Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Water molecule switching heterogeneous proton-coupled electron transfer pathway†
Zhonghuan
Liu
b,
Wei
Peng
ae,
Yuhan
Lin
ae,
Xinyu
Lin
b,
Shikang
Yin
b,
Shuhan
Jia
b,
Dongge
Ma
 d,
Yan
Yan
d,
Yan
Yan
 *b,
Peng
Zhou
*b,
Peng
Zhou
 *c,
Wanhong
Ma
*ae and
Jincai
Zhao
*c,
Wanhong
Ma
*ae and
Jincai
Zhao
 ae
ae
aKey Laboratory of Photochemistry, Beijing National Laboratory for Molecular Sciences, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190, PR China. E-mail: whma@iccas.ac.cn
bInstitute of Green Chemistry and Chemical Technology, School of Chemistry and Chemical Engineering, Jiangsu University, Zhenjiang 212013, PR China. E-mail: dgy5212004@163.com
cDepartment of Electrical Engineering and Computer Science, University of Michigan, Ann Arbor, MI 48109, USA. E-mail: dpzhou@umich.edu
dDepartment of Chemistry, College of Chemistry and Materials Engineering, Beijing Technology and Business University, Beijing 100048, China
eUniversity of Chinese Academy of Sciences, Beijing, 100049, China
First published on 3rd April 2023
Abstract
Figuring out the specific pathway of semiconductor-mediated proton-coupled electron transfer (PCET) driven by light is essential to solar energy conversion systems. In this work, we reveal that the amount of adsorbed water molecules determines the photo-induced PCET pathway on the TiO2 surface through systematic kinetic solvent isotope effect (KSIE) experiments. At low water content (<1.7 wt%), the photo-induced single-proton/single-electron transfer on TiO2 nanoparticles follows a stepwise PT/ET pathway with the formation of high-energy H+/D+–O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C or H+/D+–O–C intermediates, resulting in an inverse KSIE (H/D) ∼0.5 with tBu3ArO· and KSIE (H/D) ∼1 with TEMPO in methanol-d0/d4 systems. However, at high water content (>2 wt%), the PCET reaction follows a concerted pathway with a lower energy barrier, leading to normal KSIEs (H/D) ≥ 2 with both reagents. In situ ATR-FTIR observation and DFT calculations suggest that water molecules' existence significantly lowers the proton/electron transfer energy barrier, which coincides with our experimental observations.
C or H+/D+–O–C intermediates, resulting in an inverse KSIE (H/D) ∼0.5 with tBu3ArO· and KSIE (H/D) ∼1 with TEMPO in methanol-d0/d4 systems. However, at high water content (>2 wt%), the PCET reaction follows a concerted pathway with a lower energy barrier, leading to normal KSIEs (H/D) ≥ 2 with both reagents. In situ ATR-FTIR observation and DFT calculations suggest that water molecules' existence significantly lowers the proton/electron transfer energy barrier, which coincides with our experimental observations.
Introduction
Proton-coupled electron transfer (PCET) reactions are pervasive in natural energy transfer processes, including photosynthesis, nitrogen fixation, and respiration.1–4 Metal oxide semiconductor (e.g., ZnO, TiO2, and SnO2) nanoparticle-mediated photo-induced electron transfer has been directly proven to be a proton-coupled process.5 Figuring out the internal relation between specific intermediate reaction pathways and the interfacial chemical environment is critical to the profound understanding and future design of targeted semiconductor nanoparticle-based photocatalytic systems with high efficiency.Typically, under continuous light irradiation, metastable photo-induced electrons can be generated and constantly trapped on oxide semiconductor (e.g., TiO2) nanoparticles in small-molecule hole-scavengers (e.g., H2O, amines, or alcohols).6,7 Stabilized by protons (H+), photo-induced electrons (ecb−) on TiO2 nanoparticles were proved to be trapped at the outer surface as Ti4c3+ species,8 which can be consumed in situ or ex situ as reactive reducing reagents to accomplish many valuable and challenging chemical reactions without extra high-temperature and high-pressure conditions.9–11 The corresponding intermediate reaction pathway directly determines such a liquid/solid heterogeneous PCET process driven by photo-excitation. Compared to the stepwise electron-transfer followed by the proton-transfer (ET/PT) pathway and vice versa (PT/ET), concerted gain or loss of coupled ecb− and H+ (CPET) typically favors redox reactions. The advantage of such a reaction pathway is that it avoids high-energy intermediates caused by localized charge accumulation,12–14 which makes it consistently exhibit a lower reaction energy barrier.13 Generally, the PCET pathway selectivity was long considered only determined by the proton concentration and specific activation path built by the reactive sites in reaction systems.15 Previous observations have revealed that the reduction potential of ecb− varies sharply with the proton concentration near TiO2 electrodes, which exhibit different reactivities.16 Our previous work further distinguished the