Open Access Article
Open Access ArticleReaxFF MD and detailed reaction kinetic study on the thermal cracking and partial combustion of anisole: a biomass model tar compound
Ming Hua,
Shanhui Zhao *b and
Yonghao Luoc
*b and
Yonghao Luoc
aEverbright Greentech Technology Services (Jiangsu) Ltd, Nanjing, 210000, China
bSchool of Energy and Power Engineering, Nanjing Institute of Technology, Nanjing, 211167, China. E-mail: shhzhaoseu@163.com
cShanhai Jiao Tong University, Shanghai, 200240, China
First published on 12th December 2023
Abstract
Methods of partial oxidation for biomass tar conversion were studied based on their detailed reaction mechanism. The good accuracy of the modeling results compared with the experimental data indicate that the model was reasonable. Anisole was chosen as the tar model component for partial combustion with equivalence ratios (ER) from 0 to 0.8. The results show that oxygen promotes the pyrolysis of anisole and thereby the tar conversion rate. An appropriate amount of oxygen could crack tar into flammable small-molecule gases (H2, CO) and inhibit the generation of polycyclic aromatic hydrocarbon (PAH) compounds. In addition to the introduction of active free radicals, partial oxidation could also improve tar cracking by exothermic oxidation to produce amounts of heat. Typical PAH production was studied based on the rate of product formation (ROP). The results show that active radicals, such as H and OH, promote tar cracking. A detailed reaction pathway for tar conversion was built. Staged oxygen supply benefited the cracking of tar into small-molecule gases and inhibited the formation of PAHs.
1. Introduction
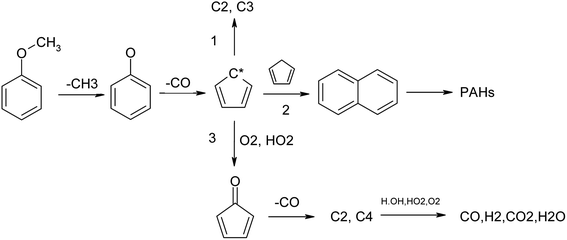
Climate change and environmental pollution have become important problems that restrict social development. The development and utilization of renewable energy technology has gradually become the focus of the global society. Biomass gasification power generation technology is a promising method of biomass energy utilization. At present, the high tar content in biomass gasification is one of the bottlenecks restricting the development and industrialization of the gasification technology.1,2 Tar in gasification syngas is composed of a variety of oxygen-containing and oxygen-free organic hydrocarbon components with high boiling point and other characteristics, such as strong corrosion and strong pollution.3,4 Condensation in the transmission pipeline results in transmission pipeline blockage and corrosion. A very high tar content may even cause corrosion, breakdown of equipment and affect the application of gasification syngas.5 Besides, since tar has higher calorific value, employing absorption and filtration will cause considerable energy waste.6,7 Therefore, seeking efficient and clean tar removal technology is key to the commercialization of gasification technology. Aromatic hydrocarbon components, including benzene, phenol, toluene, styrene, naphthalene, phenanthrene, pyrene and their derivatives, are the most difficult to be converted and removed from biomass tar.8 The main sources of aromatic hydrocarbon tar are the pyrolytic fragments of lignin and their polymerization in the high-temperature zone of the gasifier. The main source of aromatic hydrocarbons in biomass pyrolysis tar is the pyrolysis product lignin,9,10 which has three basic structural units, namely syringyl lignin (S-lignin), guajacyl lignin (G-lignin) and hydroxy-phenyl lignin (H-lignin),11–13 as shown in Fig. 1. The basic structure is an aromatic ring with a methoxyl group as the side chain.14,15 Therefore, guaiacol and anisole, which have methoxyl groups on their aromatic ring, are usually adopted as model tar compounds.16,17There are several methods for tar reduction, such as mechanical methods, thermal cracking, partial oxidation, catalytic conversion and so on.18,19 Among these, non-catalytic partial oxidation is quite an effective method that can realize high tar reduction ratios. The two-staged gasifier at the Technical University of Denmark has been reported to operate with a very low tar content in the syngas. The crucial part is the separation of pyrolysis and the gasification stage for partial oxidation to realize a primary tar conversion ratio of 99%.20 The Xylowatt gasifier has a similar structure, and the homogenous partial combustion zone could reach a tar conversion ratio of 99.5%. Partial oxidation is a key process in biomass gasification, which provides the energy for biomass pyrolysis, tar cracking and char gasification.
Brandt et al.21 reported that a 100 kW two-staged gasifier could achieve a syngas tar content as low as 15 mg Nm−3 by combining partial oxidation and charcoal bed reduction. The tar content after partial oxidation of the pyrolysis gas could be reduced to 3000 mg kg−1 dry wood and 500 mg kg−1 straw.22 A suitable supply of air and good mixing of the air with the pyrolysis gas were crucial for the results. Marda et al.23 investigated the non-catalytic partial oxidation of bio-oil for the production of hydrogen. High yields of CO and H2 were achieved, respectively up to 50–70% and around 25%. The rate of bio-oil carbon to gas conversion by non-catalytic partial oxidation was between 85–95% under optimal conditions, proving that non-catalytic partial oxidation has high potential for bio-oil or tar conversion.
However, research on the detailed kinetic mechanism of thermal cracking and partial oxidation by modeling is still lacking. Most kinetic models available currently are based on the combustion of traditional fuels. Norinaga et al.24 investigated the partial oxidation of hot coke oven gas, which contained 31 aromatic tar compounds (the smallest was benzene, and the largest was coronene). The mechanism developed by Richter and Howard25 was proposed as the kinetic mechanism. Gerun et al.26 proposed a 13-reaction mechanism involving phenol, benzene, naphthalene and soot. The reduced mechanism was coupled with the commercial CFD software Fluent. However, more work focusing on the unconventional biomass tar compounds is needed to discover the detailed conversion mechanism of biomass tar.
