Open Access Article
Open Access ArticleBirch fractionation in γ-valerolactone with the emphasis on pulp properties: prehydrolysis, acid-catalyzed, and alkaline-catalyzed concept†
Marianna
Granatier
 ,
Huy Quang
Lê
,
Huy Quang
Lê
 ,
Eva Carmona
González
and
Herbert
Sixta
,
Eva Carmona
González
and
Herbert
Sixta
 *
*
School of Chemical Engineering, Department of Biosystems and Bioproducts, Aalto University, Vuorimiehentie 1, 02150 Espoo, Finland. E-mail: herbert.sixta@aalto.fi
First published on 4th November 2022
Abstract
Organosolv pulping represents an alternative to the traditional production of pulp, cellulosic fibrous material, allowing the utilization of other pulping streams. Pulping of resistant wood species such as Finnish silver birch (Betula pendula) is generally challenging for an uncatalyzed organosolv system. In this study, we investigate two concepts of organosolv γ-valerolactone (GVL) pulping for the production of dissolving pulp. Silver birch sawdust was fractionated in both a one-step process with sulfuric acid or sodium hydroxide-catalyzed liquor and a two-step process with a prehydrolysis step followed by an alkaline GVL digestion step. The selectivity of delignification and hemicellulose removal in one-stage alkaline-GVL pulping was less efficient compared to uncatalyzed GVL pulping due to the partial consumption of sodium hydroxide solution in the GVL pulping liquor, which hydrolyzed to 4-hydroxyvaleric acid (4-HVA) under alkaline conditions according to the equilibrium conditions. Acid-catalyzed GVL pulping at higher temperature (180 °C) resulted in a pure cellulose fraction with a low amount of lignin and hemicelluloses (<5%), but at the expense of pulp yield. Prehydrolysis removed 50% of the original hemicellulose content, but the overall performance of two-stage pulping did not improve pulping selectivity and thus pulp qualities. Optimum conditions for one-stage acid-catalyzed GVL pulping were identified as 150 °C, 120 min, and 5–10 kg H2SO4 per t for the production of dissolving pulp with specifications and macromolecular properties comparable to those of an acid sulfite dissolving pulp.
Sustainability spotlightThe research of gamma-valerolactone (GVL) biorefinery aligns with UN SDG 13 (climate change) and 15 (forests). High sulfur emissions and underutilization of pulping side streams resulting in low product yield make traditional pulping ineffective. It has been confirmed that pulp biorefineries contribute to achieving the 1.5 °C target for climate change mitigation. GVL as a solvent in biomass conversion has drawn significant attention due to its green and renewable nature. The results from this work provide the basis for designing an optimal GVL pulping process producing high-quality paper or dissolving grade pulp with the simultaneous full utilization of side products. Our work represents an extensive insight into organosolv GVL pulping chemistry, which, we believe, would draw significant attention from different reader groups. |
1 Introduction
Shifting interest from fossil-based raw materials as well as thoughtful use of arable land and natural resources toward more sustainable solutions makes the lignocellulosic material an excellent choice to produce bio-based commodities. Moreover, the heavy use of crude oil significantly increases anthropogenic carbon dioxide, contributing to global warming. Nonetheless, biorefining of annual crops (e.g., bamboo), agricultural waste (e.g., straw and stalks), or woody biomass, could maintain the balance between produced and consumed CO2.1 Particularly the latter emerges as the most sustainable source thanks to the more homogenous properties, higher availability, and better transportability.2Woody biomass is divided into hardwood and softwood trees. Although the composition considerably deviates between species, woody biomass generally comprises 40–50% of cellulose, 25–30% of hemicellulose, 15–30% of lignin, and 2–10% of extractives,3 which all are raw materials for value-added products. However, only cellulose remains the main profit source in the form of paper pulp or dissolving pulp in a common pulp mill. The other two lignocellulosic streams, hemicellulose, and lignin are mostly combusted for the generation of energy, with a few exceptions (e.g. LignoBoost®). The underutilization of hemicellulose and lignin could be partially assigned to current pulping technologies (kraft and acid sulfite), which impede their full recovery.4,5
The annual global chemical pulp production accounts for more than 126 Mt divided between kraft (80%) and sulfite pulp (20%).6 Despite comprising only a small fraction of the total global pulp production, the demand for dissolving pulp has been constantly increasing in recent years (production of 10 Mt in 2021).7 Typical applications of dissolving pulp include regenerated cellulose fibers, microcrystalline cellulose, cellulose esters, and ethers. Dissolving wood pulp (DWP) is commercially produced by acid sulfite (AS) and pre-hydrolysis kraft (PHK) processes, with the latter dominating the market share. Despite the limitation in raw materials and inefficient chemical recovery, AS used to be the preferred technique for DWP production. However, the extensive adaption of a prehydrolysis stage before kraft cooking resulted in the technology gaining popularity and being favored in modern DWP mills.8–10 Pre-hydrolysis (PH) enhanced the production of high purity dissolving pulp because alkaline treatment alone was not sufficiently chemically selective toward resistant short-chain hemicelluloses. Originally, PH employed water, however, generating the vast amount of prehydrolysate proved to be challenging during the recovery of dissolved products. Moreover, the reactive low molecular weight lignin in prehydrolysis liquor (PHL), which tends to precipitate and deposit on the piping inner surface, poses a serious operational challenge. Additionally, the recovery would require the installation of the evaporation unit which would considerably increase the investment and operational costs. The problem was solved by the development of Visbatch® technology, which unites the steam prehydrolysis and displacement cooking.11
Whereas both techniques are well established, the environmental issues arising from the use of sulfur-based compounds are significant. Moreover, both processes, especially kraft, suffer from the limitation of valorization of the isolated biomass components: lignin is considerably chemically altered with covalently bound sulfur, and hemicellulose is often degraded to an extent beyond its efficient recovery in usable form. Therefore, a sustainable pulping technology should aim at a sulfur-free process with a satisfactory yield of easily bleachable pulp and a possibility to valorize side products.10,12 By using the most efficient nucleophiles, kraft (hydrogen sulfide) and AS (hydrogen sulfite) are unsurpassable aqueous-based processes. A breakthrough can only be achieved by introducing (an) organic solvent(s) to the pulping environment. Organosolv pulping emerged as a potential alternative thanks to the effective fractionation of lignocellulosic material into its components while facilitating their recovery and valorization. The selected sulfur-free organic solvents should exhibit excellent lignin dissolution capacity.4,13