binding energy difference that reflects energy levels of electron trapping states on TiO2 nanoparticles between proton (1.3 eV) and proton-free (1.8 eV) systems by synchrotron radiation UV photoelectron spectroscopy.17 Furthermore, hybrid functional periodic density functional theory (DFT) calculations elucidate the impact of proton-coupled defects on the bond dissociation-free energies (BDFEs) of O–H bonds on anatase TiO2 surfaces, which are directly related to interfacial PCET thermochemistry.18 In dye-sensitized solar cells, the PCET model has been applied in monitoring geometric parameters, excitation, and electronic structures of free and Ti4+-bound squaraine dye solar cells.19 However, in a proton-excessive PCET system, e.g., the most common solar energy conversion path of H2O → H2 or CO2 + H2O → CxHyOz involving water participation, the impact of the aqueous interfacial chemical environment on the photo-induced PCET reaction pathway on semiconductor nanoparticles remains unclear. For instance, in some photo-reduction scenarios (e.g., dehalogenation of aromatic halides, H2 production, and CO2 reduction), reaction efficiencies on semiconductor nanoparticle catalysts in H2O/organic mixtures were reported to be much higher than those in the pure organic phase.20–22 Although the effect of water as a direct reactant on photocatalytic performances has been reported in many terms, we still lack a consensual in-depth understanding of the cause of such phenomena. Therefore, determining whether these water-enhanced performances were directly achieved by regulating the PCET pathway is of utmost importance.
Results and discussion
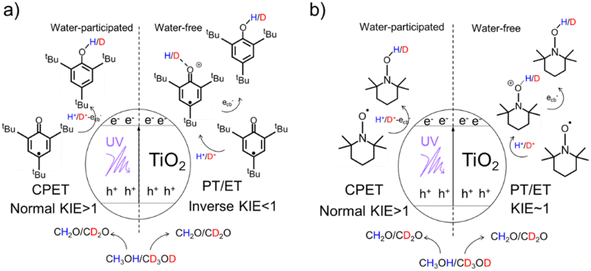
Herein, by using a 2,4,6-tri-tert-butylphenoxyl radical (tBu3ArO·) and 2,2,6,6-tetramethyl-piperidin-1-yl-oxyl (TEMPO) as single-electron/single-proton reagents and isotopically labeled methanol-d0/d4 as both the electron/proton donor and solvent, a series of delicate kinetic solvent isotope effect (KSIE (H/D)) experiments were carried out on TiO2 nanoparticles as catalysts (Scheme 1). We found an unexpected switch of the photo-induced PCET pathway from the stepwise PT/ET to CPET manipulated by a trace amount of adsorbed water molecules on TiO2 nanoparticles. In situ attenuated total reflection Fourier transform infrared spectroscopy (ATR-FTIR) observations confirmed a strong interaction between water molecules and photo-induced surface protons (mainly from the oxidation of methanol solvent) in the presence of ecb−. Such an interreaction directly affects the bond-dissociation of these protons from the TiO2 surface and favors proton-coupled reduction via a CPET pathway. Density functional theory (DFT) calculations were further employed to investigate the impact of water molecules on the proton bond-dissociation and electron transfer on the TiO2 surface.Determining the PCET pathway by KSIE (H/D) measurements
We first performed the KSIE (H/D) experiments on TiO2 nanoparticles under UV with the well-reported single-electron/single-proton radical reduction of tBu3ArO· (tBu3ArO· + H+ + ecb− → tBu3ArOH) and TEMPO (TEMPO + H+ + ecb− → TEMPOH)15,23 by using isotopically labeled methanol-d0/d4 as the H/D solvent and electron/proton donor (CH3OH + hvb+ → CH2O + H+). 1H NMR spectra (Figure S1†) and mass spectra (Figure S2†) confirm no other major products except for the reduction product. All measurements were carried out under anaerobic conditions.The KSIE (H/D) was determined using concentration-time profiles of tBu3ArO·/TEMPO that were in situ monitored in methanol-d0/d4 (H/D systems) by electron spin resonance (ESR) spectroscopy (for details of the KSIE experiments, see the ESI†). Control experiments were performed without TiO2 nanoparticles, confirming no reaction without a catalyst (Figure S3†). The commonly used commercial TiO2 nanoparticles (anatase-phase, without further treatment) were employed as a typical semiconductor nanoparticle catalyst. A direct normal KSIE (H/D) of 2.18 was observed with tBu3ArO· between methanol-d0/d4 systems (Figure S4†), suggesting a one-step CPET reaction pathway on pristine TiO2 nanoparticles.24 Moreover, with TEMPO as the reagent, a normal KSIE (H/D) of 3.73 was observed (Figure S5†). Our observation is consistent with the previously reported result by the Mayer group5 that the single-electron/single-proton transfer induced by photo-generated H+/ecb− on TiO2 and ZnO nanoparticles follows a concerted pathway. However, when freshly high-temperature (773 K) calcined TiO2 nanoparticles were used as catalysts under otherwise identical conditions, the slower reaction kinetics of tBu3ArO· in methanol-d0 compared to methanol-d4 were observed with an unexpected inverse KSIE (H/D) of 0.34 (Figure S6†), indicating that the PCET reaction pathway was significantly changed. Noticeably, only when atomic hybrid modes adjacent to –H/D coordination