Open Access Article
Open Access ArticleCooperativity between sodium ions and water molecules facilitates lipid mobility in model cell membranes†
Madhurima
Chattopadhyay
 *,
Emilia
Krok
*,
Emilia
Krok
 ,
Hanna
Orlikowska-Rzeznik
,
Hanna
Orlikowska-Rzeznik
 and
Lukasz
Piatkowski
and
Lukasz
Piatkowski
 *
*
Institute of Physics, Poznan University of Technology, Piotrowo 3, 60-965 Poznan, Poland. E-mail: madhurima.chattopadhyay@put.poznan.pl; lukasz.j.piatkowski@put.poznan.pl
First published on 22nd March 2023
Abstract
Cellular membranes are surrounded by an aqueous buffer solution containing various ions, which influence the hydration layer of the lipid head groups. At the same time, water molecules hydrating the lipids play a major role in facilitating the organisation and dynamics of membrane lipids. Employing fluorescence microscopy imaging and fluorescence recovery after photobleaching measurements, we demonstrate that the cooperativity between water and sodium (Na+) ions is crucial to maintain lipid mobility upon the removal of the outer hydration layer of the lipid membrane. Under similar hydration conditions, lipid diffusion ceases in the absence of Na+ ions. We find that Na+ ions (and similarly K+ ions) strengthen the water clathrate cage around the lipid phosphocholine headgroup and thus prevent its breaking upon removal of bulk water. Intriguingly, Ca2+ and Mg2+ do not show this effect. In this article, we provide a detailed molecular-level picture of ion specific dependence of lipid mobility and membrane hydration properties.
Introduction
Biological membranes are self-assembled structures composed of various lipids embedded with proteins. They act as dynamic barriers separating intra- and extra-cellular matrices and encapsulate various subcellular organelles. A large variety of lipids in terms of chain length, chain saturation, headgroup structure, and charge is present in biomembranes. The structural lipid heterogeneity, in particular the length mismatch between the hydrophobic tails of the lipids, promotes the formation of lipid domains in response to their unfavourable interactions with the membrane aqueous hydration layer – the so-called “hydrophobic mismatch”. Saturated lipids, such as sphingomyelin (SM) along with cholesterol form more compact liquid ordered (Lo) phase domains in the sea of more fluid liquid disordered phase (Ld) composed predominantly of unsaturated lipids.1 The Lo domains are believed to be platforms for various important biological processes like the attachment of proteins, cell signalling, ion channel regulation, pathogen entry, and many more.2 The extent of phase separation in membranes is modulated not only by lipid composition and structure, but also by various other physicochemical factors, such as temperature, pH, and the ionic strength of the environment.3 The latter in particular is an important factor known to alter various structural as well as functional properties of membranes. The interactions between lipids and ions modulate the local and global properties of the lipid bilayer such as thickness, packing, phase transition temperature, acyl chain ordering, headgroup tilt or swelling,4–7 but also take part in the regulation of ion channels, and signal transduction.4,8,9 Several molecular dynamics (MD) simulations have claimed that various ions have also a profound effect on the dynamics of the lipids in the membrane.10,11 A number of ions, predominantly Na+, K+, Ca2+, Mg2+, and Cl−, are found at the membrane interface with different intracellular and extracellular concentrations. The asymmetric distribution of lipids with different charge characters, as well as the different concentrations of various ions across the membrane, generate a suitable membrane potential for biochemical reactions.12,13Ions are known to affect the structure and dynamics of the hydration layer of lipid headgroups. The presence of Na+ and Ca2+ ions significantly influences the hydration and orientation of the phosphate group of DPPC lipids.14 Song et al. showed that slowing down of water molecules present within 10 Å of the hydrophilic surface of lipid vesicles was modulated by the presence of different ions following the order of the well-known Hofmeister series.15 At the same time, water molecules hydrating the lipids play a major role in determining membrane structure, organization, and lipid dynamics.16 Intriguingly, recent MD simulations showed that hydrogen-bonded water network directly hydrating the membrane exhibits both structural and dynamical heterogeneity.17,18 Clearly, both in native as well as in biomimetic membrane systems water–lipids–ions interactions are strongly interdependent. Hence numerous endeavors were made to elucidate the exact nature of the effect of a particular ion on lipid–water interactions and the resulting alterations of the lipid bilayer properties. Yet, the existing studies are mostly limited to molecular dynamics simulations, except few experimental works,7,14 which mainly addressed the effect of salts on the lipid–water interplay in the excess of water. While most common biological conditions indeed involve full hydration, nature exhibits several phenomena of “anhydrobiosis”, where living organisms, such as tardigrades, nematodes, yeasts, bdelloid rotifer, seeds or pollens survive complete dehydration.19–21 In addition, many biochemical processes, such as cell fusion or adsorption of macromolecules, involve both local variations of ion concentration as well as local and transient membrane dehydration.22–24
Clearly, it is very important to obtain an explicit picture of how ions affect lipid–water interactions at the molecular level under different membrane hydration conditions. To date, however, the understanding of lipid–ion interplay in the presence and absence of water has remained rather poor due to the unavailability of suitable membrane hydration modulation technique. Exploiting the recently developed protocol of preparation of desiccation-tolerant membranes,16 the present work pioneers the experimental study of ion–water–lipid interactions under low hydration conditions.
Herein, using fluorescence imaging and fluorescence recovery after photobleaching (FRAP) experiments we showed how Na+ and Ca2+ ions affect the structure and lipid dynamics of phase-separated solid supported lipid bilayers (SLBs) at fully hydrated and dehydrated conditions. We addressed not only how a specific ion influences the lipid–water interactions but also focused on how the entire process of lipid dehydration modulates diffusion of phospholipids (14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PC) in membranes at various hydration conditions as well as with varying concentrations of Na+ ions. We discovered that Na+ ions play a crucial role in retaining the water hydration layer around the phosphocholine moiety in membranes subjected to dehydration. Surprisingly, Ca2+ cation, although comparable in size with Na+, does not exhibit hydration structure-promoting capabilities. This behavior of Na+ and Ca2+ ions is confronted with the activity of K+ and Mg2+ ions. As such our findings highlight the unique characteristic of Na+ ion–lipid interactions based on its specific charge density, binding affinity, and hydration energy. Our study provides molecular-level insights into a scarcely studied but important topic of the specificity of the ionic composition of membrane local environment modulating the hydration properties and lipid diffusion within the membrane.
1 PC) in membranes at various hydration conditions as well as with varying concentrations of Na+ ions. We discovered that Na+ ions play a crucial role in retaining the water hydration layer around the phosphocholine moiety in membranes subjected to dehydration. Surprisingly, Ca2+ cation, although comparable in size with Na+, does not exhibit hydration structure-promoting capabilities. This behavior of Na+ and Ca2+ ions is confronted with the activity of K+ and Mg2+ ions. As such our findings highlight the unique characteristic of Na+ ion–lipid interactions based on its specific charge density, binding affinity, and hydration energy. Our study provides molecular-level insights into a scarcely studied but important topic of the specificity of the ionic composition of membrane local environment modulating the hydration properties and lipid diffusion within the membrane.
