Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Chemoselective bond activation by unidirectional and asynchronous PCET using ketone photoredox catalysts†
Rui
Sun‡
 ,
Serge
Ruccolo‡§
,
Serge
Ruccolo‡§
 ,
Daniel L.
Nascimento‡
,
Daniel L.
Nascimento‡
 ,
Yangzhong
Qin§
,
Nathaniel
Hibbert
and
Daniel G.
Nocera
,
Yangzhong
Qin§
,
Nathaniel
Hibbert
and
Daniel G.
Nocera
 *
*
Department of Chemistry and Chemical Biology, Harvard University, 12 Oxford Street, Cambridge, MA 02138, USA. E-mail: dnocera@fas.harvard.edu
First published on 2nd November 2023
Abstract
The triplet excited states of ketones are found to effect selective H-atom abstraction from strong amide N–H bonds in the presence of weaker C–H bonds through a proton-coupled electron transfer (PCET) pathway. This chemoselectivity, which results from differences in ionization energies (IEs) between functional groups rather than bond dissociation energies (BDEs) arises from the asynchronicity between electron and proton transfer in the PCET process. We show how this strategy may be leveraged to achieve the intramolecular anti-Markovnikov hydroamidation of alkenes to form lactams using camphorquinone as an inexpensive and sustainable photocatalyst.
Introduction
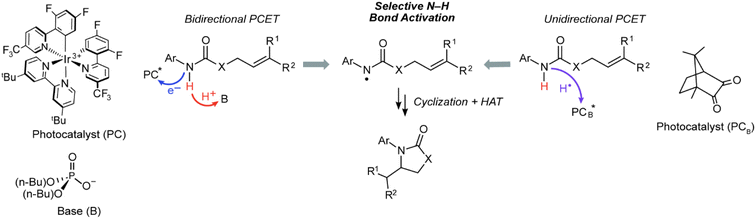
Leveraging proton-coupled electron transfer (PCET) as a foundational design element of photoredox methods has led to a powerful strategy for the selective generation of highly reactive organic intermediates such as heteroatom-centered radicals (X˙). As illustrated in Fig. 1 for the generation of a nitrogen radical from an amide, the PCET event may be described in terms of four diabatic states as accommodated by a “square scheme”.1,2 A discrete intermediate is formed at the corners of the square scheme due to stepwise electron transfer followed by proton transfer (ET-PT) or vice versa (PT-ET). The ET-PT and PT-ET paths along the edges are characterized by two transition states: one for proton transfer and one for electron transfer. Anywhere within the square scheme, PCET is characterized by a single transition state, whether the PCET pathway is synchronous (along the diagonal, e.g., hydrogen atom transfer) or asynchronous (i.e., zig-zag). Asynchronous PCET is common to bidirectional PCET, wherein the proton and electron are transferred to different acceptors. Conversely, asynchronous or synchronous PCET may occur for unidirectional PCET, wherein the proton and electron are transferred to the same acceptor.2–5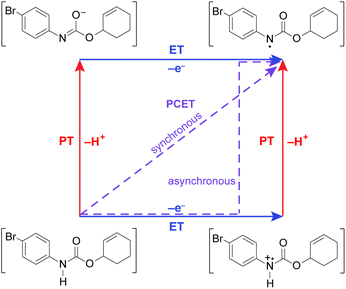 | ||
| Fig. 1 Square scheme highlighting synchronous and asynchronous PCET pathways for substrate activation. | ||
Bidirectional and unidirectional PCET mechanisms have been utilized for substrate activation. Bidirectional PCET has been particularly useful for the design of chemoselective photoredox methods to generate X˙ in organic molecules containing C–H bonds whose bond dissociation energies (BDEs) are much lower than those of the corresponding X–H bonds.6–13 For such methods (Fig. 2, left pathway), an electron is transferred to a photocatalyst (PC*), such as an Ir/Ru polypyridyl or cyclometallated complex,14–16 and the proton is accepted by either an exogenous base or basic functionality on the ligand. The bidirectional PCET pathway has been especially fruitful for the selective photogeneration of amidyl radicals (N–H BDE of ∼100 kcal mol−1),6 which may add to olefins (allylic C–H BDE of ∼83 kcal mol−1)7 to furnish anti-Markovnikov products in exceptional yields.6,17,18 Alternatively, for unidirectional PCET (Fig. 2, right pathway), the proton and electron are both transferred to the photocatalyst, PCB*. Unidirectional PCET offers the advantage of decreased molecularity and inherently higher reaction rates compared to bidirectional PCET, leading, in principle, to a higher energy efficiency. Examples of the application of unidirectional PCET include the photogeneration of halogen radicals from earth abundant metal complexes,19–22 which have been identified as key intermediates in the PCET activation of C(sp3)–H bonds for alkylation,23–26 alkenylation,27 arylation,19,28,29 acylation,19,30 and amination31,32 reactions. Notwithstanding, the activation of substrates by these compounds is predominantly dictated by thermodynamic bond strengths modulated by steric and polarity effects, leading to inferior control of chemoselectivity as compared to that achieved in bidirectional PCET systems.
