Open Access Article
Open Access ArticleSupercritical carbon dioxide/nitrogen/air extraction with multistage stripping enables selective recovery of rare earth elements from coal fly ashes†
Yaguang
Zhu‡
 ,
Guangcheng
Wang§
and
Young-Shin
Jun
,
Guangcheng
Wang§
and
Young-Shin
Jun
 *
*
Department of Energy, Environmental & Chemical Engineering, Washington University in St. Louis, St. Louis, Missouri 63130, USA. E-mail: ysjun@seas.wustl.edu; Fax: +1-314-696-1223; Tel: +1-314-935-4539 Web: https://encl.engineering.wustl.edu/
First published on 16th January 2023
Abstract
Rare earth elements (REEs) are widely used in electronic devices and renewable energy technology, but their supply is geopolitically-limited and they are extracted by environmentally unsustainable mining practices. Coal fly ash (CFA), which is mostly discarded as waste, has recently gained attention as a potential low-grade REE source, motivating the development of greener and highly specific processes for recovering and enriching REEs. Here we present a proof-of-concept for a novel REE extraction process in which supercritical fluid enhances the ability of tributyl phosphate (TBP) to selectively extract REEs directly from solid CFA matrices. For the first time, we show that supercritical nitrogen and supercritical air can work like supercritical carbon dioxide for selective extraction. Moreover, using a prototype multistage stripping process with an aqueous solution, we collected REEs with concentrations up to 21.4 mg L−1 from the extractant. Our final products contain up to 6.47% REEs, whereas the coal fly ash source initially contained only 0.0234% REEs. Using supercritical fluid, our novel process can recover valuable and critical resources from materials previously considered to be waste.
Sustainability spotlightLarge amounts of coal fly ash (CFA) deposited in landfills and wet impoundments are considered a threat to the local environment due to possible toxic element leaching. Recently, CFA has been found to be a potential source of rare earth elements (REEs), but current extraction technologies are challenged by low selectivity, organic waste production, and high energy consumption. Here, we report the use of supercritical fluids (carbon dioxide, nitrogen, and air) as greener solvents assisting a phosphonate extractant in directly and selectively extracting REEs, without energy- and material-intensive leaching. Our work shows promise to recover valuable resources from waste materials. Therefore, our work can help to realize the “Responsible Consumption and Production” of the Sustainable Development Goals (SDGs). |
Introduction
Rare earth elements (REEs) are a group of 17 chemical elements in the periodic table, specifically the 15 lanthanides plus scandium and yttrium. The wide application of REEs in computer memory, rechargeable batteries, cell phones, and fluorescent lighting manifests their indispensable roles in our daily life.1 Moreover, they are also critical to a variety of high tech applications, such as clean energy generation and catalysis, and their production is closely linked to the speed of technology development and implementation.2–4 However, due to their geopolitically-constrained supply, environmentally-unsustainable mining practices, and rapidly growing demand,3 both the United States (US) and the European Union have classified REEs as “critical materials”.5,6 To address such a limited supply, alternative domestic sources will be most welcome.7–10Recently, coal fly ash (CFA) has emerged as a promising REE resource.11,12 The average total REE concentration in CFAs has been characterized as 200–1220 ppm, and the potential annual value of the REEs that can be extracted from CFAs in the US is estimated to be $4.3 billion.12 According to American Coal Ash Association's 2019 production and use survey, approximately 79 million metric tonnes (t) of CFAs are generated annually in the US, with only 52% beneficially used and the rest discarded.13 The remaining CFAs, deposited in landfills or wet impoundments, are considered as a threat to local environment due to possible leaching of toxic elements.14,15 Notably, obtaining REEs from CFAs is less environmentally destructive and capital intensive than extraction from traditional mineral ores, because it does not generate large quantities of waste rock that is typically radioactive.11,16,17 In this regard, recovering REEs from CFAs turns waste into valuable resources with impactful environmental and societal benefits.
To successfully obtain high purities of individual REEs from mineral ores, current industrial REE extraction operations include many processes, such as alkaline roasting, acid leaching, fractional separation, ion exchange, and solvent extraction.18–20 In the initial attempt to recover REEs from CFAs, these methods were adopted first. Although previous studies have applied different methods to extract REEs from CFAs,21–23 these processes still present many challenges. First, they all require a high temperature alkaline roasting process (>400 °C), followed by an acid leaching process (using strong acid) to obtain REE-containing leachate. Their high energy and chemical demands have proven burdensome in the commercial extraction of REEs from mineral ores, and these burdens will be more severe for low grade REEs resources like CFAs.24 Notably, a strong acid is indispensable in all the REEs extraction processes. Second, an extractant that selectively complexes with REE3+ is also necessary for the extraction. For example, in the solvent extraction, di-2-ethylhexylphosphoric acid (DEHPA) was dispersed in kerosene, and together they can selectively extract REEs from the aqueous solutions.21 In addition, DEHPA-dispersed mineral oil inside a membrane was used for selectively transferring REEs from a CFA leachate to a highly acidic solution.22 However, these processes all use toxic organic solvent to disperse the extractant, and thus it is highly desirable to find environmentally-friendly solvents to replace the organic solvent. Third, and most importantly, CFAs have extremely low concentrations of REEs (<0.2%) and more than 90% major impurities (Ca, Fe, Al, Mg), so the REEs purity in the final products is only 0.5–0.7%.22 Overcoming these drawbacks requires a novel REEs extraction process that is environmentally-benign and highly selective for REEs over impurities.
