Open Access Article
Open Access ArticleElectrochemical hydrogen evolution reaction catalysed by a dinuclear cobalt complex with doubly N-confused hexaphyrin†
Risa
Takada
a,
Takashi
Nakazono
 *b,
Taiyo
Nishimura
a,
Takuya
Shiga
*b,
Taiyo
Nishimura
a,
Takuya
Shiga
 c,
Masayuki
Nihei
c,
Yusuke
Yamada
c,
Masayuki
Nihei
c,
Yusuke
Yamada
 bd and
Tohru
Wada
bd and
Tohru
Wada
 *a
*a
aDepartment of Chemistry, College of Science, Rikkyo University, 3-34-1 Nishi-Ikebukuro, Toshima-ku, Tokyo 171-8501, Japan. E-mail: twada@rikkyo.ac.jp
bResearch Center for Artificial Photosynthesis (ReCAP), Osaka Metropolitan University, 3-3-138 Sugimoto, Sumiyoshi-ku, Osaka 558-8585, Japan. E-mail: nakazono@omu.ac.jp
cDepartment of Chemistry, Faculty of Pure and Applied Sciences, University of Tsukuba, Tennodai 1-1-1, Tsukuba 305-8571, Ibaraki, Japan
dDepartment of Chemistry and Bioengineering, Graduate School of Engineering, Osaka Metropolitan University, 3-3-138 Sugimoto, Sumiyoshi-ku, Osaka 558-8585, Japan
First published on 26th May 2023
Abstract
In this study, we discovered the high catalytic activity of a dinuclear cobalt complex, Co2DNCH, supported by a doubly N-confused hexaphyrin (DNCH), a kind of ring-expanded porphyrin, for the electrochemical hydrogen evolution reaction. Co2DNCH catalysed electrochemical hydrogen evolution reaction with onset-overpotential η = 0.45 V in water at pH 7.0, and with η = 0.54 V and turnover frequency TOF = 2.27 × 104 s−1 in DMF containing Et3NHCl as a proton source. Furthermore, electrochemical measurements and density functional theory calculations elucidated that the large π-conjugated system of the DNCH ligand enables two-electron reduction centred on DNCH and hydrogen evolution at a relatively positive potential.
Introduction
Hydrogen is a non-polluting fuel with a high energy density per unit weight. It produces only water as a product following combustion and generation of electricity by a fuel cell.1,2 Hydrogen production is currently based on reforming natural gas and water–gas shift reactions, accompanied by CO2 generation. Electrochemical water reduction can be an alternative for clean hydrogen production without CO2 emissions by combination with highly effective solar photovoltaic generation.3 Although platinum is well known as an extremely active catalyst for the hydrogen evolution reaction (HER), its deposit in the earth's crust is small.4,5Therefore, the development of an HER catalyst composed of earth-abundant metals is required to realise a society based on hydrogen energy. In nature, hydrogenase containing a [FeFe] or [FeNi]-dinuclear complex as a reaction centre effectively catalyses hydrogen evolution and oxidation reactions.6,7 First-row transition metal complexes have the potential to be efficient HER catalysts. In fact, many complexes have been reported as molecular HER catalysts.8–13 In most of the previously proposed reaction pathways, HER proceeds through the hydride complex as an active species, which is formed by the reaction of an intermediate, which contains a low-valent metal ion with a vacant coordination site with a proton. The ligand stabilising such an intermediate is necessary for HER catalysts with first-row transition metal ions.
Porphyrin is a typical macrocyclic ligand whose metal complexes serve as a highly active and durable HER catalysts.14 Moore et al. previously reported that dinuclear copper(II) porphyrin, in which two macrocyclic copper(II) porphyrin molecules are doubly fused at the meso-β position, exhibits a remarkably high turnover rate (TOF = 2.0 × 106 s−1) for electrochemical HER.15 Furthermore, the triply fused Cu porphyrin reported by Sarkar et al. generates H2 with an overpotential of 320 mV lower than the non-fused monomer.16 These interesting reports inspired us to investigate dinuclear complexes supported by macrocyclic ligands.
