 Open Access Article
Open Access ArticleRecycling post-consumer PLA into acrylic acid or lactide using phosphonium ionic liquids†
Kwinten
Janssens
 a,
Wouter
Stuyck
a,
Wouter
Stuyck
 a,
Kirsten
Stiers
a,
Jens
Wéry
a,
Kirsten
Stiers
a,
Jens
Wéry
 a,
Mario
Smet
b and
Dirk E.
De Vos
a,
Mario
Smet
b and
Dirk E.
De Vos
 *a
*a
aDepartment of Microbial and Molecular Systems (M2S), Centre for Membrane Separations, Adsorption, Catalysis and Spectroscopy for Sustainable Solutions (cMACS), KU Leuven, Celestijnenlaan 200F, 3001 Leuven, Belgium. E-mail: dirk.devos@kuleuven.be
bDepartment of Chemistry, Polymer Chemistry and Materials, KU Leuven, Celestijnenlaan 200F, 3001 Leuven, Belgium
First published on 9th November 2022
Abstract
Chemical recycling of polymer waste to the original monomer (CRM) forms an important concept for a circular plastic economy. For poly(lactic acid) (PLA) this is often neglected as a result of PLA's known biodegradability. However, such degradation leads to the undesired generation of CO2, associated with a poor energy efficiency and a missed opportunity for fast circular reuse. In this work, we develop a system to recycle post-consumer PLA into either acrylic acid (AA) or lactide (LAC), depending on the presence/absence of an acidic cocatalyst and the applied reaction conditions. A detailed time profile showed that PLA is first converted into LAC and subsequently rearranged to AA under acidic conditions. Up to 50% of monomer yield was achieved in a single step, using a phosphonium ionic liquid (Bu4PBr) as the active solvent and only 5 mol% of organic cocatalyst.
Sustainability spotlightPoly(lactic acid), in short PLA, is a biobased plastic with increasing impact in the polymer industry as it is readily produced from sustainable resources and has a known biodegradability. However, polymer degradation in landfills or nature is often very slow and still results in the undesired release of CO2, which implies significant loss of valuable materials and a missed opportunity for a fast circular reuse. In this work, we propose a simple system to recycle post-consumer PLA directly into valuable polymer precursors, namely lactide and acrylic acid, using a thermally stable ionic liquid in the absence/presence of an acidic cocatalyst. This unprecedented valorization of plastic waste into valuable building blocks further improves PLA's sustainability profile. |
Introduction
Recycling our polymer waste will be a key step in the transition towards a green and sustainable future. Currently, most polymers are burned for energy recuperation or simply disposed in landfills, only then followed by mechanical recycling and chemical recycling.1,2 For chemical recycling, different strategies are known to date, but the most promising one is chemical recycling to the original monomer (CRM).3 Polymers produced from recycled monomers may emulate the physicochemical properties of their virgin relatives.3 Unfortunately, CRM does not apply to abundant polymers such as poly(olefins) or PVC, since breaking down the all-carbon polymer backbone in a controlled way to the original monomers is both thermodynamically and kinetically problematic. Some poly(esters) or poly(amides) on the other hand may more easily be transformed to the original monomers or to fairly similar small molecule products.4–8 Poly(lactones) are an interesting subcategory of poly(esters) which, in the presence of a suitable catalyst and under the right conditions, can undergo ring closing depolymerization (RCDP) back into the original lactone.3 The most established process involves the CRM of poly(caprolactone) (PCL) to ε-caprolactone using Zn or Sn catalysts which remained present in the bulk PCL matrix after polymerization by ROP (ring opening polymerization).9–11 Similar recyclability is reported for poly(butyrolactones),12,13 