Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Unlocking the holy grail of sustainable and scalable mesoporous silica using computational modelling
Tom
Stavert
 a,
Siddharth V.
Patwardhan
a,
Siddharth V.
Patwardhan
 b,
Robert
Pilling
b and
Miguel
Jorge
b,
Robert
Pilling
b and
Miguel
Jorge
 *a
*a
aDepartment of Chemical and Process Engineering, University of Strathclyde, 75 Montrose Street, Glasgow, G1 1XL, UK. E-mail: miguel.jorge@strath.ac.uk
bGreen Nanomaterials Research Group, Department of Chemical and Biological Engineering, The University of Sheffield, Mappin Street, Sheffield, S1 3JD, UK
First published on 17th February 2023
Abstract
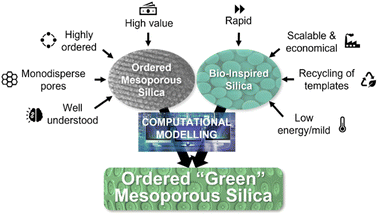
Bio-inspired methods offer a great alternative to design high-value mesoporous silica under more environmentally friendly conditions, allowing for an economical and sustainable scale-up. However, the synthesis of bio-inspired silica (BIS) is currently poorly understood, creating barriers to achieving products with comparable quality to traditional mesoporous silica. This perspective summarizes the key findings in the development of ordered mesoporous silica (OMS) and BIS synthesis, highlighting in particular the challenges faced in the development of scalable processing routes for these materials. Recent successes in improving mechanistic understanding of these syntheses using computational modelling are then presented, followed by suggestions as to how modelling may be used for predictive design of BIS with desired quality attributes. A multi-scale computational model, utilizing a combination of both ‘top-down’ and ‘bottom-up’ approaches, is argued to be critical for achieving a unified description of both BIS and OMS synthesis, allowing the potential of these materials to be fully realised.
Sustainability spotlightProducing ordered porous materials typically requires high temperature/pressure, extreme pH and harsh/toxic chemicals, and produces polluted water, thus making it highly energy and resource intensive and wasteful. Bioinspiration provides a potential solution by drawing inspiration from Nature to make the manufacturing process greener. However, this comes at a cost of less control over the ordered porous network. Achieving the best of both worlds, i.e. the ability to produce materials with ordered and controllable pore structure using the principles of green and sustainable chemistry, represents a “holy grail” in the field with the potential for transformative impact in materials discovery and manufacture. Due to their wide range of applications, this objective is aligned with the UN sustainable development goals of: clean water and sanitation (SDG 6), affordable and clean energy (SDG 7), industry, innovation and infrastructure (SDG 9), responsible consumption and production (SDG 12) and climate action (SDG 13). |
Introduction
Ordered mesoporous silica (OMS) was discovered in the 1990s by scientists at Mobil and in Japan.1–3 These materials possess well ordered, monodisperse pores on the meso-scale with a wide range of sizes and shapes. Due to the high degree of order and uniform size of their pores, OMS has incredible promise for applications such as gas separation, catalysis, drug delivery and sensors.4,5 Despite this, however, OMS is yet to see large scale commercial exploitation. This is due to the hydrothermal synthesis pathway employed for its synthesis, which is a long process involving several steps that make use of toxic or hazardous chemicals and harsh conditions, causing it to be unsustainable and uneconomic.6In order to circumvent these issues, more recent attempts to produce porous silica materials have taken on a bio-inspired approach.7,8 In nature we observe that organisms such as diatoms and sponges are capable of producing silica structures that exhibit order over hundreds of micrometers, which is rarely achieved even in synthetic conditions.9 These structures are formed at ambient temperature and neutral pH from dilute sources of silicic acid, implying that nature has found complex routes for promoting controlled silica formation (silicification) through evolution. From studying these organisms, researchers have been able to synthesise a diverse array of new silica structures with varying porosity, morphology and size under ambient conditions.9,10 However, despite these obvious advantages from an economic and environmental standpoint, some issues still face bio-inspired materials. Porous silica structures produced using bio-inspired additives typically possess amorphous pores with a broad pore size distribution, hampering their performance in comparison with OMS materials.
