Open Access Article
Open Access ArticleA high mass loading flexible electrode with a sheet-like Mn3O4/NiMoO4@NiCo LDH on a carbon cloth for supercapacitors†
Ruibin Liang‡
a,
Si Liu‡a,
Jianrong Lina,
Jingfei Daia,
Jingyi Penga,
Peiyuan Huanga,
Jianwen Chen b and
Peng Xiao
b and
Peng Xiao *a
*a
aGuangdong-Hong Kong-Macao Joint Laboratory for Intelligent Micro-Nano Optoelectronic Technology, School of Physics and Optoelectronic Engineering, Foshan University, Foshan 528225, China. E-mail: xiaopeng@fosu.edu.cn
bSchool of Electronic and Information Engineering, Foshan University, Foshan, 528000, China
First published on 15th November 2023
Abstract
Mass loading is an important parameter to evaluate the application potential of active materials in high-capacity supercapacitors. Synthesizing active materials with high mass loading is a promising strategy to improve high performance energy storage devices. Preparing electrode materials with a porous structure is of significance to overcome the disadvantages brought by high mass loading. In this work, a Mn3O4/NiMoO4@NiCo layered double hydroxide (MO/NMO/NiCo LDH) positive electrode is fabricated on a carbon cloth with a high mass loading of 20.4 mg cm−2. The MO/NMO/NiCo LDH presents as a special three-dimensional porous nanostructure and exhibits a high specific capacitance of 815 F g−1 at 1 A g−1. Impressively, the flexible supercapacitor based on the MO/NMO/NiCo LDH positive electrode and an AC negative electrode delivers a maximum energy density of 22.5 W h kg−1 and a power density of 8730 W kg−1. It also retains 60.84% of the original specific capacitance after bending to 180° 600 times. Moreover, it exhibits 76.92% capacitance retention after 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 charge/discharge cycles. These results make MO/NMO/NiCo LDH one of the most attractive candidates of positive electrode materials for high-performance flexible supercapacitors.
000 charge/discharge cycles. These results make MO/NMO/NiCo LDH one of the most attractive candidates of positive electrode materials for high-performance flexible supercapacitors.
1 Introduction
In the past decades, supercapacitors (SCs) have been widely considered as potential energy storage equipment because of their high power density, good safety, and long-life cycle.1,2 However, low energy density remains a bottleneck and developing new electrode materials is an effective approach to attain high energy density for SCs.3 It is well known that the electrochemical performance of supercapacitors is determined by the electrode materials. At present, it is difficult for a single electrode material to break through the inherent disadvantage barrier of energy storage. In recent years, a lot of attention has been given to designing and synthesizing composite electrode materials owing to the synergistic effect between different components. Barua and co-workers prepared a graphene-MOF aerogel electrode with a low mass loading of 3 mg cm−2, which provided a high specific capacitance of 98 F g−1 at 0.5 A g−1.4 The 3D-porous Ni/MnOx nanocomposite prepared by Ashassi-Sorkhabi et al. had a low mass loading of 1.65 mg cm−2 and showed a specific capacitance of 396.4 F g−1 at 0.3 A g−1. The composite also showed an excellent stability of 100% after 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles.5
000 cycles.5
Nevertheless, the electrochemical performance of the above electrode materials was achieved with a low mass loading. In fact, the mass loading is the key parameter for some electrode materials in practical applications.6 It is usually necessary to achieve an active material mass loading of 8–10 mg cm−2 to build pseudocapacitors for commercial applications.7 However, due to the poor conductivity of metal oxides, the high mass loading affects the conductivity and ion diffusion of the electrode, thereby seriously reducing the specific capacitance, rate performance, and cycle life of supercapacitors. For example, manganese dioxide (MnO2) with a thickness of less than 1 μm can exhibit ultra-high specific capacitance. However, the high mass loading of MnO2 leads to a reduction in specific capacitance due to the close-packed structures causing limited active specific area.8 Therefore, preparing SCs with high energy density and high mass loading electrodes is full of challenges.9 Electrode materials with three-dimensional (3D) porous nanostructures can ensure the rapid transport of ions and electrons through the whole electrode and mechanical stability, which provide a guarantee for its good electrochemical performance on high mass loading.
