Open Access Article
Open Access ArticleWater-soluble fluorescent chemosensor for sorbitol based on a dicationic diboronic receptor. Crystal structure and spectroscopic studies†
Julio Zamora-Moreno *a,
María K. Salomón-Flores
*a,
María K. Salomón-Flores a,
Josue Valdes-García
a,
Josue Valdes-García a,
Cristian Pinzón-Vanegas
a,
Cristian Pinzón-Vanegas a,
Diego Martínez-Oteroab,
Joaquín Barroso-Flores
a,
Diego Martínez-Oteroab,
Joaquín Barroso-Flores ab,
Raúl Villamil-Ramosc,
Miguel Á. Romero-Solano
ab,
Raúl Villamil-Ramosc,
Miguel Á. Romero-Solano a and
Alejandro Dorazco-González
a and
Alejandro Dorazco-González *a
*a
aInstitute of Chemistry, National Autonomous University of Mexico, Ciudad Universitaria, México 04510, Mexico. E-mail: julio_zm@uaem.mx; adg@unam.mx
bCentro Conjunto de Investigación en Química Sustentable, UAEM-UNAM, Instituto de Química, Universidad Nacional Autónoma de México, C. P. 50200 Toluca, Estado de México, Mexico
cCentro de Investigaciones Químicas-IICBA, Universidad Autónoma del Estado de Morelos, Av. Universidad 1001 Col. Chamilpa, Cuernavaca, Morelos C.P. 62209, Mexico
First published on 1st November 2023
Abstract
Selective recognition of saccharides by phenylboronic dyes capable of functioning in aqueous conditions is a central topic of modern supramolecular chemistry that impacts analytical sciences and biological chemistry. Herein, a new dicationic diboronic acid structure 11 was synthesized, structurally described by single-crystal X-ray diffraction, and studied in-depth as fluorescent receptor for six saccharides in pure water at pH = 7.4. This dicationic receptor 11 has been designed particularly to respond to sorbitol and involves two convergent and strongly acidified phenyl boronic acids, with a pKa of 6.6, that operate as binding sites. The addition of sorbitol in the micromolar concentration range to receptor 11 induces strong fluorescence change, but in the presence of fructose, mannitol, glucose, lactose and sucrose, only moderate optical changes are observed. This change in emission is attributed to a static complexation photoinduced electron transfer mechanism as evidenced by lifetime experiments and different spectroscopic tools. The diboronic receptor has a high affinity/selectivity to sorbitol (K = 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 M−1) over other saccharides including common interfering species such as mannitol and fructose. The results based on 1H, 11B NMR spectroscopy, high-resolution mass spectrometry and density functional theory calculations, support that sorbitol is efficiently bound to 11 in a 1
800 M−1) over other saccharides including common interfering species such as mannitol and fructose. The results based on 1H, 11B NMR spectroscopy, high-resolution mass spectrometry and density functional theory calculations, support that sorbitol is efficiently bound to 11 in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mode involving a chelating diboronate–sorbitol complexation. Since the experimental B⋯B distance (5.3 Å) in 11 is very close to the calculated distance from the DFT-optimized complex with sorbitol, the efficient binding is attributed to strong acidification and preorganization of boronic acids. These results highlight the usefulness of a new diboronic acid receptor with a strong ability for fluorescent recognition of sorbitol in physiological conditions.
1 mode involving a chelating diboronate–sorbitol complexation. Since the experimental B⋯B distance (5.3 Å) in 11 is very close to the calculated distance from the DFT-optimized complex with sorbitol, the efficient binding is attributed to strong acidification and preorganization of boronic acids. These results highlight the usefulness of a new diboronic acid receptor with a strong ability for fluorescent recognition of sorbitol in physiological conditions.
Introduction
Selective recognition of polyols such as saccharides by synthetic boronic acids-based compounds remains a relevant area in supramolecular chemistry and analytical sciences due to applications in sensing,1,2 separation,3,4 glycoprotein manipulation,5 bioimaging,6 dynamic covalent assemblies,7 clinical diagnostic of sugars-related diseases8,9 medicinal chemistry,10 and the understanding of new concepts in the molecular recognition field.11In recent decades, the use of boronic acid derivatives has been proven to be an outstanding strategy for the recognition of saccharides,12–17 glucosamine,18 catecholamines,19 nucleotides,20 ginsenosides,21 sialic acid,22 glycated hemoglobin23 and in general, 1,2-dihydroxy-substituted compounds.24
The affinity of phenylboronic acids towards 1,2-diol derivatives is primarily induced by the reversible formation of diol–boronic ester in an sp2 hybridization, which results in increasing the Lewis acidity of the boron atom and subsequent fast conversion to the diol–boronate ester with an sp3 hybridization.25,26 In principle, the binding strength of boronic acid–diol ester depends on the orientation of the target analyte's hydroxyl groups,27 the acidity of the boronic acid,2 and the influence of substituent groups that can stabilize the sp3-boronate ester.25,28 The optimal pH for tetrahedral–boronate ester formation occurs ideally at a halfway pKa value between the boronic acid and diol; however, it is well-known that this may vary depending on solvent, buffer composition, as well as the substituent groups in the boronic acid.28,29
Simple phenylboronic acid (PBA, pKa ∼ 8.8) has too low binding constants for saccharides (<250 M−1),30 to achieve greater affinity, more sophisticated receptors are required. Thus, there is interest in creating water-soluble phenylboronic acid receptors with pKa values less than physiological pH. However, this is an ongoing challenge, and it is not a trivial task.
Open-chain polyols, such as sorbitol and mannitol are biochemical metabolic intermediates and have been used as medicines. Sorbitol is among the food industry's most widely used sweetener substitutes for glucose and as thickener.31,32 Some studies have shown that sorbitol is related to secondary effects in humans.33 Among different sorbitol sensing methods, fluorescence is desired due to its known high sensitivity and rapid analytical signal.34 While the need for highly selective and efficient chemosensors for sorbitol is evident, up until now, very few examples have been described compared to fructose and glucose.35–37
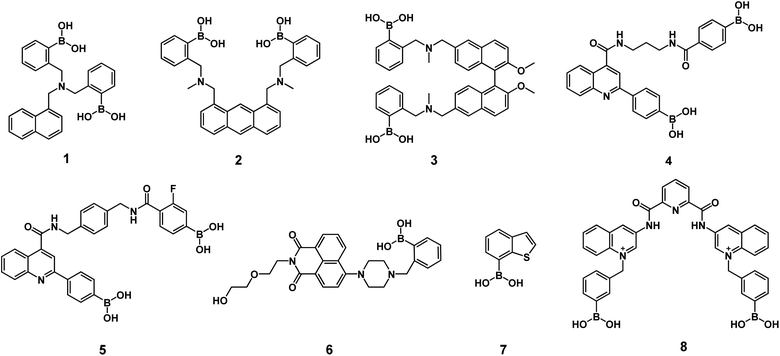
Optical recognition of sorbitol can be achieved by neutral diboronic acid dyes bearing aminomethyl groups (see Scheme 1) such as aminomethyl-naphthalene 1,38 aminomethyl-anthracene 2,39 binaphthalene-dimethanamine 3,40 carboxamide-quinoline 4-5.32,41 These fluorescent diboronic acids with amines typically show apparent binding constants between 350 and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 where the sp3-boronate ester is stabilized by the boron–nitrogen (amine) interaction. Consequently, they are suitable to sense at the micromolar concentration range, but not significantly lower. Many of these chemosensors require an organic cosolvent or basic conditions (pH > 8.0) to operate, which seriously hampers the intended applications. Furthermore, some still suffer some drawbacks such as too arduous synthesis and low selectivity to sorbitol.
000 M−1 where the sp3-boronate ester is stabilized by the boron–nitrogen (amine) interaction. Consequently, they are suitable to sense at the micromolar concentration range, but not significantly lower. Many of these chemosensors require an organic cosolvent or basic conditions (pH > 8.0) to operate, which seriously hampers the intended applications. Furthermore, some still suffer some drawbacks such as too arduous synthesis and low selectivity to sorbitol.
To the best of our knowledge, the literature features only two examples of fluorescent chemosensors for selective recognition of sorbitol in pure buffered water based on monoboronic acids bearing naphthalimide 6,42 and benzo-thiophenes 7 (ref. 43) that operate with modest affinity, (K < 4000 M−1). Overcoming these drawbacks should be feasible by using a hydrostable fluorescent diboronic receptor with pKa values close to physiological pH.
