Open Access Article
Open Access ArticleMechanocatalytic depolymerization of hemicellulose to low molecular weight oligosaccharides over an aquivion ionomer†
Jonathan Fabian
Sierra Cantor
a,
Karine
De Oliveira Vigier
 *a,
Gilles
Labat
b,
Denilson
Da Silva Perez
c and
François
Jérôme
*a,
Gilles
Labat
b,
Denilson
Da Silva Perez
c and
François
Jérôme
 *a
*a
aInstitut de Chimie des Milieux et Matériaux de Poitiers, Université de Poitiers, CNRS, 1 rue Marcel Doré, Poitiers 86073, France. E-mail: francois.jerome@univ-poitiers.ft
bFCBA, Allée de Boutaut, Bordeaux 33028, France
cCEA-Liten, 17 Av. des Martyrs, 38000 Grenoble, France
First published on 8th March 2023
Abstract
Here we report the mechanocatalytic depolymerization of hemicelluloses to low molecular weight oligosaccharides. We found that the catalyst performances were severely impacted by the water content and the residual amount of alkaline-earth/alkaline metals present in hemicellulosic samples. Under optimized conditions, water soluble oligosaccharides with DPs lower than 14 can be selectively obtained with up to 70% yield. Oligosaccharides can then be functionalized through a catalytic reductive amination, giving access to industrially relevant biobased chemicals such as hydrotropes and surfactants.
Sustainability spotlightThe growing demand for health and wellness products has boosted the research on the synthesis of oligosaccharides. With the necessity to defossilize our society, these oligosaccharides are also becoming industrially relevant building blocks for the synthesis of biobased chemicals, with applications in various end-industries. This public–private work provides the following advantages (1) biobased chemicals, (2) solvent free reactions, (3) high selectivity and (4) valorization of paper mill waste. Although this is obviously not sufficient to claim this work as sustainable, at least this work aligns with UN SDG 3 (good health and wellbeing), SDG 8 (decent work and economic growth), SDG 9 (industry, innovation, and infrastructure), SDG 12 (responsible consumption and production) and SDG 17 (parnerships for the goals). |
Oligosaccharides are an important class of compounds in our society, in particular for food and beverage applications, the largest market share of oligosaccharides.1 In this field, oligosaccharides serve as prebiotics for gut health improvement, as low calorie ingredients for patients affected by diabetes, blood pressure, intolerance to lactose, etc.2 With increasing concerns about the defossilization of our society, bio-based oligosaccharides are also rapidly becoming industrially relevant building blocks for the synthesis of specialty chemicals such as surfactants, bactericides, and thickening agents, among others, which find a wide range of applications in cosmetic, pharmaceutical and agricultural end-industries.3 The growing demand of consumers for health and wellness products, recently largely boosted by the covid pandemic, forces the chemical industry to diversify access routes to oligosaccharides.
Currently, oligosaccharides are obtained by two main routes (1) by extraction from natural resources4 and (2) by chemical5 or enzymatic synthesis.6 So far, the enzymatic route is receiving the largest interest as it potentially paves the way for a wide range of oligosaccharides with well-defined structures and properties. However, the implementation of these enzymatic processes in the chemical industry remains a difficult task due to the high dilution ratio (<5 wt% sugars in water), complex and costly downstream purification processes and low enzyme stability. All these drawbacks severely impact reactor productivities and production costs that are often not compatible with the market demand. As a result, these bioprocesses nowadays mainly address niche markets, for instance in the pharmaceutical industry where high value-added oligosaccharides, exhibiting well-defined molecular structures, are demanded.7 Alternatively, oligosaccharides can be obtained by acid-catalyzed depolymerization of polysaccharides, albeit generally at low conversions.8 Indeed, these acid-based processes unfortunately often suffer from a lack of selectivity, in particular due to the uncontrolled degradation of sugars to furanic derivatives and tar-like materials (also known as humins). Other technologies have been also explored, in particular for the production of xylo-oligosaccharides, such as autohydrolysis9 or CO2-assisted technologies (in situ acidification).10 By adjusting the temperatures and/or the pressures, it was possible to get a better control on the reaction selectivity, in particular by lowering the side formation of furanic derivatives.
Recently, we, and others, explored the mechanocatalytic depolymerization of cellulose, a concept initially introduced by Blair and Rinaldi.11 This approach relies on a synergistic effect between an acid catalyst (protonation of the glycosidic bond) and mechanical forces (lowering of exo-anomeric effects and H-bonding interactions, and change in the cellulosic chain conformation) resulting in a solvent-free depolymerization of cellulose.12 In the solid state, re-polymerization reactions also concomitantly occurred. As a result, this technology yields water soluble cello-oligosaccharides with degrees of polymerization (DP) ranging from 1 to 7.13 The absence of water/solvent and, according to the conditions, the high selectivity (no degradation of sugars and no formation of enzyme inhibitors) represent the greatest advantages of this mechanocatalytic process over current routes. Although there are now several reports on the mechanocatalytic depolymerization of cellulose, much less is known about the mechanocatalytic depolymerization of hemicelluloses, which represent an abundant and renewable class of feedstocks, which is poorly valorized nowadays. Indeed, a large part of hemicelluloses is generally solubilized in cooking liquors for pulping and is only used for energy purposes.14 The other part of hemicelluloses is associated with cellulose in paper fibres. Due to the more diversified chemical structures of hemicelluloses as compared to that of cellulose, a large library of oligosaccharides with different properties, and thus performances/applications, could be theoretically produced from them.15 To the best of our knowledge, during the writing of this study, Zhu reported the first example of mechanocatalytic depolymerization of hemicelluloses extracted from corncob.16 Using a mathematical prediction model based on Kamlet–Taft parameters, the authors revealed that dimethyl carbonate led the best impregnation procedure of H2SO4 on hemicelluloses, a pre-requisite step before mechanocatalytic depolymerisation. Under optimized conditions, xylo-oligosaccharides (XOS) with a DP lower than 6 were reported with a yield of only ∼10%. Indeed, with this procedure, XOS with DPs higher than 6 were mainly obtained. In addition, utilization of H2SO4 requires a post neutralization step, but also often makes the control of the reaction selectivity difficult due to the side formation of furfural and other degradation products, which are formed in different amounts according to the reaction conditions and the source of biomass.
