 Open Access Article
Open Access ArticleCarbon-based electrochemical biosensors as diagnostic platforms for connected decentralized healthcare
Aqsa
Khan
 ,
Emily
DeVoe
,
Emily
DeVoe
 and
Silvana
Andreescu
and
Silvana
Andreescu
 *
*
Department of Chemistry and Biomolecular Science, Clarkson University, Potsdams, NY 13699-5810, USA. E-mail: eandrees@clarkson.edu; Tel: +1 315 268 2394
First published on 6th March 2023
Abstract
Electrochemical biosensors have the potential to provide rapid and inexpensive diagnostics while moving clinical testing from centralized labs to point-of-care (POC) applications. Conductive materials functionalized with bioreceptors that remain stable and functional for measurements in real-world conditions are essential for the fabrication of electrochemical biosensors, and carbon-based nanomaterials provide the electrical, chemical, structural, and mechanical features that make them suitable for POC devices. This review details the most recent developments in the use of carbon-based nanostructures, with a focus on one-dimensional carbon nanotubes, two-dimensional graphene, and graphene oxide, their interface with biological receptors, deposition on portable, flexible, and wearable substrates, and integration on low-cost platforms for detection of clinical biomarkers. The large-scale manufacturing and implementation of microneedles as implantable and electronic tattoos as wearable devices for on-skin diagnostics, and lab-on-mouth platforms as well as the interface with mobile technologies and their potential implementation for remote POC monitoring and decentralized healthcare through cloud processing and the internet of things (IoT) are discussed with examples of applications. The review concludes with an overview of the regulatory perspectives and future trends, challenges, and opportunities for commercialization and translation of these technologies from the research lab to practice, as useful diagnostic tools for remote monitoring of patient health conditions.
1. Introduction
Electrochemical biosensors are attractive in the biomedical field due to their simple instrumentation, ease-of-use, low cost, and ability to provide healthcare management from laboratory to point-of-care testing (POCT).1 Wearable sensing systems that collect, measure, and transfer analytical data from various biofluids of the wearer via wireless communication have gained increased interest due to their potential to measure biomarkers in real-time and non-invasively. Electrochemical biosensors have made notable progress over modern laboratory-based techniques, e.g., spectrophotometry, chemiluminescence, nuclear magnetic resonance (NMR), colorimetric, fluorimetry, and mass spectroscopy (MS) that involve complex sample processing, skilled lab operators, and lack miniaturization. Unlike conventional methods, electrochemistry is not affected by turbidity or interferences from light-absorbing molecules. Moreover, the use of biomolecular receptors in the biosensing design provides the desired selectivity for measurements. Electrochemical biosensors can be fabricated at a large scale and easily integrated into POCT devices. However, while various biosensing devices have been reported, several challenges still impede their transition into practice. These include i) deviations from calibration when used in real environments, ii) lack of manufacturing practices to ensure large-scale fabrication with preserved sensing functions, iii) the stability of the bioreceptor and their effective integration with the electronic components, and iv) the availability of flexible conductive materials. A critical need for the development of wearable patient-centered biosensing devices is the availability of conductive materials functionalized with bioreceptors that remain conformable to the body, stable, and functional under strain conditions. At present few materials fulfill this role.Advancements in nanotechnology have generated a variety of materials with the desired physiochemical properties and conductivity that can be incorporated into wearables. These include carbon-based materials, conductive polymers, metal nanoparticles, and liquid metals. Among these, carbon-based nanomaterials (CBNs) are the most commonly used due to their electrical, optical, mechanical, and thermal properties, cost, and availability.2 Introduced by RN Adams in 1958,3 carbon-based materials are the dominating class of electrode materials. Their ability to promote electron transfer kinetics, stability, low ohmic resistance, good biocompatibility, and enhanced interfacial adsorption properties compared to many traditional electrochemical materials make them unique for sensing applications.4 CBNs including one-dimensional CNTs (i.e., single-walled carbon nanotubes (SWCNTs), multiwalled carbon nanotubes (MWCNTs)), two-dimensional graphene (graphene oxide (GO), reduced graphene oxide (rGO), and graphene nanoribbon (GNR)) have improved electrical and mechanical properties making them suitable for electrode modification and fabrication of wearable devices. Others, like quantum dots and fullerenes have also been applied but not as extensively used as 1D and 2D materials.
Improved performance has been achieved by interfacing carbon-based nanostructures with polymers, metallic or catalytic materials to enhance their conductivity and electrocatalytic functions and facilitate stabilization of biomolecules.5 The rich surface functionalities of hybrid structures enable them to be modified with biomolecules such as aptamers, antibodies, DNA, redox markers, RNA, nanoparticles (NPs), or be deposited in nanocomposite forms on flexible substrates to enable the fabrication of disposable, flexible, and inexpensive devices. CBNs facilitate the electron transfer by increasing the accessible surface area while maintaining the flexibility.6 Wearable biosensors can provide non-invasive real-time information of dynamically changing biomarker levels in biological fluids such as sweat, interstitial fluid (ISF), tears, and saliva.7 Significant efforts have been made to effectively integrate these materials and biomolecular receptors within low-cost supporting structures such as paper, textiles, polymer, or ceramic substrates, which is an essential step to building biomolecular structures for field deployment and implementation.2b This review focusses on the use and integration of 1D and 2D carbon-based nanomaterial hybrids for constructing wearable biosensing devices as diagnostic tools for connected personalized healthcare.
To date, reviews on CBNs for electrochemical biosensors have provided a summary of new properties of these materials and discussed their implementation in conventional laboratory-based biosensing designs and standard electrodes for personalized applications.8 Here we focus on multifunctional hybrid carbon-based materials and discuss their functionalization with bioreceptors, and integration within flexible and wearable substrates. We then discuss the possible implementation of these devices as diagnostic tools for remote monitoring of patient health conditions, and connectivity though cloud-based processing and the internet of thing (IoT). We also address manufacturing challenges, a necessary step to achieve the large scale needs to translate biosensing technologies from the lab into the market. In the first part of the review, we first discuss the properties, functionalization, and interfacing of carbon-based nanomaterials (GO, rGO, CNTs) with biological receptors. We then evaluate their deposition onto flexible and stretchable platforms and their integration with wearable electronic circuitry and the IoT health network. Finally, we discuss manufacturing by inkjet, 2D or 3D printing and the roll-to-roll fabrication on flexible electronics substrates and summarize their capabilities and potential for use at the point of need and decentralized connected healthcare. Table 1 provides examples of recent work on carbon-based materials (i.e., carbon nanotubes, graphene and hybrid carbon composites), their characteristics, applicability and integration in POC devices.
| Modified electrodes | Substrate | Analyte | Sample | Linear range | LOD | Ref. |
|---|---|---|---|---|---|---|
| Carbon nanotubes-based sensors | ||||||
| MWCNt–COOH–PB | Rubber glove | Uric-acid, glucose | Sweat | 3.58 μM, 9.10 μM | 9 | |
| G/CNTs | Textile | Glucose | ISF | 0.06 μM | 10 | |
| MWCNt–PB | Cloth-based chip | Glucose | Sweat | 0.05–1 mM | 11 | |
| Carbon paste | Microneedle | Levodopa | ISF | 0.5–3 μM | 0.5 μM | 12 |
| MCNTs-RGO/CFT | Textile | Glucose | Urine | 0–40 mM | 3.95 mM | 13 |
| MWCNT/PEDOT | Fabric | K+ | Saliva | 1–1000 nM | 1 nM | 14 |
| Graphene based sensors | ||||||
| Graphene fiber-PB | Fabric patch | Glucose | Sweat | 50 mM–1 M | 50 μM | 15 |
| Graphene ink | Flexible on-chip | Dopamine | Sweat | 5 nM | 16 | |
| PB-RGO nanofilms | Head band (fabric) | Glucose | Sweat | 7.94 μM | 17 | |
| Laser induced graphene (LIG) | Filter paper | Uric acid | Urine | 10–300 μM | 3.97 μM | 18 |
| Elastomer/graphene ink | Wearable | Na+ | Sweat | 19 | ||
| PEDOT/sulfur-doped graphene (PEDOT-G) | Wearable | Dopamine | Tears | 101 × 10–9 m | 20 | |
| Graphite nanocrystals with tetrahedral amorphous carbon (GNC-TAC) | Filter paper | Pb2+ | Urine | 0.5–700 μg L−1 | 0.15 μg L−1 | 21 |
| Graphene sponge–chitosan–PB (GS/CTS/PB) | Flexible wearable | Glucose | Sweat | 8.17–1000 μM | 2.45 μM | 22 |
| Carbon/graphite ink | Tattoo | Vitamin C | Sweat | 10–50 μM | 23 | |
| Hybrid carbon-based sensors | ||||||
| Boron-doped graphene quantum dots anchored to CNTs (BGQDs/CNTs) | Wearable | Uric acid | Sweat | 6.10–7.35 μM | 50 μM | 24 |
| Carbon nanotubes and gold nanotubes (C–Au NTs) | Flexible wearable | Urea | Sweat | 1100 mM | 0.1 mM | 25 |
| SWCNTs/ferrocene-polyaniline film/Cu (SWCNTs/F-P/Cu) | Non-invasive | Glucose | Sweat | 0.081 mM | 26 | |
| AuNPs–rGo and PtNPs–rGo | Flexible substrates | Dopamine | Urine | 0.1–20 μM (Au); 0.1–10 μM (Pt) | 75 nM and 62 nM | 27 |
| Graphene–gold NPs (GP–Au NPs) | Flexible substrate | Dopamine and uric acid | Cerebrospinal fluid | 20 nM–40.76 μM and 20–500 μM | 10 nM and 1.47 μM | 28 |
| Nafion and reduced graphene oxide enclosed carbonized silk fabric (Nafion/rGo/CSF) | Wearable electronics | Dopamine | Urine | 1 nM–30 μM | 1 nM | 29 |
| Nitrogen doped graphene (NG/PEDOT) film | Flexible wearable | Dopamine | 0.2 μM to 90 μM | 54 nM | 30 | |
| Nickel nanoparticles decorated laser induced graphene (Ni/LIG) | Glucose | 0.50–1666 μM | 0.29 μM | 31 | ||
| MIP modified polyvinylidene fluoride (PVDF)/graphene | Flexible wrist band | Lactate | Sweat | 0–20 mM | 15 mM | 32 |
| Fe/Co/rGo | Flexible wearable | Glucose | Tears | 0.1906.4 μM | 0.07 μM | 33 |
| NiO/rGO/PtE | Reusable | Linezolid drug | Urine | 0.1–90 μM | 31 μM | 34 |
| Copper oxide modified carbon nanotubes (CuO@CNTFs) | Flexible microelectrodes | Glucose | Biofluid | 13 mM | 1.4 μM | 35 |
| Porous polypropylene/carb on nanotube/polyaniline (p-PP/CNT/PANI) | Flexible mask | Respiratory rate | Human breath | 500−70 ppb | 500 ppb | 36 |
| MXene–MWCNTs | Flexible wearable | Cortisol | Sweat | 0.1 fg mL−1–1 μg mL−1 | 0.03 fg mL−1 | 37 |
| rGo–Au/SPGE | Non-invasive wearable | Glucose | Sweat | 1.25–850 μM 0.85–7.72 mM | 1.25 μM | 38 |
| Carbon fiber based-MXene–MoS2 | Flexible substrate | AA, DA, UA, microRNA | Human serum urine | 10–1000 μM, 0.5–200 μM, 0.5–150 μM | 0.89 μM, 0.23 μM, 0.35 μM 3.16 aM | 39 |
| Au–CNTs–chitosan | Disposable | β-Hydroxybutyrate | Saliva | 0.1 to 3.0 mM | 50 μM | 40 |
2. Carbon nanomaterials and hybrids for electrochemical biosensors
2.1. Properties of carbon nanomaterials and hybrids
Commonly used forms of carbon-based materials including porous carbon, graphite, graphene, quantum dots, carbon nanotubes, (e.g., SWCNTs and MWCNTs), graphitic carbon nitride, carbon nanofibers (CNFs), buckminsterfullerene (C60), nanodiamonds (NDs) and carbon nanospheres,41 summarized in Fig. 1. Their intrinsic conductivity, stability and ease of functionalization makes them ideal for electrochemical biosensors.42 Graphene and CNTs are the most used forms of carbon for electrochemical biosensing. A summary of their properties is provided below.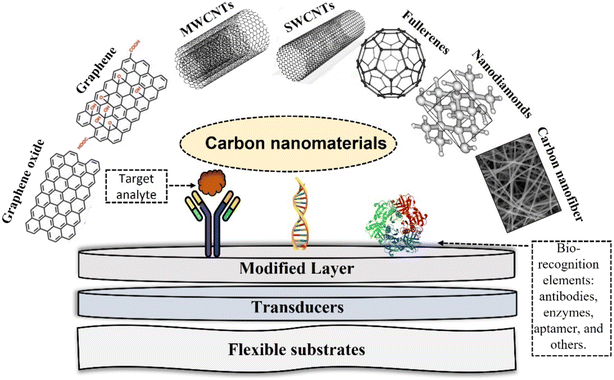 | ||
| Fig. 1 Summary of different types of carbon nanostructures for electrochemical biosensors and active biofunctionalized surface for sensing design. | ||
The key to the successful application of graphene in electrochemical biosensors lies in its ability to be interfaced with bioreceptors and be integrated within substrates that can serve as support for electrodes. GO, a highly oxidized form of chemically modified graphene, is commonly used for this purpose due to the presence of carboxylate groups that act as binding sites for biomolecules. The carbon sheet of GO has COOH on the outside along with oxygen functionalities, –OH, and epoxy (–O–) groups inside, which enhance its water solubility. The surface chemistry of GO is negatively charged due to the carboxylate groups, with partially hydrophilic regions with hydrophobic distributions at edges, capable of hydrogen and electrostatic binding. The reduced form of GO, rGO, is also commonly used due to its ability to restore conductivity and introduce defective structures into the carbon lattice. rGO is produced by treating GO under reducing conditions, through chemical, thermal or electrochemical means. 1) Chemical reduction involves the use of strong reducing agents such as hydrazine, sodium borohydride, hydroquinone, gaseous hydrogen, and strong alkaline solutions. 2) Thermal mediated reduction is produced by direct heating of GO at high temperature creating thermodynamically stable carbon oxide species. The heating of GO to 1050 °C causes the release of high temperature carbon dioxide and exfoliation in between the stacked layer of GO platelets. 3) The electrochemical treatment involves the removal of oxygen functionalities in a sodium phosphate buffer solution. Functionalized GO deposited on a variety of electrode substrates (plastic, paper, polymer etc.) showed rapid reduction within few seconds during electrochemical measurements.48Fig. 2 illustrates the common forms of graphene, GO and rGO. The carboxylate groups on GO are activated using coupling reagents such as 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDC) for further covalent attachment of biomolecules.49
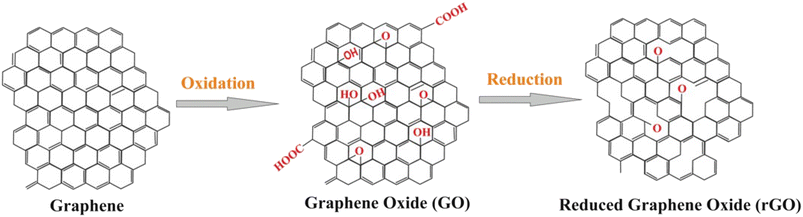 | ||
| Fig. 2 General structure of graphene, carboxyl-functionalized graphene oxide and reduced graphene oxide nanomaterials (with permission from Springer, copyright [2018]50). | ||
Activated graphene is the most commonly used electrode material in electrochemical biosensors for direct detection of electroactive molecules such as catecholamine neurotransmitters like dopamine. Dong et al., developed a 3-D loofah sponge made of a carbon/graphene aerogel, fabricated via one pot hydrothermal method and demonstrated its efficacy for the electrochemical detection of H2O2 and dopamine with detection limits of 1.2 μM and 0.25 μM, respectively.51 The activated graphene provided a large working surface area, while the sponge-like structure prevented the graphene sheets from aggregation with improved catalytic activity. Zhou et al., utilized a hybrid nitrogen-doped graphene microelectrode and demonstrated its performance for neurotransmitters sensing with high sensitivity and low detection limits of 0.69 nM for dopamine and 6.5 nM for 5-hydroxy tryptamine in real serum samples.52 The doped material provides enhanced electrocatalytic activity which results in increased performance for neurotransmitter detection.