In this work, we prepared high mass loading (20.4 mg cm−2) Mn3O4/NiMoO4@NiCo LDH (MO/NMO/NiCo LDH) on carbon cloth (CC) by electrodeposition followed by a two-step hydrothermal method. Sheet-like MO/NMO/NiCo LDH exhibited a 3D porous nanostructure. The MO/NMO/NiCo LDH electrode delivered 815 F g−1 at 1 A g−1. The MO/NMO/NiCo LDH//active carbon (AC) flexible supercapacitor (FSC) showed great potential in actual application. It exhibited an energy density of 22.5 W h kg−1 at 997.53 W kg−1. Even when the power density reached 8730 W kg−1, 1.944 W h kg−1 of energy density was retained. Importantly, the FSC keeps 60.84% of its original specific capacitance after bending to 180° 600 times. It also kept 184.62% of the original specific capacitance after 8000 charge/discharge cycles and eventually retained 76.92% after 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. The MO/NMO/NiCo LDH electrode showed potential for use in a high-performance SC with a high mass loading electrode.
000 cycles. The MO/NMO/NiCo LDH electrode showed potential for use in a high-performance SC with a high mass loading electrode.
2 Discussion
Fig. 1a shows the X-ray diffraction (XRD) pattern of MO/NMO/NiCo LDH. The diffraction peaks at 41.764°, 43.936°, 47.384°, 53.175° and 61.946° belong to the (100), (004), (102), (103) and (104) planes of CC (PDF#26-1078). The distraction peaks at 17.196°, 28.735°, 31.356°, 44.148° and 59.477° correspond to the (101), (112), (200), (220) and (224) planes of Mn3O4 (PDF#16-0154). The distraction peaks at 13.491°, 26.308°, 43.936°, 47.384°, 53.175°, 57.859, 61.946 and 63.093° accord with NiMoO4 (PDF#18-0879). The peaks at 17.196° (020), 20.049° (001), 26.819° (220), 33.674° (221), 35.036° (040), 36.399° (301), 39.507° (231) and 47.384° (340) match to NiCo LDH (PDF#48-0083). Fig. 1b and c show that massive sheet-like MO/NMO/NiCo LDH coats the surface of the CC to construct a porous structure, favoring contact between the active material and the electrolyte. This provides not only numerous ion/electron transport channels for redox reaction processing, but also ample space for material expansion/contraction, which helps maintain high specific capacitance with high mass loading and ensures good cycle stability during long charge/discharge processes. The element mapping corresponding to the scanning electron microscopy (SEM) image is shown in Fig. 1d–h and the co-existence of Ni, Co, Mo and Mn was confirmed.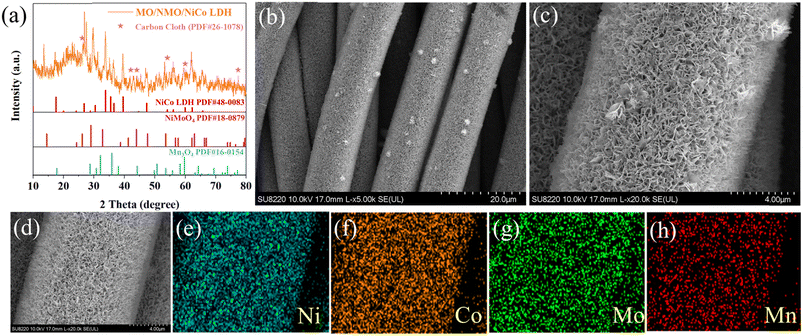 | ||
| Fig. 1 (a) The XRD pattern of MO/NMO/NiCo LDH. (b and c) SEM images of MO/NMO/NiCo LDH. (d–h) Element mapping images corresponding to the SEM. | ||
