Open Access Article
Open Access ArticleHeterometallic palladium–iron metal–organic framework as a highly active catalyst for cross-coupling reactions†
Eugenia
Miguel-Casañ‡
a,
Mohanad D.
Darawsheh‡
a,
Víctor
Fariña-Torres
b,
Iñigo J.
Vitórica-Yrezábal
c,
Eduardo
Andres-Garcia
 a,
Martín
Fañanás-Mastral
a,
Martín
Fañanás-Mastral
 *b and
Guillermo
Mínguez Espallargas
*b and
Guillermo
Mínguez Espallargas
 *a
*a
aInstituto de Ciencia Molecular (ICMol), Universidad de Valencia, C/ Catedrático José Beltrán, 2, 46980, Paterna, Spain. E-mail: guillermo.minguez@uv.es
bCentro Singular de Investigación en Química Biolóxica e Materiais Moleculares (CiQUS), Universidade de Santiago de Compostela, 15782, Santiago de Compostela, Spain. E-mail: martin.fananas@usc.es
cSchool of Chemistry, University of Manchester, Oxford Road, Manchester M13 9PL, UK
First published on 22nd November 2022
Abstract
Palladium-based metal–organic frameworks (Pd-MOFs) are an emerging class of heterogeneous catalysts extremely challenging to achieve due to the facile leaching of palladium and its tendency to be reduced. Herein, Pd(II) was successfully incorporated in the framework of a MOF denoted as MUV-22 using a solvent assisted reaction. This stable MOF, with square-octahedron (soc) topology as MIL-127, and a porosity of 710 m2 g−1, is highly active, selective, and recyclable for the Suzuki–Miyaura allylation of aryl and alkyl boronates as exemplified with the coupling between cinnamyl bromide and Me-Bpin, a typically reluctant reagent in cross-coupling reactions.
Introduction
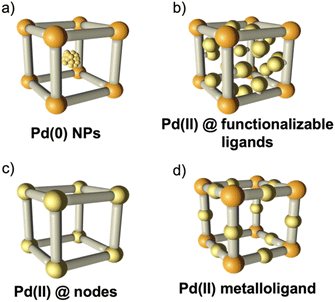
Pd(II) complexes are widely used as homogeneous catalysts in important reactions such as hydrogenation,1 coupling reactions (both carbon–carbon and carbon–heteroatoms),2 and photohydrogen production reactions,3 among others. However, given the high cost, the drawbacks related to the recyclability of homogeneous palladium complexes, and deactivation through Pd-black formation, the search of novel heterogeneous catalysts which can be recycled is of high interest.4,5 Metal–organic frameworks (MOFs), crystalline porous solids composed of metal ions or metal clusters interconnected by organic linkers, are ideal tunable platforms acting as a supporting matrix for the incorporation of catalytically active metals.6,7 Indeed, MOFs have proven to be very efficient catalysts for a large number of reactions.8–11 In this sense, MOFs containing palladium atoms (Pd-MOFs) are currently attracting tremendous interest,12–14 as they can provide a good dispersion of the exposed Pd atoms within the framework, are able to stabilize reaction intermediates,15 and promote and increase the catalytic activity.16The incorporation of Pd atoms into MOFs has been typically achieved through post-synthetic approaches, i.e. in a two-step procedure after the formation of the MOF.17,18 The most common route consists in the encapsulation of Pd nanoparticles in the pores of the MOFs (Fig. 1a), yielding stable and disperse Pd nanoparticles and avoiding their aggregation and growth,19,20 but with the limitation of blocking the channels of the MOF. An alternative approach is the use of organic ligands with anchoring groups suitable for the coordination of metal complexes (Fig. 1b),21,22 which has also proven successful in COFs;23 this can also lead to the formation of subnanometer clusters with superior catalytic performances, in comparison with standard Pd catalysts,24,25 but reduced porosity is still obtained due to the bulky organic complexes which limit the accessibility to the Pd centres. These problems can be circumvented by the incorporation of Pd as metal nodes (Fig. 1c), i.e., the formation of a Pd-MOF in which the Pd atoms form part of the framework. These can be prepared by direct synthesis26 or, as demonstrated recently, by transmetallation.27
These MOFs feature accessible Pd(II) sites, but the stability of the framework is compromised during the catalysis, as changes in the coordination sphere of the Pd centres cause rupture of the framework. A facile solution is the incorporation of the Pd centres as part of the ligands, i.e. using a metalloligand to connect the metal nodes, thus resulting in a framework with two different secondary building blocks (Fig. 1d). This was shown to be successful by Trikalitis and co-workers, who combined a Pd metalloligand with an indium trimer producing a 3D MOF, although with limited stability in solvents.28 With this approach, a more uniform distribution of the Pd centres along the material is thus achieved with no blockage of the pores. This could also reduce the instability of the framework caused by the change in the coordination sphere during catalytic cycles. However, this has rarely been achieved through the conventional synthetic methodologies,28,29 due to the tendency of Pd(II) to be reduced under these conditions, except for metalloligands where the Pd(II) centres are very inert, and thus, not useful in catalysis.