Therefore, in this paper, anisole was used as the model compound of lignin tar to study the mechanism of homogeneous cracking and partial oxidation of aromatic hydrocarbon tar in order to obtain the conversion path of typical biomass tar and provide theoretical support for the low tar biomass gasification technology.
2. Modeling method
2.1 The model building
From the perspective of fuel design research, MIT, NIST, NASA, Princeton University and other research institutions have developed some detailed reaction mechanisms for some typical fuels (benzene, CH4, ethane, cyclohexane, etc.) and emerging fuels (dimethyl ether, etc.) on the basis of experimental and theoretical research.24,27–30 These mechanisms involve hundreds of material components (including free radical fragments ranging from the smallest H atoms to the largest carbon black precursors) and thousands of chemical reactions. At present, scholars around the world are actively studying the combustion process of hydrocarbons. Frenklach et al.30 developed the process of carbon black formation and proposed the hydrogen abstraction-acetylene addition (HACA) mechanism of PAH transformation. Richter et al.28 experimentally studied the generation and transformation of PAHs in the premix combustion flame of benzene, acetylene and ethylene, which yielded carbon black particles with particle sizes less than 70 nm, and established a detailed reflection mechanism model. Richter and Howard29 proposed a simplified version of the reaction mechanism for hydrocarbon combustion in high-temperature coke oven gas and added the submodel of anisyl ether conversion mechanism proposed by M. Nowakowska et al.31 In this paper, the partial oxidation process of anisole, a model biomass tar compound, was simulated under different conditions. To describe the mechanism model of the PAH derivation process, the thermodynamic parameters proposed by M. Pecullan32 were adopted, and the FITDAT module built in Chemkin was used to generate them. The mechanism consists of 201 components and 1100 elementary reactions. The simulation was carried out using Chemkin 4.5 software.2.2 Model validation
The model of anisole conversion mechanism was coupled with the gas reaction mechanism proposed by Richter and Howard,29 and the PSR program in Chemkin 4.5 software was used to simulate the pyrolysis process of anisole at different temperatures. The simulation results were compared with the experimental data of M. Nowakowska et al.31 The simulated conditions were consistent with the experimental conditions. The experimental conditions are shown in Table 1 below.| Pyrolysis | Residence time pers | Temperature (K) | ||
|---|---|---|---|---|
| Anisole/mole% | Argon/mole% | Pressure/bar | ||
| 0.01 | 0.99 | 1 | 2 | 673–1173 |
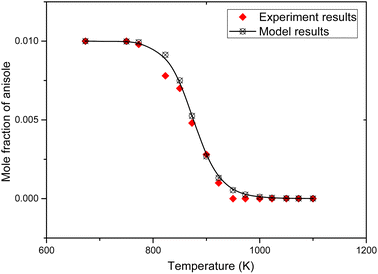
The simulation results and experimental results are shown in Fig. 2.
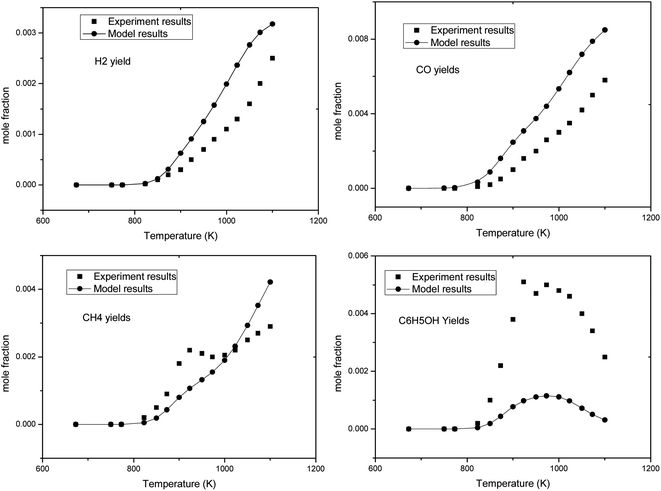
In Fig. 2, the black dots are the experimental results of the pyrolysis of anisole reported by M. Nowakowska et al.,31 and the curves are the simulation results of the corresponding working conditions simulated using the Chemkin PSR module. It can be found that this model describes the conversion of anisole pyrolysis at different temperatures. In order to fully verify the model, the formation rules of anisole pyrolysis products were simulated and compared with the experimental results, as shown in Fig. 3.
It can be seen from Fig. 3 that the simulation results of several typical gas products (H2, CO, CH4) have good agreement with the experimental results in terms of the variation trend with respect to pyrolysis temperature. Phenol is one of the important intermediate products formed in the pyrolysis of anisole. It can be seen that its yield changed with temperature, and the experimental results were good. However, there is a certain gap between the absolute value of yield at 1000 K and the experimental results. C6H5OH is produced by the initial of decomposition of anisole. The combination of C6H5O and H produces C6H5OH. In the experiments, the yield of C6H5OH is higher than that obtained by modeling. The reason is partly due to the existence of the reactor wall, which may benefit the combination of C6H5O and H. In general, this model can describe the pyrolysis process of anisole well.
2.3 Molecular dynamic modeling based on the ReaxFF method
Recently, molecular dynamic simulations based on the reactive force field (ReaxFF) have been successfully used to simulate the complex thermal reactions of hydrocarbons, such as coal, biomass and some gaseous fuels. ReaxFF has generated results consistent with quantum-mechanical calculations and experiments for numerous hydrocarbon reactions with far less computational load, indicating potential for the simulation of large reactive systems.33 Using ReaxFF, we can obtain bond cleavage, bond formation, changes in species, and further elementary reactions involved in complex thermal reactions.34 With respect to tar conversion, Chen et al.35 used the ReaxFF MD method to study the reaction of primary tar species, including phenol and xylose monomers. They found that the MD-derived activation energy was trustworthy.In this work, molecular dynamic modeling based on the ReaxFF method was used to study the thermal decomposition of anisole. A three-dimensional periodic box filled with 100 anisole molecules was built on the AMS software, with a box size of 40 Å × 40 Å × 40 Å. A set of non-isothermal simulations was performed using the NVT ensemble in the temperature range of 300–4000 K at the heating rate of 30 K ps−1, and then the temperature was maintained at 4000 K for 100 ps. In the ReaxFF simulations, the bond order cutoff and non-bonded cutoff were 0.3 and 10.0 Å, respectively. The time step was set to 0.25 fs.
