Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Efficient synthesis of new indenopyridotriazine [4.3.3]propellanes and spiroindenopyridotriazine-4H-pyran derivatives†
Monireh Rezaei and
Mohammad Bayat
and
Mohammad Bayat *
*
Department of Chemistry, Faculty of Science, Imam Khomeini International University, Qazvin, Iran. E-mail: bayat_mo@yahoo.com; m.bayat@sci.ikiu.ac.ir
First published on 27th October 2023
Abstract
The pyrido[1,2,4]triazines as substrates, generated from 1,6-diaminopyridinone derivatives and ninhydrin, were reacted with malononitrile and CH-acids to afford a new library spiro[indeno[1,2-e]pyrido[1,2-b][1,2,4]triazine-7,5′-pyran]-1,3,6′-tricarbonitrile in ethanol at reflux condition in excellent yield. Also, novel indenopyridotriazine [4.3.3]propellanes were synthesized via the reaction of pyrido[1,2,4]triazine and N-methyl-1-(methylthio)-2-nitroethenamine (NMSM) by using of HOAc in ethanol. The important aspects of this protocol are the abundance of starting materials, mild conditions, structural diversity of products, excellent yields and easy isolation of products with no chromatographic technique.
Introduction
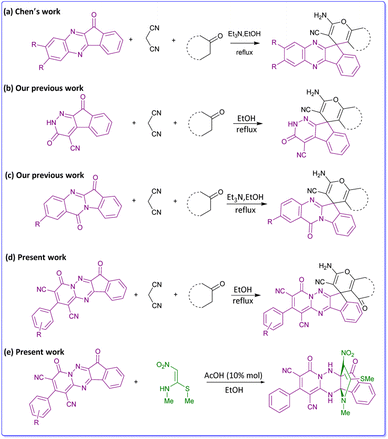
Fused nitrogen heterocycles are among the most special scaffolds in natural and synthetic products. Pyridotriazine derivatives are one of the significant fused nitrogen heterocycles which have displayed a broad range of biological properties such as anti-avian influenza virus (H5N1),1 anticancer,2 antifungal,3 anti-anxiety, anti-inflammatory,4 antibacterial,5 antioxidant,6 antimicrobial functions.7 Moreover, some examples of pyridotriazine can show optical8 and corrosion inhibition properties.9 Manifold and varied syntheses of these systems are well documented in the literature.10–12 Pyridotriazine moieties are commonly synthesized via heterocyclization reaction between 1,6-diaminopyridones and bifunctional electrophiles. As an unsymmetrical diamine, 1,6-diaminopyridone is a very active substrate for building diverse heterocyclic systems.13 The majority of the new reported methods for the construction of pyrido[1,2,4]-triazines, use reactions of 4-aryl-1,6-diamino-2-oxo-1,2-dihydro-pyridine-3,5-dicarbonitrile derivatives as a precursor with some active dicarbonyl compounds in the presence of a catalyst.14–16Diversity-Oriented Synthesis (DOS) is a new approach consists of synthetic methods to generating and developing new chemical diverse compounds libraries.17 DOS explores some promising new applications, that have the potential to significantly advance studies in drug discovery and chemical biology, so that increase the biological outcome of the molecules.18,19 Spiro compounds are a specific category of heterocycles that are extensively found in many natural products and bioactive substances.20 Pyran core-containing compounds also have a great variety of pharmaceutical potentials and biological sensors.21 In this regard, spiro-4H-pyrans are significant in organic synthesis because they have a wide spectrum of important biological effects that consist of anti-anaphylactic, antibacterial,22 antitumor, antifungal,23 and antirheumatic activities. Through multicomponent reactions,24,25 several strategies of synthesis of these compounds have been reviewed,26–28 particularly involving the reaction of active carbonyl compounds such as acenaphthoquinone, isatin or ninhydrin with 1,3-diketones, and malononitrile/ethyl cyanoacetate via a three-component reaction in the presence or absence of a catalyst.29–31 Recently, numerous publications reported the synthesis of these compounds with indeno[1,2-b]quinoxalin (Scheme 1a),32 indeno[2,1-c]pyridazine (Scheme 1b),33 indolo[2,1-b]quinazoline (Scheme 1c)34 instead of active carbonyl compounds. In our previous works, also we have reported the biological properties of spiro-4H-pyran derivatives (Scheme 1b and 1c).35–37 To extend these works, we decided to use indeno[1,2-e]pyrido[1,2-b][1,2,4]triazine as a main component in the reaction with malononitrile and CH-acids for building novel spiro-4H-pyrans (Scheme 1d). As a result, the fusion of the spiro-pyran system with the pyrido-triazine moiety could be as a privileged nucleus for many biological activities.38 We have successfully synthesized a series of compounds containing spiro-4H-pyrans attached to pyrido[1,2,4]triazine, which are completely new.
Nitrogen-containing propellane and analogs are remarkable models for the study of their synthesis, and have gained much consideration due to their pharmaceuticals.39 Many approaches have recently been reported for the synthesis of propellanes and the formation of framework of these compounds was discussed.40–44
(E)-N-Methyl-1-(methylthio)-2-nitroethenamine that have flexibility and high reactivity has shown important attention as an ambiphilic synthon and suitable framework due to the attendance of push–pull system to synthesis of numerous heterocyclic compounds and mainly exist in natural and synthetic drugs.45,46 In continuation of our effort, by using pyrido-triazine as a main component and NMSM as an ambiphilic synthon, we also investigate an efficient synthesis of indenopyridotriazine [4.3.3]propellans in the presence of HOAc in ethanol (Scheme 1e). So far, there are no reports on the synthesis of these structures.