In this work, we use a solvent-assisted reaction to prepare a robust heterometallic MOF constructed by triangular [Fe3(μ3O)(COO)6]+ secondary building units linked by square-planar Pd(II) centres. Structure solution through single crystal X-ray diffraction reveals a cubic system isoreticular to the well-known MIL-127, with pores of 2509 Å3 which are available as confirmed by N2, CO2 and CH4 sorption studies, with a BET area of 710 m2 g−1. The stability studies of this MOF reveal excellent results across wide ranges of pH, temperatures, and solvents which allow its implementation in catalysis. Finally, studies on the catalytic activity of this heterometallic MOF towards the Suzuki–Miyaura allylation of different boronic acid pinacol esters show that this MOF is an efficient catalyst for C(sp3)–C(sp3) and C(sp2)–C(sp3) couplings.
Results and discussion
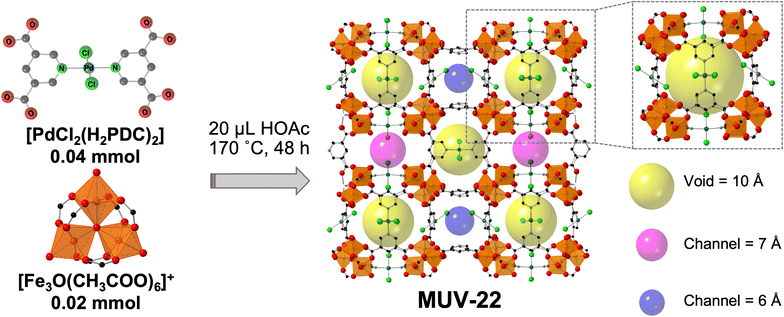
In order to demonstrate a better control on the formation of a heterometallic MOF, a mixture of the commercial cluster [Fe3O(CH3COO)6]ClO4 was reacted with the preformed palladium metalloligand PdCl2(H2PDC)2 (H4L, where H2PDC = pyridine-3,5 dicarboxylic acid). In contrast to the common approach in the preparation of MOFs, this reaction was performed in the absence of a large amount of solvent, as we have previously used for the synthesis of the elusive Fe(II) analogue of ZIF-8,30 but in the presence of only a small amount of acetic acid (20 microliters for 0.04 mmol of palladium metalloligand). The mixture was sealed under vacuum in an ampoule and heated at 170 °C for 48 h, resulting in the formation of red cubic crystals of formula [Fe3O(L)1.5(H2O)2(OH)]·solvent (denoted as MUV-22), very small but of suitable quality for structure determination via single-crystal X-ray diffraction (Fig. 2 and Section S2 in the ESI†). The use of a small amount of acetic acid, analogous to the solvent-assisted mechanochemical approach,31 is essential for the exclusive formation of MUV-22, which is the iron analogue of the reported [In3O(L)1.5(H2O)2Cl]·n(solv).28A larger amount of acetic acid yields a mixture of phases, with the formation of a polymorph of MUV-22.32 Very recently, Horike et al. have reported that solvent-free reactions with carboxylates require the formation of co-crystals,33 although we show here an alternative route through the use of a minor amount of solvent. Importantly, this methodology yields a highly crystalline material which is phase pure, as confirmed by PXRD (Fig. S3†). The bulk material was exhaustively washed with a large amount of EtOH, immersed in the solvent overnight in order to remove any unreacted starting material and the acetic acid, and finally, heated at 120 °C under vacuum overnight. The infrared (IR) spectra of the powder confirmed the absence of any unreacted starting material (Fig. S4 and S5†).