The reactive force field is crucial for modeling performance. In this work, the CHO-2016 force field, which was developed by Ashraf et al.,36 was used for all the simulations. The CHO-2016 force field overcomes the shortcomings of CHO-2008 (ref. 37) and is used extensively to study the pyrolysis or combustion of different hydrocarbon fuels. The force field has also shown good capability to describe the decomposition and combustion of single component fuels.38
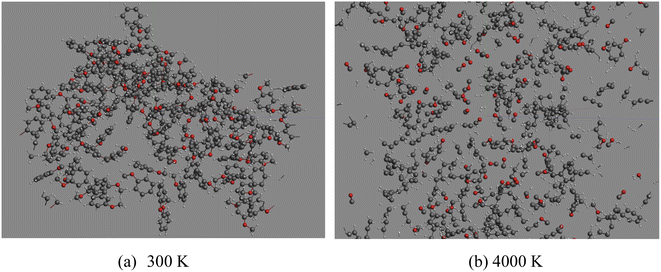
The optimized molecular configurations of anisole at 300 K and decomposition products at 4000 K are summarized in Fig. 4.
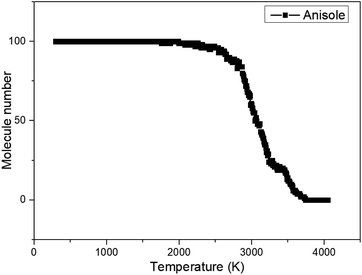
It can be seen that at 300 K, anisole is not likely to react according to the ReaxFF calculation. When the temperature reached 4000 K, anisole largely decomposed and many products, such as CO, H2, CH4, and C6H5OH, were produced, consistent with the experimental results and kinetic mechanism calculation. Based on the ReaxFF MD calculation, the variation in the number of anisole molecules with decomposition temperature was recorded, and the curve is shown in Fig. 5 below.
The plot indicates that the initial temperature of anisole decomposition is around 1976 K, which is much higher than the actual temperature recorded experimentally. The reason is that ReaxFF MD simulations typically operate at a time scale of picoseconds or nanoseconds, which is much shorter than experiments, which operate at a scale of milliseconds or seconds. To achieve reaction data in the real temperature ranges using ReaxFF MD modeling, the modeling time should be very long and comparable with the real reaction (milliseconds or seconds). Prolonged computation times are not supported by limited computational resources, especially for large molecular systems. In order to observe the chemical reaction in a short time using ReaxFF MD simulations, in practice, usually, the modeling temperature is increased to accelerate the reaction speed such that the chemical reactions occur within an extremely short period of time (picoseconds or nanoseconds). This is a reasonable approach ReaxFF MD calculations in many current applications.
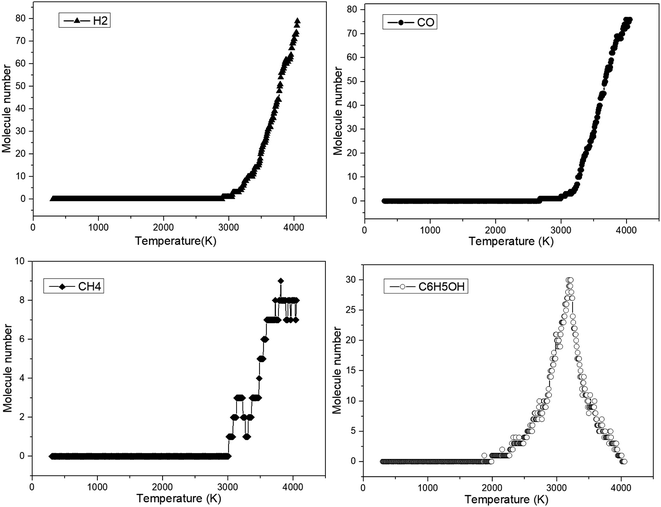
Similarly, some key products obtained during anisole decomposition were recorded, and the results are summarized in Fig. 6.
It can be indicated from Fig. 6 that phenol was initially formed at 1980 K, which is consistent with the initial decomposition temperature of anisole. This means that phenol is the initial decomposition product of anisole. With a further increase in temperature, the yield of phenol first increased to a peak value (3200 K) and then decreased, which can be attributed to secondary reaction at higher temperatures. The difference is that the initial temperature of H2, CO and CH4 is close to 3000 K, which is much higher than the initial cracking temperature of anisole. The reason is that small gaseous species are mainly derived from secondary reactions. For example, CO was mainly produced from the decarbonylation of the phenoxyl groups. In ReaxFF MD calculations, the secondary reaction usually occurs at a higher temperature, which is attributed to the short modeling time scale. Nevertheless, the formation of these small gas molecules is basically consistent with the experimental results.
