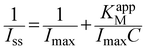Open Access Article
Open Access ArticlePreparation of electrochemical horseradish peroxidase biosensor with black phosphorene–zinc oxide nanocomposite and their applications
Feng Yang†
ab,
Yijing Ai†a,
Xiaoqing Li*c,
Lisi Wanga,
Zejun Zhanga,
Weipin Ding*b and
Wei Sun *a
*a
aKey Laboratory of Laser Technology and Optoelectronic Functional Materials of Hainan Province, Key Laboratory of Functional Materials and Photoelectrochemistry of Haikou, College of Chemistry and Chemical Engineering, Hainan Normal University, Haikou 571158, China. E-mail: sunwei@hainnu.edu.cn
bHaikou Marine Geological Survey Center, China Geological Survey, Haikou, 571127, China. E-mail: gzsdingwp@126.com
cCollege of Health Sciences, Shandong University of Traditional Chinese Medicine, Jinan, 250355, China. E-mail: l1x2q3_li@126.com
First published on 1st November 2023
Abstract
In this work, a novel and sensitive electrochemical biosensor was constructed based on a black phosphorene (BP) and nanosized zinc oxide (ZnO@BP) nanocomposite as a modifier, which was used for the immobilization of horseradish peroxidase (HRP) on a carbon ionic liquid electrode (CILE). The ZnO@BP nanocomposite was synthesized by a simple in situ hydrothermal method with stripped black phosphorus nanoplates and ZnO. The ZnO@BP and HRP-modified electrode was developed by a casting method. ZnO@BP with highly conductivity, large surface area and good biocompatibility could maintain the bioactivity of HRP and accelerate the electron transfer rate. Cyclic voltammetry was used to study the direct electrochemistry of HRP on the Nafion/HRP/ZnO@BP/CILE with the appearance of a pair of distinct redox peaks. The constructed electrochemical HRP biosensor exhibited excellent electrocatalytic effects on the reduction of trichloroacetic acid and sodium nitrite. Real samples were detected with satisfactory results, which demonstrated the potential applications of this electrochemical HRP biosensor.
1. Introduction
Two-dimensional nanomaterials have attracted extensive attention and research interest due to the large specific surface area, unique surface energy and high interfacial reaction activity, which demonstrate a wide range of applications in supercapacitance, photocatalysis, and biosensing.1–3 In the field of electrochemical biosensors, the electrode interface modified with two-dimensional nanomaterials not only exhibits high affinity and good biocompatibility for biomolecules, but also maintains the activity of biomolecules. Furthermore, the excellent electrical conductivity can significantly enhance the current signal and improve the detection sensitivity.4 In 2014, single-layer and few-layer black phosphorene (BP) nanosheets were first mechanically stripped from bulk black phosphorus crystals.5 The synthesis of BP, a new member of the two-dimensional material family, and BP-related composites with high chemical activity, excellent carrier mobility, and anisotropic electrical and thermal conductivities has become a new trend, which act as promising materials for sensing applications.6–8 For example, Niu et al. used a BP-modified glassy carbon electrode (GCE) for the sensitive voltammetric detection of rutin.9 Li et al. prepared an electrochemical biosensor based on BP and a poly(3,4-ethylenedioxythiophene)–poly(styrenesulfonate) composite with hemoglobin (Hb).10 Zhao et al. developed an Hb-biosensor based on poly-L-lysine and BP to investigate the bioactivity of Hb.11 Shi et al. synthesized nitrogen-doped carbonized polymer dots anchoring few-layer BP and constructed an electrochemical DNA sensor for the determination of Escherichia coli O157: H7.12 Xiang et al. designed a novel electrochemical nanosensor based on BP for the sensitive voltammetric detection of ochratoxin A in beer and grape juice samples.13 Ge et al. used BP, Nafion and isopropanol composite-modified GCEs for the voltammetric detection of clenbuterol in bovine meat and bovine serum samples.14 Ramalingam et al. constructed a microfluidic aptasensor based on BP and gold nanocomposite-modified screen printed electrodes for the detection of okadaic acid.15As a commonly semiconductive nanomaterial, nano-zinc oxide (ZnO) has been widely selected for the preparation of electrochemical sensors due to its good biocompatibility, high surface activity and low cost.16,17 Ding et al. used a black phosphorous quantum dot (BPQD)-doped ZnO nanoparticle-modified GCE for the detection of hydrogen peroxide with excellent electrochemical properties.18 Li et al. synthesized a BP–ZnO nanohybrid by a simple one-step co-precipitation method with enhanced visible light photocatalytic activity.19 Li et al. constructed a NO2 sensing platform based on hetero-structured ZnO–BP composites to detect NO2 gas.20
In this paper, ZnO@BP was synthesized by an in situ hydrothermal method and modified on the surface of a carbon ionic liquid electrode (CILE) as a substrate electrode. Horseradish peroxidase (HRP) was immobilized on the ZnO@BP/CILE with Nafion as a film to obtain the biosensor. The synthesis of a ZnO@BP nanocomposite and the fabrication procedure of the electrochemical biosensor are shown in Scheme 1. Due to the synergistic properties between BP and ZnO, direct electrochemical behaviors of HRP on the modified electrode exhibited a pair of quasi-reversible redox peaks. Furthermore, the modified electrode was used for the electrocatalytic reduction of trichloroacetic acid (TCA) and sodium nitrite (NaNO2) with satisfactory results.
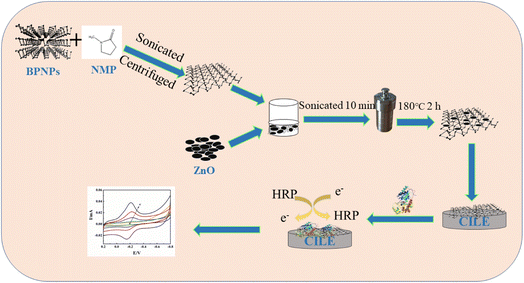 | ||
| Scheme 1 Experimental process diagram for the synthesis of ZnO@BP nanocomposite and the fabrication procedure of electrochemical biosensor. | ||
2. Experimental
2.1 Reagents
1-Hexylpyridinium hexafluorophosphate (HPPF6, >99%, Lanzhou Yulu Fine Chem. Co., Ltd., China), HRP (MW 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, Sinopharm Chem. Reagent Co., Ltd., China), black phosphorus nanoplate dispersion (BPNPs, Nanjing XFNANO Materials Tech. Co., Ltd., China), 1-methyl-2-pyrrolidone (NMP, 99.5%, Shanghai Aladdin Bio-Chem. Tech. Co., Ltd., China), TCA (Tianjin Kemiou Chem. Co., Ltd., China), nano-zinc oxide (ZnO, Nanjing XFNANO Materials Tech. Co., Ltd., China), NaNO2 (Yantai Sahe Chem, Co., Ltd., China) and graphite powder (particle size 30 μm, Shanghai Colloid Chem. Co., Ltd., China) were used as provided. The supporting electrolyte was 0.1 mol per L phosphate buffer solution (PBS) with different pH values. All the other reagents were of analytical grade, and ultra-pure water (Milli-Q, IQ-7000, Merck Millipore Co., Ltd., USA) was used throughout the experiments.
000, Sinopharm Chem. Reagent Co., Ltd., China), black phosphorus nanoplate dispersion (BPNPs, Nanjing XFNANO Materials Tech. Co., Ltd., China), 1-methyl-2-pyrrolidone (NMP, 99.5%, Shanghai Aladdin Bio-Chem. Tech. Co., Ltd., China), TCA (Tianjin Kemiou Chem. Co., Ltd., China), nano-zinc oxide (ZnO, Nanjing XFNANO Materials Tech. Co., Ltd., China), NaNO2 (Yantai Sahe Chem, Co., Ltd., China) and graphite powder (particle size 30 μm, Shanghai Colloid Chem. Co., Ltd., China) were used as provided. The supporting electrolyte was 0.1 mol per L phosphate buffer solution (PBS) with different pH values. All the other reagents were of analytical grade, and ultra-pure water (Milli-Q, IQ-7000, Merck Millipore Co., Ltd., USA) was used throughout the experiments.
