Open Access Article
Open Access ArticleThe first spectrofluorimetric protocol for sensitive quantitative analysis of bromocriptine in its pure and pharmaceutical forms: evaluation of the greenness of the method†
Shrouk G. Abdulrazik,
Tamer Z. Attia * and
Sayed M. Derayea
* and
Sayed M. Derayea
Analytical Chemistry Department, Faculty of Pharmacy, Minia University, Minia, Egypt. E-mail: tamer_zekry_a@yahoo.com
First published on 8th December 2023
Abstract
Bromocriptine mesylate, a dopamine D2 receptor agonist, has been quantitatively determined using a sensitive, precise, quick, and affordable spectrofluorimetric method. The proposed method relies on the estimation of bromocriptine native fluorescence after the optimization of different factors to improve its inherently weak fluorescence through the use of a sodium dodecyl sulphate micellar system (2% w/v). Following excitation at 238 nm, the enhanced fluorescence intensity of bromocriptine was determined at 418 nm. As compared to its native fluorescence, the bromocriptine fluorescence intensity has been greatly enhanced by about 15 fold by employing the micellar system. The plot of intensity of fluorescence versus bromocriptine concentration was linear in the range of 50–600 ng mL−1. The method was found to have a high sensitivity, as indicated by the low limit of detection and limit of quantitation values (14.57 and 44.16 ng mL−1 respectively). Without the interference of any excipient, this method was effectively employed to quantify bromocriptine in its pharmaceutical dosage form.
1. Introduction
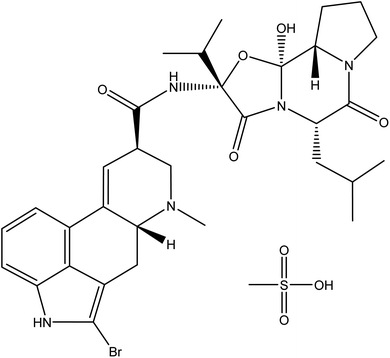
The ergot derivative, bromocriptine (Fig. 1), known as 2-bromo-alpha-ergocryptine, which acts as a dopamine D2 receptor agonist,1 is a treatment used in many cases of hormonal imbalance and plays an important role in hyperprolactinemia,2,3 infertility,4 acromegaly,5 Parkinson's disease,6 and diabetes.7 Despite its therapeutic importance, there are only a few analytical techniques available for quantitative analysis of bromocriptine mesylate, such as HPLC,8–13 voltammetry,14 and spectrophotometric methods.15 Previously published chromatographic methods require time-consuming sample pretreatment and consume large volumes of highly pure organic solvents which increase the analysis cost and have harmful impact on the environment. In addition, reported spectrophotometric methods suffer from low sensitivity as they can be applied for determination of bromocriptine over a linear range of 50 to 200 μg mL−1.Spectrofluorimetric methods of analysis are one of the most widely used methods for analysis of drugs as they have several advantages, including high sensitivity, simplicity, and low cost. Nevertheless, no fluorometric techniques have been reported for estimation of bromocriptine mesylate concentration, either in its pure form or as a pharmaceutical preparation.
The goal of this work is to evolve and validate the first spectrofluorimetric approach for determination of bromocriptine mesylate concentration with a very high sensitivity (in the range 50–600 ng mL−1) in its different forms. The proposed approach has been constructed simply to measure bromocriptine fluorescence after enhancement of the drug's very weak native fluorescence using a sodium dodecyl sulphate (SDS) micellar system in the presence of Toerell and Steinhagen buffer (pH 2.1). The developed method could be considered to be a sensitive, quick, and accurate method for determining bromocriptine mesylate concentration by utilizing all the advantages of spectrofluorimetric techniques.
2. Experimental
2.1. Apparatus
The estimation of fluorescence was performed utilizing a PerkinElmer luminescence spectrometer (LS 45; Beaconsfield, United Kingdom). A computer connected to the spectrometer running the WINLAB™ program was used for these measurements. Additionally, an AG 29 analytical balance (Glattbrug, Switzerland) and waterproof pocket pH tester (AdwaSzeged, Hungary) were utilized.2.2. Materials and reagents
• Bromocriptine mesylate (99.98%) was kindly given by Amoun Pharmaceuticals (El Obour city, Cairo, Egypt).• Dopagon® tablets containing 2.87 mg of bromocriptine mesylate per tablet (Memphis Co. for Pharma. & Chemical ind., Cairo, Egypt) were bought from Egyptian pharmacies.