were changed from sp2 to sp3, the subsequent bond-dissociation kinetic isotope effect (KIE) would appear with an inverse value, indicating the formation of a certain protonation intermediate configuration. Moreover, with TEMPO as the reagent, the KSIE (H/D) on calcined TiO2 nanoparticles was approaching ∼1 (Figure S7†) because the transformation of TEMPO into TEMPOH does not involve the hybridization change from sp2 to sp3. Control experiments have been performed with TiO2 nanoparticles calcined in an air, oxygen, or argon atmosphere. An inverse KSIE (H/D) ≤ 0.5 with tBu3ArO· and KSIE (H/D) ∼1 with TEMPO were always observed on calcined TiO2 nanoparticles regardless of atmosphere (Figures S8 and S9†). These results indicate that other effects from calcination rather than defect vacancies determine the switch of the KSIE (H/D) and corresponding PCET pathways.
The XRD result shows that the crystalline structure of anatase TiO2 nanoparticles was not changed after calcination and the crystallite diameter did not severely change (Figure S10 and Table S1†), which further excludes the impact of rutile formation as the major effect. We further compared the sedimentation behavior of a pristine/calcined TiO2 (2.5 g L−1) suspension by in situ UV-vis optical-fiber spectroscopy. As shown in Figure S11,† the light transmittance hardly changed within 90 s in both systems, suggesting that sedimentation is not the major effect for the KIE in our system.
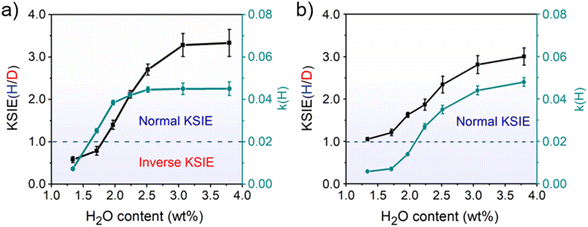
The decreased KSIE value caused by calcination would recover to the normal KSIE value after the calcined TiO2 catalyst is stored in the air for one week (Figures S12 and S13†). We argue that the loss of trace water on TiO2 nanoparticles after calcination is the determining factor for the KSIE <1. Through the in situ diffuse reflection infrared Fourier transform spectroscopy (DRIFTS) characterization of TiO2 nanoparticles during calcination (298–773 K), we found that the featured signals of adsorbed water molecules (O–H stretching vibration band at 3200 cm−1 and H–O–H bending vibration at 1642 cm−1) severely decreased until they wholly disappeared with the increasing temperature. When the temperature exceeded 773 K, the loss of dissociated water (O–H stretching vibration band at 3694/3459 cm−1 without 1642 cm−1 bending vibration) was observed (Figure S14a†). However, with the temperature further returned to room temperature, although the ambient humidity can recover a certain amount of water adsorption on TiO2 nanoparticles, it would never be able to reach the pre-calcination level (Figure S14b†), demonstrating the inevitable loss of water content in TiO2 nanoparticles after high-temperature calcination. Moreover, by using thermogravimetric analysis (TGA) measurements, we found that the water content in pristine TiO2 nanoparticles long stored in the air was ∼3.76 wt% before calcination. In contrast, after 773 K calcination and returning to room temperature, the water content of the freshly calcined TiO2 nanoparticles was significantly reduced to ∼1.33 wt% (Figure S15†). To directly demonstrate the relationship between the water content of the catalyst and the KSIE (H/D) of photo-induced PCET reactions, we prepared a series of TiO2 nanoparticle catalysts with well-controlled water contents (1.33–3.80 wt%) by treating dried TiO2 nanoparticles in airflows with different humidities (Figure S16†) (for preparation methods see the ESI†). The coverage of water layers was calculated according to the surface Ti atoms on TiO2 nanoparticles (in terms of a perfect anatase {101} surface) and is summarized in Table S2,† corresponding to an increasing water coverage from ∼ one layer to ∼ three layers. With the increasing water content of TiO2 nanoparticles, the KSIE (H/D) with tBu3ArO· significantly increased from an inverse value of 0.34 to a normal value of 3.33 (Fig. 1a). In contrast with the KSIE(H/D) with tBu3ArO·, the KSIE (H/D) with TEMPO increased from a recessive value of ∼1 to a normal value of 3 (Fig. 1b), which provides solid evidence that the KSIE (H/D) value was directly determined using the water content in the TiO2 nanoparticle catalyst. Reaction rate parameters in methanol (k(H)) with both radical reagents show a similar trend that increases with the increasing water content (Fig. 1a and b), which is consistent with the water-determined KSIE (H/D). We further employed a well-reported Fe(III)-1,10-phenanthroline spectrometric titration method (for details of the titration method see the ESI†) to quantify the oxidation of CH3OH/CD3OD on different TiO2 samples.26 As shown in Figure S17.† KIEs for methanol-d0/d4 oxidation on both dry (water content∼1.3 wt%) and wet (water content ∼ 3.8 wt%) TiO2 are identical at ∼1.3, indicating that the changed KIE value with increasing water content in the TiO2 catalyst is not from the methanol oxidation.