The main bottleneck to achieving ordered bio-inspired silica is a lack of detailed molecular-level understanding of the synthesis, and the role that these bio-inspired additives play. Computational modelling is an invaluable tool for improving our understanding of these complex interactions and has previously been used to investigate the synthesis of OMS.11 By applying this technique to probe the synthesis of BIS, we propose that a better understanding of the mechanisms that allow for controlled silicification under mild conditions can be achieved, allowing for more careful control of BIS morphology. Through this, a new class of ordered mesoporous “green” silica could be produced, combining the most desirable qualities of both OMS and BIS whilst permitting sustainable scale-up and commercial exploitation. This perspective provides a summary of recent developments in the areas of OMS and BIS. It then presents the recent progress made in computational models describing porous silica synthesis. Finally, we identify future directions for using computational tools to combine the desirable qualities of OMS and BIS.
Ordered mesoporous silica
Generally, mesoporous silicas are synthesised following the “hydrothermal” method. A surfactant (or other structure directing agent) is dissolved in a solvent (typically water) followed by a silicate precursor. Silicates and surfactant molecules interact cooperatively, self-assembling into an arrangement that mimics the structure of the final silica material (see Fig. 1). This mechanism is termed cooperative self-assembly and is accepted as by far the most common synthesis pathway for this class of materials.12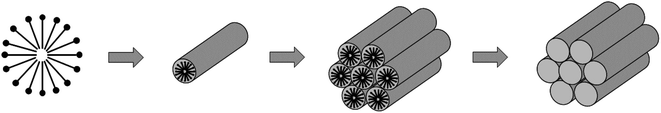 | ||
| Fig. 1 A simplified schematic of the templating mechanism that forms the ordered mesoporous material MCM-41. Initially, surfactant molecules form small micelles at the surfaces of which silica aggregates. These micelles fuse to form larger worm-like micelles and eventually a hexagonal mesostructure. Then the surfactant is removed (typically by calcination), leaving behind a porous silica structure. Based on Beck et al.1 | ||
MCM-41, the first ordered mesoporous silica material to be discovered, is produced using quaternary ammonium surfactants at high pH, forming silica with highly ordered hexagonal mesopores. The size of the pores can vary between 1.5–4 nm depending on the chain length of the surfactant used, and even larger pores of up to 10 nm were formed with the addition of an auxiliary organic, mesitylene.1 High surface areas of over 700 m2 g−1 were reported. Similar materials in this new M41S family such as MCM-48 and MCM-50 were discovered by studying the effect of changing the surfactant to silicate ratio.13 These materials also showed a high degree of order on the mesoscale, but exhibited different structures – MCM-48 has a cubic pore system and MCM-50 is lamellar. This shows that the meso-structure in MCM materials can be controlled predictably by varying surfactant concentration in accordance with the phase diagram of the surfactant used.
While cationic surfactants are extremely effective in promoting ordered mesostructures, they are expensive and toxic, prompting researchers to investigate alternative, non-ionic structure directing agents (SDAs). However, while non-ionic SDAs such as glycol ethers14 and block copolymers15 have successfully produced mesoporous silicas, extreme pH is still required to create an ordered structure. The need for charged surfactant species and extreme pH appears to result from the underlying intermolecular interactions taking place during the dynamic self-assembly of templating molecules. Attempts have been made to produce ordered silicate structures at neutral pH, and a “neutral templating route” was proposed to describe the formation of ordered hexagonal mesoporous silica, driven by hydrogen bonding interactions.16 However, evidence for this mechanism was largely indirect, and more recent experimental and simulation work has proposed that this does not in fact represent a viable description of the synthesis mechanism. Centi et al. used multi-scale molecular dynamics simulations together with experiments to show that this process is instead driven by the charge matching of ionic interactions, and that a significant proportion of silicates were in fact negatively charged.17,18
