Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Molecular cyclo-P3 complexes of the rare-earth elements via a one-pot reaction and selective reduction†
Adrian
Hauser
 a,
Luca
Münzfeld
a,
Luca
Münzfeld
 a,
Sören
Schlittenhardt
a,
Sören
Schlittenhardt
 b,
Ralf
Köppe
b,
Ralf
Köppe
 a,
Cedric
Uhlmann
a,
Cedric
Uhlmann
 a,
Ulf-Christian
Rauska
a,
Ulf-Christian
Rauska
 a,
Mario
Ruben
a,
Mario
Ruben
 bcd and
Peter W.
Roesky
bcd and
Peter W.
Roesky
 *a
*a
aInstitute of Inorganic Chemistry, Karlsruhe Institute of Technology (KIT), Engesserstraße 15, D-76131 Karlsruhe, Germany. E-mail: roesky@kit.edu
bInstitute of Nanotechnology, Karlsruhe Institute of Technology (KIT), Hermann-von-Helmholtz-Platz 1, D-76344 Eggenstein-Leopoldshafen, Germany
cCentre Européen de Science Quantique (CESQ), Institut de Science et d'Ingénierie Supramoléculaires (ISIS, UMR 7006), CNRS-Université de Strasbourg, 8 allée Gaspard Monge BP 70028, 67083 Strasbourg Cedex, France
dInstitute of Quantum Materials and Technologies (IQMT), Karlsruhe Institute of Technology, Hermann-von-Helmholtz-Platz 1, 76344 Eggenstein-Leopoldshafen, Germany
First published on 3rd February 2023
Abstract
Synthesis of new organo-lanthanide polyphosphides with an aromatic cyclo-[P4]2− moiety and a cyclo-[P3]3− moiety is presented. For this purpose, the divalent LnII-complexes [(NON)LnII(thf)2] (Ln = Sm, Yb) ((NON)2− = 4,5-bis(2,6-diisopropylphenyl-amino)-2,7-di-tert-butyl-9,9-dimethylxanthene) and trivalent LnIII-complexes [(NON)LnIIIBH4(thf)2] (Ln = Y, Sm, Dy) were used as precursors in the reduction process of white phosphorus. While using [(NON)LnII(thf)2] as a one-electron reducing agent the formation of organo-lanthanide polyphosphides with a cyclo-[P4]2− Zintl anion was observed. For comparison, we investigated a multi-electron reduction of P4 by a one-pot reaction of [(NON)LnIIIBH4(thf)2] with elemental potassium. As products molecular polyphosphides with a cyclo-[P3]3− moiety were isolated. The same compound could also be obtained by reducing the cyclo-[P4]2− Zintl anion within the coordination sphere of SmIII in [{(NON)SmIII(thf)2}2(μ-η4:η4-P4)]. Reduction of a polyphosphide within the coordination sphere of a lanthanide complex is unprecedented. Additionally, the magnetic properties of the dinuclear DyIII-compound bearing a bridging cyclo-[P3]3− moiety were investigated.
Introduction
In recent decades, the functionalization of white phosphorus (P4) using organometallic compounds has been intensively studied.1 These metal polyphosphides play an important role as intermediates in the production of organophosphorus compounds since they can circumvent the use of environmentally harmful chlorine gas.2,3 Starting with the activation of P4 using transition metals which led to the formation of various polyphosphide ring structures such as [P4]2−, [P5]− or the neutral [P6]-ring,1,4–7 this reactivity could be transferred to a variety of main group compounds in the following years.1,8–14 With the synthesis of [(Cp*)2SmIII)4P8] (Cp* = C5Me5) by reducing P4 with the solvent-free [(Cp*)2SmII], our group successfully isolated the first molecular polyphosphide of the rare earth elements in 2009 (Fig. 1a).15 This realgar-type structural motif of the [P8]4− Zintl anion was also observed shortly after via a scandium arene complex and FeI-mediated P4 activation.16,17 In the field of actinide chemistry, P4 is known to be activated by low-valent uranium and thorium species.18–23 Other molecular 4f-element compounds bearing a polyphosphide Zintl anion were synthesized either by reduction of [Cp*Fe(P5)] with different classical and non-classical divalent lanthanide precursors, or by reduction of [(Cp′′′Co)2(P2)2] (Cp′′′ = 1,2,4-tBu3C5H2) with different derivatives of samarocene, which gave access to a variety of mixed d/f-element polyphosphides.24–29 It should be noted that the latter compounds discussed here are preceded by the activation of white phosphorus with a transition metal compound. Direct activation of P4 with 4f-elements is still rather rare. It is mainly based on SET (single electron transfer) processes of classical divalent LnII-compounds, which either led to the formation of [P8]4− (Fig. 1a)15 or anti-bimetallic structural motifs with a [P4]2− Zintl anion (Fig. 1b),30 or oxidative substitution with Ln-arene complexes (Ln = La, Yb, Lu) to form 4f-element polyphosphides bearing a [P7]3− Zintl anion.16,31,32 The structural motif of a cyclo-[P3]3− polyphosphide, common for transition metal chemistry,1,33–35 is rarely encountered in rare earth compounds. The only reported example is the anti-bimetallic complex K[{(L)Y(thf)}2(μ3-η3:η3:η2-P3)] (L2− = N,N′-2,6-diisopropylphenyl-1,4-diazabutadiene), synthesised by the alkyl migration of an organosubstituted cyclo-P4R2 precursor. Thereby, the central [P3]3− Zintl anion binds at two YIII cations in a bridging coordination mode (Fig. 1c).36 In addition, there are two examples, in which a cyclo-P3 unit, which is part of a larger ligand scaffold, coordinates to a lanthanide atom. The first example, a bicyclo[4.1.0]triphosphaheptanide generated by [3 + 1] fragmentation of white phosphorus with a Lu-metallacyclopentadiene binds to the metal atom.37 The second structural example is the 4d/4f hexaphosphide [(Cp*2LnIII)2P6(Cp*Mo(CO)2)2] (Ln = Sm, Yb). Here, the P6 unit, which consists of two P–P-bridged cyclo-P3 rings, coordinates to the lanthanide ions.29 Besides structural aspects, the magnetic properties of such compounds, in particular of multinuclear Ln compounds bridged by Zintl anions, may provide new insights in the field of SMMs (single molecule magnets).38,39 | ||
| Fig. 1 Selection of various rare earth polyphosphides with different [Px]n− Zintl anions.15,30,36 | ||
Recently, organo-lanthanide compounds bridged by a [Bi6]6− Zintl anion, which promoted strong ferromagnetic interactions between lanthanides, were reported.40
Herein, we compare SET reduction of white phosphorus induced by classical divalent LnII-compounds with a multi-electron reduction induced by the in situ reaction of trivalent lanthanide complexes with potassium. As a result, new anti-bimetallic complexes of organo-lanthanide polyphosphides, which were synthesized by direct conversion of white phosphorus using di- and trivalent lanthanide compounds, were obtained. For this purpose, we stabilized the complexes by using the bulky NON ligand (NON = 4,5-bis(2,6-diisopropylphenyl-amino)-2,7-di-tert-butyl-9,9-dimethylxanthene)), which was recently employed for stabilizing metal centres in low oxidation states.41–43 Within our studies, we showcase the reduction of a polyphosphide within the coordination sphere of a lanthanide complex, which so far is unprecedented.