2.2 Apparatus
All the electrochemical experiments were performed using a CHI 1040C electrochemical workstation (Shanghai Chenhua Instrument Co., Ltd., China). Electrochemical impedance spectroscopy (EIS) was carried out using a CHI 660E electrochemical workstation (Shanghai Chenhua Instrument Co., Ltd., China). A traditional three-electrode system was used with a self-made modified electrode (Nafion/HRP/ZnO@BP/CILE) as the working electrode, Ag/AgCl (saturated KCl solution) as the reference electrode, and a platinum wire as the auxiliary electrode. Transmission electron microscopy (TEM) was performed using a JEM-2010F (JEOL, Japan) with scanning electron microscopy (SEM) using a JSM-7100F (JEOL, Japan). X-ray diffraction (XRD) experiments were conducted using a D/Max-2500V X-ray diffractometer (Rigaku, Japan) with Cu-Kα radiation. X-ray photoelectron spectroscopy (XPS) was performed using an AXIS HIS 165 spectrometer (Kratos Analytical, UK). The N2 adsorption and desorption isotherms, surface area and pore size distribution of the ZnO@BP nanocomposite were tested using an Autosorb iQ Station 2 (Quantachrome Instruments, USA) in a liquid nitrogen environment.2.3 Synthesis of ZnO@BP nanocomposite
According to the reported procedure with slight modifications,21,22 10.0 mg BPNP powder and 10.0 mL of NMP were mixed in a mortar and then ground for 10 min. The mixture was sonicated for 8 h with ice cooling and then centrifuged at 8000 rpm for 20 min to remove unexfoliated BPNPs and obtain a BP suspension. ZnO@BP was synthesized by an in situ hydrothermal method as follows:23 first, 10.0 mL 1.0 mg mL−1 nano-ZnO dispersion was added into 10.0 mL as-obtained BP suspension. The mixture solution was sonicated for 10 min and sealed into a Teflon equipped stainless steel autoclave. After heating at 180 °C for 2 h, the product was removed, washed with ethanol solution and N2 saturated ultrapure water, respectively, and dried at 60 °C for 8 h under vacuum to obtain a ZnO@BP solid powder.2.4 Construction of the modified electrode
According to the ref. 24, the mass ratio of graphite powder and HPPF6 was 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to construct a CILE, which was used as the base electrode with the electrode surface polished before each use. In a nitrogen-filled glove box, 10.0 μL of 1.0 mg mL−1 ZnO@BP suspension was casted onto the surface of the electrode and dried naturally to obtain the ZnO@BP/CILE. Then, 10.0 μL of 15.0 mg mL−1 HRP solution and 10.0 μL of 0.5% Nafion solution were applied onto the modified surface in sequence and dried at room temperature to obtain the Nafion/HRP/ZnO@BP/CILE. The same method and procedure were used to fabricate other modified electrodes for comparison.
1 to construct a CILE, which was used as the base electrode with the electrode surface polished before each use. In a nitrogen-filled glove box, 10.0 μL of 1.0 mg mL−1 ZnO@BP suspension was casted onto the surface of the electrode and dried naturally to obtain the ZnO@BP/CILE. Then, 10.0 μL of 15.0 mg mL−1 HRP solution and 10.0 μL of 0.5% Nafion solution were applied onto the modified surface in sequence and dried at room temperature to obtain the Nafion/HRP/ZnO@BP/CILE. The same method and procedure were used to fabricate other modified electrodes for comparison.
2.5 Electrochemical investigations
Electrochemical measurements were investigated in 0.1 mol L−1 PBS with different pH values ranging from 2.0 to 8.0 by cyclic voltammetry (CV) at a scan rate of 0.1 V s−1. The buffer solutions were deoxygenated by highly pure nitrogen for 20 min before the measurements. Electrochemical behaviors of different modified electrodes were analyzed in 1.0 mmol L−1 K3[Fe(CN)6] and 0.5 mol L−1 KCl solution with EIS measurements in 10.0 mmol L−1 K3[Fe(CN)6]/K4[Fe(CN)6] and 0.1 mol L−1 KCl solution in the frequency range of 0.1–105 Hz.3. Results and discussion
3.1 Characterization of ZnO@BP nanocomposite
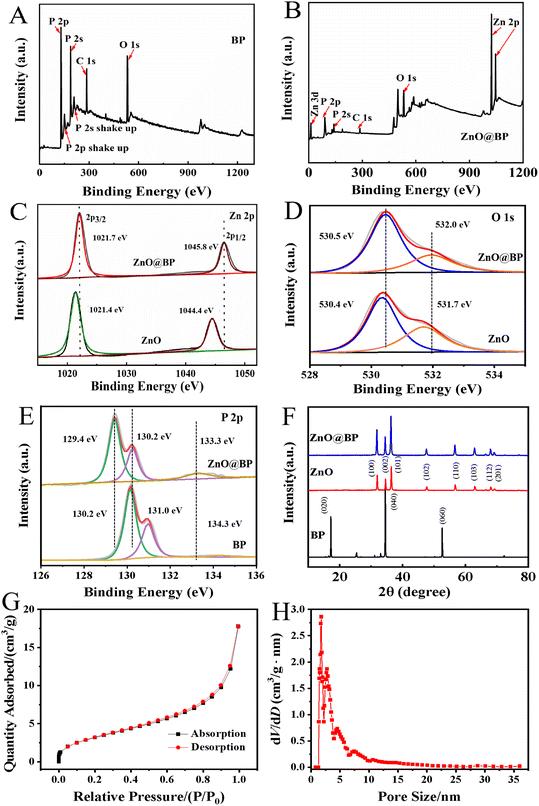
XPS was performed to illustrate the composition and chemical state of elements in materials. In Fig. 1A and B, the full survey spectra of BP and ZnO@BP confirmed the presence of O, P and Zn, in which the atom ratio of Zn 2p and P 2p was 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25. Fig. 1C showed the characteristic peaks of pure ZnO at 1021.4 eV and 1044.4 eV, which were ascribed to the binding energies of Zn 2p3/2 and Zn 2p1/2, respectively.25 Compared with pure ZnO, the characteristic peaks of Zn 2p3/2 and Zn 2p1/2 for ZnO@BP shifted 0.3 eV and 1.4 eV to a higher binding energy, respectively. Fig. 1D shows the O 1s XPS spectra of ZnO and ZnO@BP, and the peaks at 530.4 eV and 530.5 eV were assigned to the O2− ions in the Zn–O bonding of ZnO and ZnO@BP.26 Besides, the shoulder peaks at 531.7 eV and 532.0 eV were related to surface-adsorbed oxygen of materials, which also moved to higher binding energies. For XPS P 2p spectra (Fig. 1E), three characteristic peaks located at 130.2 eV, 131.0 eV and 134.3 eV for BP were attributed to P 2p3/2, P 2p1/2 and oxidized phosphorus (POx).27 The characteristic peaks of P 2p3/2 and P 2p1/2 for ZnO@BP both shifted to lower binding energies (129.4 eV and 130.2 eV, respectively), which indicated the electron transfer from ZnO to BP and strong interaction between ZnO and BP nanosheets. Besides, the peak intensity of POx increased and the binding energy changed 1.0 eV, indicating the slight oxidation of BP nanosheets during the preparation of ZnO@BP.
