Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Capillary electrophoresis mass spectrometry identifies new isomers of inositol pyrophosphates in mammalian tissues†
Danye
Qiu‡
ab,
Chunfang
Gu‡
c,
Guizhen
Liu
a,
Kevin
Ritter
a,
Verena B.
Eisenbeis
 a,
Tamara
Bittner
a,
Artiom
Gruzdev
c,
Lea
Seidel
bde,
Bertram
Bengsch
bd,
Stephen B.
Shears
a,
Tamara
Bittner
a,
Artiom
Gruzdev
c,
Lea
Seidel
bde,
Bertram
Bengsch
bd,
Stephen B.
Shears
 *c and
Henning J.
Jessen
*c and
Henning J.
Jessen
 *ab
*ab
aInstitute of Organic Chemistry, Faculty of Chemistry and Pharmacy, University of Freiburg, 79104, Freiburg, Germany
bCIBSS – Centre for Integrative Biological Signaling Studies, University of Freiburg, Germany. E-mail: Henning.jessen@oc.uni-freiburg.de
cSignal Transduction Laboratory, National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, NC 27709, USA. E-mail: shears@niehs.nih.gov
dClinic for Internal Medicine II (Gastroenterology, Hepatology, Endocrinology and Infectious Diseases), Freiburg University Medical Center, Faculty of Medicine, University of Freiburg, Freiburg, Germany
eSGBM – Spemann Graduate School of Biology and Medicine, University of Freiburg, Germany
First published on 5th December 2022
Abstract
Technical challenges have to date prevented a complete profiling of the levels of myo-inositol phosphates (InsPs) and pyrophosphates (PP-InsPs) in mammalian tissues. Here, we have deployed capillary electrophoresis mass spectrometry to identify and record the levels of InsPs and PP-InsPs in several tissues obtained from wild type mice and a newly created PPIP5K2 knockout strain. We observe that the mouse colon harbours unusually high levels of InsPs and PP-InsPs. Additionally, the PP-InsP profile is considerably more complex than previously reported for animal cells: using chemically synthesized internal stable isotope references and high-resolution mass spectra, we characterize two new PP-InsP isomers as 4/6-PP-InsP5 and 2-PP-InsP5. The latter has not previously been described in nature. The analysis of feces and the commercial mouse diet suggests that the latter is one potential source of noncanonical isomers in the colon. However, we also identify both molecules in the heart, indicating unknown synthesis pathways in mammals. We also demonstrate that the CE-MS method is sensitive enough to measure PP-InsPs from patient samples such as colon biopsies and peripheral blood mononuclear cells (PBMCs). Strikingly, PBMCs also contain 4/6-PP-InsP5 and 2-PP-InsP5. In summary, our study substantially expands PP-InsP biology in mammals.
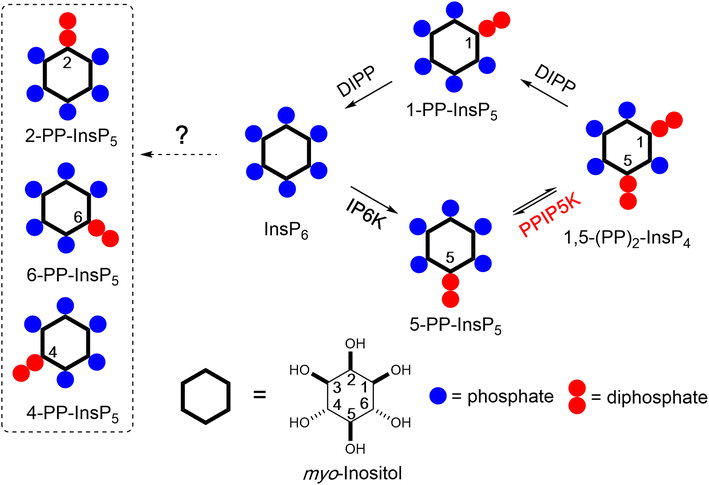
Inositol phosphates (InsPs) and pyrophosphates (PP-InsPs) are a complex signalling hub with diverse functions in eukaryotes.1–3 PP-InsPs have specialized physicochemical properties and biological functions that attract widespread interest.4–7 They occur as distinct isomers of differentially phosphorylated metabolites of InsP6 (phytic acid and phytate). The current literature suggests that in yeast and mammals these phosphorylation reactions occur selectively and successively in the 5- and 1-positions (Fig. 1) leading to 5-PP-InsP5 and 1,5-(PP)2-InsP4, respectively.8,9 In plants and slime-mold, 4/6-PP-InsP5 has been identified as the main isomer, with the absolute configuration of the biologically relevant enantiomer remaining unknown.10,11
Kinases and phosphatases that synthesize and metabolize PP-IPs are distributed throughout all eukaryotic kingdoms.7,12 In mammals, there are three isoforms of IP6Ks that add a β-phosphate at position 5 and two isoforms of PPIP5Ks that add a β-phosphate at position 1.8
Most of the research into PP-InsP turnover in mammalian cells has relied on separation by HPLC of extracts of 3H-inositol radiolabeled cells, although in more recent years a more generally accessible PAGE technique has proved useful.13,14 This body of work has consistently concluded that 5-PP-InsP5 is the most abundant PP-InsP isomer (generally <10% of InsP6 levels). The levels of 1,5-(PP)2-InsP4 and 1-PP-InsP5 are approximately 10-fold and 50-fold lower, which are below the PAGE detection limit.14–16 The relative ease with which 5-PP-InsP5 abundance can be measured has in large part driven the field's primary focus on this isomer. For example, this PP-InsP has been reported to regulate insulin signalling, exocytosis, processing body formation, intracellular protein localization, and bioenergetic homeostasis.17–22
More recently, 1,5-(PP)2-InsP4 has emerged as an independently regulated cellular signal. This facet of PP-InsP signalling first arose from kinetic assessments23 of the PPIP5K kinase domain that phosphorylates 5-PP-InsP5 to 1,5-(PP)2-InsP4 and the separate phosphatase domain that degrades 1,5-(PP)2-InsP4 back to 5-PP-InsP5 (see Fig. 1). Moreover, the phosphatase activity is inhibited by elevations in the cellular levels of inorganic phosphate (Pi), thereby enhancing net 1,5-(PP)2-InsP4 production independently of any changes in 5-InsP7 levels.23,24 As a consequence, the net kinase and phosphatase activities are tied to cellular energy and phosphate homeostasis.3,25 It has since been demonstrated that 1,5-(PP)2-InsP4 stimulates Pi efflux from mammalian cells through an interaction with an SPX domain on the transmembrane XPR1 protein.26,27 Moreover, pharmacologic inhibition of IP6Ks in mammals (rodents and monkeys), which restrains PP-InsP5 and 1,5-(PP)2-InsP4 synthesis (see Fig. 1), leads to attenuation of systemic hyperphosphatemia through inactivation of XPR1; these findings are an important milestone for potential pharmacological treatment of chronic kidney disease.28 Naturally occurring human variants of PPIP5K2 have been associated with deafness29 and keratoconus.30 Recently, [3H]inositol-radiolabeling of a hematopoietic stem cell line from a PPIP5K2−/+ mouse indicated that 1,5-(PP)2-[3H]InsP4 levels are no different from those in a typical culture medium (data for PPIP5K2−/− cells were not reported).31 In such circumstances, it has become more important to be able to accurately assay dynamic fluctuations in 1,5-(PP)2-InsP4 concentrations.