3. The partial oxidation of anisole
Employing an appropriate amount of oxygen/air injection (usually at a subchemical equivalence ratio) is an effective method for tar removal during homogeneous conversion.39,40 This is an important means used in most gasification and tar cracking technologies.41,42 B. J. Vreugdenhil et al.43 proposed that the addition of oxygen mainly had two purposes: (i) to achieve the required high temperature in the reactor through an exothermic oxidation reaction. (ii) From an elemental equilibrium point of view, this introduces extra oxygen atoms to facilitate cracking of tar into CO and H2.In the partial oxidation process, temperature, oxygen content and reaction residence time are the key parameters that affect tar conversion. In this paper, anisole has been used as the model compound of tar, and the composition formation rules of anisole were studied under different temperatures and oxygen levels. Combined with ROP and sensitivity analysis, the key reaction path of the partial oxidation transformation of tar was obtained, providing theoretical support for the partial oxidation technology for the removal of biomass tar.
3.1 Formation rules of the partial oxidation products of anisole
The conversion process of pyrolysis gas containing tar biomass was analyzed under different temperatures and oxygen equivalent conditions by using the PSR module. The key word TGIV was added, and the energy calculation was not carried out. It was considered that the temperature remains constant in the partial oxidation process. The following parameters were employed: reaction temperatures 1173 K, 1273 K, and 1373 K, oxygen equivalents at 0, 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, and 0.8, the reaction residence time was 2 s, and the pressure was 1 atm. The calculated results are shown in Fig. 7.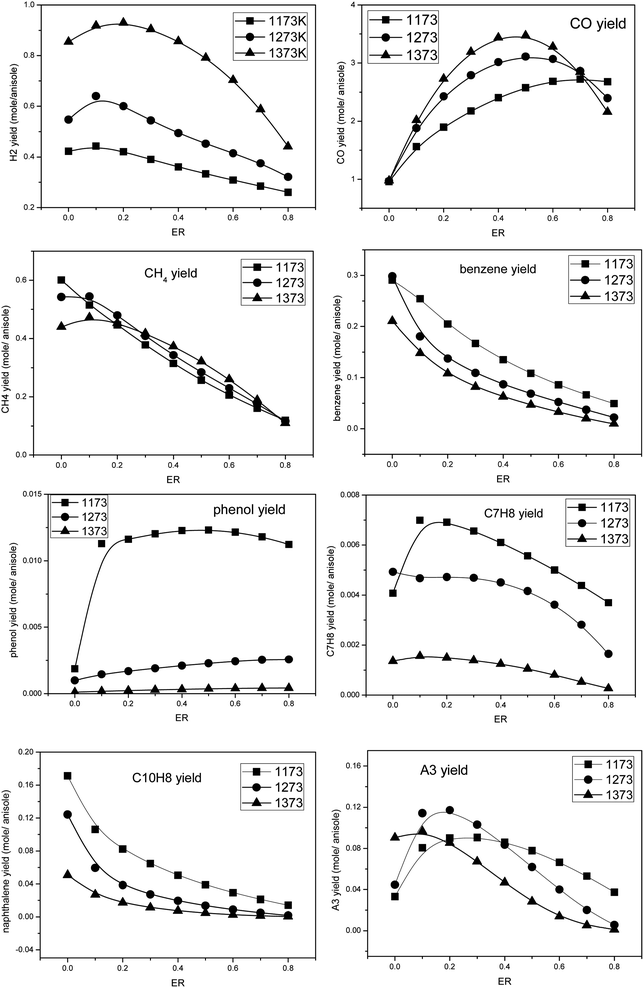 | ||
| Fig. 7 The product distributions during the partial oxidation of anisole under different temperatures and oxygen equivalents. | ||
It can be seen that temperature and amount of oxygen have an obvious influence on the formation of products in the pyrolysis process of anisole. H2 and CO were the two main flammable target products of biomass gasification, and their yields first increased and then decreased with the increase in oxygen content. At 1373 K, the H2 yield reached the maximum value at ER = 0.2, while the CO yield peaked at ER = 0.5, which may be due to the fact that the oxidation of H2 is easier. The formation law of CH4 was different from the two gases mentioned above. With the increase in oxygen content, the yield of CH4 decreased linearly, which indicates that the oxidizing atmosphere is conducive to the formation of hydrocarbon gases, such as CH4. Benzene was the smallest component among aromatic hydrocarbons, and its yield was similar to that of CH4, showing a decreasing trend with the increase in temperature and oxygen content. Naphthalene is a typical PAH tar and one of the main components of tar after partial oxidation in a biomass gasifier. It can be seen that the formation rule of naphthalene was similar to that of benzene, and the yield of naphthalene also decreased with an increase in temperature and oxygen. Phenol is a typical secondary tar; however, at 1273 K and 1373 K, the yield of phenol was low, and with the increase in oxygen content, the yield of phenol increased only slightly, which may be because the pyrolysis processes of anisole and other tars produce some phenol. Toluene is a typical secondary tar. At 1173 K, its yield peak appeared at ER = 0.1. With an increase in temperature, its yield decreased. Phenanthrene (A3) is a typical PAH tar. With an increase in temperature, the peak yield of phenanthrene (A3) shifted to the direction of low ER. These results indicate that in the partial oxidation process of anisole, a small amount of oxygen promotes the formation of macromolecular PAH tar, while in a high-oxygen atmosphere, the PAHs generated can undergo further oxidative cracking.