Results and discussion
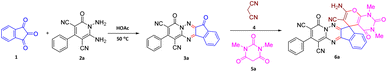
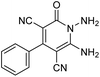
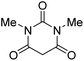
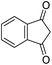
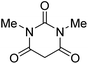
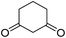
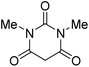
The starting material, 1,6-diaminopyridinone derivatives 2 were easily synthesised from cyanoacetohydrazide with arylbenzilidenemalononitriles via cyclocondensation reaction.47 Then reaction of ninhydrin 1 and 1,6-diamino-2-oxo-4-phenyl-1,2-dihydropyridine-3,5-dicarbonitrile derivatives 2 in acetic acid as a solvent without any catalyst led to corresponding dihydroindenopyrido[1,2,4]triazine derivatives 3. Therefore, we used compound 3 to synthesize some novel spiro[indeno[1,2-e]pyrido[1,2-b][1,2,4]triazine-7,5′-pyran]-1,3,6′-tricarbonitrile derivatives 6. Initially, we have chosen pyrido[1,2,4]triazine 3a (1 mmol), malononitrile 4 (1 mmol) and N,N-dimethylbarbituric acid 5a (1 mmol) as model substrates in numerous temperatures and solvents to acquire an optimum condition which was accomplished in ethanol without any catalyst (entry 1, Table 1).| Entry | Solvent | Catalyst | Time (h) | Temp. (°C) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions were accomplished using 1, 2a, 3a, 4, 5a (1 mmol), catalyst (0.01 mmol), and solvent (10 mL).b Isolated yield based on 6a. | |||||
| 1 | EtOH | — | 3 | 78 | 90% |
| 2 | H2O | — | 24 | 100 | No reaction |
| 3 | EtOH | HOAc | 5 | 78 | Trace |
| 4 | EtOH | Piperidine | 24 | 78 | 50% |
| 5 | EtOH | Et3N | 10 | 78 | 61% |
| 6 | MeOH | — | 10 | 64 | 45% |
| 7 | MeCN | — | 24 | 82 | 58% |
| 8 | Iso-propanol | — | 10 | 82 | 70% |
| 9 | DMF | — | 12 | 120 | 10% |
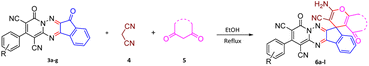
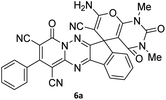
Using the results obtained from the optimized table, we succeeded in synthesizing a three-component one-pot procedure of new spiro[indeno[1,2-e]pyrido[1,2-b][1,2,4]triazine-7,5′-pyran]-1,3,6′-tricarbonitrile derivatives 6a–l from pyrido[1,2,4]triazine derivatives 3a–g, malononitrile 4 and CH-acids 5 in EtOH under catalyst-free condition (Scheme 2).
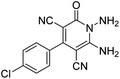
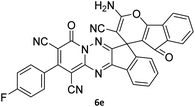
The scope of the process is shown in Table 2. The desired compounds 6a–l were obtained in excellent yields in all cases and substituent with electron-donating/withdrawing of compounds 3a–g have not been effect in this reaction.
| Entry | 1,6-Diaminopyridone | CH-acid | Product 6 | Yield (%) |
|---|---|---|---|---|
| a The reactions were performed using 1,6-diaminopyridone (1 mmol), malononitrile (1 mmol), C–H acid (1 mmol), EtOH (10 mL). | ||||
| 1 | 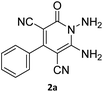 |
 |
 |
90 |
| 2 | 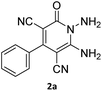 |
 |
 |
83 |
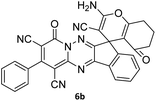
| 3 | 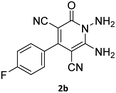 |
 |
 |
86 |
| 4 | 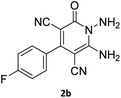 |
 |
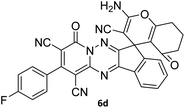 |
91 |
| 5 | 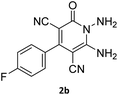 |
 |
 |
81 |
| 6 | 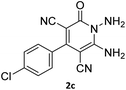 |
 |
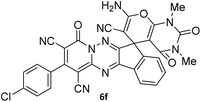 |
85 |
| 7 | 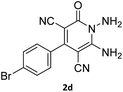 |
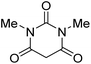 |
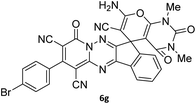 |
89 |
| 8 | 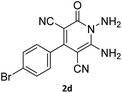 |
 |
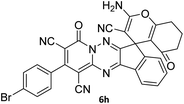 |
80 |
| 9 | 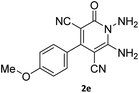 |
 |
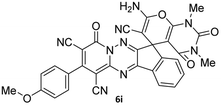 |
86 |
| 10 | 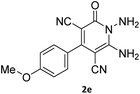 |
 |
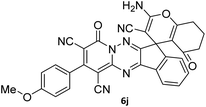 |
88 |
| 11 | 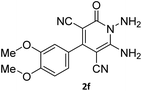 |
 |
 |
82 |
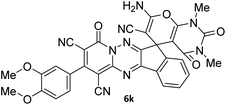
| 12 | 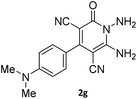 |
 |
 |
81 |
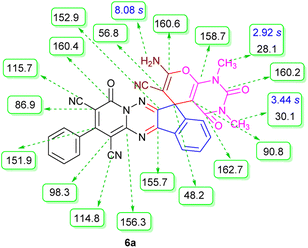
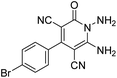
The structures of products 6a–l were elucidated from their IR, 1H NMR, 13C NMR and Mass spectra that confirmed the formation of 6a–l (see the ESI†). The 1H NMR spectrum of 6a exhibited two signals assigned as arising from two methyl groups (δ 2.92 and 3.44 ppm), signals of the aromatic moiety showed multiplets (δ 7.61–8.21 ppm), the NH2 protons (δ 8.08 ppm) that exchangeable with D2O. Chemical shifts of compound 6a were completely assigned and shown in Fig. 1. The 13C NMR spectrum of 6a recognized 29 clear resonances which is in accordance with the proposed structure. Resonances due to spiro carbon and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CNH2 appeared at δ 48.2 and 56.8 ppm respectively. Three signals at δ 86.9, 90.8 and 98.3 ppm were assigned to CCON, C–CONMe and C–CN respectively. The carbonyl group of pyridone ring was displayed at δ 160.6 ppm. The signal at δ 160.4 ppm was assigned to C–NH2. The characteristic signals of three CN were observed at δ 114.8, 115.7 and 117.0 ppm. The mass spectrum of 6a showed the molecular-ion peak at m/z 579 that confirmed the proposed structure. Absorption bands of 6a in the FT-IR spectrum displayed due to NH2, 3CN and 3CO groups at 3376, 2317, 2227, 2204, 1690, 1642, 1594 cm−1 respectively.