leading to C4 (or C8 in case of the dimer) lactone monomers.For polymers derived from smaller lactones, like poly(β-propiolactone) and poly(lactic acid) (PLA), direct CRM is not obvious due to high ring strain in the original monomer (for β-propiolactone) or to undesired side reactions at elevated depolymerization temperatures (for both β-propiolactone and lactide). Therefore, these polymers are often incinerated for energy recuperation or disposed in landfills/nature to degrade via natural processes. However, the biodegradability of PLA in nature is fairly limited, as industrial conditions and specialized enzymes are required for sufficiently fast degradation.5,6 For abovementioned polymers, CRM to the lactone monomer is only possible via a multi-step procedure requiring additional separation and purification.14,15 Alternatively, controlled depolymerization could lead to different but inherently valuable monomers e.g. acrylic acid (AA).16,17 In the case of poly(β-propiolactone), thermolysis in the presence of a tertiary amine (e.g. pentamethylenediethylenetriamine) produces AA in up to 95% yield.18 Unfortunately, production of poly(β-propiolactone) as a source of sustainable AA has not reached full commercialization due to fermentation issues.19
PLA on the other hand is nowadays widely spread in plenty of consumer products, despite its problematic biodegradation. However, if collected and sorted properly, post-consumer PLA could be an ideal renewable resource for the synthesis of biobased AA. This requires a chemical recycling method that would tolerate impurities/additives present in post-consumer PLA, e.g. hardeners like talc, which are often added for improved thermal stability for food applications. Unfortunately, such reactions are relatively unexplored in literature; the best performing system hitherto was reported by Bouwman and coworkers (2017) as part of their study of the lactide (LAC) to AA reaction.17,20 In their research, PLA was dissolved together with an active tetraphenylphosphonium bromide salt (Ph4PBr, mp 300 °C) in sulfolane at 150 °C, yielding only a modest yield of 25% AA after 16 h. In addition, a large amount of cocatalyst (42 mol% methanesulfonic acid, relative to the monomeric unit of PLA) was required to ensure sufficient depolymerization. Other studies focused on producing AA starting from lactide, achieving yields up to 70% in batch21 and 80% in continuous flow,22 but without any reference to PLA recycling.21–23 In this work, we study the catalytic conversion of end-of-life PLA to acrylic acid, with a special focus on the impact of the reaction conditions on the product distribution. Careful modifications of the reaction conditions uncovered a new way to synthesize the even more valuable lactide monomer in a single step, directly from PLA, thus creating an unprecedented CRM for recycling post-consumer waste PLA.
Results and discussion
Variation of the reaction conditions
Our first attempt to convert pure PLA to acrylic acid (AA) used an organic phosphonium bromide combined with an acid co-catalyst, like in Bouwman's work.17 However, instead of CH3SO3H, a much smaller amount of the cheap H2SO4 was used; and the high melting aromatic phosphonium bromide was replaced by Bu4PBr, which we previously used to produce AA from the dilactone lactide (LAC).21 After 2 h at 200 °C in sulfolane as the cosolvent, conversion of PLA was complete, and a mixture of products was obtained (Table 1, entry 1). AA was obtained as the major product in higher yields compared to literature, even if the acid concentration was much lower and the reaction time shorter.17 Remarkably, a mixture of lactides is obtained as the second major product, representing almost one quarter of the total mass balance. Notably, as a result of the expected racemization in this bromide-mediated depolymerization, all three forms of lactide (D/L/DL) were observed in similar amounts, and therefore combined in one product yield (ESI, product analysis†).