Open Access Article
Open Access ArticleGlobal trends and future prospects of lactic acid production from lignocellulosic biomass
Siyuan Yue ac and
Min Zhang
ac and
Min Zhang *ab
*ab
aLaboratory of Soil and Environmental Microbiology, Division of Systems Bioengineering, Department of Bioscience and Biotechnology, Faculty of Agriculture, Graduate School of Bioresources and Bioenvironmental Sciences, Kyushu University, Fukuoka 819–0395, Japan
bJiangxi Copper Technology Research Institute, Jiangxi Copper Corporation, Nanchang, Jiangxi Province 330096, China. E-mail: minzhangjt@163.com
cInstitute of Microbiology, Jiangxi Academy of Sciences, Nanchang, Jiangxi Province 330096, China
First published on 7th November 2023
Abstract
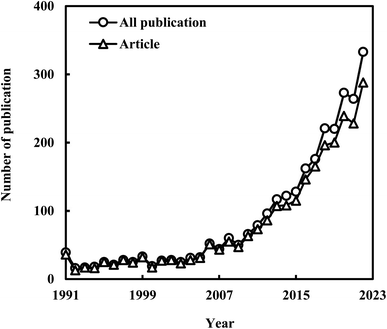
Lignocellulosic biomass (LCB) stands as a substantial and sustainable resource capable of addressing energy and environmental challenges. This study employs bibliometric analysis to investigate research trends in lactic acid (LA) production from LCB spanning the years 1991 to 2022. The analysis reveals a consistent growth trajectory with minor fluctuations in LA production from LCB. Notably, there's a significant upswing in publications since 2009. Bioresource Technology and Applied Microbiology and Biotechnology emerge as the top two journals with extensive contributions in the realm of LA production from LCB. China takes a prominent position in this research domain, boasting the highest total publication count (736), betweenness centrality value (0.30), and the number of collaborating countries (42), surpassing the USA and Japan by a considerable margin. The author keywords analysis provides valuable insights into the core themes in LA production from LCB. Furthermore, co-citation reference analysis delineates four principal domains related to LA production from LCB, with three associated with microbial conversion and one focused on chemical catalytic conversion. Additionally, this study examines commonly used LCB, microbial LA producers, and compares microbial fermentation to chemical catalytic conversion for LCB-based LA production, providing comprehensive insights into the current state of this field and suggesting future research directions.
Introduction
In a world grappling with pressing challenges such as environmental sustainability and an increasingly urgent energy crisis, the vast abundance of lignocellulosic biomass (LCB) on our planet offers a beacon of hope. Plants, which dominate terrestrial ecosystems, account for a staggering 80% or 450 gigatons of carbon to the total global biomass, making them an unparalleled wellspring of LCB resources.1 This vast reserve of renewable resources includes the fibrous components of plants, such as cellulose, hemicellulose, and lignin. LCB, sourced from a diverse array of origins, including agricultural residues, forest waste, and specialized energy crops, represents an untapped reservoir that, if effectively harnessed, could help mitigate the energy crisis that looms over us.2 The increasing interest in the production of value-added organic acids from LCB stems from the fact that these acids retain a substantial portion of critical elements like carbon and oxygen, aligning seamlessly with the high atomic economy principle.3Beyond its pivotal role in the energy landscape, lactic acid (LA), a versatile and valuable compound, holds a critical place in various industries. The majority of non-polymer grade LA and its salts find extensive use in the food industry. LA also serves as a foundational element for the synthesis of various chemicals, including acrylic acid, acetaldehyde, pyruvic acid, 2,3-pentanedione, 1,2-propanediol, LA esters, and polylactic acid.4 Polymer-grade LA plays a crucial role in biomedical research and applications, serving as an essential raw material for the production of eco-friendly polylactic acid plastics. The multifaceted utility of LA underscores its significance as a highly sought-after product in today's global market.5
To unlock the full potential of LCB and meet the increasing demand for LA, researchers have directed their focus towards two promising methodologies: microbial fermentation and chemical catalytic conversion. These pioneering approaches provide environmentally sustainable and economically viable routes for converting LCB into LA. Microbial fermentation harnesses the capabilities of microorganisms to provide a sustainable and biologically efficient method for LA production.6 Microorganisms excel at efficiently utilizing the various sugars found in LCB hydrolysates, including glucose and xylose.7 This makes them particularly well-suited for the conversion of LCB into LA. However, industrial microbial fermentation for LA production is still in its first generation, primarily utilizing starch as the raw material. Challenges must be overcome, including the hydrolysis process, as well as the separation and purification processes, in order to achieve cost-effective LA production from LCB (a second-generation substrate).8,9 Conversely, chemical catalytic conversion, utilizing catalysts and precise reaction conditions, offers an alternative pathway to convert LCB into LA.10 It can efficiently convert monosaccharides into LA11 and achieve direct catalytic conversion of cellulose and hemicellulose into LA,12,13 significantly simplifying the LCB pretreatment process. Nevertheless, the industrial application of chemical catalytic conversion for LCB-based LA production is still in its early stages, and there's a need for improvement in the selectivity and yield of catalytic conversion for complex substrates.14
Bibliometric analysis stands as a potent instrument, offering valuable insights into the trajectory and progression of research across diverse scientific domains.15–17 This paper employs bibliometric analysis to provide a comprehensive overview of current research on LA production from LCB. We focus on publication characteristics, keywords, and co-citation to uncover research trends and current standing in this field. Our objective is to offer insights into the present research landscape and potential future directions, contributing to more efficient and sustainable LA production from LCB, addressing environmental concerns, and enabling cost-effective processing.
Methods
Data sources
Scientific output data were retrieved from the Science Citation Index Expanded (SCIE) database and the 2021 Journal Citation Reports (JCR) of Web of Science on July 7th, 2023. The 2021 JCR encompasses 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 494 journals spanning 254 scientific disciplines across 111 countries/regions. For this study, specific keywords (“cellulose*” or “lignocellulose*” or “straw” or “stalk” or “stover” or “bagasse” or “corncob”) and (“lactate” or “lactic acid”) were employed to focus on the period between 1991 and 2022. To ensure precise analysis of LA production from lignocellulose materials, articles pertaining to “bacterial cellulose production” were excluded. A total of 2847 publications concerning LA production from LCB were initially identified in the SCIE. Given that articles constituted the primary document category, the subsequent analysis focused on 2576 articles.
494 journals spanning 254 scientific disciplines across 111 countries/regions. For this study, specific keywords (“cellulose*” or “lignocellulose*” or “straw” or “stalk” or “stover” or “bagasse” or “corncob”) and (“lactate” or “lactic acid”) were employed to focus on the period between 1991 and 2022. To ensure precise analysis of LA production from lignocellulose materials, articles pertaining to “bacterial cellulose production” were excluded. A total of 2847 publications concerning LA production from LCB were initially identified in the SCIE. Given that articles constituted the primary document category, the subsequent analysis focused on 2576 articles.
