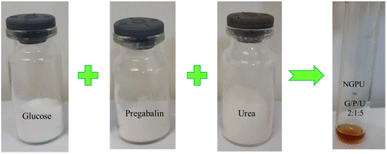
Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Facile synthesis of triazolo/benzazolo[2,1-b]quinazolinone derivatives catalyzed by a new deep eutectic mixture based on glucose, pregabalin and urea†
Parissa Naddaf Rahro,
Farhad Shirini * and
Ali Ghanadzadeh Gilani
* and
Ali Ghanadzadeh Gilani
Department of Chemistry, College of Sciences, University of Guilan, Rasht, 41335-19141, Iran. E-mail: shirini@guilan.ac.ir; fshirini@gmail.com; Fax: +98 131 3233262; Tel: +981313233262
First published on 27th October 2023
Abstract
In this study, a novel natural deep eutectic solvent was prepared from glucose, pregabalin, and urea. The prepared solvent was identified using a variety of techniques, including Fourier transform infrared spectroscopy (FT-IR), thermogravimetric analysis (TGA), derivative thermogravimetry (DTG), differential thermal analysis (DTA), and refractive index measurements (RI). The prepared deep eutectic solvent was then utilized for the one-pot synthesis of quinazolinone derivatives. The yields of the product obtained with and without the catalyst were determined, providing insights into the catalytic efficiency of the system. This protocol offers several advantages, including mild reaction conditions, easy reagent preparation, a green process, short reaction times (2–60 min), high yields (80–99%), and a straightforward procedure with the possibility of catalyst reusability.
Introduction
Easily vaporable mixtures with ecological benefits are rapidly gaining importance in modern chemistry because their use can reduce the environmental impact of organic solvents. Ionic liquids (ILs) play an important role in this area as a suitable green media and/or a promotor in organic synthesis. In these salts, poor ionic coordination and low salt melting points result from charge dispersion and/or delocalization (below 100 °C).1–4 ILs have many environmentally friendly properties, such as low vapor pressure, considerable stability under different thermal and chemical conditions ease of handling, high recyclability and low flammability.1 Therefore, ILs can play an important role as reaction and extraction media. Additionally, in some chemical reactions, the use of these liquids has enabled the production of products that are not obtainable using common organic solvents or simple post-treatment methods.5–7 Unfortunately, most of these compounds were found to be toxic, poorly biodegradable, and therefore poorly biocompatible and also showed some ecological disadvantages.8,9Deep eutectic solvents (DES) are a new class of organic solvents which can be easily prepared by the mixing and heating of appropriate hydrogen bond donor (HBD) and hydrogen bond acceptor (HBA) molecules in the correct molar ratios. These hydrogen-bonding interactions cause the melting point of these mixtures be very low even at room temperature.1,10 It should be mentioned that because of the presence of salts in many of these systems, sometimes, they are classified as a subclass of ionic liquids.
One of the interesting features of these mixtures is their simple preparation method which can be done by mixing and heating two or more species of HBD and HBA molecules until a homogeneous liquid is formed. This process is cheap and “green” in terms of the atom economy of the process.10 A variety of starting materials can be used for the preparation of DESs and natural deep eutectic solvents (NADES),11 as a new class of these mixtures, of which metabolites,12 organic acids,13,14 amino acids,15,16 sugars,17,18 choline19–21 and urea20,22 are the most important ones.
In recent years, the synthesis of biologically active compounds via green and effective methods has become an attractive topic in organic chemistry.23 Among a variety of methods multi-component reactions (MCR) are one of the most important candidates for this purpose. This is because the principles, such as atom economy, effectiveness of the reactions and simplicity of processes from doing the reactions to the separation of the products, are well respected in these methods.24,25
Quinazolinone derivatives are an interesting type of heterocyclic compound showing considerable biological and pharmacological activities, including: Antivirals and antihistamines,26 analgesic and anti-inflammatory,27 antitumor,28 anticancer29 and anti-HIV30 activities. Use as potent immunosuppressants28 is another application of these compounds.
Triazolo[2,1-b]quinazolinones and benzazolo[2,1-b]quinazolinones are important derivatives of quinazolines. A common way for the preparation of these important target molecules is the multi-component reaction of aldehydes with 3-amino-1,2,4-triazole or 2-aminobenzimidazole, and a β-diketone in the presence of a variety of catalysts.31–36
Although the use of these catalysts causes an improvement, harsh reaction conditions, long reaction times, expensive reagents, low yields of the products and the use of a large quantity of volatile organic solvents are important limitations associated with these methods. Furthermore, during most of the existing methods, the catalyst cannot be recovered or reused. Therefore, the introduction of simple, efficient and mild procedures for the synthesis of the above-mentioned derivatives of quinazolinone is still needed. Also, other studies have reported the synthesis of quinazolinone derivatives using deep eutectic solvents.37,38 Referencing these studies demonstrates that the synthesis of quinazolinone derivatives in deep eutectic solvents has been explored in the literature. This highlights the significance of the current study and its contribution to the existing knowledge in the field. Herein, we wish to introduce a new natural deep eutectic mixture which can speed-up the mentioned reactions by removing some of the aforementioned limitations.
Results and discussion
In the first stage of this study, various amounts of glucose, pregabalin, and urea were tested to prepare the desired deep eutectic mixture. The results indicated that the optimal results were achieved when these compounds were mixed in molar ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 5
5, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, and 5
2, and 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, and heated at 110 °C for 30 minutes (Table 1, entries 6, 8 and 10).