Photoexcited states of ketones are known to undergo unidirectional PCET via their conspicuous hydrogen atom transfer photochemistry, leading to their ubiquitous application as photoinitiators in polymerizations33–42 and, more recently, as catalysts for photoredox reactions.43,44 However, in contrast to the striking chemoselective activation of strong X–H bonds afforded by bidirectional PCET, much of the reactivity derived from ketone photoreagents has been limited to abstraction of weak C–H bonds adjacent to aryl or heteroatomic functionality.45 We now report that the photoexcited states of certain ketones, such as camphorquinone (CQ), are capable of selectively abstracting a strong amide X–H bond in the presence of much weaker C–H bonds, thus enabling the chemoselective generation of amidyl radicals (Fig. 2, right). Mechanistic studies establish that such chemoselectivity is the result of an asynchronous unidirectional PCET process where the quenching of a CQ excited state (CQ*) primarily correlates with the ionization energy (IE) of the substrate as opposed to its BDE. Additionally, the approach of utilizing ketone organo-photocatalysts has the added benefit of much lower toxicity in comparison to common photocatalysts based on noble metals such as Ir, the concentrations of which are strictly regulated in drug products46–50 (e.g., 0.5 ppm for parenteral administration and 5 ppm for oral administration in the case of Ir).
Results and discussion
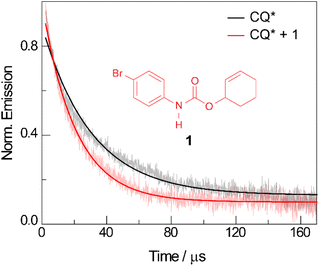
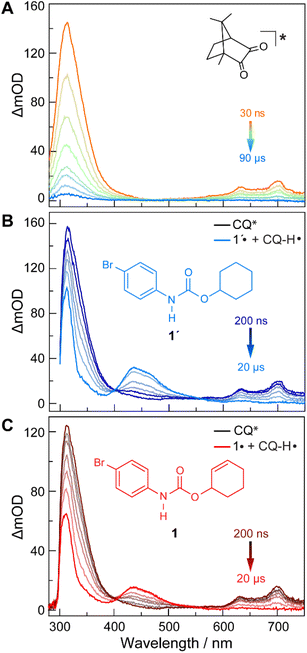
The quenching of CQ* by amide substrate is responsible for amidyl radical formation. Known amide substrates were either purchased or prepared as previously described,6 whereas new substrates were synthesized and characterized (Fig. S1–S7†) as reported in Section B of the ESI.† As shown in Fig. 3, the lifetime of CQ* (as measured by time-resolved emission kinetics at 570 nm) decreases from 30.6 μs to 20.7 μs upon addition of amide 1 (0.4 mM), implying quenching of the former by the latter. Transient absorption (TA) spectroscopy permits this reaction between amide 1 and CQ* to be directly interrogated. We focused on this initial quenching step because the subsequent steps leading to cycloamidation (i.e., cyclization and subsequent HAT to furnish the lactam) occur independently of the photocatalyst.51Fig. 4 shows the transient absorption spectra for solutions containing CQ (5 mM) alone and those containing CQ with amides 1 and 1′ (10 mM). The spectra of CQ in Fig. 4A show the relaxation of CQ*,52 while the spectra for CQ in the presence of 1′ and 1 in Fig. 4B and C, respectively, show initial absorbance dominated by the excited state of CQ at 200 ns (black traces), followed by a gradual evolution to a spectrum containing features at 430 nm and 310 nm (blue trace for 1′ and red trace for 1). The peak at 430 nm is ascribed to the amidyl radical51 while the 310 nm feature is tentatively assigned to CQ–H• due to its resemblance to the spectrum of CQ•– obtained by spectroelectrochemistry (Fig. S8B†) as well as a previously reported transient feature observed during the photoreduction of CQ by isopropanol.53 Fig. S10† shows the TA kinetic trace at 430 nm for samples containing CQ and amide substrate 1′, which is identical to substrate 1 with the exception of an olefin moiety. Substrate 1′ is strategic because it is unable to undergo cyclization upon amidyl radical formation, thus allowing for the kinetics of forward and back HAT reactions to be measured without interference from other chemical processes. From kinetic modelling of the decay of the transient absorption at 430 nm (Fig. S10†), we extract an HAT rate constant of kFH˙ = 2.9 × 107 M−1 s−1 and a back reaction rate constant of kBH˙ = 8.3 × 109 M−1 s−1, where the latter is similar to the back-electron