Content analysis
CiteSpace, developed by Chen,18 is a visual analytic tool employed in this study to analyze trends and patterns within the scholarly literature of specific disciplines during a defined timeframe. In particular, this article utilized CiteSpace Software (version 6.2.R4 Advanced (64-bit)) to generate comprehensive knowledge maps within the LA production from LCB. These maps elucidate research overviews, trends, and focal points through constructs such as co-citation networks and citation bursts. Furthermore, Gephi (version 0.9.7) was utilized to visualize networks depicting co-authors' affiliations and co-authors' country/region associations. This visualization was facilitated using the Force Atlas2 layout.16 The reported journal impact factors were sourced from the 2021 JCR. In the context of gauging the influence of institutions or countries within a specific research domain, the h-index was employed as a commonly accepted indicator. The h-index was defined based on the total articles (TA) with at least h citations each and the remaining (TA-h) articles with a maximum of ≤h citations each.16,19Results and discussions
Publication characteristics
| Journal | TP (%) | h-Index (R) | TC/TP (R) | JIF | Country |
|---|---|---|---|---|---|
| a TP: total publication; R: the rank, out of the top 10 most productive journals; TC/TP: total citation/total publication; JIF: journal impact factor. | |||||
| Bioresource Technology | 180 (6.99) | 53 (1) | 44.78 (4) | 11.4 | Netherlands |
| Applied Microbiology and Biotechnology | 48 (1.86) | 24 (2) | 35.52 (5) | 5.0 | Germany |
| Biotechnology for Biofuels | 42 (1.63) | 22 (4) | 35.10 (6) | 6.3 | UK |
| Applied Biochemistry and Biotechnology | 41 (1.59) | 19 (6) | 25.39 (7) | 3.0 | USA |
| Green Chemistry | 37 (1.44) | 23 (3) | 75.24 (1) | 9.8 | UK |
| Industrial Crops and Products | 35 (1.36) | 16 (8) | 22.89 (8) | 5.9 | Netherlands |
| Animal Feed Science and Technology | 33 (1.28) | 16 (8) | 21.12 (9) | 3.2 | Netherlands |
| Applied and Environmental Microbiology | 33 (1.28) | 21 (5) | 47.52 (3) | 4.4 | USA |
| Frontiers in Microbiology | 31 (1.20) | 13 (10) | 15.71 (10) | 5.2 | Switzerland |
| Carbohydrate Polymers | 29 (1.13) | 19 (6) | 49.52 (2) | 11.2 | UK |
Bioresource Technology stands out as the foremost journal, having published 180 (6.99%) articles. It is followed by Applied Microbiology and Biotechnology with 48 (1.86%) articles, Biotechnology for Biofuels with 42 (1.63%) and Applied Biochemistry and Biotechnology with 41 (1.59%). Through a comparative analysis of h-index, citations per article, and journal impact factor, intriguing patterns and phenomena can be unveiled. In an intriguing twist, Green Chemistry and Applied and Environmental Microbiology, though ranked fifth and eighth in terms of total publications, clinch the third and fifth spots in h-index, boasting 23 and 21 respectively. Moreover, they secure the top and third positions in citations per article values, standing at 75.24 and 47.52 correspondingly. This phenomenon underscores that Green Chemistry and Applied and Environmental Microbiology hold undisputed status as the most impactful and widely recognized journals for LA production from LCB. In a similar vein, Carbohydrate Polymers, securing the tenth rank with a modest 29 articles, manages to attain a relatively high h-index of 19 (6), an impressive citations per article value of 49.52 (2), and a substantial journal impact factor of 11.2. This outcome illuminates that while Carbohydrate Polymers may encompass a smaller quantity of LA production from LCB articles, these articles command considerable attention and acclaim.
| Institution | TP (%) | YFO | BC | CI | h-Index (R) | TC/TP (R) |
|---|---|---|---|---|---|---|
| a TP: total publication; YFO: year of first occurrence; BC: betweenness centrality; CI: collaborated institution number; R: the rank, out of the top 10 most productive institutions; TC/TP: total citation/total publication. | ||||||
| Chinese Acad. Sci., China | 92 (3.65) | 1998 | 0.27 | 108 | 31 (1) | 33.98 (6) |
| Nanjing Agr. Univ., China | 54 (2.14) | 2007 | 0.04 | 34 | 21 (2) | 24.37 (7) |
| China Agr. Univ., China | 47 (1.87) | 2006 | 0.06 | 54 | 20 (3) | 19.98 (9) |
| Beijing Forestry Univ., China | 38 (1.51) | 2011 | 0.02 | 30 | 18 (4) | 38.58 (2) |
| Nanjing Forestry Univ., China | 32 (1.27) | 2012 | 0.02 | 25 | 14 (7) | 20.09 (8) |
| Univ. Chinese Acad. Sci., China | 28 (1.11) | 2010 | 0.02 | 40 | 14 (7) | 35.32 (5) |
| Tech. Univ. Denmark, Denmark | 27 (1.07) | 1995 | 0.07 | 36 | 17 (5) | 41.78 (1) |
| East China Univ. Sci. & Technol., China | 27 (1.07) | 2013 | 0.03 | 20 | 11 (10) | 12.93 (10) |
| Univ. Georgia, USA | 25 (0.99) | 1997 | 0.01 | 18 | 14 (7) | 36.00 (3) |
| Univ. Vigo, Spain | 24 (0.95) | 1999 | 0.01 | 20 | 17 (5) | 35.58 (4) |
| Country | TP (%) | YFO | BC | CC | h-Index (R) | TC/TP (R) |
|---|---|---|---|---|---|---|
| a TP: total publication; YFO: year of first occurrence; BC: betweenness centrality; CC: collaborated country number; R: the rank, out of the top 10 most productive countries; TC/TP: total citation/total publication. | ||||||
| China | 736 (29.21) | 1997 | 0.30 | 42 | 62 (2) | 24.15 (7) |
| USA | 370 (14.68) | 1991 | 0.20 | 41 | 65 (1) | 47.5 (1) |
| Japan | 197 (7.82) | 1991 | 0.10 | 22 | 44 (3) | 29.24 (5) |
| India | 152 (6.03) | 1992 | 0.03 | 26 | 30 (6) | 21.33 (9) |
| Germany | 132 (5.24) | 1991 | 0.29 | 41 | 38 (4) | 38.71 (2) |
| Spain | 130 (5.16) | 1996 | 0.12 | 28 | 34 (5) | 31.82 (4) |
| South Korea | 102 (4.05) | 1997 | 0.01 | 16 | 28 (7) | 24.98 (6) |
| Brazil | 93 (3.69) | 1994 | 0.12 | 21 | 26 (9) | 21.6 (8) |
| Italy | 73 (2.90) | 1996 | 0.13 | 31 | 22 (10) | 19.62 (10) |
| UK | 72 (2.86) | 1991 | 0.10 | 32 | 28 (7) | 37.78 (3) |
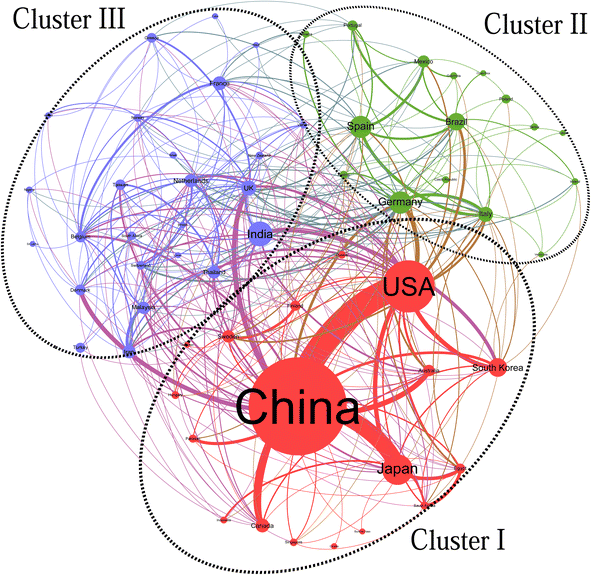
Fig. 2 illustrates three distinct clusters that vary in terms of countries/regions and publication volumes. Cluster I stands out as the largest and most intricate group, encompassing 18 countries/regions, with China, the USA, Japan, and South Korea emerging as the most prolific contributors. In Cluster II, which comprises 16 countries/regions, Germany, Spain, and Brazil serve as the central nodes. Meanwhile, Cluster III consists of 25 countries/regions, predominantly from Europe and Asia, where France, the Netherlands, the UK, and India hold central positions. In terms of collaborative efforts, the China-USA partnership claims the top spot with 58 cooperative publications. Following closely are China–Japan (38), China–Canada (14), and China–Australia (12) collaborations.