A successful strategy for the acidification of boronic acids is the insertion of positive charges into the receptor scaffold as was evidenced by Geddes in several quinolinium dyes covalently attached to monoboronic acids where pKa values dropped two units compared to the simple phenylboronic acid. These quinolinium-nucleus appended phenylboronic acids have been studied as optical sensors only for fructose, glucose and F−.44–46
Recently, we demonstrated successful applications of a set of cationic receptors based on scaffolds of 2,6-pyridinedicarboxamide containing diboronic acids such as compound 8 for recognition of saccharides in an aqueous phase at physiological pH with a selectivity towards sorbitol and glucose.47
Previous studies have shown that bisquinolinium pyridine-2,6-dicarboxamide dyes from 6-aminoquinoline possess greater solubility in water and better photophysical properties compared to 3-aminoquinoline derivatives such as isomer 8.56
Taking this into account, we surmised that an efficient sorbitol chemosensor could be achieved based on cationic diboronic acids attached to a fluorescent and semi-rigid scaffold able to orientate the boronic acids convergently.
In this study, we reported the results obtained for a water-soluble bisquinolinium pyridine-2,6-dicarboxamide salt bearing two strongly acidified boronic acids, including synthesis, crystal structure, acid–base properties, spectroscopic sensing studies of biological polyols and DFT calculations.
Results and discussion
Synthesis and structural analysis
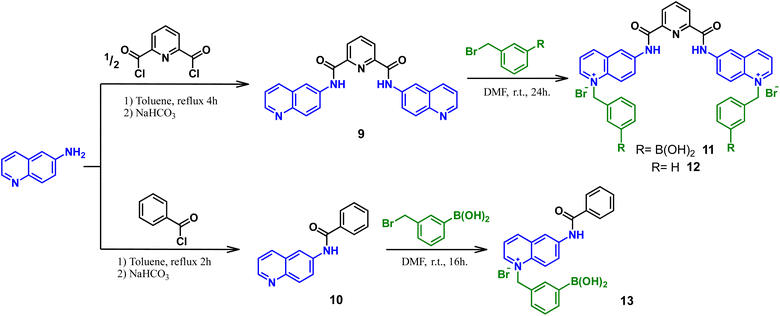
For these investigations, the bromide salt of bisquinolinium diboronic acid 11 was successfully obtained by the procedure depicted in Scheme 2.A reference compound lacking boronic acid moiety 12 and a related quinolinium mono-boronic acid 13 were also prepared for comparative purposes. Two steps were involved in the formation of compounds 11–13. First, the nucleophilic acyl substitutions were carried out by 6-aminoquinoline towards the appropriate acyl chloride in dry toluene under a N2 atmosphere, to obtain compounds 9 and 10 which were confirmed by 1H and 13C NMR-spectroscopy (Fig. S1–S4†). Subsequently, N-alkylation products were obtained as bromide salts in good yields by the prolonged treatment with the corresponding (bromomethyl)-aryl reagent at r. t. in anhydrous DMF. The salts 11–13 were obtained as pale-yellow powders and pure according to 1H, 13C, 11B NMR measurements, IR, high-resolution mass spectrometry and elemental analysis (C, H, N), see Fig. S5–S17.†
11B NMR measurements of 11 and 13 were obtained in a CD3-OD at 298 K. The spectra showed a broad signal centered at 27.4 ppm and 28.6 ppm for 11 and 13, respectively, consistent with the sp2-hybridized boron atom (Figs. S8 and S16†).48 Efforts to obtain single crystals of the bromide salt of 11 suitable for X-ray diffraction analysis were not successful. Possibly due to electrostatic repulsion between dicationic organic units, which cannot be stabilized with small anions such as bromide.
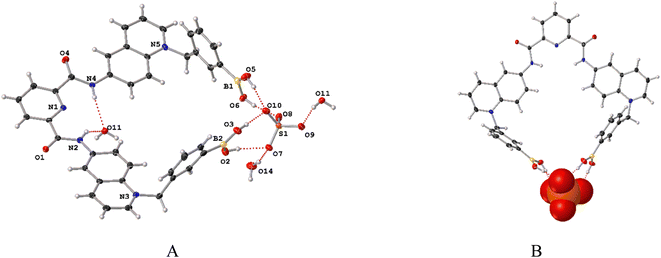
However, X-ray single-crystal structure of 11 was obtained as a sulfate trihydrated salt by an anion metathesis with NaSO4 from a mixture of H2O–DMSO (v/v, 99/1) by slow evaporation (see Table S1 in the ESI for crystallographic data†). Geometric parameters for bond lengths, angles around B atoms, hydrogen bonds and π–π stacking interactions within the crystal packing of 11-SO4 are compiled in Tables S2–S5.† Fig. 1A shows a perspective view of the molecular structure of 11-SO4 and it confirms the presence of sp2-hybridized boron atoms and trigonal geometries (Σ⦠(X–B1–X) = 360.0° and Σ⦠(X–B2–X) = 359.99°) which is consistent with its 11B NMR spectrum. Both phenylboronic acids are directed at the same side of the molecule and they are separated by a B⋯B distance of 5.30 Å which is structurally suitable for recognizing polyols in terms of the receptor preorganization.
In the reported structure of 8 that corresponds to an isomer of 11, the B⋯B distance is approximately 2.0 Å longer than in the crystal of 11. Importantly, all four –B–OH groups are oriented toward the sulfate anion, forming four hydrogen bonds B–OH⋯O as shown in Fig. 1B (Table S2†).
Clearly, the sulfate anion induces the convergence of the boronic acid groups. Thus, receptor 11 possibly could function as a receptor for oxoanions via cooperative hydrogen bonds.
The crystal of 11-SO4 is strongly hydrated; two (H2O)3 clusters are confined in the unit cell (Fig. S18†) with the consequence that the receptor cavity is occupied by a water molecule where the N–H bonds of both amide groups form two N–H⋯O hydrogen bonds. This high degree of hydration is evidence of its hydrostability in the solid state. The receptor 11-SO4 possesses a high degree of planarity between quinolinium rings and the central pyridine ring with dihedral angles between 5.54° and 8.48°. The two amide groups are directed inside the receptor cleft. This syn–syn conformation of amide groups has been usually described for crystals of pyridine-2,6-dicarboxamide pincer-like derivatives as a consequence of intramolecular H bonds of type N–H⋯Npyridine.49–53
An inspection of the crystal of 11-SO4 displays multiple π–π interactions mainly centered on the quinolinium rings and the central pyridine involving cofacial parallel stacked geometries with centroid–centroid distances ranging from 3.49 Å and 3.80 Å (Fig. S19 and Table S5†). These strong interactions can be ascribed to the positive electrostatic potential around the quinolinium rings.
Optical and acid–base properties
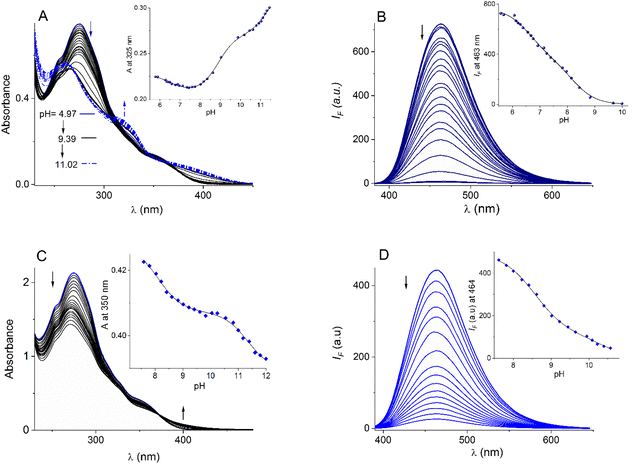
The bromide salts of cationic compounds 11–13 are soluble in buffered (10 mM MOPS pH = 7.4) pure water and follow very well the Lambert–Beer law up to 100 µM; thus, these conditions and a concentration within this range were used for further spectroscopic studies.The absorption and emission properties of bromide salts of compounds 11–13 are compiled in Table 1 and the family of UV-vis/fluorescence spectra at different pH values for 11 and 12 are displayed in Fig. 2A–D. In general, the salts 11–13 have strong absorption maxima at λ ∼ 271−274 nm with lower-energy absorption shoulders at λ ∼ 325−350 nm attributed to intraligand π → π* electronic transitions centered in the N-alkyl quinolinium fragment.54 The blue emission in these quinolinium-based compounds is typically attributed to intramolecular charge transfers (ICTs) in the excited state.55 Compound 11 possesses four ionogenic groups: two equivalent PBA moieties and two amide groups.
