Open Access Article
Open Access ArticleCatalytic enantioselective alkenylation–heteroarylation of olefins: stereoselective syntheses of 5–7 membered azacycles and oxacycles†
Zhaoming
Ma
a,
Lantian
Sun
b and
Jianrong Steve
Zhou
 *a
*a
aState Key Laboratory of Chemical Oncogenomics, Guangdong Provincial Key Laboratory of Chemical Genomics, School of Chemical Biology and Biotechnology, Peking University Shenzhen Graduate School, Room F312, 2199 Lishui Road, Nanshan District, Shenzhen 518055, China. E-mail: jrzhou@pku.edu.cn
bDepartment of Chemistry, Hong Kong Baptist University, 224 Waterloo Road, Kowloon Tong, Hong Kong, China
First published on 17th February 2023
Abstract
Catalytic enantioselective domino alkenylation–heteroarylation of nonconjugated iododienes proceeded with excellent stereoselectivity and broad scope of substrates. The reaction enables stereoselective syntheses of substituted azacycles such as piperidine, pyrrolidine azepine and dihydropyrans carrying new quaternary stereocenters. Mechanistically, C–H bonds of heterocycles were activated by lithium alkoxides via reversible deprotonation, rather than conventional palladium(II)-assisted metalation processes. Many types of heteroarenes can be used, including not only azoles (such as thiazoles, oxazoles, imidazoles and oxadiazoles), but also nonazoles (thiophene, furan and azine N-oxides).
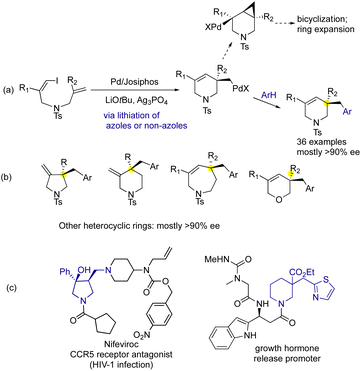
There is a resurgence of research interest in developing enantioselective domino coupling reactions initiated by Heck-type arylation of pendant alkenes.1 For example, Zhu et al.2 reported a stereoselective synthesis of stereodefined 3,3-disubstituted oxindoles via domino couplings of acroylamide ortho-triflates. The heteroarenes were limited to azoles having acidified C–H bonds such as benzothiazole, benzoxazole and 1,3,4-oxadiazole;3 they were activated via a palladium-based mechanism of nonconcerted metalation–deprotonation.4 However, analogous stereoselective domino couplings of alkenyl electrophiles proved to be much more difficult,5 due to side reactions such as Heck-type bicyclization onto the alkenyl groups after insertion to form [3.1.0]bicycles6 or subsequent ring expansion (see Fig. 1a).7
We report herein a general method for catalytic domino alkenylation–heteroarylation to readily access 5–7 membered azacycles, as well as oxacyclic dihydropyran derivatives (see Fig. 1a and b).8 These saturated azacycles, pyrrolidines and piperidines are among the most frequently used rings in medicines, including both heterocycles and carbocycles (Fig. 1b).9 For example, anti-HIV agent nifeviroc contains a trisubstituted pyrrolidine and shows an IC50 value of 2.9 nM against the CCR5 receptor, but the IC50 value of its enantiomer was only 380 nM.10 The new reaction provides azacycles carrying quaternary stereocenters at C3 or C4 positions which are difficult to prepare from other catalytic reactions.11 It also enabled asymmetric ring closure to form oxacyclic dihydropyrans (Fig. 1b).
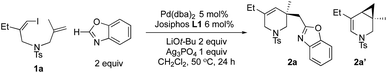
In a model study of iododiene 1a and benzoxazole, we first explored a family of Josiphos ligands12 to identify suitable ancillary ligands for palladium catalysts (see Scheme 1). In PdII complexes of Josiphos, switching from PAr2 to PCy2 (Ar = aryl; Cy = cyclohexyl) groups on the 1-ethyl sidearm is accompanied by not only electronic perturbation, but also a substantial conformational change in chelate rings formed by palladium and Josiphos, so as to avoid close contact of large PCy2 rings with the ferrocene ring.13 Consequently, all four P-substituents will undergo a substantial conformational change; hence a significant change occurs in the chiral environment surrounding the palladium centers.
