Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Towards the sustainable extraction and purification of non-animal proteins from biomass using alternative solvents
Bojan
Kopilovic†
 ,
Ana I.
Valente†
,
Ana I.
Valente†
 ,
Ana M.
Ferreira
,
Ana M.
Ferreira
 ,
Mafalda R.
Almeida
,
Ana P. M.
Tavares
,
Mara G.
Freire
,
Mafalda R.
Almeida
,
Ana P. M.
Tavares
,
Mara G.
Freire
 * and
João A. P.
Coutinho
* and
João A. P.
Coutinho
 *
*
CICECO – Aveiro Institute of Materials, Department of Chemistry, University of Aveiro, 3810-193 Aveiro, Portugal. E-mail: maragfreire@ua.pt; jcoutinho@ua.pt
First published on 3rd July 2023
Abstract
Advances towards the development of a sustainable economy must address the exploitation of bio-based products combined with the use of greener solvents and manufacturing processes that can preserve natural resources and the environment. To address the growing demand for proteins, their production must focus on non-animal sources, such as vegetal biomass and respective residues/waste, and on the use of sustainable solvents and processes for their recovery. This review provides an overview of the advances achieved in the separation and purification processes of proteins from vegetable biomass and respective residues using ionic liquids (ILs) and deep eutectic solvents (DESs) as alternative greener solvents. It begins with an overview of the ability of ILs and DESs to stabilize proteins, followed by the assessment of the extraction and separation of biomass-derived proteins assisted by ILs and DESs. Different types of non-animal biomass and respective residues are considered as protein resources, i.e., algae, plants (e.g., aloe vera and holy basil), cereals (e.g., wheat and oat), fruits (e.g., papaya, pineapple, pomegranate, and seabuckthorn berries), and vegetables (e.g., spinach, radish, and ginger). Several IL- and DES-based approaches are discussed, comprising (i) conventional solid–liquid extraction (SLE), (ii) ultrasound-assisted extraction (UAE), (iii) microwave-assisted extraction (MAE), and (iv) aqueous biphasic systems (ABSs). Finally, the economic and environmental challenges of using such alternative solvents in industrial applications are discussed, including technoeconomic analysis and life cycle assessment.
Sustainability spotlightProtein consumption is expected to double by 2050; therefore, novel non-animal protein sources are being explored to meet societal needs. These sources can include vegetal, macro- and microalgae, insects, and fungi. This work discusses the most recent advances in protein extraction from vegetable biomass and respective residues using greener solvents, namely ionic liquids and deep eutectic solvents. The economic and environmental challenges of using such alternative solvents in industrial applications are discussed. |
Introduction
In the new global economy, sustainable development has become a central ambition for the socio-economic and environmental sectors. According to the United Nations,1 an increase in the population from 7.7 to 9.7 billion people will happen in the next three decades. This increase in population and its wealth is creating an unsustainable demand for proteins to be applied in the food, textile, biotechnology, cosmetic, and pharmaceutical industries. As a result, protein consumption is predicted to double by 2050.2 To meet this demand, new sustainable protein sources need to be explored, including macro- and microalgae, insects, fungi, and plants.2 Moreover, proteins can be obtained from agro-industrial biomass (e.g., fruits, vegetables, and cereals) and agro-industrial and food waste. The use of vegetable biomass to obtain proteins offers several advantages, including high productivity, low cost, and a wide variety of protein types.3Conventional methods for protein extraction and separation from biomass usually resort to volatile organic solvents (VOCs), extreme pH and temperature, and long extraction time, which may result in low extraction yields due to protein denaturation and inactivation.3 Therefore, researchers have been focusing on developing more sustainable and effective protein extraction techniques using “greener” solvents, such as ionic liquids (ILs) and deep eutectic solvents (DESs). If properly selected, these solvents may decrease the environmental impact and improve the efficiency of the extraction/separation processes.3
ILs and DESs are widely investigated as alternative solvents to replace VOCs.4,5 If correctly designed, these solvents represent viable alternatives for extracting and separating biomass-derived proteins. In addition to improvements in protein extraction efficiency,6 they have led to improvements in protein and enzyme stability and activity.7 In some cases, these solvents also enhanced the nutritional and techno-functional properties of proteins.8
ILs are typically composed of a large and asymmetrical organic cation and an organic or inorganic anion.9 ILs are molten salts at low temperatures and can be designed by taking advantage of the large number of ion combinations available to display excellent chemical, thermal, and electrochemical stabilities, non-flammability, and negligible volatility. ILs can be designed as task-specific fluids while overcoming the limited selectivity of some VOCs.9,10 DESs are obtained by combining two or more Lewis or Brønsted acids and bases. Their formation, which comprises a significant negative deviation from the ideal solid–liquid phase behaviour, depends on strong hydrogen bonding established between one or more hydrogen bond donors (HBDs) and hydrogen bond acceptors (HBAs).11 They may be prepared with different molar ratios of the starting components. The resultant mixture has a melting point significantly lower than those of the composing individual species. As ILs, DESs are considered designer solvents because their properties can be tailored by the careful choice of the HBA and HBD and their molar ratio.12
This review aims to disclose the advances achieved in protein extraction and separation from non-animal biomass using alternative solvents, namely ILs and DESs. It begins with a brief overview of ILs and DESs, with particular attention to their ability to stabilize proteins. Then, it focuses on extracting and separating biomass-derived proteins using ILs and DESs. Different types of non-animal biomass and respective residues are discussed as protein resources, i.e., algae, plants, fruits, and vegetables.7,13–23 The IL- and DES-based approaches for protein extraction and separation overviewed correspond to (i) conventional solid–liquid extraction (SLE), (ii) ultrasound-assisted extraction (UAE), (iii) microwave-assisted extraction (MAE) and (iv) aqueous biphasic systems (ABSs) or aqueous two-phase systems (ATPS). Finally, the economic and environmental challenges of using such alternative solvents in industrial applications are discussed, as well as the need of using predicting tools to properly select these solvents.
Protein and enzyme stabilization using ILs and DESs
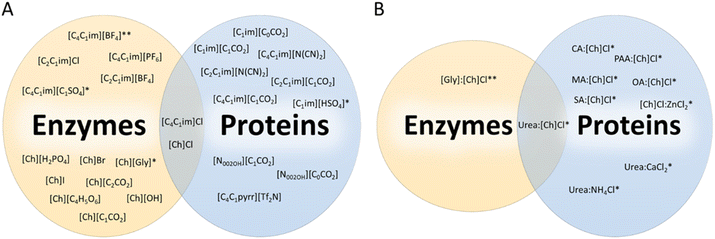
Keeping vulnerable macromolecules, such as proteins, stable is a major challenge. Moreover, denaturation and degradation are more likely to occur in products with a high protein concentration.24 Also, many proteins are susceptible to degradation due to physical and interfacial stress in the presence of solvents.25 Thus, one significant challenge is their stabilization in a wide array of products, which is highly dependent on maintaining their natural conformation. In this sense, appropriately designed ILs and DESs have emerged as promising sustainable alternatives for stabilizing proteins and keeping or improving the activity of enzymes. Interested readers can refer to the specific review articles on ILs and DESs as sustainable solvents for protein stabilization by Patel et al.26 and Almeida et al.27Tables 1 and 2 provide the names and acronyms of the ILs and DESs discussed in the current review. A schematic representation of studied alternative solvents for enzyme and protein stabilization is shown in Fig. 1.| Cation name | Abbreviation | Anion name | Abbreviation |
|---|---|---|---|
| 1-Alkyl-3-methylimidazolium | [CnC1im]+ | Acetate | [C1CO2]− |
| 1-Alkylimidazolium | [Cnim]+ | Bis(2,4,4-trimethylpentyl)phosphinate | [TMPP]− |
| 1-Butyl-1-methylpiperidinium | [C4C1pip]+ | Bis(trifluoromethane sulfonyl)imide | [Tf2N]− |
| 1-Butyl-1-methylpyridinium | [C4C1pyr]+ | Bitartarate | [C4H5O6]− |
| 1-Butyl-1-methylpyrrolidinium | [C4C1pyrr]+ | Bromide | Br− |
| 2-Hydroxyethylammonium | [N002OH]+ | Butyrate | [C3CO2]− |
| 3-(1-Tetradecyl-3-hexylimidazolium)-1-tetradecylimidazolium | [C14im-6-C14im]+ | Chloride | Cl− |
| 3-(Dimethylamino)-1-propylamine | [N011(3N)]+ | Decanoate | [C9CO2]− |
| 4-Butyl-4-methylmorpholin-4-ium | [C1C4mor]+ | Dicyanamide | [N(CN)2]− |
| Benzyldodecyldimethylammonium | [N1112(C7H7)]+ | Dihydrogen citrate | [DHC]− |
| Bis(2-hydroxyethyl)ammonium | [N00(2OH)2]+ | Dihydrogen phosphate | [H2PO4]− |
| Cholinium | [Ch]+ | Dimethylphosphate | [(C1)2PO4]− |
| Diethylamine | [N0022]+ | Formate | [C0CO2]− |
| Hexadecylpyridinium | [C16py]+ | Glycinate | [Gly]− |
| N-methyl-2-hydroxyethylammonium | [NC1-[N00(2OH)2]+ | Hexafluorophosphate | [PF6]− |
| N-butylpyridinium | [NC4pyr]+ | Hydrogensulfate | [HSO4]− |
| Prolinium | [Pro]+ | Hydroxide | OH− |
| Tetrabutylammonium | [N4444]+ | Iodide | I− |
| Tetrabutylphosphonium | [P4444]+ | Methanosulfonate | [C1SO3]− |
| Tetrabutylphosphonium | [P66614]+ | Methylsulfate | [C1SO4]− |
| Tetraethylammonium | [N2222]+ | Pentanoate | [C4CO2]− |
| Tetramethylammonium | [N1111]+ | Propionate | [C2CO2]− |
| Tri(ethyl)[2-ethoxy-2-oxoethyl]ammonium | [Et3NC2OC2]+ | Prolinate | [Pro]− |
| Tri(ethyl)[4-aminobutyl-4-oxobutyl]ammonium | [Et3NC4NC4]+ | Saccharinate | [Sac]− |
| Tetrachloroferrate | [FeCl4]− | ||
| Tetradecanoate | [C13CO2]− | ||
| Tetrafluoroborate | [BF4]− | ||
| Thiocyanate | [SCN]− | ||
| Tosylate | [Tos]− | ||
| Trifluoroacetate | [CF3CO2]− | ||
| Trifluoromethanesulfonate | [CF3SO3]− |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HBA) considered in this review
HBA) considered in this review
| HBD name | Abbreviation | HBA name | Abbreviation |
|---|---|---|---|
| Benzyl tributyl ammonium chloride | BC | 1,2-Butanediol | 12BD |
| Cholinium chloride | [Ch]Cl | 1,4-Butanediol | 14BD |
| Ethyl ammonium chloride | [EA]Cl | 2,3-Butanediol | 23BD |
| L-Carnitine | Carn | Acetic acid | AA |
| Fructose | Fru | Citric acid | CA |
| Glucose | Glu | Ethylene glycol | EG |
| Imidazole | Imi | 1,6-Hexanediol | 16HD |
| L-Maltose | Malt | L-Lactose | Lac |
| Sodium acetate | NaOA | Sorbitol | Sorb |
| Sucrose | Suc | Urea | Urea |
| Xylose | Xyl | Glycerol | Gly |
| Oxalic acid | OA | ||
| Glycolic acid | Glyc | ||
| Succinic acid | SA | ||
| Maleic acid | MA | ||
| Phenylacetic acid | PAA |
With the increasing demand for stable proteins and enzymes, various approaches for protein stabilization using ILs and DESs have been proposed. In the field of proteins found in vegetable biomass, Baker et al.28 studied the denaturation of monellin, a protein found in the fruit of the West African shrub known as serendipity berry (Dioscoreophyllum cumminsii), in water and an aqueous solution of [C4C1pyrr][Tf2N]. The presence of the IL increased the denaturation temperature of the protein from 40 °C to 100 °C.28 Several authors have demonstrated the versatility of various ILs and DESs by their ability to dissolve and stabilize zein, a prolamine protein found in maize. Biswas et al.29 studied the application of [C4C1im]Cl and [C4C1im][N(CN)2] in zein solubilization and as solvents for its chemical modification. The authors compared the IL performance with several DES mixtures, namely urea![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CaCl2, urea
CaCl2, urea![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Ch]Cl, [Ch]Cl
[Ch]Cl, [Ch]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ZnCl2, CA
ZnCl2, CA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Ch]Cl, OA
[Ch]Cl, OA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Ch]Cl, urea
[Ch]Cl, urea![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NH4Cl, SA
NH4Cl, SA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Ch]Cl, MA
[Ch]Cl, MA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Ch]Cl and PAA
[Ch]Cl and PAA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Ch]Cl. It was demonstrated that zein is soluble in the two imidazolium-based ILs in concentrations up to 15 wt% at 80 °C; however, the protein is insoluble in all the cholinium-based DESs investigated. As a succession of this work, Choi et al.30 evaluated the dissolution of zein in protic ILs (PILs). The PILs [N002OH][C0CO2] and [N002OH][C1CO2] were able to dissolve zein at a concentration of up to 70% at 150 °C by microwave treatment. However, preventing the total thermal degradation of the zein protein was not achieved. More recently, Tomlison et al.31 studied the dissolution of zein in [C4C1im][C1CO2], [C2C1im][C1CO2], [C2C1im][N(CN)2], [C4C1im]Cl, [C1im][C1CO2], [C1im][HSO4], [C1im][C0CO2] and acetic acid as a control. It was observed that zein is soluble in all ILs, except in [C1im][HSO4]. The results were explained based on the IL polarity, which is similar to water. Since the zein protein is insoluble in water, the water impact was then evaluated in the solubilization of zein using aqueous solutions of these ILs. The authors concluded that water contents up to 11.1 mol% do not affect zein solubilization. Moreover, the authors have reported that the solubility of zein is higher in the presence of acetic acid (used for comparison purposes), [C1im][C1CO2], and [C2C1im][N(CN)2].31
