Open Access Article
Open Access ArticleNMR exchange dynamics studies of metal-capped cyclodextrins reveal multiple populations of host–guest complexes in solution†
Elad
Goren
 a,
Mark A.
Iron
a,
Mark A.
Iron
 b,
Yael
Diskin-Posner
b,
Yael
Diskin-Posner
 b,
Alla
Falkovich
b,
Liat
Avram
b,
Alla
Falkovich
b,
Liat
Avram
 b and
Amnon
Bar-Shir
b and
Amnon
Bar-Shir
 *a
*a
aDepartment of Molecular Chemistry and Materials Science, Weizmann Institute of Science, Rehovot 7610001, Israel. E-mail: amnon.barshir@weizmann.ac.il
bDepartment of Chemical Research Support, Weizmann Institute of Science, Rehovot 7610001, Israel
First published on 7th September 2023
Abstract
Metal-capped molecular hosts are unique in supramolecular chemistry, benefitting from the inner cavity's hydrophobic nature and the metal center's electrochemical properties. It is shown here that the paramagnetic properties of the metals in lanthanide-capped cyclodextrins (Ln-α-CDs and Ln-β-CDs) are a convenient NMR indicator for different populations of host–guest complexes in a given solution. The paramagnetic guest exchange saturation transfer (paraGEST) method was used to study the exchange dynamics in systems composed of Ln-α-CDs or Ln-β-CDs with fluorinated guests, revealing multiple co-existing populations of host-guest complexes exclusively in solutions containing Ln-β-CDs. The enhanced spectral resolution of paraGEST, achieved by a strong pseudo contact shift induction, revealed that different molecular guests can adopt multiple orientations within Ln-β-CDs' cavities and, in contrast, only a single orientation inside Ln-α-CDs. Thus, paraGEST, which can significantly improve NMR detectability and spectral resolution of host-guest systems that experience fast exchange dynamics, is a convenient tool for studying supramolecular systems of metal-capped molecular hosts.
Introduction
Cyclodextrins (CDs) are remarkable among the water-soluble molecular hosts used in host–guest supramolecular systems.1–4 These highly water-soluble cyclic oligosaccharides have a hydrophobic cavity that allows them to form complexes with various guests, ranging from small organic molecules to proteins.5–7 The industrial-scale production of CDs, the range of cavity sizes, and their straightforward chemical modifications make CD–guest complexes attractive in many fields of science and technology. These hosts are used to overcome the poor water solubility of drugs and improve their stability in biofluids.8 In addition, they are used in electrochemical9 and optical sensing10 for capturing volatile analytes,11 and as the immobilized phase in liquid chromatography.12 CDs are utilized as building blocks in supramolecular polymers,13 molecular switches,14 and self-healing materials,15 and as reaction catalysts.16,17 Furthermore, coupling their catalytic abilities with their inherent chirality makes them attractive as enzyme mimetics.5,18 Attaching a metal center to CDs, to obtain metallo-CDs,19 evolved a whole new family of artificial metallo-enzymes, where the metal center plays a main role in both substrate binding and catalysis.20The metal center in metallo-CDs can be bound to the CD rim either by a flexible linker or a rigid bridge, with the latter forming capped metallo-CDs,21 in analogy to other metallo-cavitands.22 The features of the metal element in capped metallo-CDs and the proximity between a cavity-bound ligand (i.e., the guest) and the metal result in improved catalytic activities,23–27 and is also used for other applications.28–30 While metallo-CDs have been extensively studied, several factors can make their characterization in solutions challenging using traditional methods. For one, metallo-CDs host–guest systems are often dynamic, with a fast equilibrium between the hosted and free guest states. In addition, guest molecules may have multiple orientations inside the host's cavity,31–33 creating different carceroisomers,34,35 or they may bind the host in various host![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) guest stoichiometries36 that complicate the analysis of titration data.37 Furthermore, it was demonstrated that metallo-CD host could deform from its native CD shape or may have the metal center in different positions relative to the CD cavity.38,39 Consequently, novel and advanced methods for studying such dynamic systems are needed.
guest stoichiometries36 that complicate the analysis of titration data.37 Furthermore, it was demonstrated that metallo-CD host could deform from its native CD shape or may have the metal center in different positions relative to the CD cavity.38,39 Consequently, novel and advanced methods for studying such dynamic systems are needed.
NMR is frequently used to study and characterize host–guest systems in solutions,32 but commonly used NMR techniques cannot handle systems involving fast exchange dynamics, low binding affinities, or short relaxation times – all of which are expected for metallo-CDs. The chemical exchange saturation transfer (CEST) method, developed for MRI studies,40,41 has been transformed to study host–guest complexes with fast dynamic interactions in supramolecular systems. In such cases, the method is called guest exchange saturation transfer (GEST).42 This NMR-based approach takes advantage of the reversible, relatively fast, noncovalent interactions to: (i) identify “invisible” complexed guests in the NMR spectra, (ii) quantify exchange rates, and (iii) evaluate energy barriers.43 Using a fluorinated guest, the background-free 19F-GEST method has been recently used in the study of a variety of molecular hosts, such as cucurbit[n]urils,44 bambus[n]urils,45 deep-cavity cavitands,46 pillar[n]arenes,47 and supramolecular cages.48
Recently, 19F-GEST was extended to 19F-paraGEST (paramagnetic 19F-GEST) to study paramagnetic molecular hosts.30 In paraGEST, the resolution of the GEST spectrum (or z-spectrum) can be enhanced by increasing the chemical shift offset (Δω) between the NMR resonances of free and bound guest molecules, which is caused by the pseudo contact shift (PCS) induction capabilities of the paramagnetic element in the host. The requirement to obtain a GEST effect is that Δω > kex (where kex is the exchange rate between bound and free guest states, and Δω is defined in Hz units). Thus, the guest's proximity to a paramagnetic specie and the PCS induction capability of the metal increase Δω and, consequently, the range of detectable exchange rates. As such, paraGEST is a powerful tool to study supramolecular systems that experience fast exchange dynamics, including metallo-cavitands, and can potentially uncover host–guest interactions that other analytical tools cannot capture. Herein, we use 19F-paraGEST NMR to study host–guest systems of paramagnetic metallo-CDs, specifically lanthanide-capped cyclodextrins (Ln-α-CDs and Ln-β-CDs). This allowed us to identify at least two distinctive, coexisting 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Ln-β-CD
1 Ln-β-CD![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) guest populations in solution, having similar thermodynamic and kinetic properties. In contrast, only a single host–guest population was observed for the smaller and more symmetric Ln-α-CDs, even when using the largest possible PCS induction property. Our findings shed a light on the very different binding features of organic molecules to metal-caped α- and β-CDs in water.
