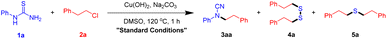Open Access Article
Open Access ArticleRapid assembly of structurally diverse cyanamides and disulfanes via base-mediated aminoalkylation of aryl thiourea†
Yongpeng Zheng ,
Jianxiao Li
,
Jianxiao Li ,
Chaorong Qi
,
Chaorong Qi ,
Wanqing Wu
,
Wanqing Wu and
Huanfeng Jiang
and
Huanfeng Jiang *
*
Key Lab of Functional Molecular Engineering of Guangdong Province, School of Chemistry and Chemical Engineering, South China University of Technology, Guangzhou 510640, P. R. China. E-mail: jianghf@scut.edu.cn
First published on 9th November 2023
Abstract
A general method for the preparation of cyanamides and disulfanes from aryl thiourea and halide through a base-mediated strategy is described. Mercaptan and N-aryl cyanamide are the key intermediates in the reaction. The current method is convenient, eco-friendly, and has high yields for the synthesis of substituted cyanamide and functional disulfanes in a one-pot procedure from readily available starting materials.
Cyanamide was first discovered as early as 1851 by Cannizzaro, and features a nucleophilic nitrogen atom bearing an electrophilic nitrile unit. Due to their unique structure (N-CN) and chemical properties, cyanamides are valuable organonitrogen compounds in pharmaceuticals, material science, and synthetic applications.1 Remarkably, cyanamide-containing compounds exhibit interesting biological activities.2 Moreover, cyanamides are frequently transformed into heterocycles through transition-metal catalyzed cycloaddition reactions.3 Among cyanamide derivatives, N-cyano-N-phenyl-p-toluenesulfonamide (NCTS) is an efficient cyanation reagent under transition metal catalytic conditions.2d
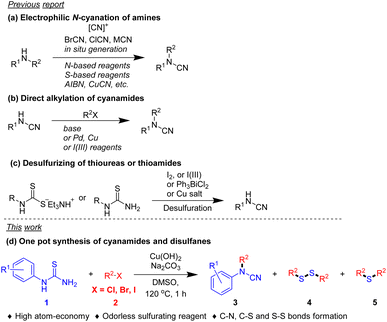
As a valuable dinitrogen resource, many elegant works have been reported for the introduction of a cyanamide group. The most widely known and extensively used strategy for the preparation of substituted cyanamides is based on the electrophilic N-cyanation of amines using cyanogen bromide (Scheme 1, Method a). However, BrCN is a very hazardous chemical which can be absorbed into the body by inhalation of its vapor and through the skin and may cause convulsions or death. Several alternative N-cyanation procedures have been developed that aim to solve that security issue in recent years.
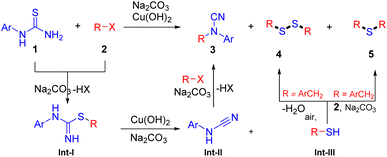
Kappe and co-workers developed a continuous-flow process for the on-demand generation of BrCN, which subsequent utilization for the construction of cyclic guanidine.4 The development of safer electrophilic cyanation reagents is another efficient approach. Alcarazo and co-workers introduce two sulfur-based reagents for the efficient electrophilic cyanation.5 In addition, AIBN6 and CuCN7 also serve as sources of nitrile under copper catalysis. The direct nucleophilic substitution of cyanamide is another straightforward method for the synthesis of substituted cyanamides (Scheme 1, Method b).8 Yet these transformations suffer from uncommon cyanamide sources. In recent years, representative methods for the preparation of substituted cyanamide that do not need a cyanide source have been developed (Scheme 1, Method c). I(III) reagents or iodine-mediated desulfurization of thioamide or dithiocarbamate are the most useful methods to obtain N-substituted cyanamides.9 Further, transition metal-catalyzed desulphurization is also an efficient method for the synthesis of cyanamides.10 It is a pity that sulfphur was abandoned in this progress. However, most of these transformations suffer from certain limitations, such as the need for excessive amounts of waste generating agents, harsh reaction conditions, poor functional group tolerance, and tedious synthetic procedures to access substrates. Hence, there is a high demand for efficient and environmentally benign new methods for the synthesis of cyanamide from readily available starting materials under mild conditions. As part of our continuing studies on green chemistry,11 we envisage that the sulfphur in thiourea could transfer to useful compounds in elegant reactions. We herein report an efficient method for the synthesis of cyanamides and disulfanes from aryl thiourea and halides in one pot procedure.
