Open Access Article
Open Access ArticleOptimization of green deep eutectic solvent (DES) extraction of Chenopodium quinoa Willd. husks saponins by response surface methodology and their antioxidant activities†
Yu-Qing Cai‡
a,
Hui Gao‡a,
Lin-Meng Songa,
Fei-Yan Taoa,
Xue-Ying Jia,
Yuan Yua,
Yu-Qing Caoa,
Shao-Jian Tang*b and
Peng Xue *a
*a
aSchool of Public Health, Weifang Medical University, Shandong 261042, PR China. E-mail: jplxp26@126.com
bSchool of Pharmacy, Weifang Medical University, Shandong 261042, PR China. E-mail: tangsj@wfmc.edu.cn; Tel: +86 0536-8462429
First published on 9th October 2023
Abstract
Quinoa saponins have outstanding activity, and there are an increasing number of extraction methods, but there are few research programs on green preparation technology. The extraction conditions of quinoa saponins with deep eutectic solvents (DESs) were optimized by single-factor experiments combined with response surface methodology. The antioxidant capacity of saponins extracted by DESs and traditional methods was evaluated by the DPPH clearance rate, iron ion chelation rate and potassium ferricyanide reducing power. The results show that the optimal DES is choline chloride: 1,2-propylene glycol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and its water content is 40%. The optimal extraction conditions were as follows: the solid-to-solvent ratio was 0.05 g mL−1, the extraction time was 89 min, and the extraction temperature was 75 °C. Under these conditions, the extraction of quinoa saponins by DES was more effective than the traditional extraction methods. The saponins extracted by DES and traditional methods were analyzed by UPLC-MS, and five main saponins were identified. Quantitative analysis by HPLC-UV showed that Q1 (m/z = 971) and Q2 (m/z = 809) had higher contents of saponins. In vitro antioxidant experiments showed that all DES saponin extracts showed good antioxidant capacity. This study provides new insight into the development and utilization of quinoa saponins.
1), and its water content is 40%. The optimal extraction conditions were as follows: the solid-to-solvent ratio was 0.05 g mL−1, the extraction time was 89 min, and the extraction temperature was 75 °C. Under these conditions, the extraction of quinoa saponins by DES was more effective than the traditional extraction methods. The saponins extracted by DES and traditional methods were analyzed by UPLC-MS, and five main saponins were identified. Quantitative analysis by HPLC-UV showed that Q1 (m/z = 971) and Q2 (m/z = 809) had higher contents of saponins. In vitro antioxidant experiments showed that all DES saponin extracts showed good antioxidant capacity. This study provides new insight into the development and utilization of quinoa saponins.
1. Introduction
Quinoa, an annual dicotyledonous plant of the amaranthus family, grows at high altitudes, has strong tolerance and adaptability to cold and arid environments, and has received extensive attention from the scientific community because of its high nutritional value.1,2 Quinoa is rich in proteins, amino acids, starch, fiber, and minerals, especially amino acids, which are rich and balanced, and its seeds have a high content of saponins and flavonol glycosides. In traditional studies, mechanical grinding is often used to remove husks. Due to the high content of saponins in the husks of quinoa seeds (approximately 3–8% of the whole plant), thus wasting a lot of saponin resources. Current in vitro and in vivo bioactivity studies have shown that quinoa saponins have a wide range of beneficial properties, including antioxidant, antidiabetic, anti-inflammatory, antimicrobial, antidiabetic, anti-inflammatory, antimicrobial and anticancer functions.3 Although quinoa saponins have many activities, the practical application of quinoa saponins has not received attention; a few of them are included in feed, and most of them are discarded, which not only results in the loss of active substances and the waste of resources but also poses a threat to the environment.4,5Deep eutectic solvents (DESs) were first proposed by Abbott in 2003 and are considered green solvents because of their advantages, such as simple synthesis, designable structure and environmental friendliness.6,7 In recent years, the use of deep eutectic solvents has been reported for the extraction of phenolic compounds in Carthamus tinctorius L., flavonoids from Pollen Typhae and polysaccharides from Camellia oleifera Abel.8 Quinoa saponin is one of the main active substances in quinoa husks, and the common extraction solvents used to extract saponin in the current study are methanol, ethanol, and water. Among the traditional extraction methods, the ultrasonic extraction method of 70% methanol and 70% ethanol had a better extraction effect. Espinoza et al. used ethanol solution as the extraction solvent to optimize the extraction conditions of quinoa saponin, and the extraction rate was greatly improved, but this method still required many organic solvents.9 These organic solvents have disadvantages such as environmental pollution, volatilization and toxicity.10 To improve the environment and reduce the pollution of organic solvents, deep eutectic solvents (DESs) have emerged as a new type of green nonpolluting extraction solvent. DESs are formed by the interaction of two or three hydrogen bond donors and hydrogen bond acceptors through hydrogen bonding or electrostatic forces and are mixtures with a lower melting point than the individual components, with a melting point of less than 100 °C.11–13 Hydrogen bond acceptors (HBAs) are mostly quaternary ammonium salts, quaternary phosphates, betaines, imidazolyl salts, etc.; hydrogen bond donors (HBDs) are mostly amides, carboxylic acids, polyols, etc. As shown in Fig. S1,† the most common DESs are based on choline chloride mixed with other ingredients, such as glycerol.14 Previously, Taco used choline chloride with glycerol to extract saponins from quinoa seeds, and the extraction rate was superior to that of conventional organic solvents. Saponins containing C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O are more conducive to the formation of hydrogen bonds, so the efficiency is increased. DES is too viscous and can lead to low extraction rate, because part of the saponin are soluble in water, the addition of water can improve the extraction rate.15 However, previous studies prepared a single DES and lacked studies on quinoa husks saponins, so the DESs prepared in this study were based on four types, acid-based, amino-based, sugar-based, and alcohol-based, with a wide variety of species, which broadened the idea of the study of plant compounds extracted by DESs. The main criteria for selecting the components of DESs in this study are that they are all easy to purchase in the market, low cost, high safety, good biodegradability, and recyclable, and the use of DESs to extract saponins from quinoa husks in this study provides a new way of thinking in the study of saponins.