guest populations in solution, having similar thermodynamic and kinetic properties. In contrast, only a single host–guest population was observed for the smaller and more symmetric Ln-α-CDs, even when using the largest possible PCS induction property. Our findings shed a light on the very different binding features of organic molecules to metal-caped α- and β-CDs in water.
Results and discussion
A family of metallo-β-CDs was synthesized28 from the corresponding 6A,6D-diamino-6A,6D-dideoxy-β-cyclodextrin (diamino-β-CD, Fig. S1–S9†), in correlation to the family of metallo-α-CDs previously synthesized and described by us (Fig. 1).30 Attaching a diethylene triamine-pentaacetic dianhydride (DTPAA) chelating moiety to either the six-membered ring diamino α-CD (Fig. 1a), or the seven-membered ring diamino β-CD (Fig. 1d), results in two different topologies of the studied Ln-CDs, as reflected from their schematic top views (Fig. 1b and e for Ln-α-CDs and Ln-β-CDs, respectively). Two series of Ln-CDs were selected for this study (Fig. 1c, f and S4–S9†) using the diamagnetic La3+ (i.e., zero PCS induction) and three paramagnetic ions (Eu3+, Ho3+ and Dy3+) with increasing PCS induction capabilities.49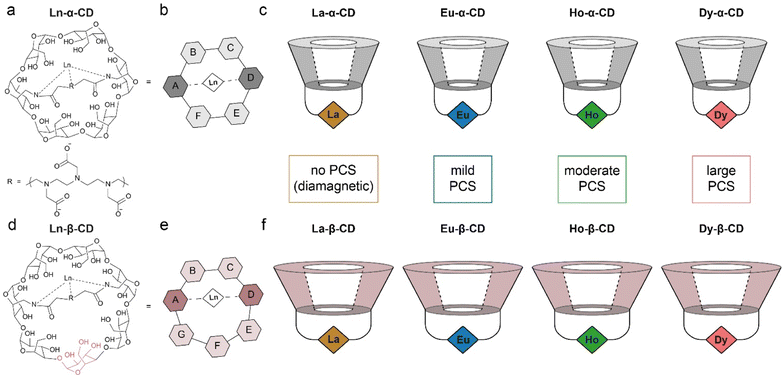 | ||
| Fig. 1 The synthesized and studied families of Ln-CDs. (a) Molecular structure and (b) schematic representation of Ln-α-CD; (c) schematics of the previously synthesized Ln-α-CDs with their relative PCS capabilities (Ln3+ ions are represented as color diamonds); (d) molecular structure and (e) schematic representation of Ln-β-CD; (f) schematics of the Ln-β-CDs synthesized with their relative PCS capabilities (Ln3+ ions are represented as color diamonds). The data in panels a–c was adapted from ref. 30. | ||
19F-paraGEST experiments were performed with Ln-α-CD hosts and 4-(trifluoromethyl)benzylamine guest (1, Fig. 2a–e). With the diamagnetic La3+ ion, which has no PCS induction capability, no 19F-paraGEST effect was observed (Fig. 2b for La-α-CD), probably because the Δω > kex condition42 is not met. Using a paramagnetic ion instead (Eu3+, Ho3+ or Dy3+, Fig. 2c–e), the PCS-induced Δω might be sufficiently large to meet the prerequisites for GEST, as we previously demonstrated for Ln-α-CDs.30 Note that the observed Δω for each 19F-paraGEST effect depends on the Ln3+ used and correlates with its Bleaney's constant.50
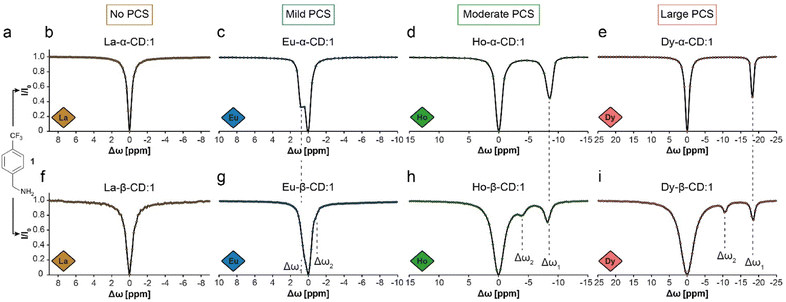 | ||
Fig. 2
19F-paraGEST z-spectra for solutions of 1 with different Ln-CDs. (a) The molecular structure of guest 1. (b–e) The 19F-paraGEST spectra obtained for 1 in the presence of different Ln-α-CD hosts. (f–i) The 19F-paraGEST spectra obtained for 1 in the presence of different Ln-β-CD hosts. All experiments were performed at 25 °C with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 host 100 host![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) guest solutions using an 11.7 T NMR spectrometer. The data in panels (b–e) was adapted from ref. 30. guest solutions using an 11.7 T NMR spectrometer. The data in panels (b–e) was adapted from ref. 30. | ||
Indeed, as we demonstrated before for metallo-α-CDs, a clear 19F-paraGEST effect was obtained for each studied paramagnetic Ln-α-CD with a specific Δω between free and bound 1. The Δω values changed based on the PCS induction abilities of the Ln3+ from +0.8 ppm in Eu-α-CD (Fig. 2c) to −8.8 ppm in Ho-α-CD (Fig. 2d) and to −18.8 ppm in Dy-α-CD (Fig. 2e), as summarized in Table 1.