Almost a century ago, ethanol was introduced as the first organic pulping solvent.14 Ethanol has become the most studied solvent that reached the first demonstration pulp mill in 1989. The process was named ALCELL®, in which the biomass was pulped in 40–50 wt% ethanol at 195 °C in a batch pressure reactor. The residual sulfur-free lignin in unbleached pulp was less condensed and resembled the original lignin in wood, therefore the commercial pulp brightness could be achieved by a standard bleaching sequence.15 The elimination of sulfur-based unpleasant odor and air emissions were the biggest environmental advantages. The ethanol pulping successfully produced dissolving pulp processed into high-quality viscose fibers.16 Despite significant environmental benefits over traditional pulping and extensive research, ALCELL® never reached its full commercialization due to the poor chemical recycling efficiency resulting from the loss reaction between solvent and wood components.15,17 Acetocell, Formacell, and MILOX were the other extensively researched fractionation processes employing organic acids (acetic acid, formic acid, performic acid, respectively). Despite the low kappa numbers, the pulp is of inferior quality to kraft pulp. Besides, these cooking chemicals pose a significant corrosion risk, demanding more resistant material, thus increasing investment costs and limiting the adaptability to existing pulping facilities.15 Organocell was developed as a response to environmental regulations in Germany to produce pulp with kraft-like properties from softwood. The process managed to reach full-scale operation in the early 90s. However, the pulp mill was unfortunately shut down due to several operating problems.15 SEW (SO2–ethanol–water) fractionation, a hybrid of organosolv and acid sulfite pulping, and clean fractionation (methyl isobutyl ketone–ethanol–water) are two of the latest promising innovations in the realm of organosolv pulping, with the former already reaching pilot operation.18,19 Homogenous or heterogeneous ionic liquid-based fractionation, ionoSolv, emerged during the last decades. In the homogenous approach, all biomass constituents are dissolved, and different fractions are then separately regenerated by the addition of anti-solvents. In the heterogeneous approach, a certain biopolymer is selectively dissolved from the biomass. The recent development of a new hybrid pretreatment replaces a water component in the ionoSolv process with a low-boiling organic solvent resulting in better delignification and production of cellulose-rich pulp.20 However, the fractionation processes based on ionic liquids lack the selective separation of celluloses and hemicelluloses. Additionally, the purification of the mixture and solvent recycling is currently unknown and requires further investigations.
In recent years, the naturally occurring cyclic ester gamma-valerolactone (GVL) has experienced substantial interest in the application for lignocellulosic biomass fractionation and conversion. Being renewable with low toxicity, low volatility, high chemical stability, and high solubility in water while staying zeotropic, GVL was first proposed as a sustainable chemical for the production of energy and carbon-based chemicals.21,22 Dumesic et al. expanded the application of GVL to the production of soluble carbohydrates from biomass by applying the pre-treatment in acid-catalyzed GVL/water solution with the subsequent ethanol production.23 Meanwhile, Luterbacher et al. aimed not only for biomass pretreatment in GVL/water but also for the simultaneous production of less condensed lignin by the addition of formaldehyde.24
However, GVL/water pulping for dissolving pulp production was firstly introduced by Fang and Sixta (2015) where a highly pure cellulose fraction could be isolated from birch wood after a single-step treatment in an aqueous solution of GVL, catalyzed by H2SO4.25 Lê et al. (2016) further developed such pulping concept to a full biorefinery, addressing the separation and valorization of the isolated and degraded biomass components, as well as evaluating the efficiency of GVL recovery from the spent liquor.26,27
GVL pulping undoubtedly exhibits potential for pulp production. Nevertheless, the pulp yield and quality can be easily influenced by cooking conditions or type of the biomass. Lê et al. (2016) achieved adequate hemicellulose removal and delignification in uncatalyzed GVL fractionation of Eucalyptus globulus,26 less recalcitrant hardwood biomass, which might not yield the same results with more resistant species such as silver birch (Betula pendula) with high amount of hemicellulose. In such cases, one-stage uncatalyzed GVL pulping might not be enough to reach DWP grade. Our research group previously explored the possibility of producing paper-grade pulp and high-quality DWP by the combination of GVL pulping and ionic liquid extraction (IONCELL-P).28 This current paper completes the product portfolio by investigating the production of rayon-grade DWP by GVL pulping.
The addition of catalyst could enhance the rate of hemicellulose hydrolysis and cleavage of lignin bonds while possibly reducing the reaction duration and temperature, and resulting in high-purity pulping streams.4 Several research groups performed the GVL fractionation or pre-treatment in an acid-catalyzed environment, but the organosolv alkaline catalysis was mostly associated with Organocell (methanol) pulping. Although there is enough evidence on catalyzed GVL pulping, the literature is still lacking systematic data about the influence of catalysts on pulp properties as well as the composition of liquid fractions.
This work investigates the possible improvements in GVL fractionation combined with wood pre-treatment and catalysis targeting the production of dissolving-grade pulp and reducing the additional purification steps. Silver birch (Betula pendula) was chosen as a raw material since it is one of the most abundant and commercially important hardwoods in Scandinavia. The yield and chemical composition of the pulps and spent liquor are determined and related to GVL treatment under specific conditions. Additionally, the impact of pulping conditions on cellulose depolymerization is evaluated by the molar mass distribution of individual pulp samples.
2 Experimentals
2.1 Materials
GVL (≥99 wt% purity) was purchased from Sigma Aldrich, sulfuric acid (95–97.0 wt%) from Merck, and sodium hydroxide pellets (≥98 wt%) from VWR Chemicals. Silver birch (Betula pendula) wood chips were supplied by Stora Enso (Finland). The wood chips were screened according to the SCAN-CM 40:01, and the accepted fractions (retaining on the Ø7 mm and Ø13 mm) were collected and stored at −20 °C. The chips were ground using a Wiley mill (Arthur H. Thomas Co., model no. 2, 1 mm screen opening) and the sawdust was collected and stored at +5 °C. The water used in the experiments was purified from demineralized water by a Millipore Synergy® UV system (water resistivity of 18.2 MΩ). The pre-hydrolysis experiments were conducted in a 10 L pilot reactor developed by the Aalto University workshop. The sawdust pulping experiments were performed in glass vials heated by a microwave reactor (Monowave 300, Anton Paar GmbH, Graz, Austria).2.2 Pre-hydrolysis of wood chips
1 kg of silver birch (Betula pendula) wood chips were pre-hydrolyzed in the 10 L reactor before the GVL pulping via steam pre-hydrolysis (PH). The pre-hydrolysis conditions were as followed: 150 °C with a liquor-to-wood ratio of 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and a P-factor of 500 (tPH = 2 h). The pre-hydrolyzed liquor (PHL) was discharged and comprehensively analyzed. The pre-hydrolyzed chips were washed in the reactor three times with fresh deionized water (7 kg for each washing), and the washing liquor was collected for further analyses. The washed pre-hydrolyzed chips were dried at 45 °C overnight, then milled to sawdust (1 mm screen opening), and stored at +5 °C.