Here, we report the mechanocatalytic depolymerization of hemicelluloses to oligosaccharides with a DP 1–9 (70% yield) using Aquivion PW98, a perfluorinated sulfonic acid ionomer, as a solid acid catalyst. As compared to the current state-of-the-art processes, we show in this report that the efficiency of the mechanocatalytic process is strongly impacted by the water content and the presence of alkaline-earth/alkaline metals naturally present in hemicelluloses. In addition, we show that the as-obtained XOS could be subjected to a catalytic reductive amination, potentially opening a route to industrially relevant biobased hydrotropes or surfactants.
First, we focused our investigations on hemicelluloses extracted from pine wood, hereafter named Pine-HEM. Pine-HEM was selected as it is quite reactive and thus constitutes an ideal substrate to get through the best conditions of the mechanocatalytic process. A more robust and industrially relevant hemicellulose extracted from pulp will be discussed later. Pine-HEM was extracted by treatment of pine wood in a pressurised reactor with hot water (160 °C) for 45 min.17 The final pH of the obtained aqueous solution was pH = 3.5, due to the cleavage of acetyl groups present in biomass. At the end of this heat treatment, the solid residue was removed and the aqueous juice containing Pine-HEM was re-concentrated by partial distillation of water, and then re-filtered, and activated carbon was added in order to adsorb lignin residues. After removal of the activated carbon by filtration, ethanol was added to the aqueous phase leading to the precipitation of Pine-HEM. The precipitated Pine-HEM was then recovered by centrifugation and thoroughly washed with ethanol. Finally, Pine-HEM was dried at 45 °C until the weight of Pine-HEM remains constant. This protocol led to Pine-HEM with a sugar composition as follows: mannan (41%), galactan (32%), glucan (15%) and xylan (12%). The water content, determined by thermogravimetric analysis (TGA), was ∼9.7% (Fig. S1†). Note that this process leaves a solid residue composed of cellulose (specialty cellulose) which is recovered for the fabrication of carboxymethylcellulose, cellulose acetate/esters or viscose, and lignin which is burned to provide energy for the process.
The mechanocatalytic depolymerisation of Pine-HEM was first investigated using optimized conditions we reported for cellulose.13a To this end, Pine-HEM was first impregnated with 6.6 wt% of H2SO4 and then ball-milled at 400 rpm in the presence of ZrO2 balls (see the ESI† for more details on the experimental procedure). The results are presented in Fig. 1. Yields of oligosaccharides were determined by SEC-HPLC and were expressed in terms of mass yield (wt%), i.e. relative to the initial mass of Pine-HEM (ESI†). After 1 h of ball-milling, oligosaccharides with a DP of 1–9 were obtained with 13% yield, confirming that Pine-HEM could be partly depolymerized using the mechanocatalytic concept. Extending the ball-milling time from 1 to 12 h led to an improvement of the oligosaccharide (DP 1–9) yields to 51%, with mono/disaccharides accounting for 6% yield (Fig. 1). A reaction time higher than 12 h did not result in a higher yield in oligosaccharides with DP < 9, but the DP 1–2 fraction was formed in a higher amount (18%) (Fig. 1). Lowering the amount of H2SO4 to 2 wt% decreased the depolymerization rate (<3% of a DP of 1–9 after 1 h vs. 13% at 6.6 wt%), confirming that the depolymerization rate of Pine-HEM was dependent on the amount of H2SO4 (Fig. 1). In contrast, increasing the amount of H2SO4 to 10 wt% led to uncontrolled degradation reactions, with the formation of unidentified soluble and insoluble black materials (Fig. S2†).
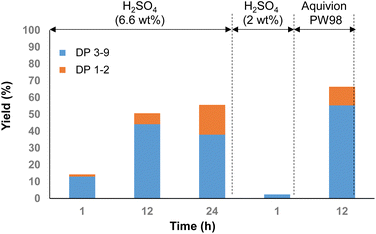 | ||
| Fig. 1 Mechanocatalytic depolymerization of Pine-HEM as a function of time, H2SO4 amount and catalyst nature. | ||
Because it was quite difficult to (i) separate H2SO4 from oligosaccharides at the end of the reaction and (ii) completely suppress the formation of coloured degradation products, Aquivion PW98 (PW = powder form, and 98 refers to the proton loading), a perfluorinated sulfonic acid polymer (0.98 mmol –SO3H g−1), was tested as a solid catalyst. We selected Aquivion PW98 because it was previously reported to be mechanically stable.11g,13a In the first set of experiments, 1.5 g of Pine-HEM was mixed with 1.5 g of Aquivion PW98 (∼15 mol% of H+ relative to glycosidic bonds) and the resulting mixture was ball-milled in a 125 mL bowl for 12 h. Under these conditions, oligosaccharides with a DP lower than 9 were successfully obtained with 66% yield. Oligosaccharides with a DP of 3–9 accounted for 55% while mono/disaccharides accounted for 11% (Fig. 1).
