Open Access Article
Open Access ArticleSolvent-free synthesis of chalcones using Mg(HSO4)2†
Ervis
Saraci
a,
Massimiliano
Andreoli
a,
Emanuele
Casali
 a,
Massimo
Verzini
b,
Maria
Argese
b,
Roberto
Fanelli
b and
Giuseppe
Zanoni
a,
Massimo
Verzini
b,
Maria
Argese
b,
Roberto
Fanelli
b and
Giuseppe
Zanoni
 *a
*a
aDepartment of Chemistry, University of Pavia, Viale Taramelli, Pavia, 12-27100, Italy. E-mail: gz@unipv.it
bFlamma S.p.A, Via Bedeschi, Chignolo D'isola, 22-24040, Italy
First published on 27th February 2023
Abstract
An innovative synthetic pathway for the synthesis of chalcones is proposed using the chemical and environmental advantages of solvent-free chemistry. The protocol was used for the synthesis of different medicinally relevant chalcones, as well as of a variety of naturally occurring chalcones, as a greener alternative to the existing methods. Finally, the solvent-free method was applied to the synthesis of bioactive drugs, such as metochalcone and elafibranor. We were also able to optimize the solid-state conditions by exploring a variety of mechanochemical conditions, including ball milling, mechanical stirring, and an ‘endless-screw’ system, which can be promising from an industrial perspective.
Sustainability spotlightThe Claisen–Schmidt aldol-like reaction was largely employed as a robust method of choice to forge chalcone moieties, which are endowed with ample pharmacological activities. However, the common use of highly corrosive and toxic NaOH places this reaction out of the green chemistry metrics. To pursue the 12 principles of green chemistry, a fusion of solvent-free synthesis and mechano-chemical processes has recently started to take hold, consistently reducing the environmental impact compared to solvent-based processes. NaOH is defined as a hazardous substance and the use of less hazardous reagents is well within the United Nations Sustainable Development Goals. The mechano-chemical process mediated by Mg(HSO4)2 is a technology that can achieve the production of chemicals through the elimination of hazardous substances, perfectly in line with UN SDG:12. |
Introduction
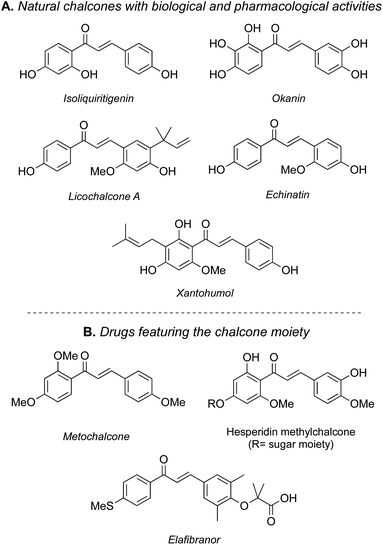
Chalcones consist of a subclass of the flavonoid family and are among many biological interesting compounds, consequently attracting research attention for decades.1 The principal sources of chalcone derivatives are plant extracts, which have been used traditionally to treat various diseases.2 Among all the bioactive properties, for example, many naturally occurring chalcones with anticancer efficacy against a variety of cancer cells have been found.3Numerous natural chalcones exist, and a great portion of them exhibit a wide range of biological activities, such as ioliquiritigenin, okanin, licochalcones a, xanthohumol, and echinatin (Fig. 1A). Moreover, several compounds have been either marketed or clinically tested, exemplifying their medical potential (Fig. 1B); for example, metochalcone has been marketed as a choleretic and diuretic drug, and hesperidin methylchalcone has a vascular protective effect. Elafibranor is an experimental medication that is being developed for the treatment of cardiometabolic diseases, including diabetes, insulin resistance, dyslipidemia, and non-alcoholic fatty liver disease (NAFLD). Elafibranor is a dual PPARα/δ agonist, and its metabolic effects have been evaluated in abdominally obese patients with either combined dyslipidemia or prediabetes. Unfortunately, it has so far been unable to have an impact in pivotal NASH trials, despite the encouraging data in earlier studies. However, a phase 3 trial is ongoing to determine its effects in primary biliary cholangitis (PBC), a chronic, autoimmune disease in which the bile ducts in the liver are gradually destroyed.