Results and discussion
Synthesis and structural characterization
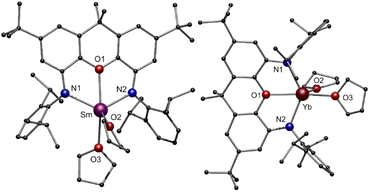
To realize the synthesis of new lanthanide polyphosphides, we initially adopted an established synthetic route in which classical divalent lanthanide compounds act as SET reagents in the activation process of P4. For this purpose, the divalent lanthanide complexes [(NON)LnII(thf)2] (Ln = Sm, Yb) (1-Ln) were first synthesised via the salt elimination reaction of [K2(NON)] and the corresponding diiodides [LnIII2(thf)2] (Ln = Sm, Yb) (Scheme 1, top). Crystals suitable for X-ray diffraction analysis could be obtained in both cases by recrystallization of the crude products from a hot n-heptane solution. Thereby, compound 1-Sm was obtained as large black crystals, while compound 1-Yb appeared as red crystals, both in yields above 60%. Complex 1-Sm crystallizes in the monoclinic space group P21, while compound 1-Yb crystallizes in the orthorhombic space group Pbca with one molecule each in the asymmetric unit. Both compounds exhibit the same structural motif in the solid state (Fig. 2). The central LnII cations are coordinated by the two amido functions of the NON ligand and by the oxygen of the xanthene backbone. Two molecules of THF saturate the coordination sphere of the lanthanide. The Sm–N bond lengths in 1-Sm (Sm–N1 2.418(7) Å, Sm–N2 2.433(7) Å) are slightly shorter compared to previously reported divalent (bis)amido SmII-compounds.44–46 Similarly, the Sm–O bond lengths are comparable to organometallic SmII-compounds known from the literature (Sm–O1 2.554(5) Å, Sm–O2 2.564(6) Å, Sm–O3 2.526(6) Å).47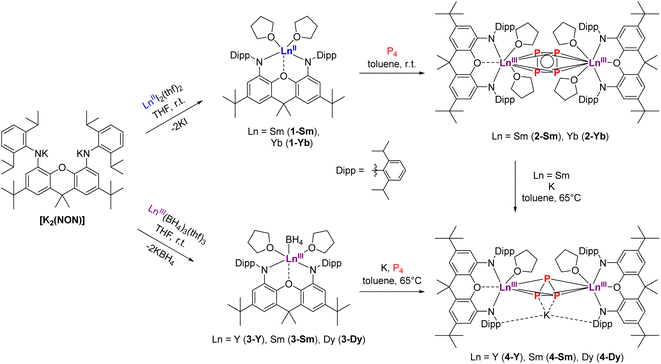 | ||
| Scheme 1 Synthesis of [(NON)LnII(thf)2] (1-Ln) and [(NON)LnIII(BH4)(thf)2] (3-Ln) as precursors for P4 activation to synthesize the anti-bimetallic cyclo-P4 (2-Ln) and cyclo-P3 complexes (4-Ln). | ||
Due to the smaller ionic radius of YbII in compound 1-Yb, the Yb–N and Yb–O distances are shortened as expected (Yb–N1 2.344(5) Å, Yb–N2 2.315(5) Å, Yb–O1 2.402(4) Å). This effect is also reflected in the N1–Ln–N2 angles, being 122.8° for 1-Sm and widened for 1-Yb to 132.0°. The NMR spectra of the compound 1-Yb (Fig S1 and S2, ESI†) in C6D6 at room temperature indicate that no coordinated THF remains on the molecule after drying the material in vacuo for several hours. All resonances can be assigned to the xanthene backbone and Dipp-groups (Dipp = diisopropylphenyl) of the ligand, respectively. Due to the paramagnetic behaviour of 1-Sm, no meaningful NMR spectra could be obtained. Elemental analysis of 1-Sm indicates that one THF molecule remains coordinated to the SmII-cation after drying the compound in vacuo.