A portfolio of additional methods has been introduced that can assay the mass levels of (PP)-InsPs in extracts of mammalian and plant cells, including using transition metals (e.g. Fe and Y) and absorbance detection (metal dye detection, MDD)32–34 and the coupling of in-line mass spectrometry to hydrophilic interaction liquid chromatography (HILIC) and metal-free C18 reversed phase columns.28,35,36 NMR detection with 13C enriched inositol is another recent and promising addition to the analytical portfolio.37 In 2020, capillary electrophoresis (CE) with mass spectrometry compatible buffers was reported for PP-InsP analytics, with only nanoliter sample consumption and accurate isomer assignment and quantitation by using stable isotope internal reference compounds.38
We now significantly expand the value of our new PP-InsP profiling techniques through our identification of substantial cellular quantities of mammalian 4/6-PP-InsP5 and 2-PP-InsP5 (see Fig. 1) based on comigration with reference compounds and high-resolution mass spectra. This conclusion is facilitated by adapting a recently developed 18O phosphate labelling approach39 in order to stereoselectively synthesize 4-PP-InsP5 to use as a heavy internal standard. Finally, it was our goal to optimize CE-MS to monitor the complete array of PP-InsPs from human patient tissues. For this work, we selected colon biopsies and peripheral blood mononuclear cells including enriched T cell subpopulations (PBMCs, CD8+). Strikingly, we also identify 4/6-PP-InsP5 and 2-PP-InsP5 in PBMCs that are particularly enriched in a CD8+ T cell preparation. Overall, this dramatic increase in the complexity of PP-InsP metabolism indicates that their biological significance has been greatly underestimated.
Results
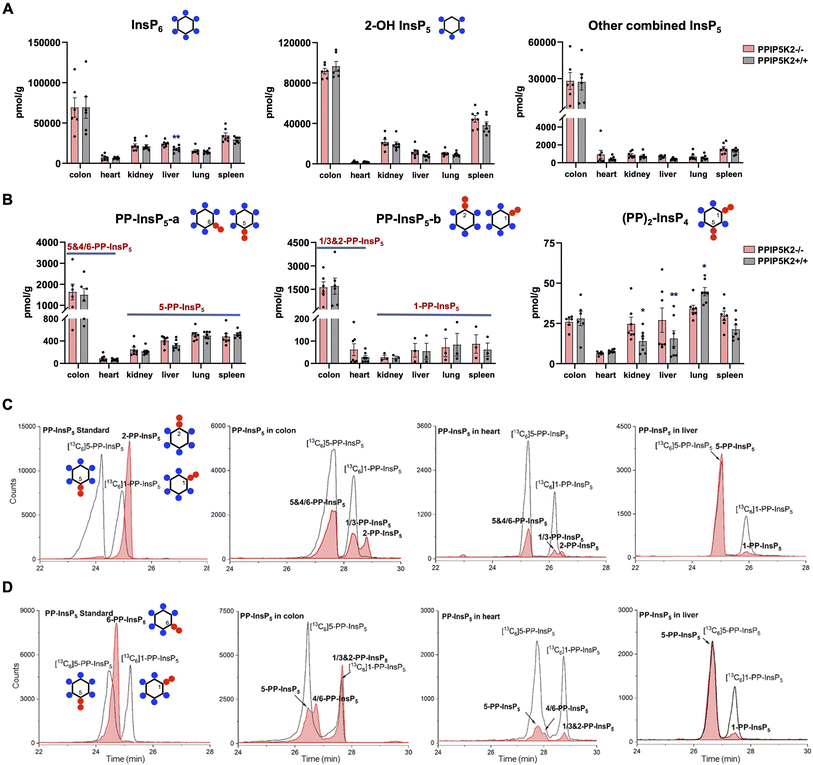
With an established protocol that uses TiO2 beads, we extracted and enriched InsPs and PP-InsPs from different mouse tissues.14,38 The enriched samples were analyzed by CE-QQQ using the same background electrolyte (35 mM ammonium acetate adjusted to pH 9.7 with NH4OH, i.e., BGE-A) that we deployed in our previous study.38 Samples were spiked with internal heavy isotope reference compounds (13C labels) of several different InsPs and PP-InsPs for assignment and quantitation. This is the first time that this method has been applied to any animal tissue for the quantification of the levels of the least abundant PP-IPs, namely 1,5-(PP)2-InsP4 and 1-PP-InsP5 (for representative examples see Fig. 2C and ESI Fig. 1†).We also used this method to compare InsP and PP-InsP levels in multiple mouse tissues, including the colon, heart, kidney, liver, lung and spleen (Fig. 2A–C). These molecules were generally least abundant in the heart. It is worth mentioning that other minor InsP5 isomers including 4/6-OH InsP5, 1/3-OH InsP5, and 5-OH InsP5 have also been identified and quantified (see representative examples obtained from the mouse colon and mouse heart; ESI Fig. 2†), while 2-OH InsP5 was always by far the predominant isomer in all investigated mouse tissues (Fig. 2A).