| λabs (log ε) | Spectrophotometric titration | λema (nm) | Fluorimetric titration | ||||
|---|---|---|---|---|---|---|---|
| pKa1-B(OH)2 | pKa2 | pKa3 | pKa1(BOH2) | pKa2 | |||
| a λex = 350 nm for 11-12; λex = 325 nm for 13. | |||||||
| 11 | 274(4.47), 350(3.87) | 6.62 ± 0.11 | 8.90 ± 0.05 | 11.34 ± 0.06 | 463 | 6.61 ± 0.07 | 8.18 ± 0.10 |
| 12 | 272(4.93), 355(4.00) | — | 8.50 ± 0.07 | 11.34 ± 0.09 | 464 | 8.37 ± 0.07 | 10.62 ± 0.21 |
| 13 | 271(4.80), 325(4.01), 350(3.80) | 8.05 ± 0.10 | 10.48 ± 0.08 | — | 473 | 7.74 ± 0.10 | 8.96 ± 0.05 |
The reference compound 12, lacking PBA moieties, has only two amide groups and monoboronic compound 13 contains one PBA moiety and one amide group. The positive charges on the quinolinium rings must acidify these groups. Thus, acid–base properties of bromide salts of 11–13 were explored by fluorescence and UV–Vis pH titrations.
For bromide salt of 12, two pKa values were estimated, pKa1 = 8.50 and pKa2 = 11.34, from UV-vis pH titration and two values were calculated from fluorescence versus pH titration, pKa1 = 8.37 and pKa2 = 10.62 (see insets Fig. 2C and D). These pKa values are similar to those we reported for the triflate salt of 12 and unambiguously assigned to amide groups.53 Total deprotonation of amide groups in 12 induced a strong decrease in emission intensity, practically generating a non-emitting species, probably by an intramolecular photoinduced electron transfer (PET).
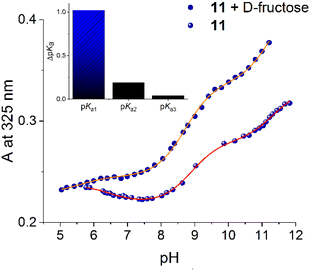
Results of UV-vis pH titration for 11 are shown in Fig. 2A. The formal fit of the absorbance (325 nm) versus pH curve to the theoretical equation allows us to estimate clearly three pKa values (pKa1 = 6.62, pKa2 = 8.90 and pKa3 = 11.34, Table 1). The two values above 8.50 can be attributed to the two amide groups compared to compound 12. The diboronic isomer 8 that we have recently reported, has pKa values less than 7.0 assigned to the boronic groups.47
The fluorimetric titration experiment shows that emission quenching of 11 is mainly controlled by the dissociation of a group with a pKa below 7.0 (Fig. 2B). The emission data at 463 nm can be very well fitted to two pKa values (see inset, Fig. 2B), pKa1 = 6.61 and pKa2 = 8.18.
The absence of a third pKa value in the fluorescence curve can be explained by the formation of a non-emitting conjugated base, after deprotonation of boronic acid and an amide group. In our experience, completely deprotonated (pH > 10) quinolinium pyridine-2,6-dicarboxamide derivatives are practically not fluorescent.47,49,56 The first pKa1 = 6.61 of 11 is comparable to those reported values for structurally related monoboronic acids appended quinolinium rings studied by Geddes which have pKa less than 7.0 assigned to the boronic groups from fluorescence data.44–46
To unambiguously assign the pKa value of boronic acid in 11, we reproduced the UV-vis pH titration in the presence of fructose (10 mM), see Fig. S20,† with the baseline expectation of having a considerable drop in the pKa value only for the corresponding boronic acid.44 Fig. 3 shows UV-vis pH profiles of 11 (25 µM) in the absence and presence of this sugar. The absorbance at 325 nm versus pH in the presence of fructose can be well fitted to three pKa values (pKa1 = 5.58, pKa2 = 8.71 and pKa3 = 11.30). Adding fructose induces a strong drop in the first pKa of 1.12 units compared to the estimated value without fructose (vide supra Table 1). In contrast, the two pKa values above 8.5 remain practically constant as is shown in inset Fig. 3, suggesting that these correspond to the two amide groups. This finding is significant because it evidences that boronic acid groups of 11 are converted in their anionic tetrahedral sugar binding forms at pH values close to the physiological value of 7.4 without interference from the ionization of the amide groups.
Fluorescent saccharide recognition
To test the affinity of 11 towards saccharides, a series of fluorescence titration experiments were performed with two open-chain polyols (sorbitol and mannitol), and “small” saccharides (fructose, galactose, glucose, myo-inositol, lactose and sucrose) at pH = 7.4. In general, adding these polyols to buffered aqueous solutions of 11 shows reproducible quenching effects with a selective peak for sorbitol.Fluorimetric titration of 11 (20 µM) with sorbitol is shown in Fig. 4A, the fluorescence intensity drops 4 times at saturation (∼0.4 mM) and the titration profile at 463 nm perfectly fits a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding mode by a nonlinear least-squares treatment using eqn (1) to give apparent binding constant of K(11-sorbitol)= (3.18 ± 0.10) × 104 M−1, where IF is the observed intensity, I0 is the intensity of the free receptor, ΔI∞ is the maximum intensity change induced by the presence of the saccharide at saturation, [G]T is the total concentration of the saccharide, and K is the apparent binding constant.57
1 binding mode by a nonlinear least-squares treatment using eqn (1) to give apparent binding constant of K(11-sorbitol)= (3.18 ± 0.10) × 104 M−1, where IF is the observed intensity, I0 is the intensity of the free receptor, ΔI∞ is the maximum intensity change induced by the presence of the saccharide at saturation, [G]T is the total concentration of the saccharide, and K is the apparent binding constant.57
 | (1) |
 | ||
| Fig. 4 (A) Changes in the emission spectra (λex = 350 nm) of buffered aqueous solution 11 (20 µM) upon the addition of increasing amounts of sorbitol (0–480 µM) at pH = 7.40. Fluorimetric titration profiles of aqueous solutions of (B) 11 and (C) 13 (20 µM in both cases) upon the addition of increasing amounts of polyols and monosaccharides at pH = 7.4. The solid lines were obtained by fitting to eqn (1). (D) Fluorimetric titrations of 11 and 12 with sorbitol. (E) Polyols used in this work. | ||
The apparent binding constants of 11 with the rest of the saccharides were estimated under the same conditions. The corresponding fluorimetric titration profiles are shown in Fig. 4B and their respective affinities are compiled in Table 2. For comparison, the data includes the apparent stability constants of PBA with saccharides at pH = 7.4, taken from the literature.30
| Analyte | 11 | 13 | PBA | ||
|---|---|---|---|---|---|
K(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
IF/I0a | K(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)b 1)b |
IF/I0a | Kc | |
| a IF/I0 indicates the quenching relative selectivity parameter at 463 nm for 11 and 474 nm for 13 in the presence of 1.0 mM of the polyol or monosaccharide.b Undetected association.c Binding constants reported in an aqueous medium at pH = 7.4, ref. 30. | |||||
| D-sorbitol | (31.83 ± 0.11) × 103 | 4.21 | (2.20 ± 0.08) × 103 | 2.16 | 370 |
| D-fructose | (4.37 ± 0.05) × 103 | 2.73 | (1.09 ± 0.06) × 103 | 1.33 | 160 |
| D-mannitol | (2.96 ± 0.12) × 103 | 2.10 | (0.92 ± 0.05) × 103 | 1.37 | 120 |
| D-galactose | (0.99 ± 0.09) × 103 | 1.26 | (0.40 ± 0.04) × 103 | 1.05 | 15 |
| D-glucose | (0.68 ± 0.04) × 103 | 1.40 | (0.20 ± 0.02) × 103 | 1.03 | 4.6 |
| Myo-inositol | (0.43 ± 0.07) × 103 | 1.05 | b | 1.01 | — |
| Lactose | (0.17 ± 0.03) × 103 | 1.03 | b | 1.01 | 1.6 |
| Sucrose | (0.07 ± 0.01) × 103 | 1.04 | b | 1.02 | 0.6 |
All systems showed the formation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 model with good quality fits (error < 10%). Noteworthy that the chemical equilibrium boronic acid–boronate ester was reached within 1.0 min after the addition of the polyol aliquot. This relatively fast equilibrium is not so common58 and probably due to the strong acidification of boronic acids. The structure of all saccharides screened is shown in Fig. 4E.