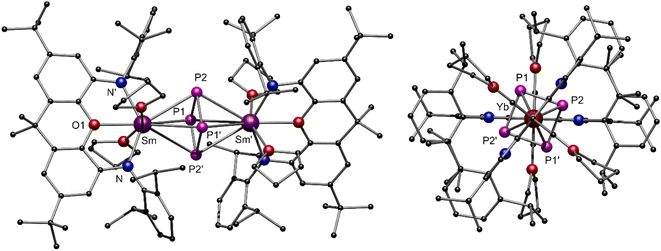
After the successful isolation of compounds 1-Ln we investigated the reductive conversion of white phosphorus via SET with these compounds (Scheme 1, top). For this purpose, the divalent complexes 1-Ln were stirred together with P4 in toluene at room temperature. Thereby deep red solutions were obtained. After filtration of insoluble residues the anti-bimetallic complexes [{(NON)LnIII(thf)2}2(μ-η4:η4-P4)] 2-Ln could be isolated as dark red (2-Sm) and dark orange (2-Yb) crystalline compounds. It should be mentioned here that the crystallization of 2-Sm was carried out from a saturated toluene solution at −10 °C, whereas the solvent had to be changed to THF for crystallization of 2-Yb. This supports the assumption that the coordinating THF remains in 1-Sm after drying in vacuo, which is required for the crystallization of 2-Sm, while no coordinating THF molecules persist in 1-Yb. The isostructural compounds crystallize in the orthorhombic space group Pban. Similar to the previously reported [{(DippForm)2SmIII}2(μ-η4:η4-P4)] (DippForm = {(2,6-iPr2C6H3)NC(H) = N(2,6-iPr2C6H3)}−) the cyclo-[P4]2− Zintl anion coordinates in a bridging η4:η4 mode between two LnIII centres and exhibits an almost perfect square shape with internal angles of 88.62(9)° and 91.38(9)° for 2-Sm and 88.73(8)° and 91.27(8)° for 2-Yb, respectively (Fig. 3). Thereby the P–P distance of 2.15 Å in 2-Ln as well as the Sm–P distances of 3.0727(8) Å and 3.0908(10) Å are in the range of reported aromatic [P4]2− Zintl anions in the coordination sphere of two SmIII-moieties.30 Corresponding Yb–P distances are slightly shorter (Yb–P1 3.0192(8) Å, Yb–P2 3.0021(8) Å) and in the range of the reported values.32 Compared to the divalent precursors 1-Ln, the Ln–N distances (Sm–N 2.360(4) Å, Yb–N 2.290(3) Å) are moderately shorter and decrease with the ionic radius of the LnIII cation as well. To illustrate the staggered arrangement of the xanthene backbones and THF ligands in 2-Ln, the top view of 2-Yb is displayed on the right side of Fig. 3. For both compounds the torsion angle of the xanthene backbones is around 53° (see Fig. S27 and S29, ESI†). Compound 2-Sm is only the second example for P4 activation which led to the formation of a 6-π-aromatic [P4]2− ring in the coordination sphere of two SmIII cations and was entirely unknown for YbIII compounds so far. NMR spectra of 2-Ln were recorded in C6D6 at room temperature. Due to the paramagnetic behaviour of complexes 2-Ln no meaningful 1H and 13C NMR spectra were obtained. In the 31P{1H} NMR spectra, however, one singlet was found in each case. The singlet at δ = 479.5 ppm for 2-Sm could be assigned to the four chemically equivalent phosphorus atoms of the cyclo-P4 moiety. Thus, the chemical shift is in a similar range to that of the previously reported [{(DippForm)2Sm}2(μ-η4:η4-P4)].30
For compound 2-Yb, the 31P{1H} NMR signal of the four phosphorus atoms is slightly shifted to higher resonances and can be detected as a singlet at δ = 382.4 ppm.
Motivated by these results, we aimed to synthesize further LnIII polyphosphides beyond Sm and Yb in order to investigate different reducing processes and to have access to a broader range of rare-earth elements for studying their physical properties. In particular, we focused on the synthesis of a dinuclear polyphosphide-bridged DyIII compound, which could potentially exhibit SMM behaviour. Although [DyI2(dme)3] is known,48 it has proven too reactive for being a suitable precursor in organometallic chemistry. Therefore, a synthetic route starting from a robust and isolable LnIII species was chosen, which subsequently should be reduced in the presence of P4 to form new molecular LnIII polyphosphides. The first step was achieved by a salt elimination reaction between [K2(NON)] and the respective [LnIII(BH4)3(thf)3] (Ln = Y, Sm, Dy) (Scheme 1, bottom). The corresponding compounds [(NON)LnIIIBH4(thf)2] 3-Ln could be isolated by recrystallization of crude materials from a hot n-heptane solution as colourless (3-Y), red (3-Sm) and light green (3-Dy) crystals in high yields. The isostructural compounds crystallize in the monoclinic space group P21/c with one molecule in the asymmetric unit.
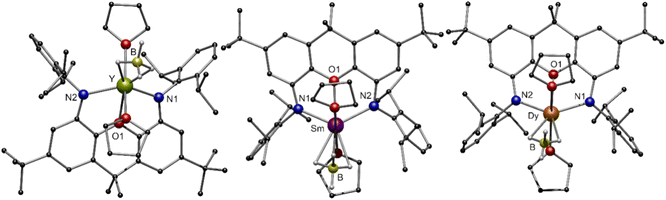
The molecular structures in the solid state of [(NON)LnIIIBH4(thf)2] 3-Ln are shown in Fig. 4. As expected, the central LnIII cation is coordinated by two amido functions and the oxygen atom of the NON ligand. Additionally, two molecules THF saturate the coordination sphere of the metal centre. Solely the coordination mode of the [BH4]− moiety, which was determined by free refinement of the hydrogen atoms, differs in 3-Ln. While the [BH4]− ligand in 3-Y and 3-Dy coordinates in an η2-H2BH2 mode, an η3-H3BH coordination mode is observed for 3-Sm. The dependence of the coordination mode of borohydrides on the ionic radii and steric demand of the applied ligands is well known in lanthanide chemistry.49,50 In the case of 3-Ln, the hapticity of [BH4]− decreases with the ionic radius of the LnIII cation with a constant steric demand of the ligand. The respective Ln–B distances are between 2.671(3) Å in 3-Sm and 2.717(3) Å in 3-Y. In the FT-IR spectra of compounds 3-Ln, different absorption patterns between 2400 cm−1 and 2100 cm−1 are observed for the different coordination modes of the [BH4]− moiety.
In the 1H{11B} NMR spectrum of 3-Y the singlet at δ = 1.13 ppm can be assigned to the four hydridic hydrogens of the [BH4]− moiety whereas no resonances for the hydrogen atoms of the borohydride can be found in the 1H NMR spectrum. All other resonances can be assigned to the NON ligand and one remaining coordinated THF molecule. The 11B NMR spectra show one broad signal at δ = −24.2 ppm for 3-Y and at δ = −38.5 ppm for 3-Sm, while remaining silent for 3-Dy due to the strong paramagnetic behaviour.