[Ch]Cl. It was demonstrated that zein is soluble in the two imidazolium-based ILs in concentrations up to 15 wt% at 80 °C; however, the protein is insoluble in all the cholinium-based DESs investigated. As a succession of this work, Choi et al.30 evaluated the dissolution of zein in protic ILs (PILs). The PILs [N002OH][C0CO2] and [N002OH][C1CO2] were able to dissolve zein at a concentration of up to 70% at 150 °C by microwave treatment. However, preventing the total thermal degradation of the zein protein was not achieved. More recently, Tomlison et al.31 studied the dissolution of zein in [C4C1im][C1CO2], [C2C1im][C1CO2], [C2C1im][N(CN)2], [C4C1im]Cl, [C1im][C1CO2], [C1im][HSO4], [C1im][C0CO2] and acetic acid as a control. It was observed that zein is soluble in all ILs, except in [C1im][HSO4]. The results were explained based on the IL polarity, which is similar to water. Since the zein protein is insoluble in water, the water impact was then evaluated in the solubilization of zein using aqueous solutions of these ILs. The authors concluded that water contents up to 11.1 mol% do not affect zein solubilization. Moreover, the authors have reported that the solubility of zein is higher in the presence of acetic acid (used for comparison purposes), [C1im][C1CO2], and [C2C1im][N(CN)2].31
In addition to proteins, enzymes have been studied as well, with reports showing enzymatic activity enhancements provided by ILs and DESs. The mushroom tyrosinase activity was studied in three ILs, namely [C4C1im][PF6], [C4C1im][BF4], and [C4C1im][C1SO4], and compared with the enzyme activity in chloroform.32,33 Higher activities were observed in chloroform and [C4C1im][PF6].33 Although the enzyme has biological activity in all ILs investigated, the activity decreases in the hydrophilic ILs [C4C1im][BF4] and [C4C1im][C1SO4]. These ILs interact with the enzyme, leading to its inactivation. Divya et al.34 explored the thermal stability and activity of two esterase domains from Mycobacterium tuberculosis in ILs constituted by the imidazolium cation, namely [C2C1im][BF4], [C2C1im]Cl, [C4C1im][BF4] and [C4C1im]Cl. The authors established that these ILs prevent protein aggregation and unfolding and increase thermal stability.34
Although good results were obtained with imidazolium-based ILs, they may raise some toxicity issues.34 Therefore, other ILs have been investigated, particularly cholinium-based ILs. Bisht et al.35 explored the effects of cholinium-based ILs, such as [Ch]Br and [Ch][Gly], on the stability of bromelain. Comparing both ILs, [Ch]Br performed better in preserving the bromelain structure. Besides the biological source of the anion of [Ch][Gly] (from the amino acid glycine), the authors concluded that the respective anion establishes stronger H-bonds with the bromelain backbone leading to the dissociation of the H-bonds within the enzyme, which is the factor responsible for the maintenance of the protein structure.35 The same research group studied the effect of [Ch]Cl, [Ch][C1CO2], [Ch][H2PO4], [Ch][C4H5O6], [Ch]I, and [Ch][OH] on the stability of bromelain.36 The authors revealed that the enzyme is less stable in [Ch][OH] but more stable in [Ch]Cl. At low concentrations, [Ch][H2PO4] and [Ch]CI can stabilize bromelain; yet, with the increase in the IL concentration, the protein structure becomes less stable.36 Also exploring biocompatible ILs, Martins et al.37 studied the stability of R-phycoerythrin in 1 M and 2 M aqueous solutions of [Ch]CI. The circular dichroism and excitation spectra of the studied samples resemble those of pure R-phycoerythrin, which is associated with the presence and maintenance of the protein structure, along with the chromophore stability.37 In the same line, Vicente et al.6 demonstrated that the crude algal extract of R-phycoerythrin, similar to pure R-phycoerythrin, is predominantly composed of α-helix. The circular dichroism spectra revealed that proteins keep their integrity throughout the entire purification process. In addition to previously mentioned proteins obtained from vegetable sources, cytochrome-c, lipase, and lysozyme stabilities in IL solutions were also reported. These proteins are commonly referred to as animal proteins; however, they can also be found in plants.38–40 The cytochrome-c tertiary structure was evaluated in the presence of the cholinium-based ILs [Ch][Pro] and [Pro][NO3]. It was concluded that the protein tertiary structure is maintained at an IL concentration of 10 mM.41
Qiao et al.42 investigated the solvation and structure of lipase in two DESs (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 [Ch]Cl
2 [Ch]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) urea and 1
urea and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 [Ch]Cl
2 [Ch]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gly) and in their aqueous solutions (1
Gly) and in their aqueous solutions (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 DES
1 DES![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water) through molecular dynamics simulations. Simulation results showed pronounced hydrogen bonding between the enzyme and the hydrogen bond donors in the DESs. For the 1
water) through molecular dynamics simulations. Simulation results showed pronounced hydrogen bonding between the enzyme and the hydrogen bond donors in the DESs. For the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 DES
1 DES![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water solutions, the results indicated that the water molecules did not replace the DES molecules at the solvation shell of the enzyme. However, for both DESs, water molecules weaken the solvation shell of lipase by reducing the enzyme-DES hydrogen bond lifetime.42 Finally, lipase remained folded in both DESs and their aqueous solutions.42 Water molecules change the surface area and conformation of the active site differently in the two DESs. The surface area of the active site of the lipase in the [Ch]Cl
water solutions, the results indicated that the water molecules did not replace the DES molecules at the solvation shell of the enzyme. However, for both DESs, water molecules weaken the solvation shell of lipase by reducing the enzyme-DES hydrogen bond lifetime.42 Finally, lipase remained folded in both DESs and their aqueous solutions.42 Water molecules change the surface area and conformation of the active site differently in the two DESs. The surface area of the active site of the lipase in the [Ch]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) urea solution decreases with the addition of water.42 However, an opposite effect occurred for the solution of [Ch]Cl
urea solution decreases with the addition of water.42 However, an opposite effect occurred for the solution of [Ch]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gly. Lipase presents a more ordered structure in both DESs, and by adding water molecules, the structure is recovered, changing back towards the structure present in the pure aqueous solution.42 In alignment with the previous work, Sanchez-Fernandez et al.25 studied the structural conformation of lysozyme in solutions with different enzyme concentrations (from 4 mg L−1 to 143 mg L−1) in the DES [Ch]Cl
Gly. Lipase presents a more ordered structure in both DESs, and by adding water molecules, the structure is recovered, changing back towards the structure present in the pure aqueous solution.42 In alignment with the previous work, Sanchez-Fernandez et al.25 studied the structural conformation of lysozyme in solutions with different enzyme concentrations (from 4 mg L−1 to 143 mg L−1) in the DES [Ch]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gly (1
Gly (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). The authors observed that lysozyme had a globular conformation in the DES, similar to the native conformation. Regarding long-term preservation, the structure and activity of the enzyme were evaluated after 40 days of storage, showing that the native conformation and activity of the enzyme were recovered after rehydration.25
2). The authors observed that lysozyme had a globular conformation in the DES, similar to the native conformation. Regarding long-term preservation, the structure and activity of the enzyme were evaluated after 40 days of storage, showing that the native conformation and activity of the enzyme were recovered after rehydration.25
ILs and DESs as alternative solvents for protein/enzyme extraction and purification
Biomass is a unique, ubiquitous, and sustainable renewable resource for producing bio-based products with wide commercial applications. Proteins and enzymes from biomass and respective residues are usually obtained after extensive, laborious, and costly downstream processing.43 Throughout the years, several extraction methods for target proteins from biomass have been reported, mainly using water and VOCs. However, the protein extraction efficiencies and yields sometimes remained low.44–46 Besides their environmental toxicity, it is well-known that organic solvents may affect the protein's stability.44–46 Several studies have reported a decrease in the activity of proteins, such as lipase and laccase, when exposed to volatile organic solvents, such as acetone, acetonitrile, DMF, ethanol, methanol, and 1-propanol, confirming the negative effect of these solvents on protein stability.47,48 Thus, developing effective, industrially viable, and environmentally friendly extraction methods for proteins from biomass while maintaining their bioactivity is highly desirable.Solid-liquid extraction (SLE) consists of the extraction and dissolution of a given compound from a solid matrix in a given solvent. In classical SLE approaches, the biomass is placed in direct contact with the solvent, and operating conditions such as temperature, extraction time, and solid–liquid ratio are optimized.49,50 Improved SLE techniques, such as microwave-assisted extraction (MAE), ultrasound-assisted extraction (UAE), and enzyme-assisted processes, have been combined with ILs and DESs to enhance the extraction performance while attempting to decrease the extraction time and amount of solvent used.49,51 UAE increases the mass transfer, being the best option when dealing with thermally sensitive compounds, while MAE allows a fast heat transfer into the solvent solution, being the best option when viscous solutions are used (e.g. pure or highly concentrated ILs and DESs solutions). After the extraction step, further use of induced precipitation, distillation, chromatography, and liquid–liquid extractions (LLE) as separation/purification techniques is usually required.52 LLE is usually performed using organic solvents immiscible with water. Compared to chromatography, liquid–liquid systems offer technological simplicity, lower operation cost, and the capability to provide high yields, improved purification factors, enhanced selectivity, and the possibility of combining the recovery and purification steps. Aiming to avoid the use of organic solvents in LLE, Albertson introduced the ABS concept for separating (bio)molecules between two water-rich phases.53 Both phases are mainly composed of water, thus affording an amenable media for (bio)molecules, including proteins. In addition to the widely studied polymer–polymer and polymer–salt ABS, Gutowski et al.54 demonstrated that ABSs could be formed by the addition of inorganic salts to aqueous solutions of hydrophilic ILs. IL-based ABSs have shown remarkable advantages when compared to more traditional polymer-based ones, namely by providing low viscous solvent media and by allowing the tailoring of extraction efficiencies and selectivity.55,56 Furthermore, it has been shown that ABS can allow the extraction and separation of the target compound in a one-pot approach.56,57
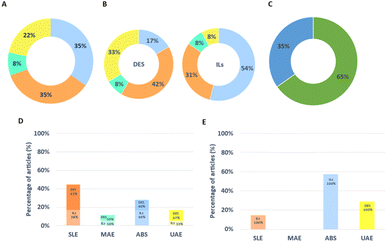
The described techniques to extract proteins from non-animal biomass, resorting to ILs and DESs as alternative solvents, are discussed in the current review. Fig. 2 shows the status of the literature related to the application of the mentioned IL- and DES-based processes in the extraction and purification of proteins and enzymes from non-animal sources. The techniques most investigated for protein and enzyme extraction and purification are SLE and ABS. These techniques have been mainly applied to proteins, accounting for 65% of the studies, contrasting with 35% of studies dealing with enzymes. In this line, SLE has been mainly applied to proteins, whereas ABSs have been mainly investigated with enzymes.
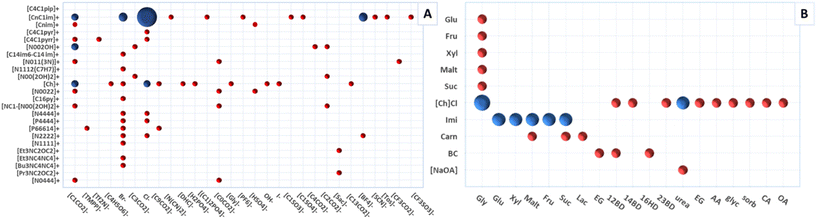
ILs and DESs used for protein extraction and purification described in this review are presented in Fig. 3A and B, respectively. According to Fig. 3A, imidazolium-based ILs are still frequently used; however, cholinium-based and PILs are also being investigated in more recent studies. Most studies focused on the use of acetate, formate, trifluoroacetate, and halide anion-based ILs. This is mainly a result of ability of these ILs to dissolve biomass and their easy synthesis and/or availability. Regarding DESs (Fig. 3B), studies reporting the use of [Ch]Cl as one of the system's components are the most numerous. This trend is explained due to the high hydrogen-bond acceptor ability of this ammonium salt, its low price, and for being considered biosafe as it is derived from vitamin B. Also, there is an apparent increasing trend in using carbohydrate-based DES. The following sections describe the application of IL- and DES-based extraction and purification processes divided by biomass type.
