Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Zn-induced electron-rich Sn catalysts enable highly efficient CO2 electroreduction to formate†
Xingxing
Tan
ab,
Shunhan
Jia
 ab,
Xinning
Song
ab,
Xinning
Song
 ab,
Xiaodong
Ma
ab,
Jiaqi
Feng
a,
Libing
Zhang
ab,
Limin
Wu
ab,
Juan
Du
c,
Aibing
Chen
ab,
Xiaodong
Ma
ab,
Jiaqi
Feng
a,
Libing
Zhang
ab,
Limin
Wu
ab,
Juan
Du
c,
Aibing
Chen
 c,
Qinggong
Zhu
c,
Qinggong
Zhu
 ab,
Xiaofu
Sun
ab,
Xiaofu
Sun
 *ab and
Buxing
Han
*ab and
Buxing
Han
 *abd
*abd
aBeijing National Laboratory for Molecular Sciences, Key Laboratory of Colloid and Interface and Thermodynamics, Center for Carbon Neutral Chemistry, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, P. R. China. E-mail: sunxiaofu@iccas.ac.cn; hanbx@iccas.ac.cn
bSchool of Chemical Sciences, University of Chinese Academy of Sciences, Beijing 100049, P. R. China
cCollege of Chemical and Pharmaceutical Engineering, Hebei University of Science and Technology, Shijiazhuang 050018, P. R. China
dShanghai Key Laboratory of Green Chemistry and Chemical Processes, School of Chemistry and Molecular Engineering, East China Normal University, Shanghai 200062, P. R. China
First published on 10th July 2023
Abstract
Renewable-energy-driven CO2 electroreduction provides a promising way to address the growing greenhouse effect issue and produce value-added chemicals. As one of the bulk chemicals, formic acid/formate has the highest revenue per mole of electrons among various products. However, the scaling up of CO2-to-formate for practical applications with high faradaic efficiency (FE) and current density is constrained by the difficulty of precisely reconciling the competing intermediates (*COOH and HCOO*). Herein, a Zn-induced electron-rich Sn electrocatalyst was reported for CO2-to-formate with high efficiency. The faradaic efficiency of formate (FEformate) could reach 96.6%, and FEformate > 90% was maintained at formate partial current density up to 625.4 mA cm−1. Detailed study indicated that catalyst reconstruction occurred during electrolysis. With appropriate electron accumulation, the electron-rich Sn catalyst could facilitate the adsorption and activation of CO2 molecules to form a 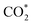 intermediate and then promoted the carbon protonation of
intermediate and then promoted the carbon protonation of 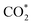 to yield a HCOO* intermediate. Afterwards, the HCOO* → HCOOH* proceeded via another proton-coupled electron transfer process, leading to high activity and selectivity for formate production.
to yield a HCOO* intermediate. Afterwards, the HCOO* → HCOOH* proceeded via another proton-coupled electron transfer process, leading to high activity and selectivity for formate production.
Introduction
The electrochemical CO2 reduction reaction (eCO2RR) to value-added chemicals and fuels utilizing renewable electricity offers a sustainable route to offset the extra carbon footprint.1–3 However, this reaction is highly energetic and unfavorable, and a thermodynamic potential of −1.90 V vs. the standard hydrogen electrode (SHE) is needed to activate CO2 to *CO2−.4 Due to the competing hydrogen evolution reaction (HER) and the similarity of the redox potentials (from −0.2 to 0.6 V vs. SHE) for all the subsequent proton-assisted processes,5,6 eCO2RR pathways generally result in a mixture of products. Different studies have aimed to understand the fundamental factors that control the product selectivity, including optimizing catalytic conditions and developing novel catalysts.7–12 The adsorption behavior of key intermediates is strongly dependent on the geometric and electronic structure of the catalyst surface.3,13–16 Although some breakthroughs have been made in improving the selectivity for a desired product, it is still in the initial stage of meeting the demands of scaling up the eCO2RR for practical applications with high faradaic efficiency (FE) and current density.Among various CO2-derived products, formic acid/formate presents the highest revenue per mole of electrons.17,18 Formic acid is a commonly used feedstock in the pharmaceutical and chemical industries.17 In addition, with its impressive energy density and convenient transportation, formic acid is also extensively studied as a promising hydrogen carrier for fuel cells.19,20 In the reaction pathway of the eCO2RR to formate, activated CO2 undergoes a proton-coupled electron transfer (PCET) process to give the HCOO* intermediate and then experiences another transfer to reduce HCOO* to HCOO−.21 This combination of processes is generally related to the intrinsic properties of the catalyst. Sn is a promising candidate toward formic acid/formate because of its favorable binding energy for HCOO*.22,23 However, Sn also shows a certain binding energy to *COOH, resulting in the generation of a CO by-product.24 A promising approach to direct the eCO2RR over Sn to the HCOO* pathway is to introduce metallic heteroatom doping to construct Sn-based catalysts, which can manipulate the electronic structure of the catalysts to facilitate both the formation and stabilization of the HCOO* intermediate.25–27 Notably, Sn-based catalysts may undergo structural evolution during the electrochemical process, and then the actual active sites will be created to trigger an efficient catalytic reaction. Therefore, it is significant to reveal the structural evolution of Sn-based catalysts and reveal active sites to achieve efficient CO2 reduction.22
Herein, we have constructed a Sn–Zn electrocatalyst (Sn–Zn–Ox) for the eCO2RR to formate. It exhibited a maximum faradaic efficiency for formate (FEformate) of 96.6% and >90% FEformate was maintained with a partial current density of formate (jformate) up to 625.4 mA cm−1. Experimental and density functional theory (DFT) calculations revealed that the reconstructed Sn sites could facilitate the adsorption and activation of CO2 molecules to form a 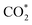 intermediate and then promoted the carbon protonation of
intermediate and then promoted the carbon protonation of 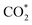 to intermediate HCOO*. Successively, HCOO* absorbed on Sn–Zn–Ox enabled H* to adsorb and react with it more accessibly, which could lower the thermodynamic barrier in the second PCET process for the formation of formate.