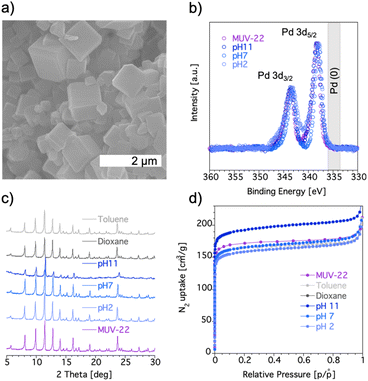
Single-crystal X-ray diffraction analysis of the as-synthesized material reveals that the red cubic crystals, shown in Fig. 3a, are isostructural with MIL-127,34 crystallizing in the space group Pm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) n, with unit cell parameters of a = b = c = 22.1118 Å (Table S1†). Structure solution reveals the presence of 6-connected trimeric [Fe3(μ3O)(COO)6]+ SBUs and tetratopic [PdCl2(PDC)2]4− metalloligands. Thus, each Fe3O(COO)6 unit acts as a six-connected node, whereas each metalloligand acts as a four-connected node, resulting in the construction of a (4,6)-connected structure with the augmented soc network (Fig. 2). The net is composed of a periodic arrangement of one kind of cages with a diameter of 10 Å (yellow spheres in Fig. 2) and two different channels with approximate diameters of 6–7 Å (pink and blue spheres in Fig. 2). The Pd metalloligands are located at the faces of a cube and connect the eight corners occupied by Fe3O trimers, each of which can be considered as a 6-c node with a trigonal prismatic geometry. Each metalloligand is composed of one palladium center coordinated by two Cl− (each disordered over two positions) and two pyridine moieties. X-ray photoelectron spectroscopy (XPS) confirms the oxidation state of the palladium ions in MUV-22 (Fig. 3b), with binding energies representative of Pd(II) (Pd 3d5/2 = 337.9 eV and Pd 3d3/2 = 343.6 eV),35 and absence of Pd(0).
n, with unit cell parameters of a = b = c = 22.1118 Å (Table S1†). Structure solution reveals the presence of 6-connected trimeric [Fe3(μ3O)(COO)6]+ SBUs and tetratopic [PdCl2(PDC)2]4− metalloligands. Thus, each Fe3O(COO)6 unit acts as a six-connected node, whereas each metalloligand acts as a four-connected node, resulting in the construction of a (4,6)-connected structure with the augmented soc network (Fig. 2). The net is composed of a periodic arrangement of one kind of cages with a diameter of 10 Å (yellow spheres in Fig. 2) and two different channels with approximate diameters of 6–7 Å (pink and blue spheres in Fig. 2). The Pd metalloligands are located at the faces of a cube and connect the eight corners occupied by Fe3O trimers, each of which can be considered as a 6-c node with a trigonal prismatic geometry. Each metalloligand is composed of one palladium center coordinated by two Cl− (each disordered over two positions) and two pyridine moieties. X-ray photoelectron spectroscopy (XPS) confirms the oxidation state of the palladium ions in MUV-22 (Fig. 3b), with binding energies representative of Pd(II) (Pd 3d5/2 = 337.9 eV and Pd 3d3/2 = 343.6 eV),35 and absence of Pd(0).
Thermogravimetric analysis (TGA) of washed MUV-22 exhibits a sharp mass loss of 20% between 25 and 50 °C, and a large plateau above 200 °C until the final decomposition at 310 °C, thus showing an expected thermal stability (Fig. S8†).
To examine the chemical stability of MUV-22, it was immersed for 24 h in different aqueous solutions with pH values ranging from 2 to 11 and in different organic solvents. The PXRD patterns of the recovered materials reveal that crystallinity is maintained under these conditions (Fig. 3c), in sharp contrast to the indium analogue, which is unstable both in organic solvents and H2O.28 Additionally, the possible degradation of the MOF was investigated examining the supernatant solution by 1H NMR spectroscopy, in order to detect the possible presence of organic ligands after 24 h immersion of MUV-22 in deuterated dioxane, toluene and different deuterated aqueous solutions of pH range between 1 and 11. As shown in Fig. S12,† no signal of the pyridine-3,5-dicarboxylic acid could be detected. Furthermore, the solid used in each treatment was also studied by measuring: (a) the porosity from N2 adsorption isotherms at 77 K, showing no significant reduction of the sorption capacity (Fig. 3d); and (b) the valence state of palladium by XPS to discard the formation of Pd(0) (Fig. 3b).