 | ||
| Scheme 1 Screening of Josiphos ligands on a model domino reaction of 1a and benzoxazole (GC yields on a 0.05 mmol scale in 0.3 mL of CH2Cl2). dba = dibenzylideneacetone. | ||
The modularity and tunability of both steric and electronic properties of Josiphos ligands are very rewarding. This allowed us to quickly identify Josiphos L1 (ref. 14) which provides desired piperideine 2a in 88% yield and 92% ee, along with a small amount of an oxidative dimer of benzoxazole. If benzoxazole was omitted, the reaction pathway sidetracked to Heck bicyclization (41% 2a′ in 87% ee) and reductive Heck cyclization (27% 2a′′). The Heck bicyclization leading to 2a′ also eliminated PdH species, which can undergo ligand exchange with alkyl palladium complex C to form 2a′′via C–H reductive elimination (see Scheme 7c below). The Heck bicyclization has no parallelism in domino coupling reactions of aryl electrophiles (see Fig. 1a). Josiphos analogues L2–L4 carrying two diarylphosphines only afforded moderate 40–70% ees. In comparison, the Josiphos series having a strongly donating PCy2 sidearm gave consistently higher ees than the former. On the other hand, the complex of L7 carrying two highly donating PCy2 donors showed poor catalytic activity and afforded a low level of stereoselectivity. It is well known that in cationic Heck-type reactions, the key step of alkene insertion is accelerated by weakly donating ligands and retarded by strongly donating phosphines.15 Axially chiral biphosphines were also tested; for example, axially chiral BINAP and Segphos furnished 58% ee and 81% ee, respectively.
During condition optimization, we noticed that other reaction parameters were also important. The choice of metal alkoxides had a remarkable impact on the outcome. Without an added alkoxide, only 16% conversion of 1a was detected without any formation of 2a (Table 1, entry 2). LiOMe led to moderate yield and ee (entry 2); LiOt-Bu, NaOMe or KOMe proved to be the best bases in terms of both chemical yields and ees (entries 4 and 5); the more basic alkoxides NaOt-Bu or KOt-Bu gave poor yields of 2a probably due to fast deprotonation and ring opening of 2-metalated benzoxazole (entries 6 and 7).16 The use of silver phosphate was essential, without which the model reaction gave a complex mixture containing 2a in 48% yield and 81% ee (entry 8). Other silver salts, Ag2CO3, AgOAc or AgOTf can also provide 2a in 60–85% yields and ∼90% ees (entries 9–11). The result suggests that these silver salts may act as halide abstractors to create a coordination site for enantiofacial olefin insertion.
| Entry | Change of conditions | Conv. of 1a (%) | Yield of 2a (%) | Yield of 2a′ (%) |
|---|---|---|---|---|
| 1 | No change | 100 | 88 (92% ee) | 9 |
| 2 | No LiOt-Bu | 16 | 0 | 0 |
| 3 | LiOMe | 100 | 63 (58% ee) | 12 |
| 4 | NaOMe | 100 | 89 (90% ee) | 0 |
| 5 | KOMe | 100 | 73 (92% ee) | 0 |
| 6 | NaOt-Bu | 33 | 12 (86% ee) | 6 |
| 7 | KOt-Bu | 92 | 26 (85% ee) | 5 |
| 8 | No Ag3PO4 | 100 | 48 (81% ee) | <5 |
| 9 | Ag2CO3 | 100 | 75 (92% ee) | 12 |
| 10 | AgOAc | 100 | 59 (94% ee) | 12 |
| 11 | AgOTf | 100 | 84 (90% ee) | 8 |
The Pd catalyst of Josiphos L1 can be applied to domino couplings of iododialkene 1a with many classes of heteroarenes (Scheme 2). We were gratified to find that the combination of lithium t-butoxide and silver phosphate enabled efficient activation of azoles including (benzo)thiazole, (benzo)oxazole, imidazoles and oxadiazole, but also nonazoles such as (benzo)thiophene,17 furan and azine N-oxides. In reactions of thiazoles (2h–i), polar groups, esters and nitrile were well tolerated. Notably, the C–H activation of C2-substituted thiazoles (2j–l) occurred regioselectively at C5 positions next to the sulfur atom. In the reactions of imidazoles (2n–o), (benzo)thiophenes (2q–t) and furan (2u), electron-withdrawing groups (e.g., ester, nitrile and trifluoroacetyl) were important to the activation and couplings of these heteroarenes. Notably, both pyridine and isoquinoline N-oxides were regioselectively activated at C2 positions to give products 2v–w in ∼70% ees under the standard conditions using L1. Switching from Ag3PO4 to AgOTf increased the stereoselectivity to >90% ees.
 | ||
| Scheme 2 Examples of azoles and non-azoles in enantioselective formation of piperidines (isolated yields from a 0.1 mmol scale in 0.5 mL of CH2Cl2). | ||
Next, we studied structural variations of (Z)-1-iodo-1,6-dienes in stereoselective formation of piperideines (Scheme 3). The alkenyl fragment can tolerate different C5-substituents (ethyl, n-hexyl, isobutyl, cyclohexyl, benzyl and phenyl) (3a–f), while the C3 substituents of the iodoalkenyl fragment can be t-butyl, i-propyl, methyl, benzyl and phenyl (3g–k). Moreover, the N-tosylamide linker can be substituted by 2-thienylsulfonamide and N,N-dimethylsulfamate (3l–m). We also attempted to prepare piperidines carrying quaternary stereocenters at C4 positions, by using 2-iodo-1,7-diene 1n. At first, its reaction with benzoxazole failed to produce product 3n in a reasonable quantity. After switching from ligand L1 to L4 possessing a large di(1-naphthyl)phosphine sidearm, we succeeded in producing piperidines 3n–p from benzoxazole, oxazole and benzothiazole in satisfactory yields and with ∼90% ee (Scheme 3).