Research tendencies and hotspots
| Keywords | TP | YFO | 91–22 R (%) | 91–98 R (%) | 99–06 R (%) | 07–14 R (%) | 15–22 R (%) |
|---|---|---|---|---|---|---|---|
| a TP: total publication; YFO: year of first occurrence; R: the rank; NA: not appear. | |||||||
| Lactic acid | 316 | 1995 | 1 (4.67) | 1 (3.56) | 1 (4.02) | 1 (5.21) | 1 (4.63) |
| Lactic acid bacteria | 77 | 1998 | 2 (1.14) | 11 (0.40) | 2 (1.28) | 4 (0.99) | 4 (1.21) |
| Polylactic acid | 75 | 2007 | 3 (1.11) | NA | NA | 2 (1.41) | 4 (1.21) |
| Lignocellulosic biomass | 67 | 2004 | 4 (0.99) | NA | 47 (0.18) | 10 (0.63) | 3 (1.25) |
| Enzymatic hydrolysis | 62 | 1999 | 5 (0.92) | NA | 7 (0.55) | 3 (1.13) | 6 (0.94) |
| Deep eutectic solvents | 61 | 2017 | 6 (0.90) | NA | NA | NA | 2 (1.34) |
| Corn stover | 45 | 1997 | 7 (0.66) | 11 (0.40) | 15 (0.37) | 7 (0.70) | 7 (0.70) |
| Bacillus coagulans | 41 | 2007 | 8 (0.61) | NA | NA | 5 (0.78) | 8 (0.66) |
| Rice straw | 41 | 2001 | 8 (0.61) | NA | 15 (0.37) | 7 (0.70) | 10 (0.64) |
| Metabolic engineering | 39 | 2010 | 10 (0.58) | NA | NA | 10 (0.63) | 8 (0.66) |
| L-Lactic acid | 36 | 2003 | 11 (0.53) | NA | 15 (0.37) | 10 (0.63) | 11 (0.55) |
| Saccharomyces cerevisiae | 36 | 2000 | 11 (0.53) | NA | 4 (0.73) | 5 (0.78) | 14 (0.46) |
| Simultaneous saccharification and fermentation | 35 | 1997 | 13 (0.52) | 11 (0.40) | 2 (1.28) | 10 (0.63) | 17 (0.40) |
| Mechanical properties | 30 | 1997 | 14 (0.44) | 11 (0.40) | 7 (0.55) | 14 (0.56) | 17 (0.40) |
| Fermentation quality | 27 | 1999 | 15 (0.40) | NA | 47 (0.18) | 39 (0.21) | 13 (0.48) |
| Lactic acid fermentation | 27 | 1992 | 15 (0.40) | 5 (0.79) | 47 (0.18) | 15 (0.49) | 17 (0.40) |
| Microbial community | 27 | 2007 | 15 (0.40) | NA | NA | 24 (0.28) | 12 (0.51) |
| Sugarcane bagasse | 26 | 2005 | 18 (0.38) | NA | 15 (0.37) | 24 (0.28) | 16 (0.44) |
| D-Lactic acid | 25 | 2006 | 19 (0.37) | NA | 47 (0.18) | 39 (0.21) | 14 (0.46) |
| Lactate dehydrogenase | 23 | 1991 | 20 (0.34) | 2 (2.77) | 4 (0.73) | 39 (0.21) | 31 (0.20) |
| Acetic acid | 22 | 2002 | 21 (0.32) | NA | 15 (0.37) | 7 (0.70) | 29 (0.22) |
| Lactobacillus plantarum | 22 | 2000 | 21 (0.32) | NA | 15 (0.37) | 65 (0.14) | 17 (0.40) |
| Wheat straw | 21 | 2002 | 23 (0.31) | NA | 15 (0.37) | 24 (0.28) | 21 (0.33) |
| Escherichia coli | 19 | 1998 | 24 (0.28) | 11 (0.40) | 15 (0.37) | 20 (0.35) | 25 (0.24) |
| Consolidated bioprocessing | 18 | 2010 | 25 (0.27) | NA | NA | 15 (0.49) | 25 (0.24) |
| Anaerobic digestion | 17 | 1995 | 26 (0.25) | 11 (0.40) | NA | 24 (0.28) | 22 (0.26) |
| Clostridium thermocellum | 16 | 1994 | 27 (0.24) | 11 (0.40) | NA | 17 (0.42) | 31 (0.20) |
| Rhizopus oryzae | 16 | 1999 | 27 (0.24) | NA | 15 (0.37) | 17 (0.42) | 39 (0.18) |
| Sugar beet pulp | 16 | 2001 | 27 (0.24) | NA | 47 (0.18) | 17 (0.42) | 31 (0.20) |
| Cellulose acetate | 15 | 1995 | 30 (0.22) | 11 (0.40) | 15 (0.37) | 65 (0.14) | 29 (0.22) |
In this paper, the data was collected by including “lactate” and “lactic acid” as essential components of the search phrase. This approach resulted in notable frequencies for terms such as “lactic acid”, “lactic acid bacteria”, “polylactic acid”, “L-lactic acid”, “lactic acid fermentation”, and “D-lactic acid”. The term “lactic acid” consistently emerges as the most frequently used keyword across all periods, boasting a frequency of 316 and a relative occurrence ranging from 3.56% to 5.21%. Ranked second among author keywords, “lactic acid bacteria” ascended from the 11th position in the years 1991–1998 to claim second place during 1999–2006, maintaining its fourth-place ranking ever since. “Polylactic acid”, holding the third position among author keywords, represents a thermoplastic polyester formed through the condensation of LA with water release. Remarkably, this keyword was absent before 2007. However, its ranking has substantially risen, securing second and fourth positions during the periods 2007–2014 and 2015–2022, respectively. As for “L-lactic acid” and “D-lactic acid”, the two isomers of LA, they did not feature before 1999. Yet, their rankings quickly ascended to 11th and 14th places, respectively, during the period 2015–2022. This trend underscores the growing research focus on the production of optically pure L- or D-LA from LCB. Additionally, “lactic acid fermentation”, ranking 15th among author keywords, made its debut in 1992 and has consistently maintained high-frequency usage. This underscores the extensive research concentration on microbial fermentation of LCB into LA.
Through the inclusion of crucial elements such as “cellulose*”, “lignocellulose*”, “straw”, “stalk”, “stover”, “bagasse”, and “corncob” in the search phrase, the focus of the analysis was achieved. Notably, author keywords like “lignocellulosic biomass”, “corn stover”, “rice straw”, “sugarcane bagasse”, “wheat straw”, and “sugar beet pulp” have garnered substantial attention in recent years. Both “lignocellulosic biomass” and “corn stover”, ranked fourth and seventh among author keywords, respectively, did not make appearances between 1991 and 1998. Nevertheless, their frequency and ranking have consistently increased in subsequent periods. The terms “rice straw”, “sugarcane bagasse”, “wheat straw”, and “sugar beet pulp” exhibit a similar pattern, with their rankings displaying certain fluctuations over the four stages. The pretreatment of LCB, a bottleneck in LA production, has garnered substantial attention from researchers. Notably, representative methods like enzymatic hydrolysis and deep eutectic solvents (DESs) have emerged as key focal points, ranking fifth and sixth, respectively. Enzymatic hydrolysis of LCB refers to the process of liberating monomeric sugars from the structural carbohydrates cellulose and hemicellulose.25 DESs, on the other hand, constitute systems formed by a eutectic mixture of Lewis or Brønsted acids and bases, which can encompass a range of anionic and/or cationic species.26 DESs made their debut in 2017, subsequently experiencing an explosive growth in related articles. This surge indicates the extensive research interest in utilizing DESs for the pretreatment of LCB.
Microbial fermentation, serving as the primary pathway for LA production from LCB, has garnered significant attention, with related author keywords attracting substantial focus. Notably, “Bacillus coagulans”, which has been reclassified as Weizmannia coagulans,27 emerged in 2007 and subsequently experienced explosive growth in related articles, securing the eighth position between 1991 and 2022. W. coagulans, a LA-forming bacterial species with a history spanning over a century since its first report, has found extensive application in the production of L-LA.28 Furthermore, author keywords like “metabolic engineering”, “Saccharomyces cerevisiae”, and “Escherichia coli” hold the 10th, 11th, and 24th rankings, respectively. Of notable significance, extensive research has been undertaken on the production of LA from LCB through metabolically engineered S. cerevisiae29,30 and E. coli.31–33 Additionally, LA often emerges as a byproduct in systems where S. cerevisiae produces ethanol from LCB.34
Ranked 21st among author keywords, “Lactobacillus plantarum” has found wide application in the production of silage through the fermentation of LCB such as corn stalk and rice straw.35,36 Additionally, metabolically engineered L. plantarum exhibits the ability to utilize xylose, achieving efficient D-LA production from LCB.37 Since its initial appearance in 1999, “fermentation quality” has consistently attracted attention, signifying a considerable body of research focused on enhancing the fermentation quality of silage feed.38 At the 27th rank among author keywords, “Clostridium thermocellum” displays the capability to degrade lignocellulosic materials to generate hydrogen, lactate, and ethanol. Moreover, it enhances the enzymatic hydrolysis of cellulosic substrates, playing a pivotal role in the consolidated bioprocessing of lignocellulose into lactate and ethanol.39,40 “Rhizopus oryzae”, first appearing in 1999, demonstrates the ability to secrete cellulase and hemicellulase, utilizing glucose, xylose, and sucrose for the production of L-LA.41 These attributes position R. oryzae as a potential candidate for generating L-LA from LCB. The fifteenth-ranked author keyword, “microbial community”, has garnered considerable attention in recent decades. This trend underscores the extensive research focus on mixed culture systems for LA or silage production through the fermentation of LCB.42,43
Ranked 13th among author keywords, “simultaneous saccharification and fermentation (SSF)” stands out as distinct from separate hydrolysis and fermentation (SHF), as it combines saccharification and fermentation processes at the same location. The SSF system offers a shorter time period and reduced feedback inhibition compared to SHF.44 Presently, this mode constitutes the primary fermentation approach for producing LA from LCB. “Anaerobic digestion”, which first appeared in 1995, disappeared during the subsequent period of 1999–2006. However, it experienced a resurgence since 2007, quickly becoming a research hotspot. Notably, LA fermentation strains are typically either obligate anaerobes or facultative anaerobes. Analyzing the author keywords provides an overview of the research on LA production from LCB.