• SDS was purchased from Oxford lab fine chemical LLP (Navghar, Vasai East, Maharashtra 401210, India), Tween 80 from El-Nasr Chemical Co. (Cairo, Egypt), and β-cyclodextrin (β-CD) from Wacker Chemie AG (Burghausen, Germany).
• HPLC grade (100%) acetonitrile, methanol, and ethanol were purchased from Fisher Company (Loughborough, United Kingdom). Other chemicals and solvents used in this work have been purchased from Biochem Company (Industrial Zone, 6th October, Giza), including sodium hydroxide, hydrochloric acid, phosphoric acid, citric acid, and sulfuric acid.
• In order to prepare the buffer solution, 1 M solutions of sodium hydroxide, phosphoric acid, and citric acid were combined. The pH of the mixture was then adjusted using 0.1 M hydrochloric acid to achieve the required range.
2.3. Standard solutions preparation
In a volumetric flask (100 mL), accurately weighed ten mg of bromocriptine mesylate was dissolved in 50 mL of methanol and diluted to the mark with methanol to prepare 100 μg mL−1 standard stock solutions which is freshly prepared. Working solutions of bromocriptine mesylate have been prepared in serial concentration from 0.5 μg mL−1 up to 6 μg mL−1.2.4. General analytical procedure
In a 10 mL volumetric flask, 1.5 mL of 2.0% SDS solution and Toerell and Steinhagen buffer solution (pH 2.1) were added, followed by addition of 1.0 mL of bromocriptine mesylate working solution (0.5–6 μg mL−1) and diluted to the mark with double distilled water, giving solutions with final concentrations over the range 50–600 ng mL−1. Following excitation at 238 nm, the fluorescence intensity was measured at 418 nm. Blank solutions were treated using the same procedure but without the drug.2.5. Preparation of pharmaceutical dosage solution
Ten tablets were ground into a powder and mixed. The amount of the mixed powder equivalent to 10 mg of the drug was accurately weighed, dissolved in a volumetric flask (100 mL) with 50 mL of methanol, and sonicated for 10 minutes. The solution was then filtered and filled to the mark with methanol. After that, the solution was further diluted with methanol to obtain working bromocriptine mesylate solutions in the range 0.5–6.0 μg mL−1. The previous general procedures were then followed.3. Results and discussion
Bromocriptine mesylate possesses a very weak native fluorescence in methanol with two excitation maxima at 238 and 308 nm and an emission at 418 nm. Measurement of the native fluorescence of investigated drug in methanol has been carried out at an excitation wavelength of 308 nm to avoid the high blank value introduced by methanol itself.It is widely recognized that adding a surfactant with a concentration higher than its critical micellar concentration could increase both the quantum yield and molar absorptivity of fluorophore fluorescence.16 This approach has been utilized to enhance the effectiveness of spectrofluorimetric techniques for a variety of analytes.17–19
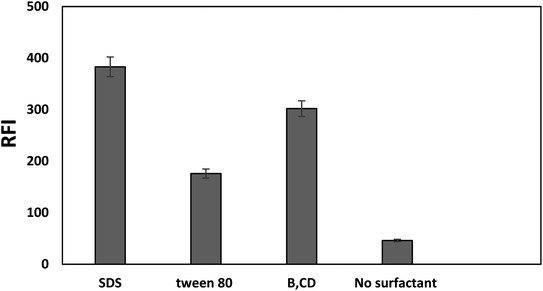
Thus, the present work aims to enhance the native weak fluorescence of bromocriptine by utilizing SDS as an anionic surfactant in aqueous media to establish a simple and highly sensitive green protocol for bromocriptine mesylate quantitation. The intensity of the enhanced fluorescence was monitored at 418 nm after excitation at 238 nm. The measurements were carried out at an excitation wavelength of 238 nm with a low blank value as water was used as the diluting solvent instead of methanol. The type of surfactant and the amount used, as well as any other factors that could influence the bromocriptine mesylate fluorescence have been thoroughly examined and optimized.