With Pine-HEM being fully soluble in water, the separation of oligosaccharides with low DPs from the reaction mixture was achieved by extraction with an ethanol–H2O mixture (9–1). It was previously shown that this procedure selectively solubilized Pine-HEM-derived oligosaccharides with DPs lower than 14.18 Using this procedure, oligosaccharides were recovered with 61% yield corresponding to 19% of oligosaccharides with a DP of 10–14 and 42% of oligosaccharides with DPs < 9 (Table 1, entry 1). In these 42% of oligosaccharides with a DP < 9, analysis by SEC-HPLC revealed 32% of oligosaccharides with a DP 3–9 and 10% of mono- and disaccharides. It corresponds to an extraction efficiency of 64% of oligosaccharides with a DP < 14. As observed above with H2SO4, extending the reaction time to 24 h did not increase the yield of the recovered oligosaccharides after extraction with ethanol–H2O (9–1), or the composition of solubilized oligosaccharides (Table 1, entry 2). This can be understood in terms of catalyst deactivation, an aspect which is discussed, and solved, later in the manuscript.
| Entry | Hemicellulose water content (wt%) | Catalyst | Time (h) | Yield of oligosaccharides soluble in ethanol–H2O (9–1)a | DP 10–14 (wt%) | DP ≤ 9 (wt%) | |
|---|---|---|---|---|---|---|---|
| DP 3–9 | DP 1–2 | ||||||
| a Mass yield of oligosaccharides solubilized in a EtOH–H20 (9–1) mixture. The rest is insoluble oligosaccharides with a DP > 14. b At 20 wt% of Aquivion PW98. | |||||||
| 1 | 9.7 | Aquivion PW98 | 12 | 61 | 19 | 32 | 10 |
| 2 | 9.7 | Aquivion PW98 | 24 | 61 | 17 | 34 | 10 |
| 3 | 4.5 | Aquivion PW98 | 24 | 73 | 28 | 32 | 13 |
| 4 | 3.5 | Aquivion PW98 | 24 | 76 | 26 | 36 | 14 |
| 5b | 9.7 | Aquivion PW98 | 24 | 18 | 9 | 5 | 4 |
Pine-HEM, and even lower molecular weight oligosaccharides, are highly hygroscopic and, from previous investigations on cellulose, it is known that water buffers mechanical forces,13a thus lowering the overall efficiency of the mechanocatalytic depolymerization process. To assess the effect of water, Pine-HEM was dried in an oven at 50 °C for different times before ball-milling in the presence of Aquivion PW98 for 24 h. The water content of dried Pine-HEM was determined by TGA. When the water content of Pine-HEM was gradually decreased from ∼9.7 to ∼3.5 wt%, the yield of ethanol–H2O (9–1) soluble oligosaccharides was concomitantly improved from 61 to 76% (Table 1, entries 2–4). As a result, the yields of recovered oligosaccharides with DPs 10–14 and ≤ 9 were also increased from 17 to 28% and 44 to 50%, respectively. Note that decreasing the water content of Pine-HEM below 3.5 wt% before the mechanocatalytic reaction was not judicious as it led to a partial degradation of Pine-HEM, as corroborated by a slight browning of the hemicellulosic sample during the drying process.
Next, we assessed the reactor productivity as a function of the mass of Pine-HEM/Aquivion PW98 introduced into the bowl (125 mL) (Fig. 2). In these experiments, the ball-milling time was stopped at 6 h in order to monitor the reaction during the whole day. The amount of ZrO2 balls introduced into the planetary ball-mill remains constant: 20 balls (φ 12 mm) in a 125 mL bowl. With 3 g of Pine-HEM/Aquivion PW98 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the reactor productivity reached 860 g of oligosaccharides with a DP ≤ 9 per m3 and per hour. Decreasing the mass introduced into the planetary ball-mill from 3 to 1.5 g logically decreased the reactor productivity by nearly a factor 2 (490 g m−3 h−1), indicating that in this 1.5–3 g window, the efficiency of the mechanocatalytic process is not affected by the mass of the sample introduced into the planetary ball-mill. However, increasing the Pine-HEM/Aquivion PW98 mass from 3 to 6 g did not result in a linear increase in the reactor productivity. Indeed, while the mass introduced into the planetary ball-mill was doubled, the reactor productivity was only increased by a factor of 1.2 (from 860 to 1080 g m−3 h−1) suggesting that the efficiency of the mechanocatalytic process dropped down when a higher mass was introduced into the planetary ball-mill. This can be understood by a lower degree of freedom of ZrO2 balls that decreased the intensity of impacts. Hence, in the following experiments, the mass of Pine-HEM + Aquivion PW 98 was kept constant at 3 g.
1), the reactor productivity reached 860 g of oligosaccharides with a DP ≤ 9 per m3 and per hour. Decreasing the mass introduced into the planetary ball-mill from 3 to 1.5 g logically decreased the reactor productivity by nearly a factor 2 (490 g m−3 h−1), indicating that in this 1.5–3 g window, the efficiency of the mechanocatalytic process is not affected by the mass of the sample introduced into the planetary ball-mill. However, increasing the Pine-HEM/Aquivion PW98 mass from 3 to 6 g did not result in a linear increase in the reactor productivity. Indeed, while the mass introduced into the planetary ball-mill was doubled, the reactor productivity was only increased by a factor of 1.2 (from 860 to 1080 g m−3 h−1) suggesting that the efficiency of the mechanocatalytic process dropped down when a higher mass was introduced into the planetary ball-mill. This can be understood by a lower degree of freedom of ZrO2 balls that decreased the intensity of impacts. Hence, in the following experiments, the mass of Pine-HEM + Aquivion PW 98 was kept constant at 3 g.
