of aldehydes 139 and 140. Aldehyde 139 was subjected to Mukaiyama aldol reaction to obtain compound 141, which was further reacted with aluminium oxide and pyridine to synthesize (E)-labda-7,11,13-trien-16,15-olide 142 in 78% yield (Scheme 16).
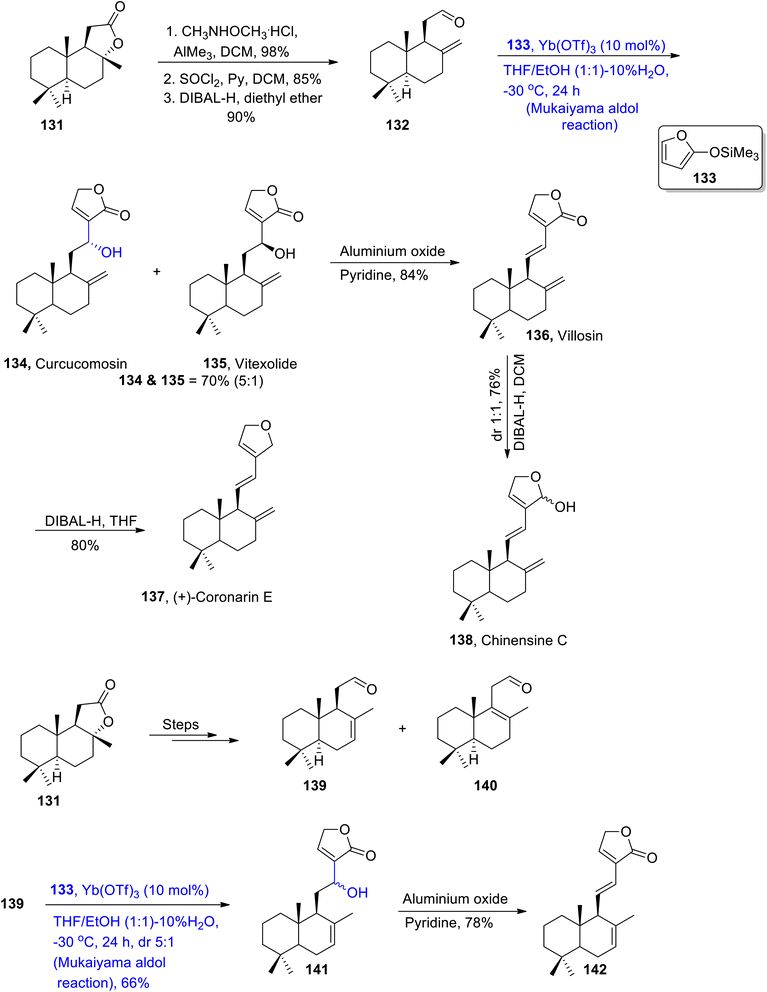 | ||
| Scheme 16 Synthesis of curcucomosin 134, vitexolide 135, villosin 136, coronarin E 137, Chinensine C 138 and (E)-labda-7,11,13-trien-16,15-olide 142. | ||
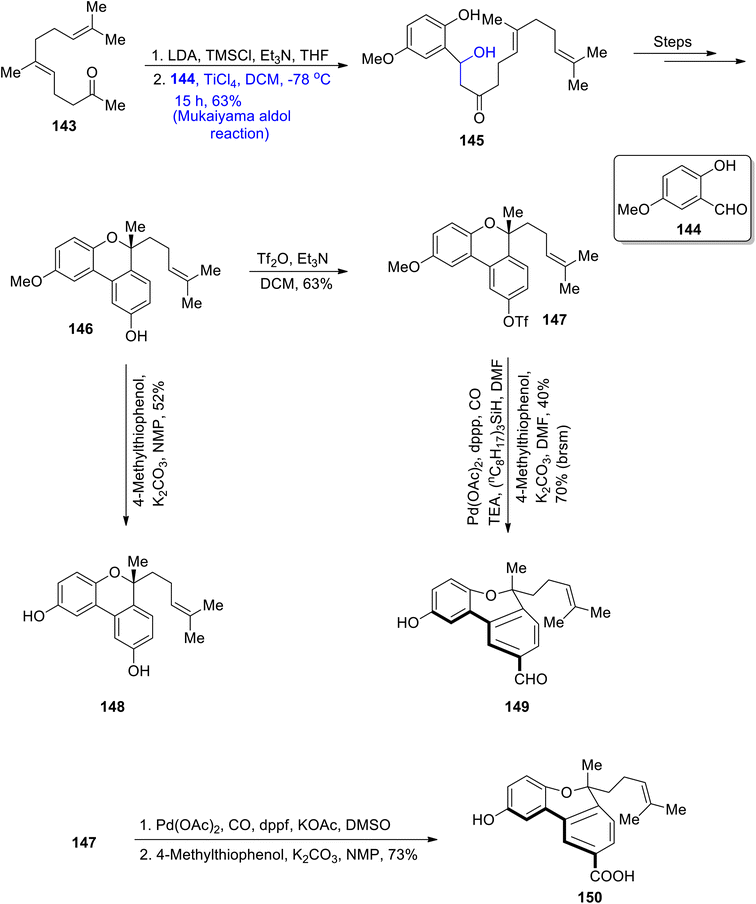
In order to treat various cancerous diseases, Ganoderma fungi has been found to play an effective role. This particular fungus is a source of extraction of a number of triterpenoids and polysaccharides.84 In a similar way, many biologically active monoterpenoids i.e. gonocochlearin A, C, D and ganocins A–C, were isolated from the Ganoderma cochlear. Many of these were found to be highly potent against acetylcholinesterase activity.85 Ganocins A–C are highly structurally complex monoterpenoids with a five-membered cyclic ring. Moreover, their structure constitutes five asymmetric/chiral centers. Beside numerous reported synthetic routes to attain these structurally and biologically diverse molecules, an efficient synthetic methodology for their synthesis was required. For this purpose, Shao et al.86 and Zhang et al.87 in 2020 reported the synthesis of these naturally occurring terpenoids by utilizing the Mukaiyama aldol reaction as a key step. In the first step, geranylacetone 143 was made to react with lithium diisopropyl amide and tert-methyl silyl chloride in the presence of triethyl amine and tetrahydrofuran, followed by its reaction with 5-methoxy salicylaldehyde 144 via titanium chloride catalyzed Mukaiyama aldol reaction. This resulted in the synthesis of compound 145 in 63% yield. The Mukaiyama product 145 was further converted to phenol 146 by undergoing a number of steps. Compound 146 was subsequently reacted with Tf2O by using triethyl amine and DCM to obtain triflate 147 in 63% yield. Then, the intermediate compounds 146 and 147 were further reacted individually i.e., compound 146 was subjected to demethylation to synthesize cochlearol T 148 in 52% yield. Similarly, compound 147 was subjected to palladium catalyzed carboxylation followed by its reaction with 4-methylthiophenol, which gave ganocochlearin C 149 in 40% yield. The intermediate compound 149 was also subjected to palladium catalyzed carboxylation in the presence of DMSO, followed by subsequent demethylation, which afforded ganocochlearin D 150 in 73% yield (Scheme 17).
Furthermore, intermediate 146 was made to react with SO2Cl2 in the presence of sodium carbonate, followed by reaction with cesium fluoride which gave dienone. Dienone was then reduced regioselectively to furnish compound 151 in 80% yield. Compound 151 was further demethylated to give ganocin B 152 and C 153 in 66% and 65% yields, respectively. Ganocin B was subsequently converted to ganocin A 154 by undergoing several steps (Scheme 18).
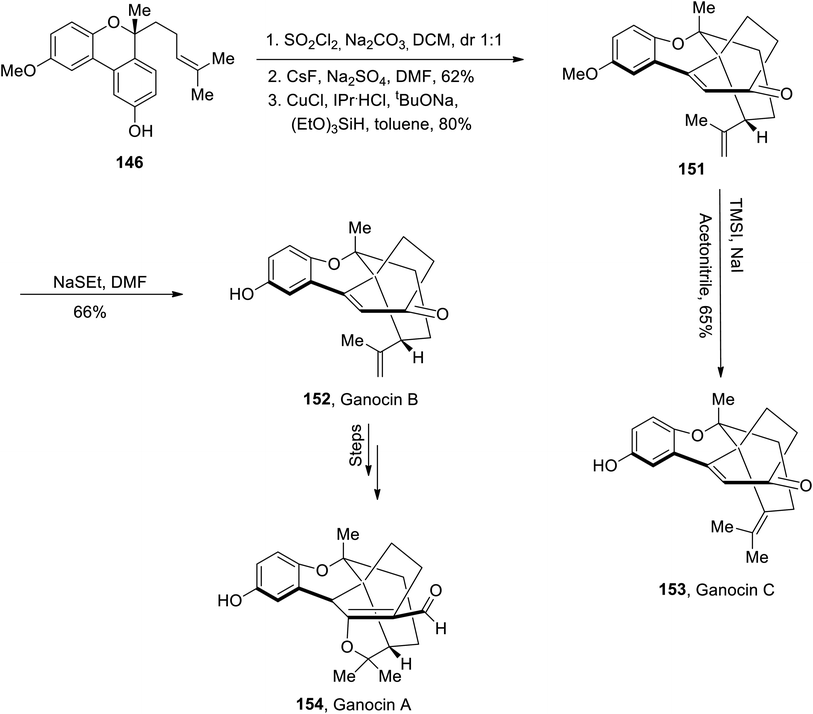
Aconitum heterophyllum is the source of many diterpenes such as campylopin,88 atropurpuran89 and Guan Fu diterpenoid A.90 Among these, atropurpuran is a non-alkaloidal diterpene whose structural framework constitutes five cyclic rings with tetracyclo and bicyclo units. Suzuki et al.91 in 2022 reported the synthesis of the highly complex structure of atropurpuran by employing the Mukaiyama aldol reaction and intramolecular Diels–Alder reaction in their strategy. The first step involved the reaction of 6-methoxy-1-tetralone 155 with hydrogen bromide utilizing aqueous hydrogen peroxide and acetic acid followed by Hartwig hydroxylation to give phenol 156 in 80% yield. The hydroxyl group was protected by benzyl bromide followed by a subsequent Mannich reaction with formaldehyde and Me2NH2Cl in acetic anhydride. The next step involved the reaction with methyl iodide in the presence of potassium cyanide and ammonium chloride to furnish nitrile 157 in 88% yield. It was then made to react with TMSOTf in triethyl amine and DCM to afford trimethyl silyl enol ether 158, which then underwent the Mukaiyama aldol reaction with acrolein 159 by utilizing Yb(OTf)3 as a catalyst in toluene or ethanol/water to synthesize adduct 160. The adduct 160 was further treated over a number of steps to obtain atropurpuran skeleton 161. This atropurpuran skeleton then resulted in the synthesis of atropurpuran diterpene 162 on treatment with Qin's intermediate or Xu's intermediate (Scheme 19).
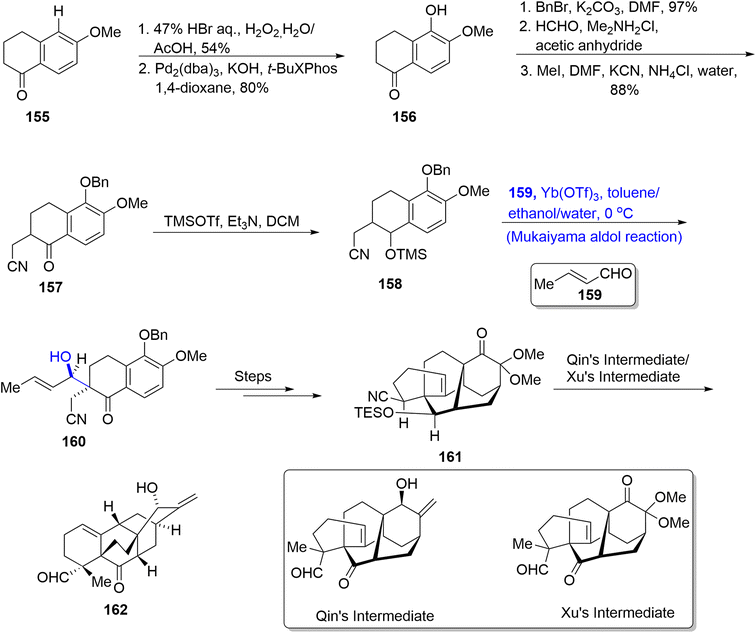
Garsubellin A has been found to be isolated from Garcinia subelliptica, a tree that is present on Okinawa Japan island.92 This naturally occurring monoterpene has wide biological significance alongside structural complexities. Their structure is composed of a cyclic framework constituting 13 carbon atoms with three quaternary carbons. They are known to boost the activity of acetylcholine transferase thus preventing the onset of Alzheimer's disease.93 Owing to their remarkable structural framework and biological significance, various synthetic routes have been reported towards their total synthesis. However, these methods resulted in racemic synthesis of target molecules. Jang et al.94 in 2022 attempted to describe the enantioselective synthesis of target molecule. The first step of their synthetic methodology involved the reaction of enone 163 with LDA and TMSCl followed by a titanium chloride catalyzed Mukaiyama aldol reaction in DCM upon treatment with aldehyde 164. As a result, the Mukaiyama aldol product 165 was obtained in 75% yield, which was then subjected to a number of steps to afford compound 166. Later, compound 166 underwent conjugate addition in the presence of Ley's conditions followed by subsequent oxidation by using DMP and DCC to furnish triketone 168 in 94% yield. It was then transformed into target molecule 169 by undergoing a number of different reactions (Scheme 20).
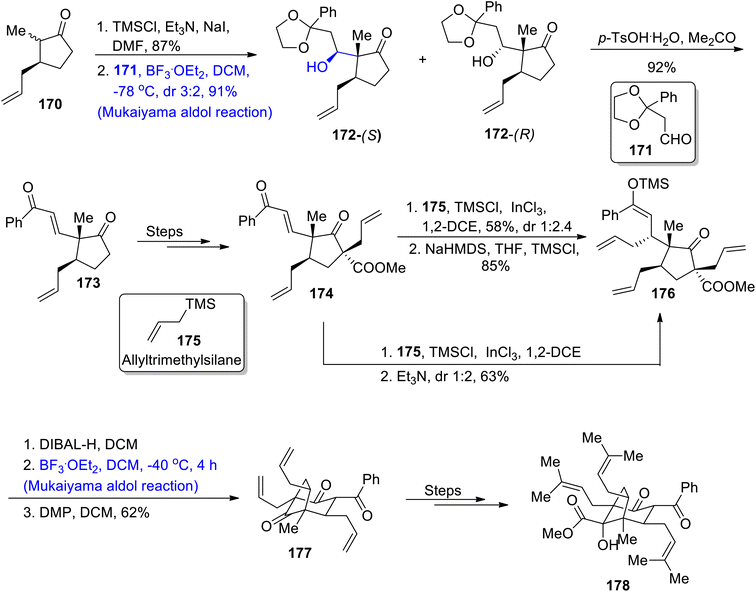
Naturally occurring polycyclic polyprenylated acylphloroglucinols belong to the class of monoterpenoids and are known to exhibit potent biological activity against a number of diseases.95 Newly discovered acylphloroglucinol monoterpenoids containing six-membered rings were derived from Hypericum patulum which includes hypatulin A and hypatulin B.96 Their structure is composed of five-membered carbon rings along with 4 asymmetric centers. Moreover, they are found to be effective against a number of bacterial strains i.e., E. coli, S. aureus and B. subtilis. As hyptaulin A was found to be easily synthesized from hyptaulin B, Leisering et al.97 in 2022 attempted to report the total synthesis of hyptaulin B by carrying out the Mukaiyama aldol reaction and photo-oxidation. The first step of synthesis involved the enolization reaction of cyclopentanone 170 followed by Mukaiyma aldol addition with aldehyde 171 to yield diastereomers 172-(S) and 172-(R) in 91% yield with diastereoselectivity ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. Both these diastereomers were further subjected to deacetalization to afford enone 173 in 92% yield. This reaction was followed by the procurement of isomer 174 in 59% yield from enone 173. Compound 174 was then subjected to Homo-Sakurai allylation followed by the addition of trimethylamine which gave substituted ester 176 in 63% yield with 1
2. Both these diastereomers were further subjected to deacetalization to afford enone 173 in 92% yield. This reaction was followed by the procurement of isomer 174 in 59% yield from enone 173. Compound 174 was then subjected to Homo-Sakurai allylation followed by the addition of trimethylamine which gave substituted ester 176 in 63% yield with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 diastereoselectivity ratio. However, Homo-Sakurai-allylation followed by treatment with sodium hexamethyldisilazide gave ester 176 in 85% yield. Ester 176 was further reduced with DIBAL-H and then subjected to Lewis acid catalyzed Mukaiyama aldol reaction in DCM. Then, oxidation was carried out with DMP, which gave triketone intermediate 177 in 62% yield. This triketone intermediate was then reacted several times, thereby leading to the synthesis of target molecule 178 (Scheme 21).
2 diastereoselectivity ratio. However, Homo-Sakurai-allylation followed by treatment with sodium hexamethyldisilazide gave ester 176 in 85% yield. Ester 176 was further reduced with DIBAL-H and then subjected to Lewis acid catalyzed Mukaiyama aldol reaction in DCM. Then, oxidation was carried out with DMP, which gave triketone intermediate 177 in 62% yield. This triketone intermediate was then reacted several times, thereby leading to the synthesis of target molecule 178 (Scheme 21).