Algae and microalgae as a source of proteins
Algae and microalgae are of high interest because of the antiviral, anti-free radical, anti-inflammatory, anti-oxidation, anti-parasitic, anti-bacterial, anti-fungal, and other functional characteristics of the compounds they have in their constitution.58 (Micro)algae composition is complex, containing diverse groups of biochemicals, such as chlorophylls,59 polysaccharides,59 γ-linolenicacid,59 β-carotene,59 and phycobiliproteins.59 Phycobiliproteins, brightly colored protein-based pigments, have a role in the receiving of light for driving photosynthesis. Phycoerythrin, allophycocyanin, and phycocyanin are the three major groups within the microalgal phycobiliprotein classification.60 The predominant pigment in the phycobiliprotein family is phycocyanin, representing 60–70% of the dry weight.61,62 Phycocyanin is commonly used as a natural colorant in the food and cosmetic industries. It can be incorporated into healthy diet products due to its physiological properties, including antioxidant, anti-inflammatory, and hepatoprotective activities.62–65 Accordingly, various researchers are developing effective platforms for the bulk production of phycocyanin-producing cultures and for its extraction from microalgae.66,67 Among these, several techniques using ILs or DESs have been developed to extract and purify proteins from algae and microalgae. A summary of these studies is shown in Table 3.| Extracted protein | Natural source | Method | IL or DES applied | References |
|---|---|---|---|---|
| a *, • Articles focusing on the extraction of phycocyanins and phycoerythrin, respectively. | ||||
| Phycobiliproteins/phycocyanins*/phycoerythrin• | Spirulina platensis | SLE | NC1-2-[N00(2OH)2][C0CO2]+NC1-2-[N00(2OH)2][C1CO2]* | 68 |
| UAE | [N00(2OH)2][C0CO2], [N00(2OH)2][C1CO2], [C4C1im]Cl | 69 | ||
| MAE/ABS | Glu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gly (1 Gly (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), Fru 1), Fru![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gly (1 Gly (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), Xyl 1), Xyl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gly (1 Gly (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), Malt 1), Malt![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gly Gly![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (1 water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5), Suc 0.5), Suc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gly (1 Gly (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)* 1)* |
72 | ||
| ABS | [C4C1im]Br, [C6C1im]Br, [C8C1im]Br*; [C4C1im]Cl*; NC1-2-[N00(2OH)2][C0CO2], NC1-2-[N00(2OH)2][C1CO2]* | 58, 68, and 70 | ||
| Gracilaria sp | SLE | [C4C1im][CF3CO2], [C4C1pyrr][C1CO2], [N111(2OH)][C1CO2], [C10C1im]Cl, [C12C1im]Cl, [C2C1im][C1CO2], [C2C1im]Cl, [C2im][C1CO2], [C4C1im][(C1)2PO4], [C4C1im][C1CO2], [C4C1im][C1SO3],[C4C1im][CF3SO3], [C4C1im][N(CN)2], [C4C1im][SCN], [C4C1im][Tos], [C4C1im]Cl, [C4C1pip]Cl, [C4C1pyr]Cl, [C4C1pyrr]Cl, [C6C1im]Cl, [C8C1im]Cl, [N111(2OH)]Cl, [N4444]Cl, [P4444]Cl* | 37 | |
| AMTPS | [C16py]Br, [N1112(C7H7)]Br, [C14mim]Cl, [C16mim]Cl, [C14im-6-C14im]Br2, [Ch][C9CO2], [Ch][C13CO2], [P4,4,4,14]Cl • | 6 | ||
| Chlorella pyrenoidosa proteins | Chlorella pyrenoidosa | SLE | [N0022][C1CO2], [N011(3N)]CF3CO2], [N0222][C1CO2], [N0444][C0CO2], [N011(3N)][C0CO2], [N0022][C0CO2], [N0022][CF3CO2], [N011(3N)][C1CO2], [N0222][C0CO2], [N0222][CF3CO2], [N0444][C1CO2], [N0444][CF3CO2]* | 73 |
| Nannochloropsis sp. proteins | Nannochloropsis sp. powder | MAE | [N1111]Cl, [Ch][C1CO2], [C2C1im]Cl, [C4C1im]Cl, [N4444][Tf2N], [NC4pyr]Cl | 74 |
Rodrigues et al.68 studied various PILs for the heated extraction of phycobiliproteins (phycocyanin, allophycocyanin and phycoerythrin) from Spirulina platensis microalgae. The authors applied NC1-2-[N00(2OH)2][C0CO2], NC1-2-[N00(2OH)2][C1CO2] and their 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) mixture, under mechanical agitation and heating. However, comparing phycobiliprotein extraction using these solvents with a sodium phosphate buffer, there was no evidence that PILs performed better than the buffer at low temperatures (20 °C, 25 °C, 30 °C, and 35 °C).68 Furthermore, no increase in phycobiliprotein extraction was observed with the increasing temperature using NC1-2-[N00(2OH)2][C0CO2], which was explained by the denaturation of the proteins by heat. Under the optimum extraction conditions (35 °C, pH 6.5, S. platensis solid
1 (v/v) mixture, under mechanical agitation and heating. However, comparing phycobiliprotein extraction using these solvents with a sodium phosphate buffer, there was no evidence that PILs performed better than the buffer at low temperatures (20 °C, 25 °C, 30 °C, and 35 °C).68 Furthermore, no increase in phycobiliprotein extraction was observed with the increasing temperature using NC1-2-[N00(2OH)2][C0CO2], which was explained by the denaturation of the proteins by heat. Under the optimum extraction conditions (35 °C, pH 6.5, S. platensis solid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) liquid ratio 0.15 g mL−1), concentration values of extracted phycobiliproteins (2.22–3.65 g L−1) from S. platensis using individually [N00(2OH)2][C1CO2], NC1-2-[N00(2OH)2][C1CO2] or a buffer solution did not differ substantially at a 95% confidence level. However, when a mixture of NC1-2-[N00(2OH)2][C0CO2] and NC1-2-[N00(2OH)2][C1CO2] 1
liquid ratio 0.15 g mL−1), concentration values of extracted phycobiliproteins (2.22–3.65 g L−1) from S. platensis using individually [N00(2OH)2][C1CO2], NC1-2-[N00(2OH)2][C1CO2] or a buffer solution did not differ substantially at a 95% confidence level. However, when a mixture of NC1-2-[N00(2OH)2][C0CO2] and NC1-2-[N00(2OH)2][C1CO2] 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) was applied under the same optimum conditions, an increase to 3.99 g L−1 in phycobiliprotein extraction was obtained. The extraction employing the IL mixture performed somewhat better than [N00(2OH)2][C0CO2], although there was again no statistical difference between these solvents at the 95% level of significance.68 In summary, this work shows that ILs/PILs are not always the best option to extract proteins from vegetable biomass compared to more conventional solvents and salt aqueous solutions.
1 (v/v) was applied under the same optimum conditions, an increase to 3.99 g L−1 in phycobiliprotein extraction was obtained. The extraction employing the IL mixture performed somewhat better than [N00(2OH)2][C0CO2], although there was again no statistical difference between these solvents at the 95% level of significance.68 In summary, this work shows that ILs/PILs are not always the best option to extract proteins from vegetable biomass compared to more conventional solvents and salt aqueous solutions.
PILs, namely [N00(2OH)2][C0CO2], [N00(2OH)2][C1CO2], and their mixture, were also used for the UAE of phycobiliproteins from S. platensis.69 Extraction conditions applied with ultrasound were 25 kHz and 25 °C for 30 min. A Design Composite Central Rotational experimental design was used to determine the impact of several parameters on the extraction of phycobiliproteins, which was maximum with the mixture [N00(2OH)2][C1CO2] + [N00(2OH)2][C0CO2] (0.75 ± 0.01 g L−1). This work contradicts the previously discussed findings in which no advantages were obtained with the use of PILs.69 One additional advantage of using PILs is their easy recovery and reuse. After the induced precipitation of phycocyanins using ammonium sulphate ((NH4)2SO4), later removed by dialysis, the same IL was reused in 3 additional extraction cycles.69
ABS were also applied to extract phycobiliproteins from S. platensis. These were constituted by [C6C1im]Br, [C8C1im]Br or [C4C1im]Br and potassium salts (tripotassium phosphate, K3PO4, K2HPO4, potassium carbonate, and K2CO3).58 The effect of pH, loading volume, algae concentration, temperature, and alkyl chain length of the IL was investigated. An extraction efficiency of 99%, a partition coefficient of 36.6, and a purification factor for phycocyanins (one phycobiliprotein) of 5.8 were obtained at an optimal pH value of 7 and 34.85 °C.58 This work is a clear example on the use of ABSs as a simultaneous extraction and purification technique. Due to the adequate pH range of phosphate buffers, ABS comprising ILs/K2HPO4 were further used for phycocyanin extraction.58 The order of ILs when used in ABSs in terms of extraction efficiency for phycocyanins was the following: [C8C1im]Br > [C6C1im]Br > [C4C1im]Br. Extraction efficiencies increased up to 90% with a purification factor of 5.8-fold using the ABS formed by [C8C1im]Br and K2HPO4. In the same line, Zhang et al.70 evaluated the partition of the protein phycocyanin in an ABS composed of K2HPO4 and [C4C1im]Cl after their extraction through successive freeze and thawing of a Spirulina powder in contact with 20 mmol L−1 Tris–HCl buffer. The effect of multiple process parameters, such as salt, IL and crude algae concentration, pH, and temperature, was studied. It was found that the phycocyanin purity in the top phase increased two times with the increase in the [C4C1im]Cl concentration up to 23 wt%, with the concentration of KH2PO4 kept at 29 wt%. The extraction efficiency continued increasing even above 23% of IL; however, the purity of phycocyanin decreased. The π–π interactions between the imidazolium cation and the aromatic residues of the proteins have been described as the driving force behind the extraction of phycocyanins.70 Moreover, electrostatic interactions seem to play also a role in the partitioning of phycocyanin, with a correlation found with the manipulation of the pH value.70 The pH value changed the partitioning behaviour, resulting in a lower purity in systems with pH 5.0, pH 6.0, pH 8.0, and pH 9.0 in comparison with pH 7.0.70 Although larger amounts of crude phycocyanin cause its accumulation in the interphase, increasing crude algae concentration from 1 to 3 mg L−1 increased the extraction efficiency and purity of phycocyanin. Further, with the temperature increase from 25 °C to 30 °C, the extraction efficiency of phycocyanin increased from 90.15% to 93.03%.70 Nevertheless, when the temperature was higher than 30 °C, the extraction yield and purity factor decreased from ∼93% to ∼92% and from ∼3.5 to 3.0, respectively.70 This behaviour could be due to stability concerns. Overall, the ABS composed of [C8C1im]Br and K2HPO4 seem to be a promising system for the extraction of phycobiliproteins due to its successful application in the separation of phycocyanin from Spirulina microalgae.58,70
Martins and co-workers37 investigated the influence of different IL families, namely imidazolium, pyridinium, pyrrolidinium, piperidinium, phosphonium, quaternary ammonium, and cholinium-based ILs, on the SLE of phycobiliproteins from red macroalgae Gracilaria sp. The effect of the [C4C1im]+ cation combined with the following anions: Cl−, [N(CN)2]−, [Tos]−, [(C1)2PO4]−, [SCN]−, [CF3SO3]−, [C1SO3]−, [CF3CO2]− and [C1CO2]− was also evaluated. The authors found that the solid–liquid ratio, IL concentration, alkyl chain length, IL cation, and IL anion play a role in extracting phycobiliprotein pigments.37 The results show that more hydrophilic ILs extract phycobiliproteins better, while ILs with lower hydrophilicity are better at extracting chlorophylls and carotenoids.37 Among the investigated ILs, the [Ch]Cl aqueous solution 0.1 M allowed extraction of 46.5% of proteins while avoiding the extraction of chlorophylls.37 From the same research group, and maintaining the biomass, another study focused on purifying R-phycoerythrin. The authors extracted phycobiliproteins from Gracilaria sp. using water and applied an aqueous micellar two-phase system (AMTPS) composed of Tergitol 15-S-7 and McIlvaine buffer with ILs as adjuvants for the purification of the target protein.6 AMTPS are liquid–liquid systems constituted by water and a surfactant.71 The evaluated ILs were the following: [C16py]Br, [N1112(C7H7)]Br, [C14mim]Cl, [C16mim]Cl, [C14im-6-C14im]Br2, [Ch][C9CO2], [Ch][C13CO2], [P44414]Cl.
During the purification process optimization, it was observed that R-phycoerythrin partitioned to the surfactant-poor phase (extraction efficiencies >60%). Furthermore, with the increasing surfactant concentration, the purity of the protein also increased. The best AMTPS for the proposed goal was the [N1,1,12,(C7H7)]Br-based one, which preserved the structural integrity of the protein. Finally, the authors recovered and reused the surfactant through ultrafiltration, developing a two-step purification process for R-phycoerythrin, where the surfactant-poor phase from the first AMTPS was re-applied in the second step.71 Food supplements and healthy diet products can be enriched with the green microalgae Chlorella.75 In this line, IL aqueous solutions were applied for the cell wall lysis and extraction of intracellular proteins from Chlorella pyrenoidosa. Wang et al.73 attempted to extract proteins by exposing the microalgae to a low-temperature and high-pressure environment to achieve cell lysis. The extraction efficiency for the used ILs follows the cation order: [N011(3N)]+ > [N0444]+ > [N0222]+ > [N0022]+, and the anion order: [C0CO2]− > [C1CO2]− > [CF3CO2]−. The system comprising [N011(3N)][C0CO2] showed the best extraction efficiency, 12.1%. Two additional extraction methods were compared, namely freeze–thawing and ultrasonication, with extraction efficiencies of 3.2% and 16%, respectively. Combining freeze–thawing with ultrasonication the protein extraction increased to 22.9%.73
Total protein extraction from Nannochloropsis sp. powder was reported in the work of Motlagh et al.74 The authors evaluated the following ILs: [N1111]Cl, [Ch][C1CO2], [C2C1im]Cl, [C4C1im]Cl, [N4444][Tf2N], and [NC4pyr]Cl. The best extraction conditions found were 0.5 g of biomass, 2% (w/v) of [Ch][C1CO2] at 40 °C for 30 min. Under these conditions, an extraction yield of 26.35% was achieved, which is more significant than the yield obtained with Soxhlet extraction performed with hexane (0.63%).