3.2 Rate of product formation (ROP) analysis
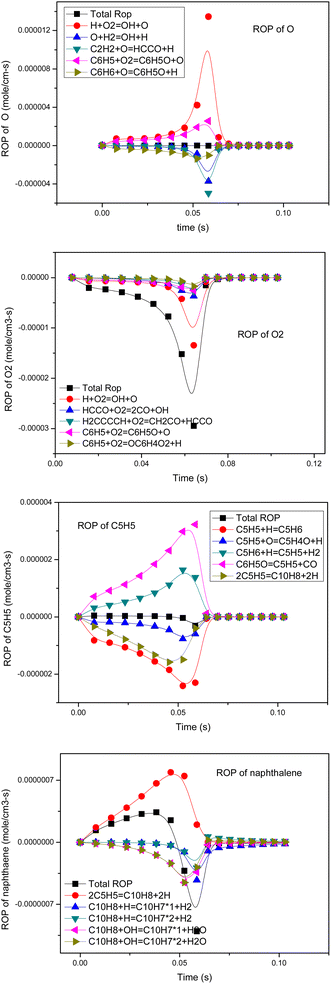
The rate of formation (ROP) coefficient reflects the influence of each elementary reaction on the corresponding product formation. A reaction with a positive ROP promotes the formation of the target product. A reaction with a negative ROP consumes the target product. Therefore, in the process of simulation, by calculating the sum of the absolute values of ROP under all calculated step sizes, the importance of this reaction in the formation of various substances in the whole conversion process was judged, and the ways of tar transformation and generation were studied and identified. Combined with sensitivity analysis, the mechanism could be simplified. The ROP analysis results of several key components of p-anisole at 900 °C and ER = 0.4 are shown in Fig. 8.In the partial oxidation process, O, O2, HO2, OH and other active radicals play a key role.
As seen in Fig. 8, the generation of O radicals mainly happens through the H + O2 = OH + O reaction, which directly comes from O2. The generated O free radicals can react directly with C2H2 and C6H6; C2H2 is an important intermediate product in the hydrocarbon combustion process and plays an important role in the generation of PAHs and carbon black through the HACA mechanism. C6H6 is the most basic aromatic hydrocarbon, which forms a phenoxyl structure under the attack of reactive oxygen radicals, and the decarbonylation of the phenoxyl structure is one of the main pathways of benzene ring cracking.44 The O2 molecule also has strong oxidation activity because it is in a triplet state, and its electronic structure is similar to free radicals. As seen in Fig. 8, O2 consumption mainly happens by the direct oxidation of the intermediate free groups, such as H, HCCO, H2CCCCH and C6H5. In the oxidation process, O2 does not only promote the cracking of macromolecular components, such as H2CCCCH and C6H5, but also generates new active radicals, such as H, OH, and O, through chain reactions to further promote the cracking and oxidation of tar.
Cyclopentadienyl (C5H5) is an important intermediate component in the combustion process of hydrocarbons and can be polymerized to form macromolecular PAHs and carbon black through the resonance-stabilized free radical binding reaction (RSR).45 From the ROP analysis, it was found that the main source of C5H5 was the decarbonylation of phenoxyl (C6H5O), and a small part came from the dehydrogenation of cyclopentadiene (C5H6). The consumption of PAHs is mainly controlled by cyclopentadienyl bimolecular polymerization, which produces a large amount of PAHs, such as naphthalene (C10H8). However, in an oxidizing atmosphere, O radicals can attack C5H5 to generate C5H4O, which can undergo further decarbonylation reactions and finally decompose into small-molecule components.
Naphthalene is a typical PAH component.41,46 It can be seen that in the partial oxidation process of anisole, the generation of naphthalene happens mainly through 2C5H5 = C10H8 + 2H, that is, the RSR mechanism of cyclopentadienyl. The cracking reaction of naphthalene occurs mainly through the reaction with H and OH free radicals, and OH is the main reaction.
Therefore, from the ROP analysis, it can be inferred that O, O2, OH and other oxygen-containing active groups play a key role in the formation and conversion of tar. In the partial oxidation by adding a certain amount of oxygen, the reaction process produces a large number of oxygen-containing active free radicals, promoting the pyrolysis of tar.
3.3 Reaction heat analysis
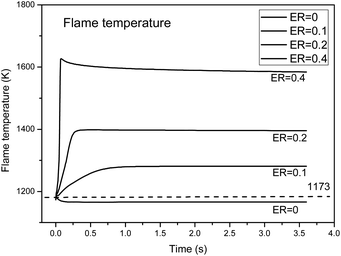
With the introduction of oxygen, partial oxidation produces a large number of active free radicals and changes the chemical reaction path. On the other hand, the oxidation reaction can generate a certain amount of heat and improve the temperature of the reaction zone. Therefore, it is necessary to analyze the energy change in the reaction process, that is, the temperature change of the reaction flame. The effect of exothermic oxidation on the partial oxidation process was considered. The ENRG keyword was added to calculate the energy of the system without considering heat loss. Fig. 9 shows the temperature field changes during the partial oxidation process under different ER (0, 0.1, 0.2, and 0.4) conditions, as calculated by the PLUG module.The results show that in an inert atmosphere, the temperature dropped in the first 0.3 s, then remained stable, and the final temperature was about 1150 K, which is about 23 K lower than the initial temperature of 1173 K. This indicates that the pyrolysis of biomass tar under an inert atmosphere is an endothermic process, so external heat supplementation is needed. Under the conditions of ER = 0.1, 0.2 and 0.4, the reaction temperature increased rapidly within the first 0.3 s, and the amplitude increased with an increase in oxygen. At ER = 0.2, the maximum temperature in the reactor was 1380 K, which is 207 K higher than the initial temperature, while at ER = 0.4, the maximum temperature in the reactor was 1594 K, which is 421 K higher than the initial temperature.
The addition of oxygen, tar and small molecules of flammable gases (H2, CO, CH4, etc.) would lead to a violent oxidation reaction, greatly increasing the temperature in parts of the oxidation zone, and at the same time, promote the generation of more active free radicals, further enhancing the pyrolysis conversion of tar.