Initially, 1-phenylthiourea (1a) and (2-chloroethyl)benzene (2a) were selected as the model substrates to screen the optimal reaction conditions, and the results are summarized in Table 1. To our delight, under the combination of Na2CO3 and Cu(OH)2 in DMSO in the open air at 120 °C for 1 h, the desired product N-phenethyl-N-phenylcyanamide (3aa) and disulfane (4a) were obtained in 95% and 79% yield, respectively (Table 1, entry 1). The yields of compounds 3 and 4 were calculated based on arylthiourea (1). In addition, thioether (5a) was also detected in trace amount by GC-MS. The reaction fails without base (entry 2), and the starting materials 1a and 2a were all recoved in yield >95%, showing that base is critical to the reaction. In this reaction, inorganic bases may be acid scavenger, promoting the nucleaddition reaction between aylthiourea and halide. The yield of cyanamide (3aa) was increased as the amount of base used in the reaction, however, the yield of disulfane was low without Cu(OH)2 (entries 3–6). Various kinds of bases were then screened, such as K2CO3, Cs2CO3, KHCO3, NaHCO3, and Et3N; among them, K salts gave the similar results to Na salts, but no desired products were determined under organic bases (entries 7–11). These results indicated that Na2CO3 is the best choice to promote the reaction. With the increase of Cu(OH)2, the yield of disulfane decreased, while the yield of cyanamide remained excellent (entries 12; see ESI† for details). These results may be due to the chelation between copper and mercaptan. Subsequently, different solvents, including water, MeCN, DMF, DMA, acetone, and toluene were estimated, and DMSO was superior to the others (entries 15–18). Furthermore, the reaction temperature and time were also optimized. It was found that increasing the temperature higher than 80 °C can obviously shorten the reaction time, and the reaction finished under 120 °C within 1 h (for more details, see ESI†).
| Entry | Base | Solvent | Yield of 3aab/% | Yield of 4ab/% | Yield of 5ab/% |
|---|---|---|---|---|---|
| a Unless otherwise note, all reactions were performed with 1a (0.20 mmol), 2a (0.48 mmol, 2.4 equiv.), base (2.5 equiv.), Cu(OH)2 (10 mol%), solvent (0.5 mL) at 120 °C for 1 h.b Yields were based on 1H NMR analysis of the crude product using CH2Br2 as an internal standard. The value in parentheses is the isolated yield of product.c Without Cu(OH)2.d Base (0.5 equiv.).e Base (1.0 equiv.).f Base (1.5 equiv.).g Base (2.0 equiv.).h Cu(OH)2 (1.0 equiv.).i 2a (2.0 equiv.).j 2a (2.2 equiv.).k At 100 °C for 12 h.l At 80 °C for 12 h. DMAc = N,N-dimethylacetamide. | |||||
| 1 | Na2CO3 | DMSO | 95 (93) | 79 (70) | Trace |
| 2 | — | DMSO | 0 | 0 | 0 |
| 3c,d | Na2CO3 | DMSO | 33 | 16 | 20 |
| 4c,e | Na2CO3 | DMSO | 65 | 30 | 32 |
| 5c,f | Na2CO3 | DMSO | 74 | 26 | 30 |
| 6c,g | Na2CO3 | DMSO | 81 | 26 | 40 |
| 7 | K2CO3 | DMSO | 97 | 70 | Trace |
| 8 | Cs2CO3 | DMSO | 70 | 50 | Trace |
| 9 | NaHCO3 | DMSO | 90 | 75 | Trace |
| 10 | KHCO3 | DMSO | 92 | 70 | Trace |
| 11 | Et3N | DMSO | 0 | 0 | 0 |
| 12h | Na2CO3 | DMSO | 97 | 0 | 0 |
| 13i | Na2CO3 | DMSO | 81 | 57 | Trace |
| 14j | Na2CO3 | DMSO | 92 | 70 | Trace |
| 15k | Na2CO3 | H2O | 0 | 0 | 0 |
| 16l | Na2CO3 | CH3CN | 76 | 30 | Trace |
| 17 | Na2CO3 | DMAc | 79 | 62 | Trace |