The water-determined KSIE (H/D) reversal directly reflects the change of the photo-induced PCET reaction pathway on TiO2 nanoparticles. Typically, the tBu3ArO· radical has three possible configurations, as shown in Scheme 2a. Type I is the O–C single bond radical structure, while type II and type III are O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond structures. Based on the reported single-crystalline structure analysis, type III is the major contributor to the stable tBu3ArO· radical, i.e., in the O
C double bond structures. Based on the reported single-crystalline structure analysis, type III is the major contributor to the stable tBu3ArO· radical, i.e., in the O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond configuration.27 Therefore, the single-proton/single-electron transfer of tBu3ArO· → tBu3ArOH is accompanied by the dissociation of the C
C double bond configuration.27 Therefore, the single-proton/single-electron transfer of tBu3ArO· → tBu3ArOH is accompanied by the dissociation of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O double bond and the hybrid configuration transition of O
O double bond and the hybrid configuration transition of O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C sp2 → O–C sp3. The inverse KIE from sp2–sp3 hybridization is a very classic phenomenon in reaction kinetics.25,28 The secondary KIE is mainly due to the difference in bending vibrations. Due to symmetry, the in-plane and out-of-plane bending of sp3 hybridized carbon atoms are equivalent. The in-plane bending vibrations of sp2 hybridized carbon or oxygen atoms are stiffer than out-of-plane bending vibrations (there is a minor site resistance for out-of-plane bending), leading to a more significant difference in zero-point energy in the O–H and O–D bonding in the reaction of the hybrid state change. In the sp3 state as the substrate, the O–D bond has lower zero-point energy and needs to cross a larger energy barrier to reach the intermediate state, while in the sp2 state it is the opposite (Fig. S18†). In this process, an inverse KIE (H/D) can only occur when the protonated intermediate of H+/D+–O
C sp2 → O–C sp3. The inverse KIE from sp2–sp3 hybridization is a very classic phenomenon in reaction kinetics.25,28 The secondary KIE is mainly due to the difference in bending vibrations. Due to symmetry, the in-plane and out-of-plane bending of sp3 hybridized carbon atoms are equivalent. The in-plane bending vibrations of sp2 hybridized carbon or oxygen atoms are stiffer than out-of-plane bending vibrations (there is a minor site resistance for out-of-plane bending), leading to a more significant difference in zero-point energy in the O–H and O–D bonding in the reaction of the hybrid state change. In the sp3 state as the substrate, the O–D bond has lower zero-point energy and needs to cross a larger energy barrier to reach the intermediate state, while in the sp2 state it is the opposite (Fig. S18†). In this process, an inverse KIE (H/D) can only occur when the protonated intermediate of H+/D+–O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C is formed and subsequently converted to the H/D–O–C product by the further ecb− transfer (Scheme 2b), reflecting a stepwise PT/ET pathway at low water content (Scheme 2b). Conversely, at high water content, tBu3ArO· was directly reduced to tBu3ArOH through one-step CPET without forming such H+/D+-O sp2 protonated intermediates, resulting in a normal KIE ≥ 2 from the direct bond-dissociation of protons from bridging-O sites on the TiO2 surface (Scheme 2b).29–31 However, due to the high electronegativity and coordination limits of the adjacent N atoms that hinder the transfer of the unpaired electron from the O atom to the carbon ring, TEMPO as another single-proton/single-electron acceptor always retains the N–O single bond free radical structure without the O
C is formed and subsequently converted to the H/D–O–C product by the further ecb− transfer (Scheme 2b), reflecting a stepwise PT/ET pathway at low water content (Scheme 2b). Conversely, at high water content, tBu3ArO· was directly reduced to tBu3ArOH through one-step CPET without forming such H+/D+-O sp2 protonated intermediates, resulting in a normal KIE ≥ 2 from the direct bond-dissociation of protons from bridging-O sites on the TiO2 surface (Scheme 2b).29–31 However, due to the high electronegativity and coordination limits of the adjacent N atoms that hinder the transfer of the unpaired electron from the O atom to the carbon ring, TEMPO as another single-proton/single-electron acceptor always retains the N–O single bond free radical structure without the O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C → O–C transition during the reaction. Thus, even with the PT intermediate formed at low water content, a KIE ∼1 rather than an inverse KIE should be observed (Scheme S2†), entirely consistent with our experimental results (Fig. 1b). Our KSIE experiments show that the participation of water can directly switch the photo-induced PCET pathway on the TiO2 nanoparticle catalyst from water-free stepwise PT/ET to water-participated CPET.