to intermediate HCOO*. Successively, HCOO* absorbed on Sn–Zn–Ox enabled H* to adsorb and react with it more accessibly, which could lower the thermodynamic barrier in the second PCET process for the formation of formate.
Results and discussion
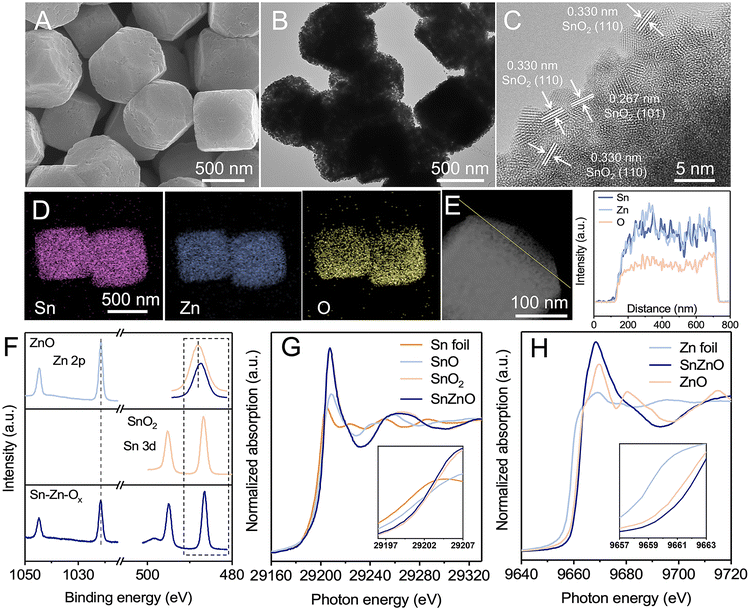
The Sn–Zn–Ox nanocomposites were synthesized using a facile coprecipitation method followed by pyrolyzing at 500 °C for 2 h in an argon atmosphere. The Sn/Zn atomic ratio of the obtained catalysts was 0.85, which was determined by inductively coupled plasma optical emission spectrometry (ICP-OES). Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) images show that Sn–Zn–Ox composites displayed a uniform truncated cubic morphology with edge lengths of about 500 nm (Fig. 1A, B, S1 and S2†). The truncated cube was composed of smaller nanoparticles and was rich in mesopores with a massive pore volume of 6.3 nm, which favored the exposure of more active sites during the eCO2RR. Only a broad diffraction peak can be observed for Sn–Zn–Ox in the X-ray diffraction (XRD) patterns (Fig. S3†), perhaps due to the small size of the granules. The high-resolution TEM (HRTEM) image shows a lattice spacing of 0.267 nm and 0.330 nm, corresponding to the (110) and (101) planes of SnO2,28 respectively (Fig. 1C). The energy dispersive X-ray (EDX) elemental mapping and line-scan analysis confirmed that Sn, Zn, and O elements were distributed uniformly over the entire architectures (Fig. 1D and E). Using the same method, we also synthesized ZnO and SnO2 for comparison. Their SEM and TEM images are shown in Fig. S4 and S5.†X-ray photoelectron spectroscopy (XPS) and X-ray absorption spectroscopy (XAS) were then performed to reveal the composition and structural information of Sn–Zn–Ox. As illustrated in Fig. 1F, the Zn 2p3/2 and Zn 2p1/2 peaks of Sn–Zn–Ox at 1021.63 eV and 1044.79 eV are slightly higher than those of Zn2+ in ZnO. The Sn 3d5/2 and Sn 3d3/2 peaks of Sn–Zn–Ox, located at 486.52 eV and 494.97 eV, shifted to a lower binding energy by about 0.21 eV compared with SnO2. The opposite shifts for Zn 2p and Sn 3d orbital peaks indicate the interaction between Zn and Sn, resulting in a modified electronic structure.29 O 1s spectra were also recorded and are shown in Fig. S6.† The peaks at 530 and 531.7 eV can be assigned to the lattice oxygen and oxygen vacancies, respectively.30 Sn–Zn–Ox showed a lower binding energy and an enlarged peak area of oxygen vacancies compared with ZnO and SnO2. The increased defect degree could improve eCO2RR activity.31 The X-ray absorption near-edge structure (XANES) spectra of Sn K-edge and Zn K-edge were obtained and are shown in Fig. 1G and H. The Sn absorption edge of Sn–Zn–Ox was analogous to the curve of SnO2, while a slight negative shift of the absorption edge position compared to SnO2 indicates a lower oxidation state of Sn in Sn–Zn–Ox. Meanwhile, the Zn absorption-edge showed an opposite shift compared with ZnO, which revealed the electron transfer from Zn to Sn in Sn–Zn–Ox.32 These results are in agreement with the XPS data.