Compared to other tissues, the colon is notable for containing substantially higher levels of InsP6 (2- to 5-fold), 2-OH-InsP5 (2- to 10-fold) and the sum of the remaining, quantitatively more minor InsP5 isomers (19- to 52-fold). The colon also contains much higher levels of PP-InsP5 isomers (Fig. 2A–C). In most of the studied tissues (kidney, liver, lung, and spleen), two baseline-resolved PP-InsP5 signals were observed (labeled ‘a’ and ‘b’), which co-eluted precisely with internal standards of [13C6]5-PP-InsP5 and [13C6]1-PP-InsP5, respectively, in each of two different BGE conditions (Fig. 2C and ESI Fig. 3A†). In these tissues, the relative proportion of 1-PP-InsP5 to 5-PP-InsP5 (approximately 1 to 7) is higher than that determined by our previous CE analysis of a line of immortalized HCT116 cells (1 to 13);40 a ratio of only 1 to 50 was previously obtained by HPLC analysis of [3H]inositol-labeled extracts of immortalized cells.41
An unexpected outcome of the EIE obtained using BGE-A was that the PP-InsP-b signals derived from the colon and heart split into two approximately equally sized peaks that are incompletely resolved; the earlier-eluting peak comigrated with an internal standard of [13C6]1-PP-InsP5 (Fig. 2C). The elution time of the second peak corresponds precisely to the elution time of a replicate sample spiked with an internal standard of 2-PP-InsP5 (ESI Fig. 4†). In addition, there is an indication that the PP-InsP-a signal derived from the colon also separates into two incompletely resolved peaks (Fig. 2C). To pursue the latter observation, we reran the samples with the background electrolyte adjusted to 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mM ammonium acetate titrated with ammonium hydroxide to pH 9.0 (i.e., BGE-B). This procedure extended the peak-to-peak resolution within the PP-InsP-a signal to the extent that its two components are also visible in the extracts prepared from the colon and heart (Fig. 2D). Note that, in contrast, the use of BGE-B did not perturb the coelution of internal standards of [13C6]5-PP-InsP5 and [13C6]1-PP-InsP5 with PP-InsP-a and PP-InsP-b signals, respectively, that were prepared from the kidney, liver, lung and spleen (Fig. 2C and D; ESI Fig. 3A and B†). However, we do not exclude that matrix effects in other tissues would blur the presence of low levels of additional PP-InsP isomers.
mM ammonium acetate titrated with ammonium hydroxide to pH 9.0 (i.e., BGE-B). This procedure extended the peak-to-peak resolution within the PP-InsP-a signal to the extent that its two components are also visible in the extracts prepared from the colon and heart (Fig. 2D). Note that, in contrast, the use of BGE-B did not perturb the coelution of internal standards of [13C6]5-PP-InsP5 and [13C6]1-PP-InsP5 with PP-InsP-a and PP-InsP-b signals, respectively, that were prepared from the kidney, liver, lung and spleen (Fig. 2C and D; ESI Fig. 3A and B†). However, we do not exclude that matrix effects in other tissues would blur the presence of low levels of additional PP-InsP isomers.
In this set of experiments with BGE-B, the first component of PP-InsP-a extracted from the colon comigrates with the internal standard of [13C6]5-PP-InsP5 and the second component of PP-InsP-a has an elution time that matches that of a standard of 6-PP-InsP5 from separate runs (Fig. 2D). Thus, we tentatively identify the second component of PP-InsP-a as 4/6-PP-InsP5 and by a process of elimination we suggest that the second component of PP-InsP-b is 2-PP-InsP5. Moreover, the proposed nature of 1/3-PP-InsP5, 2-PP-InsP5, 5-PP-InsP5 and 4/6-PP-InsP5 from the colon is also consistent with their high-resolution mass spectra collected by using a CE-qTOF system (ESI Fig. 5†). Other potential candidates with an identical mass, such as triphosphates of inositol-tetrakisphosphates (e.g. 5-PPP-InsP4), have been described so far only in vitro.42 The myo-configuration for these new PP-InsPs seems likely, since there is no prior identification of any other multiply phosphorylated inositol stereoisomers in mammals.
It is notable that in the colon we estimate that the levels of 1-PP-InsP5 (i.e., half of PP-InsP-b) and 5-PP-InsP5 (i.e., half of PP-InsP-a) are approximately equivalent (Fig. 2C and D); this observation implies that we must profoundly modify prior perceptions of 1-PP-InsP5 as a quantitatively minor constituent of mammalian cells and/or consider the possibility that the enantiomer 3-PP-InsP5 is also present. Currently applied methods do not resolve the enantiomers.
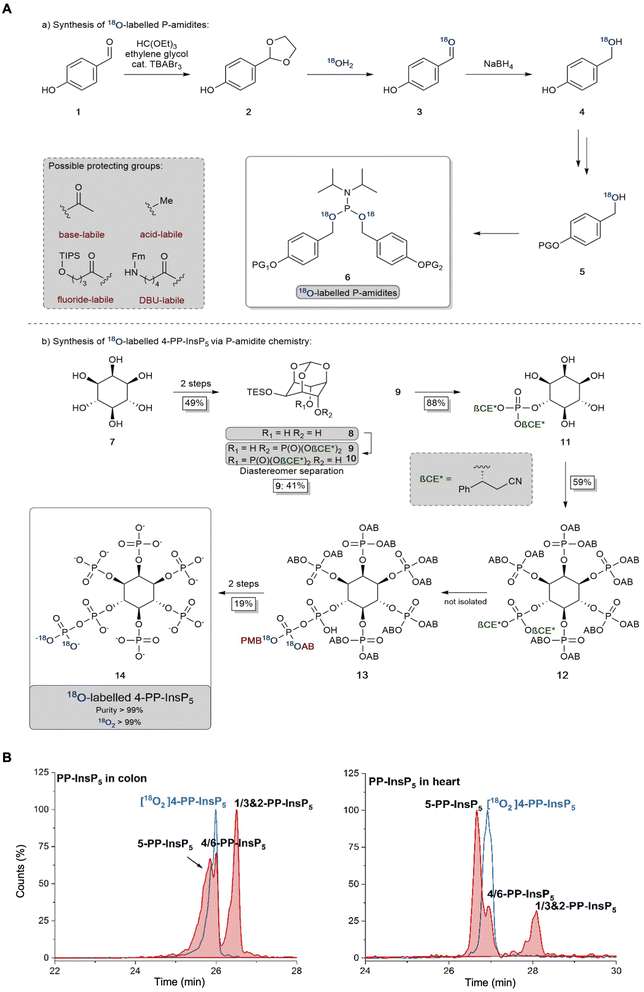
The 2-PP-InsP5 isomer has not previously been identified in any biological material, possibly because it is both unexpected and only present at relatively low levels. In contrast, 4/6-PP-InsP5 was recently discovered to be a major PP-InsP isomer in plants.10 Clearly, the latter is also a quantitatively important isomer in the mouse colon and heart (Fig. 2), and so it was particularly important to further validate its nature. Thus, we have developed a synthetic route to the preparation of enantiomerically pure [18O2]4-PP-InsP5 to deploy as an internal standard for additional chromatographic resolutions (see Fig. 3).