C → O–C transition during the reaction. Thus, even with the PT intermediate formed at low water content, a KIE ∼1 rather than an inverse KIE should be observed (Scheme S2†), entirely consistent with our experimental results (Fig. 1b). Our KSIE experiments show that the participation of water can directly switch the photo-induced PCET pathway on the TiO2 nanoparticle catalyst from water-free stepwise PT/ET to water-participated CPET.
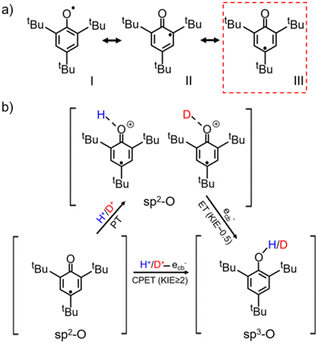 | ||
| Scheme 2 (a) Configurations of the tBu3ArO· resonance structure with type III as the major contributor;27 (b) schematic diagram of the CPET and PT/ET reaction pathways of the single-proton/single-electron transfer on TiO2 with tBu3ArO· as the acceptor. | ||
In situ ATR-FTIR experiments
Isotopic-labeled ATR-FTIR spectroscopy was further used to in situ observe water molecules on the TiO2 surface and their interaction with photo-induced protons/electrons under continuous UV irradiation. Setting the equilibrium conditions as the blank background, a positive or negative IR response reflects the intermediate species' gradual gain or loss at the methanol-d0/d4 (CH3OH/CD3OD)/H2O/TiO2 interface during the reaction. Before measurements, TiO2 nanoparticles, as the typical catalyst sample, were first calcined at 773 K for 30 minutes and then cooled to room temperature under a constant dried nitrogen flow. In the water-participated system, the calcined TiO2 sample was further treated in water vapor under a continuous nitrogen flow for 15 min, resulting in a water content of 3.80 wt%. The experiments were carried out in a nitrogen atmosphere to prevent trapped ecb− from being quenched by oxygen.As shown in Fig. 2a, when TiO2/CH3OH was used as the ATR-FTIR sample and irradiated in situ with a 365 nm LED lamp, a broad negative peak at 3272 cm−1 and signal bands at 2941 cm−1 and 2830 cm−1 emerged from the background and increased in intensity with increasing irradiation time, corresponding to the stretching vibration signals of O–H and C–H in methanol and representing the oxidation of methanol. This is further verified by the ground-state signal on the TiO2/CH3OH system. (Figure S20†). Moreover, a strong baseline upshift absorption band from 2500 cm−1 to 1100 cm−1 was observed, which is consistent with our previous observations and can be ascribed to the absorption of trapped ecb− on the TiO2 nanoparticles.17,26,32,33 These trapped ecb− were localized in the surface defect layer in the form of four-coordinated Ti4c3+ under the stabilization of protons provided by the methanol oxidation.8,17,31 When the water vapor was first introduced to TiO2 nanoparticles, a broad negative absorption of O–H at 3220 cm−1 and a mild negative peak at 1677 cm−1 corresponding to the water molecule H–O–H bending vibration emerged at the TiO2/H2O/CH3OH interface under continuous UV irradiation. These signals are accompanied by the methanol oxidation and the baseline upshift from the absorption of trapped ecb− (Fig. 2b), further verified by the ground-state water and methanol signal on TiO2 (Figure S20†). More importantly, a positive peak at 1633 cm−1 corresponding to the H–O–H bending vibration with a lower frequency (from 1677 cm−1) and a positive absorption band of O–H stretching vibration at 3594 cm−1 with a higher frequency (from 3220 cm−1) emerged. These vibrations reflect a strong interaction between water molecules and protons/ecb− from methanol oxidation on TiO2 nanoparticles. Such a strong interaction causes the stretching vibration of the water to be faster while the bending vibration slows down due to the steric effect. All these effects manifested as a change in infrared absorption wavenumbers. The negative peak at 3707 cm−1 corresponds to the terminal hydrogen on TiO2.32–34 Moreover, when the deuterium-labeled TiO2/D2O/CD3OD sample is used, the strong interaction between water and protons remains. The O–H stretching vibration peak shifts towards higher frequencies (2394 cm−1 → 2713 cm−1) and H–O–H bending vibration towards lower frequencies (1232 cm−1 → 1130 cm−1) in terms of H/D replacement effects (Fig. 2c), which is consistent with the ground-state methanol-d4 and D2O signals on TiO2 (Figure S20†). The displacement of the deuterium-labeled proton peak indicates that the proton peak is indeed produced by the interaction of water and protons. In water-free systems, similar peak shift phenomena were no longer observed on TiO2/CH3OH or TiO2/CD3OD samples (Fig. 2a and d).