After the formation of the surfactant mesophase, the solution must undergo hydrothermal treatment to improve the mesoscopic regularity of the product and to allow for further polymerization and condensation of silicates. This process takes place at elevated temperatures (between 80 and 150 °C) which must be maintained for a long period of time, often multiple days or even weeks.12 This makes the synthesis process of OMS incredibly time-consuming. After hydrothermal treatment, the surfactant template must be removed in order to obtain the porous silica material. Calcination is the most common method employed for this, as it completely removes the template by thermal decomposition. This involves heating at very high temperatures (in the case of MCM-41, 550 °C1) for several hours in order to fully break down the surfactant molecules, destroying the valuable template in the process. This method is both energy intensive and wasteful but is necessary due to the strong surfactant-silica interactions that govern the initial self-assembly process, which makes template removal by other methods challenging. Alternative methods for template removal have been proposed, however each presents its own issues, frequently resulting in incomplete template removal, disordering of pores or increased energy intensity.19,20
Bio-inspired silica
From observing organisms such as diatoms and sea sponges, we see that nature has found ways of producing silica structures of incredible complexity and beauty under ambient conditions (room temperature, neutral pH). This presents a clear opportunity to gain a better understanding of silica formation under more sustainable conditions than those present in the synthesis of traditional OMS materials. Initially, investigations into silica synthesis following a bio-inspired pathway involved using bio-molecules thought to be responsible for silica formation in nature (such as in the cell walls of diatoms) outside of their natural environment (in vitro) in order to form porous structures. Two main classes of molecules were found to be tightly embedded within the cell walls of diatoms: proteins called silaffins, and long-chain polyamines.21 These molecules were shown to promote the formation of silica nanostructures in the presence of silicic acid. In silaffins, key chemical groups were identified, namely the presence of lysine amino acid residues and polyamine moieties. In addition, long-chain polyamines were found to be present on lysine residues, and in certain silaffins, sulphate ions and quaternary ammonium groups have also been observed. Long-chain polyamines have been shown to increase the rate at which silica structures form in water.22 Much like silaffins, polyamines can self-assemble in vitro to form silica nanospheres.21The insights gained from these in vitro studies allow for consideration of which fully synthetic molecules have potential to control porous silica formation. These molecules, termed additives, have been identified as a result of having similar chemical and physical properties to bio-molecules that promote precipitation of silica. Studies using a range of polymeric bio-inspired additives were carried out including natural and synthetic additives, block co-polymers, polypeptides and dendrimers.9 Examples include ethylamines, propylamines, amino acids (Lys and Arg) and their oligopeptides (e.g. (Lys)5), polyethyleneimines (PEI, linear, and branched), polyallylamine (PAA), and various versions of poly(alkylamino methacrylate) copolymers.21 These investigations produced a variety of porous silica materials with various structures and morphologies.23 In addition, bio-inspired additives allow for control over the rate of condensation and growth of silica structures, most notably allowing for silica structures to form under ambient conditions in just seconds. This offers an advantage over traditional mesoporous silica materials that require much harsher synthesis conditions and significantly longer synthesis times. Despite this, BIS synthesis suffers from a lack of control over the structure of the final product. Some examples exist of mesoporous silica syntheses that follow bio-inspired methods using arginine-based surfactants, and milder synthesis using polyethylene glycol or a range of surfactants,24–27 however the materials produced remained poorly ordered with broad pore size distributions, thus hampering their performance compared to OMS materials that possess well ordered, monodisperse pores (see Fig. 228). A better understanding of the self-assembly and formation mechanisms is required in order to induce ordered mesoporosity in BIS and make them a viable alternative to OMS materials for many applications.