1, and a P-factor of 500 (tPH = 2 h). The pre-hydrolyzed liquor (PHL) was discharged and comprehensively analyzed. The pre-hydrolyzed chips were washed in the reactor three times with fresh deionized water (7 kg for each washing), and the washing liquor was collected for further analyses. The washed pre-hydrolyzed chips were dried at 45 °C overnight, then milled to sawdust (1 mm screen opening), and stored at +5 °C.
2.3 GVL pulping of sawdust
The pulping liquor was prepared by mixing 7.5 g of pure GVL (0.075 mol) with water and H2SO4 or NaOH to get the final acid/alkali charge ranging from 0.0002–0.018 g of H2SO4 and 0.0002–0.192 g of NaOH per 1 g of oven-dried wood (odw), and final GVL concentration of 50 wt% (Table S1†). The solutions were mixed with 1.5 g of oven-dried sawdust with a liquor![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) wood ratio (L
wood ratio (L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) W) of 10. The extractives (1.7%) were not removed from the sawdust before the reaction. The pulping was performed in a microwave reactor at a temperature ranging from 120 to 180 °C with a short heating time typically less than 1 minute, held for 120 min at the reaction temperature, and then cooled to 50 °C. After the reaction, the raw spent liquor was separated from the pulp by filtration through a borosilicate glass filter (ROBU® porosity 4). The pulp was washed with 20 g of aqueous GVL solution with a concentration of 37.5 wt% (5.9 mol L−1). The raw spent liquor and washing GVL liquor were combined (and henceforth referred to as spent liquor) for further analysis. Subsequently, the pulp was washed with 200 g of boiling water. The fully washed pulp was dried at 105 °C overnight and the yield was determined gravimetrically. Each experiment was performed in duplicate.
W) of 10. The extractives (1.7%) were not removed from the sawdust before the reaction. The pulping was performed in a microwave reactor at a temperature ranging from 120 to 180 °C with a short heating time typically less than 1 minute, held for 120 min at the reaction temperature, and then cooled to 50 °C. After the reaction, the raw spent liquor was separated from the pulp by filtration through a borosilicate glass filter (ROBU® porosity 4). The pulp was washed with 20 g of aqueous GVL solution with a concentration of 37.5 wt% (5.9 mol L−1). The raw spent liquor and washing GVL liquor were combined (and henceforth referred to as spent liquor) for further analysis. Subsequently, the pulp was washed with 200 g of boiling water. The fully washed pulp was dried at 105 °C overnight and the yield was determined gravimetrically. Each experiment was performed in duplicate.
2.4 Analytical characterization of pulping products
The total carbohydrate content in pulp and liquid samples was determined by high-performance anion exchange chromatography (HPAEC-PAD Dionex ICS-3000 system equipped with CarboPac PA20 column) following the two-step hydrolysis described in NREL/TP-510-42618 standard. Consequently, the content of cellulose and hemicellulose in wood or pulp was recalculated from neutral monosaccharides based on the Janson formula.29The molar mass distribution (MMD) of pulps was determined by Gel Permeation Chromatography (GPC) using a Dionex Ultimate 3000 HPLC module equipped with four Agilent PLgel MIXED-A columns, refractive index (Shodex DRI RI-101), and multi-angle light scattering (Viscotek/Malvern SEC/MALS 20) detectors. The pulps were subjected to a series of solvent exchanges in water, acetone, and N,N-dimethylacetamide (DMAc) and then dissolved in 90 g per L lithium chloride/N,N-dimethylacetamide (LiCl/DMAc) at room temperature under constant stirring overnight. The dissolved sample was diluted with pure DMAc to a concentration of 9 g L−1, then filtered through 0.2 μm syringe filters into a sampling vial and analyzed. Detector constants (MALS and DRI) were determined using narrow polystyrene sample (Mw = 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1, Đ = 1.04) dissolved in 0.9% LiCl in DMAc. Broad polystyrene sample (Mw = 248
000 g mol−1, Đ = 1.04) dissolved in 0.9% LiCl in DMAc. Broad polystyrene sample (Mw = 248![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1, Đ = 1.73) was used for checking the detector calibration.
000 g mol−1, Đ = 1.73) was used for checking the detector calibration.
The acid-insoluble lignin (Klason) content in pulp was determined gravimetrically following the NREL/TP-510-42618 protocol. The acid-soluble lignin in solid and liquid samples was quantified by measuring the absorbance at 205 nm (spectrophotometer Shimadzu UV-2550) and using 148 L (g cm)−1 as an extinction coefficient.26 To measure the lignin content in liquid fractions, the samples were diluted in 50% ethanol to avoid lignin precipitation.
The organic acids and furanic compounds in liquid samples were determined by High-Performance Liquid Chromatography (HPLC) using a Dionex Ultimate 3000 (Dionex, Sunnyvale, CA, USA) equipped with a Phenomenex Rezex ROA-Organic Acid column in combination with a UV diode array detector and refractive index detectors. The eluent was 0.0025 M sulfuric acid, and the temperature was set to 70 °C for the column and 55 °C for the RI detector. The formic acid was excluded from the mass balance due to the elution of other compounds at the same retention time causing the measurement inaccuracy.