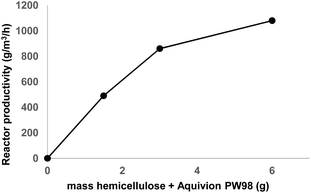 | ||
| Fig. 2 Reactor productivity as a function of the hemicellulose + Aquivion mass introduced into the planetary ball-mill. | ||
Then, the amount of Aquivion PW98 was decreased to 20 wt%, which obviously decreased the reaction rate (Table 1, entry 5). However, in this case the formation of oligosaccharides with a DP ≤ 9 plateaued at only 9%. Indeed, no improvement of the oligosaccharide yields with a DP ≤ 9 was observed by extending the reaction time, suggesting a deactivation of the Aquivion PW 98 catalyst during the reaction. To check this, Aquivion PW98 was also recycled when using a Pine-HEM/Aquivion mixture (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) of 3 g. A significant drop in the oligosaccharide yields with a DP ≤ 9 was observed, from 67% to 38% after only two catalytic cycles, supporting that Aquivion PW 98 was progressively deactivated during the mechanocatalytic depolymerisation of Pine-HEM (Fig S3†). This was further supported by determining the proton exchange capacity of fresh and spent Aquivion PW98 which dropped down from 0.96 to 0.40 and 0.09 mmol g−1 after the first and the second catalytic cycle, respectively (Table S1†). To get more insights into this aspect, ICP analysis was performed on Pine-HEM and it revealed the residual presence of Ca2+, Mg2+, K+ and Na+, with Ca2+ (74%) and K+ (18%) being the two major cations detected (Table S2†). We suggest that these cations possibly exchange with the proton present on Aquivion PW98 leading to their progressive deactivation. A rapid calculation, considering that Ca2+/Mg2+ and K+/Na+ cations can possibly exchange 2 and 1 proton, respectively, shows that the total amount of protons that could be theoretically exchanged on Aquivion PW 98 is 0.54 mmol per gram, which is about 55% of the proton exchange capacity of Aquivion PW 98. ICP experiments were tentatively performed on Aquivion PW68. A contamination with alkaline-earth/alkaline metals was clearly observed (Fig. S4†) but, unfortunately, it was not possible to determine with accuracy the level of contamination due to the lack of procedures to dissolve Aquivion PW98. Nevertheless, these results strongly suggest that the alkaline-earth/alkaline metals present in Pine-HEM partly deactivate Aquivion PW98.
1) of 3 g. A significant drop in the oligosaccharide yields with a DP ≤ 9 was observed, from 67% to 38% after only two catalytic cycles, supporting that Aquivion PW 98 was progressively deactivated during the mechanocatalytic depolymerisation of Pine-HEM (Fig S3†). This was further supported by determining the proton exchange capacity of fresh and spent Aquivion PW98 which dropped down from 0.96 to 0.40 and 0.09 mmol g−1 after the first and the second catalytic cycle, respectively (Table S1†). To get more insights into this aspect, ICP analysis was performed on Pine-HEM and it revealed the residual presence of Ca2+, Mg2+, K+ and Na+, with Ca2+ (74%) and K+ (18%) being the two major cations detected (Table S2†). We suggest that these cations possibly exchange with the proton present on Aquivion PW98 leading to their progressive deactivation. A rapid calculation, considering that Ca2+/Mg2+ and K+/Na+ cations can possibly exchange 2 and 1 proton, respectively, shows that the total amount of protons that could be theoretically exchanged on Aquivion PW 98 is 0.54 mmol per gram, which is about 55% of the proton exchange capacity of Aquivion PW 98. ICP experiments were tentatively performed on Aquivion PW68. A contamination with alkaline-earth/alkaline metals was clearly observed (Fig. S4†) but, unfortunately, it was not possible to determine with accuracy the level of contamination due to the lack of procedures to dissolve Aquivion PW98. Nevertheless, these results strongly suggest that the alkaline-earth/alkaline metals present in Pine-HEM partly deactivate Aquivion PW98.
Having all these results in our hands, we then focused our attention on the transposition and the optimization of this technology to pulp hemicellulose (obtained from hardwood kraft pulp), which is a grade of hemicellulose much more abundant, and thus, more industrially relevant than Pine-HEM. Hereafter, it will be named Pulp-HEM. Pulp industrially obtained from a paper mill is composed of about 80% of cellulose and 20% of hemicellulose. Pulp-HEM was recovered from pulp by strong alkaline extraction (NaOH) for 30 min at ambient temperature, affording a juice rich in Pulp-HEM (∼10 wt%).17 The alkaline solution was then neutralized with acetic acid to induce the precipitation of Pulp-HEM which was then recovered by centrifugation. Multiple re-dispersions in ethanol and centrifugation were performed to completely remove traces of NaOH, as it could poison the catalyst during the mechanocatalytic depolymerization reaction. Pulp-HEM was then lyophilized for 72 h. The water content of Pulp-HEM, determined by TGA, was 5 wt%. Due to the harsh acid conditions employed for the fabrication of pulp, the recovered Pulp-HEM is known to be purer, but also more recalcitrant to chemical processing, than Pine-HEM. Indeed, Pulp-HEM exhibits a much higher purity than Pine-HEM, it is composed of 99% xylan, the rest being composed of 0.5% galactan and trace amounts of glucan. Hence, from Pulp-HEM, xylo-oligosaccharides (XOS) will be obtained.