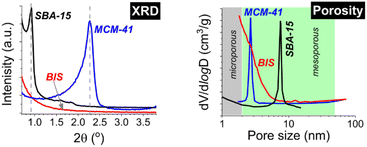 | ||
| Fig. 2 A comparison of the XRD and pore size distribution of bio-inspired silica with two archetypal ordered mesoporous silica materials, SBA-15 and MCM-41. Part of this figure is reproduced with permission from ACS Publications.28 | ||
Computational modelling of porous silica materials
The synthesis mechanism of mesoporous silica materials was originally postulated by comparing various synthesis conditions to the properties of the final product. The challenge in this approach is the complex nature of the synthesis process, which includes self-assembly, condensation reactions and phase separation all taking place simultaneously in solution.29 Computational models provide a more systematic way of describing these complex systems, giving us a clearer picture of the molecular-level interactions taking place. The first computational model of the synthesis of MCM-41 was developed in the early 2000s,30 and since then, significant advances have been made, allowing for a near complete description of this synthesis process, as well as gaining key insights that can be extrapolated to similar systems. This field was recently reviewed in detail by Jorge et al.,11 who highlighted how computational studies have contributed to the understanding of the synthesis of mesoporous silica materials, providing a better understanding of the crucial mechanism of surfactant/silica self-assembly that directly leads to a mesostructured material. One important conclusion that arose from that comprehensive review is the need to apply multi-scale computational modelling to understand these systems, since the synthesis process of mesoporous silica materials spans a wide range of time and length scales. Less consensual is the way in which this multi-scale aspect should be developed, with two distinct approaches to modelling these systems having been identified: ‘top-down’ using highly coarse-grained models fitted to a limited set of experimental data; and ‘bottom-up’, which starts from high levels of theory and builds towards progressively more simplified models.11 Below we highlight some selected and relevant examples of both of these approaches.Initial studies used “top-down” lattice Monte Carlo (MC) simulations with highly simplified intermolecular interactions to probe the phase diagrams of surfactant/solvent/silica systems that are present in the synthesis of MCM-41.30,31 Due to the simplicity of the model, only qualitative trends could be observed; however they were able to reproduce the experimentally observed phase behaviour of these systems. The model was subsequently extended to describe the phase equilibrium of periodic organosilica precursors32–35 and block copolymer-templated materials.36,37 Later, Jin et al. extended this model once again, allowing for better representation of the tetrahedral network structures present in mesoporous silica and, perhaps more importantly, allowing for silica condensation and simulation at high pH values.38 By simulating silica condensation reactions, this model was able to show that the structure of the mesophase is decided early in the synthesis. Silica condensation then “locks in” the structure, preventing further changes.
Investigations using off-lattice molecular dynamics studies were first published in 2007.39 Adopting a more “bottom-up” approach, these simulations used fully atomistic models calibrated against quantum mechanical calculations,40,41 but this limited the systems to small sizes and short times due to the computational burden of explicitly simulating all atoms. This model showed that silicates interacted more strongly with small micelles than with free surfactant monomers and also showed that silica promoted the formation of larger micelles than a reference simulation without silica, providing strong evidence for the cooperative templating pathway in OMS synthesis. Later, a simplified coarse-grained (CG) model based on the Martini 2.0 framework42 was developed,43 allowing simulations to access much larger systems and longer timescales. The model was developed by trying to reproduce both results from higher-level atomistic simulations and relevant experimental data, in what could be termed a “hybrid” between top-down and bottom-up strategies. This permitted the dynamic self-assembly of OMS at the mesoscale to be simulated, and showed that the presence of silica promoted the formation of long wormlike micelles at concentrations that would form only spherical micelles without silica present. The model was extended to include silicate oligomers44 and used to probe the phase diagram of the silica/surfactant/solvent system.45 These studies showed the importance of silica oligomers in promoting the formation of a hexagonal mesophase similar to that found in MCM-41 (Fig. 3) as well as elucidating the effect of silica charge density and benzene co-solvent addition on mesophase structure, all in qualitative agreement with experimental observations. However, this model did not explicitly include silica condensation, which is known to play an important role in OMS synthesis.
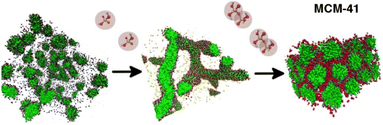 | ||
| Fig. 3 Visualisations of the coarse-grained computational model of Perez-Sanchez et al. which showed that some condensation of silicates must take place before a hexagonal mesophase can be formed. Adapted from Perez-Sanchez et al.41 | ||
Several models have been developed for describing the condensation reactions of silica using various methods including kinetic Monte Carlo (kMC), reactive ensemble MC, and Molecular Dynamics with reactive potentials.46–56 However, apart from the lattice-based study of Jin et al.38 discussed above, only one of these methods has been applied directly to templated OMS synthesis.57 Schumacher et al. developed a kMC model of silica condensation in the presence of surfactant micelles with the aim of producing realistic pore models for adsorption predictions, but because the micelles were pre-formed with a fixed cylindrical shape, few new fundamental insights into the synthesis mechanism were obtained. Recently, however, a new coarse-grained reactive silica model has been developed58 based on the MARTINI framework. This “Sticky MARTINI” model simulates the polycondensation of silicates by the association of “Virtual Sites” which are able to mimic the making and breaking of chemical bonds between silicates on-the-fly during MD simulations. As a proof-of-concept for the applicability of this new approach, the authors were able to simulate the self-assembly of a surfactant micelle in the presence of reacting silicates and the encapsulation of that micelle with a shell of condensed silica. This approach opens up huge opportunities to investigate systems relevant to porous silica synthesis at the mesoscale whilst explicitly taking into account the condensation reactions of silica species.