CNH2 appeared at δ 48.2 and 56.8 ppm respectively. Three signals at δ 86.9, 90.8 and 98.3 ppm were assigned to CCON, C–CONMe and C–CN respectively. The carbonyl group of pyridone ring was displayed at δ 160.6 ppm. The signal at δ 160.4 ppm was assigned to C–NH2. The characteristic signals of three CN were observed at δ 114.8, 115.7 and 117.0 ppm. The mass spectrum of 6a showed the molecular-ion peak at m/z 579 that confirmed the proposed structure. Absorption bands of 6a in the FT-IR spectrum displayed due to NH2, 3CN and 3CO groups at 3376, 2317, 2227, 2204, 1690, 1642, 1594 cm−1 respectively.
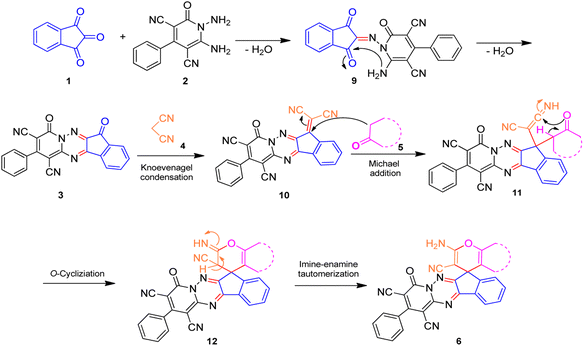
A proposed mechanism for the synthesis of spiroindenopyridotriazine 6 is illustrated in Scheme 3. First, condensation of ninhydrin 1 with 1,6-diaminopyridinone 2 leads to the formation of intermediate 9. Then nucleophilic attack of the NH2 to the carbonyl group in intermediate 9 via intramolecular cyclization gives the corresponding pyrido[1,2,4]triazine 3. Subsequently, pyrido[1,2,4]triazine 3 and malononitrile 4 participate in the Knoevenagel condensation reaction to produce the adduct 10. Next, CH-acid compound 5 attacks adduct 10 via its nucleophilic core to construct the Michael adduct 11, followed by intermolecular O-cyclization via nucleophilic addition of oxygen to the nitrile group that yields intermediate 12. In the final step, imine-enamine tautomerization of 12 leads to the final product 6.
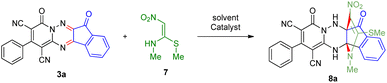
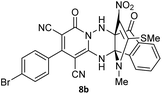
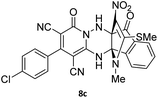
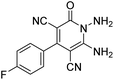
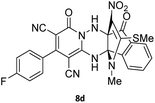
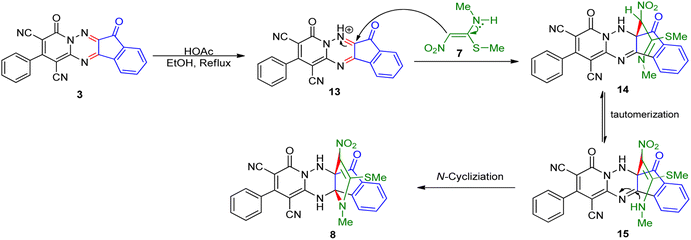
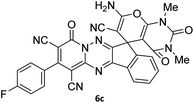
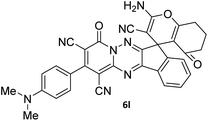
To further expand the scope of these studies, we also tested nitroketene acetals as a 1,3-N,C di-nucleophilic precursor for the preparation of indenopyridotriazine [4.3.3]propellane. By using pyrido[1,2,4]triazine 3 (containing two C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N as 1,2-dielectrophilic sources) and NMSM 7 as a nitroketene N,C-acetal in the presence of HOAc as a catalyst, the [4.3.3]propellane system, was obtained. We used different solvents and catalysts for the optimization of this reaction. As a result, without catalyst, the yield was low, so the best conditions were achieved in EtOH in the vicinity of HOAc (10 mol%) at reflux condition (Table 3). When we used compounds 3 containing electron-donating groups at high temperature conditions, the pyridone moiety was separated from the final product (TLC and NMR evidence). Thus we could synthesize the [4.3.3]propellane system 8a–d with good to high yields, when 1,6-diaminopyridone have electron-withdrawing groups. In all of these reactions, all products were obtained with high efficiency and good purity (Table 4).
N as 1,2-dielectrophilic sources) and NMSM 7 as a nitroketene N,C-acetal in the presence of HOAc as a catalyst, the [4.3.3]propellane system, was obtained. We used different solvents and catalysts for the optimization of this reaction. As a result, without catalyst, the yield was low, so the best conditions were achieved in EtOH in the vicinity of HOAc (10 mol%) at reflux condition (Table 3). When we used compounds 3 containing electron-donating groups at high temperature conditions, the pyridone moiety was separated from the final product (TLC and NMR evidence). Thus we could synthesize the [4.3.3]propellane system 8a–d with good to high yields, when 1,6-diaminopyridone have electron-withdrawing groups. In all of these reactions, all products were obtained with high efficiency and good purity (Table 4).
The low solubility of products 6 and 8 in organic solvents significantly simplified workup and purification of the products. The product is withdrawn from the reaction mixture by precipitation due to its low solubility in the solvent. However, the low solubility of these molecules made it difficult to evaluate their biological activities. Due to the low solubility of the products, NMR data could be obtained only for selected products. Just in case, it was not possible to obtain 13C NMR data for compound 6e due to insolubility, while in other cases NMR data could be obtained by increasing the number of scans.