1 model with good quality fits (error < 10%). Noteworthy that the chemical equilibrium boronic acid–boronate ester was reached within 1.0 min after the addition of the polyol aliquot. This relatively fast equilibrium is not so common58 and probably due to the strong acidification of boronic acids. The structure of all saccharides screened is shown in Fig. 4E.
Overall, sucrose, lactose, myo-inositol, glucose and galactose gave a very low quenching effect (IF/I0 < 1.4) upon the addition of 1.0 mM of polyol and low affinities were estimated. The addition of mannitol and fructose resulted in a considerable quenching effect (IF/I0 ∼ 2.5), but it was still significantly lower than that observed for sorbitol. In general, the affinity of 11 towards studied saccharides is one or two orders of magnitude lower than that estimated for sorbitol including fructose, making this chemosensor ideal for achieving selective detection of sorbitol at physiological pH.
Next, the affinity of related quinolinium mono-boronic acid 13 was evaluated with the saccharides by fluorescence; the titration profiles are shown in Fig. 4C. The affinity trend is parallel to that reported for PBA with common monosaccharides; fructose > galactose > glucose (see Table 2), which is not unexpected because it is well-known that simple monoboronic acids possess inherent selectivity to fructose.59 Regarding to sorbitol, the affinity of the monoboronic compound 13 is one order of magnitude less than that calculated for diboronic 11. These data suggest that compound 11 may involve a cooperative chelating diboronate binding sorbitol.
On the other hand, fluorescent sensing of monosaccharides by hydrophobic receptors lacking boronic acids as a result of a disaggregation mechanism has recently been reported.58 To discard this mechanism and incidentally verify the participation of the boronic acids of compound 11 in the molecular recognition, we test the effect of sorbitol on the fluorescence of reference compound 12 in the same concentration range and the same pH as that for diboronic receptor 11, and practically no effect was observed as shown in Fig. 4D.
Since compound 11 has two boronic acid-based binding points, the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding mode observed by fluorescence experiments can be rationalized as (1) chelation of the saccharides by both boronic acid groups or (2) simultaneous binding to each boronic acid group operating independently with a similar quenching effect and apparent binding constants, in this option, the estimated K values must be multiplied by a factor of 2.
1 binding mode observed by fluorescence experiments can be rationalized as (1) chelation of the saccharides by both boronic acid groups or (2) simultaneous binding to each boronic acid group operating independently with a similar quenching effect and apparent binding constants, in this option, the estimated K values must be multiplied by a factor of 2.
The first possibility is feasible, considering that compound 11 is symmetrical, semi-rigid and has both boronic acid groups oriented in the same position according to its crystalline structure. The relatively short B⋯B distance of 5.30 Å could be further shortened for the presence of flexible methylene linkers.
Fig. 5 shows the correlation between Log KPBA and Log K values for compounds 11 and 13 at pH = 7.4. For diboronic 11, there is observed a lineal correlation for saccharides, except for sorbitol which shows a strong positive deviation. Glucose also shows this positive bias but to a lesser extent. In contrast, for monoboronic 13, all saccharides, including sorbitol, have a good linear correlation which is expected for diol complexation involving only one boronic acid.
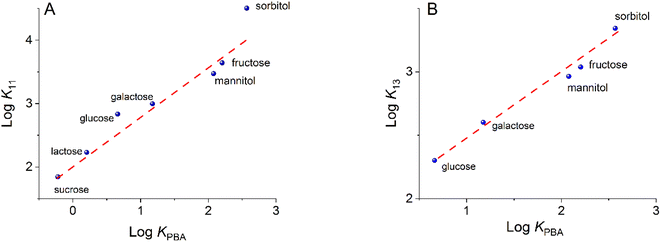 | ||
| Fig. 5 Log of apparent binding constants for 11 (A) and 13 (B) versus Log of binding constants for PBA at a pH of 7.4. Red dashed lines correspond to the slope unity. | ||
A plausible interpretation of the high affinity of 11 for sorbitol is that this diboronic compound effectively binds as a ditopic chelate. To further insight into the quenching response, the lifetimes of 11 in the absence and presence of sorbitol were measured. An aqueous solution of 11 upon excitation with a 354 nm laser exhibited a bi-exponential decay with lifetimes τ1 = 5.18 and τ2 = 2.00 ns (Fig. 6). The lifetime of solution of 11 after the addition of a concentrated solution of sorbitol (12 equiv.) has a negligible change of lifetime values and form of decay profile, indicating that static quenching is dominant in the quenching mechanism.60 Furthermore, similar lifetime values and the same shape of the spectra without shift of maxima in the fluorimetric titration of 11 with sorbitol (Fig. 2A) suggest that the transition energy is virtually the same in the two compounds and that the emission comes from the singlet state of quinolinium groups.
 | ||
| Fig. 6 Emission decay profiles of aqueous solutions of 11 in the absence and presence of 12 equiv. of sorbitol at pH = 7.4. | ||
Interaction studies by NMR and high-resolution mass spectrometry
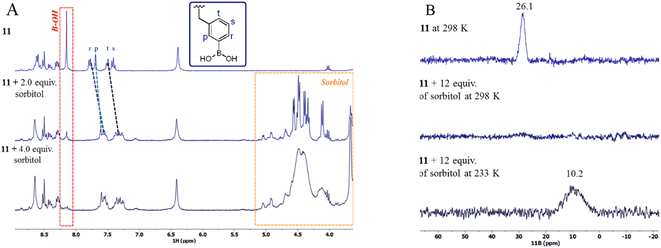
To support the formation of ester boronate of 11 with sorbitol, we performed 1H and 11B NMR spectroscopic measurements. Fig. 7A illustrates a titration experiment of 11 (2.0 mM) with sorbitol monitored by 1H NMR in DMSO-d6. Upon increasing the concentration of sorbitol (0–4.0 equiv.), only the aromatic protons (Hptsr) from phenyl boronic moieties (see Fig. 7A for the proton label) are clearly affected. This set of aromatic protons shows a considerable upfield shift of about 0.40 ppm along with the simultaneous disappearance of the protons of –B(OH)2 at 8.19 ppm (red box).In principle, this shift can be attributed to reversible boronate esterification with diol fragments from the sorbitol due to the appearance of the negative charge on the boron atom. The signals of the rest of the protons of 11 are practically unaffected. The sorbitol signals (orange box, Fig. 7A) in the titration broaden over time, possibly due to deuterium exchange with the solvent.
The change of hybridization of the B atoms in 11 from neutral trigonal-sp2 boronic acid to anionic tetrahedral-sp3 boronate generated by esterification with sorbitol was observed by 11B NMR experiments in CD3OD-D2O (Fig. 7B).
Upon the addition of 12 equiv. of sorbitol to a solution of 11 (5 mM) the initial signal for sp2-B atom at 26.1 ppm disappeared and a new broadened signal at 10.2 ppm arose for sp3-B atom. At r.t. (298 K), it was difficult to see the broadened signal of sp3-B atom; however, at a low temperature of 233 K the signal can be clearly observed as is shown in Fig. 7B (bottom). This change in the chemical shift (Δδ = 16 ppm) of the 11B NMR signals is characteristic of the formation of boronate complex with diols.48,61
Electrospray ionization (ESI) mass spectrometry has been used to study boronic acid–diol complexation.47 Next, high-resolution electrospray mass experiments were carried out in the positive mode with solutions of compound 11 in the absence and presence of sorbitol in aqueous methanol. Fig. 8A shows the ESI-MS spectrum of free compound 11, one charged state at m/z = 770.17943, for the monocationic [R + Br]+ (R = the dicationic receptor 11) is clearly observed and isotopically resolved. The signals separated by 1.0 unit match perfectly the theoretical isotopic distribution.
 | ||
| Fig. 8 ESI-HRMS spectra obtained by the positive scan of free bromide salt of 11 (A) and 11 in the presence of sorbitol (B) in neutral aqueous methanol. Insets: theoretical isotopic patterns. | ||
Fig. S21† shows the whole ESI-MS spectrum of the mixture 11 with sorbitol; the base peak at m/z = 798.2907 corresponds to one charged species for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex with formula [R(sorbitol)(H2O)4− + H]+ by exact mass and perfect coincidence with the theoretical isotopic distribution (see Fig. 8B and Table 3 entry 1). This signal has the multiplicity expected for a species containing two sp3 boronate groups and one sorbitol molecule with the elimination of four water molecules evidencing the formation of four ester bonds. Eliminating four water molecules is only possible if both groups of boronic acid participate. This species contains two boronate groups that balance the positive charges of the quinolinium rings, therefore, it is neutral and undetectable by MS. However, it can easily be cationized by trapping H+. Since the most abundant species detected by HRMS only contain one sorbitol molecule, the only possibility is the formation of a sorbitol chelate complex, which should has the chemical structure 14.