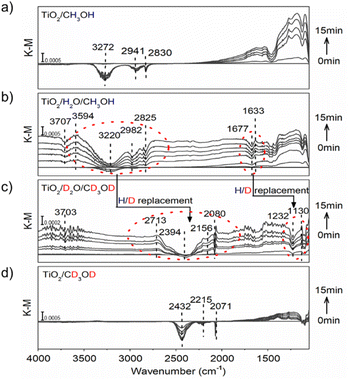 | ||
| Fig. 2 ATR-FTIR spectra in situ monitored at (a) TiO2/CH3OH; (b) TiO2/H2O/CH3OH; (c) TiO2/D2O/CD3OD; and (d) TiO2/CD3OD interface under constant 365 nm (3 W, LED) irradiation. | ||
To further confirm that the observed peak shift was due to the interaction between water molecules and proton/ecb− on the TiO2 surface, hydrochloric acid was used as an external proton donor to replace methanol, forming an H2O/HCl/TiO2 interface (pH = 6). When the light was off, the addition of HCl only induced mild water desorption from the TiO2 surface with negative peaks at 3377 cm−1 and 1644 cm−1, and no positive peaks were observed (Figure S19b†), indicating that water molecules were hardly affected by only the presence of additional protons. However, when the light was on, the negative peak at 1704 cm−1 and positive peak at 1663 cm−1 that correspond to the shift of H–O–H bending vibration towards lower frequencies were immediately observed, as well as the negative band at 3333 cm−1 and the emerging positive band at 3555 cm−1 that correspond to the shift of O–H stretching vibration towards higher frequencies (Figure S19d†), which is identical to the observed phenomenon at the TiO2/H2O/CH3OH interface. Moreover, a broad negative band at around 2964 cm−1 emerged, which can be ascribed to the destruction of hydrogen-bonds between water molecules, further demonstrating the changed water molecules in the presence of protons and photo-induced ecb−. Without the addition of HCl, no other peaks appeared except for the molecular water H–O–H bending vibration at 1640 cm−1 and the molecular water O–H stretching vibration at 3210 cm−1 under constant irradiation (Figure S19a†). We further reduced pH to 5 and repeated the ATR-FTIR experiment. As shown in Figure S19e,† the observed phenomenon is consistent with pH = 6. Furthermore, it offers a more significant blueshift signal of –OH stretching vibration and a redshift signal of H–O–H bending vibration. Such a result indicates that high proton concentrations accelerate the formation of hydrated protons, which is consistent with our conclusion. Our ATR-FTIR results solidly confirm a strong interaction between water and protons/ecb− intermediates on the TiO2 surface, possibly achieved by destroying hydrogen bonds between water or forming hydrated protons35 which would undoubtedly affect the subsequent PCET dynamics.
Typically, protons tend to be localized at bridging –O atoms on TiO2 nanoparticles. At the same time, the trapping of ecb− at neighboring Ti atoms on TiO2 nanoparticles under UV in the presence of methanol to quench photo-induced holes occurs.31 In that case, a stepwise PT/ET route with the formation of high-energy protonation intermediates was inevitable. However, according to our observations in this work, surface protons/ecb− can form a strong interaction with water (in the form of hydrogen bonds or hydrated protons), serving as a reaction relay to favor the concerted transfer of proton/ecb− pairs by avoiding the formation of high-energy intermediates. In other words, the presence of water lowered the reaction energy barrier of photo-induced PCET on TiO2 nanoparticles by switching the reaction pathway from stepwise PT/ET to CPET.