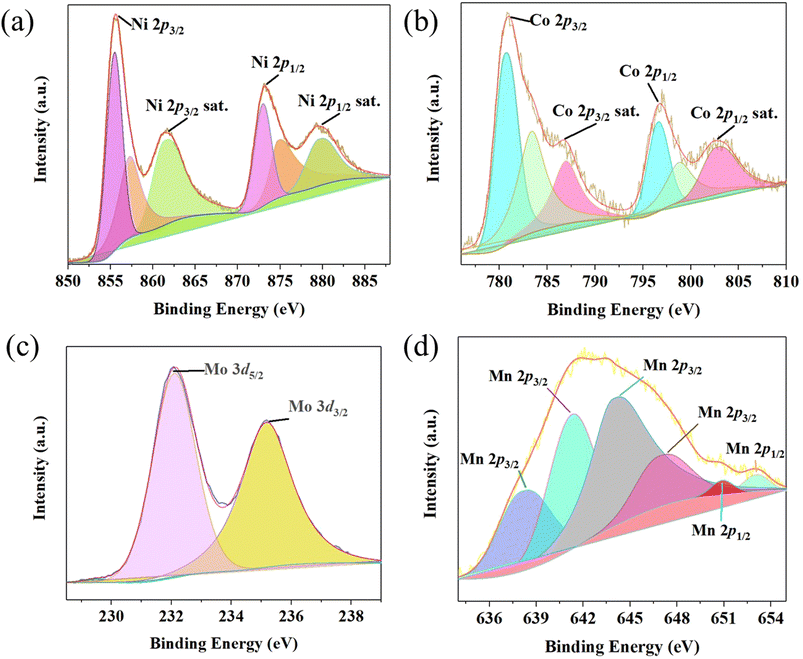
The X-ray photoelectron spectroscopy (XPS) spectrum of MO/NMO/NiCo LDH is shown in Fig. S1† and includes Ni, Co, Mn, Mo, O and C. As shown in Fig. 2a, there are two major peaks and two satellite peaks in the Ni 2p XPS spectrum.10 The peaks at 855.46 eV and 857.26 eV accord with Ni 2p3/2. The peaks at 872.95 eV and 874.77 eV originate from Ni 2p1/2. The peaks at 861.39 eV (Ni 2p3/2 sat.) and 879.58 eV (Ni 2p1/2 sat.) are satellite peaks. This proves the existence of Ni2+.11 Fig. 2b shows that the Co 2p3/2 peaks (780.61 eV and 783.37 eV) and Co 2p1/2 peaks (796.59 eV and 798.79 eV) contribute to the major peaks of the Co 2p spectrum and the peaks at 786.94 eV and 802.66 eV correspond to the satellite peaks. The existence of Co2+ is further confirmed by the binding energy difference between the peaks of Co 2p3/2 (781.03 eV) and Co 2p1/2 (797.01 eV), which is near 16.00 eV. As shown in the Mo 3d spectrum (Fig. 2c), the existence of Mo6+ is revealed by the peaks at 232.10 eV (Mo 3d5/2) and 235.17 eV (Mo 3d3/2).12 Fig. 2d shows the XPS spectrum of Mn 2p. The peaks at 638.05 eV (Mn2+ 2p3/2), 643.89 eV (Mn2+ 2p3/2) and 650.89 eV (Mn2+ 2p1/2) are from Mn2+. The peak at 646.83 eV corresponds to Mn 2p3/2. The binding energy difference between 641.26 eV and 653.05 eV is 11.79 eV, which proves the existence of Mn3O4.
Fig. 3a shows the cyclic voltammetry (CV) curves of each sample at the scan rate of 30 mV s−1. All the CV curves show redox peaks, which means that energy is stored by redox reaction.13 In addition, the CV area of MO/NMO/NiCo LDH is obviously larger than those of MO/NMO and MO/NiCo LDH, indicating that MO/NMO/NiCo LDH has better capacitance performance than the other samples. Fig. 3b shows the galvanostatic charge/discharge (GCD) curves at 1 A g−1 of the synthesized samples. Due to the energy storage advantages of composite materials and porous structure, the MO/NMO/NiCo LDH positive electrode displays a high specific capacitance of 815 F g−1, which is much larger than those of MO/NMO (525 F g−1) and MO/NiCo LDH (135 F g−1). As shown in Fig. 3c, all electrochemical impedance spectroscopy (EIS) curves consist of a quasi-semicircle (representing the charge transfer resistance (Rct) caused by redox reaction) and an oblique line (revealing the diffusion behavior of ions).14,15 The EIS curve of MO/NMO/NiCo LDH displays a smaller diameter semicircle and a larger slope in the linear part, which means that MO/NMO/NiCo LDH possesses a smaller Rct and faster ion-diffusion rate than the other samples.