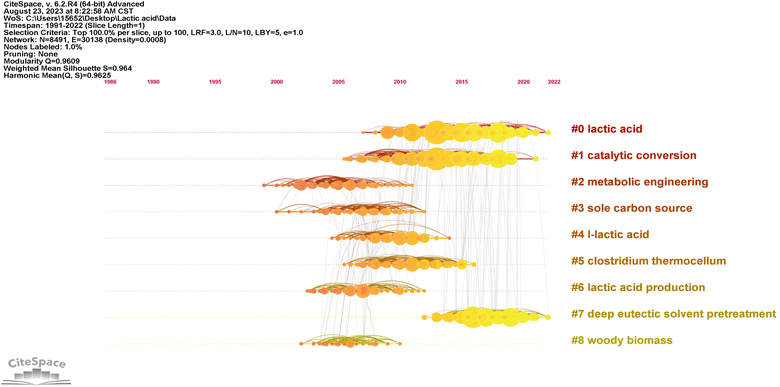 | ||
| Fig. 3 Timeline-based reference network visualization of lactic acid production from lignocellulosic biomass (lighter colors indicate closer time, darker colors indicate distant time). | ||
| Cluster ID | Size | Silhouette | Mean (year) | Latent semantic indexing (LSI) |
|---|---|---|---|---|
| 0 | 374 | 0.933 | 2015 | Lactic acid; lactic acid production; Bacillus coagulans; lignocellulosic biomass; L-lactic acid production |
| 1 | 330 | 0.902 | 2012 | Lactic acid; catalytic conversion; direct conversion; organic acid; levulinic acid |
| 2 | 217 | 0.959 | 2005 | Metabolic engineering; thermophilic bacterium; high yield; ethanologenic bacteria; engineering biocatalyst |
| 3 | 183 | 0.931 | 2007 | Sole carbon source; other organic nutrient; recombinant cellulolytic Bacillus subtilis; one-step production; ethanol production |
| 4 | 182 | 0.918 | 2009 | L-Lactic acid; efficient production; Bacillus coagulans; lactic acid; biomass-derived xylose |
| 5 | 154 | 0.983 | 2010 | Clostridium thermocellum; hydrogen production; major catabolic pathway; linking genome content; production yield |
| 6 | 150 | 0.956 | 2007 | Lactic acid production; lactic acid; new trend; renewable biomass; recycled paper sludge |
| 7 | 148 | 0.986 | 2017 | Deep eutectic solvent pretreatment; wheat straw; acidic deep eutectic solvent pretreatment; bioethanol production; enhanced enzymatic saccharification |
| 8 | 146 | 0.973 | 2006 | Woody biomass; sweet sorghum stalk; fuel ethanol production; cellulosic material; yeast fermentation |
The research trend identified concerns substrate pretreatment and degradation consisting of four clusters with the average publication timeframe spanning from 2006 to 2017. The clusters were characterized by their silhouette score, size, and mean year of publication. Cluster #3, designated as “sole carbon source” (silhouette score = 0.931; 183; 2007), highlights enhancing LA fermentation via efficient LCB pretreatment. Bacillus strains show promise for LA production. Recombinant cellulolytic B. subtilis integrates cellulose hydrolysis and fermentation, simplifying the process for potential ethanol production and comprehensive biorefinery approaches.49 Cluster #6, identified as “lactic acid production” (silhouette score = 0.956; 150; 2007), reflects a modern approach to LA production. It emphasizes efficient pretreatment and degradation of LCB, particularly recycled paper sludge, to enhance sustainable LA fermentation from various sources, promoting eco-friendly and resource-efficient processes.50,51 Cluster #8, denoted as “woody biomass” (silhouette score = 0.973; 146; 2006), signifies the focus on woody biomass pretreatment for LA production, employing techniques such as hot water, sulfuric acid, enzymatic degradation, and ammonia recycle percolation.52–54 Cluster #7, labeled as “deep eutectic solvent pretreatment” (silhouette score = 0.986; 148; 2017), the most recent cluster for substrate pretreatment. Typically, DES is created by blending quaternary ammonium halide salts with a neutral organic hydrogen bond donor, resulting in a complex of halide ions and solvent molecules.55 Prior research has demonstrated the effective pretreatment capabilities of DES on lignocellulosic materials. It facilitates the efficient separation of cellulose and lignin,56,57 establishing a basis for subsequent enzymatic cellulose hydrolysis to yield sugars and microbial fermentation for LA production.22,58
The microbial selection and engineering can be classified into two distinct clusters. Cluster #2, named “metabolic engineering” (silhouette score = 0.959; 217; 2005), signifies the substantial research conducted on producing LA from LCB using genetically modified strains. The isolation of acid-tolerant, thermophilic strains such as B. coagulans,59 and the application of metabolic engineering to modify strains such as S. cerevisiae60 and L. delbrueckii,61 have both proven effective in facilitating microbial strains to utilize LCB for LA production. Cluster #5, designated “Clostridium thermocellum” (silhouette score = 0.983; 154; 2010), has gained prominence for its remarkable capacity to efficiently break down LCB. Researchers have conducted extensive studies on the metabolic pathways of C. thermocellum,62 employing genetic engineering to enhance LA and ethanol production. This approach has proven highly efficient in the production of LA and ethanol from LCB.63,64
The research on the fermentation process optimization consists of two clusters, with the average publication years of 2009 and 2015, respectively. Cluster #4, referred to as “L-lactic acid” (silhouette score = 0.918; 182; 2009), highlights the importance of refining the fermentation process for the efficient production of L-LA from LCB. By optimizing fermentation parameters, such as pH control and fermentation mode, and employing strains like B. coagulans and Lactobacillus sp., a significant enhancement is observed in the conversion of pentose and hexose obtained from LCB into high-purity L-LA.65–67 Cluster #0, labeled as “lactic acid” (silhouette score = 0.933; 374; 2015), represents the largest cluster in the production of LA from LCB. The prior research achievements, including the adoption of SSF,68 detoxification of lignocellulosic hydrolysates,69 and the production of LA under non-sterile conditions,70 have collectively facilitated the economically viable and efficient fermentation of LA from LCB.