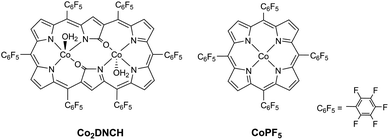
Herein, we focused on dinuclear cobalt complexes with doubly N-confused hexaphyrin (1.1.1.1.1) (Co2DNCH, Fig. 1), which is a ring-expanded porphyrin. Cobalt porphyrin complexes have long been studied as highly active in electrochemical HER in both water and organic solvents.17–20Co2DNCH was firstly synthesised by Furuta, Kim and Osuka,21 however, the catalytic activity for HER had never been reported. We have reported Co2DNCH shows high catalytic activity for photochemical water oxidation for the first time.22 A free base DNCH (H4DNCH) with a Hückel aromatic 26π-conjugated system is capable of multi-electron reduction at more positive potential than porphyrins with 18π-conjugated systems.23,24 It can accommodate two first-row transition-metal ions. Co2DNCH is expected to be a significant HER catalyst due to these characteristics.
In this paper, we report for the first time that Co2DNCH serves as a highly efficient HER catalyst. The HER catalytic activity of Co2DNCH was studied in water and in N,N-dimethylformamide (DMF). Moreover, the redox properties and HER catalytic activity of Co2DNCH were compared with those of CoPF5 (PF5 = meso-tetrakis(pentafluorophenyl)porphyrinato, Fig. 1) with the same C6F5 substituents. The catalytic reaction mechanism is discussed based on the results of electrochemical, spectroelectrochemical and density functional theory (DFT) measurements.
Results and discussion
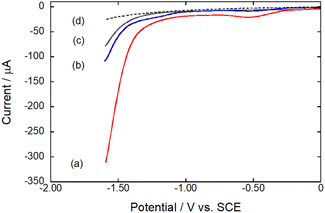
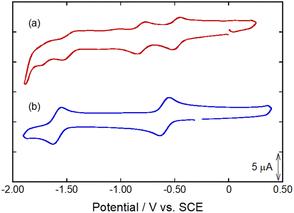
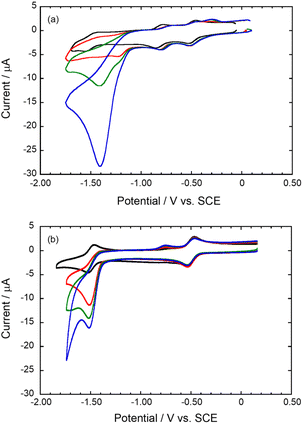
The HER catalytic activity of Co2DNCH and CoPF5 in water was investigated by linear sweep voltammetry (LSV, Fig. 2). LSVs were performed using a glassy carbon electrode modified with each catalyst and a multi-walled carbon nanotube (MW-CNT; Preparation method of the modified electrode is described in the Experimental section), because the catalyst is insoluble in water. The LSVs showed a large cathodic current derived from HER at pH 7.0 (Fig. 2). The onset potential of hydrogen evolution (EHER) of Co2DNCH was −1.1 V (vs. SCE), which was 350 mV more positive than that of CoPF5 (EHER = −1.45 V). EHER of Co2DNCH decreased with increasing pH of the solution, where the slopes were −59 mV pH−1 (Fig. S1 and S2†). The onset-overpotentials of Co2DNCH and CoPF5 were η = 0.45 and 0.80 V, respectively. After LSV using the Co2DCNCH-modified electrode, the electrode was washed with acetone. The LSV using the electrode was almost consistent with that using a glassy carbon electrode modified with only MW-CNT. Thus, the catalyst was not decomposed and functioned as a molecular catalyst (Fig. S3 and S4†). The controlled-potential electrolysis at −1.1 V using Co2DNCH or CoPF5 modified electrodes in phosphate buffer (pH 7) evolved hydrogen (Fig. S5†) with turnover numbers (TON) = 703 for Co2DNCH and TON = 125 for CoPF5 in 50 min. Both of the Faraday efficiencies were more than 98%.The LSVs using the modified electrodes showed only a broad reduction wave derived from the redox reaction of the complexes at −0.5 V. To investigate the detailed redox behaviour of Co2DNCH, we performed cyclic voltammetry (CV) of the complexes in DMF (Fig. 3 and Table S1†). The CV of Co2DNCH showed three reversible waves in E1/2 = −0.47, −0.80 and −1.49 V, and an irreversible redox wave at Epc = −1.65 V in the potential range between 0 V and −2.00 V. Alternatively, two redox waves were observed in the CV of CoPF5 at E1/2 = −0.6 and −1.59 V under the same conditions. These indicate that the two metal centres and the large π-conjugated system of Co2DNCH enable the four-electron storage at more positive potentials than −2.00 V.