2.2. Surface activation
The high surface area and abundant functional groups of carbon-based nanomaterials make them attractive platforms for the immobilization of biomolecular receptors such as Ab, enzymes, nucleic acids, and proteins (Fig. 3A). The vast majority of studies have utilized graphene, SWCNTs and MWCNTs, surface-activated or in hybrid-composite forms with polymers and other materials such as metal or metal oxide NPs and ionic liquids to enhance their binding properties and electrochemical performance. Activated carbon has increased porosity and surface area and the surface treatment can be customized by selecting the activating agent to fit the desired application.59 Typical activation procedures involve the use of strong oxidizing gases and agents like phosphoric acid to produce pores through etching and add oxygen-containing functional groups onto the carbon structure, creating active sites that can easily attach biomolecules.60 Tunable porosity and surface chemistry was achieved by activating hydrochar with phosphoric acid. The procedure involves mixing hydrochar with phosphoric acid for 8 hours at room temperature, drying at 110 °C followed by activation under static air for 1 hour.61 These materials can be used to create composites for sensing.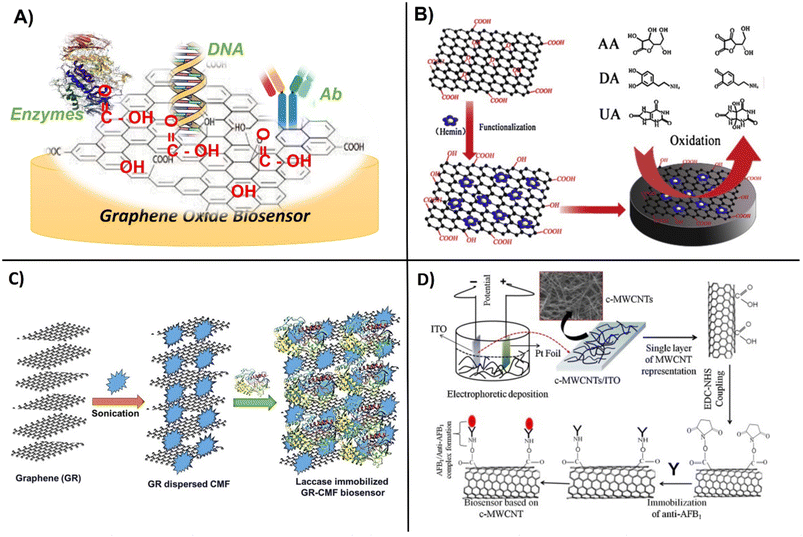 | ||
| Fig. 3 Schematic showing, A) general design of GO modification with enzymes, DNA and Abs, B) functionalization of graphene oxide with hemin for enhanced oxidation of ascorbic acid, dopamine and uric acid (with permission from Elsevier, copyright [2014]65), C) immobilization of laccase within graphene and cellulose microfibers (with permission from Springer, copyright [2017]70), and D) carboxylation of CNTs followed by functionalization with monoclonal Abs for the detection of aflatoxin-B1 (with permission from Elsevier, copyright [2013]69). | ||
Surface activation of CNTs by acid treatment generates functional groups that can further facilitate biomolecule binding.53 Further, site defects and edge effects provide enhanced reactivity of graphene and CNTs due to higher density of functional groups on their active sites.62 Surface activation is known to increase the surface/volume ratio and improve the sensing performance. For example, the electrochemical pre-treatment of carbon fiber microelectrodes (CFMEs) in 0.1 M NaOH at a potential of 1 V for 600 s was shown to improve the sensitivity of the CFMEs, enabling ultrasensitive measurements of dopamine in the brain of zebrafish embryos in the physiological nanomolar concentration range.63
2.3. Biofunctionalization
Carbon nanostructures are generally seen as biocompatible and highly suitable for biomolecule grafting. This can be achieved through simple adsorption via forces such as weak van der Waals, electrostatic, hydrophobic, and hydrogen bonding, with adsorption varying with the type of receptor molecule.64 This process is favored by positively charged molecules and those with conjugated π-bonds that can form π-π stacking with the aromatic residues of proteins. Zou et al., utilized the π–π stacking interaction between graphene oxide and hemin (H–GO) to increase electrochemical performance and enable the simultaneous detection of dopamine (LOD = 0.17 μM), ascorbic acid (LOD = 0.3 μM) and uric acid (LOD = 0.17 μM) respectively (Fig. 3B).65 Aromatic compounds such as pyrene and perylene tetracarboxylic acid (PTCA) molecules have also been used as anchors for the binding of bioreceptors via π–π-stacking. PTCA-stacked graphene worked as a redox mediator to facilitate the immobilization of aptamers with increasing sensitivity of electrochemical aptasensors. Yali et al., demonstrated the use of graphene/perylene tetracarboxylic acid (GPD) as a novel redox probe with improved conductivity and electrochemical active area. The GPD probe was shown to improve the electron transfer due to the large delocalized face-to-face surface interaction of π–π-stacking. The aptasensor exhibited high sensitivity with a detection limit of 200 fM.66 Activated forms of carbon are more easily able to form conjugates with biomolecules, but their properties vary with the size, charge, and nature of the biomolecule. In a study comparing binding interactions between GO and DNA, it was found that shorter DNA is adsorbed tighter and more rapidly to graphene than longer DNA and that adsorption is favored at a low pH and a higher ionic strength.67 Chemically reducing carbon with hydrazine provided enhanced surface area, good reusability and a low detection limit of 0.37 pM of SARS-CoV19, as compared to methods such as electrical, thermal, or catalytic reduction.68 An immunosensor based on covalently functionalized MWCNTs deposited on indium tin oxide (ITO) modified with monoclonal aflatoxin-B1 Ab enabled detection of aflatoxin-B1, with a sensitivity of 95.2 μA ng−1 mL cm−2 and a LOD of 0.08 ng mL−1 (Fig. 3D).69The surface properties, solubility, functionality, and binding efficiency of biomolecules on carbon nanomaterials have been significantly improved by coating or co-entrapment with polymeric layers. The immobilization of polymers and biomolecules on graphene can be achieved by weak noncovalent interactions via electrostatic, π–π stacking, and van der Waals forces. For example, laccase was effectively immobilized within a graphene–cellulose microfiber composite which enabled detection of catechol in the concentration range 0.2–209.7 μM (Fig. 3C).70
Covalent attachment of polymers such as chitosan, polyvinyl alcohol (PVA), and polyethylene glycol (PEG) to GO via the residual oxygen-containing functional groups has also been demonstrated, shown to improve processing and make nano-GO highly dispersible and chemically stable in physiological solutions. The covalent functionalization of a few-layers graphene or carbon nanosheets with PVA through EDC esterification improves solubility of the graphene nanomaterials71 and facilitates uniform deposition. PEG-GO is the preferred material for biosensing measurements in biological environments and physiological conditions. The use of PEG layers in a graphene-based field effect transistor with aptamer receptors enabled selective real-time detection of a cancer biomarker, prostate specific antigen, in physiological medium.72 The aptamer receptor was regenerated for multiple uses. The functionalization of GO with colamine and PEG cross-linker was shown to improve biomolecule adhesion and coating density of DNA for DNA biosensing as compared to a silanization procedure using (3-aminopropyl) trietoxysilane.73 The multistep functionalization of GO with PEG enhanced the surface coverage and robustness of the biosensor while improving applicability and performance, particularly those designed for in vivo applications. A preferred polymer for enhancing enzyme immobilization on carbon nanostructures is chitosan. Yang et al., developed a uric acid biosensor by using a chitosan/CNT dispersion cross-linked with glutaraldehyde and drop casted on a 3D super-aligned CNT array electrode. The advantage of this procedure is that the CNTs in the 3D array maintained their structure and electronic conductivity while providing a high surface area for enzyme immobilization. A detection limit of 1 μM uric acid was reported. This platform can be broadly implemented for point-of-care testing of other biomolecules.74
Examples of commonly used procedures to attach enzymes, DNA, and Ab to carbon-based nanostructured electrodes though PEG and EDC activation are shown (Fig. 4).49,73 Polymer-supported rGO prepared using poly(3,4-ethylenedioxythiophene)polystyrene sulfonate (PEDOT:PSS) demonstrated high efficiency for the electrochemical detection of DNA hybridization. The PEDOT:PSS/rGO composite was prepared in a lightweight sponge-like structure and was highly conductive. When used as electrode material, PEDOT:PSS/rGO composite enabled electrochemical detection of DNA hybridization with a sensitivity of 1 fM.75 Other procedures work on the modification of GO with proteins such as glutathione peroxidase using EDC and Nafion to construct a glutathione biosensor with an LOD of 0.9 nM as reported.76 Lee et al., developed semiconducting single-walled carbon nanotubes (sc-SWCNTs) fibers as wearable electrochemical biosensors for glucose monitoring above 0.5 μM. The sc-SWCNTs are difficult to work due to needing an improved separation method that limit their practical application.77
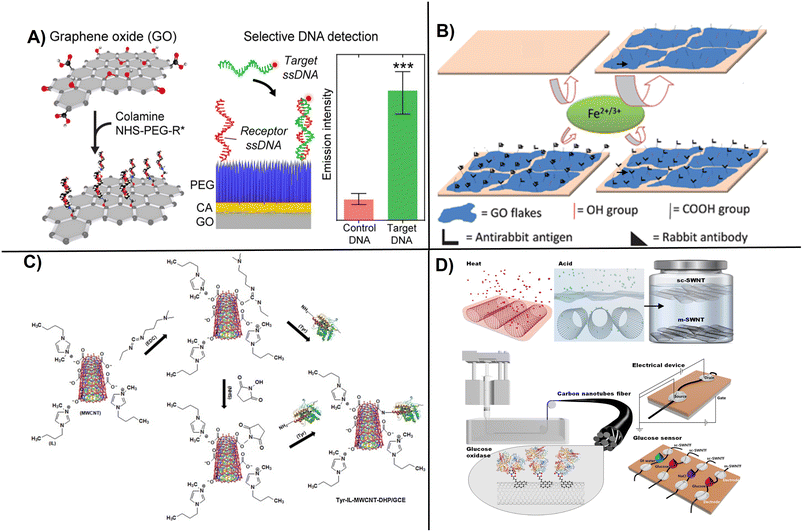 | ||
| Fig. 4 Examples of surface functionalization and electrochemical device fabrication procedures showing: A) PEG-modified graphite layer for DNA coupling (with permission from ACS, copyright [2021]73), B) covalently immobilized Ab on GO flakes onto a glassy carbon electrode surface for label-free sensing (with permission from RSC, copyright [2011]49), C) functionalization of MWCNTs with tyrosinase using 1-butyl-methylimidazonium chloride ionic liquid (with permission from Elsevier, copyright [2013]78), and D) fabrication of fiber-based glucose biosensor involving surface activation by acid treatment and immobilization of glucose oxidase on modified SWCNTs for glucose sensing (with permission from Elsevier, copyright [2020]77). | ||
3. Integration in portable low-cost substrates
3.1. Paper-based electroanalytical devices
Paper is an interesting material for POC devices due to its high surface area, porosity, ease of modification, and affordability. Electrochemical paper-based analytical devices (ePDAs) were introduced more than a decade ago, providing several inherent advantages such as ease of use, low volume per sample, portability, manufacturability, disposability, and low cost.79 ePADs combine the advantages of paper-based microfluidic devices through capillary action with electrochemical detection. Dungchai et al., 2009 reported the first electrochemical paper-based device by depositing a highly conductive track onto a paper substrate, using a carbon-based ink containing Prussian blue as a mediator.80 Fabrication of paper electrodes involves modification of the cellulosic structure to impart conductivity and hydrophobicity in order to facilitate migration of reagents and enable monitoring of electrochemical processes on paper. Therefore, the selection of a suitable material to impact conductivity and the functionalization steps are highly important to develop low-cost POC diagnostics. Carbon has been used as a convenient conductive material to create conductive tracks.Along with carbon, a variety of different materials including metals, polymers, ionic liquids, and metal NPs have also been incorporated to create conductive structures, while maintaining simplicity and low cost. Fabrication of an ePADs typically involves the use of various printing techniques including inject printing, screen printing, stencil printing and photolithography, many of which require development of biocompatible inks of specific rheology to enable printing. Carbon inks have been prepared by mixing graphite, chitosan, glycerol, and enzyme before screen printing on polyethylene terephthalate (PET) substrate to design a disposable sensor for uric acid and catechol as reported.81 This section summarizes the recent advancements of carbon-based materials to design conductive ePADs for electrochemical POC testing along with their working performance and applications.
One of the first coupling paper designs of electrochemistry was based on a three-electrode system for simultaneous detection of glucose, lactate, and uric acid. The biosensor was fabricated from a carbon ink deposited on Whatman grade 1 filter paper to create working, counter, and reference electrodes (WE, CE and RE) and their connections (Fig. 5A). The WE ink contained oxidase enzymes that specifically recognized each target analyte and detection was accomplished by electrochemically measuring the enzymatically produced hydrogen peroxide. The ink contained Prussian blue (PB; FeIII4[FeII(CN)6]3) as mediator for the reduction of H2O2. The RE contained Ag/AgCl as a pseudo-reference.80 This study demonstrated the successful integration of carbon ink for creating functional conductive ePAD for POC monitoring. The electrochemical sensor was further combined with a colorimetric unit for dual lab-on-a-chip screening of gold and iron.82 In other configurations, ePADs have been deposited on top of a screen-printed electrode for sample pre-concentration (Fig. 5B). These sensors have demonstrated applicability for the detection of dopamine in serum with the concentration range 1–100 μM and an LOD of 0.37 μM using square-wave voltammetric technique.83 The method provided selectivity against ascorbic acid and uric acid by integrating the anionic surfactant of sodium dodecyl sulfate impregnated in one layer of the device which shifts the oxidation peak of dopamine to more negative values. Other works explored the use of pyrolyzed paper as a conductive substrate for ePAD design. A microscopic study of three types of papers, 3 mM chromatography, imaging card, and multipurpose printing paper, demonstrates a fibrous cellulose structure preserved in all cases after pyrolysis (Fig. 5C). The pyrolyzed papers were used as electrode materials and showed high surface area and quasi-reversible behavior for Fe(CN)63−/Fe(CN)64− redox couple. The largest electroactive area and detection performance was obtained for the 3MM chromatographic paper.84 The procedure enabled the immobilization of urate oxidase enzyme that catalyzes the oxidation of uric acid to allenoate, reaching detection in the concentration range of 0.001–0.833 mM. Such low-cost platforms can be easily interfaced with cellphone connected electroanalyzers and are suitable for applications in remote and low-resourced communities.
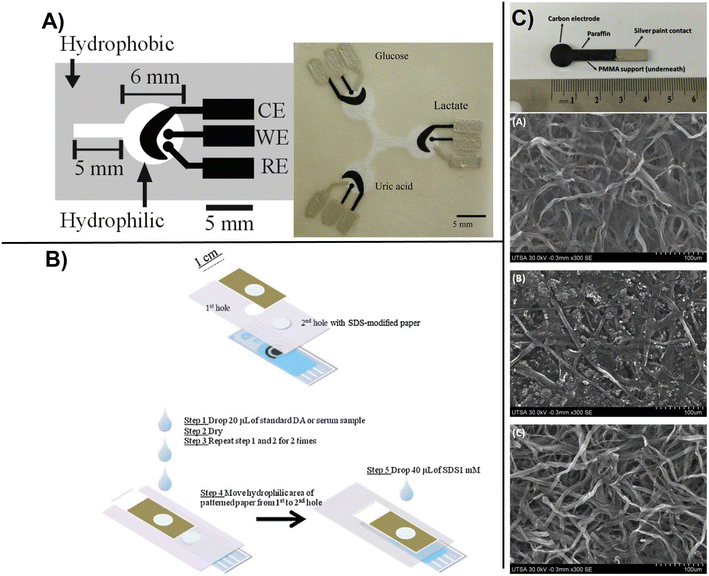 | ||
| Fig. 5 Basic design of paper based electrochemical sensors showing: A), printed carbon-based paper electrode with WE, working electrode; RE, reference electrode and CE, counter electrode for detection of glucose, lactate, and uric acid. The silver electrodes and contact pads are made from Ag/AgCl paste with the black electrode portions being the PB-modified carbon electrodes. The device size is 4 cm × 4 cm (with permission from ACS, copyright [2009]80), B) ePAD-screen printed electrode configuration for the determination of DA where the patterned paper was used for sample pre-concentration, and improved selectivity using SDS to shift the operating potential to lower values preventing interferences (with permission from Elsevier, copyright [2012]83), and C) carbon electrodes made from pyrolized paper deposited on paraffin and sealed with a silver paint including SEM micrographs of the working carbon electrodes obtained from pyrolysis of imaging card paper (A), multipurpose printing paper (B), and 3MM chromatography paper and (C), showing a microfibrillar structure (with permission from Elsevier, copyright [2016]84). | ||
A simple method to fabricate an ePAD with 2 cells and 4-working electrodes on paper surface using an inexpensive craft cutter was reported for multiplexed determination of pharmaceutical compounds (ascorbic acid, paracetamol, and caffeine), following to the fabrication steps showed in (Fig. 6).85 The interaction of graphene nanomaterials, including graphene oxide, reduced graphene oxide and few layer graphene, with different biomolecules have been discussed and additional details can be found in ref. 86.
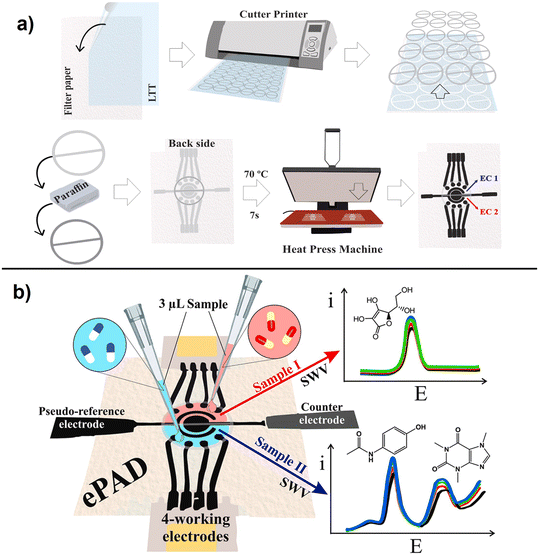 | ||
| Fig. 6 Schematic representation of multiplexed (4 WE) ePAD fabrication showing a), steps in the fabrication process using a cutter printer involving wax barrier production and b), illustration of the integrated ePAD for multi-analyte detection using SWV(with permission of Elsevier, copyright [2019]85). | ||
Improved sensing can be achieved by functionalization with conductive polymers, metal oxides and metal sulfides. Flexible self-standing graphene-based paper electrodes have been designed by using GO functionalized with zinc sulfide nanostructures (GO/ZnS) via thermal reduction provided increased electrochemical activity for oxidation of dopamine, enabling detection in 0.1–2300 μM concentration range (Fig. 7a).87 Flexible electrodes have been fabricated by coating graphene paper with electrocatalytic 2D gold nanoclusters. The process involved transfer of 2D nanoclusters at an oil densely packed to a GO paper substrate which led to formation of a uniformly monolayer of AuNPs (Fig. 7b). The performance of these sensors was demonstrated for the electrochemical sensing of glucose and H2O2 secreted by living cells.88 These approaches open a new avenue to control and systematically study the sensing interface for the next generation miniaturized and flexible freestanding bioelectronics.
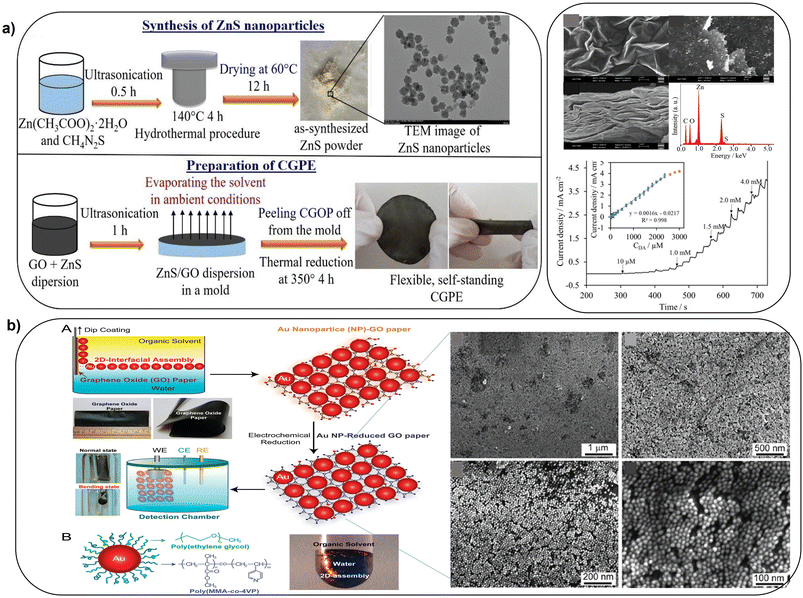 | ||
| Fig. 7 Schematic illustration of: a), synthesis of ZnS NPs and deposition of GO and ZnO as a flexible standalone CGPE sensor (with permission from Wiley, copyright [2021]87) and b), the fabrication of freestanding hybrid electrodes from 2D-assembly of AuNPs and GO-modified paper, with SEM images of 2D-assembly of AuNPs coated on GO paper at different magnifications (with permission from ACS, copyright [2012]88). | ||
In another work, flexible freestanding rGO-based ePADs were fabricated by direct electrochemical deposition of bimetallic sulfides (NiCo2S4) under vacuum filtration onto the paper substrate, enabling the simultaneous detection of ascorbic acid and folic acid. Bimetallic sulfides can be used for device fabrication due to their porous structure, self-doping ability, high electrochemical activity and active site density, providing superior performance as compared to monometallic sulfides.89 Flexible NiCo2S4 based rGO paper electrodes exhibited a very low detection limit (ascorbic acid ∼3.0 × 10−8 M, folic acid ∼1.6 × 10−9 M) due to its large electrochemically active surface area.90 Yan et al., designed an ePAD by modifying rGO with thionine and gold nanoparticles (rGO/Thi/Au NPs). The platform was used for the detection of cancer antigen based on the strong immunocomplex formation between the CA125 Ab and CA125 antigen which caused a reduction of current response of thionine, directly relating with the concentration of analyte. A LOD of 0.01 U mL−1, good linearity (0.1–200 U mL−1) and good correlation with the traditional Elisa procedure were reported.91 Sadkate et al., designed a non-enzymatic sensor by modifying a GO-based ePAD with a cobalt phthalocyanine-ionic liquid composite. The sensor was used for the electrochemical detection for glucose oxidation within the 0.01–1.3 mM concentration range with LOD of 0.67 μM respectively.92
Performance of ePADs vary with the porosity, material type and the modification procedure. Integration of CNTs for ePADs fabrication takes an advantage of high surface area, improved internal structure, and chemical stability. Valentine et al., studied the effect of the porosity of paper before and after functionalization with CNTs-Nafion using different fabrication methods (i.e., drop casting, laser scrubbing and origami) to establish the effect of network porosity on the senor performance for the detection of glucose. The results indicate that changing porosity of the paper can lead to an almost two fold increase in sensitivity (Fig. 8A).93 Novell et al., designed a simple CNTs-paper based potentiometric sensor for the electrochemical detection of K+, NH4+, and pH with performance comparable to classical solid-state ion-selective electrodes.94 In the same way A non-enzymatic SWCNT-based ePAD for the detection of glucose was fabricated by wax printing and micropatterning on nanocellulose surface. The method provided good electrochemical conductivity and mechanical flexibility due to the strong interconnection between the nanotubes layer and the porous cellulose membrane. To design a hybrid conjugation, gold nanoparticles were further deposited on the SWCNT to enhance the electrocatalytic performance. Designed sensor provided a sensitivity of 240 μA mM−1 cm2 with the LOD of 148 μM as shown (Fig. 8B).95 Modification of bacterial cellulose with CNTs provides flexibility and smart stretch-resistant for self-wearable platforms as recently demonstrated with a water sensor for smart diapers (Fig. 8C).96 Handling of fluid in an ePAD design is very important for accurate measurements. A hydrophobic barrier was drawn by pattering (photolithography, wax printing, inject printing, stamping, screen printing etc.) the paper surface to create a barrier for liquid confinement and ensuring controlled flow of reagents to the reaction zone.97 Another demonstration of ePAD fabrication involve the development of a CNT-based conductive ink inkjet-printed directly on the paper surface using a standard office-based printer. Additionally, a hydrophobic barrier was created on paper by depositing mineral oil onto the desired surface (Fig. 8D). The printed electrodes were tested for the detection of dopamine and iron.98 As demonstrated by these examples, carbon based conductive materials have several notable advantages and can be easily incorporated in ePADs using straightforward printing.