| Entry | Ionic liquida | Sulfolane | Type cocatalyst | Catalyst | Time | Temp. | Xb (%) | AAc (%) | Acrylatesc (%) | LACc,d (%) | Lactic acidc (%) | Bromo-PA'sc,e (%) | Oligomersc (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a 0.5 mmol PLA, reaction in a glass vial, 500 rpm, inert atmosphere (Ar flushed). b Conversion of PLA. c Product yield, determined via1H NMR of the crude reaction mixture, expressed relative to monomeric unit of PLA. d Combined yield of lactides: L/D lactide forming a racemic mixture and DL- or meso-lactide. e Combined yield of 2/3-bromopropanoic acids. f Mainly chloropropanoic acids. g Product yields are corrected for the 5 mol% 2-bromopropanoic acid cocatalyst. Details on catalytic reaction and product analysis can be found in the ESI. | |||||||||||||
| 1 | Bu 4 PBr (3 eq.) | 2.5 mL | H 2 SO 4 | 5 mol% | 2 h | 200 °C | 99 | 33 | 7 | 24 | 9 | 4 | 8 |
| 2 | Bu4PBr (3 eq.) | 2.5 mL | H2SO4 | 5 mol% | 4 h | 200 °C | 99 | 45 | 2 | <1 | 7 | 2 | 2 |
| 3 | Bu4PBr (3 eq.) | 2.5 mL | H2SO4 | 5 mol% | 2 h | 220 °C | 99 | 46 | 1 | <1 | 6 | 1 | 1 |
| 4 | Bu4PBr (3 eq.) | 2.0 mL | H2SO4 | 5 mol% | 2 h | 200 °C | 99 | 40 | 8 | 19 | 10 | 3 | 9 |
| 5 | Bu4PBr (3 eq.) | 1.0 mL | H2SO4 | 5 mol% | 2 h | 200 °C | 99 | 45 | 7 | 10 | 8 | 2 | 8 |
| 6 | Bu4PBr (3 eq.) | — | H2SO4 | 5 mol% | 2 h | 200 °C | 99 | 48 | 4 | <1 | 3 | <1 | 5 |
| 7 | Bu4PBr (2 eq.) | 2.5 mL | H2SO4 | 5 mol% | 2 h | 200 °C | 98 | 25 | 8 | 36 | 9 | 3 | 9 |
| 8 | Bu4PBr (1 eq.) | 2.5 mL | H2SO4 | 5 mol% | 2 h | 200 °C | 97 | 12 | 7 | 44 | 8 | 3 | 8 |
| 9 | — | 2.5 mL | H2SO4 | 5 mol% | 2 h | 200 °C | 68 | <1 | <1 | <1 | 1 | 1 | 2 |
| 10 | Et4PBr (3 eq.) | 2.5 mL | H2SO4 | 5 mol% | 2 h | 200 °C | 98 | 24 | 7 | 7 | 35 | 4 | 8 |
| 11 | Bu4PCl (3 eq.) | 2.5 mL | H2SO4 | 5 mol% | 2 h | 200 °C | 99 | 28 | 4 | 6 | 23 | 20f | 4 |
| 12 | Bu4PBr (3 eq.) | 2.5 mL | HBr 48 wt% | 5 mol% | 2 h | 200 °C | 98 | 23 | 8 | 38 | 5 | 2 | 9 |
| 13 | Bu 4 PBr (3 eq.) | 2.5 mL | C 16 H 33 Br | 5 mol% | 2 h | 200 °C | 99 | 42 | 10 | 27 | 9 | 4 | 5 |
| 14 | Bu4PBr (3 eq.) | 2.5 mL | 2-BrPAg | 5 mol% | 2 h | 200 °C | 90 | 41 | 9 | 8 | 10 | 11 | 5 |
| 15 | Bu4PBr (3 eq.) | 2.5 mL | ZnBr2 | 5 mol% | 2 h | 200 °C | 88 | 6 | 4 | 48 | 6 | 0 | 4 |
| 16 | Bu 4 PBr (3 eq.) | 2.5 mL | — | 5 mol% | 2 h | 200 °C | 96 | 4 | 3 | 50 | 13 | 0 | 3 |
| 17 | Bu4PBr (3 eq.) | 2.5 mL | C16H33Br | 2.5 mol% | 2 h | 200 °C | 90 | 18 | 6 | 33 | 12 | 5 | 5 |
| 18 | Bu4PBr (3 eq.) | 2.5 mL | C16H33Br | 10 mol% | 2 h | 200 °C | 99 | 50 | 6 | 8 | 11 | 9 | 0 |
Starting from this benchmark reaction (2 h at 200 °C), we varied reaction time and temperature to study their effect on the product distribution (entry 1–3). Special attention was given to the formation of AA or LAC, since these two products have the highest added value for future polymer applications, namely as biobased poly(acrylates) or as recycled PLA through CRM. After longer reaction times or at higher temperatures (4 h at 200 °C or 2 h at 220 °C), all observed products, including lactide, but with the exception of lactic acid, were fully converted into AA, or lost as volatiles, e.g. acetaldehyde, ethylene or CO2. These undesired fragmentation products are not further considered in this work. As will be confirmed by the time-dependent data in Fig. 1, this indicates that at least part of the starting material first reacts to lactide, which is consecutively transformed to AA.21 The enhanced production of AA at higher temperatures is in agreement with literature;23 at lower temperatures the PLA conversion was found almost complete, but more intermediate products were observed and yields of AA and lactide are lower.