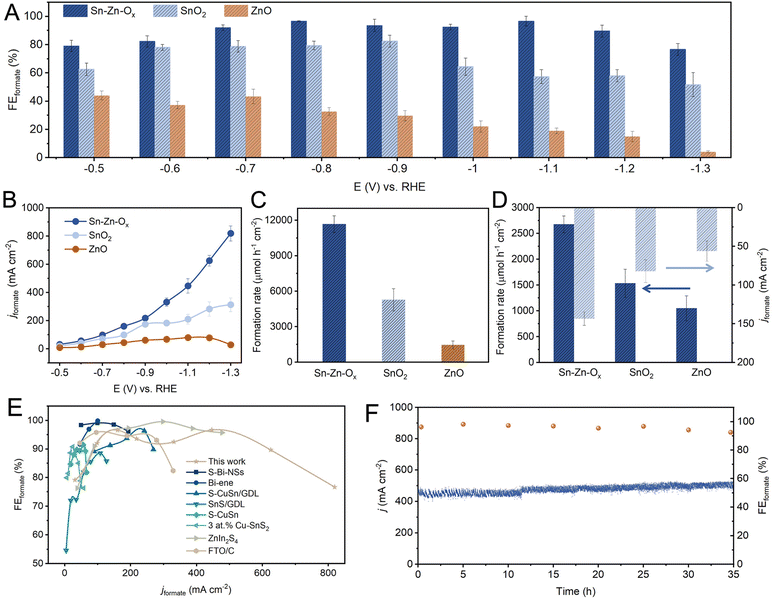
The eCO2RR performances were investigated in a flow cell using 1 M KOH as electrolyte. The gaseous and liquid products were analyzed by gas chromatography (GC) and 1H nuclear magnetic resonance (NMR) spectroscopy, respectively. Formate was the only liquid product and its FEs at different potentials are shown in Fig. 2A. The FEformate could be maintained above 90% over Sn–Zn–Ox in a wide potential window of −0.7 to −1.2 V vs. the reversible hydrogen electrode (RHE), and a maximum FEformate of 96.6% can be achieved at −0.8 V vs. RHE. However, the maximum FEformate of the as-synthesized SnO2 and ZnO was only 79.4% and 43.4%, and H2 and CO were detected as the by-products (Fig. S7–S11†).
The partial current density of formate was plotted and is shown in Fig. 2B. A high jformate of 625.4 mA cm−1 was achieved over Sn–Zn–Ox at −1.2 V vs. RHE with a high FEformate above 90%, which is much higher than that of SnO2 and ZnO. When jformate increased to 819.6 mA cm−1, the FEformate still maintained above 76%. Moreover, the Sn–Zn–Ox catalyst exhibited a high formation rate of formate of 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 667.4 μmol h−1 cm−2 at −1.2 V vs. RHE, which was 2.2 and 8.0 times higher than that of SnO2 and ZnO, respectively (Fig. 2C). The electrochemically active surface area (ECSA) was further assessed according to the double-layer capacitance (Cdl) (Fig. S12 and S13†). As shown in Fig. 2D, the ECSA-normalized jformate and formate formation rates were calculated and the value over the Sn–Zn–Ox catalyst was still the highest, indicating its high intrinsic activity. The attained activity of CO2-to-formate can be competitive with those of the best catalysts reported, as the high FEformate and large jformate were both available over Sn–Zn–Ox (Fig. 2E). In addition, an average FEformate of >90% was maintained during continuous electrolysis for 35 h at −1.1 V vs. RHE, demonstrating the long-term stability of the Sn–Zn–Ox catalyst (Fig. 2F).
667.4 μmol h−1 cm−2 at −1.2 V vs. RHE, which was 2.2 and 8.0 times higher than that of SnO2 and ZnO, respectively (Fig. 2C). The electrochemically active surface area (ECSA) was further assessed according to the double-layer capacitance (Cdl) (Fig. S12 and S13†). As shown in Fig. 2D, the ECSA-normalized jformate and formate formation rates were calculated and the value over the Sn–Zn–Ox catalyst was still the highest, indicating its high intrinsic activity. The attained activity of CO2-to-formate can be competitive with those of the best catalysts reported, as the high FEformate and large jformate were both available over Sn–Zn–Ox (Fig. 2E). In addition, an average FEformate of >90% was maintained during continuous electrolysis for 35 h at −1.1 V vs. RHE, demonstrating the long-term stability of the Sn–Zn–Ox catalyst (Fig. 2F).