| 18 | Na2CO3 | DMF | 91 | 71 | Trace |
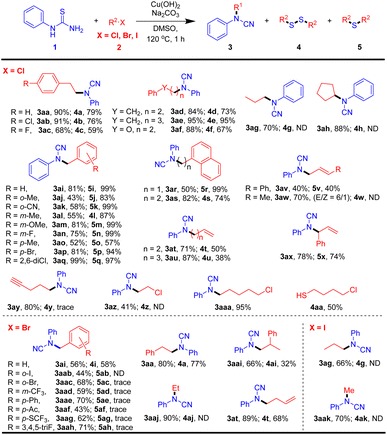
With the optimal reaction conditions in hand, we then tried to explore the generality and limitations of the protocol. First, various halides were investigated and the results are summarized in Table 2. Long-chain chloroalkanes are good substrates for the preparation of corresponding cyanamides (3ad–3ae) in 84% to 95% yields and disulfanes (4d–4e) in 73% to 95% yields. Phenol ether is tolerant in the reaction, and the corresponding cyanamide (3af) and disulfane (4f) were obtained in high yields. Simple primary chloroalkane and steric-hindrance halides can also offer corresponding products (3ag, 3ah) in 70% and 88% yields, respectively. Benzyl chloride and its alkyl, alkoxy, halo, and cyan substituted derivatives will react smoothly and produce cyanamides (3ai–3aq) in moderate to good yields. To our surprise, except for 3-methylbenzyl chloride affording disulfane 4l, the other benzyl chloride derivatives produce thioethers (5i–5q) in excellent yields under standard conditions. Halides with a terminal or internal C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond are suitable partners and give the desired products (3av–3ax, 4v–4x) in moderate yields. Particularly, 5-chloropent-1-yne is also perfectly compatible under this reaction condition, and the corresponding cyanamide (3ay) was obtained in an 80% yield. To our delight, dichloroalkanes are acceptable for the preparation of halo-substituted cyanamides (3az–3aaa) and halo-substituted disulfane products (4aa). Benzyl bromide and its electron-withdrawing or donating derivatives can offer moderate yields of cyanamide products (3ai, and 3aab–3aah). To our confusion, sulfane products were not easily obtained in these cases, and olyl dibenzyldisulfane (4i) was obtained in 58% isolated yield. Moreover, bromoethane can produce cyanamide in excellent yield (90%) under standard reaction conditions. Furthermore, 1-iodopropane and methyl iodide successfully provide corresponding products (3ag and 3aak) in yields of 66% and 70%, respectively. These results indicated that chloride, bromide, and iodo hydrocarbons are all available materials for the corresponding cyanamide products.
C double bond are suitable partners and give the desired products (3av–3ax, 4v–4x) in moderate yields. Particularly, 5-chloropent-1-yne is also perfectly compatible under this reaction condition, and the corresponding cyanamide (3ay) was obtained in an 80% yield. To our delight, dichloroalkanes are acceptable for the preparation of halo-substituted cyanamides (3az–3aaa) and halo-substituted disulfane products (4aa). Benzyl bromide and its electron-withdrawing or donating derivatives can offer moderate yields of cyanamide products (3ai, and 3aab–3aah). To our confusion, sulfane products were not easily obtained in these cases, and olyl dibenzyldisulfane (4i) was obtained in 58% isolated yield. Moreover, bromoethane can produce cyanamide in excellent yield (90%) under standard reaction conditions. Furthermore, 1-iodopropane and methyl iodide successfully provide corresponding products (3ag and 3aak) in yields of 66% and 70%, respectively. These results indicated that chloride, bromide, and iodo hydrocarbons are all available materials for the corresponding cyanamide products.