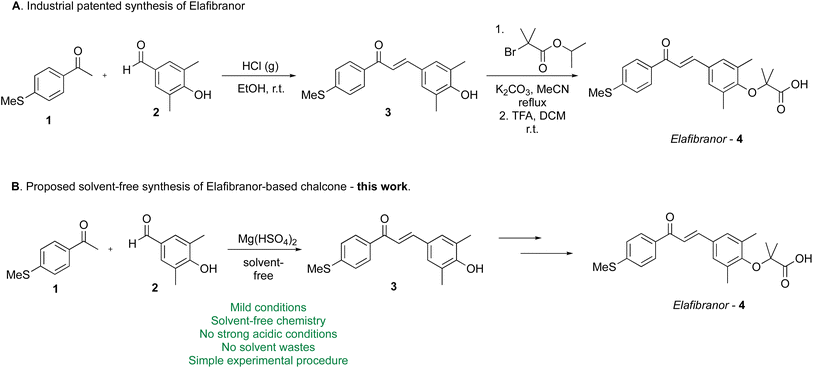
The chalcone core of elafibranor has been synthesized by using the venerable Claisen–Schmidt condensation under both acidic (HClg–EtOH)4a and basic catalysis (K2CO3)4b or by using NaOH4c (Scheme 1A).
The Claisen–Schmidt aldol-like reaction is recognized as a robust method of choice for forging the chalcone moiety and consequently, green, catalytic versions, including solvent-free protocols, have been developed and reviewed.5 To pursue the 12 principles of green chemistry,6 merging solvent-free syntheses and mechano-chemical processes can display improved green chemistry metrics and lower environmental impacts compared to solvent-based processes.7 There are several benefits related to solvent-free reactions, including a reduction in cost, energy consumption, reaction time, and pollution, as well as a simplification of the experimental procedures, work-up techniques, and labour savings.8 The first mechanochemical reactions were performed by grinding reactants together in a mortar with a pestle, an approach that is sometimes referred to as “grindstone chemistry”.9 While this technique does not require specialized equipment and is therefore feasible in any laboratory, it suffers from some limitations, especially reproducibility, as it is dependent on the physical strength of the operator. For this reason, during the last few years, mechanochemical synthesis using ball milling has become a practical and sustainable technique that enables solvent-free organic transformations.10
The advantages of mechanochemical approaches, i.e. the avoidance of potentially harmful organic solvents, shorter reaction times, and operational simplicity, have created a significant demand for their application in pharmaceutical synthesis.11 However, one of the main drawbacks of mechanochemical synthesis is the lack of ability to perform milling reactions in a continuous fashion. Indeed, continuous mechanochemistry is an advanced tool for the synthesis of organic compounds and other complex molecules. Currently, the most efficient way to perform “flow mechanochemistry” is using the screw extrusion reactor, which has been successfully employed for different organic reactions, including the Claisen–Schmidt chalcone synthesis.12 However, the use of highly corrosive and toxic NaOH is outside of the green chemistry metrics of chalcone and therefore, elafibranor synthesis.6b Herein, we report the first solvent-free general chalcones preparation method using a home-made screw reactor in the presence of a benign, readily-available and eco-friendly catalyst, such as magnesium hydrogen sulfate (vide infra). The versatility of the procedure was demonstrated by the preparation of the drug metochalcone, which has been approved for clinical use as a choleretic and diuretic agent, and a few natural occurring chalcones, such as isoliquiritigenin.