25. Fig. 1C showed the characteristic peaks of pure ZnO at 1021.4 eV and 1044.4 eV, which were ascribed to the binding energies of Zn 2p3/2 and Zn 2p1/2, respectively.25 Compared with pure ZnO, the characteristic peaks of Zn 2p3/2 and Zn 2p1/2 for ZnO@BP shifted 0.3 eV and 1.4 eV to a higher binding energy, respectively. Fig. 1D shows the O 1s XPS spectra of ZnO and ZnO@BP, and the peaks at 530.4 eV and 530.5 eV were assigned to the O2− ions in the Zn–O bonding of ZnO and ZnO@BP.26 Besides, the shoulder peaks at 531.7 eV and 532.0 eV were related to surface-adsorbed oxygen of materials, which also moved to higher binding energies. For XPS P 2p spectra (Fig. 1E), three characteristic peaks located at 130.2 eV, 131.0 eV and 134.3 eV for BP were attributed to P 2p3/2, P 2p1/2 and oxidized phosphorus (POx).27 The characteristic peaks of P 2p3/2 and P 2p1/2 for ZnO@BP both shifted to lower binding energies (129.4 eV and 130.2 eV, respectively), which indicated the electron transfer from ZnO to BP and strong interaction between ZnO and BP nanosheets. Besides, the peak intensity of POx increased and the binding energy changed 1.0 eV, indicating the slight oxidation of BP nanosheets during the preparation of ZnO@BP.
Fig. 1F further shows the XRD pattern of nano-ZnO, BP and ZnO@BP nanocomposites. As for pure nano-ZnO particles, XRD analysis showed that the reflections at 2θ were 31.89°, 34.57°, 36.48°, 47.70°, 56.89°, 63.03°, 68.11°, and 69.17°, which corresponded to the (100), (002), (101), (102), (110), (103), (112) and (201) crystal planes of single-phase ZnO with the wurtzite structure (JCPDS file no. 36-1451).28,29 For BP, three strong peaks at 16.92°, 34.22° and 52.34° were assigned to the (020), (040) and (060) planes of BP (JCPDS file no. 47-1626), respectively. The weak peaks of BP in Fig. 1F might correspond to a slightly distorted orthorhombic structure of BP.30 Due to the low doping amount of BP, the typical diffraction peak of BP could not be detected in ZnO@BP. The lattice parameters of ZnO@BP were very similar to those of pure ZnO, indicating that the influence of BP lattice on the lattice parameters of ZnO was negligible. It was inferred that ZnO was only dispersed on the surface of BP and maintained good crystal lattice.19
The textural properties of ZnO@BP were further studied by the N2 adsorption–desorption analyses. As shown in Fig. 1G, the adsorption–desorption isotherm profile was described as type IV, which indicated that ZnO@BP has abundant mesoporous and macroporous structures with a BET specific surface area of 11.7 m2 g−1. The pore distribution plot is depicted in Fig. 1H, and ZnO@BP exhibits a pore size distribution from 1.3 to 3.5 nm with a peak at 1.76 nm. The presence of mesoporous and macroporous structures of ZnO@BP was ascribed to the immobilization of HRP.
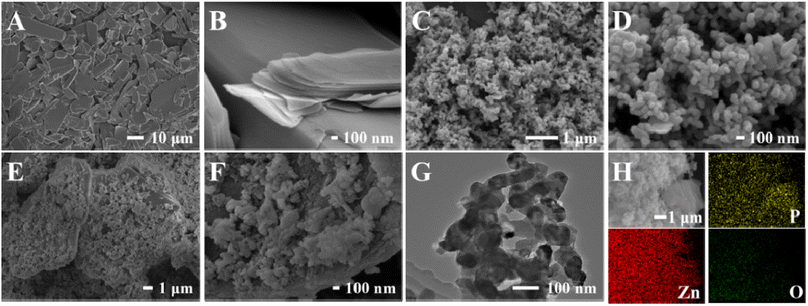
Fig. 2 shows the SEM and TEM images of BPNPs, ZnO and ZnO@BP nanocomposites. It could be observed that the BPNPs presented a multilayered sheet-like structure (Fig. 2A and B) and ZnO appeared as nanoparticles with some aggregations (Fig. 2C and D). Fig. 2E and F show the typical SEM images of ZnO@BP nanocomposites at different amplifications, which indicated that the existence of BP reduced the stacking density of ZnO nanoparticles. The TEM image (Fig. 2G) indicated that ZnO nanoparticles were successfully loaded on the BP nanosheets. The elemental mapping images of P, Zn and O (Fig. 2H) indicated that all the elements were uniformly distributed on the surface of ZnO@BP.
3.2 Electrochemical characterizations
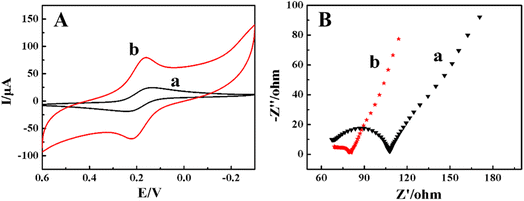
Using 1.0 mmol L−1 K3[Fe(CN)6] and 0.5 mol L−1 KCl mixture as the electrochemical probe, electrochemical responses of different modified electrodes were investigated by CV. As shown in Fig. 3, on the CILE (curve a) a pair of reversible redox peak appeared with a cathodic peak current (Ipc) of 19.80 μA and an anodic peak current (Ipa) of 17.80 μA. The anodic peak potential (Epa) and cathodic peak potential (Epc) were located at 0.249 V and 0.164 V with the peak-to-peak separation (ΔEp) of 85 mV. As for the ZnO@BP/CILE (curve b), the Ipc and Ipa were increased to 48.12 μA and 43.16 μA, respectively, which were 2.43 and 2.42 times than that of the bare CILE with a ΔEp value decreased to 65 mV, which indicated that the redox reaction of [Fe(CN)6]3−/4− became more reversible.31 Therefore, the modification of ZnO@BP on the CILE surface significantly improved the interfacial conductivity of the modified electrode, accelerated the electron transfer rate of [Fe(CN)6]3−/4−, and improved the electrochemical response signal.EIS is commonly used to investigate the electron–hole separation efficiency.32 The electron transfer resistance (Ret) depends on the semicircle domains of impedance spectra and can control the electron-transfer kinetics of the redox probe on the electrode surface. As shown in Fig. 3B, on the bare CILE (curve a) Ret was estimated to be 22 Ω, which was due to the high conductivity of the CILE. As for the ZnO@BP/CILE (curve b), the Ret was approximately 10 Ω, which was lower than that of the bare CILE, indicating that the ZnO@BP nanocomposite present on the surface of CILE provided a faster electron transfer process at the interface due to the high conductivity. The corresponding electrochemical parameters are listed in Table 1 for comparison. Based on the Randles–Sevcik equation,33,34 the effective electrode surface area of the ZnO@BP/CILE was calculated as 0.207 cm2, which was 1.64 times larger than that of the CILE (0.126 cm2). Therefore, the presence of ZnO@BP nanocomposite can not only provide a large effective surface area, but also improve the interfacial conductivity. The synergistic effects resulted in an increase in the current response of electrochemical probes with a more reversible electrode process.