1 complex with formula [R(sorbitol)(H2O)4− + H]+ by exact mass and perfect coincidence with the theoretical isotopic distribution (see Fig. 8B and Table 3 entry 1). This signal has the multiplicity expected for a species containing two sp3 boronate groups and one sorbitol molecule with the elimination of four water molecules evidencing the formation of four ester bonds. Eliminating four water molecules is only possible if both groups of boronic acid participate. This species contains two boronate groups that balance the positive charges of the quinolinium rings, therefore, it is neutral and undetectable by MS. However, it can easily be cationized by trapping H+. Since the most abundant species detected by HRMS only contain one sorbitol molecule, the only possibility is the formation of a sorbitol chelate complex, which should has the chemical structure 14.
| Species | Formula | Theoretical | Observed | |
|---|---|---|---|---|
| 14 | [R(sorbitol)(H2O)4− + H]+ | C45H38B2N5O8+ | 798.29010 | 798.290702 |
| 15 | [R(sorbitol)(H2O)4− + Br]+ | C45H39B2N5O8Br+ | 878.21626 | 878.217742 |
| 16 | [R(sorbitol)(H2O)4−(OH)2 + K]+ | C45H40B2N5O10K+ | 912.28584 | 912.285400 |
Two less abundant one-charged species are also seen at m/z = 878.2177 and m/z = 912.2854 (see Table 3, entries 2-3). The first species matches [R(sorbitol)(H2O)4− + Br]+ involving one sorbitol bound two trigonal sp2 boronic groups through four ester bonds, the one charge and mass are consistent for the presence of a Br− anion; this complex should have structure 15.
The species at m/z = 912.2854 corresponds to [R(sorbitol)(H2O)4−(OH)2 + K]+ with one sorbitol bound two anionic hydroxo sp3-boronate complexes, which are formed by the addition of a hydroxyl anion and four ester bounds as is shown in 16.
All the species detected by masses correspond to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex with a chelate binding mode, which could explain the tight affinity to sorbitol (Scheme 3).
1 complex with a chelate binding mode, which could explain the tight affinity to sorbitol (Scheme 3).
The assignments of signals of all species observed containing the dicationic compound 11-sorbitol complex along with their exact experimental/calculated masses are shown in Table 3.
Considering the results of pH titration and the most intense peak from HRMS the species 14 involving chelated sp3-boronate esters seems more plausible.
DFT calculations
To gain further insights into the recognition mode of 11 toward sorbitol, DFT calculations were performed for the species 14. The geometry was optimized at the ωB97X-D/LANL2DZ level of theory with water as an implicit solvent under the SMD continuum solvation model. The complex showed positive vibrational frequencies which indicates the presence of a minimum on the potential energy surface, and therefore it represents a physically accessible conformation. Sorbitol coordinates three –OH groups for each boronic acid as is shown in Fig. 9.It is noteworthy that the calculated B⋯B distance for the optimized macrocycle resulted from the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex is 5.53 Å which is very close to 5.30 Å experimental value.
1 complex is 5.53 Å which is very close to 5.30 Å experimental value.
Discussion affinity towards sorbitol by recent boronic acids-based receptors
Table 4 collects apparent binding constants of sorbitol complexes for recent mono and diboronic receptors. An analysis of these binding data shows that receptor 11 has an affinity one to two orders of magnitude higher than that reported for PBA (Table 2 and Fig. 5), as well as monoboronic acid receptors 1, 6 and 7 in water at pH = 7.4. This tight affinity can be assigned to the stronger Lewis acidity of dicationic 11 involving a pKa value ∼6.6 compared to pKa = 8.8 of PBA or pKa > 7.0 of 6-7.The neutral diboronic acid receptors 1, 2 and 4 based on amine dyes possess considerably lower affinities than receptor 11, despite being studied in less competitive media such as aqueous mixtures with organic co-solvents. Neutral diboronic receptors 3 and 5 based on amine and quinoline, respectively, show close behavior to 11 but in basic conditions and in aqueous organic mixtures. The isomer 8, recently reported by us, has an affinity three times lower than 12 despite having similar values to pKa.
This effect probably reflects a cooperative chelating complexation with the participation of both boronic acids in 11; it is not unexpected because the distance B⋯B in 11 is less (∼2 Å) than that observed in the crystal of isomer 8. The dicationic nature and acidity of the boronic acids in 11 make this compound useful for optical recognition of sorbitol at physiological pH.
The reduction of the pKa value of phenylboronic acids is critical to achieving higher performance of receptor 11 in aqueous phase; however, other structural features are also significant such as the inclusion of two-point recognition, the preorganization of binding sites, and rigidity of bisquinolinium pyridine-2,6-dicarboxamide scaffold.62
Conclusions
We have introduced a new fluorescent cationic diboronic acid-based receptor 11 for the optical recognition of sugars with hydrostability, photostability, and strong ability for sorbitol optical sensing in micromolar concentration range in pure water at physiological pH. Under these conditions, the addition of sorbitol exhibits an efficient and fast quenching response of 11 with pronounced affinity (K = 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 M−1) and selectivity over other common saccharides such as fructose, mannitol, glucose, lactose and sucrose. In terms of reactivity and chemical structure, diboronic receptor 11 possesses two strongly acidified boronic groups, with pKa = 6.6, oriented towards the same plane with a proximity B⋯B of 5.3 Å as evidenced by its X-ray crystal structure. Lifetime measurements and fluorescence spectroscopy display that the quenching process could be explained as a static complexation PET mechanism. 1H, 11B NMR spectroscopy, HRMS measurements and DFT calculations show that sorbitol is efficiently bound to 11 in a 1
800 M−1) and selectivity over other common saccharides such as fructose, mannitol, glucose, lactose and sucrose. In terms of reactivity and chemical structure, diboronic receptor 11 possesses two strongly acidified boronic groups, with pKa = 6.6, oriented towards the same plane with a proximity B⋯B of 5.3 Å as evidenced by its X-ray crystal structure. Lifetime measurements and fluorescence spectroscopy display that the quenching process could be explained as a static complexation PET mechanism. 1H, 11B NMR spectroscopy, HRMS measurements and DFT calculations show that sorbitol is efficiently bound to 11 in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 model involving a chelating sp3 boronate–diol complexation.
1 model involving a chelating sp3 boronate–diol complexation.
The Log of the apparent binding constants for 11 with the studied saccharides correlates linearly with the Log of binding constants with simple phenylboronic acid; except for sorbitol which shows a strong positive deviation due to the chelating binding mode.
Among all the boronic acid receptors reported for sorbitol in aqueous media, diboronic 11 has the highest affinity.
Overall, these results further highlight the use of a new chemical approach of water-soluble diboronic acid receptors based on a cationic bisquinolinium pyridine-2,6-dicarboxamide scaffold with analytical applications for the fluorescent sensing of saccharides in aqueous media.