Inspired by our results collected with Pine-HEM, Pulp-HEM (1.5 g) and Aquivion PW98 (1.5 g) were first mixed together and ball-milled at 400 rpm. The results are presented in Table 2. In contrast to Pine-HEM, Pulp-HEM is not soluble in water, which also reflects its much higher recalcitrance. Hence, the amount of water soluble XOS was directly taken as an indicator to assess the efficiency of the mechanocatalytic process. Note that only XOS with a DP lower than 14 are soluble in water (Fig. S5†). From 1 to 12 h, the yield of water soluble XOS linearly increased to reach 59% (Table 2, entry 1). The kinetic profile of the reaction is provided in Fig S6.† Analysis of the water soluble fraction by SEC-HPLC confirmed the depolymerization of Pulp-HEM with the formation of XOS with a DP of 10–14 in 19% yield and XOS with a DP ≤ 9 in 40% yield. This 40% is composed of XOS (DP 3–9) with 32% yield and the yield of mono/disaccharide 8%. As observed above with Pine-HEM, extending the ball-milling time to 24 h did not result in a further increase of the yield of water soluble XOS (Table 2, entry 2). This aspect is rationalized later. Water soluble XOS were then analyzed in more detail to check their structure. First, analyses of water-soluble XOS were performed by 13C NMR in d6-DMSO. NMR spectra were very similar to those of standards of sugars dimers such as cellobiose, xylobiose, melibiose, etc. The peaks corresponding to the terminal anomeric carbons of XOS were clearly visible at ∼98 ppm and ∼93 ppm for the β and α anomers, respectively. Internal anomeric carbon was also assigned with a typical chemical shift at ∼103 ppm (Fig S7†). Using a DEPT sequence, –CH groups (70–78 ppm) were also distinguished from –CH2 groups (62–67 ppm) (Fig. S8†). One should note that no signal was observed at about 170–190 ppm, confirming the absence of carbonyl groups on XOS, a result in line with the absence of sugar degradation products, as also corroborated by the recovery of a white sample. Terminal anomeric protons (α anomer) were also clearly identified in the 1H NMR spectrum of the recovered XOS, with typical chemical shifts at ∼5 ppm. Terminal anomeric protons from the β anomer and internal anomeric protons overlap at ∼4.2 ppm, as confirmed by an HSQC sequence (Fig S9†). The very intense signal peaks corresponding to terminal α-anomeric protons (similar to that of –CH and –CH2 groups) strongly supported the deep depolymerization of Pulp-HEM.
Unfortunately, XOS are highly hygroscopic and 1H NMR could not be used to confirm the average DP due to an overlap of XOS signals (–CH and –CH2) with that of residual water. For this reason, XOS were also analyzed by mass spectrometry. The ESI mass spectra of the water-soluble XOS revealed a series of monocationized pseudomolecular peaks with sequential m/z increments of 132 units that correspond to additional anhydroxylose units (Fig. S10†). Furthermore, in line with HPLC-SEC, only DPs up to 10 were identified.
During the mechanocatalytic reaction, repolymerization reactions may also occur concomitantly, explaining the recovery of low molecular weight XOS and not monomeric xylose. To assess the occurrence of reversion reactions, xylose was ball-milled with Aquivion PW98 for 12 h at 400 rpm. Analysis by SEC-HPLC revealed the formation of XOS in 47% yield, confirming that reversion reactions also concomitantly occurred during the mechanocataytic reaction (Fig S11†).
We know from the results on Pine-HEM that the water content of hemicellulose impacts, to some extent, the mechanocatalytic efficiency. Hence, Pulp-HEM was lyophilized a second time to decrease the water content from 5 to 3.5 wt%. As observed above with Pine-HEM, it again results in an improvement of the yield of water-soluble XOS from 59 to 79% (Table 2, entry 3).
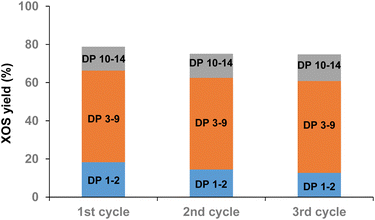
Next, by means of ICP analysis, the amount of alkaline-earth/alkaline metals present in Pulp-HEM was determined. Thanks to multiple re-dispersions in ethanol/water and centrifugation, the amount of alkaline-earth/alkaline metals (Ca2+, Mg2+, K+ and Na+) found in Pulp-HEM was about 4 times lower than in Pine-HEM (2.969 vs. 11.315 g kg−1) (Table S3†). As a result, Aquivion PW98 was much less subjected to deactivation and it was successfully recycled at least three times (Fig. 3), although Aquivion PW98 becomes slightly yellowish after use. The amount of alkaline-earth/alkaline metals in Pulp-HEM can be further decreased to only 0.1 g kg−1 by washing Pulp-HEM with a diluted HCl solution (0.12 M) at room temperature (Table S4†). After drying, the water content of HCl-treated Pulp-HEM was similar to that above, i.e. 3.5 wt%. Note that this treatment of Pulp-HEM with diluted HCl did not lead to any depolymerization, as suggested by SEC-HPLC analysis. Furthermore, ball-milling of Pulp-HEM treated with HCl for 12 h, but without Aquivion PW98, did not yield any water soluble XOS, confirming that (1) HCl did not remain entrapped within the hemicellulosic structure and (2) HCl was too diluted to alter the chemical structure of Pulp-HEM at room temperature. Interestingly, this pre-treatment of Pulp-HEM with aqueous HCl led to an even higher yield of water soluble XOS (86%, Table 2, entry 4). The as-obtained water xylo-oligosaccharides were composed of XOS with 18% of DP 10–14, 53% with DP 3–9 and 15% in mono/disaccharides. This result further demonstrates that the removal of alkaline-earth/alkaline metals present in hemicellulose is essential to avoid a decrease in the catalytic performances of Aquivion PW 98.
In the last set of experiments, the amount of Aquivion PW98 was varied. The results are presented in Fig. 4 and all data were collected after 12 h of ball-milling. Pulp-HEM with a water content of 3.5 wt% was used. As expected, an increase of the catalyst amount from 20 to 75 wt% (i.e. 2.5 to 9.7 mol% H+ relative to glycosidic bonds) linearly increased the yield of water soluble XOS, indicating that the rate of depolymerization was governed by Aquivion PW98. However, an increase of the amount of Aquivion PW98 to 100 wt% did not result in a further increase in the yield of water soluble XOS, which plateaued at about 80% after 12 h.