Mechanochemistry and catalyst optimization
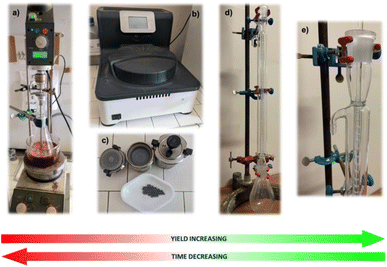
With the goal to develop a new synthesis procedure for elafibranor under green chemistry protocols, we optimized the first step of the synthetic sequence, namely, the assembling of the chalcone core via Claisen–Schmidt condensation under mechanochemical flow conditions. Catalyst optimization was initially carried out using solvent-free chemistry by systematically varying the catalyst, mole ratio, reaction time, and temperature (the relevant details are given in Table S1 in the ESI†). We found that mixing 4-methylthio acetophenone 1 (100 mol%) and aldehyde 2 (120 mol%) both as solids and Mg(HSO4)2 (200 mol%) under mechanical stirring at 200 rpm (Fig. 2a) at 50 °C for 30 minutes could afford the corresponding chalcone 3 as a brown solid with a 100% conversion. Mg(HSO4) was removed by performing a water–EtOAc extraction, in addition to the use of the organic phase of activated charcoal for removing the coloured impurities. The solid obtained by EtOAc evaporation was then crystalized using toluene to afford the desired chalcone 3 in an 82% isolated yield as a pale, yellow crystal. These experiments demonstrated that the mixing phase is crucial for full conversion of the reagents; therefore, with a suitable catalyst in hand, we then set our focus to the optimization of the mechanochemical device, namely by switching from a ball-mill system to a home-made screw reactor (Fig. 2a).We initially decided to explore the ball-mill mixing mode to take advantage of the impact and friction between the balls and the mixture of solid reagents. After exploring several experimental conditions, such as different configurations, and changing the powder-to-ball ratio, rotation speed, and heating times or ramps, a diminished conversion of 80% with decreased isolated yields of 60–75% of chalcone 3 were obtained.
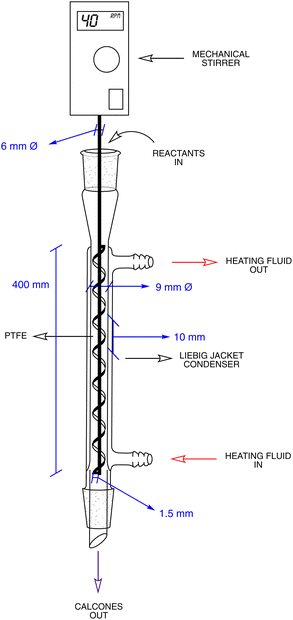
The inferior ball-mill yields led us to explore the performance of a home-made screw reactor as an alternative option (Fig. 2d, e and 3). This consisted of a jacketed single-screw reactor with a vertical alignment consisting of a Liebig condenser with a glass jacket with a 9 mm inner diameter and a 400 mm long glass rod 6 mm in diameter upon which a PTFE spiral screw with a 1.5 mm diameter and 10 mm of propeller pitch was wound. This leaves hardly any interstice between the jacket wall and the screw threads, thus maximizing the corresponding friction. The inlet and outlet ports of the jacket were connected to a constant temperature circulation heating bath.
In a typical experiment, 4-methylthio acetophenone 1 (100 mol%), aldehyde 2 (120 mol%), and Mg(HSO4)2 (200 mol%), were fed as a solid mixture and the conditions to maximize the yield were obtained by systematically varying the temperature, recycles, residence time, and screw rotation speed. Unfortunately, despite numerous efforts, the chemical conversion never exceeded 40% under the optimized conditions, i.e., temperature of 50 °C, screw rotation speed of 40 rpm (see Table S5 in the ESI†), residence time of 360 s. This was in stark contrast to what is reported in the literature for a Claisen–Schmidt reaction carried out in a screw reactor between 4-methyl benzaldehyde and acetophenone, in which the reaction was carried out in a home-made screw reactor and took place in 89 s with a yield of 92%.13 We ran the same reaction under our catalytic conditions and the corresponding a,b-unsaturated enone 5 was obtained in a 45% isolated yield with a conversion of 57% (Chart 1).