| Ln | Eu | Ho | Dy | |||
|---|---|---|---|---|---|---|
| Δω1 | Δω2 | Δω1 | Δω2 | Δω1 | Δω2 | |
| Ln-α-CD:1 | 0.8 | — | −8.8 | — | −18.8 | — |
| Ln-β-CD:1 | 0.5 | −0.8 | −8.2 | −3.8 | −18.6 | −10.9 |
With Ln-β-CDs however (Fig. 2f–i), two 19F-paraGEST effects were detected for the paramagnetic ions (marked as Δω1 and Δω2, Table 1), implying two different populations of bound guest complexes with two distinctive chemical shifts (i.e., ω1 and ω2). While this phenomenon is small for Eu-β-CD (Fig. 2g), it becomes more pronounced with two sharp and spectrally resolved 19F-paraGEST peaks for Ho-β-CD (Fig. 2h) and Dy-β-CD (Fig. 2i). Moreover, the separation between the two detected effects increases as the PCS induction increases (Fig. 2g–i, going from Eu3+ to Dy3+). The chemical shift offset of the first effect, Δω1, is similar to the observed in the corresponding Ln-α-CD, indicating a similar binding mode, which was previously demonstrated to involve the amine group of 1 facing the lanthanide center in Ln-α-CDs.30 For example, Δω1 = −18.6 ppm for 1 in Dy-β-CD (Fig. 2i) and Δω1 = −18.8 ppm for 1 in Dy-α-CD (Fig. 2e). The same observation was obtained when comparing Eu-β-CD:1 (Δω1 = 0.5 ppm, Fig. 2g) to Eu-α-CD:1 (Δω1 = 0.8 ppm, Fig. 2c) and Ho-β-CD:1 (Δω1 = −8.2 ppm, Fig. 2h) to Ho-α-CD:1 (Δω1 = −8.8 ppm, Fig. 2d). Thus, we concluded that the second 19F-paraGEST effect for each Ln-β-CD, which possesses a different Δω value relative to the corresponding Ln-α-CD, implies on a different bound guest geometry of 1 with Ln-β-CDs.
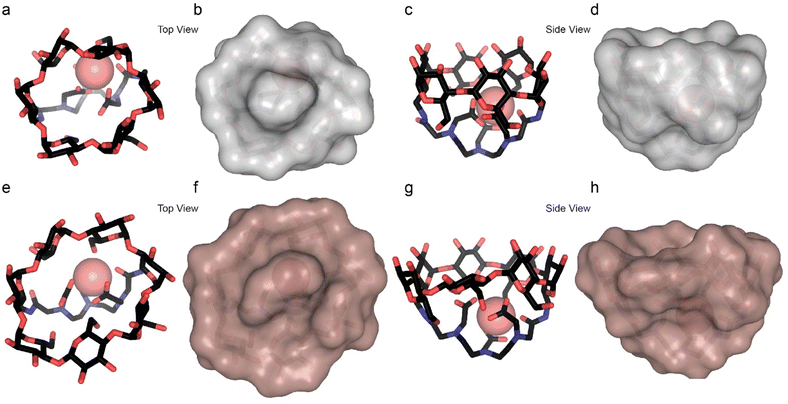
To further elaborate on the source for this difference, the structures of the two Dy-CDs were modeled computationally (Fig. 3) using Grimme and coworkers' GFN2-xTB semiempirical method.51 Dy-α-CD forms a round and bowl-like structure with a relatively small and confined interior (Fig. 3a–d) that limits the access of the guest to the lanthanide center. Meanwhile, Dy-β-CD, which is constructed with an extra sugar unit (Fig. 1d), possesses an elongated and bigger cavity (Fig. 3e–h) that may allow multiple binding modes. This could explain how only one species is observed by 19F-paraGEST for the smaller Ln-α-CDs, while Ln-β-CDs, with their larger, asymmetric cavity, can host the guest in two (or more) ways. Note here that only the Dy3+ system was modelled and the other ions are expected to give similar results, as all the lanthanide ions tend to behave similarly. Similar observations were reported for computational modelling of related metallo-CDs.39
To test the nature of the observed 19F-paraGEST phenomenon for Ln-β-CDs, three other fluorobenzylamine guests with different substitution patterns were considered and studied (2–4, Fig. 4). As previously shown for Dy-α-CD with the same fluorinated guests,30 only a single 19F-paraGEST readout was observed for all three studied guests (Fig. 4b–d), similarly to the obtained with 1 (Fig. 2e). On the other hand, two 19F-paraGEST peaks were observed for Dy-β-CD (Fig. 4e–g), in agreement with the obtained for guest 1 (Fig. 2i). Repeatedly, the Δω1 values obtained for 2–4 with Dy-β-CD (Table S1†) are similar to the values obtained with Dy-α-CD, indicating a similar binding mode. Overall, these results show that for the different molecular guests examined (1–4, Fig. 2 and 4), when a paramagnetic Ln-α-CD was used as the metallo-CD host, a single 19F-paraGEST peak was obtained, and when a paramagnetic Ln-β-CD was used, two distinctive 19F-paraGEST effects are clearly resolved.