| Electrodes | Ipc (μA) | Ipa (μA) | Epc (mV) | Epa (mV) | ΔE (mV) | Ret (Ω) |
|---|---|---|---|---|---|---|
| CILE | 19.80 | 17.80 | 0.164 | 0.249 | 85 | 22 |
| ZnO@BP/CILE | 48.12 | 43.16 | 0.159 | 0.224 | 65 | 10 |
3.3 Direct electrochemistry
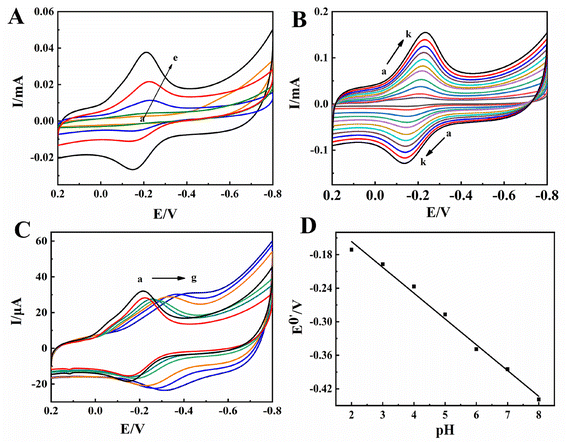
In 0.1 mol L−1 pH 2.0 PBS, the electrochemical behaviors of different modified electrodes were investigated by CV with curves shown in Fig. 4A, no voltammetric responses were observed on the bare CILE (curve a) and Nafion/ZnO@BP/CILE (curve b), indicating that no electrochemical reaction took place on the electrodes in the potential range of −0.8 to 0.2 V. On the Nafion/HRP/CILE (curve c), a pair of redox peaks appeared with Ipc as 5.456 μA and Ipa as 3.521 μA, indicating that the electron transfer of HRP had occurred on the electrode surface. Epa and Epc were located at −0.234 V and −0.142 V with ΔEp as 92 mV and Ipa/Ipc as 0.65, demonstrating a quasi-reversible electrode process.35 On the Nafion/HRP/ZnO/CILE (curve d), a larger redox peak current appeared with the Ipc and Ipa increased to 16.07 μA and 11.03 μA, respectively, indicating that the presence of ZnO could improve the response. While on the Nafion/HRP/ZnO@BP/CILE (curve e), a more reversible redox peak appeared with the largest redox currents and a well-defined peak shape. The current value of Ipc (22.40 μA) and Ipa (16.85 μA) were 4.11 and 4.79 times than those of the Nafion/HRP/CILE, respectively, revealing that ZnO@BP could promote the electron transfer of HRP effectively. It was attributed to the synergistic effects of the ZnO@BP nanocomposite with a larger effective surface area, high conductivity, increased interfacial roughness, more exposed active sites and good biocompatibility, which promoted the direct electron transfer rate and improved the electrochemical response signal. Epc and Epa were located at −0.210 V and −0.148 V with ΔE as 62 mV and the formal peak potential [E0′ = (Epa + Epc)/2] as −0.179 V, which was characteristic of HRP Fe(III)/Fe(II) redox couples.36 The results indicated that the HRP molecules maintained the active structure in the composite film with direct electron transfer of HRP accelerated by the ZnO@BP nanocomposite on the substrate electrode.The influence of scan rate in the range of 0.05 to 1.0 V s−1 on the direct electrochemical behavior of HRP was also investigated by CV with the results shown in Fig. 4B. It can be found that the redox peak current gradually increased and the peak potential slightly shifted with the increase in scanning rate. The redox peak potential had a linear relationship with ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ν and the corresponding linear regression equations were calculated as Epa (V) = 0.0492
ν and the corresponding linear regression equations were calculated as Epa (V) = 0.0492![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ν − 0.1117 (γ = 0.995) and Epc (V) = −0.0525
ν − 0.1117 (γ = 0.995) and Epc (V) = −0.0525![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ν − 0.2537 (γ = 0.993). According to Laviron's equation,37,38 the electron transfer number (n) and the electron transfer coefficient (α) were 0.926 and 0.484. Then, the reaction rate constant (ks) can be further calculated as 5.93 s−1, which was much larger than previous reports of 1.27 s−1,39 0.14 s−1,40 and 1.585 s−1.41 Therefore, the existence of the ZnO@BP nanocomposite can provide a biocompatible interface on the electrode surface, which made HRP undergo a single-electron transfer reaction on the modified electrode with the accelerated electron transfer rate. The redox peak current gradually increased with the scan rate, and there was a linear relationship between the peak currents and scan rate. The linear regression equations were obtained as Ipc (μA) = 76.83ν (V s−1) + 3.075 (γ = 0.998) and Ipa (μA) = − 63.12ν (V s−1) − 1.761 (γ = 0.998), indicating that the electrochemical reaction at the electrode interface was an adsorption control process. According to the formula Q = nAFΓ*,42 Γ* was calculated as 5.33 × 10−9 mol cm−2, and the total amount of HRP on the modified electrode interface was 2.98 × 10−8 mol cm−2. Therefore, 17.9% HRP molecules on the electrode surface participated in the electrode reaction process, which confirmed that the ZnO@BP nanocomposite could make more than one layer of HRP take part in the electrode reaction. Therefore, the ZnO@BP nanocomposite can not only offer a large surface area and high conductivity, but also improve the adsorption and the loading of HRP with the electron transfer rate accelerated. The good electrical conductivity and two-dimensional structure of BP were advantageous to quick charge transfer and ZnO loading, and the effective surface area of ZnO offered more activity sites for HRP binding.
ν − 0.2537 (γ = 0.993). According to Laviron's equation,37,38 the electron transfer number (n) and the electron transfer coefficient (α) were 0.926 and 0.484. Then, the reaction rate constant (ks) can be further calculated as 5.93 s−1, which was much larger than previous reports of 1.27 s−1,39 0.14 s−1,40 and 1.585 s−1.41 Therefore, the existence of the ZnO@BP nanocomposite can provide a biocompatible interface on the electrode surface, which made HRP undergo a single-electron transfer reaction on the modified electrode with the accelerated electron transfer rate. The redox peak current gradually increased with the scan rate, and there was a linear relationship between the peak currents and scan rate. The linear regression equations were obtained as Ipc (μA) = 76.83ν (V s−1) + 3.075 (γ = 0.998) and Ipa (μA) = − 63.12ν (V s−1) − 1.761 (γ = 0.998), indicating that the electrochemical reaction at the electrode interface was an adsorption control process. According to the formula Q = nAFΓ*,42 Γ* was calculated as 5.33 × 10−9 mol cm−2, and the total amount of HRP on the modified electrode interface was 2.98 × 10−8 mol cm−2. Therefore, 17.9% HRP molecules on the electrode surface participated in the electrode reaction process, which confirmed that the ZnO@BP nanocomposite could make more than one layer of HRP take part in the electrode reaction. Therefore, the ZnO@BP nanocomposite can not only offer a large surface area and high conductivity, but also improve the adsorption and the loading of HRP with the electron transfer rate accelerated. The good electrical conductivity and two-dimensional structure of BP were advantageous to quick charge transfer and ZnO loading, and the effective surface area of ZnO offered more activity sites for HRP binding.
The influence of different pH buffers on the direct electrochemical behavior of HRP in PBS was checked. As shown in Fig. 4C, with the increase in pH from 2.0 to 8.0, the redox peak potential gradually shifted to the negative direction. The formal peak potential (E0′) has a good linear relationship with pH (Fig. 4D) with the linear regression equation as E0′ (V) = − 0.0461pH − 0.0643 (γ = 0.991), which showed that the formal peak potential increased by 46.1 mV for each additional pH unit. For the reversible system, the theoretical value of the slope value was 59.0 mV pH−1 (298 K) for a single proton-coupled reversible one-electron transfer process.43,44 The electrochemical reduction process of HRP may be expressed using the equation: HRP Fe(III) + H+ + e− ↔ HRP Fe(II).45 When the pH was 2.0, the cathodic peak current reached the maximum and the electrochemical response signal was the most obvious. This may be attributed to the heme iron and the amino acids around HRP were influenced by the protonation of transligands or the protonation of water molecules coordinated to the heme iron.46,47 In a pH 2.0 buffer, more hydrogen ions can be provided to traverse the Nafion film and involve in the following electrode reaction. Besides, the Nafion film can protect the sensing interface composed of HRP and ZnO@BP. Therefore, pH 2.0 was selected as the optimal condition for all experiments.