Experimental section
Bromide salts of 11–13 were prepared according to modified procedures reported previously.47,53 The detailed procedure of intermediaries 9-10 and bromide salt of 12, previously reported by us, is described in the ESI.†Synthesis of 3,3′-((pyridine-2,6-dicarbonyl)bis(azanediyl))bis(1-(3-boronobenzyl)quinolin-1-ium) dibromide, 11
The compound 9 (100.0 mg, 0.24 mmol) was dissolved in 5 mL of anhydrous DMF. Then, 2.2 equiv. of 3-(bromomethyl)-phenylboronic acid (90% purity, 125.2 mg, 0.52 mmol) were added and stirred for 24 h at r. t. under an N2 atmosphere. A yellow sticky precipitate was obtained, and 1.0 mL of anhydrous DMF was added. The mixture was slurred for 2 h. Subsequently, 12.0 mL of ethyl acetate was added and stirred vigorously for 30 min, then cooled in an ice bath. The pale-yellow solid was isolated by vacuum filtration and washed with a 5.0 mL portion of a DMF/ethyl acetate mixture (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4), and with 10 mL of diethyl ether. The solid was placed in a flask and 11 was extracted with 40 mL of deionized water. The volatiles were removed in a rotary evaporator at NMT 50 °C and the almost white solid was dried under reduced pressure to obtain the bromide salt of 11 (73%, 147.8 mg). Single crystals for X-ray diffraction analysis were obtained as sulfate salt by slow evaporation from a mixture of H2O–DMSO (v/v, 99/1).
4), and with 10 mL of diethyl ether. The solid was placed in a flask and 11 was extracted with 40 mL of deionized water. The volatiles were removed in a rotary evaporator at NMT 50 °C and the almost white solid was dried under reduced pressure to obtain the bromide salt of 11 (73%, 147.8 mg). Single crystals for X-ray diffraction analysis were obtained as sulfate salt by slow evaporation from a mixture of H2O–DMSO (v/v, 99/1).
1H NMR (300 MHz, 298 K, DMSO-d6); δ 11.61 (s, 2H, NH(amide)), 9.62 (d, 3JHH = 5.7 Hz, 2H, Hj), 9.22 (3JHH = 8.7 Hz, 2H, Hh), 8.61–8.50 (m, 6H, Hi, m, l) 8.41 (m, 1H, Ha) 8.30 (pseudo-t, 3JHH = 6.9 Hz, 2H, Hb), 8.19 (broad-s, 4H, BOH), 7.78 (d, 3JHH = 7.2 Hz, 2H, Hr), 7.68 (s, 2H, Hp), 7.48 (d, 3JHH = 7.5 Hz, 2H, Ht), 7.40 (t, 3JHH = 7.5 Hz, 2H, Hs), 6.37 (s, 4H, CH2). 13C{1H} NMR (100 MHz, 298 K, DMSO-d6); δ 163.1 (carbonyl-Cd), 149.2 (Cj), 148.5 (Cc), 148.0 (Ch), 142.0 (Ca), 139.4 (Ce), 135.6 (Cq), 135.3 (Ck), 135.0 (Cr), 133.5 (Co), 132.9 (Cp), 131.7 (Cg), 130.6 (Ci), 129.6 (Ct), 128.9 (Cs), 126.6 (Cm), 123.4 (Cb), 120.8 (Cl), 118.5 (Cf), 60.7 (methylene-Cn); 11B NMR (160.5 MHz, 298 K, CD3OD) δ 27.4 (broad signal); HRMS-ESI+ (m/z): calculated for [C39H33B2N5O6Br]+: 768.179484, found: 768.179957. ATR-IR (cm−1): 3218br, 1546s, 1378s, 1333s, 771m and 714m. Anal calcd. for C39H33B2Br2N5O6: C, 55.16; H, 3.92; N, 8.25. Found: C, 55.11; H, 3.98; N, 8.20.
Synthesis of 6-benzamido-1-(3-boronobenzyl)quinolin-1-ium bromide, 13
Compound 10 (100.0 mg, 0.40 mmol) was dissolved in 4.0 mL of anhydrous DMF. Then, 1.1 equiv. of 3-(bromomethyl)-phenylboronic acid (90% purity, 105.8 mg, 0.44 mmol) were added and stirred for 16 h. at r. t. under an N2 atmosphere. The reaction turned yellow instantly, and that color was retained throughout the reaction time. The volume of the yellow solution was halved under vacuum at 40 °C in a rotary evaporator. Subsequently, 10 mL of cold ethyl acetate was added, forming a slurring precipitate at low temperature (ice bath) for 2 h. This precipitate was isolated by vacuum filtration and washed twice with cold ethyl acetate and diethyl ether at r. t. The yellow solid was dried under reduced pressure to obtain the bromide salt of 13 (91% mg). Anal calcd. for C23H20BBrN2O3: C, 56.65; H, 4.35; N, 6.05. Found: C, 56.47; H, 4.48; N, 6.0.1H NMR (400 MHz, 298 K, D2O/CD3CN); δ 9.17 (dd, 3JHH = 6.0 Hz, 4JHH = 1.6 Hz, 1H), 9.97 (d, 3JHH = 8.4 Hz, 1H), 8.59 (d, 3JHH = 2.4 Hz, 1H), 8.23 (d, 3JHH = 9.6 Hz, 1H), 8.15 (dd, 3JHH = 9.6 Hz, 3JHH = 2.4 Hz, 1H), 7.97 (dd, 3JHH = 8.4 Hz, 3JHH = 6.0 Hz, 1H), 7.76 (d, 3JHH = 6.8 Hz, 2H), 7.69 (d, 3JHH = 6.8 Hz, 1H), 7.61 (s, 1H), 7.49 (t, 3JHH = 7.4 Hz, 1H), 7.41–7.36 (m, 4H), 6.11 (s, 2H). 13C{1H} NMR (100.6 MHz, 298 K, DMSO-d6); δ 169.4, 148.8, 148.6, 140.1, 135.8, 135.6, 134.0, 133.7, 133.4, 133.2, 132.1, 130.9, 130.6, 129.9, 129.7, 128.5, 123.4, 120.6, 119.6, 62.0; 11B NMR (160 MHz, 297 K, CD3OD); δ 28.6; HRMS-ESI+ (m/z): calculated for [C23H20BN2O3]+ 383.15615, found 383.15663. ATR-IR ν (cm−1): 3354br, 1667m, 1548s, 1357s, 1330s, 1257s, 705s and 637m.
The hydrogen atoms of the C–H bonds were placed in idealized positions whereas the hydrogen atoms from the N–H and O–H moieties were localized from the difference electron density map and their position was refined with Uiso tied to the parent atom with distance restraints (DFIX).
Compound 11-SO4 presents positional disorder in DMSO solvent in two positions whit a ratio 95/5. The model of two positions was refined using geometry (SAME) and Uij restraints (SIMU, RIGU) implemented in SHELXL. The occupancy of two molecules of water was refined with free variables shown occupancy of 71% and 49% respectively. The molecular graphics were prepared using OLEX.67 Crystallographic data for crystal structure has been deposited with the Cambridge Crystallographic Data Centre no 2281178.† X-ray crystallographic data in CIF format are available in ESI.†
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by grants: PAPIIT-UNAM-220023 and CONACYT PRONACES-160671. We thank M. Sc. Eréndira García Rios, M. Sc. Lucero Mayra Ríos Ruiz, M. Sc. Lucía del Carmen Márquez Alonso, M. Sc. Lizbeth Triana Cruz, M. Sc. Hortensia Segura Silva, Dra. Beatriz Quiroz-García, Dra. Adriana Romo Pérez, and M. Sc. Elizabeth Huerta Salazar for technical assistance. J. Z. M. is grateful to DGAPA-UNAM for a postdoctoral scholarship. M. K. S. F., J. V. G. and C. P. V. are grateful to CONAHCyT for scholarships 848759, 848787 and 812567 respectively. J. B. F. is grateful to DGTIC-UNAM for the use of the supercomputer Miztli.References
- G. T. Williams, J. L. Kedge and J. S. Fossey, Molecular Boronic Acid-Based Saccharide Sensors, ACS Sens., 2021, 6, 1508–1528 CAS.
- X. Zhang, G. Liu, Z. Ning and G. Xing, Boronic acid-based chemical sensors for saccharides, Carbohydr. Res., 2017, 452, 129–148 CAS.
- S. D. Bull, M. G. Davidson, J. M. H. V. A. N. D. E. N. Elsen, J. S. Fossey, A. T. a Jenkins, Y. Jiang, Y. Kubo, F. Marken, K. Sakurai, J. Zhao and T. D. James, Exploiting the Reversible Covalent Bonding of Boronic Acids: Recognition, Sensing, and Assembly, Acc. Chem. Res., 2013, 46, 312–326 CAS.