To directly relate the enhanced selectivity of formate to the influence of the Zn component in the Sn–Zn–Ox catalyst, Zn(II) species was selectively removed from Sn–Zn–Ox by the acid-washing method and used for comparison. After the acid-washing process for 1 h, the Sn/Zn atomic ratio was increased to 4.35. The truncated cubic morphology was still maintained without obvious structural collapse, and Sn, Zn, and O elements were dispersed evenly in the sample (Fig. S14†). However, the sample after removal of Zn species showed much lower CO2-to-formate performance than Sn–Zn–Ox (Fig. S15–S17†), indicating the critical role of Zn species in the Sn–Zn–Ox catalyst.
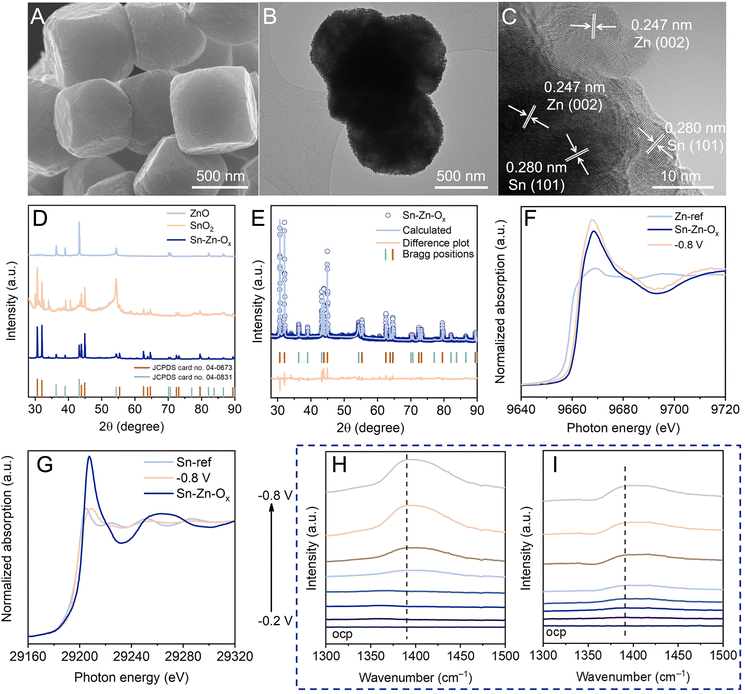
The structural evolution during the eCO2RR was investigated to gain insight into eCO2RR enhancement. As revealed by SEM and TEM images (Fig. 3A and B), the catalyst maintained the truncated cubic morphology without obvious structural collapse. Sn, Zn, and O elements still existed and were dispersed evenly in Sn–Zn–Ox after the eCO2RR (Fig. S18†). The HRTEM image displays clear lattice spacings of Sn(101) and Zn(002) planes,27,33 indicating the reduction of Sn–Zn–Ox during the eCO2RR (Fig. 3C). The diffraction peaks of Sn (JCPDS 04-0673) and Zn (JCPDS 04-0831) could be detected from quasi-in situ XRD measurement (Fig. 3D). According to the Rietveld refinement analysis of the XRD data, the contents of Sn (JCPDS 04-0673) and Zn (JCPDS 04-0831) were estimated to be 85.28% and 14.72% in Sn–Zn–Ox after the eCO2RR (Fig. 3E). From this apparent difference in the content of the two phases, it could be speculated that oxidized Sn exhibited a greater reduction degree than oxidized Zn. Further investigation of the structural evolution was conducted by in situ XANES to eliminate interference from air oxidation (Fig. S19†). At the applied potential, the Zn K-edge was shifted to lower energy located between that of the Zn foil (Zn0) reference and Sn–Zn–Ox (Fig. 3F). After the eCO2RR, similar features to Sn were detected, where a lower-energy shift of the Sn absorption edge was observed in Sn–Zn–Ox (Fig. 3G), implying a slightly lower valence state of Sn in Sn–Zn–Ox compared to Sn foil.27,34 According to the above results, the Sn oxides in Sn–Zn–Ox was reduced to metallic Sn during eCO2RR. However, the change in the oxidation state of Zn was relatively small, resulting in more electron accumulation on Sn. It contributes to CO2 activation and HCOO* intermediate adsorption, leading to enhanced eCO2RR performance.
In situ ATR-SEIRAS measurements were performed to monitor possible reaction intermediates. According to Fig. 3H and I, the IR band at 1390 cm−1 associated with O–C–O vibration in the bidentate HCOO* intermediate was monitored,35,36 and its intensities increased with the increasing potential. This is in agreement with the trend in formate formation rates. Moreover, the band intensity of HCOO* over Sn–Zn–Ox was stronger than that over SnO2. This phenomenon is consistent with the results of CO2-to-formate performance, implying that the HCOO* intermediate was the main factor in the generation of formate.37 The sharp contrast suggested that the introduction of Zn played an important role in promoting the HCOO* intermediate production.35
The dissociation of H2O in an alkaline environment is a sluggish step, which can be detrimental to the PECT processes during the eCO2RR to formate. Therefore, a catalyst with optimal water dissociation is required to ensure the proton-feeding rate in the eCO2RR to formate. As shown in Fig. S20,† a negative IR band at 1630 cm−1 ascribed to adsorbed H2O was detected.38 The band intensity of Sn–Zn–Ox was stronger than that of SnO2, indicating that the introduction of Zn could accelerate the activation of H2O. As the cathodic potential was applied, H2O molecules underwent activation to yield protons for the further protonation of *CO2 to form the HCOO* intermediate, which was confirmed using a stronger IR band for the HCOO* intermediate. These results illustrated that Sn–Zn–Ox favored the formation and stabilization of the HCOO* intermediate, which contributed to the enhanced eCO2RR performance.