The porous nature of MUV-22 was assessed by N2 sorption at 77 K. Activation of MUV-22 was successfully achieved by treating the washed material at 120 °C overnight. Fig. 3d displays the N2 adsorption isotherm, a typical type I isotherm with a plateau at 180 cm3 g−1. The obtained accessible surface area was calculated using the Brunauer–Emmett–Teller (BET) model, giving a value of 710 m2 g−1 which is comparable with the reported values of the In analogue MOF (795 m2 g−1),28 and a pore volume of 0.323 cm3 g−1. The pore size distribution was calculated using the corrected Horvath–Kawazoe method,36 exposing a narrow distribution of microporosity (4.6–5.39) Å with the maximum peak at 4.85 Å. The CO2 and CH4 gravimetric sorption isotherms, shown in Fig. S15 and S16,† reveal a total uptake of 7.2 and 4.8 mmol g−1 at 18 bar, respectively. The isosteric heats of adsorption (qst) were calculated through the Clausius–Clapeyron equation, resulting in 22.6 kJ mol−1 (for CO2) and 15.1 kJ mol−1 (for CH4), highlighting a higher affinity of MUV-22 toward carbon dioxide (see Fig. S17†). These values are slightly smaller than those found in [In3O(L)1.5(H2O)2Cl] (29.8 and 19.6 kJ mol−1 for CO2 and CH4, respectively), indicating a weaker interaction of the framework with the gas likely due to the use of a different metal.28
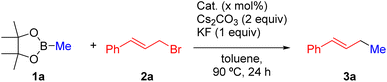
Given the high chemical stability of MUV-22, its porous nature, and the easy accessibility to the Pd(II) centers, we investigated its catalytic activity in allylic alkylation reactions. Pd-catalyzed allylic alkylation represents a highly versatile tool in chemical synthesis and has been used in the preparation of many natural products and biologically active compounds.37 Although it has been extremely useful for the construction of C–C bonds using several organometallic reagents such as organoaluminum or Grignard reagents,38 the Pd-catalyzed allylation of alkylboron compounds has been rarely reported.39 Consequently, we decided to explore the catalytic activity of MUV-22 in the reaction between methylboronic acid pinacol ester (1a), a typically reluctant reagent in cross-coupling reactions, and cinnamyl bromide (2a). After screening of several reaction parameters (see the ESI, Tables S2–S4†), we found that MUV-22 efficiently promotes the reaction in the presence of Cs2CO3 and KF in toluene at 90 °C (Table 1, entry 1). Under these conditions, (E)-1-phenyl-1-butene (3a) was cleanly formed in 58% yield.40 Importantly, product 3a was not formed in the absence of a catalyst (entry 2). In addition, MUV-22 shows better catalytic performance than homogeneous Pd(OAc)2 (entry 3), heterogeneous Pd(OH)2/C (entry 4) or even Pd-PEPPSI-IPr (entry 5) which is the state-of-the-art catalyst for the homogeneous Pd-catalyzed coupling between allyl bromides and alkyl boronic esters.39
To check a possible cooperative bimetallic effect in MUV-22 in which the Fe atom might activate the allylic substrate via Lewis acid catalysis,41 we carried out several control experiments using the corresponding Pd and Fe monomers and a combination of both (entries 6–8). The reaction took place in the presence of Pd monomer, although 3a was obtained in a diminished yield (entry 6). The Fe monomer also showed some catalytic activity, but the coupling product was formed in a much lower yield (entry 7). Interestingly, combination of both monomers led to a similar result than the one obtained for the Pd monomer, providing the coupling product in lower yield than the one obtained with MUV-22 (entry 8). Although bimetallic catalysis cannot be discarded at this point, these results suggest that the superior catalytic activity of MUV-22 might be due to a structural confinement effect. Furthermore, comparison of the catalytic activity in the coupling of Me-Bpin with a larger substrate (i.e. (E)-2-(3-bromoprop-1-en-1-yl)naphthalene) demonstrates a more drastic difference in selectivity (45% yield with MUV-22vs. 18% yield with the mixture of the monomers).