transfer rate constant measured for the Ir/base-catalyzed system (kBET = 7.9 × 109 M−1 s−1).51 We note that there is negligible ground-state hydrogen bonding between CQ and amide 1, as the association constant between the two was determined to be Ka = 2.4 ± 0.2 M−1 by 1H NMR spectroscopy (Fig. S11†). This implies that the chemoselectivity for amidyl radical formation is intrinsic to the excited state reactivity of CQ*.In order to differentiate between stepwise and concerted mechanisms in the generation of amidyl radical by CQ*, we investigated the relative quenching of CQ* by a series of Ph–XH (X = NH, S, and O) compounds and their X-methylated derivatives. Since all the Ph–XH substrates show irreversible oxidation waves, we used the gas-phase ionization energies (IEs) of these compounds as a measure of their oxidation potential, as has been previously discussed for asynchronous PCET pathways.54 Table S1† lists the calculated quenching rate constants (kq) for these compounds determined from Stern–Volmer plots (Fig. S12†) along with their IEs, X–H BDEs, and pKa values in DMSO. If a PT-ET mechanism were operative, a correlation between kq and pKa is expected since the quenching would be governed by proton transfer. However, this is not the case. We observe that the kq values correlate with IEs, signifying the importance of ET character in the quenching process. To confirm that the quenching of CQ* by Ph–XH substrates leads to X–H bond homolysis, we employed TA spectroscopy to study the reaction between CQ (10 mM) and phenol (20 mM) in DCM. Under these conditions, we observed the clear formation of phenoxyl radical with features at 380 and 400 nm (Fig. S9†).55
To further delineate between the stepwise ET-PT and concerted asynchronous PCET pathways, we first note that CQ* has an oxidation potential of 0.33 V vs. Fc+/Fc, based on a E(CQ/CQ•−) = −1.90 V vs. Fc+/Fc (Fig. S8A†) and the previously reported excited state energy of 2.23 eV for CQ* at 77 K.56 As CQ* is a far weaker outer-sphere photooxidant than the Ir catalyst (oxidation potential of 0.85 V),51 which is not quenched by the amide substrate in the absence of base,6 a stepwise ET-PT pathway for amidyl radical formation is unfeasible based on the redox potential of CQ*. This is further corroborated by a comparison of the Stern–Volmer constants (KSV) for acetanilide and N-methylacetanilide. The latter is expected to have a similar or lower oxidation potential for outer-sphere ET when compared to the former; however, only the former possesses a proton that can engage in a PCET process. As shown in Fig. 5, the Stern–Volmer constant for N-methylacetanilide (KSV = 28(38) M−1, kq = 9 (12) × 105 M−1 s−1) is two orders of magnitude lower than for acetanilide [KSV = 1841(121) M−1, kq = 6.0 (0.4) × 107 M−1 s−1], which suggests that the quenching is not dictated purely by an ET process followed by PT. This is further supported by a KIE of kH/kD = 1.33 (0.13) between acetanilide and acetanilide-d, suggesting proton involvement in the quenching process. We note that a similar KIE exists for the quenching rates of CQ* by phenol [kq = 3.18 (0.14) × 109 M−1 s−1] and phenol-d6 [kq = 2.07 (0.13) × 109 M−1 s−1] in DCM, wherein we measured a KIE of kH/kD = 1.54 (0.06). Additionally, we found that 1,2,4,5-tetramethylbenzene (1,2,4,5-TMB) does not quench CQ* though its IE (8.1 eV)57 is approximately in line with the Ph–XH compounds in Table S1.† For a stepwise process wherein ET is followed by PT, we would expect activation of 1,2,4,5-TMB. However, in contrast to 1,2,4,5-TMB, ET originates from a site carrying proton for the Ph–XH substrates listed in Table S1.† For these substrates, the pKa of the proton is expected to decrease substantially with oxidation and hence consistent with pKa contributing to the kinetics of the overall quenching.