Fig. 2 displays the fluorescence spectrum of bromocriptine mesylate in the SDS system. By comparison to the relative fluorescence intensity of a methanolic solution of SDS-free bromocriptine mesylate, it was found that the fluorescence intensity in the presence of SDS was increased by about 15 fold.
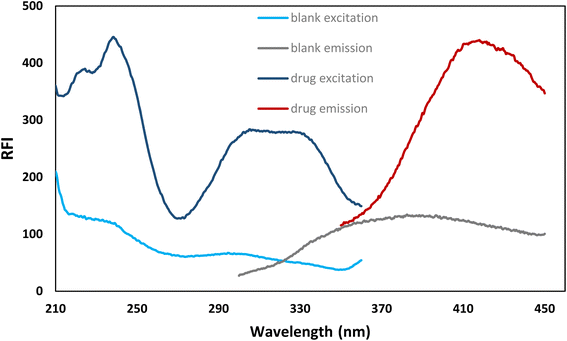 | ||
| Fig. 2 The excitation and emission spectra of bromocriptine mesylate (500 ng mL−1) in the presence of 2% SDS and a blank. | ||
3.1. Optimization of the experimental conditions
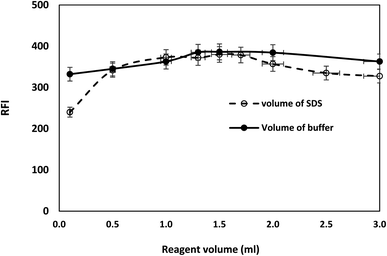
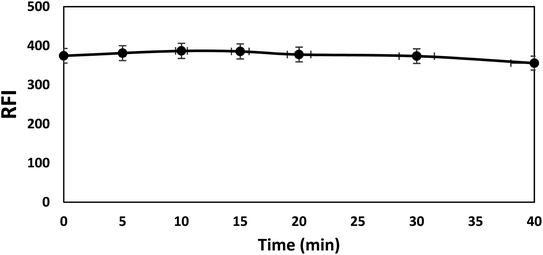
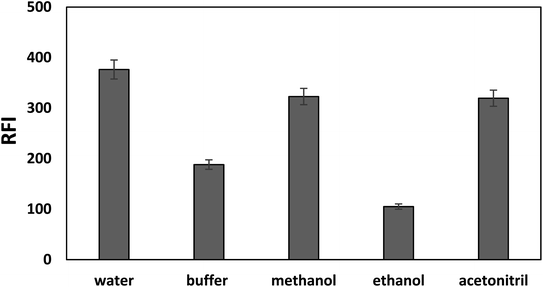
The fluorescence intensity of bromocriptine mesylate has been investigated by changing one experimental parameter whilst the other parameters were kept constant.Furthermore, the effects of using different acids (hydrochloric acid, sulfuric acid, and acetic acid) were checked and compared with Toerell and Steinhagen buffer (pH 2.1). Compared to acetic acid, stronger acids such as hydrochloric and sulfuric acid have higher protonation ability and thus resulted in a higher enhancement in the relative fluorescence intensity of the drug examined with SDS. However, the fluorescence intensities when using these acids were still lower than that achieved by using Toerell and Steinhagen buffer. In addition, different concentrations of hydrochloric acid (0.01–1.0 M) were also studied to investigate the effect of pH values lower than 2 (Fig. 4). It was observed that the relative fluorescence intensity value obtained using Toerell and Steinhagen buffer (pH 2.1) was also higher than that obtained with hydrochloric acid at all the studied concentrations. The slight decrease in the fluorescence intensity at low pH values (<2.0) may be due to the protonation of the indole nitrogen. This protonation can disrupt the electronic structure and conjugation within the indole aromatic system, resulting in decreased fluorescence efficiency. Protonation of the indole nitrogen can also lead to increased internal conversion or non-radiative decay processes, reducing the number of excited states and thus reducing the fluorescence intensity.