3, and heated at 110 °C for 30 minutes (Table 1, entries 6, 8 and 10).
| Entry | Mixture | Molar ratio | Appearance at room temperature |
|---|---|---|---|
| 1 | Glucose/pregabalin/urea | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Solid |
| 2 | Glucose/pregabalin/urea | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Solid |
| 3 | Glucose/pregabalin/urea | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
Solid |
| 4 | Glucose/pregabalin/urea | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
Solid |
| 5 | Glucose/pregabalin/urea | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
Solid |
| 6 | Glucose/pregabalin/urea | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
Clear liquid |
| 7 | Glucose/pregabalin/urea | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
Sticky paste |
| 8 | Glucose/pregabalin/urea (NGPU) | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
Clear liquid |
| 9 | Glucose/pregabalin/urea | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
Solid |
| 10 | Glucose/pregabalin/urea | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
Clear liquid |
| 11 | Glucose/pregabalin/urea | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
Solid |
| 12 | Glucose/pregabalin/urea | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
Solid |
To determine the best ratio in terms of catalytic properties, the catalytic properties of the deep eutectic mixtures were tested in the synthesis of triazolo/benzazolo[2,1-b]quinazolinone, as shown in Tables 2 and 4. The deep eutectic mixture with a molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (referred to as NGPU in this article) demonstrated the best result (Tables 2, entry 3 and 4, entry 4).
5 (referred to as NGPU in this article) demonstrated the best result (Tables 2, entry 3 and 4, entry 4).
| Entry | Catalyst (mol%) | Solvent | Temperature (°C) | Time (min) | Conversion (%) |
|---|---|---|---|---|---|
| 1 | NGPU (2) | — | 120 | 40 | 100 |
| 2 | NGPU (3) | — | 120 | 40 | Mixture of products |
| 3 | NGPU (4) | — | 120 | 20 | 100 |
| 4 | NGPU (8) | — | 120 | 43 | 100 |
| 5 | NGPU (8) | — | 100 | 40 | Not completed |
| 6 | NGPU (4) | — | 70 | 56 | Not completed |
| 7 | NGPU (4) | EtOH | Reflux | 50 | Not completed |
| 8 | NGPU (4) | CH3CN | Reflux | 50 | Not completed |
| 9 | NGPU (4) | H2O | Reflux | 50 | Not completed |
| 10 | Glucose/pregabalin/urea (4) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
— | 120 | 35 | 100 |
| 11 | Glucose/pregabalin/urea (4) 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
— | 120 | 40 | 100 |
| 12 | Pregabalin (4) | — | 120 | 100 | Not completed |
| 13 | Glucose (4) | — | 120 | 100 | Not completed |
| 14 | Urea (4) | — | 120 | 100 | Mixture of products |
| 15 | — | — | 120 | 100 | Trace |
After preparation, the selected deep eutectic mixture (NGPU) was identified using methods commonly used to identify this group of mixtures. The results of the identification are described in the next section.
Characterization of the catalyst
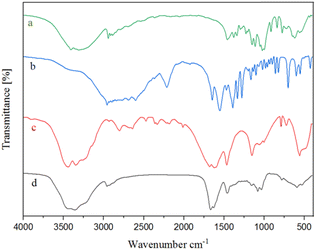
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O tensile vibrations, respectively. Furthermore, the absorption peaks around 1034 and 1079 cm−1 are related to the C–N bending vibrations of pregabalin and urea.
O tensile vibrations, respectively. Furthermore, the absorption peaks around 1034 and 1079 cm−1 are related to the C–N bending vibrations of pregabalin and urea.
Furthermore, shifts of certain peaks and weakening of the vibrations of several functional groups were also observed and these changes were attributed to the formation of hydrogen bonds between the components of NGPU and the resulting formation of NGPU.
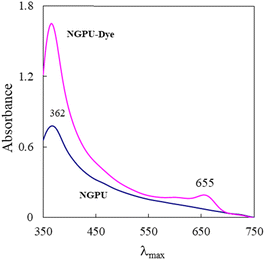
Fig. 4 shows the visible absorption spectrum of oxazine 1 perchlorate (OX1) in a prepared deep eutectic solvent. The absorption spectrum of the pure NGPU is overplotted for comparison purposes. The spectrum of the dye typically possesses an intense band, which is neighbored by a shoulder at shorter wavelengths.
Fig. 5 shows the variation in maximum absorption wavelength of oxazine 1 perchlorate (a solvatochromic dye) as a function of solvent polarity (dielectric constant). As can be seen, a relatively good correlation was observed between λmax values and the selected solvent, and a regression of 0.9925 was obtained for this scale. The dye exhibited an absorption maximum at 655 nm in the DES. According to the polarity curve, the prepared DES shows a high polarity and its dielectric constant was estimated to be about ε = 71.5.
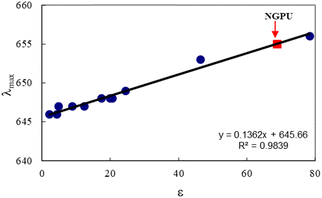 | ||
| Fig. 5 Variation of absorption maximum wavelength (λmax) of OX1 dye (2 × 10−5 M) as a function of the solvent dielectric constant, ε, at room temperature; the selected solvents were mainly low polarity and polar protic solvents, i.e. 1,4-dioxane, diethyl ether, chloroform, dichloromethane, t-butanol, 1-butanol, 2-propanol, acetone, ethanol, water (the dielectric data are taken from ref. 42). | ||
The absorption spectrum of oxazine 1 in NGPU is red shifted as compared to the dye spectra in normal organic solvents (non-polar and low polarity media). The red shift observed for the dye in the prepared DES indicates strong intermolecular interactions between the dye molecules and the polar DES medium. Although based on the previous report,43 oxazine-1 was considered as a poor polarity indicator. However, the spectral profile shows a regular variation on going from the non-polar or low polarity solvents to the polar protic solvents.
Catalytic activity
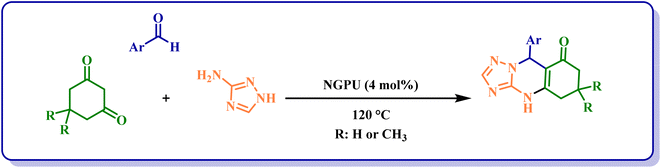
After the identification of the produced NGPU, its activity in the promotion of the synthesis of triazolo[2,1-b]quinazolinones and benzazolo[2,1-b] derivatives was investigated. In the first step, the one pot three-component reaction of 4-chlorobenzaldehyde, dimedone and 3-amino-1,2,4-triazole was selected as a model to study the influence of different conditions on it and the obtained results (Table 2) clarified that the suitable conditions for obtaining the best results is as shown in Scheme 1. Based on the obtained data, we should pay our attention to the following points: (a) the reaction cannot proceed in the presence of starting materials used for the preparation of NGPU (entries 12–14); (b) the reaction in the solvent or at lower temperatures remains incomplete even after prolonged heating (entries 5–9) and (c) a variety of molar ratios of glucose, pregabalin and urea were used for the preparation of NGPU and the one with the molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 led to the best results in 4 mol%. It should be mentioned that, because we used catalytic amounts of the prepared reagent, in this study the term deep eutectic mixture (DEM) is used instead of deep eutectic solvent (DES).