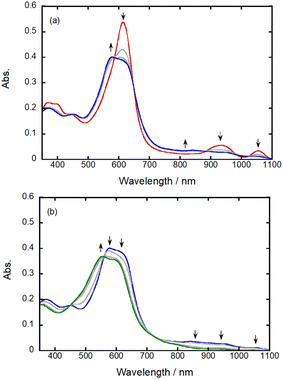
Spectroelectrochemical measurements of Co2DNCH were performed to assign redox waves (Fig. 4). In the UV-vis spectrum of Co2DNCH in DMF, the Soret-like band was observed at 614 nm, and the Q-like bands were observed at 933 and 1054 nm.25 When the Co2DNCH was reduced at −0.6 V, the absorbance of the Soret-like band at 614 nm decreased, and a new absorption band appeared at 580 nm. Meanwhile, the Q-like bands at 933 and 1054 nm disappeared, and a broad absorption band around 850–1050 nm appeared. This new broad band is consistent with that of π radical species previously reported for DNCH analogues,26,27 indicating that the first reduction of Co2DNCH is assigned to the DNCH-centred reduction to form [Co2(DNCH˙−)]−. Further electrolysis at −1.0 V caused the disappearance of the broad absorption band around 850–1050 nm. Thus, the second reduction reaction is also most likely to be the DNCH-based reaction.
In previous studies, the spin state of Co2DNCH was expected to be S = 1/2 (low spin state) per cobalt ion; however, no experimental evidence has been reported to support this hypothesis.28 The temperature dependence of the magnetic susceptibility for Co2DNCH (Fig. S6†) was measured to determine the spin state of Co2DNCH. The χMT value of Co2DNCH is 5.58 emu mol−1 K at room temperature, which decreases upon cooling (0.43 emu mol−1 K at 1.8 K). The χMT value at room temperature is close to those expected for magnetically isolated two Co2+ ions with an S = 3/2 state.29 Therefore, we assigned the spin state, and the oxidation number of the cobalt ions in Co2DNCH was S = 3/2 (HS) per Co2+ ion. The decrease of χMT values at lower temperatures suggests antiferromagnetic interactions between two Co2+ ions. Co2+ porphyrines, which are the analogue of Co2DNCH, are generally low spin and insensitive to axial ligands because of small electron exchange energy in high-spin Co2+ as compared to that in high-spin Fe2+.30 High-spin state of Co2DNCH would be arising from the weak ligand field and distorted pyramidal structure composed of the O atoms of the DNCH ring and axial OH2.
To theoretically support this assignment, we performed the DFT calculation at the uB3LYP/Lanl2DZ (for Co) and 6-31G* (for the other elements) levels of theory with a simplified model of Co2DNCH and its reduced forms in which all pentafluorophenyl groups were replaced by H atoms and aqua ligands were omitted to reduce computational cost (see the DFT calculation section of ESI†). The bond lengths and angles of the optimised Co2DNCH structure are comparable to those of the previously reported single-crystal structure (Fig. S7, S8, and Table S2†). The most stable spin state of Co2DNCH as the ground state was estimated to be septet (2S + 1 = 7) (Table S3†), which is consistent with the result of the magnetic susceptibility measurement. The spin densities of Co2DNCH and its reduced forms of one and two electrons were visualised (Fig. S9†). The spin density on the two Co ions and DNCH ligand of the one-electron reduced state remains and increases, respectively, compared with those of the initial state, strongly supporting the assignment of the first reduction reaction to the ligand-based one (Table S4†). The octet is the most stable spin state of the one-electron reduced form, which results from two high-spin Co2+ ions (S = 3/2 per a Co2+) and DNCH radical (S = 1/2). The spin density on the DNCH of the two-electron-reduced form has almost disappeared.