O are more conducive to the formation of hydrogen bonds, so the efficiency is increased. DES is too viscous and can lead to low extraction rate, because part of the saponin are soluble in water, the addition of water can improve the extraction rate.15 However, previous studies prepared a single DES and lacked studies on quinoa husks saponins, so the DESs prepared in this study were based on four types, acid-based, amino-based, sugar-based, and alcohol-based, with a wide variety of species, which broadened the idea of the study of plant compounds extracted by DESs. The main criteria for selecting the components of DESs in this study are that they are all easy to purchase in the market, low cost, high safety, good biodegradability, and recyclable, and the use of DESs to extract saponins from quinoa husks in this study provides a new way of thinking in the study of saponins.
2. Materials and methods
2.1 Chemicals
Chenopodium quinoa Willd. husks were obtained from Ulanqab (Inner Mongolia, China). Q1 (3-O-β-D-glucopyranosyl-(1 → 3)-α-L-arabino-pyranosyl-phytolaccagenic acid 28-O-β-D-glucopyranosyl) and Q2 (3-O-α-L-arab-inopyranosyl phytolaccagenic acid 28-O-β-D-glucopyranosyl ester) (NMR, purity ≥ 98%) saponin controls were pre-prepared by laboratory. Choline chloride, betaine, citric acid, urea, glucose, 1,2-propylene glycol, glycerol, vanillin, perchloric acid, ethanol, DPPH, ferrous chloride and ferric chloride were purchased from China National Pharmaceutical Industry Co., Ltd. (Beijing, China). All chemicals were of analytical grade unless they were specially mentioned. Methanol and acetonitrile were of chromatographic purity and purchased from China National Pharmaceutical Industry Co., Ltd. (Beijing, China).2.2 Apparatus and instruments
The HPLC LC-20AT was obtained from Shimadzu Corp. (Japan). The UPLC-Q-Exactive was purchased from Thermo Fisher (Waltham, MA, USA). Freeze-drying system FDU-1200 was from Shanghai Airon Co. (Shanghai, China). The enzyme labeling instrument 1510 was from Thermo Fisher (Waltham, MA, USA). Solid phase extractor LC-CQ-24Y was purchased from Shanghai Bangxi Instrument Technology (Shanghai, China).2.3 Qualitative determination of saponins
The electrospray ionization (ESI) mass spectrometry (MS) data were recorded on an Thermo Fisher Scientific UPLC-Q-Exactiveinstrument with ACQUITY UPLC BEH C18 column (3.0 × 100 mm, 1.7 μm). The UPLC conditions for the UPLC-MS analysis were as follows: column temperature: 40 °C; injection volume: 4 μL; mobile phase A, acetonitrile; mobile phase B, 0.1% ammonium acetate solution; gradient elution (mobile phase A concentration): 0–5 min: 10% B, 5–10 min: 10–28% B, 10–15 min: 28–35% B, 15–20 min: 35–40% B, 20–25 min: 40–45% B, 25–30 min: 45–85% B, 30–32 min: 85–10% B, 32–37 min: 10% B; flow rate was 0.3 mL min−1.16The ESI parameters were as follows: isolation window was 4.0 m/z, AGC target was 3 × 106, the carrier gas (N2), sheath gas flow rate: 35 arb, the column oven was 30 °C, data were collected in negative ion mode [M−H]−, scans were conducted over 100–1500 m/z, the spray voltage was 3.5 kV, the capillary voltage was 50 V, and the capillary temperature was 320 °C.17
2.4 Quantitative determination of saponins
Q1 and Q2 were selected to draw the standard curve. Configure the working solutions of Q1 and Q2 standards 10 μg mL−1, 50 μg mL−1, 300 μg mL−1, 500 μg mL−1, 750 μg mL−1 and 1500 μg mL−1 respectively. Pipette 1 mL of the above working solution separately and pass through 0.45 μm microporous filter membrane. Elution was carried out according to the above chromatographic conditions and the peak area integral values were recorded. The standard curve is established with concentration as abscissa and peak area integral value as ordinate.