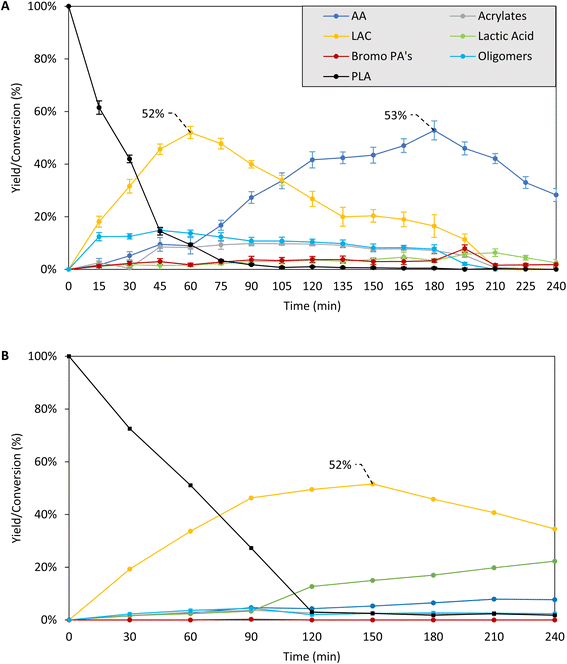 | ||
| Fig. 1 Time profile for PLA depolymerization in the presence (A) or absence (B) of cocatalyst (based on Table 1, entry 13 and 16). Reaction conditions: 0.5 mmol PLA (based on monomer), (A) 5 mol% C16H33Br cocatalyst, (B) no cocatalyst, 1.5 mmol Bu4PBr, 2.5 mL sulfolane, 0–4 h, 200 °C, Ar flushed. Product yield, determined via1H NMR of the crude reaction mixture, expressed relative to monomeric unit of PLA. LAC = combined yield of lactides. Bromo PA's = combined yield of 2/3-bromopropanoic acids. Detailed product distributions can be found in Fig. S6 and S7.† Reactions in A were performed in 5 fold, indicating high reproducibility. | ||
Before addressing in detail the reaction network, first the role and necessity of several constituents of the reaction mixture were briefly examined. The addition of sulfolane as cosolvent is not strictly necessary if AA is the desired product, as lowering the amount actually leads to gradually increasing AA yields of up to 50% (entry 4–6). The fact that the reaction performed well in the absence of sulfolane (entry 6) could be beneficial when considering scale-up, since the yield of AA could be increased by continuous evaporation of AA from the non-volatile ionic liquid.22 On the other hand, higher mass balances observed in the presence of sulfolane indicate partial stabilization of the reaction intermediates, retarding the formation of undesired volatile dead end products. Lowering the amount of IL (which is a reactive solvent) with constant cosolvent volume, resulted in a drop in AA yield, associated with an increase in lactide intermediates remaining after 2 h at 200 °C (entries 1, 7–9). Changing the type of IL did not yield better results, with the use of tetraethylphosphonium bromide resulting in high amounts of lactic acid, presumably due to the higher water content of the IL (entry 10). In the presence of tetrabutylphosphonium chloride large amounts of chloropropanoic acids were detected due to insufficiently fast dehydrochlorination of these intermediates to AA (entry 11).
In an attempt to further improve the AA yields, Brønsted and Lewis acidic cocatalysts were screened (entry 12–15). Organobromides were also tested as they are known to in situ generate HBr via dehydrobromination.22,23 For HBr, similar balanced product distributions are observed as for the initial H2SO4 benchmark reaction (entry 1 vs. 12). Remarkably, in the presence of organobromides (entry 13–14), significantly higher amounts of AA are observed than with the inorganic Brønsted acids. The slow release of anhydrous HBr via dehydrobromination allows for milder rearrangement conditions in the initial stage and stronger acidity towards the end of reaction (vide infra for the time profile of the reaction, Fig. 1A). The Lewis acid ZnBr2 was also tested in a 5 mol% cocatalyst concentration, to verify whether it could play a similar role as in the reverse RCDP of poly(caprolactone). However, it had no added benefit compared to the reaction in the absence of an acid cocatalyst (entry 15). In fact, the reaction without added acid co-catalyst yielded the largest amount of lactides (50%, entry 16) of all reactions reported in Table 1. Varying the cocatalyst concentration did not lead to significant improvements in AA yield; the observed 50% AA yield in the parameter variation was not exceeded even with twice the amount of acid cocatalyst (entry 18).