Furthermore, the septet state is more stable than the nonet state by 25.8 kcal mol−1. These results of DFT calculations support the claim that consecutive two-step ligand-based reductions of Co2DNCH provide [Co2(DNCH2−)]2−, in which two electrons make the spin pair in DNCH. A similar redox behaviour of H4DNCH was reported by Furuta et al.24
To investigate the catalytic activities of HER in organic solutions and these reaction mechanisms, CVs of Co2DNCH and CoPF5 in the presence of proton sources were performed in DMF. Influences of pKa of proton sources on the catalytic current and redox waves in CV provide important information to elucidate the reaction mechanism.31 We used triethylammonium chloride (Et3NHCl, pKDMFa = 9.2), acetic acid (AcOH, pKDMFa = 13.5) and phenol (PhOH, pKDMFa = 18.0) as proton sources.32,33 When 2.0, 5.0 and 10.0 equiv. of Et3NHCl as the strongest acid in the three were gradually added to the DMF solution of Co2DNCH, the catalytic current arising from HER increased at a negative potential greater than −1.2 V, which was between the second and third reduction potentials of Co2DNCH in the absence of a proton source (Fig. 5a). The overpotential was determined by eqn (1) and (2) based on CVs.34
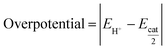 | (1) |
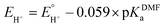 | (2) |
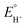 is the equilibrium electrode potential of HER in DMF, Ecat/2 is the potential at half of the catalytic current (icat), and pKDMFa is pKa in DMF of the proton source used.
is the equilibrium electrode potential of HER in DMF, Ecat/2 is the potential at half of the catalytic current (icat), and pKDMFa is pKa in DMF of the proton source used. 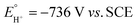 , which was calculated from
, which was calculated from 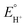 determined by Mayer and co-workers (−0.662 V vs. Fc+/0),35 and pKDMFa = 9.2 for Et3NHCl33 were used for eqn (2). Overpotential of Co2DNCH was calculated to be 0.54 V. In the case that the electron transfer between catalyst molecules and electrodes is sufficiently fast, the substrate concentration is sufficiently high, and the catalytic current is limited only by the chemical reaction in solution, the pseudo-first-order rate constant kobs, which corresponds to the TOF of HER catalyst, can be calculated using eqn (3).36,37
determined by Mayer and co-workers (−0.662 V vs. Fc+/0),35 and pKDMFa = 9.2 for Et3NHCl33 were used for eqn (2). Overpotential of Co2DNCH was calculated to be 0.54 V. In the case that the electron transfer between catalyst molecules and electrodes is sufficiently fast, the substrate concentration is sufficiently high, and the catalytic current is limited only by the chemical reaction in solution, the pseudo-first-order rate constant kobs, which corresponds to the TOF of HER catalyst, can be calculated using eqn (3).36,37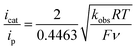 | (3) |
The addition of Et3NHCl to the solution slightly affected the first and second redox waves of Co2DNCH, and the potential of the catalytic wave shifted significantly more positively than the third redox wave in the absence of Et3NHCl. Protonation rate accompanying reduction of the complex may be slow compared with the scan rate of CV. We, therefore, performed the CVs of Co2DNCH at slow scan rate (5 mV s−1) (Fig. S13a†). An irreversible reduction wave appeared at −0.29 V with the addition of Et3NHCl, suggesting protonation of Co2DNCH. When CV was performed at a faster scan rate (100 mV s−1), the same reduction wave was observed at the same potential, although the current was smaller (Fig. S14†). Thus, the first protonation process is expected to be slow. This new reduction wave is not consistent with that of H4DNCH, nor is it due to the formation of a new species by the coordination of Cl− ions (Fig. S15†). The second and third waves at −0.42 and −0.75 V were reversible. Catalytic current was increased from −1.2 V, which unaffected by decrease of scan rate. These observations suggest the [CoCoH(DNCH)]3− reacts with Et3NHCl to form H2 and HER proceeds via (EC)EE(EC) process. When PhOH was used as a proton source, the reversibility of first and second waves at −0.42 and −0.75 V maintained and a new one-electron reduction wave was observed at −1.2 V with a small catalytic current at −1.6 V (Fig. S16a†). This CV changes mean that three-electron reduced form react with a proton to form [CoCoH(DNCH)]2−. [Co(CoH)(DNCH)]2− did not react with PhOH as a weak acid, and the four-electron reduced form [Co(CoH)(DNCH)]3− yielded hydrogen. The shoulder waves at −1.2 and −1.3 V in the CV of Co2DNCH containing 200 equiv. of AcOH would evoke the existence of dihydride species, but there is no clear evidence to support this hypothesis (Fig. S16b†). When PhOH and AcOH was used as proton sources, decreases of scan rates resulted in no significant changes of CVs.
