Open Access Article
Open Access ArticleMapping the effect of configuration and protecting group pattern on glycosyl acceptor reactivity†
Jacob M. A.
van Hengst
a,
Rik J. C.
Hellemons
a,
Wouter A.
Remmerswaal
a,
Koen N. A.
van de Vrande
a,
Thomas
Hansen
 ab,
Stefan
van der Vorm
ab,
Stefan
van der Vorm
 a,
Hermen S.
Overkleeft
a,
Hermen S.
Overkleeft
 a,
Gijsbert A.
van der Marel
a,
Gijsbert A.
van der Marel
 a and
Jeroen D. C.
Codée
a and
Jeroen D. C.
Codée
 *a
*a
aLeiden University, Leiden Institute of Chemistry, Einsteinweg 55, 2333 CC Leiden, The Netherlands. E-mail: jcodee@chem.leidenuniv.nl
bDepartment of Theoretical Chemistry, Amsterdam Institute of Molecular and Life Sciences (AIMMS), Amsterdam Center for Multiscale Modeling (ACMM), Vrije Universiteit Amsterdam, De Boelelaan 1083, 1081 HV Amsterdam, The Netherlands
First published on 13th January 2023
Abstract
The reactivity of the acceptor alcohol can have a tremendous influence on the outcome of a glycosylation reaction, both in terms of yield and stereoselectivity. Through a systematic survey of 67 acceptor alcohols in glycosylation reactions with two glucosyl donors we here reveal how the reactivity of a carbohydrate acceptor depends on its configuration and substitution pattern. The study shows how the functional groups flanking the acceptor alcohol influence the reactivity of the alcohol and show that both the nature and relative orientation play an essential role. The empiric acceptor reactivity guidelines revealed here will aid in the rational optimization of glycosylation reactions and be an important tool in the assembly of oligosaccharides.
Introduction
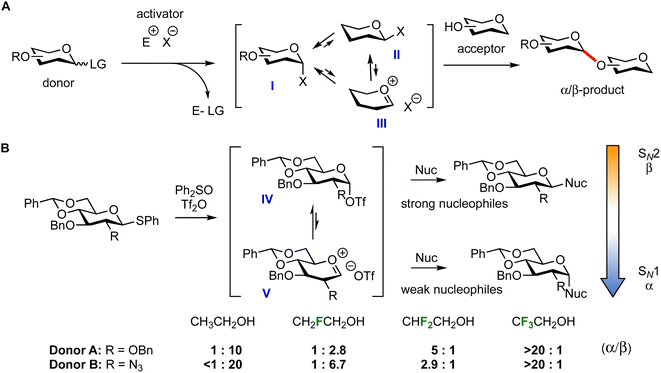
The optimization of glycosylation reactions in the context of oligosaccharide total synthesis is typically done in a target-oriented approach and despite decades of research no universal guidelines exist to ensure general stereoselective and high yielding glycosylations.1–5 Many different protocols for the synthesis of oligosaccharides have been developed, with most of them taking the approach depicted in Fig. 1A. First, a donor with a latent leaving group (LG) is activated with an activator (E–X) to generate a set of reactive species (I–III). Most commonly electrophiles are used featuring a triflate leaving group (X− = triflate, TfO−) to form a mixture of α- and β-triflates, of which the α-triflate (I), having the anomeric triflate in an axial orientation to benefit from a stabilizing anomeric effect, is generally the most stable. The covalent triflates are in equilibrium with more reactive (solvent separated) oxocarbenium ions (III). The incoming nucleophile, the glycosyl acceptor, can react with these electrophiles to form a glycosidic bond.6 The outcome of a glycosylation reaction, in terms of both stereoselectivity and yield, depends on many variables. Both external factors such as temperature, solvent, concentration and activator as well as intrinsic properties of the donor1–4,7,8 and the acceptor9–14 play a decisive role. The impact of functional and protecting groups on the reactivity of donor glycosides has been thoroughly investigated. The relative reactivity of numerous thioglycosides has been determined,15–17 and many covalent reactive species have been observed and characterized by variable temperature NMR,18 while the reactivity of oxocarbenium-like intermediates has been probed via a combination of experiments, computational chemistry and spectroscopy.14,19–21 Thanks to systematic mechanistic studies, the effect of both stereochemistry and protecting group pattern on the reactivity of the donor is well documented. The factors influencing the reactivity of the glycosyl acceptor are less well understood, because systematic studies investigating the effect of the reactivity of the acceptor on the glycosylation outcome are much more scarce.9–11,22–27 In the development of new glycosylation methodology, quite often, a seemingly random range of acceptors is screened to probe protecting group compatibility and clear structure–reactivity–stereoselectivity relationships cannot be determined because the structures of the acceptors vary too much. Using a set of model nucleophiles of gradually changing nucleophilicity – ethanol/2-fluoroethanol/2,2-difluoroethanol/2,2,2-trifluoroethanol – we have previously established how the stereoselectivity of glycosylation reactions of benzylidene protected glucose and glucosazide donors, A and B respectively, depends on the reactivity of the incoming nucleophile (Fig. 1B).9 The stereoselectivity in this system changes from high β-selectivity for the reactive nucleophiles to high α-selectivity for the less reactive nucleophiles. To account for this reactivity–stereoselectivity relationship we reasoned that the most reactive acceptors (ethanol, 2-fluoroethanol) can displace the most stable α-triflates (IV), while the weaker nucleophiles require a more reactive electrophile: the β-triflate or a contact or solvent separated oxocarbenium ion triflate pair (V).12 The triflates generated from the glucosazide donor B are more stable than their glucose counterparts (as a result of the electron-withdrawing nature of the C-2-azide) and therefore the glycosylations of this donor proceed with higher β-/lower α-selectivity.8 The direct reactivity–stereoselectivity relationship in this glycosylation system has allowed us to use this as a measure for the reactivity of various carbohydrate acceptors.11 In an initial structure–reactivity study we established that the reactivity of a glucosyl acceptor – and thus the stereoselectivity in glycosylation reactions – can be judiciously tuned by installing the appropriate protecting group pattern. It was found that changing a single benzyl group in an acceptor glucoside for a benzoate (effectively changing only two protons for an oxygen atom) can render a non-stereoselective glycosylation reaction completely α-selective.11We here map the reactivity/selectivity of a broad