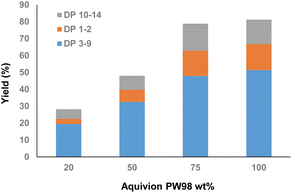 | ||
| Fig. 4 Effect of the Aquivion PW98 amount (wt%) as a function of Pulp-HEM solubility in water after 12 h of mechanocatalytic depolymerization. | ||
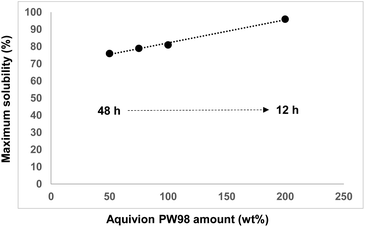
To get more insight into this aspect, the depolymerization of Pulp-HEM in water was monitored as a function of the ball-milling time, using 50 wt% of Aquivion PW98. The kinetic profile is provided in the Fig S12.† It confirms that the yield of water soluble XOS plateaued at about 80% after 48 h of ball-milling. Considering that deactivation of Aquivion PW98 is strongly limited thanks to the low amount of alkaline-earth/alkaline metals in Pulp-HEM, we ascribed this result to an effect of water. Indeed, low molecular weight XOS are highly hygroscopic. Hence, we monitored the evolution of the water content of hemicellulose/XOS during the mechanocatalytic depolymerization. As anticipated, using 50 wt% of Aquivion PW98, the water content increased from 3.5 (12 h) to 6 wt% (48 h), which unavoidably impacts the efficiency of the mechanocatalytic process. Ideally, the higher the depolymerization rate, the less time the Pulp-HEM and XOS have to adsorb water. To support this claim, the maximum yield of water soluble XOS was checked as a function of the Aquivion PW98 amount. As shown in Fig. 5, when the amount of Aquivion PW98 was gradually increased from 50 to 100 and then 200 wt%, it sped up the depolymerization of Pulp-HEM (time reduced from 48 to 12 h) which was accompanied by a concomitant increase in the maximum yield of water soluble XOS from 75 to more than 96%. This result further supports a competition between the depolymerization rate of Pulp-HEM and the water adsorption rate of the sample.
Although XOS can be used as is in the food industry,15 we next evaluated their potential as a starting organic building block for the synthesis of industrially relevant biobased chemicals. To this end, we explored the catalytic reductive amination of XOS with alkyl amines to produce hydrotropes (with short chain alkyl amines) or surfactants (with long chain alkyl amines).15,19 These chemicals are of utmost importance in our society, notably for the manufacture of safer biocide agents with applications in many end-industries (cosmetic, food, anti-fouling, etc…). In this context, XOS were mixed with an alkyl primary amine (n-butyl, n-octyl or n-dodecylamine) and heated at 50 °C under 50 bar of hydrogen in the presence of a Pt/C catalyst. Note that the combination of Pt/C and H2 has been preferred over the routinely employed NaBH3CN or NaBH(OAc)3,20 because it does lead to the formation of salts. The reductive amination was monitored by mass spectrometry. More information on the experimental procedure is provided in the ESI.† In the case of n-butyl amine, the alkyl amine was used both as a solvent and as a reactant (50 fold excess) (Fig. 6). Indeed, XOS are soluble in short chain alkyl amines (<C8-alkyl chain). After 24 h of reaction, mass spectrometry confirmed the successful and complete reductive amination of the terminal position of XOS (Fig. S13†). This result was further confirmed by the disappearance of the signals of the anomeric protons at ∼ 4–5 and 93–98 ppm in the 13C and 1H NMR spectra, respectively (Fig. S14†). No apparent depolymerisation was noticed by mass spectrometry, suggesting that the glycosidic linkages were not hydrolyzed during reductive amination, a noticeable advantage as compared to the more common catalytic glycosylation of oligosaccharides with fatty alcohols for which an important depolymerization occurs. Starting from n-dodecylamine, the reaction is more complex, as XOS are poorly miscible in fatty amines. To overcome this problem, catalytic reductive amination was performed in 2-propanol (9.8 wt% of XOS and 3.7 eq. of n-dodecylamine) to homogeneously dissolve the mixture (Fig. 6). To our delight, in this solvent, reductive amination was successful and complete (according to mass spectrometry, Fig. S15†) after 48 h of reaction yielding the corresponding amphiphilic aminated XOS as a white powder, thus providing straightforward access to biobased surfactants, the biggest market share of amino products.
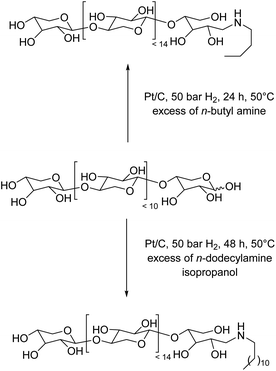 | ||
| Fig. 6 Pt/C-catalyzed reductive amination of XOS with n-alkylamines. For the sake of clarity, only linear structures of XOS are shown. | ||
Conclusions
We showed here that hemicelluloses can be mechanocatalytically depolymerized in the presence of Aquivion PW98, thus complementing the scope of lignocellulosic biomass waste for the synthesis of industrially relevant low molecular weight oligosaccharides. In this work, we highlighted that the amount of alkaline-earth/alkaline metals present in hemicelluloses poison, to some extent, Aquivion PW98, while the water content of hemicellulose (as well as the water intake during depolymerisation) impacts the efficiency of the mechanocatalytic process. Under optimized conditions, industrially abundant Pulp-HEM (water content 3.5 wt%) was depolymerized to water soluble low molecular weight XOS (DP < 14) with 70% yield. Advantageously, the chemical composition of XOS (DP 10–14, DP 3–9 and DP 1–2) can be adjusted by tuning the mechanocatalytic reaction time. As compared to the more classical acid-catalyzed depolymerisation routes of polysaccharides, this work opens a path for the depolymerisation of hemicelluloses to low molecular weight oligosaccharides (1) without any solvent and (2) in a very selective way, as no formation of furanic or degradation products was observed. Despite these noticeable advantages, we would like to bring to the reader's attention that a full life cycle analysis should now be performed to assess the sustainability of this approach and to verify whether or not it is really better than the current acid catalytic pathways.Finally, we demonstrated that water soluble XOS can be successfully used as organic building blocks for the synthesis of industrially relevant chemicals. Indeed, catalytic reductive amination is feasible at the terminal position of XOS opening a potential route to biobased hydrotropes and surfactants.