To investigate the third reduction process that occurs at −1.2 V in the presence of AcOH, the kinetic isotope effect (KIE) was calculated using eqn (4).38
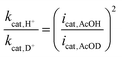 | (4) |
When the shoulder peak of catalytic current at −1.2 V was adopted in the presence of AcOH or AcOD as icat (Fig. S17†), KIE was 1.2, indicating that this process involves protonation. Therefore, [Co2(DNCH2−)]2− is reduced at −1.2 V followed by protonation to give [Co(CoH)(DNCH)]2−, which reacts with H+ to produce H2. The relatively small KIE value (1.2) suggests that a part of [Co(CoH)DNCH]2− generated at −1.2 V produces H2 because the HER of [Co(CoH)DNCH]2− with AcOH is slow. In contrast, reactions at −1.9 V showed relatively large KIE values: 2.7 for AcOH/AcOD because the hydrogen evolution rates of the reactions of [Co(CoH)(DNCH)]3− with AcOH are much faster than those of [Co(CoH)(DNCH)]2−.
Based on the observations above, we propose the HER mechanism of Co2DNCH in organic solution (Scheme 1). When Et3NHCl was used as the strongest acid in the three, the first reduction of Co2DNCH followed by protonation forms the hydride intermediate [CoCoH(DNCH)]. Stepwise two-electron reduction of the hydride intermediate give the reaction active species [CoCoH(DNCH)]2−. Following one-electron reduction to form [CoCoH(DNCH)]3− as a reaction active species, which reacts with a proton to produce H2. In the case of AcOH and PhOH used, three-electron reduced form, [Co2(DNCH)]3−, is protonated to give [CoCoH(DNCH)]2−, which slowly reacts with AcOH as a relatively strong acid. Further reduction of [Co(CoH)(DNCH)]2− to [Co(CoH)(DNCH)]3− causes fast hydrogen evolution even with PhOH (Scheme S1†). In the case of water, the second reduction of Co2DNCH may be a CPET process due to the large mobility of the proton in water.
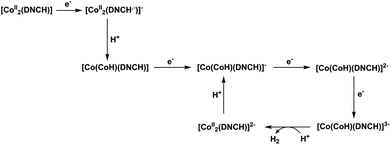 | ||
| Scheme 1 Proposed mechanism of HER catalysed by Co2DNCH in the presence of Et3NHCl as a proton source. | ||
Co2DNCH has a large π-conjugated system of DNCH allowing the ligand to accommodate two electrons. In the case of relatively acidic conditions, multi-electron reduction of the hydride intermediate provides highly active species of HER. Furthermore, the high basicity of the resultant two-electron-reduced form of Co2DNCH enables protonation and further reduction to generate the catalytic active species at relatively positive potential even when relatively week acid is used. On the other hand, CoPF5 requires a more negative potential for the reduction accompanying a protonation and hydrogen evolution. Therefore, the large π-conjugated system of DNCH is advantageous to HER.
Experimental
Synthesis
All solvents and reagents were of the highest quality available and were used as received. Co2DNCH was synthesised according to literature21 and characterized by ESI-MS and elemental analysis. CoPF5 was prepared using the literature method.39Instrumentation
Conclusions
In this paper, we have reported the catalytic activity of Co2DNCH for electrochemical hydrogen evolution reaction (HER) in water and DMF. Co2DNCH catalysed HER with η = 0.45 V in water and with η = 0.54 V and TOF = 2.27 × 104 s−1 in DMF. The catalytic activity of Co2DNCH is much higher than that of CoPF5. CVs and DFT calculations show that the stepwise two-electron reduction occurring at DNCH promotes protonation of the Co centre of Co2DNCH even when relatively week acid is used. Therefore, the large π-conjugation system of DNCH is highly useful for electrochemical HER catalysts. This study provided new insights into the research regions of electrochemical HER and ring-expanded porphyrin complexes.Conflicts of interest
There were no conflicts to declare.Acknowledgements
This work was supported by JSPS Early Career Scientists (Grant 20K15303), Grant-in-Aid for Scientific Research (C) (Grant 18K05158) and Grant-in-Aid for Scientific Research (B) (Grant 22H01871). This work was also supported by JSPS KAKENHI (Grant 20H05116) in Scientific Research on Innovative Areas ‘Innovations for Light-Energy Conversion (I4LEC)’, JGC-S Scholarship Foundation, and ENEOS Tonengeneral Research/Development Encouragement & Scholarship Foundation. The authors would like to applicate for Prof. Masahiro Yamanaka of Rikkyo Univ. to support DFT study.Notes and references
- A. Kudo and Y. Miseki, Chem. Soc. Rev., 2009, 38, 253–278 RSC.