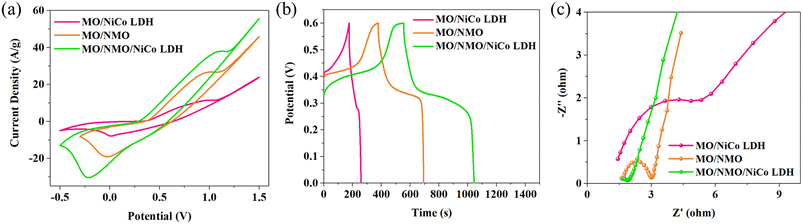 | ||
| Fig. 3 The curves of MO/NMO/NiCo LDH, MO/NMO/NiCo LDH, and MO/NMO/NiCo LDH: (a) the CV curve at 30 mV s−1, (b) GCD curve at 1 A g−1, and (c) EIS curve. | ||
Recently, mass loading has been an important parameter to evaluate the application potential of an active material in a high capacity supercapacitor. Synthesizing an active material with high mass loading is still a promising strategy to improve high performance energy storage devices, though high mass loading leads to a sacrifice in electrochemical performance. Therefore, we investigated the electrochemical properties of MO/NMO/NiCo LDH with different mass loadings. It can be easily seen in Fig. S2† that the CV curve area and discharge time increase with the mass loading. In other words, the electrochemical performance of MO/NMO/NiCo LDH is enhanced instead of sacrificed with a high mass loading of 20.4 mg cm−2. This reveals that MO/NMO/NiCo LDH has great application potential in high-capacity supercapacitors. Table 1 shows the specific capacitances and mass loadings of reported electrode materials. Compared to the reported materials,16–20 the MO/NMO/NiCo LDH electrode in our work exhibited higher specific capacitance and higher mass loading. However, MO/NMO/NiCo LDH and MO/NMO/NiCo LDH show worse specific capacitance, which may be due to the poor conductivity and low ion diffusion brought by high mass loading.
| Electrode | Specific capacitance | Mass loading | Reference |
|---|---|---|---|
| Co–Ni LDH | 811 F g−1 | 3.59 mg cm−2 | 16 |
| Graphene films | 340 F g−1 | 4.8 mg cm−2 | 17 |
| Polyaniline-graphite | 607 F g−1 | 5.89 mg cm−2 | 18 |
| N-Doped porous carbon | 457 F g−1 | 1 mg cm−2 | 19 |
| Ni–Co–S | 640 F g−1 | 8.84 mg cm−2 | 20 |
| MO/NMO | 525 F g−1 | 15 mg cm−2 | This work |
| MO/NiCo LDH | 135 F g−1 | 13.8 mg cm−2 | This work |
| MO/NMO/NiCo LDH | 815 F g−1 | 20.4 mg cm−2 | This work |
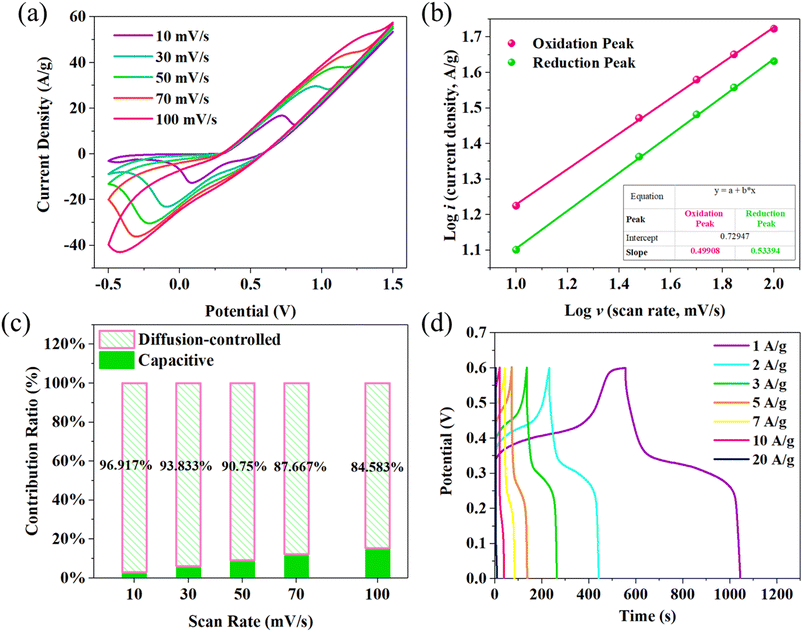
Fig. 4a shows the CV curves of MO/NMO/NiCo LDH at different scan rates with the mass loading of 20.4 mg cm−2. When the scan rate reaches 100 mV s−1, both the oxidation peak and reduction peak are still visible, revealing the good energy storage behavior of MO/NMO/NiCo LDH. To confirm the pseudocapacitance behavior of MO/NMO/NiCo LDH, kinetic calculations were performed according to the CV curves. The relationship between the scan rate (v) and peak current (i) is described by the following formulas,21
| i = avb |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) i = b i = b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v + log v + log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) i–log
i–log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v curve. As shown in Fig. 4b, the b values of the oxidation peak and reduction peak are 0.49908 and 0.53394, respectively, which are close to 0.5. This means that diffusion-controlled behavior accounts for the major proportion and the time of ions diffusing into the lattices is shortened.22 The contribution ratio of capacitive and diffusion-controlled behavior was ascertained according to the following equation,23
v curve. As shown in Fig. 4b, the b values of the oxidation peak and reduction peak are 0.49908 and 0.53394, respectively, which are close to 0.5. This means that diffusion-controlled behavior accounts for the major proportion and the time of ions diffusing into the lattices is shortened.22 The contribution ratio of capacitive and diffusion-controlled behavior was ascertained according to the following equation,23| i(v) = k1ν + k2ν1/2 |
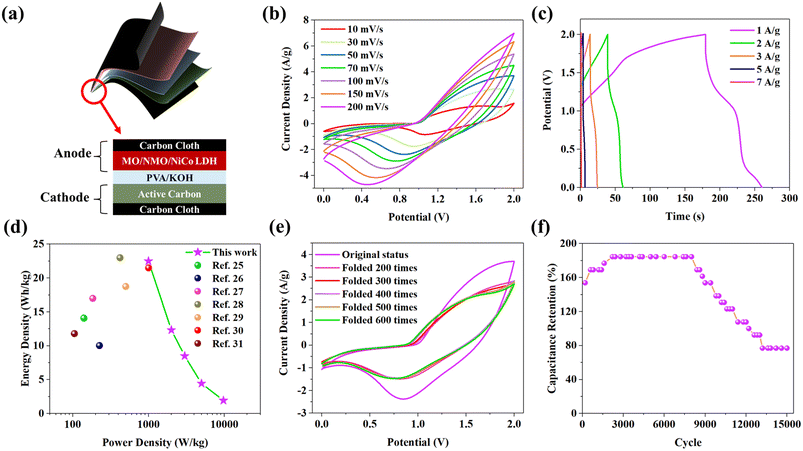