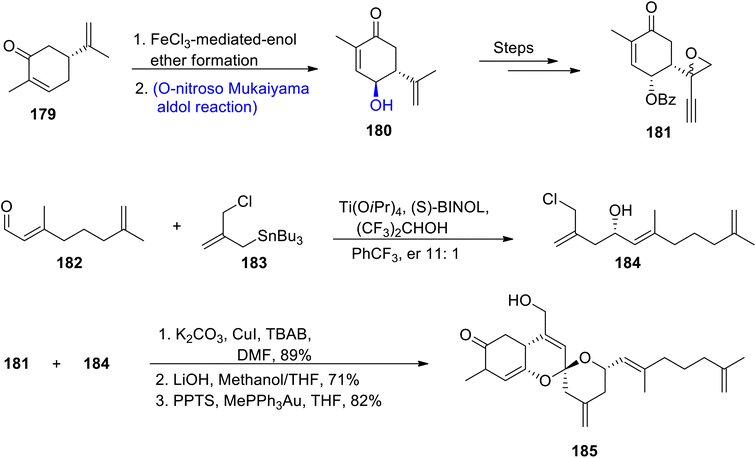
Owing to the wide range of biological activities exhibited by naturally occurring spiroketals, continuous efforts towards their total syntheses have been reported by various researchers. Alotaketal A is one of the naturally occurring marine spiroketal that has been found to be obtained from Hamiegra species. They are included in the sesterpenoid family as they have a didehydro spiroketal moiety in their structure.98,99 Lee et al.100 in 2020 attempted to describe the total synthesis of alotketal A by involving the Mukaiyama aldol reaction and epoxide rearrangement along with the gold-mediated spiroketalization approach. The synthesis began with the reaction of R-curvane 179 via iron chloride catalyzed formation of enol ether, which resulted in the synthesis of hydroxy curvane 180 via O-nitroso Mukaiyama aldol reaction. Hydroxy curvane 180 further underwent a number of reactions to synthesize coupling fragment 181. In the next step, aldehyde 182 was made to react with compound 183 in the presence of Ti(OiPr)4, (S)-BINOL, (CF3)2CHOH and PhCF3 to obtain coupling fragment 184 in 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 enantioselective ratio. Fragments 181 and 184 were finally coupled by using copper iodide followed by treatment with lithium hydroxide in methanol and gold-mediated-spiroketalization resulted in the synthesis of target molecule alotaketal A 185 in 82% yield (Scheme 22).
1 enantioselective ratio. Fragments 181 and 184 were finally coupled by using copper iodide followed by treatment with lithium hydroxide in methanol and gold-mediated-spiroketalization resulted in the synthesis of target molecule alotaketal A 185 in 82% yield (Scheme 22).
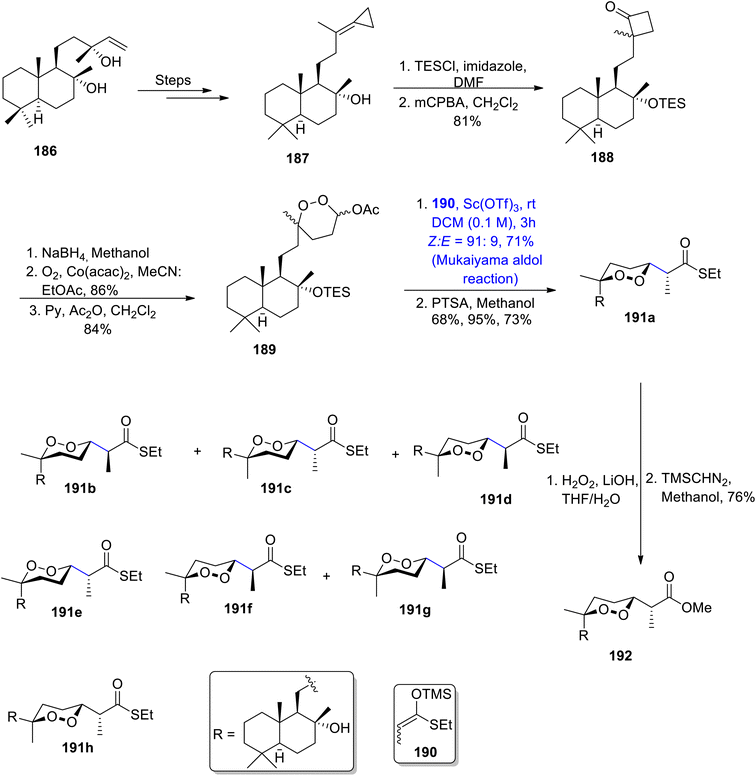
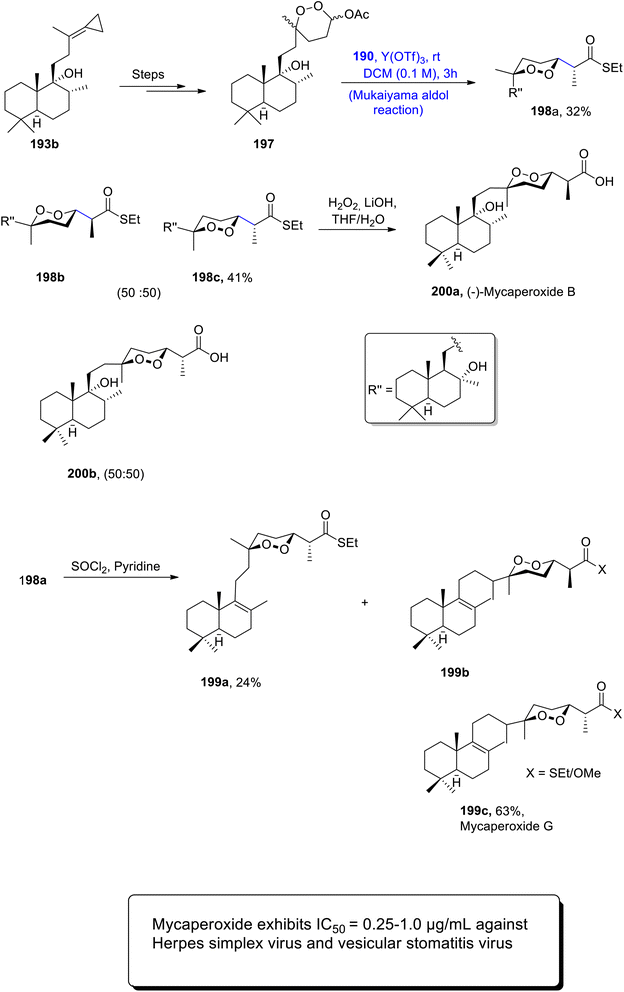
Most of the seawater norsesterpene metabolites belong to the family of naturally occurring endoperoxides. Various members of this family have been known to be isolated from different sponges i.e., Diacarnus, Sigmosceptrella, prianos or Mycale. Similarly, Mycale sponges are a source of different mycaperoxides which exhibit significant biological activity against a number of bacterial and viral diseases.101,102 They constitute 1,2-dioxane ring substituted propionic acid along with a sesquiterpene subunit in their structure. Various researchers have attempted to describe the total synthesis of these naturally occurring endoperoxides, but a concise and efficient stereoselective route has not been developed yet. Thus, Kerim et al.103 in 2022 synthesized various members of the mycaperoxide class for the first time. The synthesis initiated with the generation of cyclopropylidene 187 by treating sclareol 186 over a number of steps. Cyclopropylidene was then subjected to protection with TESCl, followed by reaction with meta-chloroperoxybenzoic acid to give compound 188 in 81% yield. Compound 188 was further converted to peroxyacetal 189 in 84% yield, by undergoing three different steps. In the next step, peroxyacetal 189 underwent the Mukaiyama aldol reaction upon treatment with silyl enol ether 190 by using Sc(OTf)3 as catalyst and dichloromethane as solvent. A subsequent reaction with p-toluene sulfonic acid in the presence of methanol gave a mixture of different diastereomers i.e., 191a, 191b, 191c and 191d. Diastereomer 191a was further reacted with hydrogen peroxide and lithium hydroxide using THF/H2O as a solvent. Thus, mycaperoxide D methyl ester 192 was obtained in 76% yield after reaction with TMSCHN2 in methanol (Scheme 23).
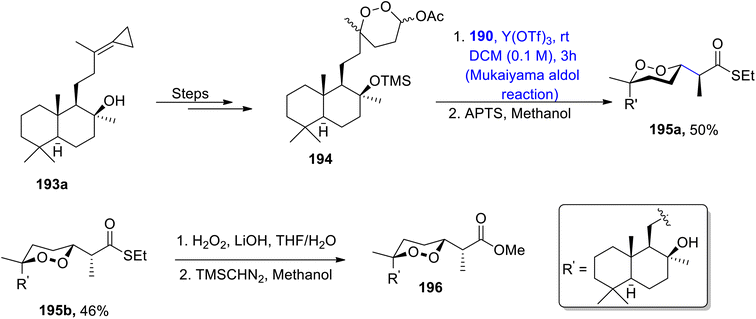
A series of similar steps were adopted to synthesize mycaperoxide C methyl ester 196. For this purpose, compound 193a was converted to peroxyacetal 194 in 82% yield. Peroxyacetal 194 later underwent reaction with silyl enol ether 190 by using a Y(OTf)3 catalyzed Mukaiyama aldol reaction in DCM solvent, followed by reaction with APTS, which gave a mixture of diastereomers 195a and 195b in 96% yield. 195b was then treated further to obtain mycaperoxide C methyl ester 196 by employing similar steps to those described earlier for the synthesis of mycaperoxide D (Scheme 24).
The Mukaiyama aldol reaction was further employed to synthesize mycaperoxides B and G. For this purpose, compound 193b was converted to peroxyacetal 197 by undergoing several steps. Peroxyacetal was then subjected to Y(OTf)3 catalyzed Mukaiyama aldol reaction upon treatment with silyl enol ether 190 to furnish aldol adducts 198a in 32% yield with 198b and 198c in a racemic mixture. Adduct 198a was further made to react with SOCl2 and pyridine to synthesize mycaperoxide G 199c in 63% yield along with 199a and 199b. Similarly, adduct 198c underwent a reaction with hydrogen peroxide in the presence of lithium hydroxide and THF/H2O, which gave mycaperoxide B 200a and compound 200b in a racemic mixture (Scheme 25).
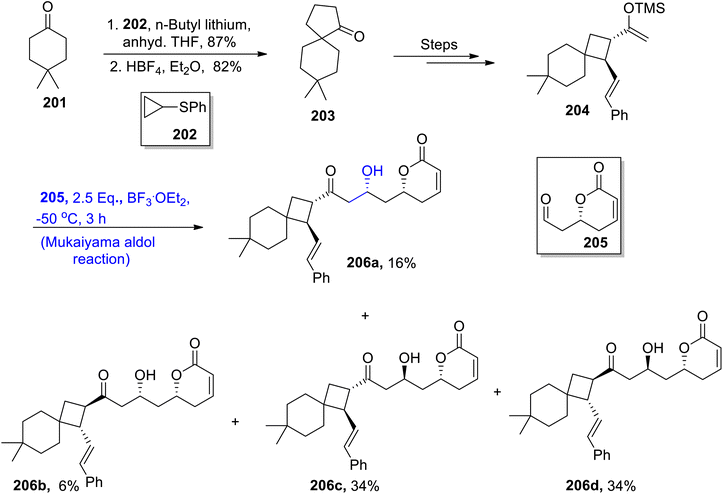
Leaves of Cryptocarya laevigata are a source of a wide range of cryptolaevillactones (CLs). They belong to a class of naturally occurring monoterpenoids whose structural framework comprises a spiro-nonane moiety.104 Considering the versatility of these cryptolaevillactones, Miura et al.105 in 2022 reported the synthesis of representative members of CLs 206a–206d by employing the Mukaiyama aldol reaction as the crucial step. The first step of synthesis involved the reaction of cyclohexanone 201 with cyclopropyl phenyl sulfide 202 in the presence of butyl lithium and anhydrous THF followed by treatment with HBF4 in diethyl ether via Trost's and Undheim's method to obtain spiro ketone 203 in 82% yield. Spiro ketone 203 was further treated a number of times to synthesize silyl enol ether 204. Silyl enol ether 204 was subjected to react with chiral aldehyde 205 via Lewis acid catalyzed Mukaiyama aldol reaction to obtain cryptolaevillactones 206a, 206b, 206c and 206d in 16%, 16%, 34% and 34% yields, respectively (Scheme 26).
2.4. Synthesis of alkaloid-based natural products
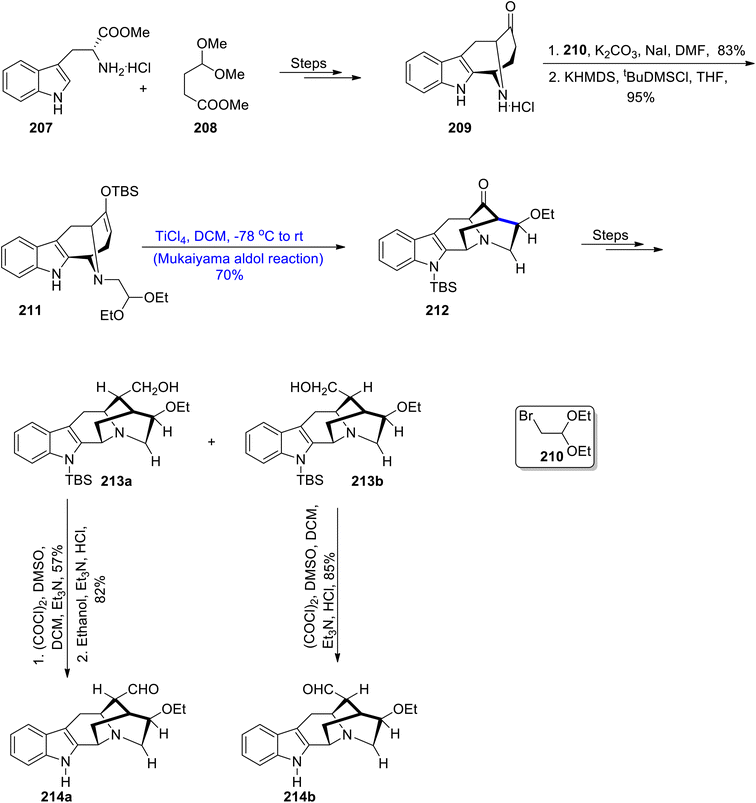
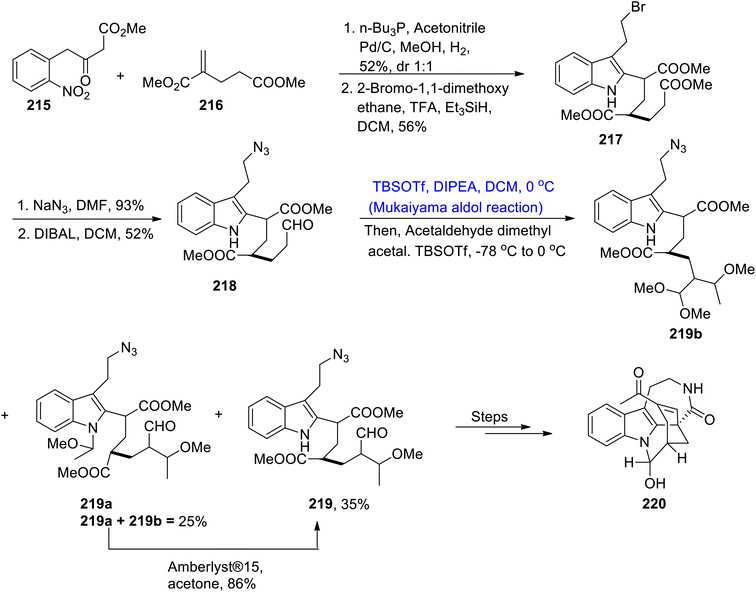
The Rauvolfia Vomitaria plant is an isolation source of rauvomines A and B, which are monoterpenoid indole alkaloids. Rauvomine A constitutes a chlorine atom at carbon number 20 and an extra cyclopropane ring is present in the skeleton of rauvomine B.106 These indole alkaloids have wide medicinal applications and they are found to be highly potent against inflammatory diseases. Owing to their unparalleled biological applications and structural diversity, they have attracted the interest of various researchers. Most recently, Wu et al.107 reported the brief and efficient total synthesis of these compounds by employing the Mukaiyama aldol reaction as one of its key steps. In this regard, compounds 207 and 208 were coupled and reacted in a number of steps, thus leading to the synthesis of ketone 209 in excellent yields. In the next step, ketone 209 was made to react with compound 210 by utilizing potassium carbonate and dimethylformamide followed by its reaction with potassium hexamethyl disilazide and tert-butyl dimethyl silyl chloride in tetrahydrofuran to obtain tert-butyldimethylsilyl ether 211 in 95% yield. In the next step, ether 211 was subjected to the Mukaiyama aldol reaction upon treatment with titanium chloride in dichloromethane at −78 °C to room temperature. This reaction resulted in the synthesis of aldol adduct 212 in 70% yield, thereby synthesizing the five-membered cyclic ring of the target molecule. The compound 212 was then reacted in the presence of different conditions to obtain the central ring of rauvomines 213a and 213b, respectively. Compounds 213a and 213b were further individually reacted with (COCl)2, dimethylsulfoxide, trimethylamine followed by treatment with hydrochloric acid to obtain the dextrorotary and levorotatory stereoisomers of compounds 214a and 214b in 82% and 85% yields, respectively. In this way, total synthesis of rauvomines can be carried out by employing this synthetic approach, which has been utilized to synthesize a highly complex ring in its structure (Scheme 27).Various types of indole alkaloids can be extracted from Tabernaemontana corymbosa plants.108 Specifically, vobasinyl iboga-type and chippiine-type indole alkaloids are found to be highly active against cancerous diseases. Kam and colleagues carried out the isolation of tronocarpine in the beginning of 21st century.109 Tronocarpine holds significant importance in the chippiine-type alkaloids family. Its structure is composed of five-membered cyclic rings, constituting lactams and chiral centers. Owing to the importance of the tronocarpine alkaloid, various synthetic schemes have been proposed by different researchers in recent years. However, previous reported methodologies were too long and time consuming. Hence, Nakayama et al.110 in 2021 demonstrated the efficient methodology for its synthesis. The first step of the synthesis involved the coupling reaction between 215 and α,β-unsaturated ester 216 in the presence of n-Bu3P, acetonitrile and methanol followed by hydrogenation and addition reactions, which led to the synthesis of compound 217 in 56% yield. The compound 217 was further treated with sodium azide in DMF solvent followed by subsequent reduction with DIBAL, which gave aldehyde 218 in 52% yield. Aldehyde 218 was then subjected to Mukaiyama aldol reaction conditions in the presence of TBSOTf, DIPEA and dichloromethane at 0 °C to obtain silyl enol ether. In the following step, acetaldehyde dimethyl acetal and TBSOTf were added to the reaction mixture, which resulted in 35% yield of compound 219 along with 25% yield of by-products i.e., 219a and 219b. By-products 219a and 219b were then treated with Amberlyst®15 in acetone to obtain 219 in 86% yield. Compound 219 was further reacted via several steps to obtain an excellent yield of tronocarpine alkaloid 220 (Scheme 28).