In addition to ILs, DESs were also used as extraction agents of proteins from algal biomass. Rathnasamy et al.72 evaluated various ABSs comprising DESs, namely Glu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gly (1
Gly (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), Fru
1), Fru![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gly (1
Gly (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), Xyl
1), Xyl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gly (1
Gly (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), Malt
1), Malt![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gly
Gly![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (1
water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5) and Suc
0.5) and Suc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gly (1
Gly (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and different salts (Na2SO4, Na2CO3, and K2HPO4) or PEG 6000 to extract phycocyanin from Spirulina powder. The maximum recovery yield was 5.8 mg g−1, and a response surface methodology (RSM) was applied to optimize an MAE with the same ABS as the solvent. Under the optimal conditions, the amount of phycocyanin increased to 85 g mL−1. The anti-bacterial activity of the ultrapure fractions of phycocyanin was finally evaluated, showing that phycocyanin was highly active against Escherichia coli and Enterobacter aerogenes.72
1), and different salts (Na2SO4, Na2CO3, and K2HPO4) or PEG 6000 to extract phycocyanin from Spirulina powder. The maximum recovery yield was 5.8 mg g−1, and a response surface methodology (RSM) was applied to optimize an MAE with the same ABS as the solvent. Under the optimal conditions, the amount of phycocyanin increased to 85 g mL−1. The anti-bacterial activity of the ultrapure fractions of phycocyanin was finally evaluated, showing that phycocyanin was highly active against Escherichia coli and Enterobacter aerogenes.72
In summary, the most promising systems reported for the extraction of proteins from algae are mainly based on imidazolium ILs, but this is strongly biased by the limited number of studies reported so far. A trend is however foreseen, where these ILs are being replaced by greener alternatives, namely cholinium-based ILs and PILs. Despite the relevance of algae-based biomass, few studies have been found in what concerns the extraction of proteins from this source using DESs.
Plants and cereals as a source of proteins
Proteins are abundant in plants and cereals. Table 4 reports the studies identified with the use of ILs and DESs, being organized by the source of biomass and methods used.| Protein | Source | Method | IL or DES applied | References |
|---|---|---|---|---|
| Plant | ||||
| Aloe proteins | Aloe vera leaf | ABS | [C4C1im][BF4] | 76 |
| Holy basil peptides | Ocimum tenuriflorum seeds | UAE/ABS | Carn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Malt (1 Malt (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), BC 1), BC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EG (1 EG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), BC 1), BC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12BD (1 12BD (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), BC 1), BC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16HD (1 16HD (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), Carn 1), Carn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Suc (1 Suc (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), Carn 1), Carn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Lac (1 Lac (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
52 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Cereals | ||||
| Primrose proteins | Oenothera biennis cake | SLE | [Ch]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gly (1 Gly (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
79 |
| Rapeseed proteins | Brassica napus cake | SLE | [Ch]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gly (1 Gly (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
79 |
| Sodom apple protease (enzyme) | Calotropis procera rhizome | UAE | Imi![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gluc (2 Gluc (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), Imi 1), Imi![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Xyl (2 Xyl (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), Imi 1), Imi![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Malt (2 Malt (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), Imi 1), Imi![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fru (2 Fru (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), Imie 1), Imie![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Suc (2 Suc (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
21 |
| Oat proteins | Avena sativa grain | SLE | [Ch]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12BD (1 12BD (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 1 1; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2; 1 2; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3; 1 3; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 1 1; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 1 1; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), [Ch]Cl 1), [Ch]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14BD (1 14BD (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 1 1; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2; 1 2; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3; 1 3; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 1 1; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 1 1; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), [Ch]Cl 1), [Ch]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23BD (1 23BD (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 1 1; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2; 1 2; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3; 1 3; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 1 1; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; 1 1; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
80 |
| Wheat-esterase (enzyme) | Wheat flour | ABS | [C4C1im][BF4] | 14 |
Aloe vera L. has long been a source of nutraceutical, medical and cosmetic compounds.13 Aloe polysaccharides and proteins were extracted and purified simultaneously using ABSs composed of [C4C1im][BF4] and inorganic salts.76 Preliminary studies were conducted to compare the performance of several ABSs, namely PEG-, surfactant-, alcohol- and IL-based ABSs. The results obtained show that the extraction efficiency of IL-ABSs was higher than that of the other studied ABSs. Furthermore, in the range between 25 °C and 50 °C, lower temperatures resulted in higher extraction of polysaccharides and proteins. Adding an additional electrolyte to the system, namely NaCl, boosted the extraction efficiency of proteins from 75% to 95%. The best performing ABS, i.e., the one composed of [C4C1im][BF4]/NaH2PO4, was able to extract 93.12% of polysaccharides and 95.85% of proteins to the salt-rich phase and IL-rich phase, respectively.76
Esterases, which are an additional example of enzymes with a wide variety of applications, are commonly used for the degradation of natural materials and industrial pollutants. Furthermore, since these enzymes can hydrolyse esters into organophosphorus compounds, conjugates composed of esterases and monosulfonate tetraphenyl porphyrin have been reported as sensing materials.77 Jiang et al.14 found an alternative and less expensive source of plant esterase in wheat, soybean, corn, rice, and other grains. The commercial production of plant-esterase is quite costly, and developing a low-cost extraction method is thus necessary. Jiang and coworkers14 optimized the use of ABS for the separation of wheat-esterase using [C4C1im][BF4] and inorganic salts (H2PO4, K2HPO4, (NH4)2SO4, and MgSO4). Increasing the concentration of [C4C1im][BF4] did not influence the residual enzyme partitioning, unlike the enzyme distribution coefficient, which increased up to 20 wt% in comparison to the protein distribution coefficient in all cases. These results confirm that wheat esterase is more likely to distribute to the IL-rich phase than residual proteins. These differences between wheat esterase and residual proteins can be attributed to each protein's molecular weight, shape, volume, and surface area.78
Karthiraj et al.52 investigated the integration of extraction and purification of bioactive peptides from holy basil (Ocimum tenuriflorum) seeds using ABSs composed of DESs (Carn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Malt (1
Malt (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), BC
1), BC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EG (1
EG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), BC
1), BC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12BD (1
12BD (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), BC
1), BC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16HD (1
16HD (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), Carn
1), Carn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Suc (1
Suc (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), Carn
1), Carn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Lac (1
Lac (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)) and salts (K2HPO4 and Na2SO4) in ultrasound-assisted liquid-phase microextraction (UA-LPME). The peptides were obtained by enzymatic hydrolysis with pepsin before the extraction step was applied. The best DESs to form an ABS were Carn
1)) and salts (K2HPO4 and Na2SO4) in ultrasound-assisted liquid-phase microextraction (UA-LPME). The peptides were obtained by enzymatic hydrolysis with pepsin before the extraction step was applied. The best DESs to form an ABS were Carn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Malt, Carn
Malt, Carn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Suc, and BC
Suc, and BC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16HD, exhibiting a good phase ratio with Na2SO4. Regarding the partition coefficient, the enriched peptides were present in higher values in the Carn
16HD, exhibiting a good phase ratio with Na2SO4. Regarding the partition coefficient, the enriched peptides were present in higher values in the Carn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Malt, followed by Carn
Malt, followed by Carn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Lac and BC
Lac and BC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16HD enriched phases. Carn
16HD enriched phases. Carn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Malt was applied with Na2SO4 in the UA-LPME process. Under the optimal conditions, a yield of 85% (peptide recovery yield of 16 mg mL−1) was achieved.
Malt was applied with Na2SO4 in the UA-LPME process. Under the optimal conditions, a yield of 85% (peptide recovery yield of 16 mg mL−1) was achieved.
DESs were also applied in the extraction of a vegetable protein via pretreatment of evening primrose cakes (Oenothera biennis).79 The by-product obtained during oilseed processing was studied with the addition of [Ch]Cl.79 Three extraction temperatures −60 °C, 100 °C and 140 °C – were evaluated. The rise of temperature resulted in an increase of protein precipitate to over four-fold (8.4 and 34.2 g/100 g, from 60 °C to 140 °C, respectively). The authors showed the characteristic polypeptide bands in SDS-PAGE in the precipitate samples at 60 °C and 100 °C; however, some denaturation of the proteins occurred at 140 °C.79 This work also reported that evening primrose cake proteins could be an alternative to soy proteins, the most currently used protein vegetable source.
DESs combining imidazole and carbohydrates (glucose, fructose, xylose, maltose, and sucrose) were used to extract protease (calotropin) from sodom apple in a two-step process.21 After addressing the density, viscosity, conductivity and refractive index of the studied DESs, their phase-forming ability with PEG (4000, 6000, 8000) and salts (Na2SO4, K2HPO4, and Na2CO3) for the formation of ABSs was evaluated. Optimized conditions for the extraction of calotropin were obtained using RSM, and then the selective partitioning of protease was achieved by ultrasonic irradiation at 35 °C for 10 min, followed by phase separation using centrifugation. Additionally, the obtained DES-rich phase was recovered and reused by back extraction with NaCl aqueous solution (15% w/v).21 A maximum protein recovery was obtained using UA, namely 98 μg mL−1 (of sodom apple latex).21
More recently, Yue et al.80 reported an extraction method for oat proteins using DESs without pH adjustment. The extraction was performed in DESs (formed from [Ch]Cl and different isomers of butanediol (12BD; 14BD; and 23BD)) and in DES/water binary mixtures. Among the initial eighteen formulations, six of them were identified as the best to extract proteins from fresh oat under the optimal conditions (90 min of extraction at 80 °C). The results obtained were independent of the presence of water. Moreover, it was observed that the proteins extracted with DESs presented a higher amount of hydrophilic amino acid residues, whereas, with the addition of water, the presence of hydrophobic amino acid residues increased. The authors discussed that the extracted proteins presented a rigid structure that the addition of water could prevent. Furthermore, it was found that the alcohol isomer can influence the flexibility/stability of the protein structure. The extract obtained with the [Ch]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14BD
14BD![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water ternary mixture (1
water ternary mixture (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) presented a higher protein content and better solubility and stability.80
1) presented a higher protein content and better solubility and stability.80
Contrary to what was observed with algae, DESs have been mainly used for extracting proteins from plants and cereals, with [Ch]Cl as the main HBA investigated. Despite the promising results reported, further research using ILs should be conducted to confirm as well the efficiency of these solvents in the extraction and purification of proteins from plants and cereals. The unique IL investigated to date is [C4C1im][BF4], which has been shown to be unstable in water and has been raising some environmental and health concerns.81 Given the plethora of ILs available today, biobased ILs must be investigated in this field as well.