The identified research trend focuses on chemical catalytic conversion, represented by a cluster with an average publication year of 2012. Cluster #1, denoted as “catalytic conversion” (silhouette score = 0.902; 330; 2012), the second-largest cluster with 330 publications, underscores the increasing attention towards the direct catalytic conversion of LCB to LA and its derivatives, signifying a relatively recent area of significant interest. Chemical catalysis can be broadly categorized into photochemical catalysis,71 biocatalysis,72 acid–base catalysis, metal catalysis,73 and more. It typically involves acid–base or metal catalysis to transform LCB into LA under specific temperature, pressure, and atmospheric conditions.74 Importantly, the transition of LCB into LA encompasses sequential stages such as LCB pretreatment, cellulose and hemicellulose hydrolysis, and catalytic conversion of monosaccharides into LA (Fig. 4).74–76 The conversion of glucose and xylose, the primary monosaccharides in LCB hydrolysates, into LA entails a series of catalytic processes. Glucose's catalytic conversion involves its isomerization into fructose, retro-aldol condensation of fructose to produce dihydroxyacetone, followed by dehydration, hydration, and isomerization, resulting in LA production.3,76 Conversely, xylose's catalytic conversion includes retro-aldol condensation, yielding dihydroxyacetone. Dihydroxyacetone then undergoes tautomeric isomerization and dehydration to produce 2-hydroxypropenal, followed by keto–enol tautomeric isomerization, hydration, and isomerization, ultimately resulting in LA production.75
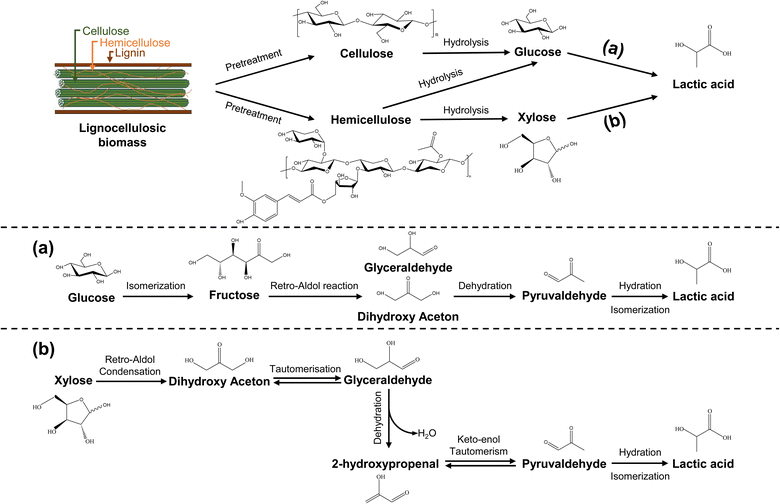 | ||
| Fig. 4 Proposed reaction mechanisms for the conversion of lignocellulosic biomass to lactic acid, including (a) the transition of glucose to lactic acid and (b) the transition of xylose to lactic acid (adapted from ref. 75 and 76). | ||
The inception of this field initially focused on achieving the homogeneous catalytic conversion of triose sugars into LA derivatives.77 Pioneering research in this domain established the foundation for the direct catalytic synthesis of LA from LCB.78,79 Numerous metal cations, including Pb(II), Al(III), Bi(III), In(III), Zn(II), Sn(II), Co(II), Ni(II), Fe(II), and Mn(II), have been recognized for their catalytic roles in converting cellulose into LA. Notably, Pb(II) demonstrated an exceptional LA yield of 68% under anaerobic conditions (N2 atmosphere of 3 MPa, temperature: 463 K, time: 4 hours).12 In a similar vein, Tang et al. (2014) reported the efficacy of homogeneous vanadyl cations as catalysts for transforming ball-milled cellulose into LA, achieving a yield of 54% under anaerobic conditions (N2 atmosphere of 2 MPa, temperature: 453 K, time: 2 hours).80 However, aerobic conditions led to the formation of formic acid instead of LA. Recent research underscores the effectiveness of dual-metal cations like Al(III)–Sn(II), Al(III)–In(III), Al(III)–Mn(II), Al(III)–Cu(II), and Al(III)–Ni(II) over single metal cations for cellulose-to-LA conversions. Among them, Al(III)–Sn(II) (ratio = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) displayed optimal performance, yielding 65% LA from ball-milled cellulose under anaerobic conditions (N2 atmosphere of 3 MPa, temperature: 463 K, time: 2 hours).3 Additionally, the direct separation of LA from synthetic solutions derived from LCB has become a noteworthy subject of investigation.81
1) displayed optimal performance, yielding 65% LA from ball-milled cellulose under anaerobic conditions (N2 atmosphere of 3 MPa, temperature: 463 K, time: 2 hours).3 Additionally, the direct separation of LA from synthetic solutions derived from LCB has become a noteworthy subject of investigation.81
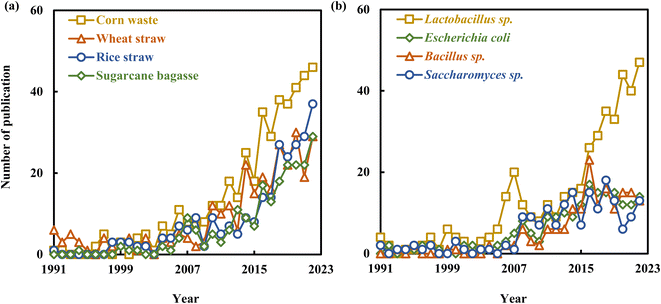 | ||
| Fig. 5 Growth trends of hotpot-related articles of lactic acid production from lignocellulosic biomass: (a) raw materials, (b) microorganisms from 1991 to 2022. | ||
Corn waste, including corn stalks and cobs, has been the subject of growing interest in recent years, with a total of 437 articles published on the topic. Liu et al. (2015) achieved a remarkable L-LA yield of 0.715 g per g-cellulose by employing engineered Pediococcus acidilactici with detoxified corn stalk as the substrate.84 Corn stalk hemicellulose derivatives were processed to produce LA, achieving a high yield of 0.796 g per g-hemicellulose with 90% selectivity through MgO catalysis.13 The number of related articles on wheat straw increased from 6 in 1991 to 29 in 2022, with a total of 296 articles published on the topic. A recently discovered strain, B. coagulans IPE22, has demonstrated impressive capabilities in fermenting pentose, hexose, and cellobiose, achieving a LA yield of 0.461 g-LA per g-dry wheat straw.69 Research related to rice straw has seen a continuous increase in recent years, with the number of relevant articles reaching 37 in 2022. Kuo et al. (2015) documented the development of an innovative engineered strain, L. paracasei 7BL, characterized by high inhibitor tolerance and the production of optically pure L-LA. This strain achieved an impressive productivity rate of 5.27 g L−1 h−1 when utilizing detoxified rice straw hydrolysate as its substrate.85 These studies collectively highlight the extensive utilization of LCB in both fermentation and chemical catalysis for LA production.
Bacteria that produce LA from LCB primarily fall into three categories: lactic acid bacteria (LAB, mainly Lactobacillus sp.), Bacillus strains (mainly B. coagulans), and E. coli. The number of articles related to Lactobacillus sp. has seen a significant increase, rising from 4 in 1991 to 47 in 2022, with a total of 425 articles published on the topic. Lactobacillus sp. can be classified into either homofermentative or heterofermentative types based on the different end products of fermentation. L. plantarum is a homofermentative bacterium that primarily produces LA through the pentose phosphate pathway. However, like many strains within the Lactobacillus genus, it produces racemic mixture of LA, containing both the L- and D-LA enantiomers.37,86 The engineered L. plantarum (with a deficient L-lactate dehydrogenase gene or expressing L-lactate oxidase gene) has been extensively utilized for the production of D-LA, exhibiting excellent utilization capabilities for pentoses and hexoses, as well as high D-LA yields.37,87,88 Research related to Bacillus strains did not appear until 1993, and it reached a maximum of 23 articles per year, accumulating a total of 167 articles on the topic. B. coagulans has the capability to grow and ferment both hexoses and pentoses present in LCB, yielding high-purity L-LA under non-sterilized conditions. These characteristics have continually enabled B. coagulans to achieve new breakthroughs in utilizing LCB for L-LA production.68,89 E. coli, owing to its rapid metabolism of hexoses and pentoses, coupled with its simple nutritional requirements, engineered E. coli demonstrates significant potential for efficient LA production from LCB.31 Nevertheless, efforts are needed to enhance its LA productivity and acid tolerance.90
Wild-type yeasts typically produce minimal LA as a primary fermentation product. However, because of their robust acid resistance and the simplicity of their cultivation medium, significant efforts have been invested in engineering yeasts to enhance LA production.5 Novy et al. (2017) reported the utilization of S. cerevisiae IBB14LA1_5 for L-LA production, achieving impressive LA yields of 0.67 g per g-glucose and 0.80 g per g-xylose. This study demonstrates that the engineered strain holds significant promise for L-LA production from LCB.91 Sornlek et al. (2022) conducted genetic engineering on S. cerevisiae, which involved the integration of the D-lactate dehydrogenase gene from Leuconostoc mesenteroides, the deletion of gpd1, gpd2, and adh1 genes to reduce glycerol and ethanol production, and hybridization with the weak acid-tolerant S. cerevisiae BCC39850 strain. The engineered strain, when subjected to SSF using alkaline-pretreated sugarcane bagasse, achieved an impressive D-LA yield of 0.33 g per g-glucan.30 This outcome underscores its potential for the production of industrially valuable products. The genetic-engineering approaches have been exploited in a big way for the improvement of LA yield and optical purity by various microbial producers. Furthermore, mixed culture systems, which harness consortia of microorganisms for fermentation, present a promising alternative to monocultures for intricate bio-transformations. They offer inherent advantages, including the distribution of metabolic burdens through division of labor, improved efficiency in converting complex substrates, and modularity.27,92 The co-culture of B. coagulans and L. rhamnosus for LA production from cassava bagasse resulted in significantly improved LA concentration, productivity, and yield, reaching 112.5 g L−1, 2.74 g L−1 h−1, and 0.88 g g−1, respectively, surpassing the outcomes of mono-culturing each bacterium.93 Collectively, previous studies indicate that genetic engineering approaches and mixed culture systems play a significant role in enhancing both the productivity and yield of LA.