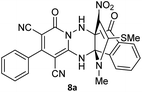
The structures of products 8a–d were deduced from their IR, 1H NMR, 13C NMR and Mass spectral data. The 1H NMR spectrum of 8a showed characteristic two signals of the N–H proton at δ = 8.25 and 9.64 ppm (exchangeable with D2O). Aromatic protons appeared at δ 7.49–8.61 ppm. The NCH3 and SCH3 groups exhibited two sharp singlets at 3.40 and 2.61 ppm, respectively. 13C NMR spectra revealed a signal at δ = 191.3 ppm for C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O carbon of the ninhydrin moiety and signals corresponding to the C–SMe, C–NO2, C–NMe groups were observed at about 160.2, 124.3 and 87.9 ppm respectively. Two nitrile groups were observed at 114.7 and 116.1 ppm. The IR spectrum of 8a displayed vibrational stretching bands for NH (3441 and 3170 cm−1), CN (2224 cm−1), C
O carbon of the ninhydrin moiety and signals corresponding to the C–SMe, C–NO2, C–NMe groups were observed at about 160.2, 124.3 and 87.9 ppm respectively. Two nitrile groups were observed at 114.7 and 116.1 ppm. The IR spectrum of 8a displayed vibrational stretching bands for NH (3441 and 3170 cm−1), CN (2224 cm−1), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (1729, 1674 cm−1) and NO2 (1544 and 1388 cm−1) groups. Mass data also supported product 8a (see the ESI†). A probable reaction mechanism was suggested as shown in Scheme 4. First, pyrido[1,2,4]triazine 3 reacts with HOAc to give protonated triazolopyridinone 13 which reacts with NMSM 7 to generate the intermediate 14. The intermediate 14 undergoes successive tautomerization followed by N-cyclization via nucleophilic addition of nitrogen of NMSM that leads to the formation of desired products 8.
O (1729, 1674 cm−1) and NO2 (1544 and 1388 cm−1) groups. Mass data also supported product 8a (see the ESI†). A probable reaction mechanism was suggested as shown in Scheme 4. First, pyrido[1,2,4]triazine 3 reacts with HOAc to give protonated triazolopyridinone 13 which reacts with NMSM 7 to generate the intermediate 14. The intermediate 14 undergoes successive tautomerization followed by N-cyclization via nucleophilic addition of nitrogen of NMSM that leads to the formation of desired products 8.
Conclusion
We have developed the synthesis of structurally diverse products from a simple reaction followed by cyclization steps and appendage diversity. This study describes a convenient protocol for the synthesis of novel spiro[indeno[1,2-e]pyrido[1,2-b][1,2,4]triazine-7,5′-pyran]-1,3,6′-tricarbonitrile via the reaction of pyrido[1,2,4]triazine with malononitrile and CH-acids under reflux condition in ethanol. Also, we have expanded an easy one-pot reaction for the synthesis of indenopyridotriazine [4.3.3]propellanes from the reaction of pyrido[1,2,4]triazine and NMSM in HOAc as a catalyst. The diverse structures that we synthesized in this study are completely new and no reports of them have been presented, and the biological evaluations of these derivatives are underway.Experimental
Materials
All commercially available chemical compounds and other solvents in this study were purchased from Merck and Aldrich chemical companies. The NMR spectra for synthesized compounds were acquired using a Bruker DRX-300 AVANCE instrument (300 MHz for 1H and 75.4 MHz for 13C) with DMSO-d6 as the deuterated solvent. Chemical shifts were reported in ppm (δ) relative to the internal TMS, and the coupling constant (J) given in hertz (Hz). FT-IR spectra and melting points of all the compounds were measured with a Bruker Tensor 27 spectrometer and electrothermal 9100 apparatus, respectively. Mass spectra were given by an Agilent 5975C VL MSD with a Triple-Axis detector operating at an ionization potential of 70 eV.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), after the reaction was completed; the reaction mixture was cooled to room temperature. Then, the solid was separated by filtration, washed twice times with water, and was subsequently washed with Et2O. The precipitate was dried in 130 °C to obtain crude product 3 without recrystallization.48
1), after the reaction was completed; the reaction mixture was cooled to room temperature. Then, the solid was separated by filtration, washed twice times with water, and was subsequently washed with Et2O. The precipitate was dried in 130 °C to obtain crude product 3 without recrystallization.48![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1473 (C
O), 1473 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), cm−1; 1H NMR (300 MHz, DMSO-d6) δ: 8.33–8.36 (1H, m, ArH), 8.18–8.20 (1H, m, ArH), 8.08–8.10 (2H, m, ArH), 7.65–7.68 (5H, m, ArH); 13C NMR (75.4 MHz, DMSO-d6) δ: 160.7, 183.6 (C
C), cm−1; 1H NMR (300 MHz, DMSO-d6) δ: 8.33–8.36 (1H, m, ArH), 8.18–8.20 (1H, m, ArH), 8.08–8.10 (2H, m, ArH), 7.65–7.68 (5H, m, ArH); 13C NMR (75.4 MHz, DMSO-d6) δ: 160.7, 183.6 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 156.2, 160.5 (C
O), 156.2, 160.5 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 151.2, 148.3, 141.0, 138.5, 138.1, 137.2, 133.7, 131.6, 129.4, 128.8, 125.9, 125.6, 115.35, 114.4 (Ar), 91.7, 99.8 (CN).
N), 151.2, 148.3, 141.0, 138.5, 138.1, 137.2, 133.7, 131.6, 129.4, 128.8, 125.9, 125.6, 115.35, 114.4 (Ar), 91.7, 99.8 (CN).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 152.9 (C
N), 152.9 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–N), 150.0 (CC
N–N), 150.0 (CC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 151.9, 138.9, 134.2, 131.8, 131.4, 131.2, 129.3, 128.7, 126.5, 124.9 (Ar), 117.0, 115.7, 114.8 (3CN), 98.3 (C–CN), 90.8 (C–CONMe), 86.9 (CCON), 56.8 (C
N), 151.9, 138.9, 134.2, 131.8, 131.4, 131.2, 129.3, 128.7, 126.5, 124.9 (Ar), 117.0, 115.7, 114.8 (3CN), 98.3 (C–CN), 90.8 (C–CONMe), 86.9 (CCON), 56.8 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CNH2), 48.2 (Cspiro), 30.1 (NCH3), 28.1 (NCH3); MS (EI, 70 eV): m/z (%) = 579 (12) [M]+, 515 (12), 423 (30), 254 (23), 156 (100), 129 (31), 83 (38), 56 (69).