Time profile & proposed reaction network
In a second stage, we performed a detailed time profile in combination with the supplementary analysis of putative intermediates to propose the overall reaction network and depolymerization mechanism (Fig. 1, Scheme 1). To acquire more insight in the relations between the lactide and acrylic acid yields, the time profile of the reaction at 200 °C was analyzed in detail, based on the optimized results in the parameter variation, both in the presence of 5 mol% hexadecyl bromide and in the absence of an acid releasing cocatalyst (Table 1, entry 13 and 16 – Fig. 1, S5 and S6†). At the early stages of the depolymerization, PLA is mainly converted to lactides (LAC, yellow), followed by a second reaction step to acrylic acid (AA, dark blue). Neither product exceeds the 50% yield cap as they can be subsequently converted: whereas LAC is converted to AA, AA itself further degrades to smaller compounds like acetaldehyde, ethylene, CO/CO2.21,23 Presumably, the organobromide slowly decomposes to HBr and the corresponding hexadecene, thus allowing for mildly acidic conditions at the start of the reaction, which accelerates the depolymerization compared to the case in the absence of cocatalyst. In a later stage, dehydrobromination is complete, resulting in a higher overall acidity in the reaction medium, which aids in the conversion of the lactide intermediates to acrylic acid. In the absence of a cocatalyst, the depolymerization of PLA is significantly slower and only lactides are formed with a slight decomposition to AA after 4 hours. A similar plateau in LAC yield is observed, with no increase in LAC after 2 h, accompanied by a slow decomposition into lactic acid and acrylic acid. 31P-NMR of the reaction mixture before and after 4 h (both in the presence and absence of acid cocatalyst) at 200 °C showed no significant changes, indicating high thermal stability of the ionic liquid for long reaction times (Fig. S4†).21–24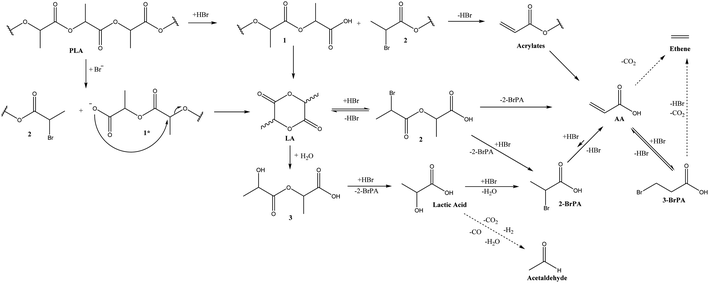 | ||
| Scheme 1 Proposed reaction network. (1) oligomers, (1*) oligomers in deprotonated form, (2) bromopropionates, (3) lactates. The proposed network was partially based on the literature of the LAC to AA reaction, adapted from multiple resources.21,23,26,27 | ||
Control experiments on the formation of lactic acid proved that its formation predominantly takes place via a hydrolytic ring opening of the lactide, as the depolymerization in the presence of additional water leads to an increased lactic acid formation (Fig. S8-A†), while rehydration of the acrylic acid is not possible (Fig. S8-B†). Remarkably, while lactic acid is readily dehydrated using acid zeolites,19 its dehydration is actually much more impaired in Bu4PBr in the presence of an acid cocatalyst. It is assumed that the water formed throughout dehydration actually hampers subsequent dehydration reactions (Fig. S9†), as was previously observed for the dehydration of polyol waste streams (containing up to 20 wt% water) in the same ionic liquid.25 Therefore, it is suggested to perform PLA recycling as much as possible in the absence of water or under continuous removal of water to avoid undesired hydrolysis towards the kinetically more inert lactic acid. Verification of the dehydrobromination mechanism using putative intermediates indicates a fast decomposition of both 2- and 3-bromopropanoic acid, with 3-BrPA reaching a thermodynamic equilibrium with AA after 5 minutes (Fig. S10†). Our observations from the time profiles and reactions with putative intermediates are in line with the known insights in the conversion of LAC to AA (Scheme 1).21,23 In the absence of water, reaction