Conflicts of interest
Authors declare no conflict of interest.Notes and references
- (a) S. I. Mussato and I. M. Mancilha, Carbohydr. Polym., 2007, 68(3), 587–597 CrossRef; (b) D. Han, J. Zulewska, K. Xiong and Z. N. Yang, Crit. Rev. Food Sci. Nutr., 2022 DOI:10.1080/10408398.2022.2139218 , Latest article;; (c) https://www.factmr.com/report/427/polysaccharides-oligosaccharides-market, access on November 29th, 2022.
- (a) N. Echegary, S. Yegin, M. Kumar, A. Hassoun, P. C. B. Campagnol and J. M. Lorenzo, Crit. Rev. Food Sci. Nutr., 2022 DOI:10.1080/10408398.2022.2081963 , Latest article;; (b) N. Liu, H. Wang, Z. Yang, K. Zhao, S. Li and N. He, Food Funct., 2022, 13(13), 6875–6893 RSC; (c) N. Zhang, M. Jin, K. Wang, Z. Zhang, N. P. Shah and H. Wei, Carbohydr. Polym., 2022, 284, 119043 CrossRef CAS PubMed.
- (a) R. Deutschmann and R. F. H. Dekker, Biotechnol. Adv., 2012, 30(6), 1627–1640 CrossRef CAS PubMed; (b) J. W. Agger and B. Zeuner, Curr. Opin. Biotechnol., 2022, 78, 102842 CrossRef CAS PubMed; (c) J. D. Cushen, K. Shanmuganathan, D. W. Janes, C. Grant Willson and C. J. Ellison, ACS Macro Lett., 2014, 3(9), 839–844 CrossRef CAS PubMed.
- (a) M. Faseleh Jahromi, J. Boo Liang, N. Abdullah, Y. Meng Goh, R. Ebrahimi and P. Shokryazdan, BioResources, 2016, 11(1), 674–695 Search PubMed; (b) X. Kou, C. Mao, B. Xie, X. Li, Z. Xue and Z. Zhang, J. Food Nutr. Res., 2016, 55(4), 313–324 CAS; (c) S. Sophonputtanaphoca, C. Pridam, J. Chinnak, M. Nathong and P. Juntipwong, Agric. Nat. Resour., 2018, 52(2), 169–175 Search PubMed; (d) K.-L. Cheong, H.-M. Qiu, H. Du, Y. Liu and B. Muhammad Khan, Molecules, 2018, 23(10), 2451 CrossRef PubMed; (e) A. Zykwinska, M.-H. Boiffard, H. Kontkanen, J. Buchert, J.-F. Thibault and E. Bonnin, J. Agric. Food Chem., 2008, 56(19), 8926–8935 CrossRef CAS PubMed.
- (a) P. H. Seeberger and D. B. Werz, Nat. Rev. Drug Discovery, 2005, 751–763 CrossRef CAS PubMed; (b) S. K. Bouhall and S. J. Sucheck, J. Carbohydr. Chem., 2014, 33(7–8), 347–367 CrossRef CAS PubMed; (c) P. H. Seeberger, Chem. Soc. Rev., 2008, 37, 19–28 RSC; (d) R. Pal, A. Das and N. Jayaraman, Pure Appl. Chem., 2019, 91(9), 1451–1470 CrossRef CAS; (e) M. Panza, S. G. Pistorio, K. J. Stine and A. V. Demchenko, Chem. Rev., 2018, 118(17), 8105–8150 CrossRef CAS PubMed; (f) M. M. Nielsen and C. M. Pedersen, Chem. Rev., 2018, 118(17), 8285–8358 CrossRef CAS PubMed.
- (a) C. Bucke, J. Chem. Technol. Biotechnol., 1996, 67, 217–220 CrossRef CAS; (b) M. Benkoulouche, R. Fauré, M. Remaud-Siméon and C. Moulis, I. André, Interface Focus, 2019, 9, 20180069 CrossRef PubMed; (c) G. Perugino, A. Trincone, M. Rossi and M. Moracci, Trends Biotechnol., 2004, 22(1), 31–37 CrossRef CAS PubMed; (d) Y. Ichikawa, G. C. Look and C.-H. Wong, Anal. Biochem., 1992, 202(2), 215–238 CrossRef CAS PubMed; (e) P. Monsan and F. Paul, FEMS Microbiol. Rev., 1995, 16(2–3), 187–192 CrossRef CAS; (f) M. Filice and M. Marciello, Curr. Org. Chem., 2013, 17(7), 701–718 CrossRef CAS; (g) T. Murata and T. Usui, Biosci., Biotechnol., Biochem., 2006, 70(5), 1049–1059 CrossRef CAS PubMed.