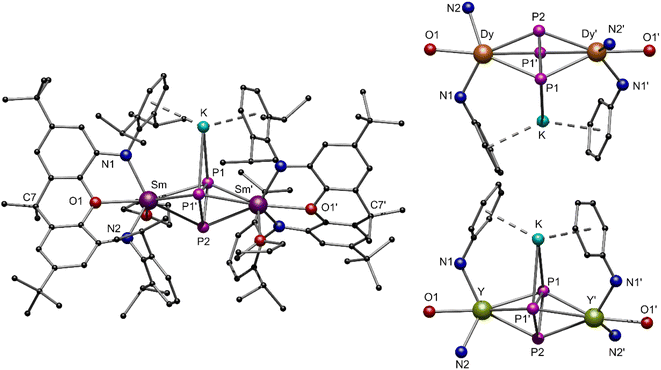
To reach the aim for new Ln polyphosphides beyond Sm and Yb, we applied the trivalent compounds 3-Ln in one-pot reactions with an excess of potassium and P4 at elevated temperatures in toluene (Scheme 1, bottom). After filtration, [K{(NON)Ln(thf)}2(μ3-η3:η3:η2-P3)] 4-Ln could be isolated as colourless (4-Y), red (4-Sm) and yellow (4-Dy) crystals at −10 °C from a saturated toluene solution. X-ray diffraction analysis revealed a bridging cyclo-[P3]3− unit in a μ-η3:η3 coordination mode as the central building block, which displays an almost perfect triangular shape with internal angles between 58.97(4)° and 62.04(5)° for P1–P2–P1′ and P1–P1′–P2, respectively (Fig. 5). The formation of 4-Ln shows that the multi-electron reduction applied here results in a different product. The P–P bond lengths within 4-Ln range from 2.160(2) Å to 2.238(2) Å, consistent with literature values of bridging cyclo-[P3]3− polyphosphides.34,36 Here, the P1–P1′ bond is always slightly elongated compared to the P1–P2 bond due to the coordination of P1 to the potassium ion (K–P1 ∼ 3.18 Å). The Ln–CtP3 bond distances (CtP3 = centroid of the cyclo-[P3] moiety) in 4-Ln decrease with the radius of the respective rare earth ion. This in turn affects the K–Ctphenyl distances, which thus also follow this trend (4-Sm: K–Ctphenyl 2.8112(6) Å to 4-Dy: K–Ctphenyl 2.7727(4) Å). The coordination of the potassium ion by the two phenyl rings of the Dipp-moieties further leads to the folding of the originally planar xanthene backbone along the Ln–O1–C7 axis by about 35° (see Fig. S34, S36 and S38, ESI†). Using the specific example of 4-Sm, the Sm–CtP3 distance of 2.6030(7) Å can be directly compared with the Sm–CtP4 distance 2-Sm. The latter is slightly longer with 2.6787(7) Å, which is due to the higher charge density in the cyclo-[P3]3− compared to the cyclo-[P4]2− polyanion in an otherwise similar chemical environment. The resulting stronger electrostatic interactions lead to a shorter Sm–CtP3 distance.
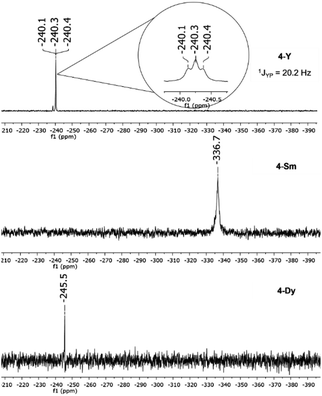
The 31P{1H} NMR spectra of compounds 4-Dy and 4-Sm show one singlet each for the cyclo-[P3]3− moiety at δ = −245.5 ppm (4-Dy) and δ = −336.7 ppm (4-Sm) (Fig. 6). The resonance of the cyclo-[P3]3− compound 4-Sm is thus considerably shifted to higher field frequencies compared to the corresponding cyclo-[P4]2− compound 2-Sm. For compound 4-Y a triplet is seen in the 31P{1H} NMR spectrum at δ = −240.3 ppm with a coupling constant of 1JYP = 20.2 Hz (Fig. 6), which is slightly higher than that of the reported complex K[{(L)Y(thf)}2(μ3-η3:η3:η2-P3)] (1JYP = 16.3 Hz).36
To get further insights in the formation of the [P3]3− Zintl anion in 4-Ln, we first investigated the reduction of 2-Sm with potassium in the NMR scale (Scheme 1, right). After a few hours at 65 °C, the 31P NMR resonance at 479.5 ppm in C6D6, which is assigned to the [P4]2− unit of 2-Sm (ESI, Fig. S3†), vanished and a new resonance at −320.8 ppm appeared. After removing C6D6in vacuo and resolving the residue in thf-d8, the resonance was detected at −336.7 ppm, which is consistent with the chemical shift of the isolated compound 4-Sm from the one pot reaction of 3-Sm with potassium and P4 (Fig. 6). This was further confirmed by the reaction of 2-Sm with potassium in a preparative scale with subsequent crystallisation of 4-Sm. In the 31P{1H} NMR spectrum no further resonances, which could be assigned to possible by-products, were detected. Hence, the reactivity of 2-Sm with potassium clearly shows that the [P4]2− can be reduced to a [P3]3− Zintl anion in the coordination sphere of two SmIII cations. Therefore, we anticipate that during the formation of 4-Ln, P4 is reduced to a [P4]2− moiety first and subsequently reduced to a [P3]3− Zintl anion. However, isolation of the [P4]2− species from the one-pot reaction of compounds 3-Ln was not possible even with variation of stoichiometry and temperature.