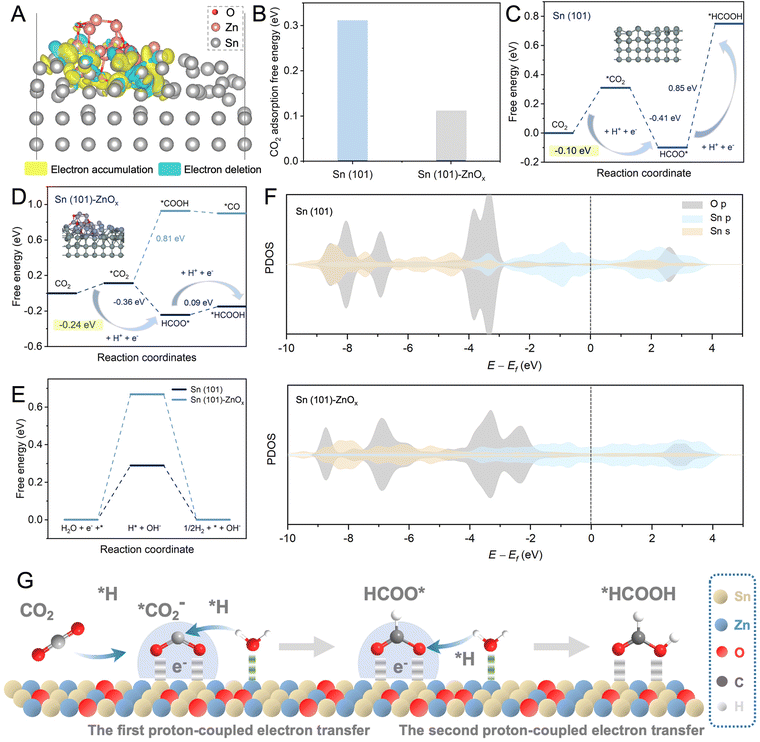
In addition, DFT calculations were performed to elucidate the mechanism for enhanced activity and selectivity of the eCO2RR. According to the catalyst characterization data and structural optimization, Sn(101) and Sn(101)–ZnOx models were constructed to represent SnO2 and Sn–Zn–Ox, respectively. The detailed data about the computational structure models and relevant parameters are shown in Fig. S21–S24.† The electronic structure and interactions of Sn(101)–ZnOx were investigated using the calculated charge density distribution. As shown in Fig. 4A, the charge density was depleted around Zn atoms and accumulated around Sn atoms, revealing the electron transfer from Zn atoms to Sn atoms and resulting in electron-rich Sn atoms. CO2 binding capability is a prerequisite for the eCO2RR. As shown in Fig. 4B, the CO2 adsorption free energy on Sn(101)–ZnOx was much lower than that on Sn(101), which was in agreement with the results of the CO2 adsorption isotherms in Fig. S25.† The above results indicate preferable CO2 adsorption on electron-rich Sn in the Sn–Zn–Ox catalyst.
Fig. 4C displays the Gibbs free energy profiles for the pathway of the eCO2RR to formate on Sn(101). As the first step, the CO2 activation process (CO2 → *CO2) is essential for the formation of the key intermediate HCOO* in the eCO2RR to formate.39,40 The formation of *CO2 on Sn (101) was endergonic, and the high free energy of *CO2 formation (0.31 eV) was not conducive to HCOO* generation. By contrast, Sn(101)–ZnOx showed a lower energy barrier (0.11 eV) for *CO2 formation (Fig. 4D), which was favorable for the subsequent hydrogenation reaction to form the HCOO* intermediate. This makes the free-energy step involved in the first PECT toward formate formation more thermodynamically accessible for Sn(101)–ZnOx. The process of HCOO* undergoing the second PECT to form *HCOOH was the rate-determining step (RDS) for the HCOOH pathway on Sn(101). The Gibbs free energy for this RDS was found to be up to 0.85 eV. Sn(101)–ZnOx could effectively reduce the free energy of *HCOOH formation to 0.09 eV and convert the RDS into *CO2 → HCOO*. The changed RDS pathway led to a decrease in the energy barrier for HCOOH formation on Sn(101)–ZnOx. These results indicate that the Sn–Zn–Ox catalyst with electron-rich Sn enabled a promotion in the formation of formate compared to SnO2. Furthermore, Sn(101)–ZnOx presented a significantly higher free energy barrier for *COOH formation than for HCOO* formation, suggesting that the HCOOH pathway was more thermodynamically favorable than the CO pathway.41 This clarified the high selectivity of Sn(101)–ZnOx toward formate formation. In addition, Sn(101)–ZnOx showed a higher energy barrier for the generation of *H intermediates compared to Sn(101) (Fig. 4E), indicating that the HER was inhibited on Sn(101)–ZnOx.