is predominantly catalyzed by the anhydrous HBr, where the Br− is expected to function as a nucleophile to perform a substitution at the C2 position, facilitated by the protonation of the acyloxy group. This results in a cleaved polymer chain with a carboxylic acid and a 2-bromopropionate polymer. Subsequent dehydrobromination results in the observed acrylates. If the acid cocatalyst is absent, Br− substitution might result in a carboxylate, which could perform backbiting into LAC like in the base catalyzed hydrolysis of PLA reported in literature (1*).26,27 The key difference here would be the use of an anhydrous nucleophile (Br−vs. OH−), providing a more stable environment for LAC, making it less prone to subsequent ring opening. If water would be present in the system, this would lead to hydrolysis leading to lactic acid derivates which would only partially be transformed into the desired acrylic acid (Fig. S9†). Cascade reactions in the presence of an acid cocatalyst will result in HBr substitution followed by fast dehydrobromination (Fig. S10†) and eventually lead to AA as the final product. Extending the reaction time or increasing the reaction temperature will result in further fragmentation of the products into undesired end-products through decarbonylation or decarboxylation.23 An overall reaction network for the depolymerization of PLA is proposed in Scheme 1.
Post-consumer PLA & reaction scale-up
The optimized catalytic systems were subsequently applied to the direct valorization of post-consumer PLA waste. As mentioned previously, depending on the application, additives are often present in the PLA product to achieve the desired physicochemical properties, e.g. to increase their thermal stability. Here, we valorized post-consumer PLA, containing approximately 70 wt% PLA and 30 wt% mineral filler (talc), as was determined by thermogravimetric analysis (TGA, Fig. S2†) and powder X-ray diffraction (PXRD, Fig. S3†). The waste PLA was subjected to depolymerization in the absence of a cocatalyst in order to maximize LAC yield, and in the presence of hexadecyl bromide (C16Br) or 2-bromopropanoic acid (2-BrPA) to obtain AA in high quantities (Fig. 2). In the ideal scenario, it would be beneficial to work with the Bu4PBr IL as the only solvent. This would simplify the system, allowing for efficient work-up through distillation of the desired products under reduced pressure (as the IL has no vapor pressure). Keeping in mind that for future applications it would be ideal to work in a continuous setup, the amount of sulfolane cosolvent was reduced drastically. In all three cases the cosolvent amount could be lowered by a factor 5 (Fig. 2). However, the CRM of PLA to LAC does require some cosolvent to ensure enough stability of the lactides and to avoid undesired ring opening to lactic/bromopropanoic acid. In the presence of the organobromide cocatalysts, on the other hand, a maximum of 42% AA yield is obtained directly from PLA waste, even in the absence of cosolvent. Similarly to previous observations on virgin PLA, high amounts of sulfolane tend to stabilize the lactide intermediates, while lactic acid should be considered as an undesired end product, only partially and slowly dehydrating to the desired AA (vide supra, Fig. S9†). In order to even further simplify the overall reaction mixture, the use of 2-BrPA is the most convenient as it will not lead to build-up of new side products when scaling up, since its dehydrobromination yields an additional acrylic acid. Vacuum distillation allows for the continuous extraction and isolation of AA, directly from the reaction matrix, in high purity (>95% selectivity, Fig. S5†). Unfortunately, this isolation technique was found too challenging for LAC due to its low vapor pressure and unstable nature in the absence of sulfolane. A potential alternative could be the continuous extraction with an apolar antisolvent, e.g. dodecane or toluene.24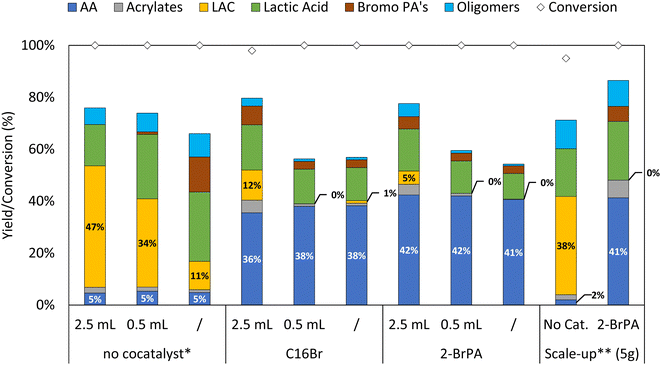 | ||