DFT calculations
To obtain a better insight into the bonding situation of the compounds under discussion, we performed quantum chemical DFT calculations on the molecules 2-Sm and 4-Sm (symmetries S4 (2-Sm) and C2 (4-Sm), def-SV(P) for all atoms, ecp for Sm3+,51 RI-BP86,52–54 program system TURBOMOLE).55 The existence of real local minima was confirmed by frequency calculations using the aoforce module. The calculated structural data are in good agreement with the experimentally determined data. As in other lanthanide complexes,15 we find that the bonding from the rare earth cations to polyphosphide ligands can be described primarily as ionic bonds. For this reason, the anionic cyclo-[P3]3− and cyclo-[P4]2− ligands were also calculated as purely ionic model compounds K3P3 and K2P4 (point symmetries C1 and D4h) and compared with the situation in these complexes. The investigation of the structural data is supported by shared electron numbers (SEN) from population analyses based on occupation numbers according to the method of Ahlrichs and Heizmann that serve as measures for covalent bonding.56,57 For 2-Sm, the P–P bond distance and the SEN are calculated to be 2.202 Å and 1.31, respectively. The values obtained for P42− in K2P4 are in good agreement with this finding (bond distance 2.200 Å, SEN 1.27). To evaluate the aromaticity, multi-center SEN(P4) of both particles were determined; the results support slight aromatic bonding (2-Sm: SEN(P4) 0.1; K2P4: SEN(P4) 0.08). Moreover, this behavior is supported by the NICS values of 2-Sm (NICS(0) +3.2, NICS(1) −4.5 ppm). For 4-Sm the P–P bond distances were calculated to be 2.244 and 2.292 Å, the SEN (P–P) were determined to be 1.20 and 1.12. The principal setup of the model compound K3P3 is comparable to that of 4-Sm: the P3 unit is surrounded by the strongly positive charged potassium cations in a complicated three dimensional manner due to their electrostatic repulsions. Still, in K3P3 the structural parameters nicely reflect those in 4-Sm: P–P bond distances are calculated to be 2.213 and 2.306 Å, the SEN (P–P) are determined to be 1.23 and 1.09. Comparison of the P–P bond distances and SEN in 2-Sm with those of 4-Sm furthermore supports the idea of a slight aromatic character in the former system. To sum up, in both compounds 2-Sm and 4-Sm the cyclo-[P3]3− and cyclo-[P4]2− units are best described as polyphosphide anions stabilized by counterions through mainly polar bonding contributions.Magnetic measurements of 4-Dy
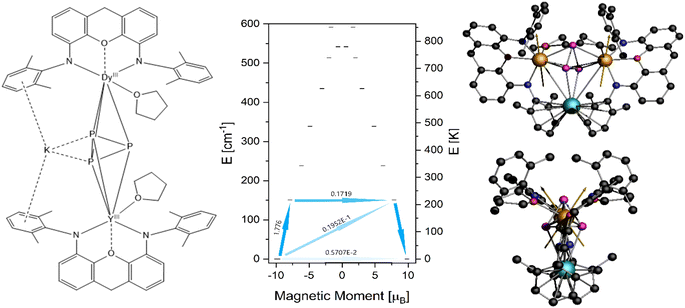
The highly anisotropic nature of trivalent lanthanide ions caused by strong spin–orbit coupling has led to a plethora of LnIII coordination compounds showing SMM behaviour.58,59 The rise of single molecular magnetism over the past decades is based on the promising applicability of SMMs in different quantum technological fields.60–62 Especially DyIII due to its strong magnetic moment (J = 15/2) and it being a Kramers ion is often reported to exhibit slow magnetic relaxation. We, therefore, investigated the magnetic properties of 4-Dy by means of SQUID magnetometry alongside ab initio CASSCF calculations. Full details of the methodology can be found in the ESI.†Our first attempt at calculating the molecular properties was performed following a common approach, replacing one DyIII with a diamagnetic YIII and keeping the molecular structure as it is obtained from XRD methods. For 4-Dy, however, the CASSCF procedure did not converge and we therefore opted to carry out the calculations based on a fictional Dy–Y compound (4-Dy*) in which we replaced the peripheral methyl and tert-butyl groups on the NON ligands with hydrogen atoms, as well as the iso-propyl groups on the DIPP groups with methyl (Fig. 7, left). The results of the single ion calculation showed a strongly axial ground state characterised by gx = 0.01, gy = 0.02 and gz = 19.64 with the wave function being mainly composed of mJ = 15/2, but showing some admixing of mJ = 11/2 (Table S12, ESI†). This mixing suggests that quantum tunnelling within the ground state is possible, which is also confirmed by the relatively high transition probability of the ground state (Fig. 7, middle). The first excited Kramers doublet is found at 151.0 cm−1 (217.25 K) with g-values gx = 0.36, gy = 0.67 and gz = 15.76. The main magnetic axis is only tilted 1.79° in respect to that of the ground state (Fig. 7, right), which can be an indicator that magnetic relaxation might occur via higher excited states. However, the state's wave function shows strong admixing (19%) of mJ = 9/2, which lets us assume that relaxation will mainly occur through this state (Table S13, ESI†).
The static magnetic behaviour of 4-Dy was tested on a sample in a flame sealed NMR tube upon cooling from 300 K to 2 K under an applied field of 0.1 T and through measurements of the molar magnetisation up to 7 T at temperatures 2, 3, 4 and 5 K. The behaviour of the susceptibility product χmolT against temperature shows a slow decrease followed by a strong drop around 10 K (Fig. 8), suggesting antiferromagnetic coupling of the two DyIII ions. Note that the absolute values of χmolT and the magnetisation given in Fig. 8 have been scaled up in order to allow simulations, vide infra. The observed room temperature value of χmolT was 20.16 cm3 K mol−1, which is only about 71% of the expected value for two uncoupled DyIII ions of 28.34 cm3 K mol−1. We believe that this error is introduced through sample preparation, as we have experienced it before and the handling of very small amounts of sample inside a glove box can lead to big percentage errors very quickly.64 However, in further discussion of dynamic behaviour and simulation of the data, introducing a correction factor as we did does not influence the results. Employing the crystal field parameters obtained from the single ion CASSCF calculation (Table S13†) rotated onto a common reference frame, we performed a simultaneous fit of χmolT(T) and M(H) using a single isotropic exchange. An almost perfect fit for χmolT(T) and good agreement around lower fields for M(H) was obtained with Jiso = −2.96 × 10−2 cm−1 (employing a -2J formalism), confirming our initial assumption of antiferromagnetic interactions.