panel of glycosyl acceptors, varying in the position of the free hydroxyl group on the ring, the relative stereochemistry of the neighbouring functional groups as well as the nature of the protecting/functional groups. We have systematically surveyed D-glucose, D-glucosamine, D-mannose, and D-galactose C-2, C-3, or C-4 hydroxyl acceptors. To limit the steric effects when comparing different ether/ester protecting groups, sterically similar benzyl and benzoyl groups were used. We have probed double stereodifferentiation effects in glycosylations of D- and L-rhamnose and D- and L-fucose systems. Finally, we have designed and surveyed a series of model “stripped” carbohydrate-like acceptors and glycerol alcohols to serve as (non-chiral) benchmark acceptor systems. Using this extensive set of acceptors, we have been able to establish structure–reactivity guidelines that can be used to rationally tune the reactivity of glycosyl acceptors to optimize glycosylation stereoselectivity.
Results and discussion
For this study, we generated 67 acceptors (see ESI† for the synthesis of the acceptors that have not been published previously) and glycosylated these with both donor A and donor B. To this end, we transformed donor A/B into the corresponding triflates using the diphenyl sulfoxide/triflic anhydride couple after which the acceptors were added and allowed to react at −40 °C. Tables 1–6 summarize the results obtained, with the results of the glucose series (acceptors 1–18) in Table 1, the mannose series (19–29) in Table 2, the galactose series (30–41) in Table 3, the rhamnose series (42–48) in Table 4, the fucose series (49–56) in Table 5 and a set of model acceptors (57–67) in Table 6.| Acceptor | Structure | Donor A | Donor B | ||||
|---|---|---|---|---|---|---|---|
| Product (yield%) |
α![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) βb βb |
β (%) | Product (yield%) |
α![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) βb βb |
β (%) | ||
| a Taken from ref. 11. b The anomeric ratio was determined using NMR of the product mixtures, isolated by size exclusion chromatography, see ESI for details. | |||||||
| 1 |
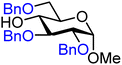
|
1A (82) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
50 | 1B (88) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
88 |
| 2 |
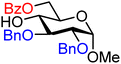
|
2A (92) | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
20 | 2B (67) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 1.1 |
52 |
| 3 |
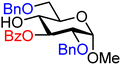
|
3A (95) | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0 | 3B (77) | 6.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
13 |
| 4 |
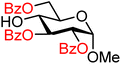
|
4A (91) | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0 | 4B (69) | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0 |
| 5 |
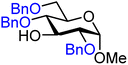
|
5A (78) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.7 2.7 |
73 | 5B (70) | <1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
100 |
| 6 |
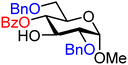
|
6A (98) | 2.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
28 | 6B (99) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
83 |
| 7 |
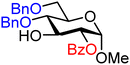
|
7A (99) | 1.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
36 | 7B (93) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
80 |
| 8 |
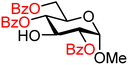
|
8A (100) | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0 | 8B (83) | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0 |
| 9 |
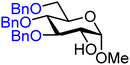
|
9A (76) | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
10 | 9B (66) | 1.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
38 |
| 10 |
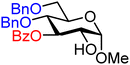
|
10A (78) | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0 | 10B (82) | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
14 |
| 11 |
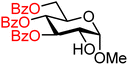
|
11A (85) | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0 | 11B (92) | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
7 |
| 12 |
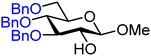
|
12A (96) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.9 1.9 |
66 | 12B (78) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
86 |
| 13 |
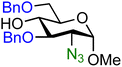
|
13A (94) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 1.1 |
52 | 13B (100) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.3 3.3 |
77 |
| 14 |
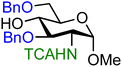
|
14A (81) | 1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
48 | 14B (100) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5 3.5 |
78 |
| 15 |
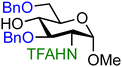
|
15A (82) | 1.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
43 | 15B (100) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 2.5 |
71 |
| 16 |
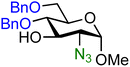
|
16A (83) | 1.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
38 | 16B (85) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 2.5 |
71 |
| 17 |
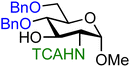
|
17A (65) | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
8 | 17B (63) | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
25 |
| 18 |
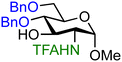
|
17B (96) | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0 | 18B (100) | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
8 |
| Acceptor | Structure | Donor A | Donor B | ||||
|---|---|---|---|---|---|---|---|
| Product (yield%) |
α![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) βb βb |
β (%) | Product (yield%) |
α![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) βb βb |
β (%) | ||
| a Taken from ref. 11. b The anomeric ratio was determined using NMR of the product mixtures, isolated by size exclusion chromatography, see ESI for details. | |||||||
| 19 |