Results and discussion
Impact of Na+ ions on the structure of SLBs
To understand how the ion–lipid–water interactions relate to the structure and lipid dynamics in biomimetic cell membranes, SLBs were reconstructed from 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PC, egg SM and cholesterol and characterized with fluorescence imaging and FRAP experiments. At room temperature, the prepared lipid membranes undergo a prominent phase separation due to the considerable difference in 14
1 PC, egg SM and cholesterol and characterized with fluorescence imaging and FRAP experiments. At room temperature, the prepared lipid membranes undergo a prominent phase separation due to the considerable difference in 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PC and SM hydrophobic chain lengths and packing. Unsaturated 14
1 PC and SM hydrophobic chain lengths and packing. Unsaturated 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
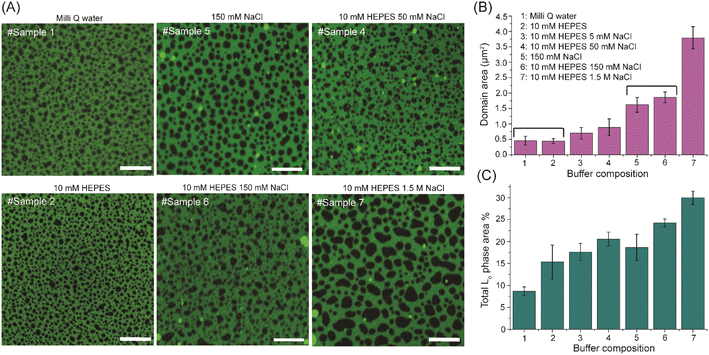
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PC forms Ld phase, saturated SM forms Lo domains, while cholesterol partitions in both phases, with a strong preference for the Lo phase. The membranes were prepared either in Milli-Q water or in a buffer with the addition of 5 mM up to 1.5 M of NaCl. As the pH of the buffer has a prominent effect on the phase separation in lipid membranes,25 10 mM HEPES buffer was used to keep the pH of the medium constant at pH = 5.2, equal to that of Milli-Q water (see Experimental section in ESI†). Representative confocal images of the phase-separated SLBs prepared in Milli-Q water as well as in buffers of different composition are shown in Fig. 1A. Qualitatively, the size of Lo domains (black patches) for SLB prepared in Milli-Q water (sample #1) is the same as for the SLB prepared in 10 mM HEPES buffer (sample #2). Similarly, the domain size of SLB prepared in 150 mM NaCl solution (sample #5) is very similar to that prepared in 10 mM HEPES–150 mM NaCl buffer (sample #6). These observations indicate that HEPES salt itself does not have a noticeable effect on the phase separation of lipids in the reconstructed SLBs. At the same time, the average domain size in SLBs hydrated with buffer containing NaCl (sample #6) is significantly higher than for the SLBs hydrated without the addition of NaCl (sample #2). Quantitative analysis confirms the strong dependence of the phase separation architecture on the NaCl content – the average domain area and the total Lo phase area % (percentage of the total image area covered by the Lo phase) increase significantly with an increase in NaCl concentration (Fig. 1B and C). The average size of domains for SLBs prepared in Milli-Q and in 10 mM HEPES–1.5 M NaCl buffer are 0.45 ± 0.15 μm2 and 3.79 ± 0.36 μm2 respectively, showing over an eightfold increase. Similarly, the percentage of area occupied by the Lo phase domains increases with an increase in NaCl concentration (Fig. 1C). Between SLBs prepared in Milli-Q and in 10 mM HEPES–1.5 M NaCl buffer the area occupied by the Lo phase increases over 3 times.
1 PC forms Ld phase, saturated SM forms Lo domains, while cholesterol partitions in both phases, with a strong preference for the Lo phase. The membranes were prepared either in Milli-Q water or in a buffer with the addition of 5 mM up to 1.5 M of NaCl. As the pH of the buffer has a prominent effect on the phase separation in lipid membranes,25 10 mM HEPES buffer was used to keep the pH of the medium constant at pH = 5.2, equal to that of Milli-Q water (see Experimental section in ESI†). Representative confocal images of the phase-separated SLBs prepared in Milli-Q water as well as in buffers of different composition are shown in Fig. 1A. Qualitatively, the size of Lo domains (black patches) for SLB prepared in Milli-Q water (sample #1) is the same as for the SLB prepared in 10 mM HEPES buffer (sample #2). Similarly, the domain size of SLB prepared in 150 mM NaCl solution (sample #5) is very similar to that prepared in 10 mM HEPES–150 mM NaCl buffer (sample #6). These observations indicate that HEPES salt itself does not have a noticeable effect on the phase separation of lipids in the reconstructed SLBs. At the same time, the average domain size in SLBs hydrated with buffer containing NaCl (sample #6) is significantly higher than for the SLBs hydrated without the addition of NaCl (sample #2). Quantitative analysis confirms the strong dependence of the phase separation architecture on the NaCl content – the average domain area and the total Lo phase area % (percentage of the total image area covered by the Lo phase) increase significantly with an increase in NaCl concentration (Fig. 1B and C). The average size of domains for SLBs prepared in Milli-Q and in 10 mM HEPES–1.5 M NaCl buffer are 0.45 ± 0.15 μm2 and 3.79 ± 0.36 μm2 respectively, showing over an eightfold increase. Similarly, the percentage of area occupied by the Lo phase domains increases with an increase in NaCl concentration (Fig. 1C). Between SLBs prepared in Milli-Q and in 10 mM HEPES–1.5 M NaCl buffer the area occupied by the Lo phase increases over 3 times.
To further corroborate that the increase in average domain area is caused solely by the addition of NaCl, we prepared SLB in Milli-Q water and imaged it before and after replacing the water by 10 mM HEPES–150 mM NaCl buffer (Fig. S1A†). Directly upon buffer replacement, the domain size did not increase considerably, but when imaging after ∼20 hours, significantly (nearly 4 times) bigger domains were present. The difference in domain area (∼4×) is fully consistent with domain area variation observed in membranes prepared directly in Milli-Q water and in HEPES/NaCl buffer (sample #1 vs. sample #6, Fig. 1B). For the reference sample, which was kept in Milli-Q water, the domains grew merely 1.5 times over the same time span. Likewise, the area occupied by the Lo domains increased by ∼68% upon buffer change, whereas for the reference SLB it remained unchanged (Fig. S1B†). The two quantities, the average domain area and the percentage of the total area occupied by the Lo phase, are very much related, but a comparison of the two can provide more information about the system. The total area % occupied by the Lo phase may increase from merging of the small lipid clusters (below the diffraction limit) into bigger, resolvable entities. Likewise, the average domain area also increases when domains merge into bigger ones and/or with the inflow of unresolvable SM entities dissolved within the Ld phase. However, increase of the average domain area does not necessarily guarantee the increase of the total Lo phase area. If only the average domain area increases, but not the total Lo area %, only merging of Lo domains takes place. On the other hand, if only total Lo phase area increases but the average domain size remains the same, formation of new, resolvable domains would be the dominant process.
With an increase in NaCl concentration, both the average domain area and percentage of the area occupied by the Lo phase increase, which unambiguously confirms the presence of unresolvable Lo phase entities within the Ld phase, which upon addition of NaCl and enhancement of phase separation, cluster and merge into bigger (resolvable) domains. In contrast, at lower salt concentration, saturated and unsaturated lipids have stronger tendency to be mixed i.e. more SM entities remain unresolvable. At the experimental pH (∼5.2), HEPES acts as a monoanionic species. Hence the solution around SLBs contains Na+, Cl− and HEPES− ions. MD simulations showed that Cl− hardly penetrates into the bilayer due to its larger size compared to Na+. Instead, Cl− ions remain mostly in the water phase and weakly interact with the choline group of PC lipids.26 Analogously, HEPES− is also expected to be prevalent in the aqueous phase without much interaction with the membrane. This is consistent with our observation of no significant difference in domain size for SLBs prepared in the presence and absence of HEPES for a constant NaCl concentration (Fig. 1, samples #1 vs. #2 and #5 vs. #6). Evidently, Na+ ions are the key players that influence the degree of phase separation in SLBs.