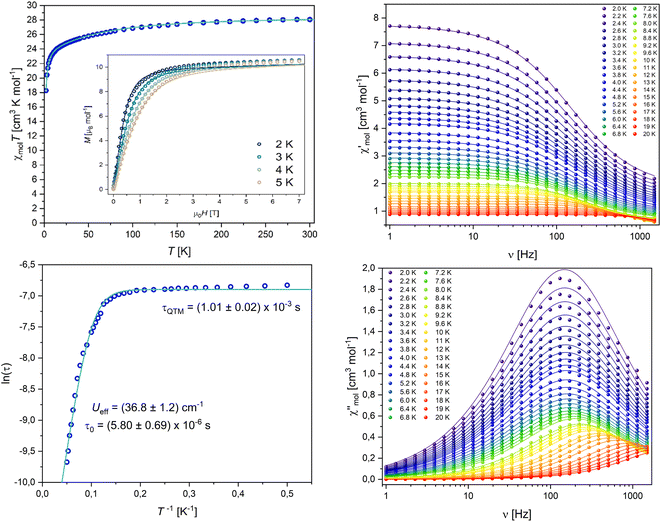 | ||
| Fig. 8 (Top left) Temperature product of the molar susceptibility vs. temperature and molar magnetisation vs. field of 4-Dy, solid lines are best fits obtained with PHI, experimental data points are scaled to allow for simulation.63 (Bottom left) Arrhenius plot; (top and bottom right) in-phase and out-of-phase susceptibilities vs. frequency of 4-Dy, solid lines are the results of a simultaneous fit to a generalised Debye model. | ||
The dynamic behaviour of 4-Dy was tested at low temperatures under application of an oscillating magnetic field (3.5 Oe). A peak in the frequency-dependent out-of-phase susceptibility was observed around 200 Hz at 2 K (Fig. 8, right). Upon the application of additional DC fields, the signal vanished quickly. Simultaneous fits of the in-phase and out-of-phase susceptibilities to a generalised Debye model (all fit parameters can be found in Table S14, ESI†) and subsequent Arrhenius analysis of the relaxation times gave an effective energy barrier Ueff = 36.8 cm−1 and relaxation times τ0 = 5.80 × 10−6 s, τQTM = 1.01 × 10−3 s. Note that we performed the Arrhenius fit employing eqn (1), leaving out the commonly employed CTn Raman-term, as we already obtained a very good fit employing only Orbach and QTM processes.
τ−1 = τ0−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−Ueff/kBT) + τQTM−1 exp(−Ueff/kBT) + τQTM−1 | (1) |
We estimated the blocking temperature TB as the temperature at which τ = 100 s and found TB = 3.17 K. This is in good agreement with hysteresis loops that we recorded, in which the hysteresis is slightly open at 2 K but essentially closed already at 3 K (Fig. S42, ESI†). The experimental energy barrier of 36.8 cm−1 seems extremely low, given the expected relaxation pathway through the first excited state at 151 cm−1. However, it has been shown before that single ion CASSCF calculations often fall short when predicting the relaxation barrier of polynuclear compounds. Dey and Rajaraman have proposed an empirical model to estimate the barrier height of Dy2 SMMs (2):65
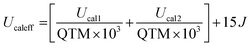 | (2) |
The most impressive relaxation barriers to this day have been achieved in DyIII complexes bearing aromatic Cp derived ligands due to their axial ligand fields being beneficial in stabilising the magnetic moment.66–68 The [P4]2− and [P3]3− Zintl ions might give similar results in the future when being paired with other ligands. We believe the rather poor SMM behavior is a consequence of the NON-ligand producing an unfavorable ligand field for DyIII as well as causing misalignment of the easy axes. As this is the first report of a polyphosphide containing SMM we are excited to see what other possibilities for SMMs these structural motifs will bring in the future.
Conclusions
In summary, we have demonstrated that the activation of white phosphorus, on the one hand, with classical divalent lanthanide compounds 1-Ln (Ln = Sm, Yb) via SET, and, on the other hand, with the trivalent lanthanide borohydride compounds 3-Ln (Ln = Y, Sm, Dy) in the presence of potassium via a multi-electron reduction leads selectively to different products. As products, organo-lanthanide polyphosphides with an aromatic cyclo-[P4]2− (2-Ln, Ln = Sm, Yb) as well as a cyclo-[P3]3− (4-Ln, Ln = Y, Sm, Dy) Zintl anion were isolated and characterized. The synthetic route of a one-pot reaction allowed the isolation of f-element polyphosphides beyond utilization of relatively stable, classical divalent Sm and Yb compounds. This led to the characterization of the first polyphosphide bridged dinuclear DyIII SMM (4-Dy). Furthermore, we have shown the reduction of the cyclo-[P4]2− moiety to form a cyclo-[P3]3− Zintl anion in the coordination sphere of two lanthanides by reacting 2-Sm with potassium.Data availability
All synthetic protocols, spectroscopic data, supplementary figures and tables, magnetic data, data from quantum chemical calculations and detailed crystallographic information can be found in the ESI.† Crystallographic data are available via the Cambridge Crystallographic Data Centre (CCDC): 2224069–2224077, and 2235392.Author contributions
AH, CU, and UCR synthesized and analyzed all compounds with support from LM. LM conducted X-ray experiments. SS and MR conducted and interpreted magnetic measurements and carried out the ab initio CASSCF calculations and interpreted the results. RK performed and analyzed quantum chemical calculations. PWR proposed the idea, supervised the work, and interpreted the results. All authors contributed to the preparation of the manuscript.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
Deutsche Forschungsgemeinschaft (DFG) is acknowledged for financial support within the Reinhart Koselleck-Projekt 440644676, RO 2008/19-1. The authors acknowledge support from the state of Baden-Württemberg through bwHPC and DFG through grant no INST 40/575-1 FUGG (JUSTUS 2 cluster).Notes and references
- L. Giusti, V. R. Landaeta, M. Vanni, J. A. Kelly, R. Wolf and M. Caporali, Coord. Chem. Rev., 2021, 441, 213927 CrossRef CAS
.
-
M. Caporali, M. Serrano-Ruiz and M. Peruzzini, in Chemistry Beyond Chlorine, ed. P. Tundo, L.-N. He, E. Lokteva and C. Mota, Springer International Publishing, Cham, 2016, pp. 97–136 Search PubMed
.
- L. Xu, Y. Chi, S. Du, W.-X. Zhang and Z. Xi, Angew. Chem., Int. Ed., 2016, 55, 9187–9190 CrossRef CAS PubMed
.