2.5 DES preparation
DESs were prepared by combining hydrogen bond donors (HBDs) and hydrogen bond acceptors (HBAs) in different molar ratios using the heating and stirring method as described in the literature.10 The heating method is the most commonly used synthetic method because it does not require purification and has a simple operation procedure. DESs were prepared by the heating and stirring method by weighing a certain molar ratio of HBD and HBA, mixing them in a beaker, adding a magnetic stirrer, adding an appropriate amount of water, heating in a water bath at 70 °C and stirring magnetically until a clear and transparent liquid was formed. A total of 42 DESs were prepared in this study. Choline chloride and betaine were used for HBD, and citric acid, urea, glucose, 1,2-propylene glycol, and glycerol were used for HBA. Information on the prepared DESs is shown in Table 1.| Type | No. | DESs | Mole ratio |
|---|---|---|---|
| Acid-based DESs | DES1 | Choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) citric acid citric acid |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 1 1; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2; 1 2; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3; 1 3; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
| DES2 | Betaine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) citric acid citric acid |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 1 1; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2; 1 2; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3; 1 3; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
|
| Amamine-based DESs | DES3 | Choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) urea urea |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 1 1; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2; 1 2; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3; 1 3; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
| DES4 | Betaine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) urea urea |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 1 1; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2; 1 2; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3; 1 3; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
|
| Sugar-based DESs | DES5 | Choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glucose glucose |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 1 1; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2; 1 2; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3; 1 3; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4; 2 4; 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| DES6 | Betaine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glucose glucose |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 1 1; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2; 1 2; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3; 1 3; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4; 2 4; 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
|
| Alcohol-based DESs | DES7 | Choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,2-propylene glycol 1,2-propylene glycol |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 1 1; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2; 1 2; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3; 1 3; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
| DES8 | Betaine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,2-propylene glycol 1,2-propylene glycol |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 1 1; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2; 1 2; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3; 1 3; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
|
| DES9 | Choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glycerol glycerol |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 1 1; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2; 1 2; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3; 1 3; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
|
| DES10 | Betaine![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) glycerol glycerol |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 1 1; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2; 1 2; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3; 1 3; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
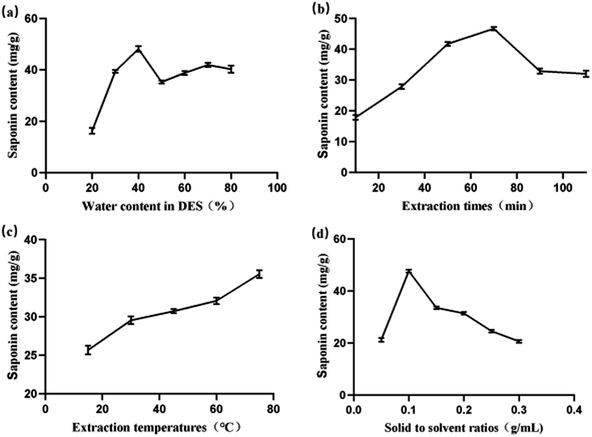
2.6 Screening of the water content of DESs
The water content of DESs is an important factor affecting the extraction effect of target components, therefore, the effect of different water content of DESs was examined. Different water contents (20, 30, 40, 50, 60, 70 and 80%) were examined using uniform solid-to-solvent ratios, extraction temperature and extraction time to finalize the optimum water content.2.7 HLB solid-phase extraction column treatment
In the traditional method, the separation and purification of saponins are generally carried out using macroporous resins. But the manual filling of the column is cumbersome and has low accuracy and a long operation time. HLB column was used in this study as a solid-phase extraction method for the purification of saponins. According to Jeong's method,12 the HLB column was processed as follows:The HLB column was activated with 12 mL of deionized water and 12 mL of methanol. The diluted samples were kept in the HLB column for 10 min and then passed through the HLB at a flow rate of 1 drop per s. The DES was recovered by freeze-drying effluent liquid. Then the HLB column was rinsed with methanol, the eluate was collected, and the saponins were obtained by evaporation.