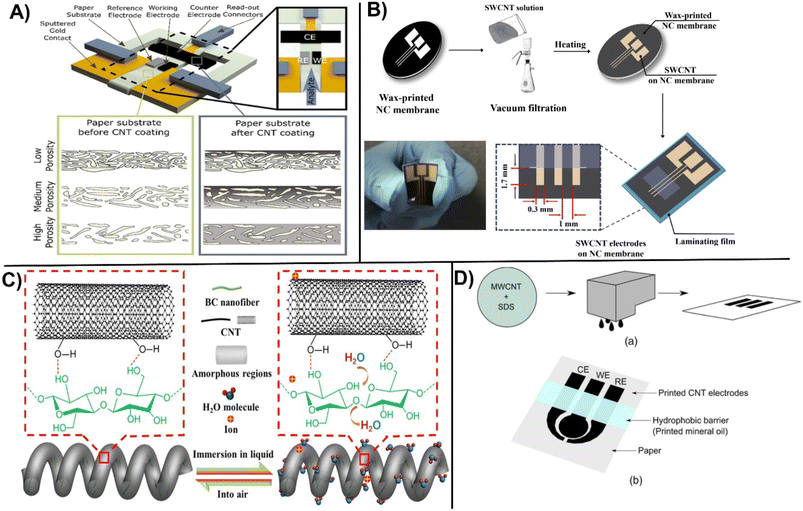 | ||
| Fig. 8 A) Fabrication of paper-based sensor before and after CNT modification and electrode design studying the influence of porosity on the CNT coating (with permission from ACS, copyright [2020]93). B) Micropatterning on SWCNT patterning on NC membrane to fabricate a flexible SWCNT electrodes (with permission from Elsevier, copyright [2018]95), and C) immobilization of CNTs on bacterial nanocellulose for creating stretchable helical fibers (with permission from ACS, copyright [2022]96), and D) fabrication of inkjet-printed CNT-based paper sensor involving: (a) preparation of CNT ink, followed by inkjet-printing the ink, and (b) printing a hydrophobic barrier on top (with permission from ECS, copyright [2015]98). | ||
3.2. Disposable screen-printing platforms
Screen printing is the first, most frequently used method for designing low cost electrodes. Screen printing carbon electrodes (SPCEs) are fabricated by sequentially jetting conductive inks with the desired size and geometry on a low-cost and eco-friendly substrate (i.e., such as silk, nylon or even paper). The sensors are most often fabricated as an integrated three electrodes system of working, counter, and reference electrode. Introducing carbon-based nanomaterials into screen printing inks offers an economical, simple, and reproducible way to fabricate nanostructured sensing interfaces. The procedure involves optimization of suitable inks and substrate templates as well as deposition conditions to ensure homogeneity and uniformity of the ink on the substrate. The scalability, low cost and the ability to multiplex electrodes for various biomarkers makes screen printing an attractive manufacturing methods for large scale diagnostics, with possibility for implementation at industrial scale.99 A SPCE electrode fabricated by printing of a graphite ink over a paper adhesive enabled direct measurement of melatonin oxidation in the concentration range between 10–100 μM without any electrode modification.100 With careful optimization, bioreceptors can be included in printing inks and be deposited automatically by printing.Modification of SPCE electrodes, carbon-based nanostructures, and bioreceptors, particularly enzymes, is well established. Materials such as graphene or CNTs have been reported to enhance the surface area and facilitate enzyme attachment, also enhancing electron transfer and increasing sensitivity and stability.101 Enzyme immobilization is generally accomplished with the help of conductive polymers or biopolymers, or with chemical immobilization procedures. Most enzyme sensors are dedicated to blood glucose sensing. A glucose SPCE-based biosensor was fabricated by first coating the surface of SPCEs with graphene-poly(3,4-ethylenedioxythiophene):polystyrene sulfonic acid (GP-PEDOT:PSS) nanocomposites, followed by the immobilization of the glucose oxidase (GOD) enzyme with (GP-PEDOT:PSS) by crosslinking with glutaraldehyde. Designed graphene based (GOD/GP-PEDOT:PSS) conductive electrodes were 13 times more sensitive than the ones without graphene (GOD/PEDOT:PSS) with the LOD of ∼0.3 μM.102
Using a unique form factor, Hu et al., designed a screen-printed electrode for glucose detection in blood using a porous graphene aerogel composite with Prussian blue (PB) and immobilized glucose oxidase (GOx). The combination of graphene aerogel increased conductivity and catalytic performance, enabling measurements of glucose with a linear range between 0.5–6.0 mM with LOD 0.15 mM. The preparation of the biosensor followed a typical screen-printing process with the last layer being a graphene-PB aerogel with GOx immobilized in chitosan (Fig. 9A).103 Other approaches involve the use of catalytic GO-based materials for non-enzymatic glucose detection. In recent example, a copper-reduced GO-modified SPCE electrode was designed for non-enzymatic measurement of glucose oxidation, achieving a linearity range between 0.10–12.5 mM, an LOD of 65 μM, and a sensitivity of 172 μA mM−1 cm−2 (Fig. 9B).104 To improve detection sensitivity, MWCNTs can be modified with perylene tetracarboxylic acid to electrochemically amplify the signal as reported.82
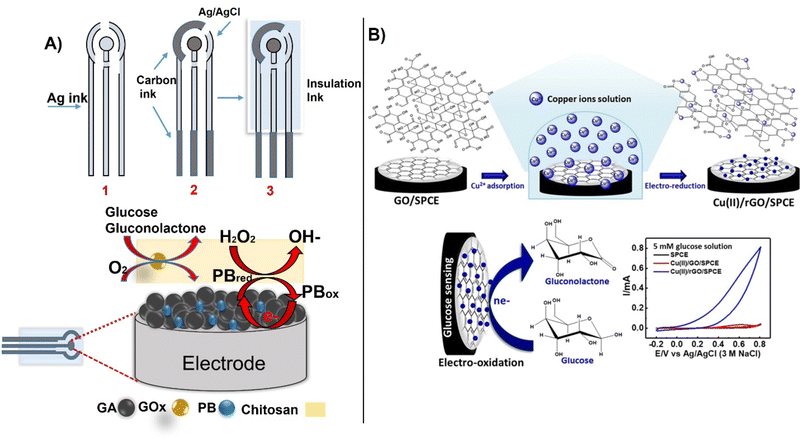 | ||
| Fig. 9 The sequential process for preparing SPCE electrodes for glucose detection by printing, (A) with an example of functionalization of the working electrode with GOx immobilized within graphene-PB aerogel composites,(reproduced from Springer,103 copyright [2022]) and (B) non-enzymatic detection using Cu–rGO deposited on SPCE (with permission from Springer, copyright [2021]104). | ||
Recently, an enzyme based electrochemical biosensor was fabricated by electrochemical deposition of hybrid silver-based graphene oxide (Ag–rGO) and used for the detection of urea in urine. The hybrid electrodes offered a 12 folds increase in sensitivity (47.598 μm−1 M−1) and a LOD of 0.1623 μM.105 Given their improved performance, carbon-based hybrid materials have been further employed for the detection of several clinically relevant compounds such as salivary uric acid,106 tyrosine,107 SARS- CoV-2,108 viruses,109 and DNA,110 respectively. In the same way, these materials are provided useful platforms for the detection of viruses,111 bacteria,112 heavy metals113 and antimicrobial resistance (AMR).114 Bachmann et al., developed a label-free electrochemical sensor for point-of-care detection of AMR for better management of patient monitoring during antibiotic therapy as an alternative to culture-based methods.114 The method involves immobilization of peptide nucleic acid probes on the electrodes surface via electrochemically reduced diazonium cations activated with EDC/NHS via electrochemical impedance spectroscopy. SPCEs are useful platforms for biomarker detection through affinity recognition involving immuno and aptamer recognition, enabling detection of cancer antigens, tumor biomarkers115 and cardiac diseases like C-reactive protein116 and troponin.117 A C-reactive protein (CPR) based on label-free SPCE immunosensing with CPR antibodies displayed a linear range of 0.5–100.03 ng ml−1 and an LOD of 0.036 ng ml−1.116 A disposable diazo-sulfonamide modified SPCE with DNA enabled measurements of aberrant microRNA expression in urine samples from diabetic kidney disease patents and control subjects based on miR-192 expression relative to miR-191 at levels comparable with the conventional PCR technique (Fig. 10).118 An SPCE biosensor strip containing β-hydroxybutyrate dehydrogenase (HBD) enzyme, o-toluidine blue O mediator, and the nicotinamide adenine dinucleotide (NAD+) as an HBD cofactor on an SPCE with CNTs and gold substrate has been developed for non-invasive monitoring of salivary ketone e.g., β-hydroxybutyrate, (HB) and wellness applications.119 These types of sensors have potential to be used for personalized decentralized measurements of salivary biomarkers for different health applications. More advanced SPCE measurements couple electrochemical detection with microfluidics. In a recent example, an electrochemical aptasensor integrated within a herringbone-embedded microfluidic chip was designed for the detection of carcinoembryonic antigen (CEA), a widely used clinical tumor marker. The SPCE was modified with a hemin-coated CNT-decorated Ti3C2 MXene nanosheet, where the MXene was used to improve dispersion and maintain electrochemical performance of the CNTs. The functionalized SPCE was embedded into a microfluidic chip to facilitate the interaction between the immobilized aptamer and the CEA in the sample. The platform showed the capability to measure CEA with an LOD of 2.88 pg ml−1 within the range of 10–1 × 106 pg ml−1. Such technologies could be adapted for further use in clinical settings.
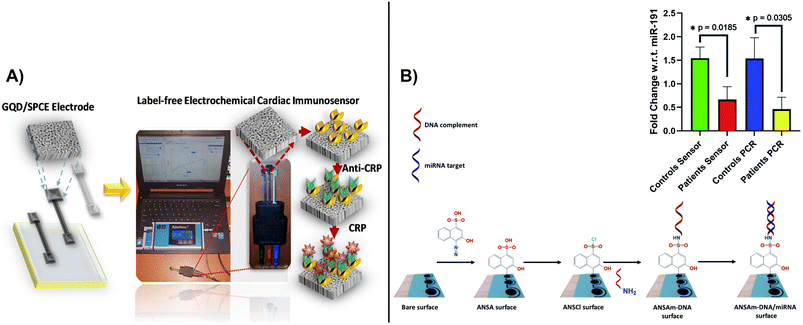 | ||
| Fig. 10 A) Fabrication of SPCE immunosensor and portable electrochemical detection set-up for detection of C-reactive protein (CRP)(with permission from ACS, copyright [2021]116), and B) modification of SPE with diazo-sulfonamide modified and DNA for detection of microRNA expression in samples from diabetic kidney disease patents, as compared to controls (inset shows comparative results between sensor data and RT-qPCR) (with permission from RSC, copyright [2021]118). | ||
3.3. Electronic tattoos for on skin diagnostics
Electronic diagnostic tattoos (e-tattoos) are a new wave of devices with potential for personalized medicine.120 Customized e-tattoos are generally made by patterning or printing conductive materials on supporting substrates or as standalone substrate-free films. The main requirements for e-tattoo development are: i) skin compatibility, ii) stretch-resistant electrical conductivity, iii) flexibility, and iv) mechanical durability.121 Carbon nanostructures have the required characteristics to be used as a conductive interface for epidermal e-tattoos due to their electrical conductivity and high mechanical strength, making them ideally suited for skin-conformed electronics. Most e-tattoos developed to date demonstrate capabilities for monitoring heart rate, temperature, or electrophysiological activity. An example of a CNT-based lightweight and deformable e-tattoo was reported using CNTs in conjunction with porous silk nanofiber. When attached to dermal surfaces, these devices enabled temperature monitoring, real-time electrophysiology, and drug delivery.122 Only a few examples of e-tattoos are developed as biosensors for molecular diagnostics for monitoring disease biomarkers.An e-tattoo developed using Pt-decorated CNTs deposited on a gallium-based liquid metal composite demonstrated potential as a skin-attached wearable biosensor for measuring oxidase enzyme substrates, i.e., lactate, glucose, and ethanol (Fig. 11A).120 Another example of skin-attachable electrochemical sensor for glucose and pH in human perspiration has been developed by coating CoWO4/CNT and polyaniline/CNT nanocomposite onto CNT-AuNS electrodes with a chlorinated silver nanowire as a reference electrode.123 To obtain a skin attachable device, the patterned electrodes were encapsulated within a sticky silbione led. Silbione was found to provide superior adhesion behavior, high biocompatibility and mechanical stability, which makes it an ideal material for wearables. Sensitivities of 10.89 mA mM cm and 71.44 mV pH−1 for glucose and pH with a stability of 10 days and 30% stretchability were reported (Fig. 11B). Wang et al.,124 reported a flexible and wearable biosensor design based on one-step laser synthesis and functionalization of platinum nanostructures within 3D porous graphene for multiplexed analysis of glucose and pH for in situ perspiration to facilitate diabetes management in a non-invasive manner (Fig. 11C). The dual functional biosensor provided a sensitivity of 67.64 μA mM−1 cm−2 for glucose and pH 72.4 mV pH−1.124
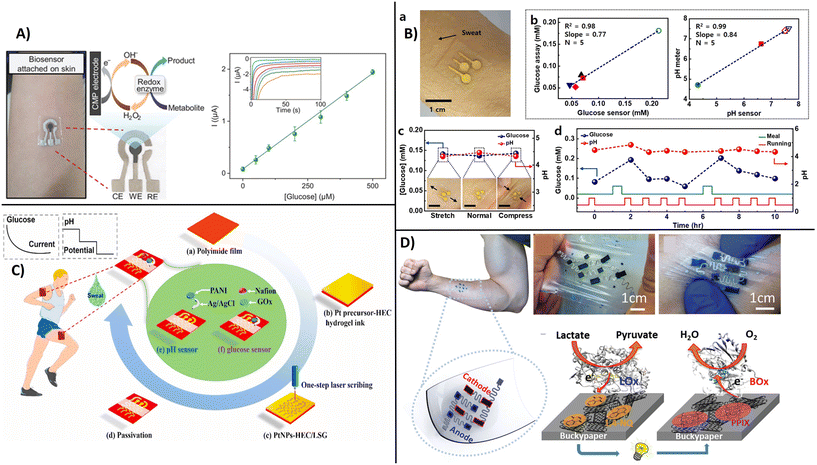 | ||
| Fig. 11 Example of smart headband for in situ measurement of perspiration, A) e-tattoo biosensor on skin for measuring glucose, ethanol, and lactate with example of chronoamperometric measurements and calibration curve for glucose (with permission from Wiley, copyright [2022]120), B) electrochemical sensor attached to the skin for glucose and pH measurements (a and b), over time and under mechanical deformation (c), before and after meal (d), (with permission from ACS, copyright [2018]123), C) flexible design of electrochemical biosensor attached to the skin for glucose and pH measurements by using one step scribing design of multi-layered working electrode (with permission from Elsevier, copyright [2023]), and D) flexible CNTs-based buckypaper lactate biofuel cell for autonomous wearable electronics (with permission from Wiley, copyright [2019]127). | ||
Platforms such as this enable personalized diagnostics and physiological monitoring. Further development and implementation of e-tattoos can advance patient specific on-skin diagnostics. In the future, several improvements are needed to enable autonomous and continuous operation such as the development of sampling collection and integration of energy harvesting systems. An example of a sweat collection patch has been reported recently, consisting of an analysis chamber for measurements of sweat conductivity and the [Na+] and [Cl−] of samples.125 With respect to energy harvesting, most currently developed e-tattoos rely on power sources using conventional energy devices to enable operation. A new direction to realize truly autonomous wearables is to couple the sensing system with a bioenergy microgrid such as those relying on human activity to harvest energy input, creating an autonomously integrated on-body wearable.126 In this example, the energy requirements for the microgrid e-textile are harvested from the sliding motion between the arms. Several examples of printed carbon-based nanostructured patches that function as an integrated biofuel cell for self-sustained power have been developed and can be found in literature, such as a stretchable lactate/O2 biofuel cell using buckypaper composed of CNTs as electrode material (Fig. 11D).127 However, it must be noted that development of most of these devices is restricted to “proof-of-concept” measurements with little or no market or clinical validation. Reasons for the lack of commercial success are the difficulties in continuous access of bio-fluids, variations in flow rates and measurements parameters, lack of biofluid replenishment rate at the sensor surface, and possible biofouling or contamination. Fig. 11 provides an example of various wearable tattoos for monitoring of different biomarkers in sweat. The next section discusses the design of CBMs and their implementation in implantable microelectrodes and for personalized healthcare.
3.4. Carbon-based wearable microneedles and implantable microelectrodes
Wearable micro-electrochemical sensors are well suited for non-invasive monitoring and personalized healthcare.128 A recent development in this field is to use micro-sized array electrodes and microneedles that penetrate skin and can measure biomarkers in interstitial fluid. Compared to analysis of biomarkers on the surface of skin, measurements in the interstitial fluid using microneedles provides continuous monitoring and higher accuracy measurements, e.g., as compared to analysis of surface biofluids like sweat. The interstitial fluid in the subcutaneous tissue can be easily accessed and measured through painless insertion of microneedles. The interstitial fluid contains biologically relevant biomarkers found in blood, providing an alternative measure to direct blood analysis. Another benefit is the reduced biofouling, although mechanical friction and potential damage of the sensing layer can occur during skin penetration.128bThe small size characteristics of the microneedles functioning as epidermal patches allows them to be applied on different locations of the body and be multiplexed for measuring multiple analytes.129 However, the microneedle size and shape relate to performance and conform to wearer. Direct correlation was reported between the tip length and the number of microelectrodes inserted and pain/discomfort to patient.128a Typical size dimensions of microelectrode arrays are tip radius of 5–80 μm with a conical, base diameter between 100–200 μm with a cylindrical or pyramidal geometry to facilitate the insertion, and tip lengths ∼600 μm. An integrated wirelessly operated microneedle array enabled continuous real-time monitoring of glucose, lactate, and alcohol with LODs of 0.32, 0.15 and 0.5 mM respectively, through the epidermic-inserted microneedle tip.129 The structure of the microarray electrode for the real-time monitoring of lactate and ethanol with the sensor patch placed on the arm of the wearer are shown in (Fig. 12A). This low-cost sensor was constructed from nine distinct sub-components that included a disposal sensing unit with oxidase enzymes immobilized within poly-o-phenylenediamine (POPDA) and a reusable electronic unit.