 | ||
| Scheme 3 Stereoselective formation of 6-membered piperideines and piperidines via domino couplings (isolated yields on a 0.1 mmol scale in 0.5 mL of CH2Cl2). Th = 2-thienyl. | ||
Seven-membered azepane derivatives are important motifs in medicines,18 but catalytic asymmetric syntheses for these azacycles are still limited to date.19 Thus, the Pd catalyst of Josiphos L4 also promoted domino coupling of (Z)-1-iodo-1,7-diene 1q with benzothiazole via a rare 7-exo-trig cyclization (Scheme 4).20 Asymmetric formation of tetrahydroazepine 3q was achieved in 70% yield and 84% ee (or 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 er). In contrast, the Pd/L1 catalyst failed to produce a significant amount of product 3q. The domino couplings using L4 proceeded well with 5-phenyloxazole and 3-cyanobenzothiophene, too (3t–3x).
8 er). In contrast, the Pd/L1 catalyst failed to produce a significant amount of product 3q. The domino couplings using L4 proceeded well with 5-phenyloxazole and 3-cyanobenzothiophene, too (3t–3x).
 | ||
| Scheme 4 Stereoselective formation of tetrahydroazepines via domino couplings of 1q (isolated yields on a 0.1 mmol scale in 0.5 mL of CH2Cl2). | ||
Furthermore, we successfully used the new method for stereoselective construction of trisubstituted pyrrolidines. Thus, a reaction of 2-iodo-1,6-diene 4a with benzoxazole generated 5a in 49% yield and 84% ee. After switching ligand L1 to Josiphos L2, a satisfactory result of 84% yield and 92% ee resulted. In this model reaction, Josiphos L3 and L4 also provided excellent yields and 90% ee (Scheme 5a). LiOtBu was important, without which only 26% conversion of 4a was detected, without any production of 5a. Additionally, when Ag3PO4 was omitted, the reaction afforded 5a in 31% yield and 71% ee, together with some premature coupling (26% yield of 5a′), hinting that the silver salt played a crucial role in halide abstraction to create a “vacant” site for enantiofacial olefin insertion.
The enantioselective formation of pyrrolidines tolerated well structural variations in iododienes (Scheme 5b), for example, ethyl and benzyl groups on olefinic units (5b–c) and acid-labile 2-thienylsulfonamide and N,N-dimethylsulfamate linkers (5d–e). Azoles such as benzoxazole, benzothiazole and 1,3,4-oxadiazole coupled well (5fi). Single crystals of 5d were obtained via vapor diffusion of hexane into a solution in DCM. X-ray crystallography thus established its absolute configuration to be 3R.†
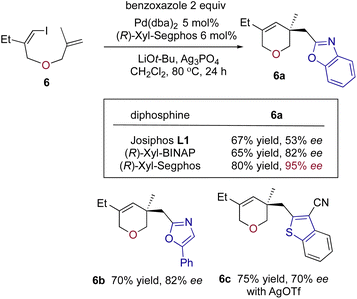
We also attempted to prepare oxacycle 6avia a domino coupling of iodo-1,6-diene 6 (Scheme 6). Initially, we found that the Pd/L1 catalyst only provided 6a in a moderate yield and 53% ee, but after changing the ancillary ligand to (R)-xyl-Segphos, the result was readily improved to 80% yield and 95% ee. Thus, the Pd/xyl-Segphos catalyst efficiently enabled stereoselective formation of other oxacycles 6b–c from 5-phenyloxazole and 3-cyanobenzothiophene. The six-membered oxacycles are present in some medicines as core structures.21
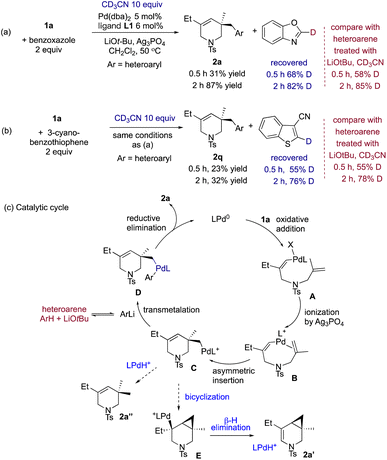
To gain insights into the activation of heteroarenes under catalytic conditions, we added 10 equiv. of CD3CN (pKa 31 in DMSO) to catalytic domino couplings of 1a with benzoxazole and 3-cyanobenzothiophene. Several observations were made. (a) 1a and benzoxazole (pKa 25 in DMSO) reacted efficiently and almost full conversion was reached to give 86% yield of 2a after 2 h, while the recovered benzoxazole was 82% deuterated at the C2 position (see Scheme 7a). Treatment of benzoxazole with LiOtBu alone resulted in a comparable level of deuteration (85% after 2 h). A similar phenomenon was seen with deuteration of 3-cyanobenzothiophene (see Scheme 7b). (b) Parent thiophene and benzothiophene failed to participate in domino coupling; they did not undergo deuteration by LiOtBu and CD3CN (<5%), either. Therefore, the electron-withdrawing groups are important for acidifying C–H bonds of (benzo)thiophenes and furan to allow reversible deprotonation to occur (e.g., 2q–u in Scheme 2). (c) Ag2CO3 complexes ligated by biarylphosphines were reported by others to catalyze