2.8 Experimental design
| Factors | Levels | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| Extraction time A (min) | 50 | 70 | 90 |
| Extraction temperature B (°C) | 45 | 60 | 75 |
| Solid-to-solvent ratios C (g mL−1) | 0.05 | 0.1 | 0.15 |
2.9 Determination of antioxidant activity
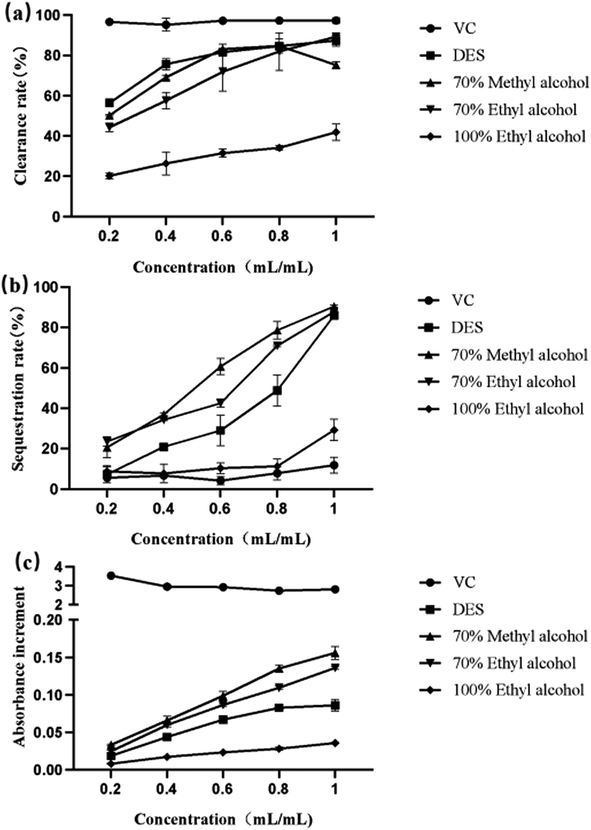
DES extract, 70% methanol extract, 70% ethanol extract, 100% ethanol extract, and 1 mmol per L VC solution using the same conditions for saponin extraction were configured into 0.20, 0.40, 0.60, 0.80, and 1.00 mL per mL solutions. VC solution was used as a positive control.
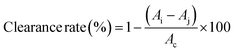 | (1) |
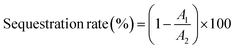 | (2) |
| Absorbance increment = Ai − A0 | (3) |
2.10 Statistical analysis
All experiments were conducted in triplicate. Data analysis was performed using SPSS Statistics version 25. The experiment data were analyzed statistically with Design-expert 13. Analysis of variance (ANOVA) was performed for calculations and modeling of optimal conditions. Values of p < 0.05 were regarded as significant.3. Results and analysis
3.1 Qualitative determination of saponins
The extracted saponins were characterized by ultrahigh-performance liquid chromatography-mass spectrometry (UPLC-MS) analysis, and UPLC-MS has higher sensitivity and a lower detection limit, and more saponins can be detected in the same sample.22 As shown in Fig. 1, although the peak times of the main saponins extracted from DES and 70% methanol and 70% ethanol were slightly different, the types of saponins were similar. The 100% ethanol extract had fewer saponin types, which was related to the low extraction rate.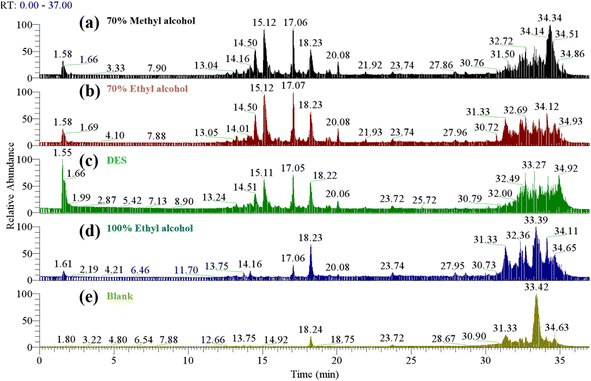 | ||
| Fig. 1 The total ion chromatogram of 70% methanol extract (a), 70% ethanol extract (b), DES extract (c) and 100% methanol extract (d), blank control (e). | ||
According to UPLC-MS analysis, the saponins extracted by the four extraction methods were hederagenin or phytolaccagenic acid saponins. According to the determined mass-charge ratio, the ion fragment information of the main saponins is shown in Fig. S2.†![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23 The mass-charge ratio is different from that of Dini because the negative ion mode [M−H]− was adopted in this study.23 The substance names are shown in Table S1,† which is consistent with the study.24 The retention time is different from the study of Colson, possibly because the elution procedure of UPLC-MS is different.25 In a previous study by Taco, a choline chlorine–glycerin–water system was used to extract quinoa saponins, and compared with traditional solvents, the main types of saponins identified were consistent with those in this study.15 The study of Taco on the thermal stability of DESs showed that green solvents were more stable than traditional solvent extracts of quinoa saponins, so DESs could replace traditional organic solvents to extract quinoa husks saponins.
23 The mass-charge ratio is different from that of Dini because the negative ion mode [M−H]− was adopted in this study.23 The substance names are shown in Table S1,† which is consistent with the study.24 The retention time is different from the study of Colson, possibly because the elution procedure of UPLC-MS is different.25 In a previous study by Taco, a choline chlorine–glycerin–water system was used to extract quinoa saponins, and compared with traditional solvents, the main types of saponins identified were consistent with those in this study.15 The study of Taco on the thermal stability of DESs showed that green solvents were more stable than traditional solvent extracts of quinoa saponins, so DESs could replace traditional organic solvents to extract quinoa husks saponins.