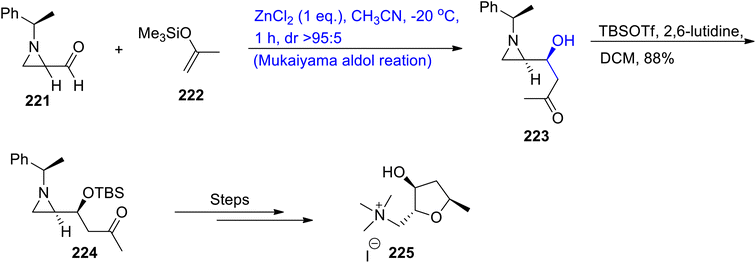
Sirivastava and Ha111 in 2021 developed a novel approach for the Mukaiyama aldol reaction by employing chiral substituents. A highly stereoselective synthesis of aziridine-2-carboxaldehyde was carried out by employing the Mukaiyama aldol reaction between chiral 1-(α-methylbenzyl)-aziridine-2-carboxylaldehyde and a diverse range of enol silanes by employing zinc chloride as a catalyst. Most of the alkaloids are known to be synthesized from β-(aziridin-2-yl)-β-hydroxy ketones i.e. (−)-epiallo-isomuscarine, epi-galantinic acid and the (2S,3R,5S)-enigmol analog. Sirivastava also reported the total synthesis of these alkaloids. The first step involved the reaction between carboxyl aldehyde 221 and chiral enol silyl compound 222 via a zinc chloride-mediated-Mukaiyama aldol reaction in acetonitrile to obtain aldol adduct 223 with a diastereoselectivity ratio of more than 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. Compound 223 was then made to react with TBSOTf and 2,6-lutidine in the presence of DCM to obtain compound 224 in 88% yield. Compound 224 was then reacted over several steps to synthesize (−)-epiallo-isomuscarine 225 (Scheme 29).
5. Compound 223 was then made to react with TBSOTf and 2,6-lutidine in the presence of DCM to obtain compound 224 in 88% yield. Compound 224 was then reacted over several steps to synthesize (−)-epiallo-isomuscarine 225 (Scheme 29).
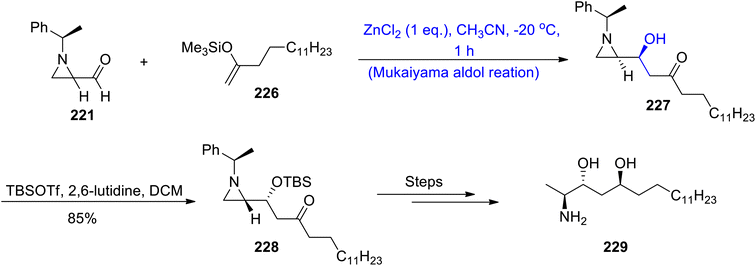
Substituted chiral aldehyde 221 was made to react with trimethylsilyl enol ether 226 by using zinc chloride as the catalyst in acetonitrile to furnish aldol adduct 227. The aldol adduct 227 was reacted with TBSOTf and 2,6-lutidine utilizing DCM which resulted in the synthesis of compound 228 in 85% yield. Finally, (2S,3R,5S)-enigmol analog 229 was synthesized by treating compound 228 over several steps (Scheme 30).
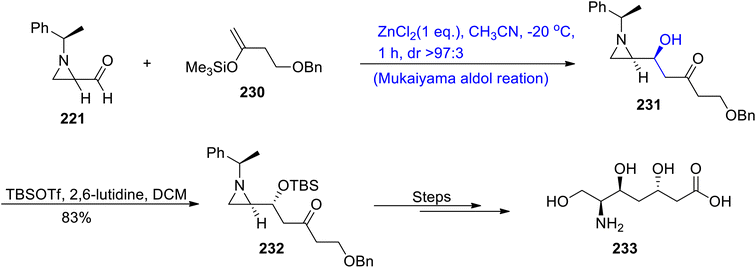
Substituted chiral aldehyde 221 underwent a zinc chloride catalyzed Mukaiyama aldol reaction with trimethyl silyl enol ether 230 to furnish aldol adduct 231 in a diastereoselectivity ratio of more than 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. Compound 231 was reacted with TBSOTf and 2,6-lutidine in dichloromethane to afford compound 232 in 83% yield. Compound 232 was further made to react in a number of steps to synthesize epi-galantic acid 233 (Scheme 31).
3. Compound 231 was reacted with TBSOTf and 2,6-lutidine in dichloromethane to afford compound 232 in 83% yield. Compound 232 was further made to react in a number of steps to synthesize epi-galantic acid 233 (Scheme 31).
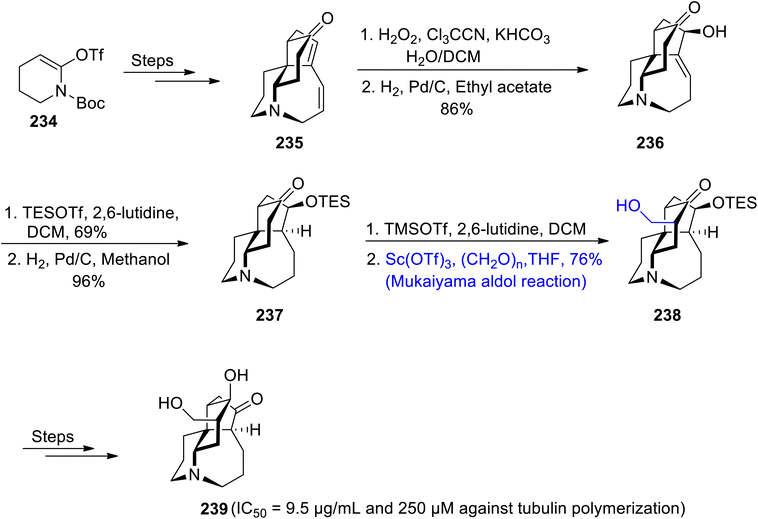
Kobayashi along with colleagues isolated lycopodium alkaloids i.e., lyconesidine A and B from Lycopodium chinese. These are naturally occurring alkaloids, which are comprised of Fawcettimine alkaloids type cyclic moiety in their structural framework.112,113 They are found to be effective in inhibiting the proliferation of cancerous cells, thus behaving as anti-mitotic agents. Compounds with anti-cancerous potential have been widely investigated.114,115 Specifically, Lyconsesidine B has been found to interrupt the polymerization process of tubulin with IC50 = 250 μM. Taking into account the structural diversity and anti-cancerous potential of Lyconesidine B, Kurose et al.116 in 2020 furnished the total synthesis of this naturally occurring alkaloid by employing the Mukaiyama aldol reaction as one of its key steps. At the onset of synthesis, enol triflate 234 was reacted over a number of steps to synthesize the tetracyclic ring of lyconesidine B 235. Tetracyclic ring containing compound 235 was further treated via epoxidation in the presence of hydrogen peroxide, Cl3CCN and potassium carbonate followed by opening of the epoxide ring in the presence of H2, and Pd/C to obtain compound 236 in 86% yield. Compound 236 was then made to react with TESOTf and 2,6-lutidine followed by reduction utilizing palladium to obtain compound 237 in 96% yield. It was then reacted with TMSOTf and 2,6-lutidine in dichloromethane followed by a scandium triflate catalyzed Mukaiyama aldol reaction with aldehyde in tetrahydrofuran, which resulted in the synthesis of β-hydroxyketone 238 in 76% yield. β-Hydroxyketone 238 later underwent a number of reactions to obtain lyconesidine B 239 (Scheme 32).
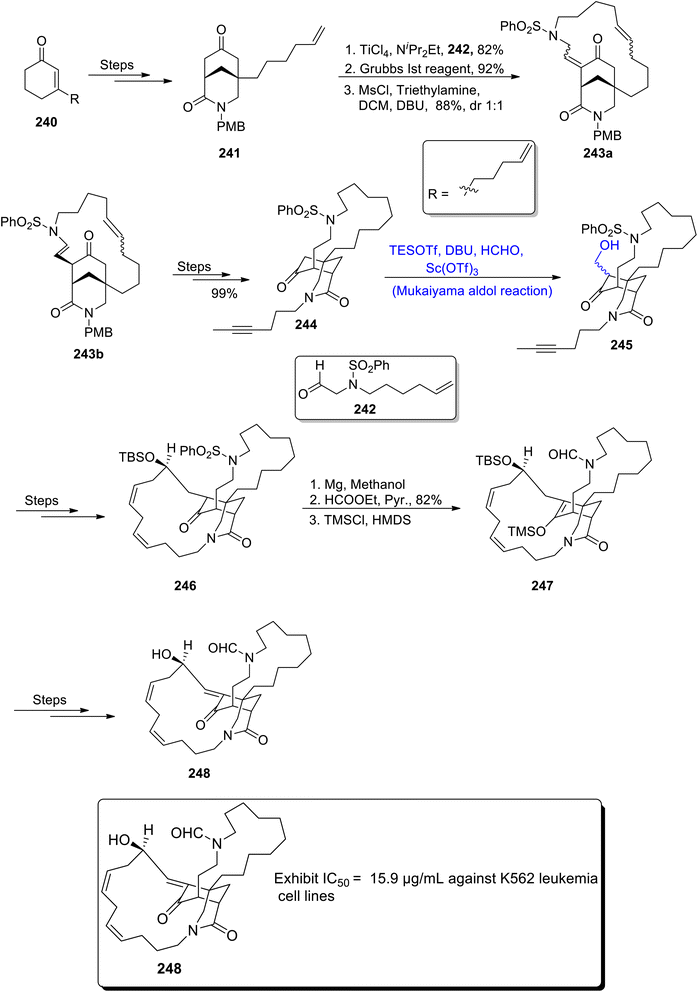
Haliclona species, which belong to the sea-water sponge genus, are a source of various naturally occurring organic compounds.117 These naturally occurring metabolites have been found to be highly potent against cancerous, bacterial and viral diseases.118 Similarly, seawater cyclic alkaloid, haliclonin A, was obtained from Haliclona sp. by Shin and colleagues. In order to attain biologically active nitrogen containing organic compounds via synthetic methodology, Luo et al.,119 in 2021 reported the efficient synthetic protocol for the synthesis of haliclonin A. The synthesis began with the attainment of nonane core based compound 241 by using enone 240 as precursor. Compound 241 was further subjected to react with compound 242 in the presence of titanium chloride followed by reaction with Grubbs reagent to obtain product in 92% yield. It was then reacted with mesityl chloride and trimethylamine in dichloromethane and DBU to obtain a diastereoselective mixture of 243a and 243b in 88% yield with dr = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The racemic mixture was then treated over a number of steps to synthesize ketone based compound 244. Compound 244 was further subjected to treat with formaldehyde via an Sc(OTf)3 catalyzed Mukaiyama aldol reaction in the presence of DBU and TESOTf to obtain Mukaiyama aldol product 245. The Mukaiyama aldol product 245 was then treated over a number of steps, which led to the synthesis of compound 246. The next step involved the treatment with magnesium in the presence of methanol followed by treatment with ethyl formate and pyridine to obtain diamide compound in 82% yield. Compound 247 was then obtained by the reaction of TMSI and HDMS. Compound 247 later underwent a number of reactions to synthesize haliclonin A 248 in 82% yield (Scheme 33).
1. The racemic mixture was then treated over a number of steps to synthesize ketone based compound 244. Compound 244 was further subjected to treat with formaldehyde via an Sc(OTf)3 catalyzed Mukaiyama aldol reaction in the presence of DBU and TESOTf to obtain Mukaiyama aldol product 245. The Mukaiyama aldol product 245 was then treated over a number of steps, which led to the synthesis of compound 246. The next step involved the treatment with magnesium in the presence of methanol followed by treatment with ethyl formate and pyridine to obtain diamide compound in 82% yield. Compound 247 was then obtained by the reaction of TMSI and HDMS. Compound 247 later underwent a number of reactions to synthesize haliclonin A 248 in 82% yield (Scheme 33).
2.5. Synthesis of naturally occurring metabolites
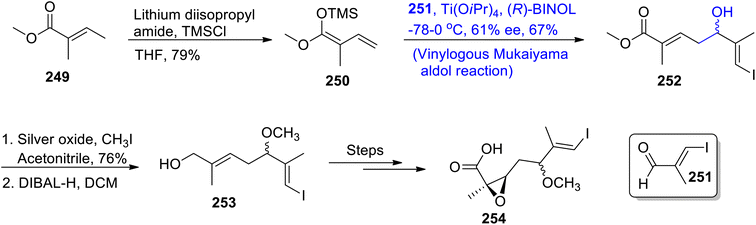
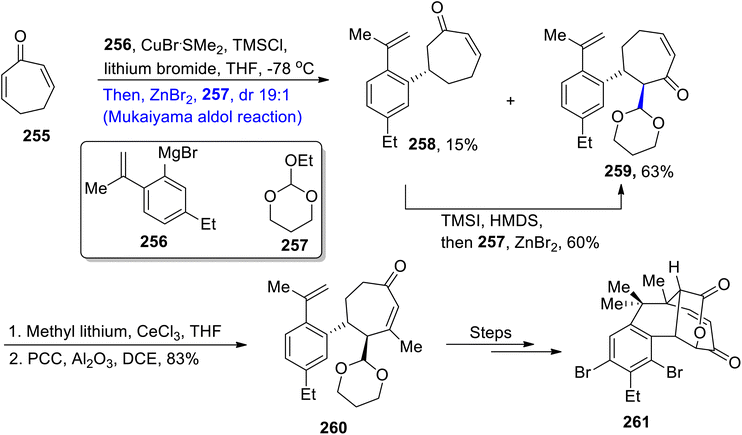
Nannocystin along with its natural counterpart is known to be isolated from the Nannocystis species. They are biologically active myxobacteria secondary metabolites that are used in the inhibition and proliferation of cancer cell lines.120,121 Their structure is composed of 21 atoms along with nine stereocenters. Their giant structure with significant medicinal applications inspired numerous efforts to achieve their total synthesis. Zhang et al.122 in 2021 carried out their total synthesis by making use of the Keck asymmetric vinylogous Mukaiyama aldol reaction to obtain component 254 which was then coupled with other components to synthesize nannocystin A. In their synthetic scheme, unsaturated ester 249 was made to react with LDA and TMSCl in tetrahydrofuran to obtain trimethyl silyl enol ether 250 in 79% yield. Compound 250 was further subjected to the Mukaiyama aldol reaction by treating it with aldehyde 251 in the presence of Ti(OiPr)4 and (R)-BINOL, which resulted in aldol adduct 252 with 67% yield. It was observed that utilization of the chiral (R)-BINOL reagent resulted in the highly enantioselective synthesis of aldol adducts. Moreover, (R)-BINOL can be easily recovered after the reaction as it does not incorporate within the reactants. In the next step, compound 252 was made to react with silver oxide and methyl iodide in acetonitrile followed by subsequent reduction, which gave allylic alcohol 253. Allylic alcohol 253 was then reacted over a number of steps to synthesize component 254 of the target molecule (Scheme 34).Salimabromide is a significant naturally occurring myxobacteria metabolite that has been extracted from plesiocystis clade of sea-water myxobacteria. It was found to be active against Arthobacter crystallopoietes. This metabolite contains fused cyclic rings with four consistent chiral centers.123,124 The structural and biological significance of salimabromide with its scarce availability in nature urged researchers to develop an efficient methodology for its synthesis. In order to contribute towards the design of facile methodology for the synthesis of salimabromide, Lu et al.125 in 2022 employed the Mukaiyama aldol reaction as a major step in their synthetic approach. They treated unsaturated lactone 255 with Grignard reagent 256 and compound 257 in the presence of CuBr·SMe2, TMSCl, lithium bromide and THF at −78 °C via the Maukaiyama aldol reaction, which gave compound 258 in 15% yield and aldol adduct 259 in 63% yield with a diastereoselectivity ratio of more than 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Aldol adduct 259 was further reacted with methyl lithium and CeCl3 in THF followed by oxidation in the presence of PCC, which resulted in the synthesis of cycloheptenone 260 in 83% yield. Compound 260 was then further treated via different steps to obtain target molecule 261 (Scheme 35).
1. Aldol adduct 259 was further reacted with methyl lithium and CeCl3 in THF followed by oxidation in the presence of PCC, which resulted in the synthesis of cycloheptenone 260 in 83% yield. Compound 260 was then further treated via different steps to obtain target molecule 261 (Scheme 35).