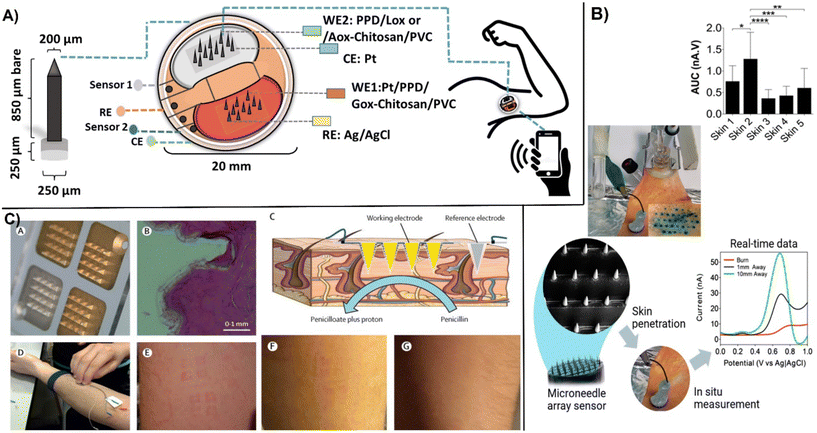 | ||
| Fig. 12 Examples of microneedle microarray biosensors: A) images of microneedle tip used for in vivo monitoring of glucose, lactate, and ethanol in the interstitial fluid. The sensor patch was placed to the arm of the wearer (reproduced from Springer ref. 129 copyright [2022]); B) ex vivo electrochemical measurements in the skin of excised porcine showing penetration of the microneedles visualized by methylene blue staining by DPV measurements. Linear time-dependent plot at 0.7 V and variation of the oxidative peak across five skin samples with statistical measurements one-way ANOVA with Tukey's multiple comparisons test were made for n = 5 animals (with permission from ACS, copyright [2019]131); C) microneedle array created by metalized electrodes consisting of three independent Au-coated working electrodes (150 nm) and Ag/AgCl (150 nm) functioning as the reference electrode (A) showing base of microneedles used, (B), showing cross-section of human skin after application (C), for penicillin measurement, (D) and application to the forearm under pressure, (E) time lapsed of left marks after wearing microneedles and, (F) after 1 h removal and, (G) 12 h after removal (with permission from Elsevier, copyright [2019]132). | ||
Enzyme-based electrochemical sensors require efficient immobilization and connectivity between the enzyme and electrodes. Many electrochemical enzyme biosensors are second generation devices that involve the use of electron mediators to facilitate the contact between the active sites and the electrode surface. In wearable devices, mediators are ideally immobilized on the electrode surface along with the enzyme. In the reported work, POPDA had a unique ability to work as a redox mediator and enzyme immobilization material, catalyzing the oxygen reduction without the use of additional mediators.130 Designed sensor demonstrated on-body performance for monitoring the three analytes with accuracies falling within 20–30% of reference methods. In microarray sensors, sensing elements typically consist of polymeric layers such as PPD, biopolymers like agarose, or poly-lactic acid that contain bioreceptor molecules deposited onto conductive micro-tip arrays. Carbon nanostructures have been used as conductive materials for dermal biosensing. Poly-lactic acid loaded with 6% wt. MWCNTs on conical microneedles with a tip diameter of 40 μm, a base diameter of 250 μm, and an accessible height of 870 μm was found as a suitable material for the construction of a microneedle array produced by micro modeling (Fig. 12B). The biosensor enabled real-time monitoring of ascorbic acid with an LOD of 180 μM by using DPV and its functionality was effectively demonstrated in a burn wound model.131 The first evaluation of microneedle biosensors in human volunteers was reported in 2019.132 Two microneedle β-lactam biosensors (one control) were applied to the participant's forearms to measure phenoxymethylpenicillin to obtain real-time individualized antibiotic monitoring. The microelectrode array consisted of four independent electrodes system. After metallising the electrodes with an adhesion coating of chromium. There of them are coated with Au to work as a working electrodes while fourth electrode is metallized with Ag and chloridised to Ag/AgCl to work as a reference electrode (Fig. 12C). The working electrodes were modified by electrodeposition with iridium oxide (IrOx) to measure pH changes with a hydrogel layer containing β-lactamase from Enterobacter cloacae. Prior to application, the electrodes were sterilized with cobalt-60 gamma radiation. The volunteers wore the microneedle biosensor for up to 6 h while being dosed with phenoxymethylpenicillin. The results of in-human trial demonstrated that the microneedle biosensors can continuously monitor antibiotic concentrations at comparative levels with conventional microdialysis standard monitoring technology.132 More details on the current development status and challenges in developing microneedle-based electrochemical sensors can be found in several recent reviews.128,133
Along with microneedle technologies, a large variety of carbon-based microelectrodes have been developed for monitoring neurotransmitters. The wide majority are carbon fiber microelectrodes used to detect neurotransmitters to understand their role and evaluate treatment of diseases such as cancer, Parkinson's, and Alzheimer's,134 which destroy the tissue of the brain, impairing movement and memory.135 Modification of microelectrodes with carbon nanomaterials has been shown to improve the sensitivity of these microelectrodes, enhance electron transfer rate, and increase conductivity.136 The performance of these electrodes can be enhanced by carefully selecting the required customized shape and size of carbon nanostructures, e.g., nanotips, cavity nanopipettes, etc.137 Nano-sized electrodes can be prepared by nanoprinting method via flame etching, designed electrodes can be inserted in the synapses for studying cell exocytosis.138 Growing carbon nanospikes on tungsten and niobium metal wires enabled mass production of nano-tip electrodes for sensitive detection of dopamine, serotonin, ascorbic acid, and 3,4-dihydroxyphenylacetic acid (DOPAC) (Fig. 13A) shows the SEM images of tungsten and niobium wires with carbon nanospikes (CNSs) modification. Selected CNSs exhibited dense and defect rich surface behaviour and can be easily functionalized. Layers of CNSs were grown on metal wired by using plasma enhanced chemical vapor deposition. The etched tungsten wire displayed tapered conical tips while niobium wires were thin and long. Both showed excellent sensitivity for real time detection of neurotransmitters. Fast scan cyclic voltammetric (FSCV) measurements were demonstrated for ascorbic acid, DOPAC, serotonin and adenosine (Fig. 13B). This method has potential for mass production of CNS based microelectrodes for neurotransmitter monitoring.
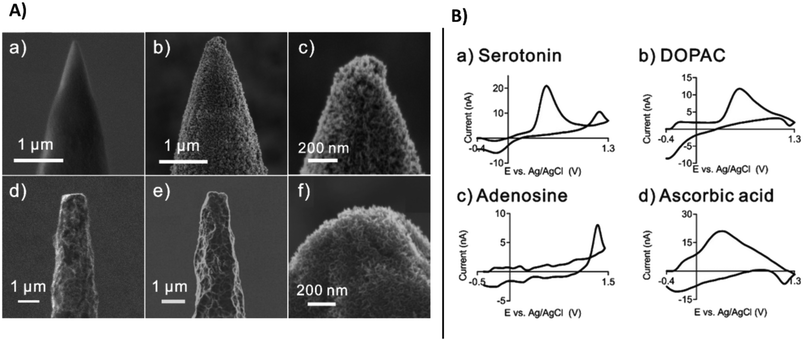 | ||
| Fig. 13 A), SEM images of tungsten and niobium wires before and after modification with CNSs: (a) the etched tungsten wire, (b) CNSs grown on the tungsten wire, (c) enlarged image of the CNSs on the tungsten wire, (d) the etched niobium wire, (e) the etched niobium wire, and (f) a more enlarged image of the CNSs on the niobium wire. B), fast scan cyclic voltammetric FSCV measurements of neurochemicals using CNS nanoelectrodes, (a) serotonin, (b) DOPAC, (c) adenosine and (d) ascorbic acid (with permission from RSC, copyright [2022]139). | ||
Huang et al., produced a flexible enzyme-based electrode with MWCNTs with immobilized glucose oxidase for blood glucose monitoring with a sensitivity of 25 nA mM−1 for diabetes management. Designed electrodes showed improved electron transport efficiency, excellent stability though entrapment, and no enzyme leaching.134 Using two-photon lithography followed by pyrolysis, 3D-printed carbon spheres and cones with electroactive surfaces enabled detection of dopamine at levels as low as 11 ± 1 nM and 10 ± 2 nM, respectively. This pretreatment procedure allows for customizable geometry of the electrode while maintaining high resolution, electroactive surfaces due to pyrolyzed carbon, allowance of a free-standing structure without the need for a large base, and great reproducibility.140 Xiao et al., developed a 7 mm by 25 μm Pt nanoparticle with a reduced graphene oxide-functionalized microelectrode array for dopamine monitoring during deep brain stimulation in Parkinson disease rat models with a detection limit of 50 nM and sensitivity of 8.251 pA μM−1. Such biosensing tools have the potential to provide a better understanding of the mechanism with increased therapeutic efficacy.141
Other types of microbiosensors have been designed for detection of cytokines which are small soluble proteins involved with inflammatory response and cell proliferation. These devices can be used for diagnosis and monitoring of cancers and other inflammatory diseases. Qi et al., designed an electrochemical sensor using graphene oxide nanosheets covalently bonded to a gold surface and 4-aminophenyl phosphoryl choline as an antifouling agent for detection of cytokine interleukin-6 (IL-6). The immunosensor enabled in vivo monitoring of IL-6 with an LOD of 1 pg mL−1 in both RAW cells and live mice.45b Shen et al., developed an electrochemical sensor using a glassy carbon rod for measuring multiple cytokines (IL-1β, IL-6, and TNF-α) within Parkinson disease mice models. An LOD of 5 pg mL−1 for each protein was reported. This sensor allowed for obtaining quantifiable data on inflammatory cytokines comparable to ELISA, showing better sensitivity and deploy ability, opening possibilities for the development of brain chips for early detection of different biomarkers.135
CFMEs have been used for measuring rapid changes in neurotransmitters because of their high sensitivity, small size, and excellent electrochemical behavior. The most popular application of CFMEs is for direct detection of neurotransmitters, and associated processes within the brain. Carbon fiber has several advantages such as small size and compatibility with biological compounds. Carbon fibers have less than 10 μm in diameter are extremely sensitive for measurements in implantable conditions, also causing less tissue damage as compared to other conventional electrodes.142 Detection of the dopamine neurotransmitter in vivo has also been achieved with a CFMEs bundle functionalized with tyrosinase and catalytically active oxidase mimetic nanoparticles, enabling the detection of dopamine at levels as low as 1 nM with a linear range of 0.01–220 μM, sensitivity of 14.2 nA μM−1, and a response time of less than 8 seconds.143 The procedure has been extended to monitoring lactate and glutamate for understanding their behavior in ischemia/reperfusion studies.144 Other microbiosensors were functionalized with aptamers for detection through bioaffinity recognition. Hou et al., developed an alkyl chain-functionalized CFMEs with non-covalently immobilized aptamer cholesterol amphiphiles for detection of neurotransmitters in vivo.145 In the same way Seven et al., developed a nanoporous CFE through heat treatment for the electrochemical detection of H2O2 and dopamine in tissue analysis with the potential for use as probes for in vivo studies due to catalytic activity and low LODs of 0.57 μM and 35.6 nM, respectively.146 Chang et al., modified CFMEs with electrodeposited graphene oxide for detection of dopamine (LOD = 11 nM, sensitivity = 41 ± 2 nA μM−1) and tested the functionality of the electrode in electro-stimulated brain slices of mice as a demonstration of use in tissue. This was a first demonstration of electrodeposited graphene oxide onto the CFMEs to enhance the sensitivity for in vivo measurements.147 The achieved high carbon-to-oxygen ratio favors conductivity and electron transfer rate due to more adsorption sites with enhanced oxygen functionalities147 to produce a concentration-dependent current change.146 The simultaneous detection of serotonin and dopamine in vivo via DPV was performed using a CFMEs array functionalized with diazonium salts and SWCNTs, which improved selectivity for pharmacological and physiological applications.148
3.5. Non-invasive lab-in-a-mouth biosensors for salivary biomarkers
Several examples of POC-based electrochemical biosensors were developed for the non-invasive monitoring of biomarkers in saliva. The concept called lab-in-a mouth, or cavitas sensors is a relatively new development to the field promising real time monitoring of biochemical information from the oral cavity.149 An example is a cell-phone connected pacifier-type biosensor connected to wireless electronics was reported for measurements of salivary biomarkers.150 The biosensor consisted of a screen printed electrode modified with chitosan and glucose oxidase. The electrode was connected to the pacifier using a 3D printed customized cell. In addition to glucose bioelectronics pacifiers have also been developed to real time monitoring of salivary electrolytes.151 This device consisted of ion-selective sensors and flexible microfluidic channels. Other devices have been developed in form of a mouthguard biosensor, placed on a tooth with the help of a cellulose acetate membrane and used for the in vivo measurement of glucose in saliva.152 The biosensor measured glucose concentrations within the range of 1.75–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 μM, which covers the typical levels in saliva, between 20–2002 μM. Using a similar concept but with the biosensor attached to a toothbrush, Liu et al. detected glucose in the concentration range from 0.18 mM to 5.22 mM within 5 min.153 The biosensor was fabricated using a carbon graphite ink with glucose oxidase (immobilized using 2% glutaraldehyde) as working electrode and a Ag/AgCl ink as reference electrode painted on a toothbrush. More deals on micro/nanodevices for biomarkers detection in saliva and applications in stomatology can be found in recent reviews.149,154
000 μM, which covers the typical levels in saliva, between 20–2002 μM. Using a similar concept but with the biosensor attached to a toothbrush, Liu et al. detected glucose in the concentration range from 0.18 mM to 5.22 mM within 5 min.153 The biosensor was fabricated using a carbon graphite ink with glucose oxidase (immobilized using 2% glutaraldehyde) as working electrode and a Ag/AgCl ink as reference electrode painted on a toothbrush. More deals on micro/nanodevices for biomarkers detection in saliva and applications in stomatology can be found in recent reviews.149,154
4. Large scale manufacturing of low-cost carbon-based diagnostic devices
One important requirement for the implementation of carbon-based wearables on a commercial scale is the ability to manufacture high quantity devices at a low cost and with high reproducibility for consumer use. The success of manufacturing relies on the selection of suitable materials and substrates as well as the method of device fabrication. As discussed earlier, carbon nanostructures have great potential to be used as a platform for bioelectronic devices design. Wearable POC device can be fabricated on flexible and inexpensive substrates like plastic,155 textiles,156 paper tattoos,157 and elastomers,157,158 which have the capability to directly contact human skin. Key design requirements and benefits offered by carbon nanostructures are the material compatibility, mechanical and structural stability, scalability for large scale application, as well as the ability to be functionalized with receptor molecules for biomarker detection. To ensure functionality and accuracy of analysis, the manufacturing protocol should be made without affecting the intrinsic properties or the recognition and detection functions of the active interface. Carbon-based nanostructures, particular CNTs and graphene, can be deposited on flexible platforms such as fabric, paper, or skin-conformable tattoos. They can be used in conjunction with other materials such as fibers of bacterial nanocellulose,96 ZnS,87 or AuNPs88 and be interfaced with biomolecules to create multifunctional hybrid films. These multicomponent structures are relatively complex and their fabrication on an industrial scale is still challenging. Methods such as printing, dipping, and drying, chemical vapor deposition (CVD), and photolithography have been explored to manufacture low-cost biosensors. Several examples are provided in this section.A CVD-based fabrication procedure used to create transparent, stretchable, and wearable graphene e-tattoo sensors is shown in Fig. 14A. The procedure involves a series of steps starting with the growth of graphene on copper foil by atomic pressure chemical vapor deposition (CVD) followed by dry patterning and coating of poly-methyl methacrylate (PMMA) polymer precursor and baking it to obtain a film of ∼460 nm on the graphene area.159b The copper layer was further etched away to create a graphene film on PMMA that constitutes the actual substrate of the e-tattoos. The sensors have been fabricated as an open mesh structure to facilitate long-term conformability with skin for applications as an e-tattoo for the noninvasive monitoring of skin temperature, hydration, electrocardiogram (ECG), electromyogram (EMG), and electroencephalogram (EEG). Similar designs have been reported by combining carbon-based nanomaterials with Ag nanoparticles to effectively increase conductivity and enable measurements of ECG and EMG.160 In this case, polyisobutylene-b-poly(oxyethylene)-b-polyisobutylene triblock copolymer was used as a dispersant for AgNPs and different shapes of carbon nanomaterials: carbon black, CNTs, and graphene. The sensor displayed high stability even after 5000 repetitions of 50% tension–tension fatigue testing and low resistance of 4.1 × 10 Ω sq−1. Kirev et al., reported a multistep fabrication protocol for manufacturing GETs161 as a solution for ‘high end low-cost’ wearables. The method involves transfer of graphene onto tattoo paper by CVD growth and formation of a multilayer graphene stack followed by contact and transfer of the GET from paper to skin using a soft adhesive conductive tape. The sensor placed on skin was tested for monitoring skin hydration, temperature, EEGs, EMGs, and ECGs. Shirhatti et al., fabricated a flexible wearable sensor using a laser-etch process to pattern gold interdigitated electrodes drop-cased with an ultrathin layer of graphene nanosheets and demonstrated its use for monitoring hearth rate, hydration, temperature, and breathing rate when the sensor was placed on skin.162
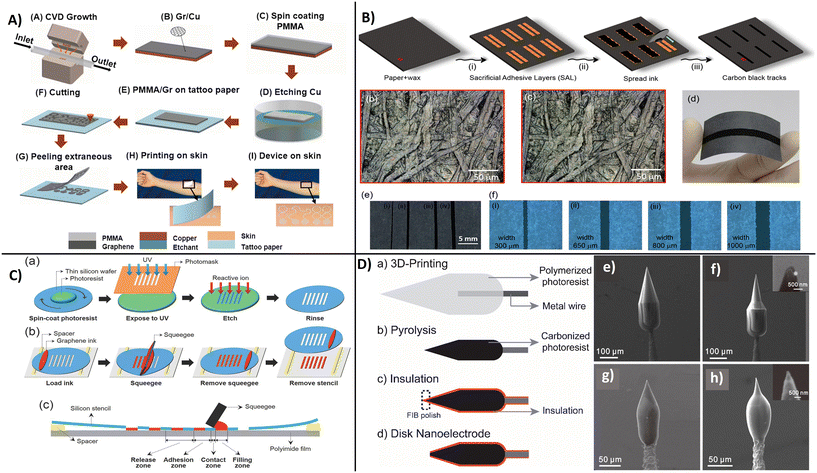 | ||
| Fig. 14 Examples of manufacturing procedures for carbon-based electrodes illustrating: A) CVD-based fabrication procedure of on-skin graphene e-tattoos (GET) showing CVD disposition of graphene on copper foil (A and B), followed by PMMA coating (C), copper etching (D) and cutting (E), and transfer of the graphene/PMMA on tattoo paper substrate (F and G), pealing (H) and placement on the skin (I) (with permission from ACS, copyright [2017]159a). B) Screen printing fabrication of a fully printed carbon black-based wearable sensor by: addition of sacrificial adhesive layers (i), deposition and spread of the carbon black ink (ii), and the cure of the layers at 60 °C (iii), followed by the removal of the adhesive layers; with scanning microscope images (b) before and (c) after the removal of the sacrificial adhesive layer and photographs of the flexible paper-based device (d–f) with different widths (with permission from ACS, copyright [2017]165). C) Fabrication of high resolution thin silicon stencil using a conventional photolithography technique showing the screen printing process with deposition of graphene ink (a and b) and cross-sectional picture of the printed electrode (c) (with permission from Wiley, copyright [2014]164), and D) fabrication of 3D-printed carbon nanoelectrodes (a–d). With SEM images of printed structures, before pyrolysis of 3D-printed electrodes with normal conical geometry (e) and sharper conical geometry (f), and after pyrolysis: carbonized electrodes (g and h) (with permission from ACS, copyright [2020]170). | ||
A straightforward fabrication procedure is to use printing, e.g., inkjet or screen printing via deposition of inks on substrates. The benefit of using printing is the low-cost mass production capability as well as the high resolution and versatility of the process. Sensors can be printed at ambient temperature which is compatible with biomolecules.163 Printing requires the development of inks of characteristic formulation and viscosities. Carbon nanomaterials such as graphene and CNTs are well suited for creating conductive inks due to their mechanical and electrical properties. In ink-jet printing, a single droplet of conductive ink can be printed very precisely on a suitable substrate with low material consumption, enabling low-cost high-volume printing of wearable sensors.164 Santhiago et al., reported a scalable fully printed paper-based wearable sensor using a conductive ink based on carbon black and Prussian blue as an electron mediator (Fig. 14B). The printed pattern had a resolution of 500 μm in width and high folding stability of over 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles.165 Hyun et al., achieved increased resolution patterning of graphene with shapes as narrow as 40 μm by screen printing using a silicon stencil and viscosity-controlled inks. This strategy, summarized in Fig. 14C, enables manufacturing of flexible electrodes for printed electronics.164 Most reported sensors are used to monitor physiochemical signals including breathing, hearth rate, blood pressure, etc. Examples of physiochemical signals of pulse and breathing rate monitoring by a wearable strain sensor were reported with functionalized carbon nanostructures,166 CNTs,167 and graphene.168 It's evident that carbon-based wearable electrodes demonstrate viability for biomonitoring (EEG, EMG, ECG, etc.)169 but the selection of suitable materials, flexible substrates, and their effective structural design is highly important for high-performance diagnostics for healthcare applications. A new approach for producing microelectrode sensors is to use 3D printing by direct laser writing which enables fabrication of customized implantable electrodes.170 The group of J. Venton has reported a method to 3D printing free-standing carbon nanoelectrodes for in vivo monitoring of neurotransmitters.170 The fabrication process, illustrated in Fig. 14D, involves deposition of IP-S photoresist on a metal wire immobilized on a silicon chip, pyrolysis of the polymer structure producing a glassy carbon-like structure followed by insulation with a layer of Al2O2 (100 nm) by atomic layer deposition (ALD) and milling of the end of the tip by focused ion beam (FIB), resulting in a disk shape carbon electrodes. The diameter of the pyrolyzed tip was ∼260 nm, while the disk electrode was ∼600 nm with geometries shown in the SEM images in Fig. 14D. The nanoelectrode was able to determine dopamine within a linear range of 10–50 μm and functionality was demonstrated with the electrode implanted in adult fruit fly.