3.2 Quantitative determination of saponins
According to the results of HPLC analysis in Fig. S3,† Q1 and Q2 were the major saponin elements in all tested quinoa husks samples regardless of whether the quinoa saponins were extracted using conventional or green solvents, so the extracted saponins were quantified using Q1 and Q2 saponin controls. According to the analysis of the literature, the mass-charge ratio m/z = 971 for Q1 and m/z = 809 for Q2 and the ionic peak of the saponin fragment are shown in Fig. S2(c).†![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23,24
23,24
3.3 Deep eutectic solvent screening
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) had the highest saponin extraction rate. Because DES5, DES6, DES7, and DES8, these DESs themselves will react with the saponin chromogenic reaction, and the vanillin glacial acetic acid-perchloric acid colorimetric method will produce errors. Therefore, these are determined using the 3.3.2 method for saponin content.
4) had the highest saponin extraction rate. Because DES5, DES6, DES7, and DES8, these DESs themselves will react with the saponin chromogenic reaction, and the vanillin glacial acetic acid-perchloric acid colorimetric method will produce errors. Therefore, these are determined using the 3.3.2 method for saponin content.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 719, R2 = 0.989 was obtained after measurement, and the Q2 standard curve Y3 = 329063X3 + 65
719, R2 = 0.989 was obtained after measurement, and the Q2 standard curve Y3 = 329063X3 + 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 472, R2 = 0.9962 was obtained after measurement. DES extracts were treated as in section 3.3.1. We determined the contents of the saponins extracted from DES4 (1
472, R2 = 0.9962 was obtained after measurement. DES extracts were treated as in section 3.3.1. We determined the contents of the saponins extracted from DES4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4), DES5, DES6, DES7, and DES8 using liquid chromatography and the results showed that DES7 (1
4), DES5, DES6, DES7, and DES8 using liquid chromatography and the results showed that DES7 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) had the highest saponin extraction rate.
1) had the highest saponin extraction rate.3.4 Determination of DES water content
DES has a strong viscosity. To make DES easy to handle, water is usually added to reduce the viscosity of DES but also changes the polarity of DES.26 Different polarities of the solvent on quinoa saponin solubility are different, and adding the appropriate amount of water can improve the saponin extraction rate. For example, ethanol aqueous solution with higher polarity is more effective than pure ethanol. In the study of Taco, two DESs, choline–glycerin chloride or choline–glycerin–water, were used to extract quinoa saponins.15 Comparing the two extraction methods, it was found that the extraction rate of saponins in the choline–glycerin–water system was higher. This indicates that the saponin extraction rate can be improved mainly by increasing the solvent polarity.14 However, adding too much water will destroy the supramolecular structure consisting of hydrogen bonds between DES, so according to the experimental results shown in Fig. 2(a), we finally determined DES7 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) with a water content of 40% for subsequent experimental studies.
1) with a water content of 40% for subsequent experimental studies.
3.5 Determination of one-way tests
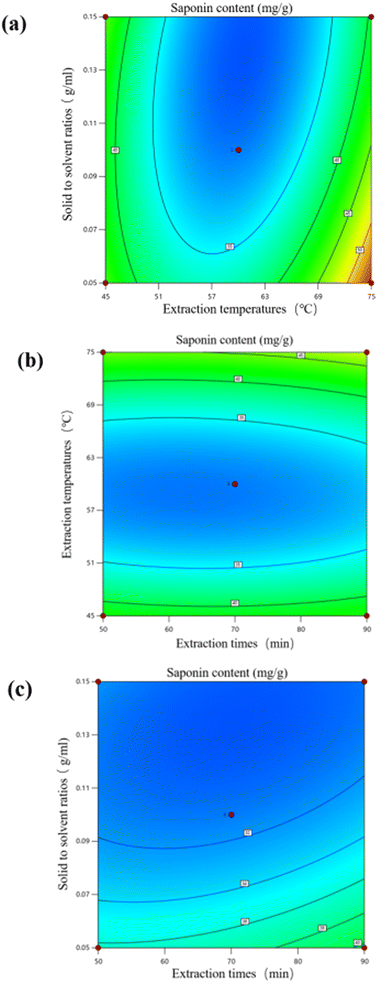
3.6 Response surface optimization test
| D = 31.24 + 0.8031A + 1.83B − 3.45C + 0.4935AB − 1.24AC − 3.8BC + 1.11A2 + 12.17B2 + 2.51C2 |