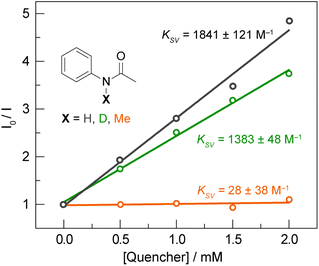 | ||
Fig. 5 Stern–Volmer plot for the quenching of CQ (1 mM) by acetanilide ( black), deutero-acetanilide ( black), deutero-acetanilide ( green) and N-methylacetanilide ( green) and N-methylacetanilide ( orange) in DCM. orange) in DCM. | ||
Taken together, the quenching and kinetic isotope effects are most consistent with a concerted asynchronous PCET pathway with a transition state that is predominantly ET in character, but does not involve the generation of distinct, oxidized intermediate preceding proton transfer (as shown in the asynchronous pathway delineated in Fig. 1). This mechanism explains the chemoselectivity for amide N–H bond activation over allylic C–H bonds, since the IEs for the former are much lower than those for the latter (e.g., 8.2 eV for 4′-fluoroacetanilide vs. 8.9–9.1 eV for cyclohexene),57 in addition to being less acidic. Furthermore, we note that a concerted asynchronous PCET between amide and carbon nitride may also account for the background hydroamidation reactivity observed with carbon nitride photocatalysts in the absence of base.58
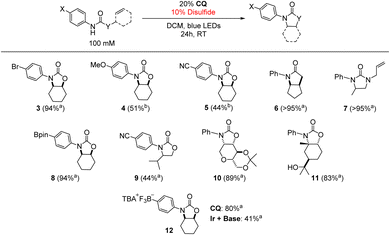
To demonstrate the synthetic utility of the pathway shown in Fig. 2 (right), we sought to establish whether CQ itself can serve as a competent photocatalyst in intramolecular hydroamidation reactions in the absence of an exogenous base. As shown in Entry 1 of Table 1, cyclized product 3 can be formed from 1 in 94% yield after 24 h of blue LED irradiation using 20% CQ and 10% phenyl disulfide (PhSSPh) as a hydrogen atom shuttle to facilitate turnover of CQ–H• and intercept, through the intermediacy of thiophenol, the transient carbon-centered radical formed after cyclization of the amidyl radical. We measured a quantum yield of Φ = 0.1 for this reaction, which is on par with that of the Ir-catalysed reaction. The omission of disulfide (entry 2) or its replacement with thiol (entry 3) led to significantly diminished yields, consistent with previous observations under Ir-catalyzed conditions.51,59 Attenuated yield was also observed for the methoxy-substituted substrate 2 (Entry 4), which has been shown to undergo cyclization at a rate that is three orders of magnitude slower upon amidyl radical formation when compared to 1.51 However, by switching from PhSSPh to 2,4,6-triisopropylphenyl disulfide [(TripS)2], we observed significantly improved yields for substrate 2 (entry 5). This superior performance of [(TripS)2] as compared to its phenyl congener has previously been documented in photoredox reactions,18,60 and is possibly due to steric protection afforded by the isopropyl moieties leading to a higher steady-state concentration of thiyl radical via retardation of disulfide formation. Finally, we investigated the performance of CQ-mediated hydroamidation in acetonitrile (MeCN), a highly polar solvent. The original method relying on bidirectional PCET using an outer-sphere Ir photooxidant and a phosphate base necessitated the coalescence of Ir*, base, and amide substrate in order to generate the amidyl radical. This is aided by ion pairing between the cationic Ir photooxidant and anionic phosphate base in DCM,51 which can be disrupted by a highly polar solvent. Since CQ is a neutral species which does not rely on ion pairing effects for its unidirectional PCET activity, we posited that it could deliver superior yields in MeCN. Indeed, as shown in entries 6 and 7, the use of CQ results in a yield that was ∼3× higher than that with the Ir/base system; switching from PhSSPh to (TripS)2 further resulted in a substantial increase in the yield to 43% (entry 8).