Computational modelling studies of bioinspired silica synthesis haven so far been much more limited. Some studies have examined the self-assembly of silaffin molecules without including silica,59 while other studies that included silica have focused on relatively simple amine-based surfactants without including the reactive features of the system.17,18,60 As discussed previously, these simulations have shown that the formation of mesostructures for both polyamine and alkylamine surfactants is driven by electrostatic interactions,17,18 rather than by weaker hydrogen bonding as originally postulated.16 This may explain why the degree of order of porous silica materials is lowered as we approach neutral pH, since the driving force for order decreases with the concentration of charged species in the system; however, more detailed studies are needed to verify this hypothesis.
Conclusions and outlook
OMS materials offer huge potential in a wide variety of applications, but their effectiveness is currently hampered by an environmentally damaging and uneconomic synthesis process. A bio-inspired approach for producing porous silica appears to fix many of these issues, however these materials possess disordered, amorphous pores hindering their performance in many applications. While OMS and BIS were developed independently, we recently identified unified mechanisms which govern all families of silicas.23 This means that the design rules and mechanisms from one family of silica can be transferred to design more complex materials. Despite this, the role that bio-inspired additives play is still poorly understood from a mechanistic perspective. A huge amount has been learned about OMS synthesis by using computational modelling, allowing for a nearly complete description of its synthesis, but little work has hitherto been carried out addressing BIS synthesis.There is currently no computational model that is capable of describing the self-assembly of the supramolecular template that governs the final structure of porous silica materials, whilst simultaneously describing silica polycondensation reactions. However, recent advances in computational modelling of silica self-assembly and condensation reactions now allow for this model to be fully realised. Such a model could provide a full description of the synthesis of not only traditional OMS materials, but also BIS. This powerful tool could greatly enhance our understanding of the underlying intermolecular interactions that allow these eco-friendly and adaptable materials to form, allowing for predictions to be made as to how ordered silica materials can be synthesized more economically and under environmentally friendly conditions. The introduction of a multi-scale reactive model into the manufacture and design of these important nanomaterials will signify a paradigm shift away from a trial-and-error based approach to a more rational, computational design strategy. In this way, a new class of ordered “green” mesoporous silica may be developed, combining the high degree of order of traditional OMS materials and the mild synthesis conditions of BIS (Fig. 4).
The development of a computational model that fully describes the synthesis of mesoporous silicates is a particular challenge due to the multitude of complex interactions that govern their formation, including supramolecular self-assembly, silica condensation reactions, phase transitions and nucleation. Although the computational studies discussed here and elsewhere11 have been able to provide insights into various aspects of porous silica synthesis, no study has been able to entirely probe the synthesis process. One important reason for this is due to the length and time scales relevant to silica synthesis, which prohibit classical atomistic models from fully exploring the dynamic templating mechanism. This necessitates using heavily simplified “coarse-grained” models to explore the synthesis mechanism of these materials, either working from a “top-down” approach as used in early lattice Monte Carlo models30 or the more recent “bottom-up” approach.43 Regardless of the approach taken, these “multi-scale” models can be challenging to develop, requiring thorough validation against high-level theory, experimental data or both. Furthermore, while the Sticky MARTINI reactive model offers great potential for achieving a full description of OMS synthesis with an unprecedented level of detail,58 applying it to simulations involving surfactant self-assembly is not straightforward as the rate of silica condensation must be carefully coupled to the kinetics of the system. Therefore, it is clear that novel and creative approaches must be taken in future attempts to model these materials.