- O. J. Scherer, J. Vondung and G. Wolmershäuser, Angew. Chem., Int. Ed., 1989, 28, 1355–1357 CrossRef
.
- B. M. Cossairt, N. A. Piro and C. C. Cummins, Chem. Rev., 2010, 110, 4164–4177 CrossRef CAS PubMed
.
- M. Caporali, L. Gonsalvi, A. Rossin and M. Peruzzini, Chem. Rev., 2010, 110, 4178–4235 CrossRef CAS PubMed
.
- M. Peruzzini, L. Gonsalvi and A. Romerosa, Chem. Soc. Rev., 2005, 34, 1038–1047 RSC
.
- M. Scheer, G. Balázs and A. Seitz, Chem. Rev., 2010, 110, 4236–4256 CrossRef CAS PubMed
.
- S. Khan, S. S. Sen and H. W. Roesky, Chem. Commun., 2012, 48, 2169–2179 RSC
.
- N. A. Giffin and J. D. Masuda, Coord. Chem. Rev., 2011, 255, 1342–1359 CrossRef CAS
.
- M. Donath, K. Schwedtmann, T. Schneider, F. Hennersdorf, A. Bauzá, A. Frontera and J. J. Weigand, Nat. Chem., 2022, 14, 384–391 CrossRef CAS PubMed
.
- J. W. Dube, C. M. E. Graham, C. L. B. Macdonald, Z. D. Brown, P. P. Power and P. J. Ragogna, Eur. J. Chem., 2014, 20, 6739–6744 CrossRef CAS PubMed
.
- B. Goswami, T. J. Feuerstein, R. Yadav, S. Lebedkin, P. J. Boden, S. T. Steiger, G. Niedner-Schatteburg, M. Gerhards, M. M. Kappes and P. W. Roesky, Chem.–Eur. J., 2021, 27, 15110–15119 CrossRef PubMed
.
- X. Sun, A. Hinz and P. W. Roesky, CCS Chem., 2022, 4, 1843–1849 CrossRef CAS
.
- S. N. Konchenko, N. A. Pushkarevsky, M. T. Gamer, R. Köppe, H. Schnöckel and P. W. Roesky, J. Am. Chem. Soc., 2009, 131, 5740–5741 CrossRef CAS PubMed
.
- W. Huang and P. L. Diaconescu, Chem. Commun., 2012, 48, 2216–2218 RSC
.
- F. Spitzer, C. Graßl, G. Balázs, E. M. Zolnhofer, K. Meyer and M. Scheer, Angew. Chem., Int. Ed., 2016, 55, 4340–4344 CrossRef CAS PubMed
.
- D. Patel, F. Tuna, E. J. L. McInnes, W. Lewis, A. J. Blake and S. T. Liddle, Angew. Chem., Int. Ed., 2013, 52, 13334–13337 CrossRef CAS PubMed
.
- B. M. Gardner, F. Tuna, E. J. L. McInnes, J. McMaster, W. Lewis, A. J. Blake and S. T. Liddle, Angew. Chem., Int. Ed., 2015, 54, 7068–7072 CrossRef CAS PubMed
.
- A. S. P. Frey, F. G. N. Cloke, P. B. Hitchcock and J. C. Green, New J. Chem., 2011, 35, 2022–2026 RSC
.
- W. Fang, I. Douair, A. Hauser, K. Li, Y. Zhao, W. Roesky Peter, S. Wang, L. Maron and C. Zhu, CCS Chem., 2022, 4, 2630–2638 CrossRef CAS
.
- O. J. Scherer, B. Werner, G. Heckmann and G. Wolmershäuser, Angew. Chem., Int. Ed., 1991, 30, 553–555 CrossRef
.
- A. Formanuik, F. Ortu, R. Beekmeyer, A. Kerridge, R. W. Adams and D. P. Mills, Dalton Trans., 2016, 45, 2390–2393 RSC
.
- T. Li, J. Wiecko, N. A. Pushkarevsky, M. T. Gamer, R. Köppe, S. N. Konchenko, M. Scheer and P. W. Roesky, Angew. Chem., Int. Ed., 2011, 50, 9491–9495 CrossRef CAS PubMed
.
- T. Li, M. T. Gamer, M. Scheer, S. N. Konchenko and P. W. Roesky, Chem. Commun., 2013, 49, 2183–2185 RSC
.
- T. Li, N. Arleth, M. T. Gamer, R. Köppe, T. Augenstein, F. Dielmann, M. Scheer, S. N. Konchenko and P. W. Roesky, Inorg. Chem., 2013, 52, 14231–14236 CrossRef CAS PubMed
.
- N. Reinfandt, N. Michenfelder, C. Schoo, R. Yadav, S. Reichl, S. N. Konchenko, A. N. Unterreiner, M. Scheer and P. W. Roesky, Eur. J. Chem., 2021, 27, 7862–7871 CrossRef CAS PubMed
.
- C. Schoo, S. Bestgen, M. Schmidt, S. N. Konchenko, M. Scheer and P. W. Roesky, Chem. Commun., 2016, 52, 13217–13220 RSC
.
- N. Arleth, M. T. Gamer, R. Köppe, N. A. Pushkarevsky, S. N. Konchenko, M. Fleischmann, M. Bodensteiner, M. Scheer and P. W. Roesky, Chem. Sci., 2015, 6, 7179–7184 RSC
.
- C. Schoo, S. Bestgen, R. Köppe, S. N. Konchenko and P. W. Roesky, Chem. Commun., 2018, 54, 4770–4773 RSC
.
- W. Huang and P. L. Diaconescu, Eur. J. Inorg. Chem., 2013, 2013, 4090–4096 CrossRef CAS
.
- A. N. Selikhov, T. V. Mahrova, A. V. Cherkasov, G. K. Fukin, E. Kirillov, C. Alvarez Lamsfus, L. Maron and A. A. Trifonov, Organometallics, 2016, 35, 2401–2409 CrossRef CAS
.
- Y. Nakanishi, Y. Ishida and H. Kawaguchi, Inorg. Chem., 2016, 55, 3967–3973 CrossRef CAS PubMed
.