To reveal the application potential of high mass loading MO/NMO/NiCo LDH in a flexible supercapacitor, a FSC was assembled using MO/NMO/NiCo LDH as the anode, AC/CC as the cathode and polyvinyl alcohol (PVA)/KOH as the gel electrolyte in a sandwich structure, as shown in Fig. 5a. Fig. S4a† shows that the CV curve at 2.0 V displays no obvious polarization, which means the FSC can work stably at 2.0 V.24 Fig. 5b shows the CV curves of the FSC at different scan rates from 10 mV s−1 to 200 mV s−1. The redox peaks can be easily seen at the high scan rate of 200 mV s−1, revealing the outstanding rate performance of the FSC. Fig. 5c shows the GCD curves of the FSC at different current densities and the corresponding specific capacitances of each GCD curve are 40.5 (1 A g−1), 22.2 (2 A g−1), 15.3 (3 A g−1), 8 (5 A g−1) and 3.5 F g−1 (7 A g−1). Fig. 5d displays the relationship between the energy density and the power density of the FSC. The maximum energy density and power density of the FSC are 22.5 W h kg−1 and 8730 W kg−1. It can be clearly seen that the FSC in this work possesses better performance than those of other reported supercapacitors, including the HPC-4//HPC-4 all-solid-state symmetric supercapacitor,25 PANI@CNFs//PANI@CNFs flexible supercapacitor,26 BIC-Co3O4//BIC-Co3O4 symmetric cell,27 APC4/1//APC4/1,28 PANI/GF//PANI/GF device,29 Ni33/ZIF-67/rGO20//Ni33/ZIF-67/rGO20 all-solid-state supercapacitor30 and L-Ti3C2Tx/E-ANF based flexible supercapacitor.31
Whether the FSC can work normally after deformation is an important indicator to measure its application potential in flexible devices. Fig. 5e shows the CV curves under different bending conditions. The CV curves under bending conditions show little difference and are obviously smaller in curve area than that of the original status. The corresponding area data of each curve are shown in Table S1.† The decrease in the CV curve area means that the performance of the FSC weakens during folding. This can be further proved by the GCD test. Fig. S4b† shows that the GCD curves under bending addition show little difference in shape to the original, which means that the FSC can work stably under bending conditions. In addition, the discharging time of each status is 81.2 s (original status, 40.5 F g−1), 56.6 s (folded 200 times, 28.3 F g−1), 54 s (folded 300 times, 27 F g−1), 52.4 s (folded 400 times, 26.2 F g−1), 50.2 s (folded 500 times, 25.1 F g−1) and 49.4 s (folded 600 times, 24.7 F g−1). In another words, the FSC kept 60.84% of the original specific capacitance after bending 600 times. The reduction of the CV curve area and discharging time may be attributed to the exfoliation of active material from the substrate. It is clearly shown in Fig. S4c† that the EIS curves under bending conditions possess smaller slopes than the original. To simulate the scenario of practical application, the long-term cycle performances of the FSC are evaluated at 5 A g−1, and Fig. 5f shows the relationship between capacitance retention and cycles. The FSC possessed a high capacitance retention of 184.62% after 8000 GCD cycles and also retained 76.92% of the original specific capacitance after 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. The superior capacitance retention is due to the special structure of MO/NMO/NiCo LDH. During the high-current charge/discharge process, the 3D porous nanostructures of MO/NMO/NiCo LDH provide a large number of transport channels for electrons/ions as well as reactive active sites, which ensure the smooth progress of the redox reaction. Meanwhile, there is ample space for material expansion/contraction, greatly improving the cycle stability during long charge/discharge processes.