deuteration of azoles, indoles and thiophenes using D2O or CH3OD.22 However, Ag3PO4 or Ag2CO3 together with Josiphos L1 only effected very low levels of deuteration in DCM after 2 h (2% and 11% for two heteroarenes). Thus, silver(I) heteroaryl complexes are probably not responsible for the activation and coupling of heteroarenes under our conditions. (d) Palladium(II) complexes were known to activate azoles and nonazoles via several different mechanisms, such as concerted metalation–deprotonation (CMD)23 nonconcerted metalation–deprotonation4a,b and electrophilic CMD,24 depending on the nature of (hetero)arenes, ancillary ligands and conditions (such as bases and solvents). However, the extent of deuteration of the heteroarenes recovered from the two catalytic whole reactions (see Scheme 7) agreed well with those from control reactions with CD3CN and LiOtBu in DCM. Thus, palladium(II) complexes did not play a significant role in activation of heteroarenes under our catalytic conditions. Thus, we concluded that the deuteration results point to a mechanistic scenario in which LiOtBu promoted reversible formation of lithiated heteroarenes.
In a productive catalytic cycle (see Scheme 7c), silver phosphate or carbonate abstracted the halide ion from oxidative-addition complex A to produce cationic alkenyl complex B. It quickly underwent asymmetric olefin insertion to form alkyl palladium species C which, in turn, was intercepted by a lithio heteroarene. Final C–C reductive elimination of species D completed the catalytic cycle of domino coupling. The lithiation, we believe, may the rate-limiting step, at least in reactions of furans, thiophenes and benzothiophenes. When complex C was not trapped by an organometallic reagent, it was sidetracked to bicyclization forming side product 2a′. The Heck bicyclization forming 2a′ also eliminated PdH species, which can undergo ligand exchange with alkyl palladium complex C and subsequent C–H reductive elimination to form 2a′′ (see Scheme 7c below).
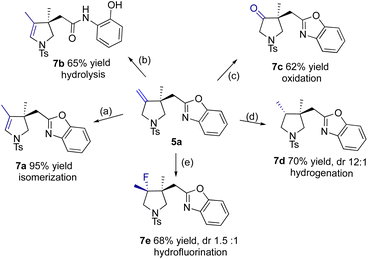
As a showcase of synthetic utility, product 5a was readily converted to other compounds via transformations of its olefinic group (see Scheme 8). (a) Stirring 5a with Co2(CO)8 in refluxing xylene led to olefin isomerization to a more stable isomer, 2-pyrroline 7avia an allyl cobalt hydride species.25 (b) Treatment with in situ formed KPPh2, however, resulted in olefinic isomerization with ring opening of benzoxazole (7b).26 (c) Moreover, RuCl3-catalyzed oxidative cleavage using NaIO4 (ref. 27) provided ketone 7c under mild conditions. (d) Catalytic hydrogenation over Pd/C occurred facioselectively to give 7d in a dr of 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, using the benzoxazole ring as a directing group. The configuration of cis-3,4-dimethyl pyrrolidine was established with NOESY analysis. (e) Iron-catalyzed radical hydrofluorination of 5a under Boger's conditions28 produced a regioselectively Markovnikov adducts 7e (as two distereomers in a ratio of 1.5
1, using the benzoxazole ring as a directing group. The configuration of cis-3,4-dimethyl pyrrolidine was established with NOESY analysis. (e) Iron-catalyzed radical hydrofluorination of 5a under Boger's conditions28 produced a regioselectively Markovnikov adducts 7e (as two distereomers in a ratio of 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
In conclusion, we report enantioselective domino alkenylation–heteroarylation reactions in excellent enantioselectivity. The reactions produced 5–7 membered azacycles (pyrrolidine, piperidine and tetrahydroazepine) containing new quaternary stereocenters, which are not easily accessible from other methods. We found that many types of heteroarenes, both azoles and nonazoles, are suitable substrates, including (benzo)thiazole, (benzo)oxazole, imidazole, oxadiazole, (benzo)thiophene, furan and azine N-oxides. Mechanistically, deuteration experiments indicated that both azoles and nonazoles were activated by regioselective, reversible lithiation by LiOtBu. This mechanism is distinct from nCDM previously reported by Zhu et al. in catalytic arylation–heteroarylation.