With the increase in NaCl concentration, we observed that the extent of phase separation increases, which can be explained by more favourable PC–PC and/or SM–SM interactions and less favourable PC–SM interactions. Due to the densely packed structure of the Lo phase, Na+ ions preferentially interact (in terms of intercalation between the lipid headgroups) with the Ld phase.26,27 Hence, it is reasonable to assume that the PC–PC interactions are affected much more than the SM–SM interactions upon increase in Na+ concentration. Both, PC and SM have choline moieties and consequently their headgroup hydration properties should be rather similar. However, PC and SM have distinct structural difference in their hydrophobic chain saturation – the two hydrocarbon chains of SM are saturated, whereas 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PC has unsaturated hydrocarbon chains. The lipid tail saturation is one of the main determinants of the packing and order of the membrane. Consequently, SM forms densely packed, highly ordered and thicker Lo phase with preferential partitioning of cholesterol, and PC forms more fluid Ld phase with looser packing. This inter-phase height mismatch, enhanced by the intrinsic hydrocarbon chain length difference between SM and 14
1 PC has unsaturated hydrocarbon chains. The lipid tail saturation is one of the main determinants of the packing and order of the membrane. Consequently, SM forms densely packed, highly ordered and thicker Lo phase with preferential partitioning of cholesterol, and PC forms more fluid Ld phase with looser packing. This inter-phase height mismatch, enhanced by the intrinsic hydrocarbon chain length difference between SM and 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PC (16 vs. 14 carbons), leads to the exposure of part of SM hydrophobic tail to water and results in energetically unfavourable interactions at the boundary zone between PC and SM molecules. This hydrophobic mismatch affects the local hydration properties between the interfacing PC and SM molecules, but it also likely influences how Na+ ions interact with these two lipids. In case PC–SM interactions would be strongly affected by the addition of NaCl, we would expect to see a change in circularity parameter of the domains. However, we do not observe any changes to the Lo phase domains shape and circularity as a function of salt concentration (Fig. S2†). Therefore we conclude that the PC–PC interactions are the ones that are most sensitive to the presence of Na+ ions. This is understandable – when Na+ ions bind to PC lipids, first the electrostatic repulsion between the headgroups is screened by the Na+ ions and second, the van der Waals interactions between hydrophobic tails increase,28,29 altogether making the PC–PC interactions more favourable. Our observations are consistent with the general understanding that the increase in NaCl concentration leads to a decrease of the area per lipid, both in case of saturated and unsaturated lipids. This conclusion comes from experimental10,30,31 and theoretical works,10,31–35 for saturated,36 partially saturated,32,33 and unsaturated lipids.31,35,37 Using molecular dynamics simulations Böckmann et al. showed that the area per POPC (unsaturated lipid) decreases from 0.655 ± 0.011 nm2 in the absence of NaCl to 0.625 ± 0.011 nm2 at 50 mM, and finally to 0.606 ± 0.009 nm2 at 220 mM of NaCl,10 consistent with other works on POPC membranes.32,33 A similar trend was observed for unsaturated DOPC lipid bilayers.35,37 These findings were corroborated experimentally using small angle X-ray diffraction (SAXD)30 and heat capacity measurements10 on POPC bilayer, as well as more recent fluorescence correlation spectroscopy measurements on DOPC membranes.31 In parallel, thickening of the POPC bilayer by 1.1 Å and 2.2 Å was observed with the addition of 50 mM and 220 mM NaCl respectively.36 These observations are generally attributed to the reduction of electrostatic repulsion between the lipid head groups, which leads to more favourable interactions between the PC lipids (PC–PC affinity increases) and consequently to the enhancement in phase separation. The decrease in area per lipid and thickening of the membrane is associated with the tighter packing of lipids within the Ld phase and potentially could lead to a displacement of cholesterol molecules and to a change in cholesterol partitioning. To verify this hypothesis we performed additional experiment using fluorescently labeled cholesterol (TopFluor cholesterol) as a function of NaCl concentration (see Fig. S3A and B† for representative confocal images). We found that there is a small decrease, on the order of ∼8–10% (see Fig. S3C†), in partitioning of cholesterol in the Ld phase when increasing NaCl content. The observed decrease in cholesterol partitioning is fully consistent with the decrease in area per lipid and thickening of the membrane and thus somewhat tighter packing of lipids within the Ld phase with increasing NaCl content in the membrane hydration buffer.
1 PC (16 vs. 14 carbons), leads to the exposure of part of SM hydrophobic tail to water and results in energetically unfavourable interactions at the boundary zone between PC and SM molecules. This hydrophobic mismatch affects the local hydration properties between the interfacing PC and SM molecules, but it also likely influences how Na+ ions interact with these two lipids. In case PC–SM interactions would be strongly affected by the addition of NaCl, we would expect to see a change in circularity parameter of the domains. However, we do not observe any changes to the Lo phase domains shape and circularity as a function of salt concentration (Fig. S2†). Therefore we conclude that the PC–PC interactions are the ones that are most sensitive to the presence of Na+ ions. This is understandable – when Na+ ions bind to PC lipids, first the electrostatic repulsion between the headgroups is screened by the Na+ ions and second, the van der Waals interactions between hydrophobic tails increase,28,29 altogether making the PC–PC interactions more favourable. Our observations are consistent with the general understanding that the increase in NaCl concentration leads to a decrease of the area per lipid, both in case of saturated and unsaturated lipids. This conclusion comes from experimental10,30,31 and theoretical works,10,31–35 for saturated,36 partially saturated,32,33 and unsaturated lipids.31,35,37 Using molecular dynamics simulations Böckmann et al. showed that the area per POPC (unsaturated lipid) decreases from 0.655 ± 0.011 nm2 in the absence of NaCl to 0.625 ± 0.011 nm2 at 50 mM, and finally to 0.606 ± 0.009 nm2 at 220 mM of NaCl,10 consistent with other works on POPC membranes.32,33 A similar trend was observed for unsaturated DOPC lipid bilayers.35,37 These findings were corroborated experimentally using small angle X-ray diffraction (SAXD)30 and heat capacity measurements10 on POPC bilayer, as well as more recent fluorescence correlation spectroscopy measurements on DOPC membranes.31 In parallel, thickening of the POPC bilayer by 1.1 Å and 2.2 Å was observed with the addition of 50 mM and 220 mM NaCl respectively.36 These observations are generally attributed to the reduction of electrostatic repulsion between the lipid head groups, which leads to more favourable interactions between the PC lipids (PC–PC affinity increases) and consequently to the enhancement in phase separation. The decrease in area per lipid and thickening of the membrane is associated with the tighter packing of lipids within the Ld phase and potentially could lead to a displacement of cholesterol molecules and to a change in cholesterol partitioning. To verify this hypothesis we performed additional experiment using fluorescently labeled cholesterol (TopFluor cholesterol) as a function of NaCl concentration (see Fig. S3A and B† for representative confocal images). We found that there is a small decrease, on the order of ∼8–10% (see Fig. S3C†), in partitioning of cholesterol in the Ld phase when increasing NaCl content. The observed decrease in cholesterol partitioning is fully consistent with the decrease in area per lipid and thickening of the membrane and thus somewhat tighter packing of lipids within the Ld phase with increasing NaCl content in the membrane hydration buffer.