We have also recorded 1,5-(PP)2-InsP4 levels in mouse tissues (Fig. 2B). These varied over a 5-fold range, with the lowest levels in the heart and the highest in the lung; as far as we are aware, no previous study has provided such data. This accomplishment enabled us to determine the impact upon 1,5-(PP)2-InsP4 levels in a newly created PPIP5K2 knockout mouse (ESI Fig. 6 and 7†). No abnormal phenotype in the KO was observed. The litter size and gender distribution were not modified. No gross anatomical phenotype was observed during tissue collection. Food intake or energy expenditure (metabolic rate and physical activity) were unchanged (ESI Fig. 8†). We did not conduct any behavioral phenotyping.
The knockout only resulted in a statistically significant reduction in 1,5-(PP)2-InsP4 levels in the lung tissue (Fig. 2B). In fact, 1,5-(PP)2-InsP4 levels trended higher in several PPIP5K2 knockout tissues compared to the wild-type, and in the kidney and liver this effect was statistically significant. Although this might initially seem a counter-intuitive outcome, it is possible that in these two tissues the loss of the PPIP5K2 1,5-(PP)2-InsP4-phosphatase domain may have a larger metabolic effect than the loss of the 5-PP-InsP5 kinase domain. The knockout did not elicit a statistically significant impact on 1,5-(PP)2-InsP4 levels in either the colon or the heart. The observation of tissue dependent variability in PP-InsP signaling brought about by PPIP5K2 knockout may depend in part on the extent to which PPIP5K1 compensates for the deletion of PPIP5K2 catalytic activity, although no such effect was evident in the liver (ESI Fig. 6 and 7†). Note also that the PPIP5K2 KO did not have off-target effects on any of the other InsPs and PP-InsPs analyzed in this study (Fig. 2A and B), except that InsP6 was increased in the PPIP5K KO liver.
We could not derive sufficient purified amounts of the putative 4/6-PP-InsP5 for NMR analysis to further corroborate the identity of this isomer. So instead, we generated a reference compound with a heavy isotope label to serve as an internal standard for CE-MS. We reasoned that the comigration of this compound under different separation conditions would serve as a strong indication that it is indeed 4/6-PP-InsP5 in its myo-configuration. The enzymes for plant 4/6-PP-InsP5 synthesis are not yet known and so an enzymatic synthesis starting from InsP6 of the reference compound with 13C labels was not possible.43 A fully chemical synthesis from expensive 13C glucose in a multi-step linear approach was deemed not feasible.37 We thus relied on our recently developed 18O phosphate labeling approach in which the expensive isotopic label can be introduced in the penultimate step of the synthesis.39
In brief, 18O labeled phosphoramidites (P-amidites) with high 18O/16O ratios are key to the synthesis. These high ratios can be obtained by the strategy shown in Fig. 3A(a). Para-hydroxybenzaldehyde is transformed into its acetal 2, which is then hydrolyzed in the presence of 99% 18O enriched water. The aldehyde 3 is directly reduced to stable alcohol 4, which can then be protected on the phenol with diverse protecting groups (in the case described here simply acetate giving the acetoxybenzyl (AB) protecting group). The alcohols 5 are then transformed into P-amidites of the general structure 6, enabling diverse protecting group patterns and high 18O enrichment. The inositol structure is assembled as reported previously,44–47 as shown in Fig. 3A(b). While strictly a desymmetrization was not required and the generation of racemic 4/6-PP-InsP5 would have been sufficient, we still generated the enantiomerically pure compound for potential future applications. Desymmetrization was achieved from intermediate protected diol 8, which was reacted with an unsymmetric P-amidite containing chiral protecting groups (β-CE*, an arylated enantiomerically pure variant of the β-cyanoethyl protecting group). The obtained diastereomeric mixture was separated and then the inositol protecting groups were removed giving pentaol 11. 11 was phosphorylated to protected InsP612 with orthogonal protecting groups (β-CE*) in the 4-position.44 Selective deprotection in that position then enables the introduction of the 18O labeled phosphate bearing two 18O oxygen atoms (M + 4). Global deprotection gave [18O]2 4-PP-InsP514 in 99% purity with >99% isotopic enrichment as determined by CE-MS. This reference compound was then dissolved in water and its concentration was determined by quantitative 1H- and 31P-NMR.
Fig. 3B demonstrates the first application of this newly generated isotopologue. Briefly, both colon and heart samples were spiked with the new reference and we utilized the optimized BGE-B that is capable of 5-PP-InsP5 and 4/6-PP-InsP5 separation. Masses were recorded and an identical migration of the unknown analyte with our reference in the same matrix was found, strongly suggesting that it is indeed 4/6-PP-InsP5 that has been measured for the first time in mammalian tissues.