DFT calculations
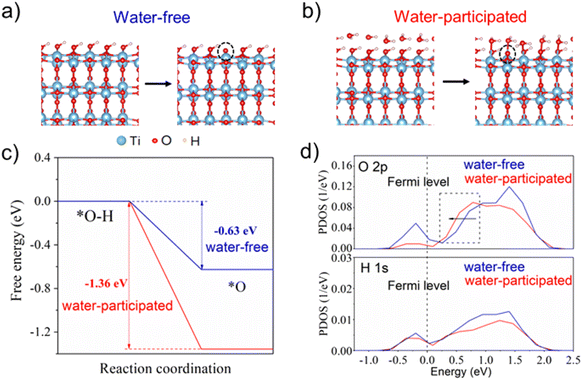
DFT calculations were further employed to investigate the impact of water molecules on the single-proton/single-electron transfer on the TiO2 surface (for details of the computational methods, see the ESI†). As shown in Fig. 3a, the calculated free energy profiles of electron and proton transfer suggest that the existence of water molecules on the TiO2 surface can significantly promote the transfer of electrons and protons from the surface –OH group due to more negative reaction energy (−1.36 eV) in comparison with the water-free system (−0.63 eV). The projected density of states (PDOS) profiles of the surface –OH group further indicate that the edge line of the main peak of O 2p states in the –OH group with a water layer on the TiO2 surface is closer to the Fermi level than that without a water layer, which is often active in the catalysis reaction. This also suggests the higher reaction activity of the –OH group in the presence of a water layer on the TiO2 surface (Fig. 3b). As a result, the surface –OH group with neighboring water is more active than that without water, which coincides with our experimental observations.Conclusion
An unexpected water-controlled switch of the photo-induced single-proton/single-electron transfer pathway on TiO2 nanoparticles was discovered. On dried TiO2 nanoparticles (water content <1.7 wt%, ∼monolayer coverage), the photo-induced PCET follows a stepwise PT/ET pathway with the formation of a high-energy protonation intermediate, resulting in an inverse KSIE (H/D) ∼0.5 with tBu3ArO· and KSIE (H/D) ∼1 with TEMPO in methanol-d0/d4 systems. However, in the presence of a trace amount of water (>2 wt% in TiO2, ∼two layer coverage), the single-proton/single-electron transfer reaction on TiO2 nanoparticles follows a concerted pathway with a lower energy barrier, leading to a normal KSIE (H/D) ≥2 with both reagents. Our work is the first to clarify the genuine impact of water on the PCET pathway responsible for the performance enhancement in water-participated systems in terms of PCET, which dispels previous confusion on this issue and can be used for the design of efficient semiconductor-based photocatalytic systems.Data availability
The data supporting the findings of this study are available within the article and its ESI† files. All other relevant source data are available from the corresponding author upon request following the data management specifications of Jiangsu University and University of Michigan. Source data are provided with this paper.Author contributions
Z. Liu: investigation, data curation, and writing of the original draft. W. Peng, Y. Lin, X. Lin, S. Yin, S. Jia, and D. Ma: resources. Y. Yan and P. Zhou: writing-review. Y. Yan, W. Ma, and J. Zhao: conceptualization, supervision, writing-review and editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge the financial support of the National Natural Science Foundation of China (Grant No. 21776117, 22176192, and 21806060).References
- E. Tsui, A. J. Metrano, Y. Tsuchiya and R. R. Knowles, Angew. Chem., Int. Ed., 2020, 59, 11845–11849 CrossRef CAS PubMed
.
- G. J. Choi, Q. Zhu, D. C. Miller, C. J. Gu and R. R. Knowles, Nature, 2016, 539, 268–271 CrossRef CAS PubMed
.
- K. T. Tarantino, P. Liu and R. R. Knowles, J. Am. Chem. Soc., 2013, 135, 10022–10025 CrossRef CAS PubMed
.
- J. Shangguan, A. J. R. Hensley, M. V. Gradiski, N. Pfriem, J. Mcewen, R. H. Morris and Y. H. C. Chin, ACS Catal., 2019, 9, 1763–1778 CrossRef CAS
.
- J. N. Schrauben, R. Hayoun, C. N. Valdez, M. Braten, L. Fridley and J. M. Mayer, Science, 2012, 336, 1298–1301 CrossRef CAS PubMed
.
- C. C. Mercado, F. J. Knorr and J. L. McHale, J. Phys. Chem. C, 2008, 112, 12786–12794 CrossRef
.
- C. C. D. Zhao, C. Yu, W. Ma and J. Zhao, J. Phys. Chem. C, 2009, 113, 13160–13165 CrossRef
.
- F. G. Santomauro, A. Lubcke, J. Rittmann, E. Baldini, A. Ferrer, M. Silatani, P. Zimmermann, S. Grubel, J. A. Johnson, S. O. Mariager, P. Beaud, D. Grolimund, C. Borca, G. Ingold, S. L. Johnson and M. Chergui, Sci. Rep., 2015, 5, 14834 CrossRef CAS PubMed
.
- C. N. Valdez, M. Braten, A. Soria, D. R. Gamelin and J. M. Mayer, J. Am. Chem. Soc., 2013, 135, 8492–8495 CrossRef CAS PubMed
.
- S. Qiao, Y. Zhou, H. Hao, X. Liu, L. Zhang and W. Wang, Green Chem., 2019, 21, 6585–6589 RSC
.
- S. Zeng, L. Zhang, W. Wang, D. Shao and H. Hao, Phys. Chem. Chem. Phys., 2017, 19, 29053–29056 RSC
.