| Entry | X Group | Differences from standard conditions listed above | Yielda (%) |
|---|---|---|---|
| a Yield determined by 1H NMR spectroscopy. b Same conditions as the published procedure, ref. 6, with 10% PhSSPh in place of 20% PhSH for consistency with CQ-mediated conditions. The phosphate base is [NMeBu3][OP(O)(n-BuO)2]. Trip = 2,4,6-triisopropylphenyl. | |||
| 1 | Br | None | 94 |
| 2 | Br | No PhSSPh | 9 |
| 3 | Br | PhSH instead of PhSSPh | 45 |
| 4 | OMe | None | 32 |
| 5 | OMe | (TripS)2 in place of PhSSPh | 51 |
| 6 | Br | MeCN in place of DCM | 14 |
| 7 | Br | Ir photooxidant + phosphate baseb, MeCN in place of DCM | <5 |
| 8 | Br | (TripS)2 in place of PhSSPh, MeCN in place of DCM | 43 |
To investigate the stability of CQ in the reaction and verify its role as the active photocatalyst,51 we measured the yield of cyclized product 4 and compared with the amount of CQ remaining by 1H NMR spectroscopy. Fig. S13† (red traces, (TripS)2 as disulfide) shows that no increase in product yield was observed after CQ was completely consumed. Analysis of the reaction mixture after photolysis by mass spectrometry (ESI-MS) revealed the formation of hydroxycamphor and camphanediol as two possible CQ decomposition products. However, ca. 20% product formation did occur when ca. 95% CQ was consumed in the presence of (TripS)2. This could be due to the presence of intermediate photoproducts (e.g., thioamide species)51 which can undergo further photolysis to yield the lactam. With PhSSPh as the disulfide, a much slower reaction was observed (Fig. S13,† black traces), consistent with the lower yield shown in Table 1.
Given the ubiquity of ketones as HAT photoinitiators, we sought to establish whether the selective generation of amidyl radicals via activation of the amide N–H bond in the presence of weak C–H bonds might be a general phenomenon. To this end, we used the cycloamidation reaction as an assay for amidyl radical generation. Although CQ remained the highest yielding ketone among those examined, a wide range of mono- and diketones gave significant yields of the cyclized product 3 (Fig. S14†), as determined by 1H NMR spectroscopy. Surprisingly, several commonly employed photoinitiators, which have been extensively studied for their propensity to readily undergo C–H abstraction, such as diacetyl61–63 and acetophenone64–66 gave significant yields of product, with the balance of the reaction being accounted for by unreacted starting material. These results demonstrate that chemoselectivity in hydroamidation photoredox transformations promoted by the PCET chemistry of triplet ketones is not limited to CQ.
To confirm the generality of the CQ-catalyzed hydroamidation reaction, we tested multiple substrates under the optimized conditions in Table 1. As shown in Table 2, a variety of alkene-bearing amides undergo hydroamidation under CQ photocatalysis. For more challenging substrates, (TripS)2 may be used in place of PhSSPh to improve the yield. Of note, Lewis acidic functionality, such as the pinacolboranyl (Bpin) moiety, was well-tolerated. Finally, CQ achieved twice the yield of the Ir + base combination in the reaction of an anionic substrate containing a trifluoroborate functional group, further highlighting the distinct reactivity of a unidirectional PCET catalyst under conditions where ion pairing between the cationic Ir photooxidant and anionic phosphate base can be disrupted. These results are of synthetic relevance as the Bpin and trifluoroborate functional groups are commonly found in nucleophilic substrates for Suzuki–Miyaura cross-coupling reactions.67–69 Currently, N-alkyl derivatives are not amenable towards cyclization under these conditions as no quenching of CQ* was observed via Stern–Volmer experiments with N-alkyl amides.
| a PhSSPh used as the disulfide. b (TripS)2 used as the disulfide. Yields determined by 1H NMR spectroscopy using 1,4-bis(trifluoromethyl)benzene or 1,3,5-tris(trifluoromethyl)benzene as an internal standard. [NMeBu3][OP(O)(n-BuO)2]used as base. Structures of the dominant diastereomers (as determined by crude 1H NMR spectroscopy with reference to previously reported spectra6) are drawn where appropriate. |
|---|
|
Conclusions
The excited states of ketones exhibit an inherent selectivity for amide N–H activation over weaker C–H bonds, as confirmed by Stern–Volmer and transient absorption experiments. This selectivity results from an asynchronous PCET reaction mechanism where the reactivity is largely dictated by the ionization energy of the functional group. This mechanism may be exploited to catalyze the intramolecular hydroamidation of alkenes under photoredox conditions with ketones including camphorquinone, which has the added benefit of being an inexpensive and non-toxic diketone,70 leading to a greener reaction method.Data availability
The ESI includes experimental for the preparation of new substrates and their 1H NMR spectra, 2D NMR spectra supporting assignment of relative stereochemistry for certain substrates, electrochemistry of CQ, transient absorption spectra and kinetics traces, 1H NMR spectra for investigating ground state H-bonding between CQ and 1, Stern–Volmer plots, photochemical results using various ketones, and details for quantum yield measurement.†Author contributions
RS, SR, DLN, and NH performed synthesis, reactivity studies, and characterization. RS and SR performed Stern–Volmer and (spectro)electrochemical measurements. YQ performed time-resolved laser experiments. RS, SG and DGN designed experiments. RS and DGN wrote the manuscript. DGN acquired funding for the studies reported herein.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the National Science Foundation (NSF) Division of Chemistry under the grant number CHE-2243724.References
- D. G. Nocera, J. Am. Chem. Soc., 2022, 144, 1069–1081 CrossRef CAS PubMed.