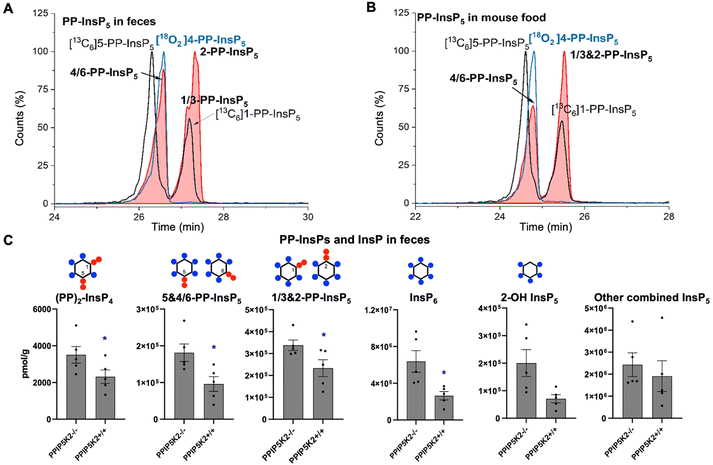
To understand the complexity of the profiles of InsPs and PP-InsPs in the colon in an organismal context, we additionally analyzed mouse feces and found them to contain very high levels of most analytes (Fig. 4A and C, ESI Fig. 9†). Moreover, neither PP-InsP peak co-eluted precisely with internal standards of either 5-PP-InsP5 or 1-PP-InsP5 again pointing towards the existence of both 4/6-PP-InsP5 and 2-PP-InsP5. In fact, the two new isomers are the most abundant analytes we detect (Fig. 4A). Interestingly, the PPIP5K knockout contained increased levels of all analytes in feces. We excluded that this was due to differences in food intake (ESI Fig. 8†). It may be possible that such changes are caused by different expression of digestive enzymes of PP-InsPs.
We next investigated if the mouse laboratory diet might contribute to the unprecedented complexity of the colonic PP-InsP profile. We provided mice with the “Rodent NIH-31 Open Formula Autoclavable Diet”, much of which is of plant origin. This is significant because recent work has determined that the quantitatively most important PP-InsP isomer in plants is one that had previously been overlooked, namely 4/6-PP-InsP5.10 Indeed, our internal standards allowed us to conclude that large amounts of 4/6-PP-InsP5 were present in the mouse diet, although a precise quantification was hindered by insufficient separation of the 4/6- and 5-PP-InsP5 peaks from the PP-InsP5-a peak (Fig. 4B). Nevertheless, the latter was smaller than the PP-InsP5-b peak, which likely comprises a mixture of 1-PP-InsP5 and 2-PP-InsP5.
Our results present the possibility that the diet might be the source of the colon's unusually high levels of InsP6 and PP-InsPs, as well as the more complex PP-InsP profile. Furthermore, 2-OH InsP5 is the minor InsP5 isomer in mouse feces and also in mouse food (ESI Fig. 10†), in contrast to it being the major InsP5 in the colon. This result suggests that the exceptional PP-InsPs and InsP6 profile in the colon are not due to contamination from feces during sample preparation. In this case, endocytosis of dietary InsP6 and PP-InsPs by colonic epithelial cells should be considered as a viable possibility.
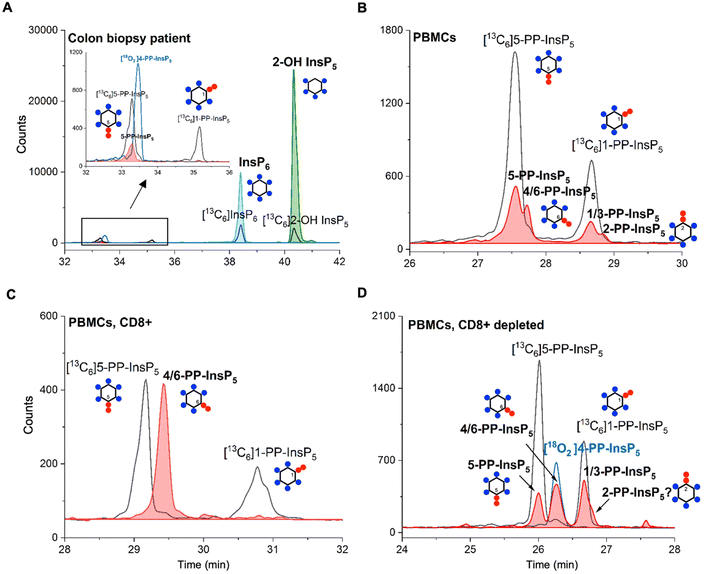
Finally, in order to demonstrate the sensitivity of the method and its potential in translational research, we obtained human samples for enrichment and profiling. We analyzed one 18 mg wet tissue colon biopsy, which was sufficient to profile the main PP-InsP and InsP contents (Fig. 5A). Only canonical isomers were identified, i.e. 5-PP-InsP5, InsP6, and 2-OH InsP5. We additionally analyzed peripheral blood mononuclear cells (PBMCs; Fig. 5B) from donors, and also CD8+ T-cells enriched from the PBMC pool by FACS (see the ESI†). Strikingly, in one such enriched sample, we identified 4/6-PP-InsP5 as the sole PP-InsP isomer (Fig. 5C). Of note, the CD8+ depleted PBMC pool (Fig. 5D) also contained 4/6-PP-InsP5 as well as 5-PP-InsP5 and the latter was identified as the minor isomer. Moreover, a peak comigrating with 2-PP-InsP5 was identified in PBMCs (Fig. 5B) and can be tentatively assigned to a shoulder of the peak of the CD8+ depleted fraction (Fig. 5D). CD8+ enrichment did not provide enough material for analysis in all samples studied, so it remains unclear whether the surprising 4/6-PP-InsP5 enrichment is generally found in CD8+ cells from different donors. However, our analysis now firmly establishes that this new isomer is of mammalian origin.