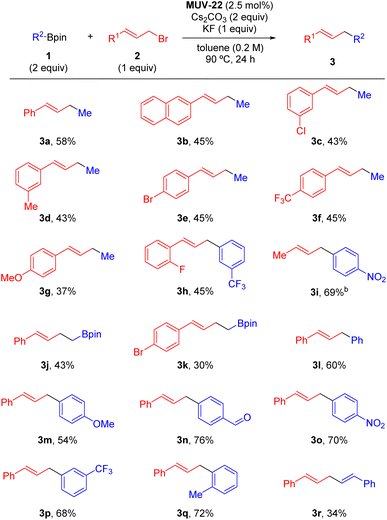
Having established optimized conditions, we set out to explore the scope of this transformation (Scheme 1). MUV-22 proved to be an efficient catalyst for the coupling of a range of allyl bromides bearing aromatic groups with electronically distinct substituents at different positions (3a–3h). Crotyl bromide was also an efficient coupling partner although in this case product 3i was obtained as a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of regioisomers. Other alkyl boronic esters such as diborylmethane could be used as illustrated with the synthesis of homoallylboronates 3j and 3k. However, alkyl-Bpin reagents bearing hydrogen atoms in the β-position did not undergo this transformation likely due to competitive β-hydride elimination pathways (see Section S6.6 in the ESI†). MUV-22 also proved to be an efficient catalyst for the allylation of C(sp2) nucleophiles. In this context, Ph-Bpin (3l) and other aryl boronic esters bearing para- (3m–3o), meta- (3p) and ortho-substituted rings (3q) were coupled with cinnamyl bromide in good yields. Additionally, the skipped diene 3r could also be synthesized under MUV-22 heterogeneous catalysis conditions by using the corresponding alkenyl boronic ester.
1 mixture of regioisomers. Other alkyl boronic esters such as diborylmethane could be used as illustrated with the synthesis of homoallylboronates 3j and 3k. However, alkyl-Bpin reagents bearing hydrogen atoms in the β-position did not undergo this transformation likely due to competitive β-hydride elimination pathways (see Section S6.6 in the ESI†). MUV-22 also proved to be an efficient catalyst for the allylation of C(sp2) nucleophiles. In this context, Ph-Bpin (3l) and other aryl boronic esters bearing para- (3m–3o), meta- (3p) and ortho-substituted rings (3q) were coupled with cinnamyl bromide in good yields. Additionally, the skipped diene 3r could also be synthesized under MUV-22 heterogeneous catalysis conditions by using the corresponding alkenyl boronic ester.
Recycling experiments of MUV-22 showed that the MOF still had remarkable catalytic efficiency after 4 cycles. Kinetic studies (Fig. 4a) revealed that the rate and yield of the reaction are essentially maintained between these runs, which demonstrates that MUV-22 is robust and maintains its activity. Furthermore, we analyzed the chemical stability of the MOF recovered after the catalysis, i.e.MUV-22-PC (see Section S7 in the ESI†). PXRD studies revealed that the crystallinity of the MOF is maintained in the recovered catalyst (Fig. 4b), as also its porous nature (Fig. S62†). The particle morphology and crystal sizes were examined through SEM (Fig. S60†) and TEM (Fig. S61†) revealing similar results for MUV-22 and MUV-22-PC, discarding in the last one the formation of nanoparticles. However, EDS studies showed that Cl− ions were replaced by F− ions in the coordination sphere of the Pd atoms (Fig. S59†), which was also confirmed with XPS (Fig. S58†). Interestingly, mixing of MUV-22 with KF in toluene at 90 °C did not result in such a process (Fig. S63†). These results suggest that this Cl/F ligand exchange might occur in some of the Pd intermediates that are generated during the catalytic cycle,42 further indicating that the reaction happens in the inside of the MOF crystal. In addition, although a possible Pd–N bond cleavage cannot be ruled out, the absence of Pd(0) by XPS (Fig. S57†) suggests a non-dissociation of the metalloligand, in agreement with previous studies from Amatore, Jutand and others.43
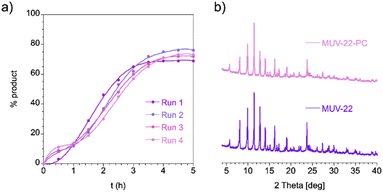 | ||
| Fig. 4 (a) Recycling of MUV-22 in the formation of product 3l; (b) PXRD patterns of MUV-22 and MUV-22-PC. | ||
Finally, the coupling between (4-formylphenyl)boronic acid pinacol ester and cinnamyl bromide (i.e. formation of 3n) was used to test the heterogeneous nature of the catalyst, through a leaching test based on a hot filtration experiment (see section S6.4 in the ESI† for details). Treatment of the filtrate with more Cs2CO3 under the same reaction conditions did not lead to any further formation of the product, thus establishing that no palladium leaches out of the MOF and excluding any homogeneous pathway.