000 cycles. The superior capacitance retention is due to the special structure of MO/NMO/NiCo LDH. During the high-current charge/discharge process, the 3D porous nanostructures of MO/NMO/NiCo LDH provide a large number of transport channels for electrons/ions as well as reactive active sites, which ensure the smooth progress of the redox reaction. Meanwhile, there is ample space for material expansion/contraction, greatly improving the cycle stability during long charge/discharge processes.
3 Conclusion
The mass loading of electrode materials is a key parameter for their practical application in energy storage devices. Research on preparing electrode materials with high mass loading is the basis for developing high performance and high-capacity supercapacitors. In this work, the MO/NMO/NiCo LDH positive electrode with porous nanostructures and high loading (20.4 mg cm−2) was successfully synthesized by electrodeposition on carbon cloth followed by hydrothermal method. The specific porous nanostructures of MO/NMO/NiCo LDH provided numerous ion/electron transport channels for redox reaction processing, enhancing the electrochemical performance. The MO/NMO/NiCo LDH positive electrode possessed good specific capacitance (815 F g−1 at 1 A g−1) and good rate performance (redox peaks visible at 100 mV s−1). The maximum energy density and power density of the MO/NMO/NiCo LDH//AC FSC were 22.5 W h kg−1 and 8730 W kg−1. The FSC retained 60.84% of its original specific capacitance after bending to 180° 600 times. In addition, it kept 76.92% of its original specific capacitance after 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 GCD cycles. These results show that the MO/NMO/NiCo LDH positive electrode has great potential in application in high mass loading flexible supercapacitors, as it meets the development and needs of flexible devices. We believe this work can give some guidance in the preparation of high loading and high-capacity flexible supercapacitors.
000 GCD cycles. These results show that the MO/NMO/NiCo LDH positive electrode has great potential in application in high mass loading flexible supercapacitors, as it meets the development and needs of flexible devices. We believe this work can give some guidance in the preparation of high loading and high-capacity flexible supercapacitors.
4 Experimental details
4.1 Reagent
Urea (99%, AR) and KOH (90%, AR) were acquired from Shanghai trial. NiCl2·6H2O (99%, AR), CoCl2·6H2O (99%, AR), MnCl2·4H2O (99.99%, metals basis) and NaCl (99%, metals basis) were bought from Macklin. PVA (Mw ∼ 145![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) and (NH4)6Mo7O24 (Mw ∼ 1235.86) were from Aladdin.
000) and (NH4)6Mo7O24 (Mw ∼ 1235.86) were from Aladdin.
4.2 Synthesis of MO on CC
A piece of CC (1 cm × 2 cm) was cleaned with deionized water and absolute ethyl alcohol under ultrasonic processing for 30 min. Then, the CC was dried at 60 °C for 12 h.The electrochemical growth of MO was processed by the cyclic voltammetry (CV) method, modified from Raut's work.10 5 mM MnCl2·4H2O and 5 mM NaCl were dissolved in 50 mL deionized water to prepare the precursor solution. The electrodeposition was performed in a three-electrode system, with the CC as the working electrode with a soak area of 1 cm × 1 cm, a platinum plate as the counter electrode, and a saturated calomel electrode as the reference electrode. The CV process was performed in the potential window of 0–1 V at 10 mV s−1 for 4 cycles. After the deposition, the electrodeposited MO was cleaned and dried for 6 h. The mass of MO deposited on the CC was 7.4 mg.