Supercritical fluid (SCF) extraction has emerged as a promising option because SCFs have little environmental impact, are non-flammable, and facilitate the mass transfer of extractants.25 Applying SCF can reduce the usage of organic solvent, and we also expect that it can improve the selective recovery of REEs from CFAs. To selectively extract REEs from a solid matrix, studies have explored using extractants to complex with REE3+ ions under supercritical carbon dioxide (scCO2).26,27 Tributyl phosphate–nitric acid (TBP–HNO3) has shown selective extraction of REEs. This extractant was prepared by contacting pure TBP with concentrated HNO3. A current hypothesis for the extraction mechanism in a scCO2 system is that TBP selectively chelates with the neutral salt formed by REE3+ and NO3−.27,28 Although scCO2 with TBP has successfully and selectively extracted REEs from high concentration REE resources (such as pure REE oxides),26,29–31 REE-rich sources (e.g., bastnaesite, monazite, NiMH batteries, and NdFeB magnets),28,32,33 and phosphogypsum (REE concentration up to 0.6%),34 its performance has not been studied with CFAs, which have extremely low REE concentrations (<0.2%). In addition, studies have used scCO2 extraction with a flow-through setup to remove toxic heavy metals from CFAs,35,36 but they did not show the capability to selectively separate REE3+ from other ions to recover valuable resources. Also, how much impurity can be extracted was not provided. Thus, separation of REEs from impurities during or after SCF extraction with TBP–HNO3 needs more systematic investigations.
Furthermore, previous studies notably tested only CO2 as the supercritical fluid. In these studies, scCO2 (critical temperature (Tc) = 31 °C, critical pressure (Pc) = 73.8 bar) offers several advantages, such as safety, abundance, and low cost. An outstanding question is whether the supercritical state of more accessible gases, such as nitrogen (Tc = −147 °C and Pc = 34.0 bar) or air (Tc = −141 °C and Pc = 37.9 bar), can also be used in the extraction and whether they can achieve a similar efficiency to scCO2.
Herein, we present a novel extraction process that uses SCF to directly and selectively extract REEs from a solid CFA matrix. This proof-of-concept study aims to investigate the feasibility of selective extraction of REEs from CFAs using SCF with little interference from impurities. We achieved excellent extraction efficiencies, between 66 and 79%, for all REEs, and found that scCO2 can decrease the concentrations of impurities in the final product, especially Ca, Mg, and Al. In previous studies, much emphasis was placed on the flow and heat properties of supercritical nitrogen and supercritical air,37,38 but they have not been tested as green solvents. Moving beyond CO2, our work is the first report to demonstrate that more common and accessible SCF sources, such as nitrogen and air, can also assist TBP to extract REEs with high efficiency and separate impurities. Moreover, we applied a multistage stripping process to collect REEs and further separate REEs and impurities to increase the purity of REEs in our collected solutions. Our extraction process replaces the toxic organic solvent used in most existing techniques with SCF to make the process “greener”. In addition, by combining SCF extraction with the multistage stripping, our process showed a higher selectivity for REEs over impurities than achieved by conventional organic solvent extraction methods. This study offers new promising SCF choices (nitrogen and air) and provides useful insights into selectivity in SCF extraction, enabling future greener processes in REE recovery from unconventional resources.
Experimental
Materials
The coal fly ash in our study came from a power plant in Missouri, burning coal from the Powder River Basin (PRB). Deionized water (DI water, resistivity ≥ 18.2 MΩ cm) was obtained from a Barnstead Ultrapure Water System (D11931, Thermo Scientific). ACS grade tributyl phosphate (TBP) and nitric acid were purchased from VWR.Supercritical fluid extraction and multistage stripping process
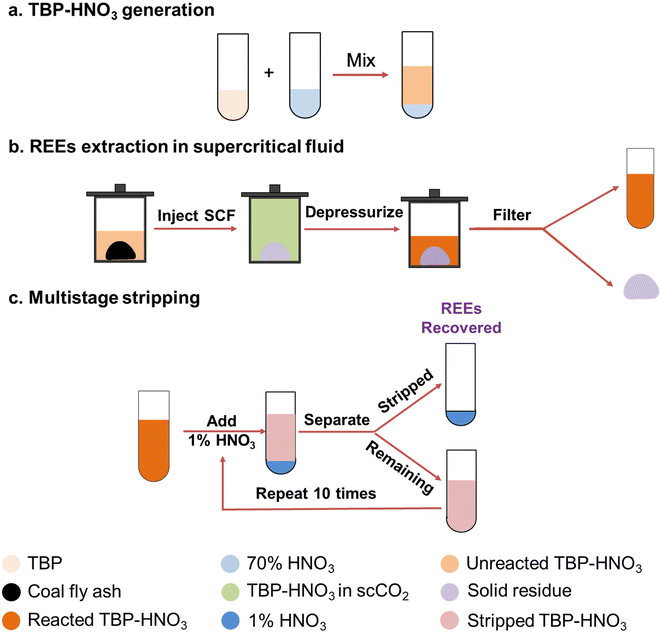
Equal volumes of TBP and 70% nitric acid were mixed and allowed to react and settle. The upper layer, the extractant TBP–HNO3 used in this work, was pipetted off (see Fig. 1). To determine the molar ratio of TBP and HNO3 in the extractant, acid–base titration was used. The molar ratio was TBP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HNO3 = 1
HNO3 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.67, and this value is close to the molar ratios of the TBP–HNO3 complex reported elsewhere.28,29 We loaded 2 g of CFA, along with 20 mL TBP–HNO3, into a reactor (250 mL, Parr Instrument Co., IL). The CO2, N2, and air used in our study were purchased from Airgas USA, LLC, MO. The gases were pressurized to 150 bar by a syringe pump (Teledyne ISCO, Inc., Lincoln, NE) and then injected into the reactor, whose temperature was controlled at 50 °C. After 2 h of extraction, the reactor was cooled to room temperature and then depressurized. The reacted TBP–HNO3 was obtained by filtering out the solid residues. These residues were then rinsed with ethanol and DI water to remove any remaining solution and prepared for further characterization. Triplicate experiments were conducted for each condition.
1.67, and this value is close to the molar ratios of the TBP–HNO3 complex reported elsewhere.28,29 We loaded 2 g of CFA, along with 20 mL TBP–HNO3, into a reactor (250 mL, Parr Instrument Co., IL). The CO2, N2, and air used in our study were purchased from Airgas USA, LLC, MO. The gases were pressurized to 150 bar by a syringe pump (Teledyne ISCO, Inc., Lincoln, NE) and then injected into the reactor, whose temperature was controlled at 50 °C. After 2 h of extraction, the reactor was cooled to room temperature and then depressurized. The reacted TBP–HNO3 was obtained by filtering out the solid residues. These residues were then rinsed with ethanol and DI water to remove any remaining solution and prepared for further characterization. Triplicate experiments were conducted for each condition.