000 cycles.165 Hyun et al., achieved increased resolution patterning of graphene with shapes as narrow as 40 μm by screen printing using a silicon stencil and viscosity-controlled inks. This strategy, summarized in Fig. 14C, enables manufacturing of flexible electrodes for printed electronics.164 Most reported sensors are used to monitor physiochemical signals including breathing, hearth rate, blood pressure, etc. Examples of physiochemical signals of pulse and breathing rate monitoring by a wearable strain sensor were reported with functionalized carbon nanostructures,166 CNTs,167 and graphene.168 It's evident that carbon-based wearable electrodes demonstrate viability for biomonitoring (EEG, EMG, ECG, etc.)169 but the selection of suitable materials, flexible substrates, and their effective structural design is highly important for high-performance diagnostics for healthcare applications. A new approach for producing microelectrode sensors is to use 3D printing by direct laser writing which enables fabrication of customized implantable electrodes.170 The group of J. Venton has reported a method to 3D printing free-standing carbon nanoelectrodes for in vivo monitoring of neurotransmitters.170 The fabrication process, illustrated in Fig. 14D, involves deposition of IP-S photoresist on a metal wire immobilized on a silicon chip, pyrolysis of the polymer structure producing a glassy carbon-like structure followed by insulation with a layer of Al2O2 (100 nm) by atomic layer deposition (ALD) and milling of the end of the tip by focused ion beam (FIB), resulting in a disk shape carbon electrodes. The diameter of the pyrolyzed tip was ∼260 nm, while the disk electrode was ∼600 nm with geometries shown in the SEM images in Fig. 14D. The nanoelectrode was able to determine dopamine within a linear range of 10–50 μm and functionality was demonstrated with the electrode implanted in adult fruit fly.
5. Biosensing connectivity for remote monitoring and decentralized healthcare
With a few exceptions, the clinical diagnostic system is largely centralized and provides limited options for personalized testing and therapy monitoring. Modern healthcare indicates a clear trend towards decentralized healthcare by promoting mobile, individualized, and predictive medicine. The concept of mobile health – mHealth or eHealth, using portable biomedical devices, represents a paradigm shift from the current practice. In mHealth, a portable sensing device is connected to a smart wireless communication system such as a cell phone. The integrated sensor should be able to monitor and continuously provide health-related signals and track physiological parameters related to motion, physical activity, or biochemical markers of disease. While several existing technologies and on-body wearables measure signals such as heart rate, temperature, or hydration levels, devices that can detect biological signals and provide continuous monitoring of disease biomarkers for health monitoring are still limited, despite the massive wearable technology market. Some of the existing challenges are related to the rigidity/bulkiness of electronic transducers, while others are due to the stability of bioreceptors, or the functionality of the measuring system. Biological molecules are affected by the environment, pH, temperature, and storage conditions, and biosensors can lose their functionality over time. Other limitations are related to calibration accuracy and reliability of measurements, e.g., possible drifts in calibration, particularly when these are placed in real-world conditions and are intended for long-term use.Integration of portable biosensing systems with the cloud-based processing and the internet of things (IoT) is important for advancing the concept of smart and connected health.171 The IoT enables device communication and use of data to coordinate decisions by sharing information.172 A challenge in mobile crowdsensing for ensuring reliable IoT in healthcare is ensuring tamper resistance of the biosensed data to ensure data integrity, privacy, and trustworthiness.173 Smartphones and tablets can be interfaced with low-cost portable sensing devices for monitoring biomarkers in biological fluids. Communication through mobile apps empowers patients to monitor medical conditions and treatment, and the medical professionals to manage and make decisions about the patient's health from any location. However, the ability of the system to be used as a diagnostics tool for eHealth relies on the successful integration of the sensing and data processing components to enable automated data collection, transmission, processing, and visualization for health management purposes. The main components of a typical remote patient monitoring system are illustrated in Fig. 15 which include: 1) the sensing system with data acquisition and transmission capabilities, 2) data processing and cloud computing unit with stored calibrations and health-related correlations, and 3) data visualization and diagnostics. Another requirement for the successful implementation of mobile eHealth systems also requires the use of low power sources and communication protocols, which accounts for a significant power consumption in sensing devices.174 Development and effective implementation of such systems involves strong collaboration between biosensing experts with data/computer scientists and system engineers. In the future, biosensors for eHealth could be used as diagnostic support tools, adding to the already available clinical decision infrastructure in healthcare to improve diagnostic and patient outcomes.175
 | ||
| Fig. 15 Components of an electronic remote patient monitoring system (eHealth): biosensing unit, data center and diagnostic and outcome. | ||
6. Considerations for commercialization and translation to practice
To be widely adopted, biosensors must meet several criteria: i) meet quality standards in accordance with FDA criteria, ii) be inexpensive, accessible, and scalable, iii) easy to use to allow medical professionals to quickly interpret the results and provide timely intervention, iv) provide accurate and reliable data. In addition, v) such devices should have no or limited sample preparation and (vi) require no reagents, or reagents should be integrated within the sensing unit. To address this requirement, efforts have been made to couple microfluidic platforms, biochips or fluidic disks containing cartridges with stored reagents and rotating channels to precisely mix reagents and perform chemical reactions in confined volumes, automating sample preparation, and minimizing reagents cost.176 The large scale production of single use disposable POC devices still requires improvement in the material design, particularly with respect to biocompatibility. Given the potential large scale use of these devices, development on novel biocompatible and sustainable materials is still needed to prevent the generation of harmful waste.177 Lastly, transition of mobile biosensors into real-world applications is subject to FDA approval178 and policy considerations regarding the design, performance, data security, and confidentiality.179 The World Health Organization (WHO) has a set of recommended criteria for developing low-cost diagnostics to be “affordable, sensitive, specific, user-friendly, rapid, robust, and equipment free” (ASSURE), and most such emerging technologies follow these criteria.The translation of these biosensors into a commercial product requires, in addition to technology development, several market and business considerations, specifically: 1) market need identified based on whether the technology offers a solution to an existing need or an improvement over existing products, 2) market size and share determined by the number of potential users, and the competitive landscape, 3) readiness for commercialization determined by a technology readiness level (TRL) done at several critical points during the technology development and testing. POC devices also require manufacturing, storage, and packaging. It should be noted that the development of most POC devices is realized in academic labs, while transfer of the technology into industry requires business, intellectual property protection, collaboration with industry and entrepreneurial endeavors. The framework for market entry readiness involves all aspects of technology development as well as business including: technology, market, regulatory, commercial and management readiness. One of the biggest challenges of translating POCs from labs to industry is the gap between academic research and industry, with most innovations in academic labs being at the proof of concept and TRL levels. Most commercial POC biosensors today are lateral flow test strips with nucleic acids and antibodies-based detection. The most successful electrochemical biosensor remains the glucometer, whose success has been driven by the large and growing diabetes market need and size,180 While advances in wearable, subcutaneous and microanalysis-based POC designs have been made and some are beginning to be commercialized, such systems still require improvements in accuracy and reliability for long term monitoring. The status of the commercial POC platforms for diabetes monitoring has been discussed and can be consulted in a recent review.180
7. Conclusions and future outlook
This review has provided an overview of the status of carbon-based electrochemical biosensors as diagnostic platforms and POC devices for healthcare. Carbonaceous-based materials have been widely used to design cost-effective devices and their integration with wireless communication devices has provided exciting opportunities for biomarker detection for decentralized healthcare.181 From the material perspective, carbon materials and nanostructures like graphene are some of the most suitable for high performance POC and wearable biosensors due to their flexibility and sub-nanometer thickness, conforming to the curvature of skin.161 Significant progress has been made in manufacturing electrochemical biosensors based on active and functional carbon nanostructures. Functionalizing carbon nanomaterials and creating hybrid structures with biomolecules has improved sensitivity and selectivity while lowering the detection limits and providing a good foundation for measuring relevant biomarkers levels, advancing their use for biomedical applications. Their integration in portable low-cost and wearable substrates has also been demonstrated with additive manufacturing methods such as printing, enabling large-scale production with increased reproducibility. This review summarized the various designs and performances reported over the last 3–5 years focusing on paper-based devices, electronic tattoos, implantable and wearable microelectrodes, and interstitial microneedles. The continuous innovations in materials design and additive manufacturing have enabled the development of a wide variety of carbon-based devices that are flexible and adaptable for biomarker detection in human biofluids (e.g., sweat, tears).44b,182 Moreover, the growing electronic health network and computing capabilities have advanced the development of apps-based monitoring and connectivity for eHealth.Existing commercially available eHealth-connected devices are physical sensors that measure physical health indicators such as heart rate and blood oxygen. Despite significant progress, few electrochemical biological sensors have been implemented into clinical practice (with the exception of the widely used glucometers); many still require development to enhance their stability, selectivity and detection performance in realistic environments to meet the needs of accurate and effective healthcare in modern society. Their capabilities to measure health indicators, diagnose diseases, and provide real-time feedback to optimize therapy makes them particularly suited for personalized healthcare. However, future commercial implementation requires strong partnerships with industry and advancement of the technology to more mature readiness levels, beyond proof-of-concept (TRL2–3) to prototype testing and validation (TRL5–6), and ultimately their proven operation in the clinical field (TRL9). The recent successful implementation of POC for rapid diagnostics of COVID-19 (ref. 183) demonstrate that such technologies are needed and can be successfully deployed when there is a strong societal, market and technology need.
With the emergence of digital health and communication networks and the focus of healthcare towards decentralization, we envision that a large range of low-cost diagnostic devices will be needed to provide real-time monitoring for health management in the future. However, their widespread adoption still requires further development and testing for improving accuracy and performance when used on patients. The future developments and challenges concerning biosensors for digital health will include:
(1) Further optimization to increase stability and functionality from hours to several months or years with on-body sensors tested in realistic monitoring conditions.
(2) Strategies to address biofouling issues by developing novel electrode materials and coatings.
(3) Selective multi-analyte detection protocols and strategies to remove interferences to enable reliable and selective measurements in complex biofluids.
(4) Development of calibration protocols for inclusion in electronic software. As with other biosensing technology, the calibration is essential in order to ensure accuracy of analysis and mitigate calibration challenges due to changes in mass transfer conditions, calibration drifts with sequential measurements, and possible alterations of the electrode surface that are common with wearable and implantable biosensors. Performing pre-post analysis calibration, validation strategies with established methods and designing protocols that take into account potential drifts are recommended to ensure accuracy of analysis. Other approaches could be to use self-reference sensors to compensate for fouling or fluctuations that could affect the accuracy of the signals. The adoption of chemometrics and machine learning could provide the necessary data analytics to improve accuracy and reliability of measurements, especially for multiplexed detection.
(5) Manufacturability, biocompatibility, stability of use, and cost considerations by incorporating criteria for material selection, large scale fabrication, and adaptability in early prototype development. The ability to adjust the design ‘on-the-fly’ and adapt protocol to address individual needs is essential, particularly for personalized bioelectronics133 such as wearables, microneedle-based interstitial sensors, and e-tattoos. Biocompatibility, mechanical durability, and intrinsic conductivity are additional criteria that should be delivered to meet FDA requirements. Biointegration, stretchability, and safe contact with body tissues is highly desirable for implantable and on-body sensors for achieving compliant human-sensing interfaces. Further developments in flexible electronics, the use of additive manufacturing methods, and appropriate tools are envisioned to improve fabrication and commercialization at a large scale.
(6) Integration of sensing systems with existing electronic infrastructure in a single platform to enable connectivity with the IoT/cloud computing networks with the possibility to be accessible from any location remotely. These can facilitate data collection, transmission, and diagnostics by monitoring the evolution of specific biomarkers at relevant levels for disease diagnostics, treatment, or health conditions. Real-time capabilities could be connected with drug delivery systems to provide personalized feedback on therapy, such as achieving a closed-loop glucose-insulin for diabetes management.
(7) Large scale clinical trials with independent multi-laboratory validations and comparability using conventional clinical analysis laboratories is needed to establish performance in relevant conditions and accelerate adoption.
In conclusion, carbon-based materials are important to the advancement of bioelectronic devices for decentralized health. This review showed that carbon-based electrochemical biosensors provide great selectivity and sensitivity for biomarker detection while providing advantages such as ease-of-use, low cost, and adaptability to be used as on-body wearables compared to other methods such as spectroscopy or chromatography. Further development needs include improving robustness, accuracy, long term operability, manufacturability, and stability to provide real-time specific and effective data for disease monitoring. Due to the wide range and interdisciplinary, further developments require convergent research and collaboration across chemistry, biology, data analytics, engineering, biomedical, and clinical fields. Collaboration between researchers, business, entrepreneurs, regulatory agencies and investors is also needed to advance commercialization and transfer these devices from the lab to the consumer for everyday use. These developments could open up a new approach to medical biosensing and eHealth to improve healthcare, enable connectivity, and reduce healthcare costs for managing health conditions in the community.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was funded by the National Science Foundation: NSF [grant number 2042544]. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.References
- H. C. Ates, P. Q. Nguyen, L. Gonzalez-Macia, E. Morales-Narvaz, F. Guder, J. J. Collins and C. Dincer, End-to-end design of wearable sensors, Nat. Rev. Mater., 2022, 887–907 CrossRef.
- (a) S. Fu, Y. Zhu, Y. Zhang, M. Zhang, Y. Zhang, L. Qiao, N. Yin, K. Song, M. Liu and D. Wang, Recent advances in carbon nanomaterials-based electrochemical sensors for phenolic compounds detection, Microchem. J., 2021, 171, 106776 CrossRef CAS; (b) Ö. Erdem, E. Derin, S. Zeibi Shirejini, K. Sagdic, E. G. Yilmaz, S. Yildiz, G. A. Akceoglu and F. Inci, Carbon-Based Nanomaterials and Sensing Tools for Wearable Health Monitoring Devices, Adv. Mater. Technol., 2022, 7(3), 2100572 CrossRef.
- R. N. Adams, Carbon Paste Electrodes, Anal. Chem., 1958, 30(9), 1576–1576 CrossRef CAS.
- (a) S. Pérez, M. L. Farré and D. Barceló, Analysis, behavior and ecotoxicity of carbon-based nanomaterials in the aquatic environment, TrAC, Trends Anal. Chem., 2009, 28(6), 820–832 CrossRef; (b) X. Yang, B. Feng, X. He, F. Li, Y. Ding and J. Fei, Carbon nanomaterial based electrochemical sensors for biogenic amines, Microchim. Acta, 2013, 180(11), 935–956 CrossRef CAS.
- E.-M. Kirchner and T. Hirsch, Recent developments in carbon-based two-dimensional materials: synthesis and modification aspects for electrochemical sensors, Microchim. Acta, 2020, 187(8), 1–21 CrossRef.
- I. Jeerapan and N. J. C. Ma, Challenges and opportunities of carbon nanomaterials for biofuel cells and supercapacitors: personalized energy for futuristic self-sustainable devices, C, 2019, 5(4), 62 CAS.
- J. Heikenfeld, A. Jajack, B. Feldman, S. W. Granger, S. Gaitonde, G. Begtrup and B. A. Katchman, Accessing analytes in biofluids for peripheral biochemical monitoring, Nat. Biotechnol., 2019, 37(4), 407–419 CrossRef CAS.
- Z. Y. Wang and Z. H. Dai, Carbon nanomaterial-based electrochemical biosensors: an overview, Nanoscale, 2015, 7(15), 6420–6431 RSC.
- Z. Li, Y. Wang, Z. Fan, Y. Sun, Y. Sun, Y. Yang, Y. Zhang, J. Ma, Z. Wang and Z. Zhu, A Dual-Function Wearable Electrochemical Sensor for Uric Acid and Glucose Sensing in Sweat, Biosensors., 2023, 13(1), 105 CrossRef CAS PubMed.
- Y. Yao, J. Chen, Y. Guo, T. Lv, Z. Chen, N. Li, S. Cao, B. Chen and T. Chen, Integration of interstitial fluid extraction and glucose detection in one device for wearable non-invasive blood glucose sensors, Biosens. Bioelectron., 2021, 179, 113078 CrossRef CAS.
- L. Zheng, Y. Liu and C. Zhang, A sample-to-answer, wearable cloth-based electrochemical sensor (WCECS) for point-of-care detection of glucose in sweat, Sens. Actuators, B, 2021, 343, 130131 CrossRef CAS.
- K. Y. Goud, C. Moonla, R. K. Mishra, C. Yu, R. Narayan, I. Litvan and J. Wang, Wearable electrochemical microneedle sensor for continuous monitoring of levodopa: toward Parkinson management, ACS Sens., 2019, 4(8), 2196–2204 CrossRef CAS.
- S. Fan, W. Chang, C. Fei, Z. Zhang, B. Hou, Z. Shi, H. Wang and Y. Hui, Stretchable and bendable textile matrix based on cellulose fibers for wearable self-powered glucose biosensors, Cellulose, 2022, 29(16), 8919–8935 CrossRef CAS.