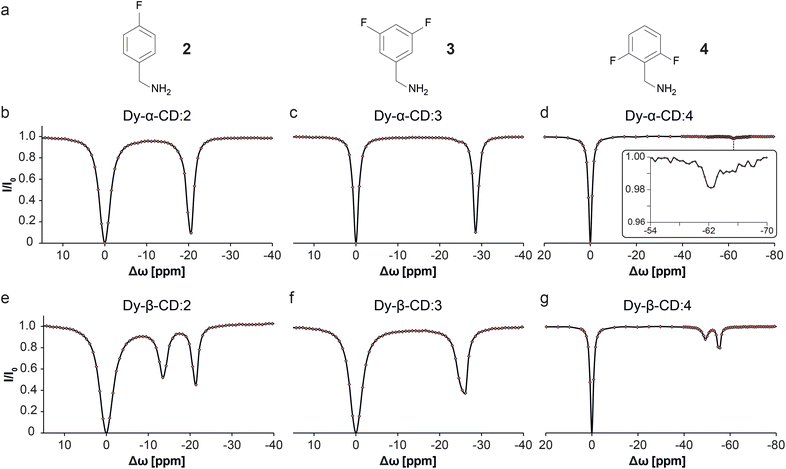 | ||
| Fig. 4 19F-paraGEST z-spectra for solutions of Dy-α-CD or Dy-β-CD with different guest molecules. (a) The molecular structures of guests 2, 3, and 4. (b–g) Z-spectra of the indicated host (as noted in each panel) and the corresponding guest. The data in panels b–d was adapted from ref. 30. | ||
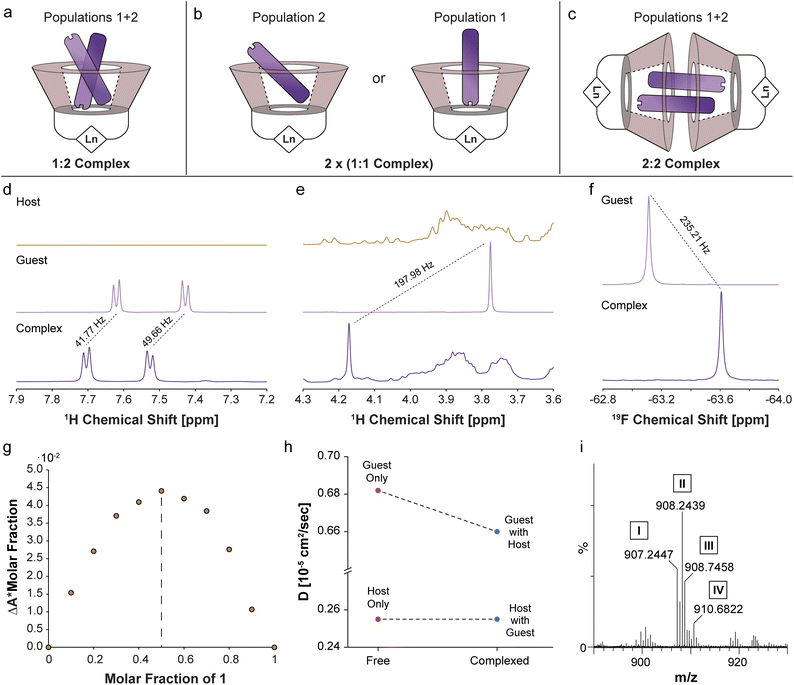
There are three potential sources for the two 19F-paraGEST signals obtained for complexes with Ln-β-CDs: (i) a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complex with two different bound guest geometries within a single host molecule (Fig. 5a and S10a†), (ii) two 1
2 complex with two different bound guest geometries within a single host molecule (Fig. 5a and S10a†), (ii) two 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 carceroisomers, where the guest is bound in a different orientation (Fig. 5b and S10b†), or (iii) a 2
1 carceroisomers, where the guest is bound in a different orientation (Fig. 5b and S10b†), or (iii) a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complex of host dimer and two guest molecules in two orientations (Fig. 5c and S10c†). After confirming the complexation of 1 to La-β-CD using 1H- and 19F-NMR (Fig. 5d–f), we plotted a Job plot to determine the host
2 complex of host dimer and two guest molecules in two orientations (Fig. 5c and S10c†). After confirming the complexation of 1 to La-β-CD using 1H- and 19F-NMR (Fig. 5d–f), we plotted a Job plot to determine the host![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) guest complex ratio, by measuring the UV-vis absorption for a series of solutions with different molar fractions of La-β-CD and 1 (Fig. S11 and Table S2†).52,53 The Job's plot (Fig. 5g) shows a maximum at a molar fraction R = 0.5, which means that a 1
guest complex ratio, by measuring the UV-vis absorption for a series of solutions with different molar fractions of La-β-CD and 1 (Fig. S11 and Table S2†).52,53 The Job's plot (Fig. 5g) shows a maximum at a molar fraction R = 0.5, which means that a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry is observed. While this rules out option (i), the other two options (ii and iii) have 1
1 stoichiometry is observed. While this rules out option (i), the other two options (ii and iii) have 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometries and could still explain the measurements. It should be pointed out that the Job's plot analysis might be limited in some cases, and other tools for analyzing UV-Vis absorption spectra can also be considered.54
1 stoichiometries and could still explain the measurements. It should be pointed out that the Job's plot analysis might be limited in some cases, and other tools for analyzing UV-Vis absorption spectra can also be considered.54
Diffusion NMR is ideal for differentiating between monomeric, dimeric, or other multimeric assemblies of host![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) guest complexes in solutions.55 A clear reduction in the diffusion coefficient of 1 (Dguest) was observed in the presence of the La-β-CD host (Fig. 5h and Tables S3, S4†), indicating the formation of a La-β-CD:1 inclusion complex. On the other hand, the diffusion coefficient of La-β-CD (Dhost) was not affected by the addition of guest 1, eliminating the possibility of the formation of a dimeric 2
guest complexes in solutions.55 A clear reduction in the diffusion coefficient of 1 (Dguest) was observed in the presence of the La-β-CD host (Fig. 5h and Tables S3, S4†), indicating the formation of a La-β-CD:1 inclusion complex. On the other hand, the diffusion coefficient of La-β-CD (Dhost) was not affected by the addition of guest 1, eliminating the possibility of the formation of a dimeric 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 host
2 host![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) guest complex (option iii, Fig. 5c and S10c†). From the extracted Dhost (0.255 × 10−5 cm2 s−1) and Dguest (0.682 × 10−5 cm2 s−1) values and the molecular weights of La-β-CD (Mhost = 1626.2 g mol−1) and 1 (Mguest = 75.15 g mol−1), the host
guest complex (option iii, Fig. 5c and S10c†). From the extracted Dhost (0.255 × 10−5 cm2 s−1) and Dguest (0.682 × 10−5 cm2 s−1) values and the molecular weights of La-β-CD (Mhost = 1626.2 g mol−1) and 1 (Mguest = 75.15 g mol−1), the host![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) guest stoichiometry56 was found to be 1
guest stoichiometry56 was found to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in the La-β-CD:1 complex, which means option ii (Fig. 5b and S10b†) describes the system. Additionally, this was confirmed by mass spectrometry (MS) analyses of Eu-β-CD:1 (Fig. 5i and S12a†) and Dy-β-CD:1 (Fig. S12b†), which indicated the formation of only 1
1 in the La-β-CD:1 complex, which means option ii (Fig. 5b and S10b†) describes the system. Additionally, this was confirmed by mass spectrometry (MS) analyses of Eu-β-CD:1 (Fig. 5i and S12a†) and Dy-β-CD:1 (Fig. S12b†), which indicated the formation of only 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Ln-β-CD:1 complexes with no evidence of other host