3.4 Electrocatalytic performance
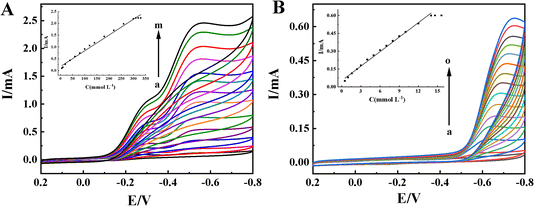
TCA is widely used in industry, agriculture, biochemistry and public health fields, which is an organohalide environmental pollutant and has been proven to be carcinogenic and poses potential risks to human health.48,49 Nitrates and nitrites are food preservative and extensively used in the food industry. At a concentration exceeding the safe levels, they pose a wide variety of health risks.50 Therefore, it is important to develop sensitive electrochemical methods for TCA and NaNO2 detection. It is well known that the redox protein-based electrochemical sensors show excellent electrocatalytic ability toward the reduction of TCA and NaNO2.51,52The electrocatalytic behaviors of the Nafion/HRP/ZnO@BP/CILE to TCA were investigated with CV curves of different concentrations of TCA recorded in Fig. 5A. With the addition of TCA, the reduction peaks current at −0.55 V gradually increased, which was a typical feature of the TCA catalytic reaction. When the TCA concentration was in the range of 0.6 to 300.0 mmol L−1, the reduction peak currents (Iss) had a linear relationship with the TCA concentration, and the linear regression equation was Iss (mA) = 0.0067C (mmol L−1) + 0.168 (γ = 0.991) with the detection limit as 0.2 mmol L−1 (3S/N). When the TCA concentration was greater than 300.0 mmol L−1, the reduction peak current remained basically stable, indicating a typical Michaelis–Menten kinetic reaction mechanism. The apparent Michaelis constant (KappM) can be calculated according to the electrochemical expression of the Lineweaver–Burk equation:53
In the formula, Iss is the steady-state current after adding the substrate, C is the concentration of the substrate, and Imax is the maximum current measured under the saturated substrate state. Using the double reciprocal plot method (1/Ip ∼ 1/[TCA]), the apparent Michaelis constant of this catalytic reaction could be calculated as 0.14 mmol L−1, which was smaller than the previous reports, as listed in Table 2. It is well known that the smaller KappM value showed the higher catalytic ability.39 Therefore, the HRP-modified electrode based on the ZnO@BP-modified CILE had a high catalytic activity for TCA.
| Modified electrode | Linear range (mmol L−1) | Detection limit (mmol L−1) | KappM (mmol L−1) | Ref. |
|---|---|---|---|---|
| a AFIL: amino functionalized ionic liquid; LDH: layered double hydroxides; GCE: glassy carbon electrode; GR: graphene, CTS: chitosan; ELDH: exfoliated Co2Al layered double hydroxide; HAp@CNF: hydroxyapatite doped carbon nanofiber. | ||||
| AFIL–LDH–Hb/GCE | 0.8–430.0 | 0.19 | 1.43 | 39 |
| CTS/Hb/GR–CuS/CILE | 1.0–64.0 | 0.20 | 6.30 | 40 |
| CTS/GR–LDH–C3N4–Hb/CILE | 0.2–36.0 | 0.05 | 3.30 | 45 |
| Hb/ZnO–MWCNTs/Nafion/GCE | 1.0–82.6 | 0.80 | — | 46 |
| CTS/ELDH–GR–Hb/CILE | 5.0–360 | 1.51 | 7.90 | 49 |
| Nafion/Mb–HAp@CNF/CILE | 6.0–180.0 | 2.00 | 224 | 54 |
| HA–HRP–CdS–IL/CILE | 1.6–18.0 | 0.53 | 0.36 | 55 |
| CTS/TiO2–Hb/CILE | 0.8–32.0 | 0.26 | 1.75 | 56 |
| Nafion/HRP/ZnO@BP/CILE | 0.6–300.0 | 0.20 | 0.14 | This work |
Fig. 5B shows the cyclic voltammograms of the Nafion/HRP/ZnO@BP/CILE in different concentrations of NaNO2 with a reduction peak appearing at −0.62 V (vs. SCE). When the concentration of NaNO2 was in the range of 0.5 to 14.6 mmol L−1, Iss had a good linear relationship with the concentration of NaNO2 (inset of Fig. 5B) and the linear regression equation was Iss (mA) = 0.0406C (mmol L−1) + 0.0523 (γ = 0.995) with the detection limit as 0.167 mmol L−1 (3S/N). When the concentration of NaNO2 reached 14.6 mmol L−1, the reduction peak current remained basically unchanged, and then KappM of the catalytic reaction of the Nafion/HRP/ZnO@BP/CILE can be calculated as 5.96 mmol L−1.
The electrocatalytic activities of the Nafion/HRP/ZnO@BP/CILE and other sensors toward TCA and NaNO2 were compared, and the results are presented in Tables 2 and 3, which indicated the superior activity of the Nafion/HRP/ZnO@BP/CILE to some of the previously reported sensors with a lower detection limit and a wider linear range. All the results concluded that the ZnO@BP nanocomposite provided better biocompatibility and higher enzymatic ability of HRP for TCA and NaNO2 detection, which was attributed to the comprehensive synergy between ZnO and BP.