3 Results and discussion
3.1 One-stage acid and alkaline pulping in 50% aqueous GVL solution
In our previous research, Lê et al. (2016)26 studied the optimal uncatalyzed pulping conditions on Eucalyptus globulus sawdust in an aqueous GVL solution employing the reaction conditions of 180 °C and 120 min in a series of experiments with varying GVL concentrations. The concentration of 50–60 wt% GVL provided the maximum delignification and adequate hemicellulose removal.26 Adopting the proven conditions (180 °C, 120 min, and 50 wt% GVL), we aimed to expand the cooking conditions on birch by introducing an acid or alkaline catalyst and evaluate its effect on pulp properties. The uncatalyzed GVL pulping was used as a reference trial.Alkali-organosolv processes are less common than auto- or acid-catalyzed ones. Organocell process was developed in response to repeatedly failing softwood pulp production by acid-organosolv pulping. The delignification proceeded in two steps: (a) the chips impregnation with 50% methanol (170–190 °C, 20–40 min), (b) pulping in 35% methanol and NaOH (170 °C, 40–60 min). The process underwent several conceptual changes (including the anthraquinone addition) until it reached the full-scale mill operation. However, shortly after opening, the plant closed due to several operating problems.13,15 In this study, we focused on one-step alkaline-GVL pulping employing NaOH and higher reaction temperature. The reactions with low concentrations of NaOH yielded pulp with properties comparable to the reference sample, but the pulp quality deteriorated with the increasing NaOH charge (Fig. 1a and 2a). The macromolecular properties were mostly preserved (Mw ≈ 300 kDa) but with a relatively high degree of heterogeneity (Table 1). The acidic and slightly neutral initial pH of pulping liquors (Table S1†) at lower alkali concentrations indicates that acid-catalyzed reactions dominated in hemicellulose and lignin removal. At high dose of NaOH (192 kg t−1), the pH of pulping liquor was slightly alkaline (pH 8.6) suggesting that the proton concentration was too low for acidic hydrolysis reactions. Simultaneously, the concentration of free hydroxyl ions at pH 8.6 was not sufficient to cleave the lignin bonds, which require pH > 11. Possible shift of the equilibrium of GVL to 4-hydroxyvaleric acid, catalyzed by higher alkalinity,22 could reduce the delignification capability of GVL. Additionally, an S-free nucleophile effective in the neutral or slightly acidic pH range would be required to further improve the efficiency and selectivity of delignification. However, as the overall alkaline pulping performance did not improve in comparison with uncatalyzed reaction, further investigation for optimization was not considered at this point.
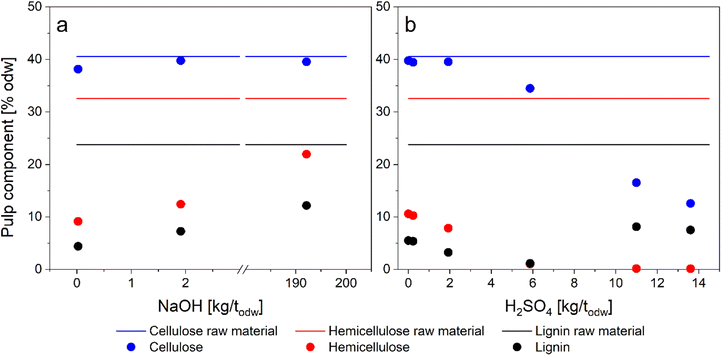 | ||
Fig. 1 The composition of pulp in (a) alkaline catalyzed; (b) acid catalyzed GVL pulping. Reaction conditions: 180 °C, 120 min, L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) W = 10. Raw material: W = 10. Raw material:  cellulose, cellulose,  hemicellulose, hemicellulose,  lignin; GVL pulping: lignin; GVL pulping:  cellulose, cellulose,  hemicellulose, hemicellulose,  lignin. The plotted data are summarized in ESI Table S2.† lignin. The plotted data are summarized in ESI Table S2.† | ||
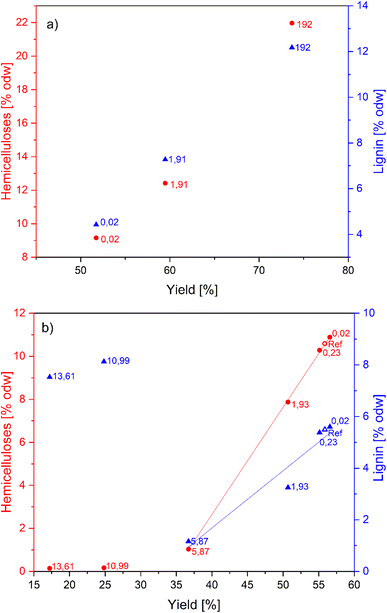 | ||
Fig. 2 The selectivity graph for pulp in (a) alkaline-catalyzed; (b) acid-catalyzed GVL pulping. Reaction conditions: 180 °C, 120 min, L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) W = 10. The digits represent the NaOH or H2SO4 in kg todw−1. The plotted data are summarized in ESI Table S2.† W = 10. The digits represent the NaOH or H2SO4 in kg todw−1. The plotted data are summarized in ESI Table S2.† | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) W 10 kg l−1, 50 wt% GVL
W 10 kg l−1, 50 wt% GVL
| Samplea | H2SO4 [kg t−1] | NaOH [kg t−1] | Chemical composition [% odp] | M w [kDa] | M n [kDa] | PDI | DP < 100 [%] | DP > 2000 [%] | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Cb | Hc | Ld | ||||||||
| a The analysis of fully bleached commercial pulps followed the same protocol as the selected pulp samples. b Cellulose. c Hemicellulose. d Lignin. e Uncatalyzed GVL cook with ionic liquid post treatment of fully bleached birch pulp (15% water content). f Uncatalyzed GVL cook. g The conditions for entry 11 are further discussed on Section 3.2. | ||||||||||
| PHK birch | — | — | 94.0 | 5.5 | 0.5 | 152 | 56 | 2.7 | 5.4 | 9.7 |
| PHK eucalyptus | — | — | 95.4 | 4.1 | 0.5 | 229 | 79 | 2.9 | 23.5 | 3.2 |
| AS beech | — | — | 94.6 | 4.9 | 0.4 | 208 | 40 | 5.2 | 9.9 | 22.7 |
| AS spruce | — | — | 96.1 | 3.5 | 0.4 | 164 | 47 | 3.4 | 14.4 | 6.6 |
| GVL-IPe (ref. 28) | — | — | 96.5 | 2.1 | 1.0 | 685 | 201 | 3.4 | 0.4 | 56.5 |
| Referencef | 0 | 0 | 71.2 | 19.0 | 9.8 | 322 | 71 | 4.5 | 2.7 | 38.5 |
| 1 | 0.02 | 0 | 70.9 | 19.2 | 9.9 | 323 | 65 | 4.9 | 4.6 | 38.0 |
| 2 | 0.2 | 0 | 71.6 | 18.6 | 9.8 | 304 | 57 | 5.3 | 7.3 | 36.5 |
| 3 | 1.9 | 0 | 78.1 | 15.5 | 6.4 | 317 | 62 | 5.1 | 4.6 | 38.1 |
| 11g | 6.1 | 0 | 93.8 | 2.9 | 3.2 | 54 | 15 | 3.5 | 34.3 | 2.0 |
| 7 | 0 | 0.02 | 73.8 | 17.7 | 8.6 | 291 | 148 | 2.0 | — | 29.6 |
| 8 | 0 | 1.9 | 67.0 | 20.8 | 12.2 | 269 | 297 | 0.9 | 11.4 | 31.8 |
| 9 | 0 | 192.1 | 53.7 | 29.8 | 16.5 | 323 | 59 | 5.5 | 6.6 | 38.6 |
Acid catalysis is well-known in (GVL) organosolv pulping. The acid primarily targets the glycosidic bonds in carbohydrates leading to hemicellulose depolymerization and solubilization. Delignification (predominantly cleavage of β-O-4 bonds) is promoted at higher hydrogen ion concentration, which can be accomplished by the addition of strong acids. Nevertheless, lignin depolymerization and repolymerization (condensation) facilitated by acid significantly alter the chemical structure. As a result, the hemicelluloses were completely hydrolyzed at high acid concentrations (>10 kg t−1), but simultaneous lignin condensation and extreme cellulose degradation were unavoidable (Fig. 1b). However, at a low acid charge (<6 kg t−1), the delignification and hemicellulose removal almost linearly correlate with increasing H2SO4 concentration (Fig. 2b). The macromolecular properties of acid-catalyzed pulps were well preserved. The mass balances of individual cooks are summarized in ESI (Table S2†). The highly acid-catalyzed pulping produced pulp with properties close to dissolving pulp, but at the expense of cellulose yield. Therefore, further optimization experiments and results adjusting the H2SO4 concentration and reaction temperature are discussed in the following section.