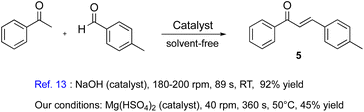 | ||
| Chart 1 Comparison between the catalytic performance of Mg(HSO4)2 and NaOH under screw reactor conditions. | ||
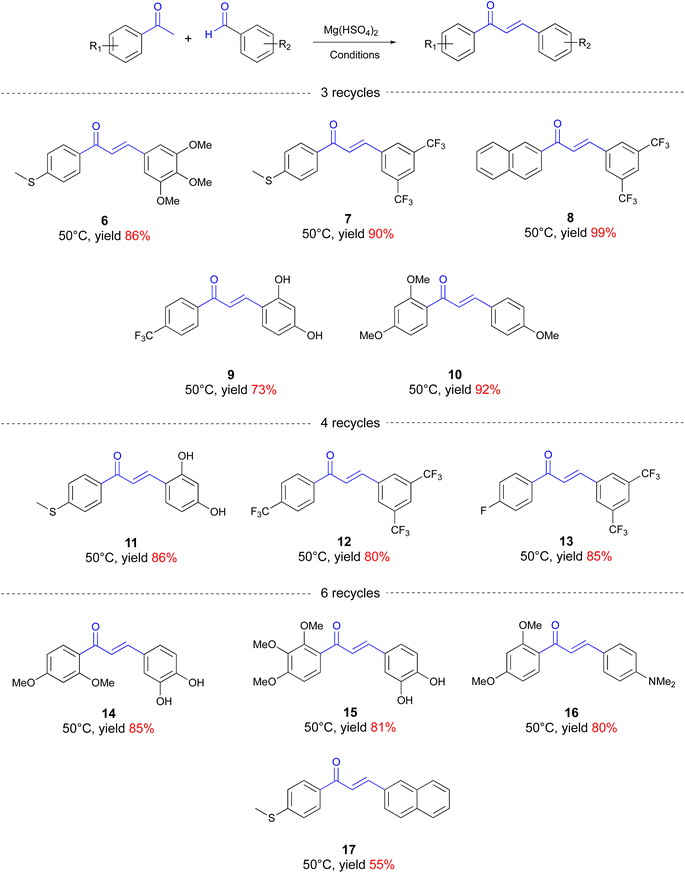
A plausible explanation for the difference could be in the catalyst used, which was NaOH for the literature reaction, but Mg(HSO4)2 in our case, with the latter, characterized by a lower catalytic efficiency. However, we discovered that when just resubjecting the whole mixture, without adding a new catalyst, and without purification, to three recycles, the conversion jumped to 100% with an isolated yield of 95% of chalcone 3 after a simple crystallization with hot toluene (vide infra). With the optimized procedure in hand, we next examined the reaction scope using Mg(HSO4)2 under our screw reactor conditions. All the reactions were performed on a 0.2 mmol scale, using 100 mol% equivalent of ketone, 120 mol% equivalent of aldehyde, and 200 mol% equivalent of magnesium catalyst (Chart 2). The procedure involved a premixing of the three components to form either a solid mixture or a paste, depending on whether the ketone or the aldehyde was solid or liquid. After the indicated number of recycles, the solid was generally crystallized from toluene (see ESI† for more details).
A variety of acetophenones and benzaldehydes were screened, with different substitution patterns in terms of steric effects and electronics. Overall, with our methodology, a variety of functional groups could be tolerated with different substitution patterns on both aldehydes and ketones, including thiomethyl, methoxy and trifluromethy groups, as well as free hydroxy groups or dimethylamino groups on the aldehydes. As expected, the effect of the substituents reverberated on the kinetics of the whole process, which could be divided into three classes of substrates: those that require 3 recycles to ensure 100% conversion (from 6 to 10), those that require 4 recycles (from 11 to 13), and those, the less reactive, that require up to 6 recycles (from 14 to 17). The yields are generally very high, from 99% for 8 to 55% for 17, the less reactive chalcone with an average yield of 83%. Interestingly, chalcone 10 was indeed the drug metochalcone (Fig. 1B), which was obtained under our solvent-free condition, with a 92% isolated yield after only 3 recycling procedures.