Conclusions
In conclusion, we have shown a solvent-assisted reaction to incorporate accessible Pd(II) centres in the framework of a novel MOF, denoted as MUV-22, using a Pd-metalloligand. The regular disposition of the Pd(II) atoms, combined with the high chemical stability and the available space provided by the porous framework, makes MUV-22 an interesting material for catalytic applications. In this sense, the activity of MUV-22 in the Suzuki–Miyaura allylation has been explored. We have observed that this material acts as an efficient heterogeneous catalyst for the C–C bond formation between a range of alkyl, aryl and alkenyl boronates with different allyl bromides. We have shown that MUV-22 catalyzes this reaction maintaining the crystallinity and the activity for several cycles. The robustness of this Pd/Fe MOF, combined with its high catalytic activity, paves the way for new opportunities in heterogeneous catalysis. Therefore, this is an example of how mixed-metal MOFs offer the possibility of incorporating multiple functionalities and take MOFs into account as heterogeneous catalysts.44 Further studies on its use in other cross-coupling reactions are currently underway.Experimental
Synthesis of MUV-22
MUV-22 was synthesized using a solvent-assisted method. [Fe3O(CH3COO)6](ClO4)·nH2O (14 mg, 0.020 mmol) and trans-[Pd2Cl2(H2PDC)2] (20 mg, 0.039 mmol) were initially briefly grounded. The mixture was placed in a glass tube and acetic acid (20 μL, 0.350 mmol) was added. The tube was flash-frozen with liquid nitrogen and sealed after a cycle of vacuum. Ten of such tubes were heated for 48 h at 170 °C (heating and cooling rates of 6 °C h−1). The resulting mixture was washed with ethanol (3 × 25 mL) and then with methanol (6 × 25 mL) for 2 days. Yield: 140 mg (67%). Phase-purity was confirmed with X-ray powder diffraction, and the heavy atom element content was checked with EDS and ICP (calc. Pd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe
Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cl ratio = 1
Cl ratio = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2; EDS: Pd
2; EDS: Pd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe
Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cl ratio = 1.0
Cl ratio = 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.1
2.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.2; ICP: Pd
2.2; ICP: Pd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe ratio of 1.0
Fe ratio of 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.8).
1.8).
Single crystal diffraction
X-ray data for compound MUV-22 were collected with a Rigaku Supernova with microfocus Mo-Kα radiation at 120 K. Data were measured, processed and reduced using the CrysAlisPro suite of programs. Absorption correction was performed using empirical methods (SCALE3 ABSPACK) based upon symmetry-equivalent reflections combined with measurements at different azimuthal angles. The crystal structure was solved and refined against all F2 values using the SHELX and Olex 2 suite of programmes. Despite the use of a highly intense X-ray source, crystals of MUV-22 only diffracted to 1.25 Å of resolution. All atoms were refined anisotropically with the exception of carbon and nitrogen atoms in order to maximize the data/parameter ratio. Chloride atoms were found to be disordered and were modelled over two positions, where atomic distances were restrained using distance restrains. The atomic displacement parameters were restrained using rigid body restrains. Hydrogen atoms were placed at calculated positions. The solvent mask protocol in Olex 2 was used to account for the remaining electron density, finding 128 electrons per formula unit, which could correspond to 13 water molecules. CCDC 2131105 contains the supplementary crystallographic data for this paper.†Gas sorption
MUV-22 crystals were activated at 120 °C under vacuum, overnight. Low-pressure single-gas nitrogen adsorption isotherms were measured in a Tristar II Plus sorptmeter (Micromeritics), at 77 K. High-pressure single-gas adsorption isotherms of CO2 and CH4 were recorded at different temperatures ranging from 283 to 333 K in an IGA-100 gravimetric gas sorption analyser (Hiden Isochema) using approximately 40 mg of MUV-22. Equilibrium conditions corresponded to 600 s interval and 0.001 mg min−1 tolerance.Catalytic activity
Data availability
The data that support the findings of this study are available in the ESI for this article.†Author contributions
E. M.-C. and M. D. D. performed the synthesis and characterization of the MOF. E. M.-C. and V. F.-T. performed the catalysis experiments. I. J. V.-Y. contributed to solution and refinement of the structure from single crystal data. E. A. G. performed the gas sorption studies. M. F.-M. and G. M. E. supervised the project and wrote the manuscript, with input from all the authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The work has been supported by the European Union (ERC-2016-CoG 724681-S-CAGE, ERC-CoG 863914-BECAME and European Regional Development Fund – ERDF), grants PID2020-117177GB-I00, and CEX2019-000919-M, funded by MCIN/AEI/10.13039/501100011033, the Generalitat Valenciana (Prometeo programme) and the Xunta de Galicia (ED431C 2022/27; Centro singular de investigación de Galicia accreditation 2019–2022, ED431G 2019/03). E. M.-C. and E. A.-G thank MICINN for a PhD fellowship (BES-2017-082451) and a Juan de la Cierva Formación fellowship (FJC2019-039015-I), respectively.References
- Q. A. Chen, Z. S. Ye, Y. Duan and Y. G. Zhou, Chem. Soc. Rev., 2013, 42, 497–511 RSC.