Data availability
Experimental and NMR data (both in pdf) have been included.Author contributions
ZM conducted all catalytic experiments and compound characterization and LS conducted derivatization reactions described in Scheme 8. JSZ drafted the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge financial support from the National Natural Science Foundation of China (22271007), Peking University Shenzhen Graduate School, State Key Laboratory of Chemical Oncogenomics, Guangdong Provincial Key Laboratory of Chemical Genomics and Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs for JSZ. Hao Xie at PKUSZ conducted X-ray diffraction and data collection.Notes and references
- (a) H. A. Döndaş, M. d. G. Retamosa and J. M. Sansano, Recent Development in Palladium-Catalyzed Domino Reactions: Access to Materials and Biologically Important Carbo- and Heterocycles, Organometallics, 2019, 38, 1828 CrossRef; (b) Y. Ping, Y. Li, J. Zhu and W. Kong, Construction of Quaternary Stereocenters by Palladium-Catalyzed Carbopalladation-Initiated Cascade Reactions, Angew. Chem., Int. Ed., 2019, 58, 1562 CrossRef CAS PubMed; (c) W. Li and J. Zhang, Recent advances in Pd-catalyzed asymmetric addition reactions, in Adv. Organomet. Chem., ed. P. J. Pérez, Academic Press, 2020, ch. 6, vol. 74, p. 325 Search PubMed; (d) R.-X. Liang and Y.-X. Jia, Aromatic π-Components for Enantioselective Heck Reactions and Heck/Anion-Capture Domino Sequences, Acc. Chem. Res., 2022, 55, 734 CrossRef CAS PubMed; (e) Q. Pan, Y. Ping and W. Kong, Nickel-Catalyzed Ligand-Controlled Selective Reductive Cyclization/Cross-Couplings, Acc. Chem. Res., 2023 DOI:10.1021/acs.accounts.2c00771; (f) H. Pellissier, Recent Developments in Enantioselective Domino Reactions. Part B: First Row Metal Catalysts, Adv. Synth. Catal., 2023 DOI:10.1002/adsc.202300002; (g) H. Pellissier, Recent Developments in Enantioselective Domino Reactions. Part A: Noble Metal Catalysts, Adv. Synth. Catal., 2023 DOI:10.1002/adsc.202201284.
- (a) W. Kong, Q. Wang and J. Zhu, Palladium-Catalyzed Enantioselective Domino Heck/Intermolecular C–H Bond Functionalization: Development and Application to the Synthesis of (+)-Esermethole, J. Am. Chem. Soc., 2015, 137, 16028 CrossRef CAS PubMed; (b) X. Bao, Q. Wang and J. Zhu, Palladium-Catalyzed Enantioselective Narasaka–Heck Reaction/Direct C−H Alkylation of Arenes: Iminoarylation of Alkenes, Angew. Chem., Int. Ed., 2017, 56, 9577 CrossRef CAS PubMed; (c) S. Tong, A. Limouni, Q. Wang, M.-X. Wang and J. Zhu, Catalytic Enantioselective Double Carbopalladation/C−H Functionalization with Statistical Amplification of Product Enantiopurity: A Convertible Linker Approach, Angew. Chem., Int. Ed., 2017, 56, 14192 CrossRef CAS PubMed.
- (a) O. René, D. Lapointe and K. Fagnou, Domino Palladium-Catalyzed Heck-Intermolecular Direct Arylation Reactions, Org. Lett., 2009, 11, 4560 CrossRef PubMed; (b) N. Zeidan, T. Beisel, R. Ross and M. Lautens, Palladium-Catalyzed Arylation/Heteroarylation of Indoles: Access to 2,3-Functionalized Indolines, Org. Lett., 2018, 20, 7332 CrossRef CAS PubMed.
- (a) J. M. Joo, B. B. Touré and D. Sames, C−H Bonds as Ubiquitous Functionality: A General Approach to Complex Arylated Imidazoles via Regioselective Sequential Arylation of All Three C−H Bonds and Regioselective N-Alkylation Enabled by SEM-Group Transposition, J. Org. Chem., 2010, 75, 4911 CrossRef CAS PubMed; (b) V. Gandon and C. Hoarau, Concerted vs Nonconcerted Metalation–Deprotonation in Orthogonal Direct C–H Arylation of Heterocycles with Halides: A Computational Study, J. Org. Chem., 2021, 86, 1769 CrossRef CAS PubMed; (c) S. Teng and J. S. Zhou, Metal-catalyzed asymmetric heteroarylation of alkenes: diverse activation mechanisms, Chem. Soc. Rev., 2022, 51, 1592 RSC.
- (a) X. Chen, J. Zhao, M. Dong, N. Yang, J. Wang, Y. Zhang, K. Liu and X. Tong, Pd(0)-Catalyzed Asymmetric Carbohalogenation: H-Bonding-Driven C(sp3)–Halogen Reductive Elimination under Mild Conditions, J. Am. Chem. Soc., 2021, 143, 1924 CrossRef CAS PubMed; (b) J.-B. Qiao, Y.-Q. Zhang, Q.-W. Yao, Z.-Z. Zhao, X. Peng and X.-Z. Shu, Enantioselective Reductive Divinylation of Unactivated Alkenes by Nickel-Catalyzed Cyclization Coupling Reaction, J. Am. Chem. Soc., 2021, 143, 12961 CrossRef CAS PubMed; (c) L. Qi, M. Dong, J. Qian, S. Yu and X. Tong, Pd0-Catalyzed Asymmetric Carbonitratation Reaction Featuring an H-Bonding-Driven Alkyl−PdII−ONO2 Reductive Elimination, Angew. Chem., Int. Ed., 2023, 62, e202215397 CAS.