CNH2), 48.2 (Cspiro), 30.1 (NCH3), 28.1 (NCH3); MS (EI, 70 eV): m/z (%) = 579 (12) [M]+, 515 (12), 423 (30), 254 (23), 156 (100), 129 (31), 83 (38), 56 (69).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 159.1 (C
N), 159.1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–N), 156.3 (
N–N), 156.3 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O), 151.8 (CC
C–O), 151.8 (CC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 156.1, 138.9, 134.2, 131.6, 131.1, 129.3, 128.7, 126.1, 125.7, 125.0 (Ar), 117.6, 115.7, 114.9 (3CN), 111.9 (C–CN), 98.3 (C–COCH2), 90.7 (CCON), 56.6 (C
N), 156.1, 138.9, 134.2, 131.6, 131.1, 129.3, 128.7, 126.1, 125.7, 125.0 (Ar), 117.6, 115.7, 114.9 (3CN), 111.9 (C–CN), 98.3 (C–COCH2), 90.7 (CCON), 56.6 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CNH2), 47.7 (Cspiro), 36.5 (O
CNH2), 47.7 (Cspiro), 36.5 (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH2), 27.3 (
C–CH2), 27.3 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH2), 20.0 (CH2); MS (EI, 70 eV): m/z (%) = 535 (8) [M]+, 436 (3), 281 (11), 236 (21), 207 (21), 149 (22), 129 (20), 84 (49), 55 (100).
C–CH2), 20.0 (CH2); MS (EI, 70 eV): m/z (%) = 535 (8) [M]+, 436 (3), 281 (11), 236 (21), 207 (21), 149 (22), 129 (20), 84 (49), 55 (100).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 152.9 (C
N), 152.9 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–N), 150.0 (CC
N–N), 150.0 (CC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 151.93, 139.01, 132.5 (d, 4JCF = 6.5 Hz), 131.35 (d, 3JCF = 8.2 Hz), 130.6, 124.9, 126.5, 117.0, 115.5 (d, 2JCF = 22 Hz, Ar), 116.7, 116.4, 114.8 (3CN), 98.4 (C–CN), 90.9 (C–CONMe), 86.9 (CCON), 56.7 (C
N), 151.93, 139.01, 132.5 (d, 4JCF = 6.5 Hz), 131.35 (d, 3JCF = 8.2 Hz), 130.6, 124.9, 126.5, 117.0, 115.5 (d, 2JCF = 22 Hz, Ar), 116.7, 116.4, 114.8 (3CN), 98.4 (C–CN), 90.9 (C–CONMe), 86.9 (CCON), 56.7 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CNH2), 48.2 (Cspiro), 30.1 (NCH3), 28.1 (NCH3); MS (EI, 70 eV): m/z (%) = 597 (5) [M]+, 441 (47), 413 (22), 254 (13), 156 (100), 129 (14), 99 (20), 66 (47).
CNH2), 48.2 (Cspiro), 30.1 (NCH3), 28.1 (NCH3); MS (EI, 70 eV): m/z (%) = 597 (5) [M]+, 441 (47), 413 (22), 254 (13), 156 (100), 129 (14), 99 (20), 66 (47).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 159.3 (C
N), 159.3 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–N), 159.1 (
N–N), 159.1 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O), 156.2 (CC
C–O), 156.2 (CC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 156.1, 151.80, 139.0, 131.5, 131.4 (d, 3JCF = 9.0 Hz), 131.1, 126.1, 125.0, 117.61, 116.5 (d, 2JCF = 22.5 Hz, ArH), 116.4, 115.7, 114.8 (3CN), 111.9 (C–CN), 98.4 (C–COCH2), 90.8 (CCON), 56.6 (C
N), 156.1, 151.80, 139.0, 131.5, 131.4 (d, 3JCF = 9.0 Hz), 131.1, 126.1, 125.0, 117.61, 116.5 (d, 2JCF = 22.5 Hz, ArH), 116.4, 115.7, 114.8 (3CN), 111.9 (C–CN), 98.4 (C–COCH2), 90.8 (CCON), 56.6 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CNH2), 47.7 (Cspiro), 36.5 (O
CNH2), 47.7 (Cspiro), 36.5 (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH2), 31.1 (
C–CH2), 31.1 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH2), 20.0 (CH2). MS (EI, 70 eV): m/z (%) = 553 (2) [M]+, 551 (20), 495 (9), 368 (100), 313 (31), 236 (37), 213 (10), 135 (15), 83 (47), 57 (63).
C–CH2), 20.0 (CH2). MS (EI, 70 eV): m/z (%) = 553 (2) [M]+, 551 (20), 495 (9), 368 (100), 313 (31), 236 (37), 213 (10), 135 (15), 83 (47), 57 (63).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 153.0 (C
N), 153.0 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–N), 151.8 (C-4), 150.0 (CC
N–N), 151.8 (C-4), 150.0 (CC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 151.3, 148.7, 138.9, 131.8, 131.4, 125.9, 122.2, 117.1, 116.0 (Ar), 115.1, 112.6, 112.0 (3CN), 97.9 (C–CN), 90.9 (C–CONMe), 86.9 (CCON), 56.49 (C
N), 151.3, 148.7, 138.9, 131.8, 131.4, 125.9, 122.2, 117.1, 116.0 (Ar), 115.1, 112.6, 112.0 (3CN), 97.9 (C–CN), 90.9 (C–CONMe), 86.9 (CCON), 56.49 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CNH2), 48.1 (Cspiro), 30.1 (NCH3), 28.1 (NCH3); MS (EI, 70 eV): m/z (%) = 575 (3) [M], 463 (3), 368 (7), 284 (17), 256 (32), 185 (22), 129 (41), 97 (55), 57 (100).
CNH2), 48.1 (Cspiro), 30.1 (NCH3), 28.1 (NCH3); MS (EI, 70 eV): m/z (%) = 575 (3) [M], 463 (3), 368 (7), 284 (17), 256 (32), 185 (22), 129 (41), 97 (55), 57 (100).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 153.0 (C
N), 153.0 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–N), 152.0 (C-4), 150.0 (CC
N–N), 152.0 (C-4), 150.0 (CC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 139.1, 133.4, 132.5, 131.8, 131.4, 130.8, 126.5, 125.0, 124.9 (Ar), 117.0, 115.6, 114.7 (3CN), 98.2 (C–CN), 90.7 (C–CONMe), 86.8 (CCON), 56.3 (C
N), 139.1, 133.4, 132.5, 131.8, 131.4, 130.8, 126.5, 125.0, 124.9 (Ar), 117.0, 115.6, 114.7 (3CN), 98.2 (C–CN), 90.7 (C–CONMe), 86.8 (CCON), 56.3 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CNH2), 48.2 (Cspiro), 30.1 (NCH3), 28.1 (NCH3); MS (EI, 70 eV): m/z (%) = 551 (11), 523 (10), 368 (15), 313 (18), 236 (27), 207 (24), 149 (56), 107 (40), 83 (55), 57 (100).