1 Ln-β-CD:1 complexes with no evidence of other host![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) guest stoichiometries. Thus, three different methods, Job's plot (Fig. 5g), diffusion NMR (Fig. 5h), and MS (Fig. 5i), suggest the formation of two different 1
guest stoichiometries. Thus, three different methods, Job's plot (Fig. 5g), diffusion NMR (Fig. 5h), and MS (Fig. 5i), suggest the formation of two different 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Ln-β-CD:1 carceroisomers in solution (Fig. 5b and S10b†) as the source for the two 19F-paraGEST effects (Fig. 2g–i and 4e–g), although these analytical tools are insensitive to the presence of two different types of such complexes (as revealed by 19F-paraGEST).
1 Ln-β-CD:1 carceroisomers in solution (Fig. 5b and S10b†) as the source for the two 19F-paraGEST effects (Fig. 2g–i and 4e–g), although these analytical tools are insensitive to the presence of two different types of such complexes (as revealed by 19F-paraGEST).
From these results, we conclude that the geometries of the two Ln-β-CD:1 populations involve two orientations or distances between the fluorine atoms of the guest and the Ln3+ ion in the host, resulting in a different Ln3+-induced PCS effect for each orientation. In contrast, the smaller cavity of Ln-α-CD (Fig. 3a–d) allows only a single guest binding orientation. Using 19F-paraGEST to measure the activation energy for guest dissociation (Ea,out, Fig. S13–S15†),43 we found that more energy is needed to dissociate 1 from Dy-α-CD (Ea,out = 66 ± 3 kJ mol−1) than from Dy-β-CD (Ea,out = 51 ± 2 and 44 ± 4 kJ mol−1 for Δω1 and Δω2, respectively). These observations imply the higher degrees of freedom possible for 1 in the larger and less symmetric cavity of Dy-β-CD (Fig. 3e–h), its weaker affinity to this seven-membered ring host (Fig. 1d and e), and support the 19F-paraGEST results.
One of the strengths of 19F-GEST is its ability to indirectly amplify NMR signals, allowing one to observe low-concentration host![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) guest complexes (relative to the dominant species in solution) and, as a result, to reveal otherwise unobservable species.45 The detection level provided by 19F-GEST depends on the guest's molar fraction (the ratio between complexed and free guest concentrations), amongst other factors such as kex or NMR relaxation properties. To determine if other undetected host–guest complexes exist at very low concentrations in solution, we increased the host
guest complexes (relative to the dominant species in solution) and, as a result, to reveal otherwise unobservable species.45 The detection level provided by 19F-GEST depends on the guest's molar fraction (the ratio between complexed and free guest concentrations), amongst other factors such as kex or NMR relaxation properties. To determine if other undetected host–guest complexes exist at very low concentrations in solution, we increased the host![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) guest ratio to 1
guest ratio to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (keeping the guest concentration constant) and compared the 19F-paraGEST spectra for Dy-α-CD and Dy-β-CD (Fig. 6). No change in the number of 19F-paraGEST peaks was observed for Dy-α-CD:1 (single peak Fig. 6a), as measured for the 1
10 (keeping the guest concentration constant) and compared the 19F-paraGEST spectra for Dy-α-CD and Dy-β-CD (Fig. 6). No change in the number of 19F-paraGEST peaks was observed for Dy-α-CD:1 (single peak Fig. 6a), as measured for the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 ratio (Fig. 2e). However, for Dy-β-CD:1 an additional 19F-paraGEST effect was observed at Δω3 = −29.3 ppm (Fig. 6b), much further upfield than Δω1 (−18.6 ppm) and Δω2 (−10.9 ppm). This points out a third host–guest population coexisting in solution, which could be an additional orientation of 1 inside the larger, less symmetric cavity of Dy-β-CD (see Fig. S10† for some possible examples of guest inclusion possibilities). The characteristics of this additional population of Dy-β-CD:1 complex should be further studied in the future (additional 1
100 ratio (Fig. 2e). However, for Dy-β-CD:1 an additional 19F-paraGEST effect was observed at Δω3 = −29.3 ppm (Fig. 6b), much further upfield than Δω1 (−18.6 ppm) and Δω2 (−10.9 ppm). This points out a third host–guest population coexisting in solution, which could be an additional orientation of 1 inside the larger, less symmetric cavity of Dy-β-CD (see Fig. S10† for some possible examples of guest inclusion possibilities). The characteristics of this additional population of Dy-β-CD:1 complex should be further studied in the future (additional 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host
1 host![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) guest complex or maybe a 1
guest complex or maybe a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 one). These findings strengthen our conclusion of multiple coexisting Ln-β-CD:1 complex populations in aqueous solutions – two major, but different, populations and at least one minor population. These findings may have implications when considering the applications of Ln-β-CD or Ln-α-CD in sensing and catalysis.