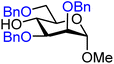
|
19A (76) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
67 | 19B (72) | <1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
100 |
| 20 |
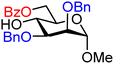
|
20A (76) | 1.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
43 | 20B (92) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
89 |
| 21 |
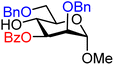
|
21A (62) | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
10 | 21B (93) | 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
40 |
| 22 |
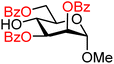
|
22A (66) | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0 | 22B (98) | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
9 |
| 23 |
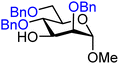
|
23A (82) | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
11 | 23B (70) | 1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
48 |
| 24 |
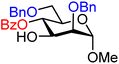
|
24A (87) | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0 | 24B (87) | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0 |
| 25 |
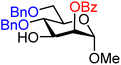
|
25A (82) | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
9 | 25B (93) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
50 |
| 26 |
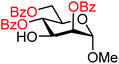
|
26A (100) | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0 | 26B (100) | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0 |
| 27 |
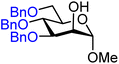
|
27A (95) | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0 | 27B (65) | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
13 |
| 28 |
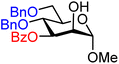
|
28A (76) | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0 | 28B (51) | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
13 |
| 29 |
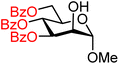
|
29A (77) | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0 | 29B (51) | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0 |
| Acceptor | Structure | Donor A | Donor B | ||||
|---|---|---|---|---|---|---|---|
| Product (yield%) |
α![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) βb βb |
β (%) | Product (yield%) |
α![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) βb βb |
β (%) | ||
| a Taken from ref. 11. b The anomeric ratio was determined using NMR of the product mixtures, isolated by size exclusion chromatography, see ESI for details. | |||||||
| 30 |
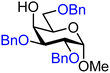
|
30A (72) | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
8 | 30B (86) | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
25 |
| 31 |
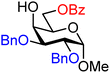
|
31A (85) | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0 | 31B (100) | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
25 |
| 32 |
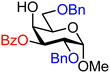
|
32A (78) | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
8 | 32B (67) | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
25 |
| 33 |
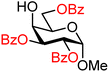
|
33A (70) | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0 | 33B (100) | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0 |
| 34 |
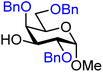
|
34A (85) | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
14 | 34B (88) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 1.3 |
57 |
| 35 |
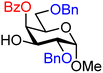
|
35A (76) | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
6 | 35B (60) | 1.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
43 |
| 36 |
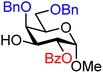
|
36A (84) | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0 | 36B (82) | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
7 |
| 37 |
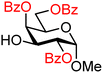
|
37A (83) | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0 | 37B (90) | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
8 |
| 38 |
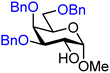
|
38A (87) | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
9 | 38B (73) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.3 1.3 |
57 |
| 39 |
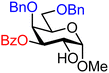
|
39A (89) | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0 | 39B (51) | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
25 |
| 40 |
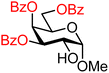
|
40A (88) | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0 | 40B (87) | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
14 |
| 41 |
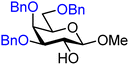
|
41A (83) | 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
40 | 41B (86) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
91 |
| Acceptor | Structure | Donor A | Donor B | ||||
|---|---|---|---|---|---|---|---|
| Product (yield%) |
α![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) βa βa |
β (%) | Product (yield%) |
α![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) βa βa |
β (%) | ||
| a The anomeric ratio was determined using NMR of the product mixtures, isolated by size exclusion chromatography, see ESI for details. | |||||||
| 42 |
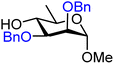
|
42A (89) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.4 2.4 |
71 | 42B (75) | <1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
100 |
| 43 |
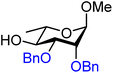
|
43A (90) | 1.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
37 | 43B (99) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
91 |
| 44 |
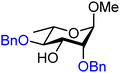
|
44A (100) | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
13 | 44B (68) | 1.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
42 |
| 45 |
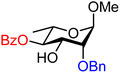
|
45A (69) | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0 | 45B (50) | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
8 |
| 46 |
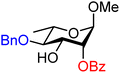
|
46A (66) | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