| a All reactions were performed with 1a (0.2 mmol), 2 (2.4 equiv.), Cu(OH)2 (10 mol%), Na2CO3 (2.5 equiv.), DMSO (0.5 mL) at 120 °C for 1 h. Yields referred to isolated yield. |
|---|
 |
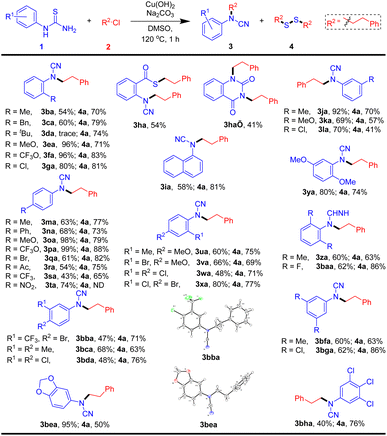
To explore further the generality of this protocol, various structurally diverse arylthioureas were then evaluated, and the representative results are summarized in Table 3. Ortho-substituted phenyl thioureas are tested first. Satisfactorily, both electron-rich (Me, Bn, MeO, and CF3O) and electron-withdrawing (Cl) substituents arylthioureas can be successfully coupled with 2a, and the corresponding cyanamide products 3ba–3ga and disulfane 4a were furnished in yields ranging from 54% to 96%. However, the high-steric-hindrance substrate 1-(2-(tert-butyl)phenyl)thiourea cannot obtain a cyanamide product. In addition, ethyl 2-thioureidobenzoate reacting with 2a will produce 3ha and 3ha′ in moderate yields. In this case, 3ha was generated via thiolysis between ethyl ester and in situ disulfane 4a. Similarly, naphthyl thiourea substrate gives the corresponding cyanamide 3ia in 58% yield and disulfane 4a in 81% yield. Meta-substituted phenyl thioureas were well tolerated under the current reaction conditions, and the cyanamide products 3ja–3la were obtained in good to excellent yields. Either electron-donating or electron-withdrawing substituents on the para site, aryl thioureas are successful in achieving the desired products. To our delight, multi-substituted alkyl, alkoxyl, and/or halogen on the phenyl ring were well accommodated, and the corresponding products were generated in yields ranging from 48 to 95%. The configurations of 3bba (ref. 12) and 3bea (ref. 13) were unequivocally confirmed by X-ray crystallographic analysis.
| a All reactions were performed with 1 (0.2 mmol), 2a (2.4 equiv.), Cu(OH)2 (10 mol%), Na2CO3 (2.5 equiv.), DMSO (0.5 mL) at 120 °C for 1 h. Yields referred to isolated yield. |
|---|
 |
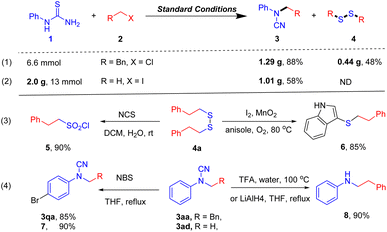
To investigate the practicality of this method, gram-scale reactions and further transformations were carried out (Scheme 2). On a 6.6 mmol scale, the desired cyanamide 3aa and disulfane 4a were isolated in 88% and 48% yield, respectively. It is noteworthy that this approach can perform at a 2 g scale with iodomethane for the synthesis of the desired methyl cyanamide product (3aak) in 58% yield. Subsequently, disulfane 4a can be readily converted to the corresponding sulfonyl chloride 5 and sulfenylindole 6 (ref. 14) in high yield. These results show that disulfane can replace mercaptan as an odorless reagent in organic synthetics. Phenyl cyanamides were easily brominated in the para site of the phenyl ring when treated with NBS in THF under reflux. It is a pity that the cyan group was eliminated when treating with TFA or LiAlH4.
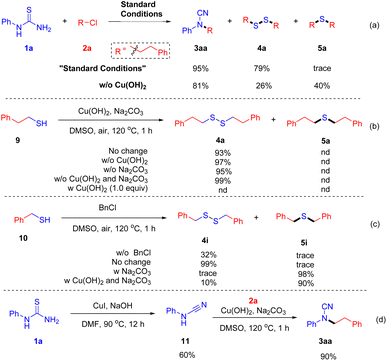
In order to further understand the reaction mechanism, some control experiments (Scheme 3) were conducted. First, the model reaction is performed under standard conditions (eqn (a)). Cyanamide (3a) and disulfane (4a) were found in the yields of 95% and 79%, respectively, and only a trace amount of thioether (5a) was detected by GC-MS. In the absence of Cu(OH)2, the yield of thioether (5a) increased dramatically. Meanwhile, trace amounts of thiol and N-phenyl cyanamide were detected in GC-MS. These results showed that Cu(OH)2 may promote the formation of disulfane (4a). Subsequently, 2-phenylethanethiol (9) was tested under various conditions (eqn (b)), and only disulfane (4a) was obtained. In the presence of 1.0 equiv. Cu(OH)2, thiol (9) is consumed, and no sulfane products are detected by GC-MS. Indicating that excess copper salt may chelate with mercaptan. Moreover, 1,2-dibenzyldisulfane (4i) is produced in high yield when benzyl mercaptan (10) is performed under air in the presence or absence of benzyl chloride (eqn (c)). However, thiother (5i) becomes the main product in the presence of a base. Indicating that base facilitates the SN2 reaction between thiol and halide. Furthermore, substituted cyanamide (3aa) was obtained successfully when treating N-phenyl cyanamide (11) with 2a under standard conditions. These results indicated that mercaptan and N-phenyl cyanamide might be the intermediates in the reaction (Scheme 4).