3.2 One-stage acid pulping of birch (f = T, [H+])
As suggested by the results in the previous section, the acid-catalyzed GVL pulping can overcome the high degree of the birch recalcitrance and provide the pulp with the desired properties. However, to avoid simultaneous pulp degradation, a series of fractionations with varying temperatures (120–180 °C) and acid charges were carried out. This experimental research is the extension of Fang and Sixta's (2015) work where they briefly evaluated the effect of GVL content, H2SO4 charge, reaction time, and temperature on pulp quality.25Despite the high acid concentration and linear correlation between increasing acid charge, hemicellulose, and lignin removal, the pulping at 120 °C underperformed (Fig. 3a). As a result, the composition and the final yield could not be accurately determined. Instead, the presented results serve as an indication point. The pulping selectivity was more prominent at 150 °C and 180 °C. The acid charge of 10 kg todw−1 at 150 °C produced the pulp with cellulose purity up to 88% (based on dry pulp), <5% odw hemicellulose, and <2% odw lignin while maintaining 43% yield. The pulping at 180 °C hydrolyzed most of the hemicelluloses but only at acid concentration < 6 kg todw−1 the cook did not suffer significant yield loss and the cellulose purity could reach 95% (on dry pulp).
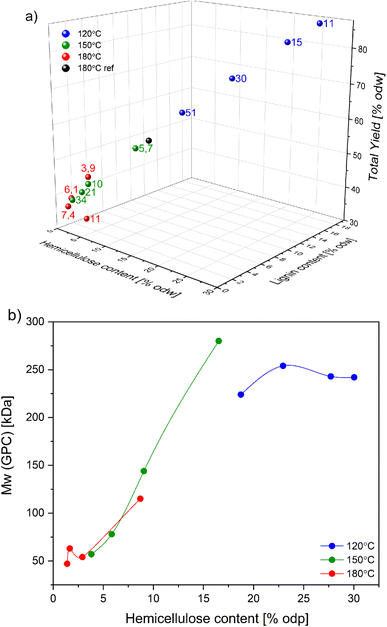 | ||
Fig. 3 (a) Selectivity graph for acid-catalyzed GVL pulping (the composition is in % odw). Reaction conditions: 120 min, L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) W = 10, 50 wt% GVL. The digits represent the H2SO4 concentration in kg todw−1; (b) purification selectivity for GVL pulping with varying acid concentration. The plotted data are summarized in Table 2 and ESI Table S3.† W = 10, 50 wt% GVL. The digits represent the H2SO4 concentration in kg todw−1; (b) purification selectivity for GVL pulping with varying acid concentration. The plotted data are summarized in Table 2 and ESI Table S3.† | ||
89–97% of the cellulose content and 73–100% of the lignin could be identified in mass balance (Table 2). The majority of the hemicelluloses were hydrolyzed to monomeric xylose, which was further dehydrated to furfural. However, the high reactivity and complicated reaction pathways of hemicelluloses and their degradation products, such as humins, impeded the complete identification in mass balances. The formation of humins is inevitable in acidic pulping and although their analysis was not the objective of this paper, the presence of humins could explain the gap in hemicellulose mass balance. The formic acid was excluded from the mass balance as the HPLC measurement indicated the elution of another compound at similar retention time, which we were unable to distinguish.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) W = 10
W = 10
| Sample | T [°C] | H2SO4 [kg t−1] | Yield [% odw] | Solid phase [% odw] | Liquid phasea [% odw] | Fur.e | OAf | Total [% odw] | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cb | Hc | Ld | C | H | L | |||||||
| a Spent liquor combined with washing water. b Cellulose. c Hemicellulose. d Lignin. e Furfural. f Organic acids (acetic acid, levulinic acid). g Uncatalyzed GVL cook. | ||||||||||||
| Wood | — | — | — | 41.6 | 32.0 | 23.8 | — | — | — | — | — | 97.4 |
| Ref.g | 180 | — | 55.8 | 39.7 | 10.6 | 5.5 | 0.3 | 11.4 | 18.7 | 0.4 | 2.0 | 91.9 |
| 10 | 180 | 3.9 | 45.3 | 37.6 ± 0.9 | 3.8 ± 0.8 | 1.9 ± 0.5 | 0.9 | 8.7 | 16.8 | 3.2 | 3.7 | 76.7 |
| 11 | 180 | 6.1 | 38.4 | 36.1 ± 2.4 | 1.1 ± 0.3 | 1.0 ± 0.2 | 2.6 | 9.3 | 17.0 | 5.1 | 4.8 | 77.2 |
| 12 | 180 | 7.4 | 36.0 | 34.2 ± 0.5 | 0.7 ± 0.2 | 1.1 ± 1.9 | 6.5 | 5.8 | 20.9 | 8.1 | 6.2 | 83.4 |
| 13 | 180 | 10.7 | 30.1 | 26.0 ± 3.6 | 0.5 ± 0.2 | 3.5 ± 1.3 | 4.1 | 8.5 | 20.5 | 12.6 | 6.1 | 81.9 |
| 14 | 150 | 5.7 | 53.4 | 39.7 ± 1.4 | 8.8 ± 0.6 | 4.9 ± 1.0 | 0.6 | 12.4 | 18.4 | 0.2 | 3.4 | 88.3 |
| 15 | 150 | 10.1 | 43.4 | 37.7 ± 0.7 | 3.9 ± 0.4 | 1.8 ± 0.3 | 1.2 | 13.4 | 20.0 | 1.1 | 6.1 | 85.3 |
| 16 | 150 | 20.6 | 40.3 | 36.0 ± 0.8 | 2.4 ± 0.5 | 1.9 ± 0.6 | 2.3 | 13.6 | 19.1 | 3.3 | 7.3 | 85.8 |
| 17 | 150 | 34.4 | 38.2 | 34.8 ± 0.7 | 1.5 ± 1.3 | 1.2 ± 1.2 | 2.2 | 9.0 | 16.2 | 5.7 | 7.7 | 78.3 |