2 one). These findings strengthen our conclusion of multiple coexisting Ln-β-CD:1 complex populations in aqueous solutions – two major, but different, populations and at least one minor population. These findings may have implications when considering the applications of Ln-β-CD or Ln-α-CD in sensing and catalysis.
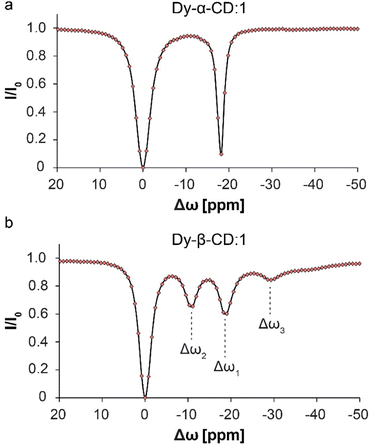 | ||
Fig. 6
19F-paraGEST z-spectra for solutions with high molar ratio of metallo-CD:1. (a) 19F-paraGEST spectrum obtained for 1 with Dy-α-CD with a single peak for bound guest at Δω1 = −18.8 ppm. The panel was adapted from ref. 30; (b) 19F-paraGEST spectrum obtained for 1 with Dy-β-CD with three resolved peaks of bound guest at Δω1 = −18.6 ppm, Δω2 = −10.9 ppm, and Δω3 = −29.3 ppm. Experiments were performed at 25 °C with solutions containing a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 host 10 host![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) guest ratio on an 11.7 T NMR spectrometer. guest ratio on an 11.7 T NMR spectrometer. | ||
Conclusions
We have synthesized two sets of metal-capped cyclodextrins – Ln-α-CDs and Ln-β-CDs – and experimentally demonstrated that the former lead to the formation of a single 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest complex in solution, while the latter form at least two different, coexisting 1
1 host–guest complex in solution, while the latter form at least two different, coexisting 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest carceroisomers (Fig. 2, 4 and 6). Computational modelling showed that the two families of Ln-CD hosts differ in the size and shape of the cavity (Fig. 3), and the larger cavity of Ln-β-CD leads to possible multiple coordination modes of the guest to the lanthanide center. We showed that commonly used analytical tools, such as UV-vis spectroscopy, diffusion NMR, and mass spectrometry, cannot distinguish between the two types of Ln-β-CD
1 host–guest carceroisomers (Fig. 2, 4 and 6). Computational modelling showed that the two families of Ln-CD hosts differ in the size and shape of the cavity (Fig. 3), and the larger cavity of Ln-β-CD leads to possible multiple coordination modes of the guest to the lanthanide center. We showed that commonly used analytical tools, such as UV-vis spectroscopy, diffusion NMR, and mass spectrometry, cannot distinguish between the two types of Ln-β-CD![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) guest complexes (Fig. 5), which were only revealed by 19F-paraGEST NMR. With this method, one can determine the kex values for the host-guest system, not attainable by other techniques, and detect very low concentrations of host–guest complexes. Using paramagnetic lanthanide ions with strong PCS capabilities (Ho3+ or Dy3+) as substituents in Ln-CDs, the multiple populations of carceroisomers could be observed for different guest molecules. These findings could be important for future designs of metallo-cavitands as metallo-enzyme mimetics, which might require different degrees of freedom for a desired substrate. Since paramagnetic CEST-MRI has been demonstrated for other paramagnetic metals, such as iron,57 nickel,58 and cobalt,59 which are attractive targets for catalysis, we envision paraGEST could be a major breakthrough in the study of host–guest interactions in metal-capped molecular cavitands.
guest complexes (Fig. 5), which were only revealed by 19F-paraGEST NMR. With this method, one can determine the kex values for the host-guest system, not attainable by other techniques, and detect very low concentrations of host–guest complexes. Using paramagnetic lanthanide ions with strong PCS capabilities (Ho3+ or Dy3+) as substituents in Ln-CDs, the multiple populations of carceroisomers could be observed for different guest molecules. These findings could be important for future designs of metallo-cavitands as metallo-enzyme mimetics, which might require different degrees of freedom for a desired substrate. Since paramagnetic CEST-MRI has been demonstrated for other paramagnetic metals, such as iron,57 nickel,58 and cobalt,59 which are attractive targets for catalysis, we envision paraGEST could be a major breakthrough in the study of host–guest interactions in metal-capped molecular cavitands.