14 | 46B (55) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
50 |
| 47 |
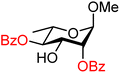
|
47A (83) | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0 | 47B (100) | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
8 |
| 48 |
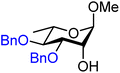
|
48A (59) | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0 | 48A (77) | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
25 |
| Acceptor | Structure | Donor A | Donor B | ||||
|---|---|---|---|---|---|---|---|
| Product (yield%) |
α![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) βa βa |
β (%) | Product (yield%) |
α![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) βa βa |
β (%) | ||
| a The anomeric ratio was determined using NMR of the product mixtures, isolated by size exclusion chromatography, see ESI for details. | |||||||
| 49 |
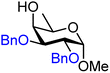
|
49A (98) | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0 | 49B (100) | 1.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
43 |
| 50 |
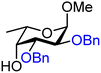
|
50A (85) | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
7 | 50B (100) | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
25 |
| 51 |
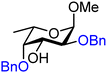
|
51A (100) | 3.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
24 | 51B (88) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
67 |
| 52 |
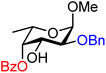
|
52A (100) | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0 | 52B (100) | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
33 |
| 53 |
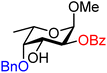
|
53A (100) | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0 | 53B (92) | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
10 |
| 54 |
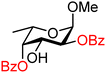
|
54A (100) | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0 | 54B (100) | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0 |
| 55 |
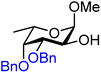
|
55A (55) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
50 | 55B (95) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.5 5.5 |
85 |
| 56 |
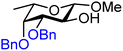
|
56A (86) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
55 | 56B (93) | <1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
100 |
| Acceptor | Structure | Donor A | Donor B | ||||
|---|---|---|---|---|---|---|---|
| Product (yield%) |
α![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) βa βa |
β (%) | Product (yield%) |
α![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) βa βa |
β (%) | ||
| a The anomeric ratio was determined using NMR of the product mixtures, isolated by size exclusion chromatography, see ESI for details. | |||||||
| 57 |
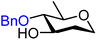
|
57A (81) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.6 1.6 |
62 | 57B (85) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 13 |
93 |
| 58 |
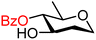
|
58A (53) | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
17 | 58B (60) | 1.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
42 |
| 59 |
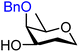
|
59A (97) | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
20 | 59B (60) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4 1.4 |
58 |
| 60 |
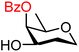
|
60A (100) | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
11 | 60B (100) | 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
40 |
| 61 |
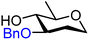
|
61A (98) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.6 1.6 |
62 | 61B (97) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 16 |
94 |
| 62 |
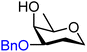
|
62A (100) | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
8 | 62B (93) | 1.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
42 |
| 63 |
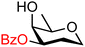
|
63A (91) | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
4 | 63B (82) | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
20 |
| 64 |
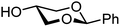
|
64A (100) | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
14 | 64B (100) | 2.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
26 |
| 65 |
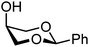
|
65A (79) | 1.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
38 | 65B (31) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
75 |
| 66 |
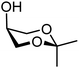
|
66A (96) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
50 | 66B (100) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
91 |
| 67 |
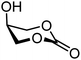
|
67A (97) | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0 | 67B (99) | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0 |
Upon analysis of the results, several trends emerge. In our previous study,11 the reactivity of glucosyl C-4-hydroxy groups was thoroughly investigated (Table 1) and it was found that the protecting groups on the C-6- and C-3-position have a significant effect on its reactivity, with the protecting group on the C-3-position having a stronger influence, due to its closer proximity. When both positions are changed from benzyl ethers to benzoyl esters, the deactivating effects work in concert leading to the following order of reactivity for the glucosyl C-4-OH acceptors: 1 > 2 >3 > 4. A similar effect is found for the mannosyl C-4-OH (Table 2 acceptors 19–22). Also in this series, the protecting group on the C-3-position has a larger influence on the reactivity of the acceptor than the protecting group on the C-6-position, and again the effects of the C-3/C-6 benzyl/benzoyl groups are additive, leading to the order of reactivity for the mannosyl C-4-OH acceptors 19 > 20 > 21 > 22. Of note, the mannosyl C-4-OH acceptors are all more reactive than their glucosyl C-4-OH equivalents.