| Run | Extraction time A (min) | Extraction temperature B (°C) | Solid-to-solvent ratios C (g mL−1) | Saponin content D (mg g−1) |
|---|---|---|---|---|
| 1 | 70 | 45 | 0.15 | 43.252 |
| 2 | 70 | 45 | 0.05 | 41.190 |
| 3 | 90 | 45 | 0.1 | 45.541 |
| 4 | 70 | 60 | 0.1 | 27.758 |
| 5 | 90 | 60 | 0.15 | 29.597 |
| 6 | 90 | 60 | 0.05 | 40.361 |
| 7 | 50 | 60 | 0.05 | 37.631 |
| 8 | 70 | 60 | 0.1 | 28.939 |
| 9 | 70 | 60 | 0.1 | 29.534 |
| 10 | 50 | 75 | 0.1 | 42.513 |
| 11 | 70 | 60 | 0.1 | 34.832 |
| 12 | 70 | 75 | 0.05 | 56.179 |
| 13 | 90 | 75 | 0.1 | 46.472 |
| 14 | 50 | 60 | 0.15 | 31.846 |
| 15 | 70 | 75 | 0.15 | 43.046 |
| 16 | 70 | 60 | 0.1 | 35.159 |
| 17 | 50 | 45 | 0.1 | 43.556 |
Analysis of variance (ANOVA) was used to assess the relationship between independent and response variables and the optimal conditions for the extraction method.28 In the response surface model for this saponin extraction rate, R2 = 0.9124, p < 0.01, the overall model reached the significance level. The lack of fit term indicates that the probability of the model predicted value not fitting the actual value is not significant, and its p = 0.4907, p > 0.05, which indicates that the lack of fit term is not significant, and this model is chosen appropriately.29 The coefficient of variation C.V. = 8.95% indicates that the model has good repeatability. According to the results in Table 4, it can be seen that the primary term C solid-to-solvent ratio reached a significant level (p < 0.05), and the effect of the secondary term B2 on the saponin extraction rate reached a highly significant level (p < 0.01). The order of the magnitude of the effect of the three factors on the saponin extraction rate was solid-to-solvent ratio (C) > extraction temperature (B) > extraction time (A).
| Source | Sum of squares | Degree of freedom | Mean square | F-value | P-value | Significant |
|---|---|---|---|---|---|---|
| a Note: *means significant. | ||||||
| Model | 873.08 | 9 | 97.01 | 8.10 | 0.0058 | p < 0.05* |
| A | 5.16 | 1 | 5.16 | 0.4309 | 0.5325 | |
| B | 26.90 | 1 | 26.90 | 2.25 | 0.1776 | |
| C | 95.35 | 1 | 95.35 | 7.96 | 0.0257 | p < 0.05* |
| AB | 0.9741 | 1 | 0.9741 | 0.0814 | 0.7837 | |
| AC | 6.20 | 1 | 6.20 | 0.5176 | 0.4952 | |
| BC | 57.72 | 1 | 57.72 | 4.82 | 0.0641 | |
| A2 | 5.18 | 1 | 5.18 | 0.4325 | 0.5318 | |
| B2 | 623.32 | 1 | 623.32 | 52.06 | 0.0002 | p < 0.05* |
| C2 | 26.43 | 1 | 26.43 | 2.21 | 0.1810 | |
| Lack of fit | 35.22 | 3 | 11.74 | 0.9664 | 0.4907 | Not significant |
Hu used ethanol to extract saponins from Eclipta prostrasta for response surface optimization experiments.30 The extraction time was 3 h, which was longer than that of this study and consumed a large amount of organic solvents. Guo reported that the extraction time of notoginseng saponins from notoginseng leaves was 1.5 hours,31 and the reflux extraction time with Soxhlet by Medina-Meza was approximately 3 hours.32 Compared with other quinoa saponin extraction methods, this study used a shorter time to avoid the deterioration of metabolites and the rise in medium temperature caused by long-term extraction. The solid-to-solvent ratios in the study of Taco were consistent with those in this study, but the extraction time was shorter and the extraction rate was lower, probably because the interaction between temperature and time was not considered in the extraction rate.15
As shown in Fig. 3, extraction temperature and solid-to-solvent ratio had the largest interaction effect on the extraction of quinoa saponins by DESs, and extraction time and extraction temperature and extraction time and solid-to-solvent ratio had the least significant interactions, which is consistent with the results of the analysis of variance (ANOVA).