To further elucidate the promoting effect of Sn(101)–ZnOx, the projected density of states (PDOS) was analyzed to explore the interaction between the O atoms in key intermediate HCOO* and the Sn atoms on catalyst models. As illustrated in Fig. 4F, Sn(101)–ZnOx shows more harmonic p–p and p–s overlaps between the O 2p and Sn 5s and 5p orbitals than Sn(101), indicating the enhancement of interactions between the active site and HCOO* intermediate after the introduction of Zn.22 In addition, the upshift of the O 2p orbital away from the Fermi level (Ef) suggests an increased antibonding state of the O atom in absorbed HCOO* on Sn(101)–ZnOx compared to that on Sn(101).42,43 This means that HCOO* absorbed on Sn(101)–ZnOx enables H* to adsorb and react with it more accessibly, leading to a decline in the Gibbs free energies of the PECT process for the formation of HCOOH. Based on the discussion above, the catalytic mechanism of Sn–Zn–Ox for the eCO2RR was outlined and is shown in Fig. 4G. First, electron-rich Sn could promote the adsorption and activation of CO2 molecules to generate *CO2. Meanwhile, the positive-valence Zn sites were more likely to drag the O atom in the absorbed H2O, which might promote the combination of H* and carbonaceous intermediates in the PCET process. Then, the lower energy barriers for the formation of HCOO* and *HCOOH are conducive to *CO2 → HCOO* → *HCOOH proceeding rapidly. Moreover, electron-rich Sn electrocatalysts induced by Zn species in Sn–Zn–Ox might suppress H2 evolution. As a consequence, the rationally constructed electron-rich Sn catalyst achieved high catalytic activity and excellent selectivity for the eCO2RR to formate.
Conclusions
In summary, Sn–Zn–Ox has been successfully prepared and used as an efficient electrocatalyst for CO2-to-formate. The highest FEformate of 96.6% could be achieved and it can maintain a high FEformate above 90% at jformate up to 625.4 mA cm−1. The in situ experimental results demonstrated the structural evolution of the catalysts and their significant role in improving the eCO2RR-to formate performance. The accumulation of electron density around Sn facilitates the activation of CO2 molecules to form a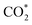 intermediate, which is conducive to the formation HCOO* species. Moreover, Sn–Zn–Ox can modulate the adsorption configuration of HCOO* by increasing the antibonding state of the O atom in absorbed HCOO*, thereby lowering the energy barrier for the PECT for HCOO* → HCOOH* and facilitating CO2-to-formate conversion. This work offers an effective strategy that coupled electronic structure manipulation and intermediate optimization for CO2 electroreduction to formate.
intermediate, which is conducive to the formation HCOO* species. Moreover, Sn–Zn–Ox can modulate the adsorption configuration of HCOO* by increasing the antibonding state of the O atom in absorbed HCOO*, thereby lowering the energy barrier for the PECT for HCOO* → HCOOH* and facilitating CO2-to-formate conversion. This work offers an effective strategy that coupled electronic structure manipulation and intermediate optimization for CO2 electroreduction to formate.
Data availability
All experimental data is available in the ESI.†Author contributions
X. X. T. performed all the experiments. S. H. J., X. N. S., X. D. M., J. Q. F, L. B. Z., and L. M. W. performed the analysis of the experimental data. J. D., A. B. C. and Q. G. Z. participated in discussions. X. F. S. and B. X. H. co-supervised the whole project. All authors discussed the results and commented on the manuscript.Conflicts of interest
The authors declare no competing financial interests.Acknowledgements
The work was supported financially by the Beijing Natural Science Foundation (J210020), National Natural Science Foundation of China (22002172 and 22121002), National Key Research and Development Program of China (2020YFA0710203), Hebei Natural Science Foundation (B2021208074) and Photon Science Center for Carbon Neutrality. The X-ray absorption spectroscopy measurements were performed at Beamline BL14W1 at the Shanghai Synchrotron Radiation Facility (SSRF) and Beamline 1W1B at the Beijing Synchrotron Radiation Facility (BSRF).Notes and references
- T. Zheng, M. Zhang, L. Wu, S. Guo, X. Liu, J. Zhao, W. Xue, J. Li, C. Liu, X. Li, Q. Jiang, J. Bao, J. Zeng, T. Yu and C. Xia, Nat. Catal., 2022, 5, 388–396 CrossRef CAS.
- Y. Xie, P. Ou, X. Wang, Z. Xu, Y. C. Li, Z. Wang, J. E. Huang, J. Wicks, C. McCallum, N. Wang, Y. Wang, T. Chen, B. T. W. Lo, D. Sinton, J. C. Yu, Y. Wang and E. H. Sargent, Nat. Catal., 2022, 5, 564–570 CrossRef CAS.