5 led to the best results in 4 mol%. It should be mentioned that, because we used catalytic amounts of the prepared reagent, in this study the term deep eutectic mixture (DEM) is used instead of deep eutectic solvent (DES).
Based on the results obtained from the above-mentioned preliminary studies, the efficiency of this protocol was studied for the reaction of a variety of aromatic aldehydes containing different types of substituents (Table 3). Using this method, high yields of the desired products were isolated in short reaction times.
| Entry | Ar | R | Product | Time (min) | Yieldb (%) | M.p. (°C) | |
|---|---|---|---|---|---|---|---|
| Found | Reported | ||||||
a Reaction conditions: aldehyde (1 mmol), 1,3-diketones (dimedone and/or 1,3-cyclohexanediones) (1 mmol) and 3-amino-1,2,4-triazole (1 mmol).b Isolated yields.c 8 mol% NGPU (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5).d Isolated yields after recrystallization. 5).d Isolated yields after recrystallization. |
|||||||
| 1 | C6H5 | CH3 | 1a | 22 | 96 | 254–257 | 252–254 (ref. 26) |
| 2 | 4-Cl-C6H4 | CH3 | 2a | 20 | 94 | 300–303 | 304–305 (ref. 44) |
| 3 | 2-Cl-C6H4c | CH3 | 3a | 14 | 88 | 286–290 | 288–290 (ref. 45) |
| 4 | 2,4-Cl2-C6H3 | CH3 | 4a | 20 | 90 | >300 | >300 (ref. 46) |
| 5 | 4-Br-C6H4 | CH3 | 5a | 30 | 85d | 292–295 | 287–288 (ref. 44) |
| 6 | 4-NO2-C6H4 | CH3 | 6a | 20 | 91 | 291–293 | 290–294 (ref. 47) |
| 7 | 3-NO2-C6H4 | CH3 | 7a | 22 | 93 | 292–294 | 290–293 (ref. 46) |
| 8 | 4-OCH3-C6H4 | CH3 | 8a | 20 | 95 | 233–235 | 231–233 (ref. 46) |
| 9 | 4-OH-C6H4 | CH3 | 9a | 18 | 97 | >300 | >300 (ref. 31) |
| 10 | 4-OH-3-OCH3-C6H3 | CH3 | 10a | 25 | 85d | 272–277 | 287–290 (ref. 48) |
| 11 | 4-CH3-C6H4 | CH3 | 11a | 20 | 93 | 262–266 | 265–267 (ref. 45) |
| 12 | 2-CH3-C6H4 | CH3 | 12a | 16 | 97 | 305–306 | 299–300 (ref. 28) |
| 13 | C6H5 | H | 13a | 24 | 98 | 298–299 | 296–300 (ref. 32) |
| 14 | 4-Cl-C6H4 | H | 14a | 60 | 80d | 294–295 | 294–296 (ref. 32) |
| 15 | 3,4,5-(OCH3)3–C6H2 | H | 15a | 4 | 98 | >300 | 297–299 (ref. 49) |
| 16 | 4-Br-C6H4 | H | 16a | 27 | 98 | >300 | 306–308 (ref. 32) |
| 17 | 4-CH3-C6H4 | H | 17a | 7 | 97 | >300 | 316–318 (ref. 50) |
| 18 | 3-NO2-C6H4 | H | 18a | 16 | 90 | 298–300 | 292–296 (ref. 32) |
| 19 | 2-CH3-C6H4 | H | 19a | 5 | 98 | >300 | New |
| 20 | 2-Br-C6H4 | H | 20a | 5 | 97 | >300 | New |
| 21 | 4-SCH3-C6H4 | H | 21a | 3 | 95 | >300 | New |
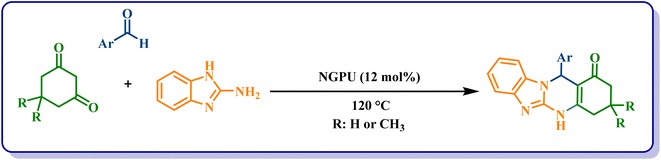
In the next step, the effectiveness of NGPU as the catalyst was studied in the preparation of benzazolo[2,1-b]quinazolinones (Scheme 2). Firstly, the reaction of 4-chlorobenzaldehyde, dimedone and 2-aminobenzimidazole was studied under the influence of different factors and the best condition was selected according to entry 4 from Table 4. Then the reaction was carried out on aromatic aldehydes containing both electron-withdrawing and electron-donating substituents and all of the named target compounds were obtained with high yields in short reaction times (Table 5).