Fruits and vegetables as a source of proteins
The main constituents of fruits and vegetables are hydrocarbons, relatively small amounts of proteins and fat, and a substantial amount of water (80–90%).82 However, latex has been reported as a protein-rich source found in thousands of plant species.83,84 For instance, biologically active compounds from papaya latex can be applied for milk-clotting, meat tenderization, antimicrobial and wound healing, among others.15,85,86 Another example is bromelain, an enzyme extracted from pineapple stem that can be used as a food supplement and in pharmaceutical formulations.87 Anti-inflammatory, antithrombotic, antiedematous, and fibrinolytic activities have been reported for bromelain, and since it inhibits both platelet aggregation and the proliferation of tumour cells, bromelain could have a medical application.88,89Table 5 summarizes the published studies with ILs and DESs in the extraction of proteins from fruits and vegetables.| Protein | Source | Method | IL or DES applied | References |
|---|---|---|---|---|
| Fruit | ||||
| Seabuckthorn berry proteins | Seabuckthorn seed meal (residue) | SLE | [Ch]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gly (1 Gly (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), [Ch]Cl 2), [Ch]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OA (1 OA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), [Ch]Cl 1), [Ch]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) urea (1 urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
93 |
| Pomegranate proteins | Punica granatum peels (residue) | PLE, UAE | [Ch]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) urea, [Ch]Cl urea, [Ch]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EG, [Ch]Cl EG, [Ch]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gly, [Ch]Cl Gly, [Ch]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AA, [Ch]Cl AA, [Ch]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gluc, [Ch]Cl Gluc, [Ch]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sorb, [Ch]Cl Sorb, [Ch]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CA, [NaOA] CA, [NaOA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) urea (all in a molar ratio of 1 urea (all in a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, where water is the 3rd component) 10, where water is the 3rd component) |
94 |
| Papain (enzyme) | Carica papaya – peels latex (residue) | ABS | [P4444]Br; [N1111]Br, [N2222]BF4, [N2222]Br, [N2222]Cl, [N4444]Br | 90 and 91 |
| Stem bromelain (enzyme) | Ananas comosus stem (residue) | ABS (AMTPS) | [C10C1im]Cl, [C12C1im]Cl, [C14C1im]Cl, [P66614][C9CO2], [P66614][TMPP], [P66614]Br | 7 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Vegetable | ||||
| RuBisCO (enzyme) | Spinach leaves | SLE | [Ch]Cl, [Ch]Br, [Ch][Ac], [Ch][DHC], [Ch][H2PO4], [C2mim]Cl, [C4mim]Cl, [C6mim]Cl, [Pr3NC2OC2][Sac], [Et3NC2OC2][Sac], [Et3NC4NC4]Br, [Bu3NC4NC4]Br | 22 |
| Protease (enzyme) | Zingiber officinale rhizomes | UAE | Imi![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gluc (2 Gluc (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), Imi 1), Imi![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Xyl (2 Xyl (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), Imi 1), Imi![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Malt (2 Malt (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), Imi 1), Imi![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fru (2 Fru (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), Imi 1), Imi![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Suc (2 Suc (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
21 |
| Radish peroxidase (enzyme) | Entire Raphanus sativus | ABS | [N002OH][C4CO2], [N002OH][C1CO2], [N002OH][C2CO2], [N00(2OH)2][C2CO2], [N002OH][C3CO2], [NC1– N002OH][C2CO2] | 20 |
Rathnasamy et al.90 attempted the extraction of papain, an enzyme derived from papaya latex (Carica papaya L.), using an ABS constituted by [P4444]Br and K2HPO4. The partition of papain from its crude fruit latex and the use of a conventional ABS were also addressed. Raising IL concentrations to an optimal amount of 150 mM led to a favourable extraction of papain. Furthermore, the increase in pH from 5 to 7.5 positively influenced the partition coefficient of papain and eliminated undesired proteins, leading to an improved purification factor in the IL-rich phase of the ABS. On the other hand, additional increases in pH above 7.5 decreased the partition coefficient of papain. The authors also found that temperatures between 20 °C and 30 °C favour papain extraction. Overall, the [P4444]Br-based ABS resulted in a yield of 96.22% and a purity factor of 9.55-fold. Also envisioning the separation of this proteolytic enzyme from papaya latex, Yu et al.91 optimized an ABS composed of PEG + NaH2PO4, but using ILs as adjuvants. Box–Behnken design and response surface methodology (BBD-RSM) were used to obtain the best conditions for papain separation.91 The most efficient system identified used 3 wt% of [N2222][BF4] as an adjuvant. The purification factor, estimated based on papain's initial specific activity, was 3.177, which was 3.0-fold more than what was obtained with the ABS without ILs (1.056). The recovered enzyme activity was 97.37%, which was 16.73% higher than the enzyme activity without the presence of ILs (80.64%). In summary, the authors demonstrated that using ILs resulted in better outcomes than using traditional methods.91
Pineapple wastes, including peel, core, leaves and crown, contain bromelain, a mixture of different cysteine proteinases.92 Bromelain mainly corresponds to pineapple stem bromelain (80%), pineapple fruit bromelain (10%) and ananain (5%), which are all found in pineapple stem. AMTPS comprising 0.3 wt% of phosphonium-based ILs were applied for the extraction of bromelain from pineapple stem. These systems showed previously an outstanding ability to maintain the pure enzyme native conformation and biological activity.7 However, in the assays with pineapple stem, a decreasing tendency of bromelain activity was observed in the following order of AMTPS: without IL > [P66614]Br > [P66614][TMPP] > [P66614]Dec > [C10C1im]Cl > [C14C1im]Cl > [C12C1im]Cl. The results clearly demonstrate a tendency among the studied IL families, namely (i) imidazolium-based ILs negatively interact with the enzyme and cause a sudden loss of activity, with values falling below 0.200, and (ii) phosphonium-based ILs led to a moderate rise in bromelain activity. The increase in the enzyme activity was achieved with only 0.3 wt% of IL.7
The SLE extraction of ribulose-1,5-biphosphate carboxylase/oxygenase (RuBisCO) from spinach leaves was studied using several IL aqueous solutions ([Ch]Cl, [Ch]Br, [Ch][C1CO2], [Ch][DHC], [Ch][DHP], [C2C1im]Cl, [C4C1im]Cl, [C6C1im]Cl, [Pr3NC2OC2][Sac], [Et3NC2OC2][Sac], [Et3NC4NC4]Br, [Bu3NC4NC4]Br).22 The authors applied a response surface methodology (RSM) to optimize the conditions for RuBisCO extraction. Under these conditions, extraction yields of 10.92 and 10.57 mg of RuBisCO per g of biomass were obtained with [Ch][Ac] and [Ch]Cl, respectively. Comparing the extraction performance obtained with [Ch][C1CO2] and [Ch]Cl with an extraction performed with ammonium hydroxide (NH4OH), it was observed that the secondary structure of RuBisCO is better preserved in IL solutions, highlighting the better performance afforded by ILs when compared to traditional solvents. Lucena et al.20 employed a sustainable technique to separate radish peroxidase from Raphanus sativus. In their study, the authors tackled the use of PILs as adjuvants using ABSs. Although the addition of PILs as an adjuvant did not facilitate the ABS phase separation, it resulted in an increase in the enzyme purification factor. In a system composed of PEG and (NH4)2SO4 without adjuvants, a purification factor of 2.54-fold was observed; when implementing PILs (5 wt%) as adjuvants a purification factor as high as 19.25-fold was obtained using [N002OH][C2CO2].20
More recently, Lin et al.93 reported a study on protein extraction from Seabuckthorn seed meal (SSM), a residue from a biotechnology industry, using DESs. SSM is a residue rich in proteins with a high percentage of essential amino acids, obtained after oil extraction from Seabuckthorn berries. These proteins have substantial hypoglycemic and anti-inflammatory effects and anti-diabetic function.95 Seabuckthorn berry proteins were extracted with the DESs [Ch]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gly (1
Gly (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), [Ch]Cl
2), [Ch]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OA (1
OA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and [Ch]Cl
1) and [Ch]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) urea (1
urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), being compared to alkaline extraction. The extract obtained after the alkaline extraction was richer in protein, namely 73.1% higher than the extracts obtained with DESs. However, the extracts with DESs presented more essential amino acids and higher total amino acid contents. Furthermore, the proteins extracted with [Ch]Cl
2), being compared to alkaline extraction. The extract obtained after the alkaline extraction was richer in protein, namely 73.1% higher than the extracts obtained with DESs. However, the extracts with DESs presented more essential amino acids and higher total amino acid contents. Furthermore, the proteins extracted with [Ch]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) urea showed the highest digestibility (54.2%), as confirmed by in vitro assays.93
urea showed the highest digestibility (54.2%), as confirmed by in vitro assays.93
Envisioning the development of greener methods to extract proteins, bioactive peptides and phenolic compounds from pomegranate peels, Hernández-Corroto et al.94 studied pressurized liquid extraction (PLE) and several DESs ([Ch]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) urea, [Ch]Cl
urea, [Ch]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EG, [Ch]Cl
EG, [Ch]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gly, [Ch]Cl
Gly, [Ch]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AA, [Ch]Cl
AA, [Ch]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gluc, [Ch]Cl
Gluc, [Ch]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sorb, [Ch]Cl
Sorb, [Ch]Cl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CA, [NaOA]
CA, [NaOA]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) urea, being prepared in the proportion of 1
urea, being prepared in the proportion of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, with water as the 3rd component), as alternative solvents. After optimization, the DES [Ch]
10, with water as the 3rd component), as alternative solvents. After optimization, the DES [Ch]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AA was identified as the best, whereas to achieve the best results, a HIFU (High Intensity Focused Ultrasound probe) with an amplitude of 60% should be used for 11 min. Further, the extracts were digested with proteolytic enzymes and their antioxidant, hypocholesterolemia, and antihypertensive capacities were evaluated. Overall, extracts and hydrolysates with high antioxidant and hypocholesterolemic properties were obtained using PLE. On the other hand, using DESs, a high antihypertensive capacity was observed in the hydrolysates. These results are a function of the extract composition. The hydrolysates obtained from the DES extracts have higher amounts of peptides, while those obtained using PLE present higher amounts of phenolic compounds. In the same line, Rathnasamy et al.21 developed a protease extraction method from ginger's rhizomes, which was already reported as a potential source of milk-coagulating cysteine protease, using ABSs containing DESs formed by imidazole and carbohydrates (glucose, fructose, xylose, maltose, and sucrose).96 The phase separation was assisted by various salts and PEG with different molecular weights.21 After optimizing the protein recovery through ultrasound-assisted liquid–liquid microextraction (UA-LLME) assisted by DESs, a maximum recovery was achieved with a DES concentration of 25% (v/v), a biomass concentration of 15% (w/v), an ultrasound temperature of 35 °C and an ultrasound time of 10 min.21
AA was identified as the best, whereas to achieve the best results, a HIFU (High Intensity Focused Ultrasound probe) with an amplitude of 60% should be used for 11 min. Further, the extracts were digested with proteolytic enzymes and their antioxidant, hypocholesterolemia, and antihypertensive capacities were evaluated. Overall, extracts and hydrolysates with high antioxidant and hypocholesterolemic properties were obtained using PLE. On the other hand, using DESs, a high antihypertensive capacity was observed in the hydrolysates. These results are a function of the extract composition. The hydrolysates obtained from the DES extracts have higher amounts of peptides, while those obtained using PLE present higher amounts of phenolic compounds. In the same line, Rathnasamy et al.21 developed a protease extraction method from ginger's rhizomes, which was already reported as a potential source of milk-coagulating cysteine protease, using ABSs containing DESs formed by imidazole and carbohydrates (glucose, fructose, xylose, maltose, and sucrose).96 The phase separation was assisted by various salts and PEG with different molecular weights.21 After optimizing the protein recovery through ultrasound-assisted liquid–liquid microextraction (UA-LLME) assisted by DESs, a maximum recovery was achieved with a DES concentration of 25% (v/v), a biomass concentration of 15% (w/v), an ultrasound temperature of 35 °C and an ultrasound time of 10 min.21
In summary, ILs and DESs are excellent alternative solvents to extract proteins and enzymes from non-animal sources. Despite the advantages of ILs and DES, such as their tunability, in most cases they need to be removed from the extract or from the target protein. Taking this into account, considerations relative to the environmental and economic impact of these alternative solvents are addressed below.
Despite the high number of ILs and DES already studied in the field of protein extraction from biomass, in general, the rationale for the solvent selection is not provided. The complexity of the protein structures, with a variety of amino acids possessing different structural features, makes finding the most appropriate solvent challenging. To simplify this task, computational approaches may be applied for the prediction of the thermodynamic properties and solvation ability of ILs and DESs.97 One possibility is the COnductor like Screening MOdel for Real Solvents (COSMO-RS), which was successfully used to select the best performing ILs for keratin dissolution.98,99 Liu et al.98 used COSMO-RS to select the top-ten ILs with potential for keratin dissolution, from more than six hundred ILs. The authors studied the logarithmic activity coefficients (ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) γ) of keratin models in ILs. Predictive σ-profiles, σ-potentials, and excess enthalpy were also used to analyse the keratin dissolution capability of ILs. The authors proposed imidazolium-based ILs as having a notably high capability for dissolving keratin, whereas ILs with tetrabutylammonium and tetrabutylphosphonium cations are not as effective. In addition, Qin et al.99 used COSMO-RS to predict the ln
γ) of keratin models in ILs. Predictive σ-profiles, σ-potentials, and excess enthalpy were also used to analyse the keratin dissolution capability of ILs. The authors proposed imidazolium-based ILs as having a notably high capability for dissolving keratin, whereas ILs with tetrabutylammonium and tetrabutylphosphonium cations are not as effective. In addition, Qin et al.99 used COSMO-RS to predict the ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) γ, σ-profiles, σ-potentials, and excess enthalpy between human hair model compounds and imidazolium-based ILs as the solvents. The authors screened 143 ILs; according to the results of this study, ILs with the 1-ethyl-3-methylimidazolium cation and acetate or diethylphosphate anions have the strongest dissolving ability for human hair. Overall, with the support of predictive approaches, such as COSMO-RS, it should be possible to screen a large number of ILs and DESs and predict their performance in protein dissolution before performing trial-and-error experimental assays.
γ, σ-profiles, σ-potentials, and excess enthalpy between human hair model compounds and imidazolium-based ILs as the solvents. The authors screened 143 ILs; according to the results of this study, ILs with the 1-ethyl-3-methylimidazolium cation and acetate or diethylphosphate anions have the strongest dissolving ability for human hair. Overall, with the support of predictive approaches, such as COSMO-RS, it should be possible to screen a large number of ILs and DESs and predict their performance in protein dissolution before performing trial-and-error experimental assays.