- R. I. Cukier and D. G. Nocera, Annu. Rev. Phys. Chem., 1998, 49, 337–369 CrossRef CAS PubMed.
- S. Y. Reece and D. G. Nocera, Annu. Rev. Biochem., 2009, 78, 673–699 CrossRef CAS PubMed.
- C. J. Chang, J. D. K. Brown, M. C. Y. Chang, E. A. Baker and D. G. Nocera, Electron Transfer in Chemistry, ed. V. Balzani, Wiley-VCH, Weinheim, Germany, 2001, vol. 3, ch. 2.4, pp. 409–461 Search PubMed.
- J. W. Darcy, B. Koronkiewicz, G. A. Parada and J. M. Mayer, Acc. Chem. Res., 2018, 51, 2391–2399 CrossRef CAS PubMed.
- D. C. Miller, G. J. Choi, H. S. Orbe and R. R. Knowles, J. Am. Chem. Soc., 2015, 137, 13492–13495 CrossRef CAS PubMed.
- S. L. Khursan, D. A. Mikhailov, V. M. Yanborisov and D. I. Borisov, React. Kinet. Catal. Lett., 1997, 61, 91–95 CrossRef CAS.
- P. R. D. Murray, J. H. Cox, N. D. Chiappini, C. B. Roos, E. A. McLoughlin, B. G. Hejna, S. T. Nguyen, H. H. Ripberger, J. M. Ganley, E. Tsui, N. Y. Shin, B. Koronkiewicz, G. Qiu and R. R. Knowles, Chem. Rev., 2021, 122, 2017–2291 CrossRef PubMed.
- Q. Zhu, D. E. Graff and R. R. Knowles, J. Am. Chem. Soc., 2018, 140, 741–747 CrossRef CAS PubMed.
- J. G. Choi, Q. Zhu, D. C. Miller, C. J. Gu and R. R. Knowles, Nature, 2016, 539, 268–271 CrossRef PubMed.
- E. Ota, H. Wang, N. L. Frye and R. R. Knowles, J. Am. Chem. Soc., 2019, 141, 1457–1462 CrossRef CAS PubMed.
- D. R. Weinberg, C. J. Gagliardi, J. F. Hull, C. F. Murphy, C. A. Kent, B. C. Westlake, A. Paul, D. H. Ess, D. G. McCafferty and T. J. Meyer, Chem. Rev., 2012, 112, 4016–4093 CrossRef CAS PubMed.
- N. Hoffmann, Eur. J. Org Chem., 2017, 2017, 1982–1992 CrossRef CAS.
- O. S. Wenger, Acc. Chem. Res., 2013, 46, 1517–1526 CrossRef CAS PubMed.
- O. S. Wenger, Coord. Chem. Rev., 2015, 282–283, 150–158 CrossRef CAS.
- N. Sinha, P. Yaltseva and O. S. Wenger, Angew. Chem., Int. Ed., 2023, 62, e202303864 CrossRef CAS PubMed.
- K. A. Margrey and D. A. A. Nicewicz, Acc. Chem. Res., 2016, 49, 1997–2006 CrossRef CAS PubMed.
- S. T. Nguyen, Q. Zhu and R. R. Knowles, ACS Catal., 2019, 9, 4502–4507 CrossRef CAS PubMed.
- B. J. Shields and A. G. Doyle, J. Am. Chem. Soc., 2016, 138, 12719–12722 CrossRef CAS PubMed.
- L. Troian-Gautier, M. D. Turlington, S. A. M. Wehlin, A. B. Maurer, M. D. Brady, W. B. Swords and G. J. Meyer, Chem. Rev., 2018, 119, 4628–4683 CrossRef PubMed.
- D. Gygi, M. I. Gonzalez, S. J. Hwang, K. T. Xia, Y. Qin, E. Johnson, F. Gygi, Y.-S. Chen and D. G. Nocera, J. Am. Chem. Soc., 2021, 143, 6060–6064 CrossRef CAS PubMed.