2.6. Synthesis of naturally occurring depsipeptides
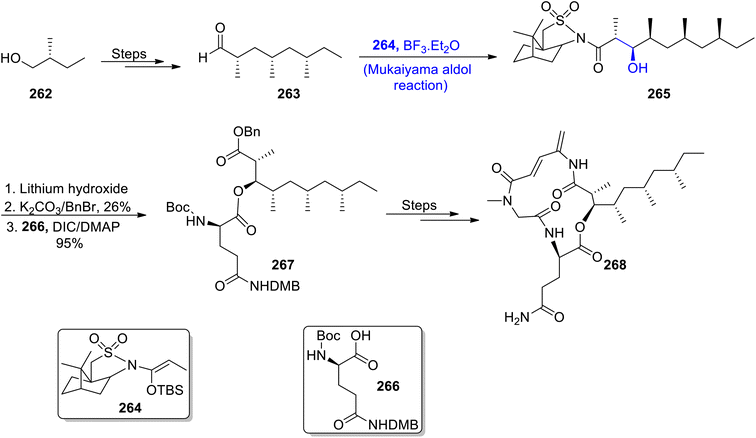
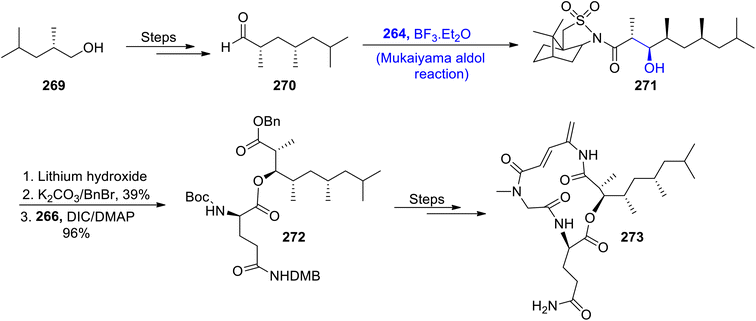
Most naturally occurring compounds have been found to inhibit the proliferation of cancerous cells. Generally, the structure of members of the rakicidin family comprises 4-amino, 2,4-pentadienolate and this moiety has been found to be effective in combating cancer cell lines effectively. Their wide biological significance and structural properties have attracted various researchers and urged them to attempt the total synthesis of members of the rakicidin family.126,127 Sea-water sponges i.e., Streptomyces species have been found to be an isolation source of rakicidin F. Han et al.128 in 2022, reported the total synthesis of this naturally occurring depsipeptide by employing the Mukaiyama aldol reaction as one of the major steps. The synthetic route commenced with the synthesis of aldehyde 263 from 2-methyl-butanol 262. Chiral aldehyde 263 was then subjected to react with silyl enol ether 264 via the Lewis acid catalyzed Mukaiyama aldol reaction to obtain aldol product 265. Aldol product 265 was further hydrolyzed in the presence of lithium hydroxide followed by reaction with benzyl bromide in the presence of potassium carbonate. Then, coupling with acid 266 resulted in the synthesis of compound 267 in 95% yield. This coupled compound 267 further underwent several reactions to furnish target molecule 268 (Scheme 36).Streptomyces species are the source of various naturally occurring secondary metabolites including rakicidin C. The depsipeptides family holds significant importance in medicinal chemistry, owing to their anti-mitotic potential. The structural framework of rakicidin C constitutes a pentadienolate moiety along with five chiral centers.129,130 Han et al.131 reported the total synthesis of naturally occurring depsipeptide i.e., rakicidin C by employing the Mukaiyama aldol reaction and Oppolzer's alkylation as key steps. The synthetic methodology commenced with the synthesis of chiral aldehyde substituted compound 270 from alcohol 269 through a number of steps. This aldehyde was then subjected to the Mukaiyama aldol reaction in the presence of boron trifluoride diethyl ether by reacting compound 270 with silyl enol ether 264, thereby giving Mukaiyama aldol product 271. The aldol product 271 was further treated with lithium hydroxide followed by protection of the benzyl group in three to four steps. The next step involved the coupling of different components upon treatment with acid 266 in the presence of DIC and DMAP to obtain coupled product 272 in 96% yield. It was then finally converted to rakicidin C 273 over a number of steps (Scheme 37).
2.7. Synthesis of miscellaneous natural products
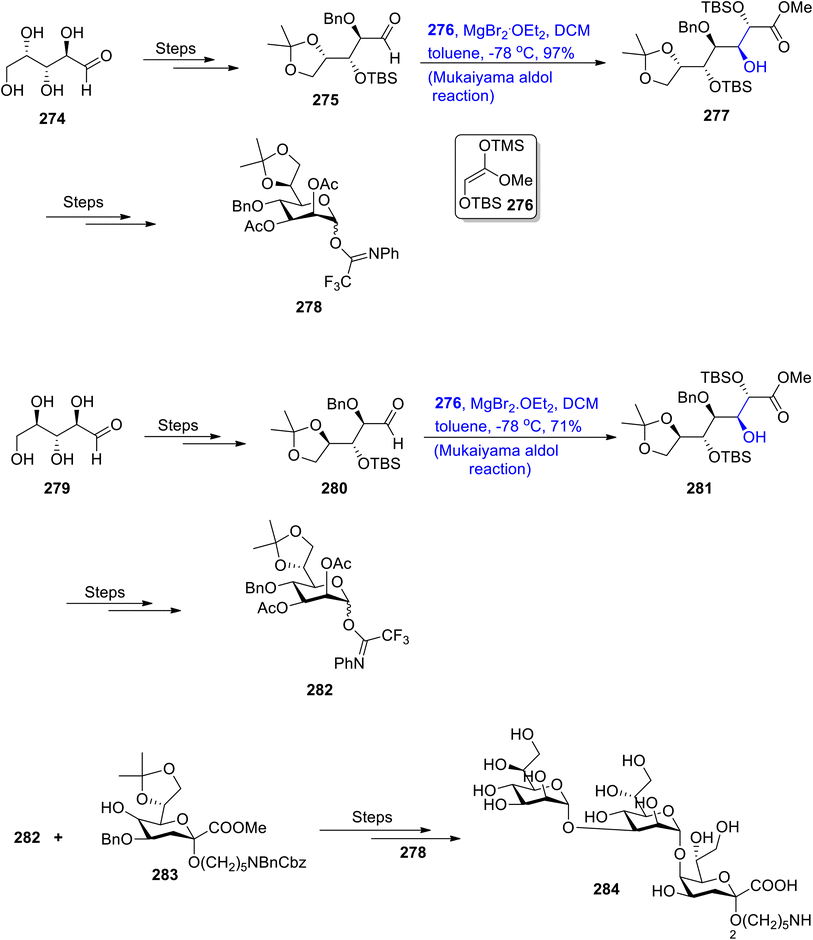
There are number of different sugars secreted by bacteria that are not present in mammals. The lipopolysaccharide layer of Gram negative bacteria has been found to constitute the carbohydrates i.e., D-glycero-D-manno-heptose (D,D-Hep) and L-glycero-D-manno-heptose (L,D-Hep). These sugars are of significant importance in medicinal chemistry as they are found to be highly active against bacterial diseases.132,133 In previous years, various synthetic schemes have been designed by different researchers by utilizing different name reactions. However, an efficient strategy for their synthesis was still required. For this purpose, Wang et al.134 in 2020 reported the total synthesis of LPS core trisaccharide 1 of V. parahemolyticus O2 by utilizing the Mukaiyama aldol reaction as one of the main steps. In order to achieve the synthesis of the L,D-Hep subunit, they reacted L-lyxose 274 via several steps to obtain chiral aldehyde 275, which was further subjected to react with silyl enol ether 276 by the Mukaiyama aldol reaction in the presence of MgBr2·OEt2, DCM and toluene. The Mukaiyama aldol product 277 was obtained in 97% yield, which was followed by treatment via several steps to give L,D-Hep subunit 278. Similarly, D,D-Hep subunit 282 was obtained by reacting D-ribose 279 through similar steps. The D,D-Hep subunit 282 was then reacted with compound 283 over a number of steps that involved coupling with subunit 278, thereby leading to the synthesis of target molecule 284 (Scheme 38).Amphidinium genus is an isolation source of about 30 members of naturally occurring macrolactones135 found in sea water. Amphidinolide F, C2 and C3 have a strong resemblance with the amphidinolide C 1 structure with only modifications in the side chain R group. Moreover, these structurally diverse, naturally occurring macrolactones have been found to inhibit the proliferation of various types of cancerous cells effectively. Owing to their significant contribution towards medicinal chemistry, amphidinolides have inspired researchers to devise efficient synthetic pathways to obtain these naturally occurring macrolactones on a large scale. In this regard, two similar yet slightly different synthetic approaches have been reported to attain amphidinolides F, C2, C1 and C3 by Ferrie et al.136 in 2022. At first, retrosynthetic analysis was carried out to obtain different fragments of the target molecule, which were then synthesized individually by using appropriate methodology. The Mukaiyama aldol reaction was carried out to obtain the C1–C9 fragment 289 in 99% yield, which is a common fragment in both synthetic approaches. In order to synthesize the C1–C9 fragment 289, chiral aldehyde 285 was reacted with silyl enol ether 286 in the presence of TMSOTf and dichloromethane via the Mukaiyama aldol reaction, which gave aldol adducts 287a and 287b in a 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75 ratio. Aldol adduct 287 was further subjected to hydrogenation followed by treatment with TBSCl by using imidazole and DMF to obtain compound 288 in 73% yield with a diastereoselectivity ratio of more than 20
75 ratio. Aldol adduct 287 was further subjected to hydrogenation followed by treatment with TBSCl by using imidazole and DMF to obtain compound 288 in 73% yield with a diastereoselectivity ratio of more than 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
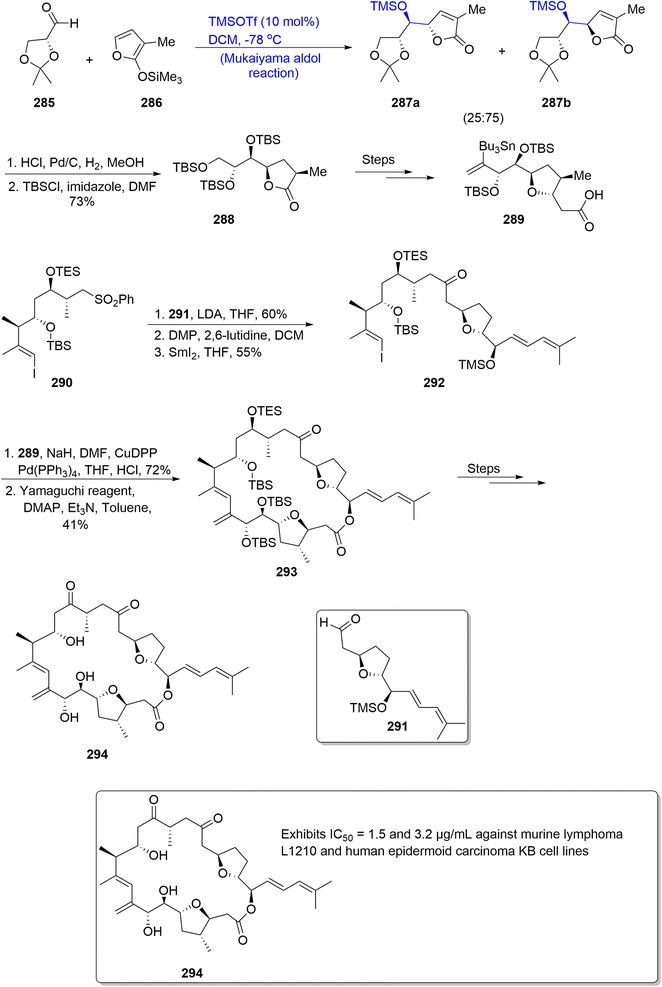
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. It was further treated over a number of steps to furnish the C1–C9 fragment 289. Similarly, other fragments C10–C17 290, C18–C34 291a were synthesized separately. In order to couple these fragments for the total synthesis of amphidinolide F, fragment 290 was made to react with fragment 291a by using lithium diisopropyl amide and THF followed by oxidation, which was carried out by employing Dess–Martin periodinane. In the next step, desulfonylation was carried out by using SmI2 to attain compound 292 in 55% yield. The next step involved the reaction of compound 292 with C1–C9 fragment 289 in the presence of sodium hydride, CuDDP and triphenyl phosphine followed by treatment with the Yamaguchi reagent, which resulted in the synthesis of macrolide 293 in 41% yield. This macrolide 293 later reacted over a number of steps to obtain amphidinolide F 294 (Scheme 39).
1. It was further treated over a number of steps to furnish the C1–C9 fragment 289. Similarly, other fragments C10–C17 290, C18–C34 291a were synthesized separately. In order to couple these fragments for the total synthesis of amphidinolide F, fragment 290 was made to react with fragment 291a by using lithium diisopropyl amide and THF followed by oxidation, which was carried out by employing Dess–Martin periodinane. In the next step, desulfonylation was carried out by using SmI2 to attain compound 292 in 55% yield. The next step involved the reaction of compound 292 with C1–C9 fragment 289 in the presence of sodium hydride, CuDDP and triphenyl phosphine followed by treatment with the Yamaguchi reagent, which resulted in the synthesis of macrolide 293 in 41% yield. This macrolide 293 later reacted over a number of steps to obtain amphidinolide F 294 (Scheme 39).
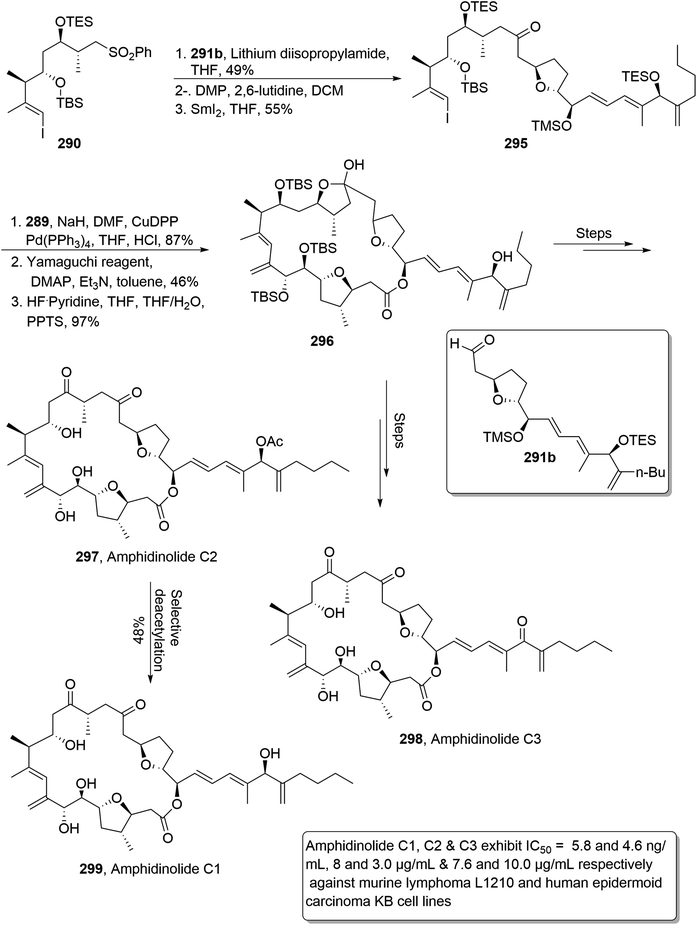
In order to synthesize amphidinolide C3, C2 and C1, the individually synthesized C10–C17 fragment 290 and C18–34 fragment 291b were reacted in the presence of LDA and tetrahydrofuran followed by Dess–Martin periodinane mediated oxidation. Further treatment with SmI2 in THF afforded compound 295 in 55% yield. Compound 310 was then made to react with C1–C9 fragment 289 under similar conditions to those employed for the synthesis of macrolide 294. Reaction with hydrogen fluoride pyridine furnished hemiketal 296 in 97% yield. Hemiketal 296 was then reacted over a number of times to convert it into amphidinolide C2 297. The newly synthesized amphidinolide C2 297 was subjected to selective deacetylation to obtain amphidinolide C1 298. Moreover, hemiketal 296 was also used to synthesize amphidinolide C3 298 by undergoing several steps (Scheme 40).
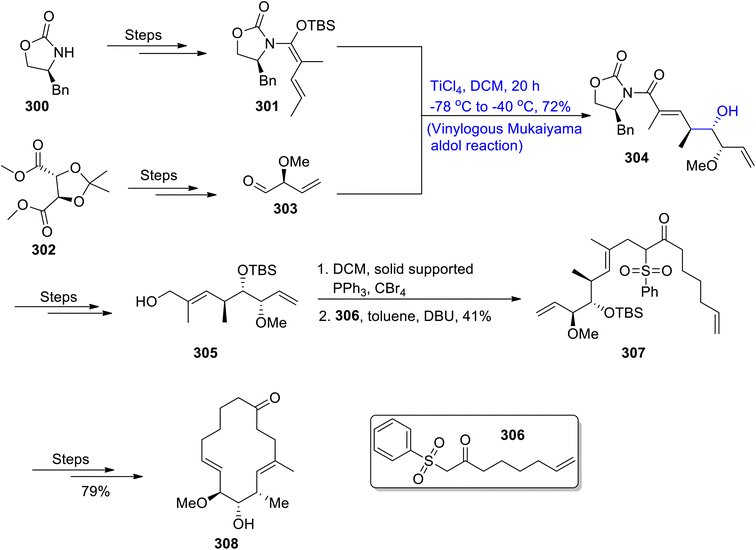
Uncontrolled proliferation of cancer cells is one of the major reasons for increasing deaths.137 There have been ongoing continuous efforts to tackle the effects of this deadly disease. In this regard, numerous natural products are found to be active against tumour cells. The hypothetical macroketone, which is an analogue of the migrastatin natural product, plays an effective role as an anti-cancerous agent. Considering the pharmacological importance of this analogue, Choudhury et al.138 in 2021, reported its total synthesis by utilizing the vinylogous Mukaiyama aldol reaction as one of its key steps. Initial steps involved the synthesis of compound 301 and aldehyde 303 from (S)-4-benzyloxazolidin-2-one 300 and 2,3-O-isopropylidene-L-tartrate 302 by known methodologies. They were then reacted together via the vinylogous Mukaiyama aldol reaction in the presence of titanium tetrachloride and dichloromethane, which gave aldol adduct 304 in 72% yield. Compound 300 was transformed into alcoholic fragment 305 by undergoing a number of steps. In the next step, alcoholic fragment 305 was made to react with carbon tetrabromide by employing triphenyl phosphine and dichloromethane followed by its reaction with β-ketosulfone 306 in toluene and DBU to obtain compound 307 in 41% yield. Compound 307 was further treated through different steps to obtain macroketone 308 (Scheme 41).