- K. Lacina, P. Skládal and T. D. James, Boronic acids for sensing and other applications - a mini-review of papers published in 2013, Chem. Cent. J., 2014, 8, 1–17 Search PubMed.
- J. S. Fossey, F. D'Hooge, J. M. H. Van Den Elsen, M. P. Pereira Morais, S. I. Pascu, S. D. Bull, F. Marken, A. T. A. Jenkins, Y. B. Jiang and T. D. James, The development of boronic acids as sensors and separation tools, Chem. Rec., 2012, 12, 464–478 CAS.
- X. Sun, W. Zhai, J. S. Fossey and T. D. James, Boronic acids for fluorescence imaging of carbohydrates, Chem. Commun., 2016, 52, 3456–3469 CAS.
- S. L. Tong, Z. Y. Tian, Y. H. Wu, Y. Yan, S. Hu and J. Yu, Crystal assembly based on 3,5-bis(2′-benzimidazole) pyridine and its complexes, Solid State Sci., 2013, 17, 6–13 CAS.
- J. S. Hansen, M. Ficker, J. F. Petersen, J. B. Christensen and T. Hoeg-Jensen, Ortho-Substituted fluorescent aryl monoboronic acid displays physiological binding of d-glucose, Tetrahedron Lett., 2013, 54, 1849–1852 CAS.
- J. N. Cambre and B. S. Sumerlin, Biomedical applications of boronic acid polymers, Polym., 2011, 52, 4631–4643 CAS.
- A. Stubelius, S. Lee and A. Almutairi, The Chemistry of Boronic Acids in Nanomaterials for Drug Delivery, Acc. Chem. Res., 2019, 52, 3108–3119 CAS.
- Z. Bian, A. Liu, Y. Li, G. Fang, Q. Yao, G. Zhang and Z. Wu, Boronic acid sensors with double recognition sites: A review, Analyst, 2020, 145, 719–744 CAS.
- C. Chen, G. El Khoury, P. Zhang, P. M. Rudd and C. R. Lowe, J. Chromatogr. A, 2016, 1444, 8–20 CAS.
- M. D. Heagy and R. K. Meka, Fluorescent and Colorimetric Probes for Carbohydrates: A Second Decade's Worth of Bright Spies for Saccharides, in Comprehensive Supramolecular Chemistry II, ed. J. L. Atwood, Elsevier, Oxford, 2017, pp. 615–647 Search PubMed.
- W. Zhai, X. Sun, T. D. James and J. S. Fossey, Boronic Acid-Based Carbohydrate Sensing, Chem. Asian J., 2015, 10, 1836–1848 CAS.
- S. Craig, Synthesis and evaluation of aryl boronic acids as fluorescent artificial receptors for biological carbohydrates, Bioorg. Chem., 2012, 40, 137–142 CAS.
- R. Hosseinzadeh, M. Mohadjerani and M. Pooryousef, Fluorene-based boronic acids as fluorescent chemosensor for monosaccharides at physiological pH, Luminescence, 2015, 30, 549–555 CAS.
- C. Hoffmann, M. Jourdain, A. Grandjean, A. Titz and G. Jung, β-Boronic Acid-Substituted Bodipy Dyes for Fluorescence Anisotropy Analysis of Carbohydrate Binding, Anal. Chem., 2022, 94, 6112–6119 CAS.
- C. R. Cooper and T. D. James, Selective D-glucosamine hydrochloride fluorescence signalling based on ammonium cation and diol recognition, Chem. Commun., 1997, 1419–1420 CAS.
- A. Chaicham, S. Sahasithiwat, T. Tuntulani and B. Tomapatanaget, Highly effective discrimination of catecholamine derivatives via FRET-on/off processes induced by the intermolecular assembly with two fluorescence sensors, Chem. Commun., 2013, 49, 9287–9289 CAS.
- M. Debiais, J. J. Vasseur and M. Smietana, Applications of the Reversible Boronic Acids/Boronate Switch to Nucleic Acids, Chem. Rec., 2022, 22, 1–16 Search PubMed.
- A. E. Hargrove, R. N. Reyes, I. Riddington, E. V. Anslyn and J. L. Sessler, Boronic acid porphyrin receptor for ginsenoside sensing, Org. Lett., 2010, 12, 4804–4807 CAS.
- P. M. Chaudhary, R. V. Murthy, R. Yadav and R. Kikkeri, A rationally designed peptidomimetic biosensor for sialic acid on cell surfaces, Chem. Commun., 2015, 51, 8112–8115 CAS.
- K. S. Ahn, J. H. Lee, J. M. Park, H. N. Choi and W. Y. Lee, Luminol chemiluminescence biosensor for glycated hemoglobin (HbA1c) in human blood samples, Biosens. Bioelectron., 2016, 75, 82–87 CAS.
- X. Huang, Y. Han, J. Li, M. Tang and G. Qing, Sensitive and specific detection of saccharide species based on fluorescence: update from 2016, Anal. Bioanal. Chem., 2023, 415, 4061–4077 CAS.
- J. A. Peters, Interactions between boric acid derivatives and saccharides in aqueous media: Structures and stabilities of resulting esters, Coord. Chem. Rev., 2014, 268, 1–22 CAS.
- G. F. Whyte, R. Vilar and R. Woscholski, Molecular recognition with boronic acids — applications in chemical biology, J. Chem. Biol., 2013, 6, 161–174 Search PubMed.
- J. Krämer, R. Kang, L. M. Grimm, L. De Cola, P. Picchetti and F. Biedermann, Molecular Probes, Chemosensors, and Nanosensors for Optical Detection of Biorelevant Molecules and Ions in Aqueous Media and Biofluids, Chem. Rev., 2022, 122, 3459–3636 Search PubMed.
- W. L. A. Brooks, C. C. Deng and B. S. Sumerlin, Structure–Reactivity Relationships in Boronic Acid–Diol Complexation, ACS Omega, 2018, 3, 17863–17870 CAS.
- J. Yan, G. Springsteen, S. Deeter and B. Wang, The relationship among pKa, pH, and binding constants in the interactions between boronic acids and diols—it is not as simple as it appears, Tetrahedron, 2004, 60, 11205–11209 CAS.
- G. Springsteen and B. Wang, A detailed examination of boronic acid-diol complexation, Tetrahedron, 2002, 58, 5291–5300 CAS.
- S. Harnsoongnoen and A. Wanthong, Real-time monitoring of sucrose, sorbitol, D-glucose and D-fructose concentration by electromagnetic sensing, Food Chem., 2017, 232, 566–570 CAS.
- G. Fang, Z. Bian, D. Liu, G. Wu, H. Wang, Z. Wu and Q. Yao, Water-soluble diboronic acid-based fluorescent sensors recognizing d-sorbitol, New J. Chem., 2019, 43, 13802–13809 CAS.
- M. S. Badiga, N. K. Jain, C. Casanova and C. S. Pitchumoni, Diarrhea in diabetics: the role of sorbitol, J. Am. Coll. Nutr., 1990, 9, 578–582 CAS.
- X. Wu, X.-X. Chen and Y. B. Jiang, Recent advances in boronic acid-based optical chemosensors, Analyst, 2017, 142, 1403–1414 CAS.
- K. Sugita, Y. Suzuki, Y. Tsuchido, S. Fujiwara, T. Hashimoto and T. Hayashita, A simple supramolecular complex of boronic acid-appended β-cyclodextrin and a fluorescent boronic acid-based probe with excellent selectivity for d-glucose in water, RSC Adv., 2022, 12, 20259–20263 CAS.
- Y. Suzuki, Y. Mizuta, A. Mikagi, T. Misawa-Suzuki, Y. Tsuchido, T. Sugaya, T. Hashimoto, K. Ema and T. Hayashita, Recognition of d -Glucose in Water with Excellent Sensitivity, Selectivity, and Chiral Selectivity Using γ-Cyclodextrin and Fluorescent Boronic Acid Inclusion Complexes Having a Pseudo-diboronic Acid Moiety, ACS Sens., 2023, 8, 218–227 CAS.
- A. Schiller, R. A. Wessling and B. Singaram, A fluorescent sensor array for saccharides based on boronic acid appended bipyridinium salts, Angew. Chem., Int. Ed., 2007, 46, 6457–6459 CAS.
- T. D. James, H. Shinmori and S. Shinkai, Novel fluorescence sensor for “small” saccharides, Chem. Commun., 1997, 71–72 CAS.