| 18 | 120 | 11.1 | 86.4 | 42.4 ± 0.6 | 25.9 ± 0.3 | 18.0 ± 0.6 | 1.4 | 7.3 | 7.1 | 0.0 | 1.0 | 103.1 |
| 19 | 120 | 14.5 | 81.3 | 42.5 ± 1.1 | 22.6 ± 2.9 | 16.0 ± 2.3 | 0.2 | 6.7 | 8.3 | 0.0 | 1.3 | 97.6 |
| 20 | 120 | 30.0 | 70.9 | 41.7 ± 1.4 | 16.3 ± 0.9 | 13.0 ± 1.0 | 0.3 | 9.1 | 11.2 | 0.0 | 3.2 | 94.7 |
| 21 | 120 | 51.1 | 61.7 | 40.7 ± 1.3 | 11.6 ± 1.1 | 9.4 ± 0.7 | 1.6 | 11.4 | 14.3 | 0.1 | 5.0 | 94.0 |
H2SO4 had a considerable effect on the molecular mass of the pulp (Fig. 3b). While the reaction at 180 °C generated short-chain almost pure cellulose, the pulp produced at 150 °C resembled the macromolecular properties of commercial dissolving pulps. In comparison to the commercial AS and PHK pulps, the overall selectivity of the catalyzed GVL pulps is lower due to the lack of strong nucleophiles. Higher polydispersity index (PDI) of GVL pulps resembles the PDI of AS pulps (Table 3), which can be attributed to random acid-catalyzed depolymerization proceeding from outer to inner cell wall. Additionally, more accessible cellulose in outer wall layers is degraded to a higher extent in the acid pulps than in PHK pulps (Fig. S1†). The molar mass of the hemicelluloses of the acid pulps is significantly lower than the molar mass of the hemicelluloses of the PHK pulps at the same DP.30–32
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) W = 10
W = 10
| Sample | NaOH [kg t−1] | Yielda [%] | Solid phase [% odw] | Liquid phaseb [% odw] | PH phasec [% odw] | Total [% odw] | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cd | He | Lf | C | H | L | Fg | Oh | C | H | L | F | O | ||||
| a The yield represents the overall yield (PH yield × pulping yield). b Spent liquor combined with washing water. c PH liquor combined with PH washing water. d Cellulose. e Hemicellulose. f Lignin. g Furfural. h Organic acids (acetic acid, levulinic acid). i Refers to uncatalyzed GVL cook with prehydrolysis. | ||||||||||||||||
| Wood | — | — | 41.6 | 32 | 23 | — | — | — | — | — | — | — | — | — | — | 97.4 |
| PH wood | — | 81.2 | 38.3 | 17 | 23 | — | — | — | — | — | — | — | — | — | — | 90.5 |
| PH-GVLi | 0 | 44.4 | 39.1 ± 0.3 | 7.9 ± 0.2 | 7.7 ± 0.1 | 0.2 | 5.2 | 17.9 | 0.4 | 0.2 | 0.2 | 6.8 | 0.7 | 1.0 | 2.7 | 90.0 |
| 22 | 0.02 | 44.2 | 39.7 ± 0.4 | 7.6 ± 0.4 | 7.2 ± 0.8 | 0.3 | 5.0 | 16.2 | 0.4 | 0.3 | 0.2 | 6.8 | 0.7 | 1.0 | 2.7 | 88.0 |
| 23 | 1.6 | 46.5 | 39.9 ± 0.3 | 8.8 ± 0.2 | 8.7 ± 0.2 | 0.2 | 4.1 | 15.1 | 0.3 | 0.4 | 0.2 | 6.8 | 0.7 | 1.0 | 2.7 | 88.8 |
| 24 | 156 | 44.1 | 38.8 ± 0.1 | 6.0 ± 0.5 | 9.6 ± 0.3 | 0.1 | 0.5 | 16.1 | 0.1 | 1.8 | 0.2 | 6.8 | 0.7 | 1.0 | 2.7 | 84.3 |
In comparison with the study of Fang and Sixta (2015),25 the extension of the pulping times could reduce the amount of H2SO4 without compromising the pulp quality.
3.3 Two-stage pre-hydrolysis + alkaline GVL pulping of birch
During the prehydrolysis, acetic acid released from deacetylated hemicelluloses (β-(1–4) bond cleavage in xylan) catalyzes the hydrolysis of the bonds between hemicelluloses, which are cleaved into oligomers and monosugars.8 The extent of hemicellulose hydrolysis is influenced by chosen technology and prehydrolysis intensity. To control the prehydrolysis intensity, the P-factor was established as a single variable expressing the PH time and temperature:where krel is the relative rate of acid-catalyzed hydrolysis of glycosidic bonds. The rate at 100 °C was chosen as unity and all other temperatures are related to it. The temperature dependance of the rate is described by an Arrhenius-type equation with an activation energy of 125.6 kJ mol−1 for xylan hydrolysis. Nevertheless, P-factor selection should consider not only the extent of hemicellulose removal, but also the chemical composition of raw material, yield, intrinsic viscosity of pulp and the extent of lignin condensation.11 Prehydrolysis increases the accessibility of the hydroxyl ion and does not interfere with a subsequent alkaline kraft pulping unless exceeding a particular intensity threshold of lignin condensation (especially for softwoods). The following alkaline stage facilitates the extraction of reactive lignin produced during prehydrolysis.