The axial galactosyl C-4-OH is significantly less reactive (Table 3 acceptor 30). When the protection groups on the C-6-position or the C-3-position of the galactose acceptors are changed from benzyl ethers to benzoyl esters (acceptors 31 and 32 respectively) the reactivity does not change significantly. Only when all protecting groups are changed to benzoyl esters, a significant effect is found and the reactivity decreases to provide highly α-selective glycosylations (acceptor 33). The C-4-hydroxyls of D- and L-rhamnose and D- and L-fucose (acceptors 42, 43, 49, 50, Tables 4 and 5) were used to investigate double stereodifferentiation effects26 in this glycosylation system. Although there are differences in stereoselectivity between the D- and L-isomers, the configuration of the acceptor seems to be more important than the absolute stereochemistry. The D-rhamnose and fucose acceptors have similar reactivity as their mannose and galactose counterparts, respectively, which is in line with what was previously found for C-4-OH glucose and C-4-OH 6-deoxyglucose acceptors.11
Regarding the reactivity of the C-3-hydroxyls, it is remarkable that the glycosylation with the glucose C-3-OH (acceptor 5) is much more β-selective than the reaction with the mannosyl, galactosyl, rhamnosyl or fucosyl C-3-OH (acceptor 23, 34, 44, and 51 respectively), which all provide similar stereoselectivity. The main structural difference that distinguishes the glucosyl C-3-OH from the other alcohols, is that this alcohol has two equatorially oriented neighbouring groups, while in mannose, galactose, rhamnose and fucose one of the neighbouring groups is axial, suggesting that this is an important factor influencing the reactivity of the acceptor. Benzoylation of the 2- and 4-position of glucose (acceptors 6 and 7) has a similar effect on the reactivity of the C-3-OH and the effects are additive (acceptor 8). While the glycosylation of all per-benzoylated acceptors in the other series (acceptors 26, 37, 47 and 54) show excellent α-selectivity, the effect of a single benzoyl group in these acceptors (as in mannosyl acceptors 24 and 25, galactosyl acceptors 35 and 36, rhamnosyl acceptors 45 and 46 and fucosyl acceptors 52 and 53) depends strongly on which position it is placed. In mannose and rhamnose, benzoylation of the equatorial C-4-OH significantly diminishes the reactivity (acceptors 24 and 45) while benzoylation of the axial C-2-alcohol has little effect on the reactivity (acceptors 25 and 46). This effect was also observed for the galactosyl and fucosyl acceptors, were benzoylation of the axial C-4-OH (acceptors 35 and 51) has a smaller effect on the reactivity of the C-3-alcohol than benzoylation of the equatorial C-2-OH (acceptors 36 and 53). These results show that the electron withdrawing effect of the benzoate esters critically depends on the orientation of this protecting group relative to the hydroxy group.
The results of the glycosylations with the C-2-OH acceptors reveal a similar trend. The reactivity of the equatorial alcohols (acceptors 9, 12, 38, 41, 55, 56) is higher than that of the axial alcohols (acceptors 27 and 48). Substitution of benzyl groups for benzoyl groups decreases the reactivity of the glucosyl, mannosyl and galactosyl C-2-OH (acceptors 9–11, 27–29 and 38–40). When regarding the reactivity of α-OMe vs. β-OMe acceptors (9vs.12, 38vs.41 and 55vs.56) it becomes clear that alcohols next to equatorial ethers are more reactive than those next to an axial ether, in line with the reactivity trend revealed above for the C-3-OH acceptors. Furthermore, the β-OMe acceptors 12, 41 and 56 have a similar reactivity as the other acceptors having the free alcohol next to two equatorial ethers (acceptors 1, 5, 19, 42, 43, 56) and the α-OMe acceptors 9 and 38 react in a similar fashion to the other acceptors having one axial and one equatorial ether (acceptors 23, 34, 44 and 51), again showing that the configuration of functional groups next to the alcohol is important for the reactivity. From all the tested acceptors, only acceptor 55 shows a higher β-selectivity than what could be expected based on the above-described configuration-reactivity trends.