- Y. Wang, Y. Wang, R. Zhu, Y. Tao, Y. Chen, Q. Liu, X. Liu and D. Wang, Woven fiber organic electrochemical transistors based on multiwalled carbon nanotube functionalized PEDOT nanowires for nondestructive detection of potassium ions, Mater. Sci. Eng. B, 2022, 278, 115657 CrossRef CAS.
- S. Cai, C. Xu, D. Jiang, M. Yuan, Q. Zhang, Z. Li and Y. Wang, Air-permeable electrode for highly sensitive and noninvasive glucose monitoring enabled by graphene fiber fabrics, Nano Energy, 2022, 93, 106904 CrossRef CAS.
- R. Muralidharan, V. Chandrashekhar, D. Butler and A. Ebrahimi, A smartphone-interfaced, flexible electrochemical biosensor based on graphene ink for selective detection of dopamine, IEEE Sens. J., 2020, 20(22), 13204–13211 CAS.
- J. Ma, Y. Du, Y. Jiang, L. Shen, H. Ma, F. Lv, Z. Cui, Y. Pan, L. Shi and N. Zhu, Wearable healthcare smart electrochemical biosensors based on co-assembled prussian blue—graphene film for glucose sensing, Microchim. Acta, 2022, 189(1), 1–9 CrossRef PubMed.
- B. Kulyk, S. O. Pereira, A. J. Fernandes, E. Fortunato, F. M. Costa and N. F. Santos, Laser-induced graphene from paper for non-enzymatic uric acid electrochemical sensing in urine, Carbon, 2022, 197, 253–263 CrossRef CAS.
- S. G. Son, H. J. Park, S.-M. Kim, S. J. Kim, M. S. Kil, J.-M. Jeong, Y. Lee, Y. Eom, S. Y. Hwang, J. Park and B. G. Choi, Ultra-fast self-healable stretchable bio-based elastomer/graphene ink using fluid dynamics process for printed wearable sweat-monitoring sensor, Chem. Eng. J., 2023, 454, 140443 CrossRef.
- W. Zhang, G. Dong, H. Feng, S. Shan, L. Huang, F. Yuan, B. Bao, L. Yan, Z. Xia, T. Lawson, J. Chen, J. Qu and Y. Liu, Wearable corneal biosensors fabricated from PEDOT functionalized sulfur-doped graphene for use in the early detection of myopia, Adv. Mater. Technol., 2020, 5(12), 2000682 CrossRef CAS.
- E. Mohagheghpour, L. Farzin, M. Malek and S. Sadjadi, A sensing strategy based on aptamers alkylated with melphalan and graphite nanocrystals in a bed of tetrahedral amorphous carbon for electrochemical detection of lead ions in human urine, Microchem. J., 2023, 184, 108206 CrossRef CAS.
- B. Li, X. Wu, C. Shi, Y. Dai, J. Zhang, W. Liu, C. Wu, Y. Zhang, X. Huang and W. Zeng, Flexible enzymatic biosensor based on graphene sponge for glucose detection in human sweat, Surf. Interfaces, 2023, 36, 102525 CrossRef CAS.
- J. R. Sempionatto, A. A. Khorshed, A. Ahmed, A. N. De Loyola e Silva, A. Barfidokht, L. Yin, K. Y. Goud, M. A. Mohamed, E. Bailey, J. May, C. Aebischer, C. Chatelle and J. Wang, Epidermal enzymatic biosensors for sweat vitamin C: Toward personalized nutrition, ACS Sens., 2020, 5(6), 1804–1813 CrossRef CAS.
- Y.-X. Wang, M. Rinawati, J.-D. Zhan, K.-Y. Lin, C.-J. Huang, K.-J. Chen, H. Mizuguchi, J.-C. Jiang, B.-J. Hwang and M.-H. Yeh, Boron-Doped Graphene Quantum Dots Anchored to Carbon Nanotubes as Noble Metal-Free Electrocatalysts of Uric Acid for a Wearable Sweat Sensor, ACS Appl. Nano Mater., 2022, 5(8), 11100–11110 CrossRef CAS.
- Y.-L. Liu, R. Liu, Y. Qin, Q.-F. Qiu, Z. Chen, S.-B. Cheng and W.-H. Huang, Flexible electrochemical urea sensor based on surface molecularly imprinted nanotubes for detection of human sweat, Anal. Chem., 2018, 90(21), 13081–13087 CrossRef CAS PubMed.
- Y. Guan, L. Liu, S. Yu, F. Lv, M. Guo, Q. Luo, S. Zhang, Z. Wang, L. Wu, Y. Lin and G. Liu, A Noninvasive Sweat Glucose Biosensor Based on Glucose Oxidase/Multiwalled Carbon Nanotubes/Ferrocene-Polyaniline Film/Cu Electrodes, Micromachines, 2022, 13(12), 2142 CrossRef PubMed.
- B. Patella, A. Sortino, F. Mazzara, G. Aiello, G. Drago, C. Torino, A. Vilasi, A. O'Riordan and R. Inguanta, Electrochemical detection of dopamine with negligible interference from ascorbic and uric acid by means of reduced graphene oxide and metals-NPs based electrodes, Anal. Chim. Acta, 2021, 1187, 339124 CrossRef CAS PubMed.
- W. He, X. Ye and T. Cui, Flexible Electrochemical Sensor With Graphene and Gold Nanoparticles to Detect Dopamine and Uric Acid, IEEE Sens. J., 2021, 21(23), 26556–26565 Search PubMed.
- W. Ji, D. Wu, W. Tang, X. Xi, Y. Su, X. Guo and R. Liu, Carbonized silk fabric-based flexible organic electrochemical transistors for highly sensitive and selective dopamine detection, Sens. Actuators, B, 2020, 304, 127414 CrossRef CAS.
- H. Teng, J. Song, G. Xu, F. Gao and X. Luo, Nitrogen-doped graphene and conducting polymer PEDOT hybrids for flexible supercapacitor and electrochemical sensor, Electrochim. Acta, 2020, 355, 136772 CrossRef CAS.
- H. Chen, Z. Mei, K. Qi, Y. Wang and R. Chen, A wearable enzyme-free glucose sensor based on nickel nanoparticles decorated laser-induced graphene, J. Electroanal. Chem., 2022, 920, 116585 CrossRef CAS.
- P. Kanokpaka, L.-Y. Chang, B.-C. Wang, T.-H. Huang, M.-J. Shih, W.-S. Hung, J.-Y. Lai, K.-C. Ho and M.-H. Yeh, Self-powered molecular imprinted polymers-based triboelectric sensor for noninvasive monitoring lactate levels in human sweat, Nano Energy, 2022, 107464 CrossRef CAS.
- F. Zhou, H. Zhao, K. Chen, S. Cao, Z. Shi and M. Lan, Flexible electrochemical sensor with Fe/Co bimetallic oxides for sensitive analysis of glucose in human tears, Anal. Chim. Acta, 2023, 340781 CrossRef CAS.
- J. A. Buledi, A. R. Solangi, A. Mallah, S. S. Hassan, S. Ameen, C. Karaman and H. Karimi-Maleh, A Reusable Nickel Oxide Reduced Graphene Oxide Modified Platinum Electrode for the Detection of Linezolid Drug, Ind. Eng. Chem. Res., 2023 DOI:10.1021/acs.iecr.2c03334.
- S. Muqaddas, M. Javed, S. Nadeem, M. A. Asghar, A. Haider, M. Ahmad, A. R. Ashraf, A. Nazir, M. Iqbal, N. Alwadai, A. Ahmad and A. Ali, Carbon Nanotube Fiber-Based Flexible Microelectrode for Electrochemical Glucose Sensors, ACS Omega, 2023, 2272–2280 CrossRef CAS.
- G. Wu, H. Du, Y. L. Cha, D. Lee, W. Kim, F. Feyzbar-Khalkhali-Nejad, T.-S. Oh, X. Zhang and D.-J. Kim, A wearable mask sensor based on polyaniline/CNT nanocomposites for monitoring ammonia gas and human breathing, Sens. Actuators, B, 2023, 375, 132858 CrossRef CAS.
- L. Tian, M. Jiang, M. Su, X. Cao, Q. Jiang, Q. Liu and C. Yu, Sweat cortisol determination utilizing MXene and multi-walled carbon nanotube nanocomposite functionalized immunosensor, Microchem. J., 2023, 185, 108172 CrossRef CAS.
- N. Gao, Z. Cai, G. Chang and Y. He, Non-invasive and wearable glucose biosensor based on gel electrolyte for detection of human sweat, J. Mater. Sci., 2023, 1–12 CAS.
- J. Zhao, C. He, W. Wu, H. Yang, L. Peng, L. Wen, Z. Hu, C. Hou and D. Huo, MXene-MoS2 carbon-fiber-based flexible electrochemical interface for multiple bioanalysis in biofluids, Chem. Eng. J., 2022, 446, 136841 CrossRef CAS.
- C. Moonla, R. Del Caño, K. Sakdaphetsiri, T. Saha, E. De la Paz, A. Düsterloh and J. J. B. Wang, Disposable screen-printed electrochemical sensing strips for rapid decentralized measurements of salivary ketone bodies: Towards therapeutic and wellness applications, Biosens. Bioelectron., 2023, 220, 114891 CrossRef CAS PubMed.
- (a) S. Dong, L. Guo, Y. Chen, Z. Zhang, Z. Yang and M. Xiang, Three-dimensional loofah sponge derived amorphous carbon−graphene aerogel via one-pot synthesis for high-performance electrochemical sensor for hydrogen peroxide and dopamine, J. Electroanal. Chem., 2022, 116236 CrossRef CAS; (b) D. Dreyer, S. Park, C. Bielawski and R. Ruoff, The chemistry of graphene oxide, Chem. Soc. Rev., 2009, 39, 3443–3447 Search PubMed.
- A. Ambrosi, C. K. Chua, A. Bonanni and M. Pumera, Electrochemistry of Graphene and Related Materials, Chem. Rev., 2014, 114(14), 7150–7188 CrossRef CAS PubMed.
- A. Hashmi, V. Nayak, K. R. B. Singh, B. Jain, M. Baid, F. Alexis and A. K. Singh, Potentialities of graphene and its allied derivatives to combat against SARS-CoV-2 infection, Mater. Today Adv., 2022, 13, 100208 CrossRef CAS PubMed.
- (a) Y. C. Qiao, X. S. Li, T. Hirtz, G. Deng, Y. H. Wei, M. R. Li, S. R. Ji, Q. Wu, J. M. Jian, F. Wu, Y. Shen, H. Tian, Y. Yang and T. L. Ren, Graphene-based wearable sensors, Nanoscale, 2019, 11(41), 18923–18945 RSC; (b) C. Huang, Z. Hao, Z. R. Wang, H. Wang, X. Z. Zhao and Y. L. Pan, An Ultraflexible and Transparent Graphene-Based Wearable Sensor for Biofluid Biomarkers Detection, Adv. Mater. Technol., 2022, 7(6) DOI:10.1002/admt.202101131.
- (a) M. Velmurugan, N. Karikalan, S.-M. Chen, Y.-H. Cheng and C. Karuppiah, Electrochemical preparation of activated graphene oxide for the simultaneous determination of hydroquinone and catechol, J. Colloid Interface Sci., 2017, 500, 54–62 CrossRef CAS PubMed; (b) M. Qi, J. Huang, H. Wei, C. Cao, S. Feng, Q. Guo, E. M. Goldys, R. Li and G. Liu, Graphene Oxide Thin Film with Dual Function Integrated into a Nanosandwich Device for in Vivo Monitoring of Interleukin-6, ACS Appl. Mater. Interfaces, 2017, 9(48), 41659–41668 CrossRef CAS PubMed.
- K. R. B. Singh, S. Rathee, G. Nagpure, J. Singh and R. P. Singh, Smart and emerging nanomaterials-based biosensor for SARS-CoV-2 detection, Mater. Lett., 2022, 307, 131092 CrossRef CAS PubMed.
- A. N. Banerjee, Graphene and its derivatives as biomedical materials: future prospects and challenges, Interface Focus, 2018, 8(3), 20170056 CrossRef PubMed.
- D. R. Dreyer, S. Park, C. W. Bielawski and R. S. Ruoff, The chemistry of graphene oxide, Chem. Soc. Rev., 2010, 39(1), 228–240 RSC.
- S. Roy, N. Soin, R. Bajpai, D. S. Misra, J. A. McLaughlin and S. S. Roy, Graphene oxide for electrochemical sensing applications, J. Mater. Chem., 2011, 21(38), 14725–14731 RSC.
- S. Priyadarsini, S. Mohanty, S. Mukherjee, S. Basu and M. Mishra, Graphene and graphene oxide as nanomaterials for medicine and biology application, J. Nanostruct. Chem., 2018, 8(2), 123–137 CrossRef CAS.
- S. Dong, L. Guo, Y. Chen, Z. Zhang, Z. Yang and M. Xiang, Three-dimensional loofah sponge derived amorphous carbon-graphene aerogel via one-pot synthesis for high-performance electrochemical sensor for hydrogen peroxide and dopamine, J. Electroanal. Chem., 2022, 911, 116236 CrossRef CAS.
- H. Zhou, R. Yu, C. Wang, G. Ran, Q. Song and J.-F. Masson, Simple multistep assembly of hybrid carbon material based microelectrode for highly sensitive detection of neurotransmitters, J. Electroanal. Chem., 2020, 863, 114082 CrossRef CAS.
- N. Yang, X. P. Chen, T. L. Ren, P. Zhang and D. G. Yang, Carbon nanotube based biosensors, Sens. Actuators, B, 2015, 207, 690–715 CrossRef CAS.
- G. Gruner, Carbon nanotube transistors for biosensing applications, Anal. Bioanal. Chem., 2006, 384(2), 322–335 CrossRef CAS PubMed.
- Z. Chen, S. M. Tabakman, A. P. Goodwin, M. G. Kattah, D. Daranciang, X. Wang, G. Zhang, X. Li, Z. Liu and P. J. Utz, Protein microarrays with carbon nanotubes as multicolor Raman labels, Nat. Biotechnol., 2008, 26(11), 1285–1292 CrossRef CAS PubMed.
- J. Malig, N. Jux, D. Kiessling, J.-J. Cid, P. Vázquez, T. Torres and D. M. Guldi, Towards Tunable Graphene/Phthalocyanine–PPV Hybrid Systems, Angew. Chem., Int. Ed., 2011, 50(15), 3561–3565 CrossRef CAS PubMed.
- C. X. Cai and J. Chen, Direct electron transfer of glucose oxidase promoted by carbon nanotubes, Anal. Biochem., 2004, 332(1), 75–83 CrossRef CAS PubMed.
- A. Thakur, R. Bharti and R. Sharma, Carbon nanotubes: Types, synthesis, cytotoxicity and applications in biomedical, Mater. Today: Proc., 2022, 50, 2256–2268 CAS.
- Z. Heidarinejad, M. H. Dehghani, M. Heidari, G. Javedan, I. Ali and M. Sillanpää, Methods for preparation and activation of activated carbon: a review, Environ. Chem. Lett., 2020, 18(2), 393–415 CrossRef CAS.
- X. Liu, S. Zuo, N. Cui and S. Wang, Investigation of ammonia/steam activation for the scalable production of high-surface area nitrogen-containing activated carbons, Carbon, 2022, 191, 581–592 CrossRef CAS.
- E. M. Chatir, A. El Hadrami, S. Ojala and R. Brahmi, Production of activated carbon with tunable porosity and surface chemistry via chemical activation of hydrochar with phosphoric acid under oxidizing atmosphere, Surf. Interfaces, 2022, 30, 101849 CrossRef CAS.
- K. P. Loh, Q. Bao, P. K. Ang and J. Yang, The chemistry of graphene, J. Mater. Chem., 2010, 20(12), 2277–2289 RSC.
- E. Dumitrescu, A. Deshpande, K. N. Wallace and S. Andreescu, Time-Dependent Monitoring of Dopamine in the Brain of Live Embryonic Zebrafish Using Electrochemically Pretreated Carbon Fiber Microelectrodes, ACS Meas. Sci. Au, 2022, 2(3), 261–270 CrossRef CAS PubMed.
- A. K. Geim, Graphene: Status and Prospects, Science, 2009, 324(5934), 1530–1534 CrossRef CAS PubMed.
- H. L. Zou, B. L. Li, H. Q. Luo and N. B. Li, A novel electrochemical biosensor based on hemin functionalized graphene oxide sheets for simultaneous determination of ascorbic acid, dopamine and uric acid, Sens. Actuators, B, 2015, 207, 535–541 CrossRef CAS.
- Y. Yuan, X. Gou, R. Yuan, Y. Chai, Y. Zhuo, X. Ye and X. Gan, Graphene-promoted 3,4,9,10-perylenetetracarboxylic acid nanocomposite as redox probe in label-free electrochemical aptasensor, Biosens. Bioelectron., 2011, 30(1), 123–127 CrossRef CAS PubMed.
- M. Wu, R. Kempaiah, P. J. J. Huang, V. Maheshwari and J. W. Liu, Adsorption and Desorption of DNA on Graphene Oxide Studied by Fluorescently Labeled Oligonucleotides, Langmuir, 2011, 27(6), 2731–2738 CrossRef CAS PubMed.
- J. Li, D. Wu, Y. Yu, T. Li, K. Li, M.-M. Xiao, Y. Li, Z.-Y. Zhang and G.-J. Zhang, Rapid and unamplified identification of COVID-19 with morpholino-modified graphene field-effect transistor nanosensor, Biosens. Bioelectron., 2021, 183, 113206 CrossRef CAS PubMed.
- C. Singh, S. Srivastava, M. A. Ali, T. K. Gupta, G. Sumana, A. Srivastava, R. B. Mathur and B. D. Malhotra, Carboxylated multiwalled carbon nanotubes based biosensor for aflatoxin detection, Sens. Actuators, B, 2013, 185, 258–264 CrossRef CAS.
- S. Palanisamy, S. K. Ramaraj, S.-M. Chen, T. C. Yang, P. Yi-Fan, T.-W. Chen, V. Velusamy and S. Selvam, A novel laccase biosensor based on laccase immobilized graphene-cellulose microfiber composite modified screen-printed carbon electrode for sensitive determination of catechol, Sci. Rep., 2017, 7(1), 1–12 CrossRef CAS.
- L. M. Veca, F. S. Lu, M. J. Meziani, L. Cao, P. Y. Zhang, G. Qi, L. W. Qu, M. Shrestha and Y. P. Sun, Polymer functionalization and solubilization of carbon nanosheets, Chem. Commun., 2009,(18), 2565–2567 RSC.
- N. Gao, T. Gao, X. Yang, X. C. Dai, W. Zhou, A. Q. Zhang and C. M. Lieber, Specific detection of biomolecules in physiological solutions using graphene transistor biosensors, Proc. Natl. Acad. Sci. U. S. A., 2016, 113(51), 14633–14638 CrossRef CAS PubMed.
- B. A. E. Lehner, D. Benz, S. A. Moshkalev, A. S. Meyer, M. A. Cotta and R. Janissen, Biocompatible Graphene Oxide Nanosheets Densely Functionalized with Biologically Active Molecules for Biosensing Applications, ACS Appl. Nano Mater., 2021, 4(8), 8334–8342 CrossRef CAS PubMed.
- M. Yang, H. Wang, P. Liu and J. Cheng, A 3D electrochemical biosensor based on Super-Aligned Carbon NanoTube array for point-of-care uric acid monitoring, Biosens. Bioelectron., 2021, 179, 113082 CrossRef CAS PubMed.
- K. D. Kiransan and E. Topcu, Conducting Polymer-Reduced Graphene Oxide Sponge Electrode for Electrochemical Detection Based on DNA Hybridization, ACS Appl. Nano Mater., 2020, 3(6), 5449–5462 CrossRef.