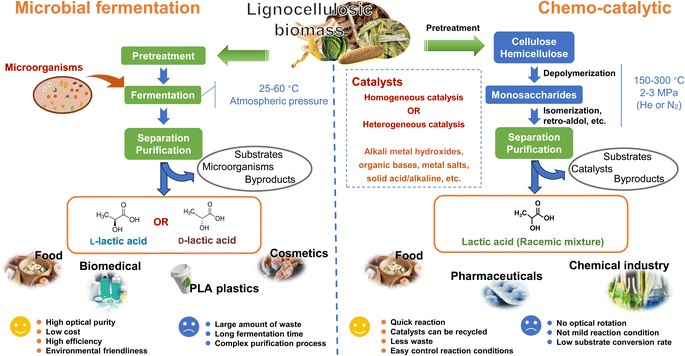 | ||
| Fig. 6 Comparative lactic acid production from lignocellulosic biomass: microbial fermentation vs. chemical catalytic conversion. | ||
Microbial fermentation generates a substantial amount of waste, including waste fermentation broth and gypsum waste.95 The formation of gypsum is primarily a result of neutralization processes. The purification of LA from the fermentation broth can be quite intricate, as the LA fermentation process often yields by-products like acetic acid and formic acid.90 Moreover, microbial fermentation tends to be time-consuming, with the fermentation process typically spanning from hours to days. In contrast, chemical catalytic conversion can overcome these problems. Chemical catalytic conversion generally takes only several hours to complete, generates fewer wastes, and allows for easy recycling of catalysts from the system.96 However, chemical catalysis has a low substrate conversion rate, and the process typically requires harsh conditions, often conducted at temperatures between 150–300 °C and pressures of 2–3 MPa.97 A recent report indicated that, under a pressure of 0.1 MPa at room temperature, glucose can be selectively converted to LA with a yield of 95.4%. However, this process takes a longer time, spanning 48 hours.98 Thus, the development of novel catalysts and the exploration of new catalytic systems would contribute to enhancing the efficiency of utilizing LCB resources in the catalytic production of LA.
Optically pure LA has been widely utilized in the production of polylactic acid, which is recognized as a crucial raw material for biomedical applications and the manufacturing of polylactic acid plastics. This application carries significant economic added value.74 The production of enantiomerically pure L-LA or D-LA depends on the microbial strains used in the fermentation process. Microbial fermentation possesses the capability to achieve high optical purity in LA production, a feat not attainable through chemical catalysis. This is a key factor in why microbial fermentation is widely utilized for LA production, despite its various limitations. Chemical catalysis can only produce a racemic mixture of L- and D-LA.83 To address this challenge, numerous studies have focused on utilizing membrane technology,99,100 porous ceramic discs,101 and high-performance chromatography102 to achieve enantiomeric resolution. However, most of these studies are still in the research stage, and the separation costs are relatively high. There is still a considerable gap to bridge before these methods can be applied on a large scale.
Based on our research findings, we have identified several promising future research directions in the field of microbial fermentation, which encompass the following key points: research is expected to continue focusing on the development and enhancement of microbial strains to improve LA production efficiency. Genetic engineering will play a pivotal role in optimizing metabolic pathways and fermentative performance. Another avenue of research involves enhancing the tolerance of microbial strains to inhibitors commonly present in LCB hydrolysates, thereby reducing the need for detoxification procedures. An emerging field of study centers on mixed microbial cultures, aiming to optimize the synergistic interactions among various microorganisms, ultimately enhancing LA production efficiency. Shifting to the realm of chemical catalytic conversion, ongoing research will emphasize the development and optimization of catalysts for the conversion of LCB into LA. This will include the exploration of novel catalyst materials and structures, as well as improvements in catalytic efficiency through catalyst modification and reaction engineering. Looking forward, there is a growing interest in integrating LA production into lignocellulosic biorefineries, aligning the production of LA with other high-value chemicals derived from biomass components like lignin. Concurrently, attention will be devoted to developing more cost-effective and environmentally friendly downstream processing methods for the separation and purification of both D- and L-LA. In conclusion, the future of LA production from LCB will require interdisciplinary collaboration across fields such as microbiology, chemistry, engineering, and environmental science. This collaborative approach will strongly emphasize sustainability, efficiency, and cost-effectiveness.
Conclusions
This paper presents a comprehensive overview of research in the field of LA production from LCB. It encompasses publication characteristics, author keywords analysis, co-citation reference analysis, and research hotspots. From 1991 to 2022, there was a significant surge in annual publications, growing from 39 to 333. Notably, Bioresource Technology and Applied Microbiology and Biotechnology emerged as the two most prolific journals in this domain. Chinese Acad. Sci., China held the top spot with the highest total publications (92), betweenness centrality value (0.27), collaborations (108), and an h-index of 31, signifying excellence in LA production from LCB. China ranks first in total publication count (736), betweenness centrality value (0.30), and the number of collaborating countries (42), reflecting its substantial influence and contributions in LA production from LCB. Author keywords analysis emphasized the pivotal role of microbial fermentation in LA production from LCB. Co-citation reference analysis delineated four key domains within LA production from LCB: substrate pretreatment and degradation, microbial selection and engineering, fermentation process optimization, and chemical catalytic conversion. Furthermore, the paper delves into commonly used LCB and microbial LA producers, highlighting the diversity of microbial and extensive studies involving various LCB sources for LA production. Moreover, the study performed a comparative analysis of microbial fermentation and chemical catalytic conversion for the production of lactic acid from LCB, elucidating their distinct strengths and weaknesses. The paper also delved into recent research endeavors aimed at addressing these challenges and underscored potential future research directions.Author contributions
All authors contributed to the conception and design of the study. Dr Min Zhang and Dr Siyuan Yue were responsible for data collection and analysis. Dr Siyuan Yue took the lead in writing the draft of the manuscript, while Dr Min Zhang reviewed and corrected it. The final manuscript was reviewed and approved by all authors.Conflicts of interest
The authors have no relevant financial or non-financial interests to disclose.Acknowledgements
Siyuan Yue acknowledges the financial support for this study from the Research and Development Special Fund Doctoral Program offered by the Jiangxi Academy of Sciences (2022YYB29).References
- Y. M. Bar-On, R. Phillips and R. Milo, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 6506–6511 CrossRef CAS PubMed.
- D. Khatiwada, P. Purohit and E. K. Ackom, Sustainability, 2019, 11, 7091 CrossRef.
- W. Deng, P. Wang, B. Wang, Y. Wang, L. Yan, Y. Li, Q. Zhang, Z. Cao and Y. Wang, Green Chem., 2018, 20, 735–744 RSC.
- P. Gallezot, Chem. Soc. Rev., 2012, 41, 1538–1558 RSC.
- M. A. Abdel-Rahman, Y. Tashiro and K. Sonomoto, Biotechnol. Adv., 2013, 31, 877–902 CrossRef CAS PubMed.
- M. A. Abdel-Rahman, Y. Tashiro and K. Sonomoto, J. Biotechnol., 2011, 156, 286–301 CrossRef CAS PubMed.
- C. Gao, C. Ma and P. Xu, Biotechnol. Adv., 2011, 29, 930–939 CrossRef CAS PubMed.
- D. Yankov, Front. Chem., 2022, 10, 823005 CrossRef CAS PubMed.
- R. A. de Oliveira, A. Komesu, C. E. Vaz Rossell, M. R. Wolf Maciel and R. Maciel Filho, Sep. Purif. Technol., 2019, 209, 26–31 CrossRef.
- J. Kim, Y.-M. Kim, V. R. Lebaka and Y.-J. Wee, Fermentation, 2022, 8, 609 CrossRef CAS.
- H. Xu, X. Ye, X. Shi, H. Zhong, D. He, B. Jin and F. Jin, Mol. Catal., 2022, 522, 112241 CrossRef CAS.
- Y. Wang, W. Deng, B. Wang, Q. Zhang, X. Wan, Z. Tang, Y. Wang, C. Zhu, Z. Cao, G. Wang and H. Wan, Nat. Commun., 2013, 4, 2141 CrossRef PubMed.
- T. He, Z. Jiang, P. Wu, J. Yi, J. Li and C. Hu, Sci. Rep., 2016, 6, 1–11 CrossRef PubMed.
- E. Nzediegwu and M. J. Dumont, Waste Biomass Valorization, 2021, 12, 2825–2851 CrossRef CAS.