3.7 Comparison of saponin extraction methods
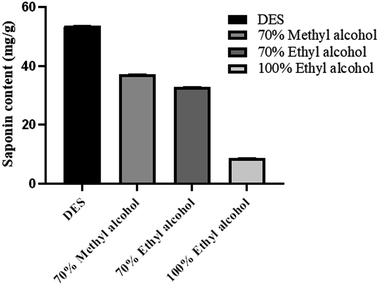
Studies have shown that DES is an effective green medium for stabilizing bioactive saponins in quinoa and has the potential to replace organic solvents.34 According to the traditional saponin extraction method, the optimized extraction conditions were used to extract quinoa saponins using 70% methanol, 70% ethanol, and 100% ethanol, and the saponin extraction rates were 37.05 ± 0.16%, 32.75 ± 0.1%, and 8.64 ± 0.1%, respectively, and the saponin extraction rate of DESs was 53.72 ± 0.1%.35 As shown in Fig. 4, compared with conventional organic solvents, the extraction effect of DESs was significantly better than that of conventional organic solvents which is consistent with Tu's study.10 This indicates that the method proposed in this study is more efficient, simple, and has better prospects for application.3.8 DES recovery
In this study, hydrophilic–lipophilic balanced adsorbents (HLB columns) were used to selectively enrich quinoa saponins with a lipophilic dammarane fraction and hydrophilic sugars.12 Solid-phase extraction was used to separate quinoa saponins and extraction solvents, and freeze-drying of the separated extraction solvents allowed the recovery of deep-eutectic solvents.3.9 Antioxidant activity results and analysis
4. Conclusion
A total of 42 green DESs were prepared in this study, and the effects of extraction conditions on the extraction rate of quinoa saponins from DESs were investigated using a one-way test combined with a response surface test. The optimum extraction process conditions obtained were an extraction time of 89 min, an extraction temperature of 75 °C, and a material-to-liquid ratio of 0.05 g mL−1. Under these conditions, the predicted saponin extraction rate was 56 mg g−1, and the actual saponin yield was 53.72 ± 0.1 mg g−1, which was not much different from the predicted value. This process will provide a theoretical basis for subsequent further optimization. Moreover, under these optimized conditions, the extraction of quinoa saponins by the DES was less time consuming and greener than that of conventional organic solvents. The new green extraction solvent prepared in this study breaks the concept that traditional organic solvents have been used to extract plant compounds, making DESs an attractive green solvent.36The results of in vitro antioxidant experiments showed that all DES saponin extracts exhibited good antioxidant capacity and showed a linear relationship with the concentration, and this study provides a reference for quinoa saponin treatment of diseases caused by oxidative stress. As shown in Table S2,† this study provides a new method for the further development and utilization of quinoa saponin.
Author contributions
Yuqing Cai: methodology, software, writing – original draft. Hui Gao: resources, writing – review & editing. Linmeng Song: methodology, data curation. Feiyan Tao: writing – review & editing, supervision, data curation. Xueying Ji: methodology. Yuan Yu: supervision. Yuqing Cao: writing – review & editing. Shaojian Tang: supervision, writing – review & editing. Peng Xue: conceptualization, funding acquisition, resources, supervision, project administration, writing – review & editing.Conflicts of interest
None.Acknowledgements
This study was supported by the National Natural Science Foundation of China (Grant No. 32102106), Innovation Team of Youth Innovation Science and Technology Plan of Shandong Province (2022KJ343) and the Natural Science Foundation of Shandong Province (ZR2020QB016). We would like to thank the Public Health Testing Center of the Weifang Medical University.References
- A. M. Filho, M. R. Pirozi, J. T. Borges, H. M. Pinheiro Sant'Ana, J. B. Chaves and J. S. Coimbra, Crit. Rev. Food Sci. Nutr., 2017, 57, 1618–1630 CrossRef PubMed.
- A. Vega-Gálvez, M. Miranda, J. Vergara, E. Uribe, L. Puente and E. A. Martínez, J. Sci. Food Agric., 2010, 90, 2541–2547 CrossRef PubMed.
- F. Yang, T. Guo, Y. Zhou, S. Han, S. Sun and F. Luo, Crit. Rev. Food Sci. Nutr., 2022, 1–15, DOI:10.1080/10408398.2022.2139219.
- G. M. Woldemichael and M. Wink, J. Agric. Food Chem., 2001, 49, 2327–2332 CrossRef CAS PubMed.
- M. S. Mora-Ocación, A. C. Morillo-Coronado and E. H. Manjarres-Hernández, Int. J. Food Sci., 2022, 2022, 7287487 Search PubMed.
- J. N. Dong, G. D. Wu, Z. Q. Dong, D. Yang, Y. K. Bo, M. An and L. S. Zhao, RSC Adv., 2021, 11, 37649–37660 RSC.
- A. P. Abbott, G. Capper, D. L. Davies, R. K. Rasheed and V. Tambyrajah, Chem. Commun., 2003, 70–71, 10.1039/b210714g.
- R. R. Zhou, J. H. Huang, D. He, Z. Y. Yi, D. Zhao, Z. Liu, S. H. Zhang and L. Q. Huang, Molecules, 2022, 27, 3132 CrossRef CAS PubMed.
- C. R. Espinoza, C. A. J. Ruiz, O. P. F. Ramos, M. A. Q. Solano, G. H. Quiñonez and N. E. S. Mallma, Acta Sci. Pol., Technol. Aliment., 2021, 20, 17–23 CAS.
- Y. J. Tu, L. N. Li, W. X. Fan, L. H. Fan, Z. T. Wang and L. Yang, Zhongguo Zhong Yao Za Zhi, 2022, 47, 6409–6416 Search PubMed.