| Modified electrode | Linear range (mmol L−1) | Detection limit (mmol L−1) | KappM (mmol L−1) | Ref. |
|---|---|---|---|---|
| a GT: graphene tube; B-GQDs: boron-doped graphene quantum dots; SGO: sulfonated graphene oxide; BPE-PEDOT:PSS: black phosphorene (BPE) and poly(3,4-ethylenedioxythiophene)–poly(styrenesulfonate) (PEDOT:PSS) hybrid. | ||||
| Nafion/Mb–HAp@CNF/CILE | 0.3–10.0 | 0.23 | 1.13 | 54 |
| Nafion/Mb/GT/CILE | 0.4–4.2 | 0.13 | 1.36 | 57 |
| Nafion/Hb/B-GQDs/CILE | 1.0–80.0 | 0.3 | 6.37 | 58 |
| Nafion–Mb–SGO–GCE | 2.0–24.5 | 1.5 | — | 59 |
| Nafion/Hb/Co3O4–CNF/CILE | 1.0–12.0 | 0.33 | — | 60 |
| Nafion/Mb–SWCNT/GCE | 0.5–5.0 | 0.95 | 6.45 | 61 |
| BPE–PEDOT:PSS–hemin/CILE | 1.0–10.5 | 0.33 | 23.31 | 62 |
| Nafion/HRP/ZnO@BP/CILE | 0.5–14.6 | 0.17 | 5.96 | This work |
3.5 Analytical applications
In terms of actual sample application, the modified electrode was used to detect the content of TCA in the medical facing peel solution (Shanghai EKEAR Bio. Tech. Co., Ltd., 35% TCA) and NaNO2 in pickled vegetables (bought from food supermarket and filtered off the mixture) by using the standard addition method. As shown in Table 4, the recovery rates were between 95.7–108.1% and 98.8–101.0% with the relative standard deviation (RSD) less than 4.0%, indicating that the proposed detection method can be used for the analysis of TCA and NaNO2 in actual samples with good application prospects.| Sample | Detected (mmol L−1) | Added (mmol L−1) | Total (mmol L−1) | Recovery (%) | RSD |
|---|---|---|---|---|---|
| Medical facial peel | 10.97 | 10.00 | 20.54 | 95.7 | 2.71 |
| 20.00 | 32.58 | 108.1 | 3.60 | ||
| 30.00 | 42.24 | 104.2 | 2.82 | ||
| Soak water of pickled vegetables | 1.12 | 2.0 | 3.14 | 101.0 | 1.93 |
| 2.5 | 3.59 | 98.8 | 2.11 | ||
| 3.0 | 4.13 | 100.3 | 1.75 |
3.6 Stability and reproducibility
The stability and reproducibility of the Nafion/HRP/ZnO@BP/CILE were investigated. After scanning continuously for 50 cycles in PBS, the cyclic voltammetric response was reduced by 2.36% as compared with the initial current. The modified electrode was stored in a refrigerator at about 4 °C for two weeks, and the redox peak current remained at 97.2% of the original value. After being placed for four weeks, the cyclic voltammetric response remained at 90.8% of its original value, which proved that the modified electrode had good stability. Three Nafion/HRP/ZnO@BP/CILE were used to detect 10.0 mmol L−1 TCA with an RSD value of 3.21%, indicating that the Nafion/HRP/ZnO@BP/CILE had excellent reproducibility for the voltammetric detection of TCA. Therefore, the good stability and excellent reproducibility of the constructed biosensor can be attributed to the high stability of the ZnO@BP nanocomposite on the modified electrode.4. Conclusion
In this work, a new and sensitive electrochemical biosensor has been prepared based on a ZnO@BP nanocomposite by an in situ hydrothermal method, which has been modified on the surface of a CILE. The electrochemical behavior of the Nafion/HRP/ZnO@BP/CILE was investigated by cyclic voltammetry with a pair of quasi-reversible redox peaks observed, indicating that the presence of the ZnO@BP nanocomposite could enhance the electron transfer rate with the advantages of large specific surface area, high electronic conductivity and good biocompatibility. The electrocatalytic behaviors to TCA and NaNO2 were further studied with the characteristics of wider linear range, lower detection limit and good stability. In addition, the proposed electrodes were used to detect the real samples with satisfactory results, which showed that the ZnO@BP nanocomposite had potential application in the field of electrochemical sensors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This project was financially supported by the China Geological Survey Project “Wetland Resources Survey of International Importance in South China” (DD20220876).References
- Y. Y. Shao, J. Wang, H. Wu, J. Liu, I. A. Aksay and Y. H. Liu, Graphene based electrochemical sensors and biosensors: a review, Electroanalysis, 2010, 22, 1027–1036, DOI:10.1002/elan.200900571.
- L. S. Oriol, L. Dominik, K. Metin, R. Aleksandra and K. Andras, Ultrasensitive photodetectors based on monolayer MoS2, Nat. Nanotechnol., 2013, 8, 497–501, DOI:10.1038/nnano.2013.100.
- Z. Zhuge, Y. H. Tang, J. W. Tao and Y. Zhao, Functionalized black phosphorus nanocomposite for biosensing, ChemElectroChem, 2019, 6, 1–6, DOI:10.1002/celc.201801439.
- S. Kumar, Y. J. Lei, N. H. Alshareef, M. A. Quevedo-Lopez and K. N. Salama, Biofunctionalized two-dimensional Ti3C2 Mxenes for ultrasensitive detection of cancer biomarker, Biosens. Bioelectron., 2018, 121, 243–249, DOI:10.1016/j.bios.2018.08.076.
- L. K. Li, Y. J. Yu, G. J. Ye, Q. Q. Ge, X. D. Qu, H. Wu, D. L. Feng, X. H. Chen and Y. B. Zhang, Black phosphorus field-effect transistors, Nat. Nanotechnol., 2014, 9, 372–377, DOI:10.1038/nnano.2014.35.
- J. H. Wu, S. L. Huang, Z. Y. Jin, J. Q. Chen, L. Hu, Y. J. Long, J. G. Lu, S. C. Ruan and Y. J. Zeng, Black phosphorus: an efficient co-catalyst for charge separation and enhanced photocatalytic hydrogen evolution, J. Mater. Sci., 2018, 53, 16557–16566, DOI:10.1007/s10853-018-2830-2.
- Y. H. Huang, L. J. Yan, B. Wang, B. Shao, Y. Y. Niu, X. P. Zhang, P. Yin, Y. Q. Ge, W. Sun and H. Zhang, Recent applications of black phosphorus and its related composites in electrochemistry and bioelectrochemistry: a mini review, Electrochem. Commun., 2021, 129, 107095, DOI:10.1016/j.elecom.2021.107095.
- S. W. Lee, F. Yang, J. K. Suh, S. J. Yang, Y. B. Lee, G. Li, H. S. Choe, A. H. Suslu, Y. B. Chen, C. Y. Ko, J. S. Park, K. Liu, J. B. Li, K. Hippalgaonkar, J. J. Urban, S. Tongay and J. Q. Wu, Anisotropic in-plane thermal conductivity of black phosphorus nanoribbons at temperatures higher than 100K, Nat. Commun., 2015, 6, 8573–8579, DOI:10.1038/ncomms9573.
- X. L. Niu, W. Z. Weng, C. X. Yin, Y. Y. Niu, G. J. Li, R. X. Dong, Y. L. Men and W. Sun, Black phosphorene modified glassy carbon electrode for the sensitive voltammetric detection of rutin, J. Electroanal. Chem., 2018, 811, 78–83, DOI:10.1016/j.jelechem.2018.01.038.
- X. Y. Li, X. L. Niu, W. S. Zhao, W. Chen, C. X. Yin, Y. L. Men, G. J. Li and W. Sun, Black phosphorene and PEDOT: PASS-modified electrode for electrochemistry of hemoglobin, Electrochem. Commun., 2018, 86, 68–71, DOI:10.1016/j.elecom.2017.11.017.
- Y. Zhao, Y. H. Zhang, Z. Zhuge, Y. H. Tang, J. W. Tao and Y. Chen, Synthesis of a poly-L-lysine/black phosphorus hybrid for biosensors, Anal. Chem., 2018, 90, 3149–3155, DOI:10.1021/acs.analchem.7b04395.
- F. Shi, B. L. Wang, L. J. Yan, Y. Y. Niu, L. S. Wang and W. Sun, In situ growth of nitrogen-doped carbonized polymer dots on black phosphorus for electrochemical DNA biosensor of Escherichia coli O157: H7, Bioelectrochemistry, 2022, 148, 108226, DOI:10.1016/j.bioelechem.2022.108226.
- Y. Xiang, M. B. Camarada, Y. P. Wen, H. Wu, J. Y. Chen, M. F. Li and X. N. Liao, Simple voltametric analyses of ochratoxin A in food samples using highly-stable and anti-fouling black phosphorene nanosensor, Electrochim. Acta, 2018, 282, 490–498, DOI:10.1016/j.electacta.2018.06.055.
- Y. Ge, M. B. Camarada, L. J. Xu, M. R. Qu, H. Liang, E. L. Zhao, M. F. Li and Y. P. Wen, A highly stable black phosphorene nanocomposite for voltametric detection of clenbuterol, Microchim. Acta, 2018, 185, 566, DOI:10.1007/s00604-018-3084-z.
- S. Ramalingam, R. Chand, C. B. Singh and A. Singh, Phosphorene-gold nanocomposite based microfluidic aptasensor for the detection of okadaic acid, Biosens. Bioelectron., 2019, 135, 14–21, DOI:10.1016/j.bios.2019.03.056.