- S. Cheraghi, M. A. Taher, H. Karimi-Maleh, F. Karimi, M. Shabani-Nooshabadi, M. Alizadeh, A. Al-Othman, N. Erk, P. K. Yegya Raman and C. Karaman, Novel enzymatic graphene oxide based biosensor for the detection of glutathione in biological body fluids, Chemosphere, 2022, 287, 132187 CrossRef CAS PubMed.
- J. Y. Lee, D.-G. Cho, S.-P. Cho, J.-H. Choi, S. J. Sung, S. Hong and W.-R. Yu, Semiconducting carbon nanotube fibers for electrochemical biosensor platforms, Mater. Des., 2020, 192, 108740 CrossRef CAS.
- F. C. Vicentini, B. C. Janegitz, C. M. A. Brett and O. Fatibello-Filho, Tyrosinase biosensor based on a glassy carbon electrode modified with multi-walled carbon nanotubes and 1-butyl-3-methylimidazolium chloride within a dihexadecylphosphate film, Sens. Actuators, B, 2013, 188, 1101–1108 CrossRef CAS.
- Z. C. Yao, P. Coatsworth, X. Shi, J. A. Zhi, L. Hu, R. Yan, F. Güderb and H.-D. Yu, Paper-based sensors for diagnostics, human activity monitoring, food safety and environmental detection, Sens. Diagn., 2022, 1, 312–342 RSC.
- W. Dungchai, O. Chailapakul and C. S. Henry, Electrochemical Detection for Paper-Based Microfluidics, Anal. Chem., 2009, 81(14), 5821–5826 CrossRef CAS PubMed.
- J. R. Camargo, T. A. Silva, G. A. Rivas and B. C. Janegitz, Novel eco-friendly water-based conductive ink for the preparation of disposable screen-printed electrodes for sensing and biosensing applications, Electrochim. Acta, 2022, 409, 139968 CrossRef CAS.
- U. Amara, K. Mahmood, S. Riaz, M. Nasir, A. Hayat, M. Hanif, M. Yaqub, D. Han, L. Niu and M. H. Nawaz, Self-assembled perylene-tetracarboxylic acid/multi-walled carbon nanotube adducts based modification of screen-printed interface for efficient enzyme immobilization towards glucose biosensing, Microchem. J., 2021, 165, 106109 CrossRef CAS.
- P. Rattanarat, W. Dungchai, W. Siangproh, O. Chailapakul and C. S. Henry, Sodium dodecyl sulfate-modified electrochemical paper-based analytical device for determination of dopamine levels in biological samples, Anal. Chim. Acta, 2012, 744, 1–7 CrossRef CAS PubMed.
- J. G. Giuliani, T. E. Benavidez, G. M. Duran, E. Vinogradova, A. Rios and C. D. Garcia, Development and Characterization of Carbon Based Electrodes from Pyrolyzed Paper for Biosensing Applications, J. Electroanal. Chem., 2016, 765, 8–15 CrossRef CAS.
- T. R. de Oliveira, W. T. Fonseca, G. de Oliveira Setti and R. C. Faria, Fast and flexible strategy to produce electrochemical paper-based analytical devices using a craft cutter printer to create wax barrier and screen-printed electrodes, Talanta, 2019, 195, 480–489 CrossRef CAS PubMed.
- V. C. Sanchez, A. Jachak, R. H. Hurt and A. B. Kane, Biological Interactions of Graphene-Family Nanomaterials: An Interdisciplinary Review, Chem. Res. Toxicol., 2012, 25(1), 15–34 Search PubMed.
- E. Erçarıkcı, Z. Aksu, E. Topçu and K. D. Kıranşan, ZnS Nanoparticles-decorated Composite Graphene Paper: A Novel Flexible Electrochemical Sensor for Detection of Dopamine, Electroanalysis, 2022, 34(1), 91–102 CrossRef.
- F. Xiao, J. Song, H. Gao, X. Zan, R. Xu and H. Duan, Coating graphene paper with 2D-assembly of electrocatalytic nanoparticles: a modular approach toward high-performance flexible electrodes, ACS Nano, 2012, 6(1), 100–110 CrossRef CAS PubMed.
- S. Peng, L. Li, C. Li, H. Tan, R. Cai, H. Yu, S. Mhaisalkar, M. Srinivasan, S. Ramakrishna and Q. Yan, In situ growth of NiCo 2 S 4 nanosheets on graphene for high-performance supercapacitors, Chem. Commun., 2013, 49(86), 10178–10180 RSC.
- E. Erçarıkcı, Z. Aksu, K. Dağcı Kıranşan and E. Topçu, Graphene paper with electrodeposited NiCo2S4 nanoparticles as a novel flexible sensor for simultaneous detection of folic acid and ascorbic acid, Diamond Relat. Mater., 2022, 121, 108713 CrossRef.
- Y. Fan, S. Shi, J. Ma and Y. Guo, A paper-based electrochemical immunosensor with reduced graphene oxide/thionine/gold nanoparticles nanocomposites modification for the detection of cancer antigen 125, Biosens. Bioelectron., 2019, 135, 1–7 CrossRef CAS PubMed.
- S. Chaiyo, E. Mehmeti, W. Siangproh, T. L. Hoang, H. P. Nguyen, O. Chailapakul and K. Kalcher, Non-enzymatic electrochemical detection of glucose with a disposable paper-based sensor using a cobalt phthalocyanine–ionic liquid–graphene composite, Biosens. Bioelectron., 2018, 102, 113–120 CrossRef CAS PubMed.
- C. J. Valentine, K. Takagishi, S. Umezu, R. Daly and M. De Volder, Paper-Based Electrochemical Sensors Using Paper as a Scaffold to Create Porous Carbon Nanotube Electrodes, ACS Appl. Mater. Interfaces, 2020, 12(27), 30680–30685 CrossRef CAS PubMed.
- M. Novell, M. Parrilla, G. A. Crespo, F. X. Rius and F. J. Andrade, Paper-Based Ion-Selective Potentiometric Sensors, Anal. Chem., 2012, 84(11), 4695–4702 CrossRef CAS.
- V.-K. Tran, E. Ko, Y. Geng, M. K. Kim, G. H. Jin, S. E. Son, W. Hur and G. H. Seong, Micro-patterning of single-walled carbon nanotubes and its surface modification with gold nanoparticles for electrochemical paper-based non-enzymatic glucose sensor, J. Electroanal. Chem., 2018, 826, 29–37 CrossRef CAS.
- Q. Q. Liang, D. Zhang, Y. C. Wu, S. Y. Chen, Z. L. Han, B. X. Wang and H. P. Wang, Self-Stretchable Fiber Liquid Sensors Made with Bacterial Cellulose/Carbon Nanotubes for Smart Diapers, ACS Appl. Mater. Interfaces, 2022, 14(18), 21319–21329 CrossRef CAS.
- H. Lim, A. T. Jafry and J. Lee, Fabrication, flow control, and applications of microfluidic paper-based analytical devices, Molecules, 2019, 24(16), 2869 CrossRef CAS PubMed.
- T. H. da Costa, E. Song, R. P. Tortorich and J.-W. Choi, A Paper-Based Electrochemical Sensor Using Inkjet-Printed Carbon Nanotube Electrodes, ECS J. Solid State Sci. Technol., 2015, 4(10), S3044–S3047 CrossRef CAS.
- (a) J. P. Metters, R. O. Kadara and C. E. Banks, New directions in screen printed electroanalytical sensors: an overview of recent developments, Analyst, 2011, 136(6), 1067–1076 RSC; (b) J. S. Stefano, L. O. Orzari, H. A. Silva-Neto, V. N. de Ataide, L. F. Mendes, W. K. T. Coltro, T. R. L. C. Paixao and B. C. Janegitz, Different approaches for fabrication of low-cost electrochemical sensors, Curr. Opin. Electrochem., 2022, 32 Search PubMed.
- R. C. O. Freitasa, L. O. Orzari, L. M. C. Ferreirac, T. R. L. C. Paixãod, W. K. T. Coltroe, F. C. Vicentinic and B. C. Janegitza, Electrochemical determination of melatonin using disposable self-adhesive inked paper electrode, J. Electroanal. Chem., 2021, 897, 115550 CrossRef.
- W.-J. Guan, Y. Li, Y.-Q. Chen, X.-B. Zhang and G.-Q. Hu, Glucose biosensor based on multi-wall carbon nanotubes and screen printed carbon electrodes, Biosens. Bioelectron., 2005, 21(3), 508–512 CrossRef CAS PubMed.
- A. Wisitsoraat, S. Pakapongpan, C. Sriprachuabwong, P. Ditsayut, P. Sritongkham, T. Lomas and A. Tuantranont, Graphene–PEDOT:PSS on screen printed carbon electrode for enzymatic biosensing, J. Electroanal. Chem., 2013, 704, 208–213 CrossRef CAS.
- T. Hu, D. Wang, J. Xu, K. Chen, X. Li, H. Yi and Z. Ni, Glucose sensing on screen-printed electrochemical electrodes based on porous graphene aerogel @prussian blue, Biomed. Microdevices, 2022, 24(1), 14 CrossRef CAS PubMed.
- S. K. Phetsang, P. Chanlek, J. Jakmunee, P. Mungkornasawakul and K. Ounnunkad, Copper/reduced graphene oxide flm modifed electrode for non-enzymatic glucose sensing application, Sci. Rep., 2021, 11(9302) DOI:10.1038/s41598-021-88747-x.
- S. N. Ashakirin, M. A. S. M. Haniff, M. H. M. Zaid, E. Mahmoudi and M. F. M. Razip Wee, Urease silver reduced graphene oxide modified screen-printed carbon electrode for urea detection, Measurement, 2022, 111058 CrossRef.
- W. Shi, J. Li, J. Wu, Q. Wei, C. Chen, N. Bao, C. Yu and H. Gu, An electrochemical biosensor based on multi-wall carbon nanotube–modified screen-printed electrode immobilized by uricase for the detection of salivary uric acid, Anal. Bioanal. Chem., 2020, 412(26), 7275–7283 CrossRef CAS PubMed.
- S. Dervin, P. Ganguly and R. S. Dahiya, Disposable Electrochemical Sensor Using Graphene Oxide–Chitosan Modified Carbon-Based Electrodes for the Detection of Tyrosine, IEEE Sens. J., 2021, 21(23), 26226–26233 CAS.
- (a) L. Fabiani, M. Saroglia, G. Galatà, R. De Santis, S. Fillo, V. Luca, G. Faggioni, N. D'Amore, E. Regalbuto, P. Salvatori, G. Terova, D. Moscone, F. Lista and F. Arduini, Magnetic beads combined with carbon black-based screen-printed electrodes for COVID-19: A reliable and miniaturized electrochemical immunosensor for SARS-CoV-2 detection in saliva, Biosens. Bioelectron., 2021, 171, 112686 CrossRef CAS PubMed; (b) M. Amouzadeh Tabrizi and P. Acedo, An Electrochemical Impedance Spectroscopy-Based Aptasensor for the Determination of SARS-CoV-2-RBD Using a Carbon Nanofiber–Gold Nanocomposite Modified Screen-Printed Electrode, Biosensors, 2022, 12(3), 142 CrossRef CAS PubMed.
- (a) S. Wang, L. Li, H. Jin, T. Yang, W. Bao, S. Huang and J. Wang, Electrochemical detection of hepatitis B and papilloma virus DNAs using SWCNT array coated with gold nanoparticles, Biosens. Bioelectron., 2013, 41, 205–210 CrossRef CAS PubMed; (b) E. Tamiya, Portable Electrochemical DNA Sensors Based on Gene Amplification Reactions to Screen and Identify Pathogen and SNPs, Sensors, 2022, 22(5), 1865 CrossRef CAS PubMed.
- (a) J. Thangphatthanarungruang, C. Chotsuwan, S. Jampasa and W. Siangproh, A new nanocomposite-based screen-printed graphene electrode for sensitive and selective detection of 8-hydroxy-2′-deoxyguanosine, FlatChem, 2022, 32, 100335 CrossRef CAS; (b) H. Nie, Z. Yang, S. Huang, Z. Wu, H. Wang, R. Yu and J. Jiang, DNA-Wrapped Carbon Nanotubes as Sensitive Electrochemical Labels in Controlled-Assembly-Mediated Signal Transduction for the Detection of Sequence-Specific DNA, Small, 2012, 8(9), 1407–1414 CrossRef CAS PubMed; (c) S. Fortunati, I. Vasini, M. Giannetto, M. Mattarozzi, A. Porchetta, A. Bertucci and M. Careri, Controlling Dynamic DNA Reactions at the Surface of Single-Walled Carbon Nanotube Electrodes to Design Hybridization Platforms with a Specific Amperometric Readout, Anal. Chem., 2022, 94(12), 5075–5083 CrossRef CAS PubMed; (d) L. D'Alton, S. Carrara, G. J. Barbante, D. Hoxley, D. J. Hayne, P. S. Francis and C. F. Hogan, A simple, low-cost instrument for electrochemiluminescence immunoassays based on a Raspberry Pi and screen-printed electrodes, Bioelectrochemistry, 2022, 108107 CrossRef PubMed.
- M. Sher, A. Faheem, W. Asghar and S. Cinti, Nano-engineered screen-printed electrodes: A dynamic tool for detection of viruses, TrAC, Trends Anal. Chem., 2021, 143, 116374 CrossRef CAS PubMed.
- N. Uria, E. Fiset, M. A. Pellitero, F. X. Muñoz, K. Rabaey and F. J. D. Campo, Immobilisation of electrochemically active bacteria on screen-printed electrodes for rapid in situ toxicity biosensing, Environ. Sci. Ecotechnology, 2020, 3, 100053 CrossRef CAS PubMed.
- Y. Hong, M. Wu, G. Chen, Z. Dai, Y. Zhang, G. Chen and X. Dong, 3D Printed Microfluidic Device with Microporous Mn2O3-Modified Screen Printed Electrode for Real-Time Determination of Heavy Metal Ions, ACS Appl. Mater. Interfaces, 2016, 8(48), 32940–32947 CrossRef CAS PubMed.
- E. A. Obaje, G. Cummins, H. Schulze, S. Mahmood, M. P. Y. Desmulliez and T. T. Bachmann, Carbon screen-printed electrodes on ceramic substrates for label-free molecular detection of antibiotic resistance, J. Interdiscip. Nanomed., 2016, 93–109 CrossRef CAS.
- (a) H. Sohrabi, N. Bolandi, A. Hemmati, S. Eyvazi, S. Ghasemzadeh, B. Baradaran, F. Oroojalian, M. R. Majidi, M. Guardia and A. Mokhtarzadeh, State-of-the-art cancer biomarker detection by portable (Bio) sensing technology: A critical review, Microchem. J., 2022, 177, 107248 CrossRef CAS; (b) G. Kabay, Y. Yin, C. K. Singh, N. Ahmad, S. Gunasekaran and M. Mutlu, Disposable electrochemical immunosensor for prostate cancer detection, Sens. Actuators, B, 2022, 360, 131667 CrossRef CAS; (c) G. Ibanez-Redin, R. H. M. Furuta, D. Wilson, F. M. Shimizu, E. M. Materon, L. Arantes, M. E. Melendez, A. L. Carvalho, R. M. Reis, M. N. Chaur, D. Goncalves and O. N. Oliveira, Jr., Screen-printed interdigitated electrodes modified with nanostructured carbon nano-onion films for detecting the cancer biomarker CA19-9, Mater. Sci. Eng., C, 2019, 99, 1502–1508 CrossRef CAS PubMed; (d) T. S. Martins, J. L. Bott-Neto, O. N. Oliveira, Jr. and S. A. S. Machado, A sandwich-type electrochemical immunosensor based on Au-rGO composite for CA15-3 tumor marker detection, Microchim. Acta, 2021, 189(1), 38 CrossRef PubMed.
- M. Lakshmanakumar, N. Nesakumar, S. Sethuraman, R. K. S, U. M. Krishnan and J. B. B. Rayappan, Fabrication of GQD-Electrodeposited Screen-Printed Carbon Electrodes for the Detection of the CRP Biomarker, ACS Omega, 2021, 6(48), 32528–32536 CrossRef CAS PubMed.
- O. Karaman, N. Özcan, C. Karaman, B. B. Yola, N. Atar and M. L. Yola, Electrochemical cardiac troponin I immunosensor based on nitrogen and boron-doped graphene quantum dots electrode platform and Ce-doped SnO2/SnS2 signal amplification, Mater. Today Chem., 2022, 23, 100666 CrossRef CAS.
- D. A. Smith, K. Simpson, M. L. Cicero, L. J. Newbury, P. Nicholas, D. J. Fraser, N. Caiger, J. E. Redman and T. Bowen, Detection of urinary microRNA biomarkers using diazo sulfonamide-modified screen printed carbon electrodes, RSC Adv., 2021, 11, 18832–18839 RSC.
- C. Moonla, R. Del Cano, K. Sakdaphetsiri, T. Saha, E. De la Paz, A. Dusterloh and J. Wang, Disposable screen-printed electrochemical sensing strips for rapid decentralized measurements of salivary ketone bodies: Towards therapeutic and wellness applications, Biosens. Bioelectron., 2022, 220, 114891 CrossRef PubMed.
- G. H. Lee, H. Woo, C. Yoon, C. Yang, J. Y. Bae, W. Kim, D. H. Lee, H. Kang, S. Han, S. K. Kang, S. Park, H. R. Kim, J. W. Jeong and S. Park, A Personalized Electronic Tattoo for Healthcare Realized by On-the-Spot Assembly of an Intrinsically Conductive and Durable Liquid-Metal Composite, Adv. Mater., 2022, 34(32), e2204159 CrossRef PubMed.
- S. Huang, Y. Liu, Y. Zhao, Z. Ren and C. F. Guo, Flexible Electronics: Stretchable Electrodes and Their Future, Adv. Funct. Mater., 2019, 29(6), 1805924 CrossRef.
- N. Gogurla, Y. Kim, S. Cho, J. Kim and S. Kim, Multifunctional and Ultrathin Electronic Tattoo for On-Skin Diagnostic and Therapeutic Applications, Adv. Mater., 2021, 33(24), e2008308 CrossRef PubMed.
- S. Y. Oh, S. Y. Hong, Y. R. Jeong, J. Yun, H. Park, S. W. Jin, G. Lee, J. H. Oh, H. Lee, S. S. Lee and J. S. Ha, Skin-Attachable, Stretchable Electrochemical Sweat Sensor for Glucose and pH Detection, ACS Appl. Mater. Interfaces, 2018, 10(16), 13729–13740 CrossRef CAS PubMed.
- Y. Wang, H. Guo, M. Yuan, J. Yu, Z. Wang and X. Chen, One-step laser synthesis platinum nanostructured 3D porous graphene: A flexible dual-functional electrochemical biosensor for glucose and pH detection in human perspiration, Talanta, 2023, 257, 124362 CrossRef CAS PubMed.
- A. S. M. Steijlen, K. M. B. Jansen, J. Bastemeijer, P. J. French and A. Bossche, Low-Cost Wearable Fluidic Sweat Collection Patch for Continuous Analyte Monitoring and Offline Analysis, Anal. Chem., 2022, 94(18), 6893–6901 CrossRef CAS PubMed.
- L. Yin, K. N. Kim, J. Lv, F. Tehrani, M. Lin, Z. Lin, J. M. Moon, J. Ma, J. Yu, S. Xu and J. Wang, A self-sustainable wearable multi-modular E-textile bioenergy microgrid system, Nat. Commun., 2021, 12(1), 1542 CrossRef CAS PubMed.