Data availability
The data that support the findings of this study are available upon a reasonable request from the corresponding author.Author contributions
E. Goren and A. Bar-Shir. designed the study. E. Goren synthesized and purified all Ln-CDs. A. Falkovich performed all MS experiments, E. Goren and A. Falkovich analyzed the MS chromatograms. E. Goren and L. Avram performed all NMR experiments (1D NMR, GEST and Diffusion) and analyzed the data shown. E. Goren collected the UV-vis data, analyzed the data and plotted the Job's plot. Y. Diskin-Posner collected and analyzed preliminary crystal structure data that wasn't include in the paper. M. A. Iron performed computational calculations based on the data collected by Y. Diskin-Posner and configured the 3D structures. E. Goren and A. Bar-Shir wrote the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Israel Science Foundation (ISF 1329/20), the Minerva Foundation, Helen and Martin Kimmel Institute for Magnetic Resonance Research, and Shimon and Golde Picker - Weizmann Annual Grant. Amnon Bar-Shir is the incumbent of the Helen and Milton A. Kimmelman Career Development Chair.References
- M. V. Rekharsky and Y. Inoue, Chem. Rev., 1998, 98, 1875–1918 CrossRef CAS PubMed.
- K. A. Connors, Chem. Rev., 1997, 97, 34 CrossRef PubMed.
- A. Harada, Acc. Chem. Res., 2001, 34, 456–464 CrossRef CAS PubMed.
- Y.-M. Zhang, Y.-H. Liu and Y. Liu, Adv. Mater., 2020, 32, 1806158 CrossRef CAS PubMed.
- R. Breslow and S. D. Dong, Chem. Rev., 1998, 98, 1997–2012 CrossRef CAS PubMed.
- J. Szejtli, Chem. Rev., 1998, 98, 1743–1754 CrossRef CAS PubMed.
- C. J. Easton and S. F. Lincoln, Chem. Soc. Rev., 1996, 25, 163–170 RSC.
- P. Jansook, N. Ogawa and T. Loftsson, Int. J. Pharm., 2018, 535, 272–284 CrossRef CAS PubMed.
- L. Tan, K.-G. Zhou, Y.-H. Zhang, H.-X. Wang, X.-D. Wang, Y.-F. Guo and H.-L. Zhang, Electrochem. Commun., 2010, 12, 557–560 CrossRef CAS.
- Q. Huang, L. Jiang, W. Liang, J. Gui, D. Xu, W. Wu, Y. Nakai, M. Nishijima, G. Fukuhara, T. Mori, Y. Inoue and C. Yang, J. Org. Chem., 2016, 81, 3430–3434 CrossRef CAS PubMed.
- M. Ceborska, K. Szwed, M. Asztemborska, M. Wszelaka-Rylik, E. Kicińska and K. Suwińska, Chem. Phys. Lett., 2015, 641, 44–50 CrossRef CAS.
- Y. Xiao, T. T. Tan and S. C. Ng, Analyst, 2011, 136, 1433–1439 RSC.
- X. Ma and H. Tian, Acc. Chem. Res., 2014, 47, 1971–1981 CrossRef CAS PubMed.
- L. Stricker, E. C. Fritz, M. Peterlechner, N. L. Doltsinis and B. J. Ravoo, J. Am. Chem. Soc., 2016, 138, 4547–4554 CrossRef CAS PubMed.
- M. Nakahata, Y. Takashima, H. Yamaguchi and A. Harada, Nat. Commun., 2011, 2, 511 CrossRef PubMed.
- R. Breslow, G. Trainor and A. Ueno, J. Am. Chem. Soc., 1983, 105, 2739–2744 CrossRef CAS.
- R. Breslow and S. Chung, Tetrahedron Lett., 1990, 31, 631–634 CrossRef CAS.
- I. Tabushi, Acc. Chem. Res., 1982, 15, 66–72 CrossRef CAS.
- R. Breslow and L. E. Overman, J. Am. Chem. Soc., 1970, 92, 1075–1077 CrossRef CAS PubMed.
- B. Zhang and R. Breslow, J. Am. Chem. Soc., 1997, 119, 1676–1681 CrossRef CAS.
- D. Armspach and D. Matt, Chem. Commun., 1999, 1073–1074, 10.1039/A902879J.
- R. Gramage-Doria, D. Armspach and D. Matt, Coord. Chem. Rev., 2013, 257, 776–816 CrossRef CAS.
- F. Ortega-Caballero, C. Rousseau, B. Christensen, T. E. Petersen and M. Bols, J. Am. Chem. Soc., 2005, 127, 3238–3239 CrossRef CAS PubMed.
- S. Guieu, E. Zaborova, Y. Blériot, G. Poli, A. Jutand, D. Madec, G. Prestat and M. Sollogoub, Angew. Chem., Int. Ed., 2010, 49, 2314–2318 CrossRef CAS PubMed.
- M. Jouffroy, R. Gramage-Doria, D. Armspach, D. Sémeril, W. Oberhauser, D. Matt and L. Toupet, Angew. Chem., Int. Ed., 2014, 53, 3937–3940 CrossRef CAS PubMed.
- B. Wang and M. Bols, Chem.Eur. J. , 2017, 23, 13766–13775 CrossRef CAS PubMed.
- J. Meijide Suárez, O. Bistri-Aslanoff, S. Roland and M. Sollogoub, ChemCatChem, 2022, 14, e202101411 CrossRef.
- M. A. Mortellaro and D. G. Nocera, J. Am. Chem. Soc., 1996, 118, 7414–7415 CrossRef CAS.
- C. M. Rudzinski, D. S. Engebretson, W. K. Hartmann and D. G. Nocera, J. Phys. Chem. A, 1998, 102, 7442–7446 CrossRef CAS.
- E. Goren, L. Avram and A. Bar-Shir, Nat. Commun., 2021, 12, 3072 CrossRef CAS PubMed.