- N. Miyaura and A. Suzuki, Chem. Rev., 1995, 95, 2457–2483 CrossRef CAS.
- B. Yan, L. Zhang, Z. Tang, M. Al-Mamun, H. Zhao and X. Su, Appl. Catal., B, 2017, 218, 743–750 CrossRef CAS.
- Y. S. Kang, Y. Lu, K. Chen, Y. Zhao, P. Wang and W. Y. Sun, Coord. Chem. Rev., 2019, 378, 262–280 CrossRef CAS.
- M. Li, D. Posevins, A. Geoffroy, C. Zhu and J. Bäckvall, Angew. Chem., Int. Ed., 2020, 59, 1992–1996 CrossRef CAS PubMed.
- S. M. J. Rogge, A. Bavykina, J. Hajek, H. Garcia, A. I. Olivos-Suarez, A. Sepúlveda-Escribano, A. Vimont, G. Clet, P. Bazin, F. Kapteijn, M. Daturi, E. V. Ramos-Fernandez, F. X. I. Llabrés i Xamena, V. Van Speybroeck and J. Gascon, Chem. Soc. Rev., 2017, 46, 3134–3184 RSC.
- A. Bavykina, N. Kolobov, I. S. Khan, J. A. Bau, A. Ramirez and J. Gascon, Chem. Rev., 2020, 120, 8468–8535 CrossRef CAS.
- A. Dhakshinamoorthy, A. M. Asiri and H. García, Trends Chem., 2020, 2, 454–466 CrossRef CAS.
- V. Pascanu, G. González Miera, A. K. Inge and B. Martín-Matute, J. Am. Chem. Soc., 2019, 141, 722–7234 CrossRef PubMed.
- C. Xu, R. Fang, R. Luque, L. Chen and Y. Li, Coord. Chem. Rev., 2019, 388, 268–292 CrossRef CAS.
- A. Corma, H. García and F. X. Llabrés I Xamena, Chem. Rev., 2010, 110, 4606–4655 CrossRef CAS.
- H. Furukawa, K. E. Cordova, M. O'Keeffe and O. M. Yaghi, Science, 2013, 341, 1230444 CrossRef.
- H. Li, M. Eddaoudi, M. O'Keeffe and O. M. Yaghi, Nature, 1999, 402, 276–279 CrossRef CAS.
- H.-C. Zhou, J. R. Long and O. M. Yaghi, Chem. Rev., 2012, 112, 673–674 CrossRef CAS PubMed.
- F. R. Fortea-Pérez, M. Mon, J. Ferrando-Soria, M. Boronat, A. Leyva-Pérez, A. Corma, J. M. Herrera, D. Osadchii, J. Gascon, D. Armentano and E. Pardo, Nat. Mater., 2017, 16, 760–766 CrossRef PubMed.
- L. Chen, Z. Gao and Y. Li, Catal. Today, 2015, 245, 122–128 CrossRef CAS.
- Z. Wang and S. M. Cohen, Chem. Soc. Rev., 2009, 38, 1315–1329 RSC.
- S. Mandal, S. Natarajan, P. Mani and A. Pankajakshan, Adv. Funct. Mater., 2021, 31, 2006291 CrossRef CAS.
- G. Lu, S. Li, Z. Guo, O. K. Farha, B. G. Hauser, X. Qi, Y. Wang, X. Wang, S. Han, X. Liu, J. S. DuChene, H. Zhang, Q. Zhang, X. Chen, J. Ma, S. C. J. Loo, W. D. Wei, Y. Yang, J. T Hupp and F. Huo, Nat. Chem., 2012, 4, 310–316 CrossRef CAS.
- S. Luo, Z. Zeng, G. Zeng, Z. Liu, R. Xiao, M. Chen, L. Tang, W. Tang, C. Lai, M. Cheng, B. Shao, Q. Liang and H. Wang, ACS Appl. Mater. Interfaces, 2019, 11, 32579–32598 CrossRef CAS PubMed.
- X. Li, R. Van Zeeland, R. V. Maligal-Ganesh, Y. Pei, G. Power, L. Stanley and W. Huang, ACS Catal., 2016, 6, 6324–6328 CrossRef CAS.
- L. Chen, S. Rangan, J. Li, H. Jiang and Y. Li, Green Chem., 2014, 16, 3978–3985 RSC.