Fasching et al. (2005)33 studied the effect of prehydrolysis followed by acid sulfite pulping, revealing that two subsequent acidic stages impaired the overall extent of delignification. The ongoing reactions during prehydrolysis (such as lignin condensation, cleavage of lignin bonds, reactions with degradation intermediates, or other chemical modifications of the lignin) hindered delignification during sulfite pulping. Therefore, Fasching et al. (2005) introduced an alkaline extraction before the sulfite stage, but the pulping performance at given extraction conditions decreased compared to two-stage and single-stage acid sulfite fractionation.33 The incorporation of the prehydrolysis stage into the GVL process could improve hemicellulose removal but considering that uncatalyzed GVL pulping is a mildly acidic process, only alkaline catalyzed GVL experiments were studied to avoid the proven under-performance of two consecutive acidic stages.
The birch chips were steam-prehydrolyzed at the intensity of P-500 since the xylan removal is the most prominent at lower P-factors. Simultaneously, higher intensity (higher P-factor) would cause lignin condensation, which could not be fully compensated by adjusting pulping conditions.11 Compared to the raw material, the hemicellulose content was halved after the prehydrolysis (Table 3). A relatively high amount of residual hemicelluloses in the chips was expected because of the high recalcitrance and the typical hemicellulose abundancy of silver birch. Alternatively, the higher P-factor could improve the hemicellulose removal at the risk of yield loss, reduced viscosity and lignin condensation.
Although the prehydrolysis hydrolyzes a small amount of the acid-soluble lignin, the most prominent delignification occurs during the GVL pulping. Neither prehydrolysis nor alkaline catalysis had a significant effect on the acceleration of the delignification of GVL pulping compared to the reference uncatalyzed conditions. Similar to one-stage alkaline pulping, NaOH undergoes the neutralization reaction with 4-hydroxyvaleric acid22 and acid products generated from GVL hydrolysis and hemicellulose degradation, respectively. As a result, even at the highest NaOH dosage, the proton concentration is too low so that no free hydroxyl ions are available for the degradation reaction. The reduced acid-hydrolytic activity due to neutralization could also explain the shift of the MMD of the pulp samples to a higher molecular weight region (Table 4). Non-catalyzed or mildly alkaline GVL cooking after pre-hydrolysis results in relatively effective delignification with little depolymerization of the cellulose. It can be assumed that more intensive prehydrolysis (higher P-factor) or the addition of S-free nucleophiles could further improve the extent of delignification.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) W = 10, 50 wt% GVL
W = 10, 50 wt% GVL
| Sample | pHSL | NaOH [kg t−1] | Chemical composition [% odp] | M w [kDa] | Mn [kDa] | PDI | DP < 100 [%] | DP > 2000 [%] | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Cb | Hc | Ld | ||||||||
| a Refers to uncatalyzed GVL cook with prehydrolysis. b Cellulose. c Hemicellulose. d Lignin. | ||||||||||
| PH-GVLa | 3.48 | 0 | 71.4 | 14.4 | 14.1 | 316 | 66 | 4.8 | 5.4 | 37.7 |
| 22 | 3.51 | 0.02 | 72.9 | 13.9 | 13.2 | 318 | 84 | 3.8 | 1.3 | 38.2 |
| 23 | 3.86 | 1.6 | 69.5 | 15.3 | 15.1 | 342 | 88 | 3.9 | 1.1 | 41.8 |
| 24 | 5.78 | 155.9 | 71.3 | 11.1 | 17.6 | 469 | 123 | 3.8 | 1.7 | 59.9 |
3.4 Suitability of proposed GVL concepts for dissolving pulp production
Dissolving pulp is characterized by a high degree of purity (α-cellulose content > 90%) with a low content of hemicellulose and lignin (<5% odw). The one-stage acid-catalyzed pulping at moderate temperature (150 °C) and low acid dosage (5–10 kg t−1) performed the best (Table S3†). Further, the best results for the two-stage pulping were achieved when the prehydrolysis was followed by low alkali GVL cook (entry 22, Table 4). Compared to commercial fully bleached PHK birch pulp, the pulp from the proposed GVL concepts still has a high content of hemicelluloses > 9.1% odp (Fig. 4a), which might lead to inferior properties of final product, and an additional purification step would be required. The higher lignin content in selected pulps (Fig. 4b) could be easily removed in later stages (oxygen delignification and totally chlorine-free bleaching) due to the lack of hexenuronic acid in GVL unbleached pulp.28 Although, the selected conditions represent the most promising outcomes, the two-stage process did not exhibit a substantial improvement considering that an extra stage would increase the complexity and expense. The one-stage GVL process proved to be a dominant concept in a new generation of dissolving pulp production.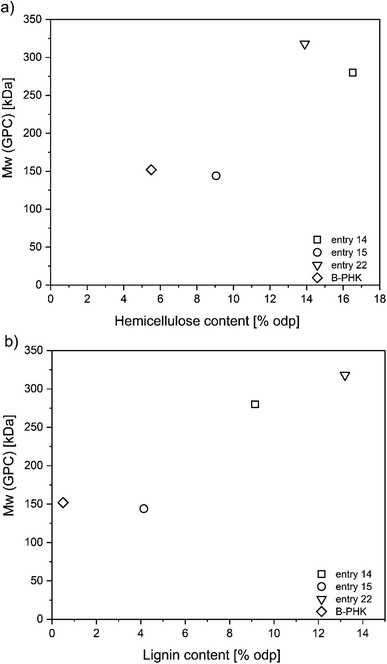 | ||
| Fig. 4 Summary of the most suitable GVL pulping conditions for dissolving pulp production as function of (a) hemicellulose removal selectivity; (b) delignification selectivity. The plotted data are summarized in Table 4 and ESI Table S3,† B-PHK = birch prehydrolysis kraft pulp. | ||
4 Conclusion
GVL is a sustainable solvent already proven to be effective in biomass fractionation mainly because it is a powerful lignin solvent. To produce a suitable rayon-grade dissolving pulp, the experimental design focused on process optimization and in-depth evaluation of the resulting pulp properties and liquid fractions. Due to the partial neutralization of NaOH in the GVL pulping liquor, alkaline catalysis was not sufficient to initiate effective delignification reactions, resulting in a pulp with inacceptable properties. Mild acid catalysis (<10 kg H2SO4 per todw) combined with a lower cooking temperature (150 °C) could overcome the recalcitrance of birch wood and enabled the production of a high yield dissolving pulp with adequate properties (4% odw of hemicelluloses and 2% odw lignin). A pre-hydrolysis step followed by alkaline GVL pulping (0.02 kg NaOH per todw) reduced the amount of hemicelluloses, but the overall performance of two-stage pulping did not improve (Table 3).This paper serves as the basis for upscaling the pulping experiments, using wood chips and addressing not only the dissolving pulp production but also the valorization of other fractionated streams.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was a part of the Academy of Finland's Flagship Programme under Project No. 318890 and 318891 (Competence Center for Materials Bioeconomy, FinnCERES).References
- G. Gellerstedt, in Wood Chemistry and Wood Biotechnology, ed. M. Ek, G. Gellerstedt and G. Henriksson, De Gruyter, Germany, 2009, ch. 1, vol. 1, pp. 1–12 Search PubMed.