Recovery and reusability of ILs and DESs
Owing to the negligible vapor pressure of ILs and most DESs, their separation from mixtures containing a pool of various compounds, among which proteins, cannot be achieved by evaporation of the solvent. On the other hand, extracted compounds from biomass are usually non-volatile and thermo-sensitive, resulting in a challenge to separate the target compound from ILs and DESs.100 These circumstances usually lead to the addition of a molecular solvent immiscible with ILs or DESs to back-extract the target compound. Most of the ILs and DESs studied in the extraction of added-value compounds from biomass are miscible with water at room temperature.69 These hydrophilic ILs may be isolated as well from the aqueous media through novel mediated phase separations, i.e., by introducing a strong salting-out species or supercritical CO2, or through back-extraction, adsorption, or membrane-based techniques. Several authors showed the recovery and reuse of ILs and DESs, without significant impact on the extraction yields.101–104 On the other hand, surfactant-like ILs that tend to form micelles in water can be separated by force field separation or membrane-based methods.105Despite that in some cases IL environmental discharge does not intentionally occurs, their recycling is considered essential from an economical point of view for process viability. Among the studies discussed here, Rodrigues et al.69 observed that PILs are excellent solvents for the extraction of S. platensis phycobiliproteins, and one of their key benefits is their ability to be recovered and reused. Phycobiliproteins were precipitated with ammonium sulfate and the PILs were employed in a fresh extraction cycle. The same PILs were reused in three consecutive cycles, during which there was a decrease in phycocyanin and allophycocyanin yields, most likely owing to the presence of ammonium sulphate, which was not entirely eliminated by dialysis. Furthermore, the presence of ammonium sulphate in the recovered PILs promoted the precipitation of the pigments. The recovery/reuse procedure appeared to have a higher impact on phycocyanin, drastically reducing its presence in the successive steps. The allophycocyanin concentration decreased from 0.80 ± 0.03 g L−1 (1st cycle) to 0.51 ± 0.02 g L−1 (3rd cycle), while the phycoerythrin concentration remained constant (0.33 g L−1).69 In the same line, Zhang et al.70 reported the recovery and reuse of [C4C1im]Cl after the extraction of phycocyanin using an IL-based ABS. The IL-rich phase was first concentrated under reduced pressure to eliminate water before being extracted into a dichloromethane solution. The IL can be recovered and reused once the organic solvent has been removed by evaporation. This work, however, did not address the performance of the recovered IL.
Regarding DESs, reusability is still a limiting step for their industrial application due to the lack of studies in this regard. Although there were no reports on studies dealing with proteins, for certain applications, such as treating lignin and catalysis, DES recovery studies are much more advanced.104,106,107 Several methods can be applied for the recovery of DESs, such as evaporation/distillation, the addition of anti-solvents, membrane filtration, crystallization, SLE, LLE, density separation, and supercritical fluid extraction.106
In terms of applications of both ILs and DESs, evaporation/distillation is the most commonly applied method; however, the fastest and simplest method is the one that involves the addition of an anti-solvent.106
Environmental and economic considerations of ILs and DESs
ILs are appealing solvents as they have shown various advantages in a plethora of chemical and biological processes. To be used as green solvents, technoeconomic analysis and life-cycle assessment of the investigated ILs and processes must be performed. A recent life-cycle assessment study by de Jesus et al.108 highlighted significant issues about the [C4C1im][BF4] toxicity, recovery and biodegradability. The studied IL presented unsatisfactory outcomes in terms of synthesis and biodegradability when compared to hexane, tetrahydrofuran, cyclopentyl methyl ether and 2-methyltetrahydrofuran. The production of [C4C1im][BF4] uses hazardous organic solvents and produces large volumes of organic waste and contaminants, while requiring a significant amount of energy. In addition, [C4C1im][BF4] was shown to be the least biodegradable solvent from the studied set of solvents. However, in terms of recovery, it performed better than hexane, tetrahydrofuran, cyclopentyl methyl ether and 2-methyltetrahydrofuran. Overall, the life-cycle assessment study revealed that when compared to other solvents, [C4C1im][BF4] had both advantages and disadvantages.By being able to tailor the IL structures and their physicochemical properties, the corresponding environmental and health impacts can be adjusted. Many biodegradable and low-toxic ILs have been proposed in the literature, primarily cholinium-based ILs and protic ILs.109,110 Some imidazolium, piperidinium, and pyridinium ILs may also be considered as readily degradable.111 Biodegradation can be designed through the functionalization of the IL structures. In brief, adding ether, carboxyl, and hydroxyl functional groups to the alkyl chain may enhance the decomposition mechanism of the initial IL structures and contribute also to lower toxicities.112 However, the benefit of hydrophilic functionalization cannot be applicable to all IL structures. The functionalization of imidazolium-based ILs with ester or amine groups did not improve their biodegradability.113 Therefore, caution must be taken into account when defining the properties of ILs according to their molecular structure.
The risk of ILs reaching the environment is not absent, and caution is required when using them. Potential reasons for this may be attributed to the contamination of the target product, leakage by drainage sources, or leakage by solvent disposal.114 Although limited quantities of released ILs will interact with the environment, it is interesting to research and consider how each IL will interact with the environment and the possible effects on it.115 For ILs to be listed as “green” alternatives to VOCs, environmental criteria such as biocompatibility, bioaccumulation, toxicity and biodegradability need to be addressed.116 It is, therefore, essential to understand the paths of biodegradation that the ILs undergo as they decompose.117,118 Metabolites released in the biodegradation process may be more toxic and bioaccumulative in the environment than the parent IL.119 The assessment of the formation, toxicity and stability of metabolites is therefore highly recommended. Since the first reported investigation of the impact of ILs on the environment, numerous toxicity and biodegradability studies have been reported, addressing the need of re-designing ILs for lower toxicity and improved biodegradability.120–123
To date, the group of ILs which have found wide application belongs to the commonly known second generation of ILs – halogen-free and stable in the presence of air and moisture, usually prepared by alkylation of a nitrogen-bearing compound yielding a first-generation halogenated IL, followed by the exchange of halide with a different anion (metathesis).124,125 Changes in the structure of second-generation ILs led to the development of task-specific ILs – third-generation ILs.126,127 These were followed by a fourth generation which is even more biodegradable, easily prepared, based on renewable resources, and less toxic.128 Thus, anions derived from proteins, amino acids, and fatty acids, among others, combined with cations such as cholinium and glycine-betaine have been investigated.129,130
The relatively claimed high cost of ILs makes these alternative solvents less attractive to be used in the extraction of proteins from biomass. However, this picture strongly depends on the IL used and the starting material for its synthesis, and on the ability to recover and reuse the solvent by a cost-effective method. For a better understanding of which ILs and processes are more affordable, technoeconomic assessments should be performed. Fig. 4 summarizes the results of a techno-economic study performed by Rana et al.,131 addressing a range of main considerations that need to be resolved in order to make IL-based biomass pretreatment processes a functional truth:
(1) decrease in IL price and volume;
(2) increase in solid load during IL pretreatment, as techno-economic analysis suggests that higher solid loads lower both the capital expenditure and the operational expense of the process;
(3) production of biocompatible ILs or novel IL-tolerant enzyme mixtures and reducing enzyme costs;
(4) design of a successful IL recovery and product recovery technology;
(5) development of an integrated method for pretreatment, downstream processing and recovery.
The price of IL differs considerably depending on the product and the seller ($1.00–800 per kg). However, if ILs are seen as components of an industrial process, it is necessary to investigate and evaluate the cost and environmental effect of the synthetic route of ILs (at the production scale). For instance, Chen et al.132 studied biomass pretreatment using triethylammonium hydrogen sulfate ([HNEt3][HSO4]). The synthesis of this recent IL needs only a basic stoichiometric mixture of two cheap starting materials: triethylamine and sulphuric acid (no more than $2.00 per kg in ton). This is a clear example that low-cost ILs can be produced and used. Finally, it is important to highlight that most studies dealing with the extraction and purification of proteins from biomass deal with aqueous solutions of ILs instead of pure ILs, decreasing the process cost.
As for the ILs, DESs must be evaluated in terms of biocompatibility, bioaccumulation, toxicity and biodegradability. The ecotoxicity of cholinium-based DESs and organic acids was evaluated by de Morais et al.133 It was verified that they present moderate toxicity, dependent on the acid concentration. Furthermore, the authors concluded that the studied DESs are more toxic than the corresponding ILs.133 Therefore, caution must be considered when applying general considerations as DESs are greener than ILs. It depends on their chemical structures of the ILs and DES being compared. In the field of DESs, the lack of studies concerning their environmental impact is even more pronounced than with ILs, and studies in this regard are urgently needed.
DESs are usually considered more economically advantageous since they can be prepared from molecules present in nature or derived from those molecules (for example, choline, urea, glycerol, and lactic acid among others). However, caution is needed since most of the time the individual components used to prepare DESs are synthesized and not recovered from natural sources. One of the main advantages of DESs is their easiness of preparation since no reaction is required as it happens with ILs: however, their individual components may need to be synthesised. Finally, DESs are always a mixture, which may turn their recovery and reuse more difficult and expensive. Overall, based on the exposure, a deeper understanding and new developments in ILs/DES are required to demonstrate if they could become sustainable alternatives for the recovery of non-animal proteins, which is further discussed in the following section.
Analysis of strengths, weaknesses, opportunities and threats (SWOT) of ILs and DESs as alternative solvents
The strengths, weaknesses, opportunities and threats (SWOT) approach gives a good image of the applicability of extraction procedures with ILs and DESs. It also provides the best quality evidence for the scalability of alternative solvents in industry. A SWOT analysis on the application of ILs and DESs as alternative solvents for the extraction of proteins from biomass is provided in Fig. 5.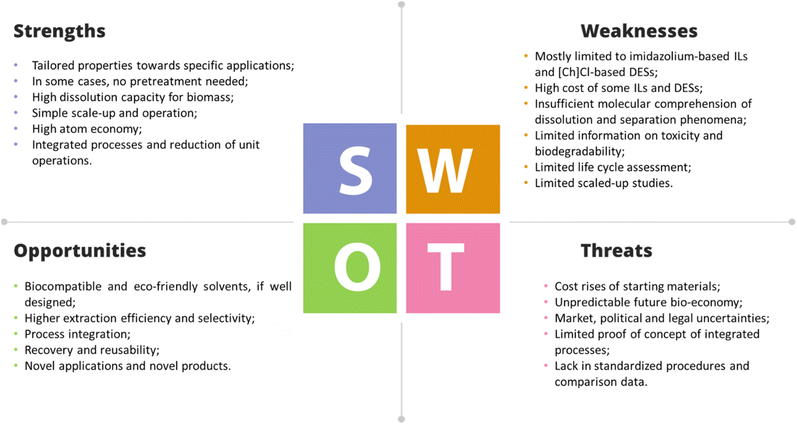 | ||
| Fig. 5 SWOT analysis on the application of alternative solvents for the extraction of proteins from biomass. | ||
Alternative solvent research in the field of biorefinery became a hot topic in 2007.134,135 Alternative solvents such as ILs and DESs can be finely tuned for a specific purpose, such as biomass protein extraction, for which they have already shown tremendous capacity. For instance, utilizing ILs can boost feed clarification, and biomolecule concentration and separation in integrated processes.26 Processes comprising liquid–liquid extractions and immobilized IL separations have been reported as having a high atom economy and other green credentials.136,137 An attractive attribute of ILs, when employed as ABS constituents, is the possibility of developing production–separation–preservation processes, which can be extended to DESs, while opening the door for viable industrial applications.138 The use of novel bio-based solvents may bring new opportunities in separation processes, avoiding hazardous and harmful conventional solvents. Using well-designed ILs and DESs with better extraction performance (e.g. extraction efficiency and selectivity) would allow the development of more sustainable processes. Still, there is the need for proof-of-concept integrated systems employing these alternative solvents, as well as standardized methods and comparison data. Moreover, a new trend of exploring the use of high pressure in extraction processes with ILs has already been established, but not yet extensively studied for proteins.73 However, there is potential for the widespread adoption of high-pressure extraction methods for proteins, along with innovative SLE techniques like MAE, UAE, and ABS, and by incorporating alternative solvents such as ILs and DESs.
Among the challenges of sustainable extraction are most certainly the growing prices of starting materials, along with uncertainties about the future evolution of the market, political, and legal environment, all of which must be considered when envisioning a bio-economy.
Significant advancements have been made in alternative solvent development in the last 20 years.139,140 Depending on their chemical structure, these solvents are accessible on a wide scale and may be envisioned for industrial applications.
Conclusions
Given their potential and renewable nature, biomass and related residues have progressively been more utilized throughout the last few decades. In this field, non-animal protein products have gained more attention as a result of increased public awareness and concerns about climate change. However, these proteins are still predominantly extracted from biomass using VOCs and lengthy processes. Therefore, in order to achieve some of the United Nations Sustainable Development Goals (SDGs), there is a need of developing cost-effective extraction and purification processes for proteins from vegetable sources.In this review, we focused on alternative solvents, namely ILs and DES, for the extraction and separation of non-animal proteins with potential application in the food, cosmetic and pharmaceutical industries. This work compiled pieces of evidence on the implementation of ILs and DESs, and their aqueous solutions, in protein extraction and separation from algae, plants (e.g. aloe vera and holy basil), cereals (e.g. wheat and oat), fruits (e.g. papaya, pineapple, pomegranate, and seabuckthorn berries), and vegetables (e.g. spinach, radish, and ginger). Several IL- and DES-based approaches were discussed, namely SLE, UAE, MAE and ABS. Overall, ILs and DESs can be tailored to improve the extraction of these proteins and they can compete with traditional VOCs. However, for these alternative solvents to be labelled as “green” solvents, more studies are needed, particularly regarding their toxicity and biodegradability, as well as in their recovery and reuse to decrease the environmental impact and costs of the developed processes. In light of the mentioned requirements, it is recommended that future research prioritizes technoeconomic analysis and life-cycle assessment. This should aim to enhance the accessibility of data, deepen the understanding regarding their environmental impact and toxicity, encourage sustainable design practices, explore options for end-of-life management, and tackle potential regulatory issues. Undertaking these efforts will certainly contribute to a more thorough and precise evaluation of ILs and DESs, while facilitating well-informed decision-making concerning their sustainable use in the long run. In addition to this, predictive models, such as COSMO-RS, can push forward the research in this field, while minimizing trial-and-error experimental assays.