| Entry | Catalyst (mol%) | Solvent | Temperature (°C) | Time (min) | Conversion (%) |
|---|---|---|---|---|---|
| 1 | NGPU (2) | — | 120 | 90 | 100 |
| 2 | NGPU (4) | — | 120 | 40 | 100 |
| 3 | NGPU (8) | — | 120 | 25 | 100 |
| 4 | NGPU (12) | — | 120 | 22 | 100 |
| 5 | NGPU (16) | — | 120 | 28 | 100 |
| 6 | NGPU (12) | — | 100 | 45 | 100 |
| 7 | NGPU (12) | EtOH | Reflux | 80 | Not completed |
| 8 | NGPU (12) | CH3CN | Reflux | 80 | Not completed |
| 9 | NGPU (12) | H2O | Reflux | 80 | Mixture of products |
| 10 | Glucose/pregabalin/urea (12) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
— | 120 | 40 | 100 |
| 11 | Glucose/pregabalin/urea (12) 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
— | 120 | 45 | 100 |
| 12 | Pregabalin (4) | — | 120 | 100 | Not completed |
| 13 | Glucose (4) | — | 120 | 100 | Not completed |
| 14 | Urea (4) | — | 120 | 100 | Mixture of products |
| 15 | — | — | 120 | 100 | Trace |
| Entry | Ar | R | Product | Time (min) | Yieldb (%) | M.p. (°C) | |
|---|---|---|---|---|---|---|---|
| Found | Reported | ||||||
| a Reaction conditions: aldehyde (1 mmol), 1,3-diketones (dimedone and/or 1,3-cyclohexanediones) (1 mmol), 2-aminobenzimidazole (1 mmol).b Isolated yields.c Isolated yields after recrystallization. | |||||||
| 1 | C6H5 | CH3 | 1b | 29 | 99 | >300 | >300 (ref. 28) |
| 2 | 4-Cl–C6H4 | CH3 | 2b | 22 | 98 | >300 | >300 (ref. 32) |
| 3 | 4-Br–C6H4 | CH3 | 3b | 33 | 98 | >300 | >300 (ref. 32) |
| 4 | 4-OH–C6H4 | CH3 | 4b | 14 | 99 | >300 | >300 (ref. 52) |
| 5 | 2,4-Cl2–C6H3 | CH3 | 5b | 27 | 98 | >300 | >300 (ref. 32) |
| 6 | 3,4,5-(OCH3)3–C6H2 | CH3 | 6b | 12 | 95 | >300 | >300 (ref. 53) |
| 7 | 4-CH3–C6H4 | CH3 | 7b | 13 | 99 | >300 | >300 (ref. 32) |
| 8 | 2-CH3–C6H4 | CH3 | 8b | 8 | 99 | >300 | >300 (ref. 28) |
| 9 | 3-NO2–C6H4 | CH3 | 9b | 18 | 85c | >300 | >300 (ref. 32) |
| 10 | C6H5 | H | 10b | 6 | 98 | >300 | 310–312 (ref. 54) |
| 11 | 2-CH3–C6H4 | H | 11b | 15 | 96 | >300 | New |
| 12 | 2-Br–C6H4 | H | 12b | 7 | 89 | >300 | New |
| 13 | 3,4,5-(OCH3)3–C6H2 | H | 13b | 2 | 99 | >300 | >300 (ref. 55) |
| 14 | 4-Pyridinebenzaldehyde | H | 14b | 18 | 95 | >300 | New |
| 15 | 4-NO2–C6H4 | H | 15b | 20 | 95 | >300 | >300 (ref. 54) |
| 16 | 4-F-C6H4 | H | 16b | 24 | 90 | >300 | >300 (ref. 54) |
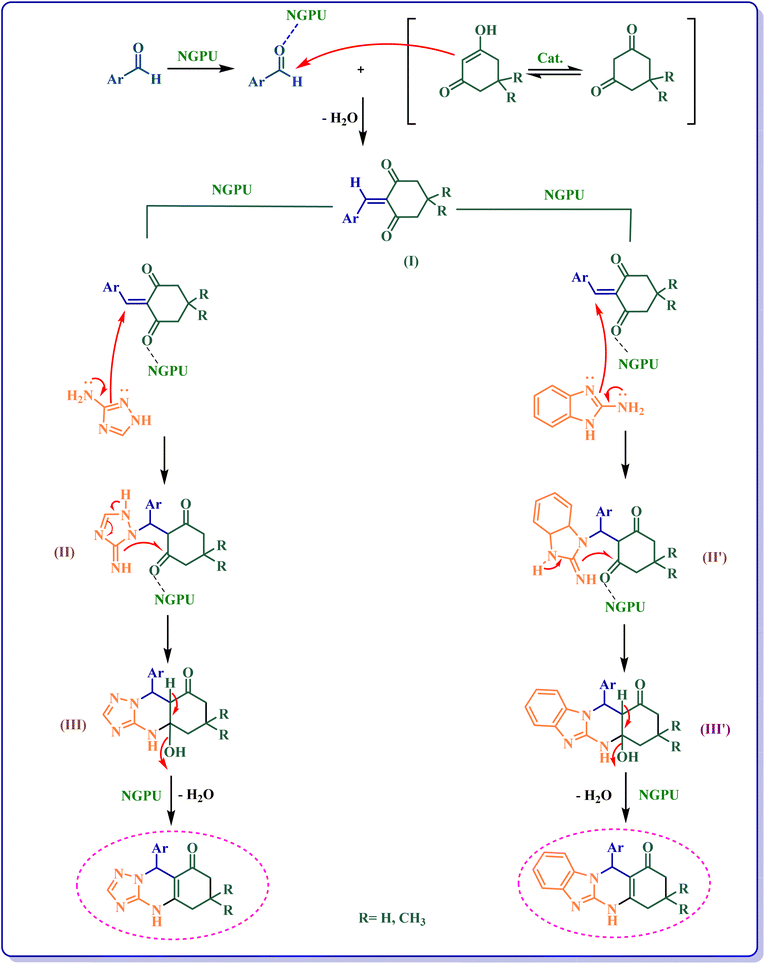
Scheme 3 shows the probable pathway of the synthesis of [1,2,4]triazoloquinazolinones and benzimidazoquinazolinones in the presence of NGPU. According to this mechanism, NGPU can activate the aldehyde against the nucleophilic attack of β-diketone leading to intermediate I. The reaction of this intermediate and nitrogen number 2 of 3-amino-1,2,4-triazole or 2-amino-benzimidazole via Michael addition produces intermediate II or II′. Then these intermediates lead to III or III′ which, by intermolecular cyclization followed by removal of a molecule of water, afford the desired products.51
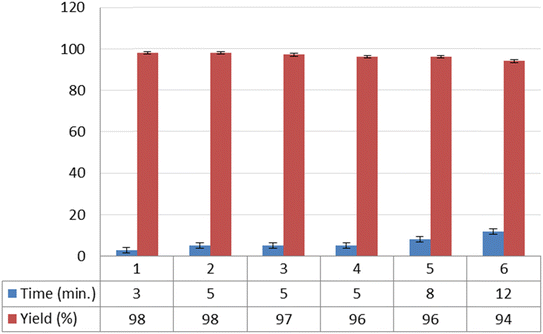
Reusability is a key feature of a catalyst and shows its compatibility with the rules of green chemistry. So to investigate this feature for NGPU, the synthesis of 9-(4-hydroxyphenyl)-6,6-dimethyl-5,6,7,9-tetrahydro-[1,2,4]triazolo [5,1-b]quinazolin-8(4H)-one was studied as the model one. At the end of the reactions, water was added and stirred for 10 min (NGPU is soluble in water), the mixture was filtered off and the solvent was evaporated from the filtrate under vacuum (70 °C). This procedure was repeated six times, and each time the desired product was obtained with an insignificant variation in reaction time and yield, a result which clarifies the practical recyclability of the catalyst (Fig. 6). The FT-IR of the same recovered and freshly prepared NGPU shows its stability under the selected conditions.