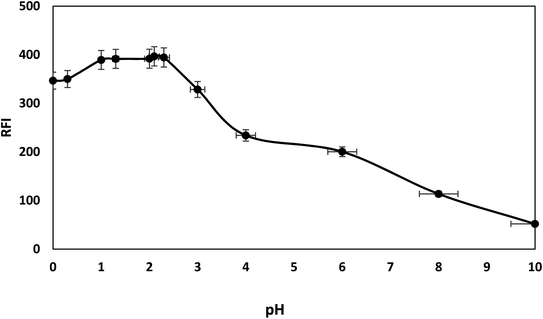 | ||
| Fig. 4 Effect of different pH levels, using Toerell and Steinhagen buffer (pH 2.0–10) and HCL (pH less than 2), on fluorescence intensity of 500 ng mL−1 bromocriptine with SDS. | ||
As a result, the optimum intensity of native bromocriptine fluorescence and micelle-enhanced fluorescence was attained using pH 2.1 Toerell and Steinhagen buffer (Fig. 4).
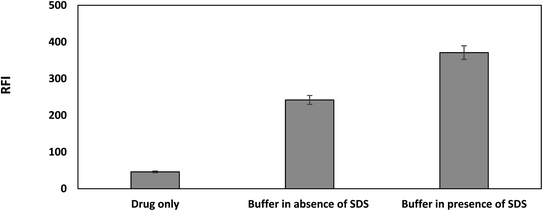
In addition, the effect of Toerell and Steinhagen buffer (pH 2.1) was investigated both with and without SDS. Fig. 5 illustrates how the use of the buffer with SDS significantly enhances the drug's relatively weak native fluorescence over the use of buffer alone. Bromocriptine has an indole ring, which is the fluorophore, and it also contains a tertiary amine on the piperidine moiety. At alkaline pH, the nitrogen atom of the tertiary amine is not ionized and carries a lone pair of electrons which could be transferred to the indole ring resulting in quenching of its fluorescence through a photoinduced electron transfer (PET) process. Upon protonation of the tertiary amine group using an acidic medium, the tertiary amine nitrogen will carry a positive charge. Thereafter, intramolecular electron transfer processes in the excited state of the drug would cease upon protonation of the tertiary amine nitrogen atom. Thus, the electron lone pair will no longer be available and consequently the PET process will be blocked, resulting in restoration of the fluorescence (ESI S1†). Moreover, the presence of a positive charge on the nitrogen atom also facilitates the electrostatic attraction between the positively charged drug molecules and the negatively charged sulfonyl group of the SDS. As a result of binding the drug to the SDS micellar system, restriction of intramolecular free rotation and increased rigidity of the drug molecules will occur, potentially increasing the drug fluorescence quantum yield.18–20
3.2. Validation of the proposed method
The developed approach has been certified in concert with the parameters specified in the International Conference on Harmonization (ICH) guidelines. Linearity, accuracy, precision, limits for quantitation and detection, and robustness are the validation parameters that have been studied.22| Parameter | Native | Micellar Enhanced |
|---|---|---|
| λex | 308 | 238 |
| λem | 418 | 418 |
| Linear range (ng mL−1) | 100–3000 | 50–600 |
| Correlation coefficient (r) | 0.9992 | 0.9996 |
| Determination coefficient (r2) | 0.9984 | 0.9993 |
| Intercept ± SD | 23.03 ± 1.85 | 14.76 ± 3.16 |
| Slope ± SD | 0.047 ± 0.001097 | 0.72 ± 0.0087 |
| LOD (ng mL−1) | 128.85 | 14.57 |
| LOQ(ng mL−1) | 390.47 | 44.16 |
| Method parameters | Concentration founda | % Recovery ± % RSDb |
|---|---|---|
| a Average of three determinations.b % RSD, % relative standard deviation. | ||
| 1 Buffer pH | ||
| 2.0 | 490.00 | 98.00 ± 0.16 |
| 2.1 | 498.95 | 99.79 ± 0.44 |
| 2.2 | 503.07 | 100.61 ± 0.26 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| 2 Buffer volume | ||
| 1.3 mL | 503.49 | 100.69 ± 0.53 |
| 1.5 mL | 493.03 | 98.6 ± 1.32 |
| 1.7 mL | 499.71 | 99.94 ± 1.21 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| 3 Volume of SDS | ||
| 1.3 mL | 500.48 | 100.09 ± 0.32 |
| 1.5 mL | 499.30 | 99.86 ± 0.14 |
| 1.7 mL | 495.59 | 99.12 ± 0.59 |
3.3. Application to the detection of bromocriptine mesylate in its pharmaceutical dosage form