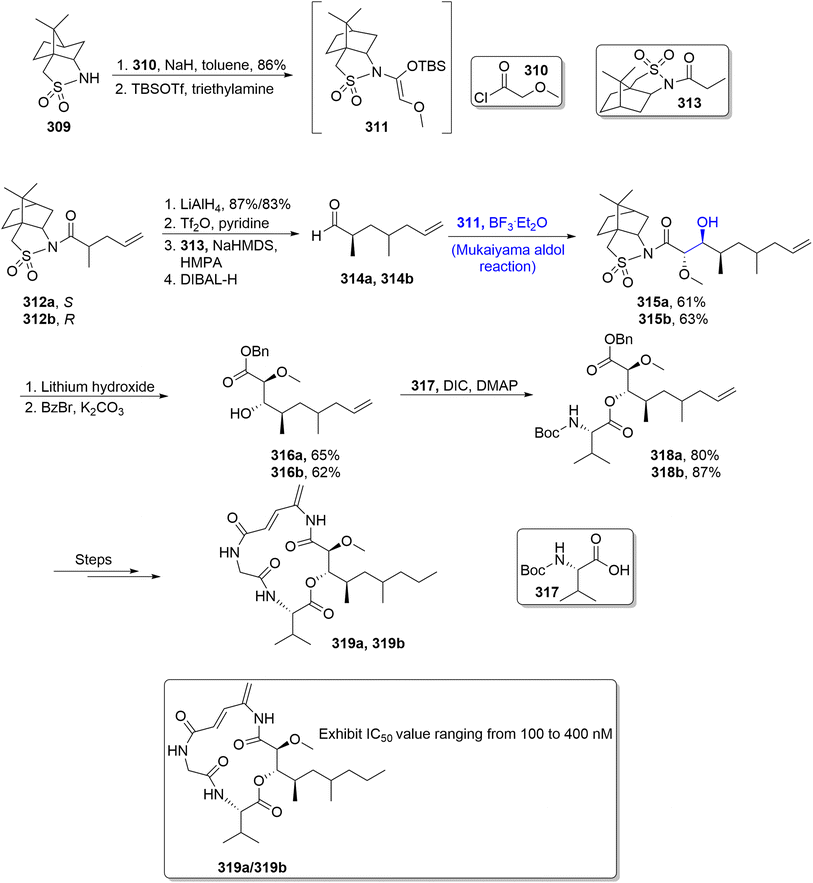
The structure of boholamide is found to be composed of a long cyclic framework containing a 4-amino-2,4-pentadienolate moiety. It is a natural product, which is isolated from the Truncatella species mollusk, and is found to exhibit potent cytotoxic activity.139 It possesses highly effective anti-tumour potential as compared to other known anti-cancerous agents i.e., rakicidin A, vinylamycin, etc. Owing to their wide anti-tumour potential, Han et al.140 in 2021 attempted to report the total synthesis of boholamide. For this purpose, compound 309 was made to react with substituted acyl chloride 310 in the presence of sodium hydride and toluene followed by its reaction with TBSOTf and trimethylamine to obtain intermediate 311. In the next step, a racemic mixture of compound 312 was subjected to reduction, followed by its treatment with Tf2O and pyridine. In the next step, reaction with camphorsultam derivative 313 and sodium hexamethyl dilsilazide and subsequent reduction resulted in a racemic mixture of compound 314. It was then subjected to a reaction with intermediate 311 via the Lewis acid catalyzed Mukaiyama aldol reaction which gave aldol adducts 315a and 315b in 61% and 63% yields, respectively. These aldol adducts were further reacted with lithium hydroxide followed by protection with benzyl bromide to afford esters 316a and 316b in 65% and 62% yields, respectively. These synthesized esters were then reacted with Boc protected L-valine 317 in the presence of DIC and dimethylaminopyridine to obtain a racemic mixture of 318a and 318b in 80% and 87% yields, respectively. These were then reacted over different steps to synthesize (S) and (R) boholamide A 319a and 319b (Scheme 42).
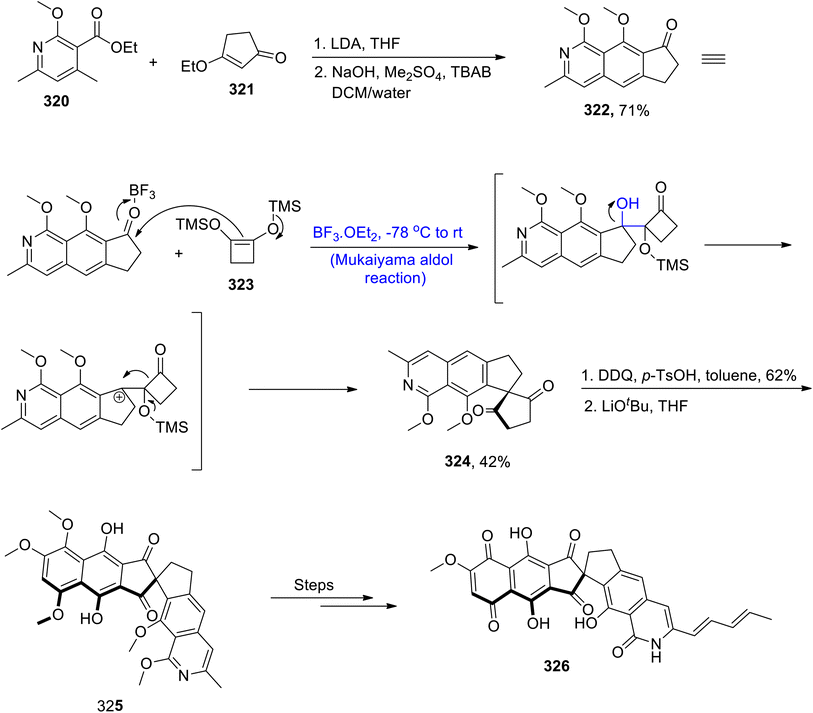
Owing to their diverse biological potential, isoquinolines hold a special place in the family of natural products. Fredericamycin A has been found to be active against bacterial and cancer diseases. It is a naturally occurring substance that has been derived from Streptomyces griseus.141 Various synthetic schemes have been reported by various researchers to synthesize the isoquinoline moiety. However, these methodologies suffer from various disadvantages of reaction conditions and stability of functional groups. In order to overcome these issues, Wang et al.142 in 2021, demonstrated the total synthesis of naturally occurring fredericamycin A by utilizing the Mukaiyama aldol reaction and Dieckmann condensation as its key steps. The first step of the synthesis involved the reaction between substituted pyridine moiety 320 and enone 321 in the presence of lithium diisopropyl amide and tetrahydrofuran followed by reaction with sodium hydroxide, Me2SO4 and TBAB to synthesize compound 322 in 71% yield. Compound 322 was then subjected to reaction with TMS enol ether 323 via a boron triflouride diethyl etherate catalyzed Mukaiyama aldol reaction, which resulted in a 42% yield of spiro diketone 324. Spiro diketone 324 was further reacted with DDQ and p-toluene sulfonic acid in toluene followed by subsequent Dieckmann condensation, which gave coupling product 325 after aromatization. Coupling product 325 was then reacted through several steps, thereby leading to the synthesis of the target molecule i.e., fredericamycin A 326 (Scheme 43).
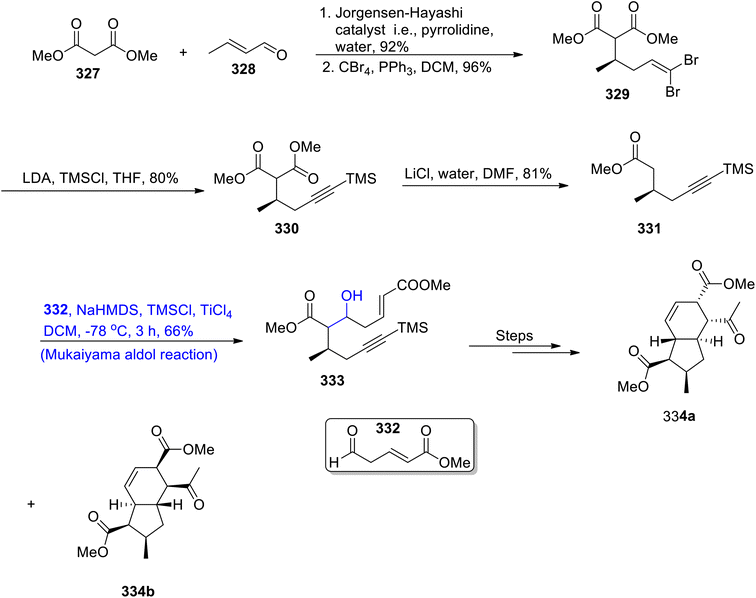
Hydrindanes are abundant in nature and hold significant importance in medicinal chemistry as they are involved in the synthesis of pharmaceutically valuable organic compounds.143 Different organic compounds such as maltophilin, clifednamides, amaminol A and ikarugamycin constitute hydrindanes. Various research groups have reported the synthesis of hydrindanes by using the Michael addition reaction. However, Sinast et al.144 reported the total synthesis of hydrindanes based clifednamides A and B by utilizing the Mukaiyama aldol reaction and Michael addition as the key steps. Streptomyces sp. JV178 are the source of these biologically active organic compounds, which are effective against division of cancer cells. In the first step of synthesis towards hydrindane, dimethyl malonate 327 and crotonaldehyde 328 were reacted in the presence of the Jorgensen–Hayashi catalyst followed by reaction with carbon tetrabromide by using triphenyl phosphine and dichloromethane, which gave compound 329 in 96% yield. Compound 329 was then reacted with lithium diisopropyl amide and trimethyl silyl chloride in tetrahydrofuran, which resulted in the synthesis of TMS alkyne 330 in 80% yield. Compound 330 was further reacted with lithium chloride and water using DMF as the solvent to furnish methyl ester 331 in 81% yield. Methyl ester 331 then underwent the titanium tetrachloride catalyzed Mukaiyama aldol reaction upon treatment with aldehyde 332 in the presence of TMSCl and sodium hexamethyl disilazide to obtain adduct 333 in 66% yield, which was reacted over number of steps, to obtain a racemic mixture of hydrindanes 334a and 334b. Hydrindane 334 can then be easily converted into clifednamies (Scheme 44).
Curvulone A and curvulone B are found to be highly biologically active as they play a significant role in combating fungal diseases as well as also inhibiting the proliferation of cancerous cells. They are isolated from the known sea-water algal species i.e., Gracilaria folifera.145 Considering the utility of the curvulone type natural product, Banoth et al.146 in 2020 carried out a brief and efficient total synthesis of curvulone by utilizing the Mukaiyama aldol reaction as one of its key steps. For this purpose, homoallylalcohol 335 was reacted with acrolein and Hoveyda Grubbs II reagent in dichloromethane to obtain unsaturated aldehyde 336 in 87% yield. Aldehyde 336 then underwent the Mukaiyama aldol reaction by reacting with trimethyl(vinyloxy)silane 337 by utilizing iodine and DCM to obtain the substituted dihydropyran ring 338 in 81% yield. Compound 338 was further subjected to reduction by employing Adam's catalyst, followed by the oxa-Michael process as a result of the reaction with tert-butoxide in tetrahydrofuran. Furthermore, Pinnick oxidation was carried out by using NaClO2, 2-methyl-2-butene, and NaH2PO4 in the presence of butanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water, which led to the synthesis of acid fragment 339 in 86% yield. In the next step, acetate compound 340 was made to react with previously synthesized fragment 339 in trifluoroacetic acid and TFAA. The resulting compound was further demethylated by utilizing Maeir's conditions i.e., AlI3, TBAI, phloroglucinol and benzene, which resulted in the synthesis of curvulone B 341 in 91% yield (Scheme 45).
water, which led to the synthesis of acid fragment 339 in 86% yield. In the next step, acetate compound 340 was made to react with previously synthesized fragment 339 in trifluoroacetic acid and TFAA. The resulting compound was further demethylated by utilizing Maeir's conditions i.e., AlI3, TBAI, phloroglucinol and benzene, which resulted in the synthesis of curvulone B 341 in 91% yield (Scheme 45).
Meayamycin B is widely utilized as a highly active modulator of the splicing factor of 3b subunit 1.147 The reported synthetic routes for meayamycin B are too long and time consuming. Thus, in order to supplement its availability, Bressin et al.148 in 2020, attempted to describe the total synthesis of meayamycin B by utilizing the Mukaiyama aldol reaction, Corey–Chaykovsky reaction, Nicolaou-type-ring opening reaction of epoxide and altered Horner–Wadsworth–Emmons Z-selective olefination as the key steps. The first step of the total synthesis involved the reduction of ester 342, followed by its reaction with phosphonate 343 and KOtBu. Furthermore, reaction with acetic acid and then with a hydrogen molecule in the presence of platinum oxide and ethanol gave lactone 344, which was transformed into fragment 345 via several steps. In the next step, ketone 346 was converted to silyl ether 347 in 90% yield, which was then made to react with acrolein by the Yb(OTf)·6H2O catalyzed Mukaiyama aldol reaction, which gave aldol adduct 348 in 70% yield. Adduct 348 was further reacted via several steps to synthesize fragment 349. Fragments 345 and 349 were then coupled via a Nitro-Grela catalyst in DCE to obtain meayamycin B 350 in 20% yield (Scheme 46).
Pharmacologically important diospongin A and B are obtained from Dioscorea spongiosa. These are pyran containing molecules which inhibit the process of the breakage of bones. Various researchers have reported the total synthesis of these natural products by utilizing the oxa-Michael addition and Prins cyclization. However, the concise and efficient total synthesis of diospongin A was reported by Vaithegi et al.149 in 2020 by using the Mukaiyama aldol reaction. For this purpose, they initiated the synthesis of the target molecule by obtaining β-alkoxy aldehyde 352 in 75% yield by reacting compound 351 through several steps. Chiral β-alkoxy aldehyde was further reacted with silyl ether 353 via a Lewis-acid catalyzed Mukaiyama aldol reaction in DCM to obtain diastereomers 354 and 355 in 71% and 14% yields, respectively, with 77![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23 and 79
23 and 79![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21. Diastereomer 354 was reacted with CSA and methanol, which resulted in the synthesis of 5-epi-diospongin A 356a in 66% yield and diospongin A 356b in 18% yield. Similarly, diastereomer 355 was further reacted with trifluoroacetic acid in the presence of DCM to obtain 356a in 69% yield and diospongin A 356b in 19% yield. Later on, compound 356a underwent Mitsunobu inversion to synthesize diospongin A 356b in 67% yield (Scheme 47).
21. Diastereomer 354 was reacted with CSA and methanol, which resulted in the synthesis of 5-epi-diospongin A 356a in 66% yield and diospongin A 356b in 18% yield. Similarly, diastereomer 355 was further reacted with trifluoroacetic acid in the presence of DCM to obtain 356a in 69% yield and diospongin A 356b in 19% yield. Later on, compound 356a underwent Mitsunobu inversion to synthesize diospongin A 356b in 67% yield (Scheme 47).
Kong et al.150 reported the efficient and concise total synthesis of grayanotoxin III with a highly enantioselective approach. For this purpose, they treated compound 357 with phosphorus tribromide in DMF and chloroform followed by its reaction with p-TsOH and HC(OMe)3 in the presence of methanol, which gave compound 358 in 54% yield. Compound 358 was then made to react with compound 359 and 360 in the presence of TMSOTf and MTBE at −78 °C via the Mukaiyama aldol reaction followed by the Hosomo–Sakurai reaction, which resulted in the synthesis of aldol adduct 361 in 58% yield. It was then transformed into compound 362 by undergoing a number of reactions. Compound 362 was further subjected to the O-Diels–Alder reaction upon treatment with compound 363, which led to the synthesis of compound 364 in 65% yield. Compound 364 was then finally treated over several steps, thereby giving target molecule 365 in 51% yield (Scheme 48).
Various natural products containing heterocyclic rings have been synthesized by numerous research groups and their evaluation against bacterial, fungal, viral and cancer cell lines has been carried out. In this regard, Moriyama et al.151 in 2021 reported the chiral total synthesis of both enantiomers of naturally occurring polymuthipyranone B by employing the chiral BINOL reagent mediated Mukaiyama aldol reaction as key step. Moreover, they carried out the synthesis of their three synthetic counterparts. Biological evaluation of these naturally occurring compounds revealed that the enantiomers of polymuthipyranone B were highly active against both cell lines of bacteria i.e., ATCC 43300 and ATCC 33591. The Mukaiyama aldol reaction was utilized for the synthesis of these biologically active scaffolds. Their synthetic scheme involved the reaction of decanal 366 with Chan's diene 367 via the Mukaiyama aldol reaction involving Ti(OiPr)4, and (S)-BINOL in tetrahydrofuran, followed by treatment with HCl, which gave β-ketoester 368a in 78% yield with 98% enantiomeric excess. 368a was further hydrolyzed with potassium hydroxide followed by its reaction with carboxylic acid 370 to carry out the incorporation of the acyl group by using the EDCL reagent in the presence of dimethylaminopyridine and dichloromethane, which furnished the (R)-enantiomer of polymuthipyranone B 371a in 48% yield with 98% enantiomeric excess. Similarly, the (S)-enantiomer of polymuthipyranone B 371b was synthesized in 50% yield with more than 98% enantiomeric excess via similar steps and by employing (R)-BINOL involving Mukaiyama aldol reaction (Scheme 49).