- G. Henriksson, E. Brännvall and H. Lennholm, in Wood Chemistry and Wood Biotechnology, ed. M. Ek, G. Gellerstedt and G. Henriksson, De Gruyter, Germany, 2009, ch. 2, vol. 1, pp. 14–44 Search PubMed.
- M. Macleod, Pap. Puu, 2007, 89, 1–7 Search PubMed.
- J. A. Ferreira and M. J. Taherzadeh, Bioresour. Technol., 2020, 299, 122695 CrossRef CAS.
- M. Kienberger, S. Maitz, T. Pichler and P. Demmelmayer, Processes, 2021, 9(5), 804 CrossRef.
- Statista, Projected production capacity of chemical pulp worldwide from 2020 to 2025 (in 1,000 metric tons), https://www.statista.com/statistics/871672/production-capacity-chemical-pulp-forecast-worldwide/, accessed August 2022 Search PubMed.
- GmbH TFY, The Fiber Year, 2022, vol. 22 Search PubMed.
- G. Schild, H. Sixta and L. Testova, Cellul. Chem. Technol., 2010, 44, 35–45 CAS.
- H. Sixta, M. Iakovlev, L. Testova, A. Roselli, M. Hummel, M. Borrega, A. van Heiningen, C. Froschauer and H. Schottenberger, Cellulose, 2013, 20, 1547–1561 CrossRef CAS.
- H. Sixta, 1 Chemical pulping (Part I), in Handbook of pulp, ed. H. SixtaWiley, Germany, 2006, pp. 2–19 Search PubMed.
- H. Sixta, 4 Chemical pulping processes (Part I), in Handbook of pulp, ed. H. Sixta, Wiley, Germany, 2006, vol. 325–327, pp. 343–344 Search PubMed.
- R. Alén, in Papermaking Science and Technology– Book 20 Biorefining of Forest Resources, ed. R. Alén, Paper Engineers’ Association, Finland, 2011, ch. 2, pp. 56–104 Search PubMed.
- P. Azadi, O. R. Inderwildi, R. Farnood and D. A. King, Renewable Sustainable Energy Rev., 2013, 21, 506–523 CrossRef CAS.
- T. Kleinert and K. Tayenthal, Angew. Chem., 1931, 44, 788–791 CrossRef CAS.
- H. L. Hergert, in Environmentally Friendly Technologies for the Pulp and Paper Industry, ed. R. A. Young and M. Akhtar, Wiley & Sons, Canada, 1st edn, 1998, ch. 1, pp. 5–67 Search PubMed.
- W. Peter and O. Höglinger, Lenzinger Ber., 1986, 61, 12–16 CAS.
- A. M. Sharazi and A. van Heiningen, Holzforschung, 2017, 71, 951–959 CAS.
- M. Iakovlev, T. Pääkkönen and A. van Heiningen, Holzforschung, 2009, 63, 779–784 CAS.
- J. J. Bozell, S. K. Black, M. Myers, D. Cahill, W. P. Miller and S. Park, Biomass Bioenergy, 2011, 35, 4197–4208 CrossRef CAS.
- M. Chen, F. Malaret, A. E. J. Firth, P. Verdia, A. R. Abouelela, Y. Chen and J. P. Hallett, Green Chem., 2020, 22, 5161–5178 RSC.
- I. T. Horváth, H. Mehdi, V. Fábos, L. Boda and L. T. Mika, Green Chem., 2008, 10, 238–242 RSC.
- M. Granatier, I. Schlapp-Hackl, Q. H. Lê, K. Nieminen, L. Pitkänen and H. Sixta, Cellulose, 2021, 28, 11567–11578 CrossRef CAS.
- J. A. Dumesic, D. M. Alonso and J. S. Luterbacher, US Pat., 9359650 B2, 2016 Search PubMed.
- L. Shuai, Y. M. Questell-Santiago and J. S. Luterbacher, Green Chem., 2016, 18, 937–943 RSC.
- W. Fang and H. Sixta, ChemSusChem, 2015, 8, 73–76 CrossRef CAS.
- Q. H. Lê, Y. Ma, M. Borrega and H. Sixta, Green Chem., 2016, 18, 5466–5476 RSC.
- Q. H. Lê, J. P. Pokki, M. Borrega, P. Uusi-Kyyny, V. Alopaeus and H. Sixta, Ind. Eng. Chem. Res., 2018, 57, 15147–15158 CrossRef.
- S. Shokri, S. Hedjazi, Q. H. Lê, A. Abdulkhani and H. Sixta, Carbohydr. Polym., 2022, 288, 119364 CrossRef CAS.
- J. Janson, Pap. Puu, 1970, 5, 323–329 Search PubMed.
- G. Jayme and A. von Köppen, Das Papier, 1950, 4, 455–462 Search PubMed.
- R. A. Young, Cellulose, 1994, 1, 107–130 CrossRef CAS.
- H. Sixta, H. Harms, S. Dapia, J. C. Parajo, J. Puls, B. Saake, H. P. Fink and T. Röder, Cellulose, 2004, 11, 73–83 CrossRef CAS.
- M. Fasching, A. Griebl, G. Kandioller, A. Zieher, H. Weber and H. Sixta, Macromol. Symp., 2005, 223, 225–238 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2su00046f |
| This journal is © The Royal Society of Chemistry 2023 |