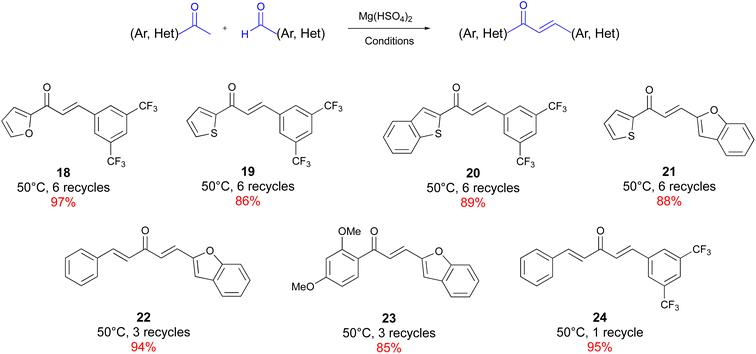
Heteroaromatic ketones and aldehydes, as well as α,β-unsaturated ketone, are sluggish substrates for this solvent-free Claisen–Schmidt condensation, affording, however, the corresponding chalcones in very good yields (Chart 3).
Heteroaromatic rings on the ketone moiety (18, 19, 20) required 6 recycling events to ensure complete conversion, while heteroaromatic aldehydes (23) displayed enhanced reactivity. As expected, the heteroaromatic reagents afforded chalcone 21 only after 7 recycles, but in a very good yield. Finally, (E)-4-phenyl-3-buten-2-one, condensed with both aromatic and a,b-unsaturated aldehydes, showed remarkable reactivity and yields (22, 24). A variety of medicinally relevant chalcones were obtained in a few hours, with a very simple protocol and with very cheap reagents. Unfortunately, some substrates react only reluctantly according to our protocol, including aliphatic ketones and aldehydes and some heteroaromatic aldehydes, such as thiophenyl carboxaldehyde and quinoline carboxaldehyde. Moreover, nitrogen-containing heterocycles are incompatible with the use of magnesium hydrogensulfate, due to the occurrence of an acid–base side reaction (see ESI† for more details).
Synthesis of naturally occurring chalcones and elafibranor
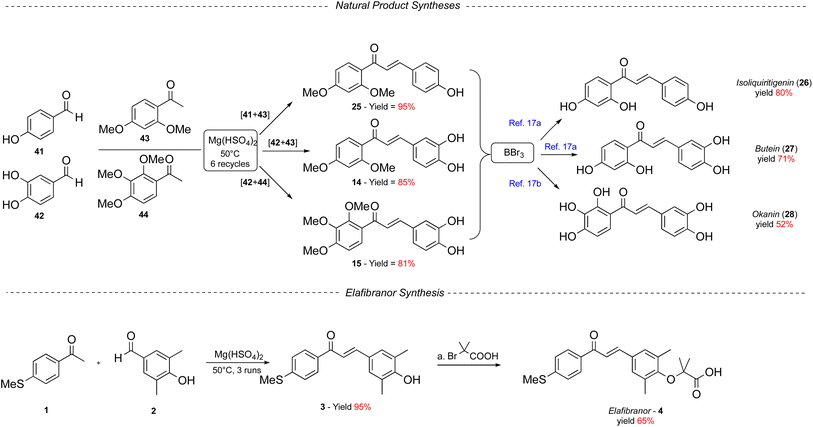
Finally, we attempted to use the magnesium hydrogensulfate solvent-free protocol for the synthesis of some naturally occurring chalcones, such as isoliquiritigenin,14 butein,15 and okanin,16 and we completed the synthesis of the antitumor drug elafibranor. Although the most logical way to perform the synthesis of the three natural products should be the reaction on the corresponding hydroxyl free ketones and aldehydes, this way proved to be unproductive. Therefore, we started with methoxy-protected ketones and hydroxyl unprotected aldehydes, all of which are commercially available (Scheme 2). In the event, aldehydes 41 and 42 reacted under our conditions, 50 °C after 3 recycles, in the presence of Mg(HSO4)2, with the methoxy-protected ketones 43 and 44, according to the combinations depicted in Scheme 2. The MeO-protected chalcones 25, 14, and 15 were thus obtained in gratifying 95%, 85%, and 81% isolated yields, respectively. BBr3 deprotection of the methoxyl ether of 25 and 15, following the literature reported procedure, provided the corresponding natural products isoliquiritigenin and okanin in 80% and 52% isolated yields.17 Finally, exposure of the protected chalcone 14 to BBr3 in DCM at −78 °C furnished butein in a 71% isolated yield.To conclude the exploration of our sustainable Claisen–Schmidt condensation, we turned our attention to the benchmark substrate chalcone 3 and completed the synthesis of elafibranor in just a single synthetic step (Scheme 2).