A multistage stripping process using 1% nitric acid was applied to selectively collect the REEs and separate them from the impurities. Specifically, 1% nitric acid was added to the reacted TBP–HNO3 in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 v/v ratio that have been experimentally determined to be the best for concentrating REEs. After 10 s of vigorous mixing, the REEs and impurities dissociated from the TBP and dissolved in the acid. After being collected by gravity separation, the diluted nitric acid containing REEs and impurities was called the stripped solution. The remaining reacted TBP–HNO3 was mixed with fresh 1% nitric acid to conduct a new stripping stage. In total, a six-stage stripping process was conducted to recover essentially all the REEs from the reacted TBP–HNO3.
10 v/v ratio that have been experimentally determined to be the best for concentrating REEs. After 10 s of vigorous mixing, the REEs and impurities dissociated from the TBP and dissolved in the acid. After being collected by gravity separation, the diluted nitric acid containing REEs and impurities was called the stripped solution. The remaining reacted TBP–HNO3 was mixed with fresh 1% nitric acid to conduct a new stripping stage. In total, a six-stage stripping process was conducted to recover essentially all the REEs from the reacted TBP–HNO3.
Characterization of solid samples
The sizes, morphologies, and elemental distributions of CFAs were characterized by SEM-EDX (ThermoFisher Quattro S Environmental Scanning Electron Microscope). We identified the mineral phase in CFA by high-resolution X-ray diffraction (HRXRD, Bruker D8 Advance X-ray diffractometer with Cu Kα radiation (λ = 1.5418 Å)). CFA and solid residues were digested by two methods, one to obtain the total elemental compositions and the other to obtain the acid-extractable REEs element compositions. In addition, the solid residue from the extraction was digested to obtain the total elemental composition and calculate the leaching efficiency, using eqn (1).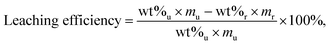 | (1) |
To quantify the total elemental composition,23 coal fly ash samples (34 ± 1 mg) were digested in a microwave digestor for 8 h at 90–100 °C in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 2 mL concentrated HF and 2 mL concentrated HNO3. Then, after complete drying, the acid-digested samples were re-digested for 8 h at 90–100 °C in a mixture of 1 mL concentrated HNO3, 1 mL 30–32% H2O2, and 5 mL DI water. After re-digestion, the samples were diluted with 1% HNO3 for further analysis. To quantify the acid-extractable REEs content,23 CFA samples (0.1–0.5 g) were digested in 10 mL concentrated HNO3 at 85–90 °C for 4 h. The digested samples were diluted with 1% HNO3 for further analysis. Triplicate digestion experiments were conducted. The concentrations of the REEs and impurities in the digested solutions were analyzed by inductively coupled plasma optical emission spectroscopy (ICP-OES, PerkinElmer Optima 7300 DV).
1 mixture of 2 mL concentrated HF and 2 mL concentrated HNO3. Then, after complete drying, the acid-digested samples were re-digested for 8 h at 90–100 °C in a mixture of 1 mL concentrated HNO3, 1 mL 30–32% H2O2, and 5 mL DI water. After re-digestion, the samples were diluted with 1% HNO3 for further analysis. To quantify the acid-extractable REEs content,23 CFA samples (0.1–0.5 g) were digested in 10 mL concentrated HNO3 at 85–90 °C for 4 h. The digested samples were diluted with 1% HNO3 for further analysis. Triplicate digestion experiments were conducted. The concentrations of the REEs and impurities in the digested solutions were analyzed by inductively coupled plasma optical emission spectroscopy (ICP-OES, PerkinElmer Optima 7300 DV).
Characterization of liquid samples
The concentration of HNO3 in the TBP–HNO3 complex was determined by acid–base titration with 0.1 M NaOH until the pH equaled 7. Then, to quantify the REE and impurity concentrations in each stripped solution collected from the six-stage stripping process under scCO2, scN2, scAir, and the heating only condition, we diluted them with 1% nitric acid and measured them using ICP-OES. The REEs purity was calculated using eqn (2): | (2) |
To study the mechanism by which SCF enhances selective extraction of REEs, the reacted TBP–HNO3 samples obtained from the extraction were digested to quantify the amounts of REEs and impurities that had complexed with TBP. Triplicate experiments were conducted. The digestion of liquid TBP–HNO3 samples was performed according to Anil et al. (2004).39 TBP–HNO3 solutions were mixed with 1 mL DI water, 2 mL concentrated HNO3, 0.4 mL 30–32% H2O2, and 0.4 mL concentrated HF. Then, an eight-step digestion was performed, lasting 1 h in total at 100 °C. After the digestion, the samples were diluted by 1% HNO3 and prepared for ICP-OES analysis.