- T. Zheng, J. Wang, Q. Wang, C. Nie, N. Smale, Z. Shi and X. Wang, Scientometrics, 2015, 105, 863–882 CrossRef.
- M. Zhang, M. Gao, S. Yue, T. Zheng, Z. Gao, X. Ma and Q. Wang, Environ. Sci. Pollut. Res., 2018, 25, 24600–24610 CrossRef PubMed.
- M. Zhang, C. Huang, J. Ni and S. Yue, Environ. Sci. Pollut. Res., 2023, 30, 109233–109249 CrossRef PubMed.
- C. Chen, J. Am. Soc. Inf. Sci. Technol., 2006, 57, 359–377 CrossRef.
- M. Zhang, Z. Gao, T. Zheng, Y. Ma, Q. Wang, M. Gao and X. Sun, J. Mater. Cycles Waste Manage., 2018, 20, 10–18 CrossRef.
- E. A. Bonch-Osmolovskaia, Microbiology, 1978, 47, 823–827 Search PubMed.
- L. Wang, B. Zhao, B. Liu, B. Yu, C. Ma, F. Su, D. Hua, Q. Li, Y. Ma and P. Xu, Bioresour. Technol., 2010, 101, 7908–7915 CrossRef CAS PubMed.
- X.-J. Shen, J.-L. Wen, Q.-Q. Mei, X. Chen, D. Sun, T.-Q. Yuan and R.-C. Sun, Green Chem., 2019, 21, 275–283 RSC.
- J. B. Binder and R. T. Raines, J. Am. Chem. Soc., 2009, 131, 1979–1985 CrossRef CAS PubMed.
- D. Chen, Z. Liu, Z. Luo, M. Webber and J. Chen, Ecol. Eng., 2016, 90, 285–293 CrossRef.
- J. J. Stickel, R. T. Elander, J. D. Mcmillan and R. Brunecky, in Bioprocessing of Renewable Resources to Commodity Bioproducts, John Wiley & Sons, Ltd, 2014, pp. 77–103 Search PubMed.
- E. L. Smith, A. P. Abbott and K. S. Ryder, Chem. Rev., 2014, 114, 11060–11082 CrossRef CAS PubMed.
- S. Yue, T. Mizoguchi, T. Kohara, M. Zhang, K. Watanabe, H. Miyamoto, Y. Tashiro and K. Sakai, Biotechnol. J., 2021, 16, 2100277 CrossRef CAS PubMed.
- J. Zhou, J. Ouyang, Q. Xu and Z. Zheng, Bioresour. Technol., 2016, 222, 431–438 CrossRef CAS PubMed.
- T. L. Turner, G. C. Zhang, S. R. Kim, V. Subramaniam, D. Steffen, C. D. Skory, J. Y. Jang, B. J. Yu and Y. S. Jin, Appl. Microbiol. Biotechnol., 2015, 99, 8023–8033 CrossRef CAS PubMed.
- W. Sornlek, K. Sae-Tang, A. Watcharawipas, S. Wongwisansri, S. Tanapongpipat, L. Eurwilaichtr, V. Champreda, W. Runguphan, P. J. Schaap and V. A. P. Martins dos Santos, J. Fungi, 2022, 8, 816 CrossRef CAS PubMed.
- B. S. Dien, N. N. Nichols and R. J. Bothast, J. Ind. Microbiol. Biotechnol., 2002, 29, 221–227 CrossRef CAS PubMed.
- S. Mazumdar, J. Bang and M. K. Oh, Appl. Biochem. Biotechnol., 2014, 172, 1938–1952 CrossRef CAS PubMed.
- S. Zhou, T. B. Causey, A. Hasona, K. T. Shanmugam and L. O. Ingram, Appl. Environ. Microbiol., 2003, 69, 399–407 CrossRef CAS PubMed.
- K. Stenberg, M. Galbe and G. Zacchi, Enzyme Microb. Technol., 2000, 26, 71–79 CrossRef CAS.
- L. Guo, Y. Lu, P. Li, L. Chen, W. Gou and C. Zhang, Front. Microbiol., 2021, 12, 1–9 Search PubMed.
- L. Mu, Z. Xie, L. Hu, G. Chen and Z. Zhang, Bioresour. Technol., 2020, 315, 123772 CrossRef CAS PubMed.
- Y. Zhang, P. V. Vadlani, A. Kumar, P. R. Hardwidge, R. Govind, T. Tanaka and A. Kondo, Appl. Microbiol. Biotechnol., 2016, 100, 279–288 CrossRef CAS PubMed.
- P. Li, S. Ji, Q. Wang, M. Qin, C. Hou and Y. Shen, Anim. Sci. J., 2017, 88, 625–632 CrossRef CAS PubMed.
- H. N. Lin, B. Bin Hu and M. J. Zhu, Int. J. Hydrogen Energy, 2016, 41, 2383–2390 CrossRef CAS.
- C. Xu, Y. Qin, Y. Li, Y. Ji, J. Huang, H. Song and J. Xu, Bioresour. Technol., 2010, 101, 9560–9569 CrossRef CAS PubMed.
- K. Saito, Y. Hasa and H. Abe, J. Biosci. Bioeng., 2012, 114, 166–169 CrossRef CAS PubMed.
- C. Zhao, L. Wang, G. Ma, X. Jiang, J. Yang, J. Lv and Y. Zhang, Animals, 2021, 11, 1–15 Search PubMed.
- H. Ren, C. Wang, W. Fan, B. Zhang, Z. Li and D. Li, Food Technol. Biotechnol., 2018, 56, 71–82 CAS.
- Y. Ren, X. Wang, Y. Li, Y. Y. Li and Q. Wang, Sustainability, 2022, 14, 1–16 CrossRef.
- M. Sabe, C. Chen, O. Sentissi, J. Deenik, D. Vancampfort, J. Firth, L. Smith, B. Stubbs, S. Rosenbaum, F. B. Schuch and M. Solmi, Front. Public Health, 2022, 10, 943435 CrossRef PubMed.
- C. Chen, J. Am. Soc. Inf. Sci. Technol., 2012, 64, 431–449 CrossRef.
- M. E. J. Newman, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 8577–8582 CrossRef CAS PubMed.
- P. J. Rousseeuw, J. Comput. Appl. Math., 1987, 20, 53–65 CrossRef.
- S. Romero, E. Merino, F. Bolívar, G. Gosset and A. Martinez, Appl. Environ. Microbiol., 2007, 73, 5190–5198 CrossRef CAS PubMed.
- L. Wang, B. Zhao, B. Liu, C. Yang, B. Yu, Q. Li, C. Ma, P. Xu and Y. Ma, Bioresour. Technol., 2010, 101, 7895–7901 CrossRef CAS PubMed.
- R. P. John, K. M. Nampoothiri and A. Pandey, Appl. Microbiol. Biotechnol., 2007, 74, 524–534 CrossRef CAS PubMed.
- A. J. Ragauskas, C. K. Williams, B. H. Davison, G. Britovsek, J. Cairney, C. A. Eckert, W. J. Frederick, J. P. Hallett, D. J. Leak, C. L. Liotta, J. R. Mielenz, R. Murphy, R. Templer and T. Tschaplinski, Science, 2006, 311, 484–489 CrossRef CAS PubMed.
- H. K. Tae and Y. Y. Lee, Bioresour. Technol., 2005, 96, 2007–2013 CrossRef PubMed.
- D. Su, J. Sun, P. Liu and Y. Lv, Chin. J. Chem. Eng., 2006, 14, 796–801 CrossRef CAS.
- Y. Liu, W. Chen, Q. Xia, B. Guo, Q. Wang, S. Liu, Y. Liu, J. Li and H. Yu, ChemSusChem, 2017, 10, 1692–1700 CrossRef CAS PubMed.
- C. Alvarez-Vasco, R. Ma, M. Quintero, M. Guo, S. Geleynse, K. K. Ramasamy, M. Wolcott and X. Zhang, Green Chem., 2016, 18, 5133–5141 RSC.
- Y. T. Tan, G. C. Ngoh and A. S. M. Chua, Bioresour. Technol., 2019, 281, 359–366 CrossRef CAS PubMed.
- C.-W. Zhang, S.-Q. Xia and P.-S. Ma, Bioresour. Technol., 2016, 219, 1–5 CrossRef CAS PubMed.
- M. A. Patel, M. S. Ou, R. Harbrucker, H. C. Aldrich, M. L. Buszko, L. O. Ingram and K. T. Shanmugam, Appl. Environ. Microbiol., 2006, 72, 3228–3235 CrossRef CAS PubMed.