- X. Chen, L. L. J. Yin, A. J. Gross, M. Le, N. J. Gutierrez, Y. Li, I. Jeerapan, F. Giroud, A. Berezovska, R. K. O'Reilly, S. Xu, S. Cosnier and J. Wang, Stretchable and Flexible Buckypaper-Based Lactate Biofuel Cell for Wearable Electronics, Adv. Funct. Mater., 2019, 29(46), 1905785 CrossRef CAS.
- (a) J. J. G. Guzmán, C. P. Rafols, M. Cuartero and G. A. Crespo, Microneedle based electrochemical (Bio)Sensing: Towards decentralized and continuous health status monitoring, TrAC, Trends Anal. Chem., 2021, 135, 116148 CrossRef; (b) J. Heikenfeld, A. Jajack, J. Rogers, P. Gutruf, L. Tian, T. Pan, R. Li, M. Khine, J. Kim, J. Wang and J. Kim, Wearable sensors: modalities, challenges, and prospects, Lab Chip, 2018, 18(2), 217–248 RSC.
- F. Tehrani, H. Teymourian, B. Wuerstle, J. Kavner, R. Patel, A. Furmidge, R. Aghavali, H. Hosseini-Toudeshki, C. Brown, F. Zhang, K. Mahato, Z. Li, A. Barfidokht, L. Yin, P. Warren, N. Huang, Z. Patel, P. P. Mercier and J. Wang, An integrated wearable microneedle array for the continuous monitoring of multiple biomarkers in interstitial fluid, Nat. Biomed. Eng., 2022, 6(11), 1214–1224 CrossRef CAS PubMed.
- (a) B. Palys, A. Bokun and J. J. E. A. Rogalski, Poly-o-phenylenediamine as redox mediator for laccase, Electrochim. Acta, 2007, 52(24), 7075–7082 CrossRef CAS; (b) S. Luo, Y. Chen, M. Zhou, C. Yao, H. Xi, Y. Kong and L. Deng, Palygorskite-poly (o-phenylenediamine) nanocomposite: An enhanced electrochemical platform for glucose biosensing, Appl. Clay Sci., 2013, 86, 59–63 CrossRef CAS.
- E. Skaria, B. A. Patel, M. S. Flint and K. W. Ng, Poly(lactic acid)/Carbon Nanotube Composite Microneedle Arrays for Dermal Biosensing, Anal. Chem., 2019, 91(7), 4436–4443 CrossRef CAS PubMed.
- T. M. Rawson, S. A. N. Gowers, D. M. E. Freeman, R. C. Wilson, S. Sharma, M. Gilchrist, A. MacGowan, A. Lovering, M. Bayliss, M. Kyriakides, P. Georgiou, A. E. G. Cass, D. O'Hare and A. H. Holmes, Microneedle biosensors for real-time, minimally invasive drug monitoring of phenoxymethylpenicillin: a first-in-human evaluation in healthy volunteers, Lancet Digital Health, 2019, 1(7), e335–e343 CrossRef PubMed.
- H. Abdullah, T. Phairatana and I. Jeerapan, Tackling the challenges of developing microneedle-based electrochemical sensors, Microchim. Acta, 2022, 189(11), 440 CrossRef CAS PubMed.
- H. Huang, T. Li, M. Jiang, C. Wei, S. Ma, D. Chen, W. Tong and X. Huang, Construction of flexible enzymatic electrode based on gradient hollow fiber membrane and multi-wall carbon tubes meshes, Biosens. Bioelectron., 2020, 152, 112001 CrossRef CAS PubMed.
- Z. Shen, J. Huang, H. Wei, H. Niu, B. Li, R. Li and G. Liu, Validation of an in vivo electrochemical immunosensing platform for simultaneous detection of multiple cytokines in Parkinson's disease mice model, Bioelectrochemistry, 2020, 134, 107532 CrossRef CAS PubMed.
- L. Pilan, Tailoring the performance of electrochemical biosensors based on carbon nanomaterials via aryldiazonium electrografting, Bioelectrochemistry, 2021, 138, 107697 CrossRef CAS PubMed.
- Z. Shao, Y. Chang and B. J. Venton, Carbon microelectrodes with customized shapes for neurotransmitter detection: A review, Anal. Chim. Acta, 2022, 1223, 340165 CrossRef CAS PubMed.
- Y. T. Li, S. H. Zhang, L. Wang, R. R. Xiao, W. Liu, X. W. Zhang, Z. Zhou, C. Amatore and W. H. Huang, Nanoelectrode for amperometric monitoring of individual vesicular exocytosis inside single synapses, Angew. Chem., Int. Ed., 2014, 53(46), 12456–12460 CAS.
- Q. Cao, Z. Shao, D. Hensley and B. J. Venton, Carbon nanospike coated nanoelectrodes for measurements of neurotransmitters, Faraday Discuss., 2022, 233, 303–314 RSC.
- C. Yang, Q. Cao, P. Puthongkham, S. T. Lee, M. Ganesana, N. V. Lavrik and B. J. Venton, 3D-Printed Carbon Electrodes for Neurotransmitter Detection, Angew. Chem., Int. Ed., 2018, 57(43), 14255–14259 CrossRef CAS PubMed.
- G. Xiao, Y. Song, Y. Zhang, Y. Xing, H. Zhao, J. Xie, S. Xu, F. Gao, M. Wang, G. Xing and X. Cai, Microelectrode Arrays Modified with Nanocomposites for Monitoring Dopamine and Spike Firings under Deep Brain Stimulation in Rat Models of Parkinson's Disease, ACS Sens., 2019, 4(8), 1992–2000 CrossRef CAS PubMed.
- M. L. Huffman and B. J. Venton, Carbon-fiber microelectrodes for in vivo applications, Analyst, 2009, 134(1), 18–24 RSC.
- J. Njagi, M. M. Chernov, J. C. Leiter and S. Andreescu, Amperometric Detection of Dopamine in Vivo with an Enzyme Based Carbon Fiber Microbiosensor, Anal. Chem., 2010, 82(3), 989–996 CrossRef CAS PubMed.
- (a) R. E. Ozel, C. Ispas, M. Ganesana, J. C. Leiter and S. Andreescu, Glutamate oxidase biosensor based on mixed ceria and titania nanoparticles for the detection of glutamate in hypoxic environments, Biosens. Bioelectron., 2014, 52, 397–402 CrossRef CAS PubMed; (b) N. P. Sardesai, M. Ganesana, A. Karimi, J. C. Leiter and S. Andreescu, Platinum-doped ceria based biosensor for in vitro and in vivo monitoring of lactate during hypoxia, Anal. Chem., 2015, 87(5), 2996–3003 CrossRef CAS PubMed.
- H. Hou, Y. Jin, H. Wei, W. Ji, Y. Xue, J. Hu, M. Zhang, Y. Jiang and L. Mao, A Generalizable and Noncovalent Strategy for Interfacing Aptamers with a Microelectrode for the Selective Sensing of Neurotransmitters In Vivo, Angew. Chem., Int. Ed., 2020, 59(43), 18996–19000 CrossRef CAS PubMed.
- F. Seven, T. Gölcez and M. Şen, Nanoporous carbon-fiber microelectrodes for sensitive detection of H2O2 and dopamine, J. Electroanal. Chem., 2020, 864, 114104 CrossRef CAS.
- Y. Chang and B. J. Venton, Optimization of graphene oxide-modified carbon-fiber microelectrode for dopamine detection, Anal. Methods, 2020, 12(22), 2893–2902 RSC.
- H. Liang, M. Zhu, H. Ye, C. Zeng, S. Wang and Y. Niu, Carbon fiber microelectrode array loaded with the diazonium salt-single-walled carbon nanotubes composites for the simultaneous monitoring of dopamine and serotonin in vivo, Anal. Chim. Acta, 2021, 1186, 339086 CrossRef CAS PubMed.
- C. Moonla, D. H. Lee, D. Rokaya, N. Rasitanon, G. Kathayat, W.-Y. Lee, J. Kim and I. Jeerapan, Review—Lab-in-a-Mouth and Advanced Point-of-Care Sensing Systems: Detecting Bioinformation from the Oral Cavity and Saliva, ECS Sensors Plus, 2022, 021603 CrossRef.
- L. Garcia-Carmona, A. Martin, J. R. Sempionatto, J. R. Moreto, M. C. Gonzalez, J. Wang and A. Escarpa, Pacifier Biosensor: Toward Noninvasive Saliva Biomarker Monitoring, Anal. Chem., 2019, 91(21), 13883–13891 CrossRef CAS PubMed.
- H. R. Lim, S. M. Lee, S. Park, C. Choi, H. Kim, J. Kim, M. Mahmood, Y. Lee, J. H. Kim and W. H. Yeo, Smart bioelectronic pacifier for real-time continuous monitoring of salivary electrolytes, Biosens. Bioelectron., 2022, 210, 114329 CrossRef CAS PubMed.
- T. Arakawa, K. Tomoto, H. Nitta, K. Toma, S. Takeuchi, T. Sekita, S. Minakuchi and K. Mitsubayashi, A Wearable Cellulose Acetate-Coated Mouthguard Biosensor for In Vivo Salivary Glucose Measurement, Anal. Chem., 2020, 92(18), 12201–12207 CrossRef CAS PubMed.
- Y. Y. Liu, W. Yue and Y. Cui, Development of an amperometric biosensor on a toothbrush for glucose, Sensors and Actuators Reports, 2023, 5, 100133 CrossRef.
- A. Sheng, L. Lin, J. Zhu, J. Zhuang, J. Li, L. Chang and H. Cheng, Micro/nanodevices for assessment and treatment in stomatology and ophthalmology, Microsyst. Nanoeng., 2021, 7, 11 CrossRef CAS PubMed.
- H. Y. Y. Nyein, W. Gao, Z. Shahpar, S. Emaminejad, S. Challa, K. Chen, H. M. Fahad, L.-C. Tai, H. Ota, R. W. Davis and A. Javey, A Wearable Electrochemical Platform for Noninvasive Simultaneous Monitoring of Ca2+ and pH, ACS Nano, 2016, 10(7), 7216–7224 CrossRef CAS PubMed.
- (a) W. Gao, S. Emaminejad, H. Y. Y. Nyein, S. Challa, K. Chen, A. Peck, H. M. Fahad, H. Ota, H. Shiraki, D. Kiriya, D.-H. Lien, G. A. Brooks, R. W. Davis and A. Javey, Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis, Nature, 2016, 529(7587), 509–514 CrossRef CAS PubMed; (b) J. R. Windmiller and J. Wang, Wearable Electrochemical Sensors and Biosensors: A Review, Electroanalysis, 2013, 25(1), 29–46 CrossRef CAS.
- A. J. Bandodkar, W. Jia and J. Wang, Tattoo-Based Wearable Electrochemical Devices: A Review, Electroanalysis, 2015, 27(3), 562–572 CrossRef CAS.
- A. J. Bandodkar, I. Jeerapan, J.-M. You, R. Nuñez-Flores and J. Wang, Highly Stretchable Fully-Printed CNT-Based Electrochemical Sensors and Biofuel Cells: Combining Intrinsic and Design-Induced Stretchability, Nano Lett., 2016, 16(1), 721–727 CrossRef CAS PubMed.
- (a) S. Kabiri Ameri, R. Ho, H. Jang, L. Tao, Y. Wang, L. Wang, D. M. Schnyer, D. Akinwande and N. Lu, Graphene Electronic Tattoo Sensors, ACS Nano, 2017, 11(8), 7634–7641 CrossRef CAS PubMed; (b) S. K. W. L. Ameri, Graphene electronic tattoo sensors for point-of-care personal health monitoring and human–machine interfaces, Information, Sensing and Energy Applications - Micro and Nano Technologies, 2020, ch. 3, pp. 59–86 Search PubMed.
- C. W. Chiu, C. Y. Huang, J. W. Li and C. L. Li, Flexible Hybrid Electronics Nanofiber Electrodes with Excellent Stretchability and Highly Stable Electrical Conductivity for Smart Clothing, ACS Appl. Mater. Interfaces, 2022, 14(37), 42441–42453 CrossRef CAS PubMed.
- D. Kireev, S. K. Ameri, A. Nederveld, J. Kampfe, H. Jang, N. Lu and D. Akinwande, Fabrication, characterization and applications of graphene electronic tattoos, Nat. Protoc., 2021, 16(5), 2395–2417 CrossRef CAS PubMed.
- V. Shirhatti, S. Nuthalapati, V. Kedambaimoole, S. Kumar, M. M. Nayak and K. Rajanna, Multifunctional Graphene Sensor Ensemble as a Smart Biomonitoring Fashion Accessory, ACS Sens., 2021, 6(12), 4325–4337 CrossRef CAS PubMed.
- (a) J. Liang, K. Tong and Q. Pei, A Water-Based Silver-Nanowire Screen-Print Ink for the Fabrication of Stretchable Conductors and Wearable Thin-Film Transistors, Adv. Mater., 2016, 28(28), 5986–5996 CrossRef CAS PubMed; (b) M. Gao, L. Li and Y. Song, Inkjet printing wearable electronic devices, J. Mater. Chem. C, 2017, 5(12), 2971–2993 RSC.
- W. J. Hyun, E. B. Secor, M. C. Hersam, C. D. Frisbie and L. F. Francis, High-Resolution Patterning of Graphene by Screen Printing with a Silicon Stencil for Highly Flexible Printed Electronics, Adv. Mater., 2015, 27(1), 109–115 CrossRef CAS PubMed.
- M. Santhiago, C. C. Correa, J. S. Bernardes, M. P. Pereira, L. J. M. Oliveira, M. Strauss and C. C. B. Bufon, Flexible and Foldable Fully-Printed Carbon Black Conductive Nanostructures on Paper for High-Performance Electronic, Electrochemical, and Wearable Devices, ACS Appl. Mater. Interfaces, 2017, 9(28), 24365–24372 CrossRef CAS PubMed.
- C. Yan, J. Wang, W. Kang, M. Cui, X. Wang, C. Y. Foo, K. J. Chee and P. S. Lee, Highly Stretchable Piezoresistive Graphene–Nanocellulose Nanopaper for Strain Sensors, Adv. Mater., 2014, 26(13), 2022–2027 CrossRef CAS PubMed.
- X. Wang, Y. Gu, Z. Xiong, Z. Cui and T. Zhang, Silk-Molded Flexible, Ultrasensitive, and Highly Stable Electronic Skin for Monitoring Human Physiological Signals, Adv. Mater., 2014, 26(9), 1336–1342 CrossRef CAS PubMed.
- (a) X. Wu, Y. Han, X. Zhang, Z. Zhou and C. Lu, Large-Area Compliant, Low-Cost, and Versatile Pressure-Sensing Platform Based on Microcrack-Designed Carbon Black@Polyurethane Sponge for Human–Machine Interfacing, Adv. Funct. Mater., 2016, 26(34), 6246–6256 CrossRef CAS; (b) C. S. Boland, U. Khan, C. Backes, A. O'Neill, J. McCauley, S. Duane, R. Shanker, Y. Liu, I. Jurewicz, A. B. Dalton and J. N. Coleman, Sensitive, High-Strain, High-Rate Bodily Motion Sensors Based on Graphene–Rubber Composites, ACS Nano, 2014, 8(9), 8819–8830 CrossRef CAS PubMed; (c) X. Li, T. Yang, Y. Yang, J. Zhu, L. Li, F. E. Alam, X. Li, K. Wang, H. Cheng, C.-T. Lin, Y. Fang and H. Zhu, Large-Area Ultrathin Graphene Films by Single-Step Marangoni Self-Assembly for Highly Sensitive Strain Sensing Application, Adv. Funct. Mater., 2016, 26(9), 1322–1329 CrossRef CAS.
- (a) T. Kim, J. Park, J. Sohn, D. Cho and S. Jeon, Bioinspired, Highly Stretchable, and Conductive Dry Adhesives Based on 1D–2D Hybrid Carbon Nanocomposites for All-in-One ECG Electrodes, ACS Nano, 2016, 10(4), 4770–4778 CrossRef CAS PubMed; (b) B. Liu, Z. Luo, W. Zhang, Q. Tu and X. Jin, Carbon nanotube-based self-adhesive polymer electrodes for wireless long-term recording of electrocardiogram signals, J. Biomater. Sci., Polym. Ed., 2016, 27(18), 1899–1908 CrossRef CAS PubMed.
- Q. Cao, M. Shin, N. V. Lavrik and B. J. Venton, 3D-Printed Carbon Nanoelectrodes for In Vivo Neurotransmitter Sensing, Nano Lett., 2020, 20(9), 6831–6836 CrossRef CAS PubMed.
- H. Moeen, Health Monitoring and Management Using Internet-of-Things (IoT) Sensing with Cloud-Based Processing: Opportunities and Challenges, 2015 IEEE International Conference on Services Computing, 2015, pp. 285–292 Search PubMed.
- L. Atzori, A. Iera and G. Morabito, The internet of things: A survey, Comput. Netw., 2010, 54(15), 2787–2805 CrossRef.
- N. Abosata, S. Al-Rubaye, G. Inalhan and C. Emmanouilidis, Internet of Things for System Integrity: A Comprehensive Survey on Security, Attacks and Countermeasures for Industrial Applications, Sensors, 2021, 21(11) DOI:10.3390/s21113654.
- T. Saba, K. Haseeb, I. Ahmed and A. Rehman, Secure and energy-efficient framework using Internet of Medical Things for e-healthcare, J. Infect. Public Health, 2020, 13(10), 1567–1575 CrossRef PubMed.
- R. T. Sutton, D. Pincock, D. C. Baumgart, D. C. Sadowski, R. N. Fedorak and K. I. Kroeker, An overview of clinical decision support systems: benefits, risks, and strategies for success, NPJ Digit. Med., 2020, 3, 17 CrossRef PubMed.
- S. Choi, M. Goryll, L. Y. M. Sin, P. K. Wong and J. Chae, Microfluidic-based biosensors toward point-of-care detection of nucleic acids and proteins, Microfluid. Nanofluid., 2011, 10(2), 231–247 CrossRef CAS PubMed.
- A. E. Ongaro, Z. Ndlovu, E. Sollier, C. Otieno, P. Ondoa, A. Street and M. Kersaudy-Kerhoas, Engineering a sustainable future for point-of-care diagnostics and single-use microfluidic devices, Lab Chip, 2022, 22(17), 3122–3137 RSC.
- FDA, Technical Considerations for Additive Manufactured Medical Devices, https://www.fda.gov/media/97633/download, 2017, Accessed 12/7/2022 Search PubMed.
- S. M. Russell and R. de la Rica, Policy Considerations for Mobile Biosensors, ACS Sens., 2018, 3(6), 1059–1068 CrossRef CAS PubMed.
- Y. Liu, X. Luo, Q. Yu, L. Ye, L. Yang and Y. Cui, Review of point-of-care platforms for diabetes: (1) sensing, Sensors and Actuators Reports, 2022, 4, 100113 CrossRef.
- Ö. Erdem, E. Derin, S. Zeibi Shirejini, K. Sagdic, E. G. Yilmaz, S. Yildiz, G. A. Akceoglu and F. Inci, Carbon-Based Nanomaterials and Sensing Tools for Wearable Health Monitoring Devices, Adv. Mater. Technol., 2022, 7(3), 2100572 CrossRef.
- Z. Hao, Y. Luo, C. Huang, Z. R. Wang, G. L. Song, Y. L. Pan, X. Z. Zhao and S. Q. Liu, An Intelligent Graphene-Based Biosensing Device for Cytokine Storm Syndrome Biomarkers Detection in Human Biofluids, Small, 2021, 17(29) DOI:10.1002/smll.202101508.
- Y. Rasmi, X. Li, J. Khan, T. Ozer and J. R. Choi, Emerging point-of-care biosensors for rapid diagnosis of COVID-19: current progress, challenges, and future prospects, Anal. Bioanal. Chem., 2021, 413(16), 4137–4159 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2023 |