- (a) Y. Zhang and E.-i. Negishi, Metal-promoted cyclization. 25. Palladium-catalyzed cascade carbometalation of alkynes and alkenes as an efficient route to cyclic and polycyclic structures, J. Am. Chem. Soc., 1989, 111, 3454 CrossRef CAS; (b) R. Grigg, U. Sakee, V. Sridharan, S. Sukirthalingam and R. Thangavelauthum, Palladium catalysed bis- and tris-cyclisations furnishing fused cyclopropyl carbo/heterocycles, Tetrahedron, 2006, 62, 9523 CrossRef CAS; (c) W. Liu and X. Tong, Pd(0)-Catalyzed Cyclizative Carboboration of n-Iodo-1,6-diene (n = 1 or 2): Access to Structurally Complex Allylboronates and Alkylboronates, Org. Lett., 2019, 21, 9396 CrossRef CAS PubMed.
- (a) Z. Owczarczyk, F. Lamaty, E. J. Vawter and E. Negishi, Apparent endo-mode cyclic carbopalladation with inversion of alkene configuration via exo-mode cyclization-cyclopropanation rearrangement, J. Am. Chem. Soc., 1992, 114, 10091 CrossRef CAS; (b) C.-W. Lee, K. S. Oh, K. S. Kim and K. H. Ahn, Suppressed β-Hydride Elimination in Palladium-Catalyzed Cascade Cyclization−Coupling Reactions: An Efficient Synthesis of 3-Arylmethylpyrrolidines, Org. Lett., 2000, 2, 1213 CrossRef CAS PubMed; (c) K. H. Kim, S. H. Kim, H. J. Lee and J. N. Kim, Palladium-Catalyzed Domino Cyclization (5-exo/3-exo), Ring-Expansion by Palladium Rearrangement, and Aromatization: An Expedient Synthesis of 4-Arylnicotinates from Morita–Baylis–Hillman Adducts, Adv. Synth. Catal., 2013, 355, 1977 CrossRef CAS.
- P. Bhutani, G. Joshi, N. Raja, N. Bachhav, P. K. Rajanna, H. Bhutani, A. T. Paul and R. Kumar, U.S. FDA Approved Drugs from 2015–June 2020: A Perspective, J. Med. Chem., 2021, 64, 2339 CrossRef CAS PubMed.
- (a) M. Aldeghi, S. Malhotra, D. L. Selwood and A. W. E. Chan, Two- and Three-dimensional Rings in Drugs, Chem. Biol. Drug Des., 2014, 83, 450 CrossRef CAS PubMed; (b) R. D. Taylor, M. MacCoss and A. D. G. Lawson, Rings in Drugs, J. Med. Chem., 2014, 57, 5845 CrossRef CAS PubMed; (c) E. Vitaku, D. T. Smith and J. T. Njardarson, Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals, J. Med. Chem., 2014, 57, 10257 CrossRef CAS PubMed.
- D. Ma, S. Yu, B. Li, L. Chen, R. Chen, K. Yu, L. Zhang, Z. Chen, D. Zhong, Z. Gong, R. Wang, H. Jiang and G. Pei, Synthesis and Biological Evaluation of 1,3,3,4-Tetrasubstituted Pyrrolidine CCR5 Receptor Antagonists. Discovery of a Potent and Orally Bioavailable Anti-HIV Agent, ChemMedChem, 2007, 2, 187 CrossRef CAS PubMed.
- G. Liu, W. Fu, X. Mu, T. Wu, M. Nie, K. Li, X. Xu and W. Tang, Pyrrolidines and piperidines bearing chiral tertiary alcohols by nickel-catalyzed enantioselective reductive cyclization of N-alkynones, Commun. Chem., 2018, 1, 90 CrossRef.
- (a) H.-U. Blaser, W. Brieden, B. Pugin, F. Spindler, M. Studer and A. Togni, Solvias Josiphos Ligands: From Discovery to Technical Applications, Top. Catal., 2002, 19, 3 CrossRef CAS; (b) H. U. Blaser, B. Pugin, F. Spindler, E. Mejía and A. Togni, Josiphos Ligands: From Discovery to Technical Applications, in Privileged Chiral Ligands and Catalysts, ed. Q.-L. Zhou, Wiley-VCH, Weinheim, 2011 Search PubMed.
- J. S. Zhou, Phosphorus Ligands, in Comprehensive Coordination Chemistry III, ed. E. C. Constable, G. Parkin and L. Que Jr, Elsevier, Oxford, 2021, p. 32 Search PubMed.