Results and discussion
Chemical nature of coal fly ash samples
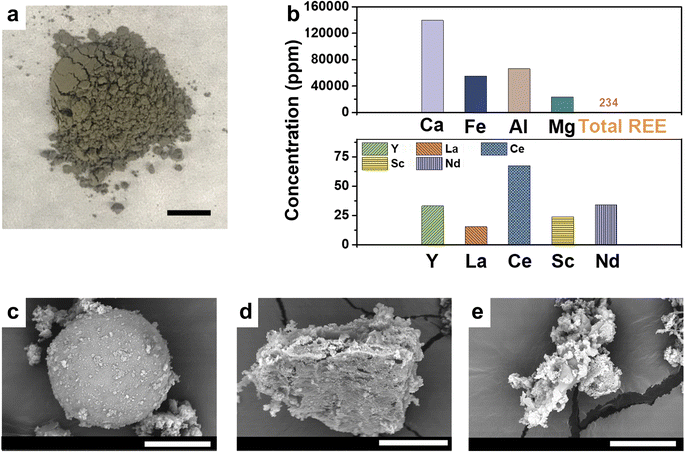
CFA samples were obtained from a power plant in Missouri that burned coal from the Powder River Basin (PRB). Fig. 2a shows dark brownish particles of the CFA used in this study. The chemical compositions of CFA samples were characterized by ICP-OES after HF–HNO3 and HNO3–H2O2 sequential digestions (Fig. 1b), and by X-ray fluorescence spectroscopy (Tables S1 and S2 in ESI†). As shown in Fig. 2b, the total REEs contents of the samples were 234 ± 2 ppm, values which are within the reported range of total REEs contents in US-based coal fly ashes.12 Cerium (Ce) was present at 60 ppm, the highest concentration among all REEs. In addition, the sample had high concentrations of Y and Nd, important elements projected to be in severely short supply by 2035.3 However, the ICP-OES results after the digestion also showed that our CFAs had a variety of high concentration impurities, including Ca (138![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 710 ppm), Fe (54
710 ppm), Fe (54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 943 ppm), Al (66
943 ppm), Al (66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 149 ppm), and Mg (23
149 ppm), and Mg (23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 306 ppm). The relatively abundant alkaline oxides (27.5% CaO and 6.7% MgO, in weight percentages) indicate that the CFAs in this study are Class C CFAs, which have been previously reported to exhibit higher REE extractability.40 The large differences in the concentrations of REEs and impurities, 2–3 orders of magnitude, clearly emphasize the outstanding challenge in selectively extracting REEs from the CFA samples.
306 ppm). The relatively abundant alkaline oxides (27.5% CaO and 6.7% MgO, in weight percentages) indicate that the CFAs in this study are Class C CFAs, which have been previously reported to exhibit higher REE extractability.40 The large differences in the concentrations of REEs and impurities, 2–3 orders of magnitude, clearly emphasize the outstanding challenge in selectively extracting REEs from the CFA samples.
In general, during coal combustion, heating above 1400 °C and rapid cooling in the post-combustion stage cause a diverse size distribution and morphology of fly ash,41,42 such as solid spheres, layered particles, and aggregated particles, as shown in Fig. 2c–e. Based on energy-dispersive X-ray (EDX) analyses, the predominant elements in the fly ash samples were silicon, calcium, aluminum, iron, and magnesium (Fig. S1†), which was consistent with ICP-OES results. The REEs' concentrations were lower than the detection limit for EDX. The CFA in our study had a complex morphology, with quartz, anhydrite, gehlenite, tricalcium aluminate, lime, and periclase being identified (Fig. S2†). A broad bump at around 20–30° 2θ suggested the presence of amorphous aluminosilicate glass. The absence of REE mineral phases indicated that REEs may adsorb or incorporate into other minerals. Thus, the degree to which these minerals can be dissolved by TBP–HNO3 under SCF affected our REEs extraction process. Notably, quartz and amorphous aluminosilicate glass are barely dissolved even under acidic conditions,40 and thus they remain as solid residues after our extraction process.
Selective extraction of REEs with scCO2, scN2, and scAir
Although scCO2-enabled extraction has been implemented for highly pure REEs oxides and post-consumer products with high concentrations of REEs,28,32,33 little is known about whether this mechanism will still work when the impurities' concentration are overwhelmingly high compared to the REEs' concentration, as in the case of CFAs. In our experiment, CFA solid samples and prepared TBP–HNO3 were loaded into a reactor, and then the SCF was injected at 50 °C and 150 bar. We found that CO2, N2, air, or their mixtures are all applicable, as long as the gas is supercritical phase. The critical temperatures and critical pressures for CO2, N2, and air are respectively 31 °C and 73.8 bar, −147 °C and 34.0 bar, and −141 °C and 37.9 bar. Because most other scCO2 extraction studies used reaction times between 1.5 and 3 h, we chose 2 h as our reaction time for appropriate comparison of achieved efficiencies.After reacting the CFAs with TBP–HNO3 under SCF conditions as Fig. 1b depicts, we calculated the concentration factor using eqn (3).
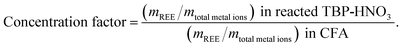 | (3) |
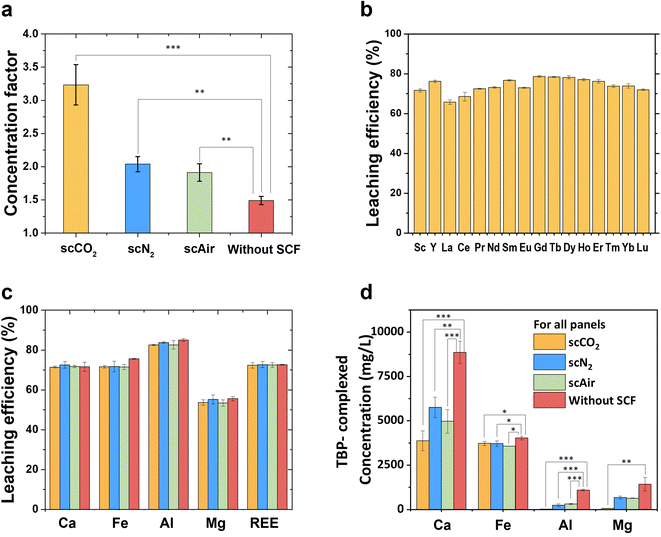
The concentrations of metal ions (including REEs) in the reacted TBP–HNO3 and CFA were obtained by digestions and ICP-OES measurements. The concentration factor reflects the selectivity of REEs over other impurities during the SCF extraction processes. To evaluate the effect of SCF on the extraction selectivity for REEs, we conducted a control experiment in which the CFAs were reacted with TBP–HNO3 at 50 °C in the absence of SCF (the “without SCF” condition). As shown in Fig. 3a, the concentration factor is 1.49 ± 0.06 for the “without SCF” condition. In a clear comparison, involving supercritical N2 and supercritical air into the extraction system can increase the concentration factor to 2.04 ± 0.11, 1.91 ± 0.13. Further, involving supercritical CO2 in the extraction system can significantly increase the concentration factor to 3.23 ± 0.30. This result demonstrates that supercritical fluids can effectively extract REE from complex CFA and can enhance the selectivity of REEs over impurities (Fig. 3a) compared with the “without SCF” condition.