- I. Romero-Muñiz, A. Mavrandonakis, P. Albacete, A. Vega, V. Briois, F. Zamora and A. E. Platero-Prats, Angew. Chem., Inter. Ed., 2020, 59, 13013–13020 CrossRef.
- L. Liu and A. Corma, Nat. Rev. Mater., 2021, 6, 244–263 CrossRef CAS.
- R. Adam, M. Mon, R. Greco, L. H. G. Kalinke, A. Vidal-Moya, A. Fernandez, R. E. P. Winpenny, A. Domenech-Carbo, A. Leyva-Perez, D. Armentano, E. Pardo and J. Ferrando-Soria, J. Am. Chem. Soc., 2019, 141, 10350–10360 CrossRef CAS.
- F. X. Llabrés i Xamena, A. Abad, A. Corma and H. Garcia, J. Catal., 2007, 250, 294–298 CrossRef.
- T. He, X.-J. Kong, J. Zhou, C. Zhao, K. Wang, X.-Q. Wu, X.-L. Lv, G.-R. Si, J.-R. Li and Z.-R. Nie, J. Am. Chem. Soc., 2021, 143, 9901–9911 CrossRef CAS.
- I. Bratsos, C. Tampaxis, I. Spanopoulos, N. Demitri, G. Charalambopoulou, D. Vourloumis, T. A. Steriotis and P. N. Trikalitis, Inorg. Chem., 2018, 57, 7244–7251 CrossRef CAS.
- V. Niel, J. M. Martinez-Agudo, M. C. Muñoz, A. B. Gaspar and J. A. Real, Inorg. Chem., 2001, 40, 3838–3839 CrossRef CAS PubMed.
- J. López-Cabrelles, J. Romero, G. Abellán, M. Giménez-Marqués, M. Palomino, S. Valencia, F. Rey and G. Mínguez Espallargas, J. Am. Chem. Soc., 2019, 141, 7173–7180 CrossRef.
- G. A. Bowmaker, Chem. Commun., 2013, 49, 334–348 RSC.
- M. D. Darawsheh, J. Mazarío, C. W. Lopes, M. Giménez-Marqués, M. E. Domine, D. M. Meira, J. Martínez, G. Mínguez Espallargas and P. Oña-Burgos, Chem.–Eur. J., 2020, 26, 13659–13667 CrossRef CAS PubMed.
- A. Hinokimoto, H. Izu, Y.-S. Wei, T. Nakajo, R. Matsuda and S. Horike, Cryst. Growth Des., 2021, 21, 6031–6036 CrossRef CAS.
- H. Chevreau, A. Permyakova, F. Nouar, P. Fabry, C. Livage, F. Ragon, A. Garcia-Marquez, T. Devic, N. Steunou, C. Serre and P. Horcajada, CrysEngComm, 2016, 18, 4094–4101 RSC.
- J. F. Moulder, W. F. Stickle, P. E. Sobol and K. D. Bomben, in Chastain, Perkin-Elmer Corporation, 1992 Search PubMed.
- L. S. Cheng and R. T. Yang, Adsorption, 1995, 1, 187–196 CrossRef CAS.
- B. M. Trost and M. L. Crawley, Chem. Rev., 2003, 103, 2921–2944 CrossRef CAS.
- E. Negishi and L. Fang, in Handbook of Organopalladium Chemistry for Organic Synthesis, ed. E. Negishi, Wiley, New York, 2002, vol. 1, ch. III.2.9 Search PubMed.
- K. Endo, T. Ohkubo, T. Ishioka and T. Shibata, J. Org. Chem., 2012, 77, 4826–4831 CrossRef CAS.
- Moderate yield is likely due to volatility of the product.
- For an example of Lewis acid activation in transition metal-catalyzed allylic substitution, see: E. Rivera-Chao, M. Mitxelena, J. A. Varela and M. Fañanás-Mastral, Angew. Chem., Int. Ed., 2019, 58, 18230–18234 CrossRef CAS.
- L. Grimaund and A. Jutand, Synthesis, 2016, 49, 1182–1189 CrossRef.
- C. Amatore, G. Le Duc and A. Jutand, Chem.–Eur. J., 2013, 19, 10082–10093 CrossRef CAS.
- M. Viciano-Chumillas, X. Liu, A. Leyva-Pérez, D. Armentano, J. Ferrando-Soria and E. Pardo, Coord. Chem. Rev., 2022, 451, 214273 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2131105. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2sc05192c |
| ‡ E. M. C. and M. D. contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2023 |