Based on the above experimental results and previous reports,15 a tentative mechanistic interpretation of the preceding observations is proposed in Scheme 4. First, base-promoted nucleoaddition of halide to arylthiourea forms isthiourea Int-I, which undergoes further intramolecular elimination in the presence of base and copper to generate cyanamide Int-II and thiol intermediate Int-III. Then halide 2 and Int-II undergo SN2 progress to afford the substituted cyanamide product (3). Meanwhile, thiol Int-III is oxidized by air to form a sulfane compound.
Conclusions
In summary, a simple method for the preparation of cyanamides and disulfanes has been established under mild reaction conditions in one pot. Many diverse substituted cyanamides and functional sulfanes are successfully prepared in moderate to excellent yields with this strategy. Importantly, the present methodology demonstrates excellent functional group compatibility, high atom- and step-economy, and mild reaction conditions. Further studies on the synthetic application of this strategy are underway in our lab.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank the National Natural Science Foundation of China (21420102003) and the Key-Area Research and Development Program of Guangdong Province (2020B010188001) for financial support.Notes and references
- For slected reviews, see: (a) D. D. Nekrasov, Synthesis and Chemical Transformations of Monoand Disubstituted Cyanamides, Russ. J. Org. Chem., 2004, 40, 1387–1402 CrossRef CAS; (b) M.-H. Larraufie, G. Maestri, M. Malacria, C. Ollivier, L. Fensterbank and E. Lacôte, The Cyanamide Moiety, Synthesis and Reactivity, Synthesis, 2012, 44, 1279–1292 CrossRef CAS; (c) M. R. R. Prabhath, L. Williams, S. V. Bhat and P. Sharma, Recent Advances in Cyanamide Chemistry: Synthesis and Applications, Molecules, 2017, 22, 615 CrossRef PubMed; (d) J. Cui, J. Song, Q. Liu, H. Liu and Y. Dong, Transition-Metal-Catalyzed Cyanation by Using an Electrophilic Cyanating Agent, N-Cyano-N-phenyl-p-toluenesulfonamide (NCTS), Chem.–Asian J., 2018, 13, 482–495 CrossRef CAS PubMed.
- (a) A. Casimiro-Garcia, J. I. Trujillo, F. Vajdos, B. Juba, M. E. Banker, A. Aulabaugh, P. Balbo, J. Bauman, J. Chrencik, J. W. Coe, R. Czerwinski, M. Dowty, J. D. Knafels, S. Kwon, L. Leung, S. Liang, R. P. Robinson, J.-B. Telliez, R. Unwalla, X. Yang and A. Thorarensen, Identification of Cyanamide-Based Janus Kinase 3 (JAK3) Covalent Inhibitors, J. Med. Chem., 2018, 61, 10665–10699 CrossRef CAS PubMed; (b) K. Bum-Erdene, I. J. Yeh, G. Gonzalez-Gutierrez, M. K. Ghozayel, K. Pollok and S. O. Meroueh, Small-Molecule Cyanamide Pan-TEAD·YAP1 Covalent Antagonists, J. Med. Chem., 2023, 66, 266–284 CrossRef CAS PubMed; (c) D. Lainé, M. Palovich, B. McCleland, E. Petitjean, I. Delhom, H. Xie, J. Deng, G. Lin, R. Davis, A. Jolit, N. Nevins, B. Zhao, J. Villa, J. Schneck, P. McDevitt, R. Midgett, C. Kmett, S. Umbrecht, B. Peck, A. B. Davis and D. Bettoun, Discovery of Novel Cyanamide-Based Inhibitors of Cathepsin C, ACS Med. Chem. Lett., 2011, 2, 142–147 CrossRef PubMed; (d) M. S. Malamas, S. Pavlopoulos, S. O. Alapafuja, S. I. Farah, A. Zvonok, K. A. Moham mad, J. West, N. T. Perry, D. N. Pelekoudas, G. Rajarshi, C. Shields, H. Chandrashekhar, J. Wood and A. Makriyannis, Design and Structure–Activity Relationships of Isothiocyanates as Potent and Selective N-Acylethanolamine-Hydrolyzing Acid Amidase Inhibitors, J. Med. Chem., 2021, 64, 5956–5972 CrossRef CAS PubMed.