- M. Chatenet, B. G. Pollet, D. R. Dekel, F. Dionigi, J. Deseure, P. Millet, R. D. Braatz, M. Z. Bazant, M. Eikerling, I. Staffell, P. Balcombe, Y. Shao-Horn and H. Schafer, Chem. Soc. Rev., 2022, 51, 4583–4762 RSC.
- X. Zou and Y. Zhang, Chem. Soc. Rev., 2015, 44, 5148–5180 RSC.
- B. Ruqia and S. I. Choi, ChemSusChem, 2018, 11, 2643–2653 CrossRef CAS PubMed.
- C. Li and J. B. Baek, ACS Omega, 2020, 5, 31–40 CrossRef CAS PubMed.
- J. C. Fontecilla-Camps, P. Amara, C. Cavazza, Y. Nicolet and A. Volbeda, Nature, 2009, 460, 814–822 CrossRef CAS PubMed.
- B. E. Barton and T. B. Rauchfuss, J. Am. Chem. Soc., 2010, 132, 14877–14885 CrossRef CAS PubMed.
- D. Brazzolotto, M. Gennari, N. Queyriaux, T. R. Simmons, J. Pécaut, S. Demeshko, F. Meyer, M. Orio, V. Artero and C. Duboc, Nat. Chem., 2016, 8, 1054–1060 CrossRef CAS PubMed.
- M. L. Helm, M. P. Stewart, R. M. Bullock, M. R. DuBois and D. L. DuBois, Science, 2011, 333, 863–866 CrossRef CAS PubMed.
- K. E. Dalle, J. Warnan, J. J. Leung, B. Reuillard, I. S. Karmel and E. Reisner, Chem. Rev., 2019, 119, 2752–2875 CrossRef CAS PubMed.
- C. Baffert, V. Artero and M. Fontecave, Inorg. Chem., 2007, 46, 1817–1824 CrossRef CAS PubMed.
- F. Gloaguen, J. D. Lawrence and T. B. Rauchfuss, J. Am. Chem. Soc., 2001, 123, 9476–9477 CrossRef CAS PubMed.
- K. Kawano, K. Yamauchi and K. Sakai, Chem. Commun., 2014, 50, 9872–9875 RSC.
- B. B. Beyene and C. H. Hung, Coord. Chem. Rev., 2020, 410, 213234 CrossRef CAS.
- D. Khusnutdinova, B. L. Wadsworth, M. Flores, A. M. Beiler, E. A. Reyes Cruz, Y. Zenkov and G. F. Moore, ACS Catal., 2018, 8, 9888–9898 CrossRef CAS.
- S. Chandra, A. S. Hazari, Q. Song, D. Hunger, N. I. Neuman, J. van Slageren, E. Klemm and B. Sarkar, ChemSusChem, 2023, 16, e202201146 CrossRef CAS PubMed.
- R. M. Kellett and T. G. Spiro, Inorg. Chem., 1985, 24, 2373–2377 CrossRef CAS.
- J. G. Kleingardner, B. Kandemir and K. L. Bren, J. Am. Chem. Soc., 2014, 136, 4–7 CrossRef CAS PubMed.
- H. M. Castro-Cruz and N. A. Macías-Ruvalcaba, Coord. Chem. Rev., 2022, 458, 214430 CrossRef CAS.
- M. Natali, A. Luisa, E. Iengo and F. Scandola, Chem. Commun., 2014, 50, 1842–1844 RSC.
- M. Suzuki, M. C. Yoon, D. Y. Kim, J. H. Kwon, H. Furuta, D. Kim and A. Osuka, Chem.–Eur. J., 2006, 12, 1754–1759 CrossRef CAS PubMed.
- T. Nakazono and T. Wada, Inorg. Chem., 2020, 60, 1284–1288 CrossRef PubMed.