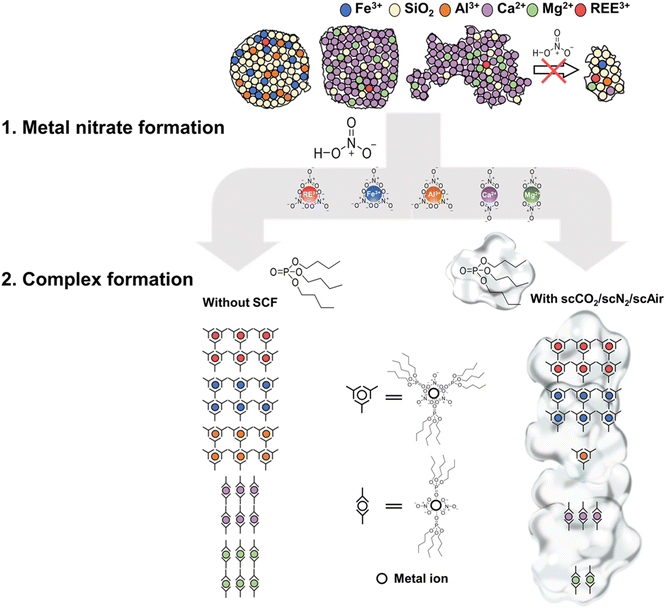
The extraction can be considered a two-step reaction. The first step of the reaction is that metal ions, including REEs and impurities, leach from CFA and react with HNO3 to form metal nitrates (metal nitrate formation in Fig. 4).33 To calculate the leaching efficiencies for all REEs (scandium, yttrium, and 17 lanthanides) with scCO2, scN2, and scAir, we have used eqn (1) and shown the results in Fig. 3b and S3.† The leaching efficiencies from the CFA sample fall in the range of 65–78%. Here, it is noteworthy that although our CFA samples contained only 0.0234% REEs and high concentrations of impurities coexisted with the REEs, the leaching efficiencies (∼70%) in this study were comparable to the leaching efficiencies (40–99%) for high purity materials containing 7–100% REEs.26,29–31 The result clearly shows that we achieved good leaching efficiency of REEs from CFAs with TBP–HNO3, even though large quantities of impurities remain. In addition to REEs, we also calculated the leaching efficiencies of major impurities, including Ca, Fe, Al, and Mg. As shown in Fig. 3c, we did not observe significant change of the leaching efficiencies for all metal ions among different conditions. Based on this result, the higher selectivity of REEs at SCF conditions was not due to the selective leaching. Then, there must be an enhanced selective complexation between REE ions and the extractant to result the enhanced selectivity under supercritical fluids.
Therefore, we investigated the second step of the extraction reaction: metal nitrates react with TBP (complex formation in Fig. 4). To quantify how many REEs/impurity metals (Ca, Fe, Al, and Mg) nitrates had complexed with TBP under different conditions, we digested the reacted TBP–HNO3 and then measured the digestion products by ICP-OES. The concentrations of complexed REE were similar among different conditions: 19.86 ± 0.45 mg L−1 (scCO2), 19.69 ± 0.62 mg L−1 (scN2), 19.24 ± 0.71 mg L−1 (scAir), and 20.23 ± 0.55 mg L−1 (without SCF). In contrast, the presence of SCF significantly affects the complexation between major impurities (Ca(NO3)2, Al(NO3)3, and Mg(NO3)2) and TBP. As Fig. 3d shows, Ca, Al, and Mg nitrates less favorably with TBP under SCF conditions than the condition without SCF, but the complexation between Fe with TBP is less affected by SCF. Wendlt and Bryant (1956) reported that the complexation capability of metal nitrates with TBP followed the series: Fe > REEs ≫ Ca > Mg > Al.43 This sequence suggests that REEs nitrates or Fe nitrate can easily complex with TBP, while calcium nitrate, magnesium nitrate, and aluminum nitrate are less reactive with TBP. Here, we observed an interesting change in TBP behavior in SCF. One possible explanation is that SCF, as a solvent, dispersed the 20 mL of TBP throughout the entire 200 mL reactor, lowering the effective concentration of TBP. However, considering the high temperature and pressure in the supercritical phase extraction, real-time measurements of extractant interactions with SCF in situ are highly challenging. Thus, while we could not provide an exact mechanisms supported by direct evidence, here we provide experimental data regarding the impacts of effective TBP concentrations on its selectivity. To provide additional data, we conducted extraction experiments using 6 g of CFA and 20 mL of TBP–HNO3 in the absence of any SCF, which was a three times lower effective TBP concentration than the original condition without SCF (2 g of CFA and 20 mL of TBP–HNO3). In other words, by increasing the amount of CFA, we decreased the effective TBP concentration. After the extraction, with a 3 times higher concentration of CFA, the concentrations of Fe and REEs were increased by more than 3 times over the original condition. In contrast, the concentrations of Ca, Mg, and Al, which are considered to weakly complex with TBP, increased by less than 2.5 times compared to the original experiment. These results suggest that lowering the effective TBP concentration could make TBP more selective for REEs and Fe over Ca, Mg, and Al. We note that, due to high solid-to-liquid ratio, it is experimentally challenging to mix 6 g CFA and 20 mL of TBP and recover the reacted TBP–HNO3 by vacuum filtration. But with SCF, a 10 times dilution can be achieved. Also, the concentration factor under high solid-to-liquid ratio is lower than the concentration factors under SCF conditions. For all the SCFs tested in our experiment, we speculated that the reactivity of the TBP might be lowered by the dilution, so that TBP would complex with highly reactive REE(NO3)3 and Fe(NO3)3, but other, less reactive metal nitrates would not complex with TBP due to its low reactivity (Fig. 5, bottom right column). We expect that future dedicated computational and spectroscopic studies can provide direct evidence of the impacts of SCF on extractants.