Pyran substituted compounds are generally found in nature,152 involving members of kavalactone family and (−)-pestalotin. Kimura and Tamura's group for the first time, isolated (−)-pestalotin from Pesalotia cryptomeriaecola and found that (−)-pestalotin work in harmony with gibberellin to promote the growth of plants. Owing to their remarkable usage, there have been numerous reports on the total synthesis of naturally occurring (−)-pestalotin. Moriyama et al.153 in 2020 reported the chiral synthesis of naturally occurring (−)-pestalotin diastereomers by using readily accessible chiral (R)-glycidol as the starting reagent. They devised two synthetic routes for the synthesis of this naturally occurring molecule which involved the catalytic asymmetric Mukaiyama aldol reaction and the catalytic diastereoselective Mukaiyama aldol reaction. The synthetic scheme began with the synthesis of (S)-α-benzyloxy aldehyde 373 from (R)-glycidol by undergoing several steps. Benzyloxy aldehyde 373 was then reacted with Chan's diene 374 via the catalytic asymmetric Mukaiyama aldol reaction by using Ti(iOPr)4, (S)-BINOL and lithium chloride, which resulted in the synthesis of aldol adducts 375a and 375b in 31% yield with high dr = 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7. Diastereomer 375a was further reacted with potassium hydroxide–methanol–water followed by treatment with Me2SO4 and acetone to obtain (1S,6S)-376 in 88% yield with a 91
7. Diastereomer 375a was further reacted with potassium hydroxide–methanol–water followed by treatment with Me2SO4 and acetone to obtain (1S,6S)-376 in 88% yield with a 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 diastereoselectivity ratio. Compound 376 was then reacted with hydrogen and palladium hydroxide/C catalyst to obtain the target molecule i.e., pestalotin 377a in 60% yield along with unnatural diastereomer 377b in trace amounts. Similarly, (−)-pestalotin was synthesized by employing the titanium chloride catalyzed Mukaiyama aldol reaction using DCM as solvent. The Mukaiyama aldol adducts 376a and 376b were then subjected to deprotection in similar conditions to synthesize pestalotin 377a (Scheme 50).
9 diastereoselectivity ratio. Compound 376 was then reacted with hydrogen and palladium hydroxide/C catalyst to obtain the target molecule i.e., pestalotin 377a in 60% yield along with unnatural diastereomer 377b in trace amounts. Similarly, (−)-pestalotin was synthesized by employing the titanium chloride catalyzed Mukaiyama aldol reaction using DCM as solvent. The Mukaiyama aldol adducts 376a and 376b were then subjected to deprotection in similar conditions to synthesize pestalotin 377a (Scheme 50).
Huffman et al.154 in 2021 attempted to report the total synthesis of merilactone A by carefully controlling chirality over the attached ring system. Fused rings having tetrahedral bridgeheads are used in the synthesis of various natural products. The concise and efficient total synthesis of naturally occurring merilactone A was reported by Huffmann et al. in 2021 by employing the Mukaiyama aldol reaction as one of its key steps. The synthesis began with the heterodimerization reaction between thioester butenolide 378 with lithium furan-2-olate 379 in tetrahydrofuran, which gave the product in a 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 diastereoselectivity ratio. The next step involved the reaction with TIPSOTf in the presence of trimethylamine and DCM followed by methylation, which was carried out by using potassium carbonate and acetone, thus giving compound 380 in 80% yield. Compound 380 was further treated with methyl vinyl ketone (MVK) via a titanium chloride catalyzed Mukaiyama–Michael reaction in the presence of dichloromethane, which led to the synthesis of compound 381 in 58% yield with a diastereoselectivity ratio of more than 20
5 diastereoselectivity ratio. The next step involved the reaction with TIPSOTf in the presence of trimethylamine and DCM followed by methylation, which was carried out by using potassium carbonate and acetone, thus giving compound 380 in 80% yield. Compound 380 was further treated with methyl vinyl ketone (MVK) via a titanium chloride catalyzed Mukaiyama–Michael reaction in the presence of dichloromethane, which led to the synthesis of compound 381 in 58% yield with a diastereoselectivity ratio of more than 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Compound 381 was then reacted over a number of reactions to synthesize target molecule 382 (Scheme 51).
1. Compound 381 was then reacted over a number of reactions to synthesize target molecule 382 (Scheme 51).
G-protein incorporated receptor 40 cells are found to be highly demonstrative in pancreatic cells, thus playing an important role in combating diabetes. MK-2305 has been found to play a leading role in the G-protein incorporated receptor 40.155 Considering the significance of MK-2305, Zhong et al.156 in 2022, reported an efficient and concise route towards its total synthesis. The first step involved the reaction between 3-chloro-4-fluorobenzotriflouride 383 and resorcinol 384 in the presence of potassium hydroxide and dimethylsulfoxide, which gave the phenol substituted compound 385 in 99% yield. Then, compound 385 was made to react with acrylonitrile by using potassium carbonate and tert-butanol via an oxy-Michael addition reaction. It was followed by treatment with trifluoroacetic acid in the presence of water via the Hoeben–Hoesch reaction, which led to the synthesis of chromanone 386 in 80% yield. Chromanone 386 was further subjected to the titanium chloride catalyzed Mukaiyama aldol reaction/elimination array, on reacting with silyl enol ether 387, which furnished compound 388 in 70% yield. It was then reacted over a number of steps, thus leading to the synthesis of MK-2305 389 (Scheme 52).
3. Conclusions
This review provides a broad overview of the syntheses of a diverse range of natural products, which involve the Mukaiyama aldol reaction as a major step in their synthetic methodologies, reported in the literature during the period 2020–2023. The aldol reaction is a Lewis-acid catalyzed reaction between silyl enol ether and carbonyl compounds which results in the synthesis of aldol adducts that are highly diastereoselective in nature and are generated in enantiomeric excess. The resulting aldol adducts are then utilized to carry out the total synthesis of various natural products. Here, a summary of the total synthesis of macrolides, polyketides, alkaloids, metabolites, terpenoids and depsipeptides, etc. utilizing the Mukaiyama aldol reaction is reported. We anticipate that our review will inspire researchers to advance further regarding the development of the Mukaiyama aldol reaction to carry out its beneficial aspects in organic synthesis.Conflicts of interest
There are no conflicts to declare.References
- T. Mukaiyama, K. Narasaka and K. Banno, New Aldol Type Reaction, Chem. Lett., 1973, 2, 1011–1014, DOI:10.1246/cl.1973.1011.
- L. Birkofer, A. Ritter and H. Vernaleken, Addition von silylierten Enolen an Benzoylchlorid und Benzaldehyd, Chem. Ber., 1966, 99, 2518–2520, DOI:10.1002/cber.19660990818.
- M. Teruaki and I. Akihiko, A Convenient Method For The Preparation Of Δ-Alkoxy-A,B-Unsaturated Aldehydes By Reaction Of Acetals With 1-Trimethylsiloxy-1,3-Butadiene, Chem. Lett., 1975, 4, 319–322, DOI:10.1246/cl.1975.319.
- I. Akihiko and M. Teruaki, New Method For The Preparation Of Polyenals From Δ-Alkoxy-A,B-Unsaturated Aldehydes, Chem. Lett., 1975, 4, 1167–1170, DOI:10.1246/cl.1975.1167.
- I. Akihiko and M. Teruaki, A New Method for the Preparation of δ-Alkoxy-α,β-unsaturated Aldehydes and Polyenals, Bull. Chem. Soc. Jpn., 1977, 50, 1161–1168, DOI:10.1246/bcsj.50.1161.
- M. Cordes and M. Kalesse, Very Recent Advances in Vinylogous Mukaiyama Aldol Reactions and Their Applications to Synthesis, Molecules, 2019, 24, 3040, DOI:10.3390/molecules24173040.
- T. Mukaiyama, K. Banno and K. Narasaka, New cross-aldol reactions. Reactions of silyl enol ethers with carbonyl compounds activated by titanium tetrachloride, J. Am. Chem. Soc., 1974, 96, 7503–7509, DOI:10.1021/ja00831a019.
- K. Saigo, M. Osaki and T. Mukaiyama, A New Method For The Preparation Of B-Hydroxyesters. The Titanium Tetrachloride-Promoted Reaction Of Ketene Alkyl Trialkylsilyl Acetals With Carbonyl Compounds, Chem. Lett., 1975, 4, 989, DOI:10.1246/cl.1975.989.
- H. Fujisawa, Y. Sasaki and T. Mukaiyama, Magnesium Bromide Diethyl Etherate Mediated Highly Diastereoselective Aldol Reaction between an Aldehyde and a Silyl Enol Ether, Chem. Lett., 2001, 30, 190–191, DOI:10.1246/cl.2001.190.
- T. Mukaiyama and K. Inomata, Reaction of Thioboronite. A Convenient Method for the Preparation of β-Hydroxyalkanethioates, Bull. Chem. Soc. Jpn., 1971, 44, 3215, DOI:10.1246/bcsj.44.3215.
- K. Inomata, M. Muraki and T. Mukaiyama, Reactions of Vinyloxyboranes. A Convenient Method for the Preparation of β-Hydroxy Thiolesters, Esters and Ketones, Bull. Chem. Soc. Jpn., 1973, 46, 1807, DOI:10.1246/bcsj.46.1807.
- T. Mukaiyama, K. Inomata and M. Muraki, Vinyloxyboranes As Synthetic Intermediates, J. Am. Chem. Soc., 1973, 95, 967, DOI:10.1021/ja00784a079.
- J. M. Lee, P. Helquist and O. Wiest, Diastereoselectivity in Lewis-acid-catalyzed Mukaiyama aldol reactions: A DFT study, J. Am. Chem. Soc., 2012, 134, 14973–14981, DOI:10.1021/ja3052975.
- H. C. Brown, R. K. Dhar, R. K. Bakshi, P. K. Pandiarajan and B. Singaram, Major Effect Of The Leaving Group In Dialkylboron Chlorides And Triflates In Controlling The Stereospecific Conversion Of Ketones Into Either [E]- Or [Z]-Enol Borinates, J. Am. Chem. Soc., 1989, 111, 3441–3442, DOI:10.1021/ja00191a058.
- A. Bernardi, C. Gennari, J. M. Goodman and I. Paterson, The Rational Design And Systematic Analysis Of Asymmetric Aldol Reactions Using Enol Borinates: Applications Of Transition State Computer Modelling, Tetrahedron: Asymmetry, 1955, 6, 2613, DOI:10.1016/0957-4166(95)00343-N.
- T. Mukaiyama, K. Banno and K. Narasaka, New cross-aldol reactions. Reactions of silyl enol ethers with carbonyl compounds activated by titanium tetrachloride, J. Am. Chem. Soc., 1974, 96, 7503–7509, DOI:10.1021/ja00831a019.
- T. Mukaiyama, Titantetrachlorid in der organischen Synthese, Angew. Chem., 1977, 89, 858–866, DOI:10.1002/ange.19770891205.
- O. Masahiro, M. Masahiro and M. Teruaki, A Convenient Method For The Preparation Of Γ-Ketosulfides From Thioacetals, Chem. Lett., 1985, 14, 1871–1874, DOI:10.1246/cl.1985.1871.
- I. Ojima and S. Inaba, Asymmetric synthesis of β-lactams. I. The reaction of dimethylketene silyl acetal with (S)-alkylidene(1-arylethyl)amines promoted by titanium tetrachloride, Tetrahedron Lett., 1980, 21, 2077–2080, DOI:10.1016/S0040-4039(00)71491-2.
- M. Oishi, S. Aratake and H. Yamamoto, Remarkable Enhancement of Catalyst Activity of Trialkylsilyl Sulfonates on the Mukaiyama Aldol Reaction: A New Approach Using Bulky Organoaluminum Cocatalysts, J. Am. Chem. Soc., 1998, 120, 8271–8272 CrossRef CAS . https://cir.nii.ac.jp/crid/1360855571114333824.
- A. Hosomi, M. Endo and H. Sakurai, Allylsilanes As Synthetic Intermediates. Ii. Syntheses Of Homoallyl Ethers From Allylsilanes And Acetals Promoted By Titanium Tetrachloride's, Chem. Lett., 1976, 941–942, DOI:10.1246/cl.1976.941.
- G. Casiraghi, L. Battistini, C. Curti, G. Rassu and F. Zanardi, The Vinylogous Aldol and Related Addition Reactions: Ten Years of Progress, Chem. Rev., 2011, 111, 3076–3154, DOI:10.1021/cr100304n.
- S. Murata, M. Suzuki and R. Noyori, Trialkylsilyl triflates. 5. A stereoselective aldol-type condensation of enol silyl ethers and acetals catalyzed by trimethylsilyl trifluoromethanesulfonate, J. Am. Chem. Soc., 1980, 102, 3248–3249, DOI:10.1021/ja00529a062.2.
- (a) A. Lubineau, Water-promoted organic reactions: aldol reaction under neutral conditions, J. Org. Chem., 1986, 51, 2142–2144, DOI:10.1021/jo00361a045; (b) T. Kitanosono and S. Kobayashi, Mukaiyama Aldol Reactions in Aqueous Media, Adv. Synth. Catal., 2013, 355, 3095–3118, DOI:10.1002/adsc.201300798.
- A. T. Nielsen and W. J. Houlihan, The Aldol Condensation, Org. React., 1968, 16, 1–438, DOI:10.1002/0471264180.or016.01.
- T. Mukaiyama, The Directed Aldol Reaction, Org. React., 1982, 28, 203–331, DOI:10.1002/0471264180.or028.03.
- A. Hosomi and H. Sakurai, Syntheses Of Γ,Δ-Unsaturated Alcohols From Allylsilanes And Carbonyl Compounds In The Presence Of Titanium Tetrachloride, Tetrahedron Lett., 1976, 17, 295–1298, DOI:10.1016/S0040-4039(00)78044-0.
- R. D. Norcross and I. Paterson, Total synthesis of bioactive marine macrolides, Chem. Rev., 1995, 95, 2041–2114, DOI:10.1021/cr00038a012.
- B. Schetter and R. Mahrwald, Aldolreaktionen In Der Total synthese Von Polyketiden, Angew. Chem., 2006, 118, 7668–7687, DOI:10.1002/ange.200602780.
- L. Cai, L. B. Seiple and Q. Li, Modular Chemical Synthesis Of Streptogramin And Lankacidin Antibiotics, Acc. Chem. Res., 2021, 54, 1891–1908, DOI:10.1021/acs.accounts.0c00894.
- A. Zampella, M. V. D'Auria, L. Minale, C. Debitus and C. Roussakis, Callipeltoside A; A Cytotoxic Aminodeoxy Sugar-Containing Macrolide of a New Type from the Marine Lithistida Sponge Callipelta sp, J. Am. Chem. Soc., 1996, 118, 11085–11088, DOI:10.1021/ja9621004.
- I. Paterson and T. Paquet T, Total Synthesis and Configurational Validation of (+)-Phorbaside A, Org. Lett., 2010, 12, 2158–2161, DOI:10.1021/ol100693c.
- S. Tabassum, A. F. Zahoor, S. Ahmad, R. Noreen, S. G. Khan and H. Ahmad, Cross-coupling reactions towards the synthesis of natural products, Mol. Diversity, 2021, 26, 647–689, DOI:10.1007/s11030-021-10195-6.
- S. Faiz, M. Yousaf, A. F. Zahoor, S. A. R. Naqvi, A. Irfan and G. Zaman, Synthetic strategies towards the synthesis of polyphenolic natural products: Pauciflorol F and isopaucifloral F: A review, Synth. Commun., 2017, 47, 1121–1135, DOI:10.1080/00397911.2017.1303510.
- S. Munawar, A. F. Zahoor, S. Ali, S. Javed, M. Irfan, K. Kotwica-Mojzych and M. Mojzych, Mitsunobu Reaction: A Powerful Tool for the Synthesis of Natural Products: A Review, Molecules, 2022, 27, 6953, DOI:10.3390/molecules27206953.
- A. Mushtaq, A. F. Zahoor, M. Bilal, S. M. Hussain, M. Irfan, R. Akhtar, A. Irfan, M. Kotwica-Mojzych and M. Mojzych, Molecules, 2023, 28, 2722, DOI:10.3390/molecules28062722.
- S. B. J. Kan, K. K.-H. Ng and I. Paterson, The Impact of the Mukaiyama Aldol Reaction in Total Synthesis, Angew. Chem., Int. Ed., 2013, 52, 9097–9108, DOI:10.1002/anie.201303914.
- M. Kalesse, M. Cordes, G. Symkenberg and H.-H. Lu, The vinylogous Mukaiyama aldol reaction (VMAR) in natural product synthesis, Nat. Prod. Rep., 2014, 31, 563–5094, 10.1039/C3NP70102F.
- C. X. Wang, R. Ding, T. Jiang, J.-S. Tang, D. Hu, G.-D. Chen, F. Lin, K.-X. Hong Yao and H. Gao, Aldgamycins J–O 16-Membered Macrolides with a Branched Octose Unit from Streptomycetes sp. and Their Antibacterial Activities, J. Nat. Prod., 2016, 79, 2446–2454, DOI:10.1021/acs.jnatprod.6b00200.
- G. A. Ellestad, M. P. Kunstmann, J. E. Lancaster, L. A. Mitscher and G. Morton, Structures Of Methyl Aldgarosides A And B Obtained From The Neutral Macrolide Antibiotic Aldgamycin E., Tetrahedron, 1967, 23, 3893–3902, DOI:10.1016/S0040-4020(01)97899-8.
- M. Hayashi, M. Ohno, K. Kinoshita, S. Satoi, M. Suzuki and K. Harada, Mycinamicins, New Macrolide Antibiotics. Isolation And Structures Of Mycinamicin Aglycones, Mycinolide IV And V, J. Antibiot., 1981, 34, 346–349, DOI:10.7164/antibiotics.34.346.