It is clear that in water-rich conditions Na+ ions modulate phase separation in lipid bilayers. The question arises whether Na+ ions have the same ability when hydration conditions are altered. To this end, SLBs with varying NaCl concentration were imaged after removing bulk buffer and equilibrating them to relative humidity (RH) of 85% (see Experimental section in ESI†). In such conditions only a single hydration layer around lipid headgroups is present, comprising of about 12 water molecules coordinated by a single PC lipid.16,38,39 No structural changes were observed for the SLBs prepared in buffer solutions of different salt concentrations apart from domain size variation similar to that observed for fully hydrated membranes (Fig. S4†). We note, however, that mechanical stability was lower for dehydrated membranes prepared in buffer with low Na+ content (see ESI, Note 1†).
Impact of Na+ ions on the dynamics of lipids in SLBs
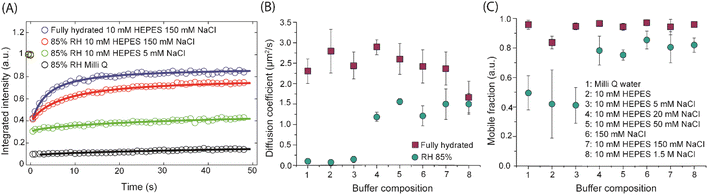
Given that structure of membranes and affinity for phase separation is strongly altered by the presence of Na+ it is expected that it may also have a prominent effect on the lateral dynamics of lipids within the membrane. In earlier FCS studies on fully hydrated, single phase POPC bilayers slowing down of lipid mobility was observed with increasing NaCl concentration.10 On the other hand, MD simulations of lipid–ion interactions in POPE bilayers suggested that presence of cations leads to the decrease in membrane fluidity, likely due to the ion-induced lipid dehydration.11 Despite a few studies investigating the role of ions affecting lipid mobility at fully hydrated conditions, the influence of ions on lipid mobility at lower hydration conditions has so far remained unexplored. We thus examined the dynamics of the Ld phase lipids both in fully hydrated and in dehydrated phase-separated membranes. Exemplary FRAP traces are shown in Fig. 2A. For fully hydrated membrane, the diffusion coefficient (D) remained similar within the error bars (confirmed by the statistical analysis – t-test) – it varied in the range of 2.3–2.8 μm2 s−1 for SLBs prepared in Milli-Q water to 10 mM HEPES–150 mM NaCl buffer. Only for 1.5 M NaCl D was reduced by about 30% of the average value for other buffer compositions, which could be explained by Na+–lipids complexations in the presence of excess NaCl.10 The mobile fraction was high (>90%) and did not change with NaCl concentration (Fig. 2C, magenta squares).While for fully hydrated membranes in the presence of Na+ ions lipid mobility is hardly affected, for dehydrated membranes the picture is drastically different. The membranes prepared in different buffers were exposed to and carefully equilibrated with the environment of high relative humidity (∼85%). Remarkably, in the absence of Na+ ions lipids become nearly immobile, D reaches very low value of <0.2 μm2 s−1 (Fig. 2B, green circles). Similarly, Ld lipids exhibit very little mobility in the membrane exposed to buffer containing low (5 mM) concentration of NaCl. However, for the membranes exposed to higher NaCl concentrations (≥20 mM), lipid mobility is significantly higher reaching value of about 1.5 μm2 s−1 (vs. ∼2.5 μm2 s−1 observed in fully hydrated membranes). A similar trend is observed for the mobile fractions (Fig. 2C, green circles). For the membranes containing little or no NaCl, mobile fractions (MF) are considerably lower (MF ∼ 45%) than for the membranes containing more NaCl (MF > 80%). Here we recall that PC lipids exposed to high RH, close to 100%, are hydrated with a single hydration shell containing about 12 water molecules.29,30,36 For the membranes equilibrated with the RH of 85%, this hydration shell starts to be affected and becomes unstable.16 Based on the changes of D, it is evident that as soon as bulk hydration is removed and the first hydration shell starts to disintegrate, the presence of Na+ ions is crucial for the lipids to maintain their mobility.
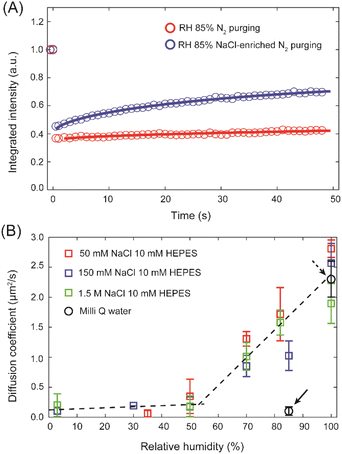
To underpin the key role of Na+ in promoting the lipid mobility after bulk dehydration, an SLB prepared in 10 mM HEPES buffer was dehydrated to 85% RH followed by 6 h of purging of wet N2 gas through a buffer solution containing 150 mM NaCl. The wet N2 gas contains tiny droplets of the buffer, which over time blend with the hydration layer of the membrane. While initially lipids in the SLB exhibited no mobility, after 6 h of purging with the N2 rich in buffer aerosol, a clear difference in the fluorescence recovery (Fig. 3A) was observed with an increase of D from 0.03 ± 0.03 μm2 s−1 to 0.24 ± 0.07 μm2 s−1. It may seem that the sole presence of Na+ ions is sufficient for the lipids to maintain their lateral mobility in water scarcity conditions. To verify such a possibility we determined the diffusion coefficients at varying membrane hydration levels for SLBs prepared in HEPES buffer with an addition of 50 mM, 150 mM and 1.5 M of NaCl. Fig. 3B shows the extracted diffusion coefficients as a function of membrane hydration level. Interestingly, the change of D follows the same trend, irrespective of the salt concentration. D drops abruptly at the initial stages of dehydration and then below approximately 50% RH it remains largely unchanged. The gradual disintegration of the lipid hydration shell leads to a sharp decline in the lateral diffusion coefficient of PC lipids, underlining that the presence of the hydration shell is absolutely necessary for the lipids to maintain their mobility at mild dehydration conditions, in agreement with our previous work.16 However, this experiment unambiguously shows that Na+ ions alone cannot facilitate lipid mobility once dehydration progresses. Instead, Na+ ions and water molecules need to work in tandem to support lipid mobility when the membrane is subjected to mild dehydration.
To clear up the molecular picture and to find an explanation for the extraordinary ability of Na+ ions to shape lipid mobility after dehydration, it is important to understand how various ions bind to PC lipids. Based on the different MD simulation studies, it is generally accepted that Na+ ions can penetrate bilayer interfacial region and localize in the vicinity of the phosphate and carbonyl oxygens of the PC headgroup.35,40 Consequently, Na+ as well as phosphate and/or carbonyl oxygens become partially dehydrated. According to the free energy of ion binding calculations, the most stable state for Na+ in the vicinity of a fully hydrated lipid bilayer, is to be fully hydrated in bulk water with a hydration coordination number of 5.41 However, Na+ can also attach to 4 water molecules and one lipid oxygen with energy higher by only 1–2 kcal mol−1 (Fig. 4B). Thus, there is little energetic penalty for the Na+ ions to be (at least partially) dehydrated. Considering other local minima of ion binding free energy, Na+ ions bound to lipid bilayer can coordinate up to 5–6 oxygen atoms, 1–3 from phosphate/carbonyl oxygen atoms from same or neighbouring lipid molecules and the rest (2–4) of the coordination is filled by water molecules. In fully hydrated conditions, the binding of the Na+ ions with PC is in a dynamic equilibrium with a maximum residence time of 10−4 s at the polar group.42 This could explain the fact that at fully hydrated condition, water plays the main role in shaping the lipid mobility with a little or no change in D upon a moderate increase in Na+ concentration. On the other hand, upon removal of the bulk water due to the unavailability of excess water the binding probability of Na+ to the lipids and the residence time of Na+ at the bilayer interface will increase. In order to keep the free energy of binding lowest, Na+ ion bound to phosphate oxygen (OP) will preferably coordinate 2–5 water molecules to fulfill its coordination instead of binding with other lipid oxygens. Further membrane dehydration leads to increased coordination of Na+ by lipids' oxygens.