To promote the practical implementation of ILs and DESs, the participation of several sectors of society is essential: academia to progress the development of greener ILs/DESs and processes; the industry that from now on has even more responsibility for the environment and if partnerships between industry and academia exist, it can be a profitable situation for both; the politics, responsible for legislation that determines how the future biorefineries can operate; and lastly, the citizens because proteins from vegetable sources should be the preferred choice over animal-derived ones.
Overall, with this review, we demonstrate the potential that non-animal biomass offers for protein production, exposing the current obstacles and opportunities, while highlighting the need to expand related studies.
Author contributions
Bojan Kopilovic and Ana I. Valente: data collection, writing and editing the original draft. Ana M. Ferreira, Mafalda R. Almeida and Ana P. M. Tavares: writing-review & editing, review, editing and manuscript checking. Mara G. Freire and João A. P. Coutinho: conceptualization, review, editing, manuscript checking and funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was developed within the scope of the project CICECO-Aveiro Institute of Materials, UIDB/50011/2020, UIDP/50011/2020 & LA/P/0006/2020, financed by national funds through the FCT/MEC (PIDDAC). This work was funded by the PRR - Recovery and Resilience Plan and by the NextGenerationEU funds at Universidade de Aveiro, through the scope of the Agenda for Business Innovation “InsectERA” (Project no. 20 with the application C644917393-00000032). Ana M. Ferreira and Ana P. M. Tavares acknowledge the FCT for the research contracts CEECIND/00361/2022 and CEECIND/2020/01867, respectively. Bojan Kopilovic and Ana I. Valente acknowledge the FCT for the PhD grants SFRH/BD/06481/2020 and SFRH/BD/08352/2021, respectively.References
- N. Alexandratos, J. Bruinsma, N. Alexandratos and J. Bruinsma, World agriculture towards 2030/2050: the 2012 revision, 2012, DOI:10.22004/AG.ECON.288998.
- A. H. Møller, M. Hammershøj, N. H. M. dos Passos, H. Tanambell, L. Stødkilde, M. Ambye-Jensen, M. Danielsen, S. K. Jensen and T. K. Dalsgaard, J. Agric. Food Chem., 2021, 69, 14341–14357 CrossRef PubMed.
- G. Franca-Oliveira, T. Fornari and B. Hernández-Ledesma, Processes, 2021, 9, 1626 CrossRef CAS.
- C. Liu, Y. Cao, W. Sun, T. Zhang, H. Wu, Q. Liu, Z. Rao and Y. Gu, RSC Sustainability, 2023, 1, 270–281 RSC.
- Y. Huang, Y. Wang, X. Guan, B. Shi, X. Wang, X. Chen, A. Fernando and X. Liu, RSC Sustainability, 2023, 1, 261–269 RSC.
- F. A. Vicente, I. S. Cardoso, M. Martins, C. V. M. Gonçalves, A. C. R. V. Dias, P. Domingues, J. A. P. Coutinho and S. P. M. Ventura, Green Chem., 2019, 21, 3816–3826 RSC.
- F. A. Vicente, L. D. Lario, A. Pessoa and S. P. M. Ventura, Process Biochem., 2016, 51, 528–534 CrossRef CAS.
- K. S. H. Eldiehy, P. Bardhan, D. Borah, M. Gohain, M. Ahmad Rather, D. Deka and M. Mandal, Fuel, 2022, 324, 124773 CrossRef CAS.
- J. C. F. Nunes, M. R. Almeida, J. L. Faria, C. G. Silva, M. C. Neves, M. G. Freire and A. P. M. Tavares, J. Solution Chem., 2022, 51, 243–278 CrossRef CAS.
- J. Zhao, G. Zhou, T. Fang, S. Ying and X. Liu, RSC Adv., 2022, 12, 16517–16529 RSC.
- C. McReynolds, A. Adrien, N. Castejon and S. C. M. Fernandes, Green Chem. Lett. Rev., 2022, 15, 383–404 CrossRef CAS.
- N. Yadav and P. Venkatesu, Phys. Chem. Chem. Phys., 2022, 24, 13474–13509 RSC.
- X. L. Chang, C. Wang, Y. Feng and Z. Liu, J. Food Eng., 2006, 75, 245–251 CrossRef CAS.
- B. Jiang, Z. Feng, C. Liu, Y. Xu, D. Li and G. Ji, J. Food Sci. Technol., 2015, 52, 2878–2885 CrossRef CAS PubMed.
- K. Hafid, J. John, T. M. Sayah, R. Domínguez, S. Becila, M. Lamri, A. L. Dib, J. M. Lorenzo and M. Gagaoua, Int. J. Biol. Macromol., 2020, 146, 798–810 CrossRef CAS PubMed.
- P. Chaiwut, P. Pintathong and S. Rawdkuen, Process Biochem., 2010, 45, 1172–1175 CrossRef CAS.
- S. Nitsawang, R. Hatti-Kaul and P. Kanasawud, Enzyme Microb. Technol., 2006, 39, 1103–1107 CrossRef CAS.
- S. Ketnawa, et al. , Asian J. Food Agro-Ind., 2009, 457–468 Search PubMed.
- Q. Cao, L. Quan, C. He, N. Li, K. Li and F. Liu, Talanta, 2008, 77, 160–165 CrossRef CAS PubMed.
- I. V. Lucena, I. V. Brandão, S. Mattedi, R. L. Souza, C. M. F. Soares, A. T. Fricks and Á. S. Lima, Fluid Phase Equilib., 2017, 452, 1–8 CrossRef CAS.
- H. B. Balaraman and S. K. Rathnasamy, Microchem. J., 2019, 150, 104132 CrossRef CAS.
- A. I. Valente, A. M. Ferreira, M. R. Almeida, A. Mohamadou, M. G. Freire and A. P. M. Tavares, Sustainable Chem., 2021, 3, 1–18 CrossRef.
- C. A. Suarez Ruiz, C. van den Berg, R. H. Wijffels and M. H. M. Eppink, Sep. Purif. Technol., 2018, 196, 254–261 CrossRef CAS.
- Advances in Protein Chemistry and Structural Biology, ed. R. Donev, Academic Press is an imprint of Elsevier, Waltham, MA, 2015, vol. 101 Search PubMed.
- A. Sanchez-Fernandez, S. Prevost and M. Wahlgren, Green Chem., 2022, 24, 4437–4442 RSC.
- R. Patel, M. Kumari and A. B. Khan, Appl. Biochem. Biotechnol., 2014, 172, 3701–3720 CrossRef CAS PubMed.
- J. S. Almeida, E. V. Capela, A. M. Loureiro, A. P. M. Tavares and M. G. Freire, ChemEngineering, 2022, 6, 51 CrossRef CAS.
- S. N. Baker, T. M. McCleskey, S. Pandey and G. A. Baker, Chem. Commun., 2004, 940–941 RSC.
- A. Biswas, R. L. Shogren, D. G. Stevenson, J. L. Willett and P. K. Bhowmik, Carbohydr. Polym., 2006, 66, 546–550 CrossRef CAS.
- H.-M. Choi and I. Kwon, Ind. Eng. Chem. Res., 2011, 50, 2452–2454 CrossRef CAS.
- S. R. Tomlinson, C. W. Kehr, M. S. Lopez, J. R. Schlup and J. L. Anthony, Ind. Eng. Chem. Res., 2014, 53, 2293–2298 CrossRef CAS.
- Z. Yang and D. A. Robb, Enzyme Microb. Technol., 1993, 15, 1030–1036 CrossRef CAS.
- Z. Yang, Y.-J. Yue and M. Xing, Biotechnol. Lett., 2007, 30, 153–158 CrossRef PubMed.
- M. B. Divya and L. Guruprasad, Spectrochim. Acta, Part A, 2020, 225, 117477 CrossRef CAS PubMed.
- M. Bisht, I. Jha and P. Venkatesu, Process Biochem., 2018, 74, 77–85 CrossRef CAS.
- P. K. Kumar, M. Bisht, P. Venkatesu, I. Bahadur and E. E. Ebenso, J. Phys. Chem. B, 2018, 122, 10435–10444 CrossRef CAS PubMed.
- M. Martins, F. A. Vieira, I. Correia, R. A. S. Ferreira, H. Abreu, J. A. P. Coutinho and S. P. M. Ventura, Green Chem., 2016, 18, 4287–4296 RSC.
- R. J. DeLange, A. N. Glazer and E. L. Smith, J. Biol. Chem., 1969, 244, 1385–1388 CrossRef CAS PubMed.
- S. Seth, D. Chakravorty, V. K. Dubey and S. Patra, Protein Expression Purif., 2014, 95, 13–21 CrossRef CAS PubMed.
- S. Wang, T. B. Ng, T. Chen, D. Lin, J. Wu, P. Rao and X. Ye, Biochem. Biophys. Res. Commun., 2005, 327, 820–827 CrossRef CAS PubMed.
- D. K. Sahoo, S. Jena, K. D. Tulsiyan, J. Dutta, S. Chakrabarty and H. S. Biswal, J. Phys. Chem. B, 2019, 123, 10100–10109 CrossRef CAS PubMed.
- Q. Qiao, J. Shi and Q. Shao, Phys. Chem. Chem. Phys., 2021, 23, 23372–23379 RSC.
- M. N. Ahmad, N. H. N. Hilmi, E. Normaya, M. A. Yarmo and K. H. K. Bulat, J. Food Sci. Technol., 2020, 57, 2852–2862 CrossRef CAS PubMed.
- R. K. Desai, M. Streefland, R. H. Wijffels and M. H. M. Eppink, Green Chem., 2014, 16, 2670–2679 RSC.
- E. Suarez Garcia, C. A. Suarez Ruiz, T. Tilaye, M. H. M. Eppink, R. H. Wijffels and C. van den Berg, Sep. Purif. Technol., 2018, 204, 56–65 CrossRef CAS.
- C. Schröder, in Ionic Liquids II, ed. B. Kirchner and E. Perlt, Springer International Publishing, Cham, 2017, pp. 127–152 Search PubMed.
- M. Z. Kamal, P. Yedavalli, M. V. Deshmukh and N. M. Rao, Protein Sci., 2013, 22, 904–915 CrossRef CAS PubMed.
- B. Rasekh, K. Khajeh, B. Ranjbar, N. Mollania, B. Almasinia and H. Tirandaz, Eng. Life Sci., 2014, 14, 442–448 CrossRef CAS.
- S. P. M. Ventura, F. A. e Silva, M. V. Quental, D. Mondal, M. G. Freire and J. A. P. Coutinho, Chem. Rev., 2017, 117, 6984–7052 CrossRef CAS PubMed.
- E. C. Achinivu, R. M. Howard, G. Li, H. Gracz and W. A. Henderson, Green Chem., 2014, 16, 1114–1119 RSC.
- B. Khadhraoui, V. Ummat, B. K. Tiwari, A. S. Fabiano-Tixier and F. Chemat, Ultrason. Sonochem., 2021, 76, 105625 CrossRef CAS PubMed.
- T. Karthiraj, B. Harish Babu and R. Senthil Kumar, Microchem. J., 2020, 157, 104883 CrossRef CAS.
- P.-Å. Albertsson, in Advances in Protein Chemistry, Elsevier, 1970, vol. 24, pp. 309–341 Search PubMed.
- K. E. Gutowski, G. A. Broker, H. D. Willauer, J. G. Huddleston, R. P. Swatloski, J. D. Holbrey and R. D. Rogers, J. Am. Chem. Soc., 2003, 125, 6632–6633 CrossRef CAS PubMed.
- C. A. Suarez Ruiz, D. P. Emmery, R. H. Wijffels, M. H. Eppink and C. van den Berg, J. Chem. Technol. Biotechnol., 2018, 93, 2774–2783 CrossRef CAS PubMed.
- S. C. Silvério, O. Rodríguez, A. P. M. Tavares, J. A. Teixeira and E. A. Macedo, J. Mol. Catal. B: Enzym., 2013, 87, 37–43 CrossRef.
- A. M. Ferreira and M. G. Freire, Bol. – Soc. Port. Quim., 2015, 139, 23 CAS.
- Y.-K. Chang, P.-L. Show, J. C.-W. Lan, J.-C. Tsai and C.-R. Huang, Bioresour. Technol., 2018, 270, 320–327 CrossRef CAS PubMed.
- J. Cichoński and G. Chrzanowski, Molecules, 2022, 27, 8852 CrossRef PubMed.
- S. T. Silveira, J. F. M. Burkert, J. A. V. Costa, C. A. V. Burkert and S. J. Kalil, Bioresour. Technol., 2007, 98, 1629–1634 CrossRef CAS PubMed.
- Z. Khan, P. Bhadouria and P. Bisen, Curr. Pharm. Biotechnol., 2005, 6, 373–379 CAS.
- A. Kulshreshtha, A. Zacharia, U. Jarouliya, P. Bhadauriya, G. Prasad and P. Bisen, Curr. Pharm. Biotechnol., 2008, 9, 400–405 CAS.
- Ma. C. Santiago-Santos, T. Ponce-Noyola, R. Olvera-Ramirez, J. Ortega-López and R. O. Cañizares-Villanueva, Process Biochem., 2004, 39, 2047–2052 CrossRef CAS.
- V. B. Bhat and K. M. Madyastha, Biochem. Biophys. Res. Commun., 2000, 275, 20–25 CrossRef CAS PubMed.