Current models for OMS and BIS synthesis can be improved by adopting a hybrid approach, which combines advantages of ‘top-down’ and ‘bottom-up’ approaches. In this way a model is developed using high levels of theory, but can also reproduce key experimental data at different levels of resolution (e.g. micelle structure at the atomistic level, phase diagrams at the CG level). This approach has been utilized by Jorge et al. to develop a new atomistic force-field for organosilicon molecules which was parametrized and validated using experimental data in combination with theoretical calculations.61 If this methodology is extended to develop coarse-grained models for silica synthesis whilst maintaining “anchors” in both theory and experimental data, a reliable and widely applicable model can be obtained for investigating the synthesis mechanisms of not only porous silica, but templated materials in general.
While the computational modelling of BIS is still in its infancy, there is great potential to take the lessons learned from modelling OMS and apply this to a broad variety of BIS synthesis processes. The challenge here lies in both the diversity of systems following bio-inspired approaches and the lack of theoretical understanding of these processes versus traditional OMS synthesis routes. This necessitates the development of complex multi-component models with limited support from experiment and theory, adding to the challenge of these simulation approaches. It is clear, however, that addressing these challenges is imperative to fully understand the synthesis pathways which lead to high value porous materials that are sustainable, economical and scalable to manufacture.
As described, the hybrid approach is well suited to probing complex multi-scale, multi-component, reactive assembly systems. In doing so it transforms model development into an explicit juggling act, ensuring alignment and best use of its dual anchors. This in turn requires particular and careful thinking as to how best to bring specific experiments and computation together. Thus, unlocking the holy grail of scalable and sustainable mesoporous silica rests not only on model type and design but also on effective collaboration between experimentalists and modellers, which in turn demands good communication and shared understanding.
Finally, it is worth a brief consideration of the even wider research context, where model development and mechanistic elucidation sit alongside complementary drivers including new material discovery, lead optimisation, advanced analytics, characterisation methods and data–driven reaction space mapping. Articulating, designing, and pursuing research in this dynamic interdependent landscape in a strategic, rather than potluck fashion, can only serve to focus resources and accelerate success.
Author contributions
Tom Stavert: investigation, writing – original draft, visualization, Siddharth V. Patwardhan: conceptualization, resources, writing – review & editing, supervision, project administration, funding acquisition, Robert Pilling: conceptualization, writing – review & editing, Miguel Jorge: conceptualization, resources, writing – review & editing, supervision, project administration, funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
RP and SP thank funding from EPSRC grants (EP/P006892/1, EP/V051458/1, EP/R025983/1) to support this research. TS is grateful to EPSRC for a DTP studentship (EP/T517938/1, studentship ref. 2606787).Notes and references
- J. S. Beck, J. C. Vartuli, W. J. Roth, M. E. Leonowicz, C. T. Kresge, K. D. Schmitt, C. T. W. Chu, D. H. Olson, E. W. Sheppard, S. B. McCullen, J. B. Higgins and J. L. Schlenker, J. Am. Chem. Soc., 1992, 114, 10834–10843 CrossRef CAS.
- C. T. Kresge and W. J. Roth, Chem. Soc. Rev., 2013, 42, 3663–3670 RSC.
- S. Inagaki, Y. Fukushima and K. Kuroda, J. Chem. Soc., Chem. Commun., 1993, 680–682 RSC.
- J. G. Croissant, Y. Fatieiev, A. Almalik and N. M. Khashab, Adv. Healthcare Mater., 2018, 7, 1700831 CrossRef PubMed.
- P. Kumar and V. Guliants, Microporous Mesoporous Mater., 2010, 132, 1–14 CrossRef CAS.
- M. Vallet-Regí, M. Colilla, I. Izquierdo-Barba and M. Manzano, Molecules, 2018, 23, 47 CrossRef PubMed.
- S. V. Patwardhan and S. S. Staniland, Green Nanomaterials, IOP Publishing, 2019 Search PubMed.
- S. V. Patwardhan, J. R. H. Manning and M. Chiacchia, Curr. Opin. Green Sustainable Chem., 2018, 12, 110–116 CrossRef.
- S. V. Patwardhan, Chem. Commun., 2011, 47, 7567–7582 RSC.
- S. V. Patwardhan, S. J. Clarson and C. C. Perry, Chem. Commun., 2005, 1113–1121 RSC.
- M. Jorge, A. W. Milne, O. N. Sobek, A. Centi, G. Pérez-Sánchez and J. R. B. Gomes, Mol. Simul., 2018, 44, 435–452 CrossRef CAS.