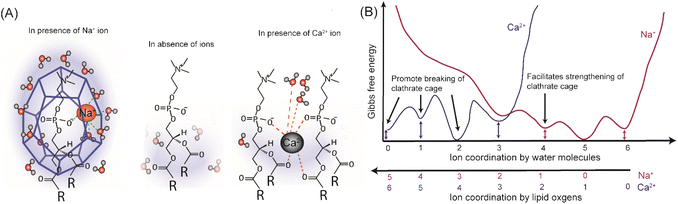 | ||
| Fig. 4 (A) Illustrative pictures of the hydration structures of PC headgroups equilibrated to 85% RH after removal of bulk water in the absence of ions as well as in the presence of Na+ and Ca2+ ions, (B) comparative, simplified schematic diagram of free energies of ion hydration for different coordination scenarios (by water molecules and lipid oxygens) for Na+ and Ca2+ ion. The scheme is based on the MD simulations data of free energies for Na+ and Ca2+ ions at various lipid and water coordination by Yang et al.41 | ||
It is widely accepted that for zwitterionic PC lipids, the first hydration shell around the phosphocholine moiety, contains ∼6–7 water molecules.16 This hydration shell is often referred to as water clathrate cage/structure. However, given its highly dynamic nature it should be viewed rather as a fluid hydration shell. This hydration layer is held in place through OP–H2O H-bonds and van der Waals interactions.29 The water molecules attached to OP-bound Na+ ion, being polarized and slower,43 also bind to other water molecules through H-bonds. Thus, with the introduction of coulombic interactions between OP–Na+–H2O, together with the OP–H2O and H2O–H2O hydrogen bonds, the PC–Na+–water clathrate complex becomes stronger than simple PC–water clathrate complex. In other words, sodium ions strengthen and stabilise the hydration structure around the PC moiety, preventing immediate disintegration of this hydration shell upon bulk dehydration (Fig. 4A). As the membrane becomes dehydrated further, coulombic interactions between lipids, Na+ ions and water molecules fail in stabilising the hydration layer leading to a sharp decline in lipid mobility. The same trajectory of changes in D, regardless of Na+ concentration, highlights that the role of Na+ ions is only related to holding the adequate number of water molecules around the phosphocholine group, but not controlling the lipid mobility directly. Consequently, Na+ ions themselves do not promote lipid mobility at very low hydration conditions (≤50% RH). Hence, it has to be emphasized that it is not just Na+ ions that maintain lipid mobility after the removal of bulk water but the perfect balance between sodium and hydration play the key role. Both sodium and water molecules complement each other to promote lipid mobility after the removal of the outer hydration layer of the lipids. On the other hand, in the absence of Na+ ions, the hydration structure, bound by only weak van der Waals interactions and H-bonds, falls apart already after the removal of bulk water, resulting in very low lipid mobility at 85% RH (Fig. 4A).
Impact of Ca2+, Mg2+ and K+ ions on the dynamics of lipids in SLBs
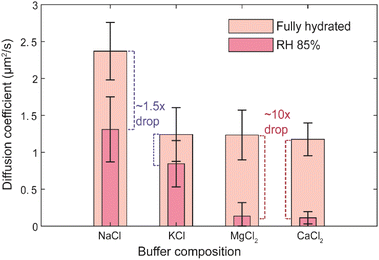
An additional reason for high mobility at ∼85% RH could be that the presence of ions polarizes the water around the lipid headgroups, which in turn shields the electrostatic repulsion between adjacent lipids better. Naturally, in that case, divalent ions, due to higher charge density, should be more efficient in supporting lipid mobility after dehydration. To verify this possibility, we measured the lateral diffusion of lipids at fully hydrated and dehydrated conditions for SLBs prepared in 10 mM HEPES buffer containing 150 mM of CaCl2. Ca2+ is a divalent ion but has a very similar ionic radius to Na+. The average domain size in the SLB prepared in buffer containing 150 mM of CaCl2 was 0.82 ± 0.13 μm2, which is smaller (approximately by a factor of 2) than the average domain size in SLB prepared with 150 mM of NaCl (Fig. S5†).The D value at fully hydrated condition was found to be 1.18 ± 0.22 μm2 s−1, which is around half of the D observed for the same concentration of Na+ ions. Consistently, the mobile fraction was also slightly lower (∼85%) than that in the presence of NaCl. Strikingly, after the removal of bulk water, lipid mobility was almost ceased already at 85% RH (D = 0.11 ± 0.08 μm2 s−1, ∼10 times drop), similar to the SLB hydrated with Milli-Q water (Fig. 5). This indicates that Ca2+ ions are unable to contribute to the stabilisation of the hydration layer around lipid headgroups. Previous studies showed that the binding constant of Ca2+ to the membrane is much higher than for Na+ and that Ca2+ preferentially binds to the lipid oxygens rather than to water molecules, leading to dehydration of the phosphate region.14 Similar conclusions have been reached in infrared studies on bulk lipid paste in different hydration conditions and containing various ions.44 Recent MD simulations showed that the lowest free energy state for Ca2+ is when it binds to 4 lipid oxygen and 2 water molecules to have its coordination number of 6 filled (see Fig. 4B).41 The other energy minima, 2–4 kcal mol−1 higher in energy than the global minimum, correspond to binding with 3–5 lipid oxygens with total coordination number 4–6, leaving the number of water molecules attached to Ca2+ close to 0. This in return leads to destabilisation of the complex hydration structure, which can no longer anchor to the phosphate oxygens. Hence, it is of no surprise that in the presence of Ca2+ ions the hydration shell disintegrates immediately after bulk water removal causing very low lipid mobility already at 85% RH. Moreover, preferential binding of Ca2+ to phosphate and carbonyl oxygens of lipids promotes the formation of Ca2+ complex with more than one lipid,7,45,46 which also explains the lower (approximately 2-fold) diffusion coefficient in the presence of 150 mM of Ca2+, already in full hydration conditions. Last but not least, the divergent action of CaCl2 with respect to NaCl also confirms that Cl− has no noticeable effect on the dynamics of the membrane constituents, in agreement with the MD simulation, which showed that Cl− being larger in size mostly resides in the bulk water.40
Evidently, strengthening of lipid hydration structure in the presence of Na+ is not related directly to the size or charge of the cation, but it is the hydration energy and the lipid binding energy of these ions that play the key role here. To further corroborate this conclusion we measured D of Ld lipids in membranes hydrated with the buffer containing another two biologically relevant ions K+ and Mg2+ (10 mM HEPES–150 mM KCl and 10 mM HEPES–10 mM MgCl2), but for which very different hydration energies have been reported (for representative confocal images of these membranes see Fig. S5†). K+ ions have similar hydration energy and lipid binding affinity as Na+ ions,41 thus one could expect that the lipid diffusion could also be promoted by the presence of K+. On contrary, for Mg2+, which has very high energy barrier (>25 kcal) to bind with a single lipid oxygen in transition from the fully hydrated state (in comparison to only 1–2 kcal energy barrier in case of Na+ and K+), one would expect similar behavior as for Ca2+ or for the membrane with no salt present in the buffer, that is low lipid mobility in the absence of bulk water. The experimental data fully confirm these expectations – after the removal of bulk water in the presence of K+ lipid mobility was mere ∼1.5 times lower, whereas for Mg2+ over 10 times lower diffusion coefficient was measured (Fig. 5). Clearly, K+ has similar effect on the lipid mobility upon membrane dehydration as Na+, whereas the activity of Mg2+ resembles that of Ca2+. It should be noted that though in the presence of divalent cations Ca2+ and Mg2+ lipid diffusion drops down to a very low value upon removal of bulk water, the underlying mechanism of action is likely different. Mg2+ does not bind to the membrane lipids, thus it neither stabilize nor disrupt the lipid hydration shell. On the other hand, Ca2+ having stronger lipid oxygen binding affinity than to water molecules, it destabilizes the lipid hydration structure. Altogether our observations confirm the importance of the free energy difference between the lipid-bound states and fully water-coordinated state of the investigated cations in determining the ability to support lipid mobility at reduced hydration conditions. We note here, that at fully hydrated conditions, lipid mobility was found to be consistently smaller in the presence of KCl, MgCl2 and CaCl2 (∼1.3 μm2 s−1) compared to NaCl (2.3 μm2 s−1), however the origin of this difference is unclear.