4.3 Preparation of NMO on MO
1 mM NiCl2·6H2O and 0.2 mM (NH4)6Mo7O24 were dissolved into 30 mL deionized water and transferred into a 100 mL Teflon autoclave with the MO immersed. The solution was heated at 150 °C for 10 h. The sample (MO/NMO) was cleaned and dried at 60 °C for 12 h. The mass of the NMO on MO was 7.6 mg.4.4 Synthesis of MO/NMO/NiCo LDH
1 mM NiCl2·6H2O, 2 mM CoCl2·6H2O and 7 mM urea were dissolved into 30 mL deionized water. The obtained solution with MO/NMO immersed was heated at 120 °C for 10 h. The MO/NMO/NiCo LDH was cleaned and dried at 60 °C for 12 h. The NiCo LDH synthesized on MO/NMO was 5.4 mg.The total mass of MO/NMO/NiCo LDH was 20.4 mg cm−2. In addition, for comparison, MO/NMO and MO/NiCo LDH were synthesized by the same process.
4.5 Assembled supercapacitor
The MO/NMO/NiCo LDH//AC FSC was assembled using MO/NMO/NiCo LDH as the anode, AC as the cathode and PVA/KOH as the gel electrolyte. PVA/KOH was prepared by adding 3 g PVA to 30 mL of deionized water and stirring at 85 °C until the solution became clear. 10 mL of 3 M KOH was added into the solution under stirring and PVA/KOH was obtained.4.6 Characterization methods
The X-ray diffraction patterns (XRD, Smartlab 9 kW) were detected in the Bragg's angle (2θ) range of 10–80° by Cu Kα radiation. X-ray photoelectron spectroscopy (XPS, Nexsa) was performed to verify the chemical states of the prepared samples. Field-emission scanning electron microscopy (FESEM, Hitachi, SU8010) was utilized to obtain the morphology.4.7 Electrochemical measurement
The electrochemical performance was studied by performing CV, galvanostatic charge–discharge (GCD), and electrochemical impedance spectroscopy (EIS) on a IVIUM electrochemical workstation. The three-electrode system consisted of the synthesized electrode as the working electrode, HgO/Hg as the reference electrode and platinum plate as the counter electrode in 3 M KOH solution.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was supported by Guangdong Science and Technology Plan (No. 2022A0505020022), Research Fund of Guangdong-Hong Kong-Macao Joint Laboratory for Intelligent Micro-Nano Optoelectronic Technology (No. 2020B1212030010), Guangdong Provincial Key Laboratory of Semiconductor Micro Display (2020B121202003) and Youth project of Guangdong Foshan joint fund of Guangdong Natural Science Foundation (Grant No. 2020A1515110601).References
- H. J. Zhou, G. Y. Zhu, S. Y. Dong, P. Liu, Y. Y. Lu, Z. Zhou, S. Cao, Y. Z. Zhang and H. Pang, Adv. Mater., 2023, 35, 2211523 CrossRef CAS PubMed
.
- C. L. Liu, Y. Bai, W. T. Li, F. Y. Yang, G. X. Zhang and H. Pang, Angew. Chem., Int. Ed., 2022, 61, e202116282 CrossRef CAS PubMed
.
- X. F. Zhang, X. D. Liu, R. Z. Yan, J. Q. Yang, Y. Liu and S. L. Dong, J. Mater. Chem. C, 2020, 8, 2008–2013 RSC
.
- A. Barua, P. Mehra and A. Paul, ACS Appl. Energy Mater., 2021, 4, 14249–14259 CrossRef CAS
.
- H. Ashassi-Sorkhabi and P. La’le Badakhshan, Appl. Surf. Sci., 2017, 419, 165–176 CrossRef CAS
.
- Y. M. He, W. J. Chen, X. D. Li, Z. X. Zhang, J. C. Fu, C. H. Zhao and E. Q. Xie, ACS Nano, 2013, 7, 174–182 CrossRef CAS PubMed
.
- Z. H. Huang, Y. Song, D. Y. Feng, Z. Sun, X. Q. Sun and X.-X. Liu, ACS Nano, 2018, 12, 3557–3567 CrossRef CAS PubMed
.
- D. J. Wu, S. H. Xu, C. Zhang, Y. P. Zhu, D. Y. Xiong, R. Huang, R. J. Qi, L. W. Wang and P. K. Chu, J. Mater. Chem. A, 2016, 4, 11317–11329 RSC
.
- L. Chen, F. Wang, Z. W. Tian, H. T. Guo, C. Y. Cai, Q. J. Wu, H. J. Du, K. M. Liu, Z. F. Hao, S. J. He, G. G. Duan and S. H. Jiang, Small, 2022, 18, 2201307 CrossRef CAS PubMed
.
- L. Wan, J. X. Liu J, Y. Zhang, J. Chen, C. Du and M. J. Xie, Int. J. Hydrogen Energy, 2020, 45, 4521–4533 CrossRef CAS
.