- Y. Wan and D. Zhao, Chem. Rev., 2007, 107, 2821–2860 CrossRef CAS PubMed.
- J. C. Vartuli, K. D. Schmitt, C. T. Kresge, W. J. Roth, M. E. Leonowicz, S. B. McCullen, S. D. Hellring, J. S. Beck and J. L. Schlenker, Chem. Mater., 1994, 6, 2317–2326 CrossRef CAS.
- G. S. Attard, J. C. Glyde and C. G. Göltner, Nature, 1995, 378, 366–368 CrossRef CAS.
- D. Zhao, Q. Huo, J. Feng, B. F. Chmelka and G. D. Stucky, J. Am. Chem. Soc., 1998, 120, 6024–6036 CrossRef CAS.
- P. T. Tanev and T. J. Pinnavaia, Science, 1995, 267, 865–867 CrossRef CAS PubMed.
- A. Centi and M. Jorge, Langmuir, 2016, 32, 7228–7240 CrossRef CAS PubMed.
- A. Centi, J. R. H. Manning, V. Srivastava, S. van Meurs, S. V. Patwardhan and M. Jorge, Mater. Horiz., 2019, 6, 1027–1033 RSC.
- C. Gérardin, J. Reboul, M. Bonne and B. Lebeau, Chem. Soc. Rev., 2013, 42, 4217–4255 RSC.
- J. R. H. Manning, T. W. S. Yip, A. Centi, M. Jorge and S. V. Patwardhan, ChemSusChem, 2017, 10, 1683–1691 CrossRef CAS PubMed.
- S. V. Patwardhan and S. S. Staniland, Green Nanotechnol., 2019, 9 Search PubMed.
- T. Mizutani, H. Nagase, N. Fujiwara and H. Ogoshi, Bull. Chem. Soc. Jpn., 1998, 71, 2017–2022 CrossRef CAS.
- J. R. H. Manning, C. Brambila and S. V. Patwardhan, Mol. Syst. Des. Eng., 2021, 6, 170–196 RSC.
- T. Coradin, C. Roux and J. Livage, J. Mater. Chem., 2002, 12, 1242–1244 RSC.
- Q. Sun, T. P. M. Beelen, R. A. van Santen, S. Hazelaar, E. G. Vrieling and W. W. C. Gieskes, J. Phys. Chem. B, 2002, 106, 11539–11548 CrossRef CAS.
- J. Li, L. Xu, B. Yang, Z. Bao, W. Pan and S. Li, Mater. Sci. Eng., C, 2015, 55, 367–372 CrossRef CAS PubMed.
- O. V. Gorbunova, O. N. Baklanova and T. I. Gulyaeva, Microporous Mesoporous Mater., 2020, 307, 110468 CrossRef CAS.
- A. M. Ewlad-Ahmed, M. A. Morris, S. V. Patwardhan and L. T. Gibson, Environ. Sci. Technol., 2012, 46, 13354–13360 CrossRef CAS PubMed.
- S. M. Auerbach, W. Fan and P. A. Monson, Int. Rev. Phys. Chem., 2015, 34, 35–70 Search PubMed.
- F. R. Siperstein and K. E. Gubbins, Mol. Simul., 2001, 27, 339–352 CrossRef CAS.
- F. R. Siperstein and K. E. Gubbins, Langmuir, 2003, 19, 2049–2057 CrossRef CAS.
- A. Patti, A. D. Mackie and F. R. Siperstein, Langmuir, 2007, 23, 6771–6780 CrossRef CAS PubMed.
- A. Patti, F. R. Siperstein and A. D. Mackie, J. Phys. Chem. C, 2007, 111, 16035–16044 CrossRef CAS.
- A. Patti, A. D. Mackie and F. R. Siperstein, J. Mater. Chem., 2009, 19, 7848–7855 RSC.
- A. Patti, A. D. Mackie, V. Zelenak and F. R. Siperstein, J. Mater. Chem., 2009, 19, 724–732 RSC.
- S. Bhattacharya and K. E. Gubbins, J. Chem. Phys., 2005, 123, 134907 CrossRef PubMed.
- S. Bhattacharya, B. Coasne, F. R. Hung and K. E. Gubbins, Langmuir, 2009, 25, 5802–5813 CrossRef CAS PubMed.