- M. Hayashi, H. OHara, M. Ohno, H. Sakakibara, S. Satoi, K. Harada and M. Suzuki, Mycinamicins; New Macrolide Antibiotics. Isolation And Structures Of New 16-Membered Aglycones, Mycinolide IV and Protomycinolide IV, J. Antibiot., 1981, 34, 1075–1077, DOI:10.7164/antibiotics.34.1075.
- B. Herle, G. Spath, L. Schreyer and A. Furstne, Total Synthesis of Mycinolide IV and Path-Scouting for Aldgamycin N. Total Synthesis, Angew. Chem., Int. Ed., 2021, 60, 7893–7899, DOI:10.1002/ange.202016475.
- I. Paterson, S. M. Dalby, J. C. Roberts, G. J. Naylor, E. A. Guzmán, R. Isbrucker, T. P. Pitts, P. Linley, D. Divlianska, J. K. Reed and A. E. Wright, Leiodermatolide, A Potent Antimitotic Macrolide From The Marine Sponge Leiodermatium Sp., Angew. Chem., Int. Ed., 2011, 123, 3277–3281, DOI:10.1002/ange.201007719.
- M. A. Jordan and L. Wilson, Microtubules As A Target For Anticancer Drugs, Nat. Rev. Cancer, 2004, 4, 253–265 CrossRef CAS PubMed . https://www.nature.com/articles/nrc1317.
- K. H. Altmann and J. Gertsch, Anticancer Drugs from Nature – Natural Products as a Unique Source of New MicrotubuleStabilizing Agents, Nat. Prod. Rep., 2007, 24, 327–357, 10.1039/B515619J.
- Y. K. Siu, J. Roane and M. J. Krische, Total Synthesis of Leiodermatolide A via Transfer Hydrogenative Allylation, Crotylation, and Propargylation: Polyketide Construction beyond Discrete Allyl- or Allenylmetal Reagents, J. Am. Chem. Soc., 2021, 143, 10590–10595, DOI:10.1021/jacs.1c06062.
- L. Laureti, L. Song, S. Huang, C. Corre, P. Leblond, G. L. Challis and B. Aigle, Identification Of A Bioactive 51-Membered Macrolide Complex By Activation Of A Silent Polyketide Synthase In Streptomyces Ambofaciens, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 6258–6263, DOI:10.1073/pnas.1019077108.
- B. Aigle, G. Challis, L. Laureti, L. Song and P. Leblond, Stambomycin and Derivatives, Their Production and Their Use as Drugs, US pat. 20120142622A1, 2012, https://agris.fao.org/agris-search/search.do?recordID=FR20210176390.
- A. T. Keatinge-Clay, Stereocontrol Within Polyketide Assembly Lines, Nat. Prod. Rep., 2016, 33, 141–149, 10.1039/C5NP00092K.
- J. Lim, V. Chintalapudi, H. G. Gudmundsson, M. Tran, A. Bernasconi, A. Blanco, L. Song, G. L. Challis and E. A. Anderson, Synthesis Of The C1–C27 Fragment Of Stambomycin D Validates Modular Polyketide Synthase-Based Stereochemical Assignments, Org. Lett., 2021, 23, 7439–7444, DOI:10.1021/acs.orglett.1c02650.
- R. E. Taylor, Tedanolide And The Evolution Of Polyketideinhibitors Of Eukaryotic Protein Synthesis, Nat. Prod. Rep., 2008, 25, 854–861, 10.1039/B805700C.
- A. B. Smith III, C. M. Adams, S. A. Lodise Barbosa and A. P. Degnan, Total Synthesis of (+)-13-Deoxytedanolide, J. Am. Chem. Soc., 2003, 125, 350–351, DOI:10.1021/ja0289649.
- D. Lucke and M. Kalesse, Synthesis of Desepoxy-Tedanolide C, Chem.–Eur. J., 2021, 27, 7085–7089, DOI:10.1002/chem.202100553.
- C. L. Ventola, The Antibiotic Resistance Crisis, Part 1: Causes And Threats, Pharmacol. Ther., 2015, 40, 277–283 Search PubMed . https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4378521.
- S. Baker, A Return To The Pre-Antimicrobial Era?, Science, 2015, 347, 1064–1066, DOI:10.1126/science.aaa2868.
- K. H. Jang, S. J. Nam, J. B. Locke, C. A. Kauffman, D. S. Beatty, L. A. Paul and W. Fenical, Anthracimycin, A Potent Anthrax Antibiotic From A Marine-Derived Actinomycete, Angew. Chem., Int. Ed., 2013, 125, 7822–7824, DOI:10.1002/ange.201302749.
- P. Tian, W. Ye, X. Zhang, Y. Tong, P. Y. Qian and R. Tong, Ten-Step Asymmetric Total Syntheses Of Potent Antibiotics Anthracimycin And Anthracimycin B, Chem. Sci., 2022, 13, 12776–12781, 10.1039/D2SC05049H.
- K. Kubota, K. Nakahara, T. Ohtsuka, Y. Yoshida, J. Kawaguchi, Y. Fujita, Y. Ozeki, A. Hara, C. Yoshimura, H. Furukawa, H. Haruyama, K. Ichikawa, M. Yamashita, T. Matsuoka and I. Iijima, Identification of 2′-Phosphodiesterase, Which Plays a Role in the 2-5A System Regulated by Interferon, J. Biol. Chem., 2004, 279, 37832 CrossRef CAS PubMed.
- M. S. Maier, A. Shemet and D. Trauner, Assembling The Carbon Skeleton Of A-74528, Chem. Sci., 2022, 13, 8395–8400, 10.1039/D2SC01366E.
- M. Horikawa, M. Shimazu, M. Aibe, H. Kaku, M. Inai and T. Tsunoda, A Role Of Uroleuconaphins, Polyketide Red Pigments In Aphid, As A Chemopreventor In The Host Defense System Against Infection With Entomopathogenic Fungi, J. Antibiot., 2018, 71, 992–999 CrossRef CAS PubMed . https://www.nature.com/articles/s41429-018-0093-4.
- M. Horikawa, M. Inai, Y. Oguri, E. Kuroda, M. Tanaka, S. Suzuki, T. Ito, S. Takahashi, H. Kaku and T. Tsunoda, Isolation and Total Syntheses of Cytotoxic Cryptolactones A1, A2, B1, and B2: α,β-Unsaturated δ-Lactones from a Cryptomyzus sp. Aphid, J. Nat. Prod., 2014, 77, 2459–2464, DOI:10.1021/np500542x.
- M. Inai, Y. Oguri, M. Horikava, H. Kaku, S. Suzuki, K. Kitamura and T. Tsunoda, Total Syntheses and Cytotoxic Evaluations of Cryptolactones A1, A2, B1, B2, and Their Derivatives. Total Syntheses and Cytotoxic Evaluations of Cryptolactones A1, A2, B1, B2, and Their Derivatives, Chem. Pharm. Bull., 2020, 68, 380–383 CrossRef PubMed.
- E. Gäumann, R. Hütter, W. Keller-Schierlein, L. Neipp, V. Prelog and H. Zähner, Stoffwechselprodukte von actnomyceten. Mitteilung. Lankamycin und lankacidin, Helv. Chim. Acta, 1960, 43, 601–606 CrossRef.
- S. Harada, E. Higashide, T. Fugono and T. Kishi, Isolation and Structures of T-2636 Antibiotics, Tetrahedron Lett., 1969, 10, 2239–2244, DOI:10.1016/S0040-4039(01)88131-4.
- L. Cai, Y. Yao, S. K. Yeon and L. B. Seiple, Modular Approaches To Lankacidin Antibiotics, J. Am. Chem. Soc., 2020, 142, 15116–15126, DOI:10.1021/jacs.0c06648.
- S. Kondo, Y. Horiuchi, M. Hamada, T. Takeuchi and H. Umezawa, A New Antitumor Antibiotic, Bactobolin Produced By Pseudomonas, J. Antibiot., 1979, 32, 1069–1071, DOI:10.7164/antibiotics.32.1069.
- M. Ishizuka, S. Fukasawa, T. Masuda, J. Sato, N. Kanbayashi, T. Takeuchi and H. Umezawa, Antitumor Effect Of Bactobolin And Its Influence On Mouse Immune System And Hematopoietic Cells, J. Antibiot., 1980, 33, 1054–1106, DOI:10.7164/antibiotics.33.1054.
- P. Vojackova, L. Michalska, M. Necas, D. Shcherbakov, E. C. Bottger, J. Sponer, J. E. Sponer and J. Svenda, Stereocontrolled Synthesis Of (−)-Bactobolin A, J. Am. Chem. Soc., 2020, 142, 7306–7311, DOI:10.1021/jacs.0c01554.
- A. J. Watson, L. J. Fuller, D. J. Jeenes and D. B. Archer, Homologs Of Aflatoxin Biosynthesis Genes And Sequence Of AFIR In Aspergillus Oryzae And Aspergillus Sojae, Appl. Environ. Microbiol., 1999, 65, 307–310, DOI:10.1128/AEM.65.1.307-310.1999.
- K. Fukunaga, T. Misato, I. Ishii and M. Asakawa, Blasticidin, A New Anti-Phytopathogenic Fungal Substance. Part I, Bull. Agric. Chem. Soc. Jpn., 1955, 19, 181–188, DOI:10.1080/03758397.1955.10857286.
- S. Sakuda, M. Ono, K. Furihata, J. Nakayama, A. Suzuki and A. Isogai, Aflastatin A, A Novel Inhibitor Of Aflatoxin Production Of Aspergillus Parasiticus, From Streptomyces, J. Am. Chem. Soc., 1996, 118, 7855–7856, DOI:10.1021/ja960899d.
- D. A. Evans, J. J. Beiger, J. D. Burch, P. H. Fuller, F. Glorius, E. Kattnig, D. A. Thaisrivongs, W. C. Trenkle, J. M. Young and J. Zhang, Total Synthesis of Aflastatin A, J. Am. Chem. Soc., 2022, 144, 19953–19972, DOI:10.1021/jacs.2c08244.
- M. Tori, K. Nakashima and Y. Asakawa, Sesquiterpenes And A Phenolic Compound From The Liverwort Omphalanthus Filiformis, Phytochemistry, 1995, 38, 651–653, DOI:10.1016/0031-9422(94)00682-J.
- R. Chen, D. Qiu, X. Lei, Y. Niu, Y. Hua, H. Peng, T. Zeng and Y. Zhang, Total Synthesis and Assignment of the Absolute Configuration of (+)-Omphalic Acid, Org. Lett., 2021, 23, 6972–6976, DOI:10.1021/acs.orglett.1c02599.
- Q. W. Shi, X. H. Su and H. Kiyota, Chemical and Pharmacological Research of the Plants in Genus Euphorbia, Chem. Rev., 2008, 108, 4295–4327, DOI:10.1021/cr078350s.
- A. Vasas, A. D. Rédei, D. Csupor, J. Molnár and J. Hohmann, Diterpenes from European Euphorbia Species Serving as Prototypes for Natural-Product-Based Drug Discovery, Eur. J. Org Chem., 2012, 27, 5115–5130, DOI:10.1002/ejoc.201200733.
- M. J. Classen, M. N. A. Bocker, R. Roth, W. M. Amberg and E. M. Carreira, Enantioselective Total Synthesis of (+)-Euphorikanin A, J. Am. Chem. Soc., 2021, 143, 8261–8265, DOI:10.1021/jacs.1c04210.
- H. B. Wang, X. Y. Wang, L. P. Liu, G. W. Qin and T. G. Kang, Tigliane Diterpenoids From The Euphorbiaceae And Thymelaeaceae Families, Chem. Rev., 2015, 115, 2975–3011, DOI:10.1021/cr200397n.
- C. Cui, B. G. Dwyer, C. Liu, D. Abegg, Z. J. Cai, D. G. Hoch, X. Yin, N. Qiu, J. Q. Liu, A. Adibekian and M. Dai, Total Synthesis and Target Identification of the Curcusone Diterpenes, J. Am. Chem. Soc., 2021, 143, 4379–4386, DOI:10.1021/jacs.1c00557.
- H. Itokawa and H. Morita, Cytotoxic and Antifungal Diterpenes from the Seeds of Alpinia galangal, Planta Med., 1988, 54, 117–120, DOI:10.1055/s-2006-962365.
- K. Dimas, C. Demetzos, M. Marsellos, R. Sotiriadou, M. Malamas and D. Kokkinopoulos, Cytotoxic Activity of Labdane Type Diterpenes Against Human Leukemic Cell Lines in vitro, Planta Med., 1988, 64, 208–211, DOI:10.1055/s-2006-957410.
- A. Adamkiewicz, I. Weglarz, A. Butkiewicz, M. Woyciechowska and J. Mlynarski, Lewis Acid-Catalyzed Stereoselective α-Addition of Chiral Aldehydes to Cyclic Dienol Silanes: Aqueous Synthesis of Chiral Butenolides, Adv. Synth. Catal., 2020, 362, 667–678, DOI:10.1002/adsc.201901218.
- R. A. Hill and J. D. Connolly, Triterpenoids, Nat. Prod. Rep., 2013, 30, 1028–1065, 10.1039/C3NP70032A.
- X. R. Peng, J. Q. Liu, L. S. Wan, X. N. Li, Y. X. Yanand and M. H. Qiu, Four New Polycyclic Meroterpenoids from Ganoderma cochlear, Org. Lett., 2014, 16, 5262–5265, DOI:10.1021/ol5023189.
- H. Shao, X. Gao, Z. T. Wang, Z. Gao and Y. M. Zhao, Divergent Biomimetic Total Syntheses of Ganocins A–C, Ganocochlearins C-D and Cochlearol T, Angew. Chem., 2020, 132, 7489–7494, DOI:10.1002/ange.202000677.
- F. Zhang and Y. M. Zhao, Divergent Total Syntheses Of Six Ganoderma Meroterpenoids: A Bioinspired Two-Phase Strategy, Synlett, 2020, 31, 1551–1554, DOI:10.1055/s-0040-1707898.
- F. P. Wang and L. P. Yan, Campylopin From Delphinium Campylocentrum, The First Hetidane C20-Diterpene, Suggests A New Alkaloid Biogenetic Pathway, Tetrahedron, 2007, 63, 1417–1420, DOI:10.1016/j.tet.2006.11.078.
- P. Tang, Q. H. Chen and F. P. Wang, Atropurpuran, A Novel Diterpene With An Unprecedented Pentacyclic Cage Skeleton, From Aconitum Hemsleyanum Var. Atropurpureum, Tetrahedron Lett., 2009, 50, 460–462, DOI:10.1016/j.tetlet.2008.11.028.
- C. H. Yang, X. C. Wang, Q. F. Tang, W. Y. Liu and J. H. Liu, A New Diterpenoid And A New Diterpenoid Alkaloid From Aconitum Coreanum, Helv. Chim. Acta, 2008, 91(4), 759–765, DOI:10.1002/hlca.200890078.
- T. Suzuki, T. Koyama, K. Nakanishi, S. Kobayashi and K. Tanino, Formal Total Synthesis of Atropurpuran, J. Org. Chem., 2020, 85, 10125–10135, DOI:10.1021/acs.joc.0c01462.
- Y. Fukuyama, A. Kuwayama and H. Minami, Garsubellin A Novel Polyprenylated Phloroglucin Derivative, Increasing Choline Acetyltransferase (CHAT) Activity In Postnatal Rat Septal Neuron Cultures, Chem. Pharm. Bull., 1997, 45, 947–949, DOI:10.1248/cpb.45.947.
- K. S. MacMillan, J. Naidoo, J. Liang, L. Melito, N. S. Williams, L. Morlock, P. J. Huntington, S. J. Estill, J. Longgood, G. L. Becker, S. L. McKnight, A. A. Pieper, J. K. De Brabander and J. M. Ready, Development of Proneurogenic, Neuroprotective Small Molecules, J. Am. Chem. Soc., 2011, 133, 1428–1437, DOI:10.1021/ja108211.
- D. Jang, M. Choi, J. Chen and C. Lee, Evolution Of A Synthetic Strategy For Garsubellin A, Chem.–Eur. J., 2022, 28, e202202383, DOI:10.1002/chem.202202383.
- R. Ciochina and R. B. Grossman, Polycyclic Polyprenylated Acylphloroglucinols, Chem. Rev., 2006, 106, 3963–3986, DOI:10.1021/cr0500582.
- A. I. Gurevich, V. N. Dobrynin, M. N. Kolosov, S. A. Popravko, I. D. Ryabova, B. K. Chernov, N. A. Derbentseva, B. E. Aizenman and A. D. Garagulya, Hyperforin An Antibiotic From Hypericum Perforatum, Antibiotiki., 1971, 16, 510–513 CAS . https://pubmed.ncbi.nlm.nih.gov/5000144/.
- S. Leisering, S. Ponath, K. Shakeri, A. Mavroskoufis, M. Kleoff, P. Vobnaker, S. Steinhauer, M. Weber and M. Christmann, Synthesis Of 3-Epi-Hypatulin B Featuring A Late-Stage Photooxidation In Flow, Org. Lett., 2022, 24, 4305–4309, DOI:10.1021/acs.orglett.2c00689.
- F. M. Zhang, S. Y. Zhang and Y. Q. Tu, Recent Progress In The Isolation, Bioactivity, Biosynthesis, And Total Synthesis Of Natural Spiroketals, Nat. Prod. Rep., 2018, 35, 75–104, 10.1039/C7NP00043J.
- R. Forestieri, C. E. Merchant, N. J. de Voogd, T. Matainaho, T. J. Kieffer and R. Andersen, Alotaketals A. And B., Sesterterpenoids From The Marine Sponge Hamigera Species That Activate The Camp Cell Signaling Pathway, Org. Lett., 2009, 11, 5166–5169, DOI:10.1021/ol902066e.
- J. Lee, J. Kim and H. Y. Lee, Au(I)-Catalyzed Cyclization of Epoxyalkynes to Allylic Alcohol Containing Spiroketals and Application to the Total Synthesis of (−)-Alotaketal A, Org. Lett., 2020, 22, 4073–4077, DOI:10.1021/acs.orglett.0c01130.