- M. C. Reddy, J. Subhashini, S. V. K. Mahipal, V. B. Bhat, P. Srinivas Reddy, G. Kiranmai, K. M. Madyastha and P. Reddanna, Biochem. Biophys. Res. Commun., 2003, 304, 385–392 CrossRef CAS PubMed.
- M. G. de Morais and J. A. V. Costa, Biotechnol. Lett., 2007, 29, 1349–1352 CrossRef PubMed.
- C.-Y. Wang, C.-C. Fu and Y.-C. Liu, Biochem. Eng. J., 2007, 37, 21–25 CrossRef CAS.
- R. D. P. Rodrigues, P. F. de Lima, R. S. de Santiago-Aguiar and M. V. P. Rocha, Algal Res., 2019, 38, 101391 CrossRef.
- R. D. P. Rodrigues, F. C. de Castro, R. S. de Santiago-Aguiar and M. V. P. Rocha, Algal Res., 2018, 31, 454–462 CrossRef.
- X. Zhang, F. Zhang, G. Luo, S. Yang and D. Wang, J. Food Nutr. Res., 2015, 3, 15–19 CrossRef CAS.
- F. A. Vicente, L. P. Malpiedi, F. A. e Silva, A. Pessoa, J. A. P. Coutinho and S. P. M. Ventura, Sep. Purif. Technol., 2014, 135, 259–267 CrossRef CAS.
- S. K. Rathnasamy, D. sri Rajendran, H. B. Balaraman and G. Viswanathan, Algal Res., 2019, 44, 101709 CrossRef.
- X. Wang and X. Zhang, Bioresour. Technol., 2012, 126, 307–313 CrossRef CAS PubMed.
- S. R. Motlagh, A. A. Elgharbawy, R. Khezri, R. Harun and R. Omar, Biomass Convers. Biorefin., 2021 DOI:10.1007/s13399-021-01972-2.
- H. J. Morris, A. Almarales, O. Carrillo and R. C. Bermúdez, Bioresour. Technol., 2008, 99, 7723–7729 CrossRef CAS PubMed.
- Z. Tan, F. Li, X. Xu and J. Xing, Desalination, 2012, 286, 389–393 CrossRef CAS.
- H. Zhiyong, Y. Yuan and Y. Lv, Food Sci., 2003,(8), 135–137 Search PubMed.
- S. Dreyer, P. Salim and U. Kragl, Biochem. Eng. J., 2009, 46, 176–185 CrossRef CAS.
- A. Grudniewska, E. M. de Melo, A. Chan, R. Gniłka, F. Boratyński and A. S. Matharu, ACS Sustainable Chem. Eng., 2018, 6, 15791–15800 CrossRef CAS.
- J. Yue, Z. Zhu, J. Yi, Y. Lan, B. Chen and J. Rao, Food Hydrocolloids, 2021, 112, 106330 CrossRef CAS.
- M. G. Freire, C. M. S. S. Neves, I. M. Marrucho, J. A. P. Coutinho and A. M. Fernandes, J. Phys. Chem. A, 2010, 114, 3744–3749 CrossRef CAS PubMed.
- N. Mirabella, V. Castellani and S. Sala, J. Cleaner Prod., 2014, 65, 28–41 CrossRef.
- B. D. Farrell, D. E. Dussourd and C. Mitter, Am. Nat., 1991, 138, 881–900 CrossRef.
- D. E. Dussourd and R. F. Denno, Ecology, 1991, 72, 1383–1396 CrossRef.
- R. N. F. Moreira Filho, N. F. Vasconcelos, F. K. Andrade, M. F. Rosa and R. S. Vieira, Colloids Surf., B, 2020, 194, 111222 CrossRef CAS PubMed.
- R. T. Basting, F. R. Gonçalves, F. M. G. França, F. L. B. do Amaral and F. M. Flório, J. Clin. Pediatr. Dent., 2016, 40, 62–68 CrossRef PubMed.
- Z. I. M. Arshad, A. Amid, F. Yusof, I. Jaswir, K. Ahmad and S. P. Loke, Appl. Microbiol. Biotechnol., 2014, 98, 7283–7297 CrossRef CAS PubMed.
- D. F. Coelho, E. Silveira, A. Pessoa Junior and E. B. Tambourgi, Bioprocess Biosyst. Eng., 2013, 36, 185–192 CrossRef CAS PubMed.
- Tropical Fruits: from Cultivation to Consumption and Health Benefits, Pineapple, ed. C. S. Bogsan and S. D. Todorov, Nova Science Publishers, New York, 2018 Search PubMed.
- S. Rathnasamy and R. Kumaresan, Int. J. Eng. Technol., 2013, 5(2), 1934–1941 Search PubMed.
- L. Yu, H. Zhang, L. Yang and K. Tian, Protein Expression Purif., 2019, 156, 8–16 CrossRef CAS PubMed.
- K. Z. Nadzirah, et al. , Int. Food Res. J., 2013, 43, 43–46 Search PubMed.
- J. Lin, H. Xiang, D. Sun-Waterhouse, C. Cui and W. Wang, Food Sci. Hum. Wellness, 2022, 11, 1028–1035 CrossRef CAS.
- E. Hernández-Corroto, M. Plaza, M. L. Marina and M. C. García, Innovative Food Sci. Emerging Technol., 2020, 60, 102314 CrossRef.
- L. M. Bal, V. Meda, S. N. Naik and S. Satya, Food Res. Int., 2011, 44, 1718–1727 CrossRef CAS.
- M. M. Hashim, D. Mingsheng, M. F. Iqbal and C. Xiaohong, Phytochemistry, 2011, 72, 458–464 CrossRef CAS PubMed.
- L. Jiřiště and M. Klajmon, J. Phys. Chem. B, 2022, 126, 3717–3736 CrossRef PubMed.
- X. Liu, Y. Nie, Y. Liu, S. Zhang and A. L. Skov, ACS Sustainable Chem. Eng., 2018, 6, 17314–17322 CrossRef CAS.
- C. Qin, H. Gao, X. Liu, X. Li, Y. Xie, Y. Bai and Y. Nie, J. Mol. Liq., 2022, 349, 118094 CrossRef CAS.
- M. M. Pereira, J. A. P. Coutinho and M. G. Freire, in Green Chemistry Series, ed. R. Bogel-Lukasik, Royal Society of Chemistry, Cambridge, 2015, pp. 227–257 Search PubMed.
- A. F. M. Cláudio, A. M. Ferreira, M. G. Freire and J. A. P. Coutinho, Green Chem., 2013, 15, 2002 RSC.
- S. Y. Lee, I. Khoiroh, T. C. Ling and P. L. Show, Sep. Purif. Technol., 2017, 179, 152–160 CrossRef CAS.
- E. Alvarez-Guerra, S. P. M. Ventura, J. A. P. Coutinho and A. Irabien, Fluid Phase Equilib., 2014, 371, 67–74 CrossRef CAS.
- A. Isci and M. Kaltschmitt, Biomass Convers. Biorefin., 2022, 12, 197–226 CrossRef.
- N. L. Mai, K. Ahn and Y.-M. Koo, Process Biochem., 2014, 49, 872–881 CrossRef CAS.
- V. Sharma, M.-L. Tsai, C.-W. Chen, P.-P. Sun, A. K. Patel, R. R. Singhania, P. Nargotra and C.-D. Dong, Bioresour. Technol., 2022, 360, 127631 CrossRef CAS PubMed.
- A. Satlewal, R. Agrawal, S. Bhagia, J. Sangoro and A. J. Ragauskas, Biotechnol. Adv., 2018, 36, 2032–2050 CrossRef CAS PubMed.
- S. S. de Jesus and R. Maciel Filho, Renewable Sustainable Energy Rev., 2022, 157, 112039 CrossRef CAS.
- M. Petkovic, J. L. Ferguson, H. Q. N. Gunaratne, R. Ferreira, M. C. Leitão, K. R. Seddon, L. P. N. Rebelo and C. S. Pereira, Green Chem., 2010, 12, 643 RSC.
- B. Peric, J. Sierra, E. Martí, R. Cruañas, M. A. Garau, J. Arning, U. Bottin-Weber and S. Stolte, J. Hazard. Mater., 2013, 261, 99–105 CrossRef CAS PubMed.
- J. Neumann, S. Steudte, C.-W. Cho, J. Thöming and S. Stolte, Green Chem., 2014, 16, 2174–2184 RSC.
- A. Jordan and N. Gathergood, Chem. Soc. Rev., 2015, 44, 8200–8237 RSC.
- R. G. Gore, L. Myles, M. Spulak, I. Beadham, T. M. Garcia, S. J. Connon and N. Gathergood, Green Chem., 2013, 15, 2747 RSC.
- Ionic Liquids in the Biorefinery Concept: Challenges and Perspectives, ed. R. Bogel-Lukasik, Royal Society of Chemistry, Cambridge, 2015 Search PubMed.
- X.-Y. Li, S.-H. Zeng, W.-H. Zhang, L. Liu, S. Ma and J.-J. Wang, Environ. Toxicol., 2013, 28, 207–214 CrossRef CAS PubMed.
- N. Sun, H. Rodríguez, M. Rahman and R. D. Rogers, Chem. Commun., 2011, 47, 1405–1421 RSC.
- K. M. Docherty, J. K. Dixon and C. F. Kulpa Jr, Biodegradation, 2007, 18, 481–493 CrossRef CAS PubMed.
- S. Stolte, S. Abdulkarim, J. Arning, A.-K. Blomeyer-Nienstedt, U. Bottin-Weber, M. Matzke, J. Ranke, B. Jastorff and J. Thöming, Green Chem., 2008, 10, 214–224 RSC.
- A. B. A. Boxall, C. J. Sinclair, K. Fenner, D. Kolpin and S. J. Maund, Environ. Sci. Technol., 2004, 38, 368A–375A CrossRef CAS PubMed.
- B. Jastorff, R. Störmann, J. Ranke, K. Mölter, F. Stock, B. Oberheitmann, W. Hoffmann, J. Hoffmann, M. Nüchter, B. Ondruschka and J. Filser, Green Chem., 2003, 5, 136–142 RSC.
- D. Coleman and N. Gathergood, Chem. Soc. Rev., 2010, 39, 600 RSC.
- M. Cvjetko Bubalo, K. Radošević, I. Radojčić Redovniković, J. Halambek and V. Gaurina Srček, Ecotoxicol. Environ. Saf., 2014, 99, 1–12 CrossRef CAS PubMed.
- M. Petkovic, K. R. Seddon, L. P. N. Rebelo and C. Silva Pereira, Chem. Soc. Rev., 2011, 40, 1383–1403 RSC.
- Green Chemistry: Fundamentals and Applications, ed. S. C. Ameta and R. Ameta, Apple Academic Press, 0 edn, 2013 Search PubMed.
- J.-M. Lévêque, J. Estager, M. Draye, G. Cravotto, L. Boffa and W. Bonrath, Monatsh. Chem., 2007, 138, 1103–1113 CrossRef.
- J. H. Davis Jr, Chem. Lett., 2004, 33, 1072–1077 CrossRef.
- C. Baudequin, D. Brégeon, J. Levillain, F. Guillen, J.-C. Plaquevent and A.-C. Gaumont, Tetrahedron: Asymmetry, 2005, 16, 3921–3945 CrossRef CAS.
- S. Şahin and E. Kurtulbaş, Biomass Convers. Biorefin., 2022, 12, 341–349 CrossRef.
- J. J. Parajó, I. P. E. Macário, Y. De Gaetano, L. Dupont, J. Salgado, J. L. Pereira, F. J. M. Gonçalves, A. Mohamadou and S. P. M. Ventura, Ecotoxicol. Environ. Saf., 2019, 184, 109580 CrossRef PubMed.
- S. Miao, R. Atkin and G. Warr, Green Chem., 2022, 24, 7281–7304 RSC.
- M. S. Rana, S. Bhushan, S. K. Prajapati, Preethi and S. Kavitha, in Food Waste to Valuable Resources, Elsevier, 2020, pp. 325–342 Search PubMed.
- L. Chen, M. Sharifzadeh, N. Mac Dowell, T. Welton, N. Shah and J. P. Hallett, Green Chem., 2014, 16, 3098–3106 RSC.
- P. de Morais, F. Gonçalves, J. A. P. Coutinho and S. P. M. Ventura, ACS Sustainable Chem. Eng., 2015, 3, 3398–3404 CrossRef CAS.
- A. Stark, Energy Environ. Sci., 2011, 4, 19–32 RSC.
- H. Passos, M. G. Freire and J. A. P. Coutinho, Green Chem., 2014, 16, 4786–4815 RSC.
- Green Solvents II: Properties and Applications of Ionic Liquids, ed. A. Mohammad and Dr Inamuddin, Springer Netherlands, Dordrecht, 2012 Search PubMed.
- Handbook of Green Chemistry, ed. P. T. Anastas and R. H. Crabtree, Wiley-VCH, Weinheim, 2009 Search PubMed.
- M. V. Quental, A. Q. Pedro, P. Pereira, M. Sharma, J. A. Queiroz, J. A. P. Coutinho, F. Sousa and M. G. Freire, ACS Sustainable Chem. Eng., 2019, 7, 9439–9448 CrossRef CAS.
- J. Liu, X. Li and K. H. Row, J. Mol. Liq., 2022, 362, 119654 CrossRef CAS.
- T. Welton, Biophys. Rev., 2018, 10, 691–706 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2023 |