- L. Jin, S. M. Auerbach and P. A. Monson, Langmuir, 2013, 29, 766–780 CrossRef CAS PubMed.
- M. Jorge, J. R. B. Gomes, M. N. D. S. Cordeiro and N. A. Seaton, J. Am. Chem. Soc., 2007, 129, 15414–15415 CrossRef CAS PubMed.
- J. R. B. Gomes, M. N. D. S. Cordeiro and M. Jorge, Geochim. Cosmochim. Acta, 2008, 72, 4421–4439 CrossRef CAS.
- M. Jorge, J. R. B. Gomes, M. N. D. S. Cordeiro and N. A. Seaton, J. Phys. Chem. B, 2009, 113, 708–718 CrossRef CAS PubMed.
- S. J. Marrink, H. J. Risselada, S. Yefimov, D. P. Tieleman and A. H. de Vries, J. Phys. Chem. B, 2007, 111, 7812–7824 CrossRef CAS PubMed.
- G. Pérez-Sánchez, J. R. B. Gomes and M. Jorge, Langmuir, 2013, 29, 2387–2396 CrossRef PubMed.
- G. Pérez-Sánchez, S.-C. Chien, J. R. B. Gomes, M. N. D. S. Cordeiro, S. M. Auerbach, P. A. Monson and M. Jorge, Chem. Mater., 2016, 28, 2715–2727 CrossRef.
- S.-C. Chien, G. Pérez-Sánchez, J. R. B. Gomes, M. N. D. S. Cordeiro, M. Jorge, S. M. Auerbach and P. A. Monson, J. Phys. Chem. C, 2017, 121, 4564–4575 CrossRef CAS.
- B. P. Feuston and S. H. Garofalini, J. Chem. Phys., 1988, 89, 5818–5824 CrossRef CAS.
- G. J. McIntosh, Phys. Chem. Chem. Phys., 2013, 15, 17496–17509 RSC.
- X.-Q. Zhang, T. T. Trinh, R. A. van Santen and A. P. J. Jansen, J. Phys. Chem. C, 2011, 115, 9561–9567 CrossRef CAS.
- X.-Q. Zhang, R. A. van Santen and A. P. J. Jansen, Phys. Chem. Chem. Phys., 2012, 14, 11969–11973 RSC.
- M. G. Wu and M. W. Deem, J. Chem. Phys., 2002, 116, 2125–2137 CrossRef CAS.
- Z. Jing, L. Xin and H. Sun, Phys. Chem. Chem. Phys., 2015, 17, 25421–25428 RSC.
- N. Z. Rao and L. D. Gelb, J. Phys. Chem. B, 2004, 108, 12418–12428 CrossRef CAS.
- A. Malani, S. M. Auerbach and P. A. Monson, J. Phys. Chem. Lett., 2010, 1, 3219–3224 CrossRef CAS.
- A. Malani, S. M. Auerbach and P. A. Monson, J. Phys. Chem. C, 2011, 115, 15988–16000 CrossRef CAS.
- V. M. Burlakov, G. A. D. Briggs, A. P. Sutton and Y. Tsukahara, Phys. Rev. Lett., 2001, 86, 3052–3055 CrossRef CAS PubMed.
- Y. Tu and J. Tersoff, Phys. Rev. Lett., 2000, 84, 4393–4396 CrossRef CAS PubMed.
- C. Schumacher, J. Gonzalez, P. A. Wright and N. A. Seaton, J. Phys. Chem. B, 2006, 110, 319–333 CrossRef CAS PubMed.
- A. P. Carvalho, S. M. Santos, G. Pérez-Sánchez, J. D. Gouveia, J. R. B. Gomes and M. Jorge, npj Comput. Mater., 2022, 8, 1–13 CrossRef.
- L. Lenoci and P. J. Camp, J. Am. Chem. Soc., 2006, 128, 10111–10117 CrossRef CAS PubMed.
- H. Eckert, M. Montagna, A. Dianat, R. Gutierrez, M. Bobeth and G. Cuniberti, BMC Materials, 2020, 2, 6 CrossRef.
- M. Jorge, A. W. Milne, M. C. Barrera and J. R. B. Gomes, ACS Phys. Chem. Au, 2021, 1, 54–69 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2023 |