Conclusions
We have applied CE-MS profiling to delineate a more sophisticated picture of InsP and PP-InsP distributions in metazoan samples. Thus, inositol pyrophosphate signalling appears even more complex than previously thought. The CE-MS method also has sufficient sensitivity to profile for the first time biopsies from human patients and PBMCs including isolated CD8+ T-cells from human blood. We obtain several unexpected results based on the high separation efficiency of capillary electrophoresis that have gone undetected with recently developed LC-MS approaches.28,35,36 In particular, we identify very high levels of PP-InsPs in colon tissue, which are potentially endocytosed from the laboratory diet, including large quantities of the putative noncanonical 4/6 and 2-PP-InsP5 isomers. Our data therefore represent a paradigm shift in our understanding of dietary influences upon PP-InsP metabolism and signaling in the colon. While 4/6-PP-InsP5 and 2-PP-InsP5 in the colon could possibly originate from the endocytosis of food constituents, this phenomenon cannot apply to heart samples as well as human PBMCs. Consequently, it appears that 4/6-PP-InsP5 and 2-PP-InsP5 can also be synthesized by mammals.Our new isomer assignments are based on the exact mass determination and exact comigration with standards of both PP-InsPs, including a novel synthetic 4-PP-InsP5 bearing two 18O oxygen isotope labels. Future studies must now address the enantiomeric identity of the new metazoan 4/6-PP-InsP5 as well as a complete structural assignment of 2-PP-InsP5 by NMR to firmly establish myo-configuration and exclude other potential constitutional isomers of the same mass and identical migration during CE. The colonic uptake, dynamic regulation, unknown enzymology and functions of these new isomers will be productive directions for future research, including their presence in the central nervous system. With the ability to profile PP-InsPs from human biopsies and blood samples, their establishment as potential disease biomarkers will also become an important future endeavor.
Data availability
Additional data can be found in the ESI.†Author contributions
DQ and CG designed, performed, and evaluated analytical experiments. CG was responsible for the animal experiments. SBS supervised animal experiments. GL and VE performed biopsy extractions and analytics. KR conducted the chemical synthesis. TB and LS isolated and extracted PBMCs. BB supervised human tissue extractions and designed experiments. HJJ and SBS conceived the idea of the project and designed experiments. HJJ, DQ, CG, and SBS wrote the manuscript. All authors provided feedback on experimental design and contributed to manuscript revisions.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was supported by the German Research Foundation (DFG) under Germany's Excellence Strategy (CIBSS – EXC-2189 – Project ID 390939984) and DFG Grant JE 572/4-1. HJJ and GL acknowledge funding from the Volkswagen Foundation (VW Momentum Grant 98604). This research was also supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences. The authors wish to thank Dorothea Fiedler (Leibniz Institut für Molekulare Pharmakologie, Berlin) and particularly Minh Nguyen Trung und Robert Harmel for providing 13C-labeled reference compounds.References
- R. F. Irvine and M. J. Schell, Back in the water: The return of the inositol phosphates, Nat. Rev. Mol. Cell Biol., 2001, 2, 327 CrossRef CAS PubMed.
- D. Laha, P. Portela-Torres, Y. Desfougeres and A. Saiardi, Inositol phosphate kinases in the eukaryote landscape, Adv. Biol. Regul., 2021, 79, 100782 CrossRef CAS PubMed.
- S. B. Shears, Intimate connections: Inositol pyrophosphates at the interface of metabolic regulation and cell signaling, J. Cell. Physiol., 2018, 233, 1897 CrossRef CAS PubMed.
- S. Lee, M. G. Kim, H. Ahn and S. Kim, Inositol Pyrophosphates: Signaling Molecules with Pleiotropic Actions in Mammals, Molecules, 2020, 25, 2208 CrossRef CAS PubMed.
- S. B. Shears and H. Wang, Metabolism and Functions of Inositol Pyrophosphates: Insights Gained from the Application of Synthetic Analogues, Molecules, 2020, 25, 4515 CrossRef CAS PubMed.
- N. W. Brown, A. M. Marmelstein and D. Fiedler, Chemical tools for interrogating inositol pyrophosphate structure and function, Chem. Soc. Rev., 2016, 45, 6311 RSC.
- M. Nguyen Trung, D. Furkert and D. Fiedler, Versatile signaling mechanisms of inositol pyrophosphates, Curr. Opin. Chem. Biol., 2022, 70, 102177 CrossRef CAS PubMed.
- S. B. Shears, Inositol pyrophosphates: why so many phosphates?, Adv. Biol. Regul., 2015, 57, 203 CrossRef CAS.
- T. A. Randall, C. Gu, X. Li, H. Wang and S. B. Shears, A two-way switch for inositol pyrophosphate signaling: Evolutionary history and biological significance of a unique, bifunctional kinase/phosphatase, Adv. Biol. Regul., 2020, 75, 100674 CrossRef.
- E. Riemer, D. Qiu, D. Laha, R. K. Harmel, P. Gaugler, V. Gaugler, M. Frei, M.-R. Hajirezaei, N. P. Laha, L. Krusenbaum, R. Schneider, A. Saiardi, D. Fiedler, H. J. Jessen, G. Schaaf and R. F. H. Giehl, ITPK1 is an InsP6/ADP phosphotransferase that controls phosphate signaling in Arabidopsis, Mol. Plant, 2021, 14, 1864 CrossRef CAS.
- Y. Desfougères, P. Portela-Torres, D. Qiu, T. M. Livermore, R. K. Harmel, F. Borghi, H. J. Jessen, D. Fiedler and A. Saiardi, The inositol pyrophosphate metabolism of Dictyostelium discoideum does not regulate inorganic polyphosphate (polyP) synthesis, Adv. Biol. Regul., 2022, 83, 100835 CrossRef PubMed.
- R. S. Kilari, J. D. Weaver, S. B. Shears and S. T. Safrany, Understanding inositol pyrophosphate metabolism and function: kinetic characterization of the DIPPs, FEBS Lett., 2013, 587, 3464 CrossRef CAS PubMed.
- O. Losito, Z. Szijgyarto, A. C. Resnick and A. Saiardi, Inositol Pyrophosphates and Their Unique Metabolic Complexity: Analysis by Gel Electrophoresis, PLoS One, 2009, 4, e5580 CrossRef PubMed.
- M. S. C. Wilson, S. J. Bulley, F. Pisani, R. F. Irvine and A. Saiardi, A novel method for the purification of inositol phosphates from biological samples reveals that no phytate is present in human plasma or urine, Open Biol., 2015, 5, 150014 CrossRef.
- F. Pisani, T. Livermore, G. Rose, J. R. Chubb, M. Gaspari and A. Saiardi, Analysis of Dictyostelium discoideum Inositol Pyrophosphate Metabolism by Gel Electrophoresis, PLoS One, 2014, 9, e85533 CrossRef PubMed.