CNH2), 48.2 (Cspiro), 30.1 (NCH3), 28.1 (NCH3); MS (EI, 70 eV): m/z (%) = 551 (11), 523 (10), 368 (15), 313 (18), 236 (27), 207 (24), 149 (56), 107 (40), 83 (55), 57 (100).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 159.2 (C
N), 159.2 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–N), 156.3 (
N–N), 156.3 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O), 156.2 (C-4), 151.8 (CC
C–O), 156.2 (C-4), 151.8 (CC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 152.7, 139.0, 133.4, 132.4, 131.5, 131.1, 130.8, 126.1, 125.0 (Ar), 117.6, 115.6, 114.8 (3CN), 111.9, 98.2 (C–COCH2), 90.6 (CCON), 56.5 (
N), 152.7, 139.0, 133.4, 132.4, 131.5, 131.1, 130.8, 126.1, 125.0 (Ar), 117.6, 115.6, 114.8 (3CN), 111.9, 98.2 (C–COCH2), 90.6 (CCON), 56.5 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CN), 47.7 (Cspiro), 36.5 (O
C–CN), 47.7 (Cspiro), 36.5 (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH2), 31.1 (
C–CH2), 31.1 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH2), 20.0 (CH2).
C–CH2), 20.0 (CH2).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 155.6 (C
N), 155.6 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–N), 152.9 (C-4), 150.0 (CC
N–N), 152.9 (C-4), 150.0 (CC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 151.9, 138.8, 131.8, 131.4, 130.7, 130.3, 126.5, 126.0, 124.8 (Ar), 117.0, 116.0, 114.7 (3CN), 98.0 (C–CN), 90.8 (C–CONMe), 86.9 (CCON), 56.8 (C
N), 151.9, 138.8, 131.8, 131.4, 130.7, 130.3, 126.5, 126.0, 124.8 (Ar), 117.0, 116.0, 114.7 (3CN), 98.0 (C–CN), 90.8 (C–CONMe), 86.9 (CCON), 56.8 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CNH2), 55.9 (OCH3), 48.2 (Cspiro), 30.1 (NCH3), 28.1 (NCH3).
CNH2), 55.9 (OCH3), 48.2 (Cspiro), 30.1 (NCH3), 28.1 (NCH3).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 160.0 (C
N), 160.0 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–N), 159.1 (
N–N), 159.1 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O), 156.4 (C-4), 151.7 (CC
C–O), 156.4 (C-4), 151.7 (CC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 156.1, 138.8, 131.6, 131.1, 130.7, 126.0, 124.9, 117.6, 116.0 (Ar), 115.1, 114.7, 114.3 (3CN), 111.9 (C–CN), 98.0 (C–COCH2), 90.8 (CCON), 56.7 (C
N), 156.1, 138.8, 131.6, 131.1, 130.7, 126.0, 124.9, 117.6, 116.0 (Ar), 115.1, 114.7, 114.3 (3CN), 111.9 (C–CN), 98.0 (C–COCH2), 90.8 (CCON), 56.7 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CNH2), 55.9 (OCH3), 47.6 (Cspiro), 36.5 (O
CNH2), 55.9 (OCH3), 47.6 (Cspiro), 36.5 (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH2), 31.1 (
C–CH2), 31.1 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH2), 20.0 (CH2).
C–CH2), 20.0 (CH2).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 152.9 (C
N), 152.9 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–N), 150.0 (CC
N–N), 150.0 (CC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 151.8, 151.3, 148.8, 138.8, 131.9, 131.4, 126.5, 125.9, 124.8, 122.2, 116.0, 112.7 (Ar), 117.0, 115.0, 112.1 (3CN), 97.9 (C–CN), 90.9 (C–CONMe), 86.9 (CCON), 56.9 (C
N), 151.8, 151.3, 148.8, 138.8, 131.9, 131.4, 126.5, 125.9, 124.8, 122.2, 116.0, 112.7 (Ar), 117.0, 115.0, 112.1 (3CN), 97.9 (C–CN), 90.9 (C–CONMe), 86.9 (CCON), 56.9 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CNH2), 56.1 (OCH3), 48.2 (Cspiro), 30.1 (NCH3), 28.1 (NCH3).
CNH2), 56.1 (OCH3), 48.2 (Cspiro), 30.1 (NCH3), 28.1 (NCH3).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 159.1 (C
N), 159.1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–N), 156.6 (
N–N), 156.6 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O), 156.1 (C-4), 151.7 (CC
C–O), 156.1 (C-4), 151.7 (CC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 152.3, 138.7, 131.7, 131.0, 130.7, 126.0, 124.8, 119.9, 117.6 (Ar), 116.6, 115.5, 111.9 (3CN), 111.7 (C–CN), 96.7 (C–COCH2), 90.3 (CCON), 56.7 (C
N), 152.3, 138.7, 131.7, 131.0, 130.7, 126.0, 124.8, 119.9, 117.6 (Ar), 116.6, 115.5, 111.9 (3CN), 111.7 (C–CN), 96.7 (C–COCH2), 90.3 (CCON), 56.7 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CNH2), 47.6 (Cspiro), 36.6 (NMe2), 36.5 (O
CNH2), 47.6 (Cspiro), 36.6 (NMe2), 36.5 (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH2), 27.3 (
C–CH2), 27.3 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CH2), 20.0 (CH2).
C–CH2), 20.0 (CH2).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 164.3 (
O), 164.3 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CNNH), 160.2 (C–SMe), 157.5 (O
CNNH), 160.2 (C–SMe), 157.5 (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–N), 155.6 (
C–N), 155.6 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C-3), 149.5, 137.7, 134.4, 133.9, 131.1, 131.1, 129.1, 129.1, 128.6, 125.5 (ArH), 124.3 (C–NO2), 116.1, 114.7, (2CN), 92.1 (NH–C–CO), 87.9 (C–NMe), 78.5 (C–CN), 65.8 (C–CN), 31.4 (NCH3), 17.7 (SCH3); MS (EI, 70 eV): m/z (%) = 523 (4) [M]+, 477 (65), 429 (100), 375 (23), 288 (84), 195 (43), 148 (49), 84 (61), 55 (73).