The effect of different protected amino groups becomes clear from the series of glucosamine acceptors (13–19, Table 1). The C-4-OH glucosamine acceptors were studied as these have been reported to be very poor nucleophiles.23 We found that, in line with the negligible effect of a C-2-O-benzoate on the reactivity of the glucose C-4-OH, the nature of the C-2-amino functionality has little effect on the reactivity of the glucosamine C-4-OH acceptors (13, 14, 15). The nucleophilicity of the C-3-OH however is strongly influenced by the neighboring C-2-nitrogen group. The reactivity of glucosazide acceptor 16 appears to be similar to the reactivity of C-2-O-benzoyl glucose 7. Protecting the glucosamine amine group with a trichloroacetyl or trifluoroacetyl group decreases the reactivity of the flanking C-3-OH more strongly, with the trifluoroacetyl group having the largest effect, providing highly α-selective glucosylation reactions.
Finally, two sets of model acceptors were introduced to probe the effect of a single neighbouring group on the reactivity of the alcohol acceptors. The first set comprises a set of ‘stripped’ carbohydrate acceptors with a single substituent next to the alcohol. The experimental data obtained with these nucleophiles show the same reactivity–stereoselectivity trends found for the carbohydrate acceptors above: equatorial acceptors are more reactive than axial acceptors (57vs.59 and 61vs.62), alcohols next to equatorial benzyl ethers are more nucleophilic than those next to axial benzyl ethers (57 and 61vs.59) and equatorial esters decrease the nucleophilicity much more than axial esters as compared to corresponding ethers (57vs.58 and 59vs.60). The second set of model acceptors consist of four glycerol C-2-OH acceptors, which were designed to investigate the effect of different protecting groups on non-chiral cyclic acceptors containing a secondary alcohol next to two protected oxygen atoms. Not surprisingly, the reactivity of the glycerol alcohols depends strongly on the protecting groups. The isopropylidene protected acceptor (66) is the most reactive, followed by the cis-benzylidene protected acceptor (65), the trans-benzylidene protected acceptor (64) and finally the carbonate protected acceptor (67). The unusual high reactivity of the axial hydroxyl groups in 65 with respect to its equatorial counterpart 64 can, at least in part, be accounted for by the internal hydrogen bonds of the alcohol with the ring oxygens, rendering the axial alcohol more electron rich.
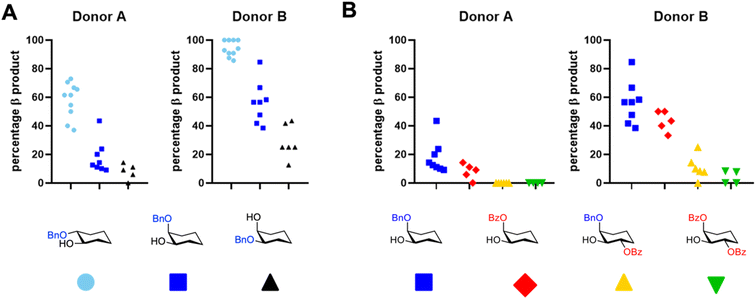
To graphically summarize the structure–reactivity relationships for the large collection of acceptors, we divided them in groups based on their configuration and protecting group pattern and plotted their reactivity, as measured by the percentage β-product with donor A and donor B (see Fig. 2). Fig. 2A shows the importance of the configuration of the alcohol and its direct neighbour(s) on the reactivity of the acceptor: equatorial acceptors are generally more reactive than axial acceptors (light blue circles and blue squares vs. black triangles) and an axial neighbour is more disarming than an equatorial neighbour (light blue circles vs. blue squares). Fig. 2B shows the effect of the orientation of a benzoyl group on the reactivity of the acceptors. When the neighbouring benzoyl is axial (red diamonds) the effect on the reactivity is smaller than that of an equatorial benzoyl (yellow triangles). Benzyl protected acceptors with one axial neighboring OBn or OMe group (blue squares) and acceptors with one axial and one equatorial benzoyl group (green inverted triangles) are provided as a reference.