Table 6 compares the results obtained from the synthesis of 9-(4-hydroxyphenyl)-6,6-dimethyl-5,6,7,9-tetrahydro-[1,2,4] triazolo[5,1-b]quinazolin-8(4H)-one and 12-(4-hydroxyphenyl)-3,3-dimethyl-3,4,5,12-tetrahydrobenzo[4,5]imidazo[2,1-b] quinazolin-1(2H)-one in the presence of NGPU with some of the previously reported catalysts. This comparison is good evidence to accept that the present method is superior in terms of the efficiency, reaction times and the catalyst amounts.
| Product | Catalyst (mol%) | Reaction conditions | Time (min) | Yield (%) |
|---|---|---|---|---|
| a This work. | ||||
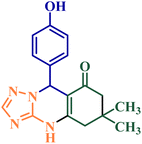 |
[DABCO](SO3H)2(HSO4)2 (0.02 mmol)32 | Solvent free/100 °C | 120 | 90 |
| NH2SO3H (50 mol%)56 | CH3CN/reflux | 60 | 89 | |
| [C4(H-DABCO)2][HSO4]4 (16 mg)33 | Solvent free/90 °C | 28 | 87 | |
| SBA–Pr–SO3H (5 mg)34 | Solvent free/r.t | 10 | 85 | |
| [(DABCO)2C3H5OH] 2Cl (5.7 mol%)31 | Solvent free/100 °C | 40 | 95 | |
NGPU (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) (4 mol%)a 5) (4 mol%)a |
120 °C | 18 | 95 | |
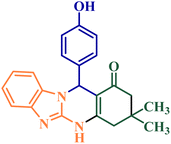 |
[DABCO](SO3H)2(HSO4)2 (0.02 mmol)32 | Solvent free/100 °C | 120 | 90 |
| NH2SO3H (0.05 mmol)56 | CH3CN/reflux | 20 | 90 | |
| Na+-MMT-[pmim]HSO4 (50 mg)35 | Solvent free/110 °C | 60 | 91 | |
| SBA–Pr–SO3H (5 mg)34 | Solvent free/r.t | 15 | 87 | |
| Fe3O4@chitosan (2 mg)36 | Ethanol/40 °C | 135 | 95 | |
NGPU (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) (12 mol%)a 5) (12 mol%)a |
120 °C | 14 | 99 | |
Conclusions
In this study, a novel natural deep eutectic mixture (DEM) was prepared from glucose, urea, and pregabalin in three different ratios. The catalytic activity of the DEM was then investigated in a one-pot synthesis of triazolo[2,1-b]quinazolinone and benzazolo[2,1-b]quinazolinone derivatives. The study found that the optimal ratio of the DEM for the synthesis of triazolo/benzazolo[2,1-b]quinazolinone was 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (NGPU), which was named after the components used in its preparation. This finding highlights the importance of optimizing the ratio of the components in the preparation of the DEM for specific applications.
5 (NGPU), which was named after the components used in its preparation. This finding highlights the importance of optimizing the ratio of the components in the preparation of the DEM for specific applications.
The reaction was also tested under conditions without a catalyst, and the results were compared with the results obtained using the NGPU catalyst. The current protocol is based on the use of readily available natural components for the synthesis of the catalyst, simple and direct work-up procedures, use of a reusable and biodegradable catalyst, short reaction times, high purity, and high yields of the desired product, all major advantages of this protocol. Further studies are currently underway in our laboratory to investigate additional responses that may be mediated by this natural deep eutectic mixture.
Experimental
Material
Chemicals were purchased from the Fluka, Merck and Aldrich Chemical Companies. Yields refer to the isolated products. Products were characterized by their physical constants and spectral analysis techniques. All the reactions were monitored by thin layer chromatography (TLC) on silica-gel polygram SILG/UV 254 plates using UV light as a means of detection.Instrumentation
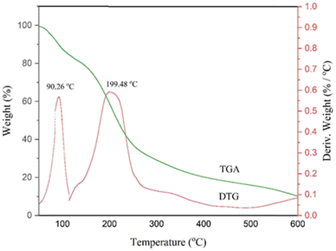
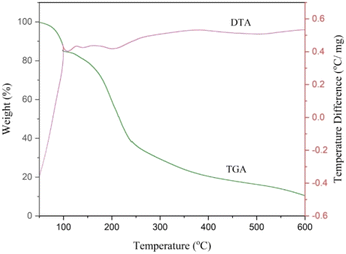
FT-IR spectra were recorded on a VERTEX 70 (Brucker, Germany) instrument using KBr pellets for samples ranging from 4000 to 400 cm−1. 1H NMR and 13C NMR were performed on 400 and 500 MHz Bruker Avance in DMSO-d6 using TMS as the internal standard. Melting points were determined using an IA9100 electric heating apparatus (Germany). Thermogravimetric analysis (TGA), derivative thermogravimetry (DTG) and differential thermal analysis (DTA) were performed on a Polymer Laboratories PL-TGA thermal analyzer. The sample was heated from 25 to 600 °C with a gradient of 20 °C min−1 under an Ar atmosphere (America). Refractive index measurements of the samples were made at a wavelength of 589 nm using a calibrated semi-automatic digital refractometer (model CETI) with a manufacturer-specified uncertainty of 0.0002 nD (Germany). The device was calibrated with HPLC water and acetone before use. According to various measurements, the refractive index measurement uncertainty was estimated to be 0.0005 nD. The instrument temperature was held constant and measured with an uncertainty of 0.02 K. The absorption spectra were recorded over the wavelength range 200–800 nm using a Cary double beam UV-visible spectrophotometer (model 100) at room temperature (Australia). The uncertainty in the measured wavelength was found to be 0.1 nm. Quartz cells were used for measurements in a solution vial = 1 cm.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 was heated in a test tube at 110 °C under constant stirring for 30 min. During this period of time, the requested deep eutectic mixture (NGPU) appeared as a clear and homogeneous liquid and was collected without further purification (Scheme 4). The obtained product was characterized using FT-IR, TGA, DTG, DTA, RI and UV-vis spectra techniques.