| Fig. 2 Post-consumer PLA depolymerization with different cosolvent amounts, including gram scale experiment. Reaction conditions: 0.5 mmol post-consumer PLA (70 wt%), 5 mol% cocatalyst, 3 equivalents Bu4PBr, 0–2.5 mL sulfolane, 3 h, 200 °C, Ar flushed. * Reaction in the absence of cocatalyst, for 2 h, 200 °C. ** Scale-up experiment (50 mmol PLA, 5.14 gram), 25 mL sulfolane (in the absence of cocatalyst), performed in a 100 mL flask. Product yield, determined via1H NMR of the crude reaction mixture, expressed relative to monomeric unit of PLA (selected time profiles can be found in Fig. S11†). | ||
Lastly, as the goal is to recycle plastic waste, the system should be able to run on a large scale. For this, the reaction was also performed in a batch setup on a 100-fold increased scale, converting over 5 g of polymer waste into new monomers. To our delight, this resulted in virtually identical results as those obtained on a smaller scale, with similar time profiles as for virgin PLA (Fig. S11†). In a first stage, the PLA is rapidly converted into lactide, followed by a subsequent rearrangement towards the acrylic acid reaching the highest acrylic acid yield of 41% after 3 h. On the other hand, in the absence of cocatalyst the lactide is slowly formed with a maximum yield of 38% after 2 h at 200 °C. Current efforts are devoted to expanding this unprecedented CRM technology of PLA recycling from a batch towards a continuous process at industrial scale.
Conclusion
In conclusion, we have developed a method to recycle post-consumer poly(lactic acid) (PLA) towards high value monomers by carefully balancing the reaction parameters. Depending on the applied conditions, it is possible to produce either acrylic acid (AA) or lactide (LAC) in 50% yield via a one pot conversion of PLA using a Bu4PBr ionic liquid as the active solvent, in the presence or absence of an acid cocatalyst, respectively. With this work we have uncovered an unprecedented way to perform chemical recycling of waste PLA directly back into the original monomer on a 5 gram scale. Future efforts on this technology should be dedicated to scaling up this technology from a batch setup to a high throughput, continuous process.Author contributions
K. J. and K. S. performed the PLA recycling experiments. K. J. and W. S. wrote the manuscript and performed the scale-up experiments. All authors helped in the conceptualization and critical reading of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
K. J. and D. D. V. are grateful to the FWO (G0D0518N and SBO project ‘Triple Cycle’) and Catalisti (MOT 2 program) for funding. We also thank Mr Galahad O' Rourke and Mr Vincent Lemmens for helping with the TGA and XRD measurements, respectively.References
- PlasticsEurope, Plastics – The Facts 2021. An analysis of European Plastics Production, Demand and Waste Data, https://plasticseurope.org/wp-content/uploads/2021/12/Plastics-the-Facts-2021-web-final.pdf Search PubMed.
- PlasticsEurope, The Circular Economy for Plastics. A European Overview, https://plasticseurope.org/wp-content/uploads/2022/06/PlasticsEurope-CircularityReport-<?pdb_no 2022?>2022<?pdb END?>_2804-Light.pdf Search PubMed.
- G. W. Coates and Y. D. Y. L. Getzler, Nat. Rev. Mater., 2020, 5, 501–516 CrossRef.
- B. Geyer, G. Lorenz and A. Kandelbauer, eXPRESS Polym. Lett., 2016, 10, 559–586 CrossRef CAS.