C-3), 149.5, 137.7, 134.4, 133.9, 131.1, 131.1, 129.1, 129.1, 128.6, 125.5 (ArH), 124.3 (C–NO2), 116.1, 114.7, (2CN), 92.1 (NH–C–CO), 87.9 (C–NMe), 78.5 (C–CN), 65.8 (C–CN), 31.4 (NCH3), 17.7 (SCH3); MS (EI, 70 eV): m/z (%) = 523 (4) [M]+, 477 (65), 429 (100), 375 (23), 288 (84), 195 (43), 148 (49), 84 (61), 55 (73).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 164.4 (
O), 164.4 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CNNH), 159.2 (C–SMe), 157.4 (O
CNNH), 159.2 (C–SMe), 157.4 (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–N), 155.7 (
C–N), 155.7 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C-3), 149.4, 137.7, 133.9, 133.6, 132.3, 131.1, 130.8, 129.3, 125.5, 124.8 (ArH), 124.3 (C–NO2), 116.0, 114.6 (2CN), 92.0 (NH–C–CO), 87.9 (C–NMe), 78.4 (C–CN), 65.8 (C–CN), 31.5 (NCH3), 17.7 (SCH3); MS (EI, 70 eV): m/z (%) = 557 (7), 509 (12), 427 (3), 376 (2), 288 (19), 195 (12), 148 (64), 101 (76), 77 (24), 55 (100).
C-3), 149.4, 137.7, 133.9, 133.6, 132.3, 131.1, 130.8, 129.3, 125.5, 124.8 (ArH), 124.3 (C–NO2), 116.0, 114.6 (2CN), 92.0 (NH–C–CO), 87.9 (C–NMe), 78.4 (C–CN), 65.8 (C–CN), 31.5 (NCH3), 17.7 (SCH3); MS (EI, 70 eV): m/z (%) = 557 (7), 509 (12), 427 (3), 376 (2), 288 (19), 195 (12), 148 (64), 101 (76), 77 (24), 55 (100).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 164.2 (
O), 164.2 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CNNH), 159.7 (C–SMe), 155.7 (O
CNNH), 159.7 (C–SMe), 155.7 (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–N), 155.2 (
C–N), 155.2 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C-3), 150.0, 142.4, 135.8, 135.3, 133.4, 132.5, 130.6, 129.3, 125.7, 123.5 (ArH), 123.1 (C–NO2), 116.2, 115.2, (2CN), 98.3 (NH–C–CO), 89.8 (C–NMe), 74.9 (C–CN), 61.6 (C–CN), 31.7 (NCH3), 17.6 (SCH3).
C-3), 150.0, 142.4, 135.8, 135.3, 133.4, 132.5, 130.6, 129.3, 125.7, 123.5 (ArH), 123.1 (C–NO2), 116.2, 115.2, (2CN), 98.3 (NH–C–CO), 89.8 (C–NMe), 74.9 (C–CN), 61.6 (C–CN), 31.7 (NCH3), 17.6 (SCH3).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 165.3 (
O), 165.3 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CNNH), 164.1 (C–F, d, 1JCF = 246.0 Hz), 159.9 (C–SMe), 155.7 (O
CNNH), 164.1 (C–F, d, 1JCF = 246.0 Hz), 159.9 (C–SMe), 155.7 (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–N), 155.2 (
C–N), 155.2 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C-3), 150.0, 142.4, 141.0, 138.5, 132.5, 131.5, 131.3 (d, 3JCF = 8.2 Hz), 125.6 (ArH), 123.5 (C–NO2), 116.7 (d, 2JCF = 22.5 Hz, Ar), 116.4, 116.1 (2CN), 98.4 (NH–C–CO), 91.8 (C–NMe), 75.1 (C–CN), 61.7 (C–CN), 31.7 (NCH3), 17.6 (SCH3).
C-3), 150.0, 142.4, 141.0, 138.5, 132.5, 131.5, 131.3 (d, 3JCF = 8.2 Hz), 125.6 (ArH), 123.5 (C–NO2), 116.7 (d, 2JCF = 22.5 Hz, Ar), 116.4, 116.1 (2CN), 98.4 (NH–C–CO), 91.8 (C–NMe), 75.1 (C–CN), 61.7 (C–CN), 31.7 (NCH3), 17.6 (SCH3).Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
Financial support of this research from Imam Khomeini International University, Iran is gratefully acknowledged.Notes and references
- E. R. Kotb, M. M. Anwar, Y. M. Syam, O. Bagato and S. Abdel Moaz, Int. J. Pharm. Technol., 2015, 7, 8237 CAS.
- S. Cascioferro, B. Parrino, V. Spano, A. Carbone, A. Montalbano, P. Barraja, P. Diana and G. Cirrincione, Eur. J. Med. Chem., 2017, 142, 328 CrossRef CAS PubMed.
- M. A. Ibrahim, R. M. Abdel-Rahman, A. M. Abdel-Halim, S. S. Ibrahim and H. A. Allimony, J. Braz. Chem. Soc., 2009, 20, 1275 CrossRef CAS.
- P. Mullick, S. A. Khan, T. Begum, S. Verma, D. Kaushik and O. Alam, Acta Pol. Pharm. Drug Res., 2009, 66, 379 CAS.
- M. Arshad, A. R. Bhat, K. K. Hoi, I. Choi and F. Athar, Chin. Chem. Lett., 2017, 28, 1559 CrossRef CAS.
- H. Serrar, I. Marmouzi, Z. Benzekri, S. Boukhris, A. Hassikou, M. E. Faouzi and A. Souizi, Lett. Org. Chem., 2017, 14, 267 CrossRef CAS.
- R. Kumar, T. S. Sirohi, H. Singh, R. Yadav, R. K. Roy, A. Chaudhary and S. N. Pandeya, Mini-Rev. Med. Chem., 2014, 14, 168 CrossRef CAS PubMed.
- M. Khanzadeh, M. Dehghanipour, A. Darehkordi and F. Rahmani, Can. J. Phys., 2018, 96, 1288 CrossRef CAS.