- Q. Zhang, K. De Oliveira Vigier, S. Royer and F. Jérôme, Chem. Soc. Rev., 2012, 41, 7108–7146 RSC.
- K. M. Jeong, M. S. Lee, M. W. Nam, J. Zhao, Y. Jin, D. K. Lee, S. W. Kwon, J. H. Jeong and J. Lee, J. Chromatogr. A, 2015, 1424, 10–17 CrossRef CAS PubMed.
- M. Shaibuna, L. V. Theresa and K. Sreekumar, Soft Matter, 2022, 18, 2695–2721 RSC.
- M. Q. Farooq, N. M. Abbasi and J. L. Anderson, J. Chromatogr. A, 2020, 1633, 461613 CrossRef CAS PubMed.
- V. Taco, P. Savarino, S. Benali, E. Villacrés, J.-M. Raquez, P. Gerbaux, P. Duez and A. Nachtergael, J. Clean. Product., 2022, 363, 132609 CrossRef CAS.
- F. Wallace, Z. Bennadji, F. Ferreira and C. Olivaro, Phytochem. Anal., 2019, 30, 644–652 CrossRef CAS PubMed.
- Y. Wang, L. Zhao, R. Zhang, X. Yang, Y. Sun, L. Shi and P. Xue, Food Sci. Nutr., 2020, 8, 921–932 CrossRef CAS PubMed.
- C. C. Peñafiel and L. Díaz Villar, Arch. Latinoam. Nutr., 1988, 38, 113–131 Search PubMed.
- P. Xue, Y. Yao, X. S. Yang, J. Feng and G. X. Ren, J. Ginseng Res., 2017, 41, 180–187 CrossRef PubMed.
- E. J. Llorent-Martínez, P. Ortega-Barrales, G. Zengin, S. Uysal, R. Ceylan, G. O. Guler, A. Mocan and A. Aktumsek, RSC Adv., 2016, 6, 88996–89006 RSC.
- T. X. Wang, M. M. Shi and J. G. Jiang, RSC Adv., 2018, 8, 23181–23190 RSC.
- X. L. Yu and Y. He, RSC Adv., 2018, 8, 24312–24321 RSC.
- I. Dini, O. Schettino, T. Simioli and A. Dini, J. Agric. Food Chem., 2001, 49, 741–746 CrossRef CAS PubMed.
- S. Dong, X. Yang, L. Zhao, F. Zhang, Z. Hou and P. Xue, Ind. Crops Prod., 2020, 149, 112350 CrossRef CAS.
- E. Colson, P. Savarino, E. J. S. Claereboudt, G. Cabrera-Barjas, M. Deleu, L. Lins, I. Eeckhaut, P. Flammang and P. Gerbaux, Molecules, 2020, 25, 1731 CrossRef CAS PubMed.
- L. Weng and M. Toner, Phys. Chem. Chem. Phys., 2018, 20, 22455–22462 RSC.
- S. S. Patil, A. Pathak and V. K. Rathod, Ultrason. Sonochem., 2021, 70, 105267 CrossRef CAS PubMed.
- D. Allouss, Y. Essamlali, O. Amadine, A. Chakir and M. Zahouily, RSC Adv., 2019, 9, 37858–37869 RSC.
- B. Ahmed, Y. Ziyad, V. Mohamed, D. Johan, D. Bieke, M. Kristiaan, H. Debby and Y. Vander, Anal. Methods, 2016, 8, 6107–6114 RSC.
- T. Hu, Y. Y. Guo, Q. F. Zhou, X. K. Zhong, L. Zhu, J. H. Piao, J. Chen and J. G. Jiang, J. Food Sci., 2012, 77, C975–C982 CrossRef CAS PubMed.
- Z. Guo, Z. Luo, S. Wu, C. Yang, T. Xiao and Y. Zhao, Molecules, 2023, 28, 3915 CrossRef CAS PubMed.
- I. G. Medina-Meza, N. A. Aluwi, S. R. Saunders and G. M. Ganjyal, J. Agric. Food Chem., 2016, 64, 8583–8591 CrossRef CAS PubMed.
- Y. J. Tan, G. S. Zhou, S. Guo, H. Yan, J. Zhang, Z. H. Zhu, X. Q. Shi, S. J. Yue, Y. P. Tang, S. L. Huang, G. P. Peng and J. A. Duan, RSC Adv., 2018, 8, 40748–40759 RSC.
- Y. C. Wu, P. Wu, Y. B. Li, T. C. Liu, L. Zhang and Y. H. Zhou, RSC Adv., 2018, 8, 15069–15077 RSC.
- C. Li, Y. Ma, X. Zhi and G. Peng, Food Sci. Biotechnol., 2023, 32, 319–328 CrossRef CAS PubMed.
- Y. Yue, Q. Huang, Y. Fu and J. Chang, RSC Adv., 2020, 10, 23403–23409 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra05949a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2023 |