3.4 Tar cracking and partial oxidation reaction path analysis
Based on the above conclusions, the main pyrolysis reaction path of anisole can be as follows (Fig. 10).Anisole can lose the methyl group and form a phenol group under certain temperatures and conditions, and further lose CO to form a cyclopentadienyl group. Another conversion route is the direct removal of the methoxyl group of phenol to form C6H5, which is the main conversion method of anisole, accounting for more than 70%. Cyclopentadiene is further converted into C2H2, C3H5 and other small molecules under the action of hydrogen. Under an inert atmosphere, the cyclopentadiene radicals can polymerize to produce naphthalene. The conversion of naphthalene can happen in two ways: pyrolysis and polymerization. Pyrolysis mainly occurs with the addition of oxygen to remove CO, while the polymerization route follows the mechanism of HACA. In the first step, acenaphthene is generated, and further polymerization occurs to produce higher-molecular-weight PAHs. The presence of oxygen promotes the circumferential oxygenation reaction of PAHs, thus increasing their cracking rate and generating more small-molecular gas components. However, the H radicals produced in the HACA process are largely consumed in the presence of oxygen, so the polymerization reaction is also strengthened to a certain extent.
Therefore, the conversion characteristics of tar under partial oxidation conditions are as follows: that the amount of tar is greatly reduced, while the structure of residual tar is more stable and its properties are more stubborn; further, most of them become tertiary tar.47
It is worth noting that in the process of biomass partial oxidation, oxygen has a multi-stage effect. At the beginning of the reaction, oxygen reacts with small molecule gases, giving off heat and forming active free radicals at the same time, thus promoting tar cracking. In the middle stage of the reaction, a certain amount of oxygen free radicals react with PAHs or cyclopentadiene, gradually take off CO pyrolysis. The PAH components are gradually cracked into small molecule gas products.
3.5 Effect of staged-oxygen supply on tar conversion
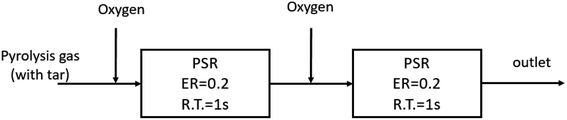
In view of the above, the oxidation cracking process of PAHs has a step-by-step characteristic. Therefore, in the actual design process of a gasifier, it is necessary to ensure that oxygen is supplied step by step through the entire homogeneous decomposition process so as to avoid the polymerization of tar at the later stage, which is caused by the consumption of oxygen by the small molecule gases at the initial stage of reaction. The hierarchical distribution oxygenation process has been applied in Carbo-V,48,49 NOTAR50,51 and other gasifiers.Based on the detailed reaction mechanism, the PSR module in Chemkin 4.5 software was used to establish a tar cracking process model with graded oxygen distribution, as shown in Fig. 11.
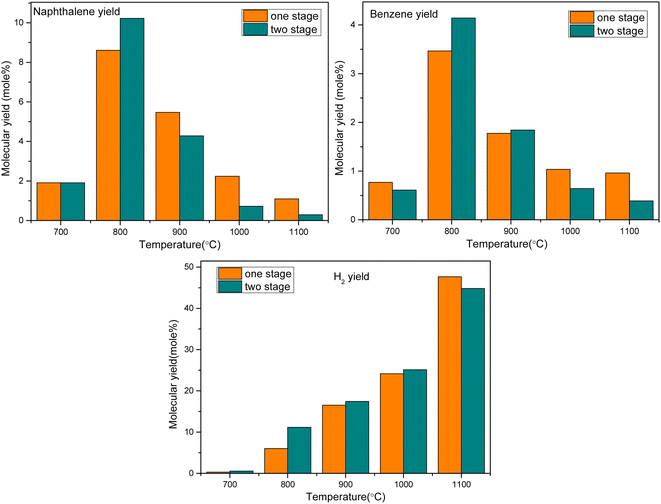
The specific parameters are as follows: the pyrolysis gas is a mixture containing anisole as the model tar compound, the single-stage oxygen distribution model is ER = 0.4, the reaction residence time R.T. = 2 s, the TGIV keyword is used, and the reaction temperature remains unchanged. In the multi-stage oxygen distribution model, two PSR modules are connected in series, and oxygen is injected in two stages at an equivalent ratio of 0.2, that is, the average oxygen injection and residence time is 1 s. The calculation results are shown in Fig. 12.
It can be seen that oxygen staging has different effects on the partial oxidation of anisole in different temperature ranges. At a lower temperature (800 °C), the yield of benzene and naphthalene can be slightly increased by oxygen fractionation. The reason is that at low temperatures, oxygen molecules are more likely to react with smaller gas components. However, from the perspective of hydrogen generation, at 800 °C, the hydrogen production rate with oxygen staging was significantly higher than that without oxygen staging. This indicates that at lower temperatures, oxygen is more likely to promote the oxidation reaction of small molecular hydrocarbons. At high temperatures, the yield of tertiary tar of aromatic hydrocarbons, such as benzene and naphthalene, decreased obviously, while the yield of hydrogen also increased rapidly. In these conditions, the oxygen molecules can directly attack the macromolecular tar compounds, enhancing the selective cracking and oxidation of tar compounds. In the whole process, under high-temperature conditions (above 1000 °C), staged-oxygen supply could obviously promote tar cracking and inhibit the generation of macromolecular PAH tar compounds.
4. Conclusion
The thermal cracking and partial oxidation processes of anisole, a model compound of lignin tar, were numerically calculated using the detailed reaction mechanism and the ReaxFF MD method. The simulation results were compared with those of M. Nowakowska et al., and the reliability of the model was verified. On this basis, the partial oxidation process of anisole is analyzed, and the factors, such as product distribution, heat change in the reaction process and oxygen staging, are discussed. The main conclusions are as follows:(1) The addition of oxygen promotes the pyrolysis of anisole. A proper amount of oxygen can crack tar into flammable small-molecule gases (H2, CO) and inhibit the generation of macromolecular PAH tar.
(2) The ROP analysis results show that O, O2, OH and other oxygen-containing active groups play a key role in the formation and transformation of tar. With the addition of a certain amount of oxygen, the partial oxidation reaction process produces a large number of oxygen-containing active free radicals, promoting the pyrolysis transformation of tar.