- K. M. K. Swamy, Y. J. Jang, M. S. Park, H. S. Koh, S. K. Lee, Y. J. Yoon and J. Yoon, A sorbitol-selective fluorescence sensor, Tetrahedron Lett., 2005, 46, 3453–3456 CAS.
- X. Liang, T. D. James and J. Zhao, 6,6′-Bis-substituted BINOL boronic acids as enantioselective and chemoselective fluorescent chemosensors for d-sorbitol, Tetrahedron, 2008, 64, 1309–1315 CAS.
- H. Wang, G. Fang, H. Wang, J. Dou, Z. Bian, Y. Li, H. Chai, Z. Wu and Q. Yao, A diboronic acid fluorescent sensor for selective recognition of d-ribose via fluorescence quenching, New J. Chem., 2019, 43, 4385–4390 CAS.
- S. Liu, H. Bai, Q. Sun, W. Zhang and J. Qian, Naphthalimide-based fluorescent photoinduced electron transfer sensors for saccharides, RSC Adv., 2015, 5, 2837–2843 CAS.
- S. Akay, W. Yang, J. Wang, L. Lin and B. Wang, Synthesis and evaluation of dual wavelength fluorescent benzo[b]thiophene boronic acid derivatives for sugar sensing, Chem. Biol. Drug Des., 2007, 70, 279–289 CAS.
- R. Badugu, J. R. Lakowicz and C. D. Geddes, Fluorescence sensors for monosaccharides based on the 6-methylquinolinium nucleus and boronic acid moiety: Potential application to ophthalmic diagnostics, Talanta, 2005, 65, 762–768 CAS.
- R. Badugu, J. R. Lakowicz and C. D. Geddes, Boronic acid fluorescent sensors for monosaccharide signaling based on the 6-methoxyquinolinium heterocyclic nucleus: Progress toward noninvasive and continuous glucose monitoring, Bioorg. Med. Chem., 2005, 13, 113–119 CAS.
- R. Badugu, J. R. Lakowicz and C. D. Geddes, A wavelength-ratiometric fluoride-sensitive probe based on the quinolinium nucleus and boronic acid moiety, Sens. Actuators, B, 2005, 104, 103–110 CAS.
- J. Valdes-García, J. Zamora-Moreno, M. K. Salomón-Flores, D. Martínez-Otero, J. Barroso-Flores, A. K. Yatsimirsky, I. J. Bazany-Rodríguez and A. Dorazco-González, Fluorescence Sensing of Monosaccharides by Bis-boronic Acids Derived from Quinolinium Dicarboxamides: Structural and Spectroscopic Studies, J. Org. Chem., 2023, 88, 2174–2189 Search PubMed.
- I. J. Bazany-Rodríguez, M. K. Salomón-Flores, A. O. Viviano-Posadas, M. A. García-Eleno, J. Barroso-Flores, D. Martínez-Otero and A. Dorazco-González, Chemosensing of neurotransmitters with selectivity and naked eye detection ofl-DOPA based on fluorescent Zn(ii)-terpyridine bearing boronic acid complexes, Dalton Trans., 2021, 50, 4255–4269 Search PubMed.
- A. O. Viviano-Posadas, U. Romero-Mendoza, I. J. Bazany-Rodríguez, R. V. Velázquez-Castillo, D. Martínez-Otero, J. M. Bautista-Renedo, N. González-Rivas, R. Galindo-Murillo, M. K. Salomón-Flores and A. Dorazco-González, Efficient fluorescent recognition of ATP/GTP by a water-soluble bisquinolinium pyridine-2,6-dicarboxamide compound. Crystal structures, spectroscopic studies and interaction mode with DNA, RSC Adv., 2022, 12, 27826–27838 CAS.
- S. O. Kang, T. S. Johnson, V. W. Day and K. Bowman-James, Pyridine-2,6-dicarboxamide pincer-based macrocycle: a versatile ligand for oxoanions, oxometallates, and transition metals, Supramol. Chem., 2018, 30, 305–314 CAS.
- P. Kumar and R. Gupta, The wonderful world of pyridine-2,6-dicarboxamide based scaffolds, Dalton Trans., 2016, 45, 18769–18783 CAS.
- A. Dorazco-González, H. Höpfl, F. Medrano and A. K. Yatsimirsky, Recognition of anions and neutral guests by dicationic pyridine-2,6-dicarboxamide receptors, J. Org. Chem., 2010, 75, 2259–2273 Search PubMed.
- I. J. Bazany-Rodríguez, D. Martínez-Otero, J. Barroso-Flores, A. K. Yatsimirsky and A. Dorazco-González, Sensitive water-soluble fluorescent chemosensor for chloride based on a bisquinolinium pyridine-dicarboxamide compound, Sens. Actuators, B, 2015, 221, 1348–1355 Search PubMed.
- W. F. Jager, T. S. Hammink, O. van den Berg and F. C. Grozema, Highly sensitive water-soluble fluorescent ph sensors based on the 7-amino-1-methylquinolinium chromophore, J. Org. Chem., 2010, 75, 2169–2178 CAS.
- K. Tanabe, Y. Suzui, M. Hasegawa and T. Kato, Full-color tunable photoluminescent ionic liquid crystals based on tripodal pyridinium, pyrimidinium, and quinolinium salts, J. Am. Chem. Soc., 2012, 134, 5652–5661 CAS.
- A. Dorazco-González, M. F. Alamo, C. Godoy-Alcántar, H. Höpfl and A. K. Yatsimirsky, Fluorescent anion sensing by bisquinolinium pyridine-2,6-dicarboxamide receptors in water, RSC Adv., 2014, 4, 455 Search PubMed.
- P. Thordarson, Determining association constants from titration experiments in supramolecular chemistry, Chem. Soc. Rev., 2011, 40, 1305–1323 CAS.
- B. M. Chapin, P. Metola, S. L. Vankayala, H. L. Woodcock, T. J. Mooibroek, V. M. Lynch, J. D. Larkin and E. V. Anslyn, Disaggregation is a Mechanism for Emission Turn-On of ortho-Aminomethylphenylboronic Acid-Based Saccharide Sensors, J. Am. Chem. Soc., 2017, 139, 5568–5578 CAS.
- X. Wu, Z. Li, X.-X. Chen, J. S. Fossey, T. D. James and Y.-B. B. Jiang, Selective sensing of saccharides using simple boronic acids and their aggregates, Chem. Soc. Rev., 2013, 42, 8032–8048 CAS.
- M. K. Salomón-Flores, C. L. Hernández-Juárez, I. J. Bazany-Rodríguez, J. Barroso-Flores, D. Martínez-Otero, R. López-Arteaga, J. Valdés-Martínez and A. Dorazco-González, Efficient fluorescent chemosensing of iodide based on a cationic meso-tetraarylporphyrin in pure water, Sens. Actuators, B, 2019, 281, 462–470 Search PubMed.
- S. Gamsey, N. A. Baxter, Z. Sharrett, D. B. Cordes, M. M. Olmstead, R. A. Wessling and B. Singaram, The effect of boronic acid-positioning in an optical glucose-sensing ensemble, Tetrahedron, 2006, 62, 6321–6331 CAS.
- S. Arimori, M. L. Bell, C. S. Oh and T. D. James, A modular fluorescence intramolecular energy transfer saccharide sensor, Org. Lett., 2002, 4, 4249–4251 CAS.
- APEX 2 Software Suite, APEX 2 Software Suite, Bruker AXS Inc., Madison, Wisconsin, USA Search PubMed.
- G. M. Sheldrick, SHELXT - Integrated space-group and crystal-structure determination, Acta Crystallogr. A, 2015, 71, 3–8 Search PubMed.
- C. B. Hübschle, G. M. Sheldrick and B. Dittrich, ShelXle: A Qt graphical user interface for SHELXL, J. Appl. Crystallogr., 2011, 44, 1281–1284 Search PubMed.
- G. M. Sheldrick, SHELXL-97, Progr. Cryst. Struct. Refinement, Univ. Göttingen Search PubMed.
- O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard and H. Puschmann, OLEX2: A complete structure solution, refinement and analysis program, J. Appl. Crystallogr., 2009, 42, 339–341 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2281178. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3ra06198a |
| This journal is © The Royal Society of Chemistry 2023 |