- X. Feng, J. Ning, D. Wang, J. C. Zhang, M. Y. Xia, Y. Wang and Y. Hao, J. Alloys Compd., 2020, 816, 152625 CrossRef CAS
.
- R. B. Liang, Y. Q. Du, J. X. Wu, X. T. Li, T. X. Liang, J. Yuan, P. Xiao and J. W. Chen, J. Solid State Chem., 2022, 307, 122845 CrossRef CAS
.
- R. B. Liang, Y. Q. Du, J. R. Lin, J. W. Chen and P. Xiao, Energy Fuels, 2022, 26, 7115–7120 CrossRef
.
- J. Z. Ji, X. X. Pan, J. H. Qin, X. M. Chen and Q. B. Zha, J. Electron. Mater., 2020, 49, 4010–4017 CrossRef
.
- L. L. Jiang, X. T. Mei, D. L. Gan, S. W. Song, X. B. Lei, F. G. Cai, Y. Q. Wang, Q. Y. Zhang, X. Lu and Z. F. Ren, Chem. –Eur. J., 2021, 27, 5761–5768 CrossRef CAS PubMed
.
- H. X. Wang, H. Yang, D. Wang, D. Y. Cheng, T. Deng, H. Y. Liu, H. B. Zhang, W. Zhang and W. T. Zheng, Nanotechnology, 2020, 31, 105402 CrossRef CAS PubMed
.
- Y. Zhao, J. Z. Liu, D. Z. Zheng, B. Wang, M. J. Hu, J. B. Sha and Y. Li, Small, 2018, 14, 1702809 CrossRef PubMed
.
- Y. J. Ye, Z. H. Huang, Y. Song, J. W. Geng, X. X. Xu and X. X. Liu, Electrochim. Acta, 2017, 240, 72–79 CrossRef CAS
.
- L. Z. Sheng, Y. Y. Zhao, B. Q. Hou, Z. P. Xiao, L. L. Jiang and Z. J. Fan, N. Carbon Mater., 2021, 36, 167–175 CrossRef
.
- Y. X. Wen, Y. P. Liu and D. Y. He, ACS Appl. Energy Mater., 2021, 4, 6531–6541 CrossRef CAS
.
- H. Liu, H. Guo, W. Q. Yao, L. W. Zhang, M. Y. Wang, T. Fan, W. H. Yang and W. Yang, Colloids Surf., A, 2020, 601, 125011 CrossRef CAS
.
- W. Lu, Z. Yuan, C. Y. Xu, J. Q. Ning, Y. J. Zhong, Z. Y. Zhang and Y. Hu, J. Mater. Chem. A, 2019, 7, 5333–5343 RSC
.
- J. Wang, J. Polleux, J. Lim and B. Dunn, J. Phys. Chem. C, 2007, 111, 14925–14931 CrossRef CAS
.
- Y. Yue, Y. L. Huang and S. W. Bian, ACS Appl. Electron. Mater., 2021, 3, 2178–2186 CrossRef CAS
.
- G. Z. Zhao, Y. J. Li, G. Zhu, J. Y. Zhu, T. Lu and L. K. Pan, Ionics, 2019, 25, 3935–3944 CrossRef CAS
.
- C. L. Hu, X. Y. Zhang, B. Liu, S. Y. Chen, X. Q. Liu, Y. M. Liu, J. Y. Liu and J. Chen, Electrochim. Acta, 2020, 338, 135846 CrossRef CAS
.
- K. J. Samdani, S. H. Kim, J. H. Park, S. H. Hong and K. T. Lee, J. Ind. Eng. Chem., 2019, 74, 96–102 CrossRef CAS
.
- M. L. Wang, T. Y. Zhang, M. Z. Cui, W. F. Liu, X. G. Liu, J. W. Zhao and J. D. Zhou, Chin. Chem. Lett., 2021, 32, 1111–1116 CrossRef CAS
.
- B. Liu, X. Y. Zhang, D. Tian, Q. Li, M. Zhong, S. Y. Chen, C. L. Hu and H. B. Ji, ACS Omega, 2020, 5, 32395–32402 CrossRef CAS PubMed
.
- S. Sundriyal, V. Shrivastav and S. Mishra S, Int. J. Hydrogen Energy, 2020, 45, 30859–30869 CrossRef CAS
.
- X. M. Yang, T. Li, X. X. He, Q. Li, J. Sun, Z. B. Lei and Z. H. Liu, J. Power Sources, 2022, 520, 230899 CrossRef CAS
.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ra06937k |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2023 |