- Z. W. Zhao, W. Lei, X. B. Zhang, B. P. Wang and H. L. Jiang, ZnO-based amperometric enzyme biosensors, Sensors, 2010, 10, 1216–1231, DOI:10.3390/s100201216.
- B. X. Gu, C. X. Xu, G. P. Zhu, S. Q. Liu, L. Y. Chen, M. L. Wang and J. J. Zhu, Layer by layer immobilized horseradish peroxidase on zinc oxide nanorods for biosensing, J. Phys. Chem. B, 2009, 113, 6553–6557, DOI:10.1021/jp900048m.
- H. C. Ding, L. Zhang, Z. R. Tang, Y. P. Dong and X. F. Chu, Black phosphorus quantum dots doped ZnO nanoparticles as efficient electrode materials for sensitive hydrogen peroxide detection, J. Electroanal. Chem., 2018, 824, 161–168, DOI:10.1016/j.jelechem.2018.07.055.
- S. T. Li, P. F. Wang, R. D. Wang, Y. F. Liu, R. S. Jing, Z. Li, Z. L. Meng, Y. Y. Liu and Q. Zhang, One-step co-precipitation method to construct black phosphorus nanosheets/ZnO nanohybrid for enhanced visible light photocatalytic activity, Appl. Surf. Sci., 2019, 497, 143682, DOI:10.1016/j.apsusc.2019.143682.
- Q. Li, Y. Cen, J. Y. Huang, X. J. Li, H. Zhang, Y. F. Geng, B. I. Yakobson, Y. Du and X. Q. Tian, Zinc oxide-black phosphorus composites for ultrasensitive nitrogen dioxide sensing, Nanoscale Horiz., 2018, 3, 525–531, 10.1039/C8NH00052B.
- J. H. Kang, J. D. Wood, S. A. Wells, J. H. Lee, X. L. Liu, K. S. Chen and M. C. Hersam, Solvent exfoliation of electronic-grade, two-dimensional black phosphorus, ACS Nano, 2015, 9, 3596–3604, DOI:10.1021/acsnano.5b01143.
- P. Yasaei, B. Kumar, T. Foroozan, C. H. Wang, M. Asadi, D. Tuschel, J. E. Indacochea, R. F. Klie and A. Salehi-Khojin, High-quality black phosphorus atomic layers by liquid-phase exfoliation, Adv. Mater., 2015, 27, 1887–1892, DOI:10.1002/adma.201405150.
- L. J. Wang, Y. F. Qi, H. Li, R. Q. Guan, F. L. Zhang, Q. F. Zhou, D. D. Wu, Z. Zhao, G. Zhou and Z. C. Sun, Au/g-C3N4 heterostructure sensitized by black phosphorus for full solar spectrum waste-to-hydrogen conversion, Sci. China Mater., 2022, 65, 974–984, DOI:10.1007/s40843-021-1833-1.
- W. Sun, Y. Z. Li, Y. Y. Duan and K. Jiao, Direct electrocatalytic oxidation of adenine and guanine on carbon ionic liquid electrode and the simultaneous determination, Biosens. Bioelectron., 2008, 24, 988–993, DOI:10.1016/j.bios.2008.07.068.
- S. Wang, B. C. Zhu, M. J. Liu, L. Y. Zhang, J. G. Yu and M. H. Zhou, Direct Z-scheme ZnO/CdS hierarchical photocatalyst for enhanced photocatalytic H2-production activity, Appl. Catal., B, 2019, 243, 19–26, DOI:10.1016/j.apcatb.2018.10.019.
- K. Kotsis and V. Staemmler, Ab initio calculations of the O1s XPS spectra of ZnO and Zn oxo compounds, Phys. Chem. Chem. Phys., 2006, 8, 1490–1498, 10.1039/B515699H.
- M. Zhu, C. Zhai, M. Fujitsuka and T. Majima, Noble metal-free near-infrared-driven photocatalyst for hydrogen production based on 2D hybrid of black phosphorus/WS2, Appl. Catal., B, 2018, 221, 645–651, DOI:10.1016/j.apcatb.2017.09.063.
- M. Akbari and S. Sharifnia, Synthesis of ZnS/ZnO nanocomposite through solution combustion method for high rate photocatalytic conversion of CO2 and CH4, Mater. Lett., 2017, 194, 110–113, DOI:10.1016/j.matlet.2017.02.020.
- Y. Al-Hadeethi, A. Umar, S. H. Al-Heniti, R. Kumar, S. H. Kim, X. Zhang and B. M. Raffah, 2D Sn-doped ZnO ultrathin nanosheet networks for enhanced acetone gas sensing application, Ceram. Int., 2017, 43, 2418–2423, DOI:10.1016/j.ceramint.2016.11.031.
- G. Abellán, C. Neiss, V. Lloret, S. Wild, J. C. Chacón-Torres, K. Werbach, F. Fedi, H. Shiozawa, A. Görling, H. Peterlik, T. Pichler, F. Hauke and A. Hirsch, Exploring the formation of black phosphorus intercalation compounds with alkali metals, Angew. Chem., Int. Ed., 2017, 56, 15267–15273, DOI:10.1002/anie.201707462.
- Y. Huang, Z. T. Han, X. Zhou, J. X. Li, X. L. Gu, Z. F. Li, W. Sun and X. L. Niu, Three-dimensional MoS2-graphene aerogel nanocomposites for electrochemical sensing of quercetin, Microchim. Acta, 2022, 189, 299, DOI:10.1007/s00604-022-05336-z.
- X. L. Cui, D. L. Jiang, P. Diao, J. X. Li, R. T. Tong and X. K. Wang, Assessing the apparent effective thickness of alkanethiol self-assembled monolayers in different concentrations of Fe (CN)63-/Fe (CN)64- by acimpedance spectroscopy, J. Electroanal. Chem., 1999, 470, 9–13, DOI:10.1016/S0022-0728(99)00201-6.
- A. Keziban, CuFe2O4/reduced graphene oxide nanocomposite decorated with gold nanoparticles as a new electrochemical sensor material for L-cysteine detection, J. Alloys Compd., 2019, 791, 391–401, DOI:10.1016/j.jallcom.2019.03.303.
- J. D. Ayad, A. Keziban, Z. B. Salih and O. Mustafa, Ag-TiO2-reduced graphene oxide hybrid film for electrochemical detection of 8-hydroxy-2′-deoxyguanosine as an oxidative DNA damage biomarker, Anal. Methods, 2020, 12, 499–506, 10.1039/C9AY02175B.
- Y. Y. Niu, R. Y. Zou, H. A. Yones, X. B. Li, X. Y. Li, X. L. Niu, Y. Chen, P. Li and W. Sun, Electrochemical behavior of horseradish peroxidase on WS2 nanosheet-modified electrode and electrocatalytic investigation, J. Chin. Chem. Soc., 2018, 65, 1127–1135, DOI:10.1002/jccs.201800054.
- X. J. Liu, T. Chen, L. F. Liu and G. X. Li, Electrochemical characteristics of heme proteins in hydroxyethylcellulose film, Sens. Actuators, B, 2006, 113, 106–111, DOI:10.1016/j.snb.2005.02.029.
- E. Laviron, The use of linear potential sweep voltammetry and of a.c. voltammetry for the study of the surface electrochemical reaction of strongly adsorbed systems and of redox modified electrodes, J. Electroanal. Chem., 1979, 100, 263–270, DOI:10.1016/S0022-0728(79)80167-9.
- E. Laviron, General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems, J. Electroanal. Chem., 1979, 101, 19–28, DOI:10.1016/S0022-0728(79)80075-3.