- M. I. Gonzalez, D. Gygi, Y. Qin, Q. Zhu, E. J. Johnson, Y.-S. Chen and D. G. Nocera, J. Am. Chem. Soc., 2022, 144, 1464–1472 CrossRef CAS PubMed.
- S. M. Treacy and T. Rovis, J. Am. Chem. Soc., 2021, 143, 2729–2735 CrossRef CAS PubMed.
- Y. C. Kang, S. M. Treacy and T. Rovis, ACS Catal., 2021, 11, 7442–7449 CrossRef CAS PubMed.
- S. Rohe, A. O. Morris, T. McCallum and L. Barriault, Angew. Chem., Int. Ed., 2018, 57, 15664–15669 CrossRef CAS PubMed.
- H. P. Deng, Q. Zhou and J. Wu, Angew. Chem., Int. Ed., 2018, 57, 12661–12665 CrossRef CAS PubMed.
- H. P. Deng, X. Z. Fan, Z. H. Chen, Q. H. Xu and J. Wu, J. Am. Chem. Soc., 2017, 139, 13579–13584 CrossRef CAS PubMed.
- M. Zidan, A. O. Morris, T. McCallum and L. Barriault, Eur. J. Org. Chem., 2020, 10, 1453–1458 CrossRef.
- M. K. Nielsen, B. J. Shields, J. Liu, M. J. Williams, M. J. Zacuto and A. G. Doyle, Angew. Chem., Int. Ed., 2017, 56, 7191–7194 CrossRef CAS PubMed.
- L. K. G. Ackerman, J. I. M. Alvarado and A. G. Doyle, J. Am. Chem. Soc., 2018, 140, 14059–14063 CrossRef CAS PubMed.
- Q. Yang, Y. H. Wang, Y. Qiao, M. Gau, P. J. Carroll, P. J. Walsh and E. J. Schelter, Science, 2021, 372, 847–852 CrossRef CAS PubMed.
- A. Hu, J. J. Guo, H. Pan and Z. Zuo, Science, 2018, 361, 668–672 CrossRef CAS PubMed.
- Y. Xu, G. Noirbent, D. Brunel, F. Liu, D. Gigmes, K. Sun, Y. Zhang, S. Liu, F. Morlet-Savary, P. Xiao, F. Dumur and J. Lalevée, Eur. Polym. J., 2020, 132, 109737 CrossRef CAS.
- S. Dadashi-Silab, S. Doran and Y. Yagci, Chem. Rev., 2016, 116, 10212–10275 CrossRef CAS PubMed.
- H. Block, A. Ledwith and A. R. Taylor, Polymer, 1971, 12, 271–288 CrossRef CAS.
- G. Amirzadeh and W. Schnabel, Makromol. Chem., 1981, 182, 2821–2835 CrossRef CAS.
- A. Kowalska, J. Sokolowski and K. Bociong, Polymers, 2021, 13, 470 CrossRef CAS PubMed.
- J. Meinwald and H. O. Klingele, J. Am. Chem. Soc., 1966, 88, 2071–2073 CrossRef CAS.
- B. M. Monroe and S. A. Weiner, J. Am. Chem. Soc., 1969, 91, 450–456 CrossRef CAS.
- K. Maruyama and T. Takahashi, Chem. Lett., 1974, 3, 467–470 CrossRef.
- O. S. Taskin, G. Yilmaz, M. A. Tasdelen and Y. Yagci, Polym. Int., 2014, 63, 902–907 CrossRef CAS.
- I. Pyszka, Z. Kucybała and J. Pączkowski, Macromol. Chem. Phys., 2004, 205, 2371–2375 CrossRef CAS.
- C. B. Tripathi, T. Ohtani, M. T. Corbett and T. Ooi, Chem. Sci., 2017, 8, 5622–5627 RSC.
- J. Mateos, S. Cuadros, A. Vega-Peñaloza and L. Dell’Amico, Synlett, 2021, 33, 116–128 Search PubMed.
- D. Ravelli, M. Fagnoni and A. Albini, Chem. Soc. Rev., 2013, 42, 97–113 RSC.
- A. J. Hunt, T. J. Farmer and J. H. Clark, Element Recovery and Sustainability, The Royal Society of Chemistry, Lindon, 2013, pp. 1–28 Search PubMed.
- P. Anastas and N. Eghbali, Chem. Soc. Rev., 2010, 39, 301–312 RSC.
- P. Anastas and M. M. Kirchhoff, Acc. Chem. Res., 2002, 35, 686–694 CrossRef CAS PubMed.