- A. Grandeury, S. Petit, G. Gouhier, V. Agasse and G. Coquerel, Tetrahedron: Asymmetry, 2003, 14, 2143–2152 CrossRef CAS.
- J. Tomeček, A. Čablová, A. Hromádková, J. Novotný, R. Marek, I. Durník, P. Kulhánek, Z. Prucková, M. Rouchal, L. Dastychová and R. Vícha, J. Org. Chem., 2021, 86, 4483–4496 CrossRef PubMed.
- R. Rutenberg, G. Leitus, E. Fallik and E. Poverenov, Chem. Commun., 2016, 52, 2565–2568 RSC.
- P. Timmerman, W. Verboom, F. C. J. M. van Veggel, J. P. M. van Duynhoven and D. N. Reinhoudt, Angew. Chem., Int. Ed., 1994, 33, 2345–2348 CrossRef.
- C. Ihm, E. Jo, J. Kim and K. Paek, Angew. Chem., Int. Ed., 2006, 45, 2056–2059 CrossRef CAS PubMed.
- P. Mura, J. Pharm. Biomed. Anal., 2014, 101, 238–250 CrossRef CAS PubMed.
- P. Thordarson, Chem. Soc. Rev., 2011, 40, 1305–1323 RSC.
- M. Guitet, P. Zhang, F. Marcelo, C. Tugny, J. Jiménez-Barbero, O. Buriez, C. Amatore, V. Mouriès-Mansuy, J.-P. Goddard, L. Fensterbank, Y. Zhang, S. Roland, M. Ménand and M. Sollogoub, Angew. Chem., Int. Ed., 2013, 52, 7213–7218 CrossRef CAS PubMed.
- P. Zhang, C. Tugny, J. Meijide Suárez, M. Guitet, E. Derat, N. Vanthuyne, Y. Zhang, O. Bistri, V. Mouriès-Mansuy, M. Ménand, S. Roland, L. Fensterbank and M. Sollogoub, Chem, 2017, 3, 174–191 CAS.
- K. M. Ward, A. H. Aletras and R. S. Balaban, J. Magn. Reson., 2000, 143, 79–87 CrossRef CAS PubMed.
- A. Bar-Shir, J. W. M. Bulte and A. A. Gilad, ACS Chem. Biol., 2015, 10, 1160–1170 CrossRef CAS PubMed.
- L. Avram and A. Bar-Shir, Org. Chem. Front., 2019, 6, 1503–1512 RSC.
- R. Shusterman-Krush, L. Grimm, L. Avram, F. Biedermann and A. Bar-Shir, Chem. Sci., 2021, 12, 865–871 RSC.
- L. Avram, M. A. Iron and A. Bar-Shir, Chem. Sci., 2016, 7, 6905–6909 RSC.
- L. Avram, V. Havel, R. Shusterman-Krush, M. A. Iron, M. Zaiss, V. Šindelář and A. Bar-Shir, Chem.Eur. J. , 2019, 25, 1687–1690 CrossRef CAS PubMed.
- L. Avram, A. D. Wishard, B. C. Gibb and A. Bar-Shir, Angew. Chem., Int. Ed., 2017, 56, 15314–15318 CrossRef CAS PubMed.
- I. Horin, S. Slovak and Y. Cohen, Chem.Eur. J. , 2023, e202301628 CrossRef CAS PubMed.
- J. Wang, L. Avram, Y. Diskin-Posner, M. J. Bialek, W. Stawski, M. Feller and R. Klajn, J. Am. Chem. Soc., 2022, 144, 21244–21254 CrossRef CAS PubMed.
- Z. K. Subha Viswanathan, K. N. Green, S. James Ratnakar and A. Dean Sherry, Chem. Rev., 2010, 110, 59 Search PubMed.
- O. A. Blackburn, R. M. Edkins, S. Faulkner, A. M. Kenwright, D. Parker, N. J. Rogers and S. Shuvaev, Dalton Trans., 2016, 45, 6782–6800 RSC.
- C. Bannwarth, S. Ehlert and S. Grimme, J. Chem. Theory Comput., 2019, 15, 1652–1671 CrossRef CAS PubMed.
- B. Rajbanshi, S. Saha, K. Das, B. K. Barman, S. Sengupta, A. Bhattacharjee and M. N. Roy, Sci. Rep., 2018, 8, 13031 CrossRef PubMed.
- J. S. Renny, L. L. Tomasevich, E. H. Tallmadge and D. B. Collum, Angew. Chem., Int. Ed., 2013, 52, 11998–12013 CrossRef CAS PubMed.
- D. Brynn Hibbert and P. Thordarson, Chem. Commun., 2016, 52, 12792–12805 RSC.
- Y. Cohen, L. Avram and L. Frish, Angew. Chem., Int. Ed., 2005, 44, 520–554 CrossRef CAS PubMed.
- A. R. Waldeck, P. W. Kuchel, A. J. Lennon and B. E. Chapman, Prog. Nucl. Magn. Reson. Spectrosc., 1997, 30, 39–68 CrossRef CAS.
- S. J. Dorazio, P. B. Tsitovich, K. E. Siters, J. A. Spernyak and J. R. Morrow, J. Am. Chem. Soc., 2011, 133, 14154–14156 CrossRef CAS PubMed.
- A. O. Olatunde, S. J. Dorazio, J. A. Spernyak and J. R. Morrow, J. Am. Chem. Soc., 2012, 134, 18503–18505 CrossRef CAS PubMed.
- S. J. Dorazio, A. O. Olatunde, J. A. Spernyak and J. R. Morrow, Chem. Commun., 2013, 49, 10025–10027 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3sc03630h |
| This journal is © The Royal Society of Chemistry 2023 |