- I. Pavlovic, D. T. Thakor, L. Bigler, M. S. Wilson, D. Laha, G. Schaaf, A. Saiardi and H. J. Jessen, Prometabolites of 5-Diphospho-myo-inositol Pentakisphosphate, Angew. Chem., Int. Ed., 2015, 54, 9622 CrossRef CAS PubMed.
- A. Chakraborty, M. A. Koldobskiy, N. T. Bello, M. Maxwell, J. J. Potter, K. R. Juluri, D. Maag, S. Kim, A. S. Huang, M. J. Dailey, M. Saleh, A. M. Snowman, T. H. Moran, E. Mezey and S. H. Snyder, Inositol Pyrophosphates Inhibit Akt Signaling, Thereby Regulating Insulin Sensitivity and Weight Gain, Cell, 2010, 143, 897 CrossRef CAS PubMed.
- T.-S. Lee, J.-Y. Lee, J. W. Kyung, Y. Yang, S. J. Park, S. Lee, I. Pavlovic, B. Kong, Y. S. Jho, H. J. Jessen, D.-H. Kweon, Y.-K. Shin, S. H. Kim, T.-Y. Yoon and S. Kim, Inositol pyrophosphates inhibit synaptotagmin-dependent exocytosis, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 8314 CrossRef CAS PubMed.
- S. Sahu, Z. Wang, X. Jiao, C. Gu, N. Jork, C. Wittwer, X. Li, S. Hostachy, D. Fiedler, H. Wang, H. J. Jessen, M. Kiledjian and S. B. Shears, InsP7 is a small-molecule regulator of NUDT3-mediated mRNA decapping and processing-body dynamics, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 19245 CrossRef CAS PubMed.
- A. Shah and R. Bhandari, IP6K1 upregulates the formation of processing bodies by influencing protein–protein interactions on the mRNA cap, J. Cell Sci., 2021, 134, jcs259117 CrossRef CAS PubMed.
- I. Pavlovic, D. T. Thakor, J. R. Vargas, C. J. McKinlay, S. Hauke, P. Anstaett, R. C. Camuña, L. Bigler, G. Gasser, C. Schultz, P. A. Wender and H. J. Jessen, Cellular delivery and photochemical release of a caged inositol-pyrophosphate induces PH-domain translocation in cellulo, Nat. Commun., 2016, 7, 10622 CrossRef CAS PubMed.
- Z. Szijgyarto, A. Garedew, C. Azevedo and A. Saiardi, Influence of Inositol Pyrophosphates on Cellular Energy Dynamics, Science, 2011, 334, 802 CrossRef CAS PubMed.
- C. Gu, H.-N. Nguyen, A. Hofer, H. J. Jessen, X. Dai, H. Wang and S. B. Shears, The Significance of the Bifunctional Kinase/Phosphatase Activities of Diphosphoinositol Pentakisphosphate Kinases (PPIP5Ks) for Coupling Inositol Pyrophosphate Cell Signaling to Cellular Phosphate Homeostasis, J. Biol. Chem., 2017, 292, 4544 CrossRef CAS PubMed.
- D. E. Dollins, W. Bai, C. Fridy Peter, C. Otto James, L. Neubauer Julie, G. Gattis Samuel, P. M. Mehta Kavi and D. York John, Vip1 is a kinase and pyrophosphatase switch that regulates inositol diphosphate signaling, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 9356 CrossRef PubMed.
- C. Gu, H.-N. Nguyen, D. Ganini, Z. Chen, J. Jessen Henning, Z. Gu, H. Wang and B. Shears Stephen, KO of 5-InsP7 kinase activity transforms the HCT116 colon cancer cell line into a hypermetabolic, growth-inhibited phenotype, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, 11968 CrossRef CAS PubMed.
- X. Li, C. Gu, S. Hostachy, S. Sahu, C. Wittwer, H. J. Jessen, D. Fiedler, H. Wang and B. Shears Stephen, Control of XPR1-dependent cellular phosphate efflux by InsP8 is an exemplar for functionally-exclusive inositol pyrophosphate signaling, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 3568 CrossRef CAS PubMed.
- M. S. Wilson, H. J. Jessen and A. Saiardi, The inositol hexakisphosphate kinases IP6K1 and -2 regulate human cellular phosphate homeostasis, including XPR1-mediated phosphate export, J. Biol. Chem., 2019, 294, 11597 CrossRef CAS PubMed.
- Y. Moritoh, S.-i. Abe, H. Akiyama, A. Kobayashi, R. Koyama, R. Hara, S. Kasai and M. Watanabe, The enzymatic activity of inositol hexakisphosphate kinase controls circulating phosphate in mammals, Nat. Commun., 2021, 12, 4847 CrossRef CAS PubMed.
- R. Yousaf, C. Gu, Z. M. Ahmed, S. N. Khan, T. B. Friedman, S. Riazuddin, S. B. Shears and S. Riazuddin, Mutations in Diphosphoinositol-Pentakisphosphate Kinase PPIP5K2 are associated with hearing loss in human and mouse, PLoS Genet., 2018, 14, e1007297 CrossRef PubMed.
- M. L. Khaled, Y. Bykhovskaya, C. Gu, A. Liu, M. D. Drewry, Z. Chen, B. A. Mysona, E. Parker, R. P. McNabb, H. Yu, X. Lu, J. Wang, X. Li, A. Al-Muammar, J. I. Rotter, L. F. Porter, A. Estes, M. A. Watsky, S. B. Smith, H. Xu, K. K. Abu-Amero, A. Kuo, S. B. Shears, Y. S. Rabinowitz and Y. Liu, PPIP5K2 and PCSK1 are Candidate Genetic Contributors to Familial Keratoconus, Sci. Rep., 2019, 9, 19406 CrossRef CAS PubMed.
- C. Du, X. Wang, Y. Wu, W. Liao, J. Xiong, Y. Zhu, C. Liu, W. Han, Y. Wang, S. Han, S. Chen, Y. Xu, S. Wang, F. Wang, K. Yang, J. Zhao and J. Wang, Renal Klotho and inorganic phosphate are extrinsic factors that antagonistically regulate hematopoietic stem cell maintenance, Cell Rep., 2022, 38, 110392 CrossRef CAS PubMed.