- B. Pinter, K. T. Smith, M. Kamitani, E. M. Zolnhofer, B. L. Tran, S. Fortier, M. Pink, G. Wu, B. C. Manor, K. Meyer, M.-H. Baik and D. J. Mindiola, J. Am. Chem. Soc., 2015, 137, 15247–15261 CrossRef CAS PubMed
.
- B. M. Cossairt and C. C. Cummins, Angew. Chem., Int. Ed., 2010, 49, 1595–1598 CrossRef CAS PubMed
.
- F. Zhang, J. Zhang, Z. Chen, L. Weng and X. Zhou, Inorg. Chem., 2019, 58, 8451–8459 CrossRef CAS PubMed
.
- S. Du, J. Yin, Y. Chi, L. Xu and W.-X. Zhang, Angew. Chem., Int. Ed., 2017, 56, 15886–15890 CrossRef CAS PubMed
.
- T. Pugh, N. F. Chilton and R. A. Layfield, Chem. Sci., 2017, 8, 2073–2080 RSC
.
- B. M. Day, F.-S. Guo and R. A. Layfield, Acc. Chem. Res., 2018, 51, 1880–1889 CrossRef CAS PubMed
.
- P. Zhang, F. Benner, N. F. Chilton and S. Demir, Chem, 2022, 8, 717–730 CAS
.
- J. Hicks, P. Vasko, J. M. Goicoechea and S. Aldridge, Nature, 2018, 557, 92–95 CrossRef CAS PubMed
.
- F. Krämer, M. S. Luff, U. Radius, F. Weigend and F. Breher, Eur. J. Inorg. Chem., 2021, 2021, 3591–3600 CrossRef
.
- L. F. Lim, M. Judd, P. Vasko, M. G. Gardiner, D. A. Pantazis, N. Cox and J. Hicks, Angew. Chem., Int. Ed., 2022, 61, e202201248 CrossRef CAS PubMed
.
- C.-L. Pan, W. Chen and J. Song, Organometallics, 2011, 30, 2252–2260 CrossRef CAS
.
- W. J. Evans, G. W. Rabe and J. W. Ziller, Organometallics, 1994, 13, 1641–1645 CrossRef CAS
.
- W. J. Evans, J. C. Brady and J. W. Ziller, Inorg. Chem., 2002, 41, 3340–3346 CrossRef CAS PubMed
.
- M. L. Cole and P. C. Junk, Chem. Commun., 2005, 2695–2697 CAS
.
- W. J. Evans, N. T. Allen and J. W. Ziller, J. Am. Chem. Soc., 2000, 122, 11749–11750 CrossRef CAS
.
- M. Visseaux and F. Bonnet, Coord. Chem. Rev., 2011, 255, 374–420 CrossRef CAS
.
- M. Ephritikhine, Chem. Rev., 1997, 97, 2193–2242 CrossRef CAS PubMed
.
- M. Dolg, H. Stoll, A. Savin and H. Preuss, Theor. Chim. Acta, 1989, 75, 173–194 CrossRef CAS
.
- J. P. Perdew, Phys. Rev. B: Condens. Matter Mater. Phys., 1986, 34, 7406 CrossRef PubMed
.
- J. P. Perdew, Phys. Rev. B: Condens. Matter Mater. Phys., 1986, 33, 8822–8824 CrossRef PubMed
.
- A. D. Becke, Phys. Rev. A: At., Mol., Opt. Phys., 1988, 38, 3098–3100 CrossRef CAS PubMed
.
-
TURBOMOLE, V7.6 2021, A development of University of Karlsruhe and Forschungszentrum Karlsruhe GmbH, 1989-2007, TURBOMOLE GmbH, 2007, available from https://www.turbomole.org Search PubMed
.
- R. Heinzmann and R. Ahlrichs, Theor. Chim. Acta, 1976, 42, 33–45 CrossRef CAS
.
- R. Ahlrichs and C. Ehrhardt, Chem. Unserer Zeit, 1985, 19, 120–124 CrossRef CAS
.
- D. N. Woodruff, R. E. P. Winpenny and R. A. Layfield, Chem. Rev., 2013, 113, 5110–5148 CrossRef CAS PubMed
.
- N. Ishikawa, M. Sugita, T. Okubo, N. Tanaka, T. Iino and Y. Kaizu, Inorg. Chem., 2003, 42, 2440–2446 CrossRef CAS PubMed
.
- C. Godfrin, A. Ferhat, R. Ballou, S. Klyatskaya, M. Ruben, W. Wernsdorfer and F. Balestro, Phys. Rev. Lett., 2017, 119, 187702 CrossRef CAS PubMed
.
- M. Urdampilleta, S. Klyatskaya, J. P. Cleuziou, M. Ruben and W. Wernsdorfer, Nat. Mater., 2011, 10, 502–506 CrossRef CAS PubMed
.
- E. Moreno-Pineda, C. Godfrin, F. Balestro, W. Wernsdorfer and M. Ruben, Chem. Soc. Rev., 2018, 47, 501–513 RSC
.
- N. F. Chilton, R. P. Anderson, L. D. Turner, A. Soncini and K. S. Murray, J. Comput. Chem., 2013, 34, 1164–1175 CrossRef CAS PubMed
.
- L. Münzfeld, X. Sun, S. Schlittenhardt, C. Schoo, A. Hauser, S. Gillhuber, F. Weigend, M. Ruben and P. W. Roesky, Chem. Sci., 2022, 13, 945–954 RSC
.
- S. Dey and G. Rajaraman, Dalton Trans., 2020, 49, 14781–14785 RSC
.
- J. D. Rinehart and J. R. Long, Chem. Sci., 2011, 2, 2078–2085 RSC
.
- C. A. Gould, K. R. McClain, D. Reta, J. G. C. Kragskow, D. A. Marchiori, E. Lachman, E.-S. Choi, J. G. Analytis, R. D. Britt, N. F. Chilton, B. G. Harvey and J. R. Long, Science, 2022, 375, 198–202 CrossRef CAS PubMed
.
- C. A. P. Goodwin, F. Ortu, D. Reta, N. F. Chilton and D. P. Mills, Nature, 2017, 548, 439–442 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Full experimental procedures, spectra, and analytical data. CCDC 2224069–2224077 and 2235392. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2sc06730g |
| This journal is © The Royal Society of Chemistry 2023 |