- D. A. Casteel, Peroxy natural products, Nat. Prod. Rep., 1992, 16, 289–312, 10.1039/NP9920900289.
- D. Z. Liu and J. K. Liu, Peroxy Natural Products, Nat. Prod. Bioprospect., 2013, 3, 161–206, DOI:10.1007/s13659-013-0042-7.
- M. D. Kerim, L. Evanno and L. Ferrie, Stereodivergent Total Syntheses of (+)-Mycaperoxides C, D, G Methyl Ester and (-)-Mycaperoxide B, Chem.–Eur. J., 2022, 29, E202203004, DOI:10.1002/chem.202203004.
- F. Tsurumi, Y. Miura, Y. Saito, K. Miyake, T. Fujie, D. J. Newman, B. R. O'Keefe, K. H. Lee and K. Nakagawa-Goto, Secondary Metabolites, Monoterpene–Polyketides Containing A Spiro[3.5]Nonane From Cryptocarya Laevigata, Org. Lett., 2018, 20, 2282–2286, DOI:10.1021/acs.orglett.8b00624.
- Y. Miura, Y. Saito and K. Nakagawa-Goto, Synthesis Of A Model Compound Of The Unique Meroterpenoids Cryptolaevilactones, Chem. Pharm. Bull., 2022, 70, 740–747, DOI:10.1248/cpb.c22-00455.
- J. Zeng, D. B. Zhang, P. P. Zhou, Q. L. Zhang, L. Zhao, J. J. Chen and K. Gao, Rauvomines A and B, Two Monoterpenoid Indole Alkaloids from Rauvolfia vomitoria, Org. Lett., 2017, 19, 3998–4001, DOI:10.1021/acs.orglett.7b01723.
- B. Wu, Z. J. Jiang, J. Tang, Z. Gao, H. Liang, B. Tang, J. Chen and K. Lei, Total Synthesis Study Of Rauvomines A And B: Construction Of The Pentacyclic Core Structure, Org. Chem. Front., 2020, 7, 1685–1689, 10.1039/C9QO01531K.
- T. S. Kam and K. Y. Loh, 5-Oxo-19,20-Dehydroervatamine From Leaves Of Tabernaemontana Corymbosa, Phytochemistry, 1993, 32, 1357–1358, DOI:10.1016/S0031-9422(00)95123-9.
- T.-S. Kam, K.-M. Sim and T.-M. Lim, Tronocarpine, a novel pentacyclic indole incorporating a seven-membered lactam moiety, Tetrahedron Lett., 2000, 41, 2733–2736, DOI:10.1016/S0040-4039(00)00250-1.
- A. Nakayama, T. Nakamura, T. Zaima, S. Fujimoto, S. Karanjit and K. Namba, Concise Total Synthesis of Tronocarpine, Angew. Chem., Int. Ed., 2021, 60, 635–639, DOI:10.1002/anie.202009966.
- N. Srivastava and H. J. Ha, Highly Efficient And Stereoselective Mukaiyama Aldol Reaction With Chiral Aziridine-2-Carboxaldehyde And Its Synthetic Applications, Asian J. Org. Chem., 2022, 11, e202100567, DOI:10.1002/ajoc.202100567.
- Y. Hirasawa, H. Morita and J. Kobayashi, Onesidines A.-C. New Alkaloids From Lycopodium Chinense, Tetrahedron, 2002, 58, 5483–5488, DOI:10.1016/S0040-4020(02)00520-3.
- Y. Hirasawa, J. Kobayashi and H. Morita, The Lycopodium Alkaloids, Heterocycles, 2009, 77, 679–729 CrossRef CAS PubMed . https://cir.nii.ac.jp/crid/1523951029759438976.
- R. Akhtar, A. F. Zahoor, A. Rasul, M. Ahmad, M. N. Anjum, M. Ajmal and Z. Raza, Design, synthesis, in-silico study and anticancer potential of novel n-4-piperazinyl-ciprofloxacin-aniline hybrids, Pak. J. Pharm. Sci., 2019, 32, 2215–2222 Search PubMed . https://www.researchgate.net/profile/Azhar-Rasul/publication/336115349.
- I. Shahzadi, A. F. Zahoor, A. Rasul, A. Mansha, S. Ahmad and Z. Raza, Synthesis, Hemolytic Studies, and In Silico Modeling of Novel Acefylline–1, 2, 4-Triazole Hybrids as Potential Anti-cancer Agents against MCF-7 and A549, ACS Omega, 2021, 6, 11943–11953, DOI:10.1021/acsomega.1c00424.
- T. Kurose, C. Tsukano, T. Nanjo and Y. Takemoto, Total Synthesis Of Lyconesidine B, A Lycopodium Alkaloid With An Oxygenated, Amine-Type Fawcettimine Core, Org. Lett., 2020, 23, 676–681, DOI:10.1021/acs.orglett.0c03816.
- J. Y. Zhu, Y. Liu, Z. J. Liu, H. Wang and H. W. Zhang, Bioactive Nitrogenous Secondary Metabolites From The Marine Sponge Genus Haliclona, Mar. Drugs, 2019, 17, 682, DOI:10.3390/md17120682.
- R. Sakai, T. Higa, C. W. Jefford, G. Benardinelli and A. Manzamine, A Novel Antitumor Alkaloid From A Sponge, J. Am. Chem. Soc., 1986, 108, 6404–6405, DOI:10.1021/ja00280a055.
- S. P. Luo, X. Z. Huang, L. D. Guo and P. Q. Huang, Catalytic Asymmetric Total Synthesis of Macrocyclic Marine Natural Product (−)-Haliclonin A, Chin. J. Chem., 2020, 38, 1723–1726, DOI:10.1002/cjoc.202000291.
- P. Krastel, S. Roggo, M. Schirle, N. T. Ross, F. Perruccio, P. Aspesi, T. Aust, K. Buntin, D. Estoppey and B. Liechty, Nannocystin A An Elongation Factor 1 Inhibitor From Myxobacteria With Differential Anti-Cancer Properties, Angew. Chem., Int. Ed., 2015, 54, 10149–10154, DOI:10.1002/anie.201505069.
- H. Hoffmann, H. Kogler, W. Heyse, H. Matter, M. Caspers, D. Schummer, C. Klemke-Jahn, A. Bauer, G. Penarier and L. Debussche, Discovery, Structure Elucidation, And Biological Characterization Of Nannocystin A, A Macrocyclic Myxobacterial Metabolite With Potent Antiproliferative Properties, Angew. Chem., Int. Ed., 2015, 54, 10145–10148, DOI:10.1002/anie.201411377.
- T. Zhang, S. Miao, M. Zhang, W. Liu, L. Wang and Y. Chen, Optimization Of Two Steps In Scale-Up Synthesis Of Nannocystin A, Mar. Drugs, 2021, 19, 198, DOI:10.3390/md19040198.
- R. Fudou, T. Iizuka, S. Sato, T. Ando, N. Shimba, S. Yamanaka and A. Haliangicin, A Novel Antifungal Metabolite Produced By A Marine Myxobacterium, J. Antibiot., 2001, 54, 153–156, DOI:10.7164/antibiotics.54.149.
- S. Felder, S. Dreisigacker, S. Kehraus, E. Neu, G. Bierbaum, P. R. Wright, D. Menche, T. F. Schäberle and G. M. König, Salimabromide: Unexpected Chemistry from the Obligate Marine Myxobacterium Enhygromyxa salina, Chem.–Eur. J., 2013, 19, 9319–9324, DOI:10.1002/chem.201301379.
- H. H. Lu, K. J. Gan, F. Q. Ni, Z. Zhang and Y. Zhu, Concise Total Synthesis Of Salimabromide, J. Am. Chem. Soc., 2022, 144, 18778–18783, DOI:10.1021/jacs.2c08337.
- D. J. Newman and G. M. Cragg GM, Natural Products As Sources Of New Drugs Over The Nearly Four Decades from 01/1981 to 09/2019, J. Nat. Prod., 2020, 83, 770–803, DOI:10.1021/acs.jnatprod.9b01285.
- S. Kitani, T. Ueguchi, Y. Igarashi, K. Leetanasaksakul, A. Thamchaipenet and T. Nihira, Rakicidin F, A New Anti Bacterial Cyclic Depsipeptide From A Marine Sponge-Derived Streptomyces Sp, J. Antibiot., 2018, 71, 139–141 CrossRef CAS PubMed . https://www.nature.com/articles/ja201792.
- F. Han, G. Liu, X. Zhao, S. Du, Y. Ding, Q. Zhang, H. Deng, L. Wang and Y. Chen, Total Synthesis And Stereochemical Assignment Of Rakicidin F., Org. Bio. Chem., 2022, 20, 4135–4140, 10.1039/D2OB00692H.
- J. Hu, D. Wunderlich, I. Sattler, X. Feng, S. Grabley and R. Thiericke, Rakicidin C, A New Cyclic Depsipeptide From Streptomyces Sp, Eur. J. Org. Chem., 2000, 19, 3353–3356, DOI:10.1002/1099-0690(200010)2000:19<3353::AID-EJOC3353>3.0.CO;2-E.
- A. L. Demain, Pharmaceutically Active Secondary Metabolites Of Microorganisms, Appl. Microbiol. Biotechnol., 1999, 52, 455–463, DOI:10.1007/s002530051546.
- F. Han, G. Liu, C. Jin, J. Wang, J. Liu, L. Wang and Y. Chen, Total Synthesis And Determination Of The Absolute Configuration Of Rakicidin C, Org. Lett., 2021, 23, 7069–7073, DOI:10.1021/acs.orglett.1c02476.
- O. Holst, in Structure Of The Lipopolysaccharide Core Region In Bacterial Lipopolysaccharide, ed. Y. A. Knirel and M. A. Valvano, Springer-Verlag, Vienna, 2011, DOI:10.1007/978-3-7091-0733-1_2.
- L. Cipolla, L. Gabrielli, D. Bini, L. Russo and N. K. Shaikh, A Critical Monosaccharide For Bacteria Viability, Nat. Prod. Rep., 2010, 27, 1618–1629, 10.1039/C004750N.
- J. Wang, J. Rong, Q. Lou, Y. Zhu and Y. Yang, Synthesis Of L-Glycero- And D-Glycero-D-Manno-Heptose Building Blocks For Stereoselective Assembly Of The Lipopolysaccharide Core Trisaccharide Of Vibrio Parahemolyticus O2, Org. Lett., 2020, 22, 8018–8022, DOI:10.1021/acs.orglett.0c02961.
- J. Kobayashi and M. Tsuda, Amphidinolides, Bioactive Macrolides From Symbiotic Marine Dinoflagellates, Nat. Prod. Rep., 2004, 21, 77–93, 10.1039/B310427N.
- L. Ferrie, I. Ciss, J. Fenneteau, S. Vallerotto, M. Seck and B. Figader, Amphidinolides F And C2: An Odyssey In Total Synthesis, J. Org. Chem., 2022, 87, 1110–1123, DOI:10.1021/acs.joc.1c02458.
- B. Wyckoff, J. G. Jones, J. S. Condeelis and J. E. Segall, A Critical Step In Metastasis: In Vivo Analysis Of Intravasation At The Primary Tumor, Cancer Res., 2000, 60, 2504–2511 Search PubMed . https://aacrjournals.org/cancerres/article/60/9/2504/507281/A-Critical-Step-in-Metastasis-In-Vivo-Analysis-of.
- R. Choudhury and D. S. Reddy, Total Synthesis Of A Hypothetical Macroketone Of Migrastatin, Eur. J. Org Chem., 2021, 21, 3050–3053, DOI:10.1002/ejoc.202100484.
- K. D. Mcbrien, R. L. Berry, S. E. Lowe, K. M. Neddermann, I. Bursuker, S. Huang, S. R. Klohr and J. E. Leet, Rakicidins, New Cytotoxic Lipopeptides From Micromonospora Sp. Fermentation, Isolation And Characterization, J. Antibiot., 1995, 48, 1446–1452, DOI:10.7164/antibiotics.48.1446.
- F. Han, G. Liu, X. Zhang, Y. Ding, L. Wang, Y. Wu, Y. Chen and Q. Zhang, Total Synthesis And Structure Revision Of Boholamide A, Org. Lett., 2021, 23, 4976–4980, DOI:10.1021/acs.orglett.1c01382.
- M. Shimura, S. Kondo, T. Nishi, E. Akita, K. Satoh and T. Hara, Isolation And Characterization Of Ericamycin, A New Antibiotic, J. Antibiot., 1966, 19, 51–55 CAS . https://cir.nii.ac.jp/crid/1572543024220844416.
- F. X. Wang, J. L. Yan, Z. Liu, T. Zhu, Y. Liu, S. C. Ren, W. X. Lv, Z. Jin and Y. R. Chi, Assembly Of Multicyclic Isoquinoline Scaffolds From Pyridines: Formal Total Synthesis Of Fredericamycin A, Chem. Sci., 2021, 12, 10259, 10.1039/D1SC02442F.
- N. U. Sata and N. Fusetani, Amaminols A and B, New Bicyclic Amino Alcohols From An Unidentified Tunicate Of The Family Polyclinidae, Tetrahedron Lett., 2000, 41, 489–492, DOI:10.1016/S0040-4039(99)02098-5.
- M. Sinast, B. Claasen, Y. Stockl, A. Greulich, A. Zens, A. Baro and S. Laschat, Synthesis of Highly Functionalized Hydrindanes via Sequential Organocatalytic Michael/Mukaiyama Aldol Addition and Telescoped Hydrozirconation/Cross-Coupling as Key Steps: En Route to the AB System of Clifednamides, J. Org. Chem., 2021, 86, 7537–7551, DOI:10.1021/acs.joc.1c00580.
- S. Takahashi, Y. Akita, T. Nakamura and H. Koshino, Total Synthesis Of Curvulone B And A Proposed Structure For Dothiorelone B; Determination Of The Configuration Of Curvulone B And Structural Revision Of Phomopsin A, Tetrahedron: Asymmetry, 2012, 23, 952–958, DOI:10.1016/j.tetasy.2012.06.009.
- S. Banoth, U. M. Choudhury, K. Marumudi, A. C. Kunwar and D. K. Mohapatra, Concise Total Synthesis Of Curvulone B., Synlett, 2021, 32, 685–688, DOI:10.1055/a-1297-6838.
- Y. Gao, A. Vogt, C. J. Forsyth and K. Koide, Comparison Of Splicing Factor 3b Inhibitors In Human Cells, ChemBioChem, 2013, 14, 49–52, DOI:10.1002/cbic.201200558.
- R. K. Bressin, S. Osman, I. Pohorilets, U. Basu and K. Koide, Total Synthesis Of Meayamycin B, J. Org. Chem., 2020, 85(7), 4637–4647, DOI:10.1021/acs.joc.9b03370.
- K. Vaithegi and K. R. Prasad, Total Synthesis of (+)-Diospongin A, Tetrahedron, 2020, 76, 131625, DOI:10.1016/j.tet.2020.131625.
- L. Kong, H. Yu, M. Deng, F. Wu, Z. Jiang and T. Luo, Total Synthesis Of (−)-Grayanotoxin III, J. Am. Chem. Soc., 2022, 144, 5268–5273, DOI:10.1021/jacs.2c01692.
- M. Moriyama, X. Liu, Y. Enoki, K. Matsumoto and Y. Tanabe, Asymmetric Total Syntheses Of Both Enantiomers Of Polymuthipyranone B And Its Unnatural Analogues: Evaluation Of Anti-MRSA Activity And Its Chiral Discrimination, Pharmaceuticals, 2021, 14, 938 CrossRef CAS PubMed . https://www.mdpi.com/1424-8247/14/9/938.
- M. T. Davies-Coleman and D. E. A. Rivett, Naturally Occurring 6-Substituted 5,6-Dihydro-A-Pyrones, Progress In The Chemistry Of Organic Natural Products, ed. W. Herz, H. Grisebach, G.W. Kirby and C. Tamm, Springer, New York, NY, USA, 1989, vol. 55, pp. 1–35, ISBN 978-3-7091-9004-3 Search PubMed.
- M. Moriyama, K. Nakata, T. Fujiwara and Y. Tanabe, Divergent Asymmetric Total Synthesis Of All Four Pestalotin Diastereomers From (R)-Glycidol, Molecules, 2020, 25, 394 CrossRef CAS PubMed . https://www.mdpi.com/1420-3049/25/2/394.
- B. J. Huffman, T. Chu, Y. Hanaki, J. J. Wong, S. Chen, K. N. Houk and R. A. Shenvi, Stereodivergent Attached-Ring Synthesis via Non-Covalent Interactions: A Short Formal Synthesis of Merrilactone A, Angew. Chem., Int. Ed., 2022, 61, e202114514, DOI:10.1002/anie.202114514.
- C. Miller, M. J. Pachanski, M. E. Kirkland, D. T. Kosinski, J. Mane, M. Bunzel, J. Cao, S. Souza, B. Thomas-Fowlkes, J. Di Salvo, A. B. Weinglas, X. Li, R. W. Myers, K. Knagge, P. E. Carrington, W. K. Hagmann and M. E. Trujillo, GPR40 Partial Agonist MK-2305 Lower Fasting Glucose In The Goto Kakizaki Rat Via Suppression Of Endogenous Glucose Production, PLoS One, 2017, 12, e0176182, DOI:10.1371/journal.pone.0176182.
- Y. L. Zhong, Y. Ji, H. Wang, X. Wang and D. R. Gauthier, Highly Enantioselective Rhodium-Catalyzed Transfer Hydrogenation of Tetrasubstituted Olefins: Application towards the Synthesis of GPR40 Agonist MK-2305, Org. Lett., 2022, 24, 3254–3258, DOI:10.1021/acs.orglett.2c01021.
| This journal is © The Royal Society of Chemistry 2023 |