- C. J. Li and B. M. Trost, Proc. Natl. Acad. Sci. U.S.A., 2008, 36, 13197–13202 CrossRef PubMed.
- C. E. Garrett and K. Prasad, Adv. Synth. Catal., 2004, 346, 889–900 CrossRef CAS.
- S. Ruccolo, Y. Qin, C. Schnedermann and D. G. Nocera, J. Am. Chem. Soc., 2018, 140, 14926–14937 CrossRef CAS PubMed.
- X. Allonas, J.-P. Fouassier, L. Angiolini and D. Caretti, Helv. Chim. Acta, 2001, 84, 2577–2588 CrossRef CAS.
- A. Singh, A. R. Scott and F. Sopchyshyn, J. Phys. Chem., 1969, 73, 2633–2643 CrossRef CAS.
- J. M. Hodgkiss, J. Rosenthal and D. G. Nocera, Handbook of Hydrogen Transfer. Physical and Chemical Aspects of Hydrogen Transfer, ed. J. T. Hynes, J. P. Klinman, H.-H. Limbach and R. L. Schowen, Wiley–VCH: Weinheim, Germany, 2006, vol. II part IV, ch. 17, pp. 503–562 Search PubMed.
- E. J. Land and M. Ebert, Trans. Faraday Soc., 1967, 63, 1181–1190 RSC.
- D. B. Larson, J. F. Arnett, A. Wahlborg and S. P. McGlynn, J. Am. Chem. Soc., 1974, 96, 6507–6508 CrossRef CAS.
- S. G. Lias, J. E. Bartmess, J. F. Liebman, J. L. Holmes, R. D. Levin and W. G. Mallard, NIST Chemistry WebBook, NIST Standard Reference Database, ed. P. J. Linstrom and W. G. Mallard, National Institute of Standards and Technology, Gaithersburg MD, 20899, (retrieved November 3, 2023) Search PubMed.
- A. J. Rieth, Y. Qin, B. C. M. Martindale and D. G. Nocera, J. Am. Chem. Soc., 2021, 143, 4646–4652 CrossRef CAS PubMed.
- N. Berg, S. Bergwinkl, P. Nuernberger, D. Horinek and R. M. Gschwind, J. Am. Chem. Soc., 2021, 143, 724–735 CrossRef CAS PubMed.
- W. You, J. M. Ganley, B. G. Ernst, C. R. Peltier, H. Y. Ko, R. A. DiStasio, R. R. Knowles and G. W. Coates, Chem. Sci., 2021, 12, 3898–3910 RSC.
- C. Y. Huang, J. Li, W. Liu and C. J. Li, Chem. Sci., 2019, 10, 5018–5024 RSC.
- R. S. J. Proctor, P. Chuentragool, A. C. Colgan and R. J. Phipps, J. Am. Chem. Soc., 2021, 143, 4928–4934 CrossRef CAS PubMed.
- J. A. Dantas, R. Echemendía, M. S. Santos, M. W. Paixão, M. A. B. Ferreira and A. G. Corrêa, J. Org. Chem., 2020, 85, 11663–11678 CrossRef CAS PubMed.
- M. W. Campbell, M. Yuan, V. C. Polites, O. Gutierrez and G. A. Molander, J. Am. Chem. Soc., 2021, 143, 3901–3910 CrossRef CAS PubMed.
- L. Han, J. B. Xia, L. You and C. Chen, Tetrahedron, 2017, 73, 3696–3701 CrossRef CAS PubMed.
- L. Capaldo and D. Ravelli, Eur. J. Org. Chem., 2017, 2017, 2056–2071 CrossRef CAS PubMed.
- N. Miyaura and A. Suzuki, Chem. Rev., 1995, 95, 2457–2483 CrossRef CAS.
- G. A. Molander and B. Biolatto, Org. Lett., 2002, 4, 1867–1870 CrossRef CAS PubMed.
- T. E. Barder and S. L. Buchwald, Org. Lett., 2004, 6, 2649–2652 CrossRef CAS PubMed.
- A. Kowalska, J. Sokolowski and K. Bociong, Polymers, 2021, 13, 470 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details, NMR spectra, (spectro)electrochemical data, transient absorption (TA) spectra, Stern–Volmer kinetics (PDF). See DOI: https://doi.org/10.1039/d3sc04362b |
| ‡ These authors contributed equally. |
| § Present address: Merck and Co., 90 East Scott Avenue, Rahway, NJ 07065, USA. |
| This journal is © The Royal Society of Chemistry 2023 |