- (a) M. Paul, Simon, Drug Dicover. Today, 1996, 1(12), 522–528 CrossRef; (b) J. Rahkila, T. Saloranta and R. Leino, in Biomass Sugars for Non-fuel Applications, 2015, ch. 6, Print ISBN 978-1-78262-113-3 Search PubMed; (c) A. Matencio, F. Caldera, C. Cecone, J. Manuel López-Nicolás and F. Trotta, Pharmaceuticals, 2020, 13(10), 281 CrossRef CAS PubMed.
- F. A. de Moura, F. T. Macagnan and L. P. da Silva, Food Sci. Technol., 2015, 275–281 CAS.
- P. Gullón, M. J. González-Muñoz and J. C. Parajó, Bioresour. Technol., 2011, 102, 6112–6119 CrossRef PubMed.
- (a) F. M. Relvas, A. R. C. Morais and R. Bogel-Lukasik, RSC Adv., 2015, 5, 73935–73944 RSC; (b) S. P. Magalhães da Silva, A. R. C. Morais and R. Bogel-Łukasik, Green Chem., 2014, 16, 238–246 RSC.
- (a) S. M. Hick, C. Griebel, D. T. Restrepo, J. H. Truitt, E. J. Buker, C. Bylda and R. G. Blair, Green Chem., 2010, 12, 468–474 RSC; (b) N. Meine, R. Rinaldi and F. Schüth, ChemSusChem, 2012, 5, 1449–1454 CrossRef CAS PubMed; (c) M. Käldström, N. Meine, C. Farès, F. Schüth and R. Rinaldi, Green Chem., 2014, 16, 3528–3538 RSC; (d) F. Schüth, R. Rinaldi, N. Meine, M. Käldström, J. Hilgert and M. D. Kaufman Rechuslski, Catal. Today, 2014, 234, 24–30 CrossRef; (e) M. Käldström, N. Meine, C. Farès, R. Rinaldi and F. Schüth, Green Chem., 2014, 16, 2454–2462 RSC; (f) F. Boissou, N. Sayoud, K. De Oliveira Vigier, A. Barakat, S. Marinkovic, B. Estrine and F. Jérôme, ChemSusChem, 2015, 8(19), 3263–3269 CrossRef CAS PubMed; (g) A. Karam, K. De Oliveira Vigier, S. Marinkovic, B. Estrine, C. Oldani and F. Jérôme, ChemSusChem, 2017, 10(18), 3604–3610 CrossRef CAS PubMed; (h) R. Schmidt, S. Fuhrmann, L. Wondraczek and A. Stolle, Powder Technol., 2016, 288, 123–131 CrossRef CAS.
- C. Loerbroks, R. Rinaldi and W. Thiel, Chem.–Eur. J., 2013, 19(48), 16282–16294 CrossRef CAS PubMed.
- (a) A. Karam, P. N. Amaniampong, J.-M. García Fernández, C. Oldani, S. Marinkovic, B. Estrine, K. De Oliveira Vigier and F. Jérôme, Front. Chem., 2018, 6, 74 CrossRef PubMed; (b) A. Shrotri, L. K. Lambert, A. Tanksale and J. Beltramini, Green Chem., 2013, 15, 2761–2768 RSC.
- R. Abejón, ChemEngineering, 2018, 2(1), 7 CrossRef.
- (a) C. D. Pinales-Márquez, R. M. Rodríguez-Jasso, R. G. Araújo, A. Loredo-Treviño, D. Nabarlatz, B. Gullón and H. A. Ruiz, Ind. Crops Prod., 2021, 162, 113274 CrossRef; (b) L. Santibáñez, C. Henríquez, R. Corro-Tejeda, S. Bernal, B. Armijo and O. Salazar, Carbohydr. Polym., 2021, 251, 117118 CrossRef PubMed; (c) F. Peng, P. Peng, F. Xu and R.-C. Sun, Biotechnol. Adv., 2012, 30(4), 879–903 CrossRef CAS PubMed; (d) C. C. de Mello Capetti, M. M. Vacilotto, A. N. G. Dabul, A. G. V. Sepulchro, V. O. A. Pellegrini and I. Polikarpov, World J. Microbiol. Biotechnol., 2021, 37, 169 CrossRef PubMed; (e) A. F. A. Carvalho, P. de Oliva Neto, D. F. da Silva and G. M. Pastore, Food Res. Int., 2013, 51(1), 75–85 CrossRef CAS.
- Y. Chen, J. Shan, Y. Cao, X. Shena, C. Tang, M. Lia, W. Zhuang, C. Zhu and H. Ying, Ind. Crops Prod., 2022, 180, 114704 CrossRef CAS.
- D. da Silva Perez, V. Bigand, F.-Z. Belmokkadem, M. Chemin, P. Huber, F. Rataboul, C. Pinel, S. Grelier, H. Cramail and M. Petit-Conil, in Valorisation des hémicelluloses: Extraction et modifications chimiques, Chimie pour la transformation durable de la ressource lignocellulosique, Collection Esprit des bois, ed S. Tatjana, Presses Universitaires de Bordeaux, 2019, ch. IV-3, pp. 71–117 Search PubMed.
- X. Yan, J. AOAC Int., 2017, 100(4), 1134–1136 CrossRef CAS PubMed.
- D. Gérard, T. Méline, M. Muzard, M. Deleu, R. Plantier-Royon and C. Rémond, Carbohydr. Res., 2020, 495, 108090 CrossRef PubMed.
- (a) D. S Dalpathado, H. Jiang, M. A. Kater and H. Desaire, Anal. Bioanal. Chem., 2005, 381(6), 1130–1137 CrossRef PubMed; (b) J. C. Gildersleeve, O. Oyelaran, J. T. Simpson and B. Allred, Bioconjugate Chem., 2008, 19(7), 1485–1490 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3su00028a |
| This journal is © The Royal Society of Chemistry 2023 |