Conclusions
In conclusion, this is the first report where a simple single-screw reactor was engineered and successfully applied for the synthesis of a variety of chalcones, which represents a common chemical scaffold of many biologically active compounds isolated from natural sources. This privileged structure has attracted research attention for over a century.1 Up to 24 different chalcones were synthetized, in good to high yields. Notably, we were able to prepare three natural products, namely isoliquiritigenin, butein, and okanin, along with two APIs, namely metochalcone and elafibranor, in a solvent-free and sustainable fashion using Mg(HSO4)2 as a catalyst. The scope and limitations of this new protocol were established. The diversity of compounds synthetized with this device combined with the high sustainability scores and easy transposition to the multigram scale open the way for the future applicability of this home-made reactor and it could represent a useful tool that, alongside 3D printing technology, could alleviate the burden of disease in developing countries. In fact, self-sufficiency in the production of APIs could translate into the increase in the local production of APIs through enabling technologies, such as flow reactions and 3D printing.18 Further modifications of the reactor for a wider range of feasibilities, including multistep flow synthesis with an aim to complete the reaction in only one run, are currently under investigation.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank Regione Lombardia – project VIPCAT (Value Added Innovative Protocols for Catalytic Transformations – CUP: E46D17000110009) and the Italian MIUR (funds PRIN 2017) for financial support.Notes and references
- (a) X. Liu and M. L. Go, Bioorg. Med. Chem., 2006, 14, 153–163 CrossRef CAS PubMed; (b) A. Modzelewska, C. Pettit, G. Achanta, N. E. Davidson, P. Huang and S. R. Khan, Bioorg. Med. Chem., 2006, 14, 3491–3495 CrossRef CAS PubMed; (c) L. Ni, C. Q. Meng and J. A. Sikorski, Expert Opin. Ther. Pat., 2004, 14, 1669–1691 CrossRef CAS; (d) B. Zhou and C. Xing, Med. Chem., 2015, 5, 388–404 Search PubMed; (e) D. I. Batovska and I. T. Todorova, Curr. Clin. Pharmacol., 2010, 5, 1–29 CrossRef CAS PubMed; (f) P. Singh, A. Anand and V. Kumar, Eur. J. Med. Chem., 2014, 85, 758–777 CrossRef CAS PubMed; (g) C. Zhuang, W. Zhang, C. Sheng, W. Zhang, C. Xing and Z. Miao, Chem. Rev., 2017, 117(12), 7762–7810 CrossRef CAS PubMed.
- (a) D. Wang, J. Liang, J. Zhang, Y. Wang and X. Chai, J. Evidence-Based Complementary Altern. Med., 2020, 3821248 Search PubMed; (b) M.-R. Zhang, K. Jiang, J.-L. Yang and Y.-P. Shi, Nat. Prod. Lett., 2019, 34, 3335–3352 CrossRef PubMed; (c) L. Bai, X. Li, L. He, Y. Zheng, H. Lu, J. Li, L. Zhong, R. Tong, Z. Jiang, J. Shi and J. Li, Am. J. Chin. Med., 2019, 47, 933–957 CrossRef CAS PubMed; (d) L. M. R. Al Muqarrabun, N. Ahmat, S. A. S. Ruzaina, N. H. Ismail and I. Sahidin, J. Ethnopharmacol., 2013, 150, 395–420 CrossRef CAS PubMed; (e) M. Boozari, S. Soltani and M. Iranshahi, Phytother. Res., 2019, 33, 546–560 CrossRef CAS PubMed; (f) N. Tawfeek, M. F. Mahmoud, D. I. Hamdan, M. Sobeh, N. Farrag, M. Wink and A. M. El-Shazly, Front. Pharmacol., 2021, 12, 593856 CrossRef CAS PubMed.