The proposed approach can be effectively applied for the estimation of the quantity of bromocriptine mesylate in its pharmaceutical dosage form (Dopagon® tablets). Statistical comparisons between the obtained results and those of another reported method15 were carried out. There were no detectable variations between the calculated and theoretical values when the results were compared at the 95% confidence level using t- and F-tests (Table 5). These results indicate that the proposed method for analyzing the quantity of bromocriptine mesylate in its pharmaceutical dosage form has good accuracy and precision.3.4. Evaluation of the greenness of the proposed method using the analytical eco-scale
The analytical method is considered green because it does not involve the use of hazardous substances, high energy consumption, or waste products. In the current study, the eco-scale score was used to assess how environmentally friendly the proposed approach is.23–25 This can be determined by giving points to each component in the method. The outcome of the analytical eco-scale evaluation is represented with a number which is calculated by subtracting penalty points from 100. As presented in Table 6, the eco-scale score of the proposed approach was higher than 75 which proves the greenness of the method.| Eco-scale | ||
|---|---|---|
| Parameters | Penalty points | |
| Reagents | Citric acid | 1 |
| Phosphoric acid | 2 | |
| NaOH | 2 | |
| SDS | 8 | |
| Water | 0 | |
| Instrument | 0 | |
| Occupational hazard | 0 | |
| Waste | 8 | |
| Total penalty points | 21 | |
| Analytical eco-scale score | 79 | |
4. Conclusions
The first spectrofluorimetric method for estimation of bromocriptine quantities in its pure and pharmaceutical dosage forms was proposed. This method relies simply on measurement of the native bromocriptine fluorescence and its fluorescence enhanced using a sodium dodecyl sulphate micellar system. The micellar enhanced spectrofluorimetric method is simple, cheap, rapid, accurate, and precise and does not require harmful and expensive solvents or derivatization steps. Thus, this method could be successfully applied in quality control laboratories.Conflicts of interest
There are no conflicts to declare.References
- D. Parkes, Bromocriptine, N. Engl. J. Med., 1979, 301(16), 873–878 CrossRef CAS PubMed.
- F. Naz, et al., Bromocriptine therapy: Review of mechanism of action, safety and tolerability, Clin. Exp. Pharmacol. Physiol., 2022, 49(8), 903–922 CrossRef CAS PubMed.
- M. O. Thorner, E. Fluckiger, and D. B. Calne, Bromocriptine. A Clinical and Pharmacological Review, Raven Press, 1980 Search PubMed.
- N. Laufer, et al., Effect of bromocriptine treatment on male infertility associated with hyperprolactinemia, Arch. Androl., 1981, 6(4), 343–346 CrossRef CAS PubMed.
- J. Abucham, Dopaminergic Treatment of Patients with Acromegaly: Still Kicking after All These Years, SciELO Brasil, 2022, pp. 275–277 Search PubMed.
- J. L. Montastruc, et al., Long-term mortality results of the randomized controlled study comparing bromocriptine to which levodopa was later added with levodopa alone in previously untreated patients with Parkinson's disease, Mov. Disord., 2001, 16(3), 511–514 CrossRef CAS PubMed.
- K. Saravanan and K. M. SUNDARAM, Effect of bromocriptine in diabetes mellitus: a review, Uttar Pradesh Journal of Zoology, 2021, 1166–1170 Search PubMed.