- S. Saitoh, N. Ishida, T. Onishi, K. Tokuhiro, E. Nagamori, K. Kitamoto and H. Takahashi, Appl. Environ. Microbiol., 2005, 71, 2789–2792 CrossRef CAS PubMed.
- M. Adsul, J. Khire, K. Bastawde and D. Gokhale, Appl. Environ. Microbiol., 2007, 73, 5055–5057 CrossRef CAS PubMed.
- L. D. Ellis, E. K. Holwerda, D. Hogsett, S. Rogers, X. Shao, T. Tschaplinski, P. Thorne and L. R. Lynd, Bioresour. Technol., 2012, 103, 293–299 CrossRef CAS PubMed.
- S. A. Tripathi, D. G. Olson, D. A. Argyros, B. B. Miller, T. F. Barrett, D. M. Murphy, J. D. McCool, A. K. Warner, V. B. Rajgarhia, L. R. Lynd, D. A. Hogsett and N. C. Caiazza, Appl. Environ. Microbiol., 2010, 76, 6591–6599 CrossRef CAS PubMed.
- A. J. Shaw, K. K. Podkaminer, S. G. Desai, J. S. Bardsley, S. R. Rogers, P. G. Thorne, D. A. Hogsett and L. R. Lynd, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 13769–13774 CrossRef CAS PubMed.
- A. Romaní, R. Yáñez, G. Garrote and J. L. Alonso, Bioresour. Technol., 2008, 99, 4247–4254 CrossRef PubMed.
- R. H. W. Maas, R. R. Bakker, M. L. A. Jansen, D. Visser, E. De Jong, G. Eggink and R. A. Weusthuis, Appl. Microbiol. Biotechnol., 2008, 78, 751–758 CrossRef CAS PubMed.
- S. L. Walton, K. M. Bischoff, A. R. P. Van Heiningen and G. P. Van Walsum, J. Ind. Microbiol. Biotechnol., 2010, 37, 823–830 CrossRef CAS PubMed.
- J. Hu, Z. Zhang, Y. Lin, S. Zhao, Y. Mei, Y. Liang and N. Peng, Bioresour. Technol., 2015, 182, 251–257 CrossRef CAS PubMed.
- Y. Zhang, X. Chen, J. Luo, B. Qi and Y. Wan, Bioresour. Technol., 2014, 158, 396–399 CrossRef CAS PubMed.
- J. Ouyang, R. Ma, Z. Zheng, C. Cai, M. Zhang and T. Jiang, Bioresour. Technol., 2013, 135, 475–480 CrossRef CAS PubMed.
- R. Cui, J. Ma, K. Liu, Z. Ali, J. Zhang, Z. Liu, X. Li, S. Yao and R. Sun, Mol. Catal., 2022, 531, 112653 CrossRef CAS.
- A. Staudt, Y. Brack, I. I. Jr and I. C. R. Leal, Mol. Catal., 2022, 528, 112464 CrossRef CAS.
- X. Peng, L. Chen and Y. Li, Mol. Catal., 2022, 529, 112568 CrossRef CAS.
- A. Bayu, A. Abudula and G. Guan, Fuel Process. Technol., 2019, 196, 106162 CrossRef CAS.
- L. Yang, J. Su, S. Carl, J. G. Lynam, X. Yang and H. Lin, Appl. Catal., B, 2015, 162, 149–157 CrossRef CAS.
- Z. Li, P. Wu, J. Pang, X. Li, S. Zhai and M. Zheng, Catalysts, 2023, 13, 545 CrossRef CAS.
- Y. Hayashi and Y. Sasaki, Chem. Commun., 2005, 2716–2718 RSC.
- M. S. Holm, S. Saravanamurugan and E. Taarning, Science, 2010, 328, 602–605 CrossRef CAS PubMed.
- F. De Clippel, M. Dusselier, R. Van Rompaey, P. Vanelderen, J. Dijkmans, E. Makshina, L. Giebeler, S. Oswald, G. V. Baron, J. F. M. Denayer, P. P. Pescarmona, P. A. Jacobs and B. F. Sels, J. Am. Chem. Soc., 2012, 134, 10089–10101 CrossRef CAS PubMed.
- Z. Tang, W. Deng, Y. Wang, E. Zhu, X. Wan, Q. Zhang and Y. Wang, ChemSusChem, 2014, 7, 1557–1567 CrossRef CAS PubMed.
- S. Xu, K. Lan, J. Li, T. He and C. Hu, Sep. Purif. Technol., 2018, 204, 281–289 CrossRef CAS.
- T. Zhao, Y. Tashiro and K. Sonomoto, Appl. Microbiol. Biotechnol., 2019, 103, 9359–9371 CrossRef CAS PubMed.
- F. H. Isikgor and C. R. Becer, Polym. Chem., 2015, 6, 4497–4559 RSC.
- G. Liu, J. Sun, J. Zhang, Y. Tu and J. Bao, Bioresour. Technol., 2015, 198, 803–810 CrossRef CAS PubMed.
- Y. C. Kuo, S. F. Yuan, C. A. Wang, Y. J. Huang, G. L. Guo and W. S. Hwang, Bioresour. Technol., 2015, 198, 651–657 CrossRef CAS PubMed.
- S. Ding and T. Tan, Process Biochem., 2006, 41, 1451–1454 CrossRef CAS.
- K. Okano, S. Yoshida, R. Yamada, T. Tanaka, C. Ogino, H. Fukuda and A. Kondo, Appl. Environ. Microbiol., 2009, 75, 7858–7861 CrossRef CAS PubMed.
- K. Okano, Y. Sato, S. Hama, T. Tanaka, H. Noda, A. Kondo and K. Honda, Biotechnol. J., 2022, 17, 2100331 CrossRef CAS PubMed.
- K. Nalawade, P. Saikia, S. Behera, K. Konde and S. Patil, Biomass Convers. Biorefin., 2023, 13, 647–660 CrossRef.
- K. Okano, T. Tanaka, C. Ogino, H. Fukuda and A. Kondo, Appl. Microbiol. Biotechnol., 2010, 85, 413–423 CrossRef CAS PubMed.
- V. Novy, B. Brunner, G. Müller and B. Nidetzky, Biotechnol. Bioeng., 2017, 114, 163–171 CrossRef CAS PubMed.
- R. L. Shahab, S. Brethauer, M. P. Davey, A. G. Smith, S. Vignolini, J. S. Luterbacher and M. H. Studer, Science, 2020, 369, eabb1214 CrossRef CAS PubMed.
- H. Chen, B. Chen, Z. Su, K. Wang, B. Wang, Y. Wang, Z. Si, Y. Wu, D. Cai and P. Qin, Ind. Crops Prod., 2020, 146, 112175 CrossRef CAS.
- Y. Huang, Y. Wang, N. Shang and P. Li, Foods, 2023, 12, 2311 CrossRef CAS PubMed.
- M. Dusselier, P. Van Wouwe, A. Dewaele, E. Makshina and B. F. Sels, Energy Environ. Sci., 2013, 6, 1415–1442 RSC.
- J. Wu, K. H. Kim, K. Jeong, D. Kim, C. S. Kim, J.-M. Ha, R. P. Chandra and J. N. Saddler, Bioresour. Technol., 2021, 324, 124664 CrossRef CAS PubMed.
- G. S. Ha, H. S. Song, D. H. Oh, M. Mba-Wright, J. M. Ha, C. J. Yoo, J. W. Choi, C. S. Kim, B. H. Jeon, H. Jeong and K. H. Kim, J. Environ. Chem. Eng., 2023, 11, 1–7 Search PubMed.
- L. Li, F. Shen, R. L. Smith and X. Qi, Green Chem., 2017, 19, 76–81 RSC.
- Q. Yang and T. S. Chung, J. Membr. Sci., 2007, 294, 127–131 CrossRef CAS.
- A. Boonpan, S. Pivsa-Art, S. Pongswat, A. Areesirisuk and P. Sirisangsawang, Energy Procedia, 2013, 34, 898–904 CrossRef CAS.
- P. Hadik, L. Kotsis, M. Eniszné-Bódogh, L. P. Szabó and E. Nagy, Sep. Purif. Technol., 2005, 41, 299–304 CrossRef CAS.
- D. M. Bai, X. M. Zhao and Z. D. Hu, Chin. J. Anal. Chem., 2001, 29, 413–415 CAS.
| This journal is © The Royal Society of Chemistry 2023 |