- C. Karthikeyan, N. S. H. N. Moorthy, S. Ramasamy, U. Vanam, E. Manivannan, D. Karunagaran and P. Trivedi, Recent Pat. Anti-Cancer Drug Discovery, 2015, 10, 97–115 CrossRef CAS PubMed.
- (a) J. Najib and K. Caumont-Bertrand, WO Patent, 200400523, 2004 Search PubMed; (b) Q. Ren, L. Deng, Z. Zhou, X. Wang, L. Hu, R. Xie and Z. Li, Bioorg. Chem., 2020, 101, 103963 CrossRef CAS PubMed; (c) J.-F. Delhomel and K. Caumont-Bertrand, US Pat., 7566737B2, 2009 Search PubMed.
- (a) G. D. Yadav and D. P. Wagh, ChemistrySelect, 2020, 5, 9059–9085 CrossRef CAS; (b) S. Siddiqui, M. U. Khan and Z. N. Siddiqui, ACS Sustainable Chem. Eng., 2017, 5, 7932–7941 CrossRef CAS.
- (a) P. T. Anastas and J. C. Warner, Green Chemistry: Theory and Practice, Oxford University Press, New York, 1998, ISBN 9780198502340 Search PubMed; (b) NaOH Is defined as a hazardous substance and the use of less dangerous reagents is perfectly within the UN's Sustainable Development Goals. Mechanochemical process mediated by Mg(HSO4)2, is a technology capable to achieve production of chemical products through the elimination of hazardous substances (UN's SDG:12), https://sdgs.un.org/topics/chemicals-and-waste.
- (a) G.-W. Wang, Chem. Soc. Rev., 2013, 42, 7668–7700 RSC; (b) T. K. Achar, A. Bose and P. Mal, Beilstein J. Org. Chem., 2017, 13, 1907–1931 CrossRef CAS PubMed; (c) S. Hwang, S. Grätz and L. Borchardt, Chem. Commun., 2022, 58, 1661–1671 RSC; (d) M. Pérez-Venegas and E. Juaristi, ACS Sustainable Chem. Eng., 2020, 8(24), 8881–8893 CrossRef; (e) V. Štrukil, Synlett, 2018, 29, 1281–1288 CrossRef; (f) S. L. James, C. J. Adams, C. Bolm, D. Braga, P. Collier, T. Friščić, F. Grepioni, K. D. Harris, G. Hyett, W. Jones, A. Krebs, J. Mack, L. Maini, A. Guy Orpen, I. P. Parkin, W. C. Shearouse, J. W. Steed and D. C. Waddell, Chem. Soc. Rev., 2012, 41, 413–447 RSC; (g) M. B. Gawande, V. D. B. Bonifácio, R. Luque, P. S. Branco and R. S. Varma, ChemSusChem, 2014, 7, 24–44 CrossRef CAS PubMed; (h) J.-L. Do and T. Friščić, Synlett, 2017, 28, 2066–2092 CrossRef CAS.