5 was heated in a test tube at 110 °C under constant stirring for 30 min. During this period of time, the requested deep eutectic mixture (NGPU) appeared as a clear and homogeneous liquid and was collected without further purification (Scheme 4). The obtained product was characterized using FT-IR, TGA, DTG, DTA, RI and UV-vis spectra techniques.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) (8 mg, 4 mol%) were mixed and stirred at 120 °C. The reaction was followed-up by TLC [EtOAc: n-hexane (3
5) (8 mg, 4 mol%) were mixed and stirred at 120 °C. The reaction was followed-up by TLC [EtOAc: n-hexane (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7)]. After completion of the reaction, the solid product was separated by the addition of water (20 mL), which solvates the catalyst, and filtration. Washing with water (10 mL) and recrystallization from ethanol (if needed) led to the requested product with a high purity. The spectral data of the new compounds are as follows.
7)]. After completion of the reaction, the solid product was separated by the addition of water (20 mL), which solvates the catalyst, and filtration. Washing with water (10 mL) and recrystallization from ethanol (if needed) led to the requested product with a high purity. The spectral data of the new compounds are as follows.
9-(2-Methyl)-5,6,7,9-tetrahydro-[1,2,4]triazolo[5,1-b] quinazolin-8(4H)-one (19a). FT-IR (KBr): ν = 3427, 3089, 2923, 1683, 1583, 1473, 1415, 1365, 1192, 738 cm−1; 1H NMR (400 MHz, DMSO-d6) σ: 1.89–2.02 (m, 2H), 2.23–2.30 (m, 2H), 2.58 (s, 3H), 2.58–2.72 (m, 2H), 6.46 (s, 1H), 7.02–7.16 (m, 4H), 7.65 (s, 1H), 11.14 (s, 1H, NH) ppm; 13C NMR (125 MHz, DMSO-d6) σ: 19.4, 21.2, 26.8, 36.8, 54.7, 107.7, 126.8, 127.3, 128.0, 130.5, 136.2, 140.8, 146.9, 150.4, 153.1, 193.8 ppm.
9-(2-Bromophenyl)-5,6,7,9-tetrahydro-[1,2,4]triazolo[5,1-b] quinazolin-8(4H)-one (20a). FT-IR (KBr): ν = 3430, 3087, 2917, 1644, 1415, 1365, 1191, 752, 534 cm−1; 1H NMR (500 MHz, DMSO-d6) σ: 1.89–1.94 (m, 2H), 2.15–2.22 (m, 2H), 2.63–2.64 (m, 2H), 6.55 (s, 1H), 7.09–7.36 (m, 3H), 7.42–7.80 (m, 2H), 11.20 (s, 1H, NH) ppm; 13C NMR (125 MHz, DMSO-d6) σ: 20.7, 26.5, 36.4, 39.1, 39.3, 58.0, 106.0, 127.9, 129.6, 132.8, 146.6, 150.1, 153.1, 193.2 ppm.
9-(4-(Methylthio)phenyl)-5,6,7,9-tetrahydro-[1,2,4]triazolo [5,1-b]quinazolin-8(4H)-one (21a). FT-IR (KBr): ν = 3424, 3088, 2912, 1645, 1587, 1480, 1363, 1186, 732 cm−1; 1H NMR (500 MHz, DMSO-d6) σ: 1.87–1.94 (m, 2H), 2.19–2.26 (m, 2H), 2.40 (s, 3H), 2.60–2.63 (m, 2H), 6.15 (s, 1H), 7.10–7.14 (m, 4H), 7.65 (s, 1H), 11.10 (s, 1H, NH) ppm; 13C NMR (125 MHz, DMSO-d6) σ: 14.6, 39.1, 39.3, 57.3, 106.5, 125.7, 127.6, 137.6, 146.7 ppm.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) (16 mg, 8 mol%) were mixed in a round bottom flask (50 mL) and stirred at 120 °C. Upon completion of the reaction, which was determined by TLC [EtOAc: n-hexane (3
5) (16 mg, 8 mol%) were mixed in a round bottom flask (50 mL) and stirred at 120 °C. Upon completion of the reaction, which was determined by TLC [EtOAc: n-hexane (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7)], water (20 mL) was added to the mixture; the solid product (which is not soluble in water) was filtered, washed with water (10 mL) and dried. The spectral data of the new compounds are as follows.
7)], water (20 mL) was added to the mixture; the solid product (which is not soluble in water) was filtered, washed with water (10 mL) and dried. The spectral data of the new compounds are as follows.
12-(2-Methyl)-3,4,5,12-tetrahydrobenzo[4,5]imidazo[2,1-b] quinazolin-1(2H)-one (11b). FT-IR (KBr): ν = 3442, 3044, 2903, 1649, 1618, 1567, 1459, 1368, 737 cm−1; 1H NMR (500 MHz, DMSO-d6) σ: 1.81–1.95 (m, 2H), 2.16–2.26 (m, 2H), 2.57 (s, 3H), 2.64–2.68 (m, 2H), 6.47 (s, 1H), 6.86–7.35 (m, 8H), 11.05 (s, 1H, NH) ppm; 13C NMR (125 MHz, DMSO-d6) σ: 18.9, 20.8, 26.6, 36.5, 39.1, 39.3, 109.4, 117.0, 120.5, 121.8, 126.3, 127.4, 130.3, 132.1, 135.3, 141.9, 145.1, 152.1, 193.2 ppm.