- Y. ELaoufir, H. Bourazmi, H. Serrar, H. Zarrok, A. Zarrouk, B. Hammouti, A. Guenbour, S. Boukhriss and H. Oudda, Pharm. Lett., 2014, 6, 526 Search PubMed.
- T. Phucho, A. Nongpiur, S. Tumtin, R. Nongrum, B. Myrboh and R. L. Nonghlaw, Arkivoc, 2008, 15, 79 Search PubMed.
- Y. Liu, X. Guo, D. Tang, J. Wang, P. Wu, J. Han and B. Chen, Chin. J. Chem., 2017, 35, 1222 CrossRef CAS.
- H. M. Ibrahim and H. Behbehani, ACS Omega, 2021, 6, 16086 CrossRef CAS PubMed.
- M. A. Ibrahim and N. M. El-Gohary, Heterocycles, 2014, 89, 1125 CrossRef CAS.
- A. Darehkordi, M. Hosseini and F. Rahmani, J. Heterocycl. Chem., 2019, 56, 1306 CrossRef CAS.
- A. Darehkordi, V. Salehi, F. Rahmani and M. Karimipour, Chem. Heterocycl. Compd., 2018, 54, 554 CrossRef CAS.
- M. Tahmasby, A. Darehkordi, M. Mohammadi and F. Nejadkhorasani, J. Mol. Struct., 2021, 1224, 129032 CrossRef CAS.
- A. Trabocchi, Diversity-Oriented Synthesis: Basics and Applications in Organic Synthesis, Drug Discovery, and Chemical Biology, Wiley and Sons, 2013 Search PubMed.
- L. Schreiber, Science, 2000, 287, 1964 CrossRef PubMed.
- E. Lenci and A. Trabocchi, Eur. J. Org Chem., 2022, 2022(29), e202200575 CrossRef CAS.
- A. Ding, M. Meazza, H. Guo, J. W. Yang and R. Rios, Chem. Soc. Rev., 2018, 47, 5946 RSC.
- M. Suthar, J. Kumbhani and K. D. Bhatt, Orient. J. Chem., 2021, 37, 1280 CAS.
- Saigal, M. Irfan, P. Khan, M. Abid and M. M. Khan, ACS Omega, 2019, 4, 16794 CrossRef CAS PubMed.
- A. Akbari, Z. Azami-Sardooei and A. Hosseini-Nia, J. Korean Chem. Soc., 2013, 57, 455 CrossRef CAS.
- M. O. Rodrigues, M. N. Eberlin and B. A. D. Neto, Chem. Rec., 2021, 10, 2762 CrossRef PubMed.
- H. G. O. Alvim, E. N. da Silva Júnior and B. A. D. Neto, RSC Adv., 2014, 4, 54282 RSC.
- Z. Tashrifi, M. Mohammadi-Khanaposhtani, H. Hamedifar, B. Larijani, S. Ansari and M. Mahdavi, Mol. Diversity, 2020, 24, 1385 CrossRef CAS PubMed.
- B. Borah, K. D. Dwivedi and L. R. Chowhan, Polycyclic Aromat. Compd., 2022, 42, 5893 CrossRef CAS.
- R. Y. Guo, Z. M. An, L. P. Mo, R. Z. Wang, H. X. Li, S. X. Wang and Z. H. Zhang, ACS Comb. Sci., 2013, 15, 557 CrossRef CAS PubMed.
- Y. He, H. Guo and J. Tian, J. Chem. Res., 2011, 35, 528 CrossRef CAS.
- P. Saluja, K. Aggarwal and J. M. Khurana, Synth. Commun., 2013, 43, 3239 CrossRef CAS.
- P. Khanna, L. Khanna, S. J. Thomas, A. M. Asiri and S. S. Panda, Curr. Org. Chem., 2018, 22, 67 CrossRef CAS.
- F. Chen, J. Zheng, M. Huang and Y. Li, Res. Chem. Intermed., 2015, 41, 5545 CrossRef CAS.
- M. Bayat and H. Hosseini, New J. Chem., 2017, 41, 14954 RSC.
- Z. Sadeghian, M. Bayat and F. Safari, Med. Chem. Res., 2021, 31, 497 CrossRef.
- F. Safari, H. Hosseini, M. Bayat and A. Ranjbar, RSC Adv., 2019, 9, 24843 RSC.
- Z. Sadeghian, M. Bayat and F. Safari, J. Mol. Struct., 2022, 1250, 131759 CrossRef CAS.
- Z. Sadeghian, M. Bayat and F. Safari, Med. Chem. Res., 2022, 31, 497 CrossRef CAS.
- E. Ezzatzadeh, S. Soleimani-Amiri, Z. Hossaini and K. Khandan Barani, Front. Chem., 2022, 10, 1001707 CrossRef CAS PubMed.
- A. M. Dilmaç, T. Wezeman, R. M. Bär and S. Bräse, Nat. Prod. Rep., 2020, 37, 224 RSC.
- N. Chen, S. Xia, M. Zou and X. Shao, Res. Chem. Intermed., 2015, 41, 5293 CrossRef CAS.
- M. B. Yadav, S. R. Bhosle and Y. T. Jeong, Tetrahedron Lett., 2022, 98, 153815 CrossRef CAS.
- K. Kato, S. Tanaka, N. Seto, K. Wada, M. Gon, S. Fa, S. Ohtani, K. Tanaka and T. Ogoshi, Chem. Commun., 2023, 59, 7080 RSC.
- I. Yavari, A. Khajeh-Khezri and M. R. Halvagar, Arabian J. Chem., 2018, 11, 188 CrossRef CAS.
- I. Yavari, A. Malekafzali and S. Skoulika, Tetrahedron Lett., 2014, 55, 3154 CrossRef CAS.
- S. Khan, H. Rahman and M. M. Khan, RSC Adv., 2019, 9, 14477 RSC.
- F. Abedinifar, B. Larijani and M. Mahdavi, RSC Adv., 2022, 12, 30436 RSC.
- H. Hosseini and M. Bayat, RSC Adv., 2018, 8, 27131 RSC.
- M. Shokoohian, N. Hazeri, M. T. Maghsoodlou and M. Lashkari, Polycyclic Aromat. Compd., 2022, 42, 1 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra06248a |
| This journal is © The Royal Society of Chemistry 2023 |