Conclusion
In conclusion, structure–reactivity relationships for a large set of glycosyl acceptors have been established, based on the stereoselectivity of these acceptors in glycosylations with two conformationally restricted glucosyl donors. The reactivity–stereoselectivity correlation is based on the premise that reactive acceptors predominantly provide the β-product via an SN2 like mechanism in which a covalent anomeric α-triflate is displaced, while less reactive acceptors give more α-product via a glycosylation proceeding with more SN1 like character. In total, 66 acceptors were tested, and this systematic series of nucleophiles has revealed the following guidelines that can be used to estimate and tune the reactivity of a given carbohydrate alcohol:(1) Equatorial acceptors are more reactive than axial alcohols.
(2) Acceptors with a neighbouring protected alcohols in an equatorial position, are more reactive than acceptors in which one of the flanking protected alcohols is axial.
(3) Benzoyl esters flanking the acceptor decrease the reactivity of the acceptor more than neighbouring benzyl ethers.
(4) The disarming effect of an equatorial benzoyl versus an equatorial benzyl ether is significantly larger than the disarming effect of an axial benzoyl versus an axial benzyl ether.
(5) For glucose, the system with only equatorial substituents, the order of reactivity for the secondary alcohols is C-3-OH > C-2-OH (β-Glc) ∼ C-4-OH > C-2-OH (α-Glc).
From the study presented here, it is apparent that the reactivity of the acceptor alcohol can have a tremendous impact on the stereochemical outcome of a glycosylation reaction. In optimizing glycosylation reactions, most attention is generally paid to the nature of the glycosyl donor and external factors such as reaction temperature and solvent. Tuning the reactivity of the acceptor provides an additional means to steer the stereochemical outcome and the empiric guidelines formulated here will allow for the rational optimization of glycosylation reactions. Tuning acceptor reactivity will be useful in optimizing both yield and stereoselectivity of a glycosylation and aid in the prevention of yield deflating side reactions, such as aglycon transfer reactions. Finally, it is expected that the systematic series of glycosylations reported here will be an important stepping stone towards the generation of a more quantitative system to determine acceptor reactivity, its relation to glycosylation stereoselectivity and understanding the different reaction paths that can be followed during a glycosylation reaction.28
Data availability
Experimental procedures for the synthesis of the acceptor alcohols, procedures for the glycosylation reactions and characterisation and NMR spectra of all products. See ESI at DOI: https://doi.org/10.1039/d2sc06139b.Author contributions
JDCC and GAM: conceptualisation; JMAH, RJCH, WAR, KNAV, TH, SV: investigation; HSO, GAM, JDCC: supervision; investigation; JMAH, JDCC: writing – original draft; JMAH, RJCH, WAR, KNAV, TH, SV, HSO, GAM, JDCC: writing-review & editing.Conflicts of interest
The authors declare no competing interests.Acknowledgements
This work was supported by an ERC-CoG (726072) and NWO VICI (VI.C.182.020) grant awarded to J. D. C. C.References
- P. R. Andreana and D. Crich, ACS Cent. Sci., 2021, 7, 1454–1462 CrossRef CAS PubMed.
- A. V. Demchenko, Handbook of Chemical Glycosylation: Advances in Stereoselectivity and Therapeutic Relevance, Wiley-VCH Verlag GmbH & Co. KGaA, 2008 Search PubMed.
- L. Bohé and D. Crich, Carbohydr. Res., 2015, 403, 48–59 CrossRef PubMed.
- C. S. Bennett, Selective Glycosylations: Synthetic Methods and Catalysts, Wiley VCH Verlag GmbH, 2017 Search PubMed.
- W.-L. Leng, H. Yao, J.-X. He and X.-W. Liu, Venturing beyond Donor-Controlled Glycosylation: New Perspectives toward Anomeric Selectivity, Acc. Chem. Res., 2018, 51(3), 628–639 CrossRef CAS PubMed.
- R. Das and B. Mukhopadhyay, ChemistryOpen, 2016, 5, 401–433 CrossRef CAS PubMed.
- S. Chatterjee, S. Moon, F. Hentschel, K. Gilmore and P. H. Seeberger, J. Am. Chem. Soc., 2018, 140, 11942–11953 CrossRef CAS PubMed.
- C.-W. Chang, M.-H. Lin, C.-H. Wu, T.-Y. Chiang and C.-C. Wang, J. Org. Chem., 2020, 85, 15945–15963 CrossRef CAS PubMed.
- S. van der Vorm, T. Hansen, H. S. Overkleeft, G. A. van der Marel and J. D. C. Codée, Chem. Sci., 2017, 8, 1867–1875 RSC.
- S. van der Vorm, H. S. Overkleeft, G. A. van der Marel and J. D. C. Codée, Stereoselectivity of Conformationally Restricted Glucosazide Donors, J. Org. Chem., 2017, 82(9), 4793–4811 CrossRef CAS PubMed.