Furthermore, it is interesting to address how Lo phase lipid mobility is affected in the presence of various cations. Therefore we determined the mobility of lipids in single (Lo) phase SLBs composed of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 SM/cholesterol and with the addition of fluorescent lipid analogue NBD–DPPE. The experiments were performed in the presence of 10 mM HEPES–150 mM NaCl and 10 mM HEPES–150 mM CaCl2 since Na+ and Ca2+ showed very different effect on the lipid mobility in case of the Ld phase. At fully hydrated conditions, both for Na+ and Ca2+ present in the buffer the D values were very similar (∼0.18 μm2 s−1) and fully consistent with the typical values obtained for the Lo phase lipids.16,47 After removal of bulk water, unlike what was observed for the Ld phase, in the presence of NaCl lipid diffusion ceases indicating that even Na+ can not assist in maintaining lipid mobility in the Lo phase. The relative (full hydration vs. 85% RH) large decrease (∼9-fold) in diffusion coefficient is very similar for both Na+ and Ca2+ (Fig. S6†). This experimental observation is understandable and corroborates the MD simulation finding – the Na+ ion binding to the more densely packed Lo phase is much less probable than it is to the loosely packed Ld phase.26
1 SM/cholesterol and with the addition of fluorescent lipid analogue NBD–DPPE. The experiments were performed in the presence of 10 mM HEPES–150 mM NaCl and 10 mM HEPES–150 mM CaCl2 since Na+ and Ca2+ showed very different effect on the lipid mobility in case of the Ld phase. At fully hydrated conditions, both for Na+ and Ca2+ present in the buffer the D values were very similar (∼0.18 μm2 s−1) and fully consistent with the typical values obtained for the Lo phase lipids.16,47 After removal of bulk water, unlike what was observed for the Ld phase, in the presence of NaCl lipid diffusion ceases indicating that even Na+ can not assist in maintaining lipid mobility in the Lo phase. The relative (full hydration vs. 85% RH) large decrease (∼9-fold) in diffusion coefficient is very similar for both Na+ and Ca2+ (Fig. S6†). This experimental observation is understandable and corroborates the MD simulation finding – the Na+ ion binding to the more densely packed Lo phase is much less probable than it is to the loosely packed Ld phase.26
Conclusions
In summary, we demonstrated that the cooperativity between water and specific ions (sodium and potassium) is an essential factor that controls lipid mobility in membranes under water depletion conditions. Na+ ions reveal their importance already in fully hydrated membranes, in which the extent of phase separation increases significantly with an increase of Na+ concentration. At the same time, in these conditions, Na+ ions have hardly any effect on the mobility of lipids. In stark contrast, the true capabilities of Na+ ions are revealed upon membrane dehydration, when they actively penetrate the inter-lipid headgroup region. There, both Na+ and K+ ions act to stabilise the hydration shell structure around the lipid headgroups, thereby facilitating lipid diffusion. However, we emphasize that the ability of these cations to promote lipid dynamics after membrane dehydration does not nullify the principal role of water in supporting lipid mobility. At very low hydration conditions, where not enough water molecules are present to form the hydration layer, Na+ and K+ ions alone fail to retain lipid mobility even at high concentration. Clearly, it is a cooperative effect, in which down to a certain dehydration level water and Na+/K+ ions work in a concerted manner in promoting lipid diffusion. The uniqueness of these monovalent cations is evident when compared to the activity of divalent Ca2+ or Mg2+ cations. Ca2+ despite having similar ionic radius to Na+, has the tendency to destabilise the hydration structure around the lipid headgroups due to its greater binding affinity to lipid oxygens than to remnant water molecules. Mg2+ ions on the other hand prefer to be fully hydrated by water and need to overcome a very high energy barrier in order to bind to the lipids. Consequently, in the presence of Ca2+ or Mg2+ lipid mobility drops to very low values upon membrane dehydration. We can thus conclude that while Na+ and K+ promote fluidity of the membrane when its hydration layer is perturbed, Ca2+ or Mg2+ rather lead to gelification (in terms of mobility) of the membrane. Importantly, confronting the activity of the studied cations it is evident that it is not the charge or the ability to polarise the local environment but purely the competition between ion hydration and ion binding to lipid oxygens that cause such a divergent activity of various cations.This work unveils the important and unique role of specific cations in modulating the membrane structure as well as lipid dynamics and emphasizes the importance of local ion composition and concentration for membrane biophysical homeostasis. Numerous reports underpin the importance of ions in various biological cell fusion events such as during viral entry, neurotransmission or fertilisation, processes where two merging lipid bilayers undergo transient membrane dehydration.24 Specifically, it has been proposed that the presence of NaCl promotes fusion of E. coli membranes and the local aggregation of the bacterial potassium channel KcsA.48 This was ascribed to Na+-induced lowering of hydration repulsive forces and modulation of the local water structure. Recently it was reported that the infection by bunyavirus depends on the local ion composition. It was shown that viral spike–membrane interactions are enhanced in the presence of locally elevated K+ concentration.49 On the other hand, the presence of Ca2+ ions is known to be one of the important factors in neurotransmission events, where also transient dehydration occurs during membrane fusion.50 It is thus feasible that depending on the particular mechanism underlying the fusion process it may involve either maintenance or a local decrease in membrane fluidity.
Our findings have also important implications for biological systems where water activity can be very low such as in cytoplasm moieties for instance Golgi apparatus or the intracellular leaflet of the membrane, which interacts strongly with the cortical actin. Another example, where low hydration is accompanied by high ion concentrations is the case of amphibian oocytes, where the cytoplasm contains approximately half of the water content compared to the nucleus, whereas the Na+ concentration is 5–10 times higher than in the cytoplasm.51
Thus overall, the presented results provide important implications and new molecular-level perspective for reviewing biochemical processes involving biomembranes subjected to transient dehydration. Yet, the challenge remains in being able to monitor locally hydration, ion composition, and lipid membrane properties in living cells.