- (a) T. K. Lane, M. H. Nguyen, B. R. D'Souza, N. A. Spahn and J. Louie, The iron-catalyzed construction of 2-aminopyrimidines from alkynenitriles and cyanamides, Chem. Commun., 2013, 49, 7735–7737 RSC; (b) J. L. Harper, S. Felten, R. M. Stolley, A. S. Hegg, P. H. Y. Cheong and J. Louie, Origins of Regio- and Chemoselectivity in Iron-PDAI-Catalyzed [2+2+2] Cycloaddition Syntheses of 4,6-Disubstituted 2-Aminopyridines, ACS Catal., 2021, 11, 14677–14687 CrossRef CAS; (c) S. Huvelle, P. Matton, C. Tran, M.-N. Rager, M. Haddad and V. Ratovelomanana-Vidal, Synthesis of Benzo[c][2,7]naphthyridinones and Benzo[c][2,6]naphthyridinones via Ruthenium-Catalyzed [2 + 2 + 2] Cycloaddition between 1,7-Diynes and Cyanamides, Org. Lett., 2022, 24, 5126–5131 CrossRef CAS PubMed; (d) N. V. Shcherbakov, E. I. Chikunova, D. Dar’in, V. Y. Kukushkin and A. Y. Dubovtsev, Redox-Neutral and Atom-Economic Route to β-Carbolines via GoldCatalyzed [4 + 2] Cycloaddition of Indolylynamides and Cyanamides, J. Org. Chem., 2021, 86, 17804–17815 CrossRef CAS PubMed; (e) K. M. Medas, R. W. Lesch, F. B. Edioma, S. P. Wrenn, V. Ndahayo and S. P. Mulcahy, Metal-Catalyzed Cyclotrimerization Reactions of Cyanamides: Synthesis of 2-Aryl-α-carbolines, Org. Lett., 2020, 22, 3135–3139 CrossRef CAS PubMed; (f) R. L. Giles, J. D. Sullivan, A. M. Steiner and R. E. Looper, Addition–Hydroamination Reactions of Propargyl Cyanamides: Rapid Access to Highly Substituted 2-Aminoimidazoles, Angew. Chem., Int. Ed., 2009, 48, 3116–3120 CrossRef CAS PubMed.
- G. Glotz, R. Lebl, D. Dallinger and C. O. Kappe, Integration of Bromine and Cyanogen Bromide Generators for the Continuous-Flow Synthesis of Cyclic Guanidines, Angew. Chem., Int. Ed., 2017, 56, 13786–13789 CrossRef CAS PubMed.
- (a) G. Talavera, J. Peña and M. Alcarazo, Dihalo(imidazolium)sulfuranes: A Versatile Platform for the Synthesis of New Electrophilic Group-Transfer Reagents, J. Am. Chem. Soc., 2015, 137, 8704–8707 CrossRef CAS PubMed; (b) X. Li, C. Golz and M. Alcarazo, 5-(Cyano)dibenzothiophenium Triflate: A Sulfur-Based Reagent for Electrophilic Cyanation and Cyanocyclizations, Angew. Chem., Int. Ed., 2019, 58, 9496–9500 CrossRef CAS PubMed.
- F. Teng, J.-T. Yu, Z. Zhou, H. Chu and J. Cheng, Copper-Catalyzed N-Cyanation of Sulfoximines by AIBN, J. Org. Chem., 2015, 80, 2822–2826 CrossRef CAS PubMed.
- F. Teng, J.-T. Yu, Y. Jiang, H. Yang and J. Cheng, A copper-mediated oxidative N-cyanation reaction, Chem. Commun., 2014, 50, 8412–8415 RSC.