(3) The reaction energy analysis shows that the addition of oxygen does not only provide the active free radicals but also releases a lot of heat through the oxidation reaction, raises the temperature of the reaction zone, and further promotes the pyrolysis of tar.
(4) The calculated results for staged-oxygen supply show that at high temperatures (above 1000 °C), using oxygen staging can promote the pyrolysis of tar, inhibit the generation of PAH tar, and selectively crack tar into small-molecule flammable gases.
Data availability
All data generated and analyzed during this study are included in this paper and appendix.Author contributions
Conceptualization and methodology: Ming Hu and Shanhui Zhao. Data collection and analysis was performed by Ming Hu and Shanhui Zhao. Figures were drawn by Ming Hu. Writing—review and editing: Yonghao Luo is all the authors read and agreed the final manuscript. Shanhui Zhao are the corresponding authors.Conflicts of interest
The author declares no competing interests.Acknowledgements
This work was supported by the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (20KJB470012) and Science Foundation of Nanjing Institute of Technology (YKJ201813).References
- S. Heidenreich and P. U. Foscolo, New concepts in biomass gasification, Prog. Energy Combust. Sci., 2015, 46, 72–95 CrossRef.
- S. Heidenreich, M. Müller, and P. U. Foscolo, Advanced Biomass Gasification: New Concepts for Efficiency Increase and Product Flexibility, Academic Press, 2016 Search PubMed.
- K. Yang, et al., Understanding the homogeneous reactions of primary tar from biomass pyrolysis by means of photoionization mass spectrometry, Energy Fuels, 2020, 34(10), 12678–12687 CrossRef CAS.
- M. L. V. Rios, et al., Reduction of tar generated during biomass gasification: A review, Biomass Bioenergy, 2018, 108, 345–370 CrossRef.
- L. P. Rabou, et al., Tar in biomass producer gas, the Energy research Centre of the Netherlands (ECN) experience: an enduring challenge, Energy Fuels, 2009, 23(12), 6189–6198 CrossRef CAS.
- C. Li and K. Suzuki, Tar property, analysis, reforming mechanism and model for biomass gasification—an overview, Renewable Sustainable Energy Rev., 2009, 13(3), 594–604 CrossRef CAS.
- M. Wang, et al., Combustion characteristics and kinetic analysis of heavy tar from continuous pyrolysis of camellia shell, Fuel Process. Technol., 2018, 176, 131–137 CrossRef CAS.
- H. N. Nguyen, M. Seemann and H. Thunman, Fate of polycyclic aromatic hydrocarbons during tertiary tar formation in steam gasification of biomass, Energy Fuels, 2018, 32(3), 3499–3509 CrossRef CAS.
- H. Kawamoto, Lignin pyrolysis reactions, J. Wood Sci., 2017, 63(2), 117–132 CrossRef CAS.
- S. Park, et al., Continuous pyrolysis of organosolv lignin and application of biochar on gasification of high density polyethylene, Appl. Energy, 2019, 255, 113801 CrossRef CAS.
- J. Ralph, C. Lapierre and W. Boerjan, Lignin structure and its engineering, Curr. Opin. Biotechnol., 2019, 56, 240–249 CrossRef CAS.
- H. dos Santos Abreu, A. M. do Nascimento and M. A. Maria, Lignin structure and wood properties, Wood Fiber Sci., 1999, 31(4), 426–433 CAS.
- B. Wang, et al., Cooperative catalysis of Co single atoms and nanoparticles enables selective CAr–OCH3 cleavage for sustainable production of lignin-based cyclohexanols, J. Energy Chem., 2023, 79, 535–549 CrossRef CAS.
- M. Lawoko, G. Henriksson and G. Gellerstedt, Structural differences between the lignin – carbohydrate complexes present in wood and in chemical pulps, Biomacromolecules, 2005, 6(6), 3467–3473 CrossRef CAS PubMed.
- P. Pal, H. Li and S. Saravanamurugan, Removal of lignin and silica from rice straw for enhanced accessibility of holocellulose for the production of high-value chemicals, Bioresour. Technol., 2022, 361, 127661 CrossRef CAS PubMed.
- N. T. T. Tran, et al., Vapor-phase hydrodeoxygenation of lignin-derived bio-oil over Al-MCM-41 supported Pd-Co and Pd-Fe catalysts, Mol. Catal., 2022, 523, 111435 CrossRef CAS.
- S. Z. Abidin, et al., Recent progress on catalyst development in biomass tar steam reforming: toluene as a biomass tar model compound, Biomass Convers. Biorefin., 2023, 1–36 Search PubMed.
- J. Fjellerup, et al., Formation, Decomposition and Cracking of Biomass Tars in Gasification, Technical University of Denmark. Department of Mechanical Engineering, 2005 Search PubMed.
- J. Han and H. Kim, The reduction and control technology of tar during biomass gasification/pyrolysis: an overview, Renewable Sustainable Energy Rev., 2008, 12(2), 397–416 CrossRef CAS.
- P. Beaty, Nexterra-Gasification of bark–A pragmatic development roadmap for gasification, in TAPPI 2007 International Conference on Renewable Energy, 2007 Search PubMed.
- P. Brandt, E. Larsen and U. Henriksen, High Tar Reduction in a Two-Stage Gasifier, Energy Fuels, 2000, 14(4), 816–819 CrossRef CAS.
- P. H. Brandt and B. Ulrik, Decomposition of tar in pyrolysis gas by partial oxidation and thermal cracking: Part 2, in 10th European Conference and Technology Exhibition Biomass for Energy and Industry, C.A.R.M.E.N., 1998, pp. 1616–1618 Search PubMed.
- J. R. Marda, et al., Non-catalytic partial oxidation of bio-oil to synthesis gas for distributed hydrogen production, Int. J. Hydrogen Energy, 2009, 34(20), 8519–8534 CrossRef CAS.