- T. R. Zhan, X. J. Wang, Y. M. Zhang, Y. Song, X. L. Liu, J. Xu and W. G. Hou, Direct electrochemistry and electrocatalysis of hemoglobin immobilized in layered double hydroxides modified with amino functionalized ionic liquid through coprecipitation technique, Sens. Actuators, B, 2015, 220, 1232–1240, DOI:10.1016/j.snb.2015.07.043.
- X. M. Feng, R. M. Li, C. H. Hu and W. H. Hou, Direct electron transfer and electrocatalysis of hemoglobin immobilized on graphene-Pt nanocomposite, J. Electroanal. Chem., 2011, 657, 28–33, DOI:10.1016/j.jelechem.2011.03.004.
- F. Shi, W. Zheng, W. Wang, F. Hou, B. Lei, Z. Sun and W. Sun, Application of graphene-copper sulfide nanocomposite modified electrode for electrochemistry and electrocatalysis of hemoglobin, Biosens. Bioelectron., 2015, 64, 131–137, DOI:10.1016/j.bios.2014.08.064.
- W. Sun, Y. Q. Guo, T. T. Li, X. M. Ju, J. Lou and C. X. Ruan, Electrochemistry of horseradish peroxidase entrapped in graphene and dsDNA composite modified carbon ionic liquid electrode, Electrochim. Acta, 2012, 75, 381–386, DOI:10.1016/j.electacta.2012.05.018.
- R. S. Nicholson and I. Shain, Theory of stationary electrode polarography for a chemical reaction coupled between two charge transfers, Anal. Chem., 1965, 37, 178–190, DOI:10.1021/2Fac60221a002.
- S. F. Wang, F. Xie and G. D. Liu, Direct electrochemistry and electrocatalysis of heme proteins on SWCNTs-CTAB modified electrodes, Talanta, 2009, 77, 1343–1350, DOI:10.1016/j.talanta.2008.09.019.
- X. J. Han, W. M. Huang, J. B. Jia, S. J. Dong and E. K. Wang, Direct electrochemistry of hemoglobin in egg-phosphatidylcholine films and its catalysis to H2O2, Biosens. Bioelectron., 2002, 17, 741–746, DOI:10.1016/S0956-5663(02)00052-0.
- T. R. Zhan, Z. W. Tan, X. J. Wang and W. G. Hou, Hemoglobin immobilized in g-C3N4 nanoparticle decorated 3D graphene-LDH network: direct electrochemistry and electrocatalysis to trichloroacetic acid, Sens. Actuators, B, 2018, 255, 149–158, DOI:10.1016/j.snb.2017.08.048.
- W. Ma and D. Tian, Direct electron transfer and electrocatalysis of hemoglobin in ZnO coated multiwalled carbon nanotubes and Nafion composite matrix, Bioelectrochemistry, 2010, 78, 106–112, DOI:10.1016/j.bioelechem.2009.08.002.
- Y. Z. Liu, R. Mao, Y. T. Tong, H. C. Lan, G. Zhang, H. J. Liu and J. H. Qu, Reductive dechlorination of trichloroacetic acid (TCAA) by electrochemical process over Pd-In/Al2O3 catalyst, Electrochim. Acta, 2017, 232, 13–21, DOI:10.1016/j.electacta.2017.02.071.
- T. R. Zhan, X. J. Wang, X. J. Li and W. G. Hou, Hemoglobin immobilized in exfoliated Co2AlLDH-graphene nanocomposite film: direct electrochemistry and electrocatalysis toward trichloroacetic acid, Sens. Actuators, B, 2016, 228, 101–108, DOI:10.1016/j.snb.2015.12.095.
- E. Wierzbicka, Novel methods of nitrate and nitrite determination: a review, J. Elem., 2020, 25, 97–106, DOI:10.5601/jelem.2019.24.3.1848.
- Y. Ding, Y. Wang and Y. Lei, Direct electrochemistry and electrocatalysis of novel single-walled carbon nanotubes-hemoglobin composite micro belts-towards the development of sensitive and mediator-free biosensor, Biosens. Bioelectron., 2010, 26, 390–397, DOI:10.1016/j.bios.2010.07.124.
- A. E. F. Nassar, J. M. Bobbitt, J. D. Stuart and J. F. Rusling, Catalytic reduction of organohalide pollutants by myoglobin in a biomembrane-like surfactant film, J. Am. Chem. Soc., 1995, 117, 10986–10993, DOI:10.1021/ja00149a022.
- K. A. Johnson and R. S. Goody, The original michaelis constant: translation of the 1913 michaelis-menten paper, Biochemistry, 2011, 50, 8264–8269, DOI:10.1021/bi201284u.
- J. Liu, W. J. Weng, H. Xie, G. L. Luo, G. J. Li, W. Sun, C. X. Ruan and X. H. Wang, Myoglobin and hydroxyapatite-doped carbon nanofiber-modified electrodes for electrochemistry and electrocatalysis, ACS Omega, 2019, 4, 15653–15659, DOI:10.1021/acsomega.9b02151.
- Z. H. Zhu, X. Li, Y. Wang, Y. Zeng, W. Sun and X. T. Huang, Direct electrochemistry and electrocatalysis of horseradish peroxidase with hyaluronic acid-ionic liquid-cadmium sulfide nanorod composite material, Anal. Chim. Acta, 2010, 670, 51–56, DOI:10.1016/j.aca.2010.04.061.
- F. Shi, W. Wang, S. Gong, B. Lei, G. Li, X. Lin, Z. Sun and W. Sun, Application of titanium dioxide nanowires for the direct electrochemistry of hemoglobin and electrocatalysis, J. Chin. Chem. Soc., 2015, 62, 554–561, DOI:10.1002/jccs.201400373.
- Y. Y. Niu, X. Y. Li, H. Xie, G. L. Luo, R. Y. Zou, Y. R. Xi, G. J. Li and W. Sun, Electrochemical performance and electrocatalytic behavior of myoglobin on graphene tube-modified electrode, J. Chin. Chem. Soc., 2020, 67, 1054–1061, DOI:10.1002/jccs.201900282.
- W. Chen, W. J. Weng, X. L. Niu, X. Y. Li, Y. L. Men, W. Sun, G. J. Li and L. F. Dong, Boron-doped graphene quantum dots modified electrode for electrochemistry and electrocatalysis of hemoglobin, J. Electroanal. Chem., 2018, 823, 137–145, DOI:10.1016/j.jelechem.2018.06.001.
- G. Y. Chen, H. Sun and S. F. Hou, Electrochemistry and electrocatalysis of myoglobin immobilized in sulfonated graphene oxide and Nafion films, Anal. Biochem., 2016, 502, 43–49, DOI:10.1016/j.ab.2016.03.003.
- H. Xie, G. L. Luo, Y. Y. Niu, W. J. Weng, Y. X. Zhao, Z. Q. Li, C. X. Ruan, G. J. Li and W. Sun, Synthesis and utilization of Co3O4 doped carbon nanofiber for fabrication of hemoglobin-based electrochemical sensor, Mater. Sci. Eng., C, 2020, 107, 110209, DOI:10.1016/j.msec.2019.110209.
- G. L. Turdean and G. Szabo, Nitrite detection in meat products samples by square-wave voltammetry at a new single walled carbon naonotubes-myoglobin modified electrode, Food Chem., 2015, 179, 325–330, DOI:10.1016/j.foodchem.2015.01.106.
- X. Y. Li, G. L. Luo, H. Xie, Y. Y. Niu, X. B. Li, R. Y. Zou, Y. R. Xi, Y. Xiong, W. Sun and G. J. Li, Voltammetric sensing performances of a carbon ionic liquid electrode modified with black phosphorene and hemin, Microchim. Acta, 2019, 186, 304–312, DOI:10.1007/s00604-019-3421-x.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2023 |