- In a preliminary antineoplastic study, compound 2a showed an IC50 value of 0.1 μM against HeLa cells.
- (a) The Mizoroki–Heck Reaction, ed. M. Oestreich, Wiley, New York, 2009 Search PubMed; (b) J. Hu, Y. Lu, Y. Li and J. Zhou, Highly Active Catalysts of Bisphosphine Oxides for Asymmetric Heck Reaction, Chem. Commun., 2013, 49, 9425 RSC; (c) C. Wu and J. Zhou, Asymmetric Intermolecular Heck Reaction of Aryl Halides, J. Am. Chem. Soc., 2014, 136, 650 CrossRef CAS PubMed; (d) S. Teng and J. S. Zhou, C–C Bond Formation Through Heck-Like Reactions, in Comprehensive Organometallic Chemistry IV, ed. G. Parkin, K. Meyer and D. O'hare, Elsevier, Oxford, 2022, p. 2 Search PubMed.
- (a) G. W. Rewcastle and A. R. Katritzky, Generation and Reactions of sp2-Carbanionic Centers in the Vicinity of Heterocyclic Nitrogen Atoms, in Adv. Heterocycl. Chem., ed. A. R. Katritzky, Academic Press, 1993, vol. 56, p. 155 Search PubMed; (b) E. Crowe, F. Hossner and M. J. Hughes, 2-Metallated Oxazoles; pKa Dependent Deuterations, NMR Studies and Palladium Catalysed Coupling, Tetrahedron, 1995, 51, 8889 CrossRef CAS; (c) C. Hilf, F. Bosold, K. Harms, M. Marsch and G. Boche, The Equilibrium Between 2-Lithium-Oxazole(-Thiazole, -Imidazole) Derivatives and Their Acyclic Isomers – A Structural Investigation, Chem. Ber., 1997, 130, 1213 CrossRef CAS.
- (a) C. Lei, X. Jin and J. Zhou, Palladium-Catalyzed Heteroarylation and Concomitant ortho-Alkylation of Aryl Iodides, Angew. Chem., Int. Ed., 2015, 54, 13397 CrossRef CAS PubMed; (b) X. Wu, C. Lei, G. Yue and J. Zhou, Palladium-Catalyzed Direct Cyclopropylation of Heterocycles, Angew. Chem., Int. Ed., 2015, 54, 9601 CrossRef CAS PubMed.
- G.-F. Zha, K. P. Rakesh, H. M. Manukumar, C. S. Shantharam and S. Long, Pharmaceutical significance of azepane based motifs for drug discovery: a critical review, Eur. J. Med. Chem., 2019, 162, 465 CrossRef CAS PubMed.
- (a) S. J. Lee and P. Beak, Asymmetric Synthesis of 4,5,6- and 3,4,5,6-Substituted Azepanes by a Highly Diastereoselective and Enantioselective Lithiation−Conjugate Addition Sequence, J. Am. Chem. Soc., 2006, 128, 2178 CrossRef CAS PubMed; (b) J.-J. Feng, T.-Y. Lin, C.-Z. Zhu, H. Wang, H.-H. Wu and J. Zhang, The Divergent Synthesis of Nitrogen Heterocycles by Rhodium(I)-Catalyzed Intermolecular Cycloadditions of Vinyl Aziridines and Alkynes, J. Am. Chem. Soc., 2016, 138, 2178 CrossRef CAS PubMed; (c) C.-Z. Zhu, J.-J. Feng and J. Zhang, Rhodium(I)-Catalyzed Intermolecular Aza-[4+3] Cycloaddition of Vinyl Aziridines and Dienes: Atom-Economical Synthesis of Enantiomerically Enriched Functionalized Azepines, Angew. Chem., Int. Ed., 2017, 56, 1351 CrossRef CAS PubMed; (d) G.-W. Wang and J. F. Bower, Modular Access to Azepines by Directed Carbonylative C–C Bond Activation of Aminocyclopropanes, J. Am. Chem. Soc., 2018, 140, 2743 CrossRef CAS PubMed; (e) W. Zawodny, S. L. Montgomery, J. R. Marshall, J. D. Finnigan, N. J. Turner and J. Clayden, Chemoenzymatic Synthesis of Substituted Azepanes by Sequential Biocatalytic Reduction and Organolithium-Mediated Rearrangement, J. Am. Chem. Soc., 2018, 140, 17872 CrossRef CAS PubMed; (f) T. Yang, X. Guo, Q. Yin and X. Zhang, Intramolecular asymmetric reductive amination: synthesis of enantioenriched dibenz[c,e]azepines, Chem. Sci., 2019, 10, 2473 RSC; (g) Y. Mao, Y. Gao and Z. Miao, Research Progress on the Asymmetric Cyclization Synthesis of Seven-Membered Rings via Transition Metal Catalysis, Chin. J. Org. Chem., 2022, 42, 1904 CrossRef; (h) M. Dong, L. Qi, J. Qian, S. Yu and X. Tong, Pd(0)-Catalyzed Asymmetric 7-Endo Hydroacyloxylative Cyclization of 1,6-Enyne Enabled by an Anion Ligand-Directed Strategy, J. Am. Chem. Soc., 2023, 145(3), 1973 CrossRef CAS PubMed.