- Q. Zang, et al., A sensitive and rapid HPLC–MS/MS method for the quantitative determination of trace amount of bromocriptine in small clinical prolactinoma tissue, J. Chromatogr. B, 2015, 989, 91–97 CrossRef CAS PubMed.
- N. H. Foda and F. El Shafie, Quantitative analysis of bromocriptine mesylate in tablet formulations by HPLC, J. Liq. Chromatogr. Relat. Technol., 1996, 19(19), 3201–3209 CrossRef CAS.
- S. Ashour and N. Kattan, New sensitive method for determination of bromocriptine in tablets by high performance liquid chromatography, Res. J. Aleppo Univ., 2013, 87, 1–10 Search PubMed.
- P. Pukngam and J. Burana-osot, Development and validation of a stability-indicating HPLC method for determination of bromocriptine mesylate in bulk drug and tablets, Curr. Pharm. Anal., 2013, 9(1), 92–101 CAS.
- P. Pukngam, Development and Validation of a Stability-Indicating Assay Method for Bromocriptine Mesylate in Bulk and Tablets, 2010 Search PubMed.
- J. Bhayji, F. Tandel, and N. Patel, Analytical Method Development and Validation for Simultaneous Estimation of Bromocriptine Mesylate and Metformin Hydrochloride with Doe Approach, 2015 Search PubMed.
- A. Radi, M. El-Shahawi and T. Elmogy, Differential pulse voltammetric determination of the dopaminergic agonist bromocriptine at glassy carbon electrode, J. Pharm. Biomed. Anal., 2005, 37(1), 195–198 CrossRef CAS PubMed.
- M. Akshay, A. Patil, and R. Gaikwad, Estimation of Bromocriptine Mesylate Ip by Uv Spectroscopy, 2021 Search PubMed.
- W. L. Hinze, et al., Micellar enhanced analytical fluorimetry, TrAC, Trends Anal. Chem., 1984, 3(8), 193–199 CrossRef CAS.
- K. M. Badr El-Din and T. Z. Attia, Spectrofluorimetric determination of certain adrenergic agonist drugs in their pure forms and pharmaceutical formulations: Content uniformity test application, Luminescence, 2017, 32(5), 706–712 CrossRef CAS PubMed.
- N. N. Atia, S. M. El-Gizawy and N. M. Hosny, Facile micelle-enhanced spectrofluorimetric method for picogram level determination of febuxostat; application in tablets and in real human plasma, Microchem. J., 2019, 147, 296–302 CrossRef CAS.
- S. M. Derayea, et al., Enhancement of the sensitivity of valacyclovir and acyclovir for their spectrofluorimetric determination in human plasma, RSC Adv., 2015, 5(96), 78920–78926 RSC.
- R. Ghonim, et al., Spectrofluorometric determination of orphenadrine, dimenhydrinate, and cinnarizine using direct and synchronous techniques with greenness assessment, Sci. Rep., 2023, 13(1), 13549 CrossRef CAS PubMed.
- L. Yu and J. M. Davis, Study of high-field dispersion in micellar electrokinetic chromatography, Electrophoresis, 1995, 16(1), 2104–2120 CrossRef CAS PubMed.
- ICH, I. Q2 (R1): Validation of analytical procedures: text and methodology, in International Conference on Harmonization, Geneva, 2005 Search PubMed.
- T. Z. Attia, S. G. Abdulrazik and S. M. Derayea, Facile spectrofluorimetric quantitation of octreotide, a synthetic peptide, in its pure form and pharmaceutical formulation; evaluation of the method's greenness, Luminescence, 2022, 37(11), 1914–1920 CrossRef CAS PubMed.
- T. Z. Attia, et al., Spectrofluorimetric determination of the anti-Covid 19 agent, remdesivir, in vials and spiked human plasma, Luminescence, 2022, 37(7), 1192–1199 CrossRef CAS PubMed.
- S. M. Derayea, et al., A feasible fluorimetric approach anchored in diaryl pyrrolone derivative for the facile analysis of milnacipran in tablets; evaluation of the method greenness, Spectrochim. Acta, Part A, 2022, 273, 121024 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra06626f |
| This journal is © The Royal Society of Chemistry 2023 |