Multistage stripping process collects REEs with high concentrations and purities
To collect REEs extracted in TBP–HNO3, we designed a multistage stripping process using 1% nitric acid, as depicted in Fig. 1c. In each stage, we added 1% nitric acid to the reacted TBP–HNO3 in a 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v ratio. This volume ratio was experimentally determined to be the best for concentrating REEs (detailed information is in ESI S1†). After vigorous mixing, the REEs and impurities have dissociated from the TBP and are dissolved into the diluted nitric acid. The 1% nitric acid, containing REEs and impurities, is then collected by gravity separation and is called “stripped solution”. The remaining reacted TBP–HNO3 is mixed with fresh 1% nitric acid for a new stripping stage. This process is repeated for five more stages, during which REEs and impurities continually dissociate from TBP and are collected by 1% nitric acid. In total, a six-stage stripping process is applied to recover essentially all the REEs from the reacted TBP–HNO3.
1 v/v ratio. This volume ratio was experimentally determined to be the best for concentrating REEs (detailed information is in ESI S1†). After vigorous mixing, the REEs and impurities have dissociated from the TBP and are dissolved into the diluted nitric acid. The 1% nitric acid, containing REEs and impurities, is then collected by gravity separation and is called “stripped solution”. The remaining reacted TBP–HNO3 is mixed with fresh 1% nitric acid for a new stripping stage. This process is repeated for five more stages, during which REEs and impurities continually dissociate from TBP and are collected by 1% nitric acid. In total, a six-stage stripping process is applied to recover essentially all the REEs from the reacted TBP–HNO3.
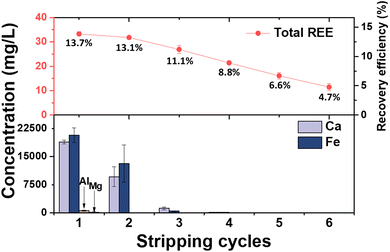
As Fig. 5 shows, REEs gradually dissociate from TBP and are then collected by 1% nitric acid (i.e., the stripping solution) in the first stage through the sixth stage. The total REEs concentrations in our first through sixth stages ranged from 11 to 35 mg L−1 under SCF conditions for the three gases (Fig. 5), values which are much higher than the reported concentrations of REEs extracted from CFAs (0.3–5.5 mg L−1) in previous studies.22,23 Interestingly, in addition to collecting REEs, we noticed that our multistage stripping process can achieve a substantial partial separation between REEs and impurities. For impurities Mg and Al, majority of them were separated from REEs during the SCF extraction. During our multistage stripping process, we only collected Mg and Al in the first and second stripping processes. Moreover, because Ca and Fe have much higher water affinity than REEs,44 and thus 94.5% of Ca and 96.7% of Fe were are preferentially removed from reacted TBP–HNO3 in the first and second stripping stages. Therefore, we could collect much lower concentrations of impurities in the remaining stripping stages to achieve a higher REE purity percentage (Table 1). As shown in Fig. 5, the first and second stripping stages are sacrificial stages in which we lost 13.7% and 13.1%, respectively, of the REEs from the CFA. We believe the two sacrificial stages are important for two reasons. First, the first and second stripping stages allowed us to significantly decrease the impurity concentrations, so in the subsequent stages we obtained more than 30% of the REEs from the CFA with much higher purity. Second, a major challenge in extracting REEs from CFA is their extremely low purity, which limits the benefits of conducting further processing. Considering that 79 million metric tonnes (t) of CFAs are generated annually in the U.S., considerable amounts of high purity REEs are available for further separation, even though 26.8% of them will be sacrificed. There is always a tradeoff between product purity and recovery efficiency. In general, by sacrificing some REEs in the first and second stripping process, we subsequently collected 31.2% of the REEs from CFA. As summarized in Table 1, these REEs had much higher concentrations and purities than the products of previous works using acid leaching, solvent extraction, and selective membrane processes to extract REEs from CFA.22,23 During the SCF extraction, ∼32% of the REEs were non-acid-extractable; thus, they did not leach from the CFA. After the multistage stripping process, <10% of the REEs remained complexed with TBP. We expect future work to further optimize the multistage stripping process to maximize recovery efficiency with high REE purity.
| Liquid emulsion membrane final liquida | Supported liquid membrane final liquida | Conventional extraction final liquida | Our work scCO2 fourth stripping solution | Our work scCO2 fifth stripping solution | Our work scCO2 sixth stripping solution | |
|---|---|---|---|---|---|---|
| a Results from a previous study by Smith et al.22 REEs purity values were calculated from eqn (2). | ||||||
| Na (μg L−1) | 333![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
4220 | 0 | 0 | 0 |
| Mg (μg L−1) | 8320 | 152 | 320 | 0 | 0 | 0 |
| Al (μg L−1) | 149![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1770 | 919![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0 | 0 | 0 |
| Fe (μg L−1) | 522 | 551 | 2100 | 313![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 802 802 |
200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 196 196 |
132![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 632 632 |
| Ca (μg L−1) | 107![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
968 | 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
246![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 556 556 |
138![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 580 580 |
74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 175 175 |
| Si (μg L−1) | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
5340 | 3450 | 0 | 0 | 0 |
| REEs (μg L−1) | 4635 | 303 | 5587 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 374 374 |
16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 088 088 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 441 441 |
| REEs purity (%) | 0.73 | 0.79 | 0.57 | 3.43 | 6.47 | 6.26 |
Given the increasing demand for and importance of REEs, alternative sources to ore-extracted products are being sought, such as CFAs. The biggest challenge here is that REEs concentrations in CFAs are much lower than the impurity concentrations. To selectively extract REEs, separation technologies, such as conventional organic solvent extraction and novel liquid membrane processes, have been explored.21–23 In the organic solvent extraction process, DEHPA was dissolved to a concentration of 10% (v/v) into kerosene, and it selectively extracted REEs from CFA leachate.21 Then, 5 M HNO3 strippant was used to recover the REEs. To enhance the kinetics during the REE stripping process, Smith et al. (2019) synthesized a strippant-in-kerosene liquid emulsion membrane system by mixing DEHPA, Span 80, 5 M HNO3, and kerosene.22 Then, to increase the selectivity for heavy REEs over light REEs, a supported liquid membrane was prepared by using vacuum filtration to impregnate a 47 mm 0.22 μm PVDF membrane with a 10% (v/v) solution of DEHPA in mineral oil.22 This membrane was used as a separator between CFA leachate and 5 M HNO3 leachate to make REEs selectively transfer through the membrane. However, the final products of these separation technologies contained less than 6000 μg L−1 REEs, and the purity was less than 1%, as shown in Table 1. In contrast, without using an organic solvent, our novel SCF extraction process can directly obtain REEs from solid phase CFAs, and it yields REEs aqueous solutions with concentrations of up to 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 374 μg L−1 and purities up to 6.47% (Table 1).