- J. Timoshenko, A. Bergmann, C. Rettenmaier, A. Herzog, R. M. Arán-Ais, H. S. Jeon, F. T. Haase, U. Hejral, P. Grosse, S. Kühl, E. M. Davis, J. Tian, O. Magnussen and B. Roldan Cuenya, Nat. Catal., 2022, 5, 259–267 CrossRef CAS.
- H. S. Shafaat and J. Y. Yang, Nat. Catal., 2021, 4, 928–933 CrossRef.
- P. Saha, S. Amanullah and A. Dey, Acc. Chem. Res., 2022, 55, 134–144 CrossRef CAS PubMed.
- Y. Wang, J. Liu and G. Zheng, Adv. Mater., 2021, 33, 2005798 CrossRef CAS PubMed.
- R. Shi, J. Guo, X. Zhang, G. I. N. Waterhouse, Z. Han, Y. Zhao, L. Shang, C. Zhou, L. Jiang and T. Zhang, Nat. Commun., 2020, 11, 3028 CrossRef CAS PubMed.
- S. S. A. Shah, T. Najam, M. Wen, S.-Q. Zang, A. Waseem and H.-L. Jiang, Small Struct., 2021, 3, 2100090 CrossRef.
- C. J. Chang, Y. A. Lai, Y. C. Chu, C. K. Peng, H. Y. Tan, C. W. Pao, Y. G. Lin, S. F. Hung, H. C. Chen and H. M. Chen, J. Am. Chem. Soc., 2023, 145, 6953–6965 CrossRef CAS.
- T. L. Soucy, W. S. Dean, J. Zhou, K. E. Rivera Cruz and C. C. L. McCrory, Acc. Chem. Res., 2022, 55, 252–261 CrossRef CAS PubMed.
- Y. Li, Z. Pei, D. Luan and X. W. D. Lou, Angew. Chem., Int. Ed. Engl., 2023, 62, e202302128 CrossRef CAS PubMed.
- X. Tan, W. Guo, S. Liu, S. Jia, L. Xu, J. Feng, X. Yan, C. Chen, Q. Zhu, X. Sun and B. Han, Chem. Sci., 2022, 13, 11918–11925 RSC.
- X. Su, Z. Jiang, J. Zhou, H. Liu, D. Zhou, H. Shang, X. Ni, Z. Peng, F. Yang, W. Chen, Z. Qi, D. Wang and Y. Wang, Nat. Commun., 2022, 13, 1322 CrossRef CAS PubMed.
- S. Kong, X. Lv, X. Wang, Z. Liu, Z. Li, B. Jia, D. Sun, C. Yang, L. Liu, A. Guan, J. Wang, G. Zheng and F. Huang, Nat. Catal., 2022, 6, 6–15 CrossRef.
- S. Liu, X. F. Lu, J. Xiao, X. Wang and X. W. D. Lou, Angew. Chem., Int. Ed., 2019, 58, 13828–13833 CrossRef CAS PubMed.
- Q. Qu, S. Ji, Y. Chen, D. Wang and Y. Li, Chem. Sci., 2021, 12, 4201–4215 RSC.
- P. Zhu and H. Wang, Nat. Catal., 2021, 4, 943–951 CrossRef CAS.
- T. Zheng, C. Liu, C. Guo, M. Zhang, X. Li, Q. Jiang, W. Xue, H. Li, A. Li, C. W. Pao, J. Xiao, C. Xia and J. Zeng, Nat. Nanotechnol., 2021, 16, 1386–1393 CrossRef CAS.
- A. Boddien and H. Junge, Nat. Nanotechnol., 2011, 6, 265–266 CrossRef CAS PubMed.
- S. Chatterjee, I. Dutta, Y. Lum, Z. Lai and K.-W. Huang, Energy Environ. Sci., 2021, 14, 1194–1246 RSC.
- G. Wang, J. Chen, Y. Ding, P. Cai, L. Yi, Y. Li, C. Tu, Y. Hou, Z. Wen and L. Dai, Chem. Soc. Rev., 2021, 50, 4993–5061 RSC.
- W. Wang, Z. Wang, R. Yang, J. Duan, Y. Liu, A. Nie, H. Li, B. Y. Xia and T. Zhai, Angew. Chem., Int. Ed., 2021, 60, 22940–22947 CrossRef CAS PubMed.
- L. Li, A. Ozden, S. Guo, A. d. A. F. P. Garci, C. Wang, M. Zhang, J. Zhang, H. Jiang, W. Wang, H. Dong, D. Sinton, E. H. Sargent and M. Zhong, Nat. Commun., 2021, 12, 5223 CrossRef CAS PubMed.
- Y. Deng, J. Zhao, S. Wang, R. Chen, J. Ding, H. J. Tsai, W. J. Zeng, S. F. Hung, W. Xu, J. Wang, F. Jaouen, X. Li, Y. Huang and B. Liu, J. Am. Chem. Soc., 2023, 145, 7242–7251 CrossRef CAS PubMed.