- (a) J. K. Ray, S. Paul, P. Ray, R. Singha, D. Y. Rao, S. Nandi and A. Anoop, Pd-catalyzed intramolecular sequential Heck cyclization and oxidation reactions: a facile pathway for the synthesis of substituted cycloheptenone evaluated using computational studies, New J. Chem., 2017, 41, 278 RSC; (b) H. Hu, Y. Peng, T. Yu, S. Cheng, S. Luo and Q. Zhu, Palladium-Catalyzed Enantioselective 7-exo-Trig Carbopalladation/Carbonylation: Cascade Reactions To Achieve Atropisomeric Dibenzo[b,d]azepin-6-ones, Org. Lett., 2021, 23, 3636 CrossRef CAS PubMed.
- M. D. Delost, D. T. Smith, B. J. Anderson and J. T. Njardarson, From Oxiranes to Oligomers: Architectures of U.S. FDA Approved Pharmaceuticals Containing Oxygen Heterocycles, J. Med. Chem., 2018, 61, 10996 CrossRef CAS PubMed.
- (a) E.-C. Li, G.-Q. Hu, Y.-X. Zhu, H.-H. Zhang, K. Shen, X.-C. Hang, C. Zhang and W. Huang, Ag2CO3-Catalyzed H/D Exchange of Five-Membered Heteroarenes at Ambient Temperature, Org. Lett., 2019, 21, 6745 CrossRef CAS PubMed; (b) A. Tlahuext-Aca and J. F. Hartwig, Site-Selective Silver-Catalyzed C–H Bond Deuteration of Five-Membered Aromatic Heterocycles and Pharmaceuticals, ACS Catal., 2021, 11, 1119 CrossRef CAS PubMed.
- (a) S. I. Gorelsky, D. Lapointe and K. Fagnou, Analysis of the Concerted Metalation-Deprotonation Mechanism in Palladium-Catalyzed Direct Arylation Across a Broad Range of Aromatic Substrates, J. Am. Chem. Soc., 2008, 130, 10848 CrossRef CAS PubMed; (b) D. Lapointe and K. Fagnou, Overview of the Mechanistic Work on the Concerted Metallation–Deprotonation Pathway, Chem. Lett., 2010, 39, 1118 CrossRef; (c) N. A. Strotman, H. R. Chobanian, Y. Guo, J. He and J. E. Wilson, Highly Regioselective Palladium-Catalyzed Direct Arylation of Oxazole at C-2 or C-5 with Aryl Bromides, Chlorides, and Triflates, Org. Lett., 2010, 12, 3578 CrossRef CAS PubMed; (d) D. L. Davies, S. A. Macgregor and C. L. McMullin, Computational Studies of Carboxylate-Assisted C–H Activation and Functionalization at Group 8–10 Transition Metal Centers, Chem. Rev., 2017, 117, 8649 CrossRef CAS PubMed.
- (a) L. Wang and B. P. Carrow, Oligothiophene Synthesis by a General C–H Activation Mechanism: Electrophilic Concerted Metalation–Deprotonation (eCMD), ACS Catal., 2019, 9, 6821 CrossRef CAS PubMed; (b) B. P. Carrow, J. Sampson and L. Wang, Base-Assisted C−H Bond Cleavage in Cross-Coupling: Recent Insights into Mechanism, Speciation, and Cooperativity, Isr. J. Chem., 2020, 60, 230 CrossRef CAS PubMed.
- L. V. R. Bonaga and M. Krafft, When the Pauson-Khand and Pauson-Khand type reactions go awry: a plethora of unexpected results, Tetrahedron, 2004, 60, 9795 CrossRef CAS.
- C. Li, Y. Huang, S. Cao, Y. Luo, Y. Zhang and G. Yang, A robust and facile method for desulfonation to amines, Org. Chem. Front., 2021, 8, 6182 RSC.
- (a) D. Yang and C. Zhang, Ruthenium-Catalyzed Oxidative Cleavage of Olefins to Aldehydes, J. Org. Chem., 2001, 66, 4814 CrossRef CAS PubMed; (b) D. F. Fernández, M. Gulías, J. L. Mascareñas and F. López, Iridium(I)-Catalyzed Intramolecular Hydrocarbonation of Alkenes: Efficient Access to Cyclic Systems Bearing Quaternary Stereocenters, Angew. Chem., Int. Ed., 2017, 56, 9541 CrossRef PubMed.
- T. J. Barker and D. L. Boger, Fe(III)/NaBH4-Mediated Free Radical Hydrofluorination of Unactivated Alkenes, J. Am. Chem. Soc., 2012, 134, 13588 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, characterization of isolated products and CIF files of crystallographic data. CCDC 2152684 (5d). For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2sc07117g |
| This journal is © The Royal Society of Chemistry 2023 |