12-(2-Bromophenyl)-3,4,5,12-tetrahydrobenzo[4,5]imidazo [2,1-b]quinazolin-1(2H)-one (12b). FT-IR (KBr): ν = 3453, 3041, 2957, 1654, 1616, 1570, 1445, 1368, 746, 543 cm−1; 1H NMR (500 MHz, DMSO-d6) σ: 1.84–1.96 (m, 2H), 2.05–2.26 (m, 2H), 2.65–2.68 (m, 2H), 6.60 (s, 1H), 6.91–7.49 (m, 8H), 11.20 (s, 1H, NH) ppm; 13C NMR (125 MHz, DMSO-d6) σ: 20.8, 26.7, 30.7, 36.5, 39.0, 79.2, 109.7, 117.1, 120.5, 128.1, 129.6, 132.1, 132.9, 141.8, 144.9, 152.7, 192.8 ppm.
12-(Pyridin-4-yl)-3,4,5,12-tetrahydrobenzo[4,5]imidazo[2,1-b] quinazolin-1(2H)-one (14b). FT-IR (KBr): ν = 3423, 3033, 2955, 1647, 1613, 1567, 1428, 1373, 1193, 743 cm−1; 1H NMR (500 MHz, DMSO-d6) σ: 1.88–2.02 (m, 2H), 2.23–2.35 (m, 2H), 2.72 (t, J = 11.1, 2H), 6.50 (s, 1H), 6.97–7.07 (m, 1H), 7.09–7.12 (m, 1H), 7.26 (d, J = 7.9, 2H), 7.30–7.32 (m, 1H), 7.42 (d, J = 7.8, 1H), 8.45–8.47 (m, 2H), 11.28 (s, 1H, NH) ppm; 13C NMR (125 MHz, DMSO-d6) σ: 21.1, 27.0, 36.7, 53.6, 110.6, 110.3, 117.6, 121.2, 122.5, 122.6, 132.2, 142.4, 145.6, 150.0, 150.3, 153.6, 193.5 ppm.
Conflicts of interest
There are no conflicts to declare.Notes and references
- M. Zabihzadeh, A. Omidi, F. Shirini, H. Tajik and M. S. N. Langarudi, J. Mol. Struct., 2020, 1206, 127730–127739 CrossRef CAS.
- M. Yarie, M. A. Zolfigol, Y. Bayat, A. Asgari, D. A. Alonso and A. Khoshnood, RSC Adv., 2016, 6, 82842–82853 RSC.
- D. Aute, A. Kshirsagar, B. Uphade and A. Gadhave, J. Chem. Sci., 2020, 132, 1–10 CrossRef.
- A. Si and A. K. Misra, Transformation of carbohydrates under green and sustainable reaction conditions, in Recent Trends in Carbohydrate Chemistry, Elsevier, 2020, p. 3 Search PubMed.
- M. Mazloumi and F. Shirini, J. Mol. Struct., 2020, 1217, 128326 CrossRef CAS.
- M. A. Zolfigol, A. Khazaei, A. R. Moosavi-Zare, J. Afsar, V. Khakyzadeh and O. Khaledian, J. Chin. Chem. Soc., 2015, 62, 398–403 CrossRef CAS.
- B. P. Reddy, K. Rajesh and V. Vijayakumar, Arabian J. Chem., 2015, 8, 138–141 CrossRef.
- I. Dindarloo Inaloo and S. Majnooni, ChemistrySelect, 2019, 4, 7811–7817 CrossRef CAS.
- X. Chen, Y. Cui, H. B. Gobeze and D. G. Kuroda, J. Phys. Chem. B, 2020, 124, 4762–4773 CrossRef CAS PubMed.
- A. Maleki, F. Hassanzadeh-Afruzi, Z. Varzi and M. S. Esmaeili, Mater. Sci. Eng., C, 2020, 109, 110502–110532 CrossRef CAS PubMed.
- Y. Liu, J. B. Friesen, J. B. McAlpine, D. C. Lankin, S.-N. Chen and G. F. Pauli, J. Nat. Prod., 2018, 81, 679–690 CrossRef CAS PubMed.
- L. I. Tomé, V. Baião, W. da Silva and C. M. Brett, Appl. Mater. Today, 2018, 10, 30–50 CrossRef.
- A. Sanchez-Fernandez, O. S. Hammond, A. J. Jackson, T. Arnold, J. Doutch and K. J. Edler, Langmuir, 2017, 33, 14304–14314 CrossRef CAS PubMed.
- A. Shishov, A. Gerasimov and A. Bulatov, Food Chem., 2022, 366, 130634 CrossRef CAS PubMed.
- M. A. Khoshdel, F. Shirini, M. S. N. Langarudi, M. Zabihzadeh and M. Biglari, New J. Chem., 2021, 45, 3138–3149 RSC.
- S. Miao, H. J. Jiang, S. Imberti, R. Atkin and G. Warr, J. Chem. Phys., 2021, 154, 214504–214512 CrossRef CAS PubMed.
- A. Shendurse and C. Khedkar, Glucose: Properties and Analysis, Encyclopedia of Food and Health, Academic Press, Oxford, 2016, p. 239 Search PubMed.
- Z. Mohammadpour, S. H. Abdollahi and A. Safavi, ACS Appl. Energy Mater., 2018, 1, 5896–5906 CrossRef CAS.
- S. Zulkefli, E. Abdulmalek and M. B. A. Rahman, Renewable Energy, 2017, 107, 36–41 CrossRef CAS.
- M. F. Lanjwani, M. Tuzen, M. Y. Khuhawar, M. R. Afshar Mogaddam and M. A. Farajzadeh, Crit. Rev. Anal. Chem., 2022, 1–14 CrossRef PubMed.