- B. Ren, G. Wen, R. Gao, D. Luo, Z. Zhang, W. Qiu, Q. Ma, X. Wang, Y. Cui, L. Ricardez-Sandoval, A. Yu and Z. Chen, Nat. Commun., 2022, 13, 2486 CrossRef CAS PubMed.
- Y. J. Ko, J. Y. Kim, W. H. Lee, M. G. Kim, T. Y. Seong, J. Park, Y. Jeong, B. K. Min, W. S. Lee, D. K. Lee and H. S. Oh, Nat. Commun., 2022, 13, 2205 CrossRef CAS.
- S. Yan, C. Peng, C. Yang, Y. Chen, J. Zhang, A. Guan, X. Lv, H. Wang, Z. Wang, T. K. Sham, Q. Han and G. Zheng, Angew. Chem., Int. Ed., 2021, 60, 25741–25745 CrossRef CAS PubMed.
- S. Liu, J. Xiao, X. F. Lu, J. Wang, X. Wang and X. W. D. Lou, Angew. Chem., Int. Ed., 2019, 58, 8499–8503 CrossRef CAS PubMed.
- G. Wen, B. Ren, M. G. Park, J. Yang, H. Dou, Z. Zhang, Y. P. Deng, Z. Bai, L. Yang, J. Gostick, G. A. Botton, Y. Hu and Z. Chen, Angew. Chem., Int. Ed., 2020, 59, 12860–12867 CrossRef CAS.
- Y. Zhang, H. Jang, X. Ge, W. Zhang, Z. Li, L. Hou, L. Zhai, X. Wei, Z. Wang, M. G. Kim, S. Liu, Q. Qin, X. Liu and J. Cho, Adv. Energy Mater., 2022, 12, 2202695 CrossRef CAS.
- H. Han, S. Jin, S. Park, Y. Kim, D. Jang, M. H. Seo and W. B. Kim, Nano Energy, 2021, 79, 105492 CrossRef CAS.
- H. Zhong, M. Ghorbani-Asl, K. H. Ly, J. Zhang, J. Ge, M. Wang, Z. Liao, D. Makarov, E. Zschech, E. Brunner, I. M. Weidinger, J. Zhang, A. V. Krasheninnikov, S. Kaskel, R. Dong and X. Feng, Nat. Commun., 2020, 11, 1409 CrossRef CAS PubMed.
- A. G. A. Mohamed, E. Zhou, Z. Zeng, J. Xie, D. Gao and Y. Wang, Adv. Sci., 2022, 9, 2104138 CrossRef CAS PubMed.
- K. Ye, Z. Zhou, J. Shao, L. Lin, D. Gao, N. Ta, R. Si, G. Wang and X. Bao, Angew. Chem., Int. Ed., 2020, 59, 4814–4821 CrossRef CAS PubMed.
- Y. Li, J. Chen, S. Chen, X. Liao, T. Zhao, F. Cheng and H. Wang, ACS Energy Lett., 2022, 7, 1454–1461 CrossRef CAS.
- C. Cao, D. D. Ma, J. F. Gu, X. Xie, G. Zeng, X. Li, S. G. Han, Q. L. Zhu, X. T. Wu and Q. Xu, Angew. Chem., Int. Ed., 2020, 59, 15014–15020 CrossRef CAS PubMed.
- X. Zhong, S. Liang, T. Yang, G. Zeng, Z. Zhong, H. Deng, L. Zhang and X. Sun, ACS Nano, 2022, 16, 19210–19219 CrossRef CAS.
- S. Chen, Z. Zhang, W. Jiang, S. Zhang, J. Zhu, L. Wang, H. Ou, S. Zaman, L. Tan, P. Zhu, E. Zhang, P. Jiang, Y. Su, D. Wang and Y. Li, J. Am. Chem. Soc., 2022, 144, 12807–12815 CrossRef CAS PubMed.
- Z. Li, B. Sun, D. Xiao, Z. Wang, Y. Liu, Z. Zheng, P. Wang, Y. Dai, H. Cheng and B. Huang, Angew. Chem., Int. Ed., 2023, 62, e202217569 CrossRef CAS PubMed.
- L. Lin, X. He, X. G. Zhang, W. Ma, B. Zhang, D. Wei, S. Xie, Q. Zhang, X. Yi and Y. Wang, Angew. Chem., Int. Ed., 2022, 62, e202214959 Search PubMed.
- F. Yang, X. Ma, W. B. Cai, P. Song and W. Xu, J. Am. Chem. Soc., 2019, 141, 20451–20459 CrossRef CAS PubMed.
- Z. Chen, X. Zhang, M. Jiao, K. Mou, X. Zhang and L. Liu, Adv. Energy Mater., 2020, 10, 5076–5080 Search PubMed.
- Z. Chen, Y. Song, J. Cai, X. Zheng, D. Han, Y. Wu, Y. Zang, S. Niu, Y. Liu, J. Zhu, X. Liu and G. Wang, Angew. Chem., Int. Ed., 2018, 57, 5076–5080 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3sc02790b |
| This journal is © The Royal Society of Chemistry 2023 |