Data availability
All relevant data supporting this article have been included in the main text and the ESI.† All original data generated during this work are available from the corresponding authors upon request.Author contributions
MC: conceptualization, methodology, investigation, formal analysis, validation, visualization, writing – original draft, writing – review and editing; EK: methodology, writing – review and editing; HOR: methodology, writing – review and editing; LP: supervision, conceptualization, validation, funding acquisition, writing – review and editing.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
L.P. acknowledges the financial support from the EMBO Installation Grant (IG 4147). M.C. and E.K. acknowledge the financial support from the First TEAM Grant No. POIR.04.04.00-00-5D32/18-00, provided by the Foundation for Polish Science (FNP). H.O.R. acknowledges financial support from the National Science Centre (Poland), grant number 2020/37/B/ST4/01785.Notes and references
- S. J. Singer and G. L. Nicolson, The Fluid Mosaic Model of the Structure of Cell Membranes, Science, 1972, 175, 720–731 CrossRef CAS PubMed.
- W. H. Binder, V. Barragan and F. M. Menger, Domains and Rafts in Lipid Membranes, Angew. Chem., Int. Ed., 2003, 42, 5802–5827 CrossRef CAS PubMed.
- R. Ohtani, Y. Anegawa, H. Watanabe, Y. Tajima, M. Kinoshita, N. Matsumori, K. Kawano, S. Yanaka, K. Kato, M. Nakamura, M. Ohba and S. Hayami, Metal Complex Lipids for Fluid–Fluid Phase Separation in Coassembled Phospholipid Membranes, Angew. Chem., Int. Ed., 2021, 60, 13603–13608 CrossRef CAS PubMed.
- R. J. Alsop, R. Maria Schober and M. C. Rheinstädter, Swelling of Phospholipid Membranes by Divalent Metal Ions Depends on the Location of the Ions in the Bilayers, Soft Matter, 2016, 12, 6737–6748 RSC.
- H. Trauble and H. Eibl, Electrostatic Effects on Lipid Phase Transitions: Membrane Structure and Ionic Environment, Proc. Natl. Acad. Sci. U. S. A., 1974, 71, 214–219 CrossRef CAS PubMed.
- J. N. Sachs, H. Nanda, H. I. Petrache and T. B. Woolf, Changes in Phosphatidylcholine Headgroup Tilt and Water Order Induced by Monovalent Salts: Molecular Dynamics Simulations, Biophys. J., 2004, 86, 3772–3782 CrossRef CAS PubMed.
- M. Sovago, G. W. H. Wurpel, M. Smits, M. Müller and M. Bonn, Calcium-Induced Phospholipid Ordering Depends on Surface Pressure, J. Am. Chem. Soc., 2007, 129, 11079–11084 CrossRef CAS PubMed.
- P. Lauger, Mechanisms of Biological Ion Transport-Carriers, Channels, and Pumps in Artificial Lipid Membranes, Angew. Chem., Int. Ed., 1985, 24, 905–923 CrossRef.
- A. Raasakka, N. C. Jones, S. V. Hoffmann and P. Kursula, Ionic Strength and Calcium Regulate Membrane Interactions of Myelin Basic Protein and the Cytoplasmic Domain of Myelin Protein Zero, Biochem. Biophys. Res. Commun., 2019, 511, 7–12 CrossRef CAS PubMed.
- R. A. Böckmann, A. Hac, T. Heimburg and H. Grubmüller, Effect of Sodium Chloride on a Lipid Bilayer, Biophys. J., 2003, 85, 1647–1655 CrossRef PubMed.
- R. Kagawa, Y. Hirano, M. Taiji, K. Yasuoka and M. Yasui, Dynamic Interactions of Cations, Water and Lipids and Influence on Membrane Fluidity, J. Membr. Sci., 2013, 435, 130–136 CrossRef CAS.
- A. A. Gurtovenko and I. Vattulainen, Lipid Transmembrane Asymmetry and Intrinsic Membrane Potential: Two Sides of the Same Coin, J. Am. Chem. Soc., 2007, 129, 5358–5359 CrossRef CAS PubMed.
- A. L. Hodgkin and P. Horowicz, The Influence of Potassium and Chloride Ions on the Membrane Potential of Single Muscle Fibers, J. Physiol., 1959, 148, 127–160 CrossRef CAS PubMed.
- N. N. Casillas-Ituarte, X. Chen, H. Castada and H. C. Allen, Na+ and Ca2+ Effect on the Hydration and Orientation of the Phosphate Group of DPPC at Air - Water and Air - Hydrated Silica Interfaces, J. Phys. Chem. B, 2010, 114, 9485–9495 CrossRef CAS PubMed.
- J. Song, J. Franck, P. Pincus, M. W. Kim and S. Han, Specific Ions Modulate Diffusion Dynamics of Hydration Water on Lipid Membrane Surfaces, J. Am. Chem. Soc., 2014, 136, 2642–2649 CrossRef CAS PubMed.
- M. Chattopadhyay, E. Krok, H. Orlikowska, P. Schwille, H. G. Franquelim and L. Piatkowski, Hydration Layer of Only a Few Molecules Controls Lipid Mobility in Biomimetic Membranes, J. Am. Chem. Soc., 2021, 143, 14551–14562 CrossRef CAS PubMed.
- C. Calero and G. Franzese, Membranes with Different Hydration Levels: The Interface between Bound and Unbound Hydration Water, J. Mol. Liq., 2019, 273, 488–496 CrossRef CAS.
- C. Calero, H. E. Stanley and G. Franzese, Structural Interpretation of the Large Slowdown of Water Dynamics at Stacked Phospholipid Membranes for Decreasing Hydration Level: All-Atom Molecular Dynamics, Materials, 2016, 9, 319 CrossRef PubMed.
- K. A. C. Madin and J. H. Crowe, Anhydrobiosis in Nematodes: Carbohydrate and Lipid Metabolism during Dehydration, J. Exp. Zool., 1975, 193, 335–342 CrossRef CAS.
- J. H. Crowe, L. M. Crowe and D. Chapman, Preservation of Membranes in Anhydrobiotic Organisms: The Role of Trehalose, Science, 1984, 223, 701–703 CrossRef CAS PubMed.
- R. Marotta, F. Leasi, A. Uggetti, C. Ricci and G. Melone, Dry and Survive: Morphological Changes during Anhydrobiosis in a Bdelloid Rotifer, J. Struct. Biol., 2010, 171, 11–17 CrossRef PubMed.
- J. Wilschut, N. DiizgiineS, R. Fraley and D. Papahadjopoulos, Studies on the Mechanism of Membrane Fusion: Kinetics of Calcium Ion Induced Fusion of Phosphatidylserine Vesicles Followed by a New Assay for Mixing of Aqueous Vesicle Contents, Biochemistry, 1980, 19, 6011–6021 CrossRef CAS PubMed.
- A. Portis, C. Newton, W. Pangborn and D. Papahadjopoulos, Studies on the Mechanism of Membrane Fusion: Evidence for an Intermembrane Ca2+-Phospholipid Complex, Synergism with Mg2+, and Inhibition by Spectrin, Biochemistry, 1979, 18, 780–790 CrossRef CAS PubMed.
- S. Aeffner, T. Reusch, B. Weinhausen and T. Salditt, Energetics of Stalk Intermediates in Membrane Fusion Are Controlled by Lipid Composition, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, E1609–E1618 CrossRef CAS PubMed.
- E. Krok, A. Batura, M. Chattopadhyay, H. Orlikowska and L. Piatkowski, Lateral Organization of Biomimetic Cell Membranes in Varying PH Conditions, J. Mol. Liq., 2022, 345, 117907 CrossRef CAS.