- (a) R. Sheldon, Introduction to Green Chemistry, Organic Synthesis and Pharmaceuticals, in Green Chemistry in the Pharmaceutical Industry, Wiley-VCH Verlag GmbH & Co., 2010, ISBN 9783527629688 Search PubMed; (b) J. Mack and S. Muthukrishnan, Solvent-Free Synthesis, in Green Techniques for Organic Synthesis and Medicinal Chemistry, Wiley-VCH Verlag GmbH & Co., 2012, ISBN 9780470711828 Search PubMed; (c) K. Tanaka and F. Toda, Chem. Rev., 2000, 100(3), 1025–1074 CrossRef CAS PubMed; (d) M. A. P. Martins, C. P. Frizzo, D. N. Moreira, L. Buriol and P. Machado, Chem. Rev., 2009, 109(9), 4140–4182 CrossRef CAS PubMed; (e) C. G. Avila-Ortiz and E. Juaristi, Molecules, 2020, 25, 3579 CrossRef CAS PubMed; (f) M. Obst and B. König, Eur. J. Org. Chem., 2018, 31, 4213–4232 CrossRef.
- L. Takacs, Chem. Soc. Rev., 2013, 42, 7649–7659 RSC.
- (a) J.-L. Do and T. Friščić, ACS Cent. Sci., 2017, 3(1), 13–19 CrossRef CAS PubMed; (b) J. G. Hernández and C. Bolm, J. Org. Chem., 2017, 82(8), 4007–4019 CrossRef PubMed; (c) J. L. Howard, Q. Cao and D. L. Browne, Chem. Sci., 2018, 9, 3080–3094 RSC; (d) J. Andersen and J. Mack, Green Chem., 2018, 20, 1435–1443 RSC; (e) T. Friščić, C. Mottillo and H. M. Titi, Angew. Chem., Int. Ed., 2020, 59, 1018–1029 CrossRef PubMed; (f) E. Calcio Gaudino, G. Grillo, M. Manzoli, S. Tabasso, S. Maccagnan and G. Cravotto, Molecules, 2022, 27, 449 CrossRef CAS PubMed.
- D. Tan, L. Loots and T. Friščić, Chem. Commun., 2016, 52, 7760–7781 RSC.
- D. E. Crawford, C. K. G. Miskimmin, A. B. Albadarin, G. Walker and S. L. James, Green Chem., 2017, 19, 1507–1518 RSC.
- B. M. Sharma, R. S. Atapalkar and A. A. Kulkarni, Green Chem., 2019, 21, 5639–5646 RSC.
- F. Peng, Q. Du, C. Peng, N. Wang, H. Tang, X. Xie, J. Shen and J. Chen, Phytother. Res., 2015, 29, 969–977 CrossRef CAS PubMed.
- K. Roh, J.-H. Lee, H. Kang, K. W. Park, Y. Song, S. Lee and J.-M. Ku, Eur. J. Med. Chem., 2020, 197, 112280 CrossRef CAS PubMed.
- (a) Y. Hou, G. Li, J. Wang, Y. Pan, K. Jiao, J. Du, R. Chen, B. Wang and N. Li, Sci. Rep., 2017, 7, 45705 CrossRef CAS PubMed; (b) Y. Naito, C. Ushiroda, K. Mizushima, R. Inoue, Z. Yasukawa, A. Abe and T. Takagi, J. Clin. Biochem. Nutr., 2020, 67, 1–9 CrossRef.
- (a) C. Sulpizio, S. T. R. Müller, Q. Zhang, L. Brecker and A. Rompel, Monatsh. Chem., 2016, 147, 1871–1881 CrossRef CAS PubMed; (b) P.-Q. Huang, Y.-H. Huang, H. Geng and J.-L. Ye, Sci. Rep., 2016, 6, 28801 CrossRef PubMed.
- (a) C. R. Sagandira, S. Nqeketo, K. Mhlana, T. Sonti, S. Gaqa and P. Watts, React. Chem. Eng., 2022, 7, 214–244 RSC; (b) I. Seoane-Viaño, S. J. Trenfield, A. W. Basit and A. Goyanes, Adv. Drug Delivery Rev., 2021, 174, 553–575 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetical procedures for known compounds and products, summary tables of optimization reactions and spectral data of all new compounds including 1H, 13C{1H} NMR spectra. See DOI: https://doi.org/10.1039/d3su00003f |
| This journal is © The Royal Society of Chemistry 2023 |