- S. van der Vorm, J. M. A. van Hengst, M. Bakker, H. S. Overkleeft, G. A. van der Marel and J. D. C. Codée, Angew. Chem., Int. Ed., 2018, 57, 8240–8244 CrossRef CAS PubMed.
- C.-W. Chang, C.-H. Wu, M.-H. Lin, P.-H. Liao, C.-C. Chang, H.-H. Chuang, S.-C. Lin, S. Lam, V. P. Verma, C. P. Hsu and C.-C. Wang, Angew. Chem., Int. Ed., 2019, 58, 16775–16779 CrossRef CAS PubMed.
- B. Hagen, S. Ali, H. S. Overkleeft, G. A. van der Marel and J. D. C. Codée, J. Org. Chem., 2017, 82, 848–868 CrossRef CAS PubMed.
- T. Hansen, H. Elferink, J. M. A. van Hengst, K. J. Houthuijs, W. A. Remmerswaal, A. Kromm, G. Berden, S. van der Vorm, A. M. Rijs, H. S. Overkleeft, D. V. Filippov, F. P. J. T. Rutjes, G. A. van der Marel, J. Martens, J. Oomens, J. D. C. Codée and T. J. Boltje, Nat. Commun., 2020, 11, 2664 CrossRef CAS PubMed.
- B. Fraser-Reid, Z. Wu, U. E. Udodong and H. Ottosson, J. Org. Chem., 1990, 55, 6068–6070 CrossRef CAS.
- N. L. Douglas, S. V. Ley, U. Lücking and S. L. Warriner, J. Chem. Soc., Perkin Trans. 1, 1998, 51–66 RSC.
- Z. Zhang, I. R. Ollmann, X.-S. Ye, R. Wischnat, T. Baasov and C.-H. Wong, J. Am. Chem. Soc., 1999, 121, 734–753 CrossRef CAS.
- T. G. Frihed, M. Bols and C. M. Pedersen, Chem. Rev., 2015, 115, 4963–5013 CrossRef CAS PubMed.
- T. Hansen, L. Lebedel, W. A. Remmerswaal, S. van der Vorm, D. P. A. Wander, M. Somers, H. S. Overkleeft, D. V. Filippov, J. Désiré, A. Mingot, Y. Bleriot, G. A. van der Marel, S. Thibaudeau and J. D. C. Codée, ACS Cent. Sci., 2019, 5, 781–788 CrossRef CAS.
- M. Marianski, E. Mucha, K. Greis, S. Moon, A. Pardo, C. Kirschbaum, D. A. Thomas, G. Meijer, G. von Helden, K. Gilmore, P. H. Seeberger and K. Pagel, Angew. Chem., Int. Ed., 2020, 59, 6166–6171 CrossRef CAS PubMed.
- A. Franconetti, A. Ardá, J. L. Asensio, Y. Blériot, S. Thibaudeau and J. Jiménez-Barbero, Acc. Chem. Res., 2021, 54, 2552–2564 CrossRef CAS.
- S. van der Vorm, T. Hansen, J. M. A. van Hengst, H. S. Overkleeft, G. A. van der Marel and J. D. C. Codée, Chem. Soc. Rev., 2019, 48, 4688–4706 RSC.
- K. L. M. Hoang and X.-W. Liu, Nat. Commun., 2014, 5, 5051 CrossRef CAS PubMed.
- S. Kaeothip, S. J. Akins and A. V. Demchenko, Carbohydr. Res., 2010, 345, 2146–2150 CrossRef CAS PubMed.
- J. Kalikanda and S. Li, Carbohydr. Res., 2011, 346, 2380–2383 CrossRef CAS PubMed.
- N. M. Spijker and C. A van Boeckel, Angew. Chem., Int. Ed. Engl., 1991, 30, 180–183 CrossRef.
- D. Crich and V. Dudkin, J. Am. Chem. Soc., 2001, 123, 6819–6825 CrossRef CAS PubMed.
- C.-W. Chang, M.-H. Lin, C.-K. Chan, K.-Y. Su, C.-H. Wu, W.-C. Lo, S. Lam, Y.-T. Cheng, P.-H. Liao, C.-H. Wong and C.-C. Wang, Angew. Chem., Int. Ed., 2021, 60, 12413–12423 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures for the synthesis of the acceptor alcohols, procedures for the glycosylation reactions and characterisation and NMR spectra of all products. See DOI: https://doi.org/10.1039/d2sc06139b |
| This journal is © The Royal Society of Chemistry 2023 |