374 μg L−1 and purities up to 6.47% (Table 1).
Currently, extracting REEs from mineral ores requires preprocessing steps (alkaline roasting and acid leaching) to turn the REE from a solid matrix into an aqueous solution. It also involves separation processes (fractional crystallization, fractional precipitation, ion exchange, and solvent extraction) to obtain high-purity individual REEs.18–20 Our study shows that our SCF extraction process could be an alternative to the acid leaching process and turn the CFA matrix into a solution containing ∼3–6% REEs. The process serves a function similar to that of acid leaching but can provide a higher purity of REEs. Notably, the REEs purity in our study is even comparable to the purity of some commercially available REEs ores.45 We expand the sources of REEs from mineral ores to previously neglected CFA, which was regarded as a waste and environmental threat. Moreover, the REEs-containing solutions obtained from our study can undergo further separation processes to produce even higher purity REEs. For example, a previous study designed a fractional precipitation method using oxalic acid to selectively precipitate REEs as REE oxalates. Fractional precipitation achieved REE purities exceeding 60%.44 This method could also be applied to selectively precipitate the REEs in our stripping solution. Thus our suggested process could be combined with fractional precipitation, ion exchange, and solvent extraction to produce higher purity REEs. Beyond showing that SCF can enhance the selective extraction of REEs from CFAs, we believe that our novel process can perform well in extracting REEs from other low-grade REEs sources, including nickel-metal hydride batteries, neodymium magnets, and acid mine drainage.28,33,46 In addition, considering that TBP has strong complexation with actinides, especially uranium and thorium,43 our extraction process can potentially be applied to recover actinides from nuclear products. In addition to REEs, our process may extract and recover heavy metals from CFA. As shown in Fig. S6A,† the extraction efficiencies for Cr, Cu, Mn, and Zn are 11.9%, 9.0, 30.9%, and 62.0%, respectively. Further, owing to their relatively weak complexation with TBP, most heavy metals are collected in the first and second stripping stages. Moreover, as shown in Fig. S6B,† the remaining heavy metal concentrations in stripping stages 4–6 are low (0–0.22 mg L−1). Their concentrations are much lower than the REEs' concentrations (11.4–21.4 mg L−1). Thus, the collected heavy metals had little impacts on the purity of the REEs collected in stripping stages 4–6.
Conclusions
Herein we show that supercritical fluids, i.e., scCO2, scN2, and scAir, can enhance the selective extraction of REEs directly from solid coal fly ash matrix. Our exploratory study is the first to demonstrate the direct application of an SCF, which both replaces harmful organic solvent and efficiently recovers valuable resources from CFA, previously considered a waste material or even an environmental threat. Although major impurities in CFAs, such as Ca, Fe, Al, and Mg, have several magnitudes higher concentrations than REEs, SCF-enhanced extraction allows us to extract REEs with greatly decreased impurity amounts in the final products. Beyond scCO2, our work also shows scN2 and scAir can be applied in the extraction process for REEs. In addition, based on chemical analysis, we found that the presence of SCFs can decrease the complexation between impurities and TBP to enhance the selectivity of REEs. After SCF extraction, we applied multistage stripping process, which can collect REEs meanwhile further decrease the impurities concentrations. Ultimately, our novel process successfully obtained final products contain up to 6.47% REEs purity from coal fly ashes, which are traditionally considered as waste and contain only 0.0234% of REEs initially.Conflicts of interest
A patent application has been submitted for the process reported here.Acknowledgements
The project was supported by Washington University's Consortium for Clean Coal Utilization. The Nano Research Facility and the Institute of Materials Science and Engineering at Washington University in St. Louis provided their facilities for the experiments. We thank Prof. Daniel Giammar to share the CFA samples. We thank Prof. James Ballard for carefully reviewing the manuscript.References
- K. Binnemans, P. T. Jones, B. Blanpain, T. Van Gerven, Y. Yang, A. Walton and M. Buchert, J. Clean. Prod., 2013, 51, 1–22 CrossRef CAS.
- B. Zhou, Z. Li and C. Chen, Minerals, 2017, 7, 203 CrossRef.
- E. Alonso, A. M. Sherman, T. J. Wallington, M. P. Everson, F. R. Field, R. Roth and R. E. Kirchain, Environ. Sci. Technol., 2012, 46, 3406–3414 CrossRef CAS PubMed.
- W. B. Group, The growing role of minerals and metals for a low carbon future, World Bank, 2017 Search PubMed.
- R. Blissett, N. Smalley and N. Rowson, Fuel, 2014, 119, 236–239 CrossRef CAS.
- K. R. Long, B. S. Van Gosen, N. K. Foley and D. Cordier, The principal Rare earth elements deposits of the United States: a summary of domestic deposits and a Global perspective, Springer, 2012 Search PubMed.
- H. D. Bandara, K. D. Field and M. H. Emmert, Green Chem., 2016, 18, 753–759 RSC.
- Y. Wu, X. Yin, Q. Zhang, W. Wang and X. Mu, Resour., Conserv. Recycl., 2014, 88, 21–31 CrossRef.
- G. A. Moldoveanu and V. G. Papangelakis, Hydrometallurgy, 2012, 117, 71–78 CrossRef.
- M. K. Jha, A. Kumari, R. Panda, J. R. Kumar, K. Yoo and J. Y. Lee, Hydrometallurgy, 2016, 165, 2–26 CrossRef CAS.