- M. N. Siddiqui, H. H. Redhwi, A. A. Al-Arfaj and D. S. Achilias, Sustainability, 2021, 13, 10528 CrossRef CAS.
- K. W. Meereboer, M. Misra and A. K. Mohanty, Green Chem., 2020, 22, 5519–5558 RSC.
- C. Jehanno, M. M. Pérez-Madrigal, J. Demarteau, H. Sardon and A. P. Dove, Polym. Chem., 2019, 10, 172–186 RSC.
- W. Stuyck, K. Janssens, M. Denayer, F. De Schouwer, R. Coeck, K. V. Bernaerts, J. Vekeman, F. De Proft and D. E. De Vos, Green Chem., 2022, 24, 6923–6930 RSC.
- H. Abe, N. Takahashi, K. J. Kim, M. Mochizuki and Y. Doi, Biomacromolecules, 2004, 5, 1606–1614 CrossRef CAS PubMed.
- H. Abe, N. Takahshi, K. J. Kim, M. Mochizuki and Y. Doi, Biomacromolecules, 2004, 5, 1480–1488 CrossRef CAS PubMed.
- K. J. Kim, Y. Doi and H. Abe, Polym. Degrad. Stab., 2008, 93, 776–785 CrossRef CAS.
- M. Hong and E. Y. X. Chen, Nat. Chem., 2016, 8, 42–49 CrossRef CAS PubMed.
- X. Tang and E. Y. X. Chen, Chem, 2019, 5, 284–312 CAS.
- V. Botvin, S. Karaseva, D. Salikova and M. Dusselier, Polym. Degrad. Stab., 2021, 183, 109427 CrossRef CAS.
- P. Majgaonkar, R. Hanich, F. Malz and R. Brüll, Chem. Eng. J., 2021, 423, 1–11 CrossRef.
- E. W. Dunn, J. R. Lamb, A. M. Lapointe and G. W. Coates, ACS Catal., 2016, 6, 8219–8223 CrossRef CAS.
- F. G. Terrade, J. van Krieken, B. J. V. Verkuijl and E. Bouwman, ChemSusChem, 2017, 10, 1904–1908 CrossRef CAS PubMed.
- C. Raith and M. Pazicky, US Pat., US2014018574A1, 2013 Search PubMed.
- E. V. Makshina, J. Canadell, J. van Krieken, E. Peeters, M. Dusselier and B. F. Sels, ChemCatChem, 2019, 11, 180–201 CrossRef CAS.
- , B. J. V. Verkuijl, et al., US Pat., US10570085B2, 2016 Search PubMed.
- M. Stalpaert, N. Peeters and D. De Vos, Catal. Sci. Technol., 2018, 8, 1468–1474 RSC.
- J. Kadar, N. Heene-Würl, S. Hahn, J. Nagengast, M. Kehrer, N. Taccardi, D. Collias, P. Dziezok, P. Wasserscheid and J. Albert, ACS Sustainable Chem. Eng., 2019, 7, 7140–7147 CrossRef CAS.
- J. Nagengast, S. Hahn, N. Taccardi, M. Kehrer, J. Kadar, D. Collias, P. Dziezok, P. Wasserscheid and J. Albert, ChemSusChem, 2018, 11, 2936–2943 CrossRef CAS PubMed.
- M. Stalpaert, K. Janssens, C. Marquez, M. Henrion, A. L. Bugaev, A. V. Soldatov and D. De Vos, ACS Catal., 2020, 10, 9401–9409 CrossRef CAS.
- K. Janssens, M. Stalpaert, M. Henrion and D. E. De Vos, Chem. Commun., 2021, 57, 6324–6327 RSC.
- S. J. de Jong, E. R. Arias, D. T. S. Rijkers, C. F. van Nostrum, J. J. Kettenes-van den Bosch and W. E. Hennink, Polymer, 2001, 42, 2795–2802 CrossRef CAS.
- P. McKeown and M. D. Jones, Sustainable Chem., 2020, 1, 1–22 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, catalytic reaction, product analysis, thermogravimetric analysis, powder X-ray diffraction and supplementary figures. See DOI: https://doi.org/10.1039/d2su00078d |
| This journal is © The Royal Society of Chemistry 2023 |