- K. Wu, T. Su, D. Hao, W. Liao, Y. Zhao, W. Ren, C. Deng and H. Lü, Chem. Commun., 2018, 54, 9579–9582 RSC.
- J. L. Pablos, P. Tiemblo, G. Ellis and T. Corrales, Polym, 2021, 13, 1050 CAS.
- O. Goli-Jolodar and F. Shirini, J. Iran. Chem. Soc., 2017, 14, 1235–1241 CrossRef CAS.
- M. R. Khodabakhshi, M. Kiamehr and R. Karimian, Polycyclic Aromat. Compd., 2022, 42, 4086–4100 CrossRef CAS.
- A. Tambe, A. Gadhave, A. Pathare and G. Shirole, Sustainable Chem. Pharm., 2021, 22, 100485 CrossRef CAS.
- S. Vibhute, D. Jamale, S. Undare, N. Valekar, G. Kolekar and P. Anbhule, Res. Chem. Intermed., 2017, 43, 4561–4574 CrossRef CAS.
- I. Khan, A. Ibrar, W. Ahmed and A. Saeed, Eur. J. Med. Chem., 2015, 90, 124–169 CrossRef CAS PubMed.
- O. Goli-Jolodar and F. Shirini, J. Iran. Chem. Soc., 2017, 14, 2275–2286 CrossRef CAS.
- J. Yang, S. Xiong, Y. Ren, T. Xiao and Y. Jiang, Org. Biomol. Chem., 2020, 18, 7174–7182 RSC.
- M. M. Heravi, L. Ranjbar, F. Derikvand, B. Alimadadi, H. A. Oskooie and F. F. Bamoharram, Mol. Diversity, 2008, 12, 181–185 CrossRef CAS PubMed.
- S. Nazari, M. Zabihzadeh, F. Shirini and H. Tajik, Polycyclic Aromat. Compd., 2023, 43, 1524–1535 CrossRef CAS.
- N. Seyyedi, F. Shirini, M. S. N. Langarudi and S. Jashnani, J. Iran. Chem. Soc., 2017, 14, 1859–1867 CrossRef CAS.
- N. Safari, F. Shirini and H. Tajik, J. Mol. Struct., 2020, 1201, 127143 CrossRef CAS.
- G. M. Ziarani, A. Badiei, Z. Aslani and N. Lashgari, Arabian J. Chem., 2015, 8, 54–61 CrossRef.
- F. Shirini, M. Mazloumi and M. Seddighi, J. Nanosci. Nanotechnol., 2018, 18, 1194–1198 CrossRef CAS PubMed.
- A. Maleki, M. Aghaei and N. Ghamari, Chem. Lett., 2015, 44, 259–261 CrossRef CAS.
- V. N. Mahire, V. E. Patel and P. P. Mahulikar, Res. Chem. Intermed., 2017, 43, 1847–1861 CrossRef CAS.
- F. Malamiri, S. Khaksar, R. Badri and E. Tahanpesar, Curr. Org. Synth., 2019, 16, 1185–1190 CrossRef CAS PubMed.
- P. Verma, N. G. Shah and S. M. Mahajani, Food Anal. Methods, 2020, 13, 2087–2101 CrossRef.
- S. A. Ghumman, S. Bashir, S. Noreen, A. M. Khan and M. Z. Malik, Int. J. Biol. Macromol., 2019, 139, 1191–1202 CrossRef CAS PubMed.
- J. M. Jones and A. N. Rollinson, Thermochim. Acta, 2013, 565, 39–45 CrossRef CAS.
- C. Reichardt and T. Welton, Solvents and Solvent Effects in Organic Chemistry, John Wiley & Sons, 2011 Search PubMed.
- A. Ghanadzadeh, A. Zeini, A. Kashef and M. Moghadam, Spectrochim. Acta, Part A, 2009, 73, 324–329 CrossRef CAS PubMed.
- M. Dashteh, S. Baghery, M. A. Zolfigol, A. Khazaei, S. Makhdoomi, M. Safaiee, J. Rakhtshah, Y. Bayat and A. Asgari, J. Phys. Chem. Solids, 2022, 160, 110322–110331 CrossRef CAS.
- H. Sharghi, J. Aboonajmi, M. Aberi and P. Shiri, J. Iran. Chem. Soc., 2018, 15, 1107–1118 CrossRef CAS.
- K. Kumari, D. Raghuvanshi and K. N. Singh, Org. Prep. Proced. Int., 2012, 44, 460–466 CrossRef CAS.
- M. Haghighat, F. Shirini and M. Golshekan, J. Mol. Struct., 2018, 1171, 168–178 CrossRef CAS.
- K. A. Shaikh, S. R. Kande and C. Khillare, IOSR J. Appl. Chem., 2014, 7, 54–58 CrossRef.
- M. Mazloumi and F. Shirini, Polycyclic Aromat. Compd., 2022, 1–15 Search PubMed.
- R. Sompalle, P. Arunachalam and S. M. Roopan, J. Mol. Liq., 2016, 224, 1348–1357 CrossRef CAS.
- M. Karimi and M. Naimi-Jamal, J. Saudi Chem. Soc., 2019, 23, 182–187 CrossRef CAS.
- F. Shirini, M. Seddighi and O. Goli-Jolodar, J. Iran. Chem. Soc., 2016, 13, 2013–2018 CrossRef CAS.
- B. Insuasty, A. Salcedo, J. Quiroga, R. Abonia, M. Nogueras, J. Cobo and S. Salido, Heterocycl. Commun., 2004, 10, 399–404 CAS.
- E. Tabrizian and A. Amoozadeh, RSC Adv., 2016, 6, 96606–96615 RSC.
- M. V. Reddy, G. C. S. Reddy and Y. T. Jeong, RSC Adv., 2015, 5, 11423–11432 RSC.
- M. M. Heravi, F. Derikvand and L. Ranjbar, Synth. Commun., 2010, 40, 677–685 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra05199d |
| This journal is © The Royal Society of Chemistry 2023 |