- K. Norinaga and J. Hayashi, Numerical simulation of the partial oxidation of hot coke oven gas with a detailed chemical kinetic model, Energy Fuels, 2010, 24(1), 165–172 CrossRef CAS.
- H. Richter and J. B. Howard, Formation and consumption of single-ring aromatic hydrocarbons and their precursors in premixed acetylene, ethylene and benzene flames, Phys. Chem. Chem. Phys., 2002, 4(11), 2038–2055 RSC.
- L. Gerun, et al., Numerical investigation of the partial oxidation in a two-stage downdraft gasifier, Fuel, 2008, 87(7), 1383–1393 CrossRef CAS.
- E. Ranzi, et al., Lumping procedures in detailed kinetic modeling of gasification, pyrolysis, partial oxidation and combustion of hydrocarbon mixtures, Prog. Energy Combust. Sci., 2001, 27(1), 99–139 CrossRef CAS.
- H. Richter, et al., Formation and consumption of single-ring aromatic hydrocarbons and their precursors in premixed acetylene, ethylene and benzene flames, Phys. Chem. Chem. Phys., 2002, 4(11), 2038–2055 RSC.
- H. Richter, et al., Detailed modeling of PAH and soot formation in a laminar premixed benzene/oxygen/argon low-pressure flame, Proc. Combust. Inst., 2005, 30(1), 1397–1405 CrossRef.
- M. Frenklach, Reaction mechanism of soot formation in flames, Phys. Chem. Chem. Phys., 2002, 4(11), 2028–2037 RSC.
- M. Nowakowska, et al., Detailed kinetic study of anisole pyrolysis and oxidation to understand tar formation during biomass combustion and gasification, Combust. Flame, 2014, 161(6), 1474–1488 CrossRef CAS.
- M. Pecullan, K. Brezinsky and I. Glassman, Pyrolysis and Oxidation of Anisole near 1000 K, J. Phys. Chem. A, 1997, 101(18), 3305–3316 CrossRef CAS.
- A. C. Van Duin, et al., ReaxFF: a reactive force field for hydrocarbons, J. Phys. Chem. A, 2001, 105(41), 9396–9409 CrossRef CAS.
- Y.-Y. Li, et al., ReaxFF study on nitrogen-transfer mechanism in the oxidation process of lignite, Fuel, 2017, 193, 331–342 CrossRef CAS.
- T. Chen, et al., High-temperature pyrolysis modeling of a thermally thick biomass particle based on an MD-derived tar cracking model, Chem. Eng. J., 2021, 417, 127923 CrossRef CAS.
- C. Ashraf and A. C. T. van Duin, Extension of the ReaxFF Combustion Force Field toward Syngas Combustion and Initial Oxidation Kinetics, J. Phys. Chem. A, 2017, 121(5), 1051–1068 CrossRef CAS PubMed.
- K. Chenoweth, A. C. Van Duin and W. A. Goddard, ReaxFF reactive force field for molecular dynamics simulations of hydrocarbon oxidation, J. Phys. Chem. A, 2008, 112(5), 1040–1053 CrossRef CAS PubMed.
- H. Kwon, et al., ReaxFF-based molecular dynamics study of bio-derived polycyclic alkanes as potential alternative jet fuels, Fuel, 2020, 279, 118548 CrossRef CAS.
- J. Ahrenfeldt, et al., The influence of partial oxidation mechanisms on tar destruction in TwoStage biomass gasification, Fuel, 2013, 112, 662–680 CrossRef CAS.
- S. Yi, et al., Experimental and numerical investigation of tar destruction under partial oxidation environment, Fuel Process. Technol., 2011, 92(8), 1513–1524 CrossRef.
- M. P. Houben, H. C. Lange and A. Steenhoven, Tar reduction through partial combustion of fuel gas, Fuel, 2005, 84(7/8), 817–824 CrossRef CAS.
- J. Han and H. Kim, The reduction and control technology of tar during biomass gasification/pyrolysis: An overview – ScienceDirect, Renewable Sustainable Energy Rev., 2008, 12(2), 397–416 CrossRef CAS.
- B. J. Vreugdenhil and R. Zwart, Tar Formation in Pyrolysis and Gasification, 2009 Search PubMed.
- N. M. Marinov, A detailed chemical kinetic model for high temperature ethanol oxidation, Int. J. Chem. Kinet., 1999, 183–220 CrossRef CAS.
- A. Jess, Mechanisms and kinetics of thermal reactions of aromatic hydrocarbons from pyrolysis of solid fuels, Fuel, 1996, 75(12), 1441–1448 CrossRef CAS.
- Y. L. Zhang, et al., Heterogeneous Cracking Reaction of Tar over Biomass Char, Using Naphthalene as Model Biomass Tar, Energy Fuels, 2014, 28(5), 3129–3137 CrossRef CAS.
- H. Nguyen, M. Seemann and H. Thunman, Fate of Polycyclic Aromatic Hydrocarbons during Tertiary Tar Formation in Steam Gasification of Biomass, Energy Fuels, 2018, 32(3), 3499–3509 CrossRef CAS.
- S. Hamel, et al., Autothermal two-stage gasification of low-density waste-derived fuels, Energy, 2007, 32(2), 95–107 CrossRef CAS.
- J. Vogels, T. Grube, and D. Stolten, Industrial Scale Hydrogen Production from Biomass via CHOREN's Unique Carbo-V-Process, 2010 Search PubMed.
- M. Milhe, et al., Autothermal and allothermal pyrolysis in a continuous fixed bed reactor, J. Anal. Appl. Pyrolysis, 2013, 102–111 CrossRef CAS.
- J. P. Damon, et al., in Environmental Benefits with Used Railway Sleepers Fueling Xylowatt's NOTAR Gasification Technology, European Biomass Conference & Exhibition, 2012 Search PubMed.
| This journal is © The Royal Society of Chemistry 2023 |