- M. Stȩpniewski, A. Bunker, M. Pasenkiewicz-Gierula, M. Karttunen and T. Róg, Effects of the Lipid Bilayer Phase State on the Water Membrane Interface, J. Phys. Chem. B, 2010, 114, 11784–11792 CrossRef PubMed.
- A. Magarkar, V. Dhawan, P. Kallinteri, T. Viitala, M. Elmowafy, T. Róg and A. Bunker, Cholesterol Level Affects Surface Charge of Lipid Membranes in Saline Solution, Sci. Rep., 2014, 4, 5005 CrossRef CAS PubMed.
- R. Friedman, Membrane–Ion Interactions, J. Membr. Biol., 2018, 251, 453–460 CrossRef CAS PubMed.
- C. C. Logisz and J. S. Hovis, Effect of Salt Concentration on Membrane Lysis Pressure, Biochim. Biophys. Acta, Biomembr., 2005, 1717, 104–108 CrossRef CAS PubMed.
- G. Pabst, A. Hodzic, J. Štrancar, S. Danner, M. Rappolt and P. Laggner, Rigidification of Neutral Lipid Bilayers in the Presence of Salts, Biophys. J., 2007, 93, 2688–2696 CrossRef CAS PubMed.
- M. Jan Akhunzada, F. D’Autilia, B. Chandramouli, N. Bhattacharjee, A. Catte, R. Di Rienzo, F. Cardarelli and G. Brancato, Interplay between Lipid Lateral Diffusion, Dye Concentration and Membrane Permeability Unveiled by a Combined Spectroscopic and Computational Study of a Model Lipid Bilayer, Sci. Rep., 2019, 9, 1508 CrossRef PubMed.
- P. Jurkiewicz, L. Cwiklik, A. Vojtíšková, P. Jungwirth and M. Hof, Structure, Dynamics, and Hydration of POPC/POPS Bilayers Suspended in NaCl, KCl, and CsCl Solutions, Biochim. Biophys. Acta, Biomembr., 2012, 1818, 609–616 CrossRef CAS PubMed.
- A. A. Gurtovenko and I. Vattulainen, Effect of NaCl and KCl on Phosphatidylcholine and Phosphatidylethanolamine Lipid Membranes: Insight from Atomic-Scale Simulations for Understanding Salt-Induced Effects in the Plasma Membrane, J. Phys. Chem. B, 2008, 112, 1953–1962 CrossRef CAS PubMed.
- A. Cordomí, O. Edholm and J. J. Perez, Effect of Ions on a Dipalmitoyl Phosphatidylcholine Bilayer. A Molecular Dynamics Simulation Study, J. Phys. Chem. B, 2008, 112, 1397–1408 CrossRef PubMed.
- R. Vácha, S. W. I. Siu, M. Petrov, R. A. Böckmann, J. Barucha-Kraszewska, P. Jurkiewicz, M. Hof, M. L. Berkowitz and P. Jungwirth, Effects of Alkali Cations and Halide Anions on the DOPC Lipid Membrane, J. Phys. Chem. A, 2009, 113, 7235–7243 CrossRef PubMed.
- S. A. Pandit, D. Bostick and M. L. Berkowitz, Molecular Dynamics Simulation of a Dipalmitoylphosphatidylcholine Bilayer with NaCl, Biophys. J., 2003, 84, 3743–3750 CrossRef CAS PubMed.
- L. F. Pineda De Castro, M. Dopson and R. Friedman, Biological Membranes in Extreme Conditions: Anionic Tetraether Lipid Membranes and Their Interactions with Sodium and Potassium, J. Phys. Chem. B, 2016, 120, 10628–10634 CrossRef CAS PubMed.
- L. Piatkowski, J. De Heij and H. J. Bakker, Probing the Distribution of Water Molecules Hydrating Lipid Membranes with Ultrafast Förster Vibrational Energy Transfer, J. Phys. Chem. B, 2013, 117, 1367–1377 CrossRef CAS PubMed.
- K. Hristova and S. H. White, Determination of the Hydrocarbon Core Structure of Fluid Dioleoylphosphocholine (DOPC) Bilayers by x-Ray Diffraction Using Specific Bromination of the Double-Bonds: Effect of Hydration, Biophys. J., 1998, 74, 2419–2433 CrossRef CAS PubMed.
- M. Pasenkiewicz-Gierula, K. Baczynski, M. Markiewicz and K. Murzyn, Computer Modelling Studies of the Bilayer/Water Interface, Biochim. Biophys. Acta, Biomembr., 2016, 1858, 2305–2321 CrossRef CAS PubMed.
- J. Yang, C. Calero, M. Bonomi and J. Martí, Specific Ion Binding at Phospholipid Membrane Surfaces, J. Chem. Theory Comput., 2015, 11, 4495–4499 CrossRef CAS PubMed.
- H. Akutsut and J. Seelig, Interaction of Metal Ions with Phosphatidylcholine Bilayer Membranes, Biochemistry, 1981, 20, 7366–7373 CrossRef PubMed.
- K. J. Tielrooij, N. Garcia-Araez, M. Bonn and H. J. Bakker, Cooperativity in Ion Hydration, Science, 2010, 328, 1006–1009 CrossRef CAS PubMed.
- H. Binder and O. Zschö, The Effect of Metal Cations on the Phase Behavior and Hydration Characteristics of Phospholipid Membranes, Chem. Phys. Lipids, 2002, 115, 39–61 CrossRef CAS PubMed.
- A. Melcrová, S. Pokorna, S. Pullanchery, M. Kohagen, P. Jurkiewicz, M. Hof, P. Jungwirth, P. S. Cremer and L. Cwiklik, The Complex Nature of Calcium Cation Interactions with Phospholipid Bilayers, Sci. Rep., 2016, 6, 1–12 CrossRef PubMed.
- R. A. Böckmann and H. Grubmüller, Multistep Binding of Divalent Cations to Phospholipid Bilayers: A Molecular Dynamics Study, Angew. Chem., Int. Ed., 2004, 43, 1021–1024 CrossRef PubMed.
- N. Kahya, D. Scherfeld, K. Bacia, B. Poolman and P. Schwille, Probing Lipid Mobility of Raft-Exhibiting Model Membranes by Fluorescence Correlation Spectroscopy, J. Biol. Chem., 2003, 278, 28109–28115 CrossRef CAS PubMed.
- M. Raja and E. Vales, Effects of Sodium Chloride on Membrane Fusion and on the Formation of Aggregates of Potassium Channel KcsA in Escherichia Coli Membrane, Biophys. Chem., 2009, 142, 46–54 CrossRef CAS PubMed.
- E. K. Punch, S. Hover, H. T. W. Blest, J. Fuller, R. Hewson, J. Fontana, J. Mankouri and J. N. Barr, Potassium Is a Trigger for Conformational Change in the Fusion Spike of an Enveloped RNA Virus, J. Biol. Chem., 2018, 293, 9937–9944 CrossRef CAS PubMed.
- J. Rizo, Molecular Mechanisms Underlying Neurotransmitter Release, Annu. Rev. Biophys., 2022, 51, 377–408 CrossRef PubMed.
- T. J. Century, I. R. Fenichel and S. B. Horowitz, The Concentrations of Water, Sodium and Potassium in the Nucleus and Cytoplasm of Amphibian Oocytes, J. Cell Sci., 1970, 7, 5–13 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and supplementary notes and figures. See DOI: https://doi.org/10.1039/d2sc06836b |
| This journal is © The Royal Society of Chemistry 2023 |