- (a) J. N. Ayres, M. W. Ashford, Y. Stöckl, V. Prudhomme, K. B. Ling, J. A. Platts and L. C. Morrill, Deoxycyanamidation of Alcohols with N-Cyano-N-phenyl-pmethylbenzenesulfonamide (NCTS), Org. Lett., 2017, 19, 3835–3838 CrossRef CAS PubMed; (b) J. N. Ayres, M. T. J. Williams, G. J. Tizzard, S. J. Coles, K. B. Ling and L. C. Morrill, Synthesis and Reactivity of N-Allenyl Cyanamides, Org. Lett., 2018, 20, 5282–5285 CrossRef CAS PubMed; (c) R. M. Stolley, W. Guo and J. Louie, Palladium-Catalyzed Arylation of Cyanamides, Org. Lett., 2012, 14, 322–325 CrossRef CAS PubMed; (d) P. Li, G. Cheng, H. Zhang, X. Xu, J. Gao and X. Cui, Copper-Catalyzed One-Pot Synthesis of Unsymmetrical Arylurea Derivatives via Tandem Reaction of Diaryliodonium Salts with N-Arylcyanamide, J. Org. Chem., 2014, 79, 8156–8162 CrossRef CAS PubMed; (e) J. Li, X. Zheng, W. Li, W. Zhou, W. Zhu and Y. Zhang, Transition-metal free N-arylation of cyanamides by diaryliodonium triflates in aqueous media, New J. Chem., 2016, 40, 77–80 RSC.
- (a) H. Ghosh, R. Yella, A. R. Ali, S. K. Sahoo and B. K. Patel, An efficient synthesis of cyanamide from amine promoted by a hypervalent iodine(III) reagent, Tetrahedron Lett., 2009, 50, 2407–2410 CrossRef CAS; (b) J. Nath, B. K. Patel, L. Jamir, U. B. Sinha and K. V. V. V. Satyanarayana, A one-pot preparation of cyanamide from dithiocarbamate using molecular iodine, Green Chem., 2009, 11, 1503–1506 RSC; (c) L. Jamir, U. B. Sinha, J. Nath and B. K. Patel, Environmentally Benign One-Pot Synthesis of Cyanamides from Dithiocarbamates Using I2 and H2O2, Synth. Commun., 2012, 42, 951–958 CrossRef CAS; (d) Y. Murata, H. Iwasa, M. Matsumura and S. Yasuike, Synthesis of Nitriles via the Iodine-Mediated Dehydrosulfurization of Thioamides, Chem. Pharm. Bull., 2020, 68, 679–681 CrossRef CAS PubMed.
- (a) S. N. M. Boddapati, N. Polam, B. R. Mutchu and H. B. Bollikolla, The synthesis of arylcyanamides: a copper-catalyzed consecutive desulfurization and C–N cross-coupling strategy, New J. Chem., 2018, 42, 918–922 RSC; (b) E. Gopi, E. Gravel and E. Doris, Triphenylbismuth Dichloride-Mediated Conversion of Thioamides to Nitriles, Eur. J. Org Chem., 2019, 2019, 4043–4045 CrossRef CAS; (c) R. Yella, V. Kavala and B. K. Patel, Bromineless Bromine as an Efficient Desulfurizing Agent for the Preparation of Cyanamides and 2-Aminothiazoles from Dithiocarbamate Salts, Synth. Commun., 2011, 41, 792–805 CrossRef CAS; (d) M. Abdoli, A. Bonardi, C. T. Supuran and R. Žalubovskis, 4-Cyanamidobenzenesulfonamide derivatives: a novel class of human and bacterial carbonic anhydrase inhibitors, Asian J. Chem., 2011, 6, 2483–2486 Search PubMed.
- (a) W. Wu and H. Jiang, Acc. Chem. Res., 2012, 45, 1736–1748 CrossRef CAS PubMed; (b) M. Hu, W. Wu and H. Jiang, ChemSusChem, 2019, 12, 2911–2935 CrossRef CAS PubMed.
- CCDC 2241702 (3bba) contains the supplementary crystallographic data for this paper.
- CCDC 2241703 (3bea) contains the supplementary crystallographic data for this paper.
- W. Li, H. Wang, S. Liu, H. Feng, E. Benassi and B. Qian, Iodine/Manganese Catalyzed Sulfenylation of Indole via Dehydrogenative Oxidative Coupling in Anisole, Adv. Synth. Catal., 2020, 362, 2666–2671 CrossRef CAS.
- S. K. Sahoo, L. Jamir, S. Guin and B. K. Patel, Copper(I)-Catalyzed Cascade Synthesis of 2-Arylsulfanylarylcyanamides, Adv. Synth. Catal., 2010, 352, 2538–2548 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and characterization of all compounds, copies of 1H and 13C NMR spectra for selected compounds. CCDC 2241702 and 2241703. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3ra06051a |
| This journal is © The Royal Society of Chemistry 2023 |