- D. R. Weinberg, C. J. Gagliardi, J. F. Hull, C. F. Murphy, C. A. Kent, B. C. Westlake, A. Paul, D. H. Ess, D. G. McCafferty and T. J. Meyer, Chem. Rev., 2012, 112, 4016–4093 CrossRef CAS PubMed
.
- R. Tyburski, T. Liu, S. D. Glover and L. Hammarstrom, J. Am. Chem. Soc., 2021, 143, 560–576 CrossRef CAS PubMed
.
- A. A. Stuchebrukhov and S. H. Schiffer, Chem. Rev., 2010, 110, 6939–6960 CrossRef PubMed
.
- C. Chen, T. Shi, W. Chang and J. Zhao, ChemCatChem, 2015, 7, 724–731 CrossRef CAS
.
- G. J. Meyer and P. Qu, Langmuir, 2001, 17, 6720–6728 CrossRef
.
- Y. Yan, W. Shi, W. Peng, Y. Lin, C. Zhang, L. Li, Y. Sun, H. Ju, J. Zhu, W. Ma and J. Zhao, Chem. Commun., 2019, 2, 88 CrossRef
.
- R. E. Warburton, J. M. Mayer and S. H. Schiffer, J. Phys. Chem. Lett., 2021, 12, 9761–9767 CrossRef CAS PubMed
.
- S. G. Aziz, O. I. Osman, S. A. Elroby, W. M. I. Hassan, A. Jedidi and R. H. Hilal, Struct. Chem., 2018, 29, 983–997 CrossRef CAS
.
- M. Lei, Z. Wang, L. Zhu, W. Nie and H. Tang, Appl. Catal., B, 2020, 261, 118236 CrossRef CAS
.
- Y. Kuramochi, M. Kamiya and H. Ishida, Inorg. Chem., 2014, 53, 3326–3332 CrossRef CAS PubMed
.
- Y. Li, P. Ren, D. Zhang, W. Qiao, D. Wang, X. Yang, X. Wen, M. H. Rummeli, H. Niemantsverdriet, J. P. Lewis, F. Besenbacher, H. Xiang, Y. Li and R. Su, ACS Catal., 2021, 11, 4338–4348 CrossRef CAS
.
- C. Wang, H. Groenzin and M. J. Shultz, J. Am. Chem. Soc., 2005, 127, 9736–9744 CrossRef CAS PubMed
.
- Y. Lin, Y. Yan, W. Peng, X. Qiao, D. Huang, H. Ji, C. Chen, W. Ma and J. Zhao, J. Phys. Chem. Lett., 2020, 11, 3941–3946 CrossRef CAS PubMed
.
- W. Chang, C. Sun, X. Pang, H. Sheng, Y. Li, H. Ji, W. Song, C. Chen, W. Ma and J. Zhao, Angew. Chem., Int. Ed., 2015, 54, 2052–2056 CrossRef CAS PubMed
.
- Y. Yan, W. Shi, Z. Yuan, S. He, D. Li, Q. Meng, H. Ji, C. Chen, W. Ma and J. Zhao, J. Am. Chem. Soc., 2017, 139, 2083–2089 CrossRef CAS PubMed
.
- V. W. Manner, T. F. Markle, J. H. Freudenthal, J. P. Roth and J. M. Mayer, Chem. Commun., 2008, 256–258 RSC
.
-
D. Dougherty and E. Anslyn, Modern Physical Organic Chemistry, 2005 Search PubMed
.
- Q. Guo, C. Zhou, Z. Ma and X. Yang, Adv. Mater., 2019, 31, e1901997 CrossRef PubMed
.
- Q. Guo, Z. Ma, C. Zhou, Z. Ren and X. Yang, Chem. Rev., 2019, 119, 11020–11041 CrossRef CAS PubMed
.
- Q. Guo, C. Zhou, Z. Ma, Z. Ren, H. Fan and X. Yang, Chem. Soc. Rev., 2016, 45, 3701–3730 RSC
.
- J. A. Moss, S. H. Szczepankiewicz and M. R. Hoffmann, J. Phys. Chem. B, 2002, 106, 2922–2927 CrossRef
.
- D. S. Warren and A. J. McQuillan, J. Phys. Chem. B, 2004, 108, 19373–19379 CrossRef CAS
.
- H. Sheng, H. Zhang, W. Song, H. Ji, W. Ma, C. Chen and J. Zhao, Angew. Chem., Int. Ed., 2015, 54, 5905–5909 CrossRef CAS PubMed
.
- Q. Yu, W. B. Carpenter, N. H. C. Lewis, A. Tokmakoff and J. M. Bowman, J. Phys. Chem. B, 2019, 123, 7214–7224 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2sc07038c |
| This journal is © The Royal Society of Chemistry 2023 |