- L. Stephens, T. Radenberg, U. Thiel, G. Vogel, K. H. Khoo, A. Dell, T. R. Jackson, P. T. Hawkins and G. W. Mayr, The detection, purification, structural characterization, and metabolism of diphosphoinositol pentakisphosphate(s) and bisdiphosphoinositol tetrakisphosphate(s), J. Biol. Chem., 1993, 268, 4009 CrossRef CAS PubMed.
- H. Lin, P. C. Fridy, A. A. Ribeiro, J. H. Choi, D. K. Barma, G. Vogel, J. R. Falck, S. B. Shears, J. D. York and G. W. Mayr, Structural Analysis and Detection of Biological Inositol Pyrophosphates Reveal That the Family of VIP/Diphosphoinositol Pentakisphosphate Kinases Are 1/3-Kinases*, J. Biol. Chem., 2009, 284, 1863 CrossRef CAS PubMed.
- D. Blüher, D. Laha, S. Thieme, A. Hofer, L. Eschen-Lippold, A. Masch, G. Balcke, I. Pavlovic, O. Nagel, A. Schonsky, R. Hinkelmann, J. Wörner, N. Parvin, R. Greiner, S. Weber, A. Tissier, M. Schutkowski, J. Lee, H. Jessen, G. Schaaf and U. Bonas, A 1-phytase type III effector interferes with plant hormone signaling, Nat. Commun., 2017, 8, 2159 CrossRef PubMed.
- M. Ito, N. Fujii, C. Wittwer, A. Sasaki, M. Tanaka, T. Bittner, H. J. Jessen, A. Saiardi, S. Takizawa and E. Nagata, Hydrophilic interaction liquid chromatography-tandem mass spectrometry for the quantitative analysis of mammalian-derived inositol poly/pyrophosphates, J. Chromatogr. A, 2018, 1573, 87 CrossRef CAS PubMed.
- A. Kobayashi, S.-i. Abe, M. Watanabe and Y. Moritoh, Liquid chromatography-mass spectrometry measurements of blood diphosphoinositol pentakisphosphate levels, J. Chromatogr. A, 2022, 1681, 463450 CrossRef CAS PubMed.
- R. K. Harmel, R. Puschmann, M. Nguyen Trung, A. Saiardi, P. Schmieder and D. Fiedler, Harnessing 13C-labeled myo-inositol to interrogate inositol phosphate messengers by NMR, Chem. Sci., 2019, 10, 5267 RSC.
- D. Qiu, M. S. Wilson, V. B. Eisenbeis, R. K. Harmel, E. Riemer, T. M. Haas, C. Wittwer, N. Jork, C. Gu, S. B. Shears, G. Schaaf, B. Kammerer, D. Fiedler, A. Saiardi and H. J. Jessen, Analysis of inositol phosphate metabolism by capillary electrophoresis electrospray ionization mass spectrometry, Nat. Commun., 2020, 11, 6035 CrossRef CAS PubMed.
- T. M. Haas, S. Mundinger, D. Qiu, N. Jork, K. Ritter, T. Durr-Mayer, A. Ripp, A. Saiardi, G. Schaaf and H. J. Jessen, Stable Isotope Phosphate Labelling of Diverse Metabolites is Enabled by a Family of (18) O-Phosphoramidites, Angew. Chem., Int. Ed., 2022, 61, e202112457 CAS.
- D. Qiu, V. B. Eisenbeis, A. Saiardi and H. J. Jessen, Absolute Quantitation of Inositol Pyrophosphates by Capillary Electrophoresis Electrospray Ionization Mass Spectrometry, J. Visualized Exp., 2021, e62847 CAS.
- C. Gu, M. S. C. Wilson, H. J. Jessen, A. Saiardi and S. B. Shears, Inositol Pyrophosphate Profiling of Two HCT116 Cell Lines Uncovers Variation in InsP8 Levels, PLoS One, 2016, 11, e0165286 CrossRef PubMed.
- P. Draškovič, A. Saiardi, R. Bhandari, A. Burton, G. Ilc, M. Kovačevič, S. H. Snyder and M. Podobnik, Inositol Hexakisphosphate Kinase Products Contain Diphosphate and Triphosphate Groups, Chem. Biol., 2008, 15, 274 CrossRef PubMed.
- R. Puschmann, R. K. Harmel and D. Fiedler, Scalable Chemoenzymatic Synthesis of Inositol Pyrophosphates, Biochemistry, 2019, 58, 3927 CrossRef CAS PubMed.
- S. Capolicchio, D. T. Thakor, A. Linden and H. J. Jessen, Synthesis of unsymmetric diphospho-inositol polyphosphates, Angew. Chem., Int. Ed., 2013, 52, 6912 CrossRef CAS PubMed.
- S. Capolicchio, H. Wang, D. T. Thakor, S. B. Shears and H. J. Jessen, Synthesis of densely phosphorylated bis-1,5-diphospho-myo-inositol tetrakisphosphate and its enantiomer by bidirectional P-anhydride formation, Angew. Chem., Int. Ed., 2014, 53, 9508 CrossRef CAS PubMed.
- T. Bittner, C. Wittwer, S. Hauke, D. Wohlwend, S. Mundinger, A. K. Dutta, D. Bezold, T. Dürr, T. Friedrich, C. Schultz and H. J. Jessen, Photolysis of Caged Inositol Pyrophosphate InsP8 Directly Modulates Intracellular Ca2+ Oscillations and Controls C2AB Domain Localization, J. Am. Chem. Soc., 2020, 142, 10606 CrossRef CAS PubMed.
- S. Hauke, A. K. Dutta, V. B. Eisenbeis, D. Bezold, T. Bittner, C. Wittwer, D. Thakor, I. Pavlovic, C. Schultz and H. J. Jessen, Photolysis of cell-permeant caged inositol pyrophosphates controls oscillations of cytosolic calcium in a β-cell line, Chem. Sci., 2019, 10, 2687 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2sc05147h |
| ‡ Contributed equally. |
| This journal is © The Royal Society of Chemistry 2023 |