- V. V. Seredin and S. Dai, Int. J. Coal Geol., 2012, 94, 67–93 CrossRef CAS.
- R. K. Taggart, J. C. Hower, G. S. Dwyer and H. Hsu-Kim, Environ. Sci. Technol., 2016, 50, 5919–5926 CrossRef CAS PubMed.
- A. C. A. Association, 2013 Coal combustion product (CCP) production & use survey report, 2019 Search PubMed.
- L. Ruhl, A. Vengosh, G. S. Dwyer, H. Hsu-Kim and A. Deonarine, Environ. Sci. Technol., 2010, 44, 9272–9278 CrossRef CAS PubMed.
- L. Ruhl, A. Vengosh, G. S. Dwyer, H. Hsu-Kim, A. Deonarine, M. Bergin and J. Kravchenko, Environ. Sci. Technol., 2009, 43, 6326–6333 CrossRef CAS PubMed.
- B. C. McLellan, G. D. Corder and S. H. Ali, Minerals, 2013, 3, 304–317 CrossRef CAS.
- S. Massari and M. Ruberti, Resour. Policy, 2013, 38, 36–43 CrossRef.
- D. Li, J. Rare Earths, 2019, 37, 468–486 CrossRef CAS.
- Z. Chour, B. Laubie, J. L. Morel, Y. Tang, R. Qiu, M.-O. Simonnot and L. Muhr, Chem. Eng. Process., 2018, 130, 208–213 CrossRef CAS.
- R. Mu, J. Chen, D. Zou, K. Li and D. Li, Sep. Purif. Technol., 2019, 209, 351–358 CrossRef CAS.
- J. F. King, R. K. Taggart, R. C. Smith, J. C. Hower and H. Hsu-Kim, Int. J. Coal Geol., 2018, 195, 75–83 CrossRef CAS.
- R. C. Smith, R. K. Taggart, J. C. Hower, M. R. Wiesner and H. Hsu-Kim, Environ. Sci. Technol., 2019, 53, 4490–4499 CrossRef CAS PubMed.
- R. K. Taggart, J. C. Hower and H. Hsu-Kim, Int. J. Coal Geol., 2018, 196, 106–114 CrossRef CAS.
- E. Vahidi and F. Zhao, in REWAS 2016, ed. B. Blanpain, Springer, New York, 2016, pp. 113–120 Search PubMed.
- C. A. Eckert, B. L. Knutson and P. G. Debenedetti, Nature, 1996, 383, 313 CrossRef CAS.
- O. Tomioka, Y. Enokida and I. Yamamoto, Sep. Sci. Technol., 2002, 37, 1153–1162 CrossRef CAS.
- R. Shimizu, K. Sawada, Y. Enokida and I. Yamamoto, J. Supercrit. Fluids, 2005, 33, 235–241 CrossRef CAS.
- Y. Yao, N. F. Farac and G. Azimi, ACS Sustainable Chem. Eng., 2017, 6, 1417–1426 CrossRef.
- D. L. Baek, R. V. Fox, M. E. Case, L. K. Sinclair, A. B. Schmidt, P. R. McIlwain, B. J. Mincher and C. M. Wai, Ind. Eng. Chem. Res., 2016, 55, 7154–7163 CrossRef CAS.
- D. Wuhua, C. Pijia and Z. Yongjun, J. Rare Earths, 2010, 28, 221–226 CrossRef.
- Z. Liyang, D. Wuhua, X. Jingming and Z. Yongjun, Chin. J. Chem. Eng., 2009, 17, 214–218 CrossRef.
- L. Sinclair, D. L. Baek, J. Thompson, J. Tester and R. V. Fox, J. Supercrit. Fluids, 2017, 124, 20–29 CrossRef CAS.
- J. Zhang, J. Anawati, Y. Yao and G. Azimi, ACS Sustainable Chem. Eng., 2018, 6, 16713–16725 CrossRef CAS.
- M. Samsonov, T. Trofimov, Y. M. Kulyako, D. Malikov and B. Myasoedov, Russ. J. Phys. Chem. B, 2016, 10, 1078–1084 CrossRef CAS.
- C. Kersch, G. F. Woerlee and G. J. Witkamp, Ind. Eng. Chem. Res., 2004, 43, 190–196 CrossRef CAS.
- N. G. Smart, T. E. Carleson, S. Elshani, S. Wang and C. M. Wai, Ind. Eng. Chem. Res., 1997, 36, 1819–1826 CrossRef CAS.
- P. Zhang, Y. Huang, B. Shen and R. Wang, Int. J. Therm. Sci., 2011, 50, 287–295 CrossRef CAS.
- H. Guo, Y. Xu, H. Chen and X. Zhou, Energy Convers. Manage., 2016, 115, 167–177 CrossRef.
- G. Anil, M. Reddy, M. Sharma, A. Kumar and T. Prakash, Chem. Anal., 2004, 49, 459–465 CAS.
- P. Liu, R. Huang and Y. Tang, Environ. Sci. Technol., 2019, 9, 5369–5377 CrossRef PubMed.
- L. B. Clarke and L. L. Sloss, Trace elements: emissions from coal combustion and gasification, IEA Coal Research London, 1992 Search PubMed.
- B. G. Kutchko and A. G. Kim, Fuel, 2006, 85, 2537–2544 CrossRef CAS.
- W. W. Wendlt and J. M. Bryant, J. Phys. Chem., 1956, 60, 1145–1146 CrossRef CAS.
- E. Jorjani and M. Shahbazi, Arab. J. Chem., 2016, 9, S1532–S1539 CrossRef CAS.
- P. Koltun and A. Tharumarajah, ISRN Metall., 2014, 2014, 907536 Search PubMed.
- F. Zhao, Z. Cong, H. Sun and D. Ren, Int. J. Coal Geol., 2007, 70, 184–192 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2su00033d |
| ‡ Current address: Andlinger Center for Energy and the Environment, Princeton University, Princeton, New Jersey, 08540, USA. |
| § Current address: Department of Chemical and Biomolecular Engineering, National University of Singapore, Singapore, 117585, Singapore. |
| This journal is © The Royal Society of Chemistry 2023 |