- H. Lei, Y. Wang, Q. Zhang and R. Cao, J. Porphyrins Phthalocyanines, 2020, 24, 1361–1371 CrossRef CAS.
- I. Mayer, K. Nakamura, A. Srinivasan, H. Furuta, H. E. Toma and K. Araki, J. Porphyrins Phthalocyanines, 2005, 9, 813–820 CrossRef CAS.
- J. H. Kwon, T. K. Ahn, M.-C. Yoon, D. Y. Kim, M. K. Koh, D. Kim, H. Furuta, M. Suzuki and A. Osuka, J. Phys. Chem. B, 2006, 110, 11683–11690 CrossRef CAS PubMed.
- T. Koide, G. Kashiwazaki, M. Suzuki, K. Furukawa, M.-C. Yoon, S. Cho, D. Kim and A. Osuka, Angew. Chem., Int. Ed., 2008, 47, 9661–9665 CrossRef CAS PubMed.
- K. Yamasumi, K. Nishimura, Y. Hisamune, Y. Nagae, T. Uchiyama, K. Kamitani, T. Hirai, M. Nishibori, S. Mori and S. Karasawa, Chem.–Eur. J., 2017, 23, 15322–15326 CrossRef CAS PubMed.
- G. Sun, E. Lei, X.-S. Liu, X.-X. Duan and C.-G. Liu, J. Mol. Model., 2018, 24, 1–9 CrossRef CAS PubMed.
- Y. Rechkemmer, F. D. Breitgoff, M. Van Der Meer, M. Atanasov, M. Hakl, M. Orlita, P. Neugebauer, F. Neese, B. Sarkar and J. Van Slageren, Nat. Commun., 2016, 7, 1–8 Search PubMed.
- V. V. Smirnov, E. K. Woller and S. G. DiMagno, Inorg. Chem., 1998, 37, 4971–4978 CrossRef CAS PubMed.
- N. Queyriaux, D. Sun, J. Fize, J. Pecaut, M. J. Field, M. Chavarot-Kerlidou and V. Artero, J. Am. Chem. Soc., 2020, 142, 274–282 CrossRef CAS PubMed.
- K. Izutsu, Acid-Base Dissociation Constants in Dipolar Aprotic Solvents, Blackwell Scientific Publication, 1990 Search PubMed.
- V. Fourmond, P. A. Jacques, M. Fontecave and V. Artero, Inorg. Chem., 2010, 49, 10338–10347 CrossRef CAS PubMed.
- A. M. Appel and M. L. Helm, ACS Catal., 2014, 4, 630–633 CrossRef CAS.
- M. L. Pegis, J. A. S. Roberts, D. J. Wasylenko, E. A. Mader, A. M. Appel and J. M. Mayer, Inorg. Chem., 2015, 54, 11883–11888 CrossRef CAS PubMed.
- E. S. Rountree, B. D. McCarthy, T. T. Eisenhart and J. L. Dempsey, Inorg. Chem., 2014, 53, 9983–10002 CrossRef CAS PubMed.
- M. Okamura, M. Kondo, R. Kuga, Y. Kurashige, T. Yanai, S. Hayami, V. K. Praneeth, M. Yoshida, K. Yoneda, S. Kawata and S. Masaoka, Nature, 2016, 530, 465–468 CrossRef CAS PubMed.
- F. Yu, D. Poole III, S. Mathew, N. Yan, J. Hessels, N. Orth, I. Ivanovic-Burmazovic and J. N. H. Reek, Angew. Chem., Int. Ed., 2018, 57, 11247–11251 CrossRef CAS PubMed.
- S. A. Jannuzzi, E. G. de Arruda, F. A. Lima, M. A. Ribeiro, C. Brinatti and A. L. Formiga, ChemistrySelect, 2016, 1, 2235–2243 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. V. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, T. A. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman and D. J. Fox, Gaussian 16, Revision B.01, Gaussian, Inc., Wallingford CT Search PubMed.
- P. J. Hay and W. R. Wadt, J. Chem. Phys., 1985, 82, 270–283 CrossRef CAS.
- W. R. Wadt and P. J. Hay, J. Chem. Phys., 1985, 82, 284–298 CrossRef CAS.
- P. J. Hay and W. R. Wadt, J. Chem. Phys., 1985, 82, 299–310 